Abstract
Objective:
Neutrophil-Lymphocyte Ratio (NLR) has been associated with inferior outcomes after lower extremity (LE) interventions. NLR has been associated with systemic inflammation and atherosclerotic burden. We examined NLR, severity of peripheral arterial disease (PAD) and outcomes following endovascular or open surgical procedures.
Methods:
Inpatients undergoing lower extremity procedures (2008 to 2016) were selected from Cerner HealthFacts® database using ICD-9 procedure codes. Disease severity was grouped into claudication, rest pain and tissue loss. Outcomes were identified using ICD-9 codes. NLR was calculated pre-and postoperatively. Chi-square analysis and multivariable logistic regression were performed. A receiver-operating curve (ROC) analysis was used to determine the cutoff for preoperative (Low < 3.65; High ≥ 3.65) and postoperative (Low < 5.96; High ≥ 5.96) NLR values.
Results:
3,687 patients were evaluated; 2,183 (59%) underwent endovascular procedures and 1,504 (41%) had open procedures. Compared with black patients, claudication was more frequent in white patients (81.7% vs. 72.7%; P < .0001), and tissue loss was less common (12.9% vs. 20.9%; P < .0001). NLR values were higher for patients with tissue loss than rest pain or claudication (4.89, 4.33 and 3.11, respectively; P < .0001). Open procedures were associated with higher postoperative NLR values than endovascular procedures (6.8 vs. 5.2; P < .0001). Mean pre- and postoperative NLR were greater in patients with more severe PAD. Multivariable analysis demonstrated that preoperative high NLR was strongly associated with in-hospital death (OR 5.4, 95% CI 1.68–17.07), cardiac complications (OR 2.9, 95% CI 1.57–5.40), amputation (OR 2.5, 95% CI 1.65–3.87), renal failure (OR 1.9, 95% CI 1.18–2.93), respiratory complications (OR 1.7, 95% CI 1.09–2.76) and prolonged length of stay (OR 1.9, 95% CI 1.89–3.71).
Conclusions:
Pre- and post-operative NLR significantly increases with disease severity for PAD, providing further evidence of NLR as a biomarker of a patient’s systemic inflammatory state. After adjusting for confounders, NLR still remained strongly associated with death and other adverse outcomes following intervention for PAD. Further study of the clinical association of NLR with other vascular disorders, such as symptomatic carotid stenosis and symptomatic and ruptured aortic aneurysmal disease, is planned to guide individualized treatment to prevent stroke or aneurysm rupture.
INTRODUCTION
Peripheral arterial disease (PAD) is a disorder that ranges from asymptomatic through intermittent claudication to rest pain and tissue loss; it affects between 8 and 12 million patients in the United States.1 Arterial territory affected is highly variable, and can range from single anatomic level involvement, such as the femoropopliteal segment, presenting most commonly with intermittent claudication, to multilevel atherosclerosis that often leads to rest pain and tissue loss. The mechanisms behind the spectrum of disease severity and anatomic level of disease are unclear, although female sex, low HDL-cholesterol and absence of diabetes has been associated with larger vessel, early-onset aortoiliac occlusive disease.2 Similarly, diabetes and renal insufficiency have been associated with a more distal, or small vessel pattern of disease; tibial atherosclerosis.3 Although intermittent claudication can be a very stable chronic symptom, progression to rest pain and tissue loss—critical limb ischemia—often leads to major amputation.4
The neutrophil-lymphocyte ratio (NLR) is a simple ratio calculated by dividing the absolute neutrophil count by the lymphocyte count in a differential sample of the complete blood count.5 The ratio could be an indicator of the degree of systemic inflammation,6 which has been linked with risk factors for atherosclerosis such as diabetes mellitus, hypertension, hyperlipidemia and endothelial dysfunction.7, 8 The link between systemic inflammation and cardiovascular disease has been demonstrated in numerous reports,9–12 and the anti-inflammatory effects of statin medications have been postulated to be an important factor in cardiovascular risk reduction in addition to their lipid-lowering properties.13–15
NLR has been proposed as an inexpensive biomarker of the inflammatory balance of a patient and has been linked to poor outcome in oncologic disciplines16–18 and cardiology with respect to post-myocardial infarction19, 20 and coronary intervention.21, 22 Despite the clear association of NLR with atherosclerosis, few reports have evaluated the value of NLR in vascular disorders,23–26 although NLR has been described as a marker for mortality in patients with critical limb ischemia undergoing infrapopliteal percutaneous interventions27 and for outcomes following lower extremity amputation.23 We hypothesized that due to the increased atherosclerotic and inflammatory burden, NLR would be associated with the severity of symptoms for patients with PAD, with higher NLR for tissue loss than rest pain and claudication. Furthermore, concordant with other studies of NLR and vascular disorders, we hypothesized that a higher NLR would be associated with poor outcomes following lower extremity procedures.
METHODS
Data source
The International Classification of Disease, Ninth Edition, Clinical Modification (ICD-9-CM) diagnosis and procedure codes were used to identify patients who underwent endovascular or open surgery for PAD between 2008 and 2015 from the Cerner Health Facts® database. Health Facts is a proprietary database comprised of electronic medical records from hospitals and hospital systems that use Cerner Corporation’s electronic health record and that choose to contribute data. Cerner de-identifies and standardizes the data before including them in Health Facts using statistical methods that are compliant with the Health Insurance Portability and Accountability Act (HIPAA). Informed patient consent was not needed as the Health Facts database is completely de-identified. The study was exempted by the Institutional Review Board at the University of Missouri.
Study population.
The study included patients undergoing elective endovascular or open procedures for PAD. Patients were excluded from the study if they were less than 21 years old at admission; had admissions during which both endovascular and open procedures were performed; had no laboratory data available, had no known codes for PAD severity, or had admissions flagged as emergent or urgent.
Covariates.
Patients’ demographics (age, sex, and race), and acute and chronic problems at the index admission (e.g., chronic heart disease, diabetes, hypertension) were examined. The Agency for Healthcare Research and Quality’s (AHRQ) Clinical Classifications Software was used to group diagnosis codes into clinically relevant groups. We used ICD-9 CM codes for the encounter to calculate the Charlson Comorbidity Index.28 We separated the overall cohort into claudication, rest pain and tissue loss groups by selecting the most severe diagnosis code.
Statistical analysis.
All analyses were performed in SAS for Windows version 9.4 (SAS Institute, Cary, NC). We calculated NLR by dividing the absolute neutrophil count by the absolute lymphocyte count. Pre-operative NLR was divided into low (< 3.65) and high (≥ 3.65) and post-operative NLR into low (< 5.96) and high (≥ 5.96) values based on the results of a receiver operating characteristic (ROC) analysis that examined length of hospital stay, using mean values for pre- and postoperative periods (see Figure 1). A chi-square statistic was used to evaluate the relationship of NLR with patient characteristics, infections, acute and chronic problems, length of stay, and complications. In addition, multivariable logistic regression was used to examine the association between NLR and any infection, length of stay > 10 days, renal failure, respiratory and cardiac complications, amputation, and in-hospital mortality after adjusting for patient characteristics. We calculated odds ratios (OR) and 95% CIs and assessed model discrimination with the c-statistic (or area under the curve), where 0.5 is no better than a coin toss and 1.0 indicates a perfect fit. Model calibration over the range of risk was assessed with the Hosmer-Lemeshow goodness-of-fit chi-square test, with p-values >.05 indicating adequate fit.
Figure 1.
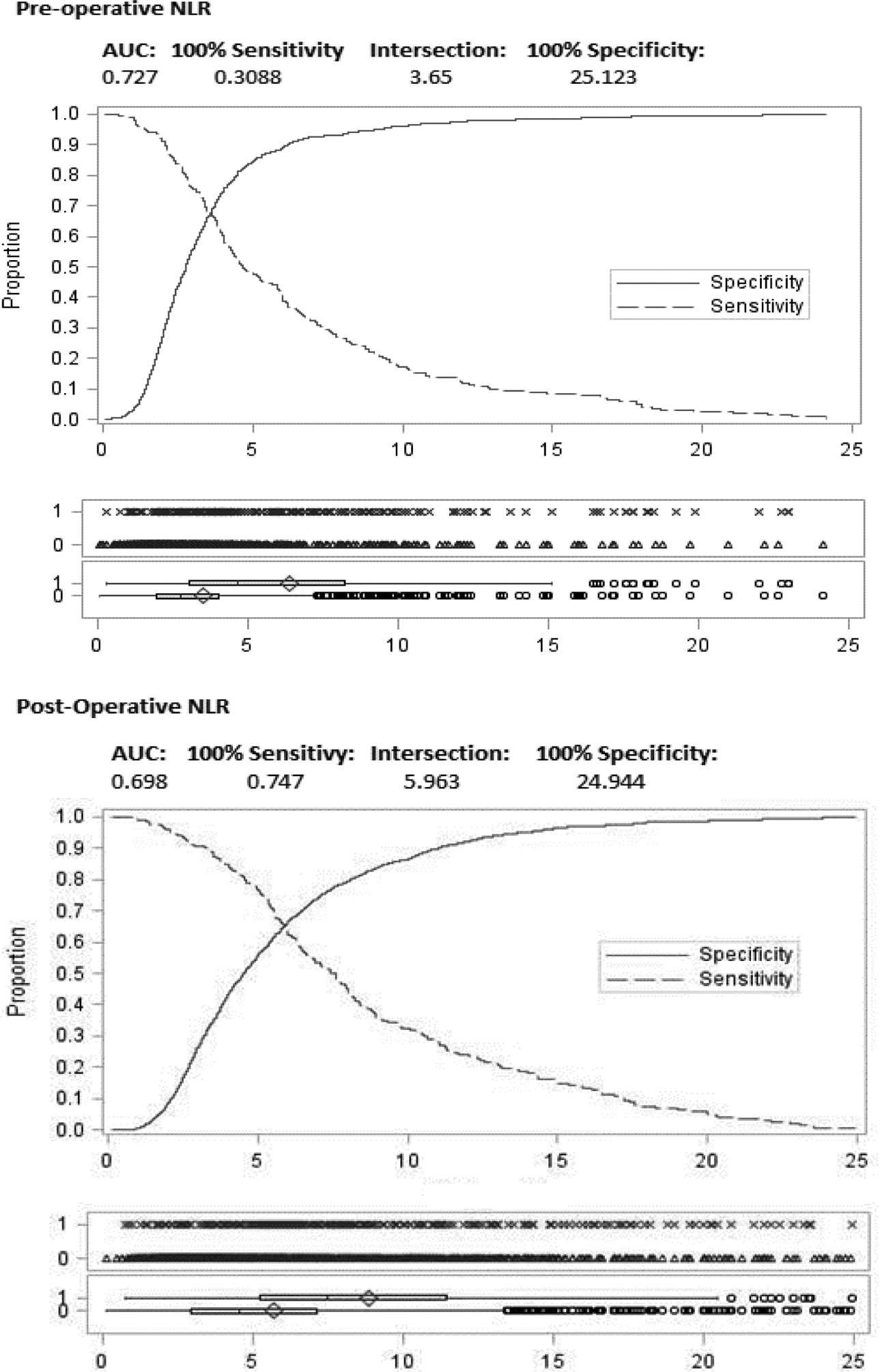
Receiver-operating curve characteristics
AUC = area under the curve; NLR = neutrophil-lymphocyte ratio
RESULTS
Demographics
A total of 3,687 patients were identified. Endovascular procedures were performed in 2,183 patients (59%) and open procedures in 1,504 patients (41%). Mean age was 68.5 and the majority of patients were male (60%) or of white race (77%). Patients were analyzed in two groups; those with preoperative NLR values and those with postoperative values and were only included if they had complete data. Outcomes refer to those occurring during the initial hospital stay.
Table 1 describes the patient sample by PAD severity. Unadjusted analysis of the groups revealed that patients with tissue loss were older than those with rest pain (71.2 vs. 68.1; P < .0001), who were in turn older than those with claudication (68.1 vs. 67; P = .02). When examining the differences in clinical presentations by race, black patients more often presented with tissue loss than with claudication (21% vs. 13%; P < .0001), whereas a greater proportion of white patients presented with claudication than tissue loss (81.7% vs. 72.7%; P < .0001).
Table 1.
Patient and encounter characteristics, by PAD severity
| PAD Severity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Claudication | Rest Pain | Tissue Lossa | |||||||
| N | % | N | % | N | % | P-valueb | P-valuec | P-valued | |
| All | 1970 | 100.00 | 534 | 100.00 | 1183 | 100.00 | -- | -- | -- |
| Age, mean (SD) | 67.0 | (10.2) | 68.1 | (10.8) | 71.2 | (11.7) | <.0001 | <.0001 | .02 |
| 21–59 | 471 | 23.91 | 119 | 22.28 | 210 | 17.75 | |||
| 60–69 | 672 | 34.11 | 178 | 33.33 | 311 | 26.29 | |||
| 70–79 | 599 | 30.41 | 149 | 27.90 | 330 | 27.90 | |||
| 80 or older | 228 | 11.57 | 88 | 16.48 | 332 | 28.06 | |||
| Race | .72 | <.0001 | .0002 | ||||||
| White | 1609 | 81.68 | 393 | 73.60 | 860 | 72.70 | |||
| Black | 256 | 12.99 | 104 | 19.48 | 248 | 20.96 | |||
| Other/unknown | 105 | 5.33 | 37 | 6.93 | 75 | 6.34 | |||
| Gender | .21 | .004 | .0005 | ||||||
| Female | 729 | 37.01 | 242 | 45.32 | 498 | 42.10 | |||
| Male | 1241 | 62.99 | 292 | 54.68 | 685 | 57.90 | |||
| Marital status | .09 | <.0001 | .08 | ||||||
| Married, life partner | 915 | 46.45 | 224 | 41.95 | 516 | 43.62 | |||
| Divorced, separated | 240 | 12.18 | 73 | 13.67 | 117 | 9.89 | |||
| Single, widowed | 594 | 30.15 | 186 | 34.83 | 448 | 37.87 | |||
| Unknown | 221 | 11.22 | 51 | 9.55 | 102 | 8.62 | |||
| Hospital bed size | .002 | .10 | .06 | ||||||
| <200 | 528 | 26.80 | 157 | 29.40 | 290 | 24.51 | |||
| 200–299 | 335 | 17.01 | 67 | 12.55 | 205 | 17.33 | |||
| 300–499 | 397 | 20.15 | 102 | 19.10 | 279 | 23.58 | |||
| 500 or more | 710 | 36.04 | 208 | 38.95 | 409 | 34.57 | |||
| Procedure type | <.0001 | <.0001 | <.0001 | ||||||
| Endovascular | 1272 | 64.57 | 232 | 43.45 | 679 | 57.40 | |||
| Open | 698 | 35.43 | 302 | 56.55 | 504 | 42.60 | |||
| Length of stay, mean (SD) | 2.27 | (4.09) | 4.10 | (4.68) | 6.39 | (7.63) | <.0001 | <.0001 | <.0001 |
| < 10 days | 1927 | 97.82 | 499 | 93.45 | 940 | 79.46 | |||
| > 10 days | 43 | 2.18 | 35 | 6.55 | 243 | 20.54 | |||
SD = Standard deviation.
Tissue loss = gangrene or ulcer
P-value based on chi-square (or t-test for continuous) comparison between PAD tissue loss (gangrene/ulcer) and rest pain.
P-value based on chi-square (or t-test for continuous) comparison between PAD tissue loss (gangrene/ulcer) and claudication.
P-value based on chi-square (or t-test for continuous) comparison between PAD rest pain and claudication..
Figure 2 demonstrates that preoperative NLR was higher in patients with rest pain than claudication (4.33 vs. 3.11; P < .0001) and higher still for those with tissue loss (4.89 vs. 3.11; P < .0001). Evaluating the trend between preoperative and postoperative NLR by procedure type and by PAD severity (Figure 3), a clear increase in NLR value is seen with increasing PAD severity (P < .0001). Open procedures were associated with higher baseline NLR values both preoperatively (4.53 vs. 3.56; P < .0001) and postoperatively (6.81 vs. 5.19; P < .0001) than endovascular procedures (Figure 3).
Figure 2.
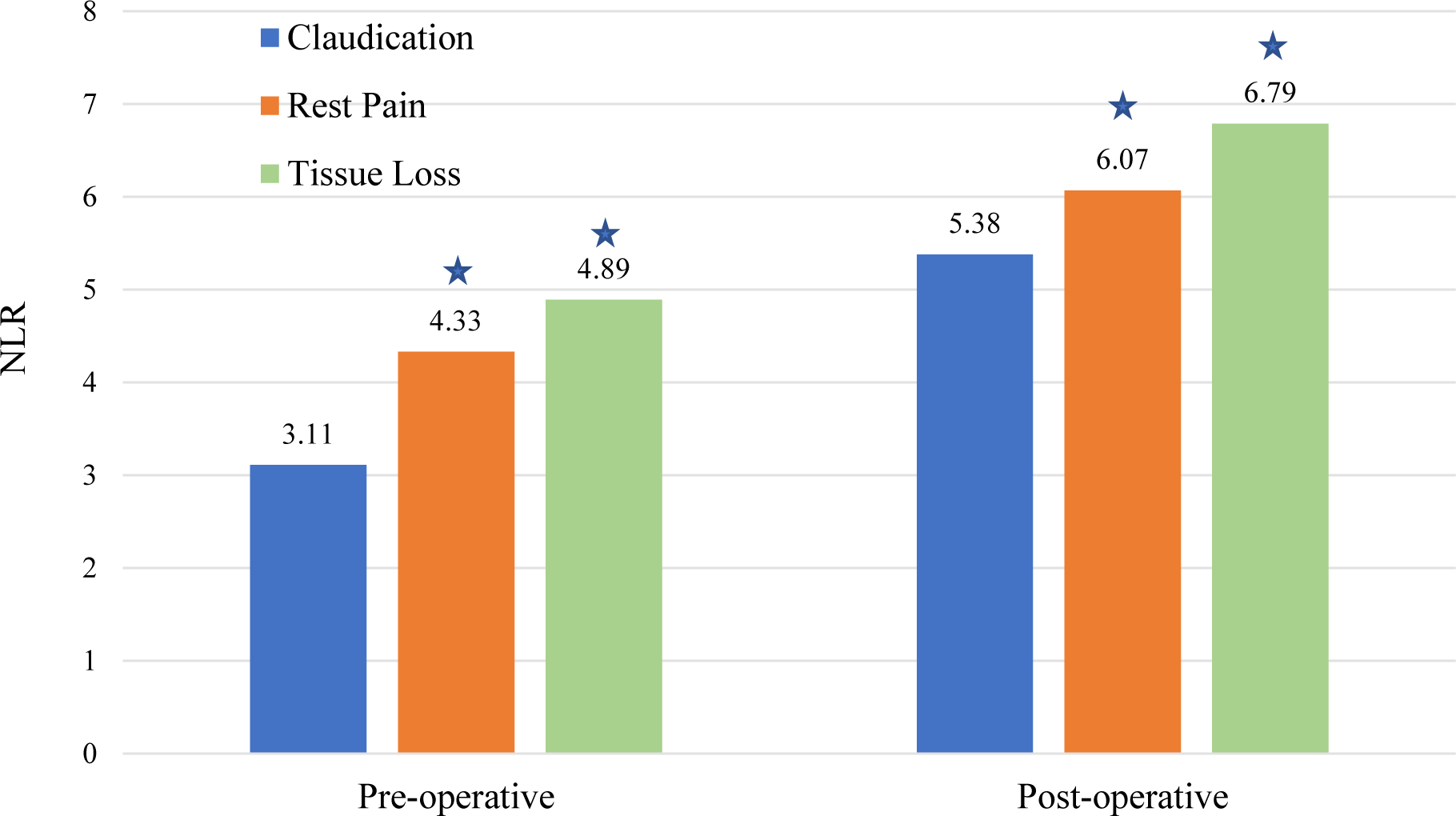
Pre- and post-operative NLR by PAD severity
 Denotes significant difference compared with claudication within each time period.
Denotes significant difference compared with claudication within each time period.
Figure 3.
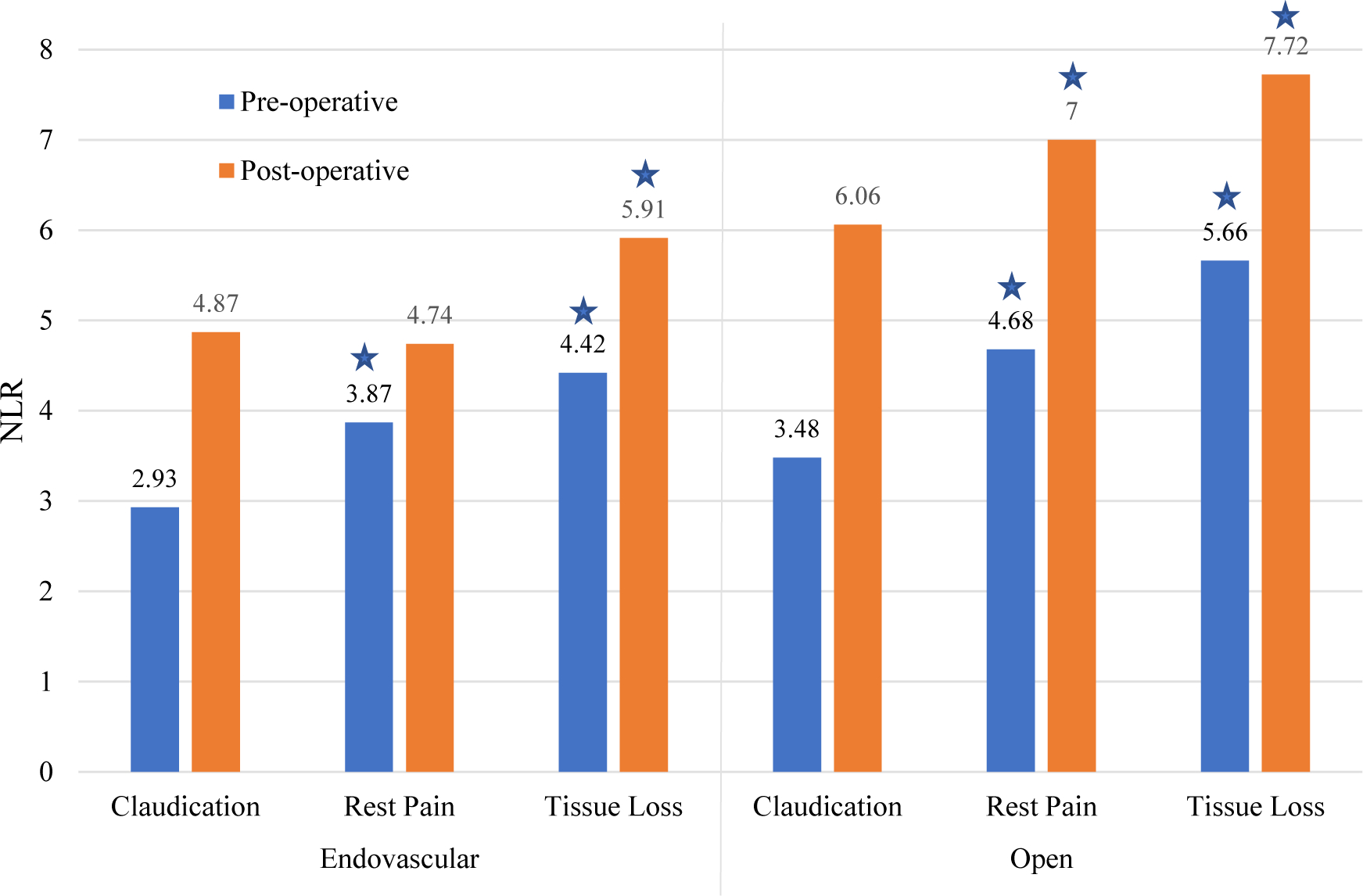
Pre- and post-operative NLR by procedure type and PAD severity.
 Denotes significant difference compared with claudication within each time period.
Denotes significant difference compared with claudication within each time period.
Table 2 details the patient characteristics for 1,455 patients with preoperative NLR levels. Unadjusted analyses demonstrate that patients with high NLR are older (70 vs. 67; p< .0001), have a higher Charlson Index (3.27 vs. 2.46; P < .0001), and more often have tissue loss (56.5% vs. 27.3%; P < .0001) compared to patients with low preoperative NLR. A higher percentage of patients with chronic kidney disease (30.6% vs. 13.7%; P < .0001) and diabetes (50.5% vs. 44.3%; P = .02) had a higher NLR. Similar findings to the preoperative NLR data were seen for the 2701 patients with postoperative NLR data (Table 3).
Table 2.
Preoperative sample characteristics by NLR level
| Overall | Low NLRa | High NLRa | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P-value | |
| Overall | 1455 | 100.00 | 922 | 63.4 | 533 | 36.6 | -- |
| Age, mean (SD) | 68.3 | (11.2) | 67.3 | (10.9) | 70.0 | (11.3) | <.0001 |
| Gender | .26 | ||||||
| Female | 557 | 38.28 | 363 | 39.37 | 194 | 36.40 | |
| Male | 898 | 61.72 | 559 | 60.63 | 339 | 63.60 | |
| Race | .72 | ||||||
| White | 1129 | 77.59 | 719 | 77.98 | 410 | 76.92 | |
| Black | 248 | 17.04 | 152 | 16.49 | 96 | 18.01 | |
| Other race | 78 | 5.36 | 51 | 5.53 | 27 | 5.07 | |
| Charlson Index, mean (SD) | 2.76 | (1.78) | 2.46 | (1.59) | 3.27 | (1.96) | <.0001 |
| Procedure type | .002 | ||||||
| Endovascular | 874 | 60.07 | 581 | 63.02 | 293 | 54.97 | |
| Open | 581 | 39.93 | 341 | 36.98 | 240 | 45.03 | |
| PAD Severity | <.0001 | ||||||
| Claudication | 712 | 48.93 | 552 | 59.87 | 160 | 30.02 | |
| Rest pain | 190 | 13.06 | 118 | 12.80 | 72 | 13.51 | |
| Tissue loss | 553 | 38.01 | 252 | 27.33 | 301 | 56.47 | |
| NLR, mean (SD) | 3.97 | (3.41) | -- | -- | -- | -- | -- |
| Pre-existing conditions | |||||||
| Chronic heart disease | 680 | 46.74 | 423 | 45.88 | 257 | 48.22 | .38 |
| Chronic kidney disease | 289 | 19.86 | 126 | 13.67 | 163 | 30.58 | <.0001 |
| Diabetes | 677 | 46.53 | 408 | 44.25 | 269 | 50.47 | .02 |
| Outcomes | |||||||
| Renal failure | 100 | 6.87 | 38 | 4.12 | 62 | 11.63 | <.0001 |
| Cardiac/MI | 53 | 3.64 | 18 | 1.95 | 35 | 6.57 | <.0001 |
| Respiratory problems | 88 | 6.05 | 41 | 4.45 | 47 | 8.82 | .0008 |
| Infection | 246 | 16.91 | 109 | 11.82 | 137 | 25.70 | <.0001 |
| Amputation | 137 | 9.42 | 40 | 4.34 | 97 | 18.20 | <.0001 |
| In-hospital death | 19 | 1.31 | 4 | 0.43 | 15 | 2.81 | .0001 |
| Length of stay, mean (SD) | 5.12 | (7.9) | 3.57 | (6.56) | 7.81 | (9.18) | <.0001 |
| Length of stay > 10 days | 223 | 15.33 | 74 | 8.03 | 149 | 27.95 | <.0001 |
Pre-operative NLR Low (< 3.65), High (≥ 3.65).
PAD = peripheral arterial disease; SD = standard deviation; MI = myocardial infarction; NLR = neutrophil to lymphocyte ratio
Table 3.
Postoperative sample characteristics by NLR level
| Overall | Low NLRa | High NLRa | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P-value | |
| Overall | 2701 | 100.0 | 939 | 34.7 | 1762 | 65.2 | -- |
| Age, mean (SD) | 68.6 | 10.9 | 67.2 | 11.2 | 69.3 | 10.7 | <.0001 |
| Gender | <.0001 | ||||||
| Female | 1070 | 39.61 | 421 | 44.83 | 649 | 36.83 | |
| Male | 1631 | 60.39 | 518 | 55.17 | 1113 | 63.17 | |
| Race | <.0001 | ||||||
| Caucasian | 2054 | 76.05 | 668 | 71.14 | 1386 | 78.66 | |
| African American | 484 | 17.92 | 199 | 21.19 | 285 | 16.17 | |
| Other race | 163 | 6.03 | 72 | 7.67 | 91 | 5.16 | |
| Charlson Index, mean (SD) | 2.50 | 1.62 | 2.24 | 1.47 | 2.64 | 1.67 | <.0001 |
| Procedure type | <.0001 | ||||||
| Endovascular | 1395 | 51.65 | 594 | 63.26 | 801 | 45.46 | |
| Open | 1306 | 48.35 | 345 | 36.74 | 961 | 54.54 | |
| PAD Severity | <.0001 | ||||||
| Claudication | 1384 | 51.24 | 569 | 60.60 | 815 | 46.25 | |
| Rest pain | 408 | 15.11 | 133 | 14.16 | 275 | 15.61 | |
| Tissue loss | 909 | 33.65 | 237 | 25.24 | 672 | 38.14 | |
| NLR, mean (SD) | 6.00 | 4.22 | -- | -- | -- | -- | -- |
| Pre-Existing Conditions | |||||||
| Chronic heart disease | 1343 | 49.72 | 447 | 47.60 | 896 | 50.85 | .10 |
| Chronic kidney disease | 443 | 16.40 | 100 | 10.65 | 343 | 19.47 | <.0001 |
| Diabetes | 1120 | 41.47 | 372 | 39.62 | 748 | 42.45 | .15 |
| Outcomes | |||||||
| Renal failure | 135 | 5.00 | 22 | 2.34 | 113 | 6.41 | <.0001 |
| Cardiac/MI | 103 | 3.81 | 29 | 3.09 | 74 | 4.20 | .15 |
| Respiratory problems | 135 | 5.00 | 24 | 2.56 | 111 | 6.30 | <.0001 |
| Infection | 313 | 11.59 | 66 | 7.03 | 247 | 14.02 | <.0001 |
| Amputation | 175 | 6.48 | 26 | 2.77 | 149 | 8.46 | <.0001 |
| In-hospital death | 20 | 0.74 | 3 | 0.32 | 17 | 0.96 | .06 |
| Length of stay, mean (sd) | 4.25 | 5.42 | 2.70 | 3.34 | 5.08 | 6.09 | <.0001 |
| Length of stay > 10 days | 266 | 9.85 | 35 | 3.73 | 231 | 13.11 | <.0001 |
Post-operative NLR Low (< 5.96), High (≥ 5.96).
PAD = peripheral arterial disease; SD = standard deviation; MI = myocardial infarction; NLR = neutrophil-lymphocyte ratio
Outcomes
Unadjusted analysis
Length of stay was longer for patients with tissue loss than rest pain or claudication (6.4 vs. 4.1 vs. 2.3 days; all P < .0001). In the patients with preoperative NLR values, a high NLR was associated with the following complications (Table 2): renal failure (11.6% vs. 4.1%; P < .0001), cardiac complications/myocardial infarction (6.6% vs. 1.9%; P < .0001), respiratory problems (8.8 vs. 4.5%; P = .0008), infection (25.7% vs. 11.8%; P < .0001), amputation (18.2% vs. 4.3%; P < .0001), in-hospital death (2.8% vs. 0.4%; P = .0001), and increased length of stay (9.2 days vs. 6.6 days; P < .0001).
Similarly, for patients with postoperative NLR data, a high NLR was also associated with the following complications (Table 3): renal failure (6.41% vs. 2.3%; P < .0001), respiratory problems (6.3 vs. 2.6%; P = .0008), infection (14.0% vs. 7.0%; P < .0001), amputation (8.5% vs. 2.8%; P < .0001) and increased length of stay (13.1 days vs. 3.7 days; P < .0001).
Multivariable analysis
We separately modeled preoperative and postoperative NLR to determine whether the association of NLR with outcome persisted through the perioperative period. Table 4 demonstrates five separate multivariable models (in-hospital death, renal failure, cardiac/MI, infection, and length of stays > 10 days) each adjusted for clinically relevant variables (age, sex, race, disease severity and pre-existing conditions) based on the non-adjusted results noted in tables 3 and 4. Preoperative high NLR was associated with in-hospital death (OR 5.4, 95% CI 1.7–17), prolonged length of stay (OR 2.6, 95% CI 1.9–3.7), cardiac complications and myocardial infarction (OR 2.9, 95% CI 1.6–5.4), and renal failure (OR 1.9, 95% CI 1.2–2.9). In the postoperative period, high NLR was associated with prolonged length of stay (OR 2.9, 95% CI 1.9–4.3), infection (OR 1.6, 95% CI 1.2–2.2), and renal failure (OR 2.0, 95% CI 1.3–3.3) (Table 4). Other model covariates that were significantly associated with the outcomes under study (in-hospital death, renal failure, cardiac/MI complications, infection and prolonged length of stay) are also detailed in Table 4.
Table 4.
Multivariable logistic regression model results
| Preoperative NLR Model | Postoperative NLR Model | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| OR | Low | High | P-value | OR | Low | High | P-value | |
| In-hospital death | ||||||||
| Chronic heart disease | 0.771 | 0.293 | 2.031 | .59 | 0.761 | 0.306 | 1.896 | .55 |
| Chronic kidney disease | 2.835 | 1.030 | 7.798 | .04 | 2.069 | 0.764 | 5.599 | .15 |
| Diabetes | 0.617 | 0.222 | 1.720 | .35 | 1.281 | 0.501 | 3.277 | .60 |
| Procedure type (open v. endo) | 1.460 | 0.556 | 3.834 | .44 | 0.673 | 0.265 | 1.711 | .40 |
| NLR (high vs. low) | 5.359 | 1.682 | 17.074 | .004 | 2.663 | 0.758 | 9.356 | .12 |
| PAD severity (rest pain v. claud.) | 2.059 | 0.551 | 7.688 | .28 | 1.394 | 0.351 | 5.538 | .63 |
| PAD severity (tissue loss v. claud.) | 0.949 | 0.277 | 3.259 | .93 | 1.439 | 0.503 | 4.121 | .49 |
| Renal failure | ||||||||
| Chronic heart disease | 1.276 | 0.815 | 1.999 | .28 | 1.213 | 0.834 | 1.765 | .31 |
| Chronic kidney disease | 4.797 | 3.006 | 7.655 | <.0001 | 4.495 | 3.063 | 6.598 | <.0001 |
| Diabetes | 1.652 | 1.007 | 2.708 | .04 | 2.089 | 1.400 | 3.117 | .0003 |
| Procedure type (open v. endo) | 1.537 | 0.983 | 2.403 | .05 | 1.471 | 1.010 | 2.143 | .04 |
| NLR (high vs. low) | 1.857 | 1.177 | 2.930 | .007 | 2.042 | 1.259 | 3.310 | .003 |
| PAD severity (rest pain v. claud.) | 1.361 | 0.616 | 3.008 | .44 | 1.576 | 0.870 | 2.857 | .13 |
| PAD severity (tissue loss v. claud.) | 2.252 | 1.266 | 4.006 | .005 | 2.072 | 1.324 | 3.243 | .001 |
| Cardiac/MI | ||||||||
| Chronic heart disease | 1.684 | 0.931 | 3.044 | .08 | 1.543 | 1.017 | 2.340 | .04 |
| Chronic kidney disease | 2.540 | 1.363 | 4.733 | .003 | 1.877 | 1.172 | 3.004 | .008 |
| Diabetes | 1.545 | 0.827 | 2.885 | .17 | 1.230 | 0.807 | 1.876 | .33 |
| Procedure type (open v. endo) | 1.520 | 0.854 | 2.705 | .15 | 1.472 | 0.972 | 2.229 | .06 |
| NLR (high vs. low) | 2.907 | 1.565 | 5.400 | .0007 | 1.057 | 0.672 | 1.664 | .80 |
| PAD severity (rest pain v. claud.) | 0.968 | 0.382 | 2.452 | .94 | 1.346 | 0.737 | 2.459 | .33 |
| PAD severity (tissue loss v. claud.) | 0.989 | 0.494 | 1.979 | .97 | 1.643 | 1.028 | 2.626 | .03 |
| Infection | ||||||||
| Chronic heart disease | 0.827 | 0.602 | 1.136 | .24 | 0.889 | 0.684 | 1.156 | .38 |
| Chronic kidney disease | 1.636 | 1.154 | 2.318 | .005 | 1.793 | 1.340 | 2.401 | <.0001 |
| Diabetes | 1.202 | 0.859 | 1.682 | .28 | 1.387 | 1.059 | 1.818 | .01 |
| Procedure type (open v. endo) | 1.219 | 0.885 | 1.678 | .22 | 1.060 | 0.815 | 1.381 | .66 |
| NLR (high vs. low) | 1.324 | 0.965 | 1.816 | .08 | 1.605 | 1.178 | 2.185 | .002 |
| PAD severity (rest pain v. claud.) | 2.779 | 1.448 | 5.335 | .002 | 2.401 | 1.480 | 3.896 | .000 |
| PAD severity (tissue loss v. claud.) | 14.06 | 8.720 | 22.685 | <.0001 | 8.972 | 6.284 | 12.81 | <.0001 |
| LOS > 10 days | ||||||||
| Chronic heart disease | 1.034 | 0.738 | 1.449 | .84 | 0.883 | 0.665 | 1.171 | .38 |
| Chronic kidney disease | 2.230 | 1.549 | 3.209 | <.0001 | 2.205 | 1.618 | 3.005 | <.0001 |
| Diabetes | 0.877 | 0.615 | 1.252 | .47 | 0.981 | 0.733 | 1.314 | .89 |
| Procedure type (open v. endo) | 2.700 | 1.919 | 3.799 | <.0001 | 1.613 | 1.209 | 2.154 | .001 |
| NLR (high v. low) | 2.645 | 1.886 | 3.709 | <.0001 | 2.896 | 1.971 | 4.255 | <.0001 |
| PAD severity (rest pain v. claud.) | 1.986 | 1.073 | 3.674 | .02 | 2.309 | 1.393 | 3.826 | .001 |
| PAD severity (tissue loss v. claud.) | 7.808 | 4.923 | 12.38 | <.0001 | 7.634 | 5.206 | 11.19 | <.0001 |
OR=odds ratio, CI=confidence interval, NLR=neutrophil to lymphocyte ratio, PAD=peripheral artery disease, MI=myocardial infarction
Patient demographics (age, race, gender) were also included in each model, but not these covariates were not included in the table.
Discussion
In this study, we found an association between increasingly severe symptoms of PAD and NLR values in a graded response from claudication to tissue loss. We also describe a strong independent association between the NLR values pre- and post-operatively and complications following lower extremity procedures. These findings serve as more evidence of the use of NLR as a marker of the inflammatory balance of a patient and may be useful when discussing and trying to mitigate individual risks of complications associated with endovascular and open procedures for PAD. The results also further validate the use of this simple blood test as a clinical indicator of disease severity.
NLR has been described as an indicator for poor outcome in oncologic and cardiovascular disorders. Adverse outcomes are linked to higher NLR, which is thought to be associated with a shift towards a systemic proinflammatory balance. Inflammation has been linked with atherosclerosis, and furthermore, the degree of inflammation correlates with the severity and outcome of disease.29 With this in mind, peripheral arterial disease provides an ideal opportunity to study the graded effect of systemic inflammation, given ranked severity of symptoms from mild claudication to rest pain and tissue loss.
Teperman et al. (2017) describe a similar study evaluating the relationship between NLR and severity of lower extremity PAD by evaluating angiographic images. NLR was divided into tertiles across the range of values and the level of anatomic obstruction classified as ‘severe’ if a >70% diameter stenosis by visual estimate was seen on peripheral angiography. The authors concluded that elevated NLR was independently associated with severe multi-level PAD.30 The association of NLR and atherosclerosis severity has been previously described in the cardiology literature and provides more evidence of a ‘dose effect’ between atherosclerotic burden, inflammation and symptoms.31
In the present study we also demonstrated a difference in NLR value between patients undergoing endovascular procedures and those undergoing open procedures who had similar symptoms (e.g. both had rest pain). Open surgical procedures are known to activate inflammatory cascades and the stress response, leading to hypothalamic-pituitary axis (HPA) stimulation of cortisol production and other metabolically-active hormones such as corticotrophin (ACTH) and growth hormone (GH).32 Endovascular procedures are generally thought to incite a lower level of overall stress response than open procedures, although the data are not definitive.33, 34 Based on these findings and those of the previously-described studies, we propose that NLR accurately reflects the balance towards a proinflammatory state, with higher values serving as a clinical measure of the severity of atherosclerotic disease burden. While this seems self-evident in that claudication patients are clinically less symptomatic than rest pain patients, it opens the discussion for wider applications for NLR in vascular disorders.
The role of NLR and disease severity in other vascular diseases, such as carotid artery stenosis, has yet to be evaluated. The clinical challenge for practitioners who manage these disorders has always been to balance the risks of the intervention with the risks of non-operative management. Park et al. (2016) describe the natural history of asymptomatic moderate carotid artery stenosis is the era of medical therapy.35 The authors followed 124 patients with carotid artery stenosis in the range 50–69% over a 5-year period with duplex ultrasound. The authors describe a progression to severe stenosis (>70%) over this timeframe in 31.8% of the cohort, with carotid intervention in 18.6% of patients. Symptom development occurred in 3% of the moderate grade carotid artery stenosis cohort over the follow-up period. Despite more sophisticated imaging techniques36, 37 and better medical management there is still no reliable method to detect which patients will progress to severe stenosis or even which patients are at risk for symptom development. NLR offers a promising and inexpensive biomarker that correlates with inflammatory and atherosclerotic burden and may allow identification of patients at high-risk for carotid stenosis progression or symptom development and lead to enhanced surveillance and possible earlier intervention.
Similarly, aortic aneurysmal disease is a vascular disorder that exhibits a relatively binary management strategy. Broadly speaking, infrarenal aortic aneurysms less than 5.5 cm are typically managed non-operatively, whereas those > 5.5 cm are considered for treatment when the operative risk is not greater than the estimated annual rupture risk.38 There are patients, however, that develop symptomatic, ruptured and rapidly enlarging aneurysms despite a standardized surveillance schedule.39 Equally as concerning are those patients who lose eligibility for endovascular repair due to aneurysmal dilation of the infrarenal neck of the aneurysm during the period of surveillance.40 NLR has previously been associated with poor outcomes in the postoperative period following both elective and ruptured abdominal aortic aneurysm (AAA) repair.24, 26 Aurelian et al. (2019) describe their findings from a comparative study of ruptured to non-ruptured infrarenal abdominal aortic aneurysm repair.41 The authors report that an NLR value > 5 was associated with higher 30-day mortality following open surgical repair and a 5 times greater possibility of AAA rupture. Higher overall NLR values were seen in patients with ruptured than non-ruptured AAA. These important findings may pave the way to more sophisticated risk prediction tools; integrating biomarkers such as NLR with traditional size-based criteria to create a more accurate individualized risk of rupture. This may, in turn, lead to early intervention in those patients deemed at highest risk of rupture who may not necessarily be at 5.5 cm or greater.
We also found a strong association between preoperative and postoperative NLR and poor outcome. The associations of preoperative NLR with adverse outcome is of prime interest, as this raises an interesting question about the ability to predict which patients will be at higher risk for complications based on NLR level. Traditional vascular surgery risk calculators have focused primarily on adverse cardiac risk. The most commonly used and well-validated online risk calculators include the National Surgical Quality Improvement Program (NSQIP), a nationally validated, risk-adjusted, outcomes-based program to measure and improve the quality of surgical care;42 the Revised Cardiac Risk Index (RCRI), a validated tool for estimating a patient’s risk of perioperative cardiac complications using patient-specific comorbidities;43 and the Vascular Study Group of New England Cardiac Risk Index (VSG-CRI), a similar risk prediction tool based upon specific comorbidities in the vascular population.44 The present study highlights the association between overall risk of complications and the preoperative NLR value for individual patients. This information, known ahead of any proposed operative treatment, might allow for a more informed discussion with the patient regarding the expected risks and complications, depending on the baseline preoperative NLR value.
NLR has been reported in a variety of different ways; ranging from arbitrary cutoffs,24, 45 tertiles or quartiles,46 and more sophisticated thresholds determined by receiver operating characteristics.47, 48 There has not been an established method for reporting NLR, making comparison across the body of literature challenging. NLR values are highly sensitive to illness and stress and represent the pro- or anti-inflammatory state of an individual. Therefore, establishing a true baseline NLR can be challenging due to the inability to control the environment and circumstances in which the NLR value was obtained.
Conclusion
The neutrophil-lymphocyte ratio exhibits an association with the PAD severity and rises in a graded fashion following endovascular or open surgery in a manner consistent with the invasive nature of the procedure. We suggest that the NLR provides a robust tool for assessing the underlying proinflammatory state of an individual. Further, an elevated NLR in the perioperative period is associated with adverse outcomes. Given the evidence underlying inflammation in the development of atherosclerosis and overall adverse outcomes, we recommend further evaluation of the NLR as a biomarker for adverse outcomes in vascular disorders and also plan to incorporate the NLR into a multimodal risk prediction array including other biomarkers of outcome such as HbA1c, albumin, ESR and CRP, for example. We propose to next study the clinical association of NLR with symptomatic carotid stenosis and symptomatic and ruptured aortic aneurysmal disease with the hope to integrate NLR with traditional risk factors to guide individualized treatment prior to stroke or aneurysm rupture.
ARTICLE HIGHLIGHTS.
Type of Research:
Retrospective analysis of prospectively collected data from the Cerner HealthFacts database
Key Findings:
In a cohort of 3,687 patients undergoing endovascular or open procedures for peripheral arterial disease (PAD), the neutrophil-to-lymphocyte ratio (NLR) positively correlated with the degree of disease severity. The NLR value was also positively correlated with the degree of surgical stress, with open procedures exhibiting higher NLR values than endovascular procedures. Most importantly, an elevated NLR value in the perioperative period was associated with all adverse outcomes, including death, following procedures for PAD.
Take home Message:
The NLR value could be used to identify patients with a proinflammatory state, who are at risk for progression of atherosclerosis and adverse outcome. Further study of the clinical use of NLR in predicting sentinel events in vascular disorders such as stroke or ruptured aortic aneurysm should be the next step for this promising biomarker.
Acknowledgments
Support from the Agency for Healthcare Research and Quality was used to fund the research reported in this publication (R24HS022140). The authors take sole responsibility in the content of this report, which does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
The authors declare no conflicts of interest
Presented at the Society for Clinical Vascular Surgery 47th Annual Symposium, March 17th–20th 2019, Boca Raton, Florida
References
- 1.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med 2008;13:209–15. [DOI] [PubMed] [Google Scholar]
- 2.Barretto S, Ballman KV, Rooke TW, Kullo IJ. Early-onset peripheral arterial occlusive disease: clinical features and determinants of disease severity and location. Vasc Med 2003;8:95–100. [DOI] [PubMed] [Google Scholar]
- 3.Deas DS Jr., Marshall AP, Bian A, Shintani A, Guzman RJ. Association of cardiovascular and biochemical risk factors with tibial artery calcification. Vasc Med 2015;20:326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farber A, Eberhardt RT. The Current State of Critical Limb Ischemia: A Systematic Review. JAMA Surg 2016;151:1070–7. [DOI] [PubMed] [Google Scholar]
- 5.Balta S, Celik T, Mikhailidis DP, Ozturk C, Demirkol S, Aparci M, et al. The Relation Between Atherosclerosis and the Neutrophil-Lymphocyte Ratio. Clin Appl Thromb Hemost 2016;22:405–11. [DOI] [PubMed] [Google Scholar]
- 6.Balta S, Demirkol S, Unlu M, Arslan Z, Celik T. Neutrophil to lymphocyte ratio may be predict of mortality in all conditions. Br J Cancer 2013;109:3125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balta S, Kurtoglu E, Kucuk U, Demirkol S, Ozturk C. Neutrophil-lymphocyte ratio as an important assessment tool. Expert Rev Cardiovasc Ther 2014;12:537–8. [DOI] [PubMed] [Google Scholar]
- 8.Karaman M, Balta S, Seyit Ahmet AY, Cakar M, Naharci I, Demirkol S, et al. The comparative effects of valsartan and amlodipine on vWf levels and N/L ratio in patients with newly diagnosed hypertension. Clin Exp Hypertens 2013;35:516–22. [DOI] [PubMed] [Google Scholar]
- 9.Moubayed SP, Heinonen TM, Tardif JC. Anti-inflammatory drugs and atherosclerosis. Curr Opin Lipidol 2007;18:638–44. [DOI] [PubMed] [Google Scholar]
- 10.Klingenberg R, Hansson GK. Treating inflammation in atherosclerotic cardiovascular disease: emerging therapies. Eur Heart J 2009;30:2838–44. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J 2010;74:213–20. [DOI] [PubMed] [Google Scholar]
- 12.van der Valk FM, van Wijk DF, Stroes ES. Novel anti-inflammatory strategies in atherosclerosis. Curr Opin Lipidol 2012;23:532–9. [DOI] [PubMed] [Google Scholar]
- 13.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 2005;4:977–87. [DOI] [PubMed] [Google Scholar]
- 14.Diamantis E, Kyriakos G, Quiles-Sanchez LV, Farmaki P, Troupis T. The Anti-Inflammatory Effects of Statins on Coronary Artery Disease: An Updated Review of the Literature. Curr Cardiol Rev 2017;13:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bu DX, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol 2011;22:165–70. [DOI] [PubMed] [Google Scholar]
- 16.Beal EW, Wei L, Ethun CG, Black SM, Dillhoff M, Salem A, et al. Elevated NLR in gallbladder cancer and cholangiocarcinoma - making bad cancers even worse: results from the US Extrahepatic Biliary Malignancy Consortium. HPB (Oxford) 2016;18:950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017;19:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol 2018;44:607–12. [DOI] [PubMed] [Google Scholar]
- 19.Dong CH, Wang ZM, Chen SY. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: A systematic review and meta-analysis. Clin Biochem 2018;52:131–6. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Cong BL, Wang M, Abdullah M, Wang XL, Zhang YH, et al. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr Med Res 2018;7:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Zhang F, Shen Y, Xu R, Chen Z, Dai Y, et al. Impact of Neutrophil to Lymphocyte Ratio (NLR) Index and Its Periprocedural Change (NLRDelta) for Percutaneous Coronary Intervention in Patients With Chronic Total Occlusion. Angiology 2017;68:640–6. [DOI] [PubMed] [Google Scholar]
- 22.Wada H, Dohi T, Miyauchi K, Shitara J, Endo H, Doi S, et al. Pre-procedural neutrophil-to-lymphocyte ratio and long-term cardiac outcomes after percutaneous coronary intervention for stable coronary artery disease. Atherosclerosis 2017;265:35–40. [DOI] [PubMed] [Google Scholar]
- 23.Pierre-Louis WS, Bath J, Mikkilineni S, Scott MC, Harlander-Locke M, Rasor Z, et al. Neutrophil to Lymphocyte Ratio as a Predictor of Outcomes after Amputation. Ann Vasc Surg 2019;54:84–91. [DOI] [PubMed] [Google Scholar]
- 24.Kordzadeh A, Malietzis G, Browne T, Prionidis I, Panayiotopoulos YP. Neutrophil to lymphocyte ratio (NLR) of five predicts 30-day morbidity in ruptured abdominal aortic aneurysms (rAAA): a retrospective cohort study. Int J Surg 2015;15:45–8. [DOI] [PubMed] [Google Scholar]
- 25.Lareyre F, Raffort J, Le D, Chan HL, Houerou TL, Cochennec F, et al. High Neutrophil to Lymphocyte Ratio Is Associated With Symptomatic and Ruptured Thoracic Aortic Aneurysm. Angiology 2018;69:686–91. [DOI] [PubMed] [Google Scholar]
- 26.Appleton ND, Bailey DM, Morris-Stiff G, Lewis MH. Neutrophil to lymphocyte ratio predicts perioperative mortality following open elective repair of abdominal aortic aneurysms. Vasc Endovascular Surg 2014;48:311–6. [DOI] [PubMed] [Google Scholar]
- 27.Chan C, Puckridge P, Ullah S, Delaney C, Spark JI. Neutrophil-lymphocyte ratio as a prognostic marker of outcome in infrapopliteal percutaneous interventions for critical limb ischemia. J Vasc Surg 2014;60:661–8. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 29.Pant S, Deshmukh A, Gurumurthy GS, Pothineni NV, Watts TE, Romeo F, et al. Inflammation and atherosclerosis--revisited. J Cardiovasc Pharmacol Ther 2014;19:170–8. [DOI] [PubMed] [Google Scholar]
- 30.Teperman J, Carruthers D, Guo Y, Barnett MP, Harris AA, Sedlis SP, et al. Relationship between neutrophil-lymphocyte ratio and severity of lower extremity peripheral artery disease. Int J Cardiol 2017;228:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misumida N, Kobayashi A, Saeed M, Fox JT, Kanei Y. Neutrophil-to-lymphocyte ratio as an independent predictor of left main and/or three-vessel disease in patients with non-ST-segment elevation myocardial infarction. Cardiovasc Revasc Med 2015;16:331–5. [DOI] [PubMed] [Google Scholar]
- 32.Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr 2013;37:21S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kataja J, Chrapek W, Kaukinen S, Pimenoff G, Salenius JP. Hormonal stress response and hemodynamic stability in patients undergoing endovascular vs. conventional abdominal aortic aneurysm repair. Scand J Surg 2007;96:236–42. [DOI] [PubMed] [Google Scholar]
- 34.Salartash K, Sternbergh WC 3rd, York JW, Money SR. Comparison of open transabdominal AAA repair with endovascular AAA repair in reduction of postoperative stress response. Ann Vasc Surg 2001;15:53–9. [DOI] [PubMed] [Google Scholar]
- 35.Park YJ, Kim DI, Kim GM, Kim DK, Kim YW. Natural History of Asymptomatic Moderate Carotid Artery Stenosis in the Era of Medical Therapy. World Neurosurg 2016;91:247–53. [DOI] [PubMed] [Google Scholar]
- 36.Kolkert JL, Meerwaldt R, Loonstra J, Schenk M, van der Palen J, van den Dungen JJ, et al. Relation between B-mode gray-scale median and clinical features of carotid stenosis vulnerability. Ann Vasc Surg 2014;28:404–10. [DOI] [PubMed] [Google Scholar]
- 37.Naylor AR, Schroeder TV, Sillesen H. Clinical and imaging features associated with an increased risk of late stroke in patients with asymptomatic carotid disease. Eur J Vasc Endovasc Surg 2014;48:633–40. [DOI] [PubMed] [Google Scholar]
- 38.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009;50:S2–49. [DOI] [PubMed] [Google Scholar]
- 39.Soto B, Vila L, Dilme JF, Escudero JR, Bellmunt S, Camacho M. Increased Peak Wall Stress, but Not Maximum Diameter, Is Associated with Symptomatic Abdominal Aortic Aneurysm. Eur J Vasc Endovasc Surg 2017;54:706–11. [DOI] [PubMed] [Google Scholar]
- 40.Dzieciuchowicz L, Tomczak J, Strauss E, Oszkinis G. Morphology-Related Limitations of Endovascular Aneurysm Repair Applicability in the Treatment of Abdominal Aortic Aneurysm in West-Central Poland. Ann Vasc Surg 2018;52:49–56. [DOI] [PubMed] [Google Scholar]
- 41.Aurelian SV, Adrian M, Andercou O, Bruno S, Alexandru O, Catalin T, et al. Neutrophil-to-Lymphocyte Ratio: A Comparative Study of Rupture to Nonruptured Infrarenal Abdominal Aortic Aneurysm. Ann Vasc Surg 2019;58:270–5. [DOI] [PubMed] [Google Scholar]
- 42.Yap MKC, Ang KF, Gonzales-Porciuncula LA, Esposo E. Validation of the American College of Surgeons Risk Calculator for preoperative risk stratification. Heart Asia 2018;10:e010993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043–9. [DOI] [PubMed] [Google Scholar]
- 44.Bertges DJ, Goodney PP, Zhao Y, Schanzer A, Nolan BW, Likosky DS, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J Vasc Surg 2010;52:674–83, 83 e1–83 e3. [DOI] [PubMed] [Google Scholar]
- 45.Bhutta H, Agha R, Wong J, Tang TY, Wilson YG, Walsh SR. Neutrophil-lymphocyte ratio predicts medium-term survival following elective major vascular surgery: a cross-sectional study. Vasc Endovascular Surg 2011;45:227–31. [DOI] [PubMed] [Google Scholar]
- 46.Zhou S, Cai B, Zhang Y, Wang L, Liu X, Xu G. The Relationship between Neutrophil-to-Lymphocyte Ratio and Aortic Arch Calcification in Ischemic Stroke Patients. J Stroke Cerebrovasc Dis 2017;26:1228–32. [DOI] [PubMed] [Google Scholar]
- 47.Yanartas M, Kalkan ME, Arslan A, Tas SG, Koksal C, Bekiroglu N, et al. Neutrophil/Lymphocyte Ratio Can Predict Postoperative Mortality in Patients with Chronic Thromboembolic Pulmonary Hypertension. Ann Thorac Cardiovasc Surg 2015;21:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oz K, Iyigun T, Karaman Z, Celik O, Akbay E, Akinc O, et al. Prognostic Value of Neutrophil to Lymphocyte Ratio and Risk Factors for Mortality in Patients with Stanford Type A Aortic Dissection. Heart Surg Forum 2017;20:E119–E23. [DOI] [PubMed] [Google Scholar]


