Abstract
The consequences of excessive fructose intake extend beyond those of metabolic disorder to changes in emotional regulation and cognitive function. Long-term consumption of fructose, particularly common when begun in adolescence, is more likely to lead to deleterious consequences than acute consumption. These long-term consequences manifest differently in males and females, suggesting a sex-divergent mechanism by which fructose can impair physiology and neural function. The purpose of the current project was to investigate a possible sex-specific mechanism by which elevated fructose consumption drives behavioral deficits and accompanying metabolic symptoms – specifically, synaptic mitochondrial function. Male and female rats were fed a high fructose diet beginning at weaning and maintained into adulthood. Measures of physiological health across the diet consumption period indicated that females were more likely to gain weight than males while both displayed increased circulating blood glucose. As adults, females fed the high fructose diet displayed increased floating behavior in the forced swim task (FST) while males exhibited increased exploratory behavior in the open field. Synaptic respiration was altered by diet in both females and males but the effect was sex-divergent – fructose-fed females had increased synaptic respiration while males showed a decrease. When exposed to an acute energetic challenge, the pattern was reversed. Taken together, these data indicate that diet-induced alterations to neural function and physiology are sex-specific and highlight the need to consider sex as a biological variable when treating metabolic disease. Furthermore, these data suggest that synaptic mitochondrial function may contribute directly to the behavioral consequences of elevated fructose consumption.
Keywords: metabolism, mitochondria, sex differences, fructose, diet, adolescence, anxiety, depression
Introduction
In the past 40 years, fructose consumption has become a standard part of the American diet due largely to the invention and introduction of added caloric sweeteners such as high fructose corn syrup into commercial food products (Marriott et al., 2009). As of the early 2000’s, added sugars accounted for approximately 16% of all caloric intake (Bray et al., 2004). Adolescents are the highest consumers of fructose, consuming as much as 21% of their daily caloric intake from added sugars (Welsh et al., 2011). The consumption of added sugars in children is positively correlated with a number of negative outcomes in both adolescence and adulthood. In the United States, adolescents have increasing rates of metabolic syndrome, obesity, and type 2 diabetes with the rise in consumption of calorically sweetened beverages and foods (Vartanian et al., 2007). Along with the emergence of pathophysiology in adolescence, excessive fructose consumption has been shown to have lasting implications into adulthood, contributing to the growing epidemic of adult metabolic syndrome worldwide (Rutledge and Adeli, 2007).
Although metabolic syndrome is a well-appreciated consequence of a diet high in fructose, emerging evidence also suggests that fructose can impact the brain and behavior (Harrell et al., 2015). Because the brain, particularly the hypothalamus, is the primary regulator of energetic homeostasis for the human body (Elmquist et al., 2005; Morton et al., 2006; Meister, 2007; Myers et al., 2008), perturbations to energetic balance have the potential to initiate neurological consequences. Previous studies in male rats have shown that a high fructose diet (HFD) initiated in adolescence alters the hypothalamic pituitary adrenal (HPA) axis transcriptome, metabolic outcomes, and increases depressive-like behavior in male rats (Harrell et al., 2015). Metabolic disruptions and associated etiology have consistently been associated with increased risk of affective-like disorders (Zhao et al., 2009; Kahl et al., 2015), and the bidirectional relationship between dysregulated metabolism and mood disorders is the subject of increased research (Musselman et al., 1998; Perlmutter et al., 2000; McIntyre et al., 2006). These findings imply that a HFD can have negative consequences on neural structure and function resulting in behavioral deficits in addition to the physiological consequences of metabolic syndrome.
In the brain, synapses are neuronal compartments that require a high amount of energy for use in neurotransmitter release and membrane potential regulation (Harris et al., 2012). Mitochondrial supply of energy and Ca2+ buffering necessitates their localization at synapses for ideal neuronal performance (Vos et al., 2010). In instances of metabolic pathophysiology, such as type 2 diabetes and metabolic syndrome, the metabolic-induced effects of oxidative stress, damage to mtDNA, and altered mitochondrial function are well characterized (Baynes, 1991; Giugliano et al., 1996; Suzuki et al., 1999). However, it is unclear how mitochondrial function at the level of the synapse may be impacted by a HFD. Because the brain metabolizes glucose as the primary source of fuel and synapses require high-energy metabolism, investigation of synaptic mitochondrial respiration following a prolonged period of dietary modification may provide insight into diet-induced alterations to affective behavior.
While extensive work has been conducted in male subjects regarding the impact of diet on neural function, it is evident that females are equally as susceptible as males to modifications following dietary intake. The beginning of puberty marks the endocrine system’s regulation of diet-induced effects including insulin sensitivity, hypertension, and lipid levels (Chen et al., 1992; Galipeau et al., 2002; Louet et al., 2004; Vasudevan et al., 2005).As women are twice as likely to experience depression and anxiety in their lifetime as men, and this trend emerges after puberty and persists for the next 30-40 years (Cyranowski et al., 2000; Ford and Erlinger, 2004), it is possible that adolescent modification to metabolic function may contribute to this sex disparity. Relatively little is known about differences in neural mitochondrial function between the sexes, however, mitochondria display prominent sex-specific and tissue specific behavior in pathophysiological states (Ventura-Clapier et al., 2017). Thus, it is possible that sex-specific consequences of diet-induced changes in mitochondrial bioenergetics at the level of the synapse drive alterations in affective-like behavior.
In this study, we hypothesized that fructose consumption beginning at weaning and continued into adulthood would result in metabolic disruptions accompanied by decreases in synaptic mitochondrial respiration. Further, we hypothesized that these alterations in mitochondrial respiration would be accompanied by increases in anxiety-like and depressive-like behavior.
Experimental Procedures
Animal Husbandry
Timed pregnant Wistar rats (n=6) were procured from Charles River (Morrisville, N.C.). All animals were housed in a temperature (20-23°C) and humidity (60%) controlled colony room in static cages. The room was kept on a 14:10 light:dark cycle. Litters were culled on post-natal day (PND) 3 to eight pups per litter (male = 4 and female = 4). This was done to ensure an equal sample size of males and females. The pups (N=46) were weaned on PND 22 and pair housed with same-sex, non-sibling cage mates for the duration of the study. Cage mates were assigned to either a control chow diet (Males n=12; Females n=10) or a HFD (Males n=14; Females n=10) on PND 25. All studies were conducted in accordance with Institutional Animal Care and Use Committee of Virginia Commonwealth University and National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Diet Tracking
Animals were either maintained on a standard chow diet or placed on a HFD beginning at PND 25 and remained on the assigned diet until their experimental end point. The chow diet was comprised of the Envigo Lab Diet 7012 (Teklad LM-485) with 5.8% kcal from fat and 0% kcal from fructose, while the HFD was 10% kcal fat and 55% kcal fructose (Research Diets D050111802), and both diets contained 19% kcal protein. Total food consumption was tracked through the duration of the experiment by weighing the total remaining food in the cage twice weekly before the food was replaced with a pre-weighed amount of new food (200g or 300g). Attention was paid to food remaining on bottom of cage and all rats received water ad libitum. Weights for each animal were taken and recorded biweekly and an average weekly weight was calculated. Utilizing average weekly weight coupled with weekly food consumption, estimated caloric efficiency (milligram weight gained per kilocalorie consumed) was calculated per animal.
Blood glucose levels were obtained from experimental subjects at 3 timepoints: end of adolescence (PND 57), end of week 10 on assigned diet (prior to behavioral testing), and end of dietary week 12 (prior to experimental endpoint). To accomplish this, a tail prick was done in the lateral tail vein using a sterile 25-gauge needle. A Freestyle Glucometer with Freestyle Lite test strips (Abbott Diabetes Care Inc., Alameda, CA) were used to obtain the reading.
Behavioral Assessments
Behavioral testing was conducted beginning after the subjects were on their designated diet for 10 full weeks at PND 95 and was conducted in order of increasing anxiogenicity of the tasks. Only one behavioral test was conducted per animal on any given day. Behavioral testing consisted of a 10-minute open field test (PND 95), a 10-minute social interaction test (PND 96), and a 10-minute forced swim test (PND 116-120). All rats were habituated to the behavioral test suite for 3 days prior to testing. The open field test, social interaction test, and forced swim test were all conducted in the middle of the rats’ light cycle under moderate illumination (100-200 lux). Behavioral testing was video recorded and tracked using Ethovision XT 13 (Noldus Information Technologies; Leesburg, VA).
Open Field Task:
The open field test serves as a measurement of anxiety-like behavior (Walsh and Cummins, 1976; Prut and Belzung, 2003) and consists of exposing the test subject to an unfamiliar square arena for ten minutes. The rat was placed in the center of a novel, 75 x 75 cm square field with 35 cm high walls (Noldus) and allowed to freely explore for 10 minutes.
Social Interaction:
A social interaction test was conducted as a measure of anxiety-like and anhedonic behavior (File and Hyde, 1978; File and Seth, 2003). In this test the experimental animal was placed in the center of the same arena used for the open field test, now containing a novel stimulus rat, and allowed to explore and interact for 10 minutes. The stimulus rat was a younger, same-sex, same-strain animal and had prior exposure to the arena. The experimental subject’s latency to interact with the stimulus rat, number of approaches to the stimulus rat, and total time spent interacting with stimulus rat were scored by hand by a treatment-blind experimenter.
Forced Swim Test:
A forced swim test (FST) was used as a measurement of depressive-like behavior (Porsolt et al., 1978). In this case a single test was used to measure depressive-like behavior (Castro et al., 2010). The rat was placed into a circular, acrylic tank (diameter = 19cm; height = 46.5cm) filled with room temperature water deep enough so that the rat could not touch the bottom of the tank and swam for 10 minutes. Time spent inactive versus actively swimming, latency to inactivity, time spent struggling, and exhibition of coping mechanisms, such as diving, were recorded. Inactivity was defined as the rat’s limbs remaining motionless for at least 0.5 seconds and struggling was defined as the rat’s head being above the water and limbs breaking the surface. Immediately following the test, rats were removed from the tank, placed in a cage on top of a heating pad, and allowed to rest for 20 minutes. Upon conclusion of the 20-minute rest period, rats were transferred into a separate room and rapidly decapitated. While the FST is often used as an indicator of depressive-like behavior, the test also served as an acute stressor for the rats. In order to assess how an acute stressor impacted measures of mitochondrial function and endocrine system function, only half of the cohort underwent the FST before euthanasia. The other half were euthanized without exposure to the test. Groups were evenly split across the four testing days.
Corticosterone Analyses
Corticosterone was measured at baseline and 30 minutes after the onset of the 10-minute forced swim test, using a commercial ELISA (sensitivity 27 pg/mL, Enzo Life Sciences, Farmingdale, NY) per manufacturer’s protocol. All samples were run in duplicate with a CV <10%. For baseline samples, rats were transferred to a testing room to acclimate prior to decapitation. Rats were then transferred to a separate room and decapitated within two minutes of handling. For the forced swim group, immediately following the 20 minutes of rest on the heating pad, rats were decapitated. Following decapitation, trunk blood was collected and allowed to clot at room temperature before the clot was removed and remaining blood was placed on ice. Blood was centrifuged (Eppendorf 5810R) at 1800 rcf for 20 minutes at 4°C. Resulting serum was stored at −80°C for use in ELISAs.
Synaptosomal Isolation
Synaptosomal isolation was adapted from a previously published protocol (Dunkley et al., 2008). Rats were euthanized via rapid decapitation without anesthesia and the whole brain was extracted. The cerebellum was removed, the brain was bisected, and the right hemisphere was placed in cold sucrose medium (320 mM Sucrose, 0.2 M EDTA, 5 mM Tris, pH 7.4) to remove excess blood. Tissue was homogenized in a 7mL Dounce glass homogenizer containing 4.5 mL cold homogenization buffer (320 mM Sucrose, 0.2 M EDTA, 50 mM dithiothreitol, 5.0 mM Tris, pH 7.4) by 5 and 6 strokes with the loose and tight plunger, respectively. The homogenate was centrifuged (Eppendorf 5810R) at 3600 rpm for 10 minutes at 4°C. The supernatant was removed and 6 mL was layered on top of a discontinuous Percoll gradient (4 ml layers of 0,3,10,15,23 % Percoll in homogenization buffer) in a 26 mL centrifuge tube and spun at 32500g for 10 minutes at 4°C (JA-20 fixed angle rotor in a Beckman Avanti J-25 centrifuge). The synaptosomes were isolated from the band between the 15% and 23% Percoll layers, diluted in Ionic Media (20 mM HEPES, 10 mM D-Glucose, 1.2 mM Na2HPO4, 1 mM MgCl2, 5 mM NaHCO3, 5 mM KCl, 140 mM NaCl, pH 7.4), and centrifuged at 15000g for 35 minutes at 4°C (JA-20 fixed angle rotor in a Beckman Avanti J-25 centrifuge). The final synaptosome pellet was collected and protein concentration was determined (Nanodrop A280, ThermoFisher Scientific). Synaptosomal protein was resuspended in ionic media for respirometry (Choi et al., 2009).
Synaptosomal Respiration
To quantify respiration, 40μg of synaptosomal protein per well was aliquoted into a 24 well cell culture microplate (Agilent Technologies, Cedar Creek, MO) coated with Poly-DLysine. Plates were centrifuged at 3400g for 30 minutes at 4°C (Eppendorf 5810R centrifuge) in order to adhere the synaptosomes to the plate. The medium was then replaced with 500μl of Seahorse XF assay media (Seahorse XF Base Medium (w/o Phenol Red), 10mM Seahorse XF Glucose, 1 mM Seahorse XF Pyruvate, 2 mM Seahorse XF L-Glutamine). The microplate was then loaded into the Seahorse XFe24 extracellular flux analyzer according to the manufacturer’s instructions. Wave Desktop 2.6 Software (Agilent) was used for data acquisition and data analysis for assays. All plates were run at 37°C and samples were run in triplicate. The measurement of oxygen consumption and extracellular acidification method is as previously described in Choi et al. (2009). Oxygen consumption rates (OCR) were determined by sequential measurement cycles consisting of a 30 second mixing time followed by a 2-minute wait time and then a 3-minute measurement period. Reagents were added in Seahorse assay media in dilutions according to manufacturer’s recommendation (2.0μM Oligomycin, 1.0μM carbonyl cyanide-p-trifluoromethox- yphenyl-hydrazon (FCCP), 0.5μM Rotenone/antimycin A per well). The first three measurements of OCR occur prior to addition of mitochondrial reagents and indicates basal respiration. Oligomycin is a complex V inhibitor and OCR following this addition indicates ATP-linked respiration (substraction of baseline OCR) and proton leak respiration (subtraction of non-mitochondrial respiration). FCCP is a protonophore and adding it will collapse the inner membrane gradient and push the electron transport chair to the maximal rate. Inhibition of complex III and I is achieved through addition of antimycin A and rotenone which will terminate electron transport chain function and demonstrate non-mitochondrial respiration. Mitochondrial reserve is calculate by subtraction of basal respiration from maximal respiration (Rose et al., 2014).
Statistical Analyses
Data were analyzed using GraphPad Prism Software (San Diego, CA). Three-way Analysis of variance (ANOVA) were run to compare diet-induced changes observed in oxygen consumption rates at baseline and following the 10-minute FST. Two-way ANOVAs were used to analyze main effects of sex or diet on behavioral outcomes (p≤0.05) using GraphPad. Sidak’s posthoc analysis was used when appropriate (p≤0.05).
Results
A HFD initiated in adolescence alters physiology in females, but not males
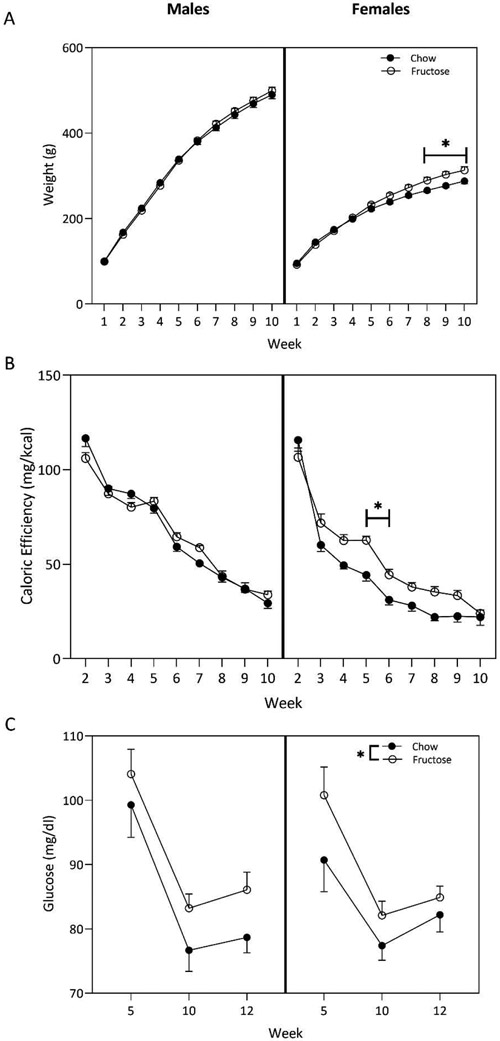
All groups, regardless of sex and diet, gained weight over the course of the 10-weeks. In females, individual 2-way ANOVAs displayed a main effect of week (F(9,180)=429.3;p<0.0001), diet (F(1,180)=27.05; p<0.0001), and a significant interaction between week and diet (F(9,180)=3.615; p=0.0004). Fructose-fed females gained more weight per week than chow-fed controls beginning at week 8 and persisting through the remainder of the 10-week dietary period (Figure 1A). In males, diet did not affect weight gain (F(1,240)=0.3417; p=0.5594).
Figure 1:
Using two-way ANOVA (diet x sex), we determined that a high-fructose diet (HFD) beginning at weaning resulted in physiological differences in adult males and females. A) Fructose-fed female rats gained more weight than their chow-fed counterparts beginning in week 8 of diet exposure. This effect was maintained throughout the 10-week consumption period. B) The HFD altered caloric efficiency in female rats during weeks 5 and 6 on the diet. Alterations were not present in male rats. The caloric efficiency of all groups decreased over time. C) Both fructose-fed males and females displayed increased blood glucose at three timepoints across the diet period compared to chow controls. Circles represent mean ± SEM. *p<0.05.
Caloric efficiency was calculated by dividing weight gained per week by the estimated amount of calories consumed per week. Caloric consumption was estimated from the total grams of food consumed per animal multiplied by the known caloric content of the respective diet (3.35kcal/gram of chow; 3.85 kcal/gram of HFD). Regardless of sex, caloric efficiency declined weekly. Diet did not alter caloric efficiency in males (p>0.05). However, in females, two-way ANOVA demonstrated a main effect of week (F(8,162)=136.4; p<0.0001) and diet (F(1.162)=34.26;p<0.0001) with a significant interaction between week and diet (F(8,162)=2.947; p=0.0042). Sidak’s multiple comparisons test revealed that this interaction was driven by weeks 5 (p=0.0013) and 6 (p=0.0492), such that fructose-fed females in week 5 and 6 were gaining more weight per calorie consumed compared to their chow-fed counterparts (Figure 1B).
Two-way ANOVA indicated that in both males (F(1,71)=5.323;p=0.024) and females (F(1,54)=4.838;p=0.0321), circulating blood glucose was increased following consumption of the HFD (p>0.05).
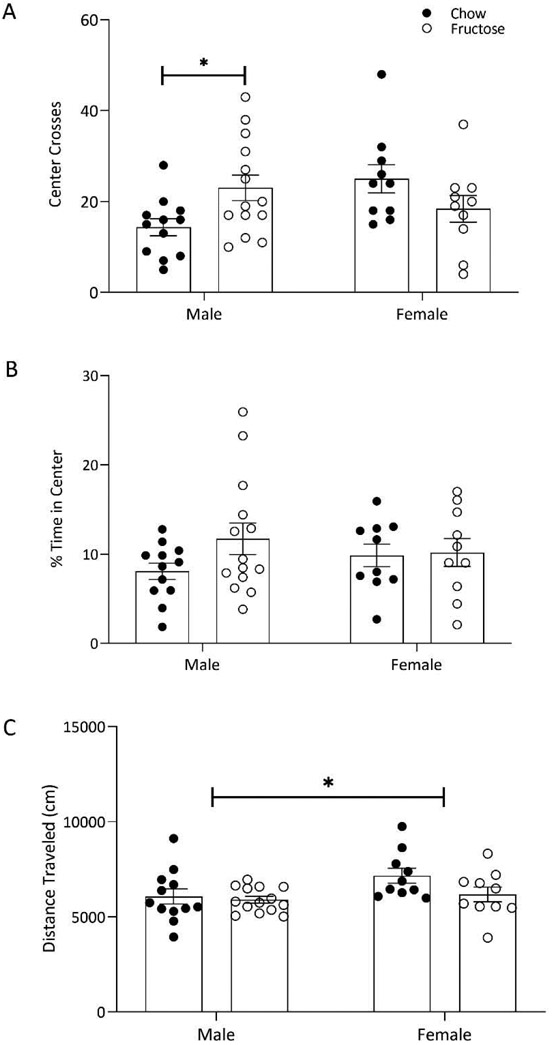
HFD decreases anxiety-like behavior in males, but not in females in the open field
In the open field test, two-way ANOVAs were conducted to assess the effect of diet on anxiety-like behavior in a 10-minute test. There was a significant diet by sex interaction on frequency of crosses into the center of the arena (F(1,42)=7.810;p=0.0078; Figure 2A). Sidak post hoc showed that fructose-fed males were crossing into the center of the arena more frequently than chow-fed controls (p=0.0412). In both males and females, diet did not alter the animal’s time spent in the center of the arena as compared to the periphery (F(1,42)=1.790;p=0.1882; Figure 2B) or the total distance traveled (cm) within the arena (F(1,42)=2.953;p=0.0931; Figure 2C). There was a main effect of sex on distance travelled with females overall travelling more than males (F(1,42)=4.241; p=0.0457; Figure 2C).
Figure 2:
Fructose consumption increased exploratory behavior in males, but not in females based on two-way ANOVA analysis (diet x sex). A) Fructose-fed males demonstrated an increased number of crosses into the center of the open field arena compared to chow-fed males. B) Percent of time spent in the center of arena relative to periphery was not altered by diet in males or females. C) Distance traveled in the open field was not modified by diet in males or females. Bars represent mean ± SEM. *p<0.05.
In both sexes, social behavior was not impacted by diet
In the social interaction test, two-way ANOVA displayed that diet and sex did not impact the latency of the experimental rats to approach the stimulus rats (diet: F(1,41)=0.6197; p=0.4357; sex: F(1,41)=2.103; p=0.1547), the number of approaches by the experimental rats to the stimulus rats (diet: F(1,41)=0.5521; p=0.4617; sex: F(1,41)=0.02579; p=0.8732), or the total time both rats spent interacting in the arena in both males and females (diet: F(1,41)=0.5610; p=0.4581; sex: F(1,41)=0.7498; p=0.3916; data not shown).
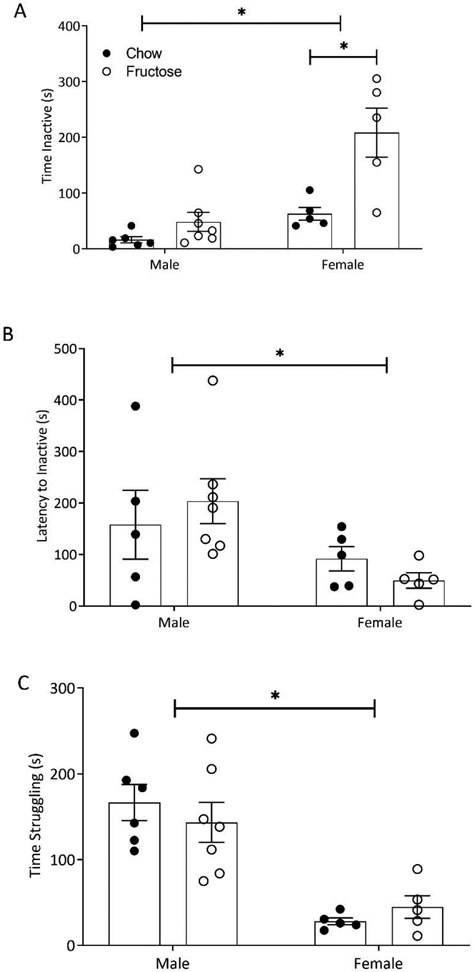
HFD increases depressive-like behavior in females
Following a 10-minute forced swim test, two-way ANOVAs were conducted assessing the impact of diet and sex on depressive-like behavior. There was a main effect of diet (F(1,19)=15.44; p=0.0009) and sex (F(1,19)=20.94; p=0.0002) as well as an interaction effect (F(1,19)=6.271; p=0.0215; Figure 3A). Sidak’s post hoc indicated that females fed the fructose diet were inactive for a significantly longer amount of time than the chow controls (p=0.0008; Figure 3A). There was a main effect of sex on the latency to inactivity (F(1,18)=6.331; p=0.0216; Figure 3B) such that females became inactive sooner than males. There was a main effect of sex on time spent struggling (F(1,19)=37.94; p<0.0001; Figure 3C) indicating that males struggled more than females.
Figure 3:
Two-Way ANOVA (diet x sex) indicated that consumption of a HFD increased floating behavior in the forced swim test (FST) in females. A) Females fed a HFD spent more time inactive than their chow-fed counterparts. Total time inactive was not modified in males. Females overall spent more time inactive compared to males. B) Latency to inactivity was not affected by diet in males or females in the FST, however, females became inactive more quickly than males. C) Total time spent struggling in the tank was elevated in males compared to females. Bars represent mean ± SEM. *p<0.05.
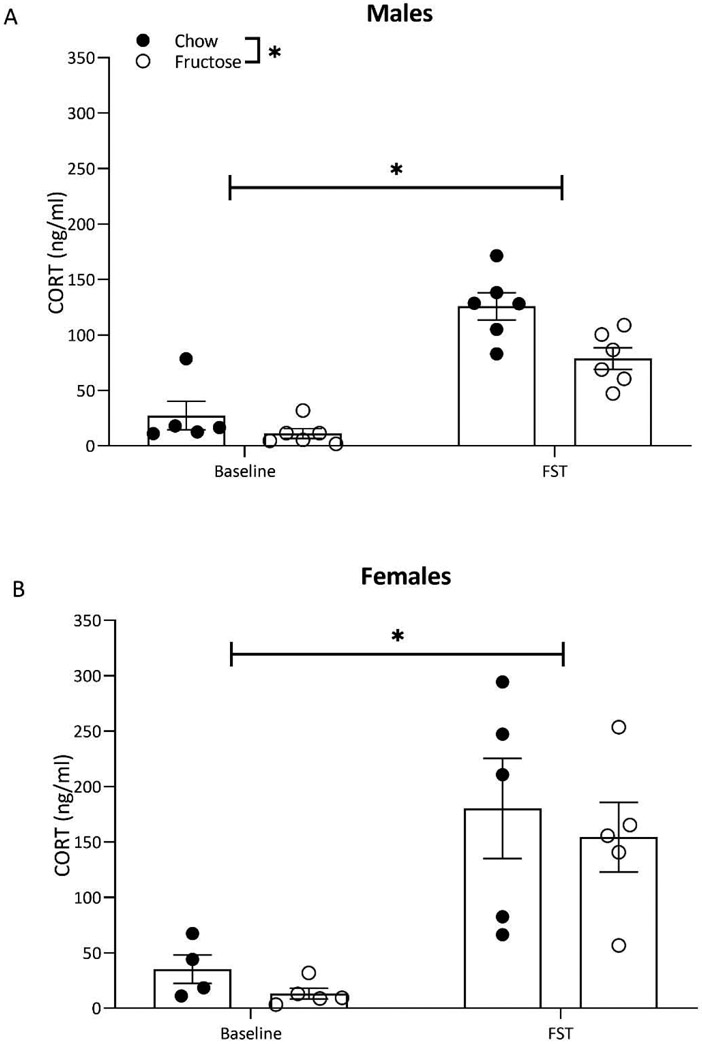
Corticosterone concentrations were altered by diet and acute stress
In males, corticosterone concentrations were impacted by the experience of the FST and diet, however, in females FST alone altered corticosterone. Two-way ANOVA displayed a main effect of diet (F(1,19)=9.64; p=0.005) and forced swim (F(1,19)=66.39; p<0.0001) on circulating corticosterone concentrations in males. Exposure to the FST resulted in significantly higher circulating corticosterone concentrations in both chow and fructose-fed males (Figure 4A). However, at both baseline and post-FST, fructose-fed males had reduced circulating corticosterone (Figure 4A). In females, two-way ANOVA revealed a main effect of forced swim exposure (F(1,15)=22.89; p=0.0002), such that females that underwent the FST displayed higher corticosterone concentrations than controls (Figure 4B). Diet did not impact corticosterone concentrations in females (F(1,15)=0.6430; p=0.4352; Figure 4B).
Figure 4:
Exposure to acute stress in the form of a 10-minute forced swim test increased blood corticosterone in both sexes based on within sex two-way ANOVA (diet x acute stress exposure). A) In males, undergoing the FST resulted in significantly increased corticosterone. In fructose-fed males, corticosterone was reduced at baseline and following the FST. B) In females, corticosterone was elevated by acute stress exposure but was not altered by diet. Bars represent mean ± SEM. *p<0.05.
HFD altered mitochondrial respiration differentially in males and females
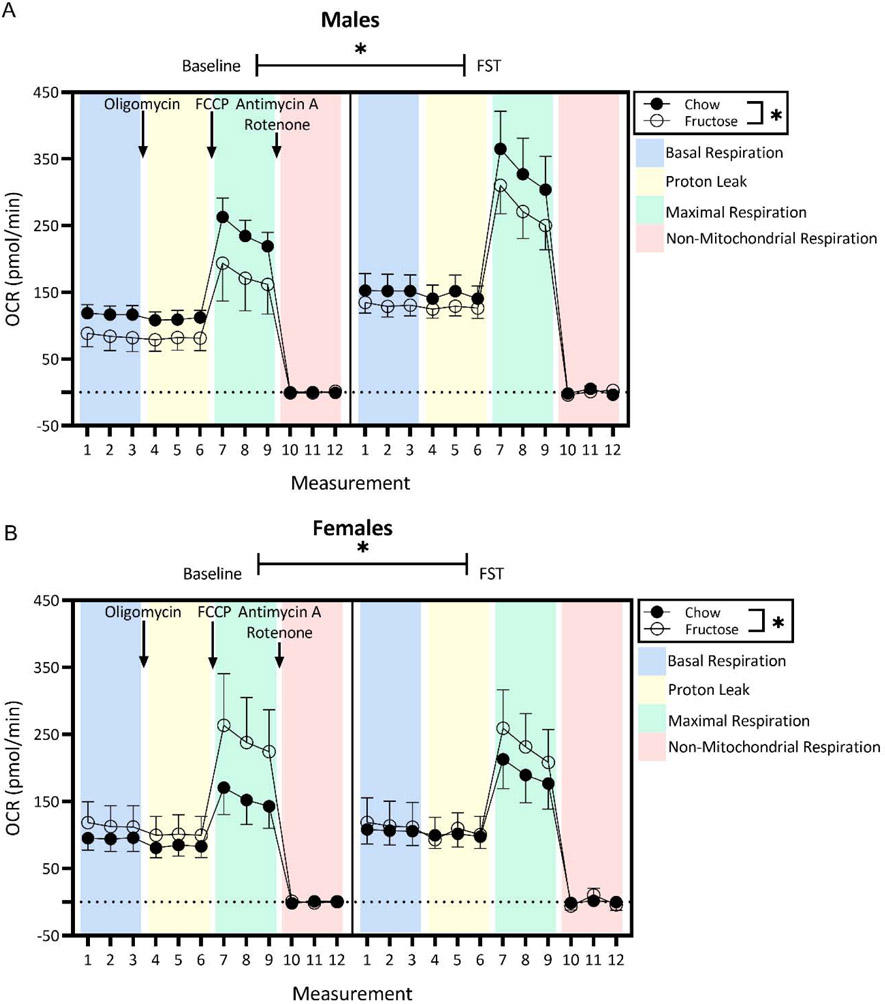
Three-way ANOVA was used to analyze the effects of measurement, diet, and FST exposure on oxygen consumption rate (OCR; pmol/min) in isolated intact synaptosomes in both males and females. In males, 3-way ANOVA displayed a main effect of measurement (F(11,180)=57.67; p<0.0001), undergoing the FST (F(11,80)=37.95; p<0.0001), and diet (F(1,180)=14.11; p=0.0002) as well as a significant interaction between measurement and FST experience (F(11,180)=2.132, p=0.0202). Regardless of whether or not males underwent the FST, fructose-fed males demonstrated decreased baseline OCR compared to their chow-fed counterparts. At the same time, regardless of dietary history, OCR were significantly increased in males who underwent the FST (Figure 5). There was no effect of diet or FST exposure on measures of mitochondrial function following treatment with oligomycin, FCCP, or rotenone and antimycin A in males (p>0.05). See Supplementary Figure 1.
Figure 5:
In both sexes, mitochondrial respiration was modified by diet, but only by FST exposure in males (three-way ANOVA with diet x stress exposure x measurement). A) In males, acute stress exposure significantly increased oxygen consumption rate (OCR) in comparison to control groups regardless of diet. Regardless of FST experience, fructose-fed males demonstrated significantly lower OCR compared to chow-fed males. B) Acute stress exposure did not affect mitochondrial respiration in females. In the baseline group, fructose-fed females had significantly increased respiration rates compared to chow-fed females. Circles represent mean ± SEM. *p<0.05. Administration of drugs for the Cell Mito Stress Test indicated by text and colored panels.
Contrastingly, in females, 3-way ANOVA demonstrated a main effect of measurement (F(11;156)= 24.16, p<0.0001) and diet (F(1,156)=5.747, p=0.0177), such that fructose-fed females demonstrated increased baseline OCR across all measurements compared to chow-fed females regardless of whether or not they underwent the FST (Figure 5). There was no effect of diet or FST exposure on measures of mitochondrial function following treatment with oligomycin, FCCP, or rotenone and antimycin A in females (p>0.05). See Supplementary Figure 2.
Discussion
Escalating incidence of metabolic syndrome in adolescents (Morrison et al., 2008; Welsh et al., 2011) and adults (Montonen et al., 2007; Sánchez-Lozada et al., 2007; Dupas et al., 2017) has driven interest in the deleterious effects of metabolic stress on behavioral and neurological outcomes. The findings in this study demonstrate that elevated consumption of fructose initiated in adolescence alters adult physiology, behavior, and synaptic function in a sex-specific manner. Both males and females displayed fructose-induced alterations in synaptic respiration, however the directionality was sex-dependent. Furthermore, females had elevated weight gain and increased inactivity in the forced swim following high fructose consumption while males exhibited increased exploratory behavior in the open field and had a dysregulated stress response. Taken together, these data suggest that consumption of a HFD manifests causes sex-specific consequences in behavior that may be driven by alterations in synaptic metabolism.
Fructose consumption modifies physiological parameters in females
Consumption of a HFD altered the physiological parameters assessed in this study in female rats, but not in male rats. Fructose-fed females gained more weight than chow-fed females beginning at 8 weeks on the diet. This is consistent with previous findings in our lab that observed an increase in weight gain in fructose-fed females after 7 weeks on the diet while males did not show exacerbated weight gain with a HFD (Hyer et al., 2019). In the current study, fructose-fed females and males showed elevated circulating glucose at three timepoints during the period of diet consumption. These data are slightly different from those observed by Hyer et. al. (2019) as females did not show increased circulating glucose. It’s possible that the weekly glucose assessment led to a more attenuated response as the values observed by Hyer et. al. (2019) are generally lower in both males and females. Here, caloric efficiency was modified by diet in weeks 5 and 6 of the paradigm in female rats, indicating that alterations in energy utilization are a possible source of the observed increases in body mass beyond that of the control-fed female rats (Schwarz et al., 1992; Cox et al., 2012). Traditionally, weight gain or alterations in caloric efficiency are the most superficial indication that metabolic alterations have occurred. The absence of modifications in body weight in the males does not confirm the absence of maladaptive qualitative changes. In fact, like females, the males fed the HFD in the current study displayed increased circulating glucose throughout the study – a diet-induced physiological change. Many studies have reported pathophysiological states indicative of metabolic syndrome following ingestion of a HFD independent of obesity (Tran et al., 2009; Harrell et al., 2015). Taken together, these data indicate that there are sex-specific changes in physiology following long-term consumption of a diet high in fructose.
Dietary modification alters mitochondrial respiration differentially in males and females
Altered physiology following consumption of a HFD was also evident in the brain. Fructose-fed males demonstrated decreased synaptosomal OCR in comparison to chow-fed counterparts, regardless of whether or not they were exposed to an acute physical challenge (FST). This may indicate that excessive consumption of fructose hindered mitochondrial bioenergetic efficiency, possibly through reduced mitochondrial population at the level of the synapse. This decrease could also be attributed to a variety of unique properties of the mitochondria (Liesa and Shirihai, 2013; Picard et al., 2015), for example mutations in tRNA mutations or mtDNA abnormalities (Patti et al., 2003; Frederick H. Wilson, Ali Hariri et al., 2004; Nishio et al., 2004) or increased oxidative stress (Cigliano et al., 2018)(Mastrocola et al., 2016). Other studies have demonstrated that a HFD disrupts insulin signaling in the brain (Agrawal and Gomez-Pinilla, 2012) which, as a potent regulator of mitochondrial biogenesis, could provide an explanation for decreased mitochondrial biogenesis as a result of excessive fructose consumption (Agrawal et al., 2016). In conjunction with one another, these studies suggest multiple mechanisms by which a HFD could decrease OCR such as altered biogenesis, mitochondrial structure, or mitochondrial efficiency. Future studies will be essential to determine the precise mechanisms that account for the effects of fructose on OCR.
Contrary to findings in males, the HFD resulted in an increase in OCR in females. Mitochondria initiate steroidogenesis, and various studies report that brain mitochondrial function is regulated by sex steroids (Gaignard et al., 2015, 2018). Estrogen has been widely reported to provide a neuroprotective effect in the face of homeostatic challenges (Arevalo et al., 2015), including in the context of metabolic disorders (Carswell et al., 2000; Toung et al., 2000). Furthermore, several studies report that the neuroprotective effects of estrogen may act through specific mitochondrial mechanisms, such as hindering excessive reactive oxygen species (ROS) production, regulation of mitochondrial Ca2+ loading, and the preservation of mitochondrial membrane integrity during times of stress (Wang et al., 2001; Nilsen, 2008). Studies conducted in both male and female Wistar rats have reported that synaptic mitochondria of female rats produce less ROS than males, resulting in less oxidative damage to mitochondria (Borrás et al., 2003). Female resilience to mitochondrial damage may be responsible for the reversal of the dietary effect evident in males.
A period of energetic demand increases mitochondrial respiration in males, but not females
In addition to diet, exposure to acute stress in the form of a FST for chow-fed rats increased mitochondrial respiration in males, but not in females. The FST constitutes a period of increased energetic demand in order to overcome the acute stress of forced swimming. Due to this increased energetic demand it is perhaps unsurprising that mitochondrial respiration increased in males. Globally, mitochondria are particularly sensitive to challenges to homeostasis and adjust their bioenergetic output accordingly (Manoli et al., 2007). Acute stress exposure leads to a migration of mitochondria to excitatory synapses, suggesting that increased respiration could be connected to an increase in number and volume of viable mitochondria within the synaptic terminal (Musazzi et al., 2019). Additionally, in response to stress, a cascade of hormones (such as corticosterone) is initiated in the CNS to mobilize substrates to meet the energetic demands of the ‘fight or flight response’. These substrates are then available for oxidation by the mitochondria. Short-term exposure to elevated levels of glucocorticoids, as demonstrated by increased corticosterone concentrations following the FST in the current study, is associated with increased mitochondrial biogenesis and other enzymatic responses of respiratory chain complexes in various tissues including the brain (Navarro et al., 2004).
In contrast, females undergoing the FST did not demonstrate significant modifications of OCR as a result of the FST. In a study investigating sex differences in mitochondrial biogenesis via mitochondrial protein synthesis in response to sprint interval training, Scalzo et al. found that mitochondrial biogenesis was higher in males than females following nine sprint interval training sessions over the course of four weeks (Scalzo et al., 2014). In conjunction with the current study, the data from Scalzo et al. suggest that synaptosomal mitochondrial OCR may not be directly impacted by exposure to high, physical energetic demand in females.
Circulating corticosterone is modified by diet in males, but not females
In males, modification of the HPA-axis was reflected via altered circulating corticosterone following the FST. Measurement of corticosterone production following the FST indicated that fructose-fed male rats produced significantly less corticosterone than their chow-fed counterparts. Unsurprisingly, both groups responded to the acute stress of the forced swim by releasing significantly more corticosterone than baseline males, regardless of diet. Both acute intake (Brindley et al., 1981, 1985) and chronic consumption of fructose have been shown to remodel the HPA axis transcriptome (Harrell et al., 2015). Excessive fructose consumptioninflicts chronic energetic stress on the HPA-axis, resulting in the HPA-axis being unable to efficiently perform its physiological role, and perhaps being less responsive to environmental stimuli. Thus, it is possible that the energetic stress brought on by the HFD initiated maladaptive changes to the HPA axis, which resulted in decreased glucocorticoid output following the acute stress stimulation from the FST in male rats (Matthews et al., 2001).
While corticosterone concentrations were not modified by diet in females, the increase in OCR of fructose-fed females undergoing the FST relative to chow-fed females provides interesting context to the lack of effect of diet on corticosterone concentrations in females. Mitochondria contain powerful antioxidant machineries that assist in the body’s defense against ROS. Studies have documented the effect of known cytoplasmic antioxidants, such as resveratrol, on serum corticosterone concentrations and depressive-like behavior (Johnson, Fournier, & Kalynchuk, 2006; Liu et al., 2014) suggesting that increased antioxidant activity can decrease depressive-like behaviors. Given the upregulation of mitochondrial respiration in the fructose-fed females in the FST, it is plausible that this resulted in higher efficacy of mitochondrial antioxidant mechanisms, leading to the lack of dietary effect on corticosterone concentrations observed in these rats.
Altered mitochondrial respiration is accompanied by sex-dependent affective-like behavior
In the open field, a validated measurement of anxiety-like behavior (Prut and Belzung, 2003), fructose-fed males showed increased exploratory behavior as evidenced by their increased number of center crosses when compared to chow-fed controls. Given that corticosterone and OCR was decreased in fructose-fed males, it’s possible that fructose-fed males exhibited a general reduction in inhibition. The overall decreased inhibition may indicate a blunted responsivity to the novelty of the open field manifesting as increased exploratory behavior. This hypothesis may be better confirmed with the inclusion of an acoustic startle test in future paradigms given the connection between sucrose avidity and startle reactivity (Desousa et al., 1998; Runke et al., 2011). By including a test such as startle response, which assesses involuntary responsivity to stimulus presentation, the impact of a HFD on inhibitory control could be further explored.
The acute physical challenge used in this study, the FST, is also a measurement of depressive-like behavior (Porsolt et al., 1978). The behavioral patterns of the rats during this acute physical challenge were assessed and fructose-fed females exhibited increased floating time, indicative of a depressive-like phenotype. As the HFD-fed females displayed increased weight gain without an increase in corticosterone, it is possible that the reduction in active swimming behavior and the increased respiration was a result of decreased motor function as opposed to increased depressive-like behavior. These data are in line with Hyer et al. (2019) that observed impaired motor function on the Rotorod task and reduced integrity of acetylcholine receptors at the neuromuscular junction following consumption of the HFD.
In conclusion, we expand on previous studies that have investigated the effects of a dietary disturbance on affective-like behaviors and mitochondrial function by including assessment of synaptic respiration and HPA responsivity in both males and females. This study implicates synaptic mitochondrial function as a potential area of intersection between energetic imbalances and behavioral modifications. The data presented herein indicate that excessive fructose consumption precipitates changes in mitochondrial respiration in the brain, and these changes diverge in a sex-specific manner. Collectively, these data provide evidence that a dietary modification is able to initiate changes at the mitochondrial level of the brain, which are accompanied by changes in behavior and neuroendocrine response. Furthermore, the differential results observed in the males and females within this study necessitate the inclusion of females in future paradigms involving dietary models and the brain.
Supplementary Material
High fructose diet causes divergent behavioral phenotypes in males and females.
Synaptic respiration is divergently altered by diet in males and females.
Acute stress differentially impacted synaptic respiration in males and females.
Diet-induced alterations to neural function and physiology are sex-specific.
Synaptic mitochondrial function may contribute to the behavioral differences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal R, Gomez-Pinilla F (2012) ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol 590:2485–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R, Noble E, Vergnes L, Ying Z, Reue K, Gomez-Pinilla F (2016) Dietary fructose aggravates the pathobiology of traumatic brain injury by influencing energy homeostasis and plasticity. J Cereb Blood Flow Metab 36:941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo M-A, Azcoitia I, Garcia-Segura LM (2015) The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci 16:17–29. [DOI] [PubMed] [Google Scholar]
- Baynes JW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–412. [DOI] [PubMed] [Google Scholar]
- Borrás C, Sastre J, García-Sala D, Lloret A, Pallardó FV, Viña J (2003) Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 34:546–552. [DOI] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79:537–543. [DOI] [PubMed] [Google Scholar]
- Brindley DN, Cooling J, Glenny HP, Burditt SL, McKechnie IS (1981) Effects of chronic modification of dietary fat and carbohydrate on the insulin, corticosterone and metabolic responses of rats fed acutely with glucose, fructose or ethanol. Biochem J 200:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley DN, Saxton J, Shahidullah H, Armstrong M (1985) Possible relationships between changes in body weight set-point and stress metabolism after treating rats chronically with D-fenfluramine. Effects of feeding rats acutely with fructose on the metabolism of corticosterone, glucose, fatty acids, glycerol an. Biochem Pharmacol 34:1265–1271. [DOI] [PubMed] [Google Scholar]
- Carswell HVO, Dominiczak AF, Macrae IM (2000) Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Circ Physiol 278:H290–H294. [DOI] [PubMed] [Google Scholar]
- Castro JE, Varea E, Márquez C, Cordero MI, Poirier G, Sandi C (2010) Role of the amygdala in antidepressant effects on hippocampal cell proliferation and survival and on depression-like behavior in the rat Giurfa M, ed. PLoS One 5:e8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Naftilan AJ, Oparil S (1992) Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 19:456–463. [DOI] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Nicholls DG (2009) Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem 109:1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigliano L, Spagnuolo MS, Crescenzo R, Cancelliere R, Iannotta L, Mazzoli A, Liverini G, Iossa S (2018) Short-Term Fructose Feeding Induces Inflammation and Oxidative Stress in the Hippocampus of Young and Adult Rats. Mol Neurobiol 55:2869–2883. [DOI] [PubMed] [Google Scholar]
- Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Havel PJ, Keim NL (2012) Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr 66:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK (2000) Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry 57:21–27. [DOI] [PubMed] [Google Scholar]
- Desousa NJ, Wunderlich GR, De Cabo C, Vaccarino FJ (1998) Individual differences in sucrose intake predict behavioral reactivity in rodent models of anxiety. Pharmacol Biochem Behav 60:841–846. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Robinson PJ (2008) A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc 3:1718–1728. [DOI] [PubMed] [Google Scholar]
- Dupas J, Feray A, Goanvec C, Guernec A, Samson N, Bougaran P, Guerrero F, Mansourati J (2017) Metabolic syndrome and hypertension resulting from fructose enriched diet in wistar rats. Biomed Res Int 2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB (2005) Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493:63–71. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JRG (1978) Can social interaction be used to measure anxiety? Br J Pharmacol 62:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P (2003) A review of 25 years of the social interaction test. Eur J Pharmacol 463:35–53. [DOI] [PubMed] [Google Scholar]
- Ford DE, Erlinger TP (2004) Depression and C-Reactive Protein in US adults. Arch Intern Med 164:1010. [DOI] [PubMed] [Google Scholar]
- Wilson Frederick H., Hariri Ali AF, Zhao Hongyu, Petersen Kitt Falk HRT, Nelson-Williams Carol KMR, Kashgarian Michael GIS, Scheinman Steven J. RPL (2004) A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. [DOI] [PMC free article] [PubMed]
- Gaignard P, Fréchou M, Liere P, Thérond P, Schumacher M, Slama A, Guennoun R (2018) Sex differences in brain mitochondrial metabolism: influence of endogenous steroids and stroke. J Neuroendocrinol 30:e12497. [DOI] [PubMed] [Google Scholar]
- Gaignard P, Savouroux S, Liere P, Pianos A, Thérond P, Schumacher M, Slama A, Guennoun R (2015) Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology 156:2893–2904. [DOI] [PubMed] [Google Scholar]
- Galipeau D, Verma S, McNeill JH (2002) Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Circ Physiol 283:H2478–H2484. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G (1996) Oxidative stress and diabetic vascular complications. Diabetes Care 19:257–267. [DOI] [PubMed] [Google Scholar]
- Harrell CS, Burgado J, Kelly SD, Johnson ZP, Neigh GN (2015) High-fructose diet during periadolescent development increases depressive-like behavior and remodels the hypothalamic transcriptome in male rats. Psychoneuroendocrinology 62:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D (2012) Synaptic energy use and supply. Neuron 75:762–777. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Schweiger U, Correll C, Muller C, Busch M-L, Bauer M, Schwarz P (2015) Depression, anxiety disorders, and metabolic syndrome in a population at risk for type 2 diabetes mellitus. Brain Behav 5:n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17:491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louet J-F, LeMay C, Mauvais-Jarvis F (2004) Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep 6:180–185. [DOI] [PubMed] [Google Scholar]
- Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, Chrousos GP (2007) Mitochondria as key components of the stress response. Trends Endocrinol Metab 18:190–198. [DOI] [PubMed] [Google Scholar]
- Marriott BP, Cole N, Lee E (2009) National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 139:1228S–1235S. [DOI] [PubMed] [Google Scholar]
- Mastrocola R, Nigro D, Cento AS, Chiazza F, Collino M, Aragno M (2016) High-fructose intake as risk factor for neurodegeneration: Key role for carboxy methyllysine accumulation in mice hippocampal neurons. Neurobiol Dis 89:65–75. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, Owens JF (2001) Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Heal Psychol 20:403–410. [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JK, Konarski JZ, Kennedy SH (2006) The effect of antidepressants on glucose homeostasis and insulin sensitivity: synthesis and mechanisms. Expert Opin Drug Saf 5:157–168. [DOI] [PubMed] [Google Scholar]
- Meister B (2007) Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav 92:263–271. [DOI] [PubMed] [Google Scholar]
- Montonen J, Järvinen R, Knekt P, Heliovaara M, Reunanen A (2007) Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr 137:1447–1454. [DOI] [PubMed] [Google Scholar]
- Morrison JA, Friedman LA, Wang P, Glueck CJ (2008) Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr 152:201–206. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443:289–295. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Sala N, Tornese P, Gallivanone F, Belloli S, Conte A, Di Grigoli G, Chen F, Ikinci A, Treccani G, Bazzini C, Castiglioni I, Nyengaard JR, Wegener G, Moresco RM, Popoli M (2019) Acute inescapable stress rapidly increases synaptic energy metabolism in prefrontal cortex and alters working memory performance. Cereb Cortex. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB (1998) The relationship of depression to cardiovascular disease. Arch Gen Psychiatry 55:580. [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Münzberg H (2008) Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, López-Cepero JM, Boveris A (2004) Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Integr Comp Physiol 286:R505–R511. [DOI] [PubMed] [Google Scholar]
- Nilsen J (2008) Estradiol and neurodegenerative oxidative stress. Front Neuroendocrinol 29:463–475. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Kanazawa A, Nagai Y, Inagaki H, Kashiwagi A (2004) Regulation and role of the mitochondrial transcription factor in the diabetic rat heart. Ann N Y Acad Sci 1011:78–85. [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ (2003) Cluster analysis and display of genome-wide expression patterns. PNAS 95:14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JB, Frishman WH, Feinstein RE (2000) Major depression as a risk factor for cardiovascular disease: therapeutic implications. Heart Dis 2:75–82. [PubMed] [Google Scholar]
- Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS, Wallace DC (2015) Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci U S A 112:E6614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33. [DOI] [PubMed] [Google Scholar]
- Rose S, Frye RE, Slattery J, Wynne R, Tippett M, Melnyk S, James SJ (2014) Oxidative stress induces mitochondrial dysfunction in a subset of autistic lymphoblastoid cell lines. Transl Psychiatry 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runke D, McIntyre DC, St-Onge V, Gilby KL (2011) Relation between startle reactivity and sucrose avidity in two rat strains bred for differential seizure susceptibility. Exp Neurol 229:259–263. [DOI] [PubMed] [Google Scholar]
- Rutledge AC, Adeli K (2007) Fructose and the metabolic syndrome : Pathophysiology and molecular mechanisms. Nutrition 65:13–23. [DOI] [PubMed] [Google Scholar]
- Sánchez-Lozada LG, Tapia E, Jiménez A, Bautista P, Cristóbal M, Nepomuceno T, Soto V, Ávila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M (2007) Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Physiol 292:F423–F429. [DOI] [PubMed] [Google Scholar]
- Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Klochak AL, Lonac MC, Paris HLR, Szallar SE, Wood LM, Peelor FF, Holmes WE, Hellerstein MK, Bell C, Hamilton KL, Miller BF (2014) Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28:2705–2714. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Schutz Y, Piolino V, Schneider H, Felber JP, Jequier E (1992) Thermogenesis in obese women: effect of fructose vs. glucose added to a meal. Am J Physiol 262:E394–401. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Hinokio Y, Komatu K, Ohtomo M, Onoda M, Hirai S, Hirai M, Hirai A, Chiba M, Kasuga S, Akai H, Toyota T (1999) Oxidative damage to mitochondrial DNA and its relationship to diabetic complications. Diabetes Res Clin Pract 45:161–168. [DOI] [PubMed] [Google Scholar]
- Toung TK, Hurn PD, Traystman RJ, Sieber FE (2000) Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke 31:2701–2706. [DOI] [PubMed] [Google Scholar]
- Tran LT, Yuen VG, McNeill JH (2009) The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem 332:145–159. [DOI] [PubMed] [Google Scholar]
- Vartanian LR, Schwartz MB, Brownell KD (2007) Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health 97:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan H, Xiang H, McNeill JH (2005) Differential regulation of insulin resistance and hypertension by sex hormones in fructose-fed male rats. Am J Physiol Circ Physiol 289:H1335–H1342. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, Garnier A (2017) Mitochondria: a central target for sex differences in pathologies. Clin Sci 131:803–822. [DOI] [PubMed] [Google Scholar]
- Vos M, Lauwers E, Verstreken P (2010) Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA (1976) The open-field test: A critical review. Psychol Bull 83:482–504. [PubMed] [Google Scholar]
- Wang J, Green PS, Simpkins JW (2001) Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J Neurochem 77:804–811. [DOI] [PubMed] [Google Scholar]
- Welsh JA, Sharma A, Cunningham SA, Vos MB (2011) Consumption of Added Sugars and Indicators of Cardiovascular Disease Risk Among US Adolescents. Circulation 123:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Ford ES, Dhingra S, Li C, Strine TW, Mokdad AH (2009) Depression and anxiety among US adults: associations with body mass index. Int J Obes 33:257–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.