Abstract
Rationale & Objective:
Intact cognition is generally a prerequisite for navigating through and completing evaluation for kidney transplantation (KT). Despite KT being contraindicated for those with severe dementia, screening for more mild forms of cognitive impairment prior to referral is rare. Candidates may have unrecognized cognitive impairment, which may prolong evaluation, elevate mortality risk, and hinder access to KT. We estimated the burden of cognitive impairment and its association with access to KT and waitlist mortality.
Study Design:
Prospective cohort study.
Setting & Participants:
3,630 participants (1/2009–6/2018) with cognitive function (Modified Mini-Mental State Examination [3MS]) measured at kidney transplant evaluation at one of two transplant centers.
Predictors:
Cognitive Impairment (3MS score <80).
Outcomes:
Listing, waitlist mortality, and KT.
Analytical Approach:
We estimated adjusted chance of listing (Cox regression), risk of waitlist mortality (competing risks regression), and KT rate (Poisson regression) by cognitive impairment. Given potential differences in etiology in cognitive impairment among those with and without diabetes, we tested whether these associations differed by diabetes status using a Wald test.
Results:
At evaluation, 6.4% of participants had cognitive impairment, which was independently associated with 25% lower chance of listing (aHR, 0.75; 95%CI, 0.61–0.91); this association did not differ by diabetes status (pinteraction=0.07). There was a nominal difference by diabetes status for the association between cognitive impairment and KT rate (pinteraction=0.05), while the association between cognitive impairment and waitlist mortality differed by diabetes status KT rates (pinteraction=0.02). Among candidates without diabetes, those with cognitive impairment were at 2.47 (95%CI,1.31–4.66) times greater risk of waitlist mortality; cognitive impairment was not associated with this outcome among candidates with diabetes.
Limitations:
Single measure of cognitive impairment.
Conclusions:
Cognitive impairment is associated with a lower chance of being placed on the waitlist, and among patients without diabetes, with increased mortality on the waitlist. Future studies should investigate whether implementation of screening for cognitive impairment improves these outcomes.
Keywords: cognitive impairment, renal transplantation, end-stage renal disease (ESRD), waitlisting, dementia, diabetes, functional dependence, dialysis, mental capacity, transplant candidate, cognitive function testing, health literacy
INTRODUCTION
Cognitive impairment is common in patients with kidney failure undergoing dialysis, ranging anywhere between 10%-80% depending on the population being studied and the test used to define it.1–5 While patients with diagnosed dementia, a state of chronic and severe cognitive impairment that affects functional ability, are contraindicated for kidney transplantation (KT),6 candidates may still have unrecognized mild cognitive impairment.7 Kidney transplant candidates are rarely screened by clinicians before referral to a transplant center for evaluation and few transplant centers assess cognitive function.8 The proportion of transplant candidates that have cognitive impairment at time of evaluation for KT, as well as the extent that it affects their functional dependence, are unclear.
Among kidney transplant candidates, intact cognition is critical for completion of transplant evaluation. Cognitive impairment leads to diminished health literacy among kidney transplant candidates,9 which may in turn spur difficulties with navigating a complex medical system and scheduling specialist appointments, leading to incomplete evaluations and a lack of access to KT.10 Furthermore, cognitive impairment diminishes capacity to manage their chronic conditions and adhere to complex medication regimens, as well as fluid and dietary restrictions, while undergoing dialysis and waiting for KT,4,11 which may impact waitlist mortality.12 There is a crucial gap in knowledge surrounding the association between cognitive impairment at KT evaluation and access to KT, as well as waitlist mortality.
To better understand cognitive impairment among patients being evaluated for KT, we leveraged a two-center prospective cohort study of dialysis patietns undergoing evaluation for transplantation (n=3,337). The main goals of this study were to assess: 1) the prevalence of cognitive impairment and the level of functional dependence among those with cognitively impairment; 2) the chance of listing for KT and rate of KT by cognitive impairment status at time of evaluation; and 3) the risk of KT waitlist mortality. Given potential differences in etiology in cognitive impairment among those with and without diabetes, we also tested whether these associations differed by diabetes status.
METHODS
Study Design
We leveraged a two-center, prospective study of 3,630 adult dialysis patients aged 18 years and older who were being evaluated for KT at the Johns Hopkins Hospital (1/2009–6/2018; n=3,337) and the University of Michigan University Hospital (1/2014–6/2018; n=293) with cognitive function measured at time of evaluation for KT (Figure S1), as described below. Participant characteristics at time of evaluation were self-reported or abstracted from medical records (age, sex, race, education, diabetes status, prior solid organ transplant (renal and non-renal), Charlson comorbidity index [CCI] adapted for dialysis13,14). Characteristics of the study population did not greatly differ from all kidney transplant candidates at Johns Hopkins Hospital based on age (mean age of 50.1 vs 53.5 for those who did not vs did participate), sex (41.1% vs 40.7% women for those who did not vs did participate), and black race (40.1% vs 44.4% in those who did vs did not participate) or from the study sample. Additionally, functional dependence was measured at time of evaluation based on self-report, including activities of daily living (ADLs) and instrumental activities of daily living (IADLs). The Johns Hopkins Institutional Review Board and the University of Michigan Institutional Review Board approved the study, and all participants provided written informed consent.
Global Cognitive Function
Global cognitive function was measured using the Modified Mini-Mental State Examation (3MS)15,16 at time of evaluation for KT (not during dialysis treatment). The 3MS was administered by trained research assistants in a private clinic room and was collected as part of a larger cohort study of aging and KT; it was solely measured for research purposes. The 3MS is a validated 15-item verbal test assessing multiple components, including psychomotor skills, memory, identification/association, orientation, and concentration/calculation. 3MS scores range from 0 to 100, where higher scores represent better cognitive function. The 3MS presents enhanced sensitivity for mild cognitive impairment in community studies over the traditional 30-point Mini-Mental State Examination (MMSE),15–17 and has a higher test-retest reliability (between 0.68 and 0.77) compared to the MMSE (between 0.48 and 0.65). Consistent with prior studies, cognitive impairment was defined as a 3MS score less than 80 (-1 SD).17–20 Providers were not aware of 3MS results at the committee listing meeting.
Descriptive Statistics by Cognitive Impairment
We generated percentages for categorical characteristics, means and standard deviations (SD) for normally distributed continuous variables, and medians and interquartile ranges (IQR) for non-normally distributed continuous variables by global cognitive impairment status among all participants at time of evaluation for KT. Additionally, we compared prevalence estimates of functional dependence by global cognitive impairment, overall and by activity. We further explored the components of the 3MS, including psychomotor skills, memory, identification/association, orientation, and concentration/calculation, to assess potential differences by diabetes status at time of evaluation.
Chance of Listing by Cognitive Impairment
Among participants not listed for KT prior to evaluation (n=3,630), unadjusted cumulative incidence was estimated at 6 months, 1 year, and 3 years using the Kaplan Meier method by global cognitive impairment status and diabetes status. Adjusted Cox proportional hazards models were used to estimate the chance of kidney transplant listing by cognitive impairment. Time to listing was defined as the time from evaluation for KT to the date of active placement on the kidney transplant waitlist or administrative censoring at the end of the study period (6/2018). Proportional hazards assumptions were confirmed by visual inspection of the complementary log-log plots and Schoenfeld residuals. The model was adjusted for age, sex, race, education, diabetes, and CCI. To test whether the association between chance of listing and cognitive impairment varied by participant age (age<65 vs. ≥65), sex (female vs. male), race (black vs. non-black), diabetes status (present vs. absent), or functional dependence (ADL/IADL: present vs. absent), an interaction between those factors and cognitive impairment was explored using a Wald test.
Risk of Waitlist Mortality by Cognitive Impairment
Among participants who were listed for KT (kidney transplant candidates) (n=2,216), Fine and Gray competing risks models21 were used to estimate unadjusted cumulative incidence of waitlist mortality by global cognitive impairment and diabetes status, accounting for KT as a competing risk. The competing risk framework was also used to estimate the adjusted risk of waitlist mortality by cognitive impairment in kidney transplant candidates. The time origin was date of listing, and kidney transplant candidates were followed to date of death; candidates were censored at the end of the study period (6/2018) if it preceded mortality or KT. Adjusted models controlled for age, sex, race, education, diabetes, and CCI. To test whether the association between risk of mortality and cognitive impairment varied by candidate age, sex, race, diabetes status, or functional dependence (ADL/IADL), an interaction between those factors and cognitive impairment was explored using a Wald test.
Rate of Transplantation by Cognitive Impairment
Additionally, among kidney transplant candidates, a competing risk framework was used to estimate unadjusted cumulative incidence of KT by global cognitive impairment status. Rate of transplantation by cognitive impairment in kidney transplant candidates was assessed using Poisson regression to generate incidence rate ratios (IRR). Person-time was calculated from the date of active kidney transplant listing to the date of KT, mortality, or censoring at the end of the study period (6/2018); the person-time did not include inactive time. All models were adjusting for age, sex, race, education, diabetes, and CCI. To test whether the association between rate of transplantation and cognitive impairment varied by candidate age, sex, race, diabetes status, or functional dependence (ADL/IADL), an interaction between those factors and cognitive impairment was explored using a Wald test.
Statistical Analyses
We estimated adjusted chance of listing (adjusted Cox regression), risk of waitlist mortality (adjusted competing risks regression), and KT rate (adjusted Poisson regression) by cognitive impairment using Stata version 15 (StataCorp, College Station, TX). Two-sided p-values < 0.05 were considered statistically significant.
Sensitivity Analyses
First, to check whether the inferences for the associations between cognitive impairment and listing, waitlist mortality, and KT rate remain robust to those with potentially “recognizable” cognitive impairment, we excluded those with a history of reported dementia or a diagnosis of dementia. Second, we further adjusted for variables that were found to be significantly different by cognitive impairment status at time of evaluation in addition to the selected a priori factors in the conceptual framework that depicts the current understanding of cognitive function and adverse outcomes in community-dwelling older adults22 and patients with kidney failure.23 Third, for chance of listing, we conducted analyses from time of dialysis initiation to listing date to compare results conducted from time of evaluation to listing to consider hypothesis that cognitively impaired patients have difficulty navigating the system. Fourth, for waitlist mortality, we conducted a sensitivity analysis censoring for KT to compare results treating it as a competing risk.
RESULTS
Study Population
Of the cohort of 3,630 dialysis patients being evaluated for KT, the median age was 56 years (interquartile range [IQR], 45–65), 41.2% were female, 45.5% were black. At time of evaluation for KT, 6.4% of participants were identified as having cognitive impairment (Table 1). Participants with cognitive impairment were more likely to be older (median age of 62 vs. 56 years, p<0.001), black (69.1% vs. 43.8%, p<0.001), have diabetes as a comorbidity (49.5% vs. 41.7%, p=0.04), and have lower educational attainment (75.5% vs. 42.3%, p<0.001), but were less likely to be female (30.0% vs. 41.9%, p<0.001) (Table 1).
Table 1.
Characteristics by global cognitive impairment among dialysis patients being evaluated for kidney transplantation (KT)
| Overall (N=3,630) |
Global Cognitive Impairment | |||
|---|---|---|---|---|
| Not Impaired (n=3,397) |
Impaired (n=233) |
p-value | ||
| Age, median years [IQR1 | 56.0 [45.1–65.1] | 55.6 [44.5–64.7] | 62.1 [54.1–69.8] | <0.001 |
| Female (%) | 41.2 | 41.9 | 30.0 | <0.001 |
| Race (%) | <0.001 | |||
| White | 47.7 | 49.4 | 21.9 | |
| Black | 45.5 | 43.8 | 69.1 | |
| Other | 6.9 | 6.7 | 9.0 | |
| High School or less (%) | 44.5 | 42.3 | 75.5 | <0.001 |
| BMI | 29.2 [6.0] | 29.3 [6.0] | 28.0 [5.9] | 0.03 |
| ADL Dependence (%) | 8.2 | 7.7 | 15.4 | <0.001 |
| IADL Dependence (%) | 20.5 | 19.4 | 36.2 | <0.001 |
| CCI, median [IQR] | 1 [0–3] | 1 [0–3] | 2[0–3] | 0.2 |
| Comorbidity (%) | ||||
| Myocardial infarction | 9.3 | 8.9 | 15.8 | 0.002 |
| Peripheral vascular disease | 6.4 | 6.2 | 8.7 | 0.2 |
| Cerebral vascular disease | 5.7 | 5.3 | 12.1 | <0.001 |
| Dementia | 0.4 | 0.4 | 1.1 | 0.2 |
| Chronic lung disease | 6.0 | 6.0 | 6.0 | 0.9 |
| Rheumatological disease | 6.7 | 6.9 | 4.4 | 0.2 |
| Peptic ulcer disease | 3.6 | 3.4 | 6.0 | 0.07 |
| Diabetes | 42.1 | 41.7 | 49.5 | 0.04 |
| Diabetes complication | 22.5 | 22.4 | 23.2 | 0.8 |
| Moderate/severe liver disease | 3.4 | 3.4 | 3.8 | 0.8 |
| Metastatic cancer | 1.0 | 1.0 | 1.1 | 0.9 |
| Leukemia | 0.3 | 0.3 | 0.0 | 0.4 |
| Lymphoma | 1.0 | 1.0 | 0.0 | 0.2 |
| HIV | 3.1 | 2.8 | 7.6 | <0.001 |
| Congestive heart failure | 14.1 | 13.7 | 21.7 | 0.02 |
| Cause of kidney failure (%) | 0.001 | |||
| Hypertension | 30.1 | 29.4 | 43.9 | |
| Diabetes | 21.0 | 21.0 | 21.1 | |
| Glomerular disease | 21.0 | 21.6 | 10.5 | |
| Other | 27.8 | 28.0 | 24.5 | |
| Years on dialysis, median [IQR] | 0.3 [0.0–1 8] | 0.2 [0.0–18] | 0.8 [0.0–3.2] | <0.001 |
| Prior kidney transplant (%) | 19.6 | 20.0 | 14.0 | 0.1 |
| Prior non-renal organ transplant (%) | 2.9 | 3.0 | 2.4 | 0.7 |
Values for continuous variables given as mean +/− standard deviation or median [interquartile range]. Participants were classified as cognitively impaired if they had a Modified Mini-Mental State Exam (3MS) score <80. Numbers and percentages at time of evaluation for kidney transplantation are presented unless otherwise indicated. Abbreviations: ADL, activities of daily living (including dependence in feeding, dressing, physical ambulation, bathing, toileting, or grooming); IADL, instrumental activities of daily living (including dependence in using the phone, shopping, cooking, house cleaning, washing, using transportation, managing money, or medications); CCI, Charlson comorbidity index adapted for dialysis.
Cognitive Impairment and Functional Dependence Burden
Notably, cognitive impairment at time of evaluation for KT was associated with functional dependence for both ADLs (15.4% vs. 7.7%, p<0.001) and IADLs (36.2% vs. 19.4%, p<0.001) (Table 2). Among the six ADL components, difficulty with physical ambulation (10.0% vs. 5.2%, p=0.004), dressing (4.7% vs. 1.4%, p<0.001), bathing (6.7% vs. 2.2%, p<0.001), and toileting (1.9% vs. 0.6%, p=0.03) were associated with cognitive impairment. All eight IADL components were associated with cognitive impairment, including difficulty shopping (24.3% vs. 9.5%, p<0.001), washing (19.5% vs. 7.7%, p<0.001), taking transportation (13.8% vs. 5.9%, p<0.001), managing medications (13.8% vs. 3.3%, p<0.001), managing money (13.4% vs. 2.6%, p<0.001), cooking (13.3% vs. 5.3%, p<0.001), house cleaning (11.0% vs. 5.1%, p<0.001), and using the phone (1.0% vs. 0.1%, p=0.007) (Table 2).
Table 2.
Functional dependence (ADLs and IADLs) and global cognitive impairment in dialysis patients being evaluated for kidney transplantation (KT)
| Overall (N=3,630) |
Global Cognitive Impairment | |||
|---|---|---|---|---|
| Not impaired (n=3,397) |
Impaired (n=233) |
p-value | ||
| ADL Dependence (Overall) | 8.2 | 7.7 | 15.4 | <0.001 |
| Feeding | 0.5 | 0.4 | 0.9 | 0.3 |
| Dressing | 1.6 | 1.4 | 4.7 | <0.001 |
| Physical Ambulation | 5.5 | 5.2 | 10.0 | 0.004 |
| Grooming | 1.4 | 1.2 | 3.3 | 0.1 |
| Toileting | 0.7 | 0.6 | 1.9 | 0.03 |
| Bathing | 2.5 | 2.2 | 6.7 | <0.001 |
| IADL Dependence (Overall) | 20.5 | 19.4 | 36.2 | <0.001 |
| Using Phone | 0.2 | 0.1 | 1.0 | 0.007 |
| Shopping | 10.4 | 9.5 | 24.3 | <0.001 |
| Cooking | 5.9 | 5.3 | 13.3 | <0.001 |
| House Cleaning | 5.5 | 5.1 | 11.0 | <0.001 |
| Washing | 8.5 | 7.7 | 19.5 | <0.001 |
| Transportation | 6.4 | 5.9 | 13.8 | <0.001 |
| Managing Medications | 3.9 | 3.3 | 13.8 | <0.001 |
| Managing Money | 3.3 | 2.6 | 13.4 | <0.001 |
ADL and IADL dependence are presented overall and by component, whereby KT candidates self-reported needing assistance with daily tasks listed. Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living.
Cognitive Function and Diabetes Status
Cognitive impairment was present in 7.3% of those with diabetes compared to 5.4% of those without diabetes (p=0.04). Median scores of the different 3MS components also differed by diabetes status, including psychomotor skills (20 vs 21 points in those with vs without diabetes; p<0.001), memory (19 vs 20 points in those with vs without diabetes with diabetes; p=0.01), and identification/association (23 vs 24 points in those with vs without diabetes; p=0.001) (Table 3). No differences in median scores were found for orientation (25 points for both groups; p=0.4) and concentration/calculation (7 points for both groups; p=0.05) by diabetes status at time of evaluation for KT (Table 3).
Table 3.
Cognitive performance scores by diabetes status at time of evaluation for KT.
| Cognitive Function | Overall (N=2,780) |
With Diabetes (n=1,166) |
Without Diabetes (n=1,614) |
|---|---|---|---|
| Cognitive Impairment (3MS<80) | 172 (6.2%) | 85 (7.3%) | 87 (5.4%) |
| 3MS Total Score | |||
| Psychomotor skills | 21 (2) | 20 (2) | 21 (1) |
| Memory | 20 (4) | 19 (4) | 20 (4) |
| Identification/Association | 24 (4) | 23 (4) | 24 (3) |
| Orientation | 25 (0) | 25 (0) | 25 (0) |
| Concentration/Calculation | 7 (0) | 7 (0) | 7 (0) |
Median unadjusted scores are presented for the Modified Mini-Mental State Exam (3MS) total score (range 0–100) and the 3MS score components, include psychomotor skills (range 0–21), memory (range 0–21), identification/association (range 0–26), orientation (range 0–25), and concentration/calculation (range 0–7). Higher scores indicate better cognitive function. Bolded scores represent statistically significant differences by diabetes status.
Cognitive Impairment and Chance of Listing
Prior to adjustment, participants who were cognitively impaired were less likely to be listed (log rank p<0.001), with a median follow-up time of 5.5 (IQR, 2.0–29.9) months since time of evaluation for KT. The median time between dialysis initiation and listing was greater among those who had cognitive impairment (11.7 [IQR, 0–38.8] months) than in those who did not have cognitive impairment (4.0 [IQR, 0–23.0] months) (p=0.008). Unadjusted cumulative incidence of listing in participants with versus without cognitive impairment was 32.0% vs. 50.5% at 6 months, 43.7% vs. 51.5% at 1 year, and 51.5% vs. 63.8% at 3 years (Table 4).
Table 4.
Cumulative incidence and associations of listing, waitlist mortality, and kidney transplantation (KT) by global cognitive impairment.
| Outcome by Global Cognitive Impairment | n | Unadjusted Cumulative incidence (%) | aHR, aSHR, aIRR* (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| 6 mo | 1 y | 3 y | ||||
| Chance of Listing | ||||||
| Overall | ||||||
| Not impaired | 233 | 50.5 | 59.1 | 63.8 | 1.00 (reference) | |
| Impaired | 3,397 | 32.0 | 43.7 | 51.5 | 0.75 (0.61–0.91) | 0.004 |
| Diabetes | ||||||
| Not impaired | 1,201 | 52.4 | 63.8 | 69.5 | 1.00 (reference) | |
| Impaired | 91 | 42.2 | 57.3 | 65.0 | 0.92 (0.70–1.22) | 0.6 |
| No Diabetes | ||||||
| Not impaired | 1,682 | 63.8 | 73.3 | 79.8 | 1.00 (reference) | |
| Impaired | 93 | 37.9 | 52.9 | 67.7 | 0.63 (0.48–0.83) | 0.001 |
| Risk of Waitlist Mortality | ||||||
| Overall | ||||||
| Not impaired | 2,101 | 0.04 | 1.4 | 9.0 | 1.00 (reference) | |
| Impaired | 115 | 0.07 | 2.2 | 14.2 | 1.35 (0.83–2.18) | 0.2 |
| Diabetes | ||||||
| Not impaired | 793 | 0.7 | 1.9 | 13.5 | 1.00 (reference) | |
| Impaired | 56 | 0.6 | 1.8 | 12.7 | 0.90 (0.48–1.70) | 0.8 |
| No Diabetes | ||||||
| Not impaired | 1,281 | 0.3 | 0.9 | 6.3 | 1.00 (reference) | |
| Impaired | 57 | 0.8 | 2.3 | 16.0 | 2.47 (1.31–4.66) | 0.01 |
| Rate of KT | ||||||
| Overall | ||||||
| Not impaired | 2,101 | 6.3 | 17.8 | 42.7 | 1.00 (reference) | |
| Impaired | 115 | 4.7 | 13.5 | 33.8 | 0.78 (0.56–1.09) | 0.1 |
| Diabetes | ||||||
| Not impaired | 793 | 4.3 | 13.0 | 31.5 | 1.00 (reference) | |
| Impaired | 56 | 4.5 | 13.7 | 33.0 | 1.12 (0.71–1.77) | 0.6 |
| No Diabetes | ||||||
| Not impaired | 1,281 | 7.8 | 20.9 | 49.8 | 1.00 (reference) | |
| Impaired | 57 | 4.9 | 13.6 | 35.1 | 0.58 (0.36–0.93) | 0.03 |
Participants were classified as cognitively impaired if they had a Modified Mini-Mental State Exam (3MS) score <80. Adjusted associations were controlled for age, sex, race, diabetes, educational attainment, and the Charlson Comorbidity Index adapted for dialysis. To explore the association between each outcome and cognitive impairment by diabetes status, an interaction term was added between cognitive impairment and diabetes status. Abbreviations: HR, hazard ratio; SHR, subdistribution hazard ratio; IRR, incidence rate ratio.
aHR for chance of listing; aSHR for risk of waitlist mortality; IRR for rate of kidney transplantation.
After adjustment, cognitively impaired participants had a 25% (adjusted hazard ratio [aHR], 0.75; 95%CI, 0.61–0.91) lower chance of listing compared to those who were not cognitively impaired (Table 4). At a borderline level of statistical significance, this association nominally differed by sex (pinteraction=0.05): among male and female particpants, the aHRs for listing in those with verus without cognitive impairment were 0.55 (95% CI, 0.38–0.80) and 0.86 (95% CI, 0.68–1.08), respectively. This association did not differ by age (pinteraction=0.4), race/ethnicity (pinteraction=0.4), diabetes status (pinteraction=0.05), or functional dependence (ADL: pinteraction=0.9, IADL: pinteraction = 0.9).
Cognitive Impairment and Risk of Waitlist Mortality
Among kidney transplant candidates (n=2,216), the median follow-up time since time of listing for transplantation was 1.6 (IQR, 0.6–2.9) years. The cumulative incidence of mortality did not differ by cognitive impairment (log rank=0.07), though cognitive impairment was associated with a 1.62-times (95% CI, 1.07–2.46) greater risk of mortality prior to adjustment. Unadjusted cumulative incidence of mortality in KT candidates with as compared to those without cognitive impairment was 0.7% vs. 0.4% at 6 months, 2.2% vs. 1.4% at 1 year, and 14.2% vs. 9.0% at 3 years (Table 4).
After adjustment, cognitive impairment was not associated with risk of waitlist mortality among kidney transplant candidates (adjusted subdistribution hazard ratio [aSHR], 1.35; 95% CI, 0.83–2.18) (Table 4). However, this association varied by diabetes status (pinteraction=0.02) (Figure 1). Specifically, among those without diabetes, cognitive impairment was associated with a 2.47-times (95%CI, 1.31–4.66) greater risk of waitlist mortality compared to those without cognitive impairment; however, among those with diabetes, there was no association between cognitive impairment and risk of waitlist mortality (aSHR, 0.90; 95%CI, 0.48–1.70) (Table 4). The association with waitlist mortality did not differ by age (pinteraction=0.3), sex (pinteraction=0.9), race (pinteraction=0.9), or functional dependence (ADL: pinteraction=0.2, IADL: pinteraction=0.9).
Figure 1. Cumulative incidence of waitlist mortality in kidney transplant (KT) candidates (n=2,216) by cognitive impairment and diabetes status.
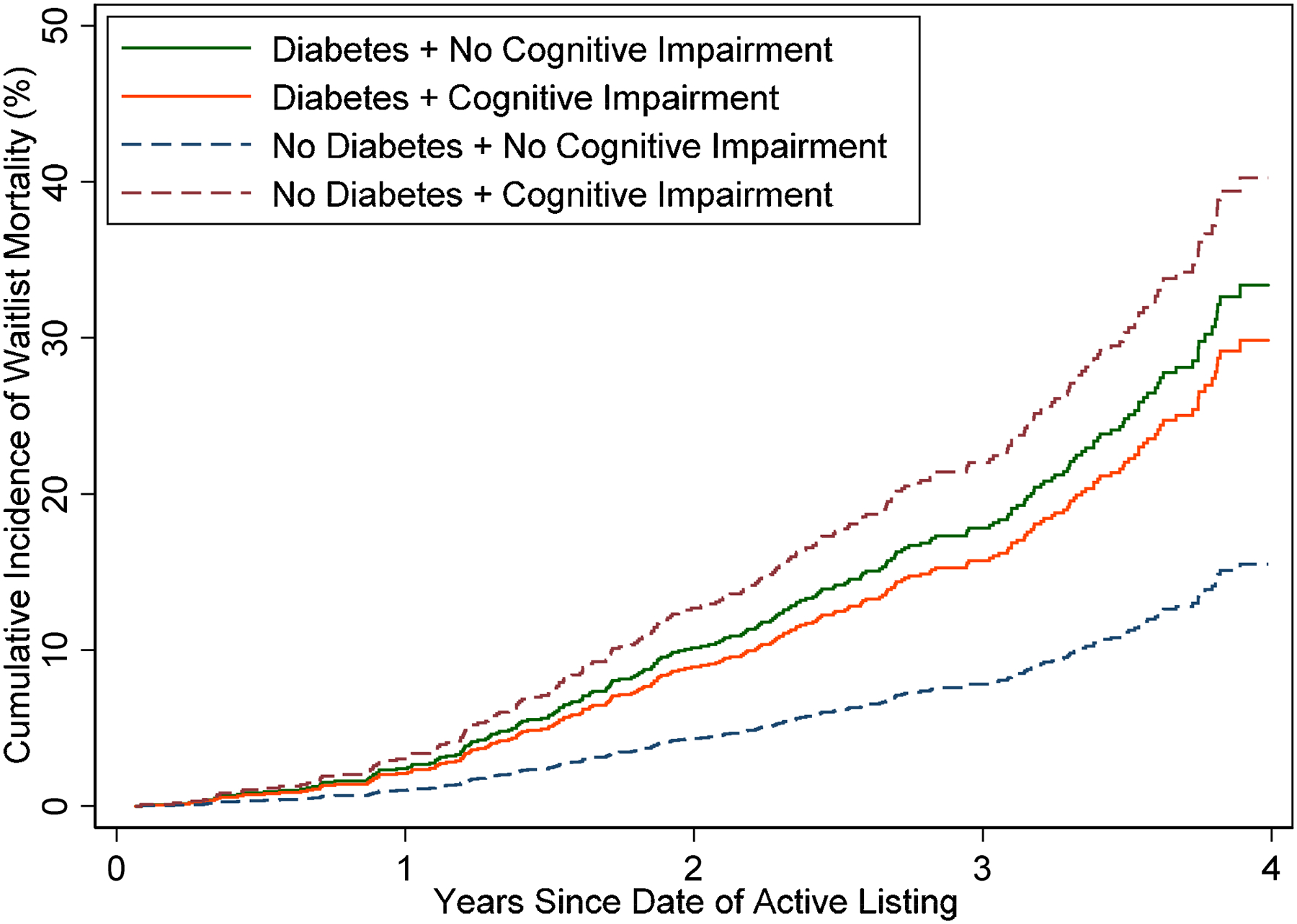
Unadjusted cumulative incidence curves are presented, with KT considered as a competing risk for waitlist mortality. To explore the association between waitlist mortality and cognitive impairment by diabetes status, an interaction term was added between cognitive impairment and diabetes status. Among those without diabetes, cognitive impairment was associated with a greater risk of waitlist mortality compared to those without cognitive impairment. Despite still having high risk of waitlist mortality, among those with diabetes, there was no association between cognitive impairment and waitlist mortality.
Cognitive Impairment and Rate of Kidney Transplantation
Among kidney transplant candidates, the median follow-up time since listing for transplantation was 1.6 (IQR, 0.6–2.9) years. The cumulative incidence of KT did not differ by cognitive impairment (log rank P = 0.1), and cognitive impairment was not associated with KT rate prior to adjustment (unadjusted incidence rate ratio [IRR], 0.74; 95% CI, 0.55–1.01). Unadjusted cumulative incidence of KT in candidates with versus without cognitive impairment was 4.7% vs. 6.3% at 6 months, 13.5% vs. 17.8% at 1 year, and 33.8% vs. 42.7% at 3 years (Table 4).
After adjustment, cognitive impairment was not associated with the rate of KT among candidates (adjusted incidence rate ratio [aIRR], 0.78; 95%CI, 0.56–1.09). However, at a borderline level of statsical significance, the association nominally varied by diabetes status (pinteraction=0.05): among those without and with diabetes, the aIRRs for KT in those with versus without cognitive impairments were 0.58 (95%CI, 0.36–0.93) and 1.12 (95% CI, 0.71–1.77), respetively (Table 4). The association did not differ by age (pinteraction=0.1), sex (pinteraction=0.8), race/ethnicity (pinteraction=0.4), and functional dependence (ADL: pinteraction=0.5, IADL: pinteraction=0.9).
Sensitivity Analyses
Despite some tests losing statistical significance, inferences based on the direction and magnitude of associations generally remained robust across sensitivity analyses, including when 1) excluding those with a history of reported dementia or a diagnosis of dementia; 2) further adjusting for statistically significant variables at time of evaluation in addition to a priori factors; 3) calculating chance of listing using time of dialysis initiation to listing date; and 4) censoring for KT when calculating waitlist mortality risk (Tables S1–4). Inferences related to rates of KT for nondiabetics were the exception to this, whereby the sensitivity analyses were less consistent with the primary analyses. For example, after adjusting for other statistically significant variables at time of evaluation, the sensitivity analysis (aIRR, 1.06; 95%CI, 0.66–1.69; p=0.8) did not reflect the same magnitude, direction, and statistical significance as the primary analysis (aIRR, 0.58, 95%CI, 0.36–0.93) (Table S2).
DISCUSSION
In this prospective cohort study of 3,360 dialysis patients being evaluated for KT, 6.4% had cognitive impairment, and prevalence of cognitive impairment was higher (7.3%) among those with diabetes. Participants with cognitive impairment were more likely to have functional dependence, particularly among all eight IADL components, including difficulty using the phone, shopping, cooking, house cleaning, washing, taking transportation, managing medications, and managing money. Additionally, cognitive impairment was associated with a 25% (HR, 0.75; 95%CI, 0.61–0.91) lower likelihood of being listed, and, at the borderline level of statistical significance the association was nominally stronger among females than among males (pinteraction=0.05). Though cognitive impairment was not associated with risk of waitlist mortality or the rate of transplantation in its own right, among those without diabetes, cognitive impairment was associated with reduced access to transplantation and a 2.47-fold (95% CI, 1.31–4.66) greater risk of mortality while on the waitlist. The same was not true among those with diabetes, such that there was neither an association between cognitive impairment and waitlist mortality (aSHR, 0.90; 95%CI, 0.48–1.70), nor between cognitive impairment and rate of KT (aIRR, 1.12; 95%CI, 0.71–1.77).
The proportion of participants with cognitive impairment at time of evaluation for KT was lower (6.4%) than has been previously reported in dialysis patients evaluated for KT (~55%)5 and among patients undergoing maintenance hemodialysis generally (71.1%).24 These difference may be attributable to different case mixes that are evaluated at different centers, different types of kidney replacement therapies, as well as the different cognitive assessment tools with varying sensitivity and specificity for identifying cognitive impairment. For example, the prior study of 349 dialysis patients who were being evaluated for KT that found about 55% of the population with cognitive impairment,5 used the Montreal Cognitive Assessment (MoCA),25 another tool to assess global cognitive functioning with a maximum score of 30 (higher scores indicating better cognitive functioning) which is recognized to have greater sensitivity as compared to the 3MS for detecting mild cognitive impairment.25,26 However, studies have recently suggested that the original recommended cutoff score of 26 in the MoCA, as was used in the previous study of patients being evaluated for KT,5 could lead to inflated rates of cognitive impairment due to false positives,27 which may present one reason for the differences in prevalence comparing both studies. Additionally, though this prior study population had a similar mean age as this study (mean=54 years), it had a greater proportion of females (58% vs. 41%) and fewer black (21% vs. 46%); prior studies have shown that prevalence of cognitive impairment differ by both sex28,29 and race.30–32 Using the 3MS, we observed a similar proportion of cognitive impairment among patients being evaluated for KT (6.4%) as those being admitted for KT (10%); though the 3MS may have identified a smaller proportion of patients compared to the MoCA, it may capture a higher-risk group of patients being evaluated for KT. These studies highlight the complexity of identifying cognitive impairment in patients treated by dialysis. Future studies should establish a valid measure of cognitive impairment among patients being evaluated for KT and should identify clinically relevant thresholds for cognitive impairment for this measure.
Our results regarding lower chance of listing in cognitively impaired dialysis patients were consistent with those found in the previous study using the MoCA.5 Though adjusted estimates were not presented for cognitive impairment identified by the MoCA, the previous findings from a single center study of 349 dialysis patients suggested that every 1-point lower MoCA score was independently associated with a 7% (aHR, 0.93; 95%CI, 0.88–0.99) reduced likelihood of listing for KT.5 In contrast, using the 3MS in a two center study of 3,360 dialysis patients, our findings suggested a stronger association between cognitive impairment and listing (aHR, 0.75; 95%CI, 0.61–0.91). We hypothesize that delaying listing may contribute to cognitive impairment given that patients would spend more time on hemodialysis, which has been shown to worsen cognitive function,33 and as we saw in this cohort, those with cognitive impairment had longer median time between dialysis initiation and listing (11.7 months) compared to those who were not cognitively impaired (4.0 months).
Furthermore, our results build upon previous findings, demonstrating that while cognitively impaired participants were more likely to be older, the association between cognitive impairment and listing were independent of participant age, and did not vary by age. These observations are particularly interesting to consider in parallel with previous studies demonstrating that as age increases in patients with kidney failure, the odds and relative rate of placement on the waitlist decreases.34,35 Importantly, unlike age, cognitive impairment is a potentially modifiable risk factor that can be prevented or improved with interventions, including cognitive training, physical exercise, blood pressure management, and prehabilitation, as has been identified in patients undergoing hemodialysis,36–39 with benefits potentially extending to cognitive tasks of activities of daily life.40,41
Among women in our study, cognitive impairment was associated with lower chance of listing. However, the same did not hold true among men. These findings support prior studies that have similarly observed sex differences in access to KT,42 and provide one potential mechanism for these sex disparities. A prior study among patients undergoing hemodialysis found that females were 1.45-fold less likely to have discussions with their providers about KT as a treatment option.42 Our results extend prior findings on sex disparities by demonstrating that cognitive impairment may have a greater impact on waitlist outcomes among females compared to males.
Our findings that cognitive impairment is associated with a 2.47-fold greater risk of mortality among those without diabetes support prior findings that suggest that cognitive impairment can exacerbate the management of existing chronic conditions, like kidney failure.11 It has been shown that cognitive impairment may hinder adherence with prescribed, and often complex, medication regimens; increase risk of adverse drug events; impair decision-making regarding treatment options; and increase cost of care.43–47
Additionally, our findings relating cognitive impairment to access to KT and risk of waitlist mortality especially among those without diabetes, is consistent with prior studies among patients undergoing hemodialysis that have shown stronger associations between dementia and risk of mortality among those without diabetes (pinteraction<0.001);48 similar findings were observed for the association with Alzheimer’s disease specifically.48 Collectively, these observations support the hypotheses that cognitive impairment may have different etiologies in dialysis patients with and without diabetes. It may also suggest that those with diabetes may already suffer from extensive cardiovascular disease burden49,50 such that any subsequent burden introduced with the addition of cognitive impairment does not augment risk stratification.
There were several limitations to this study to consider. The main limitation of this study is the use of a single instrument to define cognitive impairment. Like the MoCA, the 3MS is a validated screening tool assessing global cognitive functioning in older adults generally;15,16 however, clear thresholds for cognitive impairment using the MoCA have not yet been established for an dialysis population of all ages and education levels. Another limitation is the number of centers included, which may be vulnerable to lack of generalizability to the U.S. dialysis population being evaluated for KT. However, though the prevalence in these centers may be different than other centers, the inferences regarding the association between cognitive impairment, access to KT, and waitlist mortality are likely to remain the same. However, to our knowledge, this is the largest study to date of cognitive function, listing practices, and outcomes on the KT waitlist. This study has several notable strengths, including its large sample size, its prospective study design, as well as measurement of a validated measure of global cognitive function.15–17
In conclusion, cognitive impairment is associated with lower chance of listing, and among those without diabetes, it is associated with higher risk mortality while on the waitlist. Importantly, cognitive impairment (unlike age, sex, or race) is a potentially preventable and modifiable risk factor, as patients with kidney failure who undergo KT experience improvements in cognitive function.51–58 Therefore, optimization of cognitive function while on dialysis would likely improve outcomes in those most vulnerable. While only 6% of patients in this study had cognitive impairment, screening using the 3MS takes only 15 minutes and could help identify vulnerable groups of candidates that may benefit from further in-depth neurocognitive assessments. Thus, at time of KT referral and evaluation, transplant and nephrology providers are encouraged to consider screening and identifying patients who may benefit from cognitive treatment strategies while waiting for KT to improve associated health outcomes. Future studies are needed to investigate whether interventions designed to improve cognitive performance result in improved access to KT and decreased waitlist mortality.
Supplementary Material
Figure S1. Flow diagram of study population eligibility.
Table S1. Sensitivity analyses: cumulative incidence and associations of listing, waitlist mortality, and kidney transplantation by global cognitive impairment after excluding those with diagnosed dementia.
Table S2. Sensitivity analyses: cumulative incidence and associations of listing, waitlist mortality, and kidney transplantation by global cognitive impairment after additionally adjusting for factors associated with cognitive impairment at time of evaluation.
Table S3. Sensitivity analyses: cumulative incidence and associations of listing by global cognitive impairment utilizing time from dialysis initiation to listing.
Table S4. Sensitivity analyses: cumulative incidence and associations of waitlist mortality by global cognitive impairment, censoring for kidney transplantation (no competing risk).
Support:
Funding for this study was provided by the National Institute of Diabetes and Digestive and Kidney Disease and the National Institute of Aging: grant numbers R01AG055781 (PI: McAdams-DeMarco), R01DK114074 (PI: McAdams-DeMarco), T32DK007732 (Haugen), F32AG053025 (PI: Haugen), K01AG050699 (PI: Gross), and K24DK101828 (PI: Segev). Funders had no role in the study design, data collection, analysis, reporting, or decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTARY MATERIAL
Supplementary Material Descriptive Text for Online Delivery
Supplementary File (PDF). Figure S1; Tables S1–S4.
Financial Disclosure: The authors declare that they have no relevant financial interests.
REFERENCES
- 1.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. [DOI] [PubMed] [Google Scholar]
- 2.Kalirao P, Pederson S, Foley RN, et al. Cognitive impairment in peritoneal dialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2011;57(4):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurella Tamura M, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clinical journal of the American Society of Nephrology: CJASN. 2010;5(8):1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1997;30(1):41–49. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Montgomery RN, Bedros V, et al. Subclinical Cognitive Impairment and Listing for Kidney Transplantation. Clinical Journal of the American Society of Nephrology: CJASN. 2019:CJN.11010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams-DeMarco MA, Bae S, Chu N, et al. Dementia and Alzheimer’s Disease among Older Kidney Transplant Recipients. Journal of the American Society of Nephrology: JASN. 2017. May;28(5):1575–1583. doi: 10.1681/ASN.2016080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira AA, Weiner DE, Scott T, et al. Subcortical cognitive impairment in dialysis patients. Hemodial Int. 2007;11(3):309–314. [DOI] [PubMed] [Google Scholar]
- 8.McAdams-DeMarco MA, Van Pilsum Rasmussen SE, Chu NM, et al. Perceptions and Practices Regarding Frailty in Kidney Transplantation: Results of A National Survey. Transplantation. 2019. May 7. doi: 10.1097/TP.0000000000002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warsame F, Haugen CE, Ying H, et al. Limited health literacy and adverse outcomes among kidney transplant candidates. American Journal of Transplantation. 2019;19(2):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller-Matero LR, Hyde-Nolan ME, Eshelman A, Abouljoud M. Health literacy in patients referred for transplant: do patients have the capacity to understand? Clinical transplantation. 2015;29(4):336–342. [DOI] [PubMed] [Google Scholar]
- 11.Alzheimer’s Association. Alzheimer’s Disease Facts Figures and Figures. Alzheimer’s & Dementia. 2015;11(3):332+. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal Asher D, Ver Halen N, Cukor D. Depression and nonadherence predict mortality in hemodialysis treated end-stage renal disease patients. Hemodialysis International. 2012;16(3):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of clinical epidemiology. 1994;47(11):1245–1251. [DOI] [PubMed] [Google Scholar]
- 14.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson Comorbidity Index for use in patients with ESRD. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2003;42(1):125–132. [DOI] [PubMed] [Google Scholar]
- 15.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. Journal of Clinical Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 16.McDowell I, Kristjansson B, Hill G, Hebert R. Community screening for dementia: The mini mental state exam (MMSE) and modified mini-mental state exam (3MS) compared. Journal of clinical epidemiology. 1997;50(4):377–383. [DOI] [PubMed] [Google Scholar]
- 17.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52(11):1863–1869. [DOI] [PubMed] [Google Scholar]
- 18.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. Journal of the American Society of Nephrology: JASN. 2005;16(7):2127–2133. [DOI] [PubMed] [Google Scholar]
- 19.Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly. Results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52(6):612–619. [DOI] [PubMed] [Google Scholar]
- 20.Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. Journal of the American Society of Nephrology: JASN. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 22.Bassuk SS, Wypij D, Berkman LF. Cognitive impairment and mortality in the community-dwelling elderly. American journal of epidemiology. 2000;151(7):676–688. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Mahnken JD, Johnson DK, et al. Prevalence and correlates of cognitive impairment in kidney transplant recipients. BMC nephrology. 2017;18(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Zwieten A, Wong G, Ruospo M, et al. Prevalence and patterns of cognitive impairment in adult hemodialysis patients: the COGNITIVE-HD study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2018;33(7):1197–1206. [DOI] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 26.Tsoi KKF, Chan JYC, Hirai HW, Wong SYS, Kwok TCY. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysisReview of Cognitive Tests to Detect DementiaReview of Cognitive Tests to Detect Dementia. JAMA internal medicine. 2015;175(9):1450–1458. [DOI] [PubMed] [Google Scholar]
- 27.Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. International journal of geriatric psychiatry. 2018;33(2):379–388. [DOI] [PubMed] [Google Scholar]
- 28.Sohn D, Shpanskaya K, Lucas JE, et al. Sex Differences in Cognitive Decline in Subjects with High Likelihood of Mild Cognitive Impairment due to Alzheimer’s disease. Scientific Reports. 2018;8(1):7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espeland MA, Carmichael O, Yasar S, et al. Sex-related differences in the prevalence of cognitive impairment among overweight and obese adults with type 2 diabetes. Alzheimers Dement. 2018;14(9):1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Hayward MD, Yu Y-L. Life Course Pathways to Racial Disparities in Cognitive Impairment among Older Americans. Journal of health and social behavior. 2016;57(2):184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miles TP, Froehlich TE, Bogardus ST Jr., Inouye SK. Dementia and Race: Are There Differences Between African Americans and Caucasians? Journal of the American Geriatrics Society. 2001;49(4):477–484. [DOI] [PubMed] [Google Scholar]
- 33.McAdams-DeMarco MA, Tan J, Salter ML, et al. Frailty and Cognitive Function in Incident Hemodialysis Patients. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(12):2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6(7):1760–1767. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe RA, Ashby VB, Milford EL, et al. Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis. 2000;36(5):1025–1033. [DOI] [PubMed] [Google Scholar]
- 36.Jones RN. Cognitive Training Improves Cognitive Performance, but What Else? Journal of the American Geriatrics Society. 2018;66(4):648–649. [DOI] [PubMed] [Google Scholar]
- 37.Kjeldsen SE, Narkiewicz K, Burnier M, Oparil S. Intensive blood pressure lowering prevents mild cognitive impairment and possible dementia and slows development of white matter lesions in brain: the SPRINT Memory and Cognition IN Decreased Hypertension (SPRINT MIND) study. Blood Pressure. 2018;27(5):247–248. [DOI] [PubMed] [Google Scholar]
- 38.McAdams-DeMarco MA, Konel J, Warsame F, et al. Intradialytic Cognitive and Exercise Training May Preserve Cognitive Function. Kidney Int Rep. 2018;3(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplantation: Results from a pilot study. Clinical transplantation. 2019;33(1):e13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belleville S, Hudon C, Bier N, et al. MEMO+: Efficacy, Durability and Effect of Cognitive Training and Psychosocial Intervention in Individuals with Mild Cognitive Impairment. J Am Geriatr Soc. 2018;66(4):655–663. [DOI] [PubMed] [Google Scholar]
- 41.Rebok GW, Ball K, Guey LT, et al. Ten-Year Effects of the ACTIVE Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. Journal of the American Geriatrics Society. 2014;62(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salter ML, McAdams-Demarco MA, Law A, et al. Age and sex disparities in discussions about kidney transplantation in adults undergoing dialysis. Journal of the American Geriatrics Society. 2014;62(5):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrasco FR, Moreno A, Ridao N, et al. Kidney transplantation complications related to psychiatric or neurological disorders. Transplantation proceedings. 2009;41(6):2430–2432. [DOI] [PubMed] [Google Scholar]
- 44.Ettenhofer ML, Hinkin CH, Castellon SA, et al. Aging, neurocognition, and medication adherence in HIV infection. Am J Geriatr Psychiatry. 2009;17(4):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugarman J, McCrory DC, Hubal RC. Getting meaningful informed consent from older adults: a structured literature review of empirical research. J Am Geriatr Soc. 1998;46(4):517–524. [DOI] [PubMed] [Google Scholar]
- 46.Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney international. 2011;79(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deb A, Thornton JD, Sambamoorthi U, Innes K. Direct and indirect cost of managing alzheimer’s disease and related dementias in the United States. Expert review of pharmacoeconomics & outcomes research. 2017;17(2):189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAdams-DeMarco MA, Daubresse M, Bae S, Gross AL, Carlson MC, Segev DL. Dementia, Alzheimer’s Disease, and Mortality after Hemodialysis Initiation. Clinical Journal of the American Society of Nephrology: CJASN. 2018;13(9):1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. Jama. 1979;241(19):2035–2038. [DOI] [PubMed] [Google Scholar]
- 50.Chang YT, Wu JL, Hsu CC, Wang JD, Sung JM. Diabetes and end-stage renal disease synergistically contribute to increased incidence of cardiovascular events: a nationwide follow-up study during 1998–2009. Diabetes care. 2014;37(1):277–285. [DOI] [PubMed] [Google Scholar]
- 51.Chu NM, Gross AL, Shaffer AA, et al. Frailty and Changes in Cognitive Function after Kidney Transplantation. Journal of the American Society of Nephrology: JASN. 2019 2019. February;30(2):336–345. doi: 10.1681/ASN.2018070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joshee P, Wood AG, Wood ER, Grunfeld EA. Meta-analysis of cognitive functioning in patients following kidney transplantation. Nephrology Dialysis Transplantation. 2018. July 1;33(7):1268–1277. doi: 10.1093/ndt/gfx240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon BS, VanBuren JM, Rodrigue JR, et al. Cognitive changes associated with switching to frequent nocturnal hemodialysis or renal transplantation. BMC nephrology. 2016;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griva K, Thompson D, Jayasena D, Davenport A, Harrison M, Newman SP. Cognitive functioning pre- to post-kidney transplantation--a prospective study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21(11):3275–3282. [DOI] [PubMed] [Google Scholar]
- 55.Radic J, Ljutic D, Radic M, Kovacic V, Dodig-Curkovic K, Sain M. Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. American journal of nephrology. 2011;34(5):399–406. [DOI] [PubMed] [Google Scholar]
- 56.Kramer L, Madl C, Stockenhuber F, et al. Beneficial effect of renal transplantation on cognitive brain function. Kidney international. 1996;49(3):833–838. [DOI] [PubMed] [Google Scholar]
- 57.Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M, Debska-Slizien A, Rutkowski B. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney international. 2011;79(12):1353–1360. [DOI] [PubMed] [Google Scholar]
- 58.Koushik NS, McArthur SF, Baird AD. Adult chronic kidney disease: neurocognition in chronic renal failure. Neuropsychology review. 2010;20(1):33–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagram of study population eligibility.
Table S1. Sensitivity analyses: cumulative incidence and associations of listing, waitlist mortality, and kidney transplantation by global cognitive impairment after excluding those with diagnosed dementia.
Table S2. Sensitivity analyses: cumulative incidence and associations of listing, waitlist mortality, and kidney transplantation by global cognitive impairment after additionally adjusting for factors associated with cognitive impairment at time of evaluation.
Table S3. Sensitivity analyses: cumulative incidence and associations of listing by global cognitive impairment utilizing time from dialysis initiation to listing.
Table S4. Sensitivity analyses: cumulative incidence and associations of waitlist mortality by global cognitive impairment, censoring for kidney transplantation (no competing risk).


