Abstract
Background:
Early identification of traumatic intracranial hemorrhage (ICH) has implications for triage and intervention. Blood-based biomarkers were recently FDA approved for prediction of ICH in patients with mild TBI. We sought to determine if biomarkers measured early after injury improve prediction of mortality and clinical/radiologic outcomes compared to GCS alone in patients with moderate or severe TBI (MS-TBI).
Methods:
We measured glial-fibrillary-acidic-protein (GFAP), ubiquitin-C-terminal-hydrolase-L1 (UCH-L1), and microtubule-associated-protein-2 (MAP-2) on arrival to the ED in patients with blunt TBI enrolled in the placebo arm of the Prehospital TXA for TBI Trial (prehospital GCS 3–12, SPB > 90). Biomarkers were modeled individually and together with prehospital predictor variables [PH] (GCS, age, gender). Data was divided into a training dataset and test dataset for model derivation and evaluation. Models were evaluated for prediction of ICH, mass lesion, 48-hour and 28-day mortality, and 6-month Glasgow Outcome Scale-Extended [GOSE] and Disability Rating Scale [DRS]. AUC was evaluated in test data for PH alone, PH+individual biomarkers, and PH+3 biomarkers.
Results:
Of 243 patients with baseline samples (obtained a median 84 min after injury), prehospital GCS was 8 (IQR 5,10), 55% had ICH, and 48-hr and 28-day mortality was 7 and 13%. Poor neurologic outcome at 6 months was observed in 34% based on GOS-E ≤4, and 24% based on DRS >7. Addition of each biomarker to PH improved AUC in the majority of predictive models. GFAP+PH compared to PH alone significantly improved AUC in all models [ICH: 0.82 vs 0.64; 48-hour mortality 0.84 vs 0.71; 28-day mortality: 0.84 vs 0.66; GOSE: 0.78 vs 0.69; DRS 0.84 vs 0.81, all p<0.001].
Conclusions:
Circulating blood-based biomarkers may improve prediction neurological outcomes and mortality in patients with MS-TBI over prehospital characteristics alone. GFAP appears to be the most promising. Future evaluation in the prehospital setting is warranted.
Keywords: Biomarkers, ICH, trauma, TBI, traumatic brain injury
Background
Traumatic brain injury (TBI) is a leading cause of death (50,000/year) and disability (13.5 million) in the United States, with an estimated 2.8 million individuals seeking medical treatment for TBI per year.1 Despite a considerable body of research on TBI outcomes, current efforts to develop reliable strategies for diagnosis and prognostication are limited by dependence on symptom-based scoring systems, which are subject to clinical confounders and inter-practitioner variability.2–6 These limitations are a major obstacle in TBI research, which relies on these systems for accurate patient stratification and outcome prediction. Development of tools with greater consistency and discriminatory capacity is necessary for improved prognostication of long-term outcome, which is critical for trial inclusion, clinical decision making, and setting realistic expectations for patients and their families.
In response to these challenges, there has been increased interest in the use of circulating biomarkers of structural brain injury for TBI prognostication and classification. These biomarkers are proteins normally confined to the central nervous system which are released upon cellular injury and disruption of the blood-brain barrier.7 In February 2018, the US Food and Drug Administration approved the use of the Brain Trauma Indicator (BTI), a ubiquitin-C-terminal-hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) assay, for determining the clinical necessity of obtaining a head computed tomography (CT) scan in patients with mild TBI.8 More recently, BIO-ProTECT study showed that addition of S100 calcium-binding protein B (S100B) and GFAP to baseline patient characteristics significantly improved prognostication in moderate-to-severe TBI (MS-TBI).9 In addition, the ability of biomarkers of structural brain injury to predict intracranial hemorrhage on radiographic studies has been increasingly demonstrated.10–14 The growing evidence supporting the diagnostic and predictive value of circulating biomarkers for TBI supports further exploration of their clinical utility.
This study examined the association between early blood levels of three biomarkers (GFAP, UCH-L1, and microtubule-associated-protein-2 (MAP-2)) and predictors of clinical (48-hour and 28-day mortality, disability at 6 months based on the Extended Glasgow Outcome Scale (GOS-E) and Disability Rating Scale (DRS)) and radiological (presence of intracranial hemorrhage (ICH) on initial scan, ICH progression, Marshall CT classification scores) outcomes in patients with MS-TBI.
Methods
Study Design
We conducted a secondary analysis of data collected from patients enrolled in the placebo arm of the “Prehospital Tranexamic Acid Use for Traumatic Brain Injury” trial, a Phase II, double-blind, multicenter randomized controlled trial (ClinicalTrials.gov NCT01990768). This study was designed to examine the efficacy and safety of prehospital administration of TXA compared to placebo in patients with MS-TBI [prehospital Glasgow Coma Scale (GCS) 3–12] who were not in shock (systolic blood pressure (SBP) > 90). Subjects were enrolled according to the Food and Drug Administration (FDA) “Exception from informed consent requirements for emergency research” (21CFR50.24). Consent for continued participation was obtained from the subject or their legal authorized representative (LAR) at the earliest feasible opportunity. Opportunity for “opt out” was provided according to the requirements set forth by each site’s local regulatory board.
The trial was conducted using infrastructure of the Resuscitation Outcomes Consortium, a multicenter network funded by federal organizations in the United States and Canada to conduct clinical trials targeting cardiac arrest and life-threatening trauma.15 Approval was provided by The Institutional Review Board (IRB) for the Resuscitation Outcomes Consortium Clinical Trials Center at the University of Washington, with contingent IRB approval provided by individual sites. Funding for the parent trial was provided by the NIH National Heart, Lung, and Blood Institutes, the U.S. Department of Defense, and the University of Washington.
Study Population
The study population consisted of patients from the placebo arm (n=345) of the Prehospital TXA Use for TBI trial who were recruited by 20 trauma centers at 12 regional sites in the US and Canada between May 2015 and March 2017. The inclusion criteria for the parent trial were as follows: blunt or penetrating traumatic mechanism consistent with TBI, prehospital GCS ≤ 12 prior to administration of sedative and/or paralytic agents with at least one reactive pupil, prehospital SBP ≥ 90 mmHg, prehospital intravenous (IV) access, age ≥ 15yrs (or weight ≥ 50kg if age was unknown), and estimated time from injury to hospital arrival of <2 hours. Patients were excluded if they had any of the following: prehospital GCS=3 with no reactive pupil, estimated time from injury to start of study drug bolus dose >2 hours, unknown time of injury, clinical suspicion by EMS of seizure activity, acute MI or stroke, known history of confounding medical conditions (seizures, thromboembolic disorders or renal dialysis), CPR by EMS prior to randomization, burns >20% TBSA, suspected or known prisoners, suspected or known pregnancy, prehospital TXA or other pro-coagulant drug given prior to randomization, and subjects who activated the “opt-out” process when required by the local regulatory board. For this analysis, we included all patients in the placebo arm of the parent trial with blunt injuries for whom there were available blood samples and no missing values for prehospital variables (GCS, age, gender).
Study Procedures
Subjects meeting inclusion and exclusion criteria were enrolled by first-responders in the prehospital setting. As part of the placebo arm of the parent trial, all subjects received a bolus dose of 250mL of 0.9% sodium chloride administered in the prehospital setting, followed by a second 250mL infusion administered over 8 hours after arrival to hospital. Blood samples were obtained [upon arrival to the ED (0 hours) and at 6 hours after study randomization] and then centrifuged, aliquoted, frozen at −80°C, bar coded, and batch shipped by the clinical research staff to the Trauma Research Institute of Oregon, Oregon Health & Science University. Head CT was performed upon arrival and repeated within 24 hours for evaluation of ICH progression. Digital images of head CTs were obtained in Digital Imaging and Communication in Medicine (DICOM) format and transferred to the OHSU image repository in a de-identified manner. Head CT scans were reviewed centrally at OHSU by a neuroradiologist-trained technician using software designed to obtain computerized measurements of ICH volumes. Ten percent of scans were audited by a neuroradiologist to verify that accurate and consistent measurements were obtained. GOS-E and DRS at 6-months was available for 80% of patients enrolled in the clinical trial.
Biomarker Measurement
Serum GFAP and UCH-L1 levels were measured in duplicate for each sample using a validated enzyme-linked immunosorbent assay (ELISA) platform (Banyan Biomarkers Inc. San Diego CA) as described previously.11 Any samples yielding a signal over the quantification or calibrator range were diluted and re-assayed. Serum MAP-2 levels were measured using a standard sandwich ELISA protocol reported in a previous study.16
Outcome Measures
All 3 biomarkers (GFAP, UCH-L1, and MAP-2) were assessed individually and in combination with prehospital variables (PH: GCS, age, gender) at 0- and 24-hours. The area under the curves (AUC) for models predictive of clinical (48-hour and 28-day mortality, disability at 6 months based on the GOS-E and DRS) and radiographical outcomes (presence of ICH on initial head CT scan, ICH progression, Marshall CT classification scores) were calculated. ICH progression was defined as an increase in combined epidural, subdural, and intraparenchymal hemorrhage volumes of at least 33% and at least 1mL (measured using Analyze 12.0 software) on follow-up head CT as compared to initial head CT. Presence of mass lesion was determined according to Marshall scores calculated based on the head CT obtained on arrival.
Statistical Analysis
Data were divided randomly into a training dataset (n=122) and test dataset (n=121) for model derivation and evaluation. The training data were used to obtain model coefficients themselves. For each resulting model, the test data were used to obtain unbiased estimates of predictive performance. Simple and multiple linear regression models were created using the following predictors: PH alone, individual biomarkers alone, the combination of all 3 biomarkers, PH + individual biomarkers, and PH + all biomarkers. Models were fit for prediction of ICH on initial CT and ICH progression, mass lesion (Marshall score of 5 or 6 according to initial CT), Marshall score >1, 48-hour and 28-day mortality, 6-month GOS-E and 6-month DRS using logistic regression. Models were adjusted for the following variables: site, age (modeled as a linear spline with a knot at 45 years), gender, prehospital GCS, injury severity score, and square of the maximum head abbreviated injury severity score. Receiver operating characteristic (ROC) analysis was used to evaluate predictive ability using AUC. Confidence intervals for the AUC were estimated using the bootstrap approach with 1000 replications each. When comparing two models in which the second model contained additional predictors beyond those included in the first, a likelihood ratio test was employed.17
Results
Population
Of the 345 patients enrolled in the placebo arm of the clinical trial, 243 patients with complete biomarker and clinical data were included in this analysis. Median prehospital GCS was 8 (IQR 5,10), 55% had ICH, 48-hr and 28-day mortality was 7% and 13% respectively, and poor neurologic outcome was observed in 34% based on 6-month GOS-E ≤4 and 24% based on 6-month DRS >7. Baseline blood samples were obtained a median 84 min post-injury. Subjects were subsequently divided into training (n=122) and testing (n=121) cohorts to generate bootstrapping samples used to create the ROC curves. Baseline characteristics of these subgroups are shown in Table 1.
TABLE 1.
Characteristics of Study Sample by Training or Testing Designation
| Training data | Testing data | |
|---|---|---|
| Characteristic | (N = 122) | (N = 121) |
| Median age [IQR] – yr | 33.50 [23.00, 52.00] | 35.00 [25.00, 53.00] |
| Male sex – no. (%) | 101 (82.8) | 88 (72.7) |
| Median Prehospital Glasgow Coma Score [IQR] | 8.00 [5.00, 11.00] | 7.00 [5.00, 10.00] |
| ICH on initial CT scan – no. (%) | 62/120 (51.7) | 69/119 (58.0) |
| Mass lesion (Marshall score 5+6) – no. (%) | 16/117 (13.7) | 13/116 (11.2) |
| ICH progression – no. (%) | 13/56 (23.2) | 14/70 (20.0) |
| Mortality within 48 hours – no. (%) | 9/120 (7.5) | 8/120 (6.7) |
| Mortality within 28 days – no. (%) | 17/120 (14.2) | 13/120 (10.8) |
| GOS-E ≤ 4 – no. (%) | 38/112 (33.9) | 39/115 (33.9) |
| DRS ≥ 7 – no. (%) | 25/110 (22.7) | 29/116 (25.0) |
IQR = Interquartile range
GOS-E = Extended Glasgow Outcome Scale
DRS = Disability Rating Scale
Prediction Models of Radiographic Outcome
For prediction of ICH on initial CT scan, addition of each biomarker to PH outperformed PH alone (Table 2A, Table 2B, Figure 1). Further improvement in AUC was seen with PH + all biomarkers as compared to PH only, PH+UCH-L1 and PH+MAP-2 (all p<0.001). The predictive capacity of the PH+GFAP model was equivalent to that of PH + all biomarkers combined (AUC=0.816 vs 0.824, p=0.584). A similar pattern was observed for prediction of mass lesion defined as a Marshall score of 5 or 6 on initial CT scan (Figure 1); however, addition of UCH-L1 did not significantly improve AUC compared to PH alone (AUC=0.715 vs 0.724, p=0.258). The combination of PH + all biomarkers again outperformed PH+UCH-L1 and PH+MAP-2 (both p<0.001). PH+GFAP was equivalent to PH + all 3 biomarkers combined (p=0.31). For ICH progression, the combination of biomarkers with PH did not improve prediction. In contrast to previous radiographic outcomes, predictive power for ICH progression was reduced with the addition of GFAP to PH (AUC 0.489 vs 0.759, p=0.009).
TABLE 2A.
AUCs with 95% Confidence Intervals for Predictive Models
| Predictors in model | ICH on initial scan | Mass lesion: Marshall 5+6 | Marshall > 1 | ICH progression | 48-hr mortality | 28-day mortality | 6-month GOS-E ≤ 4 | 6-month DRS ≥ 7 |
|---|---|---|---|---|---|---|---|---|
| PH | 0.639 (0.535, 0.73) | 0.715 (0.551, 0.856) | 0.677 (0.501, 0.836) | 0.759 (0.629, 0.867) | 0.713 (0.49, 0.881) | 0.66 (0.504, 0.797) | 0.7 (0.602, 0.795) | 0.811 (0.718, 0.892) |
| UCH-L1 | 0.665 (0.556, 0.759) | 0.686 (0.539, 0.816) | 0.628 (0.467, 0.773) | 0.603 (0.423, 0.77) | 0.854 (0.702, 0.961) | 0.826 (0.704, 0.93) | 0.684 (0.567, 0.796) | 0.683 (0.556, 0.804) |
| MAP-2 | 0.736 (0.645, 0.831) | 0.831 (0.721, 0.924) | 0.777 (0.644, 0.9) | 0.543 (0.376, 0.702) | 0.804 (0.657, 0.916) | 0.768 (0.64, 0.875) | 0.645 (0.53, 0.754) | 0.652 (0.535, 0.75) |
| GFAP | 0.801 (0.714, 0.879) | 0.845 (0.714, 0.942) | 0.777 (0.624, 0.908) | 0.489 (0.31, 0.679) | 0.867 (0.686, 0.979) | 0.852 (0.724, 0.947) | 0.714 (0.609, 0.813) | 0.747 (0.625, 0.849) |
| All biomarkers | 0.8 (0.721, 0.882) | 0.869 (0.751, 0.962) | 0.82 (0.684, 0.94) | 0.486 (0.298, 0.676) | 0.862 (0.685, 0.971) | 0.85 (0.736, 0.945) | 0.714 (0.609, 0.804) | 0.738 (0.621, 0.84) |
| PH+UCH-L1 | 0.699 (0.602, 0.796) | 0.724 (0.577, 0.87) | 0.696 (0.518, 0.853) | 0.755 (0.607, 0.88) | 0.779 (0.566, 0.942) | 0.809 (0.679, 0.912) | 0.788 (0.701, 0.866) | 0.865 (0.8, 0.927) |
| PH+MAP-2 | 0.752 (0.662, 0.842) | 0.857 (0.765, 0.937) | 0.791 (0.636, 0.915) | 0.703 (0.557, 0.835) | 0.801 (0.57, 0.967) | 0.799 (0.64, 0.927) | 0.777 (0.679, 0.862) | 0.862 (0.791, 0.924) |
| PH+GFAP | 0.816 (0.742, 0.883) | 0.839 (0.736, 0.927) | 0.762 (0.61, 0.887) | 0.61 (0.427, 0.791) | 0.842 (0.61, 0.98) | 0.843 (0.696, 0.958) | 0.78 (0.683, 0.87) | 0.841 (0.747, 0.919) |
| PH+all biomarkers | 0.824 (0.747, 0.893) | 0.891 (0.802, 0.962) | 0.826 (0.699, 0.934) | 0.611 (0.408, 0.783) | 0.841 (0.599, 0.983) | 0.838 (0.682, 0.954) | 0.771 (0.673, 0.863) | 0.822 (0.728, 0.908) |
PH = Prehospital variables (GCS, age, gender)
UCH-L1 = Ubiquitin C-terminal hydrolase-L1
MAP-2 = Microtubule-associated protein 2
GFAP = Glial fibrillary acidic protein
ICH = Intracranial hemorrhage
GOS-E = Extended Glasgow Outcome Scale
DRS = Disability Rating Scale
TABLE 2B.
P-values for Testing Additional Predictive value of Biomarkers
| Models being compared | ICH on initial scan | Mass lesion: Marshall 5+6 | Marshall > 1 | ICH progression | 48-hr mortality | 28-day mortality | 6-month GOS-E ≤ 4 | 6-month DRS ≥ 7 |
|---|---|---|---|---|---|---|---|---|
| PH vs PH+UCH-L1 | 0.002 | 0.258 | 0.901 | 0.424 | 0.205 | 0.024 | 0.026 | 0.128 |
| PH vs PH+MAP-2 | <0.001 | 0.006 | 0.1 | 0.11 | 0.033 | 0.001 | 0.002 | 0.001 |
| PH vs PH+GFAP | <0.001 | 0.001 | 0.066 | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 |
| PH vs PH+all biomarkers | <0.001 | 0.003 | 0.135 | 0.078 | <0.001 | <0.001 | <0.001 | <0.001 |
| PH+UCH-L1 vs PH+all biomarkers | <0.001 | 0.002 | 0.063 | 0.045 | <0.001 | <0.001 | <0.001 | <0.001 |
| PH+MAP-2 vs PH+all biomarkers | <0.001 | 0.042 | 0.24 | 0.119 | <0.001 | <0.001 | <0.001 | <0.001 |
| PH+GFAP vs PH+all biomarkers | 0.584 | 0.31 | 0.336 | 0.979 | 0.167 | 0.967 | 0.926 | 0.365 |
PH = Prehospital variables (GCS, age, gender)
UCH-L1 = Ubiquitin C-terminal hydrolase-L1
MAP-2 = Microtubule-associated protein 2
GFAP = Glial fibrillary acidic protein
ICH = Intracranial hemorrhage
GOS-E = Extended Glasgow Outcome Scale
DRS = Disability Rating Scale
Figure 1.
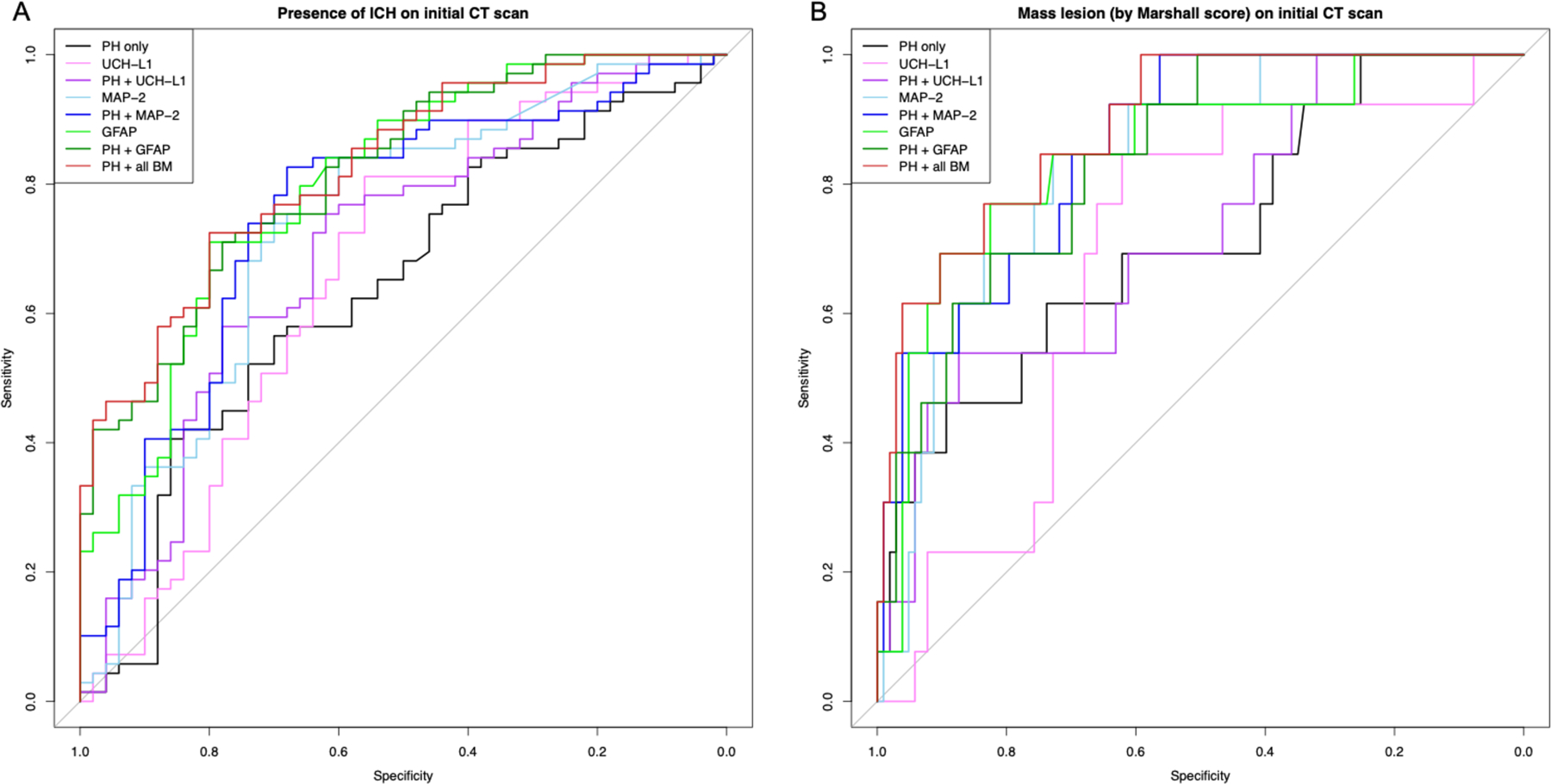
Receiver operating characteristic (ROC) curves for models predicting (A) presence of intracranial hemorrhage (ICH) on initial CT scan and (B) presence of mass lesion (Marshall scores of 5 or 6 on initial CT scan) using prehospital predictor variables (PH = GCS, age, gender), ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), microtubule-associated protein 2 (MAP-2), and glial fibrillary acidic protein (GFAP). Area under the curve (AUC) and confidence intervals were calculated using the bootstrap approach with 1000 replications.
Prediction Models of Clinical Outcomes
For prediction of 48-hour mortality (Figure 2), addition of MAP-2 and GFAP, but not UCH-L1, significantly improved upon the PH model alone, with GFAP demonstrating the highest predictive ability (AUC 0.842 vs 0.713, p<0.001). Compared to PH + all biomarkers, only PH+GFAP demonstrated equivalent predictive capacity (AUC 0.842 vs 0.841, p=0.167). All 3 biomarkers individually improved prediction of 28-day mortality compared to PH alone (Figure 2). As observed with 48-hour mortality, PH+GFAP performed best compared to PH alone (AUC 0.843 vs 0.660, p<0.001) and was equivalent to PH + all biomarkers (AUC 0.843 vs 0.838, p=0.967). For prediction of poor long-term neurologic outcome, both 6-month GOS-E and DRS followed a similar pattern, with the addition of each individual biomarker to PH significantly improving the AUC over PH alone (Figure 3). Both PH+MAP-2 and PH+UCH-L1 provided superior predictive capacity to PH + all biomarkers for 6-month GOS-E and DRS (all p<0.001), but this was not observed for PH+GFAP (GOS-E p=0.926, DRS p=0.365).
Figure 2.
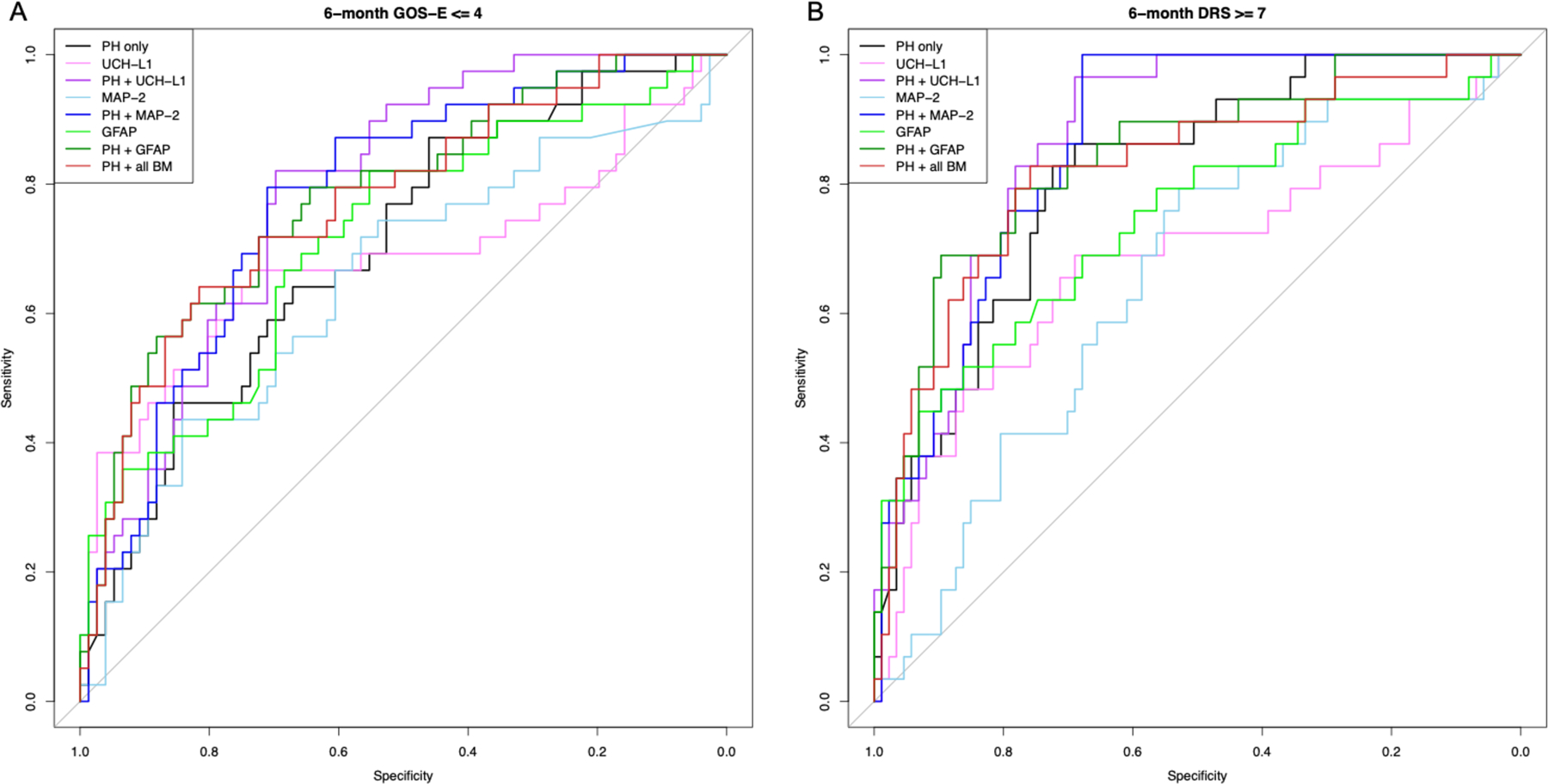
Receiver operating characteristic (ROC) curves for models predicting (A) 48-hour mortality and (B) 28-day mortality using prehospital predictor variables (PH = GCS, age, gender), ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), microtubule-associated protein 2 (MAP-2), and glial fibrillary acidic protein (GFAP). Area under the curve (AUC) and confidence intervals were calculated using the bootstrap approach with 1000 replications.
Figure 3.
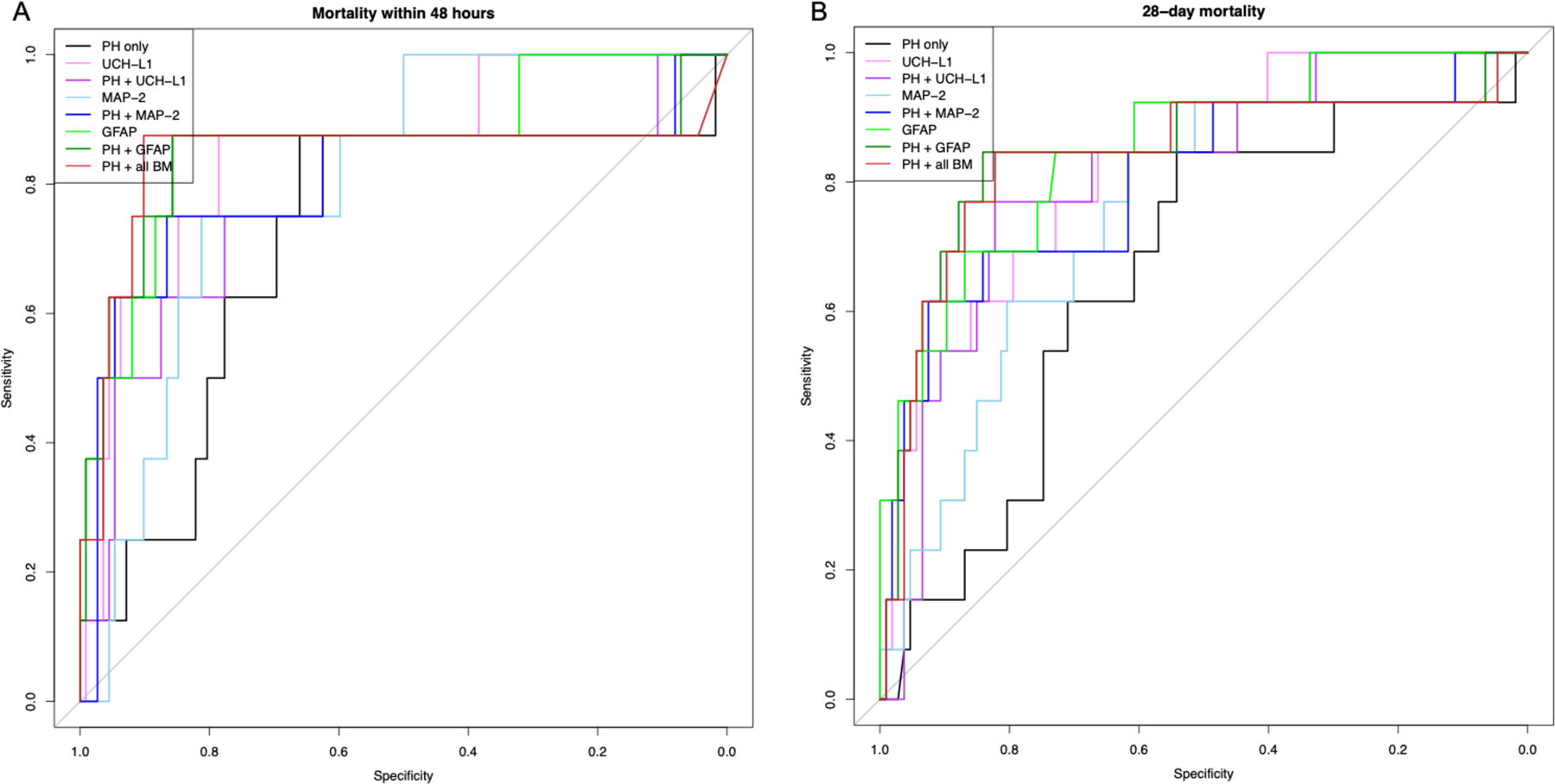
Receiver operating characteristic (ROC) curves for models predicting (A) 6-month Extended Glasgow Outcome Scale (GOS-E) and (B) 6-month Disability Rating Scale (DRS) using prehospital predictor variables (PH = GCS, age, gender), ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), microtubule-associated protein 2 (MAP-2), and glial fibrillary acidic protein (GFAP). Area under the curve (AUC) and confidence intervals were calculated using the bootstrap approach with 1000 replications.
Discussion
In this study, we examined the ability of three circulating biomarkers (GFAP, UCH-L1, and MAP-2) to predict radiologic findings on CT scan, long-term neurologic outcome, and mortality in patients with MS-TBI. We chose these 3 biomarkers, primarily based on previous studies demonstrating their predictive value. GFAP, an astrocyte-specific intermediate filament released upon disruption of the blood-brain barrier, has shown the most promising results for detecting the presence of CT abnormalities in patients with TBI, distinguishing diffuse from focal brain injury, and predicting clinical outcome.10,12,18–21 S100B, another marker of glial injury, has been previously examined in multiple settings. However, we chose to examine GFAP over S100B based on the fact that elevations in S100B have been observed in patients with isolated extracranial injuries and thus it is subject to confounding in patients with multisystem injuries.22 In addition, S100B has not been shown not improve discrimination when combined with GFAP.9 Due to its early peak, we also chose to examine the neuronal marker UCH-L1, as it has previously been shown to have predictive capacity as a biomarker for TBI.13,23,24 Finally, we examined MAP-2, a neuronal marker with specificity for dendritic injury that has been identified as a promising serum marker in the acute phase of ischemic brain injury, but has not been well-studied in patients with moderate or severe TBI.25–27 In one study of MAP-2 levels measured in serum obtained at 6-months post-injury, MAP-2 levels were higher in patients with severe TBI compared to normal controls and correlated with 6-month outcome, suggesting potential utility of this biomarker for extended post-injury monitoring.28 Furthermore, cerebrospinal fluid levels of MAP-2 have demonstrated exceptional capacity for prediction of TBI severity and mortality.16,18 Our investigation represents the earliest known measurement of serum MAP-2 levels in patients with moderate or severe TBI, with results demonstrating the predictive capacity of this biomarker for measures of severity and outcome.
In 2017, a systematic review found positive associations between GFAP and acute trauma-related intracranial lesions on head CT in 24/27 studies with additional evidence supporting the ability of GFAP to distinguish between mass lesions and diffuse injury.10 This correlation has also been observed for UCH-L1, however, UCH-L1 has been shown to be more highly associated with diffuse injury, whereas GFAP appears to be associated with mass lesions.14 More recently, Mahan et al. found that GFAP levels outperformed both UCH-L1, S100B, and the combination of all three biomarkers for prediction of positive head CT in trauma patients with mild, moderate, and severe TBI.12 With the recent FDA approval of a UCH-L1 and GFAP assay for evaluating the clinical necessity of imaging studies in patients with mild TBI, there has been increasing interest in the broader clinical applications of biomarkers for TBI. This study adds to the current body of literature by combining the most promising biomarkers to date with clinical data to improve prediction of injury and outcome. In this study in patients with moderate-to-severe TBI, we found that the combination of biomarker values (UCH-L1, MAP-2 and GFAP, obtained upon hospital arrival) with patient characteristics (prehospital GCS, age, gender) improved prediction of both CT abnormalities and clinical outcomes compared to patient characteristics alone. While admission GCS is currently used for both classification and prognosis of TBI, it is unable to distinguish between the heterogeneous patterns of injury observed in TBI and can be influenced by alterations in consciousness secondary to factors such as hypotension, intoxication, shock, sedation or intubation.2–5 Furthermore, GCS is subject to significant inter-practitioner variability, especially among less-experienced users.6
Our results also support the utility of using circulating biomarkers associated with neuronal injury to improve long-term neurologic outcome prediction in MS-TBI. While GCS has been shown to reliably predict 24-hour mortality, it has limited ability to predict long-term outcome.4,5 GFAP has been shown to enhance prediction of death and poor neurological outcome up to 6 months post-injury in both univariate and multivariate models.19,20 This prognostic capacity has been observed in measurements obtained up to 5 days post-injury in patients with severe TBI.19,23 Although previous evidence suggested that UCH-L1 might outperform GFAP as an early point of care test due to its rapid post-injury rise in mild-moderate TBI, we found that early GFAP values consistently surpassed UCH-L1 for prognostication in patients with moderate and severe TBI.23 Nevertheless, there is robust evidence for the use of UCH-L1 for prediction of post-injury complications, mortality, and 6-month GOS.24,28
While our study supported the predictive utility of early serum MAP-2 levels in the majority of models, this biomarker demonstrated the weakest performance in comparison to GFAP and UCH-L1. While previous investigations have shown promise for prediction of mortality, these studies have predominantly measured CSF levels of MAP-2. This may explain why our MAP-2 results were less robust than for GFAP and UCH-L1, which have been better studied in serum.16,28
Overall, our findings using biomarkers measured very early after injury support the recently published results of the BIO-ProTECT study, in which GFAP improved outcome prediction over baseline patient variables alone. Our results also confirm the observation that increased levels of GFAP and UCH-L1 are associated with increased likelihood of poor outcome at 6 months.9 However, in contrast to BIO-ProTECT, our results suggest that UCH-LI also improves outcome prediction over clinical characteristics alone. This difference may be due to the fact that the predictor model used in BIO-ProTECT included the Rotterdam score, a radiographic measure of injury severity, in addition to the clinical parameters of age, gender, and GCS evaluated in our study. Furthermore, blood samples were obtained an average of 3.32 hours post-injury in the BIO-ProTECT study versus 1.4 hours in our analysis, demonstrating that predictive value is maintained when biomarker levels are measured within 2 hours of MS-TBI.
This study has a number of limitations that are important to consider. While the Prehospital TXA for TBI trial attempted to enroll patients with isolated TBI, patients with extracranial injuries remained eligible for inclusion and we did not evaluate the impact of this on biomarker levels. However, a sub-analysis of isolated head injury performed in the BIO-ProTECT study did not significantly affect the predictive capacity of GFAP and UCH-L1. Secondly, evaluation of predictive capacity was based on comparison to prehospital characteristics instead of IMPACT score, which has demonstrated superiority for prognostication.29–33 In future analyses, we intend to repeat these evaluations using IMPACT scores. Furthermore, patients on baseline anticoagulant therapy were not excluded. The relationship between anticoagulation and circulating biomarker levels in the context of TBI has not been established, and future investigation may be warranted to clarify this interaction. Finally, this study excluded patients with hypotension, penetrating TBI, GCS=3 with no reactive pupils, administration of CPR by EMS, and history of seizures, MI, or stroke, thus limiting the application of our findings in these populations.
In conclusion, our results suggest that serum levels of GFAP, UCH-L1, and MAP-2 may improve the ability to predict the presence of CT lesions prior to imaging over clinical characteristics alone in patients with MS-TBI, with GFAP appearing most promising. This has significant utility for initial triage of patients with TBI and identification of patients for clinical trial enrollment. Our results also demonstrate improved prediction of long-term outcome (based on 6-month GOS-E and DRS) using a combination of biomarker levels and prehospital characteristics. Further investigation is warranted to explore the utility of biomarkers in the prehospital setting and define appropriate cutoff ranges.
Conflicts of Interest and Source of Funding:
The authors hold no relevant conflicts of interest.
Funding was provided by the following R01 awards:
Blood Based Biomarkers of Injury and Outcome in the Prehospital TXA for TBI Trial
Source: NHLBI 1RO1HL126585–01, Grants.gov GRANT11652928
Prehospital Tranexamic Acid Use for Traumatic Brain Injury
Source: Department of Defense ERMS# 13335004; Resuscitations Outcome Consortium
Contributor Information
Taylor Anderson, Oregon Health & Science University.
Jun Hwang, University of Washington.
Myrna Munar, Oregon State University.
Linda Papa, Orlando Regional Medical Center.
Holly E. Hinson, Oregon Health & Science University.
Allison Vaughan, Duke University Medical Center.
Susan Rowell, Duke University Medical Center; Oregon Health & Science University.
References
- 1.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017. March 17;66(9):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, et al. Clinical trials in head injury. J Neurotrauma. 2002. May;19(5):503–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frowein RA. Classification of coma. Acta Neurochir (Wien). 1976. March;34(1–4):5–10. [DOI] [PubMed] [Google Scholar]
- 4.Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JDF, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008. August 5;5(8):e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balestreri M, Czosnyka M, Chatfield DA, Steiner LA, Schmidt EA, Smielewski P, Matta B, Pickard JD. Predictive value of Glasgow Coma Scale after brain trauma: change in trend over the past ten years. J Neurol Neurosurg Psychiatry. 2004;75(1):161–162. [PMC free article] [PubMed] [Google Scholar]
- 6.Bledsoe BE, Casey MJ, Feldman J, Johnson L, Diel S, Forred W, Gorman C. Glasgow Coma Scale Scoring is Often Inaccurate. Prehospital Disaster Med. 2015. February;30(1):46–53. [DOI] [PubMed] [Google Scholar]
- 7.Dadas A, Washington J, Diaz-Arrastia R, Janigro D. Biomarkers in traumatic brain injury (TBI): a review. Neuropsychiatr Dis Treat. 2018. November 8;14:2989–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Evaluation of automatic class III designation for Banyan Brain Trauma Indicator.
- 9.Frankel M, Fan L, Yeatts SD, Jeromin A, Vos PE, Wagner AK, Wolf BJ, Pauls Q, Lunney M, Merck LH, et al. Association of Very Early Serum Levels of S100B, Glial Fibrillary Acidic Protein, Ubiquitin C-Terminal Hydrolase-L1, and Spectrin Breakdown Product with Outcome in ProTECT III. J Neurotrauma. 2019. October 15;36(20):2863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luoto TM, Raj R, Posti JP, Gardner AJ, Panenka WJ, Iverson GL. A Systematic Review of the Usefulness of Glial Fibrillary Acidic Protein for Predicting Acute Intracranial Lesions following Head Trauma. Front Neurol [Internet]. 2017. [cited 2019 Oct 26];8 Available from: https://www.frontiersin.org/articles/10.3389/fneur.2017.00652/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazarian JJ, Biberthaler P, Welch RD, Lewis LM, Barzo P, Bogner-Flatz V, Gunnar Brolinson P, Büki A, Chen JY, Christenson RH, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol. 2018;17(9):782–9. [DOI] [PubMed] [Google Scholar]
- 12.Mahan MY, Thorpe M, Ahmadi A, Abdallah T, Casey H, Sturtevant D, Judge-Yoakam S, Hoover C, Rafter D, Miner J, et al. Glial Fibrillary Acidic Protein (GFAP) Outperforms S100 Calcium-Binding Protein B (S100B) and Ubiquitin C-Terminal Hydrolase L1 (UCH-L1) as Predictor for Positive Computed Tomography of the Head in Trauma Subjects. World Neurosurg. 2019. August 1;128:e434–44. [DOI] [PubMed] [Google Scholar]
- 13.Papa L, Lewis LM, Silvestri S, Falk JL, Giordano P, Brophy GM, Demery JA, Liu MC, Mo J, Akinyi L, et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg. 2012. May;72(5):1335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondello S, Papa L, Buki A, Bullock MR, Czeiter E, Tortella FC, Wang KK, Hayes RL. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care Lond Engl. 2011. June 24;15(3):R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyt DB. Post hoc ergo propter hoc: the story of the Resuscitation Outcomes Consortium. J Trauma Acute Care Surg. 2013. January;74(1):8–16. [DOI] [PubMed] [Google Scholar]
- 16.Papa L, Robicsek SA, Brophy GM, Wang KKW, Hannay HJ, Heaton S, Schmalfuss I, Gabrielli A, Hayes RL, Robertson CS. Temporal Profile of Microtubule-Associated Protein 2: A Novel Indicator of Diffuse Brain Injury Severity and Early Mortality after Brain Trauma. J Neurotrauma. 2018. 01;35(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepe MS, Kerr KF, Longton G, Wang Z. Testing for improvement in prediction model performance. Stat Med. 2013. April 30;32(9):1467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papa L, Robertson CS, Wang KKW, Brophy GM, Hannay HJ, Heaton S, Schmalfuss I, Gabrielli A, Hayes RL, Robicsek SA. Biomarkers improve clinical outcome predictors of mortality following non-penetrating severe traumatic brain injury. Neurocrit Care. 2015. February;22(1):52–64. [DOI] [PubMed] [Google Scholar]
- 19.Lei J, Gao G, Feng J, Jin Y, Wang C, Mao Q, Jiang J. Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: a prospective cohort study. Crit Care Lond Engl. 2015. October 12;19:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vos PE, Jacobs B, Andriessen TMJC, Lamers KJB, Borm GF, Beems T, Edwards M, Rosmalen CF, Vissers JLM. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology. 2010. November 16;75(20):1786–93. [DOI] [PubMed] [Google Scholar]
- 21.Brunkhorst R, Pfeilschifter W, Foerch C. Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage-pathophysiological background and clinical findings. Transl Stroke Res. 2010. December;1(4):246–51. [DOI] [PubMed] [Google Scholar]
- 22.Undén J, Bellner J, Eneroth M, Alling C, Ingebrigtsen T, Romner B. Raised serum S100B levels after acute bone fractures without cerebral injury. J Trauma. 2005. January;58(1):59–61. [DOI] [PubMed] [Google Scholar]
- 23.Papa L, Brophy GM, Welch RD, Lewis LM, Braga CF, Tan CN, Ameli NJ, Lopez MA, Haeussler CA, Giordano DIM, et al. Time Course and Diagnostic Accuracy of Glial and Neuronal Blood Biomarkers GFAP and UCH-L1 in a Large Cohort of Trauma Patients With and Without Mild Traumatic Brain Injury. JAMA Neurol. 2016. May 1;73(5):551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papa L, Akinyi L, Liu MC, Pineda JA, Tepas JJ, Oli MW, Zheng W, Robinson G, Robicsek SA, Gabrielli A, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010. January;38(1):138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao X, Deng P, Xu ZC, Chen J. Moderate Traumatic Brain Injury Causes Acute Dendritic and Synaptic Degeneration in the Hippocampal Dentate Gyrus. PLOS ONE. 2011. September 13;6(9):e24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park D, Joo SS, Lee HJ, Choi K-C, Kim SU, Kim Y-B. Microtubule-associated protein 2, an early blood marker of ischemic brain injury. J Neurosci Res. 2012. February;90(2):461–7. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa K, Matsumoto M, Niinobe M, Mikoshiba K, Hata R, Ueda H, Handa N, Fukunaga R, Isaka Y, Kimura K, et al. Microtubule-associated protein 2 as a sensitive marker for cerebral ischemic damage—Immunohistochemical investigation of dendritic damage. Neuroscience. 1989. January 1;31(2):401–11. [DOI] [PubMed] [Google Scholar]
- 28.Mondello S, Gabrielli A, Catani S, D’Ippolito M, Jeromin A, Ciaramella A, Bossù P, Schmid K, Tortella F, Wang KKW, et al. Increased levels of serum MAP-2 at 6-months correlate with improved outcome in survivors of severe traumatic brain injury. Brain Inj. 2012. December 1;26(13–14):1629–35. [DOI] [PubMed] [Google Scholar]
- 29.Marmarou A, Lu J, Butcher I, McHugh GS, Murray GD, Steyerberg EW, Mushkudiani NA, Choi S, Maas AIR. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. J Neurotrauma. 2007. February;24(2):270–80. [DOI] [PubMed] [Google Scholar]
- 30.Maas AIR, Steyerberg EW, Butcher I, Dammers R, Lu J, Marmarou A, Mushkudiani NA, McHugh GS, Murray GD. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007. February;24(2):303–14. [DOI] [PubMed] [Google Scholar]
- 31.McHugh GS, Engel DC, Butcher I, Steyerberg EW, Lu J, Mushkudiani N, Hernández AV, Marmarou A, Maas AIR, Murray GD. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007. February;24(2):287–93. [DOI] [PubMed] [Google Scholar]
- 32.Van Beek JGM, Mushkudiani NA, Steyerberg EW, Butcher I, McHugh GS, Lu J, Marmarou A, Murray GD, Maas AIR. Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007. February;24(2):315–28. [DOI] [PubMed] [Google Scholar]
- 33.Egea-Guerrero JJ, Rodríguez-Rodríguez A, Gordillo-Escobar E, Fernández-Delgado E, Martínez-Roldán Á, Roldán-Reina Á, Durán-Martínez P, de Vega-Ríos E, Freire-Aragón MD, Vilches-Arenas Á, et al. IMPACT Score for Traumatic Brain Injury: Validation of the Prognostic Tool in a Spanish Cohort. J Head Trauma Rehabil. 2018. February;33(1):46. [DOI] [PubMed] [Google Scholar]


