Abstract
Objective:
To characterize cervical and ocular vestibular evoked myogenic potential (c- and oVEMP) responses using an impulse hammer (IH) in adults and pediatrics at standardized force levels and evaluate: the relationship of force level on VEMP amplitude, sternocleidomastoid (SCM) contraction on cVEMP amplitude, required number of tap stimuli, and subject comfort. Using these data, optimal testing parameters were selected.
Study Design:
Prospective study.
Setting:
Tertiary referral center.
Patients:
Seventy- eight healthy adults, adolescents, and children with no hearing or vestibular deficits.
Interventions:
All subjects received c- and oVEMP testing using IH and 500 Hz tone burst air conduction stimuli. Adults received hard, medium, and soft force levels. Adolescents and children received medium and soft force levels. A comfort questionnaire was administered pre- and post-testing.
Main Outcome Measures:
IH VEMP response parameters (response rates, latency, cVEMP pre-stimulus SCM EMG, and peak-to-peak amplitude) were assessed per force level. Subjective reporting for patient comfort was also assessed.
Results:
VEMP response rates ranged from to 92 – 100%. Force had a linear relationship with VEMP amplitude. SCM contraction had a linear relationship with raw cVEMP amplitude; however, dissipated with amplitude normalization. Force level did not impact the number of taps needed. A minimum peak force of 15 – 20 N, accounting for SCM contraction, and using a lower EMG monitoring limit for cVEMP is recommended to elicit reliable responses.
Conclusions:
Overall, IH VEMP is appropriate and comfortable to use in adults and pediatrics and can be useful when an air conduction stimulus is contraindicated or not preferred.
INTRODUCTION
Reflex hammer cervical and ocular vestibular evoked myogenic potentials (c- and oVEMP, respectively) are minimally performed in adults and undocumented in pediatrics but has advantages over traditional air conducted (AC) stimuli. Typically, reflex hammer VEMP amplitudes are larger, present in compromised middle ears, and generated quickly (1–3). However, optimal stimulus parameters are unknown. Identifying ideal testing techniques would improve reflex hammer VEMP utility.
VEMP responses are short-latency myogenic potentials generated in response to high intensity sound or bone vibration. The cVEMP, an inhibitory response from the sternocleidomastoid muscle (SCM) (4–5), infers integrity of the saccule and inferior vestibular nerve (4;6). The oVEMP, an excitatory response from the inferior oblique muscle (7), assesses the utricle and potentially saccular inputs, and superior vestibular nerve (6–8). While AC VEMP is commonly used (9),altered middle ear function could attenuate the stimulus and abolish AC VEMP responses despite intact otolith function (10–11). Additionally, high intensity AC stimuli increases unsafe sound exposure when equivalent ear canal volumes (ECV) are ≤ 0.8 mL (12). Given that children have a higher incidence of middle ear issues (13) and smaller ECVs (14), bone conducted VEMP is beneficial.
Reflex hammers stimulate the otolith organs via bone conduction and yield robust cVEMP responses in adults with conductive hearing loss (1). Repeatable hammer c- and oVEMP responses have also been noted in healthy adults (15;2) while responses are absent in adults with vestibular pathology (2–3), with comparable response rates to AC VEMP (16). In adults, reflex hammer c- and oVEMP reliability is excellent (3). This is attributed to its higher force output compared to B-71 responses (17–18). Lastly, using a reflex hammer is efficient. Tapping the forehead stimulates left and right otoliths simultaneously and equally (18), generating bilateral responses.
However, there are pitfalls to using a reflex hammer. Force level, monitoring of SCM contraction, and the number of taps required, are not established across investigations, proving it difficult to replicate findings or generalize to clinical populations. Hammer force level is minimally reported (1–2) or standardized (15). Hammer force likely impacts VEMP amplitude and increases variability, particularly if taps are inconsistent. A linear relationship between force and c- and oVEMP amplitude is hypothesized, where harder forces elicit larger amplitudes due to increased energy reaching the otoliths.
Secondly, monitoring SCM contraction during reflex hammer cVEMP testing has not been addressed. A relatively linear relationship between SCM contraction and AC cVEMP amplitude is known (5;19). Accounting for SCM contraction is important to rule out poor or absent responses related to varying or low muscle contraction. This is influential in children who may have difficulty sustaining neck elevation. For hammer VEMP, a linear relationship between SCM contraction and cVEMP amplitude is hypothesized but will dissipate with amplitude normalization.
Thirdly, the number of taps required to obtain a reliable VEMP response is unknown. Reported number of taps vary between 10 to 200 taps (3;15–16). Higher force levels could be more effective in activating otolith afferents compared to lower force levels. As such, the number of taps needed to obtain reliable c- and oVEMP responses are hypothesized to decrease as force increases.
Lastly, it is unknown if reflex hammer VEMP is a comfortable stimulus. This is relevant in pediatrics, especially if a high force level is required to obtain reliable responses. It is hypothesized that pediatrics and adults can tolerate c- and oVEMP hammer testing.
Reflex hammer VEMPs are beneficial assessments; however, investigation about best practice is needed. This study was separated into two phases. For phase 1, the purpose was to: 1) characterize c- and oVEMP responses using an impulse hammer (IH) to quantify and standardize force level, 2) determine the relationship between force level and VEMP peak-to-peak amplitude, 3) determine the relationship between SCM contraction and cVEMP peak-to-peak amplitude, 4) determine the minimum number of taps needed to obtain reliable VEMP responses, and 5) determine subjective comfort. Using these data, the purpose of phase 2 was to develop an optimized test protocol that would yield high response rates. By using optimized parameters, it was hypothesized that reliable hammer VEMP responses could be obtained.
MATERIALS AND METHODS
Phase 1
Subjects
Thirteen children (mean 6.8 years; range 4 – 9; 7 males), 14 adolescents (mean 13.4 years; range 10 – 9; 4 males) and 21 adults (mean 29.4 years; range 20 – 40; 8 males) with normal hearing and tympanometry participated. Tympanometry (GSI Tympstar, Grason-Stradler, Eden Prairie, MN, USA) was considered normal if peak admittance was ≥ 0.2 mmhos and peak pressure was between −100 and 30 daPa. Subjects received a hearing screening at 1000, 2000, and 4000 Hz, using 25 dB HL as a pass/fail criterion (20). Subjects with history of otologic surgery, balance/dizziness, neurological disorders, abnormal tympanometry or failed hearing screening were excluded. Informed consent (and photo as needed) was obtained from all subjects for testing approved by the Institutional Review Board at Boys Town National Research Hospital.
VEMP Measurements
Impulse Hammer
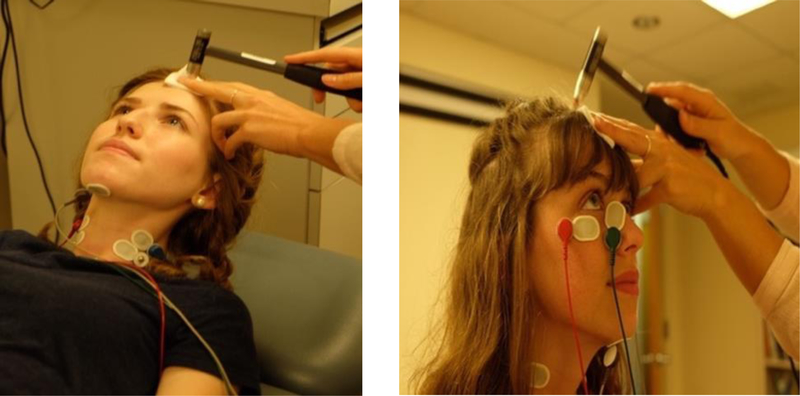
Stimuli were delivered using a Piezotronics IH with integrated ICP quartz force sensor (Model 086C01; PCB Corporation, Depew, New York, USA) to quantify the peak force level (in Newtons) per tap. IH c- and oVEMPs were recorded using a 2- channel Intelligent Hearing Systems 1.30 OptiAmp differential amplifier (Miami, FL, USA). Electromyography (EMG) was pre-processed using 5k gain and 10– 500 Hz bandpass filter. During c- and oVEMP testing, the examiner delivered manual taps at an estimated position of Fz (forehead midline, at the hairline) through a gauze pad at a rate of 2 taps/sec (Figure 1). To assess how force affects the VEMP response, adults received taps delivered at a soft (2 – 20 N; 126 – 146 dB pFL), medium (21 – 40 N; 146 – 152 dB pFL) and hard (41 – 60 N; 152 – 155 dB pFL) force level range. Due to discomfort in response to hard force levels reported by adults, only soft and medium were delivered to children and adolescents. Two trials of 30 taps were recorded for each force. In real-time, the data collection software registered the peak force of each tap and indicated whether the desired force level was met within the chosen range (e.g., 2– 20; 21– 40; 41– 60 N). Taps outside the desired force levels were excluded from analysis. See Supplemental Digital Content 1 for impulse hammer specifications and verification.
Figure 1.
Cervical (left) and Ocular (right) VEMP testing using an impulse hammer at an estimated position of Fz (midline of the forehead, at the hairline).
For the analysis, the corresponding left and right EMG and peak force level were collected using a Fireface UCX soundcard (RME, Germany) and custom software. The saved EMG files were imported into Matlab (MathWorks, Natick, MA; Version 2016a) where unrectified EMG waveforms were averaged sequentially. The examiner verified the peak force level (Figure 2) and measured c- and oVEMP latencies and peak-to-peak amplitudes for each averaged waveform. To estimate the level of SCM contraction for each cVEMP response, the root-mean-squared (RMS) of the pre-stimulus (−30 to −10 ms) EMG activity was calculated post-collection. The cVEMP corrected peak-to-peak amplitude was calculated by dividing the raw amplitude/RMS pre-stimulus EMG (24).
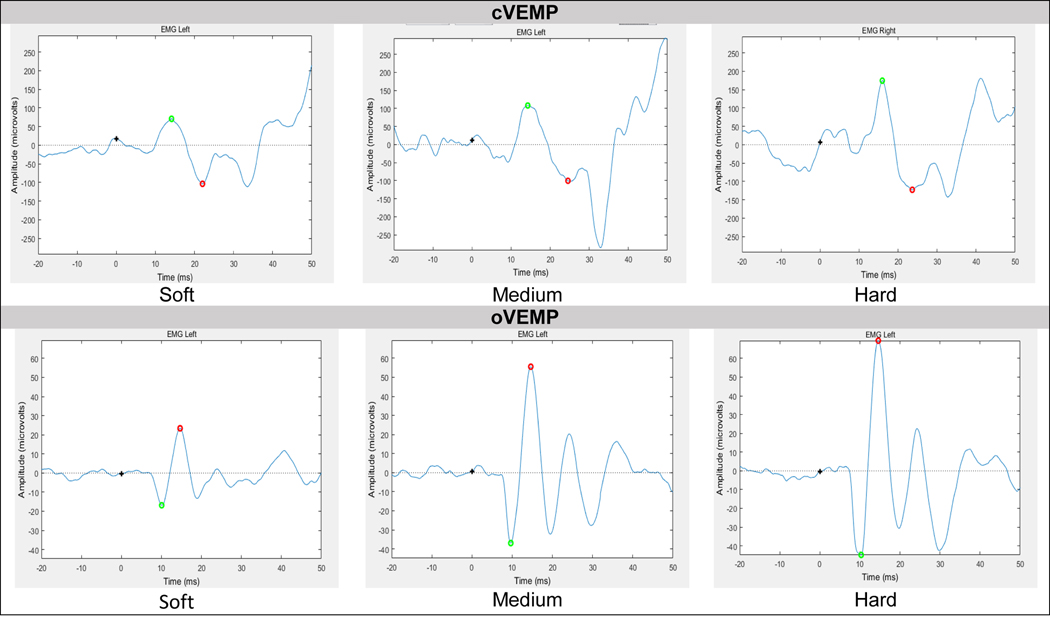
Figure 2.
Example of raw cervical (top) and ocular (bottom) VEMP response per force level (N) in one subject.
Air Conduction
AC c- and oVEMP measurements were obtained using an ICS Chartr 200 Evoked Potential System (GN Otometrics, Taastrup, DK). Stimuli were presented at 125 dB pSPL if the ECV was > 0.8 mL and at 120 dB pSPL if ≤ 0.8 mL (12). Stimuli were 500 Hz tone bursts presented in condensation, repetition of rate of 5.1 per second (Blackman gating window, 1-cycle rise/fall time, 0-cycle plateau; 4 ms duration; 5–500 band-pass filter). Seventy-five sweeps were averaged. Pre-set by the manufacturer, EMG monitoring (50 – 300 μV range) was performed throughout collection using the RMS of the total EMG activity.
Electrode Placement and Testing Position for Both Stimuli
The cVEMP electrode montage included an active electrode on the left and right SCM belly, a ground electrode under the chin and a reference electrode on the manubrium of the sternum (Figure 1, left). For AC, a separate EMG monitoring electrode was placed directly below the active electrode. For cVEMP, subjects were supine and lifted their head in the midline position for bilateral contraction. Parameters were p13/n23 latencies (ms), p13/n23 peak-to-peak amplitude (μV), and RMS EMG (μV).
The oVEMP electrode montage included an active electrode under the left and right eye (pupil-center and shifted laterally, thus on the belly of the inferior oblique muscle), a reference electrode on the right inner canthus and a ground electrode under the chin. This is an adapted version of the belly-tendon oVEMP electrode montage (25–26; Figure 1, right). During oVEMP testing, subjects were seated upright. Children and adolescents watched a video adhered to the wall at 30- degrees up- gaze. Parameters included n10/p16 latencies (ms) and n10/p16 peak-to-peak amplitude (μV).
Comfort Questionnaire
Subjects reported their physical comfort pre-post IH VEMP testing via a questionnaire. The examiner presented the questionnaire and read each question. Subjects rated their pain level from 0 (minimum pain) to 10 (maximum pain) on a visual analog scale. Subjects also indicated their preferred VEMP method (i.e., IH, AC, neither, or both) and if they would consider IH testing again, if needed (yes/no).
Phase 2
Subjects
Seven children (mean, 5.6 years; range, 4–9; 4 males), 9 adolescents (mean, 14.8 years; range, 10–19; 4 males) and 14 adults (mean, 28.6 years; range, 21–39; 7 males) participated. Two children, 3 adolescents, and 3 adults from phase 1, participated in phase 2.
VEMP Measurements
Using results from phase 1, an optimal IH VEMP procedure was developed. Taps were delivered at a soft-to-medium force level (10 – 30 N; equivalent to 140 – 149 dB pFL) based on high response rates using these levels. While the hard force yielded excellent response rates in adults, it was not chosen due to subject discomfort. SCM EMG was monitored using a pre-stimulus EMG RMS minimum of 100 μV (equivalent to 80 μV mean rectified). A 100 μV minimum is a conservative limit to avoid missed or asymmetrical responses associated with weak contractions (24;27). All remaining IH and AC c- and oVEMP procedures were the same as described above.
Statistical Analysis
Left versus right VEMP characteristics were compared using a paired samples t-test with Bonferroni p-value correction for multiple comparisons. Student-t test and one-way analysis of variance (ANOVA) was completed to compare VEMP response characteristics. Tukey’s honestly significant difference was used for post-hoc testing. Linear regression evaluated relationships between force and VEMP amplitudes and SCM EMG and cVEMP amplitudes. Pre-to-post questionnaire data was compared using Chi-square.
RESULTS
IH VEMP Responses
There was no significant difference between left/right side for all IH and AC VEMP outcomes (Supplemental Digital Content 2). Therefore, with the exception of response rates, left/right ear data were averaged for analyses. Adults and adolescents had 100% c- and oVEMP response rates regardless of peak force level while children had 92 – 100%. Collectively, IH cVEMP response rates were comparable to AC, while IH oVEMP response rates were higher than AC oVEMP (Table 1).
Table 1.
Response Rates for Impulse Hammer and Air Conduction Stimuli.
| Impulse Hammer | Force Level | Adults | Adolescents | Children | ALL |
|---|---|---|---|---|---|
| cVEMP | Hard | 100% (42/42) | Not performed | 100% (42/42) | |
| Mean Peak Force | 46.61 N | ||||
| Medium | 100% (42/42) | 100% (28/28) | 100% (26/26) | 100% (96/96) | |
| Mean Peak Force | 27.42 N | 27.48 N | 26.40 N | ||
| Soft | 100% (42/42) | 100% (28/28) | 96% (25/26) | 98% (95/96) | |
| Mean Peak Force | 12.32 N | 12.91 N | 11.50 N | ||
| oVEMP | Hard | 100% (42/42) | Not performed | 100% (42/42) | |
| Mean Peak Force | 46.15 N | ||||
| Medium | 100% (42/42) | 100% (28/28) | 92% (24/26) | 98% (94/96) | |
| Mean Peak Force | 27.98 N | 29.06 N | 28.29 N | ||
| Soft | 100% (42/42) | 100% (28/28) | 100% (26/26) | 100% (96/96) | |
| Mean Peak Force | 13.17 N | 12.52 N | 12.87 N | ||
| Air Conduction | Stimulus | Adults | Adolescents | Children | ALL |
| cVEMP | 500 Hz TB | 100% (42/42) | 100% (28/28) | 100% (26/26) | 100% (96/96) |
| oVEMP | 95% (40/42) | 92% (26/28) | 88% (22/26) | 91% (88/96) | |
Abbreviations: cVEMP, cervical vestibular evoked myogenic potential; oVEMP, ocular vestibular evoked myogenic potential; N, Newtons; TB, tone burst.
In Table 2 are the averaged IH VEMP outcomes per age group. To assess the relationship between age and IH VEMP outcomes, correlation analyses were performed. There was no significant relationship between age and cVEMP p13 latency [soft (r= −.074, p= .622); medium (r= −.171, p= .244)], pre-stimulus EMG [soft (r= −.11, p= .426); medium (r= −.03, p= .812)] and corrected peak-to-peak amplitudes [soft (r= −.05, p= .102); medium (r= −.18, p= .080)]. However, there was a significant positive relationship between age and n23 peak latency regardless of force [soft (r= .375, p= .009); medium (r= .568, p= .001)], indicating that n23 latency increases with age. No significant relationships were identified between age and oVEMP n10 [soft (r= .120, p= .421); medium (r= −.037, p= .809)], p16 [soft (r= −.007, p= .965); medium (r= −.167, p= .272)] or peak-to-peak oVEMP amplitude [soft (r= .09, p= .521); medium (r= .06, p= .644)].
Table 2.
Mean (SD) Impulse Hammer Cervical and Ocular VEMP Response Characteristics per Force Level and per Group.
| Cervical VEMP | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hard Force Level (41 – 60 N) | Medium Force Level (21 – 40 N) | Soft Force Level (2 – 20 N) | |||||||||||||
| Group | p13 | n23 | *AMP | EMG | *CA | p13 | n23 | *AMP | EMG | *CA | p13 | n23 | *AMP | EMG | *CA |
| Adults | 13.80 (2.14) | 20.73 (3.73) | 422.27 (186.90) | 243.14 (111.1) | 1.87 (0.80) | 13.88 (1.86) | 21.67 (2.87) | 299.61 (112.67) | 249.09 (97.94) | 1.23 (0.47) | 15.41 (2.88) | 23.76 (2.86) | 199.77 (78.16) | 236.11 (87.86) | 0.88 (0.22) |
| Adolescents | Not performed | 14.23 (2.04) | 21.57 (3.59) | 364.00 (281.10) | 232.04 (79.91) | 1.62 (0.54) | 16.44 (1.73) | 22.02 (1.50) | 204.62 (119.92) | 206.81 (98.49) | 0.91 (0.41) | ||||
| Children | Not performed | 14.67 (.89) | 20.14 (1.74) | 423.15 (329.62) | 249.87 (94.42) | 1.70 (1.04) | 15.39 (1.21) | 21.20 (1.65) | 298.78 (163.03) | 249.62 (67.44) | 1.19 (0.50) | ||||
| Ocular VEMP | |||||||||||||||
| Hard Force Level (41 – 60 N) | Medium Force Level (21 – 40 N) | Soft Force Level (2 – 20 N) | |||||||||||||
| n10 | p16 | *AMP | n10 | p16 | *AMP | n10 | p16 | *AMP | |||||||
| Adults | 10.10 (1.15) | 14.86 (1.21) | 53.83 (35.89) | 11.05 (1.29) | 15.86 (1.39) | 37.71 (25.76) | 13.25 (1.59) | 17.87 (2.01) | 22.57 (13.82) | ||||||
| Adolescents | Not performed | 11.32 (2.33) | 16.45 (2.17) | 30.64 (18.73) | 12.52 (1.65) | 17.69 (1.57) | 16.37 (9.90) | ||||||||
| Children | Not performed | 11.15 (.69) | 16.47 (.42) | 29.75 (20.52) | 12.45 (1.65) | 17.54 (0.81) | 17.15 (9.37) | ||||||||
Abbreviations: AMP, uncorrected peak-to-peak amplitude; EMG, electromyography; CA, corrected peak-to-peak amplitude; VEMP, vestibular evoked myogenic potential. p13/n23 and n10/p16 latencies recorded in milliseconds; AMP and EMG recorded in microvolts. EMG represents the average RMS using a 100-microvolt minimum; CA (RMS) = AMP divided by average EMG (RMS). CAs can vary depending on whether mean rectified or RMS EMG is used. RMS is typically 1.25 times larger than rectified, resulting in smaller CAs
Denotes a significant mean increase in cervical and ocular VEMP amplitude as force level increased (p < .05) for adults, adolescents and children.
Relationship Between Force Level and VEMP Amplitude
There was a stepwise growth in c- and oVEMP peak-to-peak amplitude as peak force level increased for each age group (Figure 2 demonstrates example waveforms for one subject). Soft force levels generated significantly lower cVEMP amplitudes compared to medium force for children [uncorrected amplitude: t(12)= 2.66; p= .021; corrected amplitude: t(12)= 3.09; p= .004] and adolescents [uncorrected amplitude: t(13)= 4.24; p< .001; corrected amplitude: t(13)= 3.06, p= .004]. A similar trend was noted for adults [uncorrected amplitude: F(2, 60)= 16.00; p< .001; corrected amplitude: F(2, 60)= 17.71; p< .001]. Soft force amplitudes were lower compared to medium (uncorrected amplitude: p= .033; corrected amplitude: p= .024) and hard (uncorrected amplitude: p< .001; corrected amplitude: p< .001); medium force amplitudes were significantly lower than hard force amplitudes (uncorrected amplitude: p= .011; corrected amplitude: p= .002).
For oVEMP, lower oVEMP amplitudes using a soft versus medium peak force level were evidenced in children [t(12)= 2.39; p= .028] and adolescents [t(13)= 2.55; p= .019]. A similar trend was noted for adults [F(2, 60= 11.7; p< .001). Soft force amplitudes were lower compared to medium (p= .028) and hard (p< .001), medium force amplitudes were significantly lower than hard force amplitudes (p= .025).
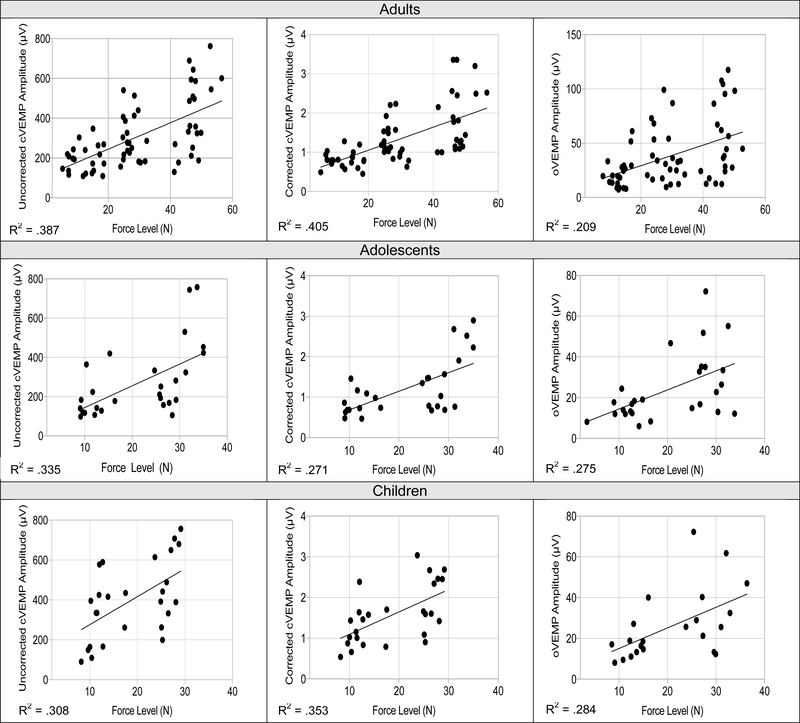
Shown in Figure 3, regression analyses revealed a linear relationship between peak force (X) and VEMP amplitude (Y) per age group for cVEMP peak-to-peak amplitude in adults (uncorrected amplitude: R2= .387, p<. 001; corrected amplitude: R2= .405, p< .001), adolescents (uncorrected amplitude: R2= .335, p= .002; corrected amplitude: R2= .271, p< .001), and children (uncorrected amplitude: R2= .308, p= .003; corrected amplitude: R2= .353, p= .001). A similar positive linear relationship between peak force and oVEMP amplitudes was noted for adults (R2= .209, p< .001), adolescents (R2= .275, p= .005), and children (R2= .284, p= .011). See Supplemental Digital Content 3 for regression equations.
Figure 3.
Significant linear relationships between impulse hammer force and peak-to-peak cervical (uncorrected and corrected) and ocular VEMP amplitudes (μv) for adults (top), adolescents (middle), and children (bottom). Force (N) is a significant predictor for cervical and ocular VEMP amplitude (all p-values > .05).
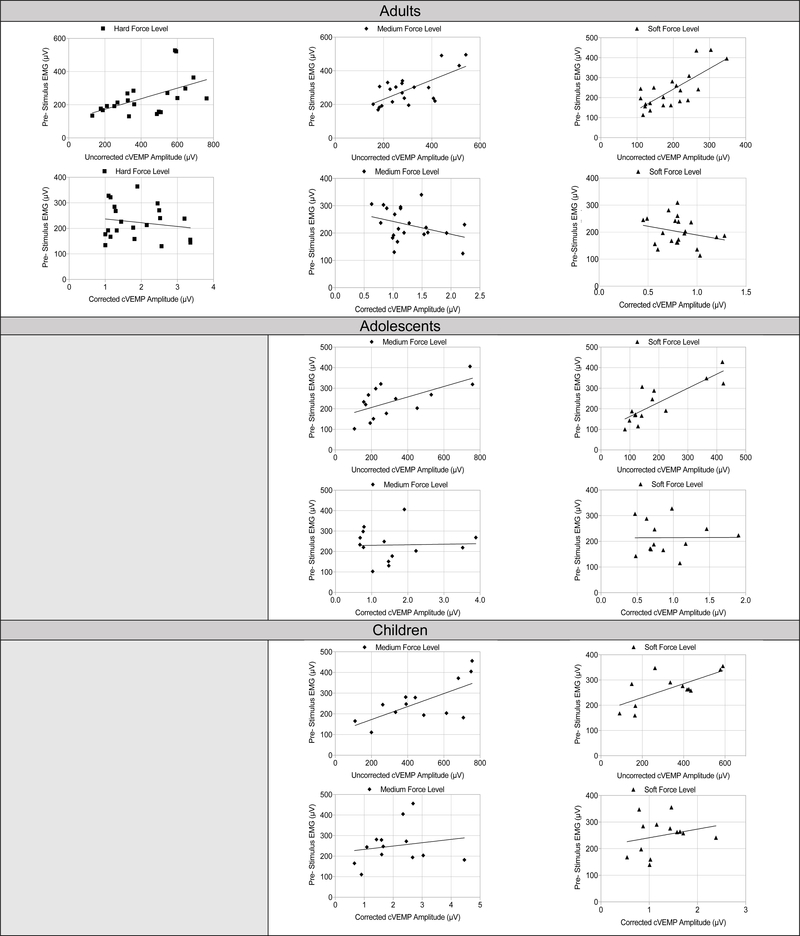
Relationship Between SCM EMG and IH cVEMP Amplitude
Shown in Figure 4, IH cVEMP amplitudes are influenced by the level of SCM contraction. There was a significant linear relationship between the pre- stimulus EMG level and uncorrected cVEMP peak-to-peak amplitudes for children [soft (R2 .493, p = .011; medium (R2= .483, p= .008)] and adolescents [soft (R2= .421, p= .012; medium (R2= .430, p= .011)]. Similar results were found for adults regardless of force level [soft (R2= .566, p <001); medium (R2= .253, p= .020); hard (R2= .382, p= .013)], suggesting that higher SCM contractions lead to higher cVEMP peak-to-peak amplitudes. However, as anticipated, when amplitude normalization was completed, the relationships became non-significant for children [soft (R2= .082, p= .343); medium (R2= .032, p= .559)], adolescents [soft (R2= .021, p= .945); medium (R2= .001, p= .908)], and adults [soft (R2= .075, p= .227); medium (R2= .109, p= .143); hard (R2= .076; p= .227)].
Figure 4.
Relationship between uncorrected and corrected cVEMP amplitude per force level. There were significant linear relationships between uncorrected cVEMP amplitudes and pre-stimulus EMG level at each hammer force. When correcting for EMG, the relationship was no longer significant per age group and force level.
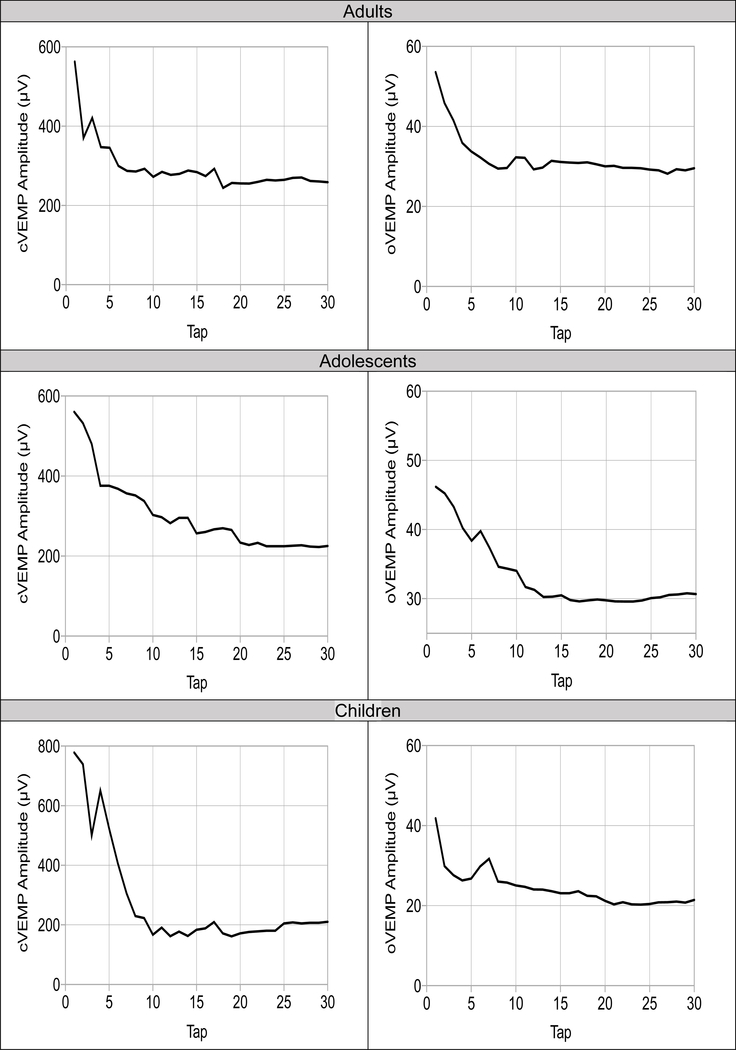
Number of IH Taps
Per subject, single trial VEMP peak-to-peak amplitude responses were sequentially averaged to determine the number of taps required to observe minimal variability (as measured by a standard deviation of 2.0 or less) in VEMP peak-to-peak amplitudes. An average number of taps was then derived for each age group per force level to compare how many taps were needed per force level. On average, for cVEMP in children, responses stabilized by tap 18 (soft) and 20 (medium) with no significant difference between number of taps needed for soft versus medium force [t(12)= 1.01, p= .309]. In adolescents, responses stabilized by tap 17 (soft) and 18 (medium) with no significant difference in number of taps needed for soft versus medium force [t(13)= 0.88, p= .309]. In adults, there was no significant difference in the average number of taps needed across the 3 force levels (F= 1.23, p= .677) as responses stabilized by tap 17 (soft), 20 (medium), and 18 (hard).
For oVEMP, there was no significant difference between number of taps needed between either force level in children [t(12)= 1.01, p= .880) and adolescents [t(13)= 1.32, p= .092). On average, oVEMP responses stabilized by tap 14 (soft and medium) in children and tap 15 (soft) and 13 (medium) in adolescents. In adults, responses stabilized by tap 13 (soft and medium) and tap 15 (hard) and not significantly different per force level (F= .242, p= .353).
Comfort Questionnaire
For all subjects, there was no significant difference in pain levels pre-to-post IH testing (pre= .20, post= .395; p= .109) and no difference in the preference of VEMP method [X2(2) = 2.213; p= .331). The majority of subjects preferred either method (n=19/48), 17 preferred AC, and 12 preferred IH. While 1 child did not agree, 98% percent would consider IH testing again.
IH VEMP Responses Using Optimized Parameters
IH response rates were 100% for c- and oVEMP per age group. Similar to phase 1, the average number of taps required was ~20 taps for cVEMP and ~15 taps for oVEMP for each group (Figure 5). See Supplementary Digital Content 4 for comparison of AC and IH VEMP responses. Collectively, findings suggest that with the exception of larger oVEMP amplitudes when using an IH, all other VEMP outcomes were unchanged when using an IH or AC across age groups and gender.
Figure 5.
VEMP response as a function of number taps for 10 – 30 N force level. On average, cervical (left) and ocular (right) VEMP response stabilizes by tap 20 and 15, respectively for all groups.
DISCUSSION
VEMP testing using a reflex hammer has benefits; however, information about best practices are limited. Until this investigation, VEMP using a hammer stimulus has not been explored in pediatrics. As hypothesized, our IH c- and oVEMP response rates agreed with previous work in adults using AC (15;28). An IH generates otolith responses because tapping the forehead produces vibratory waves that travel across the skull causing small head accelerations. This movement results in otolith hair cell deflections and respective afferent activation (2–3;17). Bone vibration also preferentially activates otolith afferents in comparison to semicircular canal (18;29), justifying bone conduction VEMP for otolith assessment.
Despite the value of reflex hammer VEMP, normative responses and associated factors that can affect the response are limited. In the present study, IH c- and oVEMP responses in adults and pediatrics at different force levels were characterized. While previous studies have used an inertial triggered reflex hammer, the force delivered between-and-within subjects is not evaluated or minimally indicated (15) and varies in force. Because the stimulus is delivered manually, inconsistent force is delivered with each tap, increasing response variability. Quantifying force is necessary to interpret amplitude responses, as reported by Iwasaki et al. (17), who opted to not report their hammer data because the output could not be sufficiently controlled. In support of our hypothesis there is a significant increase in c- and oVEMP amplitude as peak force level increases’ therefore, justifying the need to account for force level within-and-between individuals.
Using our optimized protocol (10 – 30 N), c- and oVEMP response rates were 100%. The examiners (AIR; SAC) provided an average peak force level of ~15 N (~143 dB pFL) for oVEMP and ~20 N (~146 dB pFL) for cVEMP when testing subjects. While others using a Mini-shaker (28; 30) or B-71 (28) have reported lower force levels (e.g., 131– 136 dB pFL), our force levels align with Taylor et al. 2014 (i.e., 24 N; 147 dB pFL; 31). Therefore, we recommend a minimum of ~15 N for oVEMP and ~20 N for cVEMP. These IH forces yield high response rates, yet are comfortable for children and adults.
In conjunction with saccular afferent activation and some utricular inputs (18, 32), cVEMP responses rely on adequate SCM contraction (5;19;24). With increased SCM contraction, inhibition grows, resulting in larger cVEMPs (33). Supportive of our hypothesis, uncorrected IH cVEMP amplitude is highly influenced by SCM contraction level, suggesting the need for normalization when using an IH (21;27). Additionally, EMG monitoring using a minimum of 100 μV RMS was used in phase 2 and response rates improved for children from 96% to 100%. Collectively, these findings suggest that both amplitude normalization and EMG monitoring are warranted for cVEMP when using a hammer stimulus.
The number of taps required for VEMP when using a hammer stimulus has not been widely explored. Identifying the minimum number of taps is critical for efficiency, reliability, and comfort. It was hypothesized that greater force would require significantly fewer taps; however, our findings did not support this. Regardless of force level, a minimum of ~15 – 20 taps were needed. Because fewer taps may introduce greater error in estimating the amplitude and contraction level (for cVEMP), repeating trials to confirm reproducibility is recommended.
We found that IH VEMP is comfortable, as hypothesized. A larger number of children (n= 12/20) and adolescents (n= 5/23) preferred IH versus AC. To the authors’ knowledge there are no known cutoffs for bone conduction safety; however, accounting for physical comfort and safety should be considered. While IH VEMP testing using our recommended level was tolerated by 100% of subjects with no change in pain level from pre—post testing [pre= .033, post= .066; t= .570, p= .572), the risk of ecchymosis or potential subdural bleeding could be increased with greater hammer force levels. This may be especially relevant for high risk populations (i.e., children with widened subdural space (34), elderly who are being treated with anticoagulants (35)). Due to the large variability when manually delivering hammer taps, real-time feedback about the force being delivered is an advantage for monitoring patient safety and comfort.
Lastly, IH oVEMP amplitudes were significantly larger compared to AC VEMPs across age groups (Supplementary Digital Content, 4). Similar trends have been noted by others (1;3;28;36). Bone conduction is thought to stimulate the utricular afferent pathway more effectively than AC, while AC may be a better stimulus to recruit saccular afferent activation (28). While we did not observe statistical significance between IH and AC cVEMP, IH stimuli yielded similar if not larger amplitude responses compared to AC.
Our data also showed no significant influence of age on IH or AC VEMP amplitudes (Supplementary Digital Content 4). While this is consistent with other studies using a hammer stimulus in adults [20 – 80 years] (28), our lack of an observed age effect is likely due to our younger age range. Age related changes in amplitude are not typically seen until the fifth decade of life (37–38).
When interpreting our phase 1 data we observed a linear relationship between cVEMP n23 latency and age, where the n23 increased with age. This has been a consistent finding in the pediatric cVEMP literature and attributed to the n23 receiving contribution from the musculotendinous junction (39) and the shorter SCM observed in children (40). As compared to other reflex hammer investigations (2;18;31), our IH c-and oVEMP latencies were longer. This is attributed to differences and variability in force level used across investigations. Lastly, and in line with other investigations, we did not show an effect of gender on IH and AC c-and oVEMP peak-to-peak amplitudes (21;37) or latencies (21;41) (Supplementary Digital Content, 4).
Despite its usefulness, there are limitations to IH VEMP. Foremost, identifying present cVEMP responses can be unclear. Later occurring waves (i.e., n2) can merge with the cVEMP response or be interpreted as the response itself given its large amplitude and similar morphology (1; 21–22). Other investigators who have used a hammer stimulus reported the influence of the n2; however, they differentiated the cVEMP from the n2 by confirming a clear distinction between the two peaks and noted that the cVEMP n23 had consistently earlier latencies as compared to the n2, which more often occurred within 30 – 38 ms (21;23). In patients with known bilateral vestibular loss, their EMG responses only consisted of late negativity like what was seen in normal subjects, indicating that these later waveforms are not vestibular in nature and should not be interpreted as cVEMP responses (21). Clinicians should be cognizant of the influence of the n2 when using an IH for cVEMP and establish criteria for which constitutes a response.
Secondly, for vestibular disorders such as semicircular canal dehiscence, a hammer stimulus has reduced sensitivity (66%) and specificity (3.5%) compared to a 500 Hz AC stimulus (100% sensitivity/specificity; 42). Reductions in cVEMP thresholds are less notable as compared to AC (23). Because the IH is not frequency specific but stimulates a broad frequency spectrum, manipulation of single frequencies or tuning cannot be readily adjusted. Despite these limitations, IH VEMP is considered an effective stimulus to diagnose vestibular hypofunction (22).
CONCLUSION
IH c- and oVEMP responses can be obtained in adults and pediatrics. A peak force level of ~15 N (oVEMP) and ~20 N (cVEMP), yields excellent response rates and is a comfortable stimulus. A minimum of 100 μV RMS ensures adequate SCM contraction and a minimum of 15 – 20 taps is recommended to minimize variability.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM109023 and by the National Institute on Deafness and Other Communication Disorders under award numbers R03DC015318, T35DC 008757, 5T32DC00013–36. The authors would also like to thank Dr. Kendra Schmid, Ph.D. for her statistical consultation and Dr. Robert Burkard, Ph.D., for his consultation on bone conduction calibration.
Source of funding:
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM109023 and by the National Institute on Deafness and Other Communication Disorders under award numbers R03DC015318, T35DC 008757, 5T32DC00013–36.
AIR and EM received funding from the NIH/NIDCD for this work.
KLJ does consulting regarding vestibular testing through Audiology Systems and receives funding from the NIH/NIDCD.
Footnotes
Conflicts of Interest:
All other authors (DF, TC, SAC, MLA) have no potential conflicts of interest to be disclosed.
REFERNCE LIST
- 1.Halmagyi GM, Yavor RA, Colebatch JG. Tapping the head activates the vestibular system: A new use for the clinical reflex hammer. Neurology 1995;45:1927–1929. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki S, McGarvie L, Halmagyi G, Burgess A, Kim J, Colebatch J, et al. Head taps evoke a crossed vestibulo-ocular reflex. Neurology 2007;68:1227–1229. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol 2010;31:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colebatch JG, Halmagyi GM. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology 1992;42:1635–1636. [DOI] [PubMed] [Google Scholar]
- 5.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 1994;57:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol 2010;121:132–144. [DOI] [PubMed] [Google Scholar]
- 7.Todd NP, Rosengren SW, Colebatch JG. Ocular vestibular evoked myogenic potentials (oVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol 2007;118:381–390. [DOI] [PubMed] [Google Scholar]
- 8.Weber KP, Rosengren SM, Michels R, et al. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. J Physiol 2012;590:3091–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papathanasiou ES, Murofushi T, Akin FW, et al. International guidelines for the clinical application of cervical vestibular evoked myogenic potentials: An expert consensus report. Clin Neurophysiol 2014;125:658–666. [DOI] [PubMed] [Google Scholar]
- 10.Bath AP, Harris N, McEwan J, Yardley MP. Effect of conductive hearing loss on the vestibulo-collic reflex. Clin Otolaryngol Allied Sci 1999; 24:181–183. [DOI] [PubMed] [Google Scholar]
- 11.Zhou G, Poe D, Gopen Q. Clinical use of vestibular evoked myogenic potentials in the evaluation of patients with air-bone gaps. Otol Neurotol 2012;33:1368–1374. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez AI, Thomas MLA, Fitzpatrick D, et al. Effects of high sound exposure during air-conducted vestibular evoked myogenic potential testing in children and young adults. Ear Hear 2018;39:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou G, Dargie J, Dornan B, et al. Clinical uses of cervical vestibular-evoked myogenic potential testing in pediatric patients. Medicine(Baltimore) 2014;93, e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nozza RJ, Bluestone CD, Kardatzke D, et al. Towards the validation of aural acoustic immittance measures for diagnosis of middle ear effusion in children. Ear Hear 1992;13:442–453. [DOI] [PubMed] [Google Scholar]
- 15.MacDougall HG, Holden J, Rosengren SM, Chiarovano E. μVEMP: A Portable Interface to Record Vestibular Evoked Myogenic Potentials (VEMPs) With a Smart Phone or Tablet. Front Neurol 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang TL, Young YH . Comparison of tone burst and tapping evocation of myogenic potentials in patients with chronic otitis media. Ear Hear 2003;24(3):191–194. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, Macdougall HG, Halmagyi GM, et al. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol 2008;119(9):2135–2147. [DOI] [PubMed] [Google Scholar]
- 18.Curthoys IS, Vulovic V, Burgess AM, Manzari L, Sokolic L, Pogson J, et al. Neural basis of new clinical vestibular tests: otolithic neural responses to sound and vibration. Clin Exp Pharmacol Physiol 2014;41(5):371–80. [DOI] [PubMed] [Google Scholar]
- 19.Akin FW, Murnane OD, Panus PC, et al. The influence of voluntary tonic EMG level on the vestibular-evoked myogenic potential. J of Rehab Res & Develop 2004; 41:473–480. [DOI] [PubMed] [Google Scholar]
- 20.American Speech-Language-Hearing Association. Guidelines for Audiologic Screening [Guidelines] 1997; Available from www.asha.org/policy.
- 21.Rosengren SM, Todd NPM, Colebatch JG. Vestibular evoked myogenic potentials evoked by brief interaural head acceleration: properties and possible origin. J Appl Physiol 2009;107:841–852. [DOI] [PubMed] [Google Scholar]
- 22.Rosengren SM, Colebatch JG, Young AS, Govender A, Welgampola MS. Vestibular evoked myogenic potentials in practice: Methods, pitfalls and clinical applications. Clinical Neurophysiology Practice 2019;4:47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welgampola MS, Myrie OA, Minor LB, Carey JP. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurol 2008;70:464–472. [DOI] [PubMed] [Google Scholar]
- 24.McCaslin DL, Fowler A, Jacobson GP. Amplitude normalization reduces cervical vestibular evoked myogenic potential (cVEMP) amplitude asymmetries in normal subjects: Proof of concept. J Am Acad Audiol 2014;25:268–277. [DOI] [PubMed] [Google Scholar]
- 25.Sandhu JS, George SR, Rea PA. The effect of electrode positioning on the ocular vestibular evoked myogenic potential to air-conducted sound. Clin Neurophysiol 2013;124:1232–1236. [DOI] [PubMed] [Google Scholar]
- 26.Makowiec K, McCaslin DL, Jacobson GP, Hatton K, Leea J. Effect of Electrode Montage and Head Position on Air-Conducted Ocular Vestibular Evoked Myogenic Potential. Amer J of Audiol 2017;26:180–188. [DOI] [PubMed] [Google Scholar]
- 27.Rosengren SM. Effects of muscle contraction on cervical vestibular evoked myogenic potentials in normal subjects. Clin Neurophysiol 2015;126(11):2198–2206. [DOI] [PubMed] [Google Scholar]
- 28.Rosengren SM, Govender S, Colebatch JG. Ocular and cervical vestibular evoked myogenic potentials produced by air- and bone conducted stimuli: Comparative properties and effects of age. Clin Neurophysiol 2011;122: 2282–2289. [DOI] [PubMed] [Google Scholar]
- 29.Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res 2006;175:256–267. [DOI] [PubMed] [Google Scholar]
- 30.Todd NPM, Rosengren SM, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by impulsive transmastoid accelerations. Clin Neurophysiol 2008;119(7):1638–81. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RL, Blaivie C, Bom AP, Holmeslet B, Pansell T, Brantberg K, Welgampola MS. Ocular vestibular-evoked myogenic potentials (oVEMP) to skull taps in normal and dehiscent ears: mechanisms and markers of superior canal dehiscence Exp Brain Res 2014; 232(4):1073–84. [DOI] [PubMed] [Google Scholar]
- 32.Uchino Y, Keisuke K. Differences between otolith- and semicircular canal-activated neural circuitry in the vestibular system. Neurosci Res 2011; 71:315–327. [DOI] [PubMed] [Google Scholar]
- 33.Colebatch J, Rothwell JC. Motor unit excitability changes mediating vestibulocollic reflexes in the sternocleidomastoid muscle. Clin Neurophysiol 2004;115(11):2567–73. [DOI] [PubMed] [Google Scholar]
- 34.Aoki N, Masuzawa H. Infantile acute subdural hematoma: clinical analysis of 26 cases. J Neurosurg 1984; 61:273–280. [DOI] [PubMed] [Google Scholar]
- 35.De Bonis P, Trevisi G, de Waure C, et al. Antiplatelet/anticoagulant agents and chronic subdural hematoma in the elderly. PLoS One 2013; 8:e68732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNerney KM, Burkard RF. The vestibular evoked myogenic potential (VEMP): air- versus bone-conducted stimuli. Ear Hear 2011; 32(6):e6–e15. [DOI] [PubMed] [Google Scholar]
- 37.Ochi K, Ohashi T. Age- related changes in the vestibular-evoked myogenic potentials. Otolaryngol Head Neck Surg 2003;129:655–659. [DOI] [PubMed] [Google Scholar]
- 38.Welgampola MS, Colebatch JG. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol 2001;112(11):1971–1990. [DOI] [PubMed] [Google Scholar]
- 39.Sheykholeslami K. Vestibular-evoked myogenic potentials: do we know all the basics? Aud Vest Res 2015; 24(2):51–53. [Google Scholar]
- 40.Maes L, Dhooge I, D’haenens W, Bockstael A, Keppler H, Philips B, Swinnen F, Vinck BM. The effect of age on the sinusoidal harmonic acceleration test, pseudorandom rotation test, velocity step test, caloric test, and vestibular-evoked myogenic potential test. Ear Hear 2010;31:84–94. [DOI] [PubMed] [Google Scholar]
- 41.Basta D, Todt I, Ernst A. Normative data for P1/N1-latencies of vestibular evoked myogenic potentials induced by air- or bone-conducted tone bursts. Clin Neurophysiol 2005;116:2216–2219. [DOI] [PubMed] [Google Scholar]
- 42.Janky KL, Nguyen KD, Welgampola M, Zuniga GM, Carey JP. Air-conducted oVEMPs provide the best separation between intact and superior canal dehiscent labyrinths. Oto Neurotol 2013;34:127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.