Abstract
Rationale & Objective
Cytokine release syndrome is a well-known complication of chimeric antigen receptor T-cell (CAR-T) therapy and can lead to multiorgan dysfunction. However, the nephrotoxicity of CAR-T therapy is unknown. We aimed to characterize the occurrence, cause, and outcomes of acute kidney injury (AKI), along with the occurrence of electrolyte abnormalities, among adults with diffuse large B-cell lymphoma receiving CAR-T therapy.
Study Design
Case series.
Setting & Participants
We reviewed the course of 78 adults receiving CAR-T therapy with axicabtagene ciloleucel or tisagenlecleucel at 2 major cancer centers between October 2017 and February 2019. Baseline demographics, comorbid conditions, medications, and laboratory values were obtained from electronic health records. AKI was defined using KDIGO (Kidney Disease: Improving Global Outcomes) criteria. The cause, clinical course, and outcome of AKI events and electrolyte abnormalities in the first 30 days after CAR-T infusion were characterized using data contained in electronic health records.
Results
Among 78 patients receiving CAR-T therapy, cytokine release syndrome occurred in 85%, of whom 62% were treated with tocilizumab. AKI occurred in 15 patients (19%): 8 had decreased kidney perfusion, 6 developed acute tubular necrosis, and 1 patient had urinary obstruction related to disease progression. Those with acute tubular necrosis and obstruction had the longest lengths of stay and highest 60-day mortality. Electrolyte abnormalities were common; hypophosphatemia, hypokalemia, and hyponatremia occurred in 75%, 56%, and 51% of patients, respectively.
Limitations
Small sample size; AKI adjudicated by retrospective chart review; lack of biopsy data.
Conclusions
In this case series of patients with diffuse large B-cell lymphoma receiving CAR-T therapy, AKI and electrolyte abnormalities occurred commonly in the context of cytokine release syndrome.
Chimeric antigen receptor (CAR) T-cell (CAR-T) therapy has been a breakthrough in cancer treatment, inducing durable remissions in patients with hematologic malignancies that were previously refractory to other therapies.1–3 CAR-T cells are created by genetically engineering an individual patient’s T cells to express CARs, which are capable of recognizing and attaching to specific surface markers.4–6 CAR-T cells are expanded ex vivo into hundreds of millions of cells and then reinfused into the patient, leading to immune targeting of their respective antigen (Fig 1). Initially approved for relapsed and refractory acute lymphoblastic leukemia (ALL) in pediatric and young adult patients in April 2017,7 tisagenlecleucel (Kymriah, Novartis Pharmaceuticals) was subsequently approved for the treatment of diffuse large B-cell lymphoma (DLBCL) in May 2018.8 Axicabtagene ciloleucel (Yescarta, Gilead Co) was approved in October 2017 for aggressive B-cell lymphomas.1,9,10 Alternative CAR-T cells and engineered T cells targeting different tumor antigens are being developed for solid tumors and multiple myeloma.3 As a result, the number of patients receiving CAR-T therapy is likely to increase dramatically in the near future.11–15
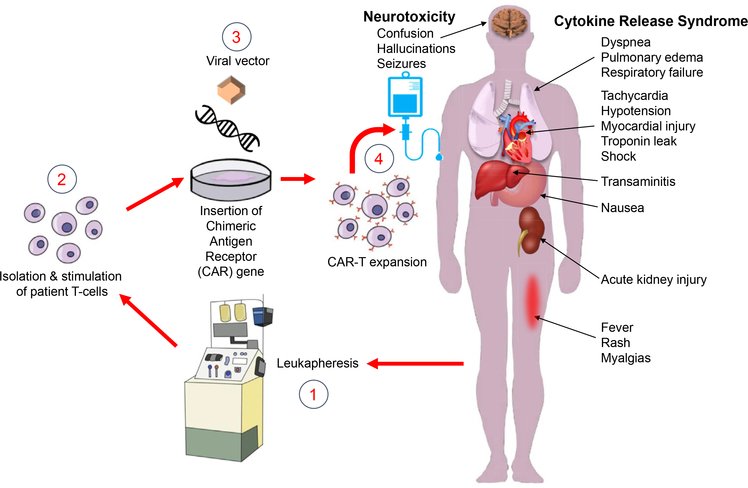
Figure 1.
Chimeric antigen receptor (CAR)-T cell (CAR-T) therapy and its complications. CAR-T therapy involves separating out a patient’s T cells using apheresis and then genetically engineering the cells to produce receptors on their surfaces, called CARs. CARs are fusion proteins of an antigen-recognition domain from a monoclonal antibody and 1 or more T-cell receptors. They allow T cells to recognize and attach to specific proteins, namely tumor antigens. T cells are expanded into hundreds of millions of cells and then infused back into the patient, selectively destroying chemotherapy-resistant cancer cells. Patients receiving CAR-T are at risk for developing cytokine release syndrome, an inflammatory response that occurs secondary to cytokine release by infused CAR-T cells. Cytokine release syndrome is characterized by fevers, hypotension, tachycardia, elevated inflammatory marker levels, and end-organ damage, including acute kidney injury and neurotoxicity. Image ©2019 Dr Xavier Vela Parada, and appears courtesy of the copyright holder.
Despite its success at inducing remission, CAR-T therapy is associated with significant toxicity in clinical trials, and fatal outcomes due to severe cytokine release syndrome and neurotoxicity have been reported.8,9,16,17 Cytokine release syndrome has been observed in >90% of patients with B-cell ALL and non-Hodgkin B-cell lymphoma in clinical trials for CD19 CAR-T, with rates differing by product and underlying disease.8,17,18 Cytokine release syndrome often manifests with high fever, fatigue, and body aches, typically occurring 1 to 5 days after the initial infusion. However, in its most extreme form, it can progress to capillary leak, vascular collapse, and multiorgan failure, including acute kidney injury (AKI; Fig 1). Patients at high risk for severe cytokine release syndrome are administered tocilizumab (Actemra, Roche), a human monoclonal antibody that targets the interleukin 6 (IL-6) receptor, with or without glucocorticoid steroids.19
Extremely limited information exists regarding adverse kidney manifestations or electrolyte disorders in patients receiving CAR-T therapy, with existing data derived from clinical trials rather than real-world practice and mostly limited to the pediatric population with ALL.20,21 We present a series in adults with DLBCL, focusing on the incidence, clinical features, timing, cause, and outcomes of AKI and electrolyte disorders after CAR-T therapy at 2 major cancer centers in Boston, MA.
Methods
Patient Population
We reviewed all patients with DLBCL treated with CAR-T therapy (commercial product axicabtagene ciloleucel or tisagenlecleucel) as part of standard of care at Massachusetts General Hospital Cancer Center and Dana-Farber Cancer Institute between October 2017 and February 2019. All clinical details of patients were elicited from the electronic health record and included demographics, comorbid conditions, chemotherapy in the 60 days before CAR-T therapy administration, medications, and postinfusion treatment complications such as cytokine release syndrome and concurrent adverse events. Comorbid conditions such as diabetes mellitus, hypertension, cirrhosis, coronary artery disease, and congestive heart failure were ascertained based on detailed manual review of each patient’s medical record. A patient was determined to have any of these diagnoses if they were listed in the chart by a provider, or if the patient was taking a medication used to treat the condition (eg, antihypertensives). The Research Patient Data Registry, a centralized data registry that stores clinical information about patients seen within the Partners Healthcare System, was queried to obtain daily laboratory data, including levels of serum creatinine (Scr), serum electrolytes, and inflammatory markers obtained within the first 30 days after CAR-T administration.
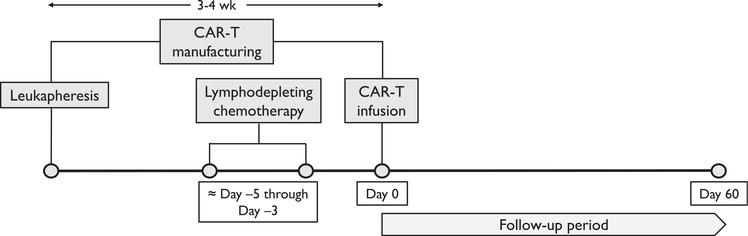
CAR-T therapy involves separating out a patient’s T cells using apheresis and then genetically engineering the cells to produce receptors on their surfaces, called CARs. CARs are fusion proteins of an antigen-binding domain from a monoclonal antibody and 1 or more T-cell receptors. The T cells are expanded into hundreds of millions of cells and then infused back into the patient, selectively destroying chemotherapy-resistant cancer cells. Before CAR-T infusion, patients received lymphodepleting chemotherapy in the form of cyclophosphamide and fludarabine (usually 3–6 days before CAR-T therapy), which promotes in vivo expansion of CAR-T cells and improves their efficacy (Fig 2).22,23 The majority (85%) received 500 mg/m2 of cyclophosphamide, though 12 patients (15%) received 250 mg/m2. In addition to lymphodepleting therapy, most patients received prophylaxis for tumor lysis syndrome; in our series, 65 of 78 (83%) received prophylactic allopurinol, while 2 patients received rasburicase the day before CAR-T infusion.
Figure 2.
Timeline of leukapheresis, leukodepletion, and chimeric antigen receptor therapy T-cell (CAR-T) infusion.
Baseline Scr level was defined as the measurement closest to and preceding CAR-T administration and was adjudicated through chart review by 2 nephrologists (S.G. and H.S.). In the event that there was a disagreement, a third nephrologist (M.E.S.) resolved disagreements. There were no missing data for baseline Scr values, and none of the patients were dialysis dependent at baseline. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.24 Baseline chronic kidney disease (CKD) was defined as eGFR < 60 mL/min/1.73 m2 for 90 or more days before CAR-T therapy. AKI was defined as 1.5-fold or greater increase in Scr level from baseline within 30 days of CAR-T therapy or requirement for kidney replacement therapy (KRT). AKI severity was graded by using KDIGO (Kidney Disease: Improving Global Outcome) criteria. These stages match the grading system of the Common Terminology Criteria for Adverse Events (CTCAE).25
AKI cause was categorized as 1 of 3 primary causes: (1) decreased kidney perfusion from any cause, including cytokine release syndrome or volume depletion, with resolution of AKI within 72 hours in response to intravenous fluids and hemodynamic support; (2) acute tubular necrosis (ATN) if AKI lasted longer than 72 hours or the patient required KRT; and (3) obstructive AKI if the patient had radiographic evidence of bilateral ureteral or urethral obstruction leading to AKI. Cases were adjudicated by 2 nephrologists (S.G. and H.S.). A third nephrologist (M.E.S.) resolved any disagreements. Clinically significant thresholds, which corresponded to CTCAE grade 2 or higher, were used to define electrolyte abnormalities. Clinically significant hyponatremia was defined as serum sodium level < 130 mEq/L; hypokalemia, as serum potassium level < 3.0 mEq/L; and hypophosphatemia, as serum phosphorus level < 2.0 mg/dL.
Tumor burden was approximated using levels of serum lactate dehydrogenase before lymphodepletion. The grades of cytokine release syndrome and neurotoxicity were adjudicated based on regular clinical assessments by the primary oncology team during the hospitalization. Cytokine release syndrome and neurotoxicity grading were extracted from the chart, as determined by the treating oncologist and using a common scale at both institutions (Table S1).25,26 Third-spacing was defined as new or worsening ascites, pleural effusions, or subcutaneous edema on either radiologic imaging or clinical examination. Length of admission and death during the first 60 days after CAR-T administration were recorded by chart review, with no missing data.
Statistical Analysis
Baseline characteristics were described using mean ± standard deviation for normally distributed continuous variables, median and interquartile range (IQR) for nonnormally distributed continuous variables, and count and percentage for categorical variables. Univariable logistic regression was used to determine the association between continuous variables and the incidence of AKI, while Fisher exact test was used to characterize the association between categorical variables and incidence of AKI. All analyses were performed using SAS, version 9.4 (SAS Corp), and STATA, version 13 (StataCorp). A 2-sided P < 0.05 was considered significant.
This study was approved by the Institutional Review Board at Dana-Farber Cancer Center and Massachusetts General Hospital. Because of the retrospective nature of the study, the need for informed consent was waived.
Results
Baseline Characteristics
A total of 78 patients with DLBCL received CAR-T therapy at Massachusetts General Hospital and Dana-Farber Cancer Institute. Baseline characteristics are summarized in Table 1. Mean age of patients was 60 ± 13 years. The majority (64%) were men, and most (85%) were white. Baseline comorbid conditions included hypertension (20%), diabetes mellitus (12%), coronary artery disease (8%), congestive heart failure (5%), and CKD (3%).
Table 1.
Baseline Characteristics of Patients With and Without AKI After CAR-T Therapy
| All Patients (N = 78) | AKI (n = 15) | No AKI (n = 63) | P | |
|---|---|---|---|---|
| Age, y | 60 ± 13 | 60 ± 12 | 60 ± 14 | 0.9 |
| Baseline Scr, mg/dL | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.8 ± 0.2 | 0.5 |
| Baseline eGFR, mL/min/1.73 m2 | 109 ± 17 | 108 ± 18 | 109 ± 17 | 0.8 |
| Male sex | 50 (64%) | 10 (67%) | 40 (63%) | 0.8 |
| Race | 0.8 | |||
| White | 66 (85%) | 13 (87%) | 53 (84%) | |
| Black | 1 (1%) | 0 (0%) | 1 (2%) | |
| Hispanic | 3 (4%) | 0 (0%) | 3 (5%) | |
| Asian | 2 (2%) | 0 (0%) | 2 (3%) | |
| Other/unknown | 6 (8%) | 2 (13%) | 4 (6%) | |
| Cirrhosis | 1 (1%) | 1 (7%) | 0 (0%) | 0.2 |
| Hypertension | 20 (26%) | 4 (27%) | 16 (25%) | 0.9 |
| Diabetes | 9 (12%) | 3 (20%) | 6 (10%) | 0.4 |
| Coronary artery disease | 6 (8%) | 2 (13%) | 4 (6%) | 0.05 |
| Congestive heart failure | 4 (5%) | 1 (7%) | 3 (5%) | 0.3 |
| ACEi/ARB use | 7 (9%) | 1 (7%) | 6 (10%) | 0.7 |
| CAR type | 0.8 | |||
| Axicabtagene ciloleucel (Yescarta) | 69 (88%) | 14 (93%) | 55 (87%) | |
| Tisgenlecleucel (Kymriah) | 9 (12%) | 1 (7%) | 8 (13%) | |
| Lactate dehydrogenase, U/L | 379 ± 316 | 540 ± 531 | 340 ± 228 | 0.05 |
Note: Values presented as mean ± standard deviation or count (percent).
Abbreviations: ACEi, angiotensin converting-enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; CAR, chimeric antigen receptor; CAR-T, chimeric antigen receptor T-cell; eGFR, estimated glomerular filtration rate; Scr, serum creatinine.
In the 60 days preceding CAR-T administration, 27 (35%) patients received chemotherapy, including gemcitabine, cisplatin, oxaliplatin, cyclophosphamide, ibrutinib, and ifosfamide. Before CAR-T therapy, all patients received lymphodepleting chemotherapy consisting of cyclophosphamide and fludarabine. One patient also received rituximab. All patients received commercial standard-of-care CAR-T products. Most (88%) patients received axicabtagene ciloleucel, and the rest (12%) received tisagenlecleucel.
Clinical Outcomes
The majority of patients (n = 50; 64%) were administered CAR-T therapy 1 day after hospital admission. There were 14 (18%) patients who received CAR-T therapy more than 72 hours after admission.
Before CAR-T infusion, 3 patients had AKI, of whom 2 had AKI from decreased kidney perfusion that preceded the onset of lymphodepleting chemotherapy. Peak Scr level in patient 1 (AKI stage 1) was 6 days before CAR-T treatment and Scr level peaked in patient 2 (stage 2) 10 days before CAR-T administration. AKI in both patients resolved with intravenous fluid at the time of lymphodepleting therapy, with Scr level improving to baseline by the time CAR-T therapy was administered.
The third patient had ATN that was likely secondary to prolonged decreased kidney perfusion and capillary leak; the AKI had partially resolved by the time of CAR-T administration. From a baseline of 1.4 mg/dL, Scr level peaked to 2.0 mg/dL (stage 1) the day before CAR-T administration and improved to 1.6 mg/dL at the time of CAR-T therapy. This patient later had a second episode of AKI following CAR-T therapy.
The incidence of cytokine release syndrome and neurotoxicity was 85% and 67%, respectively. The severity of cytokine release syndrome and neurotoxicity are shown in Table 2. Tocilizumab was administered to 62% of patients; dexamethasone, to 49%; and 42% received both. Of the 67 patients with cytokine release syndrome, 30 had manifestations of third-spacing.
Table 2.
Outcomes After CAR-T Administration
| Outcome | All Patients (N = 78) |
|---|---|
| Cytokine release syndrome | 66 (85%) |
| None | 12 (15%) |
| Grade 1 | 28 (36%) |
| Grade 2 | 28 (36%) |
| Grade 3 | 8 (10%) |
| Grade 4 | 2 (3%) |
| Neurotoxicity | 52 (67%) |
| None | 26 (33%) |
| Grade 1 | 17 (22%) |
| Grade 2 | 13 (17%) |
| Grade 3 | 22 (28%) |
| Tocilizumab | 48 (62%) |
| Dexamethasone | 38 (49%) |
| Response to therapy | |
| Remission/positive response | 43 (55%) |
| Progression | 28 (36%) |
| Unable to gauge response | 3 (4%) |
| Death during admission for CAR-T | 4 (5%) |
Abbreviation: CAR-T, chimeric antigen receptor therapy.
Among the 15 (19%) patients with AKI after CAR-T therapy, 7 had stage 1, 2 had stage 2, and 6 had stage 3 AKI (Table 3). Three of 6 with stage 3 AKI required KRT. For patients with AKI, Scr level peaked at a median of 10 (IQR, 6–12) days after administration of CAR-T therapy. In univariable analyses (Table 1), there were no significant differences in baseline demographics or comorbid conditions between patients who experienced AKI and those who did not. However, patients with AKI had higher prelymphodepletion lactate dehydrogenase levels, suggesting that greater tumor burden at baseline may be a risk factor for AKI. Two patients had eGFR < 60mL/min/1.73 m2 at baseline, and both experienced AKI after CAR-T therapy. Patients who had high grades of cytokine release syndrome (grades 3–4) had 9.8-fold higher odds of developing AKI (95% confidence interval, 2.3–41.8) compared with patients with milder grades of cytokine release syndrome after CAR-T therapy. AKI rates were not statistically different between those receiving axicabtagene ciloleucel (20%) compared with those receiving tisagenlecleucel (11%; P = 0.8). Of 15 patients with AKI, 4 (27%) had a documented infection (bacterial pneumonia, urinary tract infection, fungal empyema, or Clostridium difficile infection). Of the 63 patients without AKI, 19 (30%) had a documented infection.
Table 3.
Rates of AKI by Stage and Underlying Cause Among Patients Receiving CAR-T Therapy
| Parameter | Value |
|---|---|
| Baseline Scr, mg/dLa | |
| Patients without AKI (n = 63) | 0.8 [0.6–0.9] |
| Patients with AKI (n = 15) | 0.8 [0.6–1.0] |
| Peak Scr, mg/dLa | |
| Patients without AKI (n = 63) | 0.9 [0.8–1.1] |
| Patients with AKI (n = 15) | 1.4 [1.1–2.1] |
| Time to peak, da | |
| Patients without AKI (n = 63) | 5 [4–9] |
| Patients with AKI (n = 15) | 10 [6–12] |
| Incidence of AKI by KDIGO stage | |
| Any AKI stageb | 15/78 (19%) |
| AKI stage 1 | 7/78 (9%) |
| AKI stage 2 | 2/78 (3%) |
| AKI stage 3 | 6/78 (8%) |
| Cause | |
| Decreased kidney perfusion (n = 8) | |
| No cytokine release syndrome | 1/8 |
| Cytokine release syndrome stage 1 | 3/8 |
| Cytokine release syndrome stage 2 | 3/8 |
| Cytokine release syndrome stage 3 | 1/8 |
| Acute tubular necrosis (n = 6) | |
| Cytokine release syndrome stage 1 | 0/6 |
| Cytokine release syndrome stage 2 | 1/6 |
| Cytokine release syndrome stage 3 | 2/6 |
| Cytokine release syndrome stage 4 | 3/6 |
| Urinary obstruction (n = 1) | |
| No cytokine release syndromec | 1/1 |
Abbreviations: AKI, acute kidney injury; ATN, acute tubular necrosis; CAR-T, chimeric antigen receptor T cell; KDIGO, Kidney Disease: Improving Global Outcomes; Scr, serum creatinine.
Median [interquartile range] and count (percentage).
Cause of AKI by stage: stage 1, 6 from decreased kidney perfusion, 1 from ATN; stage 2, 2 from decreased kidney perfusion, AKI stage 3, 5 from ATN, 1 from obstruction.
Resulted from diffuse large B-cell lymphoma progression.
Cause of AKI After CAR-T Therapy
Figures S1A–F and S2A–I show the Scr level trajectory and clinical course of each of the 15 patients who developed AKI within the first 30 days after CAR-T therapy. Eight patients (Fig S2A–H) had AKI related to decreased kidney perfusion; each episode occurred within the first week after CAR-T therapy and was stage 1 (n = 6) or 2 (n = 2). The corresponding association with cytokine release syndrome severity stages, by AKI cause, is shown in Table 3. Four of these patients received supportive care alone (eg, intravenous fluids and avoidance of nephrotoxic agents), while the remaining 4 patients received tocilizumab and/or dexamethasone. Six patients developed ATN (Fig S1A–F) with prolonged episodes of AKI despite supportive measures; these patients had more severe AKI (5 of 6 patients had stage 3 AKI; 3 required KRT). Patients with ATN tended to have higher grade cytokine release syndrome (Table 3). The final case of AKI had progressive DLBCL and malignant bilateral ureteral obstruction leading to AKI (Fig S2I). All 3 patients who required KRT ultimately died in the hospital within 30 days; 2 within 24 hours of initiating KRT (Fig S1A, C, and F). None of the patients underwent kidney biopsy. Median uric acid level of patients with AKI was 3.7 (IQR, 2.5–5) mg/dL, with levels peaking to 4.5 (IQR, 3.8–5.5) mg/dL over a median of 11 (IQR, 10–13) days. Uric acid levels increased mildly in 7 patients, decreased in 7 patients, and doubled in only 1 patient (whose course is presented in Fig S1E), who had a peak uric acid level of 9.6 mg/dL that coincided with the time of AKI.
Association Between AKI and Length of Stay and 60-Day Mortality
During 60 days of follow-up, 11 patients died, 8 from progression of underlying cancer, 2 from cytokine release syndrome leading to cardiovascular collapse, and 1 attributed to a combination of cytokine release syndrome and cancer progression.
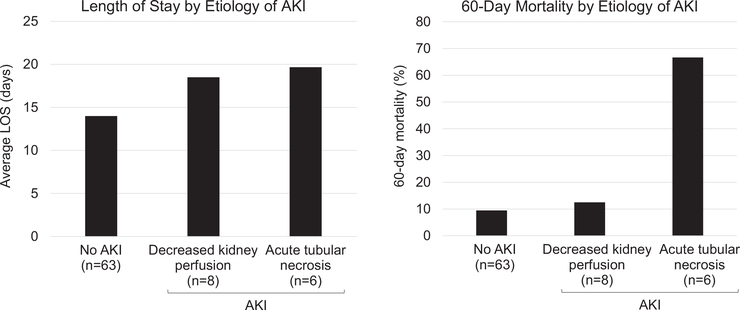
As shown in Figure 3, among patients with AKI related to decreased kidney perfusion, average length of stay was 19 days and 60-day mortality was 13%. This was similar to the average length of stay and 60-day mortality in patients who did not experience AKI. Average length of stay in days after the start of the AKI event was also similar among those with decreased kidney perfusion and those with ATN (Fig S3). Among patients with ATN, average length of stay was 19.7 days; however, 60-day mortality was 67% (Figure 3). Of the 2 patients who survived beyond 60 days, neither had a return of Scr level to baseline. Approximately 1 month after CAR-T therapy, patient 4 (Fig S1D) had an Scr level of 1.8 mg/dL, and patient 5 (Fig S1E) had an Scr level of 1.5 mg/dL.
Figure 3.
Acute kidney injury (AKI) etiology and length of stay (LOS) and mortality. LOS is duration of initial hospital admission. The patient with AKI due to malignant bilateral ureteral obstruction is excluded from the analysis.
Changes in Electrolytes and Inflammatory Markers
During the lymphodepletion phase, hyponatremia, hypokalemia, and hypophosphatemia occurred in a minority of patients (Table S2), but incidence and severity were higher after CAR-T therapy. Baseline and nadir values, as well as time to nadir (in days), for serum sodium, potassium, and phosphorus levels are presented in Table 4. Hypophosphatemia was the most common abnormality; following CAR-T therapy, 51% of patients experienced a decline in phosphorous levels to <2mg/dL, and 18%, to <1.5mg/dL. Hyponatremia (sodium < 130 mEq/L) occurred in 12 (15%) patients, with 1 (1%) patient experiencing a serum sodium level < 125 mEq/L. No patient experienced a potassium level < 3.0 mEq/L, though a value < 3.5 mEq/L was observed in 43 (54%) patients. Median time to nadir for electrolyte abnormalities was 5 to 6 days from the time of CAR-T administration and 8 to 9 days from the completion of lymphodepleting chemotherapy (Table 4). There were no standardized protocols for potassium and phosphorus repletion; these were repleted at the discretion of the primary oncology team. Median peak ferritin level was 1,673 (IQR, 863–3,703) ng/mL, and median peak C-reactive protein (CRP) level was 68 (IQR, 3–141) mg/dL. Ferritin and CRP levels doubled from baseline in 43% and 44%, respectively.
Table 4.
Electrolyte Abnormalities and Inflammatory Marker Changes After CAR-T Therapy
| Laboratory Findings | Value |
|---|---|
| Sodium | |
| Sodium concentration, mEq/L | |
| Baseline | 139 [137–142] |
| Nadir | 134 [132–136] |
| Time to nadir sodium concentration, d | 6 [3–8] |
| Nadir sodium concentration | |
| 135 mEq/L | 40 (51%) |
| <130 mEq/L | 12 (15%) |
| <125 mEq/L | 1 (1%) |
| Potassium | |
| Potassium concentration, mEq/L | |
| Baseline | 3.9 [3.8–4.1] |
| Nadir | 3.4 [3.3–3.7] |
| Time to nadir potassium concentration, d | 6 [4–9] |
| Nadir potassium concentration | |
| <3.5 mEq/L | 43 (54%) |
| <3 mEq/L | 0 (0%) |
| Phosphorus | |
| Phosphorus concentration, mg/dL | |
| Baseline | 3.2 [2.8–3.5] |
| Nadir | 1.9 [1.6–2.4] |
| Time to nadir phosphorus concentration, d | 5 [3–8] |
| Nadir phosphorus concentration | |
| <2.5 mg/dL | 59 (76%) |
| <2 mg/dL | 40 (51%) |
| <1.5 mg/dL | 14 (18%) |
| <1 mg/dL | 2 (3%) |
| Ferritin | |
| Ferritin concentration, ng/mL | |
| Baseline | 731 [456–1,525] |
| Peak | 1,673 [863–3,703] |
| Time to peak ferritin concentration, d | 8 [6–11] |
| Percent with doubling of ferritin | 43% |
| CRP | |
| CRP, mg/L | |
| Baseline | 14.0 [3.3–46.8] |
| Peak | 67.8 [2.5–141.2] |
| Time to peak CRP concentration, d | 4 [3–6] |
| Percent with doubling of CRP | 44% |
Unless otherwise indicated, values given as median [interquartile range]. Abbreviations: CAR-T, chimeric antigen receptor T-cell; CRP, C-reactive protein.
Discussion
CAR-T therapy is a major breakthrough in anticancer therapy. However, it also has a unique toxicity profile and is increasingly becoming recognized as a cause of AKI. We found that most cases of AKI occurred 6 to 10 days after CAR-T therapy for DLBCL, coincident with cytokine release syndrome. Higher grades of cytokine release syndrome (grades 3–4) were more likely to be associated with AKI. Most cases of AKI appeared to be secondary to decreased kidney perfusion and reversed with hemodynamic support, while others had features that were more consistent with ATN. Urinary obstruction was a rare contributor to AKI in this cohort but occurred in a patient with progressive DLBCL. Dialysis-requiring AKI (n = 3; 4%) was uniformly fatal.
We did not identify any specific factors that were associated with higher risk for AKI; however, both patients with baseline CKD developed AKI after CAR-T therapy. Although not statistically significant, the rate of AKI was higher in patients receiving axicabtagene ciloleucel compared with those receiving tisagenlecleucel. Axicabtagene ciloleucel’s costimulatory domain, cluster of differentiation of 28, leads to faster rates of cell expansion and higher rates of cytokine release syndrome.2,9,10,27 Different CAR-T products have different rates of cytokine release syndrome because their distinct costimulatory domains lead to differential rates of cell expansion and activation. Thus, going forward, it will be important to recognize that the rate and severity of AKI are likely to differ based on both patient characteristics and differences in CAR-T products. An analysis of 39 pediatric patients with relapsed/refractory ALL who were treated with CAR-T therapy revealed that 18 (46%) developed AKI related to grade 3 to 4 cytokine release syndrome.21 However, AKI occurrences were mostly mild, with only 9 of these 39 patients developing stage 2 or 3 AKI, with none requiring KRT. Our findings suggest that adults may be more susceptible to severe AKI related to cytokine release syndrome, though this needs to be validated in a larger study.
In addition to clinical characteristics, certain biomarkers may be associated with the development of cytokine release syndrome and possibly AKI. Levels of markers of inflammation such as ferritin and CRP increased with cytokine release syndrome, with nearly half the patients experiencing a doubling of serum ferritin and CRP levels. However, there was significant heterogeneity in the timing of increase in inflammatory marker levels, with values often peaking after Scr level, rendering them unreliable biomarkers of AKI in this cohort (Table 3). Teachey et al28 measured serum cytokines and clinical biomarkers in 51 CAR-T–treated patients with ALL at multiple time points after CAR-T infusion and found that specific cytokines, including interferon-γ, IL-6, soluble gp130, and soluble IL-6 receptor, were able to predict the development of severe cytokine release syndrome in the month after CAR-T infusion. Nevertheless, these findings need to be validated in larger cohorts to see if cytokine profiles also predict subsequent AKI in the setting of cytokine release syndrome. To our knowledge, urinary biomarkers of kidney injury have not been studied in this population.
Though it has been described in the literature,11,19 we did not identify any cases of AKI that could be attributable to tumor lysis syndrome. Porter et al11 described the first patient to receive CAR-T therapy for refractory chronic lymphocytic leukemia. The patient developed AKI thought to be secondary to tumor lysis syndrome 14 days after the infusion, but recovered with the administration of intravenous fluids and rasburicase. In our series, few patients had substantial elevations in uric acid levels after CAR-T therapy, likely because of the high rates of allopurinol prophylaxis.
In addition to AKI, we also observed high rates of clinically meaningful electrolyte abnormalities, including hyponatremia (sodium < 130 mEq/L) in 18% and hypophosphatemia (phosphorus < 2.0mg/dL) in 51% of patients. Hypokalemia was present in 54% of patients. All cases of hypokalemia were mild, with no one experiencing a potassium level < 3.0 mEq/L after CAR-T therapy; however, it is possible that there were fewer cases of severe hypokalemia because of close monitoring and reactive potassium repletion. In a case series of adult patients with ALL treated with CAR-T therapy (n = 51), grade 1 or higher hyponatremia (sodium < 135 mEq/L) occurred in 41% of patients.20
The mechanism for the electrolyte disorders is unclear but may be due to cortisol release, intravascular volume depletion, or IL-6–mediated increase in vasopressin secretion in the context of cytokine release syndrome.20 Notably, between 3 and 5 days before CAR-T infusion, all patients received lymphodepleting chemotherapy with fludarabine and cyclophosphamide; the latter has been associated with hyponatremia secondary to syndrome of inappropriate antidiuresis.29,30 With regard to hypophosphatemia, it is unclear whether this occurs secondary to renal wasting, gastrointestinal losses, or intracellular shift, in part because there are no data on the fractional excretion of phosphorus in the setting of hypophosphatemia after CAR-T therapy. IL-6 has been shown to increase fibroblast growth factor 23 levels in the setting of AKI and CKD,31 and therefore elevations in IL-6 levels in cytokine release syndrome may contribute to phosphaturia and hypophosphatemia. The underlying cause of hypokalemia is also unknown, but may be related to cortisol release or a global renal tubular defect; prospective study measurements of the fractional excretion of potassium could help elucidate whether hypokalemia is due to renal potassium wasting.
Our study has limitations. The observational design precludes inferences of causality. Although tocilizumab appears to be beneficial in the setting of cytokine release syndrome, the small numbers and lack of a control group limit our ability to draw conclusions about its effectiveness in preventing organ-specific adverse effects such as AKI. Kidney biopsy, the gold standard for diagnosing the underlying cause of AKI, was not performed in any of the patients. Furthermore, patients who required KRT were critically ill, with hypotension, sepsis, and exposure to other nephrotoxic medications, which may have hastened the development of AKI. Only 2 (3%) patients of the cohort had CKD at baseline; thus, the risk for AKI in patients with CKD needs to be evaluated in future studies.
In conclusion, we found that AKI and electrolyte abnormalities were common among recipients of CAR-T therapy, 60-day mortality was extremely high in patients with ATN or obstructive AKI, and requirement for KRT was uniformly fatal. Given that it often occurs in the context of cytokine release syndrome, the mechanism of AKI related to decreased kidney perfusion is likely related to third-spacing, volume depletion, and capillary leak. Prolonged decreased kidney perfusion may progress to ATN; alternatively, massive cytokine release may induce vasoconstriction and direct tubular toxicity. Future studies should aim to identify clinical predictors and novel biomarkers that can identify AKI earlier, characterize its impact on clinical outcomes, and define long-term risks for CKD in patients who develop AKI after receiving CAR-T therapy. Because the indications for CAR-T use will be expanding considerably in the near future, nephrologists will need to be prepared to assist in the management of AKI and electrolyte disorders.
Supplementary Material
Figure S1: Clinical course of patients with ATN.
Figure S2: Clinical course of patients with AKI related to decreased kidney perfusion and obstructive AKI.
Figure S3: Length of stay by cause of AKI.
Table S1: Grading systems for cytokine release syndrome and neurotoxicity.
Table S2: The incidence of electrolyte abnormalities during lymphodepletion.
Acknowledgements
We thank Dr Xavier Vela Parada for medical illustration of Figure 1.
Support: Dr Gupta receives grant support from the National Institutes of Health (NIH; F32 DC017342-02); Dr Curhan, from NIH (K24DK091417); Dr Leaf, from NIH (5K23DK10644805);and Dr Sise, from NIH (1K23DK117014-01). The funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Financial Disclosure: Dr Gupta served on the Advisory Board for AstraZeneca (dapagliflozin) and is a scientific coordinator for the ASCEND trial. Dr Curhan is a consultant for Shire, AstraZeneca, Decibel Therapeutics, OM1, Orfan, and Merck and receives royalties as an author and Section Editor for UpToDate. Dr Jacobson reports serving as a consultant for Kite, Novartis, Pfizer, Bayer, Celgene, Precision Bioscience, and Humanigen and research funding from Kite and Pfizer. Dr Frigault reports consultancy/honoraria from Kite/Gilead, Novartis, Celgene, Nkarta, Xenetic, Arcellx, and Foundation Medicine. The remaining authors declare that they have no relevant financial interests.
Contributor Information
Shruti Gupta, Division of Renal Medicine, Department of Internal Medicine, Brigham and Women’s Hospital.
Harish Seethapathy, Renal Division, Department of Internal Medicine, Massachusetts General Hospital.
Ian A. Strohbehn, Renal Division, Department of Internal Medicine, Massachusetts General Hospital
Matthew J. Frigault, Oncology Division, Department of Medicine, Massachusetts General Hospital
Elizabeth K. O’Donnell, Oncology Division, Department of Medicine, Massachusetts General Hospital
Caron A. Jacobson, Dana Farber Cancer Institute, Boston, MA
Shveta S. Motwani, Division of Renal Medicine, Department of Internal Medicine, Brigham and Women’s Hospital Dana Farber Cancer Institute, Boston, MA.
Samir M. Parikh, Division of Nephrology, Beth Israel Deaconess Medical Center, Boston, MA
Gary C. Curhan, Division of Renal Medicine, Department of Internal Medicine, Brigham and Women’s Hospital
Kerry L. Reynolds, Oncology Division, Department of Medicine, Massachusetts General Hospital
David E. Leaf, Division of Renal Medicine, Department of Internal Medicine, Brigham and Women’s Hospital
Meghan E. Sise, Renal Division, Department of Internal Medicine, Massachusetts General Hospital
References
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4): 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogba N, Arwood NM, Bartlett NL, et al. Chimeric antigen receptor T-cell therapy. J Natl Compr Cancer Netw. 2018;16(9): 1092–1106. [DOI] [PubMed] [Google Scholar]
- 6.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11(7):855–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grupp SA, Wright JF, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 11.Porter DL, Levine BL, Kalos M, Bagg A. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-Cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellebrecht CT, Bhoj VG, Nace A, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353(6295):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samaha H, Pignata A, Fousek K, et al. A homing system targets therapeutic T cells to brain cancer. Nature. 2018;561:331–33. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR-T cells: the long and winding road to solid tumors review- article. Cell Death Dis. 2018;9(3):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster S, Bishop MR, Tam C, et al. Primary analysis of Juliet: a global, pivotal, phase 2 trial of CTL019 in adult patients with relapsed or refractory diffuse large B-cell lymphoma. Blood. 2017;130:57. [Google Scholar]
- 18.FDA Briefing Document Oncologic Drugs Advisory Committee Meeting - BLA 125646 Tisagenlecleucel Novartis Pharmaceuticals Corporation. FDA; 2017. https://www.fda.gov/media/106081/download. Accessed April 9, 2019. [Google Scholar]
- 19.Jhaveri KD, Rosner MH. Chimeric antigen receptor T cell therapy and the kidney. Clin J Am Soc Nephrol. 2018;13(5): 796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon BN, Daley RJ, Horvat TZ, et al. Risk of hyponatremia and associated clinical characteristics in patients with acute lymphoblastic leukemia after CD19 targeted chimeric antigen receptor (CAR) T-cells. Blood. 2017;130:3584. [Google Scholar]
- 21.Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45(2): e124–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turtle CJ, Hanafi L-A, Berger C, et al. Addition of fludarabine to cyclophosphamide lymphodepletion improves in vivo expansion of CD19 chimeric antigen receptor-modified T cells and clinical outcome in adults with B cell acute lymphoblastic leukemia. Blood. 2015;126:3773. [Google Scholar]
- 23.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE. Bethesda, MD: National Institutes of Health, National Cancer Institute; 2017. [Google Scholar]
- 26.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxic- ities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayachandran NV, Chandrasekhara PKS, Thomas J, Agrawal S, Narsimulu G. Cyclophosphamide-associated complications: we need to be aware of SIADH and central pontine myelinolysis. Rheumatology. 2009;48(1):89–90. [DOI] [PubMed] [Google Scholar]
- 30.Bruining DM, van Roon EN, de Graaf H, Hoogendoorn M. Cyclophosphamide-induced symptomatic hyponatraemia. Neth J Med. 2011;69(4):192–195. [PubMed] [Google Scholar]
- 31.Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94(2):315–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Clinical course of patients with ATN.
Figure S2: Clinical course of patients with AKI related to decreased kidney perfusion and obstructive AKI.
Figure S3: Length of stay by cause of AKI.
Table S1: Grading systems for cytokine release syndrome and neurotoxicity.
Table S2: The incidence of electrolyte abnormalities during lymphodepletion.