Abstract
Objectives:
The role of follow-up blood cultures (FUBCs) in management of gram-negative bacteremia (GNB) is poorly understood. This study aims to determine utility of FUBCs in identifying patients with increased mortality risk.
Methods:
An observational study with a prospectively enrolled cohort of adult inpatients with GNB was conducted at Duke University Health System from 2002–2015. FUBCs were defined as blood cultures drawn from 24 hours to 7 days from initial positive blood culture.
Results:
Among 1702 patients with GNB, 1164 (68%) had FUBCs drawn. When drawn, FUBCs were positive in 20% (228/1113) of cases. FUBC acquisition was associated with lower all-cause in-hospital mortality (20% [108/538] versus 15% [176/1164], p=0.01) and attributable in-hospital mortality (15% [78/538] versus 8% [98/1164]; p<0.0001). Propensity score-weighted Cox proportional hazards models revealed that obtaining FUBCs was associated with reductions in all-cause (Hazard ratio [HR]=0.629; 95% confidence interval (CI), 0.511–0.772; p<0.0001) and attributable mortality (HR=0.628; 95% CI, 0.480–0.820; p=0.0007). Positive FUBCs were associated with increased all-cause mortality (21% [49/228] versus 11% [110/885]; p=0.0005) and attributable mortality (12% [27/228] versus 7% [61/885]; p=0.01) relative to negative FUBCs. Propensity score-weighted Cox proportional hazards models revealed that positive FUBCs were associated with increased all-cause (HR=2.099; 95% CI, 1.567–2.811; p<0.0001) and attributable mortality (HR=1.800; 95% CI, 1.245–2.603; p=0.002). In a calibration analysis, a scoring system accurately identified patients at high risk of positive FUBCs.
Conclusions:
Rates of positive FUBCs were high and identified patients at increased risk for mortality. Clinical variables can identify patients at high risk for positive FUBCs. FUBCs should be considered in the management of GNB.
Keywords: blood cultures, gram-negative bacteremia, persistent bacteremia, risk score
INTRODUCTION
Gram-negative bacteremia (GNB) is a major cause of morbidity and mortality in hospitalized patients. The estimated mortality rate due to GNB-related septic shock approaches 40% [1,2]. Identifying patients with GNB at high risk of poor outcomes could improve patient care as such patients may benefit from additional diagnostic procedures, surgical interventions, or novel treatment strategies.
The importance of follow-up blood cultures (FUBCs) in patients with Staphylococcus aureus bacteremia has been established [3,4]; however, the role of FUBCs in the treatment of patients with GNB is unclear. Prior studies reported that FUBCs added little value in the management of GNB given the low probability of culture positivity [5,6]. However, the small numbers of patients with GNB who underwent FUBCs in these studies limits the ability to draw conclusions on the optimal role of FUBCs in the management of GNB.
In the present study, we used a prospectively enrolled cohort of >1700 hospitalized patients with GNB to address the following objectives: 1) identify factors associated with FUBC acquisition; 2) determine factors associated with positive FUBCs and apply these risk factors in a prediction model for positive FUBCs; and 3) determine if positive FUBCs are associated with an increased risk for in-hospital mortality in GNB patients.
METHODS
Study Population
This is an observational study of prospectively enrolled patients. From January 1, 2002 through June 30, 2015, all adult inpatients with monomicrobial bacteremia due to gram-negative (GN) organisms at Duke University Medical Center and Duke Regional Hospital were prospectively enrolled. The study question was posed retrospectively. This study was IRB approved. Written informed consent was obtained from patients or their legal representative. If a patient expired prior to notification of blood culture results, the subjects were enrolled using an IRB-approved Notification of Decedent Research. In patients with multiple hospitalizations with GNB over the study, only the first such hospitalization was included. Patients with polymicrobial bacteremia and those patients who died within 24 hours of initial blood culture collection were excluded. Details regarding microbial speciation, antibiotic susceptibility testing, and infectious diseases consultation are described in the methods in the supplement.
Definitions
FUBC is defined as blood culture drawn from 24 hours to 7 days after the initial positive blood culture. Persistent GNB is defined as a positive FUBC with the same organism. Attributable mortality is defined as death secondary to GN infection and included all patients who died with persistent signs or symptoms of systemic infection, positive blood culture results, or a persistent focus of infection in the absence of another explanation for death. All-cause and attributable mortality refer to in-hospital mortality, which is death occurring during the hospitalization associated with the GNB episode. Additional definitions are described in the methods in the supplement.
Statistical Analysis
Baseline characteristics and clinical events are presented as means with standard deviation for continuous variables and frequencies with proportions for categorical variables. Statistical comparisons between groups for continuous variables were made with Mann-Whitney-U test. For categorical variables, comparisons were made using Pearson’s chi-square test. Binomial proportion confidence intervals were calculated using the adjusted Wald method. Further details regarding logistic regression models, propensity score-based inverse probability weighting, Cox proportional hazards models, prediction modeling, and classification and regression tree (CART) analyses are included in the supplement.
RESULTS
Patient characteristics and factors associated with FUBC acquisition
Of the 1702 patients with GNB who were enrolled during the study period, FUBCs were obtained on 1164 (68%) patients (Fig. 1). Clinical characteristics of patients who had FUBCs drawn were significantly different compared to those that did not have FUBCs drawn (Table 1). The frequency of FUBC acquisition varied by microbial species and were more commonly drawn for patients with Pseudomonas aeruginosa (80% [31/158]) and Serratia bacteremia (80% [20/101]) than in patients with Proteus bacteremia (62% [26/70]) (Fig. 2A, p=0.0003).
Figure 1.
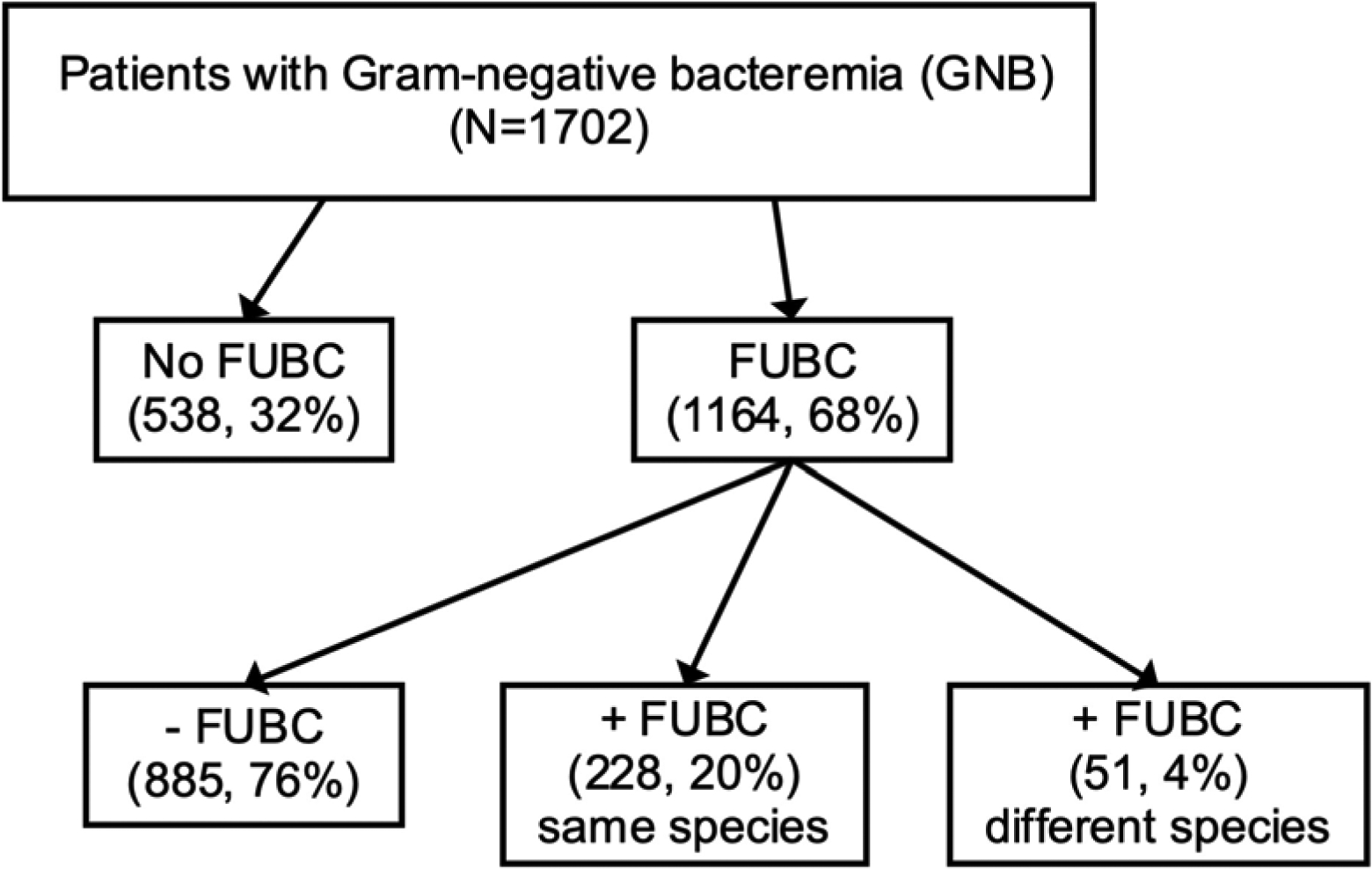
Schematic of study population. Abbreviation: FUBC – follow-up blood culture.
Table 1.
Patients with gram-negative bacteremia with and without follow-up blood cultures (FUBCs).
| Parameter | No. (%) of patients (n=1702) | p value | No. (%) of patients (n=1113) | p value | ||
|---|---|---|---|---|---|---|
| No FUBC (n=538) | FUBC (n=1164) | Negative FUBCs (n=885) | Positive FUBCs (n=228) | |||
| Mean age (yrs + SD) | 62 ± 16 | 60 ± 16 | 0.005 | 60 ± 16 | 59 ± 15 | 0.5 |
| Race | 0.16 | 0.84 | ||||
| White | 372 (69) | 750 (64) | 568 (64) | 149 (65) | ||
| Black | 141 (26) | 355 (30) | 270 (31) | 69 (30) | ||
| Other | 25 (5) | 59 (5) | 47 (5) | 10 (4) | ||
| Female | 234 (44) | 532 (46) | 0.39 | 399 (45) | 104 (46) | 0.89 |
| Medical History | ||||||
| Recent corticosteroid use | 106 (20) | 305 (26) | 0.004 | 221 (25) | 70 (31) | 0.08 |
| Neoplasm | 234 (44) | 430 (37) | 0.009 | 327 (37) | 79 (35) | 0.52 |
| Diabetes mellitus | 174 (32) | 408 (35) | 0.27 | 318 (36) | 76 (33) | 0.46 |
| Intravenous drug use | 7 (1) | 22 (2) | 0.38 | 19 (2) | 1 (<1) | 0.08 |
| Transplant recipient | 44 (8) | 188 (16) | <0.0001 | 137 (16) | 44 (19) | 0.16 |
| Rheumatologic disorder | 11 (2) | 31 (3) | 0.44 | 21 (2) | 10 (4) | 0.1 |
| HIV | 8 (2) | 19 (2) | 0.82 | 17 (2) | 2 (1) | 0.28 |
| Hemodialysis dependence | 37 (7) | 135 (12) | 0.003 | 94 (11) | 37 (16) | 0.02 |
| Site of acquistion | 0.09 | 0.11 | ||||
| Community-acquired | 389 (72) | 795 (68) | 619 (70) | 147 (65) | ||
| Hospital-acquired | 149 (28) | 369 (32) | 266 (30) | 81 (36) | ||
| Source of infection | <0.0001 | 0.0002 | ||||
| Skin/soft tissue | 29 (5) | 70 (6) | 59 (7) | 10 (4) | ||
| Endovascular | 35 (7) | 185 (16) | 120 (14) | 57 (25) | ||
| Gastrointestinal | 83 (15) | 164 (14) | 123 (14) | 30 (13) | ||
| Genitourinary | 201 (37) | 350 (30) | 281 (32) | 59 (26) | ||
| Respiratory/lung | 28 (5) | 96 (8) | 78 (9) | 14 (6) | ||
| Other | 28 (5) | 87 (8) | 56 (6) | 24 (11) | ||
| Unknown | 134 (25) | 212 (18) | 168 (19) | 34 (15) | ||
| Central venous catheter | 90 (17) | 237 (20) | 0.08 | 165 (19) | 53 (23) | 0.12 |
| Cardiac device | 30 (6) | 109 (9) | 0.008 | 76 (9) | 30 (13) | 0.04 |
| Fever | ||||||
| initial blood culture | 364 (70) | 681 (61) | 0.001 | 531 (60) | 125 (55) | 0.17 |
| follow-up blood culture | 220 (25) | 73 (32) | 0.03 | |||
| Mean APACHE-II score (SD) | 7.4 (5.4) | 7.3 (5.9) | 0.67 | 7.2 (4.8) | 7.5 (4.9) | 0.33 |
| Hypotension (SBP<90) | 152 (30) | 259 (23) | 0.002 | 188 (22) | 57 (25) | 0.26 |
| WBC count (>10) | 281 (52) | 653 (56) | 0.14 | 388 (44) | 97 (43) | 0.72 |
| Hospital Service | 0.36 | 0.13 | ||||
| Medicine | 349 (65) | 781 (67) | 608 (69) | 149 (65) | ||
| Intensive care unit | 23 (4) | 41 (4) | 34 (4) | 4 (2) | ||
| Surgery | 144 (27) | 311 (27) | 223 (25) | 66 (29) | ||
| Other | 22 (4) | 31 (3) | 20 (2) | 9 (4) | ||
| Days to effective therapy | 0.001 | 0.08 | ||||
| 0 days | 346 (64) | 729 (63) | 568 (64) | 127 (56) | ||
| 1 day | 131 (24) | 237 (20) | 168 (19) | 59 (26) | ||
| 2 days | 26 (5) | 85 (7) | 63 (7) | 19 (8) | ||
| ≥3 days | 25 (5) | 105 (9) | 79 (9) | 22 (10) | ||
| Unknown | 10 (2) | 8 (1) | 7 (<1) | 1 (<1) | ||
| Duration of effective therapy | <0.0001 | <0.0001 | ||||
| Median (range) | 13 (8–16) | 15 (13–19) | 14 (12–18) | 17 (14–24) | ||
| ≤ 7 days | 122 (23) | 139 (12) | 104 (12) | 25 (11) | ||
| 8–14 days | 227 (42) | 409 (35) | 345 (39) | 47 (21) | ||
| ≥15 days | 166 (31) | 596 (51) | 419 (47) | 154 (68) | ||
| Unknown | 23 (4) | 20 (2) | 17 (2) | 2 (<1) | ||
| Antimicrobial resistance | ||||||
| Fluoroquinolone susceptible | 411 (81) | 846 (77) | 0.06 | 638 (77) | 172 (76) | 0.87 |
| Carbapenem susceptible | 520 (97) | 1083 (93) | 0.003 | 830 (94) | 202 (89) | 0.01 |
Abbreviations: N/A – Not applicable; FUBC – follow-up blood culture; SBP - systolic blood pressire; SD – standard deviation; WBC – white blood cell count (>10 × 109/L).
Figure 2.
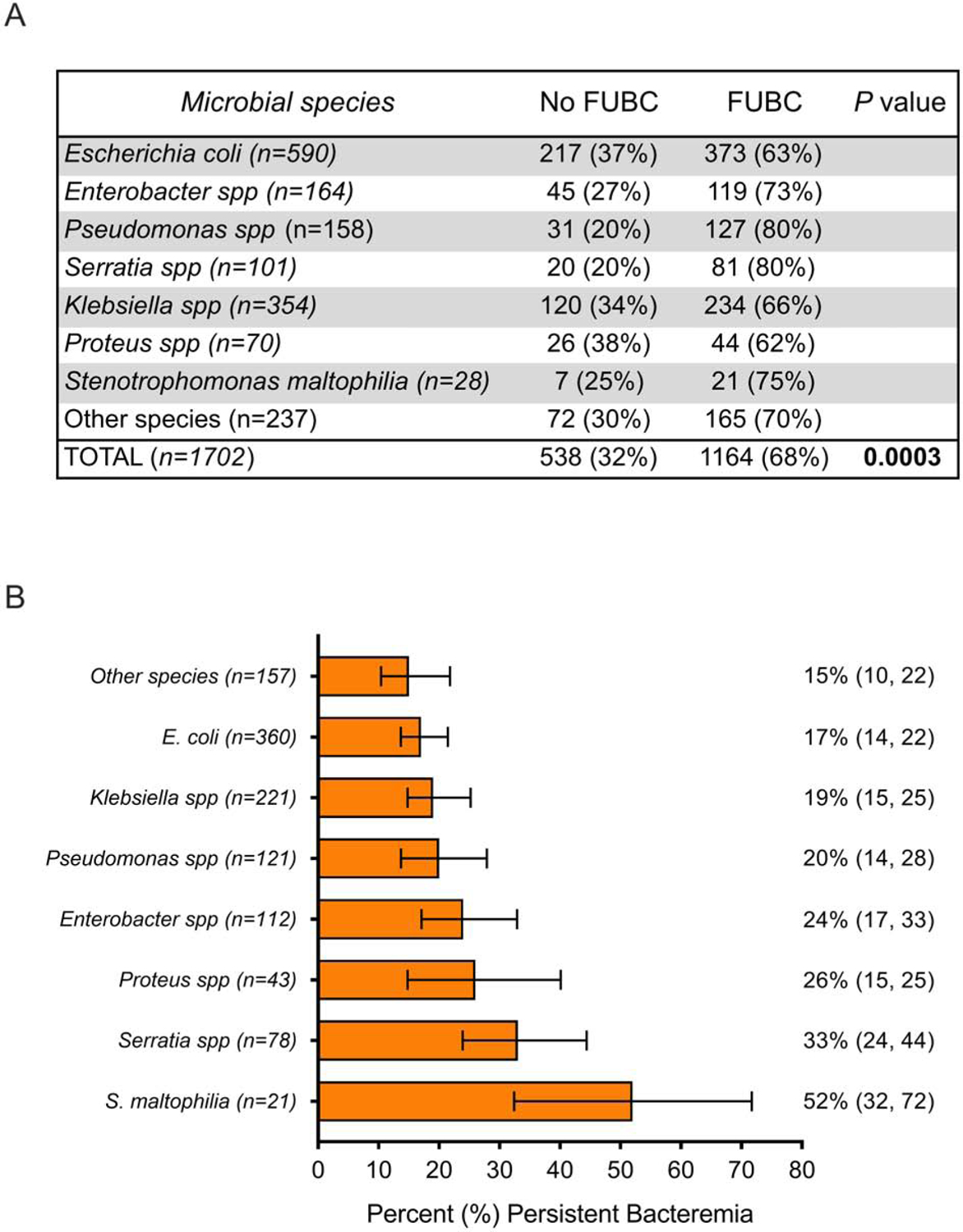
Association between bloodstream bacterial species and (A) acquisition of follow-up blood cultures and (B) frequency of FUBCs growing the same bacterial species as the original cultures in patients with gram-negative bacteremia (GNB). In (B), the 95% confidence intervals are shown in parentheses.
Infectious Diseases Consultation (IDC) prior to drawing FUBCs was not common in patients with GNB (15% [68/467]). In cases with IDC, acquisition of FUBCs (82% [56/68]) did not occur at significantly higher rate than in cases without IDC (74% [297/399]; p=0.17).
Factors associated with positive versus negative FUBCs
Of patients with FUBCs drawn, 20% (228/1164) had persistent GNB (Table 1). Compared to patients with negative FUBCs, GNB patients with persistent GNB were less likely to be on effective antibiotic therapy (64% [568/885] versus 56% [127/228]; p=0.02), more likely to have a cardiac device (13% [30/228] versus 9% [76/885]; p=0.04), more likely to be hemodialysis dependent (16% [37/228] versus 11% [94/885]; p=0.02), and more likely to have an endovascular source of infection (25% [57/228] versus 14% [120/885]; p<0.0001) (Table 1). Persistent GNB decreased as the time from initial blood culture to FUBC increased (Supplemental Figure 1). The likelihood of persistent GNB differed significantly by microbial species (Figure 2B, p=0.0005). GNB patients with FUBCs yielding a different organism than the initial culture numbered 51/1164 (4%), with 29/1164 (2%) involving a new infection and 22/1164 (2%) representing contamination (Supplemental Table 1).
Association of FUBC acquisition with patient mortality
The overall all-cause and attributable in-hospital mortality rates were 17% (284/1702) and 10% (176/1702), respectively. Patients with GNB and FUBCs had significantly lower rates of both all-cause in-hospital mortality (108/538 [20%] versus 176/1164 [15%], p=0.01) (Fig. 3A) and attributable in-hospital mortality (78/538 [15%] versus 98/1164 [8%], p<0.0001) (Fig. 3C).
Figure 3.
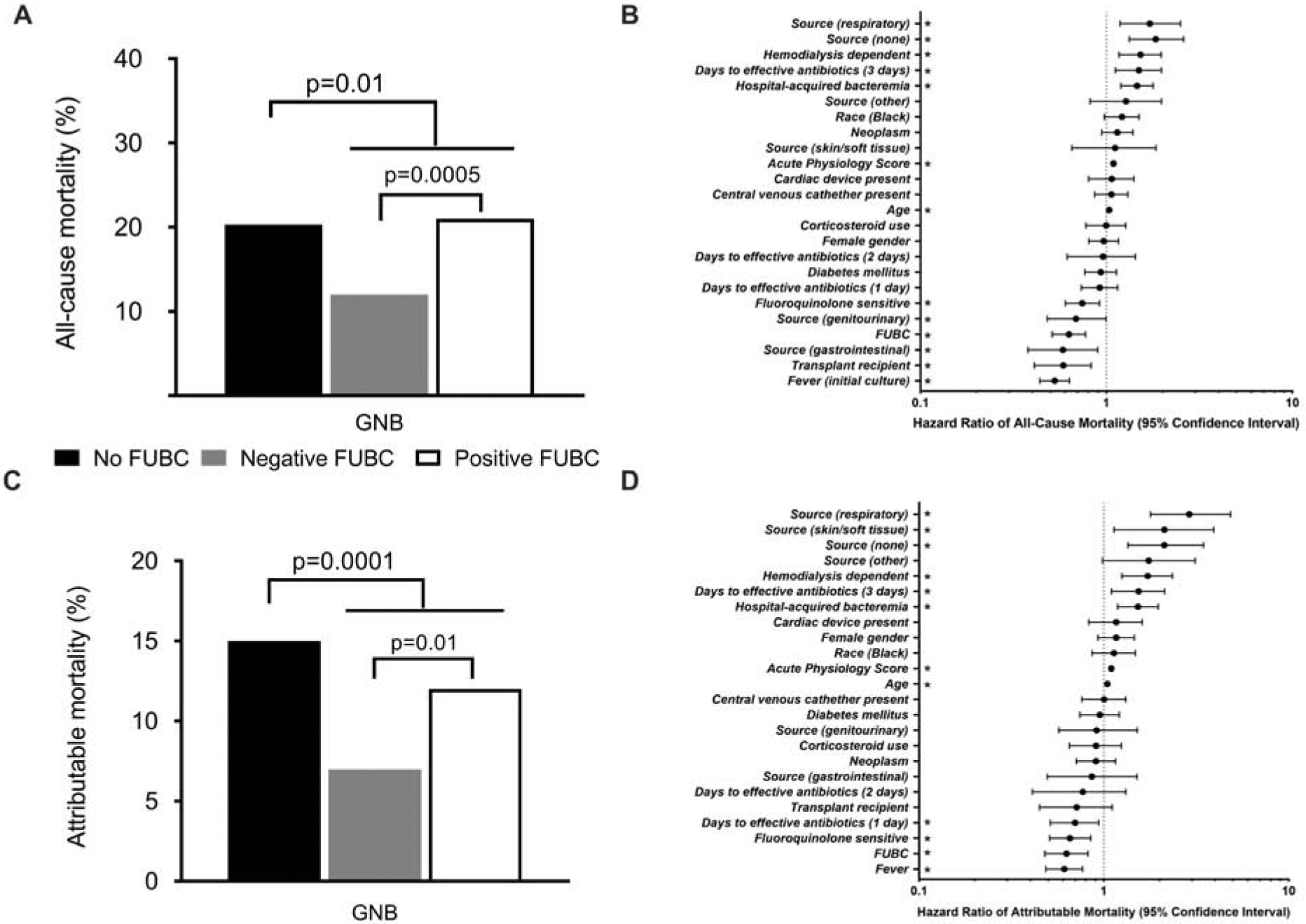
Differences in unadjusted all-cause (A) and attributable (C) in-hospital mortality in patients with gram-negative bacteremia (GNB). Differences in hazard ratios of all-cause (B) and attributable (D) in-hospital mortality in patients with GNB, as determined by propensity score weighted Cox proportional hazards analyses. * – signifies p<0.05.
A propensity score-weighted Cox proportional hazards regression model was used to evaluate differences in clinical outcomes in GNB patients with and without FUBCs (Fig 3B and 3D). Obtaining FUBCs was found to be associated with lower rates of both all-cause (HR=0.629; 95% CI, 0.511–0.772; p<0.0001) and attributable mortality (HR=0.628; 95% CI, 0.480–0.820; p=0.0007). Detailed results from the analyses are shown in Supplemental Table 2. Species-specific analyses were performed for E. coli and Klebsiella pneumoniae. In all cases, obtaining FUBCs (relative to no FUBCs) was associated with decreased all-cause mortality (Supplemental Table 3). A sensitivity analysis excluding all deaths within 48 hours of the initial blood culture showed that FUBC acquisition remained associated with decreased attributable mortality (data not shown).
Association of positive FUBCs with patient mortality
Patients with persistent GNB, relative to those with negative FUBCs, exhibited increased all-cause mortality (49/228 [21%] versus 110/885 [11%]; p=0.0005) (Fig. 3A) and attributable mortality (27/228 [12%] versus 61/885 [7%], p=0.01) (Fig. 3C). The all-cause mortality rate in GNB patients with no FUBCs drawn (20%) was similar to GNB patients with positive FUBCs (21%). A propensity score-weighted multivariable Cox proportional hazards analysis showed that persistent GNB, relative to negative FUBCs, was associated with increased attributable (HR=1.56; 95% CI, 1.13–2.16; p=0.007) and all-cause mortality (HR=1.75; 95% CI, 1.38–2.23; p<0.0001) (Supplemental Table 4).
Risk of persistent GNB among patient subpopulations
Using established methods [7,8], a risk-scoring system was constructed to estimate the probability of persistent GNB (AIMS risk score; Fig. 4A). Corresponding observed and predicted probabilities of persistent GNB are summarized in Fig. 4B. The c-statistic for the predictive model was 0.65 and was consistent with the values from the bootstrap analysis c-statistic values (median=0.66, 25%=0.65, 75%=0.68). There was no significant difference between the observed and predicted values (Hosmer-Lemeshow, p=0.45), indicating that the model was well calibrated. The predicted rate of persistent GNB was 8.6% if no risk factors were present and increased with the presence of each additional risk factor.
Figure 4.
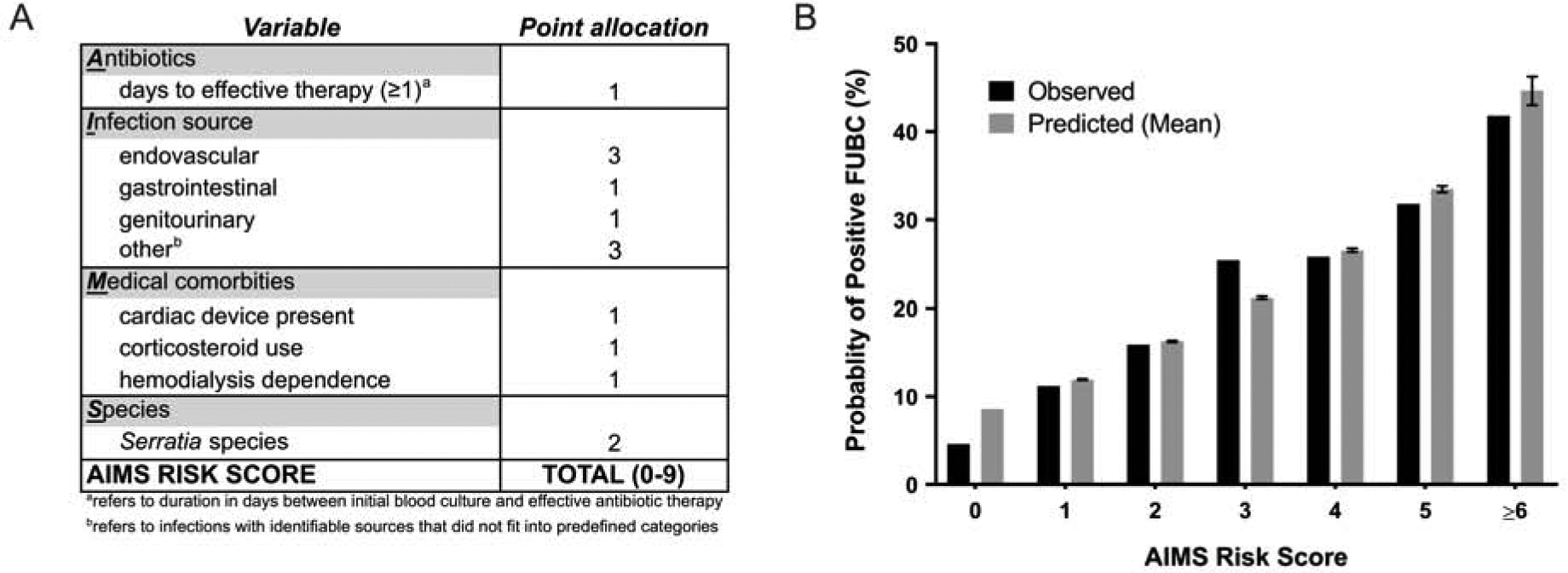
AIMS risk scoring system (A) and corresponding observed and predicted probabilities (B) of persistent gram-negative bacteremia (GNB). Error bars represent 95% confidence intervals.
In a secondary analysis, we performed a classification and regression tree (CART) analysis to identify clinical or microbial (i.e., species) breakpoints separating high and low rates of positive FUBCs in GNB. The only identified breakpoint was source of bacteremia. The group containing endovascular or ‘other’ sources of bacteremia had positive FUBCs in 32% of cases (81/257), while all other sources of bacteremia had positive FUBCs in 17% of cases (147/856; p<0.0001) (Fig. S2).
DISCUSSION
GNB is common and potentially lethal. Our study evaluated the utility of FUBCs to identify patients with GNB who are at risk for poor outcomes. The results yielded two key observations.
First, positive FUBCs in patients with GNB were common. FUBCs were positive in 20% of patients who underwent the test and 13% of the entire cohort of >1700 GNB patients. Over half of GNB patients with persistent GNB had these cultures drawn while on effective antibiotic therapy. This result contrasts with findings of previous smaller studies suggesting that FUBCs are low yield in patients receiving effective antibiotic therapy [6], and emphasizes the importance of performing FUBCs in most patients with GNB. Rates of persistent GNB in our study were higher than previously reported [6,9]. Potential explanations for this difference include variable definitions of persistent bacteremia and our tertiary care patient population with more patients at high-risk for persistent bacteremia. Such high-risk patients include those with endovascular infections (e.g., infections of central lines, cardiac devices, etc.) and bacteremia with organisms exhibiting higher rates of persistent GNB including Serratia.
Second, patients with persistent GNB are at high risk for death. In fact, patients with persistent GNB were nearly twice as likely to die than those with negative FUBCs and had similar mortality to those in whom FUBCs were not drawn. FUBCs are clinically useful to identify patients with GNB at high risk for death as positive FUBCs are potential indicators of complicated infections (e.g., uncontrolled source, metastases to distant sites, resistance to currently used antibiotics, etc.). Patients with persistent GNB could thus potentially benefit from more aggressive diagnostic and therapeutic strategies. Similarly, it is possible that the increased mortality of GNB patients without FUBCs may stem in part from missed opportunities to diagnose and manage such complicated infections.
We believe that these mortality results are valid and generalizable for several reasons. First, we found comparable rates of all-cause mortality to that of a prior study examining nosocomial GNB [10]. Second, we identified risk factors previously associated with mortality in patients with GNB including site of acquisition, source of infection, and antimicrobial resistance [11–13]. Finally, the finding that FUBCs identified patients with GNB at high risk for poor outcome remained consistent and robust throughout multiple propensity score-weighted Cox proportional hazards models designed to minimize confounding, selection bias, and survivor bias. These observations, coupled with the large sample size, suggest that the findings of the study are generalizable.
Given the potential drawbacks of FUBCs (e.g., increased resource utilization, increased costs, potential for false positives), we sought to distinguish patients at highest risk of persistent GNB. We developed a risk-scoring system called the AIMS score that uses clinical characteristics that are readily available at the time of initial positive blood culture to estimate a patient’s probability of persistent GNB. The identified AIMS score predictor variables are consistent with factors that clinicians typically associate with persistent bacteremia, including high-risk medical comorbidities, lack of effective antibiotic therapy, source of bacteremia, and certain bacteria such as Serratia. However, even in the absence of any identified characteristics (i.e., risk score 0), the predicted risk of persistent GNB is nearly 9%. Thus, clinicians should remain watchful for complications in all patients with GNB.
FUBCs were positive in approximately one in five in hospitalized patients with GNB, and when present, indicated an increased risk for mortality. FUBCs, when positive, have potential to identify patients in whom additional diagnostic or therapeutic interventions may be indicated. Conversely, negative FUBCs could help to identify low risk patients with GNB in whom antibiotic therapy could be de-escalated. For these reasons, FUBCs should be considered in the management of patients with GNB if they have any of the identified risk factors for persistent GNB identified in our AIMS scoring tool. This study highlights the need for further validation of FUBCs and the proposed scoring instrument in the management of patients with GNB. Arguments against the acquisition of FUBCs in patients with GNB have included high rate of false positive FUBCs and prolonged antibiotic use. Our false positive FUBC rate of 2% is below the maximum acceptable percentage of contaminated blood cultures (3%) according to the CLSI guidelines [14]. Although we did detect a statistically significant difference in the median duration of antibiotic use between patients who had FUBCs drawn compared to those that did not have FUBCs, this difference was minimal (2 days), and can be considered a more than reasonable exchange for the observed mortality benefit.
This study has several limitations. First, data from this study is derived from a single health system. However, since these results come from one of the largest cohorts of prospectively enrolled patients with GNB, the study makes an important contribution to our understanding of how FUBCs influence clinical management and outcomes in patients with GNB. Second, there are minimal details regarding the clinical status of the patient at the time of FUBCs (i.e. adequate source control, physician impression regarding clinical status). Thus, we cannot fully determine why blood cultures were or were not obtained. Third, additional outcomes including change in management based on FUBCs, rate of infection relapse, or increased cost associated with FUBCs were not investigated in this study. Fourth, this study takes place over a relatively long time period and so results may be subject to historical biases such as changes in the management of GNB or rates of antibiotic resistance. Finally, we have little information on low risk patients with GNB who were discharged prior to availability of the FUBC results or who were discharged from the Emergency Department and not included in the study.
Despite these limitations, this study makes several clinically impactful observations. This study reassessed the prior view that FUBCs have little clinical utility in hospitalized patients with GNB and found that rates of persistent GNB are higher than expected and that acquisition of FUBCs can identify patients at increased risk for mortality. Further studies are needed to validate the use of FUBCs as part of the routine management of high-risk patients with GNB.
Supplementary Material
Acknowledgments
Portions of these results were previously presented in San Francisco, CA, USA at IDWeek 2018 (October 5, 2018; Poster #1039).
Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 1KL2TR002554 [S.A.M] and NIH K24-AI093969 [V.G.F]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
V.G.F served as Chair of V710 Scientific Advisory Committee (Merck); has received grant support from Cerexa/Actavis/Allergan, Pfizer, Advanced Liquid Logics, NIH, MedImmune, Basilea, Karius, Contrafect, Regeneron, and Genentech; has NIH STTR/SBIR grants pending with Affinergy, Locus, and Medical Surface, Inc; has been a paid consultant for Achaogen, Astellas, Arsanis, Affinergy, Basilea, Bayer, Cerexa, Contrafect, Cubist, Debiopharm, Durata, Grifols, Genentech, MedImmune, Merck, Medicines Co., Pfizer, Novartis, Novadigm, Theravance, xBiotech, and has received honoraria from Theravance, Green Cross, and has a patent pending in sepsis diagnostics. D.v.D. has been a paid consultant for Allergan, Achaogen, Shionogi, Tetraphase, Sanofi-Pasteur, T2 Biosystems, NeuMedicine, Roche, MedImmune, Astellas, and Merck, and received grant support from NIH, Steris, and Scynexis. Travel reimbursement from IDSA, ASM and ESCMID. T.L. is on the scientific board for Motif; has been a paid consultant for Allergan, Paratek, Melinta, Nabriva, Merck, and Motif; has received grants from Motif and Merck; and has received payment for lectures for Motif and Sunovion. Other authors have no competing interests.
REFERENCES:
- [1].Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, et al. Risk factors and pathogenic significance of severe sepsis and septic shock in 2286 patients with gram-negative bacteremia. J Infect 2011;62(1):26–33. [DOI] [PubMed] [Google Scholar]
- [2].Rannikko J, Syrjanen J, Seiskari T, Aittoniemi J, Huttunen R. Sepsis-related mortality in 497 cases with blood culture-positive sepsis in an emergency department. Int J Infect Dis. 2017;58:52–7. [DOI] [PubMed] [Google Scholar]
- [3].Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312(13):1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev. 2012;25(2):362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up Blood Cultures in Gram-Negative Bacteremia: Are They Needed? Clin Infect Dis. 2017;65(11):1776–9. [DOI] [PubMed] [Google Scholar]
- [6].Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis. 2016;16:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fowler VG Jr., Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163(17):2066–72. [DOI] [PubMed] [Google Scholar]
- [8].Park LP, Chu VH, Peterson G, Skoutelis A, Lejko-Zupa T, Bouza E, et al. Validated Risk Score for Predicting 6-Month Mortality in Infective Endocarditis. J Am Heart Assoc. 2016;5(4):e003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harris PNA, Peri AM, Pelecanos AM, Hughes CM, Paterson DL, Ferguson JK. Risk factors for relapse or persistence of bacteraemia caused by Enterobacter spp.: a case-control study. Antimicrob Resist Infect Control. 2017;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–17. [DOI] [PubMed] [Google Scholar]
- [11].Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18(6):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vardakas KZ, Rafailidis PI, Konstantelias AA, Falagas ME. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J Infect. 2013;66(5):401–14. [DOI] [PubMed] [Google Scholar]
- [13].Uzun O, Akalin HE, Hayran M, Unal S. Factors influencing prognosis in bacteremia due to gram-negative organisms: evaluation of 448 episodes in a Turkish university hospital. Clin Infect Dis. 1992;15(5):866–73. [DOI] [PubMed] [Google Scholar]
- [14].Clinical Laboratory Standards Institute (CLSI). Wayne P. Principles and Procedures for Blood Cultures: Approved Guideline. CLSI Document M47-A 2007.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


