Abstract
Objective:
To conduct a scoping review of mild stroke definitions based on stroke severity assessments and/or clinical signs and symptoms reported in the literature.
Data Sources:
Electronic searches of PubMed, PsycINFO (Ovid), and CINAHL (EBSCO) databases included keyword combinations of mild stroke, minor stroke, mini stroke, mild cerebrovascular, minor cerebrovascular, transient ischemic attack, or TIA.
Study Selection:
Inclusion criteria were limited to articles published between January 2003 and February 2018. Inclusion criteria included (1) a definition of either mild or minor stroke, (2) written in English, (3) participants aged 18 years and older. Animal studies, reviews, dissertations, blogs, editorials, commentaries, case reports, newsletters, drug trials, and presentation abstracts were excluded.
Data Extraction:
Five reviewers independently screened titles and abstracts for inclusion and exclusion criteria. Two reviewers independently screened each full-text article for eligibility. The five reviewers checked the quality of the included full-text articles for accuracy. Data were extracted by two reviewers and verified by a third reviewer.
Data Synthesis:
Sixty-two studies were included in the final review. Ten unique definitions of mild stroke using stroke severity assessments were discovered, and ten different cutoff points were used with the most widely used measure to classify stroke severity – the National Institutes of Health Stroke Scale (NIHSS). Synthesis also revealed variations in stroke severity across years, time since stroke, imaging, medical indicators, clinical signs and symptoms and settings.
Conclusions:
Inconsistencies in the classification of mild stroke are evident with varying use of stroke severity assessments, measurement cut-off scores, imaging tools, and clinical or functional outcomes. Continued work is necessary to develop a consensus definition of mild stroke, which directly impacts treatment receipt, referral for services, and health service delivery.
Keywords: Mild Stroke, Minor Stroke, Mini Stroke, Stroke Classification, Stroke Outcomes
Introduction
Approximately half of the individuals with stroke may be classified as having a minor/mild stroke with non-disabling or rapidly improving symptoms.1 Furthermore, at one-month post-stroke, patients with mild stroke have an estimated 11-15% risk of experiencing a recurrent stroke.2 Relatively high prevalence of mild stroke is suggested as a result of improved medical care during initial treatment/hospitalization. For instance, treatments such as thrombectomy3 and intravenous recombinant tissue plasminogen activator (IV rtPA) therapy4 is linked to reduced stroke severity and improved functional outcomes. However, there is increasing evidence that at least one-third of patients with mild strokes have poor functional outcomes.5, 6
Patients classified with mild stroke typically do not receive rehabilitation services due to the expectant non-disabling or rapidly improving symptoms. However, they might experience deficits for months following the stroke,7, 8 resulting in unmet long-term needs following return to the community.9 Clinical guidelines and recommendations require that all individuals with stroke are assessed for rehabilitation needs.10 Early intervention by an interdisciplinary rehabilitation team can improve functional recovery following stroke.11 Prognosis for functional outcome is an influential factor on referral for post-acute stroke rehabilitation as determined by physiatrists.12 However, many assessment tools are not sensitive in determining stroke severity and rehabilitation needs following mild stroke, which further perpetuate the lack of identification and referral for needed healthcare services for persons with mild stroke.13 With the changing healthcare delivery system and the building for value-based care, provider systems are thinking strategically about products, services, and integrated solutions that improve patient outcomes while reducing costs throughout the healthcare continuum. As healthcare systems prepare for the evolving needs of their patient populations, understanding definitions and healthcare complexities (i.e., patient flow, care and resources) is essential for efficient delivery of healthcare services for patients with a stroke.
The literature describes mild stroke using a variety of terms including, but not limited to, “mild,” “minor,” “transient ischemic attack (TIA),” and “mini stroke.” Lack of a global consensus on the definition of mild stroke may result in variability in treatment across the care continuum and post-discharge outcomes. Current definitions of mild stroke vary widely in the use of clinical outcome measurement and stroke scales.14-17 TIAs have stroke-like symptoms that typically resolve within 24 hours. Studies have used various definitions of “mild” stroke, with the vast majority using scores from the National Institute of Health Stroke Scale [NIHSS],15 and the Modified Rankin Scale [mRS].17
We believe that physiatrists and rehabilitation professionals are instrumental in identifying the need and making referrals for rehabilitation services to improve functional recovery following mild stroke. Persons with mild stroke are largely not receiving needed rehabilitation therapies and services to support long-term outcomes in the current healthcare system. Not receiving needed services may be due to an unclear understanding of implications of mild stroke by healthcare providers, caregivers, and persons diagnosed with mild stroke, as well as the insensitivity of assessments to detect functional impairments in person with mild stroke.18 The lack of a global consensus of a definition may perpetuate the problem. Therefore, the objective of this study was to conduct a scoping review of mild stroke definitions based on stroke severity assessments and/or clinical signs and symptoms.
Methods
Design
A systematic scoping review of the literature was conducted to identify definitions of mild stroke. This review was not registered in PROSPERO. Scoping reviews are a form of knowledge synthesis for exploratory research questions that involve systematically searching, identifying, and integrating existing research.19 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used as the guideline for conducting this review.20
Search Strategy
In January 2017, a search was conducted by a medical librarian in PubMed, PsycINFO (Ovid), and CINAHL (EBSCO) using a combination of the following search terms: “mild stroke,” “minor stroke,” “mini stroke,” “mild cerebrovascular,” “minor cerebrovascular,” “transient ischemic attack,” or “TIA.” The results were limited to articles published between January 1, 2003 and December 31, 2016. In 2003, the American Heart Association and American Stroke Association (AHA/ASA) along with The Joint Commission developed standards for certifying Primary Stroke Centers through a Disease Specific Certification Program in the United States to develop a consistent clinical outcomes assessment and standards for stroke care.21 These efforts led to the inclusion of stroke in the International Standards for Disease Specific Care for certifying organizations outside of the US;22 therefore, we included articles from 2003 and later due to changes in care provision globally. Animal studies were excluded from the search. An updated search was conducted from January 1, 2017 to February 2018 using PubMed, PsycINFO (Ovid), and CINAHL (EBSCO). A total of 3499 records [PubMed (946), PsycInfo (1062), CINAHL (1491)] were retrieved through database searching. After the records were deduplicated, 3,422 records underwent title and abstract screening (Appendix I and II).
Screening
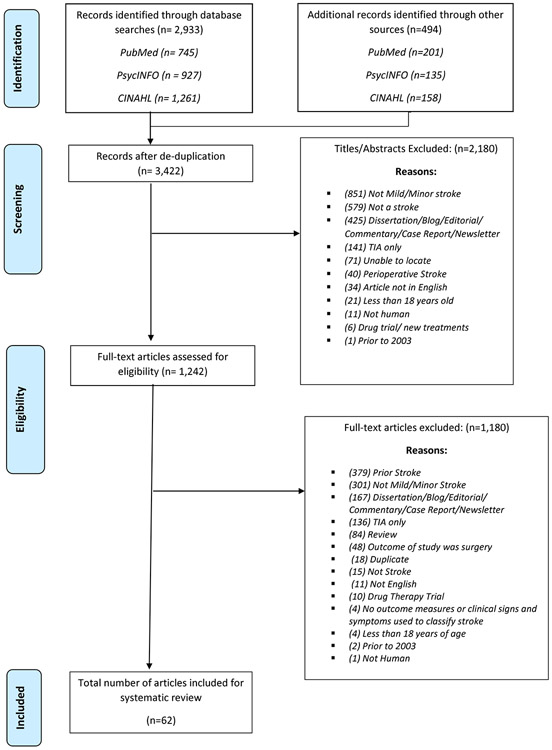
Five reviewers screened the titles and abstracts for inclusion and exclusion criteria. An article was included if it included: (1) a definition of either mild or minor stroke, (2) was written in English, and (3) included patients over the age of 18 years old. Animal studies, reviews, dissertations, blogs, editorials, commentaries, case reports, newsletters, drug trials, presentation abstracts and articles written prior to 2003 were excluded during the screening process. Of the 3,422 records that were abstract reviewed, 2,180 records were excluded, resulting in 1,242 articles eligible for full-text-review. Two reviewers independently screened each included full-text article using the same inclusion and exclusion criteria. Articles including individuals with prior history of minor or mild stroke, drug therapy trials, stenting, and that solely analyzed transient ischemic attacks were excluded. Articles were also excluded if mild/minor stroke was only an outcome of a surgery or treatment, as the primary population of the study was not mild stroke. Following the full text-review, articles that did not reach a consensus were arbitrated by a third independent reviewer and discussed by the group for consensus. Of the 1,242 articles eligible for full-text review, 1,180 articles were excluded, resulting in 62 articles included in this review. All full-text articles were re-screened by five independent reviewers to maintain accuracy and quality control.
Data Extraction
Five independent reviewers extracted the following data from each article that met the inclusion criteria: study characteristics (study design, sample size, setting and year in which the data was collected), demographic data (age, race/ethnicity, sex) and data about mild/minor stroke (terms, severity assessments, and clinical descriptions used to classify mild stroke). The data extraction form was quality checked by multiple reviewers for accuracy. The data was synthetized to understand the demographic characteristics of the studies, variations in stroke severity assessments and clinical measures, across acute and post-acute care settings, time since stroke, and years when the study were conducted. Supplemental Appendix I and II includes the search criteria.
Results
Sixty-two of the 1242 articles met the inclusion criteria and were included in the final review. The PRISMA flowchart lists the screening process, Figure 1. Cohen’s Kappa, a measurement of interrater reliability, was 0.8, signifying a substantial agreement between the reviewers for the full-text articles.23
Figure 1:
PRISMA Flowchart
Table 1 presents general information about the 62 articles included in this study. The articles span across 20 countries. Many studies were based conducted in the United States (21.0%) followed by China (12.9%), United Kingdom (11.3%), Canada (8.1%), Korea and Sweden (6.5% each), France and Taiwan (4.8% each), Denmark, India, Israel, and New Zealand (3.2% each), Australia, Bulgaria, Finland, Germany, Iran, Norway, Poland, and Spain (1.6% each). The overall sample included 82,559 participants, which included strokes of all severities. Sample sizes across each study varied, with the smallest study included only eight participants and the largest study included 27,728 participants. The study settings included the entire care continuum, from the acute hospital to the community. Thirty-six (58%) of the studies were conducted during the acute hospitalization, 12 (19.0%) in the community (i.e., that included home, outpatient, and assisted living), and 13 (21.0%) in mixed (more than one setting type) settings. Mild stroke ages were from less than 50 in 1 study (1.6%), to age 50-59 in 14 studies (22.6%), age 60-69 in 28 studies (45.2%), age 70-79 in 4 articles (6.5%), and 2 studies (3.2%) had mixed ages and 13 studies did not report age. In all the studies, 20,965 (33.2%) of the participants were male, 42,234 (66.8%) were female. Eight studies did not report the sex break-down which accounted for 22,011 participants (25.83%). One of the studies had only female participants (27,728), which contributed to the overall sex distribution.24 The years that the studies were conducted ranged from 1991 to 2016.
Table 1:
Sample Demographic Characteristics for all Included Studies
| Author/Year/Country | Sample | Setting | Sex | Study Design | Year Study Conducted |
|---|---|---|---|---|---|
| Adamit/2015/Israel | 430 | community (e.g., home, outpatient, assisted living) | NR | prospective | 2008 |
| Alt Murphy/2011/Sweden | 38 | NR | NR | NR | NR |
| Atanassova/2006/Bulgaria | 234 | acute hospital | male: 144 (61%), female: 90 (39%) | clinical survey | 2002-2004 |
| Bejot/2013/France, UK | 1650 | mixed | male: 752 (45.6%), female: 898 (54.4%) | retrospective | 2006-2010 |
| Bejot/2017/France | 1573 | acute hospital | male: 902 (57.3%), female: 671 (42.7%) | prospective | 2011-2014 |
| Bhattacharjee/2012/India | 219 | community (e.g., home, outpatient, assisted living) | male: 77 (35%), female: 142 (65%) | prospective | 2009-2010 |
| Boulos/2017/Canada | 30 | mixed | male: 17 (57%), female: 13 (43%) | prospective | NR |
| Bustren/2017/Sweden | 40 | mixed | male: 25 (62.5%), female: 15 (37.5%) | longitudinal | NR |
| Carlsson/2003/Sweden | 75 | acute hospital | male: 52 (69%), female: 23 (31%) | prospective | 1995-1997 |
| Carlsson/2004/Sweden | 15 | mixed | male: 8 (53%), female: 7 (47%) | qualitative | NR |
| Chang/2017/Korea | 455 | mixed | male: 300 (65.9%), female: 155 (34.1%) | prospective | 2012-2014 |
| Chappell/2017/UK | 264 | acute hospital | NR | prospective | 2010-2012, 2013 |
| Dabrowska-Bender/2017/Poland | 44 | acute hospital | male: 23 (52%), female: 21 (48%) | NR | 2015 |
| Daniels/2017/USA | 80 | acute hospital | male: 77 (96.25%), female: 3 (3.75%) | cohort | 2012-2014 |
| Divya/2017/India | 256 | acute hospital | male: 215 (84%), female: 41 (16%) | observational | 2013-2014 |
| Edwards/2006/USA | 219 | mixed | male: 94 (84%), female: 125 (57%) | prospective | 2001-2002 |
| Eriksson/2013/USA | 116 | mixed | male: 56 (48.3%), female: 60 (51.7%) | prospective | 2002-2006 |
| Fang/2010/Canada | 20657 | acute hospital | NR | cohort | 2003-2008 |
| Faulkner/2015/New Zealand | 55 | acute hospital | male: 29 (52.7%), female: 26 (47.3%) | randomized clinical trial | NR |
| Faulkner/2017/New Zealand | 60 | community (e.g., home, outpatient, assisted living) | male: 31 (52%), female: 29 (48%) | longitudinal | 2011 |
| Fride/2015/Israel | 163 | community (e.g., home, outpatient, assisted living) | male: 117 (71.8%), female: 46 (28.2%) | prospective | 2008-2012 |
| Gadodia/2016/USA | 1614 | acute hospital | male: 855 (53%), female: 759 (47%) | cohort | 2009-2013 |
| Ghahremanfard/2013/Iran | 100 | acute hospital | male: 52 (52%), female: 48 (48%) | cross-sectional | 2010-2011 |
| Hsieh/2017/Taiwan | 10877 | acute hospital | male: 6462 (59.4%), female: 4415 (40.6%) | retrospective | 2000-2012 |
| Joa/2017/Korea | 208 | acute hospital | male: 113 (54%), female: 95 (46%) | observational | NR |
| Jung/2015/South Korea | 3025 | acute hospital | male: 1,850 (61.2%), female: 1,175 (38.8%) |
retrospective | NR |
| Kim/2015/Korea | 80 | acute hospital | male: 54 (67.5%), female: 26 (32.5%) | prospective | 2013-2014 |
| Lin/2006/Taiwan | 522 | acute hospital | male: 324 (62%), female: 198 (38%) | prospective | 2003-2004 |
| Liu/2015/China | 211 | acute hospital | male: 155 (73.5%), female: 56 (26.5%) | prospective | 2010-2013 |
| Luengo-Fernandez/2009/UK | 591 | community (e.g., home, outpatient, assisted living) | male: 273 (46%), female: 318 (54%) | comparison | 2002-2004, 2004-2007 |
| Moustafa/2010/UK | 16 | acute hospital | male: 14 (87.5%), female: 2 (12.5%) | cohort | NR |
| Muren/2008/Norway | 30 | acute hospital | male: 17 (57%), female: 13 (43%) | prospective | 1996-2002 |
| Muus/2010/Denmark | 105 | community (e.g., home, outpatient, assisted living) | male: 63 (60%), female: 42 (40%) | longitudinal | 2005-2006 |
| Muus/2011/Denmark | 150 | acute hospital | male: 82 (55%), female: 68 (45%) | follow-up | 2003-2005 |
| Novak/2004/USA | 50 | mixed | male: 22 (44%), female: 28 (56%) | cross-sectional | NR |
| O’Brien/2010/USA | 98 | community (e.g., home, outpatient, assisted living) | male: 54 (55.1%), female: 44 (44.9%) | cross-sectional | NR |
| Ois/2009/Spain | 163 | mixed | male: 97 (59.5%), female: 66 (40.5%) | prospective | 2002-2008 |
| Pfaff/2016/Germany | 33 | acute hospital | male: 14 (42.4%) female: 19 (57.6%) | retrospective | 2010-2015 |
| Rist/2013/USA | 27728 | acute hospital | male: 0 (0%), female: 27728 (100%) | prospective | NR |
| Rochette/2007/Canada | 108 | acute hospital | NR | descriptive | 2001-2003 |
| Rozon/2015/Canada | 186 | acute hospital | male: 105 (56.4%), female: 81 (43.6%) | cohort | 2008-2011 |
| Ruuskanen/2010/Finland | 75 | acute hospital | male: 49 (65.3%), female: 26 (34.7%) | prospective | 2005-2008 |
| Sarker/2008/UK | 566 | community (e.g., home, outpatient, assisted living) | Male: 307 (54.2%), female: 259 (45.8%) | prospective | 1991-2005 |
| Seymour/2014/USA | 13 | community (e.g., home, outpatient, assisted living) | male: 9 (69.2%), female: 4 (30.8%) | cross-sectional | NR |
| Shi/2015/China | 757 | mixed | male: 513 (67.8%), female: 244 (32.2%) | retrospective | 2008-2010 |
| Shi/2016/China | 747 | mixed | male: 507 (67.9%), female: 240 (32.1%) | retrospective | 2008-2010 |
| Song/2014/China | 7455 | acute hospital | male: 4588 (61.5%), female: 2867 (38.5%) | randomized control study | 2007-2008 |
| Tellier/2011/Canada | 8 | community (e.g., home, outpatient, assisted living) | male: 2 (25%), female: 6 (75%) | cross-sectional | NR |
| Torres-Mozqueda/2008/USA | 230 | mixed | NR | prospective | NR |
| Tseng/2006/Taiwan | 360 | acute hospital | male: 207 (58%), female: 153 (42%) | prospective | 1998-1999 |
| Valdes Hernandez/2015/USA | 250 | community (e.g., home, outpatient, assisted living) | NR | longitudinal | NR |
| Villain/2017/France | 34 | acute hospital | male: 22 (64%), female: 12 (36%) | prospective | NR |
| Volonghi/2013/UK | 616 | acute hospital | male: 359 (58%), female: 257 (42%) | prospective | 2002-2007 |
| Ward/2017/UK | 57 | acute hospital | male: 32 (56%), female: 25 (44%) | NR | NR |
| Wolf/2011/USA | 53 | community (e.g., home, outpatient, assisted living) | male: 23 (43%), female: 30 (57%) | cross-sectional | NR |
| Wolf/2013/USA | 20 | acute hospital | male: 9 (45%), female: 11 (55%) | prospective | NR |
| Wolf/2017/USA | 34 | acute hospital | NR | cross-sectional | NR |
| Xue/2017/China | 438 | acute hospital | male: 230 (52.5%), female: 208 (47.5%) | prospective | 2015-2016 |
| Zhang/2014/Australia | 158 | mixed | male: 89 (56.3%), female: 69 (43.7%) | prospective | NR |
| Zhang/2017/China | 217 | acute hospital | male: 147 (67.7%), female: 70 (32.3%) | prospective | 2013-2014 |
| Zhang/2017/China | 229 | acute hospital | male: 123 (53.7%), female: 106 (46.3%) | prospective | 2015-2016 |
| Zhou/2017/China | 325 | acute hospital | male: 224 (69%), female: 101 (31%) | retrospective | 2013-2015 |
Note. NR = not reported
Mild Stroke Study Characteristics
Table 2 presents a breakdown of the 62 articles including a classification of mild stroke. Thirty-six studies used the term “mild stroke”, 18 studies used “minor stroke,” two studies used “mild/minor stroke”, four studies “minor stroke and TIA”, and two studies used “mild stroke and TIA.” The sample size for the mild stroke population was 66,095 ranging from 6 to 27,728 participants (Table 2). The mean ages of the participants were from under 50 years in one study (1.6%), age in 50s (22.6%), in 60s (45.2%), in 70s (6.5%), two studies had mixed ages (3.2%), and 13 (21.0%) studies did not include age. Sex was reported in 49 studies and included 10,192 (22.6 %) males and 34,748 (77.3 %) females. As noted above, one of the studies was completely female (27, 727) which contributed to the overall the sex distribution. The results synthetized below include the stroke severity assessments and the clinical signs or symptoms used to classify mild stroke across these studies.
Table 2:
Mild Stroke Sample Characteristics
| Author/Year/Country | Sample Size |
Age | Sex | Outcome Measures used to Classify |
Imaging, Medical Indicators, and Clinical Signs and Symptoms |
Time Since Stroke Onset |
|---|---|---|---|---|---|---|
| Adamit/2015/Israel | 249 | 68.6 ± 9.9 (50-92) | Male: 142 (57%) Female: 107 (43%) | NIHSS ≤4 | Cognition | 3 months and 6 months |
| Alt Murphy/2011/Sweden | 19 | 58.56 ± 8.85 | NR | FMA 58-64 | Motor function | Mean: 18.9 months (SD: 16.4) Range 6-63 |
| Atanassova/2006/Bulgaria | 155 | 62.31 ± 5.82 | Male: 97 (62.6%), Female: 58 (37.4%) | mRS 1-3 | CT, MRI, Doppler ultrasound | NR |
| Bejot/2013/France, UK | Dijon: 229 OXVASC : 388 | NR | Male: 752 (45.6%) Female: 898 (54.4%) | NIHSS ≤2; physician stating in medical record | NR | NR |
| Bejot/2017/France | 985 | Minor stroke: <50: 90 (17.05%) 50-65: 160 (30.30%) 65-80: 188 (35.60%) ≥80: 90 (17.05%) Mild stroke: <50: 72 (15.8%) 50-65: 132 (28.9%) 65-80: 164 (35.9%) ≥80: 89 (19.5%) | Male: 602 (61%) Female: 383 (39%) | Minor: NIHSS ≤3 Mild: NIHSS 4-9 | CT, MRI | < 4.5 hours [802 (52.2%)]; >4.5 hours [437 (28.5%)]; Unknown [297 (19.3%)] |
| Bhattacharjee/2012/India | 33 | NR | NR | mRS 1-2; NIHSS ≤5 | Cognition, Motor function | Onset, 28 days, 6 months, and 1 year follow-up |
| Boulos/2017/Canada | 30 | 63.7± 13.5 | Male: 17 (57%) Female: 13 (43%) | NIHSS≤3 | NR | 14 days |
| Bustren/2017/Sweden | 22 | 60.7 ± 11.5 | Male: 15 (68%) Female: 7 (32%) | FMA-UE mild impairment between 58 and 66 of the contralesional arm | Motor function | 3 days post stroke, 4 weeks, and 3 months post-stroke |
| Carlsson/2003/Sweden | 75 | 59.6±11.3 (30-75) | Male: 52 (69%) Female: 23 (31%) | Barthel Index 50-100 | Subtle sequelae | 1 year |
| Carlsson/2004/Sweden | 15 | 50 (30-69) | Male: 8 (53%) Female: 7 (47%) | Barthel Index 50-100 | Cognition | 1 year |
| Chang/2017/Korea | 455 | 61.27 ± 13.21 (21.4-92.1) | Male: 300 (65.9%), Female: 155 (34.1%) | NIHSS ≤5 (ischemic stroke); GCS 14-15 (hemorrhagic stroke) | Cognition, Motor function | Arrival to hospital: 24.37 (SD: 32.00) Range 1.0-159.0; and 7 days, 6 months post-stroke assessment |
| Chappell/2017/UK | 264 | 65.3 ± 11.3 | NR | NIHSS ≤4 | MRI | Baseline and 1 year follow-up, exact time NR |
| Dabrowska-Bender/2017/Poland | 23 | NR | NR | NIHSS (no ranking reported) | Depression | >1 month from study |
| Daniels/2017/USA | 78 | NR | NR | NIHSS ≤5 | MRI | Onset, upon hospital admission MRI assessment-44.9 hours (SD26.4) |
| Divya/2017/India | 256 | 65.0 ± 9.3 | Male: 215 (84%), Female: 41 (16%) | NIHSS ≤5; mRS ≤2 | CT, MRI | 3 months post |
| Edwards/2006/USA | 219 | 64.74 ± 15.87 | Male: 94 (43%), Female: 125 (57%) | NIHSS ≤5 | CT, MRI | 6 months |
| Eriksson/2013/USA | 99 | NR | NR | NIHSS ≤5 | Participation | 4-9 months; Mean 6.4 months |
| Fang/2010/Canada | 13,638 | NR | NR | CNS =8 | NR | Emergency admissions |
| Faulkner/2015/New Zealand | 27 | 65 ± 11 | Male: 15 (56%), Female: 12 (44%) | New Zealand’s TIA/stroke guidelines | Focal cerebral retinal symptoms | 7 days of symptom onset (baseline, 8 week, 12 month follow-up) |
| Faulkner/2017/New | 60 | NR | NR | NIHSS ≤5 | Blood pressure, Blood lipid | 2 weeks |
| Zealand | profile | |||||
| Fride/2015/Israel | 163 | 63.75 ± 7.7 (50-89) | Male: 117 (71.8%) Female: 46 (28.2%) | NIHSS ≤5 | Cognition | 3 months |
| Gadodia/2016/USA | 1,614 | 67 (57-77) | Male: 855 (53%), Female: 759 (47%) | NIHSS ≤5 | NR | During acute hospitalization, exact time NR |
| Ghahremanfard/2013/Iran | 15 | 61.9 ± 11.6 | Male: 9 (60%), Female: 6 (40%) | mRS ≤2 | Mean platelet volume (MPV) | Neurology clinic, exact time NR |
| Hsieh/2017/Taiwan | 7260 | NR | NR | NIHSS ≤5 | Old age, Prior hospitalization, Comorbidity, Complications | During acute hospitalization, exact time NR |
| Joa/2017/Korea | 87 | NR | NR | Korean NIHSS ≤5 | Cognition, Motor function | Beginning of rehabilitation, exact time NR |
| Jung/2015/South Korea | 3025 | NR | Male: 1,850 (61.2%) Female: 1,175 (38.8%) | NIHSS ≤4 | CT, MRI | Hospital admission (within 5 days of symptom onset) |
| Kim/2015/Korea | 80 | 63.8 ± 13.6 | Male: 54 (67.5%) Female: 26 (32.5%) | MMSE ≥24; mRS ≤3 | Cognition, Motor function | Hospital admission and each hospital day and follow-up |
| Lin/2006/Taiwan | 376 | 66.0 ± 11.6 | Male: 230 (61%) Female: 146 (39%) | NIHSS ≤3 | CT, MRI | 48 hours |
| Liu/2015/China | 211 | 60.2 ± 12.6 | Male: 155 (73.5%) Female: 56 (26.5%) | NIHSS ≤3 | MRI | 24 hours |
| Luengo-Fernandez/2009/UK | 275 | NR | NR | NIHSS ≤3 | NR | < 14 days |
| Moustafa/2010/UK | 6 | 68.3 | Male: 4 (66.7%) Female: 2 (33.3%) | NR | PET, MRI, Doppler ultrasound | 47 days (SD 31 days) |
| Muren/2008/Norway | 30 | 58.0 ± 9.0 | Male: 17 (57%) Female: 13 (43%) | NR | Cognition. Communication, Motor function, | 60 months (SD 27 months) Range 16-104 months |
| Muus/2010/Denmark | 105 | Male: 65.8 mean (range: 40-83)† Female: 66.3 mean (range: 42-87) Pooled average: 66 mean (range: 40-87) | Male: 63 (60%) Female: 42 (40%) | SSS 45-58 | Cognition, Motor function | 3 and 12 months |
| Muus/2011/Denmark | 93 | NR | NR | SSS (45-58) | Cognition, Motor function | 3, 12 and 24 months |
| Novak/2004/USA | 15 | 53.1 ± 1.6 | Male: 5 (33.3%) Female: 10 (66.7%) | mRS <3 | CT, MRI | 18.3 months (SD 4.5 months) after acute onset |
| O’Brien/2010/USA | 98 | 51.53 ± 7.74 | Male: 54 (55.1%), Female: 44 (44.9%) | NIHSS ≤3 | Cognition, Motor function | 6 months-18 months post stroke |
| Ois/2009/Spain | 163 | 71.8 ± 10.4 (45-92) | Male: 97 (59.5%) Female: 66 (40.5%) | NIHSS ≤6 | CT, MRI | 6 hours, 72 hours, 7 days, 14 days |
| Pfaff/2016/Germany | 33 | 68.0 ± 16.0 | Male: 14 (42.4%) Female: 19 (57.6%) | NIHSS ≤8 | CT, MRI | Time from onset to imaging 175 minutes (IQR 72-279); Time from onset to tPA 156 minutes IIQR 94-238) |
| Rist/2013/USA | 27,728 | 54.7 ± 7.1 | Male: 0 (0%) Female: 27,728 (100%) | mRS ≤1 | Cholesterol level | 8.4 years |
| Rochette/2007/Canada | 35 | 72.3 ± 10.5 | Male: 15 (42.9%), Female: 20 (57.1%) | CNS >8.5/11.5 | Motor function | 2-3 weeks, 3 months and 6 months |
| Rozon/2015/Canada | 186 | 63.3 ± 12.5 | Male: 105 (56.4%), Female: 81 (43.6%) | CNS >8.5/11.5; mRS ≤2 | Motor function | 1 month, 6 months, and 1 year |
| Ruuskanen/2010/Finland | 37 | Median: 62 (IQR 57-71) | Male: 24 (64.9%), Female: 13 (35.1%) | NIHSS ≤4 | Cognition | Average 4 days after onset (MD 4.00) Range 1-11 |
| Sarker/2008/UK | 259 | NR | NR | GCS >12 | CT, MRI, Cerebrospinal fluid analysis, Post mortem examination | 3 months, 1 year, yearly over 10 years |
| Seymour/2014/USA | 13 | 62.08 ± 15.10 (36-82) | Male: 9 (69.2%) Female: 4 (30.8%) | NIHSS ≤5 | Sexual functioning | 6-18 months |
| Shi/2015/China | 757 | 61.14 ± 11.56 | Male: 513 (67.8%), Female: 244 (32.2%) | NIHSS ≤3 | CT, MRI | 14 days (SD 2 days), 3 months, 6 months, and 1 year after stroke |
| Shi/2016/China | 747 | 61.0 ± 11.5 | Male: 507 (67.9%), Female: 240 (32.1%) | NIHSS ≤3 | Cognition, Depression, CT, MRI | 14 days (SD 2 days), 3 months, 6 months, and 1 year after stroke |
| Song/2014/China | 3,231 | 64.18 ± 12.31 | Male: 1999 (61.9%) Female: 1232 (38.1%) | NIHSS ≤4 | Statin use, CT, MRI | Time onset to admission: Non-statin 15.72 (SD 8.32); Statin 16.47 (SD 8.19) |
| Tellier/2011/Canada | 8 | 56.9 ± 9.2 | Male: 2 (25%) Female: 6 (75%) | CNS >8.5; mRS ≤2; | Cognition, Motor function, Participation, Mood | NR |
| Torres-Mozqueda/2008/USA | 172 | 69.3 (SE = 1.0) | Male: 94 (54.7%) Female: 78 (45.3%) | NR | CT, MRI | Acute onset, exact time NR |
| Tseng/2006/Taiwan | 193 | 64.0 ± 11.0 | Male: 116 (60%) Female: 77 (40%) | NIHSS ≤6 | CT, MRI | Onset <24 hours, exact time < 24 hours from admission in 81% of sample |
| Valdes Hernandez/2015/USA | 195 | 77.73 ± 6.42 | Male: 65 (33%) Female: 130 (67%) | MMSE ≥23 | MRI | Acute onset, NR exact time |
| Villain/2017/France | 34 | 57.52 ± 14.87 | Male: 22 (64.7%) Female: 12 (35.3%) | NIHSS ≤6 | Cognition, Depression | Outcomes within 24 hours following admission and at 3 months, exact time NR |
| Volonghi/2013/UK | 216 | 71.0 ± 12.5 | Male: 121 (56%) Female: 95 (44%) | NIHSS ≤3 | Cognition | Outcomes at 1 year and 5 year follow-up, exact time NR |
| Ward/2017/UK | 27 | 52.93 ± 9.52 | Male: 17 (63%) Female: 10 (37%) | NIHSS 1-5 | CT, MRI | Within 2 weeks of onset |
| Wolf/2011/USA | 53 | 56.2 ± 12.8 (33-51) | Male: 23 (43%) Female: 30 (57%) | NIHSS ≤5 | Cognition | Within 1 week of mild stroke |
| Wolf/2013/USA | 20 | 52.15 ± 7.43 | Male: 9 (45%) Female: 11 (55%) | NIHSS ≤5 | Cognition | Within 3 weeks post discharge from acute (Mean 21.95 days, SD 10.68), 6 months post (Mean 178.50, SD 47.90) |
| Wolf/2017/USA | 14 | 52.93 ± 9.52 | Male: 6 (43%) Female: 8 (57%) | NIHSS 1-5 | Cognition | At least 6 months post-stroke |
| Xue/2017/China | 438 | 58 (50-67) | Male: 230 (52.5%), Female: 208 (47.5%) | NIHSS ≤4 | CT, MRI, Hyperglycemia | Assessment on admission and 3 months post, exact time NR |
| Zhang/2014/Australia | 76 | 67.2 ± 10.6 | Male: 44 (57.9%), Female: 32 (42.1%) | NIHSS ≤3 | Blood pressure | Within 7 days after initial stroke |
| Zhang/2017/China | 217 | 62.4 ± 8.03 | Male: 147 (67.7%), Female: 70 (32.3%) | NIHSS ≤5 | MRI | Assessment on admission and 30 days post, exact time NR |
| Zhang/2017/China | 229 | 66.6 ± 10.7 | Male: 123 (53.7%), Female: 106 (46.3%) | NIHSS ≤3 | MRI | < 3 days after onset, thyroid tested within 24 hours from admission, MRI within 7 days |
| Zhou/2017/China | 242 | 46.0 (43-48) | Male: 168 (69.4%), Female: 74 (30.6%) | NIHSS ≤8 | CT, MRI | < 3 days after onset, mRS assessment evaluated at 14 days post |
Note.
Age only reported by sex. 6MWT = 6-minute Walk Test; CNS = Canadian Neurological Scale; GCS = Glasgow Coma Scale; FMA = Fugl-Meyer Assessment; FMA-UE = Fugl-Meyer Assessment for upper extremity; MMSE = Mini-mental State Examination; MOCA = Montreal Cognitive Assessment; mRS = Modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale; NR = not reported; SIS = Stroke Impact Scale; SSS= Scandinavian Stroke Scale.
Stroke Severity Assessments used to Classify Mild Stroke
As noted in Table 2 and 3, the stroke severity assessments for mild stroke identified from the included studies were mostly the NIHSS (n=41, 66%),14, 16, 25-63 followed by the Modified Rankin Scale, mRS (n=9, 15%),24, 27, 31, 64-69 and the Canadian Neurological Scale (n=4, 7%).68-71 Two articles each (3%) used the Barthel Index, Fugl-Meyer Assessment,72, 73 Glasgow Coma Scale,29, 74 and Mini-Mental State Exam.66, 75 New Zealand TIA Stroke Guidelines76 was used one time (2%). The articles that classified mild stroke using the NIHSS had variable cut-off scores that ranged from 2 to 9. NIHSS score of ≤5 (including the Korean NIHSS<5) was used most often in 15 articles (37%).27, 29, 31-39, 48, 56, 58, 62 This was followed by a NIHSS score of ≤3 that was used in 11 articles (27%),25, 28, 41-44, 49, 50, 54, 60, 61 NIHSS ≤4 in 6 articles (15%),14, 16, 40, 47, 51, 59 NIHSS ≤6 was used in 3 articles, (7%)45, 52, 53 and NIHSS ≤8 in 2 articles (5%).46, 63 Other NIHSS scores that included ≤2,26 range 1-5.55, 57 One study used the NIHSS to distinguish minor stroke ≤3 from mild stroke (4-9),25 and one study did not report the NIHSS cutoff score.30
Table 3:
Mild Stroke Frequency
| Country | Frequency | Percentage |
|---|---|---|
| United States | 13 | 21.0 |
| China | 8 | 12.9 |
| United Kingdom | 7 | 11.3 |
| Canada | 5 | 8.1 |
| Korea | 4 | 6.5 |
| Sweden | 4 | 6.5 |
| France | 3 | 4.8 |
| Taiwan | 3 | 4.8 |
| Denmark | 2 | 3.2 |
| India | 2 | 3.2 |
| Israel | 2 | 3.2 |
| New Zealand | 2 | 3.2 |
| Australia | 1 | 1.6 |
| Bulgaria | 1 | 1.6 |
| Finland | 1 | 1.6 |
| Germany | 1 | 1.6 |
| Iran | 1 | 1.6 |
| Norway | 1 | 1.6 |
| Poland | 1 | 1.6 |
| Spain | 1 | 1.6 |
| Outcome Measures Used to Classify | Frequency | Percentage |
| NIHSS | 42 | 61.8 |
| NIHSS No Ranking | 1 | |
| NIHSS 1-5 | 2 | |
| NIHSS<2 | 1 | |
| NIHSS<3 | 11 | |
| NIHSS<4 | 4 | |
| NIHSS<5 | 14 | |
| NIHSS<5 Korean | 1 | |
| NIHSS<6 | 5 | |
| NIHSS<7 | 1 | |
| NIHSS<8 | 2 | |
| Modified Rankin Scale | 9 | 13.2 |
| Canadian Neurologic Scale | 4 | 5.9 |
| Barthel Index | 2 | 2.9 |
| Fugl Meyer Assessment | 2 | 2.9 |
| Scandinavian Stroke Scale | 2 | 2.9 |
| Glascow Coma Scale | 2 | 2.9 |
| Mini Mental State Exam | 2 | 2.9 |
| 6 Minute Walk Test and Stroke Impact Scale | 1 | 1.5 |
| New Zealand TIA Stroke Guidelines | 1 | 1.5 |
| No Response | 1 | 1.5 |
| Clinical Signs and Symptoms | Frequency | Percentage |
| MRI | 25 | 23.4 |
| Cognition | 19 | 17.8 |
| CT Scan | 18 | 16.8 |
| Motor Functioning | 13 | 12.1 |
| No Response | 5 | 4.7 |
| Depression | 3 | 2.8 |
| Blood Pressure | 2 | 1.9 |
| Doppler Ultrasound Exam | 2 | 1.9 |
| Participation | 2 | 1.9 |
| Blood Lipid Profile | 1 | 0.9 |
| Cerebral Spinal Fluid Analysis | 1 | 0.9 |
| Cholesterol Level | 1 | 0.9 |
| Communication | 1 | 0.9 |
| Comorbidities | 1 | 0.9 |
| Complications | 1 | 0.9 |
| Discharge Timeframe | 1 | 0.9 |
| PET Scan | 1 | 0.9 |
| Focal Cerebral Retinal Symptoms | 1 | 0.9 |
| Hyperglycemia | 1 | 0.9 |
| Mean Platelet Volume | 1 | 0.9 |
| Mood | 1 | 0.9 |
| Old Age | 1 | 0.9 |
| Post Mortem Exam | 1 | 0.9 |
| Prior Hospitalization | 1 | 0.9 |
| Sexual Functioning | 1 | 0.9 |
| Statin Use | 1 | 0.9 |
| Subtle Sequalae | 1 | 0.9 |
Imaging, Clinical Signs and Symptoms, and Medical Indicators to assess Mild Stroke
One-hundred and seven clinical signs and symptoms in mild stroke were identified in the 62 articles. Imaging findings were found in most studies (n=44, 41.1%), Table 3. Imaging tools included MRI (n=25, 40%),16, 25, 31-33, 40-42, 45, 46, 49-52, 55, 56, 61-64, 67, 74, 75, 77, 78 CT scan (n=18, 29%),25, 32, 33, 39, 41, 45, 46, 49-52, 55, 59, 63, 64, 67, 74, 78 and PET scan (n=1, 2%).77 Other clinical signs and symptoms used to identify mild stroke were: cognition (n=19, 17.8%)14, 27, 29, 36, 39, 44, 47, 50, 53, 54, 56-58, 66, 69, 73, 79-81 motor function (n=13, 21%),27, 29, 39, 44, 66, 68, 69, 71, 79-83 and medical indicators such as blood pressure, doppler ultrasound exam, blood lipid profile, cerebral spinal fluid analysis, cholesterol level, focal cerebral retinal symptoms, hyperglycemia, mean platelet volume, and statin use (n=10, 16%).24, 35, 51, 59, 60, 64, 65, 74, 76, 77 Other clinical signs and symptoms used less often to classify the individual with a mild stroke were: depression (n=3, 5%),30, 50, 53 and participation (n=2, 3 %).34, 69 The remaining clinical signs and symptoms to identify mild stroke included comorbidities, complications, old age, prior hospitalization,38 postmortem exam,74 subtle sequelae,72 sexual functioning,48 mood,69 communication.79 (n=1, 2%).
Variations in Stroke Severity Assessments Across Settings
Of the 36 studies conducted in acute care, a majority of studies (n=24, 67%) used the NIHSS scale,16, 25, 30-32, 37-42, 46, 47, 51-55, 57-59, 61-63 followed by Modified Rankin Scale, mRS (n=6, 17%),32, 64-66, 68 CNS (n=3, 8%).68, 70, 71 The MMSE,66 SSS,80 New Zealand’s stroke guidelines,76 and BI72 were each used in one study, and two studies did not report any stroke severity assessment scales.77, 79 Variability in the cut off scores of NIHSS was evident, that included: ≤5 (n=7, 19%),31, 32, 37-39, 58, 62 and 1-5,55, 58 followed by ≤325, 41, 42, 54, 61 ≤416, 40, 47, 51, 59 (n=5,21%), and ≤652, 53 and ≤846, 63 (n=3, 8%). One article classified stroke as both “minor” and “mild”,25 where minor was ≤3 and mild was between 4-9. Another article did not report the NIHSS ranking.30 The five studies that used mRS score to classify mild stroke had variations in their cut off scores, ≤2,32, 65, 68 1-3,64 ≤3,66 and ≤1.24 Most studies used imaging tools such as CT, MRI, and PET (n=17, 47%).16, 25, 31, 32, 40-42, 46, 51, 52, 55, 59, 61, 62, 64, 72, 77 followed by clinical signs and symptoms included cognition, motor function, depression, age, retinal symptoms, complications, and comorbidities, and sequelae (n=15, 42%),30, 38, 39, 47, 53, 54, 57, 58, 66, 68, 71, 72, 76, 79, 80 followed by medical indicators (n=6,17%) such as Doppler ultrasound,64, 77 mean platelet volume,65 cholesterol level,24 statin use,51 hyperglycemia.59 Two studies did not report imaging or clinical signs and symptoms.37, 70
Of the 12 studies conducted in the community, most studies used the NIHSS (n=8,67%),14, 27, 35, 36, 43, 44, 48, 56 followed by the mRS (n=2, 17%),27, 69 and MMSE, CNS, GCS, and SSS (n=1, 8.3%). The most frequent NIHSS cutoff scores for included ≤5, (n=5, 63%)27, 36, 48, 56 followed by ≤3 (n=2, 25%),43, 44 and ≤4 (n=1, 13%).14 Two studies used imaging tools such as CT and MRI,74, 75 and two studies used medical indicators such as blood pressure, lipid profile, cerebrospinal fluid analysis, and post mortem examination.35, 74 Most of the studies used clinical signs and symptoms (n=8, 67%) such as cognition, mood, motor function, sexual function, and participation.14, 27, 36, 44, 48, 56, 69, 81 One study did not report clinical signs and symptoms or imaging to diagnose mild stroke.43
Of the 13 studies that were conducted across multiple settings, most studies used the NIHSS (n=9, 69%),26, 28, 29, 33, 34, 45, 49, 50, 60 and one study each used the mRS,67 GCS,29 FMA of upper extremity.83 One study did not report the stroke severity assessments used to classify mild stroke.78 The most frequent NIHSS cutoff scores used was ≤3 (n=4, 44%),28, 49, 50, 60 followed by ≤5 (n=3, 33.3%),29, 33, 34 and one study (11%)used the score of ≤645 and ≤2.26 Six studies (46%) utilized imaging such as CT and MRI,33, 45, 49, 50, 67, 78 and one study used medical indicator e.g., blood pressure.60 Only five studies, 38% used clinical signs and symptoms including cognition, depression, motor function, and participation.29, 34, 50, 73, 83 Two studies did not report imaging or clinical signs and symptoms for mild stroke.26, 28
Variations in Stroke Severity Assessments Across Years
Most studies that provided assessments for mild stroke were conducted between 2013-2017, n=39 (63%), 15 studies (24%) were conducted between 2008-2012, and 8 studies (13%) were conducted between 2003-2007.
Between 2013-2017, most studies were used NIHSS (n=32, 82%); the cutoff scores ranging from <3 to <8,14, 16, 25, 26, 28-32, 34-40, 42, 46, 48-51, 53-55, 57-63 followed by mRS (n=5, 13%),24, 32, 65, 66, 68 MMSE (n=2, 5%),66, 75 CNS,68 FMA-UE,83 New Zealand’s stroke guidelines,76 GCS.29 16 studies assessed mild stroke from clinical signs and symptoms such as cognition, motor function, depression,14, 29, 30, 34, 36, 38, 48, 50, 53, 54, 57, 58, 66, 68, 76, 83 and imaging such as CT and MRI,16, 25, 31, 32, 40, 42, 46, 49-51, 55, 59, 61-63, 75 six studies medical indicators such as hyperglycemia, blood pressure, statin use, platelet volume.24, 35, 51, 59, 60, 65
Between 2008-2012, most studies used NIHSS, n=6, 40%; the cutoff scores ranging from <3 to <6,27, 43-45, 47, 56 followed by mRS,27, 69 CNS,69, 70 and SSS,80, 81 n=2, 13%, and one study used FMA,82 GCS.74 Three studies did not report use of any outcome measures.77-79 To assess mild stroke, most studies used clinical signs and symptoms such as cognition, motor function, participation, mood, and communication (n=9, 60%),27, 44, 47, 56, 69, 79-82 followed by imaging such as CT, MRI, and PET (n=4, 27%),45, 74, 77, 78 followed by medical indicators such as doppler ultrasound exam, CSF analysis, post mortem examination (n=2, 13%).74, 77 Two studies did not report imaging, clinical signs and symptoms, or medical indicators to assess mild stroke.43, 70
Between 2003-2007, 3 studies used NIHSS (38%); the cutoff scores ranging from <3 to <6,33, 41, 52 followed by mRS64, 67 and Barthel Index72, 73 (n=2, 25%), and CNS (n=1, 13%)71 These studies mainly used CT, MRI, and doppler ultrasound (n=5, 63%),33, 41, 52, 64, 67 followed by clinical signs and symptoms such as cognition, motor function, and sequalae (n=3,38%).71-73
Variations in Stroke Severity Characteristics Across Time Since Stroke
Time Since Stroke Onset Across Years
Many studies included the time since onset, (n=49, 79%). The studies that included time from onset of stroke in less than 30 days (n=28, 45%),25, 27-29, 31, 35, 40-43, 45-47, 49-52, 55, 56, 58, 60, 61, 63, 66, 70, 71, 76, 83 1-3 months (n=9 15 %),14, 30, 32, 36, 68, 74, 77, 80, 81 > 3 months-1 year (n=7, 11%),33, 34, 44, 48, 56, 72, 73 greater than one year (n=4, 6%).24, 65, 79, 82 One-fourth of the studies did not report time since stroke (n=14, 23%).16, 25, 37-39, 53, 54, 59, 62, 64, 65, 69, 75, 78
The majority of the studies that reported onset of stroke in less than 30 days occurred in the recent time period from 2013-2017 (n=20, 32%),25, 28, 29, 31, 35, 37, 40, 42, 46, 49-51, 55, 58, 60, 62, 63, 66, 76, 83 five studies reported time from onset in less than 30 days from 2008-2012 (n=5, 8%),27, 45, 47, 56, 70 and three studies were from 2003-2007 (n=3, 5%).41, 52, 71 The nine studies with reported time from stroke onset from 1-3 months in 2008-2012 (n=4, 6%)74, 77, 80, 81 and 2013-2017 (n=5, 8 %)14, 30, 32, 36, 68 The seven studies that reported time from onset of >3 months-1 year were primarily in 2003-2007 (n=4, 6%)33, 67, 72, 73 2008-2012 (n=2, 3%)40,75 and 2013-2017 (n=1, <1%).48 Finally, the four studies that indicated stroke onset of greater than one year were 2003-2007 (n=1 2% ),67 2008-2012 (n=2, 3%)79, 82, and 2013-2017 (n=1, 2%).24
Time Since Stroke Onset and Stroke Severity
In the studies that used less than 30 days since time of stroke onset, (n=21, 34%) used the NIHSS scale to determine stroke severity,25, 28, 31, 35, 40-43, 45-47, 49, 51-53, 55, 56, 58, 60, 61, 63 CNS (n=2, 3%),70, 71 and MMSE and mRS (n=1, 2%),66 mRS and NIHSS (n=1, 2%),27 New Zealand’s TIA/stroke guidelines (n=1, 2%),76 FMA-UE (n=1, 2%),83 Glascow Coma Scale (GCS) and NIHSS (n=1, 2%).29 In the 1-3 month range from time since stroke onset, NIHSS only was (n=3, 5%),14, 30, 36 NIHSS and mRS (n=1, 2%),32 SSS (n=2, 3%),80, 81 GCS (n=1, 2%),74 CNS with mRS (n=1, 2%),68 and 1 study did not document the use of stroke severity scale but used of imaging (n=1, 2%).77 Seven studies were in the timeframe of > 3months -1 year since time of stroke onset. Of these, NIHSS was used (n=5, 8%),33, 34, 44, 48, 57 and Barthel Index (n=2, 3%).72, 73 None of the studies that were greater than one year post-stroke onset used the NIHSS, mRS (n=2, 3%),24, 67 FMA (n=1, 2%),82 and one study did not report using a stroke severity scale for determination but used clinical signs and symptoms (n=1, 2%).79 Stroke severity and no response for stroke time of onset was noted in 13 studies. These studies used NIHSS (n=8, 13%),16, 26, 37, 38, 53, 54, 59, 61 mRS (n=2, 3%)64, 65 Korean NIHSS (n=1, 2%),39 CNS and mRS (n=1, 2%),69 and MMSE (n=1, 2%).75
Time Since Onset and Imaging, Medical Indicators, and Clinical Signs and Symptoms
Variations in imaging used to make the diagnosis of mild stroke, studies with onset of stroke in less than 30 days included CT/MRI (n=9;15%),25, 40, 41, 45, 46, 49, 52, 55, 63 3 used MRI alone (n=3,5%),31, 42, 62 1 study used (n=1,2%)50 MRI,CT in combination with cognition and depression, and one study ( n=1, 2)51 look at CT, MRI and the use of statins. Three (n=3, 5%)47, 56, 58 studies used cognition as their assessment to categorize the patients. The other twelve studies that were reviewed with stroke onset in less than 30 days used various clinical and lab work combinations as follows: three ( n=3, 5%)27, 29, 66 motor function and cognition assessment, two (n=2,3%%)71, 83 motor function alone, one (n=1,2%)76 focal cerebral retinal symptoms , one (n=1, 2%)35 blood pressure and blood lipid profile; one ( n=1, 2%)60 used only blood pressure and three (n=3, 5%)28, 43, 70 studies did not identify use of imaging or how the severity of stroke was determined. For studies with onset between 1-3 months, determining severity of stroke was completed by: 1 study (n=1,2%)32 CT/MRI, one study (n=1,2%)77 used the combination of PET, MRI and doppler ultrasound, one n=1,2%)74 CT, MRI, cerebrospinal fluid analysis and post mortem examination , two studies (n=2,3%)14, 36 used cognition , one study ( n=1,2%)81 used a combination of cognition and motor function, one study ( n=1, 2%)30 utilized assessment of depression, and one ( n=1, 2%)68 used motor function alone to determine the level of stroke severity. Studies utilizing those with stroke between 3 months to 1 year only one study (n=1, 2%)33 used imaging of CT/MRI. The remaining seven studies used the following to establish stroke severity instead of imaging: three ( n=3, 5%)54, 57, 73 cognition, one ( n=1, 2%)72 subtle sequelae, one (n=1,2%)34 participation, one ( n=1,2%)44 cognition and motor function, and finally one ( n=1,2%)48 utilized sexual functioning. For studies where participants were greater than one year post stroke one study ( n=1, 2%)67 CT/MRI to determine stroke severity, one ( n=1,2%)82 used motor function, one ( n=1, 2%)24 used cholesterol level, and one (n=1,2%)79 used a combination of cognition, communication and motor function to assist with determining stroke severity.
Discussion
This scoping review identified 62 studies meeting our inclusion criteria that used a definition to classify mild stroke. Varying nomenclature was used across the studies to describe mild stroke, and there was no consistent approach for classifying mild stroke using stroke severity assessments or clinical criteria. Adding to the complexity of a concise classification system were the range of contextual factors, which may have a substantial influence on terminology and classification criteria including the timing when stroke severity was assessed across the care continuum and the country in which the study was conducted.
Developing a consistent definition of mild stroke into interdisciplinary health care practice is critical for several reasons. First, a coherent definition will facilitate an appropriate and consistent identification of individuals with mild stroke. Specifically, practitioners using consistent assessments and clinical criteria for screening stroke will be more likely to differentiate varied levels of stroke severity with an accurate identification of individuals with mild stroke. This accurate identification may increase the likelihood of individuals with mild stroke being referred to services to support their ongoing rehabilitation needs. The development of a standardized definition of mild stroke at different time points along the trajectory of stroke recovery may also have an impact in addressing the needs of individuals with mild stroke. Practitioners such as physiatrists and rehabilitation professionals who understand the breadth and depth of challenges, including functional barriers among individuals with mild stroke, may be better prepared to refer these individuals to appropriate health care and community-based services to support their long-term health and functional outcomes.
To summarize the themes in the mild stroke scoping review, many of the research articles were conducted in the United States (21%), occurred in an acute care hospital (58%) and included persons with stroke of varying ages, with 60-69 being the most common. Stroke severity assessment was determined frequently by the NIHSS (61.8%), clinical signs and symptoms primarily identified imaging (41.1%), 45% had time since onset of stroke in less than 30 days, and most studies with assessment of mild stroke were completed between 2013-2017. Synthesis also revealed variations in stroke severity across years, time since stroke, imaging, medical indicators, clinical signs and symptoms and settings. These themes suggest variability in the definitions of mild stroke.
Of note, this study discovered ten unique definitions of mild stroke using stroke severity assessments that included ten different cut-off points within the most widely used measure to classify stroke severity – the NIHSS. Several clinical signs and symptoms were reported to classify a stroke as mild. The wide degree of variability and lack of a consensus definition undoubtedly contributes to inconsistent treatments and referrals.84 Furthermore, the measures used in the literature span several domains of the International Classification of Functioning, Disability and Health (ICF).85 The ICF is an internationally accepted framework that provides a common language for conceptualizing interactions between functioning, activities, participation, and contextual influences including personal and environmental factors. Working within a framework such as the ICF is a useful approach when examining assessments for individuals with disability85 and may be particularly useful for examining stroke severity assessments in individuals’ post-stroke.86
The NIHSS is the most widely accepted tool for measuring stroke severity in the literature.15 Despite being well-accepted, the inconsistent cut-offs used to determine stroke severity contribute to misclassification which in turn may lead to variations in administering interventions and referral to health care services. The NIHSS is meant to measure stroke-specific neurological deficit and was initially developed as a research tool to measure baseline data in acute stroke clinical trials.87 Although the NIHSS is valid and reliable, it maintains a focus on body functions and structures and may be not appropriate for measuring activity and participation that are important domains for measuring severity beyond acute care. Integrating measures that represent other ICF domains may be complementary to the focus on specific neurological impairments when classifying individuals with mild stroke. Many individual items of the NIHSS have poor reliability (i.e., loss of consciousness, gaze, facial palsy, ataxia, and dysarthria).26 Using different definitions of mild/minor stroke using NIHSS, patients’ outcomes varied from favorable to less favorable.
The modified Rankin Scale (mRS) was the second most frequent stroke severity assessments used in the literature17, 88 to classify individuals with mild stroke. The mRS is a valid and reliable tool that measures level of disability from the activity domain using a 0-6 scale, which spans from no symptoms (0) to dead (6). The mRS is one of the stroke reporting measures to assess 90-day functional outcomes suggested by the American Heart Association’s Get with the Guidelines.89 Along with measuring functional outcomes, it is important to include measures of cognition, along with activity and participation that will capture an accurate depiction of post-stroke disability among individuals with mild stroke.
The authors of this study did not locate articles meeting full inclusion for analyses that used subjective perspectives of mild stroke. Although individuals with mild stroke may not always recognize the breadth or depth of their post-stroke disability,84 integrating perspectives of individuals with mild stroke on their rehabilitation needs may be critical, particularly after acute hospitalization. Additionally, caregivers may have a unique and critical perspective of mild stroke severity.86 Future research is necessary for understanding the post-acute experience among individuals with mild stroke and their caregivers. Incorporating these perspectives in a future gold-standard classification will be necessary for a comprehensive classification of mild stroke. Some studies used TIA and mild/minor stroke interchangeably. We included studies since 2003, which overlaps when the definition for TIA was changed by the American Heart Association and the American Stroke Association in 2009 to brain injury “without acute infarction.”90 Therefore, there is a likelihood that we excluded some studies that classified patients with TIA before 2009 that might be mild/minor strokes. Based on the 62 identified articles, we are able to provide a summary of mild stroke definitions using stroke severity assessments and clinical signs or symptoms, which provides the initial steps towards the development of an international consensus classification of mild stroke. Appropriately classifying individuals with mild stroke has significant implications for health care services delivery. Since mild stroke is often viewed as having “non-disabling or rapidly improving symptoms, many do not receive medical treatment, such as tPA, experience delay of imaging.4, 91 In addition, many do not receive additional health services following discharge, despite having impending disability.92 Studies have indicated that individuals with mild stroke and their caregivers feel ill-equipped following discharge home1 and have unmet service needs.69 Ultimately, it is important to address the disparities in care receipt of individuals with mild stroke to improve service delivery and post-discharge outcomes.
Limitations
We only included studies that were published in English; hence the results of this study must be interpreted cautiously. Additionally, the most frequent limitation in scoping reviews is the possibility that the review may have missed some relevant studies. This limitation may be attributed to database selection, exclusion of literature based on search terms and search time constraints.
Conclusions
Inconsistencies in the classification of mild stroke are evident in the literature. Studies vary on the use of stroke severity assessments, cut-off scores, imaging tools, and clinical outcomes to classify mild stroke. A lack of an international consensus definition of mild stroke has a direct impact on treatment received, referral for services, and health service delivery. Future work must include a Delphi study with neurologists, physiatrists, rehabilitation professionals, and other members of the stroke care team to develop an international consensus definition of mild stroke. A consensus definition will support standardized terminology, improvement in diagnosis, administration of medical interventions, appropriate referral for services, and assessment of outcomes across the care continuum – from hospital to the community – among individuals with mild stroke.
Supplementary Material
Acknowledgements:
Monique Pappadis work on this study was supported by the National Institute on Aging, Grant Numbers P30AG024832 and P30AG059301, The National Institute on Disability, Independent Living, and Rehabilitation Research, Grant Number 90DP0028, and the Agency for Healthcare Research and Quality, Grant Number R24HS022134. All other authors do not have any conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carlsson GE, Moller A, Blomstrand C. Managing an everyday life of uncertainty--a qualitative study of coping in persons with mild stroke. Disabil Rehabil. 2009;31(10):773–782. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JD, Kapral MK, Fang J, Swartz RH. Long-term morbidity and mortality in patients without early complications after stroke or transient ischemic attack. Cmaj. July 24 2017;189(29):E954–e961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Roman L, Serena J, Abilleira S, Ribo M, Millan M, Urra X, Cardona P, Lopez-Cancio E, Tomasello A, Castano C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Perez M, Goyal M, Demchuk AM, von Kummer R, Gallofre M, Davalos A. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. June 11 2015;372(24) :2296–2306. [DOI] [PubMed] [Google Scholar]
- 4.Messe SR, Khatri P, Reeves MJ, Smith EE, Saver JL, Bhatt DL, Grau-Sepulveda MV, Cox M, Peterson ED, Fonarow GC, Schwamm LH. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. October 11 2016;87(15):1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao Z, Liu M, Wang D, Wu B, Tao W, Chang X. Etiologic subtype predicts outcome in mild stroke: prospective data from a hospital stroke registry. BMC Neurol. October 24 2013;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith EE, Fonarow GC, Reeves MJ, Cox M, Olson DM, Hernandez AF, Schwamm LH. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from Get With The Guidelines-Stroke. Stroke. November 2011;42(11):3110–3115. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher HC, Bateman BT, Boden-Albala B, Berman MF, Mohr JP, Sacco RL, Pile-Spellman J. Use of thrombolysis in acute ischemic stroke: analysis of the Nationwide Inpatient Sample 1999 to 2004. Ann Emerg Med. August 2007;50(2):99–107. [DOI] [PubMed] [Google Scholar]
- 8.Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke. November 2005;36(11):2497–2499. [DOI] [PubMed] [Google Scholar]
- 9.Chen T, Zhang B, Deng Y, Fan JC, Zhang L, Song F. Long-term unmet needs after stroke: systematic review of evidence from survey studies. BMJ Open. May 19 2019;9(5):e028137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. June 2016;47(6):e98–e169. [DOI] [PubMed] [Google Scholar]
- 11.Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, Billinger SA. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke. October 2010;41(10):2402–2448. [DOI] [PubMed] [Google Scholar]
- 12.Cormier DJ, Frantz MA, Rand E, Stein J. Physiatrist referral preferences for postacute stroke rehabilitation. Medicine (Baltimore). August 2016;95(33):e4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faux SG, Arora P, Shiner CT, Thompson-Butel AG, Klein LA. Rehabilitation and education are underutilized for mild stroke and TIA sufferers. Disabil Rehabil. June 2018;40(12):1480–1484. [DOI] [PubMed] [Google Scholar]
- 14.Adamit T, Maeir A, Ben Assayag E, Bornstein NM, Korczyn AD, Katz N. Impact of first-ever mild stroke on participation at 3 and 6 month post-event: the TABASCO study. Disabil Rehabil. 2015;37(8):667–673. [DOI] [PubMed] [Google Scholar]
- 15.Brott T, Adams HP Jr., Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. July 1989;20(7):864–870. [DOI] [PubMed] [Google Scholar]
- 16.Chappell FM, Del Carmen Valdes Hernandez M, Makin SD, Shuler K, Sakka E, Dennis MS, Armitage PA, Munoz Maniega S, Wardlaw JM. Sample size considerations for trials using cerebral white matter hyperintensity progression as an intermediate outcome at 1 year after mild stroke: results of a prospective cohort study. Trials. February 21 2017;18(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. May 1988;19(5):604–607. [DOI] [PubMed] [Google Scholar]
- 18.Hand B, Page SJ, White S. Stroke Survivors Scoring Zero on the NIH Stroke Scale Score Still Exhibit Significant Motor Impairment and Functional Limitation. Stroke research and treatment. 2014;2014:462681–462681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colquhoun HL, Levac D, O'Brien KK, Straus S, Tricco AC, Perrier L, Kastner M, Moher D. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. December 2014;67(12):1291–1294. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. August 18 2009;151(4):W65–94. [DOI] [PubMed] [Google Scholar]
- 21.Gorelick PB. Primary and comprehensive stroke centers: history, value and certification criteria. J Stroke. May 2013;15(2):78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedder W. National and international quality initiatives to improve stroke care. Neurol Clin. November 2008;26(4):1191–1207, xi. [DOI] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. March 1977;33(1):159–174. [PubMed] [Google Scholar]
- 24.Rist PM, Buring JE, Kase CS, Ridker PM, Kurth T. Biomarkers and functional outcomes from ischaemic cerebral events in women: a prospective cohort study. Eur J Neurol. February 2013;20(2):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bejot Y, Guilloteau A, Joux J, Lannuzel A, Mimeau E, Mislin-Tritsch C, Fournel I, Bonithon-Kopp C. Social deprivation and stroke severity on admission: a French cohort study in Burgundy and the West Indies - Guyana region. Eur J Neurol. May 2017;24(5):694–702. [DOI] [PubMed] [Google Scholar]
- 26.Bejot Y, Mehta Z, Giroud M, Rothwell PM. Impact of completeness of ascertainment of minor stroke on stroke incidence: implications for ideal study methods. Stroke. July 2013;44(7):1796–1802. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharjee M, Vairale J, Gawali K, Dalal PM. Factors affecting burden on caregivers of stroke survivors: Population-based study in Mumbai (India). Ann Indian Acad Neurol. April 2012;15(2): 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulos MI, Murray BJ, Muir RT, Gao F, Szilagyi GM, Huroy M, Kiss A, Walters AS, Black SE, Lim AS, Swartz RH. Periodic Limb Movements and White Matter Hyperintensities in First-Ever Minor Stroke or High-Risk Transient Ischemic Attack. Sleep. March 1 2017;40(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang WH, Sohn MK, Lee J, Kim DY, Lee SG, Shin YI, Oh GJ, Lee YS, Joo MC, Han EY, Kim MS, Jang SY, Kim JH, Kim YH. Long-term functional outcomes of patients with very mild stroke: does a NIHSS score of 0 mean no disability? An interim analysis of the KOSCO study. Disabil Rehabil. May 2017;39(9):904–910. [DOI] [PubMed] [Google Scholar]
- 30.Dabrowska-Bender M, Milewska M, Golabek A, Duda-Zalewska A, Staniszewska A. The Impact of Ischemic Cerebral Stroke on the Quality of Life of Patients Based on Clinical, Social, and Psychoemotional Factors. J Stroke Cerebrovasc Dis. January 2017;26(1):101–107. [DOI] [PubMed] [Google Scholar]
- 31.Daniels SK, Pathak S, Mukhi SV, Stach CB, Morgan RO, Anderson JA. The Relationship Between Lesion Localization and Dysphagia in Acute Stroke. Dysphagia. December 2017;32(6):777–784. [DOI] [PubMed] [Google Scholar]
- 32.Divya KP, Menon RN, Varma RP, Sylaja PN, Thomas B, Kesavadas C, Sunitha J, Lekha VS, Deepak S. Post-stroke cognitive impairment - A cross-sectional comparison study between mild cognitive impairment of vascular and non-vascular etiology. J Neurol Sci. January 15 2017;372:356–362. [DOI] [PubMed] [Google Scholar]
- 33.Edwards DF, Hahn M, Baum C, Dromerick AW. The impact of mild stroke on meaningful activity and life satisfaction. J Stroke Cerebrovasc Dis. Jul-Aug 2006;15(4):151–157. [DOI] [PubMed] [Google Scholar]
- 34.Eriksson G, Carolyn Baum M, Wolf TJ, Connor LT. Perceived participation after stroke: the influence of activity retention, reintegration, and perceived recovery. Am J Occup Ther. Nov-Dec 2013;67(6):e131–138. [DOI] [PubMed] [Google Scholar]
- 35.Faulkner J, Stoner L, Lanford J, Jolliffe E, Mitchelmore A, Lambrick D. Long-Term Effect of Participation in an Early Exercise and Education Program on Clinical Outcomes and Cost Implications, in Patients with TIA and Minor, Non-Disabling Stroke. Transl Stroke Res. June 2017;8(3):220–227. [DOI] [PubMed] [Google Scholar]
- 36.Fride Y, Adamit T, Maeir A, Ben Assayag E, Bornstein NM, Korczyn AD, Katz N. What are the correlates of cognition and participation to return to work after first ever mild stroke? Top Stroke Rehabil. October 2015;22(5):317–325. [DOI] [PubMed] [Google Scholar]
- 37.Gadodia G, Rizk N, Camp D, Bryant K, Zimmerman S, Brasher C, Connelly K, Dunn J, Frankel M, Ido MS, Lugtu J, Nahab F. Presenting Symptoms and Dysphagia Screen Predict Outcome in Mild and Rapidly Improving Acute Ischemic Stroke Patients. J Stroke Cerebrovasc Dis. December 2016;25(12):2876–2881. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh CY, Lin HJ, Hu YH, Sung SF. Stroke severity may predict causes of readmission within one year in patients with first ischemic stroke event. J Neurol Sci. January 15 2017;372:21–27. [DOI] [PubMed] [Google Scholar]
- 39.Joa KL, Kim WH, Choi HY, Park CH, Kim ES, Lee SJ, Kim SY, Ko SH, Jung HY. The Effect of Sleep Disturbances on the Functional Recovery of Rehabilitation Inpatients Following Mild and Moderate Stroke. Am J Phys Med Rehabil. October 2017;96(10):734–740. [DOI] [PubMed] [Google Scholar]
- 40.Jung JM, Choi J, Eun MY, Seo WK, Cho KH, Yu S, Oh K, Hong S, Park KY. Prestroke antiplatelet agents in first-ever ischemic stroke: clinical effects. Neurology. March 17 2015;84(11):1080–1089. [DOI] [PubMed] [Google Scholar]
- 41.Lin HJ, Yeh PS, Tsai TC, Cheng TJ, Ke D, Lin KC, Ho JG, Chang CY. Differential risks of subsequent vascular events for transient ischaemic attack and minor ischaemic stroke. J Clin Neurosci. January 2007;14(1):17–21. [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Sun W, Scalzo F, Xiong Y, Zhang X, Qiu Z, Zhu W, Ma M, Liu W, Xu G, Lu G, Liebeskind DS, Liu X. Early Magnetic Resonance Imaging Predicts Early Neurological Deterioration in Acute Middle Cerebral Artery Minor Stroke. J Stroke Cerebrovasc Dis. February 2016;25(2):469–474. [DOI] [PubMed] [Google Scholar]
- 43.Luengo-Fernandez R, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol. March 2009;8(3):235–243. [DOI] [PubMed] [Google Scholar]
- 44.O'Brien AN, Wolf TJ. Determining work outcomes in mild to moderate stroke survivors. Work. 2010;36(4):441–447. [DOI] [PubMed] [Google Scholar]
- 45.Ois A, Cuadrado-Godia E, Rodriguez-Campello A, Jimenez-Conde J, Roquer J. High risk of early neurological recurrence in symptomatic carotid stenosis. Stroke. August 2009;40(8):2727–2731. [DOI] [PubMed] [Google Scholar]
- 46.Pfaff J, Herweh C, Pham M, Schonenberger S, Nagel S, Ringleb PA, Bendszus M, Mohlenbruch M. Mechanical Thrombectomy in Patients with Acute Ischemic Stroke and Lower NIHSS Scores: Recanalization Rates, Periprocedural Complications, and Clinical Outcome. AJNR Am J Neuroradiol. November 2016;37(11):2066–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruuskanen EI, Laihosalo M, Kettunen J, Losoi H, Nurmi L, Koivisto AM, Dastidar P, Ollikainen J, Jehkonen M. Predictors of discharge to home after thrombolytic treatment in right hemisphere infarct patients. J Cent Nerv Syst Dis. 2010;2:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seymour LM, Wolf TJ. Participation changes in sexual functioning after mild stroke. OTJR (Thorofare N J). Spring 2014;34(2):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi Y, Xiang Y, Yang Y, Zhang N, Wang S, Ungvari GS, Chiu HF, Tang WK, Wang Y, Zhao X, Wang Y, Wang C. Depression after minor stroke: Prevalence and predictors. J Psychosom Res. August 2015;79(2):143–147. [DOI] [PubMed] [Google Scholar]
- 50.Shi YZ, Xiang YT, Yang Y, Zhang N, Wang S, Ungvari GS, Chiu HF, Tang WK, Wang YL, Zhao XQ, Wang YJ, Wang CX. Depression after minor stroke: the association with disability and quality of life--a 1-year follow-up study. Int J Geriatr Psychiatry. April 2016;31(4):421–427. [DOI] [PubMed] [Google Scholar]
- 51.Song B, Wang Y, Zhao X, Liu L, Wang C, Wang A, Du W, Wang Y. Association between statin use and short-term outcome based on severity of ischemic stroke: a cohort study. PLoS One. 2014;9(1):e84389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tseng MC, Chang KC. Stroke severity and early recovery after first-ever ischemic stroke: results of a hospital-based study in Taiwan. Health Policy. November 2006;79(1):73–78. [DOI] [PubMed] [Google Scholar]
- 53.Villain M, Sibon I, Renou P, Poli M, Swendsen J. Very early social support following mild stroke is associated with emotional and behavioral outcomes three months later. Clin Rehabil. January 2017;31(1):135–141. [DOI] [PubMed] [Google Scholar]
- 54.Volonghi I, Pendlebury ST, Welch SJ, Mehta Z, Rothwell PM. Cognitive outcomes after acute coronary syndrome: a population based comparison with transient ischaemic attack and minor stroke. Heart. October 2013;99(20):1509–1514. [DOI] [PubMed] [Google Scholar]
- 55.Ward K, Rao P, Reilly CC, Rafferty GF, Polkey MI, Kalra L, Moxham J. Poor cough flow in acute stroke patients is associated with reduced functional residual capacity and low cough inspired volume. BMJ Open Respir Res. 2017;4(1):e000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf TJ, Barbee AR, White D. Executive dysfunction immediately after mild stroke. OTJR (Thorofare N J). Winter 2011;31(1):S23–29. [DOI] [PubMed] [Google Scholar]
- 57.Wolf TJ, Dahl A, Auen C, Doherty M. The reliability and validity of the Complex Task Performance Assessment: A performance-based assessment of executive function. Neuropsychol Rehabil. July 2017;27(5):707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf TJ, Rognstad MC. Changes in cognition following mild stroke. Neuropsychol Rehabil. 2013;23(2):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue WY, Xu YC, Wu YW, Yang M. Observation of elevated fasting blood glucose and functional outcome after ischemic stroke in patients with and without diabetes. Oncotarget. September 15 2017;8(40):67980–67989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Cadilhac DA, Churilov L, Donnan GA, O'Callaghan C, Dewey HM. Does abnormal circadian blood pressure pattern really matter in patients with transient ischemic attack or minor stroke? Stroke. March 2014;45(3):865–867. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Xie Y, Ding C, Xiao J, Tang Y, Jiang X, Shan H, Lin Y, Zhu Y, Li C, Hu D, Ling Z, Xu G, Sheng L. Subclinical hypothyroidism and risk of cerebral small vessel disease: A hospital-based observational study. Clin Endocrinol (Oxf). November 2017;87(5):581–586. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Z, Ren W, Shao B, Xu H, Cheng J, Wang Q, Gu Y, Zhu B, He J. Leukoaraiosis is Associated with Worse Short-Term Functional and Cognitive Recovery after Minor Stroke. Neurol Med Chir (Tokyo). March 15 2017;57(3):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou X, Yu F, Feng X, Wang J, Li Z, Zhan Q, Xia J. Immunity and inflammation predictors for short-term outcome of stroke in young adults. Int J Neurosci. July 2018;128(7):634–639. [DOI] [PubMed] [Google Scholar]
- 64.Atanassova PA, Semerdjieva MA, Naydenov VI, Dzhurkova AG, Traykova NI, Chalakova NT. Transient ischaemic attacks and minor strokes (analyses and clinically significant comparisons). Folia Med (Plovdiv). 2006;48(2):30–36. [PubMed] [Google Scholar]
- 65.Ghahremanfard F, Asghari N, Ghorbani R, Samaei A, Ghomi H, Tamadon M. The relationship between mean platelet volume and severity of acute ischemic brain stroke. Neurosciences (Riyadh). April 2013;18(2):147–151. [PubMed] [Google Scholar]
- 66.Kim J, Kim Y, Yang KI, Kim DE, Kim SA. The Relationship Between Sleep Disturbance and Functional Status in Mild Stroke Patients. Ann Rehabil Med. August 2015;39(4):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Novak V, Yang AC, Lepicovsky L, Goldberger AL, Lipsitz LA, Peng CK. Multimodal pressure-flow method to assess dynamics of cerebral autoregulation in stroke and hypertension. Biomed Eng Online. October 25 2004;3(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rozon J, Rochette A. Changes in life habits affected by mild stroke and their association with depressive symptoms. J Rehabil Med. June 2015;47(6):495–501. [DOI] [PubMed] [Google Scholar]
- 69.Tellier M, Rochette A, Lefebvre H. Impact of mild stroke on the quality of life of spouses. Int J Rehabil Res. September 2011;34(3):209–214. [DOI] [PubMed] [Google Scholar]
- 70.Fang J, Saposnik G, Silver FL, Kapral MK. Association between weekend hospital presentation and stroke fatality. Neurology. November 2 2010;75(18):1589–1596. [DOI] [PubMed] [Google Scholar]
- 71.Rochette A, Desrosiers J, Bravo G, St-Cyr-Tribble D, Bourget A. Changes in participation after a mild stroke: quantitative and qualitative perspectives. Top Stroke Rehabil. May-Jun 2007;14(3):59–68. [DOI] [PubMed] [Google Scholar]
- 72.Carlsson GE, Moller A, Blomstrand C. Consequences of mild stroke in persons <75 years -- a 1-year follow-up. Cerebrovasc Dis. 2003;16(4):383–388. [DOI] [PubMed] [Google Scholar]
- 73.Carlsson GE, Moller A, Blomstrand C. A qualitative study of the consequences of 'hidden dysfunctions' one year after a mild stroke in persons <75 years. Disabil Rehabil. December 2 2004;26(23):1373–1380. [DOI] [PubMed] [Google Scholar]
- 74.Sarker SJ, Heuschmann PU, Burger I, Wolfe CD, Rudd AG, Smeeton NC, Toschke AM. Predictors of survival after haemorrhagic stroke in a multi-ethnic population: the South London Stroke Register (SLSR). J Neurol Neurosurg Psychiatry. March 2008;79(3):260–265. [DOI] [PubMed] [Google Scholar]
- 75.Valdes Hernandez Mdel C, Armitage PA, Thrippleton MJ, Chappell F, Sandeman E, Munoz Maniega S, Shuler K, Wardlaw JM. Rationale, design and methodology of the image analysis protocol for studies of patients with cerebral small vessel disease and mild stroke. Brain Behav. December 2015;5(12):e00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faulkner J, McGonigal G, Woolley B, Stoner L, Wong L, Lambrick D. A randomized controlled trial to assess the psychosocial effects of early exercise engagement in patients diagnosed with transient ischaemic attack and mild, non-disabling stroke. Clin Rehabil. August 2015;29(8):783–794. [DOI] [PubMed] [Google Scholar]
- 77.Moustafa RR, Izquierdo-Garcia D, Fryer TD, Graves MJ, Rudd JH, Gillard JH, Weissberg PL, Baron JC, Warburton EA. Carotid plaque inflammation is associated with cerebral microembolism in patients with recent transient ischemic attack or stroke: a pilot study. Circ Cardiovasc Imaging. September 2010;3(5):536–541. [DOI] [PubMed] [Google Scholar]
- 78.Torres-Mozqueda F, He J, Yeh IB, Schwamm LH, Lev MH, Schaefer PW, Gonzalez RG. An acute ischemic stroke classification instrument that includes CT or MR angiography: the Boston Acute Stroke Imaging Scale. AJNR Am J Neuroradiol. June 2008;29(6):1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muren MA, Hutler M, Hooper J. Functional capacity and health-related quality of life in individuals post stroke. Top Stroke Rehabil. Jan-Feb 2008;15(1):51–58. [DOI] [PubMed] [Google Scholar]
- 80.Muus I, Christensen D, Petzold M, Harder I, Johnsen SP, Kirkevold M, Ringsberg KC. Responsiveness and sensitivity of the Stroke Specific Quality of Life Scale Danish version. Disabil Rehabil. 2011;33(25-26):2425–2433. [DOI] [PubMed] [Google Scholar]
- 81.Muus I, Petzold M, Ringsberg KC. Health-related quality of life among Danish patients 3 and 12 months after TIA or mild stroke. Scand J Caring Sci. June 2010;24(2):211–218. [DOI] [PubMed] [Google Scholar]
- 82.Alt Murphy M, Willen C, Sunnerhagen KS. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil Neural Repair. January 2011;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 83.Bustren EL, Sunnerhagen KS, Alt Murphy M. Movement Kinematics of the Ipsilesional Upper Extremity in Persons With Moderate or Mild Stroke. Neurorehabil Neural Repair. April 2017;31(4):376–386. [DOI] [PubMed] [Google Scholar]
- 84.Schwartz JK, Capo-Lugo CE, Akinwuntan AE, Roberts P, Krishnan S, Belagaje SR, Kovic M, Burns SP, Hu X, Danzl M, Devos H, Page SJ. Classification of Mild Stroke: A Mapping Review. Pm r. February 11 2019. [DOI] [PubMed] [Google Scholar]
- 85.de Kleijn-de Vrankrijker MW. The long way from the International Classification of Impairments, Disabilities and Handicaps (ICIDH) to the International Classification of Functioning, Disability and Health (ICF). Disabil Rehabil. June 3-17 2003;25(11-12):561–564. [DOI] [PubMed] [Google Scholar]
- 86.Krishnan S, Pappadis MR, Weller SC, Stearnes M, Kumar A, Ottenbacher KJ, Reistetter TA. Needs of Stroke Survivors as Perceived by Their Caregivers: A Scoping Review. Am J Phys Med Rehabil. July 2017;96(7):487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, Zivin J. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke. November 1999;30(11):2347–2354. [DOI] [PubMed] [Google Scholar]
- 88.Rankin J Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. May 1957;2(5):200–215. [DOI] [PubMed] [Google Scholar]
- 89.Howard G, Schwamm LH, Donnelly JP, Howard VJ, Jasne A, Smith EE, Rhodes JD, Kissela BM, Fonarow GC, Kleindorfer DO, Albright KC. Participation in Get With The Guidelines-Stroke and Its Association With Quality of Care for Stroke. JAMA Neurol. November 1 2018;75(11):1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. June 2009;40(6):2276–2293. [DOI] [PubMed] [Google Scholar]
- 91.Kwei KT, Liang J, Wilson N, Tuhrim S, Dhamoon M. Stroke Severity Affects Timing: Time From Stroke Code Activation to Initial Imaging is Longer in Patients With Milder Strokes. Neurologist. May 2018;23(3):79–82. [DOI] [PubMed] [Google Scholar]
- 92.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. July 24 2012;79(4):306–313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.