Abstract
Leukocyte differentials are a useful tool for assessing systemic immunological changes during pathogen infections, particularly for non-model species. To date, no study has explored how experimental infection with a common bacterial pathogen, Mycoplasma gallisepticum (MG), influences the course and strength of hematological changes in the natural songbird host, house finches. Here we experimentally inoculated house finches with MG isolates known to vary in virulence and quantified the proportions of circulating leukocytes over the entirety of infection. First, we found significant temporal effects of MG infection on the proportions of most cell types, with strong increases in heterophil and monocyte proportions during infection. Marked decreases in lymphocyte proportions also occurred during infection, though these proportional changes may simply be driven by correlated increases in other leukocytes. Second, we found significant effects of isolate virulence, with the strongest changes in cell proportions occurring in birds inoculated with the higher virulence isolates, and almost no detectable changes relative to sham treatment groups in birds inoculated with the lowest virulence isolate. Finally, we found that variation in infection severity positively predicted the proportion of circulating heterophils and lymphocytes, but the strength of these correlations was dependent on isolate. Taken together, these results indicate strong hematological changes in house finches during MG infection, with markedly different responses to MG isolates of varying virulence. These results are consistent with the possibility that evolved virulence in house finch MG results in higher degrees of immune stimulation and associated immunopathology, with potential direct benefits for MG transmission.
Keywords: leukocyte profiles, Mycoplasma gallisepticum, Haemorhous mexicanus, house finch, cellular immunity, pathogen virulence, white blood cell differential
Introduction
Host immune responses have been shown to have crucial impacts on the susceptibility and transmission of disease (Brunham, Plummer & Stephens, 1993; Schmid-Hempel, 2003; Burgner, Jamieson & Blackwell, 2006). Thus, investigating the vast number of evolutionary, ecological, and physiological factors that affect the immune function of hosts is critical in understanding disease dynamics of a system more broadly. Although the immunological responses of naturally-occurring wildlife hosts are relatively understudied, wildlife disease systems provide an exciting opportunity to understand how host immune responses vary following a pathogen’s initial emergence and evolution (e.g. Grodio et al., 2012; Duggal et al., 2014; Vinkler et al., 2018).
One well-studied wildlife disease system for understanding the complex interactions of a pathogen with its natural host is the bacterial pathogen Mycoplasma gallisepticum (MG) that affects house finches (Haemorhous mexicanus). In 1994, a novel strain of MG emerged in eastern North American populations of house finches from domestic poultry and spread rapidly throughout eastern house finch populations (Dhondt, Tessaglia & Slothower, 1998). After causing an almost 60% decline in the eastern house finch population (Hochachka & Dhondt, 2002), MG spread throughout the house finch range in the continental United States (Duckworth, Badyaev, Hill & Roberts, 2003; Staley, Bonneaud, McGraw, Vleck & Hill, 2018). MG infection causes severe conjunctivitis and rhinitis in house finches, ultimately decreasing overwinter survival rates of free-living house finches (Faustino et al., 2004; Kollias et al., 2004). Since the initial outbreak of MG, several emerging genotypic variants have been reported (Pillai et al., 2003; Tulman et al., 2012). Interestingly, MG isolates in both eastern and western populations have independently been increasing in virulence (Hawley et al., 2013; Bonneaud et al., 2018). Furthermore, there is growing evidence that host immunity is contributing to the evolution of higher virulence in this system (Bonneaud et al., 2018; Fleming-Davies et al., 2018; Leon, Fleming-Davies & Hawley, 2019), but the specific immune mechanisms that underlie this wildlife-pathogen interaction are still largely unknown. Finally, this system is particularly amenable to experimental study because house finches infected with virulent isolates in captivity do not experience direct mortality; instead, infection-induced mortality occurs in the wild when lethargic house finches are unable to find food or are depredated (Adelman, Mayer & Hawley, 2017). Thus, we can use experimental infections to readily study immunological responses to isolates of distinct virulence. Overall, this wildlife disease system is a particularly exciting one for exploring wild host immune function and its implications for host-pathogen coevolution.
Although there is an increasing interest in studying immune function of wild hosts, there are a number of challenges in developing the appropriate methodologies (Millet et al., 2007; Boughton, Joop & Armitage, 2011). One increasingly popular tool being used by ecologists to get around these limitations in wild birds is white blood cell differentials (also referred to as the leukocyte profile or leukocyte differentials) (Davis, Cook & Altizer, 2004; Krams et al., 2012; Zylberberg, 2015). This relatively simple hematological measurement, which does not require the species-specific reagents that are often absent for wild species or the terminal sampling often required to get sufficient samples from small birds, offers valuable insights into the immune responses associated with both stress and infection, with similar patterns observed across taxonomic groups (reviewed in Davis et al. 2008). Of particular interest in avian species is the ratio of heterophils to lymphocytes, or the H/L ratio (Müller, Jenni-Eiermann & Jenni, 2011; Cirule et al., 2012; Krams et al., 2012). Several studies in poultry and house finches have documented an increase in this ratio in response to handling stress and MG infection (Gross & Siegel, 1983; Branton et al., 1997; Davis et al., 2004; Davis, 2005; Al-Murrani et al., 2006; Fratto, Ezenwa & Davis, 2014). This is likely due to the importance of heterophils (functionally similar to the mammalian neutrophil) in the inflammatory and phagocytic response (Latimer et al., 1988; Harmon, 1998; Maxwell & Robertson, 1998). Circulating monocytes, which differentiate into macrophages when they migrate to specific tissues, also show a proportional increase in response to MG infection, but not to stress (Branton et al., 1997; Davis et al., 2008; Shi & Pamer, 2011). As such, relative increases in monocytes are typically used as an indicator of an active infection (Davis et al., 2008; Shi & Pamer, 2011). While prior studies have detected proportional increases in both heterophils and monocytes in free-living house finches with clinical signs of MG infection (Davis et al., 2004; Fratto et al., 2014), these field studies were correlational, making it difficult to fully determine which components of white blood cell ratios were a cause or a consequence of MG infection. Experimental infection studies are an important complement to field studies because they allow direct characterization of how house finch leukocyte profiles change over the course of MG infection, and the effects of isolate virulence on these changes.
In the following study, we attempted to answer the three following questions about the house finch immune response to experimental MG infection: (1) How do the proportions of circulating leukocytes change over the course of MG infection? (2) How do differences in isolate virulence affect the host leukocyte profile? and (3) Within isolates, do host leukocyte profiles correlate with infection severity (i.e. conjunctival pathogen load)? To answer these questions, we inoculated wild-caught but initially MG-naïve house finches with one of three isolates of MG known from prior work to vary in virulence (Hawley et al., 2013; Fleming-Davies et al., 2018) and performed white blood cell differentials at various time points over the course of infection. We selected three isolates that vary both in relative virulence (CA2009=low virulence, NC1995=low-medium, VA2013=high virulence) and in geographic origin, with the California isolate (CA2009) stemming from a distinct monophyletic clade from that of eastern North American isolates (Hochachka et al., 2013). Nonetheless, despite detectable genetic differentiation between eastern and western isolates, house finch MG isolates appear to have low overall levels of genomic (Tulman et al., 2012) and antigenic variability (Grodio et al., 2012).
Based on previous studies done in house finches and poultry (Branton et al., 1997; Davis et al., 2004; Fratto et al., 2014), we predicted that there would be an increase in the proportion of heterophils and the proportion of monocytes over the course of MG infection. Additionally, because past work has shown more virulent MG isolates to be more immunogenic in house finches (e.g. Grodio et al., 2012; Vinkler et al., 2018), we predicted that the highest virulence isolate (VA2013) in our study (Fleming-Davies et al., 2018) would induce a stronger immune response in house finches than lower virulence isolates, characterized by a higher proportion of inflammatory cells (i.e. heterophils, monocytes). Finally, we predicted that, within isolates, more severe infection (i.e., higher conjunctival pathogen loads) would correlate with a stronger pro-inflammatory response in the house finch leukocyte profile. Overall, we hoped to gain a better understanding of the role the cellular immune system plays in host-pathogen interactions with implications for disease transmission and susceptibility in this system.
Materials and Methods
Experimental Design.
In order to understand how the leukocyte profile changes over the course of infection with MG isolates of varying virulence (Questions 1 and 2), we took blood smears at various time points from house finches (n=31) experimentally inoculated with MG isolates that we show to vary in virulence (see Results), as well as uninfected sham controls (n=8). In addition, we quantified the conjunctival pathogen load of all individuals (see methods below) over the same infection period to test for correlations between infection severity and observed leukocyte profiles (Question 3).
House Finch Capture and Housing.
A total of 65 hatch-year house finches were captured in July-September 2013 in Montgomery Co., VA using cage traps and mist nets (permits: VDGIF (044569) and USFWS (MB158404–1)), for a separate study (Fleming-Davies et al., 2018). A subset of these birds (n=37) split across four treatments groups (Table 1) were used in this study. Because we opportunistically obtained blood smears from a randomly selected subset of birds during sampling for the above study, we had a slightly unequal male to female ratio across treatments (Table 1). However, sex was controlled for in all statistical models (see Statistical Analysis).
Table 1.
Treatments and sample sizes. All birds received an inoculation with media alone (sham control) or a low (CA2009), low-medium (NC1995), or high (VA2013) virulence isolate of Mycoplasma gallisepticum.
| Sham Control | Low Virulence Isolate (CA2009) | Low-Medium Virulence Isolate (NC1995) | High Virulence Isolate (VA2013) | |
|---|---|---|---|---|
| Males | 4 | 6 | 4 | 6 |
| Females | 4 | 4 | 5 | 4 |
| Total | 8 | 10 | 9 | 10 |
All 65 captured birds were initially pair-housed for a 14-day quarantine period and captured weekly to assess for clinical signs of MG stemming from field exposure prior to capture. All individuals were then blood sampled on day 14–16 post-capture and tested via ELISA (as per Hawley et al. 2011) for previous exposure to MG. All individuals that were seropositive for MG, exhibited clinical signs of MG, or were ever co-housed with an individual that exhibited clinical signs of MG were excluded from this study. All birds received ad libitum water and pelleted diet (Daily Maintenance Diet, Roudybush Inc. Woodland, CA) for the entirety of the experiment. Two weeks prior to inoculation, all birds were housed singly in wire mesh cages (18” x 18” x 36”) under constant temperature and day length (12L:12D) across three rooms. Treatments were randomized across the three captive rooms. All housing and experimental protocols were approved by Virginia Tech’s Institutional Animal Care and Use Committee prior to the start of the study.
Timeline.
Blood smears for all 39 individuals used in this study were initially taken seven days prior to inoculation to obtain a baseline measurement. At day 0, all house finches were inoculated with one of three MG isolates (Table 1) or with sterile Frey’s bacterial media with 15% swine serum (FMS) as a sham control treatment. A blood smear was taken from each individual at 4, 8, 12 or 24 hours post-inoculation (PI). Birds were randomly assigned to only one of these four early blood samples in order to avoid taking too much blood volume from these small animals. However, because we only had 2–3 individuals per treatment / time point combination, samples taken from 4–24 hours post-inoculation were all considered to be “day 0” for the purposes of this study. From then on, blood smears were taken from every individual at days 3, 7, 14, 28, and 42 PI. Sampling of infection severity (pathogen load) occurred at −7, 7, 14, 28, and 42 days PI (see methods below), while sampling of disease severity (eye score) for assessing isolate virulence occurred at −7, 3, 7, 14, 28, and 42 days PI (see methods below).
Inoculation.
Birds were bilaterally inoculated in the palpebral conjunctiva of each eye with a total of 0.04 mL of one of the four treatments (Table 1). All stock MG inocula were grown in FMS and stored at −80°C prior to use (Kleven, 2008). Both the VA2013 isolate (2013.089–15(2P) 9/13/13) and NC1995 isolate (13295–2 (5P) 1/13/10) were diluted with FMS to the approximate concentration of the CA2009 isolate (2009.061–1 (3P) 10/25/10), so birds across all MG treatments received the same approximate dose (1.87 x 107 color changing units/mL). All stock inocula were provided by D. H. Ley, North Carolina State University, College of Veterinary Medicine, Raleigh, NC, USA.
Quantifying Leukocyte Profiles.
We collected blood samples by puncturing the brachial vein of each house finch with a sterile 26-guage needle and collecting the blood in 20uL plastic EDTA coated capillary tubes. The two-slide-wedge technique was used to prepare the smears after bleeding, as per Walberg 2001. Once the smears had air-dried, they were fixed in methanol and stained using Wright-Geisma Quik Staining. Using a magnification of 1000X, one observer (NB), who was blind to each bird’s treatment, counted the first 100 white blood cells seen on an even monolayer of cells for each smear. Following Campbell 1995, white blood cells were further identified as a heterophil, lymphocyte, monocyte, eosinophil, or basophil. Once all smears had been read, the proportion of each cell type and the H/L ratio was calculated for each individual across all time points. A single observer read all of the smears for this study to limit a potential effect of observer.
To assess the repeatability of the leukocyte counts, 15 random smears were recounted by the same individual responsible for all of the original counts (NB). Pearson’s correlations were used to assess the similarity of the counts for each type across the two smears. Correlation coefficients for lymphocyte, heterophil and eosinophil proportions all exceeded 0.95. Monocyte proportions had a correlation coefficient of 0.68 and basophil proportions had a correlation coefficient of 0.55. Monocytes and basophils are the least abundant cell types (see Fig 2D, F), and as such, it is not unexpected to find a higher degree of variation in repeated counts for these two cell populations, given that different parts of the smear are randomly viewed when repeated counts are made.
Figure 2.
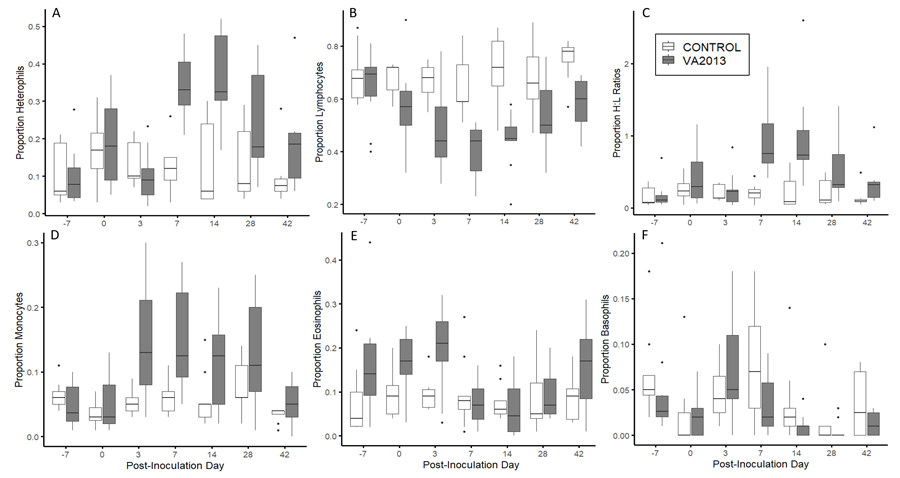
Boxplots of the proportion of circulating leukocytes in house finches inoculated with either a sham treatment (n=8; light gray) or high virulence (n=10; dark gray) isolate of Mycoplasma gallisepticum at 7 distinct time points over the course of infection. The proportion of heterophils (A), lymphocytes (B), H/L ratios (C), monocytes (D), and eosinophil (E) all showed significant interactions between post-inoculation day and treatment. Basophils (F) only showed a significant effect of post-inoculation day. Note that the y-axis differs between individual graphs.
Quantifying Infection Severity.
We quantified the presence and load of MG in the conjunctiva as a measure of infection severity via qPCR. The left and right conjunctiva of each house finch was individually swabbed for 5 seconds with a sterile cotton swab pre-dipped in tryptose phosphate buffer broth (TPB). Following swabbing, the tip of each swab was placed in 300 µl of sterile TPB (combining samples from each conjunctiva), swirled, and wrung out on the inside of the tube to remove any liquid before being discarded. All tubes were then placed on ice for transport and stored in a −20°C freezer to await DNA extractions.
The genomic DNA of all conjunctival swabs was extracted using Qiagen DNeasy 96 Blood and Tissue Kits (Qiagen, Valencia, CA). The quantitative PCR assay that was used to quantify pathogen load targeted the mgc2 gene of MG using primers and a probe, as per Grodio et. al. (2008). Each qPCR reaction contained 5 μl of DNA sample along with 7.5 μl IQ™ Supermix (2X), 0.375 μl each of a 10 μM forward and reverse primers, 0.225 μl of a 10 μM probe, and 3.25 μl of DNase-free water giving a final volume of 15 μl per reaction. All qPCR reactions for this experiment were run using a Bio-Rad CFX-98 C1000 Touch Real Time PCR System under the following cycling parameters: 95°C for 3 sec and 40 amplification cycles at 95°C for 3 sec and 60°C for 30 sec at a ramp rate of 0.5 degree/sec. Additionally, standard curves were generated using 10-fold serial dilutions of plasmid containing a 303 bp mgc2 insert (Grodio, Dhondt, O’Connell & Schat, 2008). The curve was produced using 1.15 x 102 to 1.15 x 107 copy numbers. The mgc2 copies found in both eyes of an individual were summed across sampling dates and then log10 transformed prior to analysis.
Quantifying Isolate Virulence.
We quantified the severity of visible eye lesions as a measure of isolate virulence. As per Sydenstricker et al. 2006, eye lesions were scored on a 0–3 scale that followed this criteria: 0=no detectable swelling or eversion, 1=minor swelling around the ring of the eye, 2=moderate swelling and eversion of the conjunctival tissue, 3=the eye nearly hidden by swelling and crusted exudate. All scoring was done blind to treatment. For each sampling date, lesion scores for each eye (right and left) were summed for each individual. Prior work has demonstrated that the severity of visible eye lesions is correlated with a reduction in anti-predator behaviors (Adelman et al., 2017) and higher mortality in the wild (Faustino et al., 2004). Thus, we use the severity of eye lesions as our primary metric of disease-induced mortality, or virulence, in this system (Hawley et al., 2013). As a second metric of virulence, quantitative pathogen loads (see above – Quantifying Infection Severity) were also analyzed. However, the use of pathogen loads as a metric of virulence (as done in Fleming-Davies et al., 2018) yields very similar results, because across isolates, pathogen load and virulence are strongly correlated (Hawley et al., 2013).
Statistical Analysis.
All statistical analyses were run using R (R Core team, 2015). Cell population proportions were arcsine square-root transformed prior to analysis to normalize the variance in the data (Davis et al., 2004). Heterophil:lymphocyte (H:L) ratios were not transformed, but were analyzed as calculated ratios, as per prior work (Davis et al., 2004).
Quantifying Isolate Virulence.
To confirm that the MG isolates used in this study varied in virulence as in prior work (Hawley et al., 2013; Pflaum et al., 2017), we tested whether isolate treatment influenced the degree of clinical signs that predict mortality risk in the wild (Faustino et al., 2004; Adelman et al., 2017). To assess the degree of virulence for each isolate, we took the sum of eye scores recorded for each individual across five total post-infection measurements. We then used analysis of variance to test whether total eye score over the course of infection varied with treatment (control, CA2009, NC1995, and VA2013). We performed Tukey-Kramer post-hoc tests, which controlled for multiple comparisons, to determine which pairwise treatment combinations significantly differed from each other. We did the same for conjunctival pathogen load (summing across four post-infection measurements) as a secondary metric of virulence. Pathogen loads positively covary with eye score among isolates in this system (Hawley et al., 2013), and pathogen loads have previously been used as a metric for virulence in this system (Fleming-Davies et al., 2018). Differences among isolates in total pathogen loads were analyzed in the same fashion as total eye score.
Temporal Effects of MG Infection on Leukocyte Profiles.
To determine how house finch leukocyte populations vary during the course of MG infection, we examined temporal effects of sham-inoculated control birds versus those inoculated with VA2013, the most virulent isolate used in our study (see Results). We examined only the most virulent isolate for this analysis to maximize the likelihood of detecting temporal effects with our small sample size. We ran linear mixed models on repeated measures (seven smears from each bird taken on days −7, 0, 3, 7, 14, 28, and 42 post-inoculation) of each leukocyte cell population proportion and H:L ratios. Fixed effects for each model included treatment (control or VA2013), sex, post inoculation day (PID), and all pairwise interactions. Post-inoculation day (PID) was treated as a categorical factor to account for the fact that host responses to MG infection are acute and thus non-linear over the time course examined. Individual bird ID was included as a random effect for each model to account for the non-independence of repeated measures from the same individuals. All two-way interactions between fixed effects were initially tested, but only included in final models when statistically significant at an alpha level of 0.05. However, because we were specifically interested in the interaction between treatment and PID (aka, we expected cell populations to change with time for VA2013-treated birds, but not control-treated birds), we left the interaction between treatment and PID in the final models even when non-significant.
Effects of Virulence on Leukocyte Profiles.
To examine how isolate virulence altered leukocyte populations during MG infection, we examined the effect of all treatments (control, CA2009, NC1995, or VA2013) on leukocyte proportions on day 14 post-inoculation. We focused on day 14 for the analysis of isolate effects, because temporal analyses indicated that this time period showed the peak change for most leukocyte populations (see Results). Because each individual was only represented once in this analysis, we used general linear models, with treatment, sex, and their pairwise interaction as the predictor variables. Again, pairwise interactions were removed when non-significant. For cell types with significant effects of treatment, we performed Tukey-Kramer post-hoc tests on all pairwise combinations to determine which treatments significantly differed from each other.
Within-Isolate Effects of Infection Intensity on Leukocyte Profiles.
Finally, we examined whether variation in infection intensity among individuals within isolate treatment predicted leukocyte proportions during MG infection. Here, data for control individuals and data collected prior to experimental inoculation (day 0) were excluded, because we were specifically interested in variation in infection intensity during the course of experimental infection. We used mixed linear models, with treatment (CA2009, NC1995, or VA2013), infection intensity, treatment*infection intensity (our variable of interest for determining within-isolate effects), sex, and post-inoculation day (again, treated as a categorical factor). We initially included pairwise interactions between treatment, sex, treatment and PID, but because none were significant, they were removed from final models. Because we were specifically interested in the interaction between treatment and infection intensity, which would indicate that the effect of infection intensity on leukocyte populations varies by isolate, that pairwise interaction was left in all models, even when non-significant.
Results
Correlations Among Leukocyte Proportions.
We quantified correlations among the white blood cell types examined using Pearson’s correlations. Lymphocyte proportions were found to significantly and negatively correlate with heterophil (-0.74, p<0.0001), monocyte (-0.47, p<0.0001), and eosinophil (-0.39, p<0.0001) proportions. Heterophil proportions were also found to negatively correlate with basophil proportions (-0.20, p<0.001), and positively correlate with monocyte proportions (0.22, p<0.001).
Quantifying Isolate Virulence.
As predicted based on past work (Hawley et al., 2013), we found significant effects of treatment on virulence, as measured by total eye score (F3,333.38=29.154, p<0.0001) and total pathogen load (F3,545.7=35.632, p<0.0001) across infection. Post-hoc tests indicated that all four treatments differ significantly from each other for total pathogen load (p<0.027 for all pairwise comparisons). For total eye score, VA2013 resulted in significantly higher eyes scores than all other treatments (post-hoc comparisons, all p<0.0001). Total eye scores for the NC1995 (“low-medium” virulence) treatment were significantly higher than for the control treatment (p=0.04) but did not significantly differ from the CA2009 treatment (p=0.22). Further, total eye scores for the CA2009 treatment did not significantly differ from the control sham treatment (p=0.41). Overall, consistent with past work (Hawley et al., 2013; Fleming-Davies et al., 2018), the VA2013 isolate was the most virulent of the isolates used. However, NC1995, which was previously categorized as being of medium virulence, did not significantly differ from CA2009 (low virulence) in post-hoc tests for eye score, despite qualitative differences in eye score and significant differences in pathogen load between these isolates (Figure 1). Because this isolate has been quantified as being of medium virulence in previous work (Hawley et al., 2013; Fleming-Davies et al., 2018), significantly differed from controls, unlike our low virulence isolate (CA2009), and had pathogen loads which were significantly greater than CA2009, we conservatively categorized this isolate as being of “low-medium” virulence.
Figure 1.
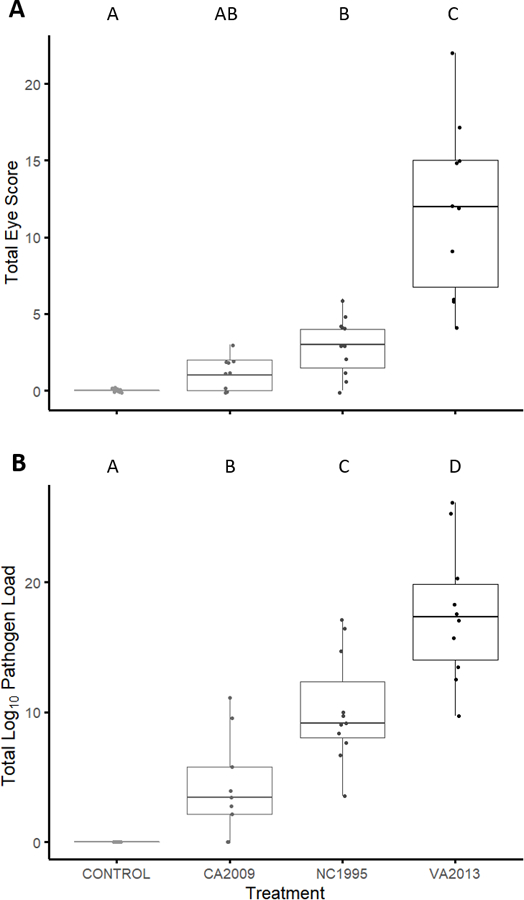
The total degree of conjunctivitis (A) and log10 pathogen load (B) produced by each treatment (control or infection with one of three Mycoplasma gallisepticum isolates). The M. gallisepticum isolates are ordered from least to most virulent (left to right) based on the results of prior work (Hawley et al., 2013). The degree of eye score was visibly scored on a 0 to 3 scale for each eye and summed within individuals (left + right) on a given sampling day, for a maximum value of 6. Here, we summed the eye score for each individual across five total measures taken post-inoculation (A). We also summed the quantitative pathogen load across four total measures taken post-inoculation (B). Distinct letters above each bar represent means that were significantly different (p<0.05) via Tukey post-hoc tests that controlled for multiple comparisons.
Temporal Effects of MG on Leukocyte Profiles.
We found strong temporal effects of infection with a virulent MG isolate (VA2013) on leukocyte populations in house finches. The interaction between PID and treatment (Control or VA2013) was a significant predictor of the proportions of heterophils (Treatment*PID: F6,94.47 = 7.83, P <0.0001), lymphocytes (Treatment*PID: F6,94.67 = 2.54, P=0.025), monocytes (Treatment*PID: F6,94.60 = 3.76, P =0.0021), eosinophils (Treatment*PID: F6,94.45 = 2.49, P=0.028), and H/L ratios (Treatment*PID: F6,94.69 = 5.47, P <0.0001). Basophil proportions showed significant effects of PID (F6,94.79 = 5.71, P <0.0001) as a main effect, but the interaction between PID and treatment was not statistically significant (F6,94.70 = 1.34, P = 0.25). The kinetics of the various cell populations were distinct in some cases, but in general, changes in cell proportions peaked between days 7 and 14 post-inoculation for infected individuals with lymphocytes showing a marked decrease, and heterophils and monocytes showing a marked increase (Fig 2). Sex was not a significant predictor of leukocyte proportions either alone (all F1,16.045 <1.51, all P > 0.39) or in interaction with treatment or PID.
Effects of Isolate Virulence on Leukocyte Profiles.
Isolate treatment significantly influenced the proportions of most cell types at day 14 post-inoculation, including heterophils (F3,34 = 8.84, P =0.00018), lymphocytes (F3,34 = 9.38, P =0.0001), monocytes (F3,34 = 2.94, P =0.046), and H/L ratios (F3,34 = 6.54, P =0.0013). There were no significant effects of treatment on the proportions of eosinophils (F3,34 = 0.169, P =0.91) or basophils (F3,34 = 1.80, P =0.16). Pairwise tests generally showed that the CA2009 (low virulence) isolate did not differ significantly from controls (Fig 3; all Post-hoc Control-CA2009 comparisons, P>0.23), suggesting that the lowest virulence isolate generally did not cause leukocyte profiles to deviate from those of sham controls. The NC1995 (low-medium virulence) and VA2013 (high virulence) isolates significantly differed from the control and CA2009 (low virulence) treatments for both heterophil and lymphocyte proportions (Post-hoc tests, all pairwise P<0.012). Finally, sex was not a significant predictor of any cell proportions on day 14 post-inoculation (F1,34 <2.85, P >0.10).
Figure 3.
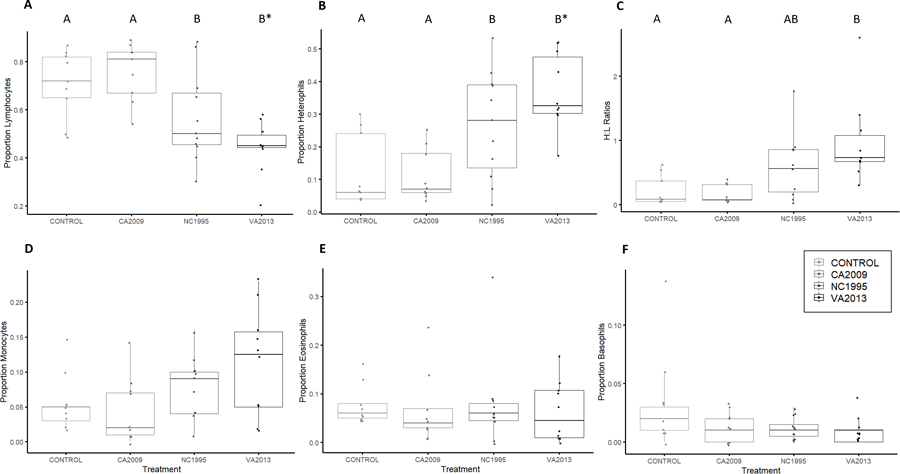
The mean proportion of lymphocytes (A), heterophils (B), H/L ratios (C), monocytes (D), eosinophils (E) and basophils (F) of house finches 14 days post-inoculation with either media alone (control) or an isolate of Mycoplasma gallisepticum with low (CA2009), low-medium (NC1995), or high (VA2013) virulence (x-axis, isolates increase in virulence from left to right). The timepoint of 14 days post-inoculation was selected as it represents the peak of infection and disease severity. Distinct letters above bars indicate means that significantly differed by pairwise post-hoc tests that controlled for multiple comparisons (p<0.05). Asterisks represent marginal insignificance. Points represent individual birds. The mean proportion of monocytes (D), eosinophils (E) and basophils (F) did not differ across isolates by pairwise post-hoc tests and thus do not show distinct letters above bars. Note that the y-axis differs between individual graphs. Although isolates are ordered from lowest to most virulent (left to right) for visual clarity, isolate was treated as categorical for all analyses.
Effect of Infection Intensity on Leukocyte Profiles.
Infection severity, measured as conjunctival pathogen loads, significantly influenced lymphocyte and heterophil proportions, but this relationship varied with isolate (treatment * infection intensity: F2,92.38 > 3.54, P = 0.033). Infection intensity was positively associated with heterophil proportions for the high (VA2013) and low-medium virulence isolates (NC1995) (Fig 4A). However, there was no relationship between infection intensity and heterophil proportions in the lowest virulence treatment (CA2009). Lymphocyte proportions generally decreased with infection intensity for the low-medium (NC1995) and high virulence (VA2013) isolates but, similar to heterophils, did not vary with infection intensity in the lowest virulence (CA2009) treatment (Fig 4B). Despite significant effects on both lymphocyte and heterophil proportions, the interaction between infection intensity and treatment did not reach statistical significance for H/L ratios (F2,101.188 = 2.47, P = 0.089). Finally, infection intensity, either alone or in interaction with treatment, did not predict proportions of any other cell type (all F < 2.54, all P > 0.08).
Figure 4.
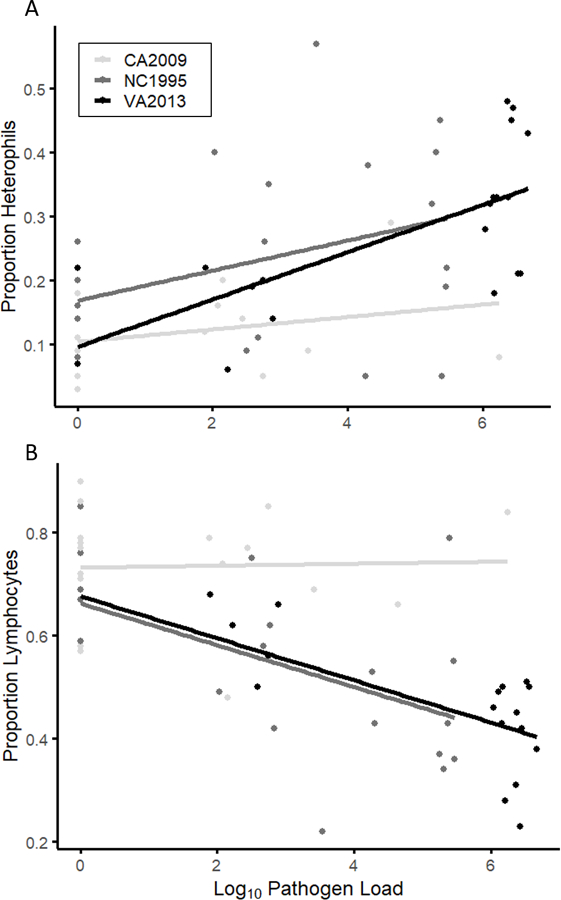
Measure of the proportion of circulating heterophils (A) and lymphocytes (B) relative to infection severity (as measured by conjunctival pathogen loads) in house finches inoculated with one of three isolates of Mycoplasma gallisepticum (see legend). Both the high virulence isolate (VA2013) and low-medium virulence isolate (NC1995) show correlations between infection severity and the proportion of circulating heterophils (A) and lymphocytes (B). In contrast, the low virulence isolate (CA2009) does not show any associations between infection severity and heterophil or lymphocyte proportions. All other leukocyte types did not significantly predict infection severity (not shown). Note that the y-axis is different for individual graphs. Here, each individual is represented multiple times as repeated measures post-infection. This was controlled for in the statistical analysis using random effects.
Discussion
Here, we performed the first experimental study of house finch leukocyte responses to a common bacterial infection, MG, which has evolved in virulence since its emergence in house finches. First, we show strong temporal effects of the cellular immune response to MG in house finches, and a cellular response characterized by increases in pro-inflammatory cell types. Second, we show variation in the strength of house finch leukocyte responses with isolate virulence, which has increased since MG emerged in house finches. Finally, we show correlations between the degree of infection severity among individuals and the strength of heterophil and lymphocyte changes, but only for the low-medium and high virulence isolates. Overall, our results indicate that house finches have a marked, pro-inflammatory cellular response to MG infection, and the strength of this response varies with the degree of infection severity both within- and among-isolates.
We first examined temporal effects of experimental MG infection on the cellular immune response in house finches by comparing leukocyte profiles of sham-inoculated finches to finches inoculated with the highest virulence isolate used in our study (VA2013). Based on previous studies (Branton et al., 1997; Davis et al., 2004) we predicted that there would be a significant increase in the proportion of monocytes and heterophils in MG-infected house finches over the course of infection. We found strong support for that prediction, as we detected significant interactions between infection treatment and time for both monocytes and heterophils, with the proportions of both cell types increasing in response to MG infection (Fig 2A, D). These cell types generally peaked at day 7 or 14 post-inoculation, which corresponds to the time point of peak conjunctivitis severity in captive house finches (Kollias et al., 2004). Previous work from Davis et al. 2004 found similar increases in monocyte and heterophil proportions when sampling wild-caught house finches with visible signs of conjunctivitis. Furthermore, studies conducted on poultry infected with MG (Kerr & Olson, 1970; Branton et al., 1997), as well as a variety of other avian bacterial infections (Harmon, 1998; Shi & Pamer, 2011), show increases in heterophil and monocyte proportions in response to infection. Our results thus corroborate these past studies and indicate the importance of heterophils and monocytes in responding to MG infection in experimentally infected house finches.
In contrast to patterns in heterophils and monocytes, a significant decrease was observed in the mean proportion of circulating lymphocytes in VA2013-infected birds. However, the proportions of lymphocytes were negatively correlated with both heterophil and monocyte proportions in our study (see Results), and thus decreases in lymphocyte proportions may simply reflect the temporal increases in those cell types. Decreases in lymphocyte proportions were not observed in free-living house finches with clinical signs of MG infection (Davis et al., 2004), but similar to our study, lymphopenia, or a relative decrease in lymphocytes, has been shown in MG-infected poultry with high H/L ratios (Kerr & Olson, 1970; Branton et al., 1997). While the detected lymphopenia in both house finches and poultry during MG infection are likely largely driven by proportional changes in other cell types, work in poultry has demonstrated lymphopenia, determined via absolute counts, can result from increases in certain stress hormones, such as corticosterone (Gross & Siegel, 1983), and corticosterone concentrations were recently shown to increase in house finches during MG infection (Love et al., 2016). Thus glucocorticoid-induced redistribution or ‘trafficking’ of lymphocytes to the lymph nodes (Mashaly et al., 1998; Dhabhar, 2002) could potentially also contribute to the observed lymphopenia during MG infection, but quantification of absolute cell counts is needed to robustly demonstrate this.
Consistent with the observed increase in the mean proportion of heterophils and decrease in the mean proportion of lymphocytes as infection peaked (Fig 2A–B), H/L ratios were shown to have strong temporal dynamics, with a peak increase occurring at day 7 post-inoculation (Fig 2C). These results are consistent with a variety of avian studies that indicate that H/L ratios increase during infection (e.g., Branton et al., 1997; Davis et al., 2008; Krams et al., 2012; Fratto et al., 2014). Since both heterophilia and lymphopenia were observed, changes in the proportion of both cell types are important in driving the increase in H/L ratios in MG-infected house finches. This is in contrast to the results of Davis et al. 2004, which indicated that only heterophil proportions were likely driving the increase in H/L ratios in free-living house finches with clinical signs of MG. However, the fact that wild-caught birds in that study were likely at different stages of infection at the time of capture may have interfered with the detection of lymphocyte responses. Thus, sampling experimentally-infected birds allowed us to capture these specific cell dynamics that may have been obscured in previous studies. Teasing apart the relative effects of stress and infection on H/L ratios is a significant challenge since they are so closely tied (reviewed in Davis et al. 2008) but will be important in future studies to understand what drives the cellular immune response to MG in house finches.
Eosinophils also showed decreases over time in VA2013-infected house finches, with a peak reduction at day 14 (Figure 2E). However, as with lymphocytes, a decrease in the relative mean proportion of eosinophils at peak infection might be a product simply of the relative increases of other cell types, particularly given that eosinophil proportions during infection were largely equivalent to those of sham-inoculated birds. The only examined cell type that did not show a significant temporal effect in our study was basophils. However, because this cell type was the rarest in our study, and thus our detections of basophils were the least repeatable, it is difficult to draw robust conclusions about this cell type.
In addition to temporal effects during infection, we examined the effects of differential isolate virulence on host leukocyte profiles. We predicted that the higher virulence isolate (VA2013) would stimulate a stronger cellular immune response characterized by a higher proportion of monocytes and heterophils than lower virulence isolates. In keeping with our prediction, we found that house finches infected with the high virulence isolate (VA2013) and the low-medium virulence isolate (NC1995) did indeed induce significantly higher mean proportions of heterophils (Fig 3A), higher H/L ratios (Fig 3C), and significantly lower mean proportions of lymphocytes (Fig 3B) in comparison to sham control house finches or those inoculated with the lowest virulence isolate examined (CA2009). The proportion of circulating monocytes also showed significant differences across treatments in our model but did not show any significant pairwise differences in post-hoc tests (Fig 3F). Our results corroborate the results of Davis et al. 2004, who showed that differences in the severity of conjunctivitis, our proxy for virulence, were correlated with the proportion of circulating heterophils and monocytes among free-living house finches with MG. Furthermore, the strength of the house finch humoral response and the strength of cytokine responses are known to correlate positively with increasing MG isolate virulence (Grodio et al., 2012; Vinkler et al., 2018). Since cytokines are important in regulating white blood cell synthesis, recruitment, and distribution (Klasing, 1994), the differential cytokine expression produced by isolates of distinct virulence might likewise affect the strength and timing of changes in leukocyte populations.
Although we saw significant effects of isolate treatment on leukocyte responses, post-hoc tests suggested that the high and low-medium virulence isolates produced largely similar cellular immune responses, despite significant differences in the degree of disease severity produced (Fig 1). Furthermore, the lowest virulence isolate produced cellular immune responses that were generally not distinguishable from the sham treatment group. This may indicate the presence of a threshold effect, whereby only the low-medium and high virulence isolates produce sufficiently high infection intensities to exceed the threshold for a strong and largely similar cellular immune response. Indeed, low virulence MG isolates are associated with lower pathogen loads than higher virulence isolates (Fig 1B; Hawley et al., 2013; Leon et al., 2019), and correspondingly, subthreshold levels of antigen are known to elicit suboptimal immune responses (Murphy & Weaver, 2017). Overall, our results and those of past work demonstrate the potential for differential interaction of the house finch immune response with isolates of distinct virulence. However, it is important to note that one caveat of our approach is that we examined all isolate differences at day 14 post-inoculation to eliminate confounding effects of temporal patterns, which may have masked isolate-specific variation that was present in the response kinetics. Further, due to the relatively small number of isolates used (n=3 total isolates), we are unable to definitively determine whether the detected differences are strictly due to differences in pathogen virulence, or another pathogen trait.
Our final goal was to examine whether individual variation in leukocyte profiles within treatments can be predicted by variation in infection severity, measured as conjunctival pathogen burden. Because the isolates vary in the average degree of conjunctival pathogen load that they produce (Hawley et al., 2013; Fig 1B), we examined relationships between pathogen load and leukocyte counts while controlling for isolate. We found that only the proportions of circulating heterophils and lymphocytes were significantly predicted by infection severity, with positive and negative correlations observed, respectively (Fig 4A–B). However, these associations were dependent on isolate, wherein significant correlations between leukocyte counts and infection severity were present only in the high and low-medium virulence treatment groups. This relationship between individual pathogen load and heterophil proportions may help explain the detected isolate-specific variation in the strength of certain cellular responses noted above. Although lymphocytes showed significant negative correlations with infection intensity, this may be entirely driven by the strong negative correlation observed between these two cell types, and thus may represent an indirect effect of the response of heterophils to increasing infection severity (Fig 4A).
However, what remains unclear is why individuals exposed to the lowest virulence isolate show no detectable association between infection severity and heterophil proportions, particularly when some individuals in the lowest virulence treatment harbored quite high pathogen loads at particular post-inoculation time points (x-axis, Fig 4). This suggests that the lack of differences among isolates is not solely a result of the conjunctival loads produced by these isolates at a single time point. For example, high pathogen loads may need to be sustained to reach potential thresholds for mounting immune responses. While the presence of a pathogen load threshold is speculative pending further study, one reason for evolving such a threshold is that heterophils, while undoubtedly offering a protective benefit against bacterial infection in their phagocytic capabilities, are also associated with certain immunopathological costs (Harmon, 1998). At lower pathogen loads, the protective benefit of heterophil responses may not outweigh the cost of increased inflammation for host fitness. On the other hand, the lack of immune responsiveness to high pathogen loads of CA2009 could represent some other unmeasured difference between the isolates used, such as antigenic differences between western versus eastern-origin isolates. However, immunoblot analyses on eastern versus western house finch MG isolates distinct from those used in this study found that sera from birds infected with two eastern isolates (NC2006 and VA1994) reacted with strong intensity to CA2006-specific antisera (Grodio et al., 2012). This suggests that the observed differences in immunogenicity of distinct isolates may not be due antigen specificity or identity, but further work is needed to determine what other traits of isolates may be important in this system.
Overall, our results indicate that house finches have a marked cellular immune response to MG infection, but the strength of this response correlates with isolate virulence, with more virulent isolates inducing a significantly stronger pro-inflammatory response than lower virulence isolates. Our results are consistent with the possibility that inflammatory responses produced during mycoplasmal conjunctivitis infection are largely immunopathological (Vinkler et al., 2018), with significant consequences for transmission and, more broadly, co-evolutionary processes in this system (Graham, Allen & Read, 2005). Increases in the severity of conjunctival pathology in experimentally infected birds are known to increase the deposition rate of MG onto feeders (Adelman et al., 2013). Thus, it may be that higher virulence isolates directly benefit when hosts up-regulate aspects of their immune function (such as the proportion of circulating heterophils), because the more severe conjunctival inflammation, tissue damage, and exudate that result can augment transmission. Further, in chickens, a misdirected inflammatory response has been found to facilitate MG invasion of the conjunctiva and upper respiratory tract (Gaunson et al., 2000, 2006; Mohammed et al., 2007). Overall, our results suggest that house finch pro-inflammatory responses, including heterophilia, are not necessarily always protective in this system and may even be influencing transmission dynamics. This potential mechanism of pathogen manipulation of inflammation may be increasingly important for the fitness of MG because house finches are known to be evolving towards partial resistance or tolerance (Bonneaud et al., 2011, 2019; Adelman et al., 2013). While our results here are limited to leukocyte profiles, future work should explore other immune metrics to enhance our mechanistic understanding of the host immune response to isolates of varying virulence. Because of the ongoing co-evolution occurring between host and pathogen in this system, understanding how the house finch immune system responds to pathogen isolates of differing virulence has critical implications for understanding disease dynamics and host-pathogen evolution more broadly.
Research Highlights.
House finches show a marked pro-inflammatory response to M. gallisepticum infection
Virulent pathogen isolates produce stronger finch white blood cell (WBC) responses
Among birds, stronger WBC responses are associated with higher infection severity
Acknowledgments
This work was funded by NIH grant 5R01GM105245 as part of the joint NIH-NSF-USDA Ecology and Evolution of Infectious Diseases program. N. Bale received research support from the Virginia Tech Biological Sciences Alumni Research Excellence Award. Additional fellowship support for A. Leon was provided by the Virginia Tech IMSD program funded through NIH-NIGMS grant R25GM072767–09. We thank Laila Kirkpatrick, M. Camille Hopkins, Sahnzi Moyers, Michal Vinkler, James Adelman and Kurt Zimmerman for their assistance with this project.
Footnotes
Disclosure Statement
The authors declare no competing interests.
References
- Adelman JS, Carter AW, Hopkins WA & Hawley DM (2013). Deposition of pathogenic Mycoplasma gallisepticum onto bird feeders: host pathology is more important than temperature-driven increases in food intake. Biology Letters, 9, 20130594–20130594. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23966599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman JS, Kirkpatrick L, Grodio JL & Hawley DM (2013). House Finch Populations Differ in Early Inflammatory Signaling and Pathogen Tolerance at the Peak of Mycoplasma gallisepticum Infection. The American Naturalist, 181, 674–689. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23594550 [DOI] [PubMed] [Google Scholar]
- Adelman JS, Mayer C & Hawley DM (2017). Infection reduces anti-predator behaviors in house finches. Journal of Avian Biology, 48, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Murrani WK, Al-Rawi AJ, Al-Hadithi MF & Al-Tikriti B (2006). Association between heterophil/lymphocyte ratio, a marker of ‘resistance’ to stress, and some production and fitness traits in chickens. British Poultry Science, 47, 443–448. Retrieved from http://www.tandfonline.com/doi/abs/10.1080/00071660600829118 [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Balenger SL, Russell AF, Zhang J, Hill GE & Edwards SV (2011). Rapid evolution of disease resistance is accompanied by functional changes in gene expression in a wild bird. Proceedings of the National Academy of Sciences, 108, 7866–7871. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3093480&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneaud C, Giraudeau M, Tardy L, Staley M, Hill GE & McGraw KJ (2018). Rapid Antagonistic Coevolution in an Emerging Pathogen and Its Vertebrate Host. Current biology : CB, 28, 2978–2983.e5. Elsevier Ltd. Retrieved from 10.1016/j.cub.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Tardy L, Giraudeau M, Hill GE, McGraw KJ & Wilson AJ (2019). Evolution of both host resistance and tolerance to an emerging bacterial pathogen. Evolution Letters, 1–11. Retrieved from http://doi.wiley.com/10.1002/evl3.133
- Boughton RK, Joop G & Armitage SAO (2011). Outdoor immunology: methodological considerations for ecologists. Functional Ecology, 25, 81–100. Retrieved from http://doi.wiley.com/10.1111/j.1365-2435.2010.01817.x [Google Scholar]
- Branton SL, May JD, Lott BD & Maslin WR (1997). Various Blood Parameters in Commercial Hens Acutely and Chronically Infected with Mycoplasma gallisepticum and Mycoplasma synoviae. Avian Diseases. [PubMed]
- Brunham RC, Plummer FA & Stephens RS (1993). Bacterial antigenic variation, host immune response, and pathogen-host coevolution. Infection and Immunity. [DOI] [PMC free article] [PubMed]
- Burgner D, Jamieson SE & Blackwell JM (2006). Genetic susceptibility to infectious diseases: big is beautiful, but will bigger be even better? Lancet Infectious Diseases. [DOI] [PMC free article] [PubMed]
- Campbell TW (1995). Avian hematology and cytology Avian hematology and cytology (pp. viii–104). Iowa State University Press. [Google Scholar]
- Cirule D, Krama T, Vrublevska J, Rantala MJ & Krams I (2012). A rapid effect of handling on counts of white blood cells in a wintering passerine bird: A more practical measure of stress? Journal of Ornithology.
- Davis AK, Maney DL & Maerz JC (2008). The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Functional Ecology.
- Davis AK (2005). Effect of handling time and repeated sampling on avian white blood cell counts. Journal of Field Ornithology, 76, 334–338. Retrieved from http://www.bioone.org/doi/abs/10.1648/0273-8570-76.4.334 [Google Scholar]
- Davis AK, Cook KC & Altizer S (2004). Leukocyte Profiles in Wild House Finches with and without Mycoplasmal Conjunctivitis, a Recently Emerged Bacterial Disease. EcoHealth, 1, 362–373. Retrieved from http://link.springer.com/10.1007/s10393-004-0134-2 [Google Scholar]
- Dhabhar FS (2002). A hassle a day may keep the doctor away: Stress and the augmentation of immune function. Integrative and Comparative Biology. [DOI] [PubMed]
- Dhondt AA, Tessaglia DL & Slothower RL (1998). Epidemic mycoplasmal conjunctivitis in house finches from eastern North America. Journal of Wildlife Diseases. [DOI] [PubMed]
- Duckworth RA, Badyaev AV, Hill GE & Roberts SR (2003). First Case of Mycoplasma gallisepticum Infection in the Western Range of the House Finch (Carpodacus mexicanus). The Auk, 120, 528–530. Retrieved from https://www.jstor.org/stable/info/10.2307/4090206 [Google Scholar]
- Duggal NK, Bosco-Lauth A, Bowen RA, Wheeler SS, Reisen WK, Felix TA, Mann BR, Romo H, Swetnam DM, Barrett ADT & Brault AC (2014). Evidence for Co-evolution of West Nile Virus and House Sparrows in North America. PLoS Neglected Tropical Diseases. [DOI] [PMC free article] [PubMed]
- Faustino CR, Jennelle CS, Connolly V, Davis AK, Swarthout EC, Dhondt AA & Cooch EG (2004). Mycoplasma gallisepticum infection dynamics in a house finch population: seasonal variation in survival, encounter and transmission rate. Journal of Animal Ecology, 73, 651–669. [Google Scholar]
- Fleming-Davies AE, Williams PD, Dhondt AA, Dobson AP, Hochachka WM, Leon AE, Ley DH, Osnas EE & Hawley DM (2018). Incomplete host immunity favors the evolution of virulence in an emergent pathogen. Science, 359, 1030–1033. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29496878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratto M, Ezenwa VO & Davis AK (2014). Infection with Mycoplasma gallisepticum buffers the effects of acute stress on innate immunity in house finches. Physiological and biochemical zoology : PBZ, 87, 257–64. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24642543 [DOI] [PubMed] [Google Scholar]
- Gaunson JE, Philip CJ, Whithear KG & Browning GF (2000). Lymphocytic infiltration in the chicken trachea in response to Mycoplasma gallisepticum infection. Microbiology. [DOI] [PubMed]
- Gaunson JE, Philip CJ, Whithear KG & Browning GF (2006). The cellular immune response in the tracheal mucosa to Mycoplasma gallisepticum in vaccinated and unvaccinated chickens in the acute and chronic stages of disease. Vaccine, 24, 2627–2633. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16406173 [DOI] [PubMed] [Google Scholar]
- Graham AL, Allen JE & Read AF (2005). Evolutionary Causes and Consequences of Immunopathology. Annual Review of Ecology, Evolution, and Systematics, 36, 373–397. Retrieved from http://www.annualreviews.org/doi/abs/10.1146/annurev.ecolsys.36.102003.152622 [Google Scholar]
- Grodio JL, Dhondt KV, O’Connell PH & Schat KA (2008). Detection and quantification of Mycoplasma gallisepticum genome load in conjunctival samples of experimentally infected house finches ( Carpodacus mexicanus ) using real-time polymerase chain reaction. Avian Pathology, 37, 385–391. Retrieved from http://www.tandfonline.com/doi/full/10.1080/03079450802216629 [DOI] [PubMed] [Google Scholar]
- Grodio JL, Hawley DM, Osnas EE, Ley DH, Dhondt KV, Dhondt AA & Schat KA (2012). Pathogenicity and immunogenicity of three Mycoplasma gallisepticum isolates in house finches (Carpodacus mexicanus). Veterinary Microbiology, 155, 53–61. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21885217 [DOI] [PubMed] [Google Scholar]
- Gross WB & Siegel HS (1983). Evaluation of the Heterophil/Lymphocyte Ratio as a Measure of Stress in Chickens. Avian Diseases. [PubMed]
- Harmon BG (1998). Avian Heterophils in Inflammation and Disease Resistance. Poultry Science. [DOI] [PubMed]
- Hawley DM, Grodio J, Frasca S, Kirkpatrick L & Ley DH (2011). Experimental infection of domestic canaries (Serinus canaria domestica) with Mycoplasma gallisepticum: a new model system for a wildlife disease. Avian pathology : journal of the W.V.P.A, 40, 321–7. Retrieved from http://www.tandfonline.com/doi/abs/10.1080/03079457.2011.571660 [DOI] [PubMed] [Google Scholar]
- Hawley DM, Osnas EE, Dobson AP, Hochachka WM, Ley DH & Dhondt AA (2013). Parallel Patterns of Increased Virulence in a Recently Emerged Wildlife Pathogen. (Read AF, Ed.)PLoS Biology, 11, e1001570 Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3665845&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka WM & Dhondt AA (2002). Density-dependent decline of host abundance resulting from a new infectious disease. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed]
- Hochachka WM, Dhondt AA, Dobson A, Hawley DM, Ley DH & Lovette IJ (2013). Multiple host transfers, but only one successful lineage in a continent-spanning emergent pathogen. Proceedings of the Royal Society B: Biological Sciences, 280, 20131068–20131068. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23843387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KM & Olson NO (1970). Pathology of Chickens Inoculated Experimentally or Contact-Infected with Mycoplasma synoviae. Avian Diseases. [PubMed]
- Klasing KC (1994). Avian leukocytic cytokines. Poultry science. [DOI] [PubMed]
- Kleven SH (2008). Control of Avian Mycoplasma Infections in Commercial Poultry. Avian Diseases Digest, 3, e1–e1. Retrieved from http://www.bioone.org/doi/abs/10.1637/8424.1 [DOI] [PubMed] [Google Scholar]
- Kollias GV, Sydenstricker KV, Kollias HW, Ley DH, Hosseini PR, Connolly V & Dhondt AA (2004). Experimental infection of house finches with Mycoplasma gallisepticum. Journal of wildlife diseases, 40, 79–86. Retrieved from http://www.bioone.org/doi/10.7589/0090-3558-40.1.79 [DOI] [PubMed] [Google Scholar]
- Krams I, Vrublevska J, Cirule D, Kivleniece I, Krama T, Rantala MJ, Sild E & Hõrak P (2012). Heterophil/lymphocyte ratios predict the magnitude of humoral immune response to a novel antigen in great tits (Parus major). Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology, 161, 422–428. Elsevier Inc. Retrieved from 10.1016/j.cbpa.2011.12.018 [DOI] [PubMed] [Google Scholar]
- Latimer KS, Tang K-N, Goodwin MA, Steffens WL & Brown J (1988). Leukocyte Changes Associated with Acute Inflammation in Chickens. Avian Diseases. [PubMed]
- Leon AE, Fleming-Davies AE & Hawley DM (2019). Host exposure history modulates the within-host advantage of virulence in a songbird-bacterium system. Scientific Reports, 9, 20348 Springer US. Retrieved from 10.1038/s41598-019-56540-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love AC, Foltz SL, Adelman JS, Moore IT & Hawley DM (2016). Changes in corticosterone concentrations and behavior during Mycoplasma gallisepticum infection in house finches (Haemorhous mexicanus). General and Comparative Endocrinology, 235, 70–77. Elsevier Inc. Retrieved from 10.1016/j.ygcen.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Mashaly MM, Trout JM, Hendricks G, Al-Dokhi LM & Gehad A (1998). The role of neuroendocrine immune interactions in the initiation of humoral immunity in chickens. Domestic Animal Endocrinology. [DOI] [PubMed]
- Maxwell MH & Robertson GW (1998). The avian heterophil leucocyte: a review. World’s Poultry Science Journal.
- Millet S, Bennett J, Lee KA, Hau M & Klasing KC (2007). Quantifying and comparing constitutive immunity across avian species. Developmental and Comparative Immunology. [DOI] [PubMed]
- Mohammed J, Frasca S, Cecchini K, Rood D, Nyaoke AC, Geary SJ & Silbart LK (2007). Chemokine and cytokine gene expression profiles in chickens inoculated with Mycoplasma gallisepticum strains Rlow or GT5. Vaccine, 25, 8611–8621. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18006123 [DOI] [PubMed] [Google Scholar]
- Müller C, Jenni-Eiermann S & Jenni L (2011). Heterophils/Lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings. Functional Ecology.
- Murphy K & Weaver C (2017). Janeway’s Immunobiology (9th Editio.). Garland Science/Taylor & Francis Group, LLC. [Google Scholar]
- Pflaum K, Tulman ER, Beaudet J, Liao X, Dhondt KV, Dhondt AA, Hawley DM, Ley DH, Kerr KM & Geary SJ (2017). Attenuated phenotype of a recent house finch-associated Mycoplasma gallisepticum isolate in domestic poultry. Infection and Immunity. [DOI] [PMC free article] [PubMed]
- Pillai SR, Mays HL, Ley DH, Luttrell P, Panangala VS, Farmer KL & Roberts SR (2003). Molecular Variability of House Finch Mycoplasma gallisepticum Isolates as Revealed by Sequencing and Restriction Fragment Length Polymorphism Analysis of the pvpA Gene. Avian Diseases. [DOI] [PubMed]
- Schmid-Hempel P (2003). Variation in immune defence as a question of evolutionary ecology. Proceedings of the Royal Society B: Biological Sciences, 270, 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C & Pamer EG (2011). Monocyte recruitment during infection and inflammation. Nature Reviews Immunology. [DOI] [PMC free article] [PubMed]
- Staley M, Bonneaud C, McGraw KJ, Vleck CM & Hill GE (2018). Detection of Mycoplasma gallisepticum in House Finches ( Haemorhous mexicanus ) from Arizona. Avian Diseases. [DOI] [PubMed]
- Sydenstricker KV, Dhondt AA, Hawley DM, Jennelle CS, Kollias HW & Kollias GV (2006). Characterization of Experimental Mycoplasma gallisepticum Infection in Captive House Finch Flocks. Avian Diseases, 50, 39–44. Retrieved from http://www.bioone.org/doi/abs/10.1637/7403-062805R.1 [DOI] [PubMed] [Google Scholar]
- Tulman ER, Liao X, Szczepanek SM, Ley DH, Kutish GF & Geary SJ (2012). Extensive variation in surface lipoprotein gene content and genomic changes associated with virulence during evolution of a novel North American house finch epizootic strain of Mycoplasma gallisepticum. Microbiology, 158, 2073–2088. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22628486 [DOI] [PubMed] [Google Scholar]
- Vinkler M, Leon AE, Kirkpatrick L, Dalloul RA & Hawley DM (2018). Differing house finch cytokine expression responses to original and evolved isolates of Mycoplasma gallisepticum. Frontiers in Immunology, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberg J (2001). White blood cell counting techniques in birds. Seminars in Avian and Exotic Pet Medicine.
- Zylberberg M (2015). Common measures of immune function vary with time of day and sampling protocol in five passerine species. Journal of Experimental Biology. [DOI] [PubMed]


