Abstract
Transition metals from manganese to zinc function as catalytic and structural cofactors for an amazing diversity of proteins and enzymes, and thus are essential for all forms of life. During infection, inflammatory host proteins limit the accessibility of multiple transition metals to invading pathogens in a process termed nutritional immunity. In order to respond to host-mediated metal starvation, bacteria employ both protein and RNA-based mechanisms to sense prevailing transition metal concentrations, that collectively regulate systems-level strategies to maintain cellular metallostasis. In this review, we discuss a number of recent advances in our understanding of how bacteria orchestrate the adaptive response to host-mediated multi-metal restriction, highlighting crosstalk among these regulatory systems.
Introduction
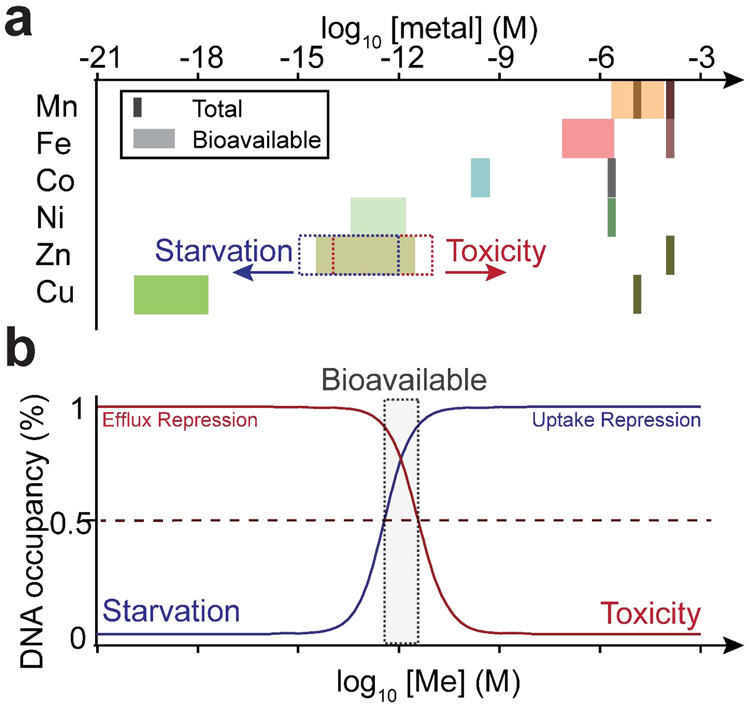
Transition metals perform myriad roles as catalytic and structural cofactors in ≈30% of proteins in a typical bacterial proteome, and thus are strongly integrated into nearly every aspect of cellular metabolism. Proper metalation of the metalloproteome is therefore crucial for enzymatic function and global metabolism, and is acutely impacted by transition metal bioavailability both inside and outside of the bacterial cell. Metallostasis is the cellular process that governs an adaptive response to both metal restriction and metal overload, a process that is particularly important in the infected host [1,2*,3]. Metallostasis is a systems-level process governed by the regulation of gene expression at both transcriptional and post-transcriptional levels to allow for metal scavenging, efflux and intracellular sequestration, ribosome remodeling and metabolic re-programming necessitated by perturbation of the metalloproteome itself. The intracellular concentrations of bioavailable metal are predicted to follow a metal competitivity index when a suite of metal-sensing metalloregulatory proteins are used as proxy for these concentrations [4**]. Bacterial cells thus appear to buffer bioavailable metal such that the free energy of metalating a given metalloprotein with Zn(II), for example, will only allow Zn(II) proteins to be metalated under normal cellular conditions (Figure 1a) [4**]. As such, large fluctuations in free metal concentration or metal activity are predicted to result in under-metalation and mis-metalation of the proteome under conditions of severe metal restriction or toxicity, respectively (Figure 1b).
Figure 1.
Metallostasis. (a) Bioavailable metal (Me) concentrations can be estimated from metal sensor affinities for their cognate metals and are tuned to the inverse of the Irving-Williams series of divalent ion-chelate stability constants. Increases in bioavailable metal (dashed red box) results in cellular toxicity, and leads to mis-metalation of coordination sites bound by less competitive metals [4**]. Decreases in bioavailable metal (dashed blue box) results in cellular starvation which may lead to under-metalation and potentially mis-metalation of the metalloproteome by more competitive metals. Total Mn concentration varies in an approximate range of Mn:Fe ratio of 0.1~1 from “Fe-centric” (light brown line) to “Mn-centric” bacteria (dark brown line) [26]. (b) Pairs of uptake and efflux metalloregulators sense cellular metal concentration which maintain bioavailable metal in the metal activity range defined by the gray box [11].
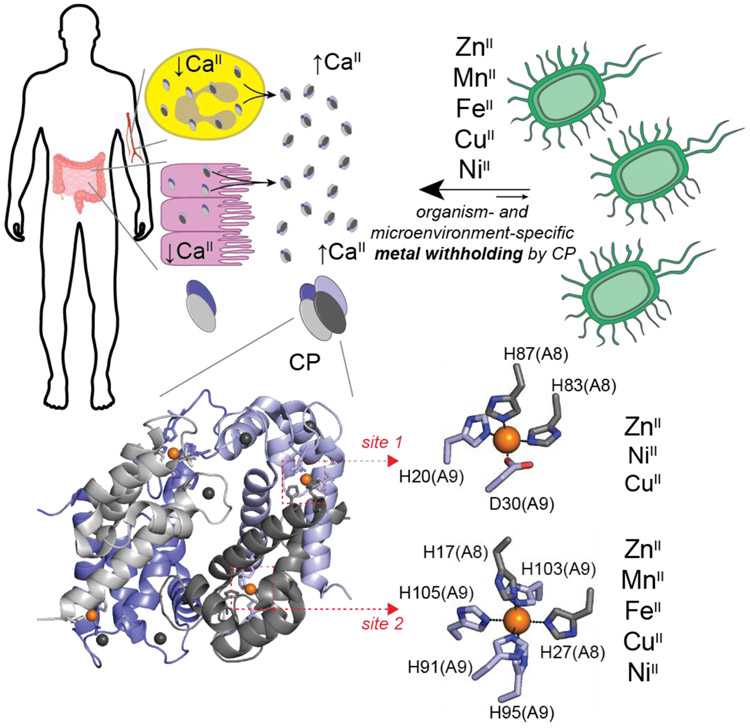
Upon bacterial infection, the vertebrate host actively disrupts metallostasis as a means to inhibit bacterial or fungal growth. On the one hand, Cu and Zn(II) poisoning in phagolysosomes of macrophages, for example, are employed by the host to combat bacterial infections [3,5]. On the other hand, host neutrophils recruited to sites of infection harbor calprotectin, Ca(II)-activated S100A8/S100A9 heterotetramer, that is secreted into the extracellular milieu to scavenge transition metals as part of the process termed nutritional immunity [6]. The multi-metal chelator protein CP has two types of transition metal binding sites; a His3Asp distorted tetrahedral site 1 that has been shown to coordinate Zn(II), Cu(II) and Ni(II) and an octahedral His6 site 2 capable of forming metal complexes with Zn(II), Cu(II), Ni(II), Fe(II) and Mn(II) (Figure 2) [2*,7].
Figure 2.
Nutritional Immunity. The human host secretes calprotectin (CP) from neutrophils (yellow) and epithelial cells (pink) in an effort to starve the invading pathogen of Zn(II), Mn(II), Fe(II), Cu(II), and Ni(II) (for a comprehensive review, see [2*]). While calprotectin is capable of chelating all metals shown, the exact withholding effect is both organism- and tissue microenvironment-dependent, as reviewed in [2*]. See text for additional details. Calprotectin is a dimer of S100A8/S100A9 heterodimers, forming an active α2β2 heterotetramer in the presence of higher Ca(II) concentrations in the extracellular milieu. Each αβ heterodimer harbors one subunit-bridging site (His3Asp, Site 1) and one broadly promiscuous, subunit-bridging divalent metal binding site (His6, Site 2) (PDB: 4GGF).
While host-mediated metal restriction may well induce specific failures in metabolism due to the loss of a single nutrient metal, even depletion of a single metal globally disrupts a finely-tuned balance among all biologically relevant transition metals in the cell, sometimes even changing the nature of the metal cofactor in a specific enzyme [8,9]. Under-metalation of a subset of Fe(II) metalloenzymes, for example, may result in unfilled Fe(II) sites in the proteome that can be occupied by more competitive metals, i.e., Zn(II). On the other hand, Zn(II) toxicity is expected to result in Zn(II) mis-metalation of sites occupied by less competitive metals, i.e., Mn(II) and Fe(II) [10,11]. As a result, perturbation of the bioavailable levels of a single metal has the capacity to disrupt many aspects of cellular metallostasis. Here we highlight recent work that reveals how host-induced transition metal starvation of bacterial pathogens extends far beyond metal uptake regulation to a global impact on cellular metabolism.
Multi-Metal Restriction by Calprotectin
The initial observation of bacterial Zn(II) restriction by the His3Asp site 1 of CP led the community to consider CP a Zn(II)-specific chelator, and subsequent work described the functional role of the His6 site 2 as involved in Mn(II) chelation (Figure 2) [2*]. More recently, Cu starvation has been observed in the fungal pathogen C. albicans treated with CP, where Zn(II), Mn(II), and Cu(II) are all sequestered by CP [7]. A documented role of CP-mediated Fe(II) sequestration was initially controversial since Fe starvation of the pathogen had long been attributed to ferric iron restriction by host iron-binding proteins lactoferrin, transferrin, and lipocalin 2 (as Fe(III)-enterobactin complexes) [12,13] at sites of infection. Indeed, Fe(III) is expected to predominate in the oxidizing conditions of the extracellular space (Figure 2). However, the observation of CP-mediated Fe sequestration in a growth medium containing a reducing agent, β-mercaptoethanol, suggested that CP-mediated Fe(II) withholding could well be important in an anaerobic or microaerophilic environment of a biofilm, for example, where Fe(II) becomes bioavailable [14]. Comprehensive follow-up studies revealed that CP itself is capable of impacting the redox equilibrium of Fe in the absence of a chemical reductant [15], and that natural reductants are in fact present at sites of infection, providing physiological support for Fe(II) withholding. For example, redox cycling phenazines, which are capable of reducing ferric iron to ferrous iron [16], are secreted by P. aeruginosa and appear to enhance the ability of CP to function in Fe(II) sequestration [17**]. Moreover, recent work that revisits prior observations of CP-mediated Zn(II) starvation in Acinetobactor baumannii confirms a role of CP in sequestration of Fe(II) [18**]. This work is consistent with findings of significant chelatable or labile Fe(II) in mice, including around sites of Acinetobacter baumannii infection [19].
Remarkably, in Borrelia burgdorferi, the causative agent of Lyme disease, CP inhibits growth in a metal-independent fashion, in a process that requires physical association of CP with the bacterium [20]. These findings collectively shift the paradigm of the major anti-microbial protein CP from a Zn(II) and/or Mn(II)-specific chelator to a molecule that is ideally suited to starve an invading pathogen of virtually any metallonutrient at a site of infection. The mechanism by which CP inhibits growth of an organism will be dependent not only the metal bioavailability in a specific niche [18**], but also the metal quota and more importantly, the metalloenzymes that must be metalated for the organism to survive myriad other host antimicrobial defenses beyond transition metal restriction [21], an excellent example of which is Mn(II)-driven superoxide dismutase activity in S. aureus [22].
The Response to Multi-Metal Starvation
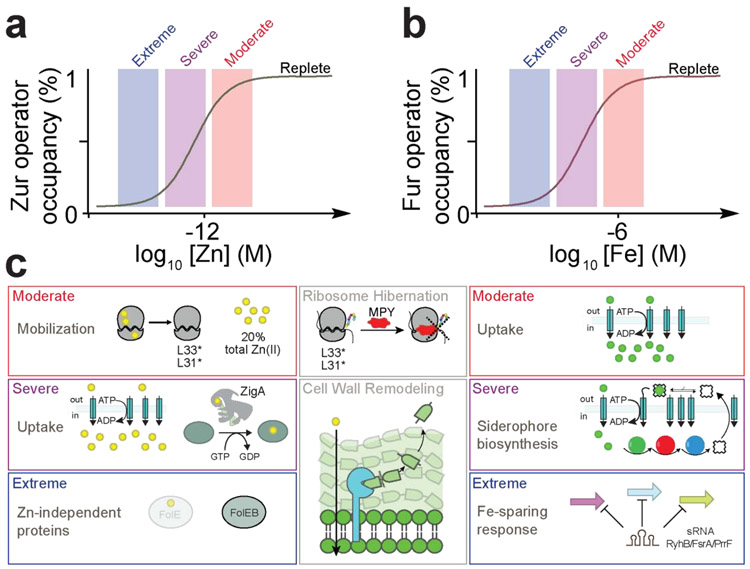
Bacteria sense fluctuations in intracellular transition metal concentrations via metal uptake and efflux regulation using both transcriptional and post-transcriptional sRNA- and RNA riboswitch-based mechanisms [1,23]. In the absence of extreme metal toxicity or restriction, a basal level expression of metal transporters allows the cell to respond to minor fluctuations in bioavailable metal [24], with the uptake repressors typically controlling the expression of a larger number of genes than efflux regulators [25]. In particular, the iron uptake repressor Fur and to a lesser extent, the zinc uptake repressor Zur, impact the integrity of a larger fraction of the proteome given the large Zn(II) and Fe quotas for many human pathogens, Streptococcus pneumoniae being a prominent exception [10,26]. Thus, the need arises for what might be considered a hierarchical or “graded” expression of these larger transcriptional regulons. Indeed, both Fur and Zur have been shown to direct a graded transcriptional response to increasing degrees of metal starvation in two organisms (Figure 3) [27-29]. This is linked to metal occupancy of an accessory metal sensing site beyond the primary sensing site [28], which in turn results in variable degrees of transcriptional repression of individual genes and allows for distinct waves of Fur/Zur dissociation as the cellular metal activity falls [27,29,30].
Figure 3.
Gradient response to metal starvation. (a) Zur and (b) Fur derepress at three distinct or “step-wise” waves of increasing degrees of Zn(II) and Fe depletion, respectively, labeled moderate, severe and extreme. (c) The Zur step-wise derepression waves are mobilization from the ribosome, extracellular uptake and intracellular allocation by putative Zn(II) metallochaperone, e.g., ZigA [18**] or ZagA [32**], and metabolic remodeling with the expression of Zn(II)-independent proteins (left) [27, 30]. The Fur step-wise derepression waves are metal uptake, siderophore production/Fe(III) capture and uptake, and Fe-sparing through a sRNA (right) [29]. As the graded response has only been observed in two organisms thus far, further studies in other bacteria are required to explore the generality of this regulatory response. Additional recently identified responses to Zn(II) starvation are mycobacterial-specific protein Y (MPY)-mediated assembly of inactive or “hibernating” antibiotic-resistant 70S ribosomes in M. tuberculosis [33*] and enhanced resistance to Zn(II) restriction via ZrlA/ShyB-dependent remodeling of the cell wall [47*,48] (center).
Intracellular Ribosomal Stores
The Zur-regulated response to incrementally increasing degrees of Zn(II) restriction starts with the mobilization of intracellular metal stores from the ribosome via expression of Zn(II)-independent paralogs that replace Zn(II)-dependent ribosomal proteins of both the small and large subunits; this avoids the initial need to produce metal acquisition systems which is energetically costly (Figure 3a) [31]. Although this phenomenon has been known for nearly two decades [31], the extent to which Zn(II) is released and/or new low-zinc ribosomes assembled has only recently been estimated as 20% of total cell-associated Zn(II) (≈190 μM) as becoming bioavailable (Figure 1b) [32**]. The impact of these ribosomal protein substitutions on translational efficiency and ribosome assembly remain largely unexplored, but some bacteria may well arrest translation entirely in what are known as “hibernating” ribosomes [33*]. The extent to which ribosomal remodeling is coincident with global changes in the cellular spectrum of transfer RNA (tRNA) modifications, known to impact decoding accuracy and rates, is also not known; however, queuosine and thiouridine biosynthesis are clearly impacted by Zn(II) and cellular sulfur availability/Fe-S cluster status, respectively, in bacterial cells [18,34,35].
Intracellular Metal Allocation and Extracellular Metal Acquisition
As metal ion availability decreases further, the cell scavenges extracellular metal by overexpressing high affinity Zn(II) importers, while also responding to a sizable flux of Zn(II) released from ribosomes (Figure 3a). If this Zn(II) were truly “free”, this might signal cellular zinc toxicity; thus, some mechanism that directs this newly mobilized metal to Zn(II)-requiring proteins becomes necessary. In B. subtilis, this second wave of Zur regulation results in the expression of a COG0523-family Zn(II) metallochaperone, ZagA, which along with A. baumannii ZigA, are excellent candidates to fulfill this role in metal allocation. These enzymes are metal-activated GTPases that bind Zn(II) with high affinity (KZn≈1011 M−1), and it has been proposed that this Zn(II) is delivered to key proteome targets as a result of transient protein-protein interactions [32**,36,37]. This Zn(II) chaperoning effectively prioritizes metabolism since it likely results in metalation of a subset of zinc metalloenzymes in the cell, thus sustaining folate biosynthesis in B. subtilis and flavin biosynthesis in A. baumannii [32**,37]. Alternatively, ZigA and related proteins may physically interact with the ribosome itself, given an evolutionary relationship to M. tuberculosis mycobacterial-specific protein Y (MPY) recruitment factor, MRF, which occurs under conditions of zinc restriction (Figure 3c) [33*].
In Streptomyces coelicolor, the putative zincophore coelibactin is expressed in the second wave of the Zn(II) starvation response (Figure 3c) and is thought to be used for Zn(II) uptake [27]. The recent discovery of opine [N-(carboxyalkyl) amino acid] metallophores capable of capturing a wide range of divalent metals, including Zn(II) [38], Co(II), Ni(II) and Fe(II) suggests that possibility that broad-spectrum metallophore systems, e.g., like yersiniabactin [39], might represent a general strategy deployed to capture whatever metal is bioavailable in the surrounding milieu to meet nutritional needs, with Zn(II) or Fe starvation a general signal to do so. S. aureus, Y. pestis and P. aeruginosa encode machinery to biosynthesize, export, and uptake these simple nicotianamine-like molecules, staphylopine, yersinopine and pseudopaline, respectively [38,40], which in the case of S. aureus, is strongly induced by CP [41]. Moreover, these metallophores outcompete CP for Zn(II) at the host-pathogen interface [42*].
Metabolic Remodeling/Metal Sparing
Finally, if nutritional needs remain unmet, a third wave of the adaptive response results in what is essentially a remodeling of cellular metabolism [18**,27,29,30]. This has historically been understood as a metal sparing response which we define as a process that lowers cellular demand for a particular metal, by prioritizing metabolism in a number of ways (Figure 3c). One strategy is to express a paralog of an obligatory single metal-dependent enzyme with one that is characterized by a relaxed metal specificity profile, or lacks catalytic metal altogether. This has been observed in enzymes of folate biosynthesis [for Zn(II)] and in glycolysis [for Mn(II)] [43,44]. Additional Zur-regulated genes that encode what appear to be paralogs of metalloenzymes are expected to provide a means to prioritize one metabolic process(es) over others under conditions of Zn(II) restriction in an organism-specific manner [45,46]. These include folate, pyrimidine, and possibly queuosine biosynthesis [46].
Remodeling of the bacterial cell wall occurs under conditions of Zn(II) starvation through Zur-regulated expression of N-acetylmuramoyl-L-alanine amidases A. baumannii ZrlA, Vibrio cholerae ShyB, and P. aeruginosa AmiA [45,47*,48]. These peptidoglycan-remodeling hydrolases are Zn(II)-dependent metallopeptidases, and increased expression of a Zn(II)-dependent enzyme under conditions of Zn(II) restriction is counterintuitive. It is unknown if this is an early-, mid- or late-stage response to zinc limitation (Figure 3c), and it remains unclear if these peptidases make the cell wall more resistant to Zn(II) limitation or simply perform a housekeeping role while their constitutively expressed counterparts are no longer active.
An important late-stage Fe-deprivation response is regulation of gene expression by a Fur-regulated sRNA, originally described for E. coli RhyB [49] and more recently characterized in B. subtilis as FsrA and in P. aeruginosa as PrrF1/PrrF2 [50,51**]. These sRNAs regulate translation as antisense RNAs to down-regulate the expression of nonessential Fe-containing enzymes and Fe-storage proteins under extreme Fe limitation, while also enhancing translation of selected genes involved in siderophore biosynthesis (Figure 3b) [49,52]. PrrF regulation in P. aeruginosa extends far beyond Fe homeostasis and impacts many other metabolic and cellular processes as a result of the extensive Fe-metalloproteome, including quorum sensing, twitching motility, branched-chain amino acid biosynthesis [53], sulfur metabolism, and phenazine biosynthesis [51]. In this way, Fe restriction remodels metabolism to prioritize processes that maintain cellular growth under these conditions.
Crosstalk Among Metallostasis Systems
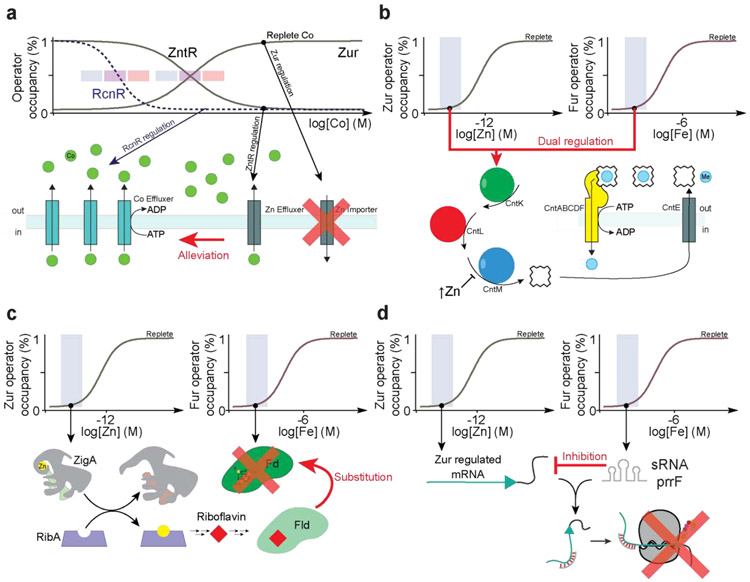
Although the divalent metal competitivity index [54] is collectively managed by metal-specific protein- and RNA riboswitch-based regulators (Figure 1a), metalloregulatory proteins can be mis-metalated by non-cognate metals, particularly under conditions of acute metal toxicity. In some cases [55], but not all [56], coordination of a non-cognate metal can drive the same in vitro allosteric response as the cognate metal, thus potentially undermining the integrity of cellular metallostasis (Figure 1). For example, in Salmonella enterica sevovar Typhimurium, acute Zn(II) toxicity transcriptionally induces a Co(II) toxicity response by the Co/Ni sensor, RcnR, while conversely, acute Co(II) toxicity triggers a Zn(II) toxicity response regulated by the Zn(II) efflux activator ZntR while also leading to repression of the Zur-regulon (Figure 4a) [11]. This type of Zn-Co crosstalk may be important for survival under acute phase metal stress, since it leverages the likelihood that metal effluxers regulated in this case by RcnR and ZntR, catalyze transport of non-cognate metals with rates that are similar to cognate metals, but with lower cellular sensitivities. Mis-metalation of Fur by Mn(II) under conditions of Mn(II) toxicity, on the other hand, is detrimental to cell viability, and is avoided by proper tuning of the coordinated responses of the Mn(II)-sensing repressor and Fur to distinct ranges of cellular metal activity (Figure 1a) [57].
Figure 4.
Metallostasis crosstalk. (a) In S. enterica serovar Typhimurium, RcnR senses Co(II) toxicity and derepresses Co(II) effluxers (cyan). Under conditions of Co(II) toxicity, Zn(II) responsive regulators ZntR and Zur also sense non-cognate Co(II) to allow for the expression of Zn(II) effluxers (gray) that are capable of effluxing non-cognate Co(II) [11]. (b) The metallophore staphylopine is produced for transition metal uptake under conditions of low Zn(II) or low Fe via Zur and Fur dual regulation [58]. Once metallostasis is restored, staphylopine dehydrogenase (CntM) is inhibited by high levels of Zn(II) to avoid toxicity [59]. (c) Fe starvation is expected to result in the loss of cellular reducing equivalents provided by ferredoxin (Fd) [62]. Flavodoxins (Fld) can functionally replace the inactivated Fds in a Fur-dependent manner, as there are Fur regulated Fld-encoding genes in B. subtilis and C. acetobutylicum [61,66]. When A. baumannii suffers Zn(II) restriction by CP, a high cellular concentration of a candidate Zn(II) metallochaperone ZigA sustains riboflavin biosynthesis, proposed to occur via metalation of the Zn(II)-dependent GTP cyclohydrolase II RibA, such that Zn(II) depletion effectively rescues a response to Fe restriction [18**]. (d) Zn(II) starvation results in the transcription of the Zur regulon. Fe starvation leads to the transcription of Fur-regulated PrrF sRNA in P. aeruginosa, which in turn inhibits the translation of some proteins encoded by the Zur regulon [51**].
Metallostasis crosstalk may have additionally evolved as a result of the need for physiological adaptation to multiple metal stresses, particularly those encountered by bacterial pathogens. For example, microenvironments that restrict Zn(II) availability may well restrict Fe, and vice versa, mediated by one or more host proteins, including CP (Figure 2). Recent work reveals that the genes encoding for the biosynthesis, efflux and uptake of the broad-spectrum metallophore staphylopine in S. aureus are cooperatively regulated by both Fur and Zur [42,58], while those for pseudopaline in P. aeruginosa have thus far only been shown to be regulated by Zur [40]. While both metallophores are capable of reversing Zn(II)-deplete growth defects [38,40,42*], it has not yet been demonstrated that they can combat Fe(II) starvation, consistent with the lower affinity of Fe(II) (log KFe≈12) vs. Zn(II) (log KZn≈15) for staphylopine [38]. In S. aureus, the staphylopine biosynthetic enzyme, staphylopine dehydrogenase (CntM), is allosterically regulated by metal concentrations in vitro, thus providing another level of regulatory control, but one that is exquisitely sensitive to the concentration and nature of the metal (Figure 4b) [59].
CP-induced co-restriction of Fe and Zn(II) in A. baumannii provides an example of integration or crosstalk in the adaptive response to global metal limitation [18**]. Fe starvation leads to the cellular depletion of Fe-S cluster proteins including the important cellular reductant ferredoxin [51]. Under these conditions, flavin-harboring flavodoxins may functionally replace ferredoxins, consistent with their relative redox potentials [60], through a Fur-mediated mechanism (Figure 4c) [61,62]. The important point here is that ZigA, required for robust de novo flavin biosynthesis in A. baumannii, is regulated by Zur, and not by Fur [18]. This may reflect a cellular need to metalate a key Zn(II) metalloenzyme of the de novo riboflavin biosynthetic pathway [18], and may well be a consequence of the uniquely large metabolic footprints of Fe and Zn as ubiquitous cofactors in cellular metabolism in a typical Fe-centric bacterium [26].
Other examples of regulatory interconnectivity beyond the response to Fe and Zn co-restriction, have recently been described. For example, the Fur-regulated sRNA PrrF1/2 induced by Fe starvation represses translation of parts of the Zur regulon in P. aeruginosa (Figure 4d) [51**]. Similarly, the S. aureus sRNA RsaC is co-transcribed with the gene encoding the Mn(II) uptake transporter MntABC in response to Mn(II) limitation; mature RsaC targets mRNAs that encode Mn(II)-cofactored superoxide dismutase, favoring synthesis of the cambialistic SOD that utilizes either Mn or Fe [8], but also proteins involved in Fe/Zn(II) transport and Fe and Zn(II) homeostasis [63]. Thus, RsaC may further integrate the adaptive response beyond multi-metal nutritional immunity in S. aureus, to host-mediated oxidative and nitrosative stressors projected to temporally present at the same cellular niche.
Concluding remarks
Bacterial metallostasis is orchestrated by the interconnected actions of a suite of regulatory proteins and metal-sensing riboswitches that collectively function as arbiters of the bioavailability of metals in cells (Figure 1), and thus exert significant control over the metalation status of the proteome [4**]. Host efforts to perturb metallostasis as a means to limit infection, in turn, significantly impact metabolic flow in the infected host, given in particular, the generally large footprints of Zn(II)- and Fe-dependent metabolic machinery [18**,51**]. Multi-metal restriction by host proteins, of which CP is just one [2*,17**], is an emerging theme at the host-pathogen interface, which occurs in the presence of other host stressors; as a result, a systems-level adaptive response, involving multiple layers of regulation and cross-talk, is required in some cases to re-wire metabolism so that essential processes run [18**,51**]. The challenge moving forward is to identify these processes, which are likely unique at specific sites of infection for a specific pathogen within a community of organisms. Multi-“omics” approaches [18**,54] and imaging strategies [64] needed to elucidate metal speciation in bacterial cells and infected tissues [65], promise new insights into this central host-defense process.
Highlights.
Maintaining cellular metal bioavailability is a tightly regulated process
Host-mediated metal sequestration via calprotectin may result in multi-metal restriction
The bacterial response to metal starvation involves intricate regulatory networks
Metal starvation elicits significant crosstalk among metallostasis systems
Overcoming metal ion imbalance involves metabolic remodeling
Acknowledgements
Work in the Giedroc laboratory on bacterial transition metal homeostasis is supported by a grant from the NIH (R35 GM118157). MRJ was supported by a graduate training fellowship in Quantitative and Chemical Biology at Indiana University (T32 GM109825, T32 GM131994). JW was supported by a grant from the National Institute of Allergy and Infectious Diseases (AI101171 to Eric Skaar, Walter Chazin and DPG), while DAC is supported by grants from the Pew, Williams and Bunge & Born Foundations.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang J, Capdevila DA, Giedroc DP: Metal Ion Homeostasis In Encyclopedia of Inorganic Chemistry III. Edited by Lu Y, Que L. Elsevier; 2019: 10.1016/B978-0-12-409547-2.14675-X. [DOI] [Google Scholar]
- 2.Zygiel EM, Nolan EM: Transition Metal Sequestration by the Host-Defense Protein Calprotectin. Annu Rev Biochem 2018, 87:621–643.* This extensive review covers the recent advances in our understanding of the structure and function of calprotectin, with an emphasis on the coordination chemistry of the transition metal binding sites.
- 3.Sheldon JR, Skaar EP: Metals as Phagocyte Antimicrobial Effectors. Curr Opin Immunol 2019, 60:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osman D, Martini MA, Foster AW, Chen J, Scott AJP, Morton RJ, Steed JW, Lurie-Luke E, Huggins TG, Lawrence AD, Deery E, Warren MJ, Chivers PT, Robinson NJ: Bacterial Sensors Define Intracellular Free Energies for Correct Enzyme Metalation. Nat Chem Biol 2019, 15:241–249.** This work experimentally validates a long standing hypothesis that bioavailable metal is tuned to the competitivity of the metal itself thus defining the dterminants for cognate metalloprotein metalation in cells.
- 5.Xu Z, Wang P, Wang H, Yu ZH, Au-Yeung HY, Hirayama T, Sun H, Yan A: Zinc Excess Increases Cellular Demand for Iron and Decreases Tolerance to Copper in Escherichia Coli. J Biol Chem 2019, doi: 10.1074/jbc.ra119.010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg ED: Nutritional Immunity. JAMA 1975, 231:39. [DOI] [PubMed] [Google Scholar]
- 7.Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC: Role of Calprotectin in Withholding Zinc and Copper from Candida Albicans. Infect Immun 2017, 86:IAI.00779–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia YM, Barwinska-Sendra A, Tarrant E, Skaar EP, Waldron KJ, Kehl-Fie TE: A Superoxide Dismutase Capable of Functioning with Iron or Manganese Promotes the Resistance of Staphylococcus Aureus to Calprotectin and Nutritional Immunity. PLoS Pathog 2017, 13:e1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobota JM, Imlay JA: Iron Enzyme Ribulose-5-Phosphate 3-Epimerase in Escherichia Coli Is Rapidly Damaged by Hydrogen Peroxide but Can Be Protected by Manganese. Proc Natl Acad Sci 2011, 108:5402–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin JE, Edmonds KA, Bruce KE, Campanello GC, Eijkelkamp BA, Brazel EB, McDevitt CA, Winkler ME, Giedroc DP: The Zinc Efflux Activator SczA Protects S Treptococcus Pneumoniae Serotype 2 D39 from Intracellular Zinc Toxicity. Mol Microbiol 2017, 104:636–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osman D, Foster AW, Chen J, Svedaite K, Steed JW, Lurie-Luke E, Huggins TG, Robinson NJ: Fine Control of Metal Concentrations Is Necessary for Cells to Discern Zinc from Cobalt. Nat Commun 2017, 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammer ND, Skaar EP: The Impact of Metal Sequestration on Staphylococcus Aureus Metabolism. Curr Opin Microbiol 2012, 15:10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachman MA, Lenio S, Schmidt L, Oyler JE, Weiser JN: Interaction of Lipocalin 2, Transferrin, and Siderophores Determines the Replicative Niche of Klebsiella Pneumoniae during Pneumonia. MBio 2012, 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashige TG, Zhang B, Krebs C, Nolan EM: Human Calprotectin Is an Iron-Sequestering Host-Defense Protein. Nat Chem Biol 2015, 11:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashige TG, Nolan EM: Human Calprotectin Affects the Redox Speciation of Iron. Metallomics 2017, 9:1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Newman DK: Redox Reactions of Phenazine Antibiotics with Ferric (Hydr)Oxides and Molecular Oxygen. Environ Sci Technol 2008, 42:2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zygiel EM, Nelson CE, Brewer LK, Oglesby-Sherrouse AG, Nolan EM: The Human Innate Immune Protein Calprotectin Induces Iron Starvation Responses in Pseudomonas Aeruginosa. J Biol Chem 2019, 294:3549–3562.** This work establishes the importance of CP in Fe(II) sequestration in an aerobic environment which is enhanced by redox cycling metabolites produced by P. aeruginosa.
- 18.Wang J, Lonergan ZR, Gonzalez-Gutierrez G, Nairn BL, Maxwell CN, Zhang Y, Andreini C, Karty JA, Chazin WJ, Trinidad JC, Skaar EP, Giedroc DP: Multi-Metal Restriction by Calprotectin Impacts De Novo Flavin Biosynthesis in Acinetobacter Baumannii. Cell Chem Biol 2019, 26:1–11.** This report explores the impact of CP sequestration on changes in the A. baumannii proteome. Novel responses are identified, and a complex interplay between CP-induced Zn(II) and Fe starvation is described.
- 19.Aron ATT, Heffern MCC, Lonergan ZRR, Vander Wal MNN, Blank BRR, Spangler B, Zhang Y, Park HMM, Stahl A, Renslo ARR, Skaar EPP, Chang CJJ: In Vivo Bioluminescence Imaging of Labile Iron Accumulation in a Murine Model of Acinetobacter Baumannii Infection. Proc Natl Acad Sci 2017, 114:12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besold AN, Culbertson EM, Nam L, Hobbs RP, Boyko A, Maxwell CN, Chazin WJ, Marques AR, Culotta VC: Antimicrobial Action of Calprotectin That Does Not Involve Metal Withholding. Metallomics 2018, 10:1728–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radin JN, Zhu J, Brazel EB, McDevitt CA, Kehl-Fie TE: Synergy between Nutritional Immunity and Independent Host Defenses Contributes to the Importance of the MntABC Manganese Transporter during Staphylococcus Aureus Infection. Infect Immun 2018, 87:e00642–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP: Nutrient Metal Sequestration by Calprotectin Inhibits Bacterial Superoxide Defense Enhancing Neutrophil Killing of Staphylococcus Aureus. Cell Host Microbe 2011, 10:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin JE, Le MT, Bhattarai N, Capdevila DA, Shen J, Winkler ME, Giedroc DP: A Mn-Sensing Riboswitch Activates Expression of a Mn2+/Ca2+ ATPase Transporter in Streptococcus. Nucleic Acids Res 2019, 47:6885–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santiago AG, Chen T-Y, Genova LA, Jung W, George Thompson AM, McEvoy MM, Chen P: Adaptor Protein Mediates Dynamic Pump Assembly for Bacterial Metal Efflux. Proc Natl Acad Sci 2017, 114:201704729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capdevila DA, Edmonds KA, Giedroc DP: Metallochaperones and Metalloregulation in Bacteria. Essays Biochem 2017, 61:177–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisher JP, Giedroc DP: Manganese Acquisition and Homeostasis at the Host-Pathogen Interface. Front Cell Infect Microbiol 2013, 3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin J-H, Jung HJ, An YJ, Cho Y-B, Cha S-S, Roe J-H: Graded Expression of Zinc-Responsive Genes through Two Regulatory Zinc-Binding Sites in Zur. Proc Natl Acad Sci 2011, 108:5045–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Z, Gabriel SE, Helmann JD: Sequential Binding and Sensing of Zn(II) by Bacillus Subtilis Zur. Nucleic Acids Res 2011, 39:9130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pi H, Helmann JD: Sequential Induction of Fur-Regulated Genes in Response to Iron Limitation in Bacillus Subtilis. Proc Natl Acad Sci 2017, 114:12785–12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin J, Helmann JD: Molecular Logic of the Zur-Regulated Zinc Deprivation Response in Bacillus Subtilis. Nat Commun 2016, 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panina EM, Mironov AA, Gelfand MS: Comparative Genomics of Bacterial Zinc Regulons: Enhanced Ion Transport, Pathogenesis, and Rearrangement of Ribosomal Proteins. Proc Natl Acad Sci 2003, 100:9912–9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandrangsu P, Huang X, Gaballa A, Helmann JD: Bacillus Subtilis FolE Is Sustained by the ZagA Zinc Metallochaperone and the Alarmone ZTP under Conditions of Zinc Deficiency. Mol Microbiol 2019, 112:751–765.** This work quantifies the extent of Zn(II) release from ribosomal stores under conditions of zinc limitation, while providing evidence that a candidate Zn(II) metallochaperone ZagA sustains folate biosynthesis by physically interaccting with GTP cyclohydrolase IA.
- 33.Li Y, Sharma MR, Koripella RK, Yang Y, Kaushal PS, Lin Q, Wade JT, Gray TA, Derbyshire KM, Agrawal RK, Ojha AK: Zinc Depletion Induces Ribosome Hibernation in Mycobacteria. Proc Natl Acad Sci U S A 2018, 115:8191–8196.* The authors identify a factor that binds to and inactivates Zur-regulated low-zinc remodeled ribosomes in M. tuberculosis.
- 34.Zheng C, Black KA, Dos Santos PC: Diverse Mechanisms of Sulfur Decoration in Bacterial Trna and Their Cellular Functions. Biomolecules 2017, 7:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng H, Zhang X, Ding M, Zhang X, Zhu Y: Transcriptome Profiles of Soybean Leaves and Roots in Response to Zinc Deficiency. Physiol Plant 2019, 167:330–351. [DOI] [PubMed] [Google Scholar]
- 36.Nairn BL, Lonergan ZR, Wang J, Braymer JJ, Zhang Y, Calcutt MW, Lisher JP, Gilston BA, Chazin WJ, de Crécy-Lagard V, Giedroc DP, Skaar EP: The Response of Acinetobacter Baumannii to Zinc Starvation. Cell Host Microbe 2016, 19:826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan MR, Wang J, Weiss A, Skaar EP, Capdevila DA, Giedroc DP: Mechanistic Insights into the Metal-Dependent Activation of Zn II -Dependent Metallochaperones. Inorg Chem 2019, 58:13661–13672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghssein G, Brutesco C, Ouerdane L, Fojcik C, Izaute A, Wang S, Hajjar C, Lobinski R, Lemaire D, Richaud P, Voulhoux R, Espaillat A, Cava F, Pignol D, Borezée-Durant E, Arnoux P: Biosynthesis of a Broad-Spectrum Nicotianamine-like Metallophore in Staphylococcus Aureus. Science 2016, 352:1105–9. [DOI] [PubMed] [Google Scholar]
- 39.EI Koh, Hung CS Parker KS, Crowley JR Giblin DE, Henderson JP: Metal Selectivity by the Virulence-Associated Yersiniabactin Metallophore System. Metallomics 2015, 7:1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lhospice S, Gomez NO, Ouerdane L, Brutesco C, Ghssein G, Hajjar C, Liratni A, Wang S, Richaud P, Bleves S, Ball G, Borezée-Durant E, Lobinski R, Pignol D, Arnoux P, Voulhoux R: Pseudomonas Aeruginosa Zinc Uptake in Chelating Environment Is Primarily Mediated by the Metallophore Pseudopaline. Sci Rep 2017, 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng H, Shen J, Edmonds KA, Luebke JL, Hickey AK, Palmer LD, Chang FJ, Bruce KA, Kehl-Fie TE, Skaar EP, Giedroc DP: Sulfide Homeostasis and Nitroxyl Intersect via Formation of Reactive Sulfur Species in Staphylococcus Aureus. mSphere 2017, 2:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grim KP, San Francisco B, Radin JN, Brazel EB, Kelliher JL, Solorzano KP, Kim PC, Mcdevitt CA, Kehl-fie TE: The Metallophore Staphylopine Enables Staphylococcus Aureus To Compete with the Host for Zinc and Overcome Nutritional Immunity. MBio 2017, 8:1–16.* This study provides evidene that the broad range metallophore staphylopine functions specifically in Zn(II) uptake in response to CP treatment.
- 43.Sankaran B, Bonnett SA, Shah K, Gabriel S, Reddy R, Schimmel P, Rodionov DA, De Crécy-Lagard V, Helmann JD, Iwata-Reuyl D, Swairjo MA: Zinc-Independent Folate Biosynthesis: Genetic, Biochemical, and Structural Investigations Reveal New Metal Dependence for GTP Cyclohydrolase IB. J Bacteriol 2009, 191:6936–6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radin JN, Kelliher JL, Solorzano PKP, Grim KP, Ramezanifard R, Slauch JM, Kehl-Fie TE: Metal-Independent Variants of Phosphoglycerate Mutase Promote Resistance to Nutritional Immunity and Retention of Glycolysis during Infection. PLOS Pathos 2019, 15:e1007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haas CE, Rodionov DA, Kropat J, Malasarn D, Merchant SS, de Crécy-Lagard V: A Subset of the Diverse COG0523 Family of Putative Metal Chaperones Is Linked to Zinc Homeostasis in All Kingdoms of Life. BMC Genomics 2009, 10:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikhaylina A, Ksibe AZ, Scanlan DJ, Blindauer CA: Bacterial Zinc Uptake Regulator Proteins and Their Regulons. Biochem Soc Trans 2018, 46:983–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lonergan ZR, Nairn BL, Wang J, Hsu Y-P, Hesse LE, Beavers WN, Chazin WJ, Trinidad JC, VanNieuwenhze MS, Giedroc DP, Skaar EP: An Acinetobacter Baumannii, Zinc-Regulated Peptidase Maintains Cell Wall Integrity during Immune-Mediated Nutrient Sequestration. Cell Rep 2019, 26:2009–2018.e6.* This study describes s Zur-regulated cell wall-remodeing zinc metallopeptidase that promotes growth under conditions of extreme Zn(II) restriction.
- 48.Murphy SG, Alvarez L, Adams MC, Liu S, Chappie JS, Cava F, Dörr T: Endopeptidase Regulation as a Novel Function of the Zur-Dependent Zinc Starvation Response. MBio 2019, 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masse E, Gottesman S: A Small RNA Regulates the Expression of Genes Involved in Iron Metabolism in Escherichia Coli. Proc Natl Acad Sci 2002, 99:4620–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khakh SK, Antelmann H, Helmann JD, Song K-B, Aguilar C, Smaldone GT, Gaballa A: The Bacillus Subtilis Iron-Sparing Response Is Mediated by a Fur-Regulated Small RNA and Three Small, Basic Proteins. Proc Natl Acad Sci 2008, 105:11927–11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson CE, Huang W, Brewer LK, Nguyen AT, Kane MA, Wilks A, Oglesby-Sherrouse AG: Proteomic Analysis of the Pseudomonas Aeruginosa Iron Starvation Response Reveals PrrF Small Regulatory RNA-Dependent Iron Regulation of Twitching Motility, Amino Acid Metabolism, and Zinc Homeostasis Proteins. J Bacteriol 2019, 201:1–23.** This work investigates the Pseudomonas aeruginosa iron starvation response using quantitative proteomics approaches, establishing crosstalk between Zn(II) and Fe homeostasis via the sRNA PrrF.
- 52.Prévost K, Salvail H, Desnoyers G, Jacques JF, Phaneuf É, Massé E: The Small RNA RyhB Activates the Translation of ShiA MRNA Encoding a Permease of Shikimate, a Compound Involved in Siderophore Synthesis. Mol Microbiol 2007, 64:1260–1273. [DOI] [PubMed] [Google Scholar]
- 53.Macomber L, Imlay JA: The Iron-Sulfur Clusters of Dehydratases Are Primary Intracellular Targets of Copper Toxicity. Proc Natl Acad Sci U S A 2009, 106:8344–8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tottey S, Waldron KJ, Firbank SJ, Reale B, Bessant C, Sato K, Cheek TR, Gray J, Banfield MJ, Dennison C, Robinson NJ: Protein-Folding Location Can Regulate Manganese-Binding versus Copper- or Zinc-Binding. Nature 2008, 455:1138–1142. [DOI] [PubMed] [Google Scholar]
- 55.Ma Z, Cowart DM, Scott RA, Giedroc DP: Molecular Insights into the Metal Selectivity of the Copper(I)-Sensing Repressor CsoR from Bacillus Subtilis. Biochemistry 2009, 48:3325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glauninger H, Zhang Y, Higgins KA, Jacobs AD, Martin JE, Fu Y, Coyne Rd HJ, Bruce KE, Maroney MJ, Clemmer DE, Capdevila DA, Giedroc DP: Metal-Dependent Allosteric Activation and Inhibition on the Same Molecular Scaffold: The Copper Sensor CopY from Streptococcus Pneumoniae. Chem Sci 2018, 9:105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Z, Faulkner MJ, Helmann JD: Origins of Specificity and Cross-Talk in Metal Ion Sensing by Bacillus Subtilis Fur. Mol Microbiol 2012, 86:1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fojcik C, Arnoux P, Ouerdane L, Aigle M, Alfonsi L, Borezée-Durant E: Independent and Cooperative Regulation of Staphylopine Biosynthesis and Trafficking by Fur and Zur. Mol Microbiol 2018, 108:159–177. [DOI] [PubMed] [Google Scholar]
- 59.Hajjar C, Fanelli R, Laffont C, Brutesco C, Cullia G, Tribout M, Nurizzo D, Borezée-Durant E, Voulhoux R, Pignol D, Lavergne J, Cavelier F, Arnoux P: Control by Metals of Staphylopine Dehydrogenase Activity during Metallophore Biosynthesis. J Am Chem Soc 2019, 141:5555–5562. [DOI] [PubMed] [Google Scholar]
- 60.Yoch DC, Valentine RC: Ferredoxins and Flavodoxins of Bacteria. Annu Rev Microbiol 1972, 26:139–162. [DOI] [PubMed] [Google Scholar]
- 61.Vasileva D, Janssen H, Honicke D, Ehrenreich A, Bahl H: Effect of Iron Limitation and Fur Gene Inactivation on the Transcriptional Profile of the Strict Anaerobe Clostridium Acetobutylicum. Microbiology 2012, 158:1918–1929. [DOI] [PubMed] [Google Scholar]
- 62.Erdner DL, Anderson DM: Ferredoxin and Flavodoxin as Biochemical Indicators of Iron Limitation during Open-Ocean Iron Enrichment. Limnol Oceanogr 1999, 44:1609–1615. [Google Scholar]
- 63.Lalaouna D, Baude J, Wu Z, Tomasini A, Chicher J, Marzi S, Vandenesch F, Romby P, Caldelari I, Moreau K: RsaC SRNA Modulates the Oxidative Stress Response of Staphylococcus Aureus during Manganese Starvation. Nucleic Acids Res 2019, 47:9871–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cassat JE, Moore JL, Wilson KJ, Stark Z, Prentice BM, Van de Plas R, Perry WJ, Zhang Y, Virostko J, Colvin DC, Rose KL, Judd AM, Reyzer ML, Spraggins JM, Grunenwald CM, Gore JC, Caprioli RM, Skaar EP: Integrated Molecular Imaging Reveals Tissue Heterogeneity Driving Host-Pathogen Interactions. Sci Transl Med 2018, 10:eaan6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perry WJ, Spraggins JM, Sheldon JR, Grunenwald CM, Heinrichs DE, Cassat JE, Skaar EP, Caprioli RM: Staphylococcus Aureus Exhibits Heterogeneous Siderophore Production within the Vertebrate Host. Proc Natl Acad Sci 2019, 116:21980–21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baichoo N, Wang T, Ye R, Helmann JD: Global Analysis of the Bacillus Subtilis Fur Regulon and the Iron Starvation Stimulon. Mol Microbiol 2002, 45:1613–1629. [DOI] [PubMed] [Google Scholar]