Abstract
Introduction:
Short sleep duration and quality are problems with many youth, and these are associated with difficulties in executive function which may interfere with self-management behaviors. Thus, our purpose was to describe subjective and objective sleep characteristics and their associations with executive function, stress and coping, adjustment, and diabetes self-management in youth with Type 1 Diabetes (T1D).
Method:
Youth with T1D (N=40, M age 13.4±1.9 years, 60% female, 77.1% non-Hispanic white, diabetes duration 7.1±4.6 years, HbA1c 8.2±1.2%) wore an actigraph and a continuous glucose monitor concurrently for 3–7 days and completed questionnaires. Descriptive and bivariate analyses were conducted.
Results:
Sleep variability was associated with higher stress, higher depressive symptoms, and more glucose variability. More consistent rest-activity rhythm timing was associated with fewer trait anxiety symptoms. More robust rhythms were associated with better diabetes self-management.
Discussion:
Providers should routinely assess sleep habits in youth, especially those with T1D. Improving consistency in sleep timing and sleep duration may be a potential therapeutic target to improve diabetes clinical outcomes.
Keywords: diabetes mellitus, type 1; sleep; diabetes self-management; youth
Introduction
Short sleep duration, defined as less than 8–9 hours per night at least three times per week, is highly prevalent among youth in the United States (Estrada, 2012; Wheaton, Jones, Cooper, & Croft, 2018). The recommended sleep duration is 9 to 12 hours per night for children 6 to 12 years of age and 8 to 10 hours per night for adolescents 13 to 18 years of age (Paruthi et al., 2016). As youth transition to adolescence, meeting this recommended sleep duration is challenging due to social demands, the use of electronic media (Cain, 2010), and biological changes (e.g., later chronotype, delayed melatonin secretion) (Crowley, 2007). Coupled with the need to rise for early school start times, youth frequently report sleep deficits (Jaser et al., 2017). Short sleep duration in youth is associated with poorer academic performance, cognitive impairments, such as deficits in executive function and impaired memory consolidation, poorer quality of life (Durmer & Dinges, 2005; Frier, 2016; Graveling & Frier, 2017; Jauch-Chara, Schmid, Hallschmid, Born, & Schultes, 2008; Wolfson, 2003), and impaired body weight regulation (Knutson & Van Cauter, 2008; Kohatsu et al., 2006; Spiegel, 1999).
Type 1 diabetes (T1D), one of the most prevalent chronic conditions in youth, affects over 200,000 children and adolescents in the United States (Centers for Disease Control and Prevention, 2017; Chiang, Kirkman, Laffel, & Peters, 2014). Further, only 17–23% of youth with T1D achieve targets for glycemic control (glycosylated hemoglobin A1C <7.5%) (Miller et al., 2015). Poor glycemic control is associated with premature macrovascular and microvascular complications (Beck et al., 2017). Self-management of T1D requires a variety of intensive daily treatment activities, including regular blood glucose monitoring and multiple daily injections (American Diabetes Association, 2019). The demands of this intensive treatment regimen regularly interfere with healthy sleep habits (Barone et al., 2015). In addition, youth typically wake up at least once per night to manage their diabetes (Perfect, Elkins, Lyle-Lahroud, & Posey, 2010).
More than two-thirds of youth with T1D short sleep duration by self-report (Estrada, 2012) and obtain significantly less sleep (Reutrakul et al., 2016) and reduced slow wave sleep compared to their peers without diabetes (Perfect et al., 2010). Reduced slow wave sleep is associated with poorer diabetes self-management, glycemic control, and diabetes quality of life (DQOL), and more daytime sleepiness in youth with T1D (McDonough et al., 2017). Short sleep duration contributes to insulin resistance and impaired glucose metabolism and is associated with poorer glycemic control in youth and young adults with T1D (Barone et al., 2015; Jaser & Ellis, 2016; Perfect et al., 2012; Reutrakul et al., 2016; Van Cauter, 2011). Longer sleep duration is associated with better diabetes self-management in youth with T1D (McDonough, Clements, DeLurgio, & Patton, 2017).
Approximately half of all hypoglycemic events occur overnight during sleep and may persist for several hours (Snogdal et al., 2012), and most episodes of hypoglycemia that occur overnight do not lead to nocturnal awakening (Graveling & Frier, 2017; Woodward, 2009). Approximately 30% of youth with T1D experience nocturnal hypoglycemia at least three times weekly, regardless of the use of multiple daily injections or insulin pumps (Woodward, 2009).
Nocturnal hypoglycemia is associated with impaired cognitive functioning, adding to the cognitive deficits that occur as a result of short sleep duration alone (Graveling & Frier, 2017). There are distinct electroencephalogram characteristics during hypoglycemia, such as higher amplitude and lower frequency (Snogdal, et al., 2012). Greater frequency of nighttime hypoglycemia worsens fear of hypoglycemia overnight, adding to sleep disruptions (Anderbro, 2015). In middle-aged adults with T1D, nighttime hypoglycemia has been linked to poorer self-reported sleep quality and poorer daily performance at work, including leaving work early and missed work days due to daytime fatigue (Fulcher et al., 2014).
An emerging trend in research is not only to examine the duration of sleep, but also the timing and regularity of sleep. Sleep variability, varying day-to-day sleep schedules, is associated with poorer glycemic control in youth with T1D (e.g., higher HbA1c levels) (Patel et al., 2018). Variability in sleep duration may reflect alterations between sleep deprivation and compensation and leads to shifts in circadian timing. Circadian rhythms represent endogenously generated oscillations in physiology that occur during a 24-hour period (Williams, McLin, Dressman, & Neubauer, 2016). The circadian timing system promotes wakefulness in the evening and sleep in the early morning (Hagenauer, Perryman, Lee, & Carskadon, 2009). The endogenous circadian period and light sensitivity of the circadian system are altered during puberty (Hagenauer, Perryman, Lee, & Carskadon, 2009; Taylor, Jenni, Acebo, & Carskadon, 2005).
Assessment of sleep characteristics (e.g., total sleep time, sleep/wake times, sleep quality) are not routinely addressed in care of many youth, including those with T1D. Understanding sleep patterns and sources of disturbances in these youth may lead to the development of interventions designed to improve sleep quality and duration and in turn, diabetes self-management, glycemia, and long-term diabetes outcomes. Thus, the purpose of this cross-sectional exploratory study was to describe subjective sleep characteristics (habits, duration, quality) and objective sleep characteristics (total sleep time [TST], sleep variability, bed time/rise time, sleep efficiency [SE], wake after sleep onset [WASO], sleep onset latency [SOL]), rest-activity rhythm (MESOR, amplitude, acrophase, and rest-activity quotient) and their associations with glycemic indices (HbA1c, variability) in youth with T1D. We also explored the relationships among objective sleep characteristics and executive function, stress and coping, diabetes self-management, and adjustment (glycemic control, glucose variability, DQOL, depressive and anxiety symptoms). Based on our literature review, we hypothesized that shorter subjective and objective sleep duration and other objective sleep characteristics (e.g., more severe sleep variability) would be associated with poorer glycemic control (e.g., higher HbA1c) and greater glycemic variability.
Material and Methods
We recruited a sample of 68 youth with T1D and their caregivers from the Yale Children’s Type 1 Diabetes Program and 40 completed the study. Youth were eligible to participate if they were between the ages of 10–16 years, diagnosed with T1D for at least 6 months, without any other major health problem, not currently participating in other intervention studies, and able to read/speak English fluently. The age range was chosen to capture the period of deteriorating glycemic control during the transition to adolescence.
Approval was obtained from the Yale Human Investigation Committee. Trained research assistants (RAs) approached youth and their caregivers during regularly scheduled visits at the Yale Children’s Type 1 Diabetes Program. After informed consent and verbal assent were obtained, youth and caregivers completed questionnaires, and the youth completed a test of executive function (Trail A and B). Youth were then given the Phillips Respironics Actiwatch2™, a wrist-worn device, to wear continuously on their non-dominant wrist for 1 week, removing only for bathing. They were instructed to depress the event marker at “lights out” and “lights on” times to demarcate time in bed. Youth who did not already use a CGM were given a Medtronic iPro™ CGM to wear continuously for the same week with the Actiwatch.
Youth completed sleep diaries daily in the mornings and evenings to track daytime sleep-related behaviors (e.g., caffeine use, exercise) and nocturnal sleep characteristics (e.g., bedtime, awakening). An RA called participants the day after enrollment to address any problems and again at the end of the week to remind them to complete the surveys and return the watch and dairy in a pre-paid mailer. Youth and caregivers received an incentive for their time to complete questionnaires and received an additional incentive for returning the watch and sleep diary.
A total of 104 youth with T1D were approached to participate. Of these, 36 declined participation due to time commitment, lack of interest, or refusal to wear a CGM. A total of 68 participants consented and completed baseline questionnaires. Of these, 40 completed all questionnaires and successfully wore the CGM and Actiwatch for 3 to 7 continuous days/nights (mean = 6.4±1.1).
Measures
Demographic data (family demographics, including income, race/ethnicity, parent education, marital status, adolescent gender) were collected from parents or guardians. Total family income was categorized as less than $40,000, $40,000 to $80,000, or greater than $80,000. Race/ethnicity was self-reported and dichotomized as white/non-Hispanic or non-white. Marital status of parents/guardians was categorized as married/partnered or single/divorced. All other scales were completed by the adolescent.
Subjective sleep characteristics were assessed using the Pittsburgh Sleep Quality Index (PSQI) of sleep parameters and the Adolescent Sleep Habits Survey (ASHS, Buysse, 1989; Shahid, Wilkinson, Marcu, & Shapiro, 2012). The PSQI is a 19-item self-report measure that assesses sleep duration and quality during the past month. Each item is grouped into 1 of 7 components and each component yields a score from 0–3, with 3 indicating poor quality. Scores have been shown to be associated with objective measures of sleep such as polysomnography, and the measure has been used in studies with youth (Megdal & Schernhammer, 2007). Component scores are summed and range from 0–21 with a higher score indicating poorer sleep quality. The total score was used in the analysis. Cronbach’s alpha in this sample was 0.87.
The ASHS was used to measure daytime sleepiness (Shahid et al., 2012). The 16-item subscale measured sleepiness during the daytime activities on a 6-point Likert scale (no problem at all to a very big problem), frequency of naps, struggle to stay awake or falling asleep in various situations (e.g., face to face conversation, watching television, eating a meal), and car accidents related to sleepiness (for those with a driver’s license). Cronbach’s alpha in this sample was 0.75.
Objective sleep characteristics were measured using the Actiwatch 2™ (Phillips Respironics). From these data, sleep/wake patterns and rest-activity rhythm were determined from the frequency of movement in 30 second intervals (Ancoli-Israel et al., 2003). Participants wore the Actigraph on their non-dominant wrist for at least 72 hours according to the recommended duration (Morgenthaler, et al., 2007). Actigraphy data from the watch were analyzed using Philips Actiware™ software to calculate TST, SE, WASO, and SOL. Youth completed a sleep diary that was used to validate bedtime and waketime. The rest-activity rhythm was computed from continuous motor activity measured from 24-hour wrist actigraphy and reflected both endogenous circadian rhythms (e.g., melatonin) and exogenous rhythms (e.g., 24-hour light-dark cycle) and the timing of sleep and activity (Brown, Smolensky, D’Alonzo, & Redman, 1990; Luik, Zuurbier, Hofman, Van Someren, & Tiemeier, 2013). From the rest-activity data for consecutive days, the rest-activity rhythm of each participant was estimated using a single component cosinor model which produced four rest-activity rhythm parameters including MESOR (24-hour rhythm-adjusted mean activity movements; higher values represent more robust movement), amplitude (measure of the extent of rhythmic change or range of activity and rest values over 24-hours), acrophase (peak alertness time; actual clock time of the peak amplitude), and rest-activity quotient (amplitude/MESOR) (Levin et al., 2005). Actigraphy has been validated for use with youth with sensitivity and specificity scores of 95.0 and 74.5, respectively (Meltzer, 2012).
Sleep variability was calculated using the SD of total sleep time across the nights, representing the variation within-subjects in sleep night-to-night (Patel et al., 2018). We used paired t tests to compare sleep data between weekdays (Monday through Thursday) and weekends (Friday and Saturday). Bivariate correlations were performed to determine the associations among objective characteristics of sleep (total sleep time and sleep variability) and outcomes (executive function, stress, diabetes self-management, and adjustment).
Executive function was measured by two tests. The Diabetes-Related Executive Functioning Scale (DREFS) is a 77-item self-report measure designed to assess 11 domains of diabetes-related executive function (Duke, Raymond, & Harris, 2014). This measure assesses planning, organizing materials, task initiation, monitoring of actions, mental flexibility, time management, emotion regulation, inhibition, distractibility, memory, and sequential task completion. Questions are answered using a 5-point Likert scale. Total scores range from 77 to 385, with higher scores indicating better executive functioning. Cronbach’s alpha in this sample was 0.87.
The Trail-Making Test (TMT) is administered by an investigator and is one of the most widely used measures of cognitive processing and executive function (Sanchez-Cubillo et al., 2009). It is sensitive to impairment in a variety of cognitive domains. It consists of two parts, A and B, each of which represent the time to completion of the tasks. The TMT-A requires an individual to draw lines sequentially connecting 25 encircled numbers on a paper and the TMT-B requires an individual to alternate between numbers and letters (e.g., 1, A, 2, B, 3, C). The score represents the amount of time required to complete the task. The average for the TMT-B is 75 seconds, with deficiencies noted > 273 seconds (Sanchez-Cubillo et al., 2009) Both parts were used in this study. Higher scores reflect poorer executive function. This measure was scored by subtracting the score of part A from part B.
Stress and coping were measured using the Responses to Stress Questionnaire (RSQ), a 67-item measure that assesses diabetes-specific stress and coping in youth (Connor Smith, 2000). The first 10 items measure the frequency of diabetes-specific stress, such as stress about revealing the diabetes diagnosis to peers, stress about poor HbA1c levels, and stress about daily management of diabetes. Diabetes-specific stress scores range from 0 to 30, with higher scores indicating greater stress. A score of 10 or higher indicates high diabetes-specific stress. The remaining 57 items measure responses to stress (coping) with a range of voluntary and involuntary response to stressors. This measure has good reliability and validity in children (Connor Smith, 2000). The Cronbach’s alpha was 0.87 in our sample.
Diabetes self-management was measured by the Self-Care Inventory (SCI), a 14-item measure of youth perceptions of their adherence to treatment recommendations for their diabetes (La Greca, 2004). A mean score is created, ranging from 1 (never do it) to 5 (always do this as recommended without fail). Higher scores reflect better diabetes self-management. The total score was used in the analysis. Cronbach’s alpha in this sample was 0.79.
Glycemic control was determined from HbA1c, and glucose variability from CGM means and variability indices. The American Diabetes Association (ADA) recommends a target HbA1c of 7.5% (58 mmol/mol) or below in youth (ADA, 2019). The majority (80%) of analyses in our sample were performed using the Bayer Diagnostics DCA2000™ (Bayer, Tarrytown, NY) that has a normal range of 4.2–6.3% (22 mmol/mol – 45 mmol/mol).
We used the Medtronic iPro 2™ Professional CGM (Medtronic MiniMed, Inc.) or the participants’ own CGM to measure glucose continuously over the monitoring period. CGM data were used to calculate low blood glucose index and high blood glucose index, measures of frequency and extent of low and high blood glucose using the blood glucose risk function, where blood glucose is measured in mg/dL (Clarke & Kovatchev, 2009). The variability of CGM was represented by blood glucose risk index. Based on Poincarè Plot with individual CGM, short- and long-term variability of CGM was measured and labeled SD1 and SD2, respectively. The area (AFE=π×SD1×SD2) and shape (SFE=SD2/SD1) of the fitting ellipse were calculated to measure overall variability and ratio of long-term variability to short-term variability.
Adjustment measures included depressive symptoms, anxiety symptoms, and DQOL. We included these three variables because they have been highly correlated in previous studies (Moreira, Soares, Cristina Maria Bouissou Morais, Teixeira, e Silva, Ana Cristina Simões, & Kummer, 2015; Pan & Yeh, 2017). For example, anxiety and depressive symptoms are highly comorbid (Coplan, Aaronson, Panthangi, & Kim, 2015). Poor quality of life is frequently associated with depressive symptoms (Verma et al., 2017). The Children’s Depression Inventory (CDI) is a 27-item self-report measure of depressive symptoms, including mood disturbance, self-evaluation, vegetative functions, and interpersonal behaviors (Kovacs, 1985). Scores range from 0 to 54. The total score was used in this analysis. Youth who scored above the threshold for depression (12 or higher) were referred to the clinic social worker or psychologist for evaluation. Cronbach’s alpha in this sample was 0.90.
The State-Trait Anxiety Inventory for Children (STAIC) was used to measure anxiety symptoms (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1970). It is one of the most widely used scales to measure anxiety symptoms in children 8 to 12 years of age, but it has also been used in youth 13 to 18 years of age (Ingerski, Anderson, Dolan, & Hood, 2010). The self-report measure contains 40 items, 20 that measure state anxiety symptoms, a transient emotional state of fear or tension, and 20 that measure trait anxiety symptoms, a tendency toward feeling anxious. Participants are asked to evaluate their state anxiety “at the moment” and their trait anxiety “in general.” Scores range from 20 to 60. Higher scores reflect more anxiety symptoms. The scale has an approximate cut point of 40, above which is suggested to detect clinically significant anxiety symptoms (Knight, Waal-Manning, & Spears, 1983) Responses are rated on a 4-point Likert scale from low to high. Cronbach’s alpha in this sample was 0.85.
DQOL was measured by the Diabetes-Specific Pediatric Quality of Life (Peds-QL™ 3.0) scale (Varni, 2003). This 28-item measure includes five subscales, including general DQOL (11 items), general T1D treatment QOL (4 items), specific T1D treatment QOL (7 items), worry (3 items), and communication (3 items). In each subscale, respondents are asked to report on how much of a problem each type has been over the past month, ranging from “never a problem” to “almost always a problem.” DQOL scores range from 0 to 100, with higher scores indicating better DQOL. Total score was used in this analysis. High reliability and validity have been established (Varni, James, Seid, & Kurtin, 2001). Cronbach’s alpha was 0.87 in this sample.
Data Analyses
Actigraphy data were scored with Actiware™ v. 5 software. Questionnaires and diary forms in a scannable format were examined for improperly filled items and missing data, scanned and committed to an MS Access database. Data were analyzed using SPSS v. 25 and SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). Prior to the analysis, data were screened for missing or out of range values and distributions of continuous variables. Descriptive statistics were calculated for all variables. The RSQ, CDI, STAIC, SCI, and HbA1c variables violated assumptions of normality; thus, nonparametric tests (Mann-Whitney U, Kruskal-Wallis, and Spearman) were used in these analyses. To examine for covariance among the variables of interest (age, gender, race, income, duration of diabetes), a series of independent t tests, one-way analyses of variance, and correlations were used for normally distributed variables.
We also examined associations between the actigraphy-based sleep variables (TST, sleep variability) and rest-activity parameters (MESOR, Amplitude, Acrophase, and rest-activity quotient) with CGM measures (overall mean glucose, low blood glucose index, high blood glucose index, and overall glucose variability).
Results
The final sample included 40 youth with T1D with a mean age of 13.4±1.9 years, 60% female, 77.1% were non-Hispanic white, and a mean duration of diabetes of 7.1 ±4.6 years. The mean glucose across all days of CGM data was 182.7 mg/dL (±34.0). Most participants (97.3%) used an insulin pump for treatment and had their own CGM (70.6%). Mean HbA1c was 8.2±1.2% (66 mmol/mol), slightly better than in a nationally representative sample (8.9%, 74 mmol/mol) (Varni et al., 2001). Participant characteristics are shown in Table 1.
Table 1.
Sample characteristics N = 40
| Characteristics | N | (%) or Mean ± SD | |
|---|---|---|---|
| Age (years) | 40 | 13.28 | ± 1.9 |
| Gender | 39 | ||
| Race/ethnicity | 35 | ||
| White, Non-Hispanic | 31 | (88.6) | |
| Non-White | 4 | (11.4) | |
| Annual Income (< 80,000/year) | 34 | (26.5) | |
| Type 1 Diabetes Profile | |||
| T1D duration (years) | 27 | 7.1 | ± 4.6 |
| A1c (%) | 40 | 8.2 | ±1.2 |
| Insulin Pump (% yes) | 39 | (97.4) | |
| Glucose Mean (CGM) a | 40 | 182.7 | ± 34.0 |
Note: For continuous variables, normally distributed data are presented as mean ± SD. Data for categorical variables are presented as n (%). Comparisons between short vs. normal sleepers were made with one-way independent t tests for normally distributed continuous variables, by the Kruskal Wallis test for continuous variables which violated assumptions of normality, and by χ2 for categorical variables. Bold P values are statistically significant.
Descriptive statistics for all variables are displayed in Table 2. Based on actigraphy data, the mean TST ranged from 303.1 – 518.3 minutes, SOL ranged from 0 – 57.9 minutes, SE ranged from 61.4 – 91.9%, WASO ranged from 21.1 – 79.9 minutes, and the sleep fragmentation index ranged from 8.5 – 33.2. Mean and median bedtimes were 11:31 PM and 11:16 PM, respectively, with a range of 9:04 PM to 2:14 AM. The mean and median wake times were 7:31 AM and 7:12 AM respectively, and wake times ranged from 5:28 AM to 11:02 AM. Only 22.5% obtained ≥ 8 hours of sleep on average. Sleep variability ranged from 18.4 min. to 226.4 min. with a mean of 75.4 min. The mean weekday wake time was 7:17 AM, which was earlier than the mean weekend wake time of 8:03 AM. Participants slept slightly, but not significantly, longer on the weekends compared to weekdays (451 minutes vs. 437 minutes).
Table 2.
Descriptive statistics for sleep characteristics, stress and coping, adherence and adjustment
| Measure | N | Mean | SD |
|---|---|---|---|
| Actigraphy | |||
| TST (min) | 40 | 436.7 | 50.1 |
| SOL (min) | 40 | 14.4 | 12.1 |
| SE (%) | 40 | 85.6 | 5.2 |
| WASO (min) | 40 | 41.2 | 14.5 |
| Sleep Fragmentation Index (% + %)1 | 40 | 17.2 | 4.9 |
| Sleep variability (min) | 40 | 75.4 | 47.7 |
| Mesor, counts | 40 | 130.0 | 41.1 |
| Amplitude, counts | 40 | 115.9 | 43.2 |
| Peak time hh:mm | 40 | 3:04 PM | 1:12 PM |
| Rest Activity Quotient | 40 | 0.90 | 0.12 |
| Sleep questionnaires | |||
| Overall Sleep Quality (PSQI)2 | 32 | 6.9 | 1.5 |
| Sleepiness | 30 | 4.4 | 2.2 |
| Executive Function | |||
| DREFS | 32 | 29.8 | 3.5 |
| Trail | 39 | 13.4 | 11.4 |
| Stress and Coping | |||
| Stress (RSQ total stress) | 32 | 10.3 | 4.2 |
| Coping (RSQ total) | 34 | 104.7 | 32.5 |
| Adherence | |||
| Self-Care Inventory | 33 | 28.5 | 5.8 |
| Adjustment | |||
| Glycemic control (A1c) | 39 | 82 | 1.2 |
| Children’s Depression Inventory | 30 | 6.7 | 7.2 |
| Diabetes Quality of Life (Peds-QL) | 61.8 | 13.7 | |
| State Anxiety | 31 | 47.7 | 3.6 |
| Trait Anxiety | 32 | 32.4 | 7.6 |
Sleep fragmentation index (movement index + fragmentation index). It includes both restlessness and fragmentation of the sleep period.
PSQI: Clinical cut off for poor sleep quality is 5 or higher. Higher scores indicate poorer sleep quality.
Youth reported a mean PSQI global sleep quality score of 6.9 (±1.5), which is above the clinical cutoff for poor sleep quality (de la Vega et al., 2015; Grandner, 2006; Woods & Scott, 2016). Participants reported high diabetes-specific stress (RSQ = 10.3±4.2), poor diabetes self-management (SCI = 28.5±5.8), and high state anxiety (STAIC = 47.7±3.6). We present the bivariate correlation matrix of objective TST and sleep variability (executive function, stress and coping, diabetes self-management, and adjustment in Table 3. Higher sleep variability was associated with higher stress (r = 0.45, p < 0.05), higher mean glucose (r = 0.34, p < 0.05), a higher high blood glucose index (HBGI) (r = 0.36, p < 0.05), higher depressive symptoms (rho = 0.38, p < 0.05), and lower trait anxiety symptoms (rho = −0.43, p < 0.05).
Table 3.
Bivariate correlations coefficients between total sleep time, sleep variability, circadian rhythm, and clinical behavioral outcomes
| Outcomes | Total sleep time | Sleep variability | MESOR | Amplitude | Acrophase | Circadian Quotient |
|---|---|---|---|---|---|---|
| Daytime sleepiness | 0.12 | −0.28 | −0.00 | −0.08 | −0.08 | −0.02 |
| Executive function | ||||||
| DREFSY | 0.07 | 0.14 | 0.21 | 0.16 | 0.29 | −0.18 |
| Trail Score (B-A) | −0.31† | −0.11 | −0.22 | −0.30† | 0.28† | −0.32† |
| Stress and Coping | ||||||
| Stress | 0.14 | 0.36* | 0.11 | 0.15 | 0.04 | 0.17 |
| Coping | −0.08 | 0.29† | 0.18 | 0.08 | 0.25 | −0.25 |
| Adherence | ||||||
| Self-Care Inventory | 0.05 | 0.07 | 0.04 | 0.26 | 0.20 | 0.40* |
| Adjustment | ||||||
| Glycemic control | 0.12 | −0.01 | 0.20 | −0.02 | −0.00 | −0.21 |
| Glucose meana | 0.03 | 0.34* | 0.15 | 0.03 | −0.10 | −0.16 |
| Overall glucose variabilitya | 0.06 | 0.30† | −0.01 | 0.02 | −0.17 | 0.14 |
| Low blood glucose indexa | 0.22 | −0.06 | −0.25 | −0.18 | −0.21 | 0.00 |
| High blood glucose indexa | 0.06 | 0.36* | −0.05 | −0.13 | 0.01 | −0.08 |
| Depressive symptoms | −0.03 | 0.38* | −0.10 | −0.23 | −0.19 | −0.29 |
| Diabetes quality of life | 0.06 | 0.25 | −0.18 | −0.09 | −0.16 | 0.05 |
| Trait Anxiety | 0.04 | −0.03 | −0.43* | −0.49** | −0.47* | −0.26 |
| State Anxiety | −0.12 | 0.21 | −0.12 | −0.20 | 0.14 | −0.21 |
Note.
p < 0.10.
bold font indicates:
p < 0.05.
p < 0.01.
p < 0.001
CGM variables
The bivariate correlations between rest-activity rhythm variables (MESOR, amplitude, acrophase, and rest-activity quotient) and clinical behavioral variables are also shown in Table 3. Those with a higher mean MESOR and a higher amplitude had lower trait anxiety symptoms (r = −0.43, p < 0.05; r = −0.49, p < 0.01). Those with a more consistent timing of the rest-activity rhythm (acrophase) had lower trait anxiety symptoms (r = −0.47, p < 0.05). Those with a more robust rhythm (rest-activity quotient) had better diabetes self-management (r = 0.40, p < 0.05). See Figure 1 for sample of a glucose variability and rest-activity rhythm analysis where we fitted the glucose values over the rest activity rhythm.
Figure 1.
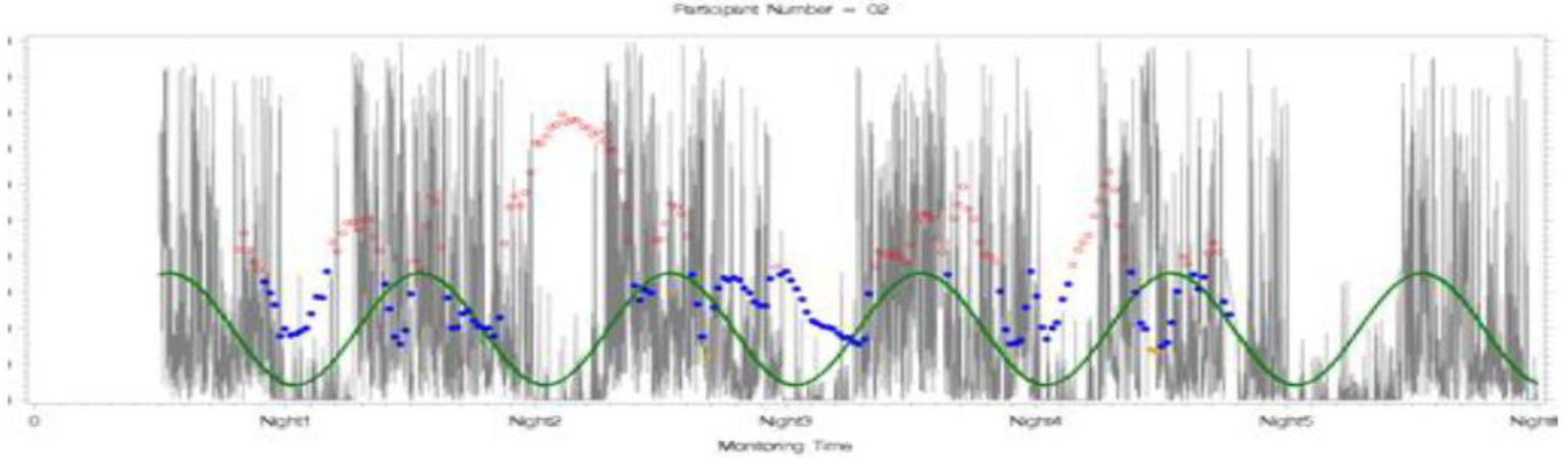
Rest-activity rhythm and glucose variabilities (CGM)
Red: high glucose values; blue: glucose levels in range; green: rest-activity rhythm. We fitted the glucose values over the rest activity rhythm.
Discussion
Our analyses confirmed previous findings and identified new important relationships among sleep characteristics, executive function, adjustment, and self-management in youth with T1D. The high prevalence of short sleep duration in our study (87.5%) was similar to other studies of similar age youth with T1D (87%) (Patel et al., 2018; Perfect, Elkins, Lyle-Lahroud, & Posey, 2010) and worse than in the general adolescent population (68.9%). Sleep variability was similar to those in a previous study of youth with T1D (Patel et al., 2018). Youth slept slightly longer on the weekends compared to weekdays with later mean wake times and bedtimes on the weekends compared with weekdays, and these differences were similar to Patel et al.’s (2018) recent study.
The associations among TST and glycemic control or other variables were not significant in our study. These have varied in other studies. Short sleep duration was associated with poorer glycemic control in previous studies of adults and youth with T1D (Borel et al., 2013; Matejko et al., 2015; Jaser & Ellis, 2016; Perfect et al., 2012; Reutrakul et al., 2016). However, the association between TST and glycemic control was not significant in other studies of adults (Barone et al., 2015; van Dijk et al., 2011) and youth with T1D (Yeshayahu & Mahmud, 2010),
The association between sleep variability and glycemic control was not significant in our study. Sleep variability was associated with glycemic control in studies of youth (Clarke & Kovatchev, 2009) and middle-aged adults (mean age 41.5 years) (Chontong, Saetung, & Reutrakul, 2016). In our study, sleep variability was associated with higher mean glucose and higher high blood glucose index (HBGI). HbA1c is retrospective and may not reflect current trends in glucose data (Pickup, Freeman, & Sutton, 2011). The associations among overall glucose variability and sleep parameters were not significant in our study, although the association between sleep variability and glucose variability did approach significance (r = 0.30, p < 0.10). Also, more severe sleep variability was associated with higher depressive symptoms and lower trait anxiety symptoms. Although the findings related to lower trait anxiety symptoms were unexpected, depressive symptoms may interfere with intraindividual changes in sleep duration, including both increases or decreases in sleep duration. Encouraging consistency in sleep duration may improve depressive symptoms in youth with T1D.
Our findings related to rest-activity rhythm indicate that those with more consistent rhythms had fewer anxiety symptoms. Also, those with a more robust rest-activity rhythm had better self-reported diabetes self-management. Although studies could not be located on rest-activity rhythm and clinical and behavioral variables in youth with diabetes, blunted rest-activity amplitude has been previously noted in children with seasonal affective disorder compared to healthy controls (Glod, Teicher, Polcari, McGreenery, & Ito, 1997).
These results should be considered in the context of the study’s limitations. The study was cross-sectional; therefore, the direction of the associations cannot be inferred. Our sample was mostly Caucasian (88.6%), higher income (73.5% > 85,000/year), female (61.5%), and from one geographic location, limiting generalizability. Nonetheless, this sample was consistent with the clinic population. The lack of significance in the associations among TST, sleep variability, and glycemic control may be due to the small number of participants. We did not collect data about whether youth were in school, so we cannot relate the findings about weekday and weekend differences to their schedules. Study participation occurred year-round and included summer and school vacations. The range of days that our participants wore the Actigraph was three to seven days with a mean of 6.4±1.1 days. While this duration may not fully capture habitual sleep patterns, current recommendations for actigraphy suggest that 72 hours is adequate for monitoring sleep (Morgenthaler, et al., 2007). There may be other factors that may have confounded the results, such as Body Mass Index (BMI), sleep apnea, and the use of self-report measures.
Based on our findings and recent recommendations from the ADA (ADA, 2019), primary care and diabetes clinicians should routinely assess sleep habits of youth with T1D. Sleep is a potentially modifiable target for intervention in this population. More sleep variability is associated with higher stress, higher depressive symptoms, and poorer glucose control as evidenced by the higher mean glucose and higher blood glucose index risk score in the current study. Improving consistency in sleep timing and duration may help to improve diabetes clinical outcomes. In a randomized controlled trial, Perfect and colleagues established that a behavioral intervention where sleep duration was extended by 30 minutes per day led to a 7.4% improvement in mean glucose levels measured by CGM (Perfect, Michelle, Frye, & Bluez, 2018). It is unknown if extending sleep duration over time is sustainable, nor what the long-term impact is on clinical outcomes. Future researchers should address this gap.
Acknowledgements:
The research described here was supported by National Institutes of Health/National Institute of Nursing Research [1P20NR014126]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflicts of interest to disclose.
Contributor Information
Kaitlyn Rechenberg, University of South Florida, College of Nursing, Tampa, Florida 33620.
Nancy Redeker, Yale University, School of Nursing and School of Medicine, West Haven, Connecticut 06477.
Henry Klar Yaggi, Yale University, School of Medicine, New Haven, Connecticut 06511.
Margaret Grey, Yale University, School of Nursing and School of Medicine, West Haven, Connecticut 06477.
References
- American Diabetes Association. (2019). Children and adolescents: Standards of medical care in diabetes—2019. Diabetes Care, 42(Supplement 1), S148–S164. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. (2019). Glycemic targets: Standards of medical care in diabetes—2019. Diabetes Care, 42(Supplement 1), S61–S70. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, & Pollak CP (2003). The role of actigraphy in the study of sleep and circadian rhythms. Sleep, 26(3), 342–392. [DOI] [PubMed] [Google Scholar]
- Anderbro T (2015). Fear of hypoglycemia: Relationship to hypoglycemic risk and psychological factors. Acta Diabetologica, 52(3), 581–589. [DOI] [PubMed] [Google Scholar]
- Barone MTU, Wey D, Schorr F, Franco DR, Carra MK, Lorenzi Filho G, & Menna-Barreto L (2015). Sleep and glycemic control in type 1 diabetes. Archives of Endocrinology & Metabolism, 59, 71–78. [DOI] [PubMed] [Google Scholar]
- Beck J, Greenwood DA, Blanton L, Bollinger ST, Butcher MK, Condon JE, … & Kolb LE (2018). 2017 National standards for diabetes self-management education and support. The Diabetes Educator, 44, 35–50. [DOI] [PubMed] [Google Scholar]
- Borel A, Pepin J, Nasse L, Baguet J, Netter S, & Benhamou P (2013). Short sleep duration measured by wrist actimetry is associated with deteriorated glycemic control in type 1 diabetes. Diabetes Care, 36(10), 2902–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Smolensky MH, D’Alonzo GE, & Redman DP (1990). Actigraphy: A means of assessing circadian patterns in human activity. Chronobiology International, 7(2), 125–133. [DOI] [PubMed] [Google Scholar]
- Buysse DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Cain N (2010). Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Medicine, 11(8), 735–742. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017). National diabetes statistics report, 2017 Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA. [Google Scholar]
- Chiang JL, Kirkman MS, Laffel LM, & Peters AL (2014). Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care, 37(7), 2034–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chontong S, Saetung S, & Reutrakul S (2016). Higher sleep variability is associated with poorer glycaemic control in patients with type 1 diabetes. Journal of Sleep Research, 25(4), 438–444. [DOI] [PubMed] [Google Scholar]
- Clarke W, & Kovatchev B (2009). Statistical tools to analyze continuous glucose monitor data. Diabetes Technology & Therapeutics, 11(S1), S–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor Smith JK (2000). Responses to stress in adolescence: Measurement of coping and involuntary stress responses. Journal of Consulting & Clinical Psychology, 68(6), 976–992. [PubMed] [Google Scholar]
- Coplan JD, Aaronson CJ, Panthangi V, & Kim Y (2015). Treating comorbid anxiety and depression: Psychosocial and pharmacological approaches. World Journal of Psychiatry, 5(4), 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ (2007). Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Medicine, 8(6), 602–612. [DOI] [PubMed] [Google Scholar]
- de la Vega R, Tomé-Pires C, Solé E, Racine M, Castarlenas E, Jensen MP, & Miró J (2015). The Pittsburgh Sleep Quality Index: Validity and factor structure in young people. Psychological Assessment, 27(4), e22. [DOI] [PubMed] [Google Scholar]
- Duke DC, Raymond JK, & Harris MA (2014). The Diabetes Related Executive Functioning Scale (DREFS): Pilot results. Children’s Health Care, 43(4), 327–344. [Google Scholar]
- Durmer J, & Dinges D (2005). Neurocognitive consequences of sleep deprivation. Seminars in Neurology, 25, 117–129. [DOI] [PubMed] [Google Scholar]
- Estrada CL (2012). Insufficient sleep in young patients with diabetes and their families. Biological Research for Nursing, 14, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frier BM (2016). Hypoglycaemia in adults with insulin-treated diabetes in the UK: Self-reported frequency and effects. Diabetic Medicine, 33(8), 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher G, Singer J, Castañeda R, Fraige Filho F, Maffei L, Snyman J, & Brod M (2014). The psychosocial and financial impact of non-severe hypoglycemic events on people with diabetes: Two international surveys. Journal of Medical Economics, 17(10), 751–761. [DOI] [PubMed] [Google Scholar]
- Glod CA, Teicher MH, Polcari A, McGreenery CE, & Ito Y (1997). Circadian rest-activity disturbances in children with seasonal affective disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 36(2), 188–195. [DOI] [PubMed] [Google Scholar]
- Grandner MA (2006). Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep and Biological Rhythms, 4(2), 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveling AJ, & Frier BM (2017). The risks of nocturnal hypoglycaemia in insulin-treated diabetes. Diabetes Research & Clinical Practice, 133, 30–39. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, & Carskadon MA (2009). Adolescent changes in the homeostatic and circadian regulation of sleep. Developmental Neuroscience, 31(4), 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerski LM, Anderson BJ, Dolan LM, & Hood KK (2010). Blood glucose monitoring and glycemic control in adolescence: Contribution of diabetes-specific responsibility and family conflict. Journal of Adolescent Health, 47(2), 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser SS, Foster NC, Nelson BA, Kittelsrud JM, DiMeglio LA, Quinn M, … T1D Exchange Clinic Network. (2017). Sleep in children with type 1 diabetes and their parents in the T1D exchange. Sleep Medicine, 39, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch-Chara K, Hallschmid M, Gais S, Schmid SM, Oltmanns KM, Colmorgen C, … & Schultes B (2007). Hypoglycemia during sleep impairs consolidation of declarative memory in type 1 diabetic and healthy humans. Diabetes Care, 30(8), 2040–2045. [DOI] [PubMed] [Google Scholar]
- Knight RG, Waal-Manning HJ, & Spears GF (1983). Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression Scale. British Journal of Clinical Psychology, 22(4), 245–249. [DOI] [PubMed] [Google Scholar]
- Knutson KL, & Van Cauter E (2008). Associations between sleep loss and increased risk of obesity and diabetes. Annals of the New York Academy of Sciences, 1129, 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu ND, Tsai R, Young T, VanGilder R, Burmeister LF, Stromquist AM, & Merchant JA (2006). Sleep duration and body mass index in a rural population. Archives of internal medicine, 166(16), 1701–1705. [DOI] [PubMed] [Google Scholar]
- Kovacs M (1985). The Children’s Depression Inventory (CDI). Psychopharmacological Bulletin, 21, 995–998. [PubMed] [Google Scholar]
- La Greca A (2004). Manual for the Self Care Inventory. Miami, FL: University of Miami, [Google Scholar]
- Levin RD, Daehler MA, Grutsch JF, Quiton J, Lis CG, Peterson C, … Huff-Adams S (2005). Circadian function in patients with advanced non-small-cell lung cancer. British Journal of Cancer, 93(11), 1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, & Tiemeier H (2013). Stability and fragmentation of the activity rhythm across the sleep-wake cycle: The importance of age, lifestyle, and mental health. Chronobiology International, 30(10), 1223–1230. [DOI] [PubMed] [Google Scholar]
- Matejko B, Kiec-Wilk B, Szopa M, Trznadel Morawska I, Malecki MT, & Klupa T (2015). Are late-night eating habits and sleep duration associated with glycemic control in adult type 1 diabetes patients treated with insulin pumps? Journal of Diabetes Investigation, 6(4), 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough RJ, Clements MA, DeLurgio SA, & Patton SR (2017). Sleep duration and its impact on adherence in adolescents with type 1 diabetes mellitus. Pediatric Diabetes, 18(4), 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megdal SP, & Schernhammer ES (2007). Correlates for poor sleepers in a Los Angeles high school. Sleep Medicine, 9, 60–63. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ (2012). Use of actigraphy for assessment in pediatric sleep research. Sleep Medicine Reviews, 16(5), 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, … & Tamborlane WV (2015). Current state of type 1 diabetes treatment in the US: Updated data from the T1D Exchange clinic registry. Diabetes care, 38(6), 971–978. [DOI] [PubMed] [Google Scholar]
- Moore M (2009). Relationships among sleepiness, sleep time, and psychological functioning in adolescents. Journal of Pediatric Psychology, 34(10), 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira JM, Soares CM, Teixeira AL, Silva AC, & Kummer AM (2015). Anxiety, depression, resilience and quality of life in children and adolescents with pre-dialysis chronic kidney disease. Pediatric Nephrology, 30(12), 2153–2162. [DOI] [PubMed] [Google Scholar]
- Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, … Maganti R (2007). Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. Sleep, 30(11), 1445–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, & Yeh C (2017). Impact of depressive/anxiety symptoms on the quality of life of adolescents with ADHD: A community-based 1-year prospective follow-up study. European Child & Adolescent Psychiatry, 26(6), 659–667. [DOI] [PubMed] [Google Scholar]
- Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, … Quan SF (2016). Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine, 12(6), 785–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NJ, Savin KL, Kahanda SN, Malow BA, Williams LA, Lochbihler G, & Jaser SS (2018). Sleep habits in adolescents with type 1 diabetes: Variability in sleep duration linked with glycemic control. Pediatric Diabetes, 19(6), 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect MM, Patel PG, Scott RE, Wheeler MD, Patel C, Griffin K, … & Quan SF (2012). Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep, 35, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect MM, Elkins GR, Lyle-Lahroud T, & Posey JR (2010). Stress and quality of sleep among individuals diagnosed with diabetes. Stress and Health: Journal of the International Society for the Investigation of Stress, 26, 61–74. [Google Scholar]
- Perfect M, Frye S, & Bluez GP (2018). The effects of a sleep extension intervention on glucose control in youth with type 1 diabetes. Diabetes, 67(S1), 849–P.29440278 [Google Scholar]
- Pickup JC, Freeman SC, & Sutton AJ (2011). Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: Meta-analysis of randomised controlled trials using individual patient data. British Medical Journal, 343, d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S, Thakkinstian A, Anothaisintawee T, Chontong S, Borel A, Perfect MM, Knutson KL (2016). Sleep characteristics in type 1 diabetes and associations with glycemic control: Systematic review and meta-analysis. Sleep Medicine, 23, 26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, & Barcelo F (2009). Construct validity of the trail making test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society, 15(3), 438–450. [DOI] [PubMed] [Google Scholar]
- Shahid A, Wilkinson K, Marcu S, & Shapiro CM (2012). STOP, THAT and one hundred other sleep scales. Springer Science & Business Media: Toronto, CA. [Google Scholar]
- Snogdal LS, Folkestad L, Elsborg R, Remvig LS, Beck-Nielsen H, Thorsteinsson B, Juhl CB (2012). Detection of hypoglycemia associated EEG changes during sleep in type 1 diabetes mellitus. Diabetes Research & Clinical Practice, 98, 91–97. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, & Van Cauter E (1999). Impact of sleep debt on metabolic and endocrine function. The Lancet, 354(9188), 1435–1439. [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1970). STAI manual for the State-Trait Anxiety Inventory. Self-Evaluation Questionnaire, 1–24. [Google Scholar]
- Van Cauter E (2011). Sleep disturbances and insulin resistance. Diabetic Medicine, 28(12), 1455–1462. [DOI] [PubMed] [Google Scholar]
- van Dijk M, Donga E, van Dijk JG, Lammers G, van Kralingen KW, Dekkers OM, Romijn JA (2011). Disturbed subjective sleep characteristics in adult patients with longstanding type 1 diabetes mellitus. Diabetologia, 54(8), 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW (2003). The PedsQL™* 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambulatory Pediatrics, 3(6), 329–341. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, & Kurtin PS (2001). PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ version 4.0 generic core scales in healthy and patient populations. Medical Care, 39(8), 800–812. [DOI] [PubMed] [Google Scholar]
- Verma SK, Luo N, Subramaniam M, Sum CF, Stahl D, Liow PH, & Chong SA (2010). Impact of depression on health related quality of life in patients with diabetes. Annals of the Academy of Medicine Singapore, 39, 913–919. [PubMed] [Google Scholar]
- Wheaton AG, Jones SE, Cooper AC, & Croft JB (2018). Short sleep duration among middle school and high school students—United States, 2015. Morbidity and Mortality Weekly Report, 67(3), 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WP, McLin DE, Dressman MA, & Neubauer DN (2016). Comparative review of approved melatonin agonists for the treatment of circadian rhythm sleep-wake disorders. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 36(9), 1028–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR (2003). Understanding adolescent’s sleep patterns and school performance: A critical appraisal. Sleep Medicine Reviews, 7(6), 491–506. [DOI] [PubMed] [Google Scholar]
- Woods HC, & Scott H (2016). # Sleepyteens: Social media use in adolescence is associated with poor sleep quality, anxiety, depression and low self-esteem. Journal of Adolescence, 51, 41–49. [DOI] [PubMed] [Google Scholar]
- Woodward A (2009). Nocturnal hypoglycaemia in type 1 diabetes—frequency and predictive factors. QJM: An International Journal of Medicine, 102(9), 603–607. [DOI] [PubMed] [Google Scholar]
- Yeshayahu Y, & Mahmud FH (2010). Altered sleep patterns in adolescents with type 1 diabetes: Implications for insulin regimen. Diabetes Care, 33(11), e142. [DOI] [PubMed] [Google Scholar]


