Abstract
Objective:
To investigate the association of daily branched-chain amino acids (BCAAs) intake with the risks of obesity and insulin resistance in children of mothers with gestational diabetes mellitus (GDM).
Methods:
Daily BCAAs intake was calculated using a validated food frequency questionnaire in 996 children of mothers with GDM. The odds ratios (ORs) (95% confidence intervals) of childhood obesity and insulin resistance were obtained using logistic regression models.
Results:
The multivariable-adjusted ORs for overweight and insulin resistance increased across quartiles of daily BCAAs intake (P for trend < 0.05). Multivariable-adjusted ORs for each 1 standard deviation increase in BCAAs intake were 1.37 (1.16–1.62) for overweight, and 1.19 (1.02–1.38) for insulin resistance. After additional adjustment of children’s daily total energy intake, the OR was still significant for overweight risk but no longer significant for insulin resistance. There were positive associations of daily leucine, isoleucine and valine intake with the risks of overweight and insulin resistance.
Conclusions:
Daily BCAAs intake was associated with increased risks of overweight and insulin resistance in children of mothers with GDM, but this association was not fully independent of children’s daily energy intake. Restriction in dietary BCAAs may help prevent childhood obesity and insulin resistance.
Keywords: Obesity, Pediatrics, Insulin Resistance, Gestational Diabetes, Branched-Chain Amino Acids
Introduction
The branched-chain amino acids (BCAAs), including leucine, isoleucine, and valine, are essential amino acids for human beings (1). BCAAs are comparatively abundant in dietary proteins, constituting up to 15% - 20% of protein intake, which increase after intake of a meal containing proteins (1). A positive association was found between a BCAA-rich diet and metabolic health, including the regulation of body weight, muscle protein synthesis, and glucose homeostasis (2, 3). Moreover, some recent human studies found that elevated serum BCAA levels were associated with weight gain, insulin resistance and glucose metabolism abnormality in adults (4, 5). Increased serum BCAA levels were also associated with insulin resistance in a non-obese insulin-resistant fructose-fed rat model (6). A prospective study further demonstrated that serum BCAAs levels predicted the future risk of diabetes (7). In children, elevations in the circulating BCAAs levels were significantly associated with obesity in children and adolescents, which may independently predict future insulin resistance (8).
One meta-analysis reported that oral BCAA supplementation exerted modest influence on the circulating leucine profile, and the total BCAAs intake level was positively associated with the risk of type 2 diabetes (9). It was also reported that reduced dietary intake of BCAAs was associated with an improvement of glucose tolerance and body composition (10, 11). However, a study in young northern Chinese adults demonstrated that the dietary BCAA ratio was inversely associated with the risks of obesity, postprandial glucose and status of inflammation (12). Nevertheless, it is unclear whether excessive BCAAs intake is a risk factor for children’s obesity and insulin resistance. We aimed to examine the association of daily BCAAs intake with the risks of overweight and insulin resistance among 996 children of mothers with gestational diabetes mellitus (GDM).
Methods
GDM screening process
Tianjin is the fourth largest city in China, only 30-min distance by train from Beijing. There are six central districts in Tianjin with about 4.3 million residents. In 1999, the Tianjin Women’s and Children’s Health Center launched an urban universal screening of GDM using the World Health Organization (WHO)’s criteria in all six central districts. The screening rate was reported to be >91% between 1999 and 2008 (13). We first invited all pregnant women (at their 26–30 gestational weeks) to participate in a one-hour oral glucose tolerance test (OGTT) with 50-g glucose load in their community health centers. Then, those with glucose reading ≥7.8 mmol/L were referred to the Tianjin Women’s and Children’s Health Center to undergo a 2-hour OGTT with 75-g glucose load. If the pregnant women met the 1999 WHO’s criteria of diabetes (fasting glucose ≥7 mmol/L or 2-h glucose ≥11.1 mmol/L) or impaired glucose tolerance (IGT) (2-h glucose ≥7.8 mmol/L and <11.1 mmol/L), they would be diagnosed as GDM (14).
Study Population
Totally 76,325 women were screened from 2005 to 2009, among whom 4644 women were diagnosed as GDM and 71,681 were free of GDM. We invited all 4644 GDM women to participate in the Tianjin Gestational Diabetes Mellitus Prevention Program (TGDMPP). From August 2009 to July 2011, a total of 1263 GDM women finished the baseline survey. A total of 996 children finished the follow-up survey, and had complete information of BMI and insulin resistance (Figure 1). The recruitment, inclusion and exclusion criteria have been described in detail elsewhere (15). We collected written informed consents from all participants, and this study was approved by the Human Subjects Committee of the Tianjin Women’s and Children’s Health Center.
Figure 1.
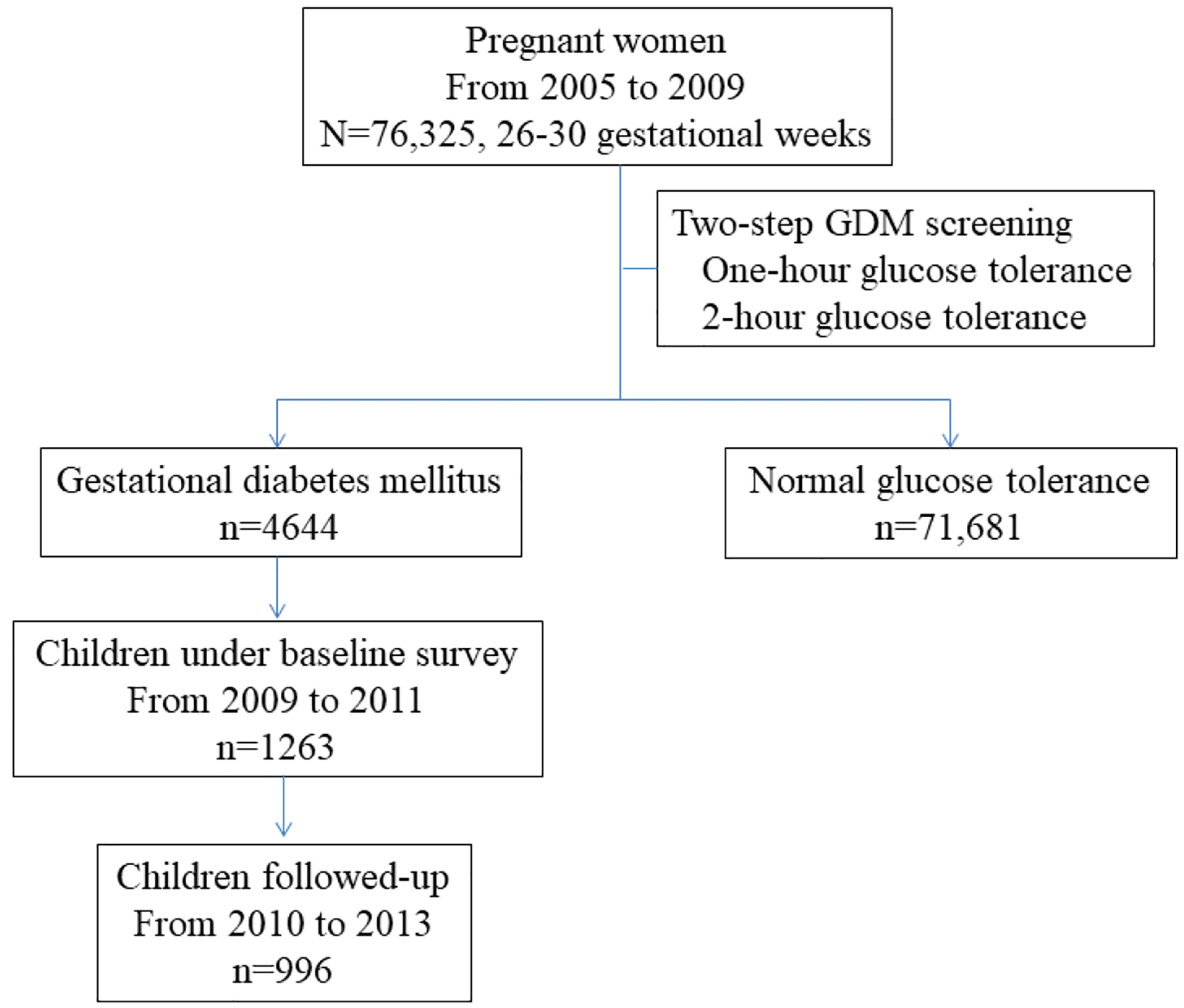
Flow chart of subjects enrollment.
Abbreviation: GDM gestational diabetes mellitus
Questionnaires and measurements
Mothers’ information was collected by a self-administered questionnaire, including socio-demographic characteristics, such as age, marital status, education (< 13 years, 13–16 years, and ≥16 years), family income (<5000 yuan/month, 5000–8000 yuan/month, and ≥8000 yuan/month), and occupation; pregnancy outcomes (pre-pregnancy weight, weight gain during pregnancy, gestational age, and the number of births in the index pregnancy); and lifestyle in the past year, such as smoking status (non-smokers, former smokers, and current smokers), passive smoking, alcohol drinking status, sitting time and leisure-time physical activity (0 min/day, 1–29 min/day, and ≥30 min/day). Children’s information was collected by another questionnaire completed by their mothers, including children’s general information, such as gender, birth date, age, birth weight, birth length, lactation (exclusive formula, mixed or exclusive breast); history of diseases and medication; and routine activities (indoor and outdoor activities, screening watching time, and sleep duration) (16). A validated food frequency questionnaire to measure the children’s frequency and quantity of intake of 35 major food groups and beverages during the past year was collected by children’s mothers. The food frequency questionnaire asked these children about their frequency of “usual” consumption of 35 food categories, with response categories never, times/year, times/month, times/week, or times/day, and quantity of average consumption of gram or milliliter per time. The performance of the food frequency questionnaire has been validated in the China National Nutrition and Health Survey in 2002 [17]. Energy and daily BCAAs intake were calculated according to a food ingredient list published in 2002 (17). Children were divided into 4 groups according to BCAAs quartiles stratified by sex and age.
All mother-child pairs underwent a physical examination. Using the standardized protocol, all participants’ height and weight were measured in light indoor clothing and without shoes by trained research doctors. Body mass index (BMI) was obtained by dividing weight in kilograms by the square of height in meters. All mothers’ pre-pregnancy BMI calculation used their self-reported pre-pregnancy weight and their measured height. Children’s BMI calculation used their body weight and height examined at the study visit. Children’s Z scores for BMI-for-age were calculated based on the WHO growth reference (18, 19). Children’s BMI was classified as normal weight, BMI <85th percentiles; overweight, 85th percentile ≤ BMI <95th percentile; and obesity, BMI ≥95th percentile, according to the WHO age- and gender-specific growth reference (18, 19).
Whole blood specimens were collected from all participants after an overnight fast of at least 8 hours. Plasma glucose was measured using an automatic analyzer (TBA-120FR; Toshiba, Japan), and insulin was measured with chemiluminescence using a Siemens ADVIA Centaur CP Immunoassay System. The homeostatic model assessment was used to estimate insulin resistance (HOMA-IR) as previously described (20), and insulin resistance was defined as the upper quartile of HOMA-IR.
Statistical analysis
The general characteristics (continuous and categorical variables) of both mothers and children according to quartiles of children’s daily BCAA intake levels were performed using the chi-square test or general linear model. Logistic regression models were used to estimate odds ratios of childhood overweight and insulin resistance according to children’s daily different BCAA intake levels. BCAAs were evaluated in the following two ways: (1) as quartiles; and (2) as a continuous variable. All analyses were adjusted for maternal age, gestational age, education and smoking status, pre-pregnancy BMI and gestational weight gain, and children’s sex, age, birth weight, and feeding status (categorical variables) (Model 1), and then children’s lifestyles including outdoor physical activity time (continuous variables), screen watching time (continuous variables), and sleep time (categorical variables) (Model 2), as well as children’s daily total energy intake (Model 3). All the statistical analyses were performed with the SPSS 25.0 for windows software package (IBM SPSS statistics 25). Two-sided P <0.05 was considered statistically significant.
Results
General characteristics of the study population are presented in Table 1. There were differences in sex composition, Z score for BMI-for-age, outdoor activity hour, daily energy intake, energy intake from fat, prevalence of overweight and insulin resistance among children with different daily BCAA intake levels (all P<0.05). Moreover, the linear regression analysis indicated that BCAAs and components were positively associated with HOMA-IR and BMI Z score (all P values <0.05, as shown in Table S1).
Table 1.
Clinical feature in children with different levels of daily branched-chain amino acids intake
| Quartiles of branched-chain amino acids | P value | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| No. of subjects | 249 | 249 | 249 | 249 | |
| Maternal characteristics | |||||
| Delivery age, years | 31.3 ± 3.64 | 30.6 ± 3.4 | 31.4 ± 3.52 | 30.7 ± 3.56 | 0.027 |
| Gestational age at delivery, weeks | 39.0 ± 1.49 | 39.2 ± 1.34 | 39.1 ± 1.52 | 38.9 ± 1.63 | 0.19 |
| Pre-pregnancy BMI, kg/m2 | 23.2 ± 3.32 | 22.7 ± 3.19 | 23.2 ± 3.19 | 23.3 ± 3.49 | 0.24 |
| Gestational weight gain, kg | 16.8 ± 6.02 | 16.2 ± 5.51 | 16.9 ± 6.00 | 16.8 ± 6.37 | 0.56 |
| Current smoker, % | 0 | 1.2 | 1.6 | 3.2 | 0.004 |
| Education, % | 0.71 | ||||
| <13 years | 23.3 | 18.9 | 24.5 | 21.3 | |
| 13–16 years | 69.1 | 75.1 | 67.9 | 69.9 | |
| ≥16 years | 7.6 | 6.0 | 7.6 | 8.8 | |
| Child characteristics | |||||
| Boy, % | 47.8 | 50.6 | 50.6 | 64.3 | <0.001 |
| Age, years | 3.07 ± 1.04 | 3.08 ± 1.08 | 3.01 ± 1.02 | 3.14 ± 1.09 | 0.58 |
| Birth weight, gram | 3540±513 | 3503±492 | 3557 ± 552 | 3571 ± 542 | 0.51 |
| Mode of infant feeding, % | 0.76 | ||||
| Exclusive breastfeeding | 45.4 | 42.6 | 38.6 | 47.0 | |
| Exclusive formula feeding | 39.4 | 48.2 | 45.4 | 37.8 | |
| Mixed feeding | 15.3 | 9.2 | 16.1 | 15.3 | |
| BMI, kg/m2 | 15.4 ± 1.22 | 15.6 ± 1.6 | 15.9 ± 1.54 | 16.2 ± 2.09 | <0.001 |
| BMI-for-age z-score | −0.07 ± 0.91 | 0.03 ± 1.12 | 0.24 ± 1.07 | 0.47 ± 1.33 | <0.001 |
| Prevalence of overweight, % | 10.0 | 17.3 | 18.1 | 24.5 | <0.001 |
| Fasting plasma glucose, mmol/l | 4.31 ± 0.4 | 4.33 ± 0.39 | 4.36 ± 0.35 | 4.38 ± 0.39 | 0.22 |
| HOMA-IR* | −0.44 ± 0.35 | −0.42 ± 0.36 | −0.42 ± 0.36 | −0.37 ± 0.38 | 0.14 |
| Insulin resistance, % | 18.9 | 24.5 | 25.3 | 28.5 | 0.015 |
| Outdoor activity, hours | 1.69 ± 0.83 | 1.61 ± 0.89 | 1.54 ± 0.88 | 1.73 ± 0.86 | 0.07 |
| Screen watching time, hours | 1.3 ± 1.04 | 1.24 ± 1.02 | 1.25 ± 1.05 | 1.39 ± 1.04 | 0.33 |
| Sleeping time, % | 0.58 | ||||
| ≤8 hours/day | 3.6 | 1.6 | 1.2 | 1.6 | |
| 9–10 hours/day | 43.4 | 45.4 | 47.4 | 49.8 | |
| ≥11 hours/day | 53.0 | 53.0 | 51.4 | 48.6 | |
| Daily nutrition intake | |||||
| Energy, kcal | 686 ± 138 | 836 ± 131 | 953 ± 138 | 1157 ± 237 | <0.001 |
| Energy intake from protein, % | 13.8 ± 2.20 | 15.7 ± 2.22 | 16.6 ± 2.18 | 17.9 ± 2.72 | <0.001 |
| Energy intake from carbohydrate, % | 53.7 ± 8.47 | 52.2 ± 7.34 | 51.6 ± 6.86 | 51.4 ± 7.00 | 0.002 |
| Energy intake from fat, % | 32.5 ± 8.26 | 32.1 ± 7.17 | 31.8 ± 6.64 | 30.6 ± 6.4. | 0.028 |
| Branched chain amino acids, mg/day | 3893 ± 702 | 5428 ± 322 | 6591 ± 363 | 8595 ± 1353 | <0.001 |
| Isoleucine, mg/day | 1021 ± 186 | 1419 ± 86 | 1718 ± 97 | 2238 ± 349 | <0.001 |
| Leucine, mg/day | 1717 ± 310 | 2404 ± 152 | 2931 ± 169 | 3833 ± 618 | <0.001 |
| Valine, mg/day | 1155 ± 211 | 1605 ± 98 | 1942 ± 111 | 2523 ± 391 | <0.001 |
BMI, body mass index; HOMA-IR, homeostasis model assessment for insulin resistance.
BMI-for-age Z score, and overweight in children were evaluated according to the age- and sex-specific growth reference issued by World Health Organization.
Data were log-transformed.
As shown in Table 2 and Figure S1, elevated levels of daily BCAA intake (assessed by quartiles) were associated with an increased risk of childhood overweight (P for trend<0.01). The multivariable-adjusted (maternal age at delivery, gestational age at delivery, education, current smoking, and children’s age, sex, birth weight, feeding status, outdoor physical activity time, screen watching time, and sleep time - Model 2) odds ratios (ORs) of childhood overweight associated with each 1 standard deviation (SD) increase in daily intake of BCAAs, isoleucine, leucine and valine were 1.37 (95% confidence interval [95% CI 1.16–1.62]), 1.38 (95% CI 1.16–1.63), 1.36 (95% CI 1.15–1.61), and 1.36 (95% CI 1.15–1.61), respectively. The association of daily intake of BCAAs, isoleucine, and leucine with the risk of childhood overweight attenuated but was still significant after further adjustment of children’s daily energy intake (multivariable-adjusted Model 3).
Table 2.
Odds ratios of overweight by different dietary intake levels of branched-chain amino acid, isoleucine, leucine, and valine
| No. of participants | No. of cases | Odds ratios (95% confidence intervals) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||
| Branched-chain amino acids | |||||
| Quartile 1 | 249 | 25 | 1 | 1 | 1 |
| Quartile 2 | 249 | 43 | 2.11 (1.22 – 3.65) | 2.19 (1.26 – 3.82) | 2.07 (1.13 – 3.82) |
| Quartile 3 | 249 | 45 | 1.97 (1.15 – 3.39) | 2.06 (1.19 – 3.57) | 1.74 (0.88 – 3.43) |
| Quartile 4 | 249 | 61 | 2.76 (1.63 – 4.67) | 2.81 (1.65 – 4.78) | 2.21 (1.05 – 4.64) |
| P value for trend | <0.001 | <0.001 | 0.11 | ||
| One SD increase | 1.38 (1.17 – 1.63) | 1.37 (1.16 – 1.62) | 1.31 (1.01 – 1.70) | ||
| Isoleucine | |||||
| Quartile 1 | 250 | 24 | 1 | 1 | 1 |
| Quartile 2 | 249 | 49 | 2.57 (1.50 – 4.43) | 2.63 (1.52 – 4.56) | 2.38 (1.31 – 4.34) |
| Quartile 3 | 250 | 43 | 1.85 (1.06 – 3.22) | 1.89 (1.08 – 3.32) | 1.51 (0.76 – 3.00) |
| Quartile 4 | 247 | 58 | 2.93 (1.71 – 5.00) | 2.90 (1.69 – 4.99) | 2.08 (0.99 – 4.40) |
| P value for trend | 0.001 | 0.001 | 0.25 | ||
| One SD increase | 1.38 (1.17 – 1.63) | 1.38 (1.16 – 1.63) | 1.30 (1.01 – 1.69) | ||
| Leucine | |||||
| Quartile 1 | 249 | 26 | 1 | 1 | 1 |
| Quartile 2 | 249 | 43 | 2.07 (1.20 – 3.56) | 2.18 (1.26 – 3.78) | 2.02 (1.10 – 3.68) |
| Quartile 3 | 250 | 45 | 1.86 (1.09 – 3.18) | 1.93 (1.12 – 3.33) | 1.56 (0.80 – 3.04) |
| Quartile 4 | 248 | 60 | 2.62 (1.55 – 4.41) | 2.66 (1.57 – 4.52) | 1.99 (0.96 – 4.12) |
| P value for trend | 0.001 | 0.001 | 0.18 | ||
| One SD increase | 1.38 (1.17 – 1.63) | 1.36 (1.15 – 1.61) | 1.32 (1.02 – 1.70) | ||
| Valine | |||||
| Quartile 1 | 249 | 25 | 1 | 1 | 1 |
| Quartile 2 | 249 | 43 | 2.12 (1.23 – 3.66) | 2.17 (1.25 – 3.77) | 2.05 (1.11 – 3.82) |
| Quartile 3 | 249 | 45 | 1.92 (1.11 – 3.30) | 2.03 (1.17 – 3.52) | 1.70 (0.85 – 3.39) |
| Quartile 4 | 249 | 61 | 2.77 (1.63 – 4.69) | 2.81 (1.65 – 4.79) | 2.21 (1.04 – 4.67) |
| P value for trend | 0.001 | <0.001 | 0.12 | ||
| One SD increase | 1.37 (1.16 – 1.61) | 1.36 (1.15 – 1.61) | 1.29 (0.99 – 1.67) | ||
Model 1 adjusted for maternal age at delivery, gestational age at delivery, education, current smoking, pre-pregnancy BMI and gestational weight gain, and children’s age, sex, birth weight, and feeding status.
Model 2 adjusted for variables in Model 1 plus children’s outdoor physical activity time, screen-watching time, and sleep time.
Model 3 adjusted for variables in Model 2 plus children’s daily energy intake.
Abbreviation: standard deviation, SD.
Multivariable-adjusted ORs of childhood insulin resistance across quartiles of daily intake of total BCAAs were 1.00, 1.39, 1.52, and 1.71 (P for trend 0.018), respectively (Model 2, Table 3; Figure S2). There were positive associations of daily intake of isoleucine, leucine and valine with the risk of childhood insulin resistance. Multivariable-adjusted (Model 2) ORs of childhood insulin resistance associated with each 1 SD increase in daily intake of BCAAs, isoleucine, leucine and valine were 1.19 (95% CI 1.02–1.38), 1.19 (95% CI 1.03–1.39), 1.19 (95% CI 1.02–1.38), and 1.19 (95% CI 1.02–1.39), respectively. The positive associations of daily intake of BCAAs, isoleucine, leucine and valine with the risk of childhood insulin resistance were no longer significant after further adjustment of children’s daily energy intake (multivariable-adjusted Model 3).
Table 3.
Odds ratios of insulin resistance by different dietary intake levels of branched-chain amino acid, isoleucine, leucine, and valine
| No. of participants | No. of cases | Odds ratios (95% confidence intervals) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||
| Branched-chain amino acids | |||||
| Quartile 1 | 249 | 47 | 1 | 1 | 1 |
| Quartile 2 | 249 | 61 | 1.37 (0.88 – 2.14) | 1.39 (0.89 – 2.17) | 1.26 (0.77 – 2.07) |
| Quartile 3 | 249 | 63 | 1.49 (0.96 – 2.32) | 1.52 (0.97 – 2.37) | 1.28 (0.74 – 2.24) |
| Quartile 4 | 249 | 71 | 1.74 (1.12 – 2.69) | 1.71 (1.10 – 2.66) | 1.43 (0.76 – 2.67) |
| P value for trend | 0.014 | 0.018 | 0.31 | ||
| One SD increase | 1.20 (1.03 – 1.39) | 1.19 (1.02 – 1.38) | 1.12 (0.89 – 1.42) | ||
| Isoleucine | |||||
| Quartile 1 | 250 | 45 | 1 | 1 | 1 |
| Quartile 2 | 249 | 64 | 1.60 (1.03 – 2.50) | 1.61 (1.03 – 2.52) | 1.48 (0.9 – 2.41) |
| Quartile 3 | 250 | 63 | 1.55 (0.99 – 2.42) | 1.55 (0.99 – 2.42) | 1.32 (0.76 – 2.31) |
| Quartile 4 | 247 | 70 | 1.82 (1.17 – 2.84) | 1.79 (1.15 – 2.79) | 1.49 (0.79 – 2.8) |
| P value for trend | 0.014 | 0.019 | 0.33 | ||
| One SD increase | 1.20 (1.04 – 1.40) | 1.19 (1.03 – 1.39) | 1.13 (0.89 – 1.43) | ||
| Leucine | |||||
| Quartile 1 | 249 | 47 | 1 | 1 | 1 |
| Quartile 2 | 249 | 61 | 1.41 (0.90 – 2.20) | 1.44 (0.92 – 2.25) | 1.31 (0.8 – 2.15) |
| Quartile 3 | 250 | 60 | 1.37 (0.88 – 2.14) | 1.38 (0.89 – 2.17) | 1.18 (0.68 – 2.05) |
| Quartile 4 | 248 | 74 | 1.83 (1.18 – 2.83) | 1.81 (1.17 – 2.81) | 1.55 (0.83 – 2.87) |
| P value for trend | 0.011 | 0.014 | 0.24 | ||
| One SD increase | 1.20 (1.03 – 1.39) | 1.19 (1.02 – 1.38) | 1.12 (0.89 – 1.41) | ||
| Valine | |||||
| Quartile 1 | 249 | 46 | 1 | 1 | 1 |
| Quartile 2 | 249 | 60 | 1.38 (0.88 – 2.16) | 1.40 (0.89 – 2.19) | 1.31 (0.79 – 2.18) |
| Quartile 3 | 250 | 66 | 1.67 (1.07 – 2.59) | 1.70 (1.09 – 2.65) | 1.53 (0.87 – 2.71) |
| Quartile 4 | 248 | 70 | 1.83 (1.18 – 2.86) | 1.81 (1.16 – 2.82) | 1.65 (0.87 – 3.14) |
| P value for trend | 0.005 | 0.006 | 0.13 | ||
| One SD increase | 1.20 (1.03 – 1.39) | 1.19 (1.02 – 1.39) | 1.12 (0.88 – 1.43) | ||
Model 1 adjusted for maternal age at delivery, gestational age at delivery, education, current smoking, pre-pregnancy BMI and gestational weight gain, and children’s age, sex, birth weight, and feeding status.
Model 2 adjusted for variables in Model 1 plus children’s outdoor physical activity time, screen-watching time, and sleep time.
Model 3 adjusted for variables in Model 2 plus children’s daily energy intake.
Abbreviation: standard deviation, SD.
Discussion
The present study indicated that daily intake of BCAAs, leucine, isoleucine and valine was associated with increased risks of overweight and insulin resistance among children of mothers with GDM; however, this association was not fully independent of children’s daily energy intake, especially for the risk of childhood insulin resistance.
The prevalence of pediatric obesity increased rapidly in recent decades worldwide (21). Many factors accounted for the rapid increase in pediatric obesity, including congenital and acquired factors. Maternal pre-pregnancy overweight and obesity, excess gestational weight gain were risk factors for the children’s obesity; other childhood factors such as elevated energy intake, elevated screen time, reduced outdoor activity were also risk factors for childhood obesity(22–24). The present study indicated that daily dietary intake of BCAAs was independently associated with increased risks of childhood overweight and insulin resistance after adjustment of these major risk factors. However, the positive association of daily dietary intake of BCAAs with the risk of childhood insulin resistance was not independent of children’s daily energy intake.
Low-fat diet or low-carbohydrate diet has been accepted as an effective intervention way for metabolic disorders (25). Recently, it is reported that protein restricted diet could help improve metabolic indexes, including obesity and insulin resistance in humans (10). Several recent studies further indicated that BCAA restriction may largely recapitulate the metabolic effects induced by the restriction of proteins (10, 26, 27). Cummings et al. pointed out that the restriction of dietary BCAAs significantly decreased body weight and adiposity, increased energy expenditure, and improved glucose tolerance and insulin sensitivity in animal experiments (28). There were very few studies on the effects of dietary BCAA restriction on metabolism in humans (29). In the present study, our data indicated dietary BCAAs intake was independently associated with childhood obesity and insulin resistance, however, this association was not fully independent of children’s daily energy intake, especially for the risk of childhood insulin resistance. Large human clinical trials are needed to assess whether dietary BCAA restriction can lose weight and improve metabolism among both adults and children, and whether above association is independent of daily energy intake.
The mechanisms mediating BCAAs and metabolic disorders were complicated. First, BCAA supplementation was shown to increase the activation of mammalian target of rapamycin (mTOR) and subsequent ribosomal protein S6 kinase (S6K) phosphorylation, which was coupled with insulin receptor substrate (IRS)-1 Ser-307 phosphorylation and decreased insulin-induced phosphoinositide 3-kinase (PI3K) activity, resulting in impaired insulin signaling (30, 31). Second, the metabolites of BCAAs were associated with the risks of obesity and insulin resistance. Researchers pointed out that 3-hydroxyisobutyrate dehydrogenase in the muscle tissue of rats decreased 50%, resulting in an elevation of catabolic intermediate of valine 3-hydroxyisobutyrate (3-HIB) (32, 33). In animals, 3-HIB is secreted from muscle cells, activates endothelial FA transport, stimulates muscle fatty acid uptake in vivo, and promotes muscle lipid accumulation and insulin resistance (34). In human studies, 3-HIB was shown to be related to insulin resistance in individuals with overweight and obesity, and changes in 3-HIB were associated with metabolic improvements with weight loss (35). Finally, Leucine supplementation led to abnormal catabolism of BCAA and the incompletely oxidized lipid species contributed to mitochondrial dysfunction in skeletal muscle in high fat diet-fed rats (36). BCAAs could also stimulate metabolic stress in islet β cells in animals (10). Impaired BCAAs catabolism may result in increased circulating levels of BCAAs that enhance their pathological effects on obesity and insulin resistance (6).
There were some strengths in our study. Our study enrolled a large sample of children of mothers with GDM. Data on a variety of confounding variables, such as the parameters of mothers before and during pregnancy; and indices of the children, including birth weight, lifestyles factors, and anthropometric indexes were collected and used in the final analysis. There were also some limitations in our study. This was a cross-sectional study, and more prospective studies should be warranted in the future. Moreover, the study samples in the present study were restricted to children of mothers with GDM, and the extrapolation of the conclusion to the whole children population should be scrupulous.
Conclusion
The present study indicated that dietary BCAAs intake was associated with increased risks of obesity and insulin resistance in children of mothers with GDM, however, the association of dietary BCAAs intake with the risk of insulin resistance was not independent of children’s daily energy intake. Thus, dietary BCAAs restriction may prevent these children from obesity and insulin resistance, and more clinical trials are needed to further verify this issue.
Supplementary Material
Research in context.
What is already known about this subject?
Several studies have reported a positive association of branched-chain amino acids via diet or plasma with the risks of obesity and insulin resistance in adults.
Question:
It is uncertain whether excessive branched-chain amino acid intake increases the risks of obesity and insulin resistance in children.
Findings:
Daily intake of branched-chain amino acids was associated with increased risks of overweight and insulin resistance in children of mothers with gestational diabetes mellitus.
What does your study add?
Our data indicated that excessive branched-chain amino acid intake increased the risks of obesity and insulin resistance in children, so restriction in nutrition ingredients such as branched-chain amino acids may be important to prevent childhood obesity and insulin resistance.
Funding source:
This study was supported by the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly program for Collaborative Research between China and Europe. Dr. Hu was partly supported by the grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100790) and the National Institute of General Medical Sciences (U54GM104940) of the National Institutes of Health.
Footnotes
Data Sharing Statement: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Duality of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Reference
- 1.Lu J, Xie G, Jia W, Jia W. Insulin resistance and the metabolism of branched-chain amino acids. Front Med 2013; 7(1): 53–59. [DOI] [PubMed] [Google Scholar]
- 2.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014; 10(12): 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomstrand E, Eliasson J, Karlsson HK, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr 2006; 136(1 Suppl): 269s–273s. [DOI] [PubMed] [Google Scholar]
- 4.Shah SH, Crosslin DR, Haynes CS, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012; 55(2): 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barcelo A, Morell-Garcia D. A randomized controlled trial: branched-chain amino acid levels and glucose metabolism in patients with obesity and sleep apnea. 2017; 26(6): 773–781. [DOI] [PubMed] [Google Scholar]
- 6.David J, Dardevet D, Mosoni L. Impaired Skeletal Muscle Branched-Chain Amino Acids Catabolism Contributes to Their Increased Circulating Levels in a Non-Obese Insulin-Resistant Fructose-Fed Rat Model. 2019; 11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17(4): 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes 2013; 8(1): 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okekunle AP, Zhang M, Wang Z, et al. Dietary branched-chain amino acids intake exhibited a different relationship with type 2 diabetes and obesity risk: a meta-analysis. Acta Diabetol 2019; 56(2): 187–195. [DOI] [PubMed] [Google Scholar]
- 10.Fontana L, Cummings NE, Arriola Apelo SI, et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep 2016; 16(2): 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halkjaer J, Olsen A, Overvad K, et al. Intake of total, animal and plant protein and subsequent changes in weight or waist circumference in European men and women: the Diogenes project. Int J Obes (Lond) 2011; 35(8): 1104–1113. [DOI] [PubMed] [Google Scholar]
- 12.Li YC, Li Y, Liu LY, et al. The Ratio of Dietary Branched-Chain Amino Acids is Associated with a Lower Prevalence of Obesity in Young Northern Chinese Adults: An Internet-Based Cross-Sectional Study. Nutrients 2015; 7(11): 9573–9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Dong L, Zhang C, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med 2011; (28): 652–657. [DOI] [PubMed] [Google Scholar]
- 14.WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus Geneva: World Health Organisation; 1999. [Google Scholar]
- 15.Hu G, Tian H, Zhang F, et al. Tianjin Gestational Diabetes Mellitus Prevention Program: Study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pract 2012; 98(3): 508–517. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Liu H, Zhang C, et al. Maternal glucose during pregnancy and after delivery in women with gestational diabetes mellitus on overweight status of their children. Biomed Res Int 2015; 2015: 543038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Yang G, Pan X. [Chinese food ingredient list (2002)]. Peking University Medical Press; 2002. [Google Scholar]
- 18.Lu J, Hou X, Zhang L, et al. Associations between clinical characteristics and chronic complications in latent autoimmune diabetes in adults and type 2 diabetes. Diabetes Metab Res Rev 2015; 31(4): 411–420. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, Xiang Y, Ji L, et al. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes 2013; 62(2): 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Zhang S, Liu H, et al. Different associations of diabetes with beta-cell dysfunction and insulin resistance among obese and nonobese Chinese women with prior gestational diabetes mellitus. Diabetes Care 2014; 37(9): 2533–2539. [DOI] [PubMed] [Google Scholar]
- 21.Afshin A, Forouzanfar MH, Reitsma MB, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017; 377(1): 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hjorth MF, Chaput JP, Ritz C, et al. Fatness predicts decreased physical activity and increased sedentary time, but not vice versa: support from a longitudinal study in 8- to 11-year-old children. Int J Obes (Lond) 2014; 38(7): 959–965. [DOI] [PubMed] [Google Scholar]
- 23.Munthali RJ, Sahibdeen V, Kagura J, et al. Genetic risk score for adult body mass index associations with childhood and adolescent weight gain in an African population. Genes Nutr 2018; 13: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballon M, Botton J, Charles MA, et al. Socioeconomic inequalities in weight, height and body mass index from birth to 5 years. Int J Obes (Lond) 2018. [DOI] [PubMed] [Google Scholar]
- 25.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008; 359(3): 229–241. [DOI] [PubMed] [Google Scholar]
- 26.Hill CM, Morrison CD. Dietary branched-chain amino acids and metabolic health: when less is more. J Physiol 2018; 596(4): 555–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccolo BD, Comerford KB, Karakas SE, Knotts TA, Fiehn O, Adams SH. Whey protein supplementation does not alter plasma branched-chained amino acid profiles but results in unique metabolomics patterns in obese women enrolled in an 8-week weight loss trial. J Nutr 2015; 145(4): 691–700. [DOI] [PubMed] [Google Scholar]
- 28.Cummings NE, Williams EM, Kasza I, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol 2018; 596(4): 623–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson JG, Hintze K, Marchant ED. Restricting branched-chain amino acids: an approach to improve metabolic health. J Physiol 2018; 596(13): 2469–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol 2006; 26(1): 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005; 54(9): 2674–2684. [DOI] [PubMed] [Google Scholar]
- 32.Roe CR, Cederbaum SD, Roe DS, Mardach R, Galindo A, Sweetman L. Isolated isobutyryl-CoA dehydrogenase deficiency: an unrecognized defect in human valine metabolism. Mol Genet Metab 1998; 65(4): 264–271. [DOI] [PubMed] [Google Scholar]
- 33.Mullen E, Ohlendieck K. Proteomic profiling of non-obese type 2 diabetic skeletal muscle. Int J Mol Med 2010; 25(3): 445–458. [DOI] [PubMed] [Google Scholar]
- 34.Jang C, Oh SF, Wada S, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 2016; 22(4): 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haufe S, Engeli S, Kaminski J, et al. Branched-chain amino acid catabolism rather than amino acids plasma concentrations is associated with diet-induced changes in insulin resistance in overweight to obese individuals. Nutr Metab Cardiovasc Dis 2017; 27(10): 858–864. [DOI] [PubMed] [Google Scholar]
- 36.Liu R, Li H, Fan W, et al. Leucine Supplementation Differently Modulates Branched-Chain Amino Acid Catabolism, Mitochondrial Function and Metabolic Profiles at the Different Stage of Insulin Resistance in Rats on High-Fat Diet. Nutrients 2017; 9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


