Abstract
Human immunodeficiency virus type 1 (HIV-1) subverts intracellular trafficking pathways to avoid its degradation and elimination, thereby enhancing its survival and spread. The molecular mechanisms involved in intracellular transport of HIV-1 are not yet fully defined. We demonstrate that the actin- binding protein lymphocyte-specific protein 1 (LSP1) interacts with the interferon-inducible protein bone marrow stromal antigen 2 (BST-2) in dendritic cells (DCs) to facilitate both endocytosis of surface-bound HIV-1 and the formation of early endosomes. Analysis of the molecular interaction between LSP1and BST-2 reveal that the N-terminus of LSP1 interacts with BST-2. Overall, we identify a novel mechanism of intracellular trafficking of HIV-1 in DCs centering on the LSP1/BST-2 complex. We also show that the HIV-1 accessory protein Vpu subverts this pathway by inducing proteasomal degradation of LSP1, augmenting cell-cell transmission of HIV-1.
Keywords: dendritic cell, endocytosis, human immunodeficiency virus (HIV), trafficking, cytoskeleton
INTRODUCTION
During various stages of the HIV-1 life cycle, virions and viral components are transported within host cells [1, 2]. The actin cytoskeletal system is known to participate in these transport processes, yet how HIV-1 can avoid intracellular degradation is not fully understood [3–6]. Dendritic cells (DCs) play a pivotal role in immune defense against viral infection [7, 8]. HIV-1 rarely establishes a productive infection in DCs; instead, these cells endocytose intact virus and store it in intraluminal vesicles that are incorporated into multivesicular bodies (MVBs), which can fuse with lysosomes and degrade the virus [7, 9–12].
Recent studies have highlighted an actin-binding protein, lymphocyte-specific protein 1 (LSP1), in the regulation of endocytosis and phagocytosis [13–17]. LSP1 is a 52-kD phosphoprotein found on the cytoplasmic surface of the plasma membrane and expressed in all human leukocytes and endothelial cells [18–22]. Structurally, LSP1 contains an acidic N-terminal domain and a C-terminal domain which is enriched with basic amino acids [14]. The highly conserved C-terminal domain is considered as a functionally important region of the LSP1 molecule as it harbors the F-actin binding site and several potential phosphorylation sites by serine and threonine kinases including mitogen-activated protein (MAP) kinase-activated protein kinase 2 (MK2) or protein kinase C (PKC) [14, 23]. The phosphorylation status of LSP1 is responsible for its linking to the actin cytoskeleton [14, 24]. LSP1 participates in the formation of filopodia, lamellipodia, ruffles, and the actin-rich cell cortex of various cell types and thereby modulates chemotaxis [14, 25–28].
The role of LSP1 in endocytosis was first described in yeast by demonstrating its localization at the endocytosis initiation sites on the plasma membrane [17]. Recently, LSP1 has been shown to localize to nascent phagocytic cups during Fcγ receptor-mediated phagocytosis [15]. Knockdown of LSP1 significantly inhibited phagocytic activity of macrophages [15]. Studies have also shown that LSP1 may play various roles during the different stages of the HIV-1 life cycle [13, 16, 29, 30]. However, the exact role of LSP1 in HIV-1 is not yet fully characterized. Smith et al. have demonstrated that LSP1 interacts with DC-SIGN and mediates internalization of HIV-1 [30]. Further they showed that LSP1 facilitates the trafficking of virus towards proteasomal degradation [30]. In addition, LSP1 serves as a scaffold protein in DC-SIGN induced formation of a signalosome complex which determines the outcome of pro-inflammatory immune responses against invading pathogens [29]. Recently, Chauhan et al. found that LSP1 plays an important role in endocytosis of HIV-1 in astrocytes [13].
HIV-1 virions bound to the cell surface of DCs are endocytosed and transferred to phagolysosomal complexes for degradation and subsequent antigen presentation [7, 8]. Bone marrow stromal cell antigen 2 (BST-2)/Tetherin, an interferon-inducible protein is known to potently restrict the release of HIV-1 from host cells by physically anchoring virions to the cell surface [31, 32]. BST-2 also mediates the endocytosis of surface accumulated HIV-1 virions and sequesters them within intracellular vesicles for subsequent lysosomal degradation [31, 32]. At intracellular HIV-1 assembly sites, BST-2 localizes at virus-containing compartment (VCC) and physically anchors virions to VCCs, subsequently recruiting components of the ESCRT complex [33]. It has been shown that BST-2 also regulates the release of extracellular vesicles by anchoring them to the cell surface [34]. Our recent study and others have demonstrated that LSP1 interacts with DC-SIGN and facilitates transport of HIV-1 to the proteasomal complex and subsequent degradation [16, 29]. Here, we show that LSP1 interacts with BST-2 and plays a key role in the endocytosis of HIV-1 in DCs. In addition, we found that LSP1 interacts with molecules of the ESCRT complex and facilitates intracellular trafficking of HIV-1 towards a phagolysosomal complex. Furthermore, we found that the HIV-1 accessory molecule Vpu can induce degradation of LSP1 and subvert its trafficking, thereby decreasing degradation of HIV-1 and enhancing its transfer to T-cells. These novel observations shed new light on how LSP1 participates in HIV pathobiology.
MATERIALS AND METHODS
Ethics Statement
Buffy coats were obtained from the Blood Transfusion Service, Massachusetts General Hospital, Boston, MA, in compliance with the Beth Israel Deaconess Medical Center Committee on Clinical Investigations (CCI) protocol #2008-P-000418/5. Buffy coats were provided at this institution for research purposes; therefore, no informed consent was further needed. In addition, buffy coats were provided without identifiers. This study was approved by Beth Israel Deaconess Medical Center’s CCI, Institutional Review Board, and Privacy Board appointed to review research involving human subjects. The experimental procedures were carried out in strict accordance with approved guidelines.
Cells, HIV-1 and constructs
Human peripheral blood mononuclear cells (PBMCs) were isolated from healthy human donor buffy coats by ficoll-paque density gradient method. Monocyte derived dendritic cells (hereafter referred as DCs) were prepared and cultured as previously described [25, 27]. Briefly, CD14+ monocytes were isolated from PBMCs, using a human CD14+ positive selection kit (StemCell Technologies, Vancouver, BC, Canada) per manufacturer’s instructions. Monocytes were cultured in complete RPMI medium, supplemented with 50 ng/ml human granulocyte macrophage colony-stimulating factor (Specific activity ≥ 1 × 107 units/mg, PeproTech, Rocky Hill, NJ) and 50 ng/ml human interleukin-4 (Specific activity ≥ 5 × 106 units/mg. PeproTech,) for 6 days. On day 3, half of the medium was replenished by the fresh medium. CD4+ T-cells (hereafter referred as T-cells) were isolated from PBMCs by negative selection using a CD4+ isolation kit (Stem Cell Technologies, Vancouver Canada). Isolated T-cells were cultured in RPMI medium containing 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin, supplemented with phytohemagglutinin (PHA-L) and interleukin-2 (IL-2). Following three days of culture, cells were maintained in complete culture medium supplemented with IL-2 (10 ng/ml). The purity of these T-cells was analyzed by checking the expression of CD3 and CD4 markers using flow cytometry and purity of isolated T-cells was 90–94%. T- cells were autologous to DCs in all experiments.
HIV-1 BaL was obtained from the NIH AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, NIH. To prepare HIV-1 stocks, PBMC derived T-cells were cultured with HIV-1 BaL for 7 days. Fresh T-cells, suspended at 1 × 106 cells/ml were added at day 7. At day 14 after initial viral inoculation, the supernatant was harvested and stored at −80 °C. HIV-1 p24 viral antigen in the supernatants was quantified by ELISA (Zeptometrix Corporation, Buffalo, NY). 293T cells were procured from American Type Culture Collection (ATCC) (Manassas, VA).
Constructs FLAG-tagged Vpu (3xFLAG-Vpu), Vpr (3xFLAG-Vpr) and Vif (3xFLAG-Vif) were kindly gifted to the lab by Dr. Nevan J. Krogan (University of San Francisco, CA) [35]. HIV-1 NL4–3, HIV-1 NL4–3ΔVpu, and HIV-1 NL4–3ΔEnvEGFP constructs were obtained from the NIH AIDS Research and Reference Reagent Program-Fisher Bioservices.
PCR was used to clone the full-length LSP1 encompassing amino acids 2–339 and the truncation mutants 2–145 and 146–339 into a modified pTriEx-1 vector at the BamH1 and EcoR1 sites for expression in E. coli. The expressed proteins contained an N-terminal 6xHis-GST tag followed by thrombin and rhinovirus 3C protease cleavage sites. Full length LSP1 was also cloned into pCMV-HA1 at the BglII and EcoR1 sites to create an N-terminal hemagglutinin (HA) tag on the expressed protein.
The mature form of BST-2 (amino acids 2–161) and a mutant lacking a putative GPI-anchor amidated serine (S161A) were cloned into the mammalian expression vector pCMV-HA1 at the BglII and EcoR1 sites. The expressed proteins contain an N-terminal HA tag. All constructs were verified by sequencing prior to transfection or transformation.
Antibodies and reagents
EEA1, Rab5, HA tag, GST tag, LSP1, Ubiquitin, HRS, STAM1, β-TrCP and FLAG antibodies were obtained from Cell Signaling Technology (Danvers, MA). Tetherin and GAPDH antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), LSP1 antibody was purchased from BD. CellTrace Far-Red DDAO-SE (C34553), and Lipofectamine 2000 were obtained from Life Technologies Corp. FITC-conjugated p24 GAG (6604665) and RD1-conjugated p24 GAG (6604667) antibodies were obtained from Beckman Coulter, Inc. (Brea, CA). Anti-GFP antibody (FITC) was obtained from Abcam (Cambridge, MA). Interferon-α was purchased from PBL Assay Science (Piscataway, NJ)
siRNA-mediated knockdown of LSP1
Small interference RNA-mediated knockdown of LSP1 was performed using LSP1 –siRNA (Santa Cruz Biotechnology) as described previously [36]. DCs were transfected with LSP1 siRNA or non-targeted (NT) siRNA as a negative control (GE Dharmacon, Lafayette, CO) by nucleofection (Lonza, Wakersville, MD) according to the manufacturer’s instructions.
Confocal microscopy
DCs or T-cells were cultured on chamber slides. They were uninfected or infected with HIV-1 BaL for 2 or 24 hours. They were fixed in 4% paraformaldehyde and blocked with 5% normal goat serum in PBS/Triton X100 (1 hour). Cells were then incubated with primary antibodies overnight at 4 °C, washed thrice with PBS, and stained with conjugated secondary antibodies for 2 hours. Subsequently, cells were washed thrice with PBS, and slides were mounted using Prolong Gold antifade with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen). Slides were examined under a Zeiss 880 Meta confocal microscope (Carl Zeiss Microimaging, LLC, Thornwood, NY), and images were acquired using ZEN2 software (Carl Zeiss). Figures were made using Adobe Photoshop CS4 software (Adobe Systems, San Jose, CA). Total 100 cells per condition were analyzed by randomly choosing 10 images containing approximately 10 cells /image.
Analyzing the role of HIV-1Vpu in the LSP1 degradation
Constructs HA tagged LSP1 (LSP1-HA) and FLAG-tagged Vpu (3xFLAG-Vpu) or Vpr (3xFLAG-Vpr) or Vif (3xFLAG-Vif) were co-transfected into 293T cells by using Lonza Nucleofector device according to the manufacturer’s instructions. After 24 hours cells were lysed and immunoprecipitated with anti-HA antibody. Interactions of LSP1 with Vpu, Vpr and Vif were analyzed with anti-FLAG antibody. To analyze effects of HIV-1Vpu in LSP1 degradation, infectious molecular clone pNL4–3 or pNL4–3ΔVpu or pNL4–3ΔEnvEGFP was nucleofected into DCs by using Lonza Nucleofector device according to the manufacturer’s instructions. After 3 days post transfection LSP1 expression levels were quantitated by Western blotting.
Western blotting and immunoprecipitation
Western blotting was performed as previously described [27]. Briefly, uninfected and HIV-1 infected/ untreated or IFN-α treated T-cells or DCs or untreated or transfected 293T cells were collected in cell lysis buffer, protein lysates were separated on NuPAGE precast gels (Life Technologies Corp.), transferred to 0.45 μm nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA), and probed with appropriate primary antibodies followed by incubation with their respective secondary antibodies. Proteins were visualized with Western Lightning Plus ECL Substrate (PerkinElmer, Waltham, MA).
For immunoprecipitation assay, untreated or HIV-1 infected or IFN-α treated DCs or T-cells or transfected 293T cells were lysed with cell lysis buffer (Cell Signaling Technology). Immunoprecipitation was performed as previously described [16].
Endocytosis assay
Untreated DCs and DCs transfected with NT-siRNAs or LSP1-specific siRNAs were incubated with HIV-1 BaL for 30 minutes at 4°C, followed by incubation at 37°C for 2 hours as described previously [37]. The cells were lysed with cell lysis buffer and analyzed for p24 using ELISA.
Assessing HIV-1 transfer by flow cytometry
To analyze the effect of LSP1 knockdown on HIV-1 transfer from DCs to T-cells, DCs (2.5× 105) transfected with NT-siRNA or LSP1-siRNA were incubated with HIV-1 BaL [20ng/ml p24 gag] for 2 hours, washed thrice in 1X PBS to remove untrapped virions, and replated. Subsequently, Far-Red-labelled autologous T-cells (1.0×106) were added. After indicated times, cells were stained for p24-FITC (HIV-1 marker) before acquiring by flow cytometry. Far-Red-labelled T-cells were analyzed for p24 co-expression by FACS analysis, from a total of 10,000 acquired events.
Statistical Analysis
Differences between groups were calculated using a standard 2-tailed Student’s t-test. P-values ≤ 0.05 were considered statistically significant.
RESULTS
LSP1 mediates intracellular trafficking of HIV-1 via interacting with BST-2 and components of ESCRT machinery
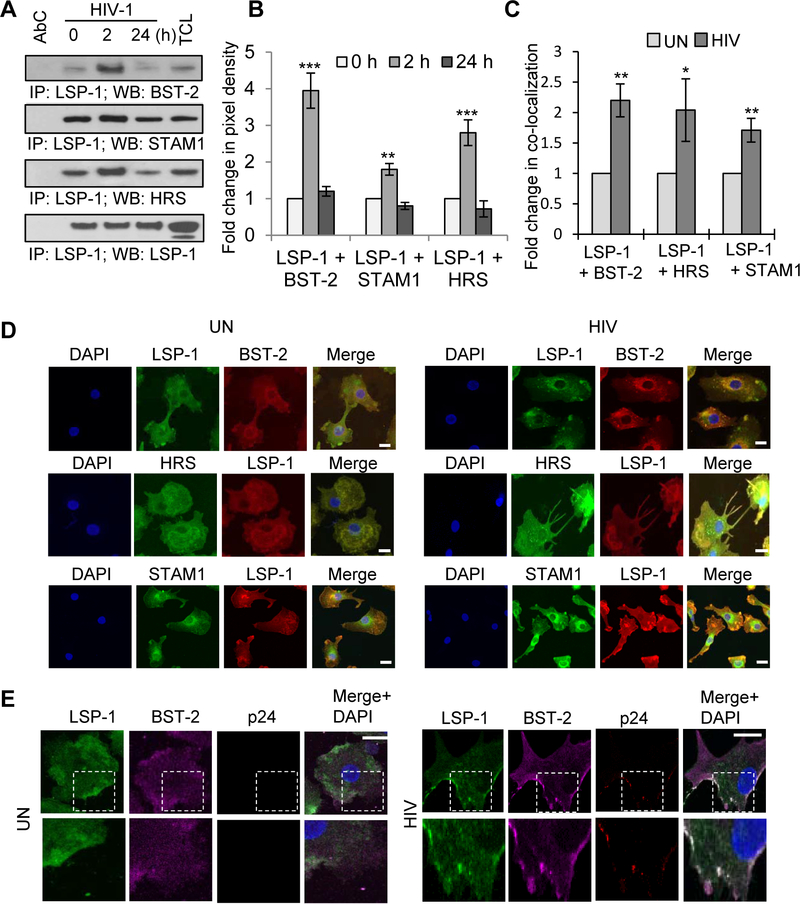
LSP1 has been shown to play a key role in DC-SIGN mediated internalization of HIV-1 by interacting with various scaffolding proteins including KSR1, CNK and Raf1 [29]. Since LSP1 also can transport HIV-1 to proteasomes, we analyzed its interaction with components of the ESCRT complex. Immunoprecipitation experiments in DCs (immature monocyte derived dendritic cells) revealed that HIV-1 induced increased association between LSP1 and Signal Transducing Adaptor Molecule 1 (STAM1) and Hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) (Fig. 1A and B). These two molecules are components of the ESCRT-0 complex that mediate the endocytic sorting of ubiquitinated membrane proteins to the lysosomal compartment for degradation [38, 39]. Intriguingly, further analysis revealed an interaction between LSP1 and BST-2; this interaction was significantly enhanced after HIV-1 infection. Confocal microscopic analyses confirmed an enhanced interaction of LSP1 with STAM1, HRS and BST-2 upon HIV-1 infection in DCs (Fig. 1C and D). To elucidate the role of LSP1/BST-2 complex in intracellular trafficking of HIV-1, we analyzed the co-localization of LSP1/BST-2 with HIV-1 p24 in HIV-1 infected DCs. Our confocal microscopic analyses revealed increased co-localization of the LSP1/BST-2 complex with HIV-1 p24, indicating that LSP1/BST-2 may participate in intracellular trafficking of HIV-1 (Fig. 1E).
Figure 1: LSP1 mediates intracellular trafficking of HIV-1 by interacting with BST-2 and ESCRT components.
(A) DCs were incubated with HIV-1 BaL (10ng/ml) for indicated time points (0–24 hours); lysed and immunoprecipitated with anti-LSP1 antibody. Interactions of LSP1 with BST-2, STAM1 and HRS were analyzed by Western blotting. LSP1 was used as loading control. Results are representative of 3 independent experiments. (B) Quantitative analysis of the Western blots in (A). The band intensity in each lane was determined by Image J software. The fold change was determined by calculating the value of each lane vs. un-infected (0). Data represent the mean ± SD of 3 independent experiments. AbC – antibody control; TCL - total cell lysate. (C) Quantitative analysis of colocalization of LSP1/BST-2, LSP1/HRS and LSP1/STAM1 interactions in DCs in (D) using Image J software. Data represents fold changes in Pearson’s correlation coefficient indices of 10 randomly chosen images per condition by normalizing to the control (UN). (D) Confocal images of LSP1 interactions with BST-2, HRS and STAM1 in DCs, incubated with or without HIV-1 for 2 hours. Scale bars = 10 μm. (E) Confocal images of LSP1/BST-2/HIV-1 p24 interaction in DCs incubated with HIV-1 for 2 hours. White square depicts area of colocalization. Results are representative of 3 independent experiments. Scale bars = 10 μm.
Molecular interactions of LSP1 and BST-2
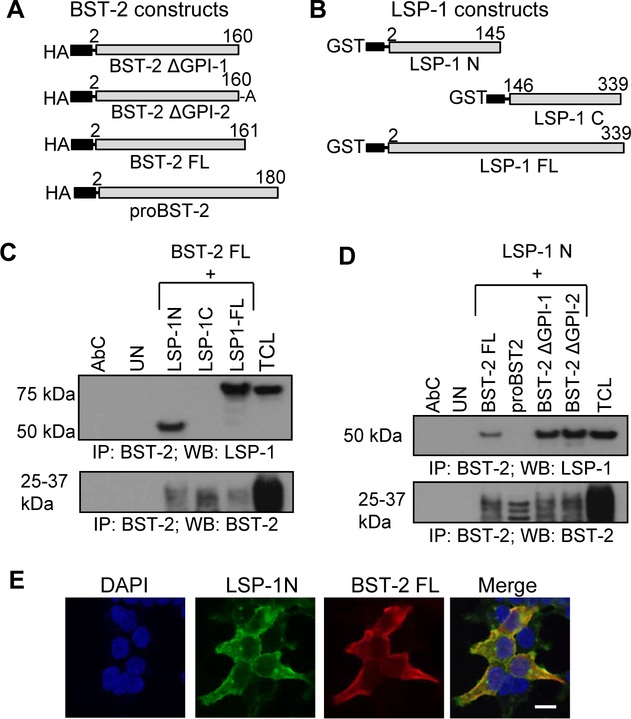
To further analyze the molecular interactions between LSP1 and BST-2, we co-transfected full length or truncated constructs of LSP1 and BST-2 (Fig. 2A and B) into 293T cells. Immunoprecipitation analysis revealed the interaction of the N-terminus of LSP1 with BST-2 FL. We did not observe any interaction between the C-terminus LSP1 with BST-2 FL (Fig. 2C). Next, to study the role of the GPI anchor of BST-2 in this interaction, we co-transfected 293T cells with BST-2 FL (aa2–161) or proBST-2 (aa2–180) or BST-2 ΔGPI or BST-2 ΔGPI mutated (aa160 S x A) and N-terminus LSP1 constructs. We observed interaction of N-terminus LSP1 with both GPI mutants of BST-2 and BST-2 FL constructs, indicating that the GPI anchor does not participate in the interaction between LSP1 and BST-2. Interestingly, we did not observe an interaction of LSP1 with proBST-2 (aa2–180) (Fig. 2D). We confirmed the LSP1 N-terminus and BST-2 FL interaction in co-transfected 293T cells by confocal microscopy (Fig. 2E).
Figure 2: Molecular interactions of LSP1 and BST-2.
(A & B) BST-2 and LSP1 constructs used for overexpression: HA-tagged BST-2 with GPI mutation by substituting A at position 161 with S (BST-2 ΔGPI-1), BST-2 with GPI mutation by deletion of A at position 161 (BST-2 ΔGPI-2), BST-2 FL (full length), proBST-2; GST-tagged LSP1 N (C-terminal deletion), LSP1 C (N-terminal deletion) and LSP1 FL (full length). (C) Interactions between BST-2 and LSP1 by immunoprecipitation in 293T cells co-transfected with BST-2 FL and LSP1 constructs (N-term, C-term, and FL). BST-2 was used as a loading control. AbC – antibody control; TCL - total cell lysate. (D) Interactions between BST-2 and LSP1 by immunoprecipitation of 293T cells co-transfected with LSP1 N and BST-2 FL, proBST-2, BST-2 ΔGPI-1 and BST-2 ΔGPI-2 constructs. BST-2 was used as a loading control. (E) Co-localization of LSP1 and BST-2 in 293T cells co-transfected with LSP1 N-term and BST-2 FL constructs and stained with anti-GST (green) and anti-HA (red) antibodies. Results are representative of 3 independent experiments. Scale bar = 10μm.
LSP1 mediates HIV-1 endocytosis in DCs
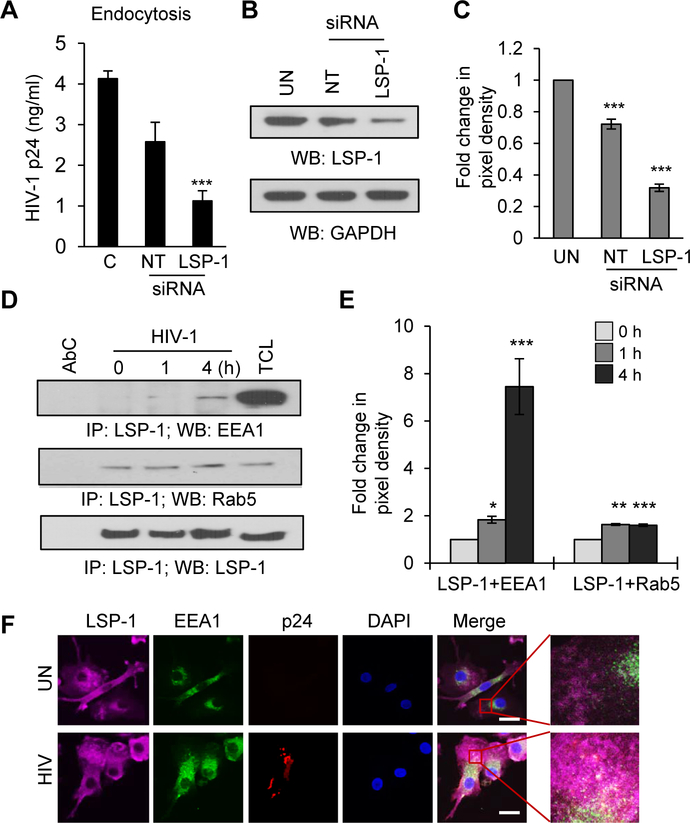
Previous studies have shown that ablation of LSP1 by siRNA significantly inhibited productive infection of HIV-1 in astrocytes, suggesting its role in HIV-1 endocytosis [40]. We thus tested the effects of LSP1 depletion on HIV-1 endocytosis in DCs. We knocked down expression of LSP1 by siRNA, and observed that HIV-1 endocytosis was significantly inhibited in LSP1 depleted cells compared to controls (Fig. 3A). LSP1 knockdown was confirmed by using Western blotting (Fig. 3B and C). Next, we analyzed the association of LSP1 with the early endosome markers EEA1 (Early Endosome Antigen) and Rab5, a regulatory guanosine triphosphatase associated with the sorting endosome (Fig. 3D and E). We observed an enhanced association of LSP1 with EEA1 and Rab5 after HIV-1 treatment. Next, we performed confocal microscopy to analyze whether LSP1 mediates transfer of HIV-1 to endosomes by co-staining of LSP1, EEA1 and HIV-1 p24 after incubating DCs with HIV-1 (Fig. 3F). We observed increased co-localization of these molecules in HIV-1 incubated cells compared to controls. These results indicate that LSP1 may be involved in the internalization of HIV-1 by mediating the formation of early endosomes in DCs.
Figure 3: LSP1 mediates HIV-1 endocytosis in DCs.
(A) Untreated DCs and DCs transfected with NT-siRNAs or LSP1-specific siRNAs were incubated with HIV-1 BaL as described in Methods. The cells were lysed and analyzed for p24 using ELISA. Data represent the mean ± SEM of 3 experiments (C=control, NT=non-targeted). (B) Representative Western blots analysis of LSP1 expression in untreated DCs (UN) and in DCs transfected with NT-siRNAs (NT) or LSP1-specific (LSP1) siRNAs. GAPDH served as a loading control. (C) Quantitative analysis of the Western blots in (B). The band intensity in each lane was determined by Image J software. The fold change was determined by considering value of un-transfected control (UN) as 1. Data represent the mean ± SD of 3 independent experiments. (D) DCs were incubated with HIV-1 BaL (10ng/ml) for indicated time points (0–4 hours); lysed and immunoprecipitated with anti-LSP1 antibody. Interactions of LSP1 with EEA1 and Rab5 were analyzed by Western blotting. LSP1 was used as loading control. AbC – antibody control; TCL - total cell lysate. (E) Quantitative analysis of the Western blots of LSP1/EEA1 and LSP1/Rab5 interactions. The band intensity in each lane was determined by Image J software. The fold change was determined by considering value of untreated control (0) as 1. Data represent the mean ± SD of 3 independent experiments. (F) Confocal images of LSP1/EEA1/HIV-1 p24 interaction in DCs incubated with or without HIV-1 for 2 hours. Scale bars = 10 μm.
Interferon-α enhances LSP1 and BST-2 interaction in both DCs and T-cells
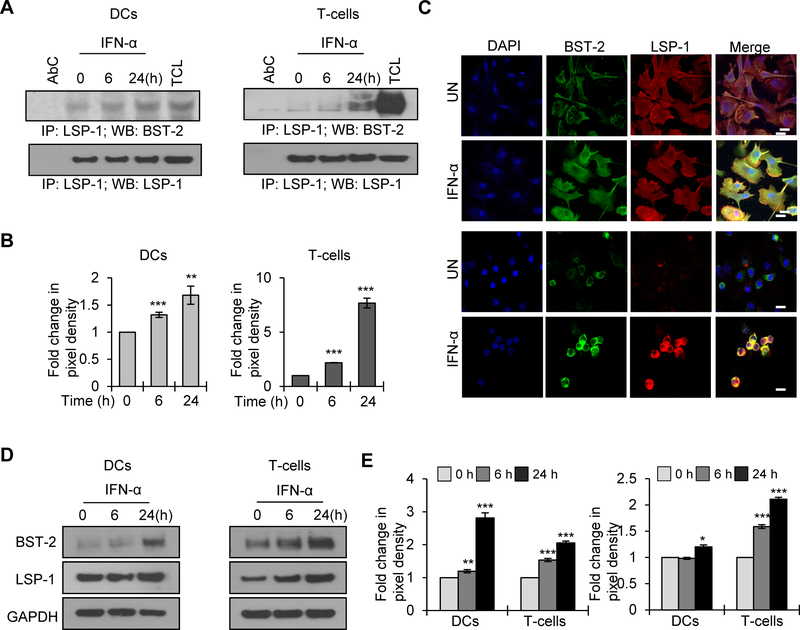
Since BST-2 is an IFN-α inducible protein, we studied the effect of INF-α on the LSP1/BST-2 interaction, in DCs and T-cells. We observed an enhanced association between LSP1/BST-2 upon IFN-α treatment in both cell types (Fig. 4A and B). We confirmed this IFN-α enhanced interaction by confocal microcopy (Fig. 4C). Further, we tested the effects of IFN-α on the expression pattern of LSP1 and BST-2 in both T-cells and DCs. As expected, we found increased expression of BST-2 upon IFN-α treatment in both cell types. Interestingly, we also observed a moderate increase in LSP1 expression in DCs and a significant increase in T-cells upon IFN-α treatment (Fig. 4D and E).
Figure 4: Interferon-α enhances LSP1 and BST-1 interaction in DCs and T-cells.
(A) DCs (left panel) and T-cells (right panel) were untreated or treated with 300U/ml of IFN-α for indicated time points (0–24 hours); lysed and immunoprecipitated with anti-LSP1 antibody. Interaction of LSP1 with BST-2 was analyzed by Western blotting. LSP1 was used as loading control. AbC – antibody control; TCL - total cell lysate. (B) Quantitative analysis of the Western blots of T-cells and DCs in (A). (C) Confocal images of BST-2 and LSP1 interactions in DCs (top panel) and T-cells (lower panel), untreated or treated with IFN-α for 24 hours. Scale bar = 10 μm. Images are representative of 3 independent experiments. (D) Cell lysates from DCs or T-cells, untreated or treated with 300U/ml of IFN-α for indicated time points were analyzed for BST-2 and LSP1 expression by Western blot analysis. GAPDH served as a loading control. (E) Quantitative analysis of the Western blots of BST-2 (left panel) and LSP1 (right panel) in DCs and T-cells (D). The band intensity in each lane was determined by Image J software. The fold change was determined by considering value of untreated (0) as 1. Data represent the mean ± SD of 3 independent experiments.
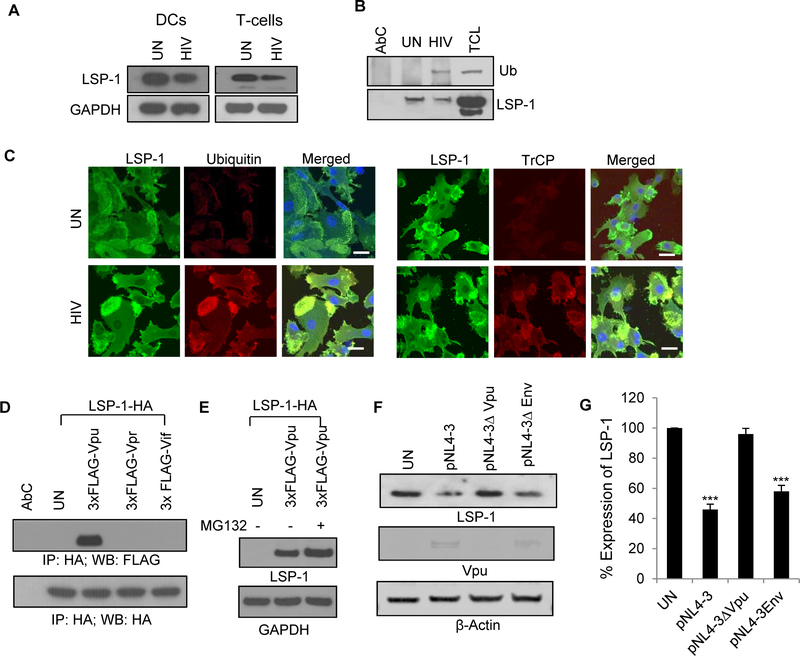
HIV-1 Vpu induces proteasomal degradation of LSP1 in DCs
HIV-1 is known to alter the host intracellular trafficking pathways by its accessory proteins such as Vif, Vpr and Vpu [41]. Furthermore, HIV-1 Vpu has been shown to induce degradation of various host resistance factors including BST-2 [41, 42]. Hence, we analyzed the expression pattern of LSP1 in HIV-1 infected DCs and T-cells. We found a significant decrease in the expression of LSP1 in HIV-1 infected cells compared to control cells (Fig. 5A). Next, we analyzed the molecular mechanisms involved in the decreased expression of LSP1 in HIV-1 infected cells. We did not observe changes in the levels of mRNA of LSP1 (data not shown). However, immunoprecipitation experiments revealed increased ubiquitination of LSP1 in HIV-1 infected DCs compared to uninfected cells (Fig. 5B). We confirmed this result using confocal microscopy, where we observed increased co-localization of ubiquitin and LSP1 in HIV-1 infected cells compared to control cells (Fig. 5C, left panel). In addition, we found enhanced colocalization of β-Trcp and LSP1 in HIV-1 infected DCs compared to uninfected cells indicating that β-TrCP may mediates ubiquitination and degradation of LSP1 in HIV-1 infected DCs (Fig. 5C, right panel). To further investigate the role of HIV-1 accessory molecules in LSP1 degradation, we co-transfected FLAG-tagged Vpu or Vpr or Vif and HA-tagged LSP1 in 293T cells and analyzed the interaction of these molecules with LSP1 by immunoprecipitation (Fig. 5D). We only observed an interaction between Vpu and LSP1. Next, to study the role of Vpu in LSP1 degradation, we cultured Vpu and LSP1 co-transfected 293T cells in the presence or absence of a proteasome inhibitor and then analyzed the level of LSP1 expression by Western blotting (Fig. 5E). We observed increased LSP1 expression in proteasome inhibitor treated cells compared to untreated cells. To further confirm the role of Vpu in LSP1 degradation, we transfected the DCs with Vpu-deleted variant of HIV-1NL4–3 (pNL4–3Δvpu) or full-length HIV-1 NL4–3 (pNL4–3) and Env-deleted variant of HIV-1 NL4–3 (pNL4–3ΔEnv EGFP) as controls and analyzed the expression levels of LSP1 in these cells after 3 days post-transfection. We observed no significant change in the expression of LSP1 in DCs infected with Vpu-deleted variant of HIV-1 compared to uninfected cells, whereas LSP1 expression level was significantly reduced in HIV-1 NL4–3 or HIV-1 NL4–3ΔEnv infected cells (Fig. 5F–G). These results indicate that HIV-1 Vpu can participate in proteasomal degradation of LSP1 in HIV-1 infected DCs and T-cells. Interestingly, it has been shown that HIV-1 Vpu also can mediate proteasomal degradation of BST-2 and thereby inhibit its intrinsic resistance against HIV-1 infection [41, 42].
Figure 5: Degradation of LSP1 by HIV-1.
(A) Cell lysates from DCs or T-cells incubated with or without HIV-1 BaL (20ng/ml) for 24 hours were analyzed for LSP1 expression by Western blot analysis. GAPDH served as a loading control. (B) DCs were incubated with or without HIV-1 BaL (20ng/ml) for 24 hours; lysed and immunoprecipitated with anti-LSP1 antibody. Interaction of LSP1 with Ubiquitin (Ub) was analyzed by Western blotting. LSP1 was used as loading control. AbC – antibody control; TCL - total cell lysate. (C) Confocal images of LSP1/Ubiquitin (left panel) and LSP1/β-TrCP (right panel) interactions in DCs incubated with or without HIV-1 for 24 hours. Scale bars = 10 μm. (D) 293T cells were co-transfected with HA tagged LSP1 (LSP1-HA) with FLAG-tagged Vpu (3xFLAG-Vpu) or Vpr (3xFLAG-Vpr) or Vif (3xFLAG-Vif); lysed and immunoprecipitated with anti-HA antibody. Interactions of LSP1 with Vpu, Vpr and Vif were analyzed with anti-FLAG antibody. HA was used as loading control. (E) Cell lysates from 293T cells co-transfected with 3xFLAG-Vpu and LSP1-HA cultured with or without proteasome inhibitor MG132 were analyzed for LSP1 expression by Western blot analysis. GAPDH served as a loading control. (F-G) DCs were nucleofected with infectious molecular clone pNL4–3 or pNL4–3ΔVpu or pNL4–3ΔEnvEGFP, and LSP1 expression levels were analyzed by Western blotting. The band intensity was quantitated by using ImageJ software and percent expression was calculated by considering untreated (UN) as 100%. Results are representative of 3 independent experiments.
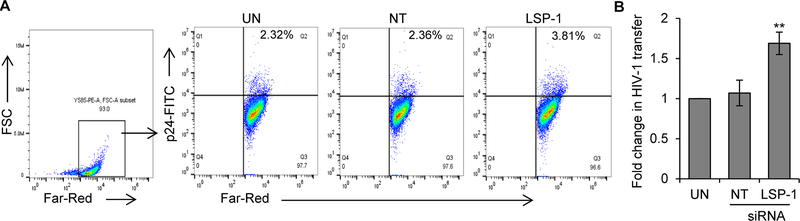
LSP1 knockdown enhances DC to T-cell HIV-1 transmission
Next, we tested the effects of LSP1 knockdown in DCs on cell-cell transmission of HIV-1 using an in vitro DC to T-cell viral transfer assay. LSP1 knocked down DCs were incubated with HIV-1 BaL for 2 hours, washed to remove unbound virus and Far Red labelled-T-cells were added at a 1:4 ratio. After 3 days, the DC and T-cell co-cultures were stained with anti-HIV-1 p24 antibody and analyzed by flow cytometry. We quantitated the HIV-1 p24 positive Far-Red-labelled T-cells in the total Far-Red-labelled T-cell population, which represents T-cells infected from DCs. We found significant increases in transfer of HIV-1 from LSP1 depleted DCs compared to controls (Fig. 6A and B).
Figure 6: Knockdown of LSP1 in DCs enhances HIV-1 transmission to T-cells.
(A) Untransfected (UN), control transfected (NT) or LSP1 knockdown DCs were incubated with HIV-1 BaL for 2 hours, washed to remove free virus. Far-Red-labelled T-cells were added. After 3 days, cells were harvested and stained with p24-FITC antibody (HIV-1 marker) and analyzed by flow cytometry. Far-Red-labelled T-cells were gated and p24 positive Far-Red-labelled T-cells were quantitated. (B) Fold change in HIV-1 transfer as described in (A) was calculated by considering untransfected as 1 from 6 independent experiments. UN – untransfected DCs; NT - non-targeted siRNA transfected DCs; LSP1 – LSP1 siRNA transfected DCs.
DISCUSSION
HIV-1 utilizes various mechanisms to exploit a cell-encoded intracellular vesicle trafficking pathway to avoid degradation and facilitate replication [9–12]. The actin binding cytoskeletal protein, LSP1, has been shown to play a central role in transporting the virus for proteasomal degradation [14, 30]. In this study, we demonstrate that LSP1 interacts with BST-2 and IFN-α further enhances this interaction. In addition, we found that a LSP1/BST-2 complex facilitates endocytosis of cell surface bound viruses. BST-2, also known as tetherin, is a restriction factor of enveloped viruses including HIV-1, and inhibits its release by tethering viral particles to the surface of infected cells [31, 32]. Although it has been well established that BST-2 could inhibit virion release, the fate of the tethered, mature virions on the infected cell surface is still not well defined. These virions are either endocytosed and transported to a phagolysosomal complex for degradation or may enhance cell-to-cell spread of infection [10]. At the cell surface, BST-2 is expressed as a dimer consisting of an N-terminal transmembrane domain, glycosylated coiled-coil extracellular region, and C-terminal glycophosphatidyl inositol (GPI) anchor [43–45]. The N-terminal cytoplasmic tail encodes a dual tyrosine motif, which is known to trigger endocytosis and activation of the transcription factor NF-κB [45–48]. In addition, this motif is implicated in clathrin-dependent endocytosis and association with the actin cytoskeletal system [45, 47]. Our structural analysis of the LSP1/BST-2 interaction revealed that it is the N-terminal domain of LSP1 which binds to BST-2. Furthermore, we hypothesize that LSP1 may bind to the dual tyrosine motif of N-terminal region of BST-2, since this site has been shown to interact with the actin cytoskeleton [45]. Intriguingly, the dual tyrosine motif of N-terminal region is also involved in clathrin mediated endocytosis [45, 47]. Hence, LSP1 interaction with BST-2 may regulate internalization of HIV-1 virions tethered to BST-2 at the cell surface. Our confocal microscopic studies revealed co-localization of a LSP1/BST-2 complex with HIV-1 p24; this further supports our hypothesis that LSP1/BST-2 is involved in HIV-1 internalization.
Previously, we have reported on the role of LSP1 in internalization of HIV-1 in DCs and others have reported this in astrocytes [16, 40]. Binding of HIV-1 to C-type lectin DC-SIGN induced its interaction with LSP1, thereby mediating the formation of a signalosome complex, which is involved in internalizing HIV-1 [29]. Our study further revealed that LSP1 interacts with the ESCRT complex, which plays a major role in intracellular vesicular trafficking. Hence, LSP1 may facilitate the transfer of endocytosed HIV-1 to the ESCRT complex, which is further transported to the phagolysosomal complex. Our study also revealed the recruitment of HRS and STAM1 to the LSP1/BST-2 complex. HRS and STAM1 are involved in endocytic sorting of ubiquitinated proteins to the lysosomal compartment for degradation [38, 39]. Interestingly, BST-2 has also been shown to associate with HRS during HIV-1 Vpu mediated degradation [38]. These results further support the finding of Smith et al., who demonstrated a role of LSP1 in transporting HIV-1 to the proteasomal complex for degradation [30].
HIV-1 can subvert intracellular trafficking pathways to avoid its degradation and enhance its spread [10, 49]. HIV-1 overcomes BST-2 mediated intrinsic resistance by promoting its down-regulation via proteasomal degradation [45, 50, 51]. Interestingly, we also observed HIV-1 Vpu induced degradation of LSP1 in both DCs and T-cells, thereby altering intracellular trafficking to avoid degradation. Downregulation of LSP1 with specific siRNA in DCs has been shown to enhance transfer of HIV-1 from DC to T-cells [30]. Consistent with these results, we observed siRNA mediated knockdown of LSP1 in DCs enhanced transmission of HIV-1 to T-cells.
In sum, here we identify a novel mechanism of intracellular trafficking of HIV-1 by demonstrating that a LSP1/BST-2 complex participates in the internalization of cell surface bound HIV-1 particles and further traffics viral particles towards proteasomal degradation via interacting with ESCRT components. Moreover, we observed that HIV-1 Vpu can subvert this pathway by inducing proteasomal degradation of LSP1 and, thereby, facilitate cell-cell transmission.
Acknowledgements
The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH (HIV-1 Ba-L; HIV-1 NL4-3, cat# 114; HIV-1 NL4-3 ΔVpu, cat# 968; HIV-1NL4-3ΔEnv EGFP, cat# 11100). HIV-1 Ba-L from Dr. Suzanne Gartner, Dr. Mikulas Popovic and Dr. Robert Gallo. We thank Dr. Nevan J. Krogan, University of San Francisco, CA for generously providing the Vpr, Vif and Vpu constructs. We thank Dr. Jerome Groopman for helpful suggestions on experimental design. The research is supported in part by grants from the National Institutes of Health [National Institute on Drug Abuse (http://www.drugabuse.gov)] 5 R21 DA040353 (to A.P.) and 5R01DA036298-04 (to J.E.G).
Abbreviations
- HIV-1
Human immunodeficiency virus-1
- LSP1
Lymphocyte-specific protein-1
- BST-2
Bone Marrow Stromal Antigen 2
- DC
Dendritic cells
- ESCRT
The endosomal sorting complexes required for transport
- GPI
Glycosylphosphatidylinositol
- KSR-1
Kinase suppressor of RAS-1
- CNK
Connector enhancer of KSR
Footnotes
Conflict of interest
No competing interests declared.
References
- 1.Cann AJ & Karn J (1989) Molecular biology of HIV: new insights into the virus life-cycle, AIDS. 3 Suppl 1, S19–34. [PubMed] [Google Scholar]
- 2.Haseltine WA (1988) Replication and pathogenesis of the AIDS virus, J Acquir Immune Defic Syndr. 1, 217–40. [PubMed] [Google Scholar]
- 3.Gaudin R, de Alencar BC, Arhel N & Benaroch P (2013) HIV trafficking in host cells: motors wanted!, Trends Cell Biol. 23, 652–62. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann M, Nikolic DS & Piguet V (2011) How HIV-1 takes advantage of the cytoskeleton during replication and cell-to-cell transmission, Viruses. 3, 1757–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naghavi MH & Goff SP (2007) Retroviral proteins that interact with the host cell cytoskeleton, Curr Opin Immunol. 19, 402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ospina Stella A & Turville S (2018) All-Round Manipulation of the Actin Cytoskeleton by HIV, Viruses. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed Z, Kawamura T, Shimada S & Piguet V (2015) The role of human dendritic cells in HIV-1 infection, J Invest Dermatol. 135, 1225–1233. [DOI] [PubMed] [Google Scholar]
- 8.Wacleche VS, Tremblay CL, Routy JP & Ancuta P (2018) The Biology of Monocytes and Dendritic Cells: Contribution to HIV Pathogenesis, Viruses. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavrois M, Neidleman J & Greene WC (2008) The achilles heel of the trojan horse model of HIV-1 trans-infection, PLoS Pathog. 4, e1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piguet V & Steinman RM (2007) The interaction of HIV with dendritic cells: outcomes and pathways, Trends Immunol. 28, 503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puryear WB & Gummuluru S (2013) Role of glycosphingolipids in dendritic cell-mediated HIV-1 trans-infection, Adv Exp Med Biol. 762, 131–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu HJ, Reuter MA & McDonald D (2008) HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells, PLoS Pathog. 4, e1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauhan A & Khandkar M (2015) Endocytosis of human immunodeficiency virus 1 (HIV-1) in astrocytes: a fiery path to its destination, Microb Pathog. 78, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jongstra-Bilen J & Jongstra J (2006) Leukocyte-specific protein 1 (LSP1): a regulator of leukocyte emigration in inflammation, Immunol Res. 35, 65–74. [DOI] [PubMed] [Google Scholar]
- 15.Maxeiner S, Shi N, Schalla C, Aydin G, Hoss M, Vogel S, Zenke M & Sechi AS (2015) Crucial role for the LSP1-myosin1e bimolecular complex in the regulation of Fcgamma receptor-driven phagocytosis, Mol Biol Cell. 26, 1652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad A, Kulkarni R, Jiang S & Groopman JE (2017) Cocaine Enhances DC to T-cell HIV-1 Transmission by Activating DC-SIGN/LARG/LSP1 Complex and Facilitating Infectious Synapse Formation, Sci Rep. 7, 40648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C & Walter P (2006) Eisosomes mark static sites of endocytosis, Nature. 439, 998–1003. [DOI] [PubMed] [Google Scholar]
- 18.Jongstra J, Ittel ME, Iscove NN & Brady G (1994) The LSP1 gene is expressed in cultured normal and transformed mouse macrophages, Mol Immunol. 31, 1125–31. [DOI] [PubMed] [Google Scholar]
- 19.Kadiyala RK, McIntyre BW & Krensky AM (1990) Molecular cloning and characterization of WP34, a phosphorylated human lymphocyte differentiation and activation antigen, Eur J Immunol. 20, 2417–23. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Guerrero A & Howard TH (1995) The actin-binding protein, lymphocyte-specific protein 1, is expressed in human leukocytes and human myeloid and lymphoid cell lines, J Immunol. 155, 3563–9. [PubMed] [Google Scholar]
- 21.Liu L, Cara DC, Kaur J, Raharjo E, Mullaly SC, Jongstra-Bilen J, Jongstra J & Kubes P (2005) LSP1 is an endothelial gatekeeper of leukocyte transendothelial migration, J Exp Med. 201, 409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palker TJ, Fong AM, Scearce RM, Patel DD & Haynes BF (1998) Developmental regulation of lymphocyte-specific protein 1 (LSP1) expression in thymus during human T-cell maturation, Hybridoma. 17, 497–507. [DOI] [PubMed] [Google Scholar]
- 23.Huang CK, Zhan L, Ai Y & Jongstra J (1997) LSP1 is the major substrate for mitogen-activated protein kinase-activated protein kinase 2 in human neutrophils, J Biol Chem. 272, 17–9. [DOI] [PubMed] [Google Scholar]
- 24.Jongstra-Bilen J, Janmey PA, Hartwig JH, Galea S & Jongstra J (1992) The lymphocyte-specific protein LSP1 binds to F-actin and to the cytoskeleton through its COOH-terminal basic domain, J Cell Biol. 118, 1443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand AR, Prasad A, Bradley RR, Deol YS, Nagaraja T, Ren X, Terwilliger EF & Ganju RK (2009) HIV-1 gp120-induced migration of dendritic cells is regulated by a novel kinase cascade involving Pyk2, p38 MAP kinase, and LSP1, Blood. 114, 3588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossain M, Qadri SM, Xu N, Su Y, Cayabyab FS, Heit B & Liu L (2015) Endothelial LSP1 Modulates Extravascular Neutrophil Chemotaxis by Regulating Nonhematopoietic Vascular PECAM-1 Expression, J Immunol. 195, 2408–16. [DOI] [PubMed] [Google Scholar]
- 27.Prasad A, Kuzontkoski PM, Shrivastava A, Zhu W, Li DY & Groopman JE (2012) Slit2N/Robo1 inhibit HIV-gp120-induced migration and podosome formation in immature dendritic cells by sequestering LSP1 and WASp, PLoS One. 7, e48854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Zhan L, Ai Y, Hannigan M, Gaestel M, Huang CK & Madri JA (2007) MAPKAPK2-mediated LSP1 phosphorylation and FMLP-induced neutrophil polarization, Biochem Biophys Res Commun. 358, 170–5. [DOI] [PubMed] [Google Scholar]
- 29.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M & Geijtenbeek TB (2009) Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori, Nat Immunol. 10, 1081–8. [DOI] [PubMed] [Google Scholar]
- 30.Smith AL, Ganesh L, Leung K, Jongstra-Bilen J, Jongstra J & Nabel GJ (2007) Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells, J Exp Med. 204, 421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neil SJ, Zang T & Bieniasz PD (2008) Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu, Nature. 451, 425–30. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Caballero D, Zang T, Ebrahimi A, McNatt MW, Gregory DA, Johnson MC & Bieniasz PD (2009) Tetherin inhibits HIV-1 release by directly tethering virions to cells, Cell. 139, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu H, Wang JJ, Qi M, Yoon JJ, Chen X, Wen X, Hammonds J, Ding L & Spearman P (2012) Tetherin/BST-2 is essential for the formation of the intracellular virus-containing compartment in HIV-infected macrophages, Cell Host Microbe. 12, 360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar JR, Manna PT, Nishimura S, Banting G & Robinson MS (2016) Tetherin is an exosomal tether, Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jager S, Kim DY, Hultquist JF, Shindo K, LaRue RS, Kwon E, Li M, Anderson BD, Yen L, Stanley D, Mahon C, Kane J, Franks-Skiba K, Cimermancic P, Burlingame A, Sali A, Craik CS, Harris RS, Gross JD & Krogan NJ (2011) Vif hijacks CBF-beta to degrade APOBEC3G and promote HIV-1 infection, Nature. 481, 371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulkarni R & Prasad A (2017) Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3, Sci Rep. 7, 14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pritschet K, Donhauser N, Schuster P, Ries M, Haupt S, Kittan NA, Korn K, Pohlmann S, Holland G, Bannert N, Bogner E & Schmidt B (2012) CD4- and dynamin-dependent endocytosis of HIV-1 into plasmacytoid dendritic cells, Virology. 423, 152–64. [DOI] [PubMed] [Google Scholar]
- 38.Janvier K, Pelchen-Matthews A, Renaud JB, Caillet M, Marsh M & Berlioz-Torrent C (2011) The ESCRT-0 component HRS is required for HIV-1 Vpu-mediated BST-2/tetherin down-regulation, PLoS Pathog. 7, e1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kojima K, Amano Y, Yoshino K, Tanaka N, Sugamura K & Takeshita T (2014) ESCRT-0 protein hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) is targeted to endosomes independently of signal-transducing adaptor molecule (STAM) and the complex formation with STAM promotes its endosomal dissociation, J Biol Chem. 289, 33296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chauhan A, Mehla R, Vijayakumar TS & Handy I (2014) Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes, Virology. 456–457, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malim MH & Emerman M (2008) HIV-1 accessory proteins--ensuring viral survival in a hostile environment, Cell Host Microbe. 3, 388–98. [DOI] [PubMed] [Google Scholar]
- 42.Goffinet C, Allespach I, Homann S, Tervo HM, Habermann A, Rupp D, Oberbremer L, Kern C, Tibroni N, Welsch S, Krijnse-Locker J, Banting G, Krausslich HG, Fackler OT & Keppler OT (2009) HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor, Cell Host Microbe. 5, 285–97. [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa J, Kaisho T, Tomizawa H, Lee BO, Kobune Y, Inazawa J, Oritani K, Itoh M, Ochi T, Ishihara K & et al. (1995) Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST-2, that may be involved in pre-B-cell growth, Genomics. 26, 527–34. [DOI] [PubMed] [Google Scholar]
- 44.Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A & Banting G (2003) BST-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology, Traffic. 4, 694–709. [DOI] [PubMed] [Google Scholar]
- 45.Mahauad-Fernandez WD & Okeoma CM (2016) The role of BST-2/Tetherin in host protection and disease manifestation, Immun Inflamm Dis. 4, 4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galao RP, Le Tortorec A, Pickering S, Kueck T & Neil SJ (2012) Innate sensing of HIV-1 assembly by Tetherin induces NFkappaB-dependent proinflammatory responses, Cell Host Microbe. 12, 633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masuyama N, Kuronita T, Tanaka R, Muto T, Hirota Y, Takigawa A, Fujita H, Aso Y, Amano J & Tanaka Y (2009) HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin, J Biol Chem. 284, 15927–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y, Doi T, Shimotohno K, Harada T, Nishida E, Hayashi H & Sugano S (2003) Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways, Oncogene. 22, 3307–18. [DOI] [PubMed] [Google Scholar]
- 49.Dvorin JD & Malim MH (2003) Intracellular trafficking of HIV-1 cores: journey to the center of the cell, Curr Top Microbiol Immunol. 281, 179–208. [DOI] [PubMed] [Google Scholar]
- 50.Fujita H, Fujimoto K, Tokunaga K & Tanaka Y (2012) Intracellular logistics of BST-2/tetherin, Curr HIV Res. 10, 321–6. [DOI] [PubMed] [Google Scholar]
- 51.Li SX, Barrett BS, Guo K & Santiago ML (2016) Tetherin/BST-2: Restriction Factor or Immunomodulator?, Curr HIV Res. 14, 235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]