Abstract
Adenoviruses (Ads) are robust vectors for therapeutic and vaccine applications, but their use can be limited by differences in their in vitro and in vivo pharmacologies. This review emphasizes that there is not just one Ad, but a whole virome of diverse viruses that can be used as therapeutics. It discusses that true vector targeting involves not only retargeting viruses, but importantly also detargeting the viruses from off-target cells.
Keywords: Ad serotypes, liver, sequestration, serotypes, gain of function, retargeting, detargeting
Introduction
Adenoviruses (Ads) have many features that make them useful as oncolytic viruses, as gene-based vaccines, or as gene therapy vectors. First and foremost, they can be produced at exceptionally high yields up to 1013 virus particles (vp) from 109 cells. Ads are also stable non-enveloped viruses that can be lyophilized for long-term storage without a cold-chain [1–5]. This is quite different from the diverse array of enveloped viral vectors that are inactivated by freeze drying. This is important for storage, but also for deployment for global applications to regions where refrigeration is not always available.
Ad vectors mediate high transduction efficiency in dividing and non-dividing cells. Ads do not actively integrate into the host genome reducing the risk of insertional oncogenesis [6], but this also limits their persistence in actively dividing cells [7,8]. On the other hand, Ad genomes can persist for years in non-dividing cells provided that an immune response is not produced against Ad or the transgene product. For example, baboons injected once with helper-dependent Ad (HD-Ad) vectors have had persistent transgene expression for more than 7 years [9].
In many head-to-head in vivo comparisons, Ads mediate higher expression and more potent vaccine effects than most other vectors [10–15]. For example, when compared to DNA or vaccinia virus as an HIV vaccine in macaques, replication-defective Ad5 (RD-Ad5) vectors generated higher immune responses and better protection [12,13]. In an example from our lab, in gene therapy for propionic acidemia, a 10-fold lower gene dose of RD-Ad5 generated equal to or higher PCCA transgene expression than the popular adeno-associated virus 8 (AAV8) vector [10].
While Ads are arguably the most potent in vivo gene expression platform, they are also well-known for their ability to provoke immune responses and for a tragic death in an early gene therapy trial for ornithine transcarbamylase deficiency [16]. This makes them highly sought as gene-based vaccines and oncolytic viruses, but has restricted their use for gene therapy. This lack of use for gene therapy is largely political rather than scientific, since newer HD-Ad vectors and polymer shielding approaches largely mitigate most of their side effects [9,17–21].
This review discusses retargeting and detargeting Ads for therapeutic and vaccine applications. This retargeting can be imposed first by physically retargeting Ad particles to different receptors. For replication-competent vectors, a second layer of targeting can be applied post-entry by controlling how Ads activate their genetic program in cell-specific ways. A third layer of control can be added by controlling transgene expression in cell or situation-specific fashions. We will delve into these technologies later in the article, but must first lay the foundations of how the genetically diverse adenovirus virome provides opportunities to start targeting efforts with viruses that are already tuned to different applications.
Adenovirus Capsid Proteins as Platforms for Physical Particle Targeting and Off-Target Interactions
There are three major capsid proteins on adenoviruses: fiber, penton base, and hexon (Fig. 1, reviewed in [22,23]). There are 36 monomers of fiber, 60 monomers of penton base, and 720 monomers of hexon on each Ad virion. There is good evidence that the fiber and penton base proteins of many Ad serotypes interact directly with cellular receptors, but little evidence showing that hexons directly target cellular receptors. One exception to this binding of Ad hexons to scavenger receptors on macrophages, Kupffer cells, and endothelial cells [24,25]. While these interactions are usually destructive to Ads in vivo [24], ectopic expression of scavenger receptors on cells in vitro can lead to productive infection [26]. A more recent observation shows that certain human Ads can bind scavenger receptor MARCO (SR-A6) for productive infection [27].
Figure 1. Cryo-Electron Microscopic Structures of Ad26.
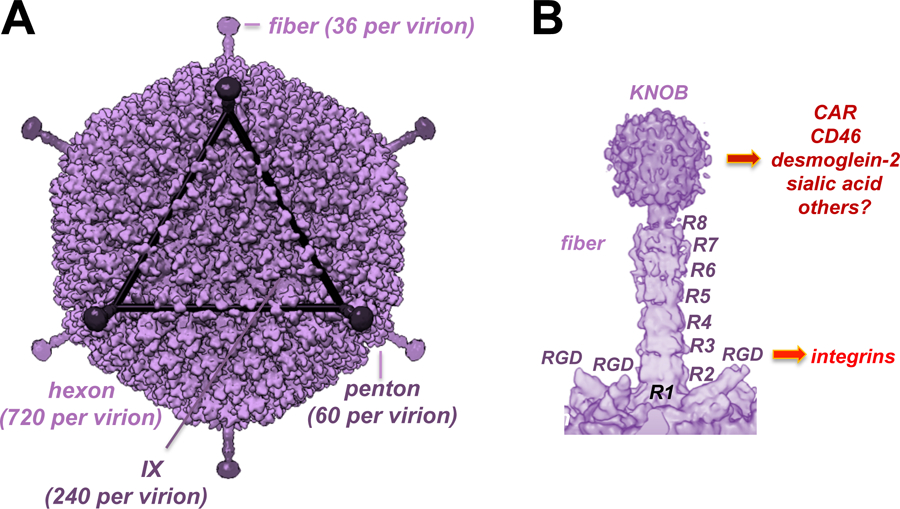
A) Full virion structure B) Fiber and penton base. R indicated fiber shaft repeats. RGD indicates arginine-glycine-aspartic acid integrin binding motifs in the penton base. Knob indicates the receptor binding portion of the Ad26 fiber trimer. Receptors bound by these capsomers are shown on the right. Adapted from [33].
There is also at least one minor protein, IX, that can also display targeting ligands. Beyond fiber, penton, IX, and hexon, all other viral proteins are hidden within the virion or are not packaged into virions. These four proteins can serve on scaffolds to display 36, 60, 240, or 720 copies of targeting ligands, respectively [28]. Low affinity ligands like peptides from library selections may not work well if displayed in low copy fibers, but might work well if displayed on more capsomers to allow avidity interactions. High affinity ligands should theoretically work on any capsomer, but data using biotinylated vectors suggests that only fiber may be good for very high affinity ligands (see below).
In vitro, Ad fiber proteins act as primary high affinity attachment ligands for these viruses provided their receptors are expressed on target cells. Three fiber monomers trimerize to form a fiber at each vertex of the icosahedral capsid (Fig. 1B). These fibers form a “knob” domain at their c-terminus that is involved with most receptor interactions. Although fibers have this same basic structure, their shaft length, flexibility, and receptor binding vary considerably. The archetype adenovirus, human Ad serotype 5 (HAdV-5, hereafter referred to as Ad5) has a knob that binds to the coxsackie and adenovirus receptor (CAR). Most human Ads have only one fiber trimer, but three others express two different fibers [29], a long fiber and a short fiber. Fastidious gastrointestinal human Ad40 and 41 from species F were the examples for having novel dual fibers. In their cases, the long fibers bound CAR, but the short fiber did not appear to have overt receptor binding functions [30,31].
Most Ads have an RGD motif in their penton base that binds to integrins [32]. This RGD motif is displayed on loops with different lengths by the different viruses [33]. Ad5’s fiber binds CAR with 10-fold higher affinity than its penton binds αv integrins [32]. Because of this affinity difference, species C viruses have been shown to first engage CAR, then bind integrins which facilitate receptor-mediated endocytosis [34]. This is how it works in vitro in a cell culture dish and perhaps on mucosa, but this staged interaction is over-written after an intravenous (IV) injection by other interactions with host factors in the blood.
Other Ads can bind to CAR, CD46, sialic acid, desmoglein-2, and perhaps other receptors (Fig. 1B, [23]). For many years, a non-CD46 additional receptor for species B viruses Ad3, Ad7, Ad11 and Ad14 was a mystery. This “receptor X” was ultimately identified as desmoglein-2 by Dr. Lieber and colleagues [35]. More recent work with Ad3 shows that its fiber binds desmoglein-2 in an unusual 1:1 stoichiometry [36,37].
Species D Ad37 is the archetype for viruses using sialic acid as a receptor [38,39]. Ad37 and most Ads do not use simple sialic acid for binding. Species D human Ad37 is also the archetype virus for causing keratoconjunctivitis. Like Ad37, species D Ad8, Ad53, Ad54, Ad56, and Ad64 are also associated with this disease [40]. Recent comparison of these virus’ utilization of sialic acid on corneal cells in vitro demonstrated that Ad8, Ad53, Ad54, and Ad64 all use this receptor [40]. In contrast, Ad56 did not.
Sialic acid binding Ads can be quite specific for certain sialic acid structures. For example, Ad37 uses sialic acid only as presented in GD1a glycans [38,39]. The relatively new species D human Ad52 joins Ad40 and Ad41 in having two fibers: a short and long one. Like Ad40 and 41, Ad52 binds CAR, but also binds sialic acid [41]. More detailed examination of this interaction shows that the short fiber of Ad52 long chains of alpha-2,8-linked polysialic acid [29].
Human species D Ad26 is in rampant use as a gene-based vaccine and as an oncolytic virus [42–50]. There is ongoing debate on this virus’ receptor utilization. Original in vitro data on artificial CAR and CD46-modified cells indicated that Ad26 did not use CAR, but instead used CD46 for infection [42]. While Ad26 infection was increased on cells expressing CD46, this infection was half as efficient CD46-utilizing species B Ads [42]. Subsequent work by our laboratory on primary human B cells showed Ad26 used CD46 and integrin, but did not use sialic acid as evidenced by a lack of effect of neuraminidase on cells [46]. A more recent study reports that Ad26 does not use CD46 and instead uses αvβ3 integrin as its primary receptor [51]. Other work showed that Ad26 binds CAR and CD46 with 20 and 50 μM affinities, respectively [52]. A recent publication showed that removal of cell-surface sialic inhibits Ad26 infection [53]. This work also co-crystalized Ad26 knob with sialic acid, thus making the argument that sialic acid is “the” Ad26 receptor [53]. Conversely, we find Ad26 knob binds CD46-D4 with 0.12 μM affinity and that it infects cells expressing CD46 in the presence or absence of sialic acid (unpublished observations). Regardless, whether Ad26 binds CD46 and/or sialic acid, its receptor binding is markedly weaker than the nM binding of most archetype viruses like Ad5, Ad35, and Ad11 for their receptors.
The affinities of these interactions vary. Ad5 fiber binds CAR with 15 nM affinity. Species B Ad11 binds CD46 with 13 n M affinity, whereas species B Ad21’s affinity is 22-fold lower at 284 nM. Some species D Ads including Ad37 use sialic acid as a receptor, some with high selectivity for the GD1a glycan [38,39]. While Ad37 does bind sialic acid, the affinity of this interaction is only 19 μM. This is similar to the affinity of many other viruses for their receptors, but it is also 1,000-fold lower than the affinity of Ad5 and Ad11 fibers for their cognate receptors [54,55].
Therefore, one might expect this low affinity fiber to do well binding receptors in static conditions (e.g. on the eye, on mucosa, after an intramuscular (IM) injection), but struggle to interact in high shear conditions (e.g. after an intravenous (IV) injection). One might also expect that a low affinity fiber might rely more on secondary interactions of penton base with integrins on surfaces. This hypothesis might also play out in a similar fashion when exogenous low affinity targeting peptides are added to Ad capsid proteins. Low affinity ligands may perform well in the lab in a tissue culture dish or under static in vivo conditions, but may fail when challenged under high shear after intravenous injections.
Ad5 fiber has 21 or 22 β-spiral repeats in its shaft making this protein approximately 37 nm in length [23,56]). While one might this this long structure would prevent any access of penton base to integrins, this long fiber has a flexible joint near its base [57] allowing it to bend out of the way for binding to αv integrins in “virus yoga” [57].
This flexibility can be appreciated by the inability to observe species C fibers in cryo-electron microscopic (cryo-EM) reconstructions [58,59]) because every fiber is in a different position on the virions and so their density gets averaged out. Most short-shafted fibers in species B and D viruses lack this flexible domain in their shafts, and so these can be observed in cryo-EM (e.g. Ad26 fiber in Fig. 1B and [33,60]). This lack of flexibility may be compensated in some viruses by having more extended loops in penton base to display their RGD motif [33]. If these short-shafted fibers bind their cognate receptor with very low affinity (i.e. Ad37 for sialic acid), penton interactions may become more dominant than fiber interactions for cell binding [46]. Likewise, if target cells lack an Ad’s cognate fiber receptor, the virus can use integrins as a fall back receptor.
To a certain degree, this can be observed based on the time allowed for the virus to infect cells in vitro. If the cell lacks CAR for example, letting Ad5 infect it for only an hour yields little infection [61]. In contrast, if the virus is allowed to bind the same cell for 24 hours, lower affinity integrin binding can occur allowing most of the cells to be infected. Similar effects can be observed when inserting cell targeting peptides into Ad proteins. If the ligand has low affinity, it may appear to fail if given a short binding time, but may succeed under extended conditions.
Types of Adenovirus Vectors
Before discussing retargeting and detargeting, we will define the different types of Ad vectors onto which these approaches may be applied. Most gene-based adenovirus vectors in the literature are replication-defective Ad (RD-Ad) adenovirus serotype 5 (Ad5) vectors (Fig. 2). Ad5 is popular in part because commercial kits to make these viruses came out in the 1990’s and no other serotype kits have been sold.
Figure 2. Schematic of Different Types of Adenovirus Vectors.
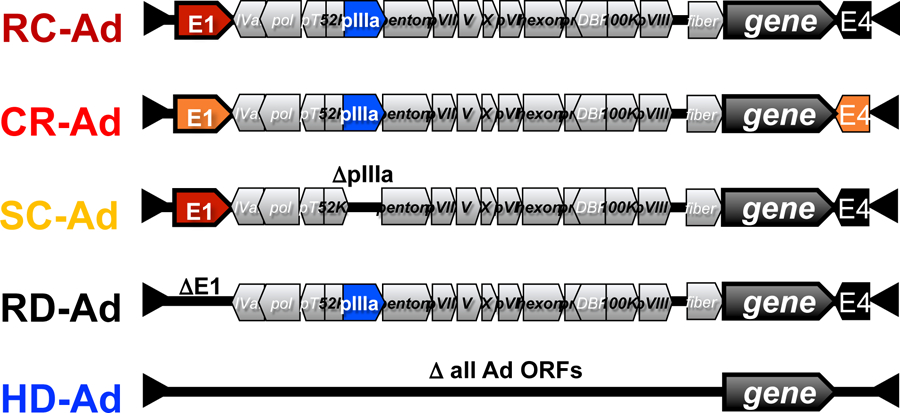
RC-Ad = replication-competent Ad. SC-Ad = single-cycle Ad. RD-Ad = replication-defective, E1-deleted Ad. HD-Ad = helper-dependent Ad.
These RD-Ad vectors have their E1 gene deleted to prevent them from replicating their DNA and making progeny viruses. This prevents the vector from killing the cell that was just modified. This also avoids causing potentially dangerous and potentially lethal Ad infections from the vector itself. An RD-Ad infects a cell, delivers its one copy of a gene, and can express robust amounts of its transgene protein. They are safe, but do not replicate transgenes or amplify transgene expression.
Much that is known about adaptive T cell responses against Ad vectors was learned with RD-Ad vectors. While the pivotal E1 gene is deleted, there can still be leaky expression from the remaining 17 or more viral open-reading frames (ORFs) and this leaky expression in transduced cells targets them for destruction by adaptive CD8 T cells [62].
Given this, helper-dependent adenoviral (HD-Ad) vectors were developed that have all Ad ORFs deleted [63–65](Fig. 2). No Ad proteins are produced and this avoids immune cells killing the transduced cells [63–65]. This reduced immunogenicity allows HD-Ad to mediate sustained liver gene therapy for longer than 7 years in non-human primates [9]. Any residual HD-Ad toxicity can be blunted by genetic and chemical shielding approaches (reviewed in [20,26,66–68]). This makes HD-Ads viable platforms for gene therapy. However, bad public relations cloud the scientific merits of these improved Ad vectors. HD-Ads are also replication-defective vectors that will not amplify transgenes. They avoid immune responses against encoded viral antigens. However, if the transgene protein itself is immunogenic, it will provoke T cells responses that will delete these modified cells [69].
While RD-Ad and HD-Ad do not amplify transgenes, an E1+ replication-competent Ad (RC-Ad) vector could infect the same cell type and replicate the same transgene many thousands-fold [70–81] (Fig. 2). In vitro, this translates into 33 to 100-fold increases in protein production [82,83]. RC-Ad vectors are indeed more potent. However, fully replication-competent Ads run the real risk of causing adenovirus infections in humans. Indeed, when live RC-Ad vaccines are used in military service members, these wild viruses are delivered in enteric-coated capsules or tablets and given orally primarily to prevent them from causing Ad respiratory infections in nurses and vaccinees [1]. More recent clinical studies of an RC-Ad4 influenza vaccine (clinical trial NCT01443936) showed that this replicating vaccine generates potent B cell and antibody responses in humans after single intranasal (IN), tonsillar, or oral delivery [84]. However, this study also showed that 60% of the volunteers that received RC-Ad by the IN route came down with respiratory Ad infections (Dr. Mark Connors, NIH, personal communication).
To take advantage of transgene DNA replication by replicating Ads, but avoid the risk of adenovirus infections, we developed single-cycle Ad (SC-Ad) vectors (Fig. 2, [11,82,83,85] and reviewed in [86]). SC-Ads retain their E1 genes to allow them to replicate their genomes, but are deleted for their pIIIa gene to block the production of infectious progeny viruses. SC-Ads replicate their genomes and transgenes as effectively as RC-Ad (up to 10,000-fold)[82]. RC- and SC-Ad produce more transgene protein than RD-Ad vectors [82]. Like RC-Ads, SC-Ads also kill the first infected cell. However, they do not generate progeny viruses, so this initial cell death is limited to the first cells infected. SC-Ads generate more robust and more persistent immune responses than either RD-Ad or RC-Ads [83]. In head-to-head comparisons against standard RD-Ad vaccines, SC-Ad produces significantly higher antibodies and better protection against influenza virus [87]. SC-Ads have also shown potency as vaccines against Ebola virus and against Clostridoides difficile after single immunization [11,88].
Conditionally-replicating Ads (CRAds) are designed primarily for cancer applications with the goal of having Ads activate specifically in cancer cells while not activating in normal cells ([89–93], and reviewed in [94]). CRAds are engineered to activate in cancer cells by replacing promiscuous E1 or E4 promoters with cancer-specific promoters or by mutating the ability of E1A or E1B proteins to block their ability to interact with pivotal cellular proteins like pRB, p53, or p300 pathways.
While CRAds are a clever post-entry strategy with demonstrated specificities, they are somewhat of an illusion when they are applied in vivo. It is true that the CRAd design can prevent the virus from activating in off-target cells and killing them directly. However, those cells will still die in vivo.
Any off-target cell that is infected by a CRAd in vivo will have leaky Ad ORF expression just like RD-Ad vectors [95]. The incoming capsid proteins and/or these leaky Ad ORF proteins will be detected by the immune system as a foreign invasion [95].. A CRAd-infected off-target cell may not die because of the direct cytotoxicity of the virus. However, it will still die, but in this case by execution by cytotoxic T lymphocytes. CRAd control may spare the host organism by reducing amplification of progeny viruses from off-target cells. But those infected host cell will still die and may provoke side effects.
This can be true for any Ad vector. If the immune system detects viral or transgene antigens, T cells will destroy that cell. HD-Ads can avoid this provided that their transgene protein is close enough to “self” to escape detection. If an HD-Ad’s transgene protein is foreign, these cells will also be destroyed by the immune system.
One final important note about testing replicating Ads. Ad DNA replication and transgene amplification is highly species-dependent [96]. Human and non-human primate cells can amplify their genomes 3,000 to 100,000-fold [82,83,97]. In contrast, most mouse lines do not allow any replication. In vivo, there can be as much as 13-fold DNA replication of Ad6 DNA in the liver after an intravenous injection [85]. In contrast, there is only 3-fold DNA replication of Ad DNA in the lungs after intranasal administration [85]. Syrian hamsters are thought to be a better model for human Ads [96]. This is true for species C Ads, but not for many other adenoviruses [98]. In Syrian hamster HaK cells, one can observe 350-fold Ad6 DNA replication [83], but markedly less in vivo. Therefore, testing RC-Ad, SC-Ad, or CRAds in most small animals will under-appreciate their potency and also their side effects.
Adenovirus Serotypes as a Diverse Palette for Physical Particle Targeting and Post-Entry Activation Targeting
There are increasing numbers of Ad serotypes and genotypes that are being discovered nearly every day. These serve as a genetically and functionally diverse palette of biologies on which to apply vector engineering and cell targeting approaches (Fig. 3). Genetic diversity in human Ads can approach 40% at the whole genome level [99,100]. This genetic diversity translates into each virus having divergent protein surfaces that are able to evade each other’s antibodies.
Figure 3. Schematic of the Human Adenovirus Virome Palette for Adenovirus Targeting.
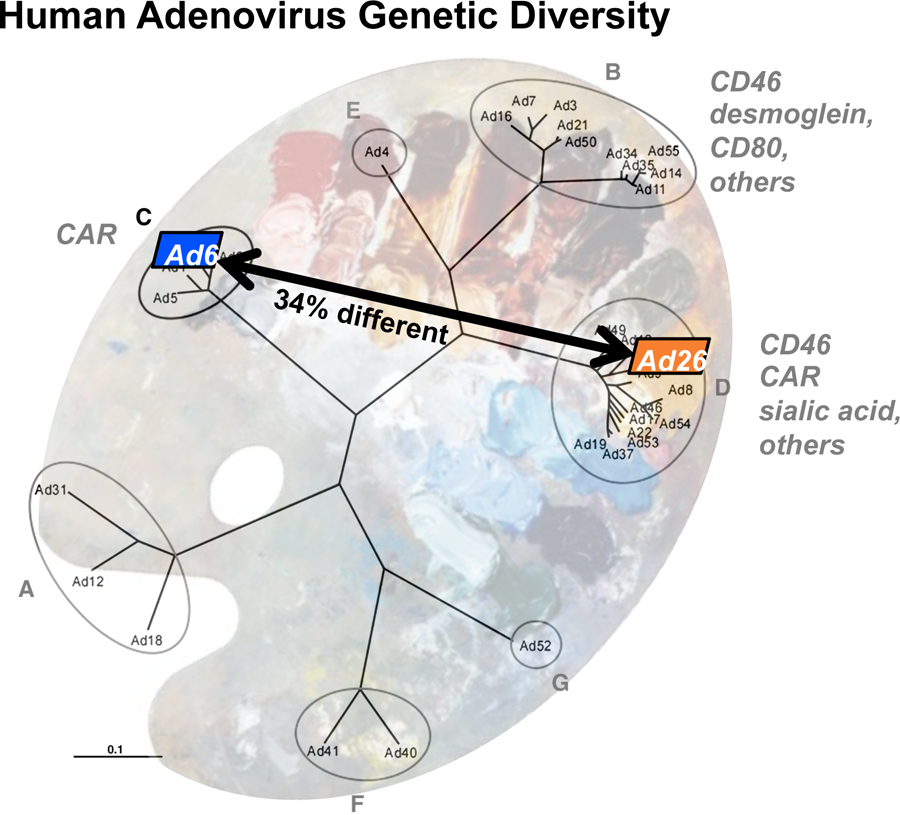
Adapted from [100] and showing whole genome difference between species C Ad6 and species D Ad26 described in [107].
We and others have delved into the biologies of other human and non-human Ads in the quest for new functionalities or to evade anti-Ad5 immunity in patients and to have non-Ad5 genetic platforms for vector engineering [23,38,42,46–48,98,101–112]. They also allow one to avoid pre-existing immune responses against certain Ads (i.e. Ad5) and to vary the Ad serotype between treatments in a shell game called serotype-switching [101,113].
Adenovirus Serotypes as a Diverse Palette for Physical Particle Vector Targeting
This diversity also translates into the evolution of viruses that naturally bind different receptors and natural differences in therapeutic potential based on this (Fig. 3). As discussed early, the fiber proteins of Ads bind CAR, sialic acid, CD46, desmoglein-2, a few others (Fig. 3 and reviewed in [23]). Archetype Ad5 virus and its species C family members Ad1, 2, 6, and 57 bind CAR. Species B Ads like Ad21 and Ad35 bind CD46. Seminal work by Dmitry Shayakhmetov in Andre Lieber’s laboratory generated some of the most potent retargeted Ads by given Ad5 CD46-binding fibers from species B Ads [102,114]. This approach has been stolen by many labs including ours. While this does retarget Ad5, it does not actually retarget Ads as a family of viruses as CD46 is already in the wheelhouse of human adenoviruses.
Different serotypes of Ad can also bind receptors indirectly by binding host proteins like vitamin K-dependent clotting factors (primarily FX and FIX), complement, natural antibodies, and other proteins that serve as “bridges” to receptors [24,115,116]. These host-derived binding proteins and their effects on Ad tropism in vivo are discussed in detail in other articles in this collection.
The cell binding proteins evolved by Ads and the host proteins that bind certain Ad serotypes can be modified by genetic or chemical engineering to physically retarget Ad particles to new receptors. These interactions can also be mutated or chemically blocked to detarget Ads from off-target tissue for therapy.
In many cases, wild Ads have been screened for utility prior to engineering. In another approach, Terri Hermiston’s group bred multiple Ads together to encourage inter-species recombination to generate better oncolytic viruses [117]. One of these viruses known as ColoAd1 that is a chimera of two species B viruses, Ad11p and Ad3, was renamed Enadenotucirev and is in human clinical trials [118].
Adenovirus Serotypes as A Diverse Palette for Post-Entry Vector Activation Targeting
Viral genetic diversity also translates into differences in the activation of different Ads after cell binding and entry has occurred. This is most relevant to Ads that retain E1 and activate DNA or full viral replication (i.e. SC-Ads, RC-Ads, and oncolytic Ads).
For example, we showed that species B, C, and D Ads infect primary human B cell cancers to different degrees, but also activate DNA replication to different degrees [45]. CD46-binding Ad11 and Ad35 infected myeloma cells 100-fold more efficiently than species C Ad5 and 6 or species D Ad26 and Ad48. While one would predict that Ad11 and Ad35 would then dominate in genome and progeny virus replication, they never activated DNA replication in these cells. In contrast, the species C and D viruses activated and amplified their genomes in these primary cells. From this, different Ads have different entry and activation biologies that can be harnessed for post-entry targeting.
We also directly compared the genetic activation programs of two of these genetically distant Ads: human species C Ad6 and species D Ad26 [107]. Ad6 and Ad26 differ by 34% at the whole genome DNA level (Fig. 4). Ad6 binds CAR, αv integrins, and FX. Ad26 binds CAR, CD46, sialic acid, αv integrins, but not FX. Despite differences in receptor utilization, but both infect human lung A549 cells. Both viruses initiate DNA replication within 12 hours with identical kinetics and both begin killing cells within 72 hours. Ad6 infected cells remain adherent until death. Ad26 infected cells detach from plates within 12 hours, but remain viable in this detached state. Quantitative PCR (qPCR) and next generation sequencing (NGS) showed that both viruses activate their early genes at 6 hours and transition to late gene activation by 12 hours.
Figure 4. mRNA Activation after Infection of Human Lung Cells with Species C Ad6 and Species D Ad26.

See main text for further information. Adapted from [107].
However, there are marked differences in how these viruses activate E1A and E1B genes and how E3A and E3B immunevasion mRNAs are activated (Fig. 4 and [107]). Differences in E1 activation could be related to differences in the sequences of their E1 promoters, but also perhaps due to differences in their ability to neutralize cellular proteins. For example, both viruses retain pRB binding motifs, but p300 and BS69 binding motifs are not conserved in Ad26. Variations in E3 mRNA expression translated into Ad6 being more effective at suppressing MHC I display on infected cells and evading extrinsic apoptosis signals than Ad26. These differences in E1 and E3 utilization likely underpin differences in the fundamental ability of these viruses to kill different cancers [45,46,98,119]. More differences are likely to be found in the diverse genetic palette of adenoviruses. This provides a wide repertoire of genetic platforms on which to apply pre-entry and post-entry vector targeting strategies.
Adenovirus Pharmacology
Retargeting and detargeting of Ads are easy in cell culture, it is like shooting fish in a barrel. Ignoring the in vivo pharmacology of Ads is however a drastic mistake: you may develop the world’s best targeted adenovirus, but if most of it is absorbed and destroyed by off-target cells and tissues, you will fail in vivo. Given this, we discuss important aspects of Ad in vivo pharmacology below before moving to retargeting and detargeting efforts.
Rapid Blood Protein and Cell Binding after Intravenous Injections
Other articles in this collection provide detailed review on these topics, so this will be a somewhat brief review with our opinions on these topics. More detailed reviews and our opinions on these topics can be found in [94,120]. When Ads are injected directly into the bloodstream, they can rapidly bind to blood proteins, platelets, red blood cells, and nucleated cells, and these fundamentally change the biodistribution of these viruses [121–127]. Because of this, Ads do not always perform as expected in vivo if these expectations are based on in vitro cell culture data.
Binding to blood clotting factors IX and X, natural antibodies, and complement can decide the fate of IV-injected Ads. Binding clotting factors can partially protect Ads from destruction in macrophages, particularly liver Kupffer cells. Species C human Ads can cloak themselves with FIX and FX and hide themselves to a certain degree from destruction by macrophages. Most other Ad serotypes do not and they can be drastically consumed and destroyed by macrophages. IX and X can also serve bridges to retarget Ads to heparin sulfate proteoglycans on cells. Binding to natural antibody IgMs can target Ads for covalent tagging by complement proteins to also target the viruses to macrophages for destruction. Uptake by macrophages and Kupffer cells and non-immune cells can trigger potentially dangerous innate immune responses and helps initiate adaptive immune responses against the virus and its transgene proteins.
Intravenously injected Ads can also bind to and activate platelets and endothelial cells [66,125,128,129]. Activation of these cells induces clotting and in extreme cases can lead to disseminated intravascular coagulation (DIC) and death [130,131]. Platelet binding can also target Ads for degradation by macrophages [125].
Systemic Distribution of Adenoviruses after Intravenous Injection: Interactions with Organs and the Reticuloendothelial System (RES)
An IV dose of Ad by most routes will usually encounter the heart and lungs before being distributed to the liver, spleen, and kidneys (reviewed in [94,120]). In mice, almost 98% of IV injected Ad5 is found in the liver 30 minutes after injection [132]. At this same dose, only about 1% of injected Ad5 can be found in either the lungs or the kidney at this dose. If the dose is increased 4-fold, Ad5 in the liver falls to 85% of injected dose and virus in the spleen and lung rises to 6 and 5% of injected dose, respectively. These are results in one strain of mice. No doubt there will be differences in relative distributions in humans. Different Ad serotypes may vary in their relative distributions, but the liver and spleen are likely to dominate all, since the RES cells housed in these tissues is evolved to absorb and neutralize particulate invaders.
The rapid distribution and sequestration of Ad after an IV injection can be easily appreciated by viewing movies of Ad5 labeled with near-infrared (NIR) fluorophores distributing in mice [133]. In these, Ad5 can be seen entering the heart within 500 milliseconds, flowing through distant arteries in 7 seconds, “blushing” the skin and tissues within 11 seconds, and then accumulating in the liver within 3 minutes of the injection [133].
The Liver as a Dominant Pharmacologic Dead End for Adenoviruses.
Approximately 1.5 liters of blood passes through the liver every minute in humans. After an IV injection and upstream absorption, Ads enter liver sinusoids where a large fraction of virions are absorbed by liver sinusoidal endothelial cells (LSECs) and Kupffer cells that line the sinusoids (reviewed in [120]). Kupffer cells are the resident macrophage of the liver. While they comprise only ~7% of liver cells, they may account for up to 90% of all of the macrophages in the body [134]. It has been estimated that liver Kupffer cells can sequester up to 98% of intravenously injected Ad5 vector in mice [135]. LSECs are also a major component of the RES, but their role in sequestration of Ads is underappreciated [136,137]. LSECs constitute ~25% of all liver cells [134]. LSECs and Kupffer cells work in concert to clear particles from the blood. Kupffer cells absorb particles up to 2 μm in diameter and LSECs absorb particles below 230 nm [134,138]. Therefore, both cells can phagocytose or pinocytose ~100 nm Ads.
Viruses that evade LSECs and Kupffer cells enter the parenchyma of the liver through fenestrations in the sinusoid wall that are large enough to pass Ads. Once inside the liver, Ads can infect hepatocytes. If the goal is to transduce hepatocytes, this is great. If the goal is to reach more distant tissues or cancer cells, this is terrible, since more of the injected dose is depleted.
Beyond the Liver
Beyond the liver, we know that a smaller, but significant fraction of Ad lands in the spleen, kidneys, and lungs after intravenous injection. As noted above, high doses of Ad5 in mice can result 6% of the injected dose landing in the spleen and 5% in the lung. If you normalize viral genomes to organ weight, the spleen absorbs Ad5 as well as the liver kilogram for kilogram [139]. This specific activity representation is helpful for understanding adenoviral biology and immune responses against these viruses. However, viral genomes per organ weight or viral genomes per host genome underestimates the magnitude of the liver as a pharmacologic sink for IV injected Ads, since it is considerably more massive than other tissues. Absorption in the spleen seems to be in large part due to uptake and sometimes transduction of macrophages. Absorption in the spleen can have drastic immunologic impacts as this organ can amplify innate and adaptive immune responses against the viruses or against their transgene products.
The kidney is another interesting organ for adenoviruses. Its natural filtering functions prevents most entry into the organ adenoviruses after IV injection. The glomerulus of the kidney actively excludes proteins above 50 kiloDaltons (kDa) from entry into the organ (reviewed in [140,141]). In addition, slit diaphragms between podocytes in the glomerulus are only 10 nm. Therefore, on paper, 100 nm, 150 megaDalton adenoviruses have little likelihood of penetrating beyond the glomerulus deeper into the organ. In addition, there is only low-level infection of glomerular cells by most Ads [141]. Transduction of cells in the parenchyma of the kidney has been reported after IV injections of different Ad serotypes or retargeted Ads [142,143]. While this is reported, it is unclear how these huge Ads break the glomerular barrier. Perhaps they overwhelm the glomerulus, form immune complexes, or perhaps enter by an unexpected route like retrograde through kidney lymphatics [144,145].
Adenoviral Vector Retargeting and Detargeting
Discussions of early efforts to retarget and detarget Ads can be found in our previous reviews [22,94,119,120,146,147]. Activity in the Ad targeting and detargeting space since 2011 can be found in the following references [19,148–208]. General strategies are summarized in a schematic (Fig. 5).
Figure 5. Schematic of Adenovirus Retargeting and Detargeting Strategies.
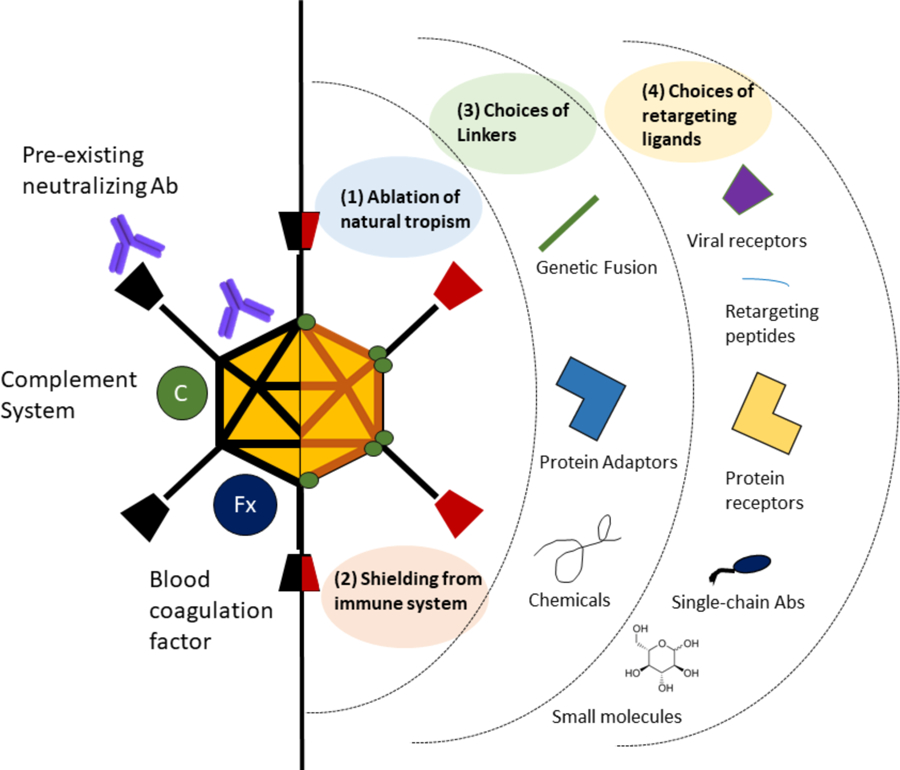
See main text for further information.
Most early and recent work has been directed at retargeting Ads to new receptors rather than detargeting them from off-target receptors and cells. Much of this early work was championed by David Curiel’s original group at the University of Alabama at Birmingham [209–215]. The reader should check out these seminal early works and follow subsequent work from Dr. Curiel and his “progeny” scientists. Other seminal work on the basic biology of Ads including retargeting, swapping fibers, and in vivo sequestration can be found in publications by Andre Lieber and Dmitry Shayakhmetov [104,114,115,216–227].
Subsequent work aimed to detarget Ad from its cognate in vitro receptors. Many great retargeted Ads have been broken on the shores of the massive absorption of most of their injected doses by the RES. We believe that we must be effective at detargeting before we can be effective at retargeting.
Evading Blood Proteins and Cells
After intravenous injection, the multivalent Velcro-like surface of Ad binds proteins and cells in the blood. Blocking these interactions are likely key to improving the ability of Ads to reach distant cells, particularly since many of these proteins target the viruses for destruction by the RES. A number of strategies can be used to detarget these cells (Fig. 5). The use of alternate Ad serotypes may avoid some of these interactions, since binding is receptor mediated. Other approaches are to genetically-delete viral ligands that bind CAR, CD46, integrin, and other interactions [228]. Another approach to evade interactions is to shield Ads with polymers like polyethylene glycol (PEG) and poly-N-(2-hydroxypropyl) methacrylamide (HPMA) [20,66,124,132,135,229–245].
Shielding with these polymers prevents interactions of Ads with blood proteins, blood cells, endothelial cells, and Kupffer cells [20,66,124,190,246,247]. Polymer shielding can also reduce innate immune responses and liver damage after IV injection. Random covalent conjugation of these polymers has the down side that they can inhibit the ability of Ads to bind receptors and unpackage in cells. This problem can be avoided in part by targeting polymer modifications to specific sites on Ad by inserting cysteines into hexon and targeting conjugation to this amino acid with maleimide [26,238,239,248].
A novel new shielding approach was tested wherein Ads were “cloaked” with silica (SiAd) [163]. This nanoparticle coating blocked the production of inflammatory cytokines and reduced production of neutralizing antibodies as well as increased virus infection after intratumoral injection.
Another innovative approach has been to insert albumin binding peptide into the hexon of Ad5 to shield it from neutralizing antibodies [249,250]. This approach allows the virus to cloak itself in albumin in the blood and may have utility to evade antibodies as well as other problematic factors in the blood.
Evading the Reticuloendothelial System
In mice, 98% of Ad5 appears to be absorbed by liver Kupffer cells and LSECs at low dose after an IV injection [132]. If you increase the dose above the sequestration threshold, virus spills into liver hepatocytes [251]. This can increase liver transduction if this is your goal, but this also has immunologic side effects. One brute force way to avoid Kupffer cells and perhaps LSECs is to “predose” the system by injecting other particles like gadolinium chloride, chlodronate liposomes, or Ad5 itself to saturate and kill Kupffer cells before injecting the therapeutic or reporter virus [135,218,252–255]. While predosing can be effective, uptake of Ad into Kupffer cells not only kills the virus, but also kills the Kupffer cells [256]. This creates a highly inflammatory milieu and results in dead Kupffer cell fragments lodging in the lung where they can provoke dangerous side effects.
Ad polymer shielding is an effective means to detarget Kupffer and LSECs [20,240,241]. Larger polymers that increase the diameter of Ad beyond the size of liver fenestrations also appeared prevent entry into the parenchyma of the liver and uptake into hepatocytes.
Changing the serotype or the hypervariable regions (HVRs) of Ads can also hide the virus from Kupffer and other cells [68,97]. For example, giving Ad5 the HVRs from Ad6 blocks its uptake by scavenger receptors, macrophages, and increases transduction of hepatocytes [68]. Similarly, deleting the large, highly-charged HVR1 of Ad5 reduces its binding to scavenger receptor MARCO (SR-A6) [27]. Conversely, giving an Ad HVRs that do not bind FX for shielding from IgM and complement can make uptake and side effects worse. For example, giving Ad5 the HVRs from Ad48 increased Kupffer cell uptake and inflammation against the virus [257].
Other approaches are to insert peptides or proteins into the HVRs of Ad [123,258–260]. Insertions into HVR5 appear able to block binding of FX to Ads. At the time, the expectation was that blocking this would prevent FX acting as a bridge to targeting heparin sulfate proteoglycans on cells like hepatocytes. In vivo data supported this paradigm, since insertion of RGD or a biotin acceptor peptide (BAP) into HVR5 markedly reduces hepatocyte transduction [123,258–260]. While this worked, the underlying hypothesis appears wrong as these FX binding insertions likely just block the ability of the virus to cloak itself in FX to avoid targeting to and destruction by Kupffer cells. If the viruses are more destroyed by Kupffer cells, hepatocyte transduction will also be reduced.
While Kupffer cells are a problem, depleting them by predosing does not reduce Ad genomes in the liver [255,256]. So Kupffer cells are not the only story. To do better, Di Paolo et al. engineered Ad5 to target multiple liver cells 5 [228]. In this work, they showed that no single intervention by itself fully detargeted the virus from the liver. While one can detarget multiple interactions, in many cases, the virus actually needs these functions to be efficient. Therefore, detargeting can come at the cost of efficacy.
Adenoviral Vector Retargeting
Genetic Insertions into Ad Capsomers
Early work on inserting ligands relied on genetically adding known small peptides to the Ad5 fiber. First proof of principle was adding a non-cell targeting epitope tag to the C-terminus of fiber [209]. Later work inserted a flag tag or an RGD motif into the non-conserved flexible loop between the H and I beta sheets of the Ad5 knob [212,213]. Many subsequent studies have inserted RGD into almost all capsomers of Ad. Most of these studies erroneously describe these as “retargeted” vectors when in fact they are simply “gain of function” vectors, since Ad already has its own RGD motif in its penton base. Placing RGD on fiber does not really retarget the virus. Rather, it just exposes the motif better for interactions with the same αv integrins that Ad already uses. More recent work has inserted more specific RGD peptides from foot and mouth disease virus to more specifically target αvβ6 integrins that are upregulated on cancer cells [261].
Other efforts have involved replacement of the trimeric fiber with heterologous trimeric proteins like bacteriophage fibritin [161,175,184,262–266] or reovirus sigma 1 protein [267–269]. Recent examples of retargeting by direct genetic introduction of ligands into Ad capsomers can be found in the following references: [19,153,155,159,165,167,174–176,184,186,188,192,193,205]. Other examples are discussed below in selected cases that provide guidance for future engineering efforts.
Choosing the best Cell Targeting Ligands for Ad Genetic Engineering
Incompatibility of Secreted Targeting Ligands
Some of the best cell targeting ligands are antibodies and other glycosylated proteins (Fig. 5). Unfortunately, these ligands are excreted through the secretory pathway where they are post-translationally modified with carbohydrates and disulfide bonds that are key to their targeting functions. This is unfortunate, because Ads are built in the reducing environment of nucleus where disulfides do not form and little glycosylation occurs. Therefore, one must usually engage in drastic ligand engineering to translate these excellent ligands from secretory tech to nuclear tech for direct genetic incorporation into Ad capsomer proteins [262]. Alternately, one can generate bridging molecules in which one end binds an Ad capsomer or tag and the other is the targeting ligand [270,271]. In contrast, chemical engineering approaches that chemically cross-link exogenous ligands to Ads can bridge the divide between the secretory and nuclear world for targeting [232,236].
Peptide Ligands and Peptide-presenting Phage Libraries
What do you do if you do not have a targeting ligand already conveniently in hand? To quote Ghostbusterstm: “Who you gonna call?”
This question actually served as part of the lead author’s post-doctoral work. To find these needed ligands, we selected cell-binding and cell-internalizing peptides from peptide-presenting phage libraries [272]. Our goals were: 1) To identify ligands without any prior knowledge of the biology or receptors of the target tissue; 2) To develop a technology that would identify ligands that bind directly to the cells of interest for direct transduction and 3) To identify cell-binding ligands that would be compatible with genetic engineering into viral gene delivery vectors.
Peptides were attractive, since they are relatively small for genetic engineering into Ads, but also can be easily produced in GMP grade by chemical synthesis for targeting non-viral vectors or Ads by bioengineering approaches.
The possibility of identifying peptide ligands for vector targeting was suggested at the time by the early use of peptide-presenting phage libraries to select peptides against proteins in vitro in ELISA plates [273–275]. These peptide-presenting phage libraries had been developed by engineering filamentous bacteriophage to display random peptides by inserting semi-random DNA into their pIII receptor binding protein (analogous to Ad fiber) or their pVIII (analogous to the hexon). This peptide discovery technology was a uniquely powerful, since the actual ligand is physically attached to the DNA that encodes it. This allowed any good peptide sequence to be inferred by sequencing the DNA.
Proof of principle was demonstrated with peptide libraries build in filamentous phage and has been followed by other approaches like ribosomal display and yeast display [276–279]. Yeast display has the advantage over bacterial libraries of being able to generate ligands with some level of carbohydrate modifications. For example, ribosome display was used to generate bifunctional designed ankyrin repeat proteins (DARPins) that retarget Ad5 to Her-2 [276].
These library technologies used to need be developed by individual labs. Phage peptide libraries have long been available commercially from companies like New England Biolabs and you can see how this availability has generated most of the cell-targeting peptides that are in the adenovirus literature. Some peptide and single-chain antibody libraries can now be purchased from other vendors. This is a great expansion of availability, but is hindered by expense and sometimes stringent material transfer agreements.
The Importance of Ligand Library Size
More importantly, peptide libraries built in bacteria can have diversities of up to 1010 members. Consider that an average antibody recognizes 6 amino acids [273–275]. Therefore, if you want a targeting peptide that might be as good as an antibody, you may want to be able to screen 6-mer peptides. Consider that a peptide library must have at least 20 members in the library to cover one amino acid position with all possible 20 amino acids. To cover 2 amino acid positions, you need a library with 202 or 400 members. To cover 4 amino acid positions, you need 204 combinations or 1.6×105 library members. To cover 6 amino acid positions, you need a library of 6.4×107 library members. If you want to do better than the needed 6 amino acids of binding surface, a library covering all combinations of 8 amino acids would need 2.56×1010 library members. At the time we searched for peptide ligands and even now, generating huge complexity libraries are still best built in bacteria. Random PCR can generate more diversity, but they are not generally easy to apply in the context of a stable genetic platform for screening.
Selection of Peptides against Mammalian Cells and Their Receptors
In the early 90’s, peptide-presenting phage libraries had been used to select peptides against proteins on plates in vitro [273–275] and it had not been used to select peptides directly on mammalian cells. We demonstrated that you could do this by selecting 12 and 20-mer peptide libraries against mammalian cells [272]. As we pursued this work, other work described selecting peptides against purified cell surface receptors [280–283], against platelets [284,285], and notably selected the now famous RGD-4C peptide [282] which is the go-to ligand for Ad vector targeting. Subsequent work described selection peptide libraries in vitro and in vivo [232,286–291]. A few of these early investigators went the full distance and translated phage-selected peptides onto gene therapy vectors. Examples include translation of phage-selected peptides into adenovirus and AAV by genetic insertion [155,213,292–294], by chemical cross-linking to adenovirus [232,295], and by incorporation of targeting peptides into vector-specific antibodies [288,296].
Ligand Context
Genetic insertion of foreign peptides into the viral capsomer proteins has been reviewed previously [22,147]. These methods can be unpredictable in terms of whether these peptide insertions would be compatible with capsomer folding and virion assembly and whether the peptide would retain its cell binding functions when grafted into this foreign protein. For example, when we have had many disastrous experiences of having peptides that either fail to work in Ad or destroy things like fiber trimerization. We took one approach to circumvent these problems by engineering a bacteriophage library that displayed random peptides already in the context of the Ad fiber HI loop between fiber H and I beta sheets [293]. We showed that we could select muscle-binding peptides from these libraries and that at least one could be grafted back into the HI loop of Ad5 to yield functional retargeting of the virus [293].
We called these “context-specific” phage libraries, but later realized that most non-enveloped viral proteins have an abundance of very similar beta sheets separated by flexible loops. Therefore, these are not so much “context-specific”, but viral capsomer “compatible”. We proved this principle by grafting these HI-loop-selected muscle-binding peptides into a similar beta sheet-loop-beta sheet structure of HVR5 in Ad5’s hexon [294]. We showed that these HVR-modified viruses had increased infection of muscle cells in vitro and in vivo. Interestingly, we showed that only one of the two peptides inserted into HVR5 detargeted Ad5 from hepatocytes in the liver. This suggests variable effects of insertions into hexon on modulating interactions with FX and perhaps Kupffer cells.
In summary, abundant data demonstrate that viable cell targeting ligands can be selected from bacterial phage libraries and can be used to retarget Ads by genetic or chemical engineering.
Direct Adenovirus Peptide Libraries
More recently, peptide libraries have been created in which random peptides are cloned directly into the Ad capsid [297–299] without the pain and suffering of having to translate phage tech into Ad tech. This technique was modified to accommodate the insertion of peptides with known affinity for cellular targets. Lupold et al. designed an Ad peptide library that had a constant binding peptide insert flanked with random linker sequences [298]. Virions could then be selected with retained binding specificity [300].
Although these Ad libraries could theoretically contain up to 109 unique peptides as bacterial plasmids, there is a huge bottleneck in converting Ad plasmids to Ad viruses in mammalian cells. If you transfect 293 cells with 10 μg of DNA and get 20 plaques, your library is 20 members and you can only cover 1 amino acid position with all 20 amino acids. In practice, with concerted effort, these Ad libraries yielded sizes of up to 2 × 105 members [297,298] allowing coverage of all combinations of a four amino acid peptide.
The library size bottleneck was more recently addressed in part by work in the Yakamoto lab [153,301]. In this approach, the transfection to plaque bottleneck with plasmids was circumvented in part. In the first iteration, full adenoviral genomes were generated by Cre-lox recombination between a fiber-modified plasmid library and Ad DNA/terminal protein complex (DNA-TPC) before transfection into mammalian cells [297]. In the second iteration, a fiber plasmid library and a fiberless Ad DNA-TPC were co-transfected into Cre-expressing 293 cells. This generated a library of approximately 104 members from approximately 106 cells and allowed selection of novel Ads with new functionalities [301].
This is a great step forward for Ad retargeting. However, total library size will still likely limit the affinity of any peptides selected from these libraries. If such a library is scaled up to Cell Factory scale with 109 cells, this may yield a library with 107 library members. This allows coverage of all combinations of 4-mer peptides, but not all combinations of 5-mers.
While RGD can be held up as a great cell binding 3-mer peptide, it is an exception rather than a rule for binding. Bigger peptides are likely better for binding. When we directly competed 12-mer vs 20-mer phage libraries against each other, the bigger library always generated better cell binding peptides (unpublished data). Even if one uses large complexity phage libraries, most peptides selected out of peptide libraries start with affinities in 10 to 100 μM level. Given this, it is standard practice to identify a lead peptide from a peptide and then generate a second mutant library based on the original peptide sequence and select this again to increase peptide affinity [272].
Therefore, direct peptide libraries in Ads are a great advance for the field. Efforts to increase library size and more importantly ligands affinity will help move these technologies forward.
Bioengineering to Covalently Attach Targeting Ligands to Ads
Another strategy to retarget Ad is to covalently attach targeting ligands to Ad capsid using mono- or bifunctional cross-linkers (Fig. 5). In this approach, amine reactive polymers bearing synthesized ligands can be cross-linked to Ad for shielding and retargeting. For example, PEG-glucose and PEG-galactose have been used to retarget Ad vaccines for intranasal immunization [229]. One end of a bifunctional polymer can be conjugated to lysines on the surface of the Ad and the other end of the polymer can be cross-linked to targeting ligand. For example, small proteins like FGF-2 and EGF and phage-selected peptides have been cross-linked to Ads using bifunctional PEG, HPMA, or other reagents [232,236,242,295,302]. Not only is this technology suitable for the display of ligands from the capsid, but native vector tropism can either be maintained or inactivated, depending on choice of amino acid targeted. This approach works best with ligands that have free single cysteines that are not tied up in disulfide bonds (i.e. FGF-2 and synthetic peptides with added cysteines). Complex disulfide-bearing ligands like antibodies can be used, but breaking these apart to liberate free cysteines can be tricky. Alternately, one can use things like Traut’s reagent to convert amines on the ligand to cysteines for maleimide reaction. An improvement on this approach uses combined genetic and chemical engineering to target PEGs to cysteines inserted into specific sites in capsomer proteins [26,238,248,303]. Other approaches have utilized not only natural amino acids such as cysteine, but also unnatural amino acids to photo-cross-link ligands onto vectors [203,304].
Polymer coupling of ligands is an excellent way to screen peptide-library generated ligands before going to the trouble of genetically engineering them into capsomer proteins [295] and to shield or detarget Ad at the same time. It can also provide two layers of particle targeting: the first by the ligand and polymer and the second by the capsomers. In the context of a replicating oncolytic or vaccine vector, progeny virions coming from the first infection can have secondary targets now that they are no longer coated by polymers. More recent examples of chemical retargeting can be found in several studies [148,149,151,156,157,164,168–171,177,178,180–182,185,194–197,203,204,207]
Adenovirus Targeting with Adaptor Proteins
Targeting Ads with high affinity proteins like antibodies is hampered by their large size and improper folding of antibodies in the reducing environment of the nucleus where Ad is assembled. To circumvent this incompatibility, molecular adapters have been designed that bind Ad capsomers on one end and target receptors with the other end. Early adaptors bound Ad5 fiber by fusing the ectodomain of CAR to FGF-2 and EGF [305]. We used the GLA domain from FX to bind the hexons from species C Ad to fuse to single-chain antibodies targeting Her-2, EGFR, and the stem cell marker ABCG2 [271].
If CAR or FX adaptors are encoded by oncolytic Ads, the incoming Ad is not targeted by the adapter unless it is added to virus as an exogenous recombinant protein. This either weakens the adaptor technology or strengthens it by allowing two stages targeting. For first stage, initial infection is mediated by whatever fiber or targeting ligands is displayed on the virus. For the second stage, the Ad is retargeted by the adaptor. Using CAR or FX may have different utilities. CAR binds 36 sites on Ad. FX adaptor binds up to 240 binding sites on Ad, so one might benefit by more avidity interactions to zipper up Ad to cellular receptors. CAR adaptor might block CAR binding during the second stage which could be good or bad. FX adaptors preserve fiber functions while still providing a second level of retargeting. This may be useful to help oncolytic Ads spread in tumors since FX targeting is not affected by excess fiber production [271].
A major hurdle to this targeting method is that the adaptor molecules rely on non-covalent protein-protein interactions for their conjugation to the Ad capsid. Naturally occurring antibodies or CAR receptors could compete for Ad binding and displace the molecular adaptors from the capsid, abolishing the vector targeting activity. For example, we originally hoped that FX adaptors would not only retarget, but would also detarget Ad from hepatocytes, but this did not work in practice [119], perhaps because of the huge amounts of FX in the blood stream competed the protein off the virus.
Other examples of Ads targeting with adaptor molecules can be found elsewhere [154,158,172,179,183,187,191,198,200,201].
Lessons Learned from Adaptor Targeting with Metabolically Biotinylated Adenoviruses
We developed a different adaptor system where biotin acceptor peptides (BAPs) were genetically fused to Ad fiber, protein IX, or inserted into hexon HVRs [28,61,306,307]. When BAP-modified viruses are produced in mammalian cells, the BAP tag is covalently biotinylated during vector production by the endogenous enzyme holocarboxylase synthetase [308] or by co-expression of bacterial BirA [61,146,308,309]. This allows biotinylated Ads to be bound to any biotinylated ligand using avidin as a bridge. Alternately, one can genetically or chemically generate a single avidin targeting ligand for a two-component targeting complex. This system forms more stable complexes than other adaptors, because of the extreme affinity of avidin for biotin (10−15 M, which is about a quarter the strength of a carbon-carbon single covalent bond).
We showed that the system is adaptable, in that Ad-Fiber-BAP could be retargeted to new receptors using biotinylated peptides, proteins, carbohydrates, DNA, antibodies, and magnets [28,61,306]. There is also the advantage that you can buy many biotinylated ligands right off the shelf. The disadvantage would come in translating to clinic, since you would need to produce two or three GMP-rated components to target: GMP Ad-BAP + GMP avidin or streptavidin + GMP biotinylated ligand or GMP-Ad-BAP + GMP avidin-ligand fusion protein.
While there are strengths and weaknesses in the Ad-BAP systems, they actually provide unique insights into 1) which capsomers work best for targeting; 2) how the biology of the ligand affects targeting; and 3) how the affinity of ligands might affect targeting on different capsomers.
We used the BAP system to directly compare targeting through the Ad fiber, protein IX and hexon capsomeres, using a variety of high-affinity ligands (antibodies, transferrin, and cholera toxin B subunit) on multiple cell types. While all of these capsomers could bind and display the same high affinity targeting ligands, the fiber protein always worked for targeting, whereas IX and hexon did not [28]. When Ads were labeled with fluorophores and observed on cells by microscopy, it appeared that some of the failed vectors were actually trapped on their receptors and recycled to the cell surface rather than released from endosomes [28]. The one exception to this was transferrin which could mediate intermediate levels of transduction when displayed on IX-BAP [307].
These results are most likely explained by differences in the biology of fiber, IX, and hexon proteins as well as the biology of ligand-receptor interactions during endosomal uptake and escape. Receptor binding is a critical first step in Ad infection, but virus release from the receptor after uptake is equally important. Ads normally accomplish this by shedding fiber and penton base in the endosome once their tasks are complete [34]. Following this, fibers and penton base are released from the virion [34,310]. In contrast, protein IX and hexon dissociate from virions 30 minutes or more after receptor binding, well after endosomolysis and cytosolic escape [311].
From this, we believe that fiber works with high affinity ligands, because it naturally releases from the virion, so the virus can escape high affinity interactions with receptors. Conversely, viruses bound to receptors with high affinity ligands on IX and hexon on the icosahedron cannot be released from the same receptors because these capsomer proteins are not shed until they are in the cytoplasm or at the nuclear membrane. Unlike the high-affinity antibody ligands, transferrin works with the icosahedral proteins because this ligand is released from its receptor at endosomal pH [312].
If this model is correct, high affinity ligands may have problems if directly inserted or coupled to IX or hexon, but may be functional if they can be designed to release from their receptors or the virion itself. This model also suggests that lower affinity ligands may not have these problems, since their kinetic off rates from receptors are likely high allowing for their spontaneous release after internalization. This is supported by observations of being able to insert low affinity peptides into hexon and have them work for retargeting [258,294].
Combined Targeting and Detargeting
The vast majority of work in this space has been devoted to retargeting Ads. This works well in vitro, but frequently fails in vivo due to the many pharmacologic and host factors that were discussed above (reviewed in [120]). Early work used pharmacologic interventions to detarget Ads. This included “predosing” discussed previously wherein a first injection of Ad or another particulate reagent like clodronate liposomes hours before therapeutic virus injection can destroy the Kupffer cells and allow the second virus to be effective. This effect can be garnered by two separate injections or by one very high dose injection. The second pharmacologic approach was to use drugs like warfarin or snake toxins to knock out FX and blood factor binding to Ad to “detarget” hepatocytes. In reality, this detargeting is really removing FX’s shielding effects and is in actuality retargeting Ad for destruction in Kupffer cells and macrophages [24,313–316]. Predosing and blocking FX binding could yield improvements in treating tumors after intravenous injections [255], but the effects were not as strong as hoped likely because the viruses were still be absorbed by other cells like endothelial cells. In addition, destroying Kupffer cells can have profound consequences including death [256,317].
We described above retargeting Ad5 to muscle while detargeting it from the liver by inserting phage-derived peptides into the virus’ hexon [294]. In this case, detargeting was largely sacrificial. The reduced liver transduction by the virus was likely mediated by blocking FX binding to hexon and de-protecting the virus from complement and destruction in Kupffer cells. Better detargeting was achieved by Shayakhmetov and colleagues by blocking uptake into hepatocytes, Kupffer cells, and endothelial cells [228], but this lacked retargeting.
More recent efforts have combined targeting and detargeting in a vector called Ad5NULL-A20 that bears capsomer mutations that block binding to CAR, αvβ3/5 integrin, and FX, but that also includes peptide A20 that targets αvβ6 integrins [318]. After high dose (1011 virus particle) intravenous injections, this virus provides remarkable 107-fold reductions in Ad genomes in the liver with other large reductions in uptake in other off-target organs. Given other work that shows that FX protects Ad5 from complement activation and Kupffer sequestration, one might expect that deleting FX binding would make the virus more susceptible to uptake and destruction in these cells [24,313–316]. This may occur even with Ad5NULL-A20, but the use of high dose injections may have helped clear this block by destroying the Kupffer cells. Regardless, this is a significant step forward for detargeting and retargeting by genetic strategies.
Conclusions and Perspectives
As the diverse in vivo biology of the adenovirus virome has been better appreciated, it has become clearer that vector pharmacology relies only in part on evolved receptor binding ligands and can be significantly influenced by interactions with host proteins. We now understand that Ads encounter progressive viral distractions and sinks in the blood and in organs that can quantitively deplete the vast majority of IV injected Ad therapeutics. Retargeting efforts after IV injection that are pursued without considering detargeting are likely doomed to failure. Avoiding the blood and vasculature is a smart way to avoid these problems for those therapies that can be delivered by other routes. If this is not possible, selections of the right Ad serotypes combined with genetic or chemical modifications of the virus holds promise to bypass these viral sinks. Once detargeted, effective retargeting strategies can be applied to Ad vectors. Whether IV administered Ads can penetrate into tissues from the blood is a separate question.
It is unclear to what degree genetic and chemical detargeting can shield the virus from blood protein binding and the host from this rapid toxicity. We speculate that chemical shielding may be better at reducing this immediate toxicity based on their general abilities to blunt binding and side effects. However, comprehensive genetic engineering of Ads that detarget certain proteins or cells may also succeed at blunting rapid and extended side effects after systemic Ad therapy.
Abbreviations
- Ad
adenovirus
- vp
virus particles
- HD-Ad
helper-dependent adenovirus
- RD-Ad
replication-defective adenovirus
- CAR
coxsackie and adenovirus receptor
- IV
intravenous
- IM
intramuscular
- RC-Ad
replication-competent adenovirus
- SC-Ad
single-cycle adenovirus
- CRAd
conditionally-replicating adenovirus
- FIX
blood clotting factor IX
- FX
blood clotting factor X
- RES
reticuloendothelial system
- NIR
near-infrared
- LSEC
liver sinusoidal endothelial cell
- HVR
hypervariable region
- PEG
polyethylene glycol
- HPMA
poly-N-(2-hydroxypropyl) methacrylamide
- BAP
biotin acceptor peptide
- GMP
good manufacturing practice
- DARPins
designed ankyrin repeat proteins
- DNA-TPC
Ad DNA/terminal protein complex
REFERENCES
- [1].Couch RB, Chanock RM, Cate TR, Lang DJ, Knight V and Huebner RJ (1963). Immunization with types 4 and 7 adenovirus by selective infection of the intestinal tract. Am Rev Respir Dis 88, SUPPL 394–403. [DOI] [PubMed] [Google Scholar]
- [2].Top FH Jr., Buescher EL, Bancroft WH and Russell PK (1971). Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. J Infect Dis 124, 155–60. [DOI] [PubMed] [Google Scholar]
- [3].Mercier GT et al. (2007). Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine 25, 8687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Croyle MA, Cheng X, Sandhu A and Wilson JM (2001). Development of novel formulations that enhance adenoviral-mediated gene expression in the lung in vitro and in vivo. Mol Ther 4, 22–8. [DOI] [PubMed] [Google Scholar]
- [5].Croyle MA, Cheng X and Wilson JM (2001). Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Ther 8, 1281–90. [DOI] [PubMed] [Google Scholar]
- [6].Jager L and Ehrhardt A (2007). Emerging adenoviral vectors for stable correction of genetic disorders. Curr Gene Ther 7, 272–83. [DOI] [PubMed] [Google Scholar]
- [7].Mitani K and Kubo S (2002). Adenovirus as an integrating vector. Curr Gene Ther 2, 135–44. [DOI] [PubMed] [Google Scholar]
- [8].Stephen SL, Montini E, Sivanandam VG, Al-Dhalimy M, Kestler HA, Finegold M, Grompe M and Kochanek S (2010). Chromosomal integration of adenoviral vector DNA in vivo. J Virol 84, 9987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brunetti-Pierri N et al. (2013). Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum Gene Ther 24, 761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guenzel AJ et al. (2013). Generation of a hypomorphic model of propionic acidemia amenable to gene therapy testing. Mol Ther 21, 1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Anguiano-Zarate SS, Matchett WE, Nehete PN, Sastry JK, Marzi A and Barry MA (2018). A Replicating Single-Cycle Adenovirus Vaccine Against Ebola Virus. J Infect Dis 218, 1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shiver JW et al. (2002). Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415, 331–5. [DOI] [PubMed] [Google Scholar]
- [13].Casimiro DR et al. (2003). Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol 77, 6305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barefoot B et al. (2008). Comparison of multiple vaccine vectors in a single heterologous prime-boost trial. Vaccine 26, 6108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Abbink P et al. (2016). Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353, 1129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM and Batshaw ML (2003). Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 80, 148–58. [DOI] [PubMed] [Google Scholar]
- [17].Rastall DP et al. (2016). Long-term, high-level hepatic secretion of acid alpha-glucosidase for Pompe disease achieved in non-human primates using helper-dependent adenovirus. Gene Ther 23, 743–752. [DOI] [PubMed] [Google Scholar]
- [18].Palmer DJ, Grove NC and Ng P (2016). Helper virus-mediated downregulation of transgene expression permits production of recalcitrant helper-dependent adenoviral vector. Mol Ther Methods Clin Dev 3, 16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ruan MZ, Cerullo V, Cela R, Clarke C, Lundgren-Akerlund E, Barry MA and Lee BH (2016). Treatment of osteoarthritis using a helper-dependent adenoviral vector retargeted to chondrocytes. Mol Ther Methods Clin Dev 3, 16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mok H, Palmer DJ, Ng P and Barry MA (2005). Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther 11, 66–79. [DOI] [PubMed] [Google Scholar]
- [21].Croyle MA, Le HT, Linse KD, Cerullo V, Toietta G, Beaudet A and Pastore L (2005). PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther 12, 579–87. [DOI] [PubMed] [Google Scholar]
- [22].Campos SK and Barry MA (2007). Current advances and future challenges in Adenoviral vector biology and targeting. Curr Gene Ther 7, 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arnberg N (2009). Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol 19, 165–78. [DOI] [PubMed] [Google Scholar]
- [24].Xu Z, Tian J, Smith JS and Byrnes AP (2008). Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol 82, 11705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Piccolo P, Vetrini F, Mithbaokar P, Grove NC, Bertin T, Palmer D, Ng P and Brunetti-Pierri N (2013). SR-A and SREC-I are Kupffer and endothelial cell receptors for helper-dependent adenoviral vectors. Mol Ther 21, 767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khare R, Reddy VS, Nemerow GR and Barry MA (2012). Identification of adenovirus serotype 5 hexon regions that interact with scavenger receptors. J Virol 86, 2293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stichling N et al. (2018). Lung macrophage scavenger receptor SR-A6 (MARCO) is an adenovirus type-specific virus entry receptor. PLoS Pathog 14, e1006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Campos SK and Barry MA (2006). Comparison of adenovirus fiber, protein IX, and hexon capsomeres as scaffolds for vector purification and cell targeting. Virology 349, 453–62. [DOI] [PubMed] [Google Scholar]
- [29].Lenman A et al. (2018). Polysialic acid is a cellular receptor for human adenovirus 52. Proc Natl Acad Sci U S A 115, E4264–E4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nakamura T, Sato K and Hamada H (2003). Reduction of natural adenovirus tropism to the liver by both ablation of fiber-coxsackievirus and adenovirus receptor interaction and use of replaceable short fiber. J Virol 77, 2512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kesisoglou F, Chamberlain JR, Schmiedlin-Ren P, Kaz A, Fleisher D, Roessler B and Zimmermann EM (2005). Chimeric Ad5 vectors expressing the short fiber of Ad41 show reduced affinity for human intestinal epithelium. Mol Pharm 2, 500–8. [DOI] [PubMed] [Google Scholar]
- [32].Wickham TJ, Mathias P, Cheresh DA and Nemerow GR (1993). Integrins avb3 or avb5 promote adenovirus internalization but not virus attachment. Cell 73, 309–319. [DOI] [PubMed] [Google Scholar]
- [33].Yu X, Veesler D, Campbell MG, Barry ME, Asturias FJ, Barry MA and Reddy VS (2017). Cryo-EM structure of human adenovirus D26 reveals the conservation of structural organization among human adenoviruses. Sci Adv 3, e1602670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Greber UF, Willetts M, Webster P and Helenius A (1993). Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75, 477–86. [DOI] [PubMed] [Google Scholar]
- [35].Wang H et al. (2011). Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med 17, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vassal-Stermann E et al. (2019). CryoEM structure of adenovirus type 3 fibre with desmoglein 2 shows an unusual mode of receptor engagement. Nat Commun 10, 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vassal-Stermann E et al. (2018). Mapping of Adenovirus of serotype 3 fibre interaction to desmoglein 2 revealed a novel ‘non-classical’ mechanism of viral receptor engagement. Sci Rep 8, 8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arnberg N, Edlund K, Kidd AH and Wadell G (2000). Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol 74, 42–8. [PMC free article] [PubMed] [Google Scholar]
- [39].Nilsson EC et al. (2011). The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med 17, 105–9. [DOI] [PubMed] [Google Scholar]
- [40].Chandra N, Frangsmyr L, Imhof S, Caraballo R, Elofsson M and Arnberg N (2019). Sialic Acid-Containing Glycans as Cellular Receptors for Ocular Human Adenoviruses: Implications for Tropism and Treatment. Viruses 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lenman A et al. (2015). Human adenovirus 52 uses sialic acid-containing glycoproteins and the coxsackie and adenovirus receptor for binding to target cells. PLoS Pathog 11, e1004657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Abbink P et al. (2007). Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 81, 4654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu J et al. (2008). Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457, 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mast TC et al. (2010). International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28, 950–7. [DOI] [PubMed] [Google Scholar]
- [45].Senac JS, Doronin K, Russell SJ, Jelinek DF, Greipp PR and Barry MA (2010). Infection and killing of multiple myeloma by adenoviruses. Hum Gene Ther 21, 179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen CY, Senac JS, Weaver EA, May SM, Jelinek DF, Greipp P, Witzig T and Barry MA (2011). Species D Adenoviruses as Oncolytics against B-cell Cancers. Clin Cancer Res 17, 6712–6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Weaver EA, Chen CY, May SM, Barry ME and Barry MA (2011). Comparison of adenoviruses as oncolytics and cancer vaccines in an immunocompetent B cell lymphoma model. Hum Gene Ther 22, 1095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Weaver EA and Barry MA (2013). Low seroprevalent species D adenovirus vectors as influenza vaccines. PLoS One 8, e73313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Baden LR et al. (2013). First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 207, 240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Barouch DH et al. (2015). Protective efficacy of adenovirus-protein vaccines against SIV challenges in rhesus monkeys. Science [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nestic D, Uil TG, Ma J, Roy S, Vellinga J, Baker AH, Custers J and Majhen D (2019). alphavbeta3 Integrin Is Required for Efficient Infection of Epithelial Cells with Human Adenovirus Type 26. J Virol 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Baker AT, Greenshields-Watson A, Coughlan L, Davies JA, Uusi-Kerttula H, Cole DK, Rizkallah PJ and Parker AL (2019). Diversity within the adenovirus fiber knob hypervariable loops influences primary receptor interactions. Nat Commun 10, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Baker AT, Mundy RM, Davies JA, Rizkallah PJ and Parker AL (2019). Human adenovirus type 26 uses sialic acid-bearing glycans as a primary cell entry receptor. Sci Adv 5, eaax3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Henry LJ, Xia D, Wilke ME, Deisenhofer J and Gerard RD (1994). Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol 68, 5239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang H et al. (2007). Identification of CD46 binding sites within the adenovirus serotype 35 fiber knob. J Virol 81, 12785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].van Raaij MJ, Mitraki A, Lavigne G and Cusack S (1999). A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 401, 935–8. [DOI] [PubMed] [Google Scholar]
- [57].Wu E and Nemerow GR (2004). Virus yoga: the role of flexibility in virus host cell recognition. Trends Microbiol 12, 162–9. [DOI] [PubMed] [Google Scholar]
- [58].Rux JJ and Burnett RM (2004). Adenovirus structure. Hum Gene Ther 15, 1167–76. [DOI] [PubMed] [Google Scholar]
- [59].Marsh MP, Campos SK, Baker ML, Chen CY, Chiu W and Barry MA (2006). Cryoelectron microscopy of protein IX-modified adenoviruses suggests a new position for the C terminus of protein IX. J Virol 80, 11881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Reddy VS, Natchiar SK, Stewart PL and Nemerow GR Crystal structure of human adenovirus at 3.5 A resolution. Science 329, 1071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Parrott MB, Adams KE, Mercier GT, Mok H, Campos SK and Barry MA (2003). Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol Ther 8, 688–700. [DOI] [PubMed] [Google Scholar]
- [62].Yang Y, Su Q and Wilson JM (1996). Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J. Virol 70, 7209–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mitani K, Graham FL, Caskey CT and Kochanek S (1995). Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proceedings of the National Academy of Sciences of the United States of America 92, 3854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Clemens PR, Kochanek S, Sunada Y, Chan S, Chen HH, Campbell KP and Caskey CT (1996). In vivo muscle gene transfer of full-length dystrophin with an adenoviral vector that lacks all viral genes. Gene Therapy 3, 965–72. [PubMed] [Google Scholar]
- [65].Fisher KJ, C.H., Burda J, Chen SJ, Wilson JM (1996). Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology 217, 11–22. [DOI] [PubMed] [Google Scholar]
- [66].Hofherr SE, Mok H, Gushiken FC, Lopez JA and Barry MA (2007). Polyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopenia. Hum Gene Ther 18, 837–48. [DOI] [PubMed] [Google Scholar]
- [67].Hofherr SE, Shashkova EV, Weaver EA, Khare R and Barry MA (2008). Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol Ther 16, 1276–82. [DOI] [PubMed] [Google Scholar]
- [68].Khare R et al. (2011). Generation of a Kupffer Cell-evading Adenovirus for Systemic and Liver-directed Gene Transfer. Mol Ther 19, 1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Weaver EA, Nehete PN, Buchl SS, Senac JS, Palmer D, Ng P, Sastry KJ and Barry MA (2009). Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS ONE 4, e5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Malkevitch N, Patterson LJ, Aldrich K, Richardson E, Alvord WG and Robert-Guroff M (2003). A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques. J Immunol 170, 4281–9. [DOI] [PubMed] [Google Scholar]
- [71].Zhao J et al. (2003). Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinants. Vaccine 21, 4022–35. [DOI] [PubMed] [Google Scholar]
- [72].Patterson LJ et al. (2004). Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol 78, 2212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Peng B et al. (2005). Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J Virol 79, 10200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhao J et al. (2005). Enhanced cellular immunity to SIV Gag following co-administration of adenoviruses encoding wild-type or mutant HIV Tat and SIV Gag. Virology 342, 1–12. [DOI] [PubMed] [Google Scholar]
- [75].Gomez-Roman VR et al. (2006). An adenovirus-based HIV subtype B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype B HIV strains. J Acquir Immune Defic Syndr 43, 270–7. [DOI] [PubMed] [Google Scholar]
- [76].Gomez-Roman VR et al. (2006). Oral delivery of replication-competent adenovirus vectors is well tolerated by SIV- and SHIV-infected rhesus macaques. Vaccine 24, 5064–72. [DOI] [PubMed] [Google Scholar]
- [77].Peng B, Voltan R, Cristillo AD, Alvord WG, Davis-Warren A, Zhou Q, Murthy KK and Robert-Guroff M (2006). Replicating Ad-recombinants encoding non-myristoylated rather than wild-type HIV Nef elicit enhanced cellular immunity. Aids 20, 2149–57. [DOI] [PubMed] [Google Scholar]
- [78].Demberg T et al. (2007). A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol 81, 3414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, Montefiori DC and Robert-Guroff M (2009). Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol 83, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Morgan C et al. (2008). The use of nonhuman primate models in HIV vaccine development. PLoS Med 5, e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Demberg T and Robert-Guroff M (2009). Mucosal immunity and protection against HIV/SIV infection: strategies and challenges for vaccine design. Int Rev Immunol 28, 20–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Crosby CM and Barry MA (2014). IIIa deleted adenovirus as a single-cycle genome replicating vector. Virology 462–463, 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Crosby CM, Nehete P, Sastry KJ and Barry MA (2015). Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. J Virol 89, 669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Matsuda K et al. (2019). Prolonged evolution of the memory B cell response induced by a replicating adenovirus-influenza H5 vaccine. Sci Immunol 4 [DOI] [PubMed] [Google Scholar]
- [85].Crosby CM and Barry MA (2017). Transgene Expression and Host Cell Responses to Replication-Defective, Single-Cycle, and Replication-Competent Adenovirus Vectors. Genes (Basel) 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Barry M (2018). Single-cycle adenovirus vectors in the current vaccine landscape. Expert Rev Vaccines 17, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Crosby CM, Matchett WE, Anguiano-Zarate SS, Parks CA, Weaver EA, Pease LR, Webby RJ and Barry MA (2017). Replicating Single-Cycle Adenovirus Vectors Generate Amplified Influenza Vaccine Responses. J Virol 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Matchett WE, Anguiano-Zarate SS and Barry MA (2019). A Replicating Single Cycle Adenovirus against Clostridium difficile. J. Infectious Dis in review [DOI] [PMC free article] [PubMed]
- [89].Bischoff JR et al. (1996). An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274, 373–6. [DOI] [PubMed] [Google Scholar]
- [90].Fueyo J et al. (2000). A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 19, 2–12. [DOI] [PubMed] [Google Scholar]
- [91].Doronin K, Kuppuswamy M, Toth K, Tollefson AE, Krajcsi P, Krougliak V and Wold WS (2001). Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J Virol 75, 3314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bauerschmitz GJ et al. (2006). Triple-targeted oncolytic adenoviruses featuring the cox2 promoter, E1A transcomplementation, and serotype chimerism for enhanced selectivity for ovarian cancer cells. Mol Ther 14, 164–74. [DOI] [PubMed] [Google Scholar]
- [93].Shashkova EV, Spencer JF, Wold WS and Doronin K (2007). Targeting Interferon-alpha Increases Antitumor Efficacy and Reduces Hepatotoxicity of E1A-mutated Spread-enhanced Oncolytic Adenovirus. Mol Ther 15, 598–607. [DOI] [PubMed] [Google Scholar]
- [94].Khare R, Chen CY, Weaver EA and Barry MA (2011). Advances and future challenges in adenoviral vector pharmacology and targeting. Curr Gene Ther 11, 241–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yang Y, Ertl HCJ and Wilson JM (1994). MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice injected with E1-deleted recombinant adenoviruses. Immunity 1, 433–42. [DOI] [PubMed] [Google Scholar]
- [96].Thomas MA, Spencer JF and Wold WS (2007). Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol Med 130, 169–83. [DOI] [PubMed] [Google Scholar]
- [97].Shashkova EV, May SM and Barry MA (2009). Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology 394, 311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chen CY, Weaver EA, Khare R, May SM and Barry MA (2011). Mining the adenovirus virome for oncolytics against multiple solid tumor types. Cancer Gene Ther 18, 744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Davison AJ, Benko M and Harrach B (2003). Genetic content and evolution of adenoviruses. J Gen Virol 84, 2895–908. [DOI] [PubMed] [Google Scholar]
- [100].Weaver EA, Hillestad ML, Khare R, Palmer D, Ng P and Barry MA (2011). Characterization of species C human adenovirus serotype 6 (Ad6). Virology 412, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Morral N, O.N.W., Rice K, Leland M, Kaplan J, Piedra PA, Zhou H, Parks RJ, Velji R, Aguilar-Cordova E, Wadsworth S, Graham FL, Kochanek S, Carey KD, Beaudet AL (1999). Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci U S A 96, 12816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G and Lieber A (2000). Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J Virol 74, 2567–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mei YF, Lindman K and Wadell G (2002). Human adenoviruses of subgenera B, C, and E with various tropisms differ in both binding to and replication in the epithelial A549 and 293 cells. Virology 295, 30–43. [DOI] [PubMed] [Google Scholar]
- [104].Gaggar A, Shayakhmetov DM and Lieber A (2003). CD46 is a cellular receptor for group B adenoviruses. Nat Med 9, 1408–12. [DOI] [PubMed] [Google Scholar]
- [105].Hemminki A et al. (2003). A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther 7, 163–73. [DOI] [PubMed] [Google Scholar]
- [106].Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM and Ertl HC (2003). Induction of CD8(+) T Cells to an HIV-1 Antigen through a Prime Boost Regimen with Heterologous E1-Deleted Adenoviral Vaccine Carriers. J Immunol 171, 6774–9. [DOI] [PubMed] [Google Scholar]
- [107].Turner MA, Middha S, Hofherr SE and Barry MA (2015). Comparison of the Life Cycles of Genetically Distant Species C and Species D Human Adenoviruses Ad6 and Ad26 in Human Cells. J Virol 89, 12401–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lasaro MO and Ertl HC (2009). New insights on adenovirus as vaccine vectors. Mol Ther 17, 1333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Baker AH, McVey JH, Waddington SN, Di Paolo NC and Shayakhmetov DM (2007). The influence of blood on in vivo adenovirus bio-distribution and transduction. Mol Ther 15, 1410–6. [DOI] [PubMed] [Google Scholar]
- [110].Strauss R et al. (2009). Epithelial phenotype confers resistance of ovarian cancer cells to oncolytic adenoviruses. Cancer Res 69, 5115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Nguyen TV, Crosby CM, Heller GJ, Mendel ZI, Barry ME and Barry MA (2018). Oncolytic adenovirus Ad657 for systemic virotherapy against prostate cancer. Oncolytic Virotherapy 7, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhang W et al. (2017). An Engineered Virus Library as a Resource for the Spectrum-wide Exploration of Virus and Vector Diversity. Cell Rep 19, 1698–1709. [DOI] [PubMed] [Google Scholar]
- [113].Lemckert AA et al. (2005). Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. J Virol 79, 9694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Shayakhmetov DM and Lieber A (2000). Dependence of adenovirus infectivity on length of the fiber shaft domain. J Virol 74, 10274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Shayakhmetov DM, Gaggar A, Ni S, Li ZY and Lieber A (2005). Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol 79, 7478–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Parker AL et al. (2006). Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 108, 2554–61. [DOI] [PubMed] [Google Scholar]
- [117].Kuhn I et al. (2008). Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS One 3, e2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Garcia-Carbonero R et al. (2017). Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J Immunother Cancer 5, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Barry MA, Weaver EA and Chen CY (2012). Mining the adenovirus “virome” for systemic oncolytics. Curr Pharm Biotechnol 13, 1804–8. [DOI] [PubMed] [Google Scholar]
- [120].Barry MA, Hofherr SE, Chen CY, Senac JS, Hillestad ML and Shashkova EV (2009). Systemic delivery of therapeutic viruses. Curr Opin Mol Ther 11, 411–20. [PubMed] [Google Scholar]
- [121].Carlisle RC et al. (2009). Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood 113, 1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Cichon G et al. (2003). Titer determination of Ad5 in blood: a cautionary note. Gene Ther 10, 1012–7. [DOI] [PubMed] [Google Scholar]
- [123].Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL and Shayakhmetov DM (2008). Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci U S A 105, 5483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lyons M et al. (2006). Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol Ther 14, 118–28. [DOI] [PubMed] [Google Scholar]
- [125].Stone D, Liu Y, Shayakhmetov D, Li ZY, Ni S and Lieber A (2007). Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of liver. J Virol [DOI] [PMC free article] [PubMed]
- [126].Waddington SN et al. (2008). Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132, 397–409. [DOI] [PubMed] [Google Scholar]
- [127].Seiradake E et al. (2009). The cell adhesion molecule “CAR” and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog 5, e1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Othman M, Labelle A, Mazzetti I, Elbatarny HS and Lillicrap D (2007). Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood 109, 2832–9. [DOI] [PubMed] [Google Scholar]
- [129].Eggerman TL, Mondoro TH, Lozier JN and Vostal JG (2002). Adenoviral vectors do not induce, inhibit, or potentiate human platelet aggregation. Hum Gene Ther 13, 125–8. [DOI] [PubMed] [Google Scholar]
- [130].Raper SE et al. (2002). A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther 13, 163–75. [DOI] [PubMed] [Google Scholar]
- [131].Schnell MA et al. (2001). Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther 3, 708–22. [DOI] [PubMed] [Google Scholar]
- [132].Green NK et al. (2004). Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther 11, 1256–63. [DOI] [PubMed] [Google Scholar]
- [133].Hofherr SE, Adams KE, Chen CY, May S, Weaver EA and Barry MA (2011). Real-time dynamic imaging of virus distribution in vivo. PLoS One 6, e17076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Jacobs F, Wisse E and De Geest B (2010). The role of liver sinusoidal cells in hepatocyte-directed gene transfer. Am J Pathol 176, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Alemany R, Suzuki K and Curiel DT (2000). Blood clearance rates of adenovirus type 5 in mice. J Gen Virol 81, 2605–9. [DOI] [PubMed] [Google Scholar]
- [136].Elvevold K, Simon-Santamaria J, Hasvold H, McCourt P, Smedsrod B and Sorensen KK (2008). Liver sinusoidal endothelial cells depend on mannose receptor-mediated recruitment of lysosomal enzymes for normal degradation capacity. Hepatology 48, 2007–15. [DOI] [PubMed] [Google Scholar]
- [137].Elvevold K, Smedsrod B and Martinez I (2008). The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol 294, G391–400. [DOI] [PubMed] [Google Scholar]
- [138].Shiratori Y, Kawase T, Shiina S, Komatsu Y and Omata M (1993). Role of hepatic sinusoidal cells in hepatic injury and fibrosis in the liver. Gastroenterol Jpn 28 Suppl 4, 102–6; discussion 112–5. [DOI] [PubMed] [Google Scholar]
- [139].Chenine AL et al. (2005). Schistosoma mansoni infection promotes SHIV clade C replication in rhesus macaques. AIDS 19, 1793–7. [DOI] [PubMed] [Google Scholar]
- [140].WareJoncas Z, Campbell JM, Martinez-Galvez G, Gendron WAC, Barry MA, Harris PC, Sussman CR and Ekker SC (2018). Precision gene editing technology and applications in nephrology. Nat Rev Nephrol [DOI] [PMC free article] [PubMed]
- [141].Rubin J, Nguyen T, Allen K, Ayasoufi K and Barry MA (2019). Comparison of Gene Delivery to the Kidney by Adenovirus, Adeno-associated Virus, and Lentiviral Vectors after Intravenous and Direct Kidney Injections. Human Gene Therapy in press [DOI] [PMC free article] [PubMed]
- [142].Diaconu I et al. (2009). Serotype chimeric and fiber-mutated adenovirus Ad5/19p-HIT for targeting renal cancer and untargeting the liver. Hum Gene Ther 20, 611–20. [DOI] [PubMed] [Google Scholar]
- [143].Denby L, Work LM, Seggern DJ, Wu E, McVey JH, Nicklin SA and Baker AH (2007). Development of renal-targeted vectors through combined in vivo phage display and capsid engineering of adenoviral fibers from serotype 19p. Mol Ther 15, 1647–54. [DOI] [PubMed] [Google Scholar]
- [144].Murphy JJ, Myint MK, Rattner WH, Klaus R and Shallow J (1958). The lymphatic system of the kidney. J Urol 80, 1–6. [DOI] [PubMed] [Google Scholar]
- [145].McIntosh GH and Morris B (1971). The lymphatics of the kidney and the formation of renal lymph. J Physiol 214, 365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Barry MA, Campos SK, Ghosh D, Adams KE, Mok H, Mercier GT and Parrott MB (2003). Biotinylated Gene Therapy Vectors. Expert Opinion in Biological Therapy 3, 926–40. [DOI] [PubMed] [Google Scholar]
- [147].Barry MA, Takahashi S and Parrott MB (2002) Selection of peptides on phage In Vector Targeting Strategies for Gene Therapy (Douglas J.a.C., D.T., ed.êds), pp. 549–579. Wiley-Liss, Inc., Hoboken, NJ. [Google Scholar]
- [148].Zeng Q, Han J, Zhao D, Gong T, Zhang Z and Sun X (2012). Protection of adenovirus from neutralizing antibody by cationic PEG derivative ionically linked to adenovirus. Int J Nanomedicine 7, 985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Yoon AR, Kasala D, Li Y, Hong J, Lee W, Jung SJ and Yun CO (2016). Antitumor effect and safety profile of systemically delivered oncolytic adenovirus complexed with EGFR-targeted PAMAM-based dendrimer in orthotopic lung tumor model. J Control Release 231, 2–16. [DOI] [PubMed] [Google Scholar]
- [150].Yoon AR, Hong J, Kim SW and Yun CO (2016). Redirecting adenovirus tropism by genetic, chemical, and mechanical modification of the adenovirus surface for cancer gene therapy. Expert Opin Drug Deliv 13, 843–58. [DOI] [PubMed] [Google Scholar]
- [151].Yao XL et al. (2012). Optimization and internalization mechanisms of PEGylated adenovirus vector with targeting peptide for cancer gene therapy. Biomacromolecules 13, 2402–9. [DOI] [PubMed] [Google Scholar]
- [152].Yamamoto Y, Nagasato M, Yoshida T and Aoki K (2017). Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci 108, 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Yamamoto Y et al. (2014). A targeting ligand enhances infectivity and cytotoxicity of an oncolytic adenovirus in human pancreatic cancer tissues. J Control Release 192, 284–93. [DOI] [PubMed] [Google Scholar]
- [154].Wu B, Mei S, Cui L, Zhao Z, Chen J, Wu T and Li G (2017). Marine Lectins DlFBL and HddSBL Fused with Soluble Coxsackie-Adenovirus Receptor Facilitate Adenovirus Infection in Cancer Cells BUT Have Different Effects on Cell Survival. Mar Drugs 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Wang D, Li W, Zhang H, Mao Q and Xia H (2014). A targeting peptide improves adenovirus-mediated transduction of a glioblastoma cell line. Oncol Rep 31, 2093–8. [DOI] [PubMed] [Google Scholar]
- [156].Wan Y, Han J, Fan G, Zhang Z, Gong T and Sun X (2013). Enzyme-responsive liposomes modified adenoviral vectors for enhanced tumor cell transduction and reduced immunogenicity. Biomaterials 34, 3020–30. [DOI] [PubMed] [Google Scholar]
- [157].Vetter A et al. (2013). Adenoviral vectors coated with PAMAM dendrimer conjugates allow CAR independent virus uptake and targeting to the EGF receptor. Mol Pharm 10, 606–18. [DOI] [PubMed] [Google Scholar]
- [158].Vasiljevic S et al. (2015). Redirecting adenoviruses to tumour cells using therapeutic antibodies: Generation of a versatile human bispecific adaptor. Mol Immunol 68, 234–43. [DOI] [PubMed] [Google Scholar]
- [159].van Erp EA, Kaliberova LN, Kaliberov SA and Curiel DT (2015). Retargeted oncolytic adenovirus displaying a single variable domain of camelid heavy-chain-only antibody in a fiber protein. Mol Ther Oncolytics 2, 15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sun Y, Lv X, Ding P, Wang L, Sun Y, Li S, Zhang H and Gao Z (2019). Exploring the functions of polymers in adenovirus-mediated gene delivery: Evading immune response and redirecting tropism. Acta Biomater [DOI] [PubMed]
- [161].Stepanenko AA and Chekhonin VP (2018). Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX. Virus Res 257, 40–51. [DOI] [PubMed] [Google Scholar]
- [162].Schmid M et al. (2018). Adenoviral vector with shield and adapter increases tumor specificity and escapes liver and immune control. Nat Commun 9, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Sapre AA et al. (2019). Silica cloaking of adenovirus enhances gene delivery while reducing immunogenicity. J Control Release 297, 48–59. [DOI] [PubMed] [Google Scholar]
- [164].Rubino FA, Oum YH, Rajaram L, Chu Y and Carrico IS (2012). Chemoselective modification of viral surfaces via bioorthogonal click chemistry. J Vis Exp, e4246. [DOI] [PMC free article] [PubMed]
- [165].Robertson S, Parker AL, Clarke C, Duffy MR, Alba R, Nicklin SA and Baker AH (2016). Retargeting FX-binding-ablated HAdV-5 to vascular cells by inclusion of the RGD-4C peptide in hexon hypervariable region 7 and the HI loop. J Gen Virol 97, 1911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Reetz J, Herchenroder O and Putzer BM (2014). Peptide-based technologies to alter adenoviral vector tropism: ways and means for systemic treatment of cancer. Viruses 6, 1540–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Puig-Saus C, Rojas LA, Laborda E, Figueras A, Alba R, Fillat C and Alemany R (2014). iRGD tumor-penetrating peptide-modified oncolytic adenovirus shows enhanced tumor transduction, intratumoral dissemination and antitumor efficacy. Gene Ther 21, 767–74. [DOI] [PubMed] [Google Scholar]
- [168].Prill JM, Subr V, Pasquarelli N, Engler T, Hoffmeister A, Kochanek S, Ulbrich K and Kreppel F (2014). Traceless bioresponsive shielding of adenovirus hexon with HPMA copolymers maintains transduction capacity in vitro and in vivo. PLoS One 9, e82716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Pearce OM, Fisher KD, Humphries J, Seymour LW, Smith A and Davis BG (2005). Glycoviruses: chemical glycosylation retargets adenoviral gene transfer. Angew Chem Int Ed Engl 44, 1057–61. [DOI] [PubMed] [Google Scholar]
- [170].Oum YH and Carrico IS (2012). Altering adenoviral tropism via click modification with ErbB specific ligands. Bioconjug Chem 23, 1370–6. [DOI] [PubMed] [Google Scholar]
- [171].Nigatu AS, Vupputuri S, Flynn N and Ramsey JD (2015). Effects of cell-penetrating peptides on transduction efficiency of PEGylated adenovirus. Biomed Pharmacother 71, 153–60. [DOI] [PubMed] [Google Scholar]
- [172].Niemann J and Kuhnel F (2020). Tumor Targeting of Oncolytic Adenoviruses Using Bispecific Adapter Proteins. Methods Mol Biol 2058, 31–49. [DOI] [PubMed] [Google Scholar]
- [173].Niemann J and Kuhnel F (2017). Oncolytic viruses: adenoviruses. Virus Genes 53, 700–706. [DOI] [PubMed] [Google Scholar]
- [174].Miura Y, Yamasaki S, Davydova J, Brown E, Aoki K, Vickers S and Yamamoto M (2013). Infectivity-selective oncolytic adenovirus developed by high-throughput screening of adenovirus-formatted library. Mol Ther 21, 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Matsui H, Sakurai F, Katayama K, Abe Y, Machitani M, Kurachi S, Tachibana M and Mizuguchi H (2013). A targeted adenovirus vector displaying a human fibronectin type III domain-based monobody in a fiber protein. Biomaterials 34, 4191–4201. [DOI] [PubMed] [Google Scholar]
- [176].MacLeod SH et al. (2012). HER3 targeting of adenovirus by fiber modification increases infection of breast cancer cells in vitro, but not following intratumoral injection in mice. Cancer Gene Ther 19, 888–98. [DOI] [PubMed] [Google Scholar]
- [177].Liu Z, Ke F, Duan C, Lan H, Li J, Gao C, Li J and Zhong Z (2013). Mannan-conjugated adenovirus enhanced gene therapy effects on murine hepatocellular carcinoma cells in vitro and in vivo. Bioconjug Chem 24, 1387–97. [DOI] [PubMed] [Google Scholar]
- [178].Li S, Chen J, Xu H, Long J, Xie X and Zhang Y (2014). The targeted transduction of MMP-overexpressing tumor cells by ACPP-HPMA copolymer-coated adenovirus conjugates. PLoS One 9, e100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Li G et al. (2015). CD47-retargeted oncolytic adenovirus armed with melanoma differentiation-associated gene-7/interleukin-24 suppresses in vivo leukemia cell growth. Oncotarget 6, 43496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Lee CH, Kasala D, Na Y, Lee MS, Kim SW, Jeong JH and Yun CO (2014). Enhanced therapeutic efficacy of an adenovirus-PEI-bile-acid complex in tumors with low coxsackie and adenovirus receptor expression. Biomaterials 35, 5505–16. [DOI] [PubMed] [Google Scholar]
- [181].Kwon OJ, Kang E, Choi JW, Kim SW and Yun CO (2013). Therapeutic targeting of chitosan-PEG-folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. J Control Release 169, 257–65. [DOI] [PubMed] [Google Scholar]
- [182].Kratzer RF, Espenlaub S, Hoffmeister A, Kron MW and Kreppel F (2017). Covalent decoration of adenovirus vector capsids with the carbohydrate epitope alphaGal does not improve vector immunogenicity, but allows to study the in vivo fate of adenovirus immunocomplexes. PLoS One 12, e0176852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Kloos A et al. (2015). PolySia-Specific Retargeting of Oncolytic Viruses Triggers Tumor-Specific Immune Responses and Facilitates Therapy of Disseminated Lung Cancer. Cancer Immunol Res 3, 751–63. [DOI] [PubMed] [Google Scholar]
- [184].Kaliberov SA, Kaliberova LN, Yan H, Kapoor V and Hallahan DE (2016). Retargeted adenoviruses for radiation-guided gene delivery. Cancer Gene Ther 23, 303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Jung SJ, Kasala D, Choi JW, Lee SH, Hwang JK, Kim SW and Yun CO (2015). Safety profiles and antitumor efficacy of oncolytic adenovirus coated with bioreducible polymer in the treatment of a CAR negative tumor model. Biomacromolecules 16, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Jonsson F, Hagedorn C and Kreppel F (2018). Combined Genetic and Chemical Capsid Modifications of Adenovirus-Based Gene Transfer Vectors for Shielding and Targeting. J Vis Exp [DOI] [PMC free article] [PubMed]
- [187].Hangalapura BN, Timares L, Oosterhoff D, Scheper RJ, Curiel DT and de Gruijl TD (2012). CD40-targeted adenoviral cancer vaccines: the long and winding road to the clinic. J Gene Med 14, 416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Guse K et al. (2012). Capsid-modified adenoviral vectors for improved muscle-directed gene therapy. Hum Gene Ther 23, 1065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Gentile CM, Borovjagin AV, Richter JR, Jani AH, Wu H, Zinn KR and Warram JM (2019). Genetic strategy to decrease complement activation with adenoviral therapies. PLoS One 14, e0215226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Fan G, Fan M, Wang Q, Jiang J, Wan Y, Gong T, Zhang Z and Sun X (2016). Bio-inspired polymer envelopes around adenoviral vectors to reduce immunogenicity and improve in vivo kinetics. Acta Biomater 30, 94–105. [DOI] [PubMed] [Google Scholar]
- [191].Dreier B et al. (2013). Development of a generic adenovirus delivery system based on structure-guided design of bispecific trimeric DARPin adapters. Proc Natl Acad Sci U S A 110, E869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].de Vrij J et al. (2012). A cathepsin-cleavage site between the adenovirus capsid protein IX and a tumor-targeting ligand improves targeted transduction. Gene Ther 19, 899–906. [DOI] [PubMed] [Google Scholar]
- [193].Coughlan L, Uusi-Kerttula H, Ma J, Degg BP, Parker AL and Baker AH (2014). Retargeting adenovirus serotype 48 fiber knob domain by peptide incorporation. Hum Gene Ther 25, 385–94. [DOI] [PubMed] [Google Scholar]
- [194].Choi JW, Park JW, Na Y, Jung SJ, Hwang JK, Choi D, Lee KG and Yun CO (2015). Using a magnetic field to redirect an oncolytic adenovirus complexed with iron oxide augments gene therapy efficacy. Biomaterials 65, 163–74. [DOI] [PubMed] [Google Scholar]
- [195].Choi JW, Kim HA, Nam K, Na Y, Yun CO and Kim S (2015). Hepatoma targeting peptide conjugated bio-reducible polymer complexed with oncolytic adenovirus for cancer gene therapy. J Control Release 220, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Choi JW, Jung SJ, Kasala D, Hwang JK, Hu J, Bae YH and Yun CO (2015). pH-sensitive oncolytic adenovirus hybrid targeting acidic tumor microenvironment and angiogenesis. J Control Release 205, 134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Chen J et al. (2016). Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy. ACS Nano 10, 11548–11560. [DOI] [PubMed] [Google Scholar]
- [198].Chen H, Zheng X, Di B, Wang D, Zhang Y, Xia H and Mao Q (2013). Aptamer modification improves the adenoviral transduction of malignant glioma cells. J Biotechnol 168, 362–6. [PubMed] [Google Scholar]
- [199].Bradshaw AC, Coughlan L, Miller AM, Alba R, van Rooijen N, Nicklin SA and Baker AH (2012). Biodistribution and inflammatory profiles of novel penton and hexon double-mutant serotype 5 adenoviruses. J Control Release 164, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Bhatia S, O’Bryan SM, Rivera AA, Curiel DT and Mathis JM (2016). CXCL12 retargeting of an adenovirus vector to cancer cells using a bispecific adapter. Oncolytic Virother 5, 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Beatty MS, Timares L and Curiel DT (2013). Augmented adenovirus transduction of murine T lymphocytes utilizing a bi-specific protein targeting murine interleukin 2 receptor. Cancer Gene Ther 20, 445–52. [DOI] [PubMed] [Google Scholar]
- [202].Beatty MS and Curiel DT (2012). Chapter two--Adenovirus strategies for tissue-specific targeting. Adv Cancer Res 115, 39–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Banerjee PS, Ostapchuk P, Hearing P and Carrico IS (2011). Unnatural amino acid incorporation onto adenoviral (Ad) coat proteins facilitates chemoselective modification and retargeting of Ad type 5 vectors. J Virol 85, 7546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Bamberger D, Hobernik D, Konhauser M, Bros M and Wich PR (2017). Surface Modification of Polysaccharide-Based Nanoparticles with PEG and Dextran and the Effects on Immune Cell Binding and Stimulatory Characteristics. Mol Pharm 14, 4403–4416. [DOI] [PubMed] [Google Scholar]
- [205].Ballard EN, Trinh VT, Hogg RT and Gerard RD (2012). Peptide targeting of adenoviral vectors to augment tumor gene transfer. Cancer Gene Ther 19, 476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Baker AT, Aguirre-Hernandez C, Hallden G and Parker AL (2018). Designer Oncolytic Adenovirus: Coming of Age. Cancers (Basel) 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Bachtarzi H, Stevenson M, Subr V, Ulbrich K, Seymour LW and Fisher KD (2011). Targeting adenovirus gene delivery to activated tumour-associated vasculature via endothelial selectins. J Control Release 150, 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Alonso-Padilla J, Papp T, Kajan GL, Benko M, Havenga M, Lemckert A, Harrach B and Baker AH (2016). Development of Novel Adenoviral Vectors to Overcome Challenges Observed With HAdV-5-based Constructs. Mol Ther 24, 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Michael S, Hong J, Curiel D and Engler J (1995). Addition of a short peptide ligand to the adenovirus fiber protein. Gene Therapy 2, 660–9. [PubMed] [Google Scholar]
- [210].Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M and Curiel DT (1996). Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol 14, 1574–8. [DOI] [PubMed] [Google Scholar]
- [211].Krasnykh VN, Mikheeva GV, Douglas JT and Curiel DT (1996). Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. Journal of Virology 70, 6839–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N and Curiel DT (1998). An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol 72, 9706–13 stably expressing the adenovirus type 5 fibre protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N and Curiel DT (1998). Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol 72, 1844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Zinn KR et al. (1998). Imaging and tissue biodistribution of 99mTc-labeled adenovirus knob (serotype 5). Gene Ther 5, 798–808. [DOI] [PubMed] [Google Scholar]
- [215].Reynolds P, Dmitriev I and Curiel D (1999). Insertion of an RGD motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Ther 6, 1336–9. [DOI] [PubMed] [Google Scholar]
- [216].Wang H et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med 17, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Fooks AR, Schadeck E, Liebert UG, Dowsett AB, Rima BK, Steward M, Stephenson JR and Wilkinson GW (1995). High-level expression of the measles virus nucleocapsid protein by using a replication-deficient adenovirus vector: induction of an MHC-1-restricted CTL response and protection in a murine model. Virology 210, 456–65. [DOI] [PubMed] [Google Scholar]
- [218].Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B and Kay MA (1997). The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol 71, 8798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Lieber A, H.C., Meuse L, Schowalter D, Kirillova I, Winther B, Kay MA (1997). The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol 71, 8798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Lieber A, H.C., Meuse L, Himeda C, Wilson C, Kay MA (1998). Inhibition of NF-kappaB activation in combination with bcl-2 expression allows for persistence of first-generation adenovirus vectors in the mouse liver. J. Virol 72, 9267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Shayakhmetov DM, P.T., Stamatoyannopoulos G, Lieber A (2000). Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J. Virol 74, 2567–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Steinwaerder DS and Lieber A (2000). Insulation from viral transcriptional regulatory elements improves inducible transgene expression from adenovirus vectors in vitro and in vivo. Gene Ther 7, 556–67. [DOI] [PubMed] [Google Scholar]
- [223].Yotnda P, Onishi H, Heslop HE, Shayakhmetov D, Lieber A, Brenner M and Davis A (2001). Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther 8, 930–7. [DOI] [PubMed] [Google Scholar]
- [224].Shayakhmetov DM, Li ZY, Ternovoi V, Gaggar A, Gharwan H and Lieber A (2003). The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J Virol 77, 3712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Shayakhmetov DM, Li ZY, Ni S and Lieber A (2004). Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol 78, 5368–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Sova P, Ren XW, Ni S, Bernt KM, Mi J, Kiviat N and Lieber A (2004). A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol Ther 9, 496–509. [DOI] [PubMed] [Google Scholar]
- [227].Shayakhmetov DM, Eberly AM, Li ZY and Lieber A (2005). Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J Virol 79, 1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Di Paolo NC, van Rooijen N and Shayakhmetov DM (2009). Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver. Mol Ther 17, 675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Weaver EA and Barry MA (2008). Effects of shielding adenoviral vectors with polyethylene glycol on vector-specific and vaccine-mediated immune responses. Hum Gene Ther 19, 1369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Chillon M, Lee JH, Fasbender A and Welsh MJ (1998). Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther 5, 995–1002. [DOI] [PubMed] [Google Scholar]
- [231].O’Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE and Francis GE (1999). PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther 10, 1349–58. [DOI] [PubMed] [Google Scholar]
- [232].Romanczuk H, Galer CE, Zabner J, Barsomian G, Wadsworth SC and O’Riordan CR (1999). Modification of an adenoviral vector with biologically selected peptides: a novel strategy for gene delivery to cells of choice [see comments]. Hum Gene Ther 10, 2615–26. [DOI] [PubMed] [Google Scholar]
- [233].Croyle MA, Yu QC and Wilson JM (2000). Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum Gene Ther 11, 1713–22. [DOI] [PubMed] [Google Scholar]
- [234].Croyle MA, Chirmule N, Zhang Y and Wilson JM (2001). “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol 75, 4792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Croyle MA, Chirmule N, Zhang Y and Wilson JM (2002). PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther 13, 1887–900. [DOI] [PubMed] [Google Scholar]
- [236].Lanciotti J, Song A, Doukas J, Sosnowski B, Pierce G, Gregory R, Wadsworth S and O’Riordan C (2003). Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol Ther 8, 99–107. [DOI] [PubMed] [Google Scholar]
- [237].Ogawara K, Rots MG, Kok RJ, Moorlag HE, Van Loenen AM, Meijer DK, Haisma HJ and Molema G (2004). A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum Gene Ther 15, 433–43. [DOI] [PubMed] [Google Scholar]
- [238].Kreppel F, Gackowski J, Schmidt E and Kochanek S (2005). Combined genetic and chemical capsid modifications enable flexible and efficient de- and retargeting of adenovirus vectors. Mol Ther 12, 107–17. [DOI] [PubMed] [Google Scholar]
- [239].Wortmann A, Vohringer S, Engler T, Corjon S, Schirmbeck R, Reimann J, Kochanek S and Kreppel F (2007). Fully Detargeted Polyethylene Glycol-coated Adenovirus Vectors Are Potent Genetic Vaccines and Escape from Pre-existing Anti-adenovirus Antibodies. Mol Ther [DOI] [PubMed]
- [240].Hofherr S, Senac JS, Chen CY, Palmer D, Ng P and Barry MA (2008). Short-term Rescue of Neonatal Lethality in a Mouse Model of Propionic Acidemia by Gene Therapy. Hum Gene Ther [DOI] [PMC free article] [PubMed]
- [241].Doronin K, Shashkova EV, May SM, Hofherr SE and Barry MA (2009). Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum Gene Ther 20, 975–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V and Seymour LW (2001). Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther 8, 341–8. [DOI] [PubMed] [Google Scholar]
- [243].Parker AL, Fisher KD, Oupicky D, Read ML, Nicklin SA, Baker AH and Seymour LW (2005). Enhanced gene transfer activity of peptide-targeted gene-delivery vectors. J Drug Target 13, 39–51. [DOI] [PubMed] [Google Scholar]
- [244].Stevenson M et al. (2006). Chick embryo lethal orphan virus can be polymer-coated and retargeted to infect mammalian cells. Gene Ther 13, 356–68. [DOI] [PubMed] [Google Scholar]
- [245].Stevenson M et al. (2007). Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via alpha6-integrins. Cancer Gene Ther 14, 335–45. [DOI] [PubMed] [Google Scholar]
- [246].Danielsson A et al. (2010). An ex vivo loop system models the toxicity and efficacy of PEGylated and unmodified adenovirus serotype 5 in whole human blood. Gene Ther 17, 752–62. [DOI] [PubMed] [Google Scholar]
- [247].Nguyen TV, Heller GJ, Barry ME, Crosby CM, Turner MA and Barry MA (2016). Evaluation of polymer shielding for adenovirus serotype 6 (Ad6) for systemic virotherapy against human prostate cancers. Mol Ther Oncolytics 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Prill JM et al. (2011). Modifications of adenovirus hexon allow for either hepatocyte detargeting or targeting with potential evasion from Kupffer cells. Mol Ther 19, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Rojas LA, Condezo GN, Moreno R, Fajardo CA, Arias-Badia M, San Martin C and Alemany R (2016). Albumin-binding adenoviruses circumvent pre-existing neutralizing antibodies upon systemic delivery. J Control Release 237, 78–88. [DOI] [PubMed] [Google Scholar]
- [250].Mato-Berciano A, Raimondi G, Maliandi MV, Alemany R, Montoliu L and Fillat C (2017). A NOTCH-sensitive uPAR-regulated oncolytic adenovirus effectively suppresses pancreatic tumor growth and triggers synergistic anticancer effects with gemcitabine and nab-paclitaxel. Oncotarget 8, 22700–22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Bristol JA, Shirley P, Idamakanti N, Kaleko M and Connelly S (2000). In vivo dose threshold effect of adenovirus-mediated factor VIII gene therapy in hemophiliac mice. Mol Ther 2, 223–32. [DOI] [PubMed] [Google Scholar]
- [252].Hardonk MJ, Dijkhuis FW, Hulstaert CE and Koudstaal J (1992). Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol 52, 296–302. [DOI] [PubMed] [Google Scholar]
- [253].Worgall S, Wolff G, Falck-Pedersen E and Crystal RG (1997). Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther 8, 37–44. [DOI] [PubMed] [Google Scholar]
- [254].Schiedner G, Hertel S, Johnston M, Dries V, van Rooijen N and Kochanek S (2003). Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther 7, 35–43. [DOI] [PubMed] [Google Scholar]
- [255].Shashkova EV, Doronin K, Senac JS and Barry MA (2008). Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res 68, 5896–904. [DOI] [PubMed] [Google Scholar]
- [256].Manickan E, Smith JS, Tian J, Eggerman TL, Lozier JN, Muller J and Byrnes AP (2006). Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol Ther 13, 108–17. [DOI] [PubMed] [Google Scholar]
- [257].Coughlan L et al. (2012). Ad5:Ad48 hexon hypervariable region substitutions lead to toxicity and increased inflammatory responses following intravenous delivery. Mol Ther 20, 2268–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Vigne E, Mahfouz I, Dedieu JF, Brie A, Perricaudet M and Yeh P (1999). RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J Virol 73, 5156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Vigant F et al. (2008). Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol Ther 16, 1474–80. [DOI] [PubMed] [Google Scholar]
- [260].Shashkova EV, May SM, Doronin K and Barry MA (2009). Expanded anticancer therapeutic window of hexon-modified oncolytic adenovirus. Mol Ther 17, 2121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Man YKS, Davies JA, Coughlan L, Pantelidou C, Blazquez-Moreno A, Marshall JF, Parker AL and Hallden G (2018). The Novel Oncolytic Adenoviral Mutant Ad5–3Delta-A20T Retargeted to alphavbeta6 Integrins Efficiently Eliminates Pancreatic Cancer Cells. Mol Cancer Ther 17, 575–587. [DOI] [PubMed] [Google Scholar]
- [262].Belousova N et al. (2003). Genetically targeted adenovirus vector directed to CD40-expressing cells. J Virol 77, 11367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Hedley SJ et al. (2006). An adenovirus vector with a chimeric fiber incorporating stabilized single chain antibody achieves targeted gene delivery. Gene Ther 13, 88–94. [DOI] [PubMed] [Google Scholar]
- [264].Kashentseva EA, Seki T, Curiel DT and Dmitriev IP (2002). Adenovirus targeting to c-erbB-2 oncoprotein by single-chain antibody fused to trimeric form of adenovirus receptor ectodomain. Cancer Res 62, 609–16. [PubMed] [Google Scholar]
- [265].Krasnykh V, Belousova N, Korokhov N, Mikheeva G and Curiel DT (2001). Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol 75, 4176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Kim JW et al. (2015). A Genetically Modified Adenoviral Vector with a Phage Display-Derived Peptide Incorporated into Fiber Fibritin Chimera Prolongs Survival in Experimental Glioma. Hum Gene Ther 26, 635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Mercier GT, Campbell JA, Chappell JD, Stehle T, Dermody TS and Barry MA (2004). A chimeric adenovirus vector encoding reovirus attachment protein sigma1 targets cells expressing junctional adhesion molecule 1. Proc Natl Acad Sci U S A 101, 6188–6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Weaver EA, Mercier GT, Gottschalk S and Barry MA (2012). T-cell-biased immune responses generated by a mucosally targeted adenovirus-sigma1 vaccine. Mucosal Immunol 5, 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Tsuruta Y et al. (2007). A mosaic fiber adenovirus serotype 5 vector containing reovirus sigma 1 and adenovirus serotype 3 knob fibers increases transduction in an ovarian cancer ex vivo system via a coxsackie and adenovirus receptor-independent pathway. Clin Cancer Res 13, 2777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V and Curiel DT (2000). Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol 74, 6875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Chen CY, May SM and Barry MA (2010). Targeting adenoviruses with factor x-single-chain antibody fusion proteins. Hum Gene Ther 21, 739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Barry MA, Dower WJ and Johnston SA (1996). Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med 2, 299–305. [DOI] [PubMed] [Google Scholar]
- [273].Cwirla SE, Peters EA, Barrett RW and Dower WJ (1990). Peptides on phage: A vast library of peptides for identifying ligands. Proceedings of the National Academy of Sciences USA 87, 6378–6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Devlin JJ, Panganiban LC and Devlin PE (1990). Random peptide libraries: a source of specific protein binding molecules. Science 249, 404–406. [DOI] [PubMed] [Google Scholar]
- [275].Scott JK and Smith GP (1990). Searching for peptide ligands with an epitope library. Science 249, 386–390. [DOI] [PubMed] [Google Scholar]
- [276].Dreier B et al. (2011). Her2-specific multivalent adapters confer designed tropism to adenovirus for gene targeting. J Mol Biol 405, 410–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Cherf GM and Cochran JR (2015). Applications of Yeast Surface Display for Protein Engineering. Methods Mol Biol 1319, 155–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Sheehan J and Marasco WA (2015). Phage and Yeast Display. Microbiol Spectr 3, AID-0028–2014. [DOI] [PubMed] [Google Scholar]
- [279].Frenzel A, Schirrmann T and Hust M (2016). Phage display-derived human antibodies in clinical development and therapy. MAbs 8, 1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Gui J, Moyana T, Malcolm B and Xiang J (1996). Identification of a decapeptide with the binding reactivity for tumor-associated TAG72 antigen from a phage displayed library. Proteins 24, 352–8. [DOI] [PubMed] [Google Scholar]
- [281].Gui J, Moyana T and Xiang J (1996). Selection of a peptide with affinity for the tumor-associated TAG72 antigen from a phage-displayed library. Biochem Biophys Res Commun 218, 414–9. [DOI] [PubMed] [Google Scholar]
- [282].Koivunen E, Gay DA and Ruoslahti E (1993). Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J Biol Chem 268, 20205–10. [PubMed] [Google Scholar]
- [283].Koivunen E, Wang B and Ruoslahti E (1995). Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology (N Y) 13, 265–70. [DOI] [PubMed] [Google Scholar]
- [284].Doorbar J and Winter G (1994). Isolation of a peptide antagonist to the thrombin receptor using phage display. Journal of Molecular Biology 244, 361–369. [DOI] [PubMed] [Google Scholar]
- [285].Fong S, Doyle LV, Devlin JJ and Doyle MV (1994). Scanning whole cells with phag-display libraries: Identification of peptide ligands that modulate cell function. Drug Development Research 33, 64–70. [Google Scholar]
- [286].Mazzucchelli L, Burritt JB, Jesaitis AJ, Nusrat A, Liang TW, Gewirtz AT, Schnell FJ and Parkos CA (1999). Cell-specific peptide binding by human neutrophils. Blood 93, 1738–48. [PubMed] [Google Scholar]
- [287].Romanov VI, Durand DB and Petrenko VA (2001). Phage display selection of peptides that affect prostate carcinoma cells attachment and invasion. Prostate 47, 239–51. [DOI] [PubMed] [Google Scholar]
- [288].Nicklin SA, White SJ, Watkins SJ, Hawkins RE and Baker AH (2000). Selective targeting of gene transfer to vascular endothelial cells by use of peptides isolated by phage display. Circulation 102, 231–7. [DOI] [PubMed] [Google Scholar]
- [289].White SJ, Nicklin SA, Sawamura T and Baker AH (2001). Identification of peptides that target the endothelial cell-specific LOX-1 receptor. Hypertension 37, 449–55. [DOI] [PubMed] [Google Scholar]
- [290].Tseng-Law J, Szalay P, Guillermo R, Kobori J, Van Epps D, Schneidkraut MJ and Deans R (1999). Identification of a peptide directed against the anti-CD34 antibody, 9C5, by phage display and its use in hematopoietic stem cell selection. Exp Hematol 27, 936–45. [DOI] [PubMed] [Google Scholar]
- [291].Eidne KA, Henery CC and Aitken RJ (2000). Selection of peptides targeting the human sperm surface using random peptide phage display identify ligands homologous to ZP3. Biol Reprod 63, 1396–402. [DOI] [PubMed] [Google Scholar]
- [292].Dmitriev IP, Kashentseva EA and Curiel DT (2002). Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J Virol 76, 6893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Ghosh D and Barry MA (2005). Selection of muscle-binding peptides from context-specific peptide-presenting phage libraries for adenoviral vector targeting. Journal of Virology 79, 13667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Nguyen TV, Anguiano-Zarate SS, Matchett WE, Barry ME and Barry MA (2018). Retargeted and detargeted adenovirus for gene delivery to the muscle. Virology 514, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Takahashi S, Mok H, Parrott MB, Marini FC 3rd, Andreeff M, Brenner MK and Barry MA (2003). Selection of chronic lymphocytic leukemia binding peptides. Cancer Res 63, 5213–7. [PubMed] [Google Scholar]
- [296].Trepel M, Grifman M, Weitzman MD and Pasqualini R (2000). Molecular adaptors for vascular-targeted adenoviral gene delivery. Hum Gene Ther 11, 1971–81. [DOI] [PubMed] [Google Scholar]
- [297].Miura Y et al. (2007). Direct selection of targeted adenovirus vectors by random peptide display on the fiber knob. Gene Ther 14, 1448–60. [DOI] [PubMed] [Google Scholar]
- [298].Lupold SE, Kudrolli TA, Chowdhury WH, Wu P and Rodriguez R (2007). A novel method for generating and screening peptides and libraries displayed on adenovirus fiber. Nucleic Acids Res 35, e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Nishimoto T et al. (2009). Oncolytic virus therapy for pancreatic cancer using the adenovirus library displaying random peptides on the fiber knob. Gene Ther 16, 669–80. [DOI] [PubMed] [Google Scholar]
- [300].Wu P, Kudrolli TA, Chowdhury WH, Liu MM, Rodriguez R and Lupold SE (2010). Adenovirus targeting to prostate-specific membrane antigen through virus-displayed, semirandom peptide library screening. Cancer Res 70, 9549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Yamamoto Y et al. (2014). Development of a novel efficient method to construct an adenovirus library displaying random peptides on the fiber knob. Mol Pharm 11, 1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Menezes KM, Mok HS and Barry MA (2006). Increased transduction of skeletal muscle cells by fibroblast growth factor-modified adenoviral vectors. Hum Gene Ther 17, 314–20. [DOI] [PubMed] [Google Scholar]
- [303].Laakkonen JP, Engler T, Romero IA, Weksler B, Couraud PO, Kreppel F and Kochanek S (2012). Transcellular targeting of fiber- and hexon-modified adenovirus vectors across the brain microvascular endothelial cells in vitro. PLoS One 7, e45977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Kita A et al. (2016). Adenovirus vector-based incorporation of a photo-cross-linkable amino acid into proteins in human primary cells and cancerous cell lines. Sci Rep 6, 36946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Ren PK, Wang F, Li HM, Li ZH and Huang Q (2006). [The construction of recombinant adenovirus expressing bifunctional fusion protein sCAR-EGF and the detection of its activity]. Sheng Wu Gong Cheng Xue Bao 22, 713–9. [PubMed] [Google Scholar]
- [306].Campos SK and Barry MA (2004). Rapid construction of capsid-modified adenoviral vectors through bacteriophage lambda red recombination. Human Gene Therapy 15, 1125–1130. [DOI] [PubMed] [Google Scholar]
- [307].Campos SK, Parrott MB and Barry MA (2004). Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Molecular Therapy 9, 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Parrott MB and Barry MA (2000). Metabolic biotinylation of recombinant proteins in mammalian cells and in mice. Mol Ther 1, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Parrott MB and Barry MA (2001). Metabolic biotinylation of secreted and cell surface proteins from mammalian cells. Biochem. Biophys. Res. Comm 281, 993–1000. [DOI] [PubMed] [Google Scholar]
- [310].Nakano MY, Boucke K, Suomalainen M, Stidwill RP and Greber UF (2000). The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J Virol 74, 7085–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Greber UF (1998). Virus assembly and disassembly: the adenovirus cysteine protease as a trigger factor. Rev Med Virol 8, 213–222. [DOI] [PubMed] [Google Scholar]
- [312].Qian ZM, Li H, Sun H and Ho K (2002). Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev 54, 561–587. [DOI] [PubMed] [Google Scholar]
- [313].Qiu Q, Xu Z, Tian J, Moitra R, Gunti S, Notkins AL and Byrnes AP (2015). Impact of Natural IgM Concentration on Gene Therapy with Adenovirus Type 5 Vectors. J Virol 89, 3412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Xu Z, Qiu Q, Tian J, Smith JS, Conenello GM, Morita T and Byrnes AP (2013). Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat Med 19, 452–7. [DOI] [PubMed] [Google Scholar]
- [315].Khare R, Hillestad ML, Xu Z, Byrnes AP and Barry MA (2013). Circulating antibodies and macrophages as modulators of adenovirus pharmacology. J Virol 87, 3678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Tian J, Xu Z, Smith JS, Hofherr SE, Barry MA and Byrnes AP (2009). Adenovirus activates complement by distinctly different mechanisms in vitro and in vivo: indirect complement activation by virions in vivo. J Virol 83, 5648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Smith JS, Tian J, Muller J and Byrnes AP (2004). Unexpected pulmonary uptake of adenovirus vectors in animals with chronic liver disease. Gene Ther 11, 431–8. [DOI] [PubMed] [Google Scholar]
- [318].Uusi-Kerttula H et al. (2018). Ad5NULL-A20: A Tropism-Modified, alphavbeta6 Integrin-Selective Oncolytic Adenovirus for Epithelial Ovarian Cancer Therapies. Clin Cancer Res 24, 4215–4224. [DOI] [PubMed] [Google Scholar]


