Abstract
Heparin resistance is an uncommon phenomenon defined as the need for high-dose unfractionated heparin (UFH) of more than 35,000 IU/day to achieve the target activated partial-thromboplastin time ratio or the failure to achieve the desired activated clotting time after a full UFH dose. This rare phenomenon is being more commonly observed in Covid-19 patients in a hypercoagulable state. We describe a Covid-19 patient confirmed by reverse-transcriptase polymerase chain reaction assay, with acute limb ischemia, who developed heparin resistance. The patient was managed by the departments of vascular surgery, anesthesia and intensive care, and the Coagulation Service and Thrombosis Research from San Raffaele Scientific Institute, Milan, Italy.
Since the recent pandemic outbreak of 2019-nCoV infection, several series have been reported showing a high mortality rate related to the virus-induced severe interstitial pneumonia.1 First, the infection was thought to be a respiratory disorder; however, with the experience acquired from several clinical presentations, it has been supposed that Covid-19 infection is a systemic disease, not limited to the lungs.2 , 3 In fact, Covid-19 patients could develop a large number of coagulation abnormalities including changes in the activated partial-thromboplastin time (aPTT), prothrombin time (PT), and increase of D-dimer.4 As a clinical implication, high rates of thrombotic events both for venous and arterial vessels been described as further complications in Covid-19 patients and an inflammatory pathway involving endothelium activation has been suggested to justify clots formation.2 , 3 , 5, 6, 7, 8
The treatment of thrombosis in the setting of a deep hypercoagulable state, such as in Covid-19 patients, may be hampered by the inability to achieve the target aPTT when treating with unfractionated heparin (UFH).9 This rare phenomenon is called heparin resistance (HR), which can be defined by the use of high doses of UFH to raise the aPTT and activated clotting time (ACT) to within therapeutically desired ranges.10
Herein is described a case of a Covid-19 patient who developed HR in the setting of acute limb ischemia associated with acroischemia. The patient signed the institutional informed consent for the procedure and the publication of his clinical information and images.
Case Report
A slightly overweight, 67-year-old male patient with no past medical history accessed emergency room with fever (37.8°C) and mild respiratory symptoms. After the diagnosis of Covid-19, he was sent home on hydroxychloroquine and azithromycin. Five days later, he was readmitted to the emergency room with worsening asthenia and fever. At physical examination, the patient presented anasarca, a respiratory rate of 32 breaths per minute, and bibasilar rales at auscultation, mainly on the right lung. Oxygen saturation in ambient air was 87%.
Computed tomography chest scan revealed bilateral ground-glass opacity and crazy paving appearance of the right lung. Complete demographic characteristics, clinical details, and laboratory findings are shown in Table I . Notably, C-reactive protein (114.1 mg/L, normal range <6 mg/L), fibrinogen (711 mg/dL, range 150–400 mg/dL) and D-dimer (1.83 μg/mL, range <0.77 μg/mL) were altered. Moreover, the association of hypoalbuminemia (33.2 g/L, 50.7% of total serum proteins) proteinuria 30 mg/dL, (normal range 0–15 mg/dL) and anasarca, was consistent with the diagnosis of nephrotic syndrome on top of Covid-19 pneumonia.
Table I.
Demographics, clinical characteristics, and laboratory findingsa
| Characteristics | Patient 1 |
|---|---|
| Age | 67 |
| Sex | Male |
| Relevant past medical history | None |
| Symptoms onset | Fever, dyspnea, fatigue, anasarca |
| Preoperative respiratory support | Noninvasive mechanical ventilation |
| LMWH prophylaxis | Enoxaparin 6,000 IU/day |
| Days from admission to thrombotic event | 5 |
| Event | Right acute limb ischemia secondary to iliac-femoro-popliteal arterial thrombosis |
| Intervention | Surgical thrombectomy |
| Intraoperative UFH to achieve ACT > 250 sec | 14,000 IU |
| Postoperative UFH infusion to achieve aPTT ratio > 2 | 2,000 IU/h |
| Admission | |
| Platelet count (×109/L) | 214,000 |
| Leukocytes (109/L) | 5.6 |
| Lymphocytes (109/L) | 0.9 |
| Hemoglobin (g/L) | 15.1 |
| Prothrombin time ratio | 0.96 |
| aPTT ratio | 1 |
| D-dimer (μg/mL) | 1.83 |
| Fibrinogen (mg/dL) | 711 |
| Preoperative | |
| Platelet count (×109/L) | 405 |
| Leukocytes (109/L) | 11.6 |
| Lymphocytes (109/L) | 0.6 |
| Hemoglobin (g/L) | 15.7 |
| Prothrombin time ratio | 1.06 |
| aPTT ratio | 0.84 |
| D-dimer (μg/mL) | >20 |
| Fibrinogen (mg/dL) | 740 |
| At last follow-up | |
| Platelet count (×109/L) | 285,000 |
| Leukocytes (109/L) | 8.4 |
| Lymphocytes (109/L) | 1.7 |
| Hemoglobin (g/L) | 13.3 |
| Prothrombin time ratio | 1.12 |
| aPTT ratio | 1.07 |
| D-dimer (μg/mL) | 1.60 |
| Fibrinogen (mg/dL) | 510 |
Reference ranges are as follows: platelet count, 150,000 to 450,000 per cubic millimeter; leukocytes, 4.8 to 10.8 per cubic liter; lymphocytes, 1 to 4.8 per cubic liter; hemoglobin, 14 to 18 grams per deciliter; normal prothrombin time ratio 0.9 to 1.18; normal activated partial-thromboplastin time, 0.75 to 1.29; D-dimer, 0.27–0.77 μg per milliliter; fibrinogen, 150 to 400 mg per deciliter.
The patient was admitted to the Covid-19 ward and treated with noninvasive ventilation, ceftriaxone, and enoxaparin 6,000 anti-Xa IU once a day as antithrombotic prophylaxis. During the first 48 hr, the patient did not show any further clinical deterioration. However, from the third day after the admission, there was a progressive increase of the platelet count (443 × 109/L), D-dimer (3.73 μg/mL), C-reactive protein (229 mg/L), and worsening of the gas exchange. Consequently the patient was intubated and moved to the Covid-19 intensive care unit (ICU).
On day 5, the patient suffered pain, numbness, and coldness of the right lower limb along with a massive augmentation of the D-dimer (>20 μg/mL). A Duplex ultrasound scan (DUS) showed complete occlusion of the right iliac and femoro-popliteal arteries, for which it performed an emergent revascularization of the lower limb under general anesthesia. A 20 G catheter was inserted in the right radial artery to monitor the invasive blood pressure. To achieve an ACT above 250 sec, 3 boluses of 1,000 IU each of intravenous UFH were required after the administration of a loading dose of 5,000 IU. To maintain the ACT target, further 6,000 IU of UFH were added. A right lower limb conventional thrombectomy was successfully performed with a Fogarty catheter using multiple accesses (femoral and below-the-knee popliteal). An intra-operative DUS control showed a biphasic flow pattern on the treated arteries. During the surgery, the sudden flattening of the arterial wave, the coldness and pallor of the right upper limb were highly suggestive of limb ischemia. The clinical suspicion was confirmed by the intra-operative DUS, which detected the thrombosis of the right brachial artery. Then, a right upper limb thrombectomy was successfully performed with restoring of radial artery patency. The patient was discharged from the operating theater with a continuous infusion of 1,000 IU/h of UFH. Notably, an aPTT ratio of 1.92 was obtained only after the increase of the UFH infusion rate to 2,000 IU/h (more than 35,000 IU/day). Nevertheless, the aPTT ratio dropped to 0.86 during the first post-operative day leading to in further increase of the amount of UFH administered. The day after, the occurrence of a reactive thrombocytosis accounted for a reduction of the aPTT ratio from 2.54 to sub-optimal values. Since the anti-factor Xa assay was below the therapeutic range (0.34 IU/mL), the UFH was shifted to 8,000 of enoxaparin twice a day. Over the 2 following days, D-dimer decrease to 0.72 μg/mL and the anti-Xa activity ranged between 0.60 and 0.77 IU/mL. During this period, a high dosage (0.25 μg/kg/min) of norepinephrine was needed to treat hypotension.
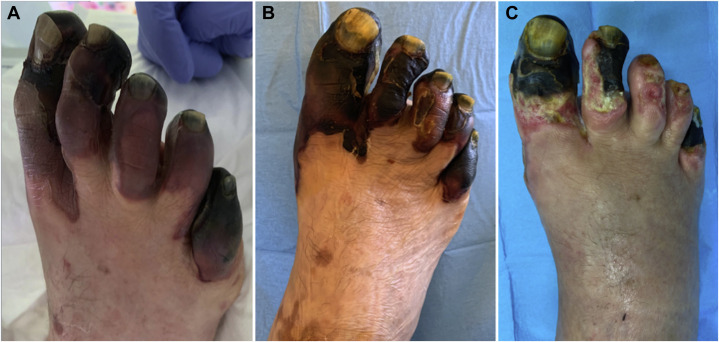
On the sixth post-op day, the patient presented a bilateral persistent acroischemia of the lower extremities, including finger/toe cyanosis, skin bulla, and dry gangrene (Fig. 1 A) with patency of lower limb arteries at the DUS. Therefore, prostaglandin E−1 infusion at the dosage of 60 mcg twice a day was administered. Five days later, the patient was discharged from the ICU to the medical Covid-19 ward and twenty days later from the hospital with oral anticoagulation.
Fig. 1.
Evolution of the acroischemic lesions. (A) At the sixth post-op day. (B) At hospital discharge. (C) At the last follow-up (2 months).
At 2 months follow-up, no further thromboembolic events occurred. A slow reduction of D-dimer levels from 3.3 μg/mL to 1.6 μg/mL was observed. Detailed laboratory findings at the last follow-up are summarized in Table I. The evolution of the acroischemic lesions, characterized by gradual improvement without the need of major amputation, is shown in Figure 1 and the patient is coming to wound care service twice a week.
Discussion
During the Covid-19 pandemic, it has become clear the hypercoagulable state of critically ill Covid-19 patients. In addition, some published epidemiologic data have shown increased thromboembolic events in those patients, such as unusual ischemic limbs and venous thromboembolism.7 , 8 Several clinical and laboratory findings are observed in hospitalized Covid-19 patients who developed thromboembolic events, including high D-dimer levels, high fibrinogen levels, and low antithrombin levels.11 In the setting of arterial limb ischemia, proper heparinization plays a key role in terms of limb salvage, major and minor amputations, and mortality. However, the inability to achieve the target aPTT when treating with UFH, known as HR, has been observed in hospitalized Covid-19 patients.9
HR is often observed during cardiovascular operations, mainly in procedures that require a cardiopulmonary bypass.12 HR is defined as the need for a high dose of UFH of more than 35,000 IU/day to achieve the target aPTT ratio or the failure to achieve the desired ACT after a full UFH dose. Predictors of HR are age (>65 years), antithrombin III (ATIII) activity less than or equal to 60%, platelets greater than 300 × 109/L, and increased factor VIII and fibrinogen levels.10 , 12 Besides the fact that the most common condition responsible for HR is the deficiency of ATIII, clinical indices for related risk factors and the mechanism underlying HR have not been fully determined.10 , 12
Covid-19 infection is an endothelial thromboinflammatory syndrome, involving the microcirculation of organs and districts which may lead to multiple organ failure and death.2 , 3 A marker of the hypercoagulable state is the increased D-dimer level which is associated with an unfavorable prognosis.13 In light of the aforementioned considerations, the adoption of a strategy involving heparin at higher than prophylactic dosages in addition to antiviral and anti-inflammatory drugs has a solid physiopathology background and may be effective in preventing venous and arterial thromboembolic episodes. Use of UFH at therapeutic dose has been advocated over the administration of low molecular weight heparin (LMWH) for several reasons, with renal failure being the only issue of relevance.11 However, in Covid-19 patients, both very high concentrations of factor VIII and fibrinogen may not allow to achieve an effective anticoagulation with a standard dose of UFH as witnessed by the subtherapeutic aPTT range described in all cases. For all the aforementioned considerations, it is reasonable that Covid-19 infection triggers, in predisposed patients, HR. Since the replacement of UFH with LMWH at therapeutic dosage was associated with a prompt decline in D-dimer levels and no new thromboembolic events, it is unlikely that the ATIII deficiency was the cause of HR.
Conclusion
The present report underscores 3 aspects of the relationship between Covid-19 infection and coagulation: first, it is a systemic endothelial disease not limited to the lungs; second, the anticoagulation cannot be managed as one-size-fits-all owing to the coexistence of several “sheds of thrombophilia” (i.e. HR, deficiency of ATIII); third, the aPTT should be integrated with the monitoring of the anti-Xa activity levels to prevent overdosage especially in patients with acute renal failure.
Footnotes
Conflicts of Interest: The authors have no relevant conflicts for the present work.
Authors' contributions: Domenico Baccellieri, Victor Bilman, Luca Apruzzi, Fabrizio Monaco, Armando D’Angelo, and Diletta Loschi contributed to the concept, analysis/interpretation of data, critical writing, and revising intellectual content. Germano Melissano and Roberto Chiesa contributed to the concept, critical writing, and revising intellectual content.
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciceri F., Beretta L., Scandroglio A.M. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llitjos J., Leclerc M., Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baccellieri D., Apruzzi L., Ardita V. The “venous perspective” in Lombardia (Italy) during the first weeks of the COVID-19 epidemic. Phlebology. 2020;35:295–296. doi: 10.1177/0268355520925727. [DOI] [PubMed] [Google Scholar]
- 8.Melissano G., Mascia D., Baccellieri D. Pattern of vascular disease in Lombardy, Italy, during the first month of the COVID-19 outbreak. J Vasc Surg. 2020;72:4–5. doi: 10.1016/j.jvs.2020.04.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beun R., Kusadasi N., Sikma M. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42:19–20. doi: 10.1111/ijlh.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durrani J., Malik F., Ali N. To be or not to be a case of heparin resistance. J Community Hosp Intern Med Perspect. 2018;8:145–148. doi: 10.1080/20009666.2018.1466599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett C.D., Moore H.B., Yaffe M.B. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: a comment. J Thromb Haemost. 2020:1–4. doi: 10.1111/jth.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawatsu S., Sasaki K., Sakatsume K. Predictors of heparin resistance before cardiovascular operations in Adults. Ann Thorac Surg. 2018;105:1316–1321. doi: 10.1016/j.athoracsur.2018.01.068. [DOI] [PubMed] [Google Scholar]
- 13.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]