Abstract
Purpose
Neoadjuvant chemotherapy (NAC) involving trastuzumab markedly increases pathologic complete response (pCR) rates in patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer. Despite increasing pCR rates, long-term survival gains are controversial owing to distinctive biologic behavior mediated by the presence of hormonal receptors (HRs) that may interact with HER2 receptors. We, therefore, investigated the differences in relative survival gain provided by neoadjuvant trastuzumab-based chemotherapy on HR positive (HR+) status of patients.
Methods
We retrospectively analyzed women with stage II or III HER2+ breast cancer who underwent NAC followed by a breast cancer surgery between 2008 and 2013. The survival benefits of adding trastuzumab to NAC were analyzed by classifying patients into HR+ and HR negative (HR−) groups.
Results
Of 666 patients included in the study, 374 (52.1%) were HR+ and 319 (47.9%) were HR−. In the HR+ group, trastuzumab treatment led to higher pCR rates and significantly better breast cancer specific survival (BCSS) and overall survival (OS) than no trastuzumab treatment. However, among patients with HR− breast cancer, trastuzumab treatment showed no statistically significant difference between BCSS and OS following multivariate analysis.
Conclusion
We found that the addition of trastuzumab to NAC improved relative survival benefit in HER2+/HR+ patients than in HER2+/HR− patients, even though the pCR rate increases were lower. Although pCR has been regarded as a surrogate marker for estimating long-term survival benefits after NAC, it alone may not translate into real long-term oncologic outcomes in particular cancer subtypes after trastuzumab-based NAC. Further longer-term evaluation of the objective survival benefit after NAC driven by a dual HER2 block according to HR status is warranted.
Keywords: Breast neoplasms; Induction chemotherapy; Receptor, ErbB-2; Survival; Trastuzumab
INTRODUCTION
The therapeutic effect of neoadjuvant chemotherapy (NAC) on early breast cancer is similar to that of adjuvant chemotherapy [1]. Currently, NAC is being individualized for patients with breast cancer according to intrinsic subtypes [2,3]. As several representative randomized controlled trials (RCTs) have demonstrated the efficacy of NAC combined with anti-human epidermal growth factor receptor 2 (HER2) agents in patients with HER2+ early breast cancer, this is currently the standard treatment for this subtype [4,5,6].
Pathologic complete response (pCR) is considered a surrogate marker for predicting long-term survival benefits in patients receiving NAC [2,7]. Although NAC with trastuzumab resulted in significantly higher pCR rates in those with HER+ breast cancer, several studies have not demonstrated a long-term overall survival (OS) benefit when patients were stratified by HR status. In addition, outcomes were based on post-hoc analyses following clinical trials [4,8,9,10,11,12,13]. Hormonal receptor positive (HR+)/ HER2+ and HR negative (HR−)/ HER2+ breast cancers are biologically distinct [14]. HR−/HER2+ cancer demonstrates more sensitivity to NAC and shows better pCR rates [15]. To the best of our knowledge, clinical trials comparing the long-term survival benefit of NAC with trastuzumab according to HR status, particularly in Asian populations, have not yet been published.
Not long after NAC with trastuzumab was approved for daily practice, pertuzumab was integrated into the standard NAC for HER2+ breast cancer for dual blockade. However, many Asian countries faced financial hurdles in reimbursing medical expenses of patients with HER + breast cancer treated with trastuzumab with or without pertuzumab. These countries must allocate budget efficiently to help patients with HER2+ breast cancer according to the clinical trials reflecting the patients’ biologic and ethnic characteristics. Initiating new RCTs for NAC without trastuzumab is not feasible for malignancies for which anti-HER2 agents are the standard treatment [16]. Therefore, a retrospective study with a fairly organized and well-maintained database is the only option for analyzing the effect of NAC plus trastuzumab on long-term survival according to HR status.
METHODS
Compliance with ethical standards
This study protocol was approved by the Institutional Review Board of the Catholic University of Korea (VC18RCSI0163). The Korean Breast Cancer Society (KBCS) prospectively collected all patient data. All participants in this study provided written informed consent for the storage of their medical information in the database and the use of this information for research purposes.
Data source and study population
Since 1996, the KBCS has been prospectively collecting patient data that were reported by surgeons at 102 general hospitals, including 41 university hospitals and 61 accredited training hospitals. Data audits were routinely performed to guarantee the quality of the database by the KBCS registration committee. Survival and cause of death were censored by the National Statistical Office of the Republic of Korea.
We selected female patients aged ≥ 20 years with HER2+ stage II or III primary breast cancer diagnosed between 2008 and 2013. HER2 positivity was defined as either 3+ overexpression as observed via immunohistochemical staining or HER2 amplification as observed via fluorescent in situ hybridization (HER2/CEP17 ratio ≥ 2.0). HR positivity was defined as >1% staining for either estrogen or progesterone receptors or both. This study included patients who received trastuzumab-based NAC and then underwent breast cancer surgery. Patients who received HER2-targeted NAC other than trastuzumab were excluded. Only patients with complete data regarding clinical and pathologic parameters were included. The final study population comprised 666 patients.
From the KBCS database, which processes personal information anonymously from the initial phase of data gathering, we extracted information regarding patient age, date of diagnosis, date of surgery, date of death, cause of death, type of surgery, clinical stage, pathologic stage, NAC regimen, treatment response (pCR or non-pCR), HR status, HER2 status, histologic grade, adjuvant hormonal therapy, and adjuvant radiotherapy. Clinical and pathologic TNM stages were classified according to the sixth classification of the American Joint Committee on Cancer.
Statistical analysis
Patient clinical characteristics were compared using the χ2 test and Student's t-test. pCR was defined as the absence of residual invasive cancer cells in the breasts and axillary lymph nodes (ypT0/is + ypN0). Survival was defined as the time interval from the date of diagnosis to death. Survival was estimated using the Kaplan-Meier method and was compared using the log-rank test (2-sided, p < 0.05). Cox proportional regression models were used to estimate the hazard ratio of breast cancer specific survival (BCSS) and OS (95% confidence interval [CI]). All statistical analyses were performed using SPSS Statistics, version 18.0 (SPSS Inc., Chicago, USA).
Data availability statement
The datasets generated or analyzed during the current study are not publicly available due to the patient privacy requirements but are available from the corresponding author upon reasonable request.
RESULTS
Patients
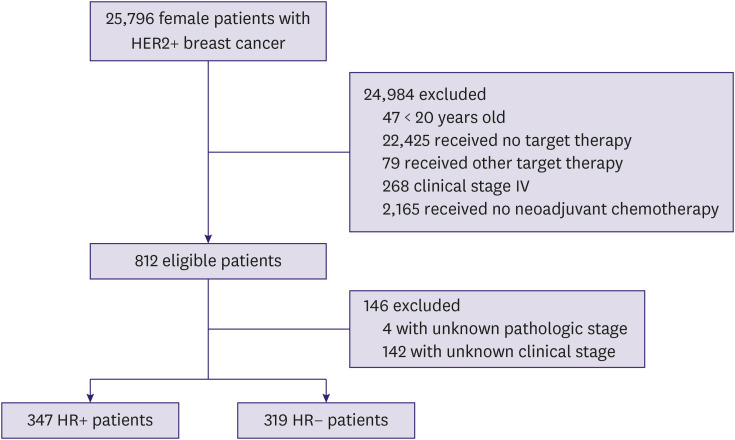
The final study population comprised 666 patients with a mean age of 48.2 ± 10.0 years and a median length of follow-up of 54 (45.6–82) months. Among 666 patients, 347 (52.1%) and 319 (47.9%) had HR+ and HR− status, respectively (Figure 1). Most patients (620; 93.1%) had invasive ductal carcinoma.
Figure 1. Flow chart of patient population. Data collection was performed between 2008 and 2013.
HER2 = human epidermal growth factor receptor 2; HR = hormone receptor.
Treatment
After surgery, all patients continued the recommended trastuzumab treatment with adjuvant chemotherapy, and 90.8% of patients in the HR+ group received adjuvant hormonal therapy. The rate of breast-conserving surgery was significantly higher in the trastuzumab-based NAC group than in the non-trastuzumab-based NAC group. Patient baseline characteristics and treatments according to HR status are depicted in Table 1.
Table 1. Patient characteristics by HR status.
| Characteristics | Total (n = 666) | HR positive (n = 347) | HR negative (n = 319) | |||||
|---|---|---|---|---|---|---|---|---|
| Trastuzumab (n = 221) | Non-trastuzumab (n = 126) | p-value | Trastuzumab (n = 208) | Non-trastuzumab (n = 111) | p-value | |||
| Age (yr) | 48.2 ± 10.0 | 45.8 ± 9.8 | 47.6 ± 8.8 | 0.156 | 49.3 ± 10.3 | 51.6 ± 10.0 | 0.058 | |
| 20–49 | 378 (56.8) | 151 (68.3) | 72 (57.1) | 0.047 | 111 (53.4) | 44 (39.6) | 0.025 | |
| ≥ 50 | 288 (43.2) | 70 (31.7) | 54 (42.9) | 97 (46.6) | 67 (60.4) | |||
| Clinical stage | 0.001 | < 0.001 | ||||||
| II | 231 (34.7) | 95 (43.0) | 32 (25.4) | 84 (40.4) | 20 (18.0) | |||
| III | 435 (65.3) | 126 (57.0) | 94 (74.6) | 124 (59.6) | 91 (82.0) | |||
| Histologic grade | 0.522 | 0.261 | ||||||
| G1/G2 | 288 (43.2) | 127 (57.5) | 63 (50.0) | 59 (28.4) | 39 (35.1) | |||
| G3 | 247 (37.1) | 65 (29.4) | 39 (31.0) | 100 (48.1) | 43 (38.7) | |||
| NA | 131 (19.7) | 29 (13.1) | 24 (19.0) | 49 (23.6) | 29 (26.1) | |||
| Histologic type | 0.373 | 0.033 | ||||||
| IDC | 620 (93.1) | 212 (95.9) | 117 (92.9) | 191 (91.8) | 100 (90.1) | |||
| ILC | 9 (1.4) | 1 (0.5) | 2 (1.6) | 1 (0.5) | 5 (4.5) | |||
| NA, other | 37 (5.6) | 8 (3.6) | 7 (5.6) | 16 (7.7) | 6 (5.4) | |||
| Surgery | 0.051 | 0.003 | ||||||
| BCS | 250 (37.5) | 92 (41.6) | 39 (31.0) | 90 (43.3) | 29 (26.1) | |||
| Mastectomy | 416 (62.5) | 129 (58.4) | 87 (69.0) | 118 (56.7) | 82 (73.9) | |||
| NAC regimen | 0.012 | 0.193 | ||||||
| T + A | 490 (73.6) | 155 (70.1) | 62 (76.2) | 151 (72.6) | 88 (79.3) | |||
| T | 153 (23.0) | 62 (28.1) | 40 (17.5) | 51 (24.5) | 18 (16.2) | |||
| NA | 23 (3.5) | 4 (1.8) | 13 (6.3) | 6 (2.9) | 5 (4.5) | |||
| Hormone therapy | 0.281 | |||||||
| Yes | 315 (90.8) | 210 (95.0) | 105 (83.3) | |||||
| No | 15 (4.3) | 8 (3.6) | 7 (5.6) | |||||
| NA | 17 (4.9) | 3 (1.4) | 14 (11.1) | |||||
| pCR rate | 15.9 | 15.8 | 5.6 | 0.006 | 26.4 | 8.1 | < 0.001 | |
Data are shown as mean ± standard deviation or number (%).
HR = hormone receptor; BCS = breast-conserving surgery; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; NA = not applicable; NAC = neoadjuvant chemotherapy; pCR = pathologic complete remission.
Pathologic complete response
The pCR was reported in 90 of 429 patients (21.0%) in the trastuzumab-based NAC group and 16 of 237 patients (6.8%) in the non-trastuzumab group (p < 0.001). Irrespective of HR status, the pCR rate was significantly higher in the trastuzumab-based NAC group than in the non-trastuzumab group (HR+: 15.8 vs. 5.6%; p = 0.006; HR: 26.4 vs. 8.1%; p < 0.001).
Survival analysis
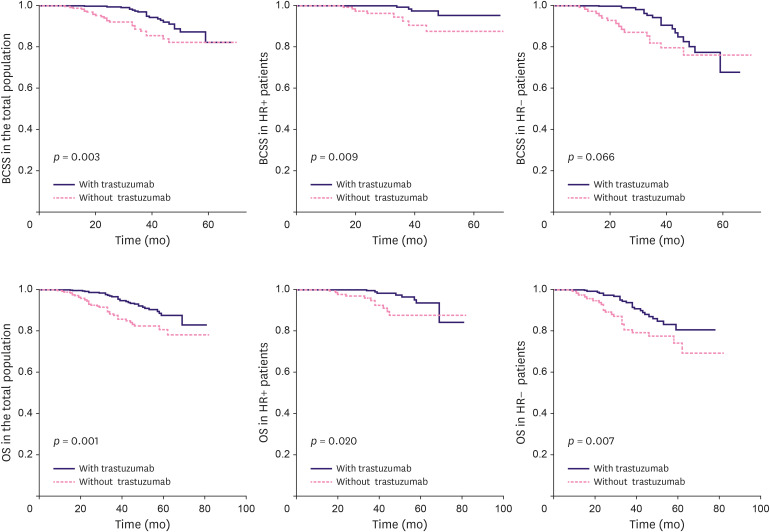
Both patients with HR+/HER2+ and HR−/HER2+ breast cancer in the trastuzumab-based NAC group had significantly better OS than those in the non-trastuzumab-based NAC group (log-rank; HR+: p = 0.020; HR−: p = 0.007). However, HR+ HER+ patients in the trastuzumab-based NAC group demonstrated significantly better BCSS than HR− HER+ patients in the non-trastuzumab-based NAC group (log-rank; HR+ HER+: p= 0.009; HR− HER+: p = 0.066) (Figure 2). Univariate Cox proportional hazard analysis showed a significant BCSS benefit following trastuzumab treatment in the HR+/HER2+ group (HR+/HER2+: hazard ratio, 0.232; 95% CI, 0.070–0.773; HR−/HER2+: hazard ratio, 0.533; 95% CI, 0.269–1.055) and an OS benefit in both the HR groups (HR+/HER2+: hazard ratio, 0.354; 95% CI, 0.142–0.884; HR−/HER2+: hazard ratio, 0.513; 95% CI, 0.290–0.910). Multivariate Cox regression analysis revealed significantly better BCSS (HR+/HER2+: hazard ratio, 0.221; 95% CI, 0.055–0.886; HR−/HER2+: hazard ratio, 0.500; 95% CI, 0.220–1.138) and relatively improved OS in HR+/HER2+ trastuzumab-based NAC group only (HR+/HER2+: hazard ratio, 0.359; 95% CI, 0.133–0.966; HR−/HER2+: hazard ratio, 0.522; 95% CI, 0.269–1.015). For HER2+patients with HR+/HER2+ breast cancer showing pCR after trastuzumab-based NAC, statistical analysis could not be conducted owing to the small number of events. HER2+Among patients without pCR, those with HR+/HER2+ breast cancer showed significantly better BCSS than those with HR−/HER2+ breast cancer (Table 2).
Figure 2. Kaplan-Meier estimates of BCSS and OS according to HR status.
BCSS = breast cancer-specific survival; HR = hormone receptor; OS = overall survival.
Table 2. Multivariate analysis of survival after adding trastuzumab to NAC according to pathologic complete remission and HR status.
| HR positive | HR negative | ||||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | ||
| All | |||||
| BCSS | 0.221 (0.055–0.886) | 0.033 | 0.500 (0.220–1.138) | 0.098 | |
| OS | 0.359 (0.133–0.966) | 0.043 | 0.522 (0.269–1.015) | 0.055 | |
| With pCR | |||||
| BCSS | NA | 0.271 (0.027–2.734) | 0.271 | ||
| OS | NA | 0.271 (0.027–2.734) | 0.271 | ||
| Without pCR | |||||
| BCSS | 0.382 (0.146–1.001) | 0.050 | 0.622 (0.337–1.147) | 0.128 | |
| OS | 0.418 (0.165–1.055) | 0.065 | 0.657 (0.360–1.202) | 0.173 | |
BCSS = breast cancer specific survival; CI = confidence interval; HR = hormone receptor; NA = not applicable; NAC = neoadjuvant chemotherapy; OS = overall survival; pCR = pathologic complete remission.
DISCUSSION
In the 2014 report of the NOAH trial, 151 patients with HR−/HER2+ breast cancer and 84 patients with HR+/HER2+ breast cancer were analyzed to determine the survival benefit of trastuzumab administered as neoadjuvant systemic therapy with chemotherapeutic agents. However, statistical head-to-head comparison according to HR positivity was not reported. In the 2010 GeparQuattro report, 261 HER2+patients with HR+/HER2+ breast cancer and 182 HER2+patients with HR−/HER2+ breast cancer were enrolled [5]. The pCR rate after trastuzumab-based NAC was significantly higher in HER2+patients with HR−/HER2+ breast cancer. However, long-term survival outcomes stratified by HR positivity were not reported. Therefore, we planned to analyze the differences in oncologic outcomes after adding trastuzumab to NAC between patients with HR+/HER2+ and HR−/ HER2+ breast cancer by comparing the hazard ratio.
In a subgroup analysis of the NOAH trial, no benefit of neoadjuvant trastuzumab was noted regarding OS in the HR+/HER2+ subgroups (HR+/HER2+: hazard ratio, 1.05; 95% CI, 0.53–2.10). However, in our study, multivariate analysis demonstrated a significant survival benefit of neoadjuvant trastuzumab in patients with HR+ status only, despite a mere 10.2% increase in pCR. This finding indicates a relatively greater benefit for patients, which reflected as improved BCSS and OS that may be expected for those with HR+/HER2+ status than for those with HR−/HER2+ status.
As the US Food and Drug Administration had accepted pCR as a surrogate marker to support the accelerated approval of chemotherapeutics, most recent trials have focused on the pCR rate [7]. Therefore, many RCTs have used pCR as a surrogate marker to determine the efficacy [8]. However, the OS benefit of a drug that is assessed using pCR at a trial level is controversial [12,17]. In the CTneoBC pooled analysis, a markedly increased pCR was not associated with a corresponding benefit in OS. A significant increase in the pCR rate with no disease-free survival or OS benefit was also demonstrated in the NeoALTTO trial with lapatinib [9]. The low OS, despite the marked increase in the pCR rate, may be related to patient heterogeneity and breast cancer intrinsic subtype [10]. There is a lack of consensus that pCR is the surrogate marker for predicting long-term survival, especially in luminal-type breast cancer [18,19]. Similar to other studies, our study demonstrated that the pCR rate was higher in the HR−/HER2+ group than in the HR+/HER2+ group. However, no comparable long-term relative survival benefit was noted among patients with HR−/HER2+ breast cancer compared with patients with HR+/HER2+ breast cancer despite the marked increase in the pCR in the former.
Although we cannot delineate the exact mechanism that differentiates our findings from those of previous trials, there are a few plausible explanations. Breast cancer in Asian individuals has a distinctive molecular genomic basis, tends to occur at a younger age, and has a worse prognosis than that in Caucasian individuals. An ongoing study revealed that Asian individuals have lower estrogen receptor (ER) gene expression within the ER+ subtypes and an increased prevalence of TP53 mutations [20]. Another example of probable genetic differences between Asians and Caucasians can be found in patterns of non-small cell lung carcinoma. The frequency of the mutation rate of epidermal growth factor receptor is higher in Asians, resulting in the need for different treatment strategies [21,22]. Unfortunately, research on neoadjuvant HER2-targeted treatment is limited owing to the low number of Asian patients with breast cancer who are enrolled in trials.
In this study, it was imperative to balance patient subsets for comparisons to avoid selection biases that might lead to erroneous conclusions. The proportion of stage II and lower histologic grades is higher in the HR+/HER2+ trastuzumab-based NAC subsets than in HR−/HER2+ counterparts. By contrast, the proportion of patients younger than 50 years was significantly higher in the HR+ HER2+ trastuzumab-based NAC group. Therefore, we compared the hazard ratio between these 2 subsets after multivariate analyses to determine comparative survival gain by patients after trastuzumab-based NAC treatment stratified by HR positivity, while mitigating the effects of unbalanced characteristics due to the inherent pitfalls of the retrospective study using a preset database.
The relative survival gain in patients with HR−/HER2+ breast cancer was not significant according to the results of the multivariate analysis in this study, possibly because of the small sample size. Another possible reason may be molecular changes observed after NAC, such as the loss of HER2 expression [23,24]. The negative phenotype conversion rate from HER2+ to HER2− after NAC was higher in the HR+/HER2+ group, implying that the molecular subtype shifts from luminal HER2+ to luminal A. This change could be translated into increased survival.
The limitations of this retrospective study included the exclusion of missing data, which is a typical inherent pitfall of large-scale databases such as ours. Starting in December 2013, the Korean government began reimbursing expenses related to trastuzumab neoadjuvant treatment combined with NAC for breast cancer. The median follow-up period was slightly less than 5 years. Although the risk of early distant metastases within 5 years after diagnosis is similar in HER2+ breast cancer irrespective of HR status, the hazard ratio of late distant metastases ≥ 5 years after diagnosis is higher in the HR− HER2+ subtype [19] Therefore, improved survival of patients with the HR+ HER2+ subtypes can be prolonged after 5 years [24]. The advantage of our study is its high consistency in the perioperative management of the patients, as the pattern of local and systemic treatments have been monitored by the Korean government to ensure that treatments recommended by international guidelines are not overlooked. Even though head-to-head and well-balanced RCTs are mandatory for definitive delineation of relative oncologic outcome gains patients with for HER2+ status with endocrine dependency, this type of RCT is not possible for 2 reasons [16]. First, omitting the neoadjuvant trastuzumab for patients with HER2+ status is impossible owing to ethical issues. Second, the dramatically reduced number of events in the pCR group requires a large number of patients to be enrolled to make statistical analysis possible.
As pCR cannot be achieved in all patients undergoing NAC with trastuzumab or pertuzumab, further research regarding molecular typing in non-pCR groups should be pursued to determine the optimal populations that may receive survival benefit from neoadjuvant target therapy. Considering that patients with HR+ status generally show better survival outcomes than patients with HR− status, survival effects of some possible molecular changes such as HER2 loss and crosstalk between HR and HER2 in patients without pCR after NAC with trastuzumab may warrant further research on various molecular subtypes.
Footnotes
Funding: This work was supported by the Korean Breast Cancer Society.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Schneider J, Gwak H.
- Data curation: Schneider J.
- Formal analysis: Gwak H.
- Supervision: Lee HJ, Nam SJ, Lee SJ, Jung JH, Jung SH, Lim ST, Jeon YW.
- Writing - original draft: Schneider J, Gwak H.
- Writing - review & editing: Schneider J, Gwak H.
References
- 1.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 3.Harbeck N, Gluz O. Neoadjuvant therapy for triple negative and HER2-positive early breast cancer. Breast. 2017;34(Suppl 1):S99–S103. doi: 10.1016/j.breast.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–647. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- 5.Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 6.Untch M, Fasching PA, Konecny GE, Hasmüller S, Lebeau A, Kreienberg R, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 7.Pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. Food and Drug Administration; 2014. [Accessed October 2rd, 2018]. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf. [Google Scholar]
- 8.Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2:751–760. doi: 10.1001/jamaoncol.2015.6113. [DOI] [PubMed] [Google Scholar]
- 9.de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15:1137–1146. doi: 10.1016/S1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
- 10.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Hurvitz S. Long-term outcomes of neoadjuvant treatment of HER2-positive breast cancer. Clin Adv Hematol Oncol. 2016;14:520–530. [PubMed] [Google Scholar]
- 12.Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 13.Rose BS, Winer EP, Mamon HJ. Perils of the pathologic complete response. J Clin Oncol. 2016;34:3959–3962. doi: 10.1200/JCO.2016.68.1718. [DOI] [PubMed] [Google Scholar]
- 14.Nitz UA, Gluz O, Christgen M, Grischke EM, Augustin D, Kuemmel S, et al. De-escalation strategies in HER2-positive early breast cancer (EBC): final analysis of the WSG-ADAPT HER2+/HR- phase II trial: efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel. Ann Oncol. 2017;28:2768–2772. doi: 10.1093/annonc/mdx494. [DOI] [PubMed] [Google Scholar]
- 15.Harbeck N. Insights into biology of luminal HER2 vs. enriched HER2 subtypes: Therapeutic implications. Breast. 2015;24(Suppl 2):S44–S48. doi: 10.1016/j.breast.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubovszky G, Horváth Z. Recent advances in the neoadjuvant treatment of breast cancer. J Breast Cancer. 2017;20:119–131. doi: 10.4048/jbc.2017.20.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 19.Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011;13:221. doi: 10.1186/bcr2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kan Z, Ding Y, Kim J, Jung HH, Chung W, Lal S, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9:1725. doi: 10.1038/s41467-018-04129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park K, Yu CJ, Kim SW, Lin MC, Sriuranpong V, Tsai CM, et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION study. JAMA Oncol. 2016;2:305–312. doi: 10.1001/jamaoncol.2015.4921. [DOI] [PubMed] [Google Scholar]
- 23.Niikura N, Tomotaki A, Miyata H, Iwamoto T, Kawai M, Anan K, et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol. 2016;27:480–487. doi: 10.1093/annonc/mdv611. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida A, Hayashi N, Suzuki K, Takimoto M, Nakamura S, Yamauchi H. Change in HER2 status after neoadjuvant chemotherapy and the prognostic impact in patients with primary breast cancer. J Surg Oncol. 2017;116:1021–1028. doi: 10.1002/jso.24762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are not publicly available due to the patient privacy requirements but are available from the corresponding author upon reasonable request.