Abstract
Phyllodes tumor (PT) of the breast is a relatively rare fibroepithelial tumor that accounts for < 1% of primary breast neoplasms. PT is classified histologically as benign, borderline, or malignant, and a malignant PT has greater potential to metastasize than benign PT. Although almost all other organs can be affected, common metastatic sites are the lung and bone via the hematogenous route. There have been several studies reporting cutaneous and soft tissue metastases of PT, though the incidence is rare. Herein, we report a very rare case of scalp metastasis of malignant PT that was diagnosed via skin biopsy and surgical excision.
Keywords: Breast, Neoplasm metastasis, Phyllodes tumor, Scalp
INTRODUCTION
Phyllodes tumors (PT) are rare, rapidly growing, fibroepithelial tumors of the breast that account for < 1% of primary breast neoplasms [1]. They originate from the periductal stroma and are composed of both epithelial and stromal components [2]. The histopathologic spectrum of PT includes classification as benign, borderline, or malignant, designated according to the World Health Organization classification based on the tumor margins, mitotic activity, nuclear pleomorphism, and stromal overgrowth and cellularity [3]. Malignant PT tends to show more local recurrence and metastasis than benign PT, although no tumor behavior predicting metastasis has been verified [4]. The most common sites of distant metastases are the lung and bone via the hematogenous route, while a few studies have reported uncommon cutaneous and soft tissue metastases [5,6]. Additionally, only 1%–2% of all scalp tumors are malignant, and metastasis to the scalp occurs predominantly from visceral tumors [7]. To our knowledge, there has been no report of distant scalp metastasis of PT without coexisting metastasis in the brain parenchyma or head and neck region. We herein describe scalp metastasis of malignant PT in a 58-year-old woman and include a review of the literature.
CASE REPORT
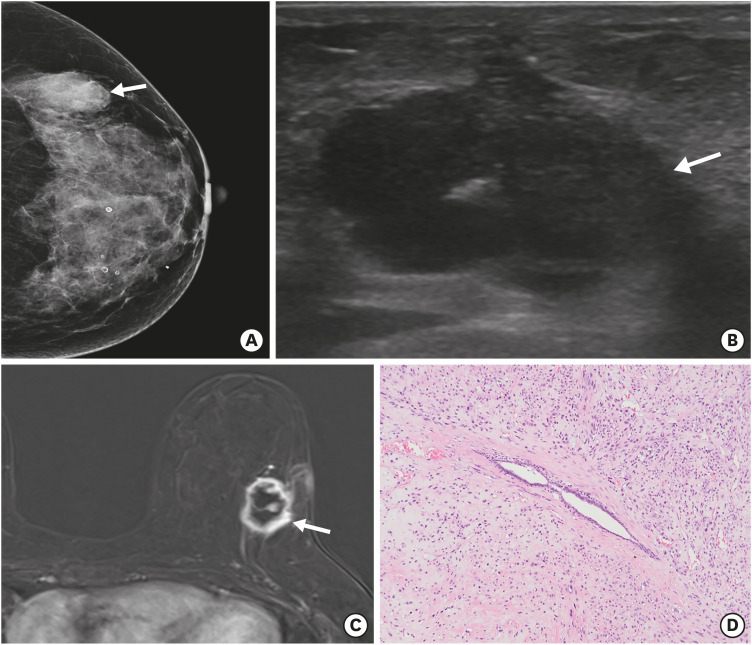
A 58-year-old woman was admitted to our hospital for evaluation of a lump in the left breast. The initial outside-hospital mammogram (Figure 1A) revealed a 3.4 × 2.0-cm mass in the upper outer quadrant of the left breast. On ultrasonography of the left breast (Figure 1B), a 3.1 × 2.1 × 2.7-cm irregular hypoechoic mass with lobulated and partially indistinct margins was evident in the upper outer quadrant (2 o'clock) of the breast. There were no significantly enlarged lymph nodes in the axillae. Ultrasound-guided core-needle biopsy was performed, and the result suggested differential diagnosis of malignant PT, undifferentiated sarcoma, or metaplastic carcinoma. Breast magnetic resonance imaging (MRI) was performed for evaluation of tumor extent. MRI of the left breast (Figure 1C) revealed an approximate 3-cm irregular rim-enhancing mass with a plateau kinetic pattern in the upper outer quadrant of the breast. The patient underwent breast-conserving surgery with negative resection margins. Microscopically, the tumor was characterized by a leaf-like pattern of cystic or cleft-like spaces, lined with epithelium (Figure 1D). The specimen also showed stromal hypercellularity, heterogeneous stromal overgrowth, marked cellular pleomorphism, and a high mitotic count of over 40 per 10 high-power-fields. Immunohistochemical (IHC) staining showed that the tumor cells were estrogen receptor-negative, negative for human epidermal growth factor receptor 2, weakly positive for progesterone receptor (< 5%), positive for Ki-67 (50%) and bcl-2, weakly positive for p63 (10%) and p53 (< 1%), and negative for cytokeratin, S-100, epithelial membrane antigen, and CD34; the specimen confirmed the diagnosis of malignant PT.
Figure 1. Malignant phyllodes tumor of the left breast at the time of initial diagnosis. (A) Craniocaudal view of the mammogram shows an indistinct high-density mass (arrow) in the left outer breast. (B) Ultrasound image shows an irregular hypoechoic mass (arrow) in the upper outer quadrant of the left breast. (C) Axial contrast-enhanced magnetic resonance image shows an irregular rim-enhancing mass (arrow) in the upper outer quadrant of the left breast. (D) Microscopically, the tumor shows a leaf-like pattern of cystic or cleft-like spaces lined with epithelium and abundant stromal cellularity (hematoxylin and eosin stain, ×100).
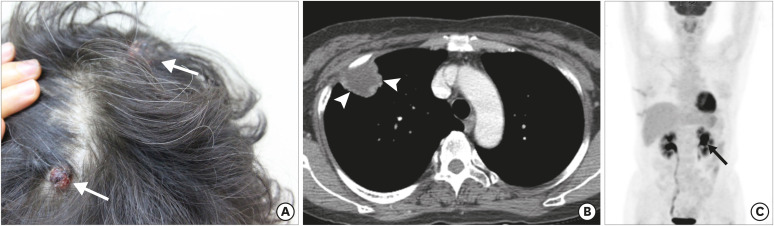
At 3 years post-surgery, the patient re-visited our hospital due to a palpable, intermittently painful mass in the scalp. On physical examination, 2 erythematous masses (maximum 1.5 cm) were detected in the right frontal and left parietal scalp (Figure 2A). Incidentally, a 3-cm mass was observed in the right upper lobe of the lung on chest computed tomography (CT) (Figure 2B). The patient underwent skin biopsy and video-assisted thoracic surgery for the lung mass to rule out primary lung malignancy or metastasis of malignant PT of the breast. The pathologic result was concurrent with metastatic malignant PT of the scalp and lung, with histological similarity and IHC correlation to the previously diagnosed malignant PT of the breast. There was no evidence of recurrence or contralateral metastasis in the breast. During the subsequent week, fluorine-18-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET-CT) was performed for systemic evaluation and an approximately 2.4 cm-hypermetabolic mass (SUVmax = 17.0) was detected in the left kidney (Figure 2C).
Figure 2. Metastases presenting as scalp masses 3 years post-surgery. (A) Two approximate 1.5 × 1.5-cm erythematous polypoid masses (arrows) in the right frontal and left parietal scalp are observed. (B) Contrast-enhanced chest CT image shows peripheral rim-enhancing mass (arrowheads) with a central necrotic portion in the apical segment of the right upper lobe. Video-assisted thoracic surgery for the lung mass revealed metastasis of malignant phyllodes tumor of the breast. 18F-FDG PET-CT was performed for systemic evaluation. (C) Maximum intensity projection reconstruction of a PET image shows additional avid FDG uptake in the left kidney (arrow); no detectable tumoral uptake in other organs is observed.
CT = computed tomography; 18F = fluorine-18; FDG = fluorodeoxyglucose; PET = positron emission tomography.
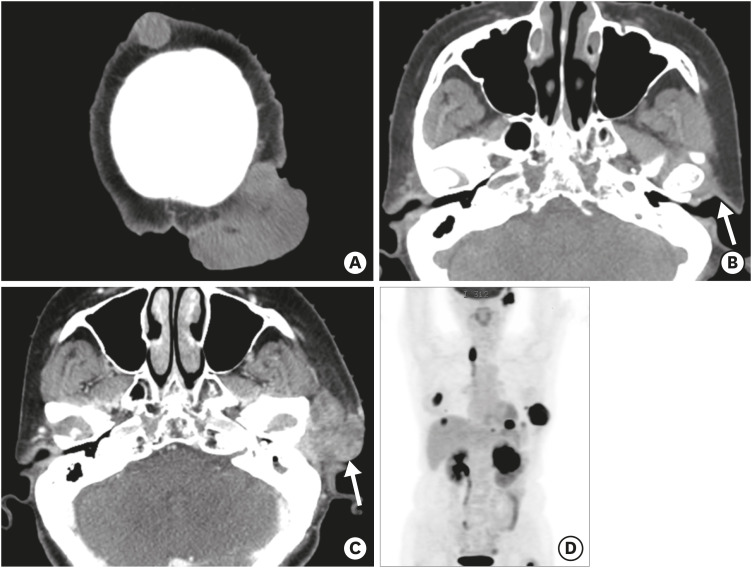
Four cycles of adriamycin (60 mg/m2) and cyclophosphamide (500 mg/m2) chemotherapy were administered, and the renal mass decreased in size. However, the scalp masses increased in size (maximum 5.6 cm, Figure 3A) without bony destruction. There was no other mass in the head and neck area on the initial CT for inclusion in the radiotherapy planning for the scalp mass (Figure 3B). After 10 rounds of radiotherapy (400 cGy), the masses were resected with a 1.5-cm safety margin. Microscopically, the specimen showed spindle-cell proliferation with abundant mitoses and nuclear pleomorphism involving the dermal and subcutaneous tissues, and the diagnosis of metastatic PT was rendered.
Figure 3. Widespread metastases of malignant phyllodes tumor. (A, B) A non-enhanced brain CT image for radiotherapy-planning shows increased size of the masses in the scalp. No abnormal mass lesion is observed in the left preauricular or parotid region (arrow in B). Eight months later, a rapid growing mass in the left preauricular area was noted. (C) Contrast-enhanced CT shows a heterogeneously enhancing mass (arrow in C) in the left parotid gland, with angioinvasion of the left external carotid artery and venous branches (not shown). (D) Maximum intensity projection reconstruction of a positron emission tomography image shows multifocal metastases in the left thoracic wall, left kidney, left lung and subphrenic space, left parotid gland, lymph nodes, and bones.
CT = computed tomography.
There was no evidence of recurrence during the first 5 months post-treatment; however, the patient complained of a new erythematous papule of the scalp, and the skin biopsy result was confirmed as recurrent metastatic PT. The patient refused additional chemotherapy. After 3 months, a rapidly growing, heterogeneously enhanced mass in the left parotid gland with angioinvasion of the left external carotid artery and venous branches with accompanying tumoral thrombi was noted on CT (Figure 3C). Additionally, PET-CT showed multifocal progression of metastases to the left thoracic wall, left kidney, left lung and subphrenic space, and skeletal system, including the left femur and ribs (Figure 3D). The patient underwent an additional 4 cycles of palliative systemic chemotherapy and radiotherapy for the parotid mass; however, the patient showed progressive disease and passed away 16 months after the initial diagnosis of distant metastases.
DISCUSSION
PT contains both epithelial and stromal components, and typically shows leaf-like structures characterized by cleft-like spaces lined with bland epithelium. PT is classified into benign, borderline, or malignant type based on an assessment of the tumor margins, mitotic activity, nuclear pleomorphism, and stromal overgrowth and cellularity. The majority (60%–75%) of PT are benign [3]. Stromal components are mainly related to metastasis or sarcomatous differentiation, while carcinomatous differentiation rarely arises from the epithelial component [8,9].
Local recurrence can occur in all categories of PT; however, inadequate resection margins are known to be significantly related to failure of local tumor control [10]. Therefore, the standard management of PT has traditionally included wide excision, generally with at least 10-mm margins. Meanwhile, the risk of metastasis is not commonly associated with surgical extent but with initial malignant potential of PT. Additionally, metastasis can appear without coexisting local recurrence [11,12]. Overall, the distant metastasis rate of PT is variable, ranging from 1.7%–27%, while the average rate of metastasis of benign PT is only 0.4% [6]. Chen et al. [13] reported that stromal cellularity, stromal overgrowth, stromal atypia, mitotic activity, tumor margin, and heterologous stromal elements were significantly correlated with metastasis of PT. In this case, the large size of the initial tumor, high stromal cellularity, severe nuclear pleomorphism, and mitotic count over 40 per 10 high-power-fields could have led to distant metastasis and a poor patient prognosis.
Metastasis occurs mostly through the hematogenous rather than lymphogenous route. The lung and skeleton are reported to be the 2 most common sites of distant metastasis of PT, but almost all other organs can be affected [6]. There are few reported cases of cutaneous and soft tissue metastases of PT because most subtypes of breast cancer with cutaneous metastases have been epithelial tumors, while the stromal component plays a significant role in metastasis of PT [5]. Additionally, cases of scalp metastasis without involvement of other proximal soft tissues or organs, which have been proven by surgical excision, are rarely reported. Ganesh et al. [14] reported a series of cases of metastatic PT, and one of their patients showed extensive brain and scalp metastases after lung metastases. However, our patient showed lung and scalp metastases without simultaneous brain parenchymal metastasis or local tumor recurrence. Additionally, unusual late onset left parotid gland metastasis with accompanying tumoral thrombi in the extracranial vessels was observed.
Wide local excision is considered as the standard initial treatment for PT, including malignant PT. Routine axillary lymph node dissection is not recommended, and the role of adjuvant radiotherapy or chemotherapy for the treatment of PT remains uncertain [13]. Once metastasis develops, prognosis is poor. For treatment of metastatic PT, multimodal therapy has been used. Standard chemotherapy has a limited role and is administered for palliation [15]. Various chemotherapeutic agents, such as ifosfamide, cyclophosphamide, cisplatin, doxorubicin, or etoposide, have been used for systemic therapy based on management of a soft tissue sarcoma [14,15]. In this case, both excision and radiotherapy after systemic chemotherapy showed poor local tumor control of scalp metastasis, and metastases in multiple organs developed despite the chemotherapy.
In summary, we report a case of distant scalp metastasis originating from primary malignant PT of the breast in the early stages of metastasis. Although the occurrence of distant metastasis in PT is rare and cutaneous metastasis is infrequent, it could be a differential diagnosis in the evaluation of a skin lesion in a patient with underlying malignant PT.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Lee HJ, Lim HS, Park MH.
- Data curation: Lee HJ, Lim HS, Lee JS.
- Formal analysis: Lee HJ.
- Investigation: Lee HJ, Lim HS.
- Project administration: Lee HJ.
- Resources: Lim HS.
- Software: Lee HJ, Lim HS.
- Supervision: Lim HS, Ki SY, Lee JE, Park MH.
- Validation: Lee JS.
- Writing - original draft: Lee HJ.
- Writing - review & editing: Lim HS, Ki SY, Lee JE, Lee JS.
References
- 1.Telli ML, Horst KC, Guardino AE, Dirbas FM, Carlson RW. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw. 2007;5:324–330. doi: 10.6004/jnccn.2007.0027. [DOI] [PubMed] [Google Scholar]
- 2.Lindquist KD, van Heerden JA, Weiland LH, Martin JK., Jr Recurrent and metastatic cystosarcoma phyllodes. Am J Surg. 1982;144:341–343. doi: 10.1016/0002-9610(82)90016-2. [DOI] [PubMed] [Google Scholar]
- 3.Tan PH, Tse GM, Lee A, Simpson JF, Hanby AM. Fibroepithelial tumours. In: Lakhani SR, Ellis IO, Schnitee SJ, Tan PH, van de Vijver MJ, editors. WHO Classification of Tumors of the Breast. 4th ed. Lyon: IARC Press; 2012. pp. 142–147. [Google Scholar]
- 4.Chaney AW, Pollack A, McNeese MD, Zagars GK, Pisters PW, Pollock RE, et al. Primary treatment of cystosarcoma phyllodes of the breast. Cancer. 2000;89:1502–1511. doi: 10.1002/1097-0142(20001001)89:7<1502::aid-cncr13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Swapp RE, Shon W, Peethambaram PP, Moran SL, Reynolds C. Cutaneous presentation of a distant metastasis of malignant phyllodes tumor. Int J Dermatol. 2012;51:72–74. doi: 10.1111/j.1365-4632.2011.04923.x. [DOI] [PubMed] [Google Scholar]
- 6.Wei J, Tan YT, Cai YC, Yuan ZY, Yang D, Wang SS, et al. Predictive factors for the local recurrence and distant metastasis of phyllodes tumors of the breast: a retrospective analysis of 192 cases at a single center. Chin J Cancer. 2014;33:492–500. doi: 10.5732/cjc.014.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulman JM, Pauli ML, Neuhaus IM, Sanchez Rodriguez R, Taravati K, Shin US, et al. The distribution of cutaneous metastases correlates with local immunologic milieu. J Am Acad Dermatol. 2016;74:470–476. doi: 10.1016/j.jaad.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleer CG, Giordano TJ, Braun T, Oberman HA. Pathologic, immunohistochemical, and molecular features of benign and malignant phyllodes tumors of the breast. Mod Pathol. 2001;14:185–190. doi: 10.1038/modpathol.3880282. [DOI] [PubMed] [Google Scholar]
- 9.Yabanoglu H, Colakoglu T, Aytac H, Parlakgumus A, Bolat F, Pourbagher A, et al. Comparison of predictive factors for the diagnosis and clinical course of phyllodes tumours of the breast. Acta Chir Belg. 2015;115:27–32. [PubMed] [Google Scholar]
- 10.Pietruszka M, Barnes L. Cystosarcoma phyllodes: a clinicopathologic analysis of 42 cases. Cancer. 1978;41:1974–1983. doi: 10.1002/1097-0142(197805)41:5<1974::aid-cncr2820410543>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Grimes MM. Cystosarcoma phyllodes of the breast: histologic features, flow cytometric analysis, and clinical correlations. Mod Pathol. 1992;5:232–239. [PubMed] [Google Scholar]
- 12.Mishra SP, Tiwary SK, Mishra M, Khanna AK. Phyllodes tumor of breast: a review article. ISRN Surg. 2013;2013:361469. doi: 10.1155/2013/361469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WH, Cheng SP, Tzen CY, Yang TL, Jeng KS, Liu CL, et al. Surgical treatment of phyllodes tumors of the breast: retrospective review of 172 cases. J Surg Oncol. 2005;91:185–194. doi: 10.1002/jso.20334. [DOI] [PubMed] [Google Scholar]
- 14.Ganesh V, Lee J, Wan BA, Rakovitch E, Vesprini D, Slodkowska E, et al. Palliative treatment of metastatic phyllodes tumors: a case series. AME Case Rep. 2017;1:9. doi: 10.21037/acr.2017.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramakant P, Selvamani, Therese MM, Paul MJ. Metastatic malignant phyllodes tumor of the breast: an aggressive disease-analysis of 7 cases. Indian J Surg Oncol. 2015;6:363–369. doi: 10.1007/s13193-015-0397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]