Abstract
Bdelloid rotifers are a group of microscopic invertebrates known for their obligate parthenogenesis and exceptional resistance to extreme environments. Their diversity and distributions are poorly studied in Asia, especially in China. In order to better understand the species distribution and diversity of bdelloid rotifers in China, a scientific surveys of habitats was conducted with 61 samples (both terrestrial and aquatic habitats) from 11 provinces and regions of China, ranging from tropics to subtropics with a specific focus on poorly sampled areas (Oriental) during September 2017 to October 2018. A total of 59 morphospecies (including subspecies) were found, of which, thirty-nine morphospecies (including one genus) are new records for China, almost doubling the number of previous records. Four rare morphospecies (Adineta cf. acuticornis Haigh, A. beysunae Örstan, Habrotrocha ligula loxoglotta De Koning and H. serpens Donner) are depicted and redescribed, and an updated checklist of Chinese bdelloids with their location and ecological information is presented. This study provides new data from a large region of China, enriching the knowledge of bdelloid biodiversity, and their global biogeography.
Keywords: bdelloids, biogeography, morphospecies, Oriental region, taxonomy
Introduction
Bdelloid rotifers are microscopic invertebrates that constitute a subclass Bdelloidea of the phylum Rotifera, known for their peculiar obligate parthenogenesis (Welch and Meselson 2000; Welch et al. 2004) and outstanding ability to withstand harsh periods through anhydrobiosis (Ricci 1998; Gladyshev and Meselson 2008). The minute size of bdelloids (from less than 160 to 500–600 µm) allows their long-distance dispersal by wind, water, and animals to access to almost all possible habitats (Bohonak and Jenkins 2003; Fenchel 2004; Kellogg and Griffin 2006). They inhabit both aquatic (mainly freshwater lakes, ponds, and streams) and terrestrial habitats (e.g., mosses, lichens, tree barks, soil and litter) (Donner 1965). Rarely, bdelloids are found in marine and brackish waters (Fontaneto et al. 2006; Demirkalp et al. 2010; Song 2014).
Analysis of Bdelloidea taxonomy characteristics is problematic because only observation of living and active specimens allows appropriate identification of species. That is why it has not been widely carried out. Furthermore, there are no readily available reagents that can be used to anesthetize them and preserve their bodies fully extended (Örstan and Plewka 2017). Untill recently, only about 460 bdelloid species have been described worldwidely (Segers 2007), but there is ample evidence that the total number of bdelloid species is at least several times greater than the current one (Fontaneto et al. 2011; Robeson et al. 2011). In addition, the intensity of taxonomic researches on bdelloid species in different regions of the world was extremely uneven, thus the species diversity varies greatly from region to region. For instance, over 300 species are known from Europe (Fontaneto et al. 2007), while only about 50 species are found in the Oriental region (Segers 2007).
In China, only 48 bdelloid morphospecies have been reported (Zhuge et al. 1998; Koste and Zhuge 1998; Yin and Xu 2016) (Table 1). The first study on the Chinese bdelloid rotifers was reported by Thorpe (1893), who found four species of Rotaria in Yangtze River area in Wuhu city, Anhui Province. After that, few fragmental reports from fresh waters and terrestrial environments in a large region of China were presented (Daday 1906; Stewart 1908; Gee 1927; Wang 1961; Bartoš 1963; Wang 1974; Gong 1983; Koste and Zhuge 1996, 1998; Zhuge et al. 1998; Yin and Xu 2016). Up to now, this taxon has not been actively studied in China comparing to Europe or even to Antarctica (Segers 2007), and the biogeography of bdelloids in South Asia is unclear, and their habitat preferences are incomplete. This study aimed to conduct a taxonomic work and evaluate the diversity of bdelloid rotifers in China, especially the poorly investigated tropical zones of the Oriental biogeographic region.
Table 1.
Checklist of bdelloid rotifers recorded from China before 2015.
| Species | Habitats | EL (m) | WT (°C) | AT (°C) | pH | Distribution and references |
|---|---|---|---|---|---|---|
| Adineta gracilis Janson, 1893 | Moss | 800–1400 | – | – | – | GD (l) |
| A. oculata (Milne, 1886) | Moss | 800–1800 | – | – | – | GD (l) |
| A. vaga (Davis, 1873) | Moss and stream | 0–1750 m | 16–18 | 26–28 | 5 | GD (f, l), TB (g, h) |
| Dissotrocha aculeata (Ehrenberg, 1832) | Pond, river and bog | 0–3650 | 20 | 20 | 6 | IM (b), HB, SD, ZJ, SC, XJ (e) TB (h), GD (l) |
| D. macrostyla (Ehrenberg, 1832) | Pond and bog | 0–3030 | 17–20 | 13.5 | 6 | JS (d), TB (h), HA (i) |
| D. macrostyla tuberculata (Gosse, 1886) | Puddle on the roadside | – | 20 | – | 7.6 | HA(k) |
| Habrotrocha angusticollis angusticollis (Murray, 1905) | Sphagnum, river, lake branch channel and puddle with aquatic plant | 0–4750 | 14–30 | 21–25.5 | 6–8.5 | ZJ (e), TB (h), HA (i, k), GD (l) |
| H. angusticollis attenuata (Murray, 1906) | Moss | – | – | – | – | GD (f) |
| H. ampulla (Murray, 1911) | River with macrophyte | – | 20 | – | 6.32 | HA (i) |
| H. collaris (Ehrenberg, 1832) | Bog, stream, lake and moss | 800–3800 | 12–19.5 | 15–25 | 6–7 | TB (h), GD (l) |
| H. constricta (Dujardin, 1841) | – | – | – | – | – | HA (j) |
| H. elegans (Milne, 1886) | Lake | 3658 | 13 | 15 | 7 | TB (h) |
| *H. flexicollis Bartoš, 1963 | Moss | – | – | – | – | GD (f) |
| H. fusca (Bryce, 1894) | Moss | – | – | – | – | GD (f) |
| H. insignis Bryce, 1915 | Moss | – | – | – | – | GD (f) |
| H. modesta Bartoš, 1963 | Moss | – | – | – | – | GD (f) |
| H. munda Bryce,1913 | Bog | 4200 | 16.5 | 13.5 | 6 | TB (h) |
| H. perforata (Murray, 1906) | Moss | – | – | – | – | GD (f) |
| H. pulchra (Murray, 1905) | Spring with attachment from meadow, stone and soil, puddle from glacier | 5700 | 17 | 11 | 8 | TB (g, h) |
| H. pusilla (Bryce, 1893) | Puddle from spring and wet moss on stone | 830–2400 | 30 | 25 | 6 | TB (h) |
| H. thienemanni Hauer, 1924 | Puddle with aquatic plant and moss, glacier | 830–5550 | 13–30 | 15–25 | 5–7 | TB (g, h) |
| H. tridens (Milne, 1886) | Moss | 600–1900 | – | – | – | GD (l) |
| Otostephanos cf. donneri (Bartoš, 1959) | Aquatic ecosystem | – | – | – | – | YN (j) |
| Macrotrachela bullata (Murray, 1906) | Stream with algae or moss | 1668–4150 | 10–18 | 19–28 | 5–6 | TB (g, h) |
| M. ehrenbergii (Janson, 1893) | Moss | 4500 | – | – | – | TB (h) |
| M. insolita De Koning, 1947 | Moss | 1000–1200 | – | – | – | GD (l) |
| M. multispinosa Thompson, 1892 | Attachments on aquatic plants, bogs and moss from grass lands | 3300 | 16 | 17 | 6 | TB (h) |
| M. musculosa (Miline, 1886) | Springs and wet moss | 4150–4500 | 6 | 11–19 | 6 | TB (h) |
| M. plicata (Bryce, 1892) | Puddles | 4400–4500 | 12–14 | 10–14 | 7 | TB (h) |
| M. papillosa Thompson, 1892 | Moss | – | – | – | – | GD (f) |
| M. punctata (Murray, 1911) | Attachment from stone and wet grass | 3800–3850 | 12 | 19 | 7 | TB (h) |
| M. quadrlcornlfera Milne, 1886 | Moss | 0–1900 | – | – | – | GD (l) |
| Mniobia tentans Donner, 1949 | Stream with algae or moss | 1668–1750 | 16–18 | 25–28 | 5 | TB (g, h) |
| Philodina citrina Ehrenberg, 1832 | Rice field, puddle, shallow and wet moss | 600–4350 | 12–27 | 10–28 | 6–7 | TB(c, h), GD (l) |
| P. erythrophthalma Ehrenberg, 1830 | Pond, pool and stream with algae | 0–3370 | 9 | 12 | 7 | HB (e), TB (c, h) |
| P. megalotrocha Ehrenberg, 1832 | Lake with macrophyte, pond, water reservoir and rice field | – | 20–26 | – | 6–8 | HB, SH, JS, ZJ (e) HA(i, k) |
| P. nemoralis Bryce, 1903 | Rice field, bog and moss | 2000–2400 | 36 | 29 | 5 | TB (h) |
| P. roseola Ehrenberg, 1832 | River, pond, marsh and moss | 0–3100 | – | – | – | IM (b) TB(c), HB, SH, JS, ZJ, HA (e) GD (l) |
| P. vorax (Janson, 1893) | Stream, spring and puddle from river or glacier | 2400–5500 | 7–17 | – | 6–8 | TB (g, h) |
| *Pleuretra similis Bartoš, 1963 | Moss | – | – | – | – | GD (f) |
| Rotaria citrina (Ehrenberg, 1838) | Rice field and pool | 0–2400 | 13–28 | – | 6 | HB (e), TB (h) |
| R. macroceros (Gosse, 1851) | Yangtze River, lake and moss | – | 25 | – | 6 | AH (a), HB (e), GD (l), HA(i, k) |
| R. macrura (Ehrenberg, 1832) | River | – | – | – | – | IM (b) |
| R. neptunia (Ehrenberg, 1830) | Pond, rice field and puddle | 0–3650 | 18–26 | – | 6–8.7 | AH (a), SH, JS, ZJ, HB, BJ, HL, LN, GS, HN, GD, GX, YN, SC (e), HA (i, k), TB (h) |
| R. rotatoria (Pallas, 1766) | Pond and rice field | 0–830 | 20–26 | – | 6–8.7 | AH (a), IM (b), SH, HB (e), TB (h), HA (i, k) |
| R. sordida (Western, 1893) | Moss; polluted lake | – | 21 | – | 7.1 | GD (f), HA(i, k) |
| R. tardigrada (Ehrenberg,1830) | Lake, polluted river and puddle | 0–3658 | 18–25 | – | 6–7 | AH (a), HA (i, k), HL, SH, GS, JS (e) TB (h) |
| R. tridens (Montet, 1915) | Bog, wet moss pool and attachment from stone | 2900–4550 | – | 15–18 | 6 | TB (c, h) |
Sources: (a) (Thorpe 1893); (b) (Daday 1906); (c) (Stewart 1908); (d) (Gee 1927); (e) (Wang 1961); (f) (Bartoš 1963); (g) (Wang 1974); (h) (Gong 1983); (i) (Koste and Zhuge 1996); (j) (Zhuge 1997); (k) (Koste and Zhuge 1998); (l) (Yin and Xu 2016). ‘cf.’ is retained for those taxa which have some differences from the nominate morphospecies, requiring further study. *: China only. Abbreviation: AH: Anhui; AT: air temperature; BJ: Beijing; EL: elevation; GD: Guangdong; GS: Gansu; GX: Guangxi; HA: Hainan; HB: Hubei; HL: Heilongjiang; HN: Hunan; IN: Inner Mongolia; JS: Jiangsu; LN: Liaoning; SD: Shandong; SC: Sichuan; SH: Shanghai; TB: Tibet; WT: water temperature; XJ: Xinjiang; YN: Yunnan; ZJ: Zhejiang.
Materials and methods
Sampling area, collection procedures and sample processing
A total of 61 samples was collected during the period from September 2017 to October 2018 in 11 provinces and regions of China across its subtropical and tropical zones at altitudes from 0–2850 m above sea level from four types of terrestrial habitat (soil, mosses, leaf litter and lichens) and four types of aquatic habitat (plankton, benthos, periphyton and dew) in fresh or brackish waters (Fig. 1, Table 2). Of these samples, eleven were collected from fresh water, six from brackish water, one from dew on leaves, thirty from mosses, ten from leaf litter, two from lichens, and one from soil with mosses.
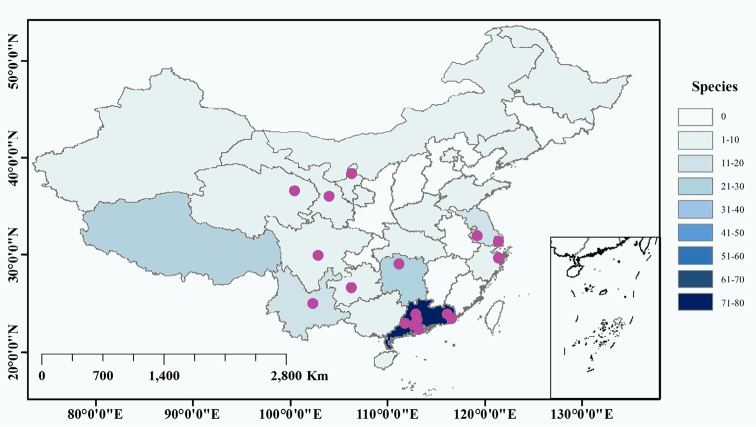
Figure 1.
Locations of the sampling sites in this study (purple circles) and species richness of bdelloid rotifers (blue) recorded between 1908 and 2018 in China.
Table 2.
Sampling locality information of this survey.
| Locality codes | Locality | Sampling date | Habitat | GPS coordinates | Elevation (m) |
|---|---|---|---|---|---|
| GD1 | Chaozhou | 18.08.2017 | Moss on concrete | 23°58'15.13"N, 116°38'12.14"E | 1136 |
| GD2 | Chaozhou | 18.08.2017 | Moss on bark | 23°58'14.93"N, 116°38'12.08E | 1139 |
| GD3 | Chaozhou | 18.08.2017 | Moss on rock | 23°58'14.99"N, 116°38'12.11"E | 1138 |
| GD4 | Chaozhou | 18.08.2017 | Moss on soil | 23°58'15.02"N, 116°38'12.09"E | 1138 |
| GD5 | Chaozhou | 18.08.2017 | Moss on rock | 23°55'59.37"N, 116°36'59.84"E | 436 |
| GD6 | Guangzhou | 05.11.2017 | Dry moss on bark | 23°06'35.11"N, 113°14'21.20"E | 10 |
| GD7 | Guangzhou | 20.09.2017 | Lotus pond | 23°07'54.80"N, 113°20'39.44"E | 16 |
| GD8 | Guangzhou | 05.11.2017 | Lotus pond | 23°07'54.80"N, 113°20'39.44"E | 16 |
| GD9 | Guangzhou | 25.10.2018 | Lotus pond | 23°07'54.80"N, 113°20'39.44"E | 16 |
| GD10 | Guangzhou | 11.06.2018 | Moss on concrete | 23°08'1.29"N, 113°20'38.81"E | 15 |
| GD11 | Guangzhou | 11.06.2018 | Soil | 23°07'51.89"N, 113°20'37.45"E | 18 |
| GD12 | Haiou island | 28.10.2017 | Water hyacinth root in brackish water | 22°58'23.36"N, 113°30'40.95"E | 4 |
| GD13 | Guangzhou | 13.06.2018 | Bamboo leaf litter | 23°18'4.12"N, 113°26'23.21"E | 214 |
| GD14 | Guangzhou | 13.06.2018 | Bamboo leaf litter | 23°18'18.99"N, 113°26'56.14"E | 152 |
| GD15 | Guangzhou | 20.06.2017 | Bottom of lotic water | 23°18'0.59"N, 113°26'27.47"E | 226 |
| GD16 | Guangzhou | 26.10.2018 | Urban river | 23°03'29.0"N, 113°24' 26.6"E | 0.75 |
| GD17 | Nanao island | 22.04.2018 | Puddle | 23°25'44.88"N, 117°01'49.56"E | 108 |
| GD18 | Nanao island | 09.01.2018 | Gracilaria lichenoides in brackish pond | 23°27‘18.13“N, 117°7'31.35"E | 170 |
| GD19 | Nanao island | 22.04.2018 | Lotic water | 23°26'38.29"N, 117°05'22.94"E | 124 |
| GD20 | Qingyuan | 12.05.2018 | Moss on concrete | 24°36'46.73"N, 112°35'57.02"E | 237 |
| GD21 | Qingyuan | 12.05.2018 | Moss on concrete | 24°36'40.72"N, 112°35'50.11"E | 143 |
| GD22 | Qingyuan | 12.05.2018 | Moss on soil | 24°36'40.44"N, 112°36'9.26"E | 142 |
| GD23 | Qingyuan | 12.05.2018 | Moss on bark | 24°36'41.29"N, 112°36'9.26"E | 175 |
| GD24 | Qiao island | 29.10.2017 | Bottom of brackish pool in mangrove | 23°27'32.41"N, 117°06'3.59"E | 53 |
| GD25 | Nanao island | 18.11.2018 | Leaf litter | 22°25'42.45"N, 113°37'51.53"E | 137 |
| GD26 | Nanao island | 18.11.2018 | Leaf litter | 23°27‘18.13“N, 117°7'31.35"E | 9 |
| GS1 | Lanzhou | 07.06.2018 | Wet moss near pond | 36°08'25.56"N, 103°41'41.18"E | 1615 |
| GZ1 | Guiyang | 24.08.2017 | Moss on rock | 26°36'1.75"N, 106°41'10.39"E | 1213 |
| GZ2 | Guiyang | 24.08.2017 | Moss on rock | 26°05'52.74"N, 105°52'55.89"E | 1170 |
| HN1 | Changde | 20.06.2017 | Moss on rock | 29°3'10.0"N, 111°40'13"E | 31 |
| HN2 | Changde | 15.09.2017 | Moss on rock | 29°3'10.0"N, 111°40'13"E | 31 |
| HN3 | Changde | 11.12.2017 | Moss on rock | 29°3'10.0"N, 111°40'13"E | 31 |
| HN4 | Changde | 12.03.2018 | Moss on rock | 29°3'10.0"N, 111°40'13"E | 31 |
| HN5 | Changde | 11.12.2017 | Aquatic plant | 29°02'23.49"N, 111°42'33.35"E | 35 |
| HN6 | Changde | 12.12.2017 | Lemna minor in river | 29°7'20.0"N, 111°39'49"E | 57 |
| HN7 | Changde | 15.09.2017 | Water sample from a pond | 29°3'10"N, 111°40'13.0"E | 30 |
| HN8 | Changde | 12.03.2018 | Moss on soil | 29°03'13.78"N, 111°40'12.69"E | 31 |
| HN9 | Changde | 11.12.2017 | Lotus pond | 29°03'3.68"N, 111°39'57.93"E | 35 |
| JS1 | Nanjing | 15.08.2018 | Bamboo leaf litter | 32°3'28.63"N, 118°45'27.47"E | 39 |
| JS2 | Nanjing | 15.08.2018 | Moss with leaf litter | 32°3'28.19"N, 118°45'24.52"E | 34 |
| NX1 | Yinchuan | 02.07.2018 | Moss from dessert (32 °C of soil surface) | 38°33'45.38"N, 106°32'0.37"E | 1128 |
| NX2 | Yinchuan | 01.07.2018 | Extremely dry Juniperus litter | 38°29'22"N, 106°12'1"E | 1109 |
| QH1 | Qinghai lake | 09.06.2018 | Wet moss on bark | 36°47'46.11"N, 101°06'18.99"E | 2850 |
| SC1 | Wawu mountain | 23.08.2017 | Wet moss on bark | 29°40'15.26"N, 102°56'53.92"E | 2105 |
| SC2 | Wawu mountain | 23.08.2017 | Wet moss on bark | 29°40'10.36"N, 102°56'53.92"E | 2105 |
| SC3 | Wawu mountain | 23.08.2017 | Wet moss on bark | 29°40'10.36"N, 102°56'53.92"E | 2100 |
| SH1 | Chongming island | 29.12.2017 | Aquatic plants in brackish water | 31°31'9.5"N, 121°56'4.3"E | 3 |
| SH2 | Chongming island | 29.12.2017 | Moss on soil in brackish marsh | 31°29'54"N, 121°55'20.7"E | 3 |
| SH3 | Chongming island | 29.12.2017 | Reed root in brackish water | 31°30'43.9"N, 121°57'27.9"E | 3 |
| SH4 | Chongming island | 29.12.2017 | Aquatic plants in brackish water | 31°31'2.9"N, 121°55'3.1"E | 2 |
| YN1 | Kunming | 01.06.2018 | Moss on concrete | 25°3'20.5"N, 102°42'8.6"E | 1889 |
| YN2 | Kunming | 01.06.2018 | Moss on soil | 25°3‘2.2"N, 102°42'5.1"E | 1908 |
| YN3 | Kunming | 01.06.2018 | Moss on concrete | 25°3‘6.1"N, 102°42'5.41"E | 1900 |
| YN4 | Kunming | 01.06.2018 | Lichens on bark | 25°8'0.2"N, 102°39'40.6"E | 1900 |
| YN5 | Kunming | 01.06.2018 | Moss on rock | 24°57'59.4"N, 102°39'35"E | 1888 |
| YN6 | Kunming | 11.10.2018 | Lichens on rock | 24°57'49.1"N, 102°37'44.6"E | 2150 |
| YN7 | Kunming | 11.10.2018 | Leaf litter | 24°57'53.2"N, 102°37'44.6"E | 2143 |
| YN8 | Kunming | 11.10.2018 | Dew on leaves | 24°57'55.5"N, 102°37'44.3"E | 2136 |
| ZJ1 | Hangzhou | 19.11.2017 | Dry moss on Torreya grandis’ bark | 30°21'42.0"N, 119°34'28"E | 305 |
| ZJ2 | Ningbo | 03.11.2018 | Bamboo leaf litter, | 29°52'40.3"N, 121°33'15.55"E | 37 |
| ZJ3 | Zhoushan | 03.11.2018 | Leaf litter | – | – |
GPS coordinates based on WGS84 system. Abbreviation: GD: Guangdong; GS: Gansu; GZ: Guizhou; HN: Hunan; JS: Jiangsu; NX: Ningxia; QH: Qinghai; SC: Sichuan; SH: Shanghai; XJ: Xinjiang; YN: Yunnan; ZJ: Zhejiang.
Based on the definition of boundary between the Palearctic and Oriental biogeographic regions in China (Norton et al. 2010), fifty-seven samples were collected in the Oriental region, while four samples (NX1, NX2, GS1, QH1) were collected in the Palearctic region (Table 2). According to the Geodetector model to partition subtropical and tropical zone in China (Dong 2017), forty-two samples were collected in the subtropical zone, while nineteen samples (GD1–19) were collected in the tropical zone (Table 2).
Samples from terrestrial habitats were placed into firmly closed paper envelopes, then dried at room temperature and stored in the envelopes for several weeks or months. Planktonic samples were obtained by filtering 1 to 5 liters of water through a plankton net with a mesh size 30 µm. Benthic ones were collected by scraping the bottom of water bodies with a 500 ml plastic bottle. Periphytic rotifers were obtained by shaking or scraping aquatic plants, then preserved in plastic bottles.
Samples from aquatic habitats were concentrated by a nylon net of 30 μm mesh size, then examined in lab immediately without fixating or anesthetization. Rotifers from mosses, lichens and leaf litter were extracted by washing the substrate with distilled water following the method of Peters (1993). Soil rotifers were extracted by the method of wet-sieving and centrifugation in a sugar gradient (Freckman 1993).
Light microscopy procedures
Rotifers isolated from waters were transferred into a Petri dish and sorted under a dark field dissecting microscope (SZX10, Olympus, Japan) with a magnification of 64×. Selected specimens were placed onto glass slides by using micropipettes, then examined alive under a microscope (BX51, Olympus, Japan) with magnification of ×200–400. All living specimens were recorded and photographed using a digital camera (Truechrome Metrics, China) with the software of TCapture. Photos and digital screenshots from videos were used for species identification and illustrations.
Species identification
Species were identified by both external morphology and anatomy using the keys of Donner (1965) and the original descriptions and redescriptions of specific species (Murray 1906; Song and Kim 2000; Yakovenko 2000a, 2000b; Kutikova 2005; Bielańska-Grajner 2013; Song 2014, 2015; Song and Min 2015; Song and Lee 2017). Drawings of some rare morphospecies were made with Adobe Illustrator CC 2018 and Photoshop CC 2017.
All rotifers were measured from screenshots of digital videos after Iakovenko et al. (2013, 2015) and Örstan (2018). Total length (TL) in the case of adinetid rotifers is the distance between the middle of the anterior rim of the head excluding rostrum, and the posterior rim of the spur pseudosegment; head length (HL) is the distance between the anterior edge of the head (posterior to the rostrum) and the anterior rim of the antennal pseudosegment, i.e., TL and HL do not include the rostrum, because it was usually bent under the head (Iakovenko et al. 2015). The head length in A. beysunae is the distance between the anterior edge of the head and an imaginary line passing through the innermost denticles of the rakes to better compare it with the original description (Örstan 2018). The number of denticles on each rake is represented formulaically using an ‘en dash’ (Örstan 2018). We counted the distal foot with the toes as a pseudosegment separate from the one carrying the spurs as Bryce (1894), Donner (1965) and Iakovenko et al. (2013, 2015) did.
Abbreviations
BW body width (when creeping)
CW corona width
FL foot length
FW foot width
HL head length
HW head width
MinNW minimal neck width
MxNW maximal neck width
NL neck length
TL total length
TrL trunk and rump length
RaL ramus length
RkW rake width
RL rump length
RW rump width
SL spur length
SSW spur pseudosegment width
TrW trophi width
Results
Species diversity
Fifty-nine morphospecies (including three subspecies) were identified in this survey (Table 3), and the bdelloids that were unidentifiable to the species level were not included in the list. Of them, thirty-nine taxa (including one genus) are new records for China, and thirty-eight species are new records for the Oriental region. The species list of Chinese bdelloid fauna has been increased from 48 to 87. Detailed information about their distribution and ecological information is reported in Tables 2, 3.
Table 3.
Bdelloid rotifers found in this study with their updated biogeographic distribution after Segers (2007).
| Species | Locality codes | Biogeographic regions |
|---|---|---|
| *Adineta cf. acuticornis Haigh, 1967 | GD6, YN5–6, SC2 | AUS, ORI# |
| *A. barbata Janson, 1893 | GD10, 14, JS1, ZJ2 | AFR, ANT, AUS, NEA, NEO, PAL, ORI# |
| *A. bartosi Wulfert, 1960 | GZ2 | PAL, ORI# |
| *A. beysunae Örstan, 2018 | GD13–14, 25–26, YN7–8, JS1 | NEA, ORI# |
| *A. cuneata Milne, 1916 | GD1–2, SC2, JS2, YN6–7 | AFR, AUS, NEA, PAL, ORI# |
| A. gracilis Janson, 1893 | HN2, QH1, JS1 | AFR, ANT, AUS, NEA, ORI, PAL |
| A. oculata (Milne, 1886) | GD7, YN3, HN1 | NEO, PAL, ORI# |
| *A. ricciae Segers & Shiel, 2005 | GD23, HN4 | AUS, ORI# |
| *A. steineri Bartoš, 1951 | GD13 | ANT, AUS, NEA, NEO, PAL, ORI# |
| A. vaga (Davis, 1873) | GZ2, HN2–4, ZJ2, YN1,7, GD5,7,13–14, 20–21,23 | AFR, ANT, AUS, NEA, NEO, ORI, PAL |
| Dissotrocha macrostyla (Ehrenberg, 1838) | HN6 | AFR, AUS, NEA, NEO, ORI, PAL |
| *Habrotrocha bidens (Gosse, 1851) | ZJ1 | AFR, AUS, NEA, NEO, ORI, PAL |
| *H. cf. spicula Bryce, 1913 | GD2 | AFR, AUS, ORI, PAL |
| H. constricta (Dujardin, 1841) | HN2 | AFR, ANT, AUS, NEA, NEO, PAC, PAL, ORI# |
| H. insignis Bryce, 1915 | GD3 | AUS, PAL, ORI# |
| *H. ligula loxoglotta De Koning, 1947 | YN5 | PAL, ORI# |
| *H. rosa Donner, 1949 | GD25 | AFR, AUS, NEA, NEO, PAL, ORI# |
| *H. serpens Donner, 1949 | GD6 | AFR, AUS, PAL, ORI# |
| *Otostephanos regalis Milne, 1916 | GD13 | AFR, PAL, ORI# |
| *Scepanotrocha semitecta Donner, 1951 | SC1 | NEO, PAL, ORI# |
| Macrotrachela bullata (Murray, 1906) | GD3–4, GZ2 | AFR, ORI, PAL |
| M. ehrenbergii (Janson, 1893) | HN7, GZ1 | AFR, AUS, NEA, NEO, ORI, PAC, PAL |
| *M. habita (Bryce, 1894) | GD6, 11,20, 22–23, YN1–3, GZ1 | AFR, ANT, AUS, NEA, NEO, ORI, PAL |
| *M. hewitti (Murray, 1911) | SH1 | AFR, PAL, ORI# |
| *M. inermis Donner, 1965 | YN4 | PAL, ORI# |
| M. insolita De Koning, 1947 | GD2, HN8 | ANT, AUS, NEA, NEO, PAL, ORI# |
| *M. latior Doner, 1951 | YN7 | PAL, ORI# |
| *M. libera Donner, 1949 | HN4 | PAL, ORI# |
| M. multispinosa multispinosa Thompson, 1892 | GD6 | AFR, AUS, NEA, NEO, ORI, PAL |
| *M. multispinosa brevispinosa (Murray, 1908) | YN5 | AFR, AUS, NEO, ORI, PAL |
| *M. nana (Bryce, 1912) | QH1 | AFR, AUS, NEA, NEO, PAL |
| M. plicata (Bryce, 1892) | SC2–3 | AFR, AUS, NEA, PAL, ORI# |
| *M. quadricornifera quadricorniferoides De Koning, 1929 | JS1, 2 | AFR, ANT, NEO, ORI, PAL |
| *M. quadricornifera scutellata Schulte, 1954 | GD13 | AUS, PAL, ORI# |
| *M. timida Milne, 1916 | SC1–3, YN7 | AFR, AUS, PAL, ORI# |
| *Philodina acuticornis Murray, 1902 | GD20–21, JS1, ZJ2 | AFR, AUS, NEA, NEO, PAL, ORI# |
| *P. cf. indica Murray, 1906 | YN4 | NEA, PAL, ORI# |
| *P. cf. proterva Milne, 1916 | GD5, YN1, 6, ZJ2 | AFR, AUS, NEA, PAL, ORI# |
| *P. childi Milne, 1916 | GD14, YN7 | PAL, ORI# |
| *P. duplicalcar (De Koning, 1947) | NX2 | PAL |
| P. megalotrocha Ehrenberg, 1832 | HN5–6, 9, GD9, 12 | AFR, AUS, NEA, NEO, ORI, PAL |
| *P. cf. parvicalcar De Koning, 1947 | SH2, GD25 | PAL, ORI# |
| *P. plena (Bryce, 1894) | QH1, YN7 | AFR, ANT, AUS, NEA, NEO, PAL, ORI# |
| *P. rapida Milne, 1916 | YN7 | AFR, NEO, PAL, ORI# |
| P. roseola Ehrenberg, 1832 | GD19 | AFR, AUS, NEA, NEO, PAL, ORI# |
| *P. rugosa Bryce, 1903 | GD20–21 | AFR, AUS, NEA, NEO, PAL, ORI# |
| *P. tenuicalcar De Koning, 1947 | NX1 | PAL |
| *P. tranquilla Wulfert, 1942 | HN2, GS1 | AUS, PAL, ORI# |
| P. vorax (Janson, 1893) | HN2 | AFR, AUS, NEA, NEO, ORI, PAL |
| *Pleuretra africana Murray, 1911 | YN2, 6 | AFR, NEO, ORI# |
| *P. brycei (Weber, 1898) | GD15, 23 | AFR, AUS, NEA, NEO, PAL, ORI# |
| Rotaria citrina (Ehrenberg, 1838) | GD16 | AFR, AUS, NEA, PAL, ORI# |
| *R. laticeps Wulfert, 1942 | GD15, 24 | AUS, PAL, ORI# |
| R. neptunia Ehrenberg, 1830 | GD16–17 | AFR, AUS, NEA, NEO, ORI, PAL |
| *R. neptunoida Harring, 1913 | GD16–17, 19 | AFR, AUS, NEA, ORI, PAL |
| R. rotatoria (Pallas, 1766) | HN5, GD8, 18, SH1, 3 | AFR, AUS, NEA, NEO, ORI, PAL |
| R. sordida (Western, 1893) | HN2, 8, YN2–3, GD13–14,26, JS1 | AFR, AUS, NEA, NEO, ORI, PAL |
| R. tardigrada (Ehrenberg, 1830) | HN9 | AFR, AUS, NEA, NEO, ORI, PAL |
| R. tridens (Montet, 1915) | HN6, 9, GD9, 12 | AUS, NEA, NEO, PAL, ORI# |
*: Taxa new for China, #: new for ORI. Abbreviations: AFR: Afrotropical region; ANT: Antarctic region; AUS: Australian region; NEA: Nearctic region; NEO: Neotropical region; ORI: Oriental region; PAC: Pacific region; PAL: Palearctic region. ‘cf.’ is retained for those taxa which have some differences from the nominate morphospecies, requiring further study. Locality codes see Table 2 for sampling information.
During our survey, five collected bdelloids have a general resemblance to known species, but also showed some dissimilar traits from previously described taxa, and they were qualified with ‘cf.’ and await further analysis. One of these doubtful species, reported as H. cf. spicula Bryce, which showed a upturned dorsal protrusion. Philodina cf. indica Murray, P. cf. proterva Milne and P. cf. parvicalcar showed relative wide range of variations in their head proportion, which need further analyses.
Among these new records, some species are very rare, and few were first found out of their type localities or habitats, e.g., Adineta beysunae Örstan and Habrotrocha ligula loxoglotta De Koning; some new morphological characteristics were observed and need to be added to the original descriptions, e.g., Adineta cf. acuticornis Haigh and Habrotrocha serpens Donner, which are redescribed and illustrated in the next section.
Species richness of bdelliods recorded between 1908 and 2018 in different provinces of China is presented in Figure 1, showing that sampling intensity greatly influenced the species diversity in different regions of China. For instance, the provinces of Guangdong, Yunnan and Hunan were the subject of 26, eight, and nine studies, which recorded 33, 18, and 16 morphospecies, respectively, whereas the provinces of Jiangsu, Zhejiang, Guizhou, Sichuan, Shanghai, Qinghai, Ningxia, and Gansu have no more than four investigations, which only recorded up to six morphospecies for each.
Redescriptions of some rare morphospecies
Phylum Rotifera Cuvier, 1817
Class Eurotatoria De Ridder, 1957
Order Adinetida Melone & Ricci, 1995
Family Adinetidae Melone & Ricci, 1995
Genus Adineta Hudson & Gosse, 1886
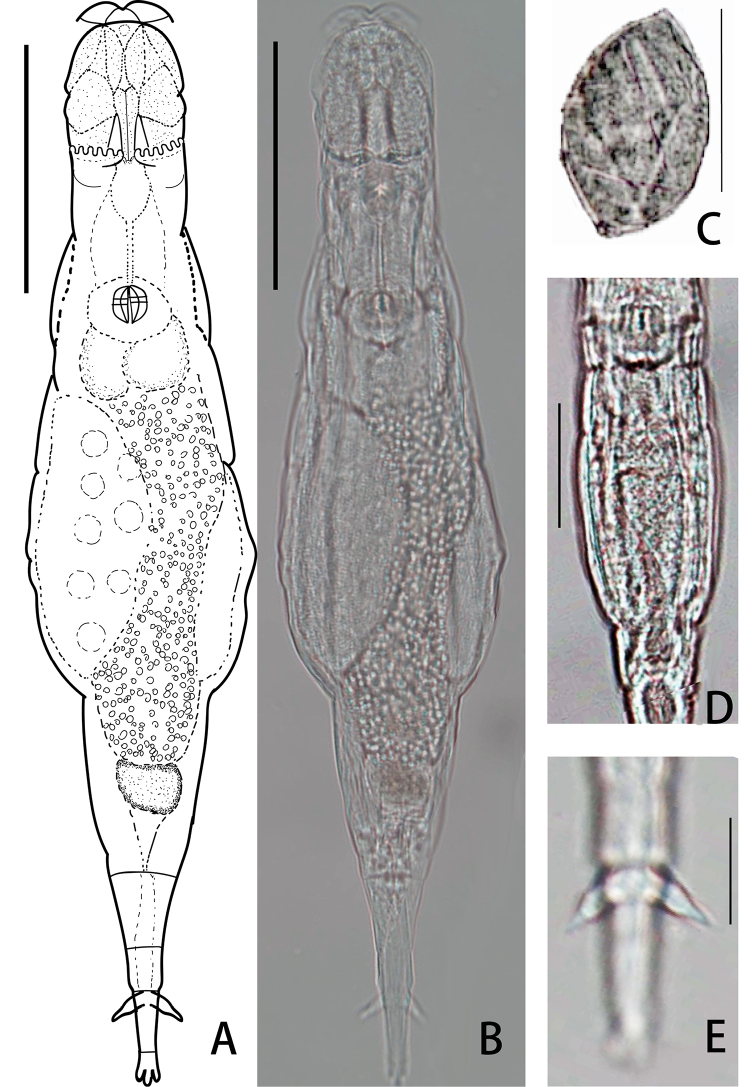
Adineta cf. acuticornis
Haigh, 1967
BBD9A6D0-B51A-56BD-9CCF-4D393202A1C0
Figure 2.
Adineta cf. acuticornis Haigh, 1967 A, B habitus, ventral view C egg D stomach lumen E spur. Scale bars: 50 μm (A–D); 10 μm (E).
Material.
Eight specimens found in mosses and two specimens found in lichens, from tropical (GD 6) and subtropical (YN 5–6, SC 2) zones (Table 2).
Description.
Body transparent and colorless, with smooth skin. No eyespots. Rostrum rather long when animal creeps and stretches out, distal rostral pseudosegment semi-circular and flattened. Rostral lamella divided into two broad sickles-like lobes, immobile, laterally elongated, no trace of cilia under the present microscope image. Small oval head, HW 63–90% of HL and 11–16% of TL, HL 15–18% of TL. Five rectangular denticles in each rake.
Neck width not distinct from head and trunk. The width of the first two pseudosegments of neck approximately equal to HW, the second neck pseudosegment much wider and swollen than the first one. Antenna of two pseudosegments, with length 56–64% of the bearing pseudosegment width. Trunk oval, BW 15–22% of TL. Rump conical, TrL 54–67% of TL. The stomach lumen very narrow and Z-shaped (Fig. 2D). Oviparous; egg oval and smooth, one knob at each pole (Fig. 2C); Vitellarium large with eight nuclei.
Foot slim and short, of four pseudosegments. Spurs long, the inner edge of the spurs almost parallel to the straight outer edge for two-thirds of its length, then a small bulge followed by a contraction and tapers to a sharp point (Fig. 2E). SL 4–8% of TL, and 143–193% of SSW. Three long and unsegmented toes. Dorsal toe longer than two ventral toes. Trophi small, round. Dental formula 2/2.
Measurements.
The detailed measurements are summarized in Table 4 with a comparison of the original data from Haigh (1967).
Table 4.
Comparison the body dimensions of Adineta acuticornis between Chinese specimens and the original description.
| Measurements | Chinese specimens | Original description |
| TL | 166–266 (227±33) | 210 |
| BW | 30–61 (44±10) | |
| HL | 30–45 (40±5) | |
| HW | 25–37 (30±4) | 30 |
| NL | 15–34 (25±6) | |
| MinNW | 16–31 (25±4) | |
| MxNW | 22–42 (31±6) | |
| RL | 20–44 (30±9) | |
| RW | 22–38 (25±7) | |
| FL | 21–32 (28±4) | |
| FW | 11–16 (13±2) | |
| SL | 11–13 (12±1) | 12 |
| SSW | 6–8 (7±1) | 9 |
| RaL | 9–12 (10±1) | |
| TrW | 5–6 (5±1) | 7.5 |
| Rake | 5–5 | |
| TL/SL | 14.4–21.6 (18.3±3.4) | 17.5 |
| TL/HW | 6.6–8.6 (7.5±0.8) | 7 |
| Rostral lamella | immobile | immobile |
| Antenna | 1/2 MNW | half neck width |
| Foot segments | 4 | 4 |
| Stomach lumen | one loop | two loops |
| Habitats | lichen and moss | damp moss on soil |
BW: body width; FL: foot length; FW: foot width; HL: head length; HW: head width; MinNW: minimal neck width; MxNW: maximal neck width; NL: neck length; RaL: ramus length; RL: rump length; RW: rump width; SL: spur length; SSW: spur pseudosegment width; TL: total length; TrW: trophi width. Measurements are given in μm.
Remarks.
Adineta acuticornis has not been found since its original description by Haigh (1967) and was considered as an endemic morphospecies of New Zealand (Shiel and Green 1996). It was found in China for the firstly time also in the Oriental biogeographic region recorded in two provinces of China in 2017 or 2018. It was recorded in damp mosses on soil face in the type locality, whereas in this study, numerous specimens were recorded in both dry and damp mosses, and two specimens in lichens on soil surface.
A distinct characteristic differentiating this morphospecies from Adineta vaga Davis is its wide and rostral lamellae which are slightly wider than the anterior head, while the rostral lamellae of A. vaga are narrower than the anterior head. It differs from Adineta glauca Wulfert by its spur shape, which is short and has a flat base, while A. glauca spur with a swollen base. This morphospecies differs from Adineta longicornis Murray by its spur shape which has bulge, while A. longicornis spur is slender and acute (Murray 1906: 5a, 5b).
The general morphology of the Chinese specimen conforms to the description of the New Zealand population, except the position of the spur contraction is closer to the tip (the contraction is in the middle of the spur in Haigh’s description) and the stomach lumen do not have distinct two loops as Haigh’s description. A comparison with Haigh’s (1967) body dimensions showed a similar body proportion (Table 4). Since there was no genetic evidence to prove it actual systematic status, we assigned ‘cf.’ (resembling original description) as the status of this find. Besides, we observed three new morphological features missed by Haigh (1967): each rake with five denticles, a larger vitellarium with eight nuclei and egg with one knob on each pole.
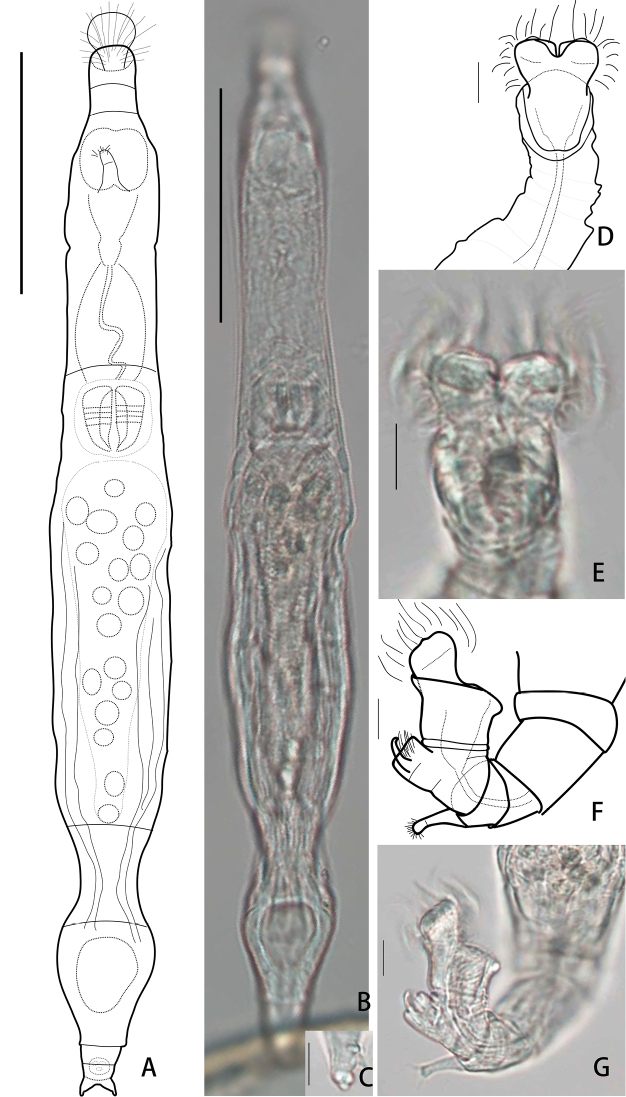
Adineta beysunae
Örstan, 2018
DB0A782F-7F55-5C75-8502-6CD9AB3A05E8
Figure 3.
Adineta beysunae Örstan, 2018 A, B habitus, dorsal view. Scale bars: 50 μm (A, B).
Material.
Numerous specimens found in leaf litter from three provinces (GD13–14, 25–26, YN7, JS1) across tropical and subtropical zones. One specimen found in dew on leaves from Southwest of China (YN8) (Table 2).
Description.
Body angulate, large and transparent. Sometimes the organs in the trunk show brown coloration. No eyespots. Rostral lamella flat and widened, with two lateral triangular auricular protrusions holding long rostral setae under them (the number of stiff under each could not be counted under microscope). Setae length varies from 11 to 30 μm. Head trapezoid, rather large and long, HW 80–110% of HLb, HLb 17–22% of TL, HW 13–20% of TL. Numbers of U-gaps denticles on rakes: 9–9 (N = 3), 10–10 (N = 4).
Neck distinct from head, the first two pseudosegments of neck narrower than HW. Trunk oval. Posterior end of the first rump pseudosegment with a pair of lateral angular knobs.
Foot of five pseudosegments with two pairs of lateral knobs on its first two pseudosegments, FL 14–22% of TL. Spurs long and sturdy, with short interspace, SL 6–8% of TL, 172–284% of SSW. Three short unsegmented toes. Ventral toe longer than two dorsal toes. Dental formula 2/2.
Measurements.
TL 289±40 μm, HLb 49±5 μm, HW 45±4 μm, FL 49±8 μm, SL 20±1 μm, SSW 10±1 μm, RkW (N = 2, with 9–9 denticles; N = 4, with 10–10 denticles) 21±1 μm, RaL (N = 14) 15.9±2 μm, TrW 7.3±1 μm.
Remarks.
This is the second report of this morphospecies since its original description by Örstan (2018) in rainwater and plant debris from the United States. In the present study, A. beysunae was found in leaf litter and dew on leaves. And interestingly, it was abundant in 60% of all leaf litter samples. Our study suggested A. beysunae might have a habitat preference for leaf litter and temporary waterbodies.
Family Habrotrochidae Harring, 1913
Genus Habrotrocha Ehrenberg, 1838
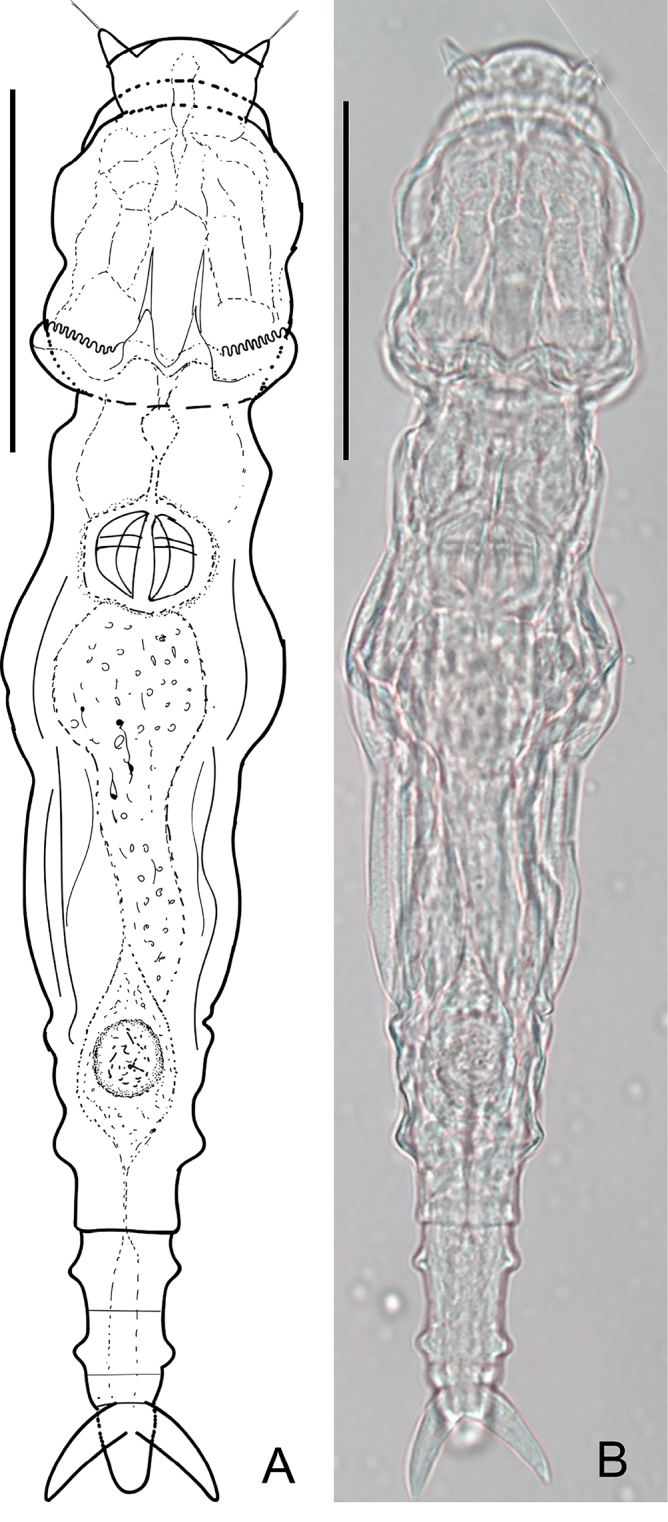
Habrotrocha ligula loxoglotta
De Koning, 1947
403B6D29-02A8-512B-B905-F01C9E43B440
Figure 4.
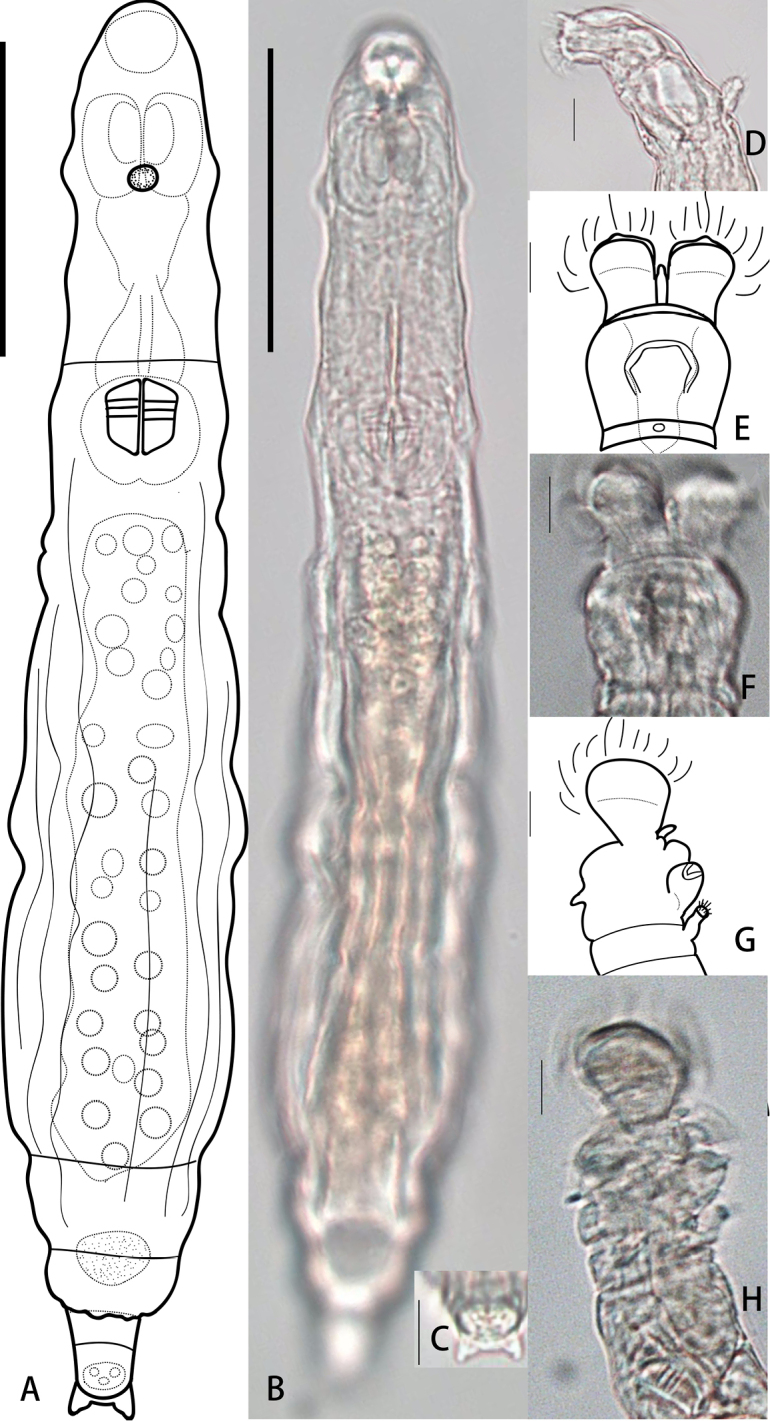
Habrotrocha ligula loxoglotta De Koning 1947 A, B habitus, creeping, dorsal view C rostrum, lateral view D, E head, dorsal view F, G head, with ligula sloping obliquely to the dorsal side, lateral view. Scale bars: 50 μm (A, B), 10 μm (C–G).
Materials.
Five specimens found in mosses on rock from Southwest China (YN5) (Table 2).
Description.
Body slender and transparent, integument smooth. Rostrum long and strongly bent ventrally. Rostral lamellae divided into two semi-circular lobes and wider than the anterior rim of rostrum. Head similar to hexagon, HW 89% of HL. Corona slightly narrower than collar, with papillae clearly seen in the middle of each trochus, CW 97% of HW. Trochal discs separated by a narrow, V-shaped sulcus, in which a cylindrical ligula bends obliquely to the dorsal side (Fig. 4F, G). A slight contraction near the tip which then forms a small papilla on the tip of the ligula, attaining the level of the discs at the inner side (Fig. 4D, E). Upper lip a flat bow. Neck also bent ventrally when animal creeps. The first pseudosegment of neck slightly narrower than the head at the corners of the mouth, not distinct from head and trunk. A pair of lateral cuticular bulges on the dorsal antenna pseudosegment. Antenna with two segments, its length 30–40% of the bearing pseudosegment width.
Trunk slender and cylindrical, TrL 59–67% of TL. Rump conical, with both pseudosegments somewhat swollen and strong arched up dorsally and roofing the foot, the posterior rim of the second pseudosegment creased, RL 8–10% of TL.
Foot short with three pseudosegments, FL 6–8% of TL. Bulbous spurs short and triangular shape, with distinct tips and wide interspace, base swollen. The width of interspace 114% of SL, 97% of the swollen width. Three stout unsegmented toes of the same length. Trophi small, dental formula 3/3.
Measurements.
TL 186±43 μm, NL 27±3 μm, TrL 119±37 μm, RL 156±2 μm, RW 22±7, FL 12±2 μm, SL 4±2 μm, RaL 13±1 μm, TrW 5.6±0.5 μm.
Remarks.
Habrotrocha ligula loxoglotta was originally described from Holland (De Koning 1947), later reported from beech-oak needle-litter in Germany, from dry mosses in France (Donner 1951) and from mosses in Austria (Kutikova 2005). In this study, it was found for the first time in China (Yunnan Province) as well as in the Oriental region.
Habrotrocha serpens
Donner, 1949
54C1875B-173F-5A64-A69D-1089550BE4AD
Figure 5.
Habrotrocha serpens Donner, 1949 A, B habitus, creeping (not fully extended), dorsal view C three toes, dorsal view D, E head, ventral view F, G head, lateral view (the second pseudosegment of rostrum contracted). Scale bars: 50 μm (A, B); 10 μm (C–G).
Materials.
Five specimens found in dry mosses on bark from southern China (GD6) (Table 2).
Description.
Body extremely slender (BW is only about 6% of TL), long and cylindrical, integument transparent and smooth. Rostrum rather long, with two pseudosegments. The first pseudosegment circular and slightly bigger than the second one which often contracted (Fig. 5B). One whole semi-circular lamella not divided into lobes, rather large, broader than the rostrum, covers the long and stiff tactile cilia. Head slender, HW 44% of HL, 22% of TL. Corona also slender, a little wider than the head, CW 107% of HW. Trochal pedicels grown together, central rounded papillae on each separated trochal discs, incline to the dorsal side. Upper lip low, narrow and without lobes, slightly arched, not covered by the incompletely extended rostrum. Lower lip spoon-shaped, strongly protrudes forward.
Neck slender. Throat very short, pharyngeal tube long, undulating before the mastax. Dorsal antenna slender, with two segments, its length 86% of the antennal pseudosegment width. Trunk slender, the two lateral sides of trunk almost parallel when animal fully extended, the last trunk segment often strongly contracted. Rump conical, with both pseudosegments swollen, arched up dorsally and roofing the foot, RL 12% of TL.
Foot very short, of four pseudosegments, FL 5% of TL. Spurs triangular and have swollen base, each with curved inner margins and a very small interspace. SL 63% of SSW. Three short unsegmented and of approximately equal length toes (Fig. 5C). Trophi large, dental formula 4/4.
Measurements.
The detailed measurements are summarized in Table 5 with a comparison of the original data from Donner (1949; 1970).
Table 5.
Comparison the body dimensions of Habrotrocha serpens between Chinese specimens and the original description from Donner (1949; 1970).
| Measurements | Chinese specimens | Donner 1949 | Donner 1970 |
| TL | 213 | 193–273 | 200 |
| BW | 18.7 | 17 | |
| HL | 42 | ||
| HW | 18.4 | ||
| CW | 19.7 | ||
| NL | 31.2 | ||
| MinNW | 17.8 | ||
| MxNW | 19.2 | ||
| RL | 26.6 | ||
| RW | 20 | ||
| FL | 12 | ||
| FW | 9.9 | ||
| SL | 3.4 | ||
| SSW | 5.4 | ||
| RaL | 14 | 12.7 | 14.8 |
| TrW | 5.9 | ||
| TL/BW | 11.4 | 11.8 |
BW: body width when creeping; FL: foot length; FW: foot width; HL: head length; HW: head width; CW: corona width; MinNW: minimal neck width; MxNW: maximal neck width; NL: neck length; RaL: ramus length; RL: rump length; RW: rump width; SL: spur length; SSW: spur pseudosegment width; TL: total length; TrW: trophi width. Measurements are given in μm.
Remarks.
The general morphology of our sample conforms with the description of the Austrian population except that the rostrum is not always fully expanded to/exceeding the upper lip in a feeding position. It may because of the second pseudosegment of rostrum often contracted. Additionally, we observed three approximately equal-lengthed toes which were not clear in Donner’s (1949) description.
This morphospecies was first described from soil from Austria by Donner (1949), and then recorded in moss and soil from Austria and Czechoslovakia (Bartoš 1951); in needle litter, Calamagrosits turf, grasses and leaf litter from Austria, Czechoslovakia, Romania, and Spain (Donner 1965, 1970). It is new for China as well as for the Oriental region.
Discussion
Taxonomy and diversity of bdelloid rotifers in China
Only 48 species were recorded in eleven studies conducted in China between 1908 and 2018 (Table 1), which implies that taxonomic and diversity researches on Chinese bdelloids are very limited. Moreover, only 65% (31 of 48) of the recoreded morphospecies were illustrated and described (e.g., Wang 1961; Bartoš 1963; Gong 1983), and many of the illustrations are inaccurate, not showing important details and the descriptions are not detailed enough to verify their identity. Besides, there are 17 morphospecies listed in the literature without any illustrations, photographs or descriptions (e.g., Koste and Zhuge 1996; Yin and Xu 2016), which need further verification. Also, some species were recorded out of their specific habitats (e.g., H. thienemanni Hauer and P. roseola Ehrenberg) and some recorded in unusual environments (e.g., Habrotrocha pulchra Murray, H. thienemanni Hauer, Mniobia tentans Donner, Macrotrachela bullata Murray, and Philodina vorax Janson were abundant in glacier over 5500 m a.s.l.) (Table 1). These ecological differences may hide potential cryptic taxa and need further studies combined with new techniques such as DNA taxonomy.
Due to a lack of insufficient taxonomic and diversity research in China, species richness is extremely uneven in different provinces of China. More morphospecies were recorded in the Tibetan Plateau (27 morphospecies) and Guangdong Province (22 morphospecies) with more samples collected (Stewart 1908; Bartoš 1963; Wang 1974; Gong 1983; Yin and Xu 2016). Four new morphospecies were reported in Guangdong, including Habrotrocha modesta Bartoš, H. flexicollis Bartoš, Pleuretra proxima Bartoš, and P. similis Bartoš. Unfortunately, they were never found again, and these are considered as disappeared ‘endemic morphospecies’ in latter researches. Research on different habitats of bdelloids were also uneven. Most studies were only focused on fragmented fresh-water bodies or mosses, but did not pay attention to other habitats such as brackish waters, soil and litter. Therefore, more studies are necessary to explore the taxonomy and diversity of bdelloid rotifers in China, especially with a focus on the areas and habitats that were not well studied.
Geographical distribution and ecological information of Chinese bdelloids
The high dispersal potential of bdelloids has supposedly led to their generally cosmopolitan distribution (Fenchel and Finlay 2004). The previous extensive sampling of bdelloids confirms that some species can be found in distant areas on different continents, but also some species can only be found in specific area (Donner 1965; Segers 2007). At present, studies of biogeography on these taxa are not comprehensive. For example, Adineta ricciae Segers and Shiel, previously considered as an Australia-endemic species, was observed in South China (the Oriental region); A. beysuanae has been described in a container filled with plant debris and rain water from the United States (Örstan 2018), and it was then found in similar or drier habitats (dew and leaf litter) from China. These findings imply that the currently described distribution of bdelloids is incomplete and may be strong influenced by the sampling effort, especially in the poorly investigated areas, such as South Asia.
With study extending to more ecological habitats, some morphospecies were found in a broader range of habitats. We observed five brackish water morphospecies: Rotaria rotatoria Pallas, R. laticeps Wulfert, R. tridens Montet, Philodina megalotrocha Ehrenberg and Macrotrachela hewitti Murray. They were found among aquatic plants or brackish temporary puddle with sediment in mangrove. Noticeably, R. rotatoria was abundant and dominated in Gracilaria lichenoides (a red alga) culture ponds, possibly because G. lichenoides could provide suitable habitats. These ecological differences seem to represent different ecological niches, which may hide some interesting phenomena of separated evolutionary lineages. For example, Adineta vaga, which occurs in the multiple types of habitats, has a large amount of cryptic diversity (Fontaneto et al. 2011).
More extensive surveys of bdelloids in Asia
More than half of the recorded morphospecies from this study (some presumed cosmopolitan) are new records for the Oriental region as well as for South Asia. As there are still considerable gaps in faunistic studies in the Oriental region, we do not yet have sufficient faunistic data to determine the true distributions of bdelloids. Our findings highlight the need for further taxonomic studies on bdelloids in Asia. Furthermore, asexual bdelloids have evolved independently in spite of being effectively sympatric, indicating that they may adapt to different ecological niches, thus the type of habitat is a key player for microscopic species diversity and evolution (Birky et al. 2005). Applications of molecular phylogeny for identification of bdelloid species would be invaluable in uncovering the actual systematic status of some euryoecious or variable morphospecies so that we may better understand the true distribution of bdelloid species.
Supplementary Material
Acknowledgements
We gratefully acknowledge Prof. Larry Liddle (Long Island University, USA), Dr. Xuejia He (Research Center for Harmful Algae and Aquatic Environment, Jinan University), Dr. Thomas Mesaglio (University of New South Wale, Australia), Dr. Xiao Ma (South China Sea Institute of Oceanology, Chinese Academy of Sciences) and Prof. Zhili He (University of Oklahoma) for their comments on the manuscript and the linguistic help. We also thank Dr. Zhiwei Liu (Jinan University) for helping on the map of the sampling localities. This research was supported by National Natural Science Foundation of China (41673080, 31601840).
Citation
Zeng Y, Wei N, Wang Q, Iakovenko NS, Li Y, Yang Y (2020) Bdelloid rotifers (Rotifera, Bdelloidea) of China: diversity and new records. ZooKeys 941: 1–23. https://doi.org/10.3897/zookeys.941.50465
References
- Bartoš E. (1951) The Czechoslovak Rotatoria of the order Bdelloidea. Věstník. Československé Zoologické Společnosti 15: 241–500. [Google Scholar]
- Bartoš E. (1963) Die Bdelloidender Moosprobenaus China and Java. Věstník. Československé Zoologické Společnosti 27: 31–42. [Google Scholar]
- Bielanska-Grajner I, Ejsmont-Karabin J, Iakovenko N. (2013) Fauna słodkowodna Polski, Zeszyt 32C: Wrotki (Rotifera, Bdelloidea). Wydawnictwo Uniwersytetu Łódzkiego, Łódz, 153 pp. [Google Scholar]
- Bohonak AJ, Jenkins DG. (2003) Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecology Letters 6(8): 783–796. 10.1046/j.1461-0248.2003.00486.x [DOI] [Google Scholar]
- Daday J. (1906) Édesvízi mikroskopi állatok Mongoliából. Mathematikai és Természettudomanyi Értesitő 24(2): 34–77. [Google Scholar]
- De Smet WH. (1998) Preparation of rotifer trophi for light and scanning electron microscopy. Hydrobiologia 387: 117–121. 10.1023/A:1017053518665 [DOI] [Google Scholar]
- Demirkalp FY, Saygi Y, Caglar SS, Gunduz E, Kilinc S. (2010) Limnological assesment on the Brakish Shallow Liman Lake from Kizilirmak Delta (Turkey). Journal of Animal and Veterinary Advances 9(16): 2132–2139. 10.3923/javaa.2010.2132.2139 [DOI] [Google Scholar]
- Dong YX, Xu Q, Yang R, Xu CD, Wang YY. (2017) Delineation of the northern border of the tropical zone of China’s mainland using Geodetector. Acta Geographica Sinca 72(1): 135–147. [in Chinese] 10.11821/dlxb201701011 [DOI] [Google Scholar]
- Donner J. (1949) Rotatorien der Humusböden. Österreichische Zoologische Zeitschrift 2(1): 117–151. [Google Scholar]
- Donner J. (1951) Rotatorien der Humusböden. III. Teil. Zoologische Jahrbücher, Abteilung für Systematik, Geographie und Biologie der Tiere 79: 614–638. [Google Scholar]
- Donner J. (1965) Ordnung Bdelloidea (Rotatoria, Rädertiere). Akademie-Verlag, Berlin, 267 pp. [Google Scholar]
- Donner J. (1970) Rotatorien aus einigen Böden und Moosen Spaniens und seiner Inseln. Revue d’Écologie et de Biologie du Sol 7: 501–532. [Google Scholar]
- Fenchel TOM, Finlay BJ. (2004) The ubiquity of small species: patterns of local and global diversity. BioScience 54(8): 777–784. 10.1641/0006-3568(2004)054[0777:TUOSSP]2.0.CO;2 [DOI]
- Fontaneto D, Melone G. (2003) On some rotifers new for the Italian fauna. Italian Journal of Zoology 70(3): 253–259. 10.1080/11250000309356526 [DOI] [Google Scholar]
- Fontaneto D, De Smet WH, Ricci C. (2006) Rotifers in saltwater environments, re-evaluation of an inconspicuous taxon. Journal of the Marine Biological Association of the United Kingdom 86(4): 623–656. 10.1017/S0025315406013531 [DOI] [Google Scholar]
- Fontaneto D, Ricci C. (2006) Spatial gradients in species diversity of microscopic. animals: the case of bdelloid rotifers at high altitude. Journal of Biogeography 33(7): 1305–1313. 10.1111/j.1365-2699.2006.01502.x [DOI] [Google Scholar]
- Fontaneto D, Herniou EA, Barraclough TG, Ricci C. (2007) On the global distribution of microscopic animals: new worldwide data on bdelloid rotifers. Zoological Studies 46(3): 336–346. [Google Scholar]
- Fontaneto D, Iakovenko N, Eyres I, Kaya M, Wyman M, Barraclough TG. (2011) Cryptic diversity in the genus Adineta Hudson & Gosse, 1886 (Rotifera: Bdelloidea: Adinetidae): a DNA taxonomy approach. Hydrobiologia 662(1): 27–33. 10.1007/s10750-010-0481-7 [DOI] [Google Scholar]
- Freckman DW, Virginia RA. (1993) Extraction of nematodes from Dry Valley Antarctic soils. Polar Biology 13(7): 483–487. 10.1007/BF00233139 [DOI] [Google Scholar]
- Gee NG. (1927) Some Chinese rotifers. Lingnan Agricultural Review 4: 43–53. [Google Scholar]
- Gladyshev E, Meselson M. (2008) Extreme resistance of bdelloid rotifers to ionizing radiation. Proceedings of the National Academy of Sciences 105(13): 5139–5144. 10.1073/pnas.0800966105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong XJ. (1983) The rotifers from the High Plateau of Tibet. In: Jiang XZ, Shen YF, Gong XJ. (Eds) Freshwater Invertebrate from Tibet.Series of the Scientific Expedition to Qinghai-Xizang Plateau. Science Press of China, Beijing, 335–424. [in Chinese]
- Haigh SB. (1967) The bdelloid rotifers of New Zealand, Part III. Journal of the Quekett Microscopical Club 30: 193–201. [Google Scholar]
- Iakovenko N S, Kašparová E, Plewka M, Janko K. (2013) Otostephanos (Rotifera, Bdelloidea, Habrotrochidae) with the description of two new species. Systematics and Biodiversity 11(4): 477–494. 10.1080/14772000.2013.857737 [DOI] [Google Scholar]
- Iakovenko N, Smykla J, Convey P, Kašparová E, Kozeretska I, Trokhymets V, Dykyy I, Plewka M, Devetter M, Duriš Z. (2015) Antarctic bdelloid rotifers: diversity, endemism and evolution. Hydrobiologia 761(1): 5–43. 10.1007/s10750-015-2463-2 [DOI] [Google Scholar]
- Kaya M, Herniou EA, Barraclough TG, Fontaneto D. (2009) A faunistic survey of bdelloid rotifers in Turkey. Zoology in the Middle East 48(1): 114–116. 10.1080/09397140.2009.10638379 [DOI] [Google Scholar]
- Koste W, Zhuge Y. (1996) A preliminary report on the occurrence of Rotifera in Hainan. Quekett Journal of Microscopy 37(8): 666–683. [Google Scholar]
- Koste W, Zhuge V. (1998) Zur Kenntnis der Rotatorienfauna (Rotifera) der Insel Hainan, China. Teil 11. Osnabrücker Naturwissenschaftliche Mitteilungen, 24: 183–222. [Google Scholar]
- Kellogg CA, Griffin DW. (2006) Aerobiology and the global transport of desert dust. Trends in Ecology and Evolution 21(11): 638–644. 10.1016/j.tree.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Kutikova LA. (2005) The bdelloid rotifers of the fauna of Russia. Russian Academy of Sciences, Proceedings of the Zoological Institute, Moscow, 305 pp. [Google Scholar]
- Murray J. (1906) Some Rotifera of the Sikkim Himalaya. Journal of the Royal Microscopical Society: 637–644. 10.1111/j.1365-2818.1906.tb00619.x [DOI]
- Norton CJ, Jin C, Wang Y, Zhang Y. (2010) Rethinking the Palearctic-oriental biogeographic boundary in Quaternary China. In: Norton CJ, Braun DR. (Eds) Asian Paleoanthropology.Springer, Dordrecht, 81–100. 10.1007/978-90-481-9094-2_7 [DOI]
- Örstan A, Plewka M. (2017) An introduction to bdelloid rotifers and their study. http://www.quekett.org/starting/microscopic-life/bdelloid-rotifers/ [accessed 25 February 2019]
- Örstan A. (2018) Taxonomic morphology of the genus Adineta (Rotifera: Bdelloidea: Adinetidae) with a new species from a suburban garden. Zootaxa 4524(2): 187–199. 10.11646/zootaxa.4524.2.3 [DOI] [PubMed] [Google Scholar]
- Peters U, Koste W, Westheide W. (1993) A quantitative method to extract moss-dwelling rotifers. Hydrobiologia 255(1): 339–341. 10.1007/BF00025857 [DOI] [Google Scholar]
- Ricci C. (1998) Anhydrobiotic capabilities of bdelloid rotifers. Hydrobiologia 387: 321–326. 10.1023/A:1017086425934 [DOI] [Google Scholar]
- Robeson MS, King AJ, Freeman KR, Birky CW, Martin AP, Schmidt SK. (2011) Soil rotifer communities are extremely diverse globally but spatially autocorrelated locally. Proceedings of the National Academy of Sciences 108(1): 4406–4410. 10.1073/pnas.1012678108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers H. (2007) Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 1564: 1–104. 10.11646/zootaxa.1564.1.1 [DOI] [Google Scholar]
- Stewart FH. (1908) Rotifers and Gastrochicha from Tibet. Records ot the Indian Museum 2: 316–323. [Google Scholar]
- Shiel R, Green J. (1996) Rotifera recorded from New Zealand, 1859–1995, with comments on zoogeography. New Zealand Journal of Zoology 23(2): 191–207. 10.1080/03014223.1996.9518078 [DOI] [Google Scholar]
- Song MO. (2014) Eight new records of monogonont and bdelloid rotifers from Korea. Journal of Species Research 3(1): 53–62. 10.12651/JSR.2014.3.1.053 [DOI] [Google Scholar]
- Song MO. (2015) New records of one monogonont and 5 bdelloid rotifers from Korea. Korean Journal of Environmental Biology 33(2): 140–147. 10.11626/KJEB.2015.33.2.140 [DOI] [Google Scholar]
- Song MO, Kim W. (2000) Bdelloid rotifers from Korea. Hydrobiologia 439(1–3): 91–101. 10.1023/A:1004181414535 [DOI] [Google Scholar]
- Song MO, Lee CH. (2017) A new and five rare bdelloids from Korea. Zootaxa 4242(3): 529–547. 10.11646/zootaxa.4242.3.6 [DOI] [PubMed] [Google Scholar]
- Song MO, Min GS. (2015) A new species and ten new records of bdelloid rotifers from Korea. Zootaxa 3964(2): 211–27. 10.11646/zootaxa.3964.2.3 [DOI] [PubMed] [Google Scholar]
- Thorpe SVG. (1893) The Rotifera of China. Journal of the Royal Microscopical Society 13(2): 145–152. 10.1111/j.1365-2818.1893.tb01272.x [DOI] [Google Scholar]
- Wang JJ. (1961) Fauna of Freshwater Rotifera of China. Science Press of China, Beijing, 288 pp. [in Chinese] [Google Scholar]
- Wang JJ. (1974) The preliminary study of Rotifera in Everest Mountain area. In: Tibetan Science Research Team of the Chinese Academy of Sciences (Eds) Scientific Expedition Reports of Everest Mountain Area, 1966–1968.Vol. 208. Biology and Alpine Physiology. Science Press of China, Beijing, 137–144. [in Chinese]
- Welch DBM, Meselson M. (2000) Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288(5469): 1211–1215. 10.1126/science.288.5469.1211 [DOI] [PubMed] [Google Scholar]
- Welch JLM, Welch DBM, Meselson M. (2004) Cytogenetic evidence for asexual evolution of bdelloid rotifers. Proceedings of the National Academy of Sciences of the United States of America 101(6): 1618–1621. 10.1073/pnas.0307677100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovenko N. (2000a) New for the fauna of Ukraine rotifers (Rotifera, Bdelloidea) of Adinetidae and Habrotrochidae families. Vestnik Zoologii 34(1–2): 11–19. [Google Scholar]
- Yakovenko N. (2000b) New for the fauna of Ukraine rotifers (Rotifera, Bdelloidea) of Philodinidae family. Vestnik Zoologii 14: 26–32. [Google Scholar]
- Yin ZW, Xu RL. (2016) The composition and distribution of moss-dwelling bdelloid rotifers in Nanling Forest Park of Guangdong. Chinese Journal of Applied and Environmental Biology 22(2): 0307–0312. [in Chinese] 10.3724/SP.J.1145.2015.07010 [DOI] [Google Scholar]
- Zhuge Y, Huang XF, Koste W. (1998) Rotifera recorded from China, 1893–1997, with remarks on their composition and distribution. International Review of Hydrobiology 83(3): 217–232. 10.1002/iroh.19980830305 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.