Abstract
Purpose
Our aim was to evaluate the frequency and SNP-SNP interactions between factor V Leiden (FVL) G1691A, prothrombin G20210A mutation, and C677T MTHFR and PAI-1 4G/5G gene polymorphisms in female IVF patients with unexplained infertility (UI) by using a multifactor dimensionality reduction (MDR) model analysis.
Methods
A total of 225 subjects were enrolled in the study. There were 105 females in UI group and 120 healthy controls. Designated SNPs were determined by using allele-specific PCR methods. The difference in thrombophilia prevalence was assessed by a chi-square test and logistic regression analysis. Four-locus SNP interaction model was tested using the MDR approach. A ten-fold cross-validation consistency (CVC) and permutation testing were performed.
Results
There was a significant difference of MTHFR C677T polymorphism frequency between the groups. Significantly less UI patients had MTHFR CC genotype (p = 0.005), while the risk allele T was more frequent (OR = 1.83, p = 0.0018). Logistic regression determined a significant association only for MTHFR C677T in our patients (TT genotype OR = 2.99). The MDR analysis confirmed the significance of a single-locus model for MTHFR C677T polymorphism (p = 0.015; OR = 2.93). However, the best, significant predictive model was the two-locus model comprising MTHFR C677T and FVL (CVC = 10/10, testing accuracy = 60.95%, p = 0.013; OR = 3.02).
Conclusion
The MTHFR C677T polymorphism was significantly associated with UI, with minor allele T being more frequent. Additionally, there was a significantly increased presence of MTHFR C677T with FVL mutation in these patients. Therefore, MTHFR and its interaction with FVL should be recognized as contributing factors in the pathogenesis of infertility.
Keywords: Epistasis, Methylenetetrahydrofolate reductase, Factor V Leiden, Prothrombin G20210A mutation, PAI-1 4G/5G, In vitro fertilization
Introduction
Duration of infertility and the lack of a previous pregnancy are associated with the risk of not having a live birth after assisted reproduction techniques (ART) treatment. A successful in vitro fertilization (IVF) process depends on a healthy endometrium and good quality embryo, so that unfavorable IVF outcomes are often due to implantation failure [1, 2].
Several hemostasis factors have been found to affect the embryo progression, endometrial remodeling, and maternal-embryo interaction during the early phases of implantation process [2–5]. It has been established that thrombophilia may negatively impact pregnancy through the thrombosis of placental blood vessels, consequently leading to decreased placental perfusion and adverse pregnancy outcomes, such as recurrent miscarriages, intrauterine growth restriction, pre-eclampsia, and premature detachment of the placenta [5–7]. Factor V Leiden (FVL) G1691A mutation and prothrombin (FII) G20210A gene mutation (PGM) are considered to confer the highest risk of thrombotic complications [8, 9].
In addition, inherited thrombophilia is proposed to be a risk factor for infertility and recurrent IVF failure, where the alteration of hemostasis factors would predispose to a poor blastocyst implantation [2, 4–6]. There are several hypothesized mechanisms for this adverse impact: interference with trophoblast differentiation and inhibition of its invasion, inadequate placentation and spiral artery remodeling, defective fibrinolysis and fibrin deposition, oxidative stress, etc. [3–6, 10, 11]. However, the evidence of a causal association between thrombophilia and infertility has been inconclusive so far [2, 11, 12].
Currently, there is not any consensus about screening or treatment of inherited thrombophilia single nucleotide polymorphisms (SNPs) in the setting of pregnant females or patients undergoing ART. Most guidelines recommend screening of only the selected groups of patients, such as those with venous thromboembolism [8, 9]. Despite this, a set of polymorphisms and mutations are tested in females considering the IVF procedure. These are most commonly FVL (rs6025), FII (rs1799963), methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism (rs1801133), and plasminogen activator inhibitor-1 (PAI-1) -675 4G/5G insertion-deletion polymorphism (rs1799889). Furthermore, prophylactic low-molecular-weight heparin (LWMH) is used empirically in an attempt to improve IVF success rates and to prevent pregnancy complications [2, 8, 9].
Although there are many potential etiologies, many women do not have any apparent cause of infertility determined. In complex diseases, the effect of a SNP is widely influenced by the interaction with other genetic variants, as well as the environmental factors [13]. Determining the interactions between multiple factors is a challenging but necessary task to increase the chances for a healthy pregnancy through the appropriate treatments. In order to do so, we applied a specific statistical approach—a multifactor dimensionality reduction (MDR) method that is a non-parametric, model-free approach, which can deal with many factors and determine epistasis in the absence of the main effects. Through the permutation testing, it selects a single model that has the best predictive ability [13, 14]. We also compared the MDR results with traditional statistical tests.
The aim of the study was to evaluate the frequency, association, and SNP-SNP interactions (epistasis) of factor V Leiden (G1691A) mutation, prothrombin G20210A mutation, C677T MTHFR, and PAI-1 -675 4G/5G polymorphisms in female IVF patients with unexplained infertility (UI), and compare to the general prevalence in population of healthy controls, by using the MDR statistical method.
Patients and methods
Study design and participants
The study was approved by the Ethics Committee of Faculty of Medicine University of Nis, Serbia (No. 12-2307-2/15, 10.03.2016) and informed written consent was obtained from all the participants. The study was performed in the period 2017–2019 at the Faculty of Medicine University of Nis and the Department of Gynecology and Obstetrics of the Clinical Center Nis, Serbia.
A total of 225 subjects were enrolled in this prospective case-control study. All the patients underwent IVF or intra-cytoplasmic sperm injection (ICSI) cycle, with a fresh embryo transfer. After thorough examination and testing, there were 105 females with unexplained cause of infertility. They were allocated into the patient group (UI group). The examinations involved gynecological ultrasonography and hysterosalpingography in all patients and hysteroscopy as required. The testing was done for all the parameters listed in the exclusion criteria. The exclusion criteria for UI group were the following: a male factor, infection, polycystic ovary syndrome, endometriosis, various uterine or fallopian tube pathologies and anatomic abnormalities (after ultrasound and hysterosalpingogram), immunological factors (acquired thrombophilia—antiphospholipid syndrome), the presence of autoantibodies (anticardiolipin antibodies, antinuclear autoantibodies, lupus anticoagulant, etc.), anti-Mullerian hormone (AMH) below 1.5 ng/ml, diabetes mellitus, thyroid dysfunction (mainly hypothyroidism, TSH value > 2.5 mIU/L), and abnormalities related to follicle-stimulating hormone (FSH), luteinizing hormone (LH), progesterone, estradiol (E2), and testosterone. The patients with the deficiencies of natural anticoagulants (protein C, protein S, and antithrombin) were also excluded.
Our control group comprised 120 healthy blood donors and volunteers, from the general population, who agreed to donate a blood sample for our study (both males and females). The samples were collected during a regular annual activity of the Institute for Blood Transfusion in Nis. Blood donors fulfilled a detailed health questionnaire and underwent a short physical examination. The subjects with acute or chronic diseases were excluded. Additionally, our healthy participants completed a short study questionnaire that focused on a family history of thrombotic events and reproductive problems. Those with a positive history were also excluded.
Genotyping
Participants’ DNAs were isolated from 200 μl of whole blood using the GeneJET Whole Blood Genomic DNA Purification Mini Kit (Thermo Fisher Scientific Inc. Haverhill, USA). The FVL G1691A mutation and PGM were determined using the multiplexed allele-specific PCR method, while MTHFR C677T was assessed by specific allele-specific PCR. The allele-specific PCR reactions were performed according to Endler et al. [15], with separately performed MTHFR for precision purposes. The PAI-1 4G/5G polymorphism was determined by the restriction fragment length polymorphism method using BslI restriction enzyme. Amplification products are of 99 bp (5G) and 98 bp (4G), while BslI cleaves the 5G polymorphism into a 77-bp and a 22-bp fragment [16]. The sequences and amounts of used primers are shown in Table 1.
Table 1.
Primer sequences and amounts used in the allele-specific PCRs
| Primer | pmol/25 μl | Sequence | Amplicon length, bp |
|---|---|---|---|
| FVL 1691G | 5 |
5′-AACAAGGACAAAATACCTGTAT- TCATC-3′ |
233 |
| FVL 1691A | 5 |
5′-GTCTGTCTGTCTCTTCAAGGAC- AAAATACCTGTATTCTTT-3′ |
246 |
| FVL common | 5 | 5′-CGCAGGAACAACACCATGAT-3′ | |
| FII 20210G | 2.5 | 5′-CACTGGGAGCATTGAGGCGC-3′ | 180 |
| FII 20210A | 10 |
5′-ATGAATAGTAATGGGAGCATT- GAGGATT-3′ |
188 |
| FII common | 10 |
5′-ATGTGTTCCGCCTGAAGAAGT- GGA-3′ |
|
| MTHFR 677C | 4 |
5′-CTCTCTCTCTGAAGGAGAAGGT- GTCTGCGGTAGC-3′ |
207 |
| MTHFR 677 T | 5 |
5′-TGAAGGAGAAGGTGTCTGCGG- GACT-3′ |
198 |
| MTHFR common | 6 | 5′-AGGACGGTGCGGTGAGAGTG-3′ | |
| PAI-1 F | 1 | 5′-CACAGAGAGAGTCTGGCCACGT-3′ | / |
| PAI-1 R | 1 | 5′-CCAACAGAGGACTCTTGGTCT-3′ | / |
PCR, polymerase chain reaction; FV, factor V; FII, factor II; MTHFR, methylenetetrahydrofolate reductase; PAI, plasminogen activator inhibitor
PCR products were generated in 25 μL containing 12.5 μl KAPA2G Fast HotStart ReadyMix PCR Kit: KR0376-v5.14 (Sigma-Aldrich Inc. St. Louis, MO, USA) and ~ 20 ng/μl DNA. Amplifications were performed in Eppendorf Master cycler ep. gradient S (Applied Biosystems, Foster City CA, USA). The PCR conditions were as follows: denaturation for 2 min at 95 °C; followed by 35 cycles at 95 °C for 15 s, 56 °C for 15 s, and 72 °C for 15 s; and final extension step of 10 min at 72 °C.
The PCR conditions for MTHFR C677T were as follows: denaturation for 2 min at 95 °C; followed by 36 cycles at 95 °C for 15 s, 62 °C for 15 s, and 72 °C for 15 s; and final extension step of 10 min at 72 °C.
The PCR conditions for PAI-1 were as follows: denaturation for 3 min at 95 °C; followed by 35 cycles at 95 °C for 30 s, 54 °C for 30 s, and 72 °C for 15 s; and final extension step of 5 min at 72 °C. Product digestion was performed using BslI restriction enzyme (New England Biolabs, Ipswich, MA, USA) at 55 °C for 1 h, and checked by the agarose gel electrophoresis.
All products were run on vertical gel electrophoresis on 8% or 10% polyacrylamide gel. After staining with ethidium bromide, the results were visualized on a Transilluminator Hoefer MacroVue UVis-20 (Amersham Biosciences Corp. Piscataway, USA).
Statistical analysis
Variables are reported as mean ± standard deviation (SD) or median ± interquartile range (IQR), as required according to the Shapiro-Wilk normality test. Deviation of allele and genotype frequency from the Hardy-Weinberg equilibrium was assessed by chi-square test. The prevalence of genotypes and alleles of thrombophilia factors was compared between patients and controls by chi-square tests or Fisher’s exact tests (two-sided). If required, post hoc analysis was performed using adjusted residuals and Bonferroni correction with a probability value of p < 0.05/6. Univariate odds ratio (OR) and 95% confidence intervals (CI) were estimated separately for each mutation. Logistic regression analysis was used to assess the associations of SNPs with UI as an outcome and to calculate the odds with 95% CI for UI between the groups.
The MDR statistical approach is a non-parametric statistical tool for detecting and modeling epistasis (gene-gene interaction). By constructing an algorithm, MDR creates new variables by pooling genotypes from multiple SNPs. The genotypes are grouped into “high risk” and “low risk,” which reduces the data into one dimension, while the endpoint variable must be dichotomous. The combination is designated as high risk if the ratio of cases to controls exceeds their overall ratio in the data set. Testing of the hypothesis on a MDR model, and its predictive ability, requires permutation testing. The dataset is divided into multiple partitions (1000) and a testing set is produced from the given data for cross-validation. A single model that has the fewest misclassified cases is selected as the best. For detailed description of the MDR method, see our references [13, 14, 17].
In our study, a four-locus SNP-SNP interaction model was tested using a MDR approach. Ten-fold cross-validation consistency (CVC) was performed, with the threshold set to 1. One- to four-factor level of interactions/model size was calculated. Significance levels were assigned to each model in the final set and hypothesis testing of the final best models was performed through permutation testing [13, 14].
In all tests, the values of p < 0.05 were considered statistically significant.
The analyses were performed using the IBM SPSS Statistics for Windows, version 25.0. (Armonk, NY: IBM Corp., USA) and non-parametric MDR software package version 3.0.2 (www.multifactordimensionalityreduction.org). The statistical significance of the MDR model was assessed by permutation testing using the MDR permutation testing software BETA version 0.4.6 [13]. Linkage disequilibrium (LD) between two alleles was calculated according to appropriate formulas [18].
Results
The average age in the patient group was 35 ± 6 (range, 26–42), with body mass index (BMI) average of 24 ± 5 kg/m2. Subjects in the control group were 27 ± 6.5 (range, 20–54) years old and with average BMI of 25 ± 4 kg/m2 (p > 0.05). All of our patients had satisfactory ovarian reserve (AMH > 1.5 ng/ml), with mean top quality embryo of 1.57 ± 1.29, and mean transferred embryo number of 2.48 ± 0.63. All participants were Caucasians of Serbian ethnicity. Tested SNPs show no deviation from Hardy-Weinberg equilibrium in all groups (p > 0.05), except for PAI-1 4G/5G in the patient group, which was p = 0.037 and not greatly removed from an equilibrium state.
Firstly, we compared the prevalence of genotypes and alleles of each thrombophilia between patients and controls (Tables 2 and 3). There was a significant difference in genotypes of MTHFR C677T polymorphism (χ2 = 12.735, p = 0.002). Post hoc analysis revealed that CC genotype made a significant contribution to this result. After calculation of adjusted residuals (AR) for each observed and expected frequency of the MTHFR genotypes, we determined the following: significantly, more subjects in the control group had CC genotype than expected (40.8%, AR = 4.86, p < 0.000), while there were less UI patients than expected with CC genotype (19.1%, AR = − 2.84, p = 0.005).
Table 2.
Distribution of SNPs genotypes between the groups
| SNP | Genotype | Patients with UI (n = 105), n (%) | Control group (n = 120), n (%) | χ2 (df = 2) or FET | p value |
|---|---|---|---|---|---|
|
FVL G1691A |
GG GA AA |
98 (93.3) 7 (6.67) 0 (0) |
116 (96.7) 4 (3.3) 0 (0) |
FET | 0.355 |
|
FII G20210A |
GG GA AA |
100 (95.2) 5 (4.7) 0 (0) |
117 (97.5) 3 (2.5) 0 (0) |
FET | 0.478 |
| MTHFR C677T |
CC CT TT |
20 (19.1) 63 (60.0) 22 (20.9) |
49 (40.8) 55 (45.8) 16 (13.3) |
12.735 | 0.002* |
| PAI-1 4G/5G |
5G/5G 4G/5G 4G/4G |
18 (17.1) 63 (60.0) 24 (22.9) |
22 (18.3) 62 (51.7) 36 (30.0) |
1.816 | 0.408 |
UI, unexplained infertility; FVL, factor V Leiden; FII, coagulation factor II; MTHFR, methylenetetrahydrofolate reductase; PAI-1, plasminogen activator inhibitor 1; χ2, chi-square; df, degrees of freedom; FET, Fisher exact test
*Significant p value
Table 3.
Distribution of SNPs alleles in study subjects
| SNP | Allele | Patients with UI (n = 105) n (%) |
Control group (n = 120) n (%) |
χ2 (df = 2) or FET | p value | OR (95% CI) |
|---|---|---|---|---|---|---|
|
FVL G1691A |
G A |
203 (96.7) 7 (3.3) |
236 (98.3) 4 (1.7) |
FET | 0.361 | 2.27 (0.655–7.851) |
|
FII G20210A |
G A |
205 (97.6) 5 (2.4) |
237 (98.8) 3 (1.3) |
FET | 0.482 | 1.93 (0.455–8.161) |
| MTHFR C677T |
C T |
103 (49.1) 107 (50.9) |
153 (63.8) 87 (36.3) |
9.8714 | 0.0018* | 1.83 (1.153–2.665) |
| PAI-1 4G/5G |
5G 4G |
99 (47.1) 111 (52.9) |
106 (44.2) 134 (55.8) |
0.400 | 0.527 | 0.89 (0.612–1.287) |
UI, unexplained infertility; FVL, factor V Leiden (G1691A); FII, coagulation factor II; MTHFR, methylenetetrahydrofolate reductase; PAI-1, plasminogen activator inhibitor 1; OR, odds ratio; CI, confidence interval; χ2, chi-square; df, degrees of freedom; FET, Fisher exact test
*Significant p value
There was also a significant difference in the frequency of C677T MTHFR alleles between the groups, with the risk allele T being more frequent in patients (OR = 1.83 (95% CI, 1.15–2.67); p = 0.0018). Dominant and recessive genetic models for MTHFR and PAI-1 polymorphisms were also analyzed. Again, the significant difference was determined for MTHFR C677T in a dominant model. There were significantly more subjects with CC genotype in the control group and more dominant, CT + TT, model of inheritance in the patients group (p = 0.0004) (OR = 2.93; 95% CI, 1.59–5.39) (Table 4).
Table 4.
Dominant and recessive genetic models of MTHFR and PAI-1 polymorphisms
| SNP | Dominant model, n (%) | Recessive model, n (%) | |||
|---|---|---|---|---|---|
| UI patients | Controls | UI patients | Controls | ||
| MTHFR C677T | CC | 20 (19.1) | 49 (40.8) | 83 (79.0) | 104 (86.7) |
| CT | 85 (80.9) | 71 (59.2) | |||
| TT | 22 (21.0) | 16 (13.3) | |||
| OR (95%CI) | 2.93 (1.59–5.39) | 1.72 (0.85–3.49) | |||
| p value of χ2 | 0.0004* | 0.128 | |||
| PAI-1 4G/5G | 5G/5G | 18 (17.1) | 22 (18.3) | 81 (77.1) | 84 (70.0) |
| 4G/5G | 87 (82.9) | 98 (81.7) | |||
| 4G/4G | 24 (22.9) | 36 (30.0) | |||
| OR (95%CI) | 1.08 (0.54–2.16) | 0.69 (0.38–1.26) | |||
| p value of χ2 | 0.816 | 0.227 | |||
SNP, single nucleotide polymorphism; UI, unexplained infertility; MTHFR, methylenetetrahydrofolate reductase; PAI-1, plasminogen activator inhibitor 1; OR, odds ratio; CI, confidence interval
*Significant p value
Results of logistic regression
Two binomial logistic regression analyses were applied to evaluate the association of UI with the presence of one or more thrombophilic SNPs. A model comprising all the genotypes of all SNPs was statistically significant (χ2 = 15.625, df = 6, p = 0.016), and correctly classified 60.9% cases, with positive predictive value of 58.1% (specificity = 66.7%, sensitivity = 53.3%). A significant association was found only for MTHFR C677T polymorphism (p = 0.004). The presence of TT genotype increased 2.99-fold the probability of UI (95% CI, 1.26–7.12; p = 0.018), while CT genotype was associated with 2.79-fold increased risk of UI (95% CI, 1.47–5.29; p = 0.002). When the referent MTHFR genotype in this model was set to be TT, the CC genotype decreased 0.33-fold the risk for UI (95% CI, 0.14–0.79; p = 0.013).
The second logistic regression analysis included only dominant and recessive models for MTHFR and PAI-1 polymorphisms, since there were no recessive genotypes for FVL and PGM. The model had the specificity of 54.2% and sensitivity of 66.7%. In the dominant MTHFR C677T model (CT + TT), the CC genotype decreased 0.36-fold the risk of UI (95% CI, 0.191–0.681; p = 0.002) (Table 5).
Table 5.
Association of thrombophilia SNPs and UI, results of the logistic regressions
| B | OR | 95% CI | p value | |
|---|---|---|---|---|
| A model with all SNPs and genotypes | ||||
| FVL (GA) | 0.583 | 1.792 | 0.457–7.026 | 0.403 |
| FII (GA) | 0.663 | 1.941 | 0.430–8.767 | 0.389 |
| MTHFR | 0.004* | |||
| MTHFR (CT) | 1.026 | 2.790 | 1.473–5.285 | 0.002* |
| MTHFR (TT) | 1.097 | 2.996 | 1.262–7.116 | 0.018* |
| MTHFR (CC)** | − 1.097 | 0.334 | 0.141–0.793 | 0.013* |
| PAI1 | 0.610 | |||
| PAI1 (4G/4G) | − 0.043 | 0.958 | 0.413–2.220 | 0.920 |
| PAI1 (4G/5G) | 0.251 | 1.286 | 0.614–2.692 | 0.505 |
| A model with dominant and recessive MTHFR and PAI-1 genotype models | ||||
| MTHFR dominant (CT + TT vs. CC) | − 1.021 | 0.360 | 0.191–0.681 | 0.002* |
| MTHFR recessive (TT vs. CC + CT) | − 0.149 | 0.862 | 0.410–1.812 | 0.695 |
| PAI-1 dominant (5G/4G + 4G/4G vs. 5G/5G) | − 0.230 | 0.795 | 0.381–1.656 | 0.540 |
| PAI-1 recessive (4G/4G vs. 5G/5G + 5G/4G) | − 0.324 | 0.723 | 0.379–1.380 | 0.325 |
UI, unexplained infertility; FVL, factor V Leiden (G1691A); PGM, prothrombin G20210A mutation; MTHFR, methylenetetrahydrofolate reductase; PAI-1, plasminogen activator inhibitor 1; B, unstandardized regression weight; OR, odds ratio; CI, confidence interval
*Significant p value, ** the same model, but with TT genotype set as a reference for comparison of other MTHFR genotypes
Results of MDR analysis
Four SNPs were included in the MDR analysis to assess their prediction potential and interactions. As expected, MTHFR C677T polymorphism was a significant single-locus model, with a perfect CVC (showing the reproducibility of the model in 100%) and a test accuracy of 60.85% (p = 0.015) (OR = 2.93 (1.59–5.39), p = 0.0004). However, the best significant predictive model was the two-locus model comprising MTHFR C677T polymorphism and FVL mutation (CVC = 10/10, TA = 60.95%, p = 0.013) (OR = 3.02 (1.63–5.59), p = 0.0003). Additionally, the three-locus model (FVL, FII, MTHFR) showed a significant risk (OR = 2.8, p = 0.001) with accuracy of 59.4%, but permutation test showed only boundary reliability for this model (p = 0.049) (Table 6).
Table 6.
SNP-SNP interaction models using MDR analysis
| Number of loci | Model | TA (%) | CV consistency | OR, p value | p value* |
|---|---|---|---|---|---|
| 1 | MTHFR | 60.89 | 10/10 | 2.930, 0.0004 | 0.015 |
| 2 | FV, MTHFR | 60.95 | 10/10 | 3.018, 0.0003 | 0.013 |
| 3 | FV, FII, MTHFR | 59.35 | 8/10 | 2.814, 0.0008 | 0.049 |
| 4 | FV, FII, MTHFR, PAI-1 | 56.19 | 10/10 | 3.000, 0.0009 | 0.221 |
SNP, single nucleotide polymorphism; MDR, multifactor dimensionality reduction; CV, cross-validation; TA, balanced accuracy testing; OR, odds ratio
*Based on 1.000 permutation testing
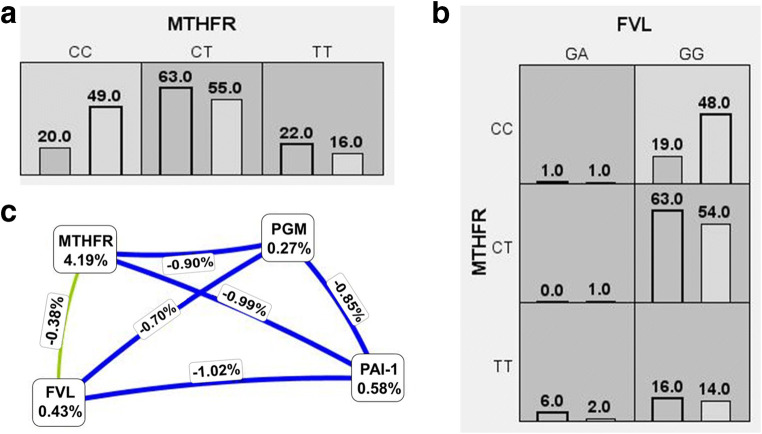
Figure 1 shows low- and high-risk genotype combinations of significant SNPs, as well as an entropy-based interaction graph. Only the subjects with CC genotype of the MTHFR gene and those with MTHFR CC plus FVL GG genotypes were at low risk for UI development.
Fig. 1.
Low- and high-risk genotype combinations of one (A) and two-locus (B) model and cluster analysis of Fruchterman-Reingold dendogram (C). High-risk genotypes are presented with the dark gray cells. Left bar in each cell corresponds to the number of patients and right bar to the number of controls. Interaction graph is based on entropy. Entropy values of the SNPs are marked in the cells, showing the main independent effects, and those on the lines represent the entropy of interaction. The independent effect is shadowed green, while blue denotes absence of interaction
As presented on the graph, only MTHFR C677T entropy (4.19%) is distinguishable from the others, which correlates with its significant independent main effect. The graphics shows the impact of tested SNPs in the following descending order of entropy: MTHFR C > T, PAI-1 5G > 4G, FVL G > A, and FII G > A. Most SNP-SNP interactions were negligible (blue lines), except for MTHFR-FVL (green line) that showed a reduced degree of redundancy or “independent effects” of the impact, decreasing by 0.38% with their combined effect.
Furthermore, we evaluated linkage equilibrium for MTHFR 677 T and FVL 1691A and determined high disequilibrium in patient group (D′ = 0.71 and r2 = 0.02) and low in the controls (D′ = 0.22 and r2 = 0.001). However, there is a discrepancy between determined coefficients which is due to the marked differences of minor alleles frequencies (very low for FVL (3.3%) and high for MTHFR (> 50%)). In other words, in 71% of patients, SNPs would be coinherited, but only the rare allele (A in FVL) could point to the concomitant presence of the more frequent minor allele in another SNP (T in MTHFR), not vice versa.
Discussion
Many genetic and environmental factors are involved in the pathogenesis of infertility. In fact, epistasis, or gene-gene and gene-environment interactions, has a crucial role in determining the phenotype of a disease. Frequency of infertility of unknown cause is relatively high, up to 30% of couples involved in ART programs [1, 6, 19]. One of the pathogenic mechanisms that has been associated with unexplained infertility is inappropriate blood vessel formation [19, 20]. Since primary trophoblast invasion requires degradation of extracellular matrix and endometrial blood vessel adaptation, an adequate engagement of coagulation and fibrinolysis is necessary. These processes must be accurately organized to provide adequate fibrin deposition that would further promote cell migration and consequent trophoblast enhancement [2, 4]. At the same time, invasion of decidual vessels is accompanied by increased tissue factor production and PAI-1 activity [21]. Unusual forms of fibrin deposition and massive perivillous fibrin deposits have been correlated with thrombophilia [22, 23]. Increased thrombin production, such as in PGM, evokes decidual cells to produce anti-angiogenic fms-like tyrosine kinase-1 factor, which inhibits extravillous trophoblast (EVT) proliferation. Shallow EVT invasion and incomplete vascular transformation result in embryonal under-perfusion [21].
As for MTHFR, the C677T polymorphism reduces MTHFR enzyme activity and TT genotype creates a thermolabile enzyme with about 30% of expected activity. This further blocks the methionine metabolism and potentially elevates homocysteine level, while decreasing folate concentrations in the plasma [2, 24]. Among others, hyperhomocysteinemia is linked with increased thrombin generation, augmented factor V activity, and impaired fibrinolytic potential [10]. Moreover, it has been suggested that folate deficiency itself underlies UI by affecting oocyte maturation and embryo implantation through the mechanisms of DNA hypomethylation and fragmentation, cell division, oxidative and nitrosative stress, proinflammatory cytokine secretion, etc. [10, 25, 26].
In this study, we found a significant association of UI with the common C677T polymorphism of the MTHFR gene. Chi-square test and logistic regressions both confirmed the C677T SNP as an important factor for fertility. The allele T increased 1.83-fold the risk for UI. The second important finding was its significant interaction with FVL G1691A mutation and mutual association with infertility. A single factor MDR model with MTHFR C677T showed reliability, high reproducibility, and a 2.9-fold increased risk for UI (p = 0.0004), while SNP-SNP interactions showed high risk in the two-component model involving FVL and MTHFR C677T, with the best accuracy of risk prediction, 60.9% (OR = 3.02, p = 0.0003). Permutation tests confirmed the significance of these SNPs models.
The polymorphisms of the MTHFR gene have been extensively studied. Our results are in accordance with the previous reports of association between MTHFR C677T polymorphism and unexplained female and male infertility and early spontaneous abortion [25, 27, 28]. Increased prevalence of CT and TT genotypes of MTHFR has been found to be a risk factor for male infertility, due to derangement of DNA integrity and methylation process, that even increased spontaneous abortion in their spouses [27]. Maternal C677T polymorphism was associated with preterm birth risk under allele contrast (T vs. C), homozygote TT, and recessive model, and low birth weight susceptibility for the same traits plus dominant model (a meta-analysis) [29].
With reference to the various results of thrombophilia prevalence, several investigators previously observed that FVL could increase the risk of infertility, while others did not have such findings [6, 12, 30]. Ricci et al. [12] found the same prevalence of FVL and FII G20210A in asymptomatic females undergoing IVF and those with spontaneous pregnancies, without any significant effect on the outcome. Similar to our study, Tanacan et al. [26] determined high heterozygous FVL frequency (18.9%) in patients with at least one failed IVF cycle who spontaneously became pregnant after proper management. However, FVL was a risk factor for very early, unexplained, recurrent miscarriage (before 10 weeks) with OR of 2.4 [31]. Interestingly, some investigators reported increased implantation rate after IVF in FVL carriers compared to non-carriers. They hypothesized that this was a genetic advantage related to faster hemostasis [13, 32].
With employment of MDR analysis, we identified a significant random association of C677T MTHFR and G1691A FVL genetic variants. As indicated in Fig. 1, an independent relationship may exist between the two SNPs that makes the best prediction of susceptibility to UI compared to other single SNPs tested.
These two SNPs are reported to be in a LD [33]. However, studying rare alleles (minor allele 5%) is difficult, since their main effect (as LD reflects the presence of mutation) and statistical power are often lacking. It is not merely because of LD, but to account for the minor allele frequencies in these patients. This may lead to exaggerated D′ values, but as r2 is very low, SNPs cannot substitute for one another. Therefore, the conclusions must be taken with caution [34].
A genetic background and environment certainly modify the expression of polymorphisms. In that sense, some studies found or were unable to find a significant influence of thrombophilic SNPs on IVF patients. For example, the MTHFR C677T polymorphism did not represent a risk factor for infertility in the Brazilian population (n = 130), even when it was homozygous [35]. On the other hand, the frequency of PAI-1 4G/5G was significantly changed among the Greek infertile females, with normal homozygous genotype being more frequent [19]. Although increased PAI-1 expression has been associated with an increased risk for infertility, repeated implantation failure, and worse pregnancy outcome [4, 11, 36], we found no significant difference in its 4G/5G genotype nor allele frequencies among our subjects.
Current guidelines do not recommend thrombophilia testing (especially polymorphisms) for infertility patients, and there is no evidence for the recommendation of anticoagulant (heparin) treatment [9]. Evaluation and management of inherited thrombophilia, especially MTHFR polymorphisms, may be beneficial for patients with UI and may even be associated with spontaneous pregnancies [26]. Importantly, anticoagulant medications may not be necessary in individuals with MTHFR minor allele T, and supplementation with folic acid should be sufficient, but nevertheless necessary treatment for UI [25, 37].
Additionally, it would be valuable to test interactions of other thrombophilia SNPs that showed significant associations with infertility and pregnancy complications, but are not routinely tested in these patients, such as those for annexin V C4/M2 haplotype, platelet glycoprotein III Leu33Pro, and FXIII Val34Leu [3, 38–40]. It would also be interesting to consider the genotype of the male partner, as there are indications that the male carrier status, and thus the embryonal genotype, may influence the process of implantation [38].
There are two limitations to our study. Firstly, our control group is a general population group, meaning that although the controls fulfilled necessary testing and questionnaire requirements in order to become blood donors and to participate in our study, they were not all confirmed to be fertile. Secondly, since the FVL and FII G20210A are mutations, their minimal allele frequency is very low, so the study power in our sample size is not enough.
Conclusion
We may draw the conclusion that MTHFR C677T polymorphism and FVL G1691A mutation are inherited thrombophilic factors associated with UI. Disturbances in folate metabolism with the MTHFR C677T predominantly affect these patients, probably through multiple pathways, not only related to its thrombotic potential. The minor allele T is more frequent among the patients. Additionally, there is a significant increased concomitant presence of MTHFR C677T and FVL mutation in UI patients. The association of these two SNPs should, therefore, be recognized at least as a potential contributing factor involved in the pathogenesis of infertility.
Authors’ contributions
J. Milenkovic and M. Milojkovic conceptualized and designed the study. Material preparation, data collection, and analyses were performed by J. Milenkovic, D. Mitic, Z. Smelcerovic, S. Vujic, and N. Bojanic. T. Jevtovic-Stoimenov coordinated and supervised data collection and processing. The first draft of the manuscript was written by J. Milenkovic and D. Stojanovic. M. Milojkovic, D. Mitic, and T. Jevtovic-Stoimenov critically reviewed the manuscript. All authors read, commented, and approved the final manuscript.
Funding information
The work is supported by the Ministry of Education, Science and Technological Development of Republic of Serbia, Project No. III41018; and the Faculty of Medicine University of Nis Serbia, Project No. 3 (2017-19) and No. (2020).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhattacharya S, Maheshwari A, Mollison J. Factors associated with failed treatment: an analysis of 121,744 women embarking on their first IVF cycles. PLoS ONE. 2013;8(12):e82249. doi: 10.1371/journal.pone.0082249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivanov P, Tomov S, Tsvyatkovska T, Konova E, Komsa-Penkova R. Thrombophilia in assisted reproductive technology — place and needs of thromboprophylaxis. In: Ivanov P, editor. Pregnancy thrombophilia - the unsuspected risk. London: Intech Open; 2013. pp. 129–158. [Google Scholar]
- 3.Patounakis G, Bergh E, Forman EJ, Tao X, Lonczak A, Franasiak JM, Treff N, Scott RT., Jr Multiple thrombophilic single nucleotide polymorphisms lack a significant effect on outcomes in fresh IVF cycles: an analysis of 1717 patients. J Assist Reprod Genet. 2016;33(1):67–73. doi: 10.1007/s10815-015-0606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye Y, Vattai A, Zhang X, Zhu J, Thaler CJ, Mahner S, Jeschke U, von Schönfeldt V. Role of plasminogen activator inhibitor type 1 in pathologies of female reproductive diseases. Int J Mol Sci. 2017;18(8):1651. doi: 10.3390/ijms18081651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redline RW. Thrombophilia and placental pathology. Clin Obstet Gynecol. 2006;49:885–894. doi: 10.1097/01.grf.0000211957.68745.6b. [DOI] [PubMed] [Google Scholar]
- 6.Safdarian L, Najmi Z, Aleyasin A, Aghahosseini M, Rashidi M, Asadollah S. Recurrent IVF failure and hereditary thrombophilia. Iran J Reprod Med. 2014;12(7):467–470. [PMC free article] [PubMed] [Google Scholar]
- 7.Kinzler WL, Prasad V. Ananth CV; New Jersey-Placental Abruption Study Investigators. The effect of maternal thrombophilia on placental abruption: histologic correlates. J Matern Fetal Neonatal Med. 2009;22(3):243–248. doi: 10.1080/14767050802551795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Royal College of Obstetricians and Gynaecologists . Thrombosis and embolism during pregnancy and the puerperium, reducing the risk (Green-top Guideline No. 37a) London: Royal College of Obstetricians and Gynaecologists; 2015. [Google Scholar]
- 9.American College of Medical Genetics ACOG Practice Bulletin No. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132(1):e18–e34. doi: 10.1097/AOG.0000000000002703. [DOI] [PubMed] [Google Scholar]
- 10.Undas A, Brozek J, Szczeklik A. Homocysteine and thrombosis: from basic science to clinical evidence. Thromb Haemost. 2005;94(5):907–915. doi: 10.1160/TH05-05-0313. [DOI] [PubMed] [Google Scholar]
- 11.Coulam CB, Jeyendran RS, Fishel LA, Roussev R. Multiple thrombophilic gene mutations rather than specific gene mutations are risk factors for recurrent miscarriage. Am J Reprod Immunol. 2006;55(5):360–368. doi: 10.1111/j.1600-0897.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- 12.Ricci G, Bogatti P, Fischer-Tamaro L, Giolo E, Luppi S, Montico M, Ronfani L, Morgutti M. Factor V Leiden and prothrombin gene G20210A mutation and in vitro fertilization: prospective cohort study. Hum Reprod. 2011;26(11):3068–3077. doi: 10.1093/humrep/der261. [DOI] [PubMed] [Google Scholar]
- 13.Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003;19(3):376–382. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- 14.Motsinger AA, Ritchie MD. Multifactor dimensionality reduction: an analysis strategy for modelling and detecting gene - gene interactions in human genetics and pharmacogenomics studies. Hum Genomics. 2006;2(5):318–328. doi: 10.1186/1479-7364-2-5-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endler G, Kyrle PA, Eichinger S, Exner M, Mannhalter C. Multiplexed mutagenically separated PCR: simultaneous single-tube detection of the factor V R506Q (G1691A), the prothrombin G20210A, and the methylenetetrahydrofolate reductase A223V (C677T) variants. Clin Chem. 2001;47:333–335. doi: 10.1093/clinchem/47.2.333. [DOI] [PubMed] [Google Scholar]
- 16.Isordia-Salas I, Leaños-Miranda A, Sainz IM, Reyes-Maldonado E, Borrayo-Sánchez G. Association of the plasminogen activator inhibitor-1 gene 4G/5G polymorphism with ST elevation acute myocardial infarction in young patients. Rev Esp Cardiol. 2009;62:365–372. doi: 10.1016/S0300-8932(09)70893-0. [DOI] [PubMed] [Google Scholar]
- 17.Moore JH, Andrews PC. Epistasis analysis using multifactor dimensionality reduction. In: Moore J, Williams S, editors. Epistasis. Methods in molecular biology (methods and protocols) New York: Humana Press; 2015. pp. 301–314. [DOI] [PubMed] [Google Scholar]
- 18.Lewontin RC. The interaction of selection and linkage. I. General considerations; heterotic models. Genetics. 1964;49(1):49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kydonopoulou K, Delkos D, Rousso D, Ilonidis G, Mandala E. Association of plasminogen activator inhibitor-type 1 (PAI-1) -675 4G/5G polymorphism with unexplained female infertility. Hippokratia. 2017;21(4):180–185. [PMC free article] [PubMed] [Google Scholar]
- 20.Lash GE, Innes BA, Drury JA, Robson SC, Quenby S, Bulmer JN. Localization of angiogenic growth factors and their receptors in the human endometrium throughout the menstrual cycle and in recurrent miscarriage. Hum Reprod. 2012;27(1):183–195. doi: 10.1093/humrep/der376. [DOI] [PubMed] [Google Scholar]
- 21.Lockwood CJ, Krikun G, Rahman M, Caze R, Buchwalder L, Schatz F. The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin Thromb Hemost. 2007;33:111–117. doi: 10.1055/s-2006-958469. [DOI] [PubMed] [Google Scholar]
- 22.Rogers BB, Momirova V, Dizon-townson D, Wenstrom K, Samuels P, Sibai B, Spong C, Caritis SN, Sorokin Y, Miodovnik M, Sullivan O, Conway MJ, Wapner DRJ. Avascular villi, increased syncytial knots, and hypervascular villi are associated with pregnancies complicated by factor V Leiden mutation. Pediatr Dev Pathol. 2010;13:341–347. doi: 10.2350/09-05-0657-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gogia N, Machin GA. Maternal thrombophilias are associated with specific placental lesions. Pediatr Dev Pathol. 2008;11:424–429. doi: 10.2350/07-09-0345.1. [DOI] [PubMed] [Google Scholar]
- 24.Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A, Červeňová T, Halašová E, Lehotský J. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. 2016;17(10):1733. doi: 10.3390/ijms17101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altmäe S, Stavreus-Evers A, Ruiz JR, Laanpere M, Syvänen T, Yngve A, Salumets A, Nilsson TK. Variations in folate pathway genes are associated with unexplained female infertility. Fertil Steril. 2010;94(1):130–137. doi: 10.1016/j.fertnstert.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Tanacan A, Beksac MS. Spontaneous pregnancies in patients with at least one failed IVF cycle after the management of autoimmune disorders, hereditary thrombophilia, and methylation disorders. JBRA Assist Reprod. 2019;23(4):361–366. doi: 10.5935/1518-0557.20190034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zhang F, Dai L. C677T polymorphism increases the risk of early spontaneous abortion. J Assist Reprod Genet. 2019;36(8):1737–1741. doi: 10.1007/s10815-019-01500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong M, Dong W, He T, Shi Z, Huang G, Ren R, Huang S, Qiu S, Yuan R. MTHFR 677C > T polymorphism increases the male infertility risk: a meta-analysis involving 26 studies. PLoS One. 2015;10(3):e0121147. doi: 10.1371/journal.pone.0121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Zhu P, Geng X, Liu Z, Cui L, Gao Z, Jiang B, Yang L. Genetic polymorphism of MTHFR C677T with preterm birth and low birth weight susceptibility: a meta-analysis. Arch Gynecol Obstet. 2017;295(5):1105–1118. doi: 10.1007/s00404-017-4322-z. [DOI] [PubMed] [Google Scholar]
- 30.Behjati R, Modarressi MH, Jeddi-Tehrani M, Dokoohaki P, Ghasemi J, Zarnani AH, Aarabi M, Memariani T, Ghaffari M, Akhondi MA. Thrombophilic mutations in Iranian patients with infertility and recurrent spontaneous abortion. Ann Hematol. 2006;85(4):268–271. doi: 10.1007/s00277-005-0021-0. [DOI] [PubMed] [Google Scholar]
- 31.Reznikoff-Etiévan MF, Cayol V, Carbonne B, Robert A, Coulet F, Milliez J. Factor V Leiden and G20210A prothrombin mutations are risk factors for very early recurrent miscarriage. BJOG. 2001;108(12):1251–1254. doi: 10.1111/j.1471-0528.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- 32.Steinvil A, Raz R, Berliner S, Steinberg DM, Zeltser D, Levran D, Shimron O, Sella T, Chodick G, Shalev V, Salomon O. Association of common thrombophilias and antiphospholipid antibodies with success rate of in vitro fertilisation. Thromb Haemost. 2012;108(6):1192–1197. doi: 10.1160/TH12-06-0381. [DOI] [PubMed] [Google Scholar]
- 33.O’Shaughnessy KM, Fu B, Ferraro F, Lewis I, Downing S, Morris NH. Factor V Leiden and thermolabile methylenetetrahydrofolate reductase gene variants in an East Anglian preeclampsia cohort. Hypertension. 1999;33:1338–1341. doi: 10.1161/01.HYP.33.6.1338. [DOI] [PubMed] [Google Scholar]
- 34.Slatkin M. Linkage disequilibrium — understanding the evolutionary past and mapping the medical future. Nat Rev Genet. 2008;9:477–485. doi: 10.1038/nrg2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soligo AG, Barini R, Annichino-Bizzacchi JM. Prevalence of the MTHFR C677T mutation in fertile and infertile women. Prevalência da mutação MTHFR C677T em mulheres férteis e inférteis. Rev Bras Ginecol Obstet. 2017;39(12):659–662. doi: 10.1055/s-0037-1606289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gris JC, Ripart-Neveu S, Maugard C, Tailland ML, Brun S, Courtieu C, Biron C, Hoffet M, Hédon B, Marès P. Respective evaluation of the prevalence of haemostasis abnormalities in unexplained primary early recurrent miscarriages. The Nimes Obstetricians and Haematologists (NOHA) Study. Thromb Haemost. 1997;77:1096–1103. doi: 10.1055/s-0038-1656119. [DOI] [PubMed] [Google Scholar]
- 37.Ata B, Urman B. Thrombophilia and assisted reproduction technology—any detrimental impact or unnecessary overuse? J Assist Reprod Genet. 2016;33:1305–1310. doi: 10.1007/s10815-016-0771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fishel S, Patel R, Lytollis A, Robinson J, Smedley M, Smith P, Camerona C, Thorntona S, Dowella K, Atkinsonb G, Shakere A, Lowed P, Kazemc R, Brettf S, Foxf A. Multicentre study of the clinical relevance of screening IVF patients for carrier status of the annexin A5 M2 haplotype. Reprod BioMed Online. 2014;29:80–87. doi: 10.1016/j.rbmo.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Diaz-Nuñez M, Rabanal A, Expósito A, Ferrando M, Quintana F, Manuel Soria J, Matorras R. Recurrent miscarriage and implantation failure of unknown cause studied by a panel of thrombophilia conditions: increased frequency of FXIII Val34Leu polymorphism. J Reprod Infertil. 2019;20:76–82. [PMC free article] [PubMed] [Google Scholar]
- 40.Vlachadis N, Tsamadias V, Vrachnis N, Kaparos G, Vitoratos N, Kouskouni E, Economou E. Associations of combined polymorphisms of the platelet membrane glycoproteins Ia and IIIa and the platelet-endothelial cell adhesion molecule-1 and P-Selectin genes with IVF implantation failures. J Obstet Gynaecol. 2017;37:363–369. doi: 10.1080/01443615.2016.1256978. [DOI] [PubMed] [Google Scholar]