Abstract
Purpose
Binovular follicles including a pair of conjoined oocytes within a common zona pellucida or their fusion in the zonal region gained some attentions due to its possible role in dizygotic twins. Although some cases in the literature been reported in which two conjoined oocytes arising from binovular follicles were mature, and injected with two separated sperm, no available evidence reported for dizygotic twin pregnancies.
Methods
A case report of a 37-year-old female patient underwent embryo transfer cycle whereby a pair of conjoined blastocysts after ICSI of a pair of conjoined oocytes was transferred.
Results
The β-hCG level was positive 15 days after embryo transfer. The subsequent pregnancy scan revealed a dizygotic pregnancy. The woman gave birth to two healthy boys in the mid of 38 weeks of gestation by cesarean section.
Conclusions
Given the insufficient evidence on how to handle conjoined oocytes, this report acknowledges the first occurrence of dizygotic twin delivery resulted from transfer of a pair of conjoined blastocysts after ICSI of a pair of conjoined oocytes. This also confirms that we should be extremely conservative in discarding any mature oocyte without sufficient data about its useless future to result in a healthy baby.
Keywords: Dizygotic twin, conjoined oocytes, binovular follicles
Introduction
Dizygotic twins (DZ) comprise approximately two-thirds of all twins and result from fertilization of two oocytes with two sperm [1, 2]. The introduction of assisted reproductive techniques (ARTs) has increased the incidence of DZ pregnancies [3].
A possible mechanism for conception in DZ is that a mature pair of conjoined oocytes within a single zona pellucida originates from binovular follicle, can be fertilized by two sperm and continue development [4–7]. Three assumptions can explain the formation of binovular follicles: (i) abnormal meiotic division can result in two oocytes within a single zona pellucida [4], (ii) fusion of two individual follicles [8], or (iii) a developmental accident due to failure of connective tissue to separate closely proximate oocytes [7]. The most probable is the latter mechanism [9, 10] as binovular follicles represent a natural polymorphism rather than a pathological phenomenon [9, 11].
Zeilmaker et al. (1983) reported the first case of a binovular complex containing two mature oocytes in humans with no reported pregnancy [7]. However, Zeilmaker et al. suggested that maturity of pair of conjoined oocytes followed by fertilization and further developmental capacity of both oocytes may be a cause of DZ. Following this, there were 24 other reports with one or two mature oocytes [12–14]. However, a pair of mature conjoined oocytes arising from binovular follicles has never been observed or proven to be a cause of dizygotic twinning [12].
We report, for the first time, a case of DZ twin livebirth from conjoined oocytes after intracytoplasmic sperm injection (ICSI).
Case report
Patient history
A 37-year-old Egyptian woman and her 42-year-old Egyptian husband with 5-year secondary infertility were seen at Al-Yasmeen fertility and gynecology center. The woman had regular menstrual cycles, but her partner has asthenozoospermia confirmed on three occasions. Two antral follicles in each ovary were seen at basal vaginal ultrasound scan in January 2018. Her AMH was 0.62 ng/ml, while her basal hormone concentrations were 9.55 mIU/ml FSH, 8.01 IU/ml LH, and 52.31 pg/ml estradiol on cycle day 2.
Ovarian stimulation and ICSI
The couple was counseled for ICSI after appropriate investigations. The long protocol regimen was used for pituitary downregulation using GnRH analogue (Lucrin®, Abbott, Rungis Cedex, France). Ovulation induction was undertaken using recombinant human follicle stimulating hormone (FSH) (Gonal-F®, Serono, Geneva, Switzerland), 300 IU daily. After 10 days of ovarian stimulation, the patient’s peak estradiol level was 1430 pg/ml, and her progesterone level was 0.7 ng/ml. Recombinant human chorionic gonadotrophin (HCG) (OvitrelleR, Serono, Geneva, Switzerland) was administered subcutaneously for final oocyte maturation, with 6 oocytes retrieved after 35 h.
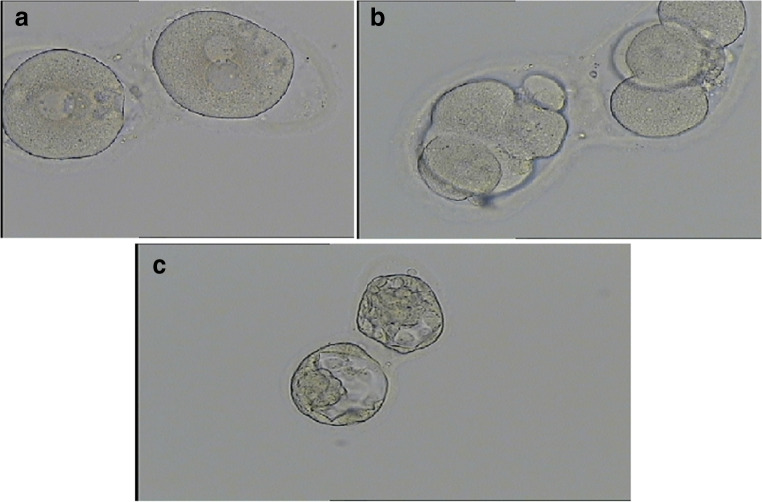
Five oocytes were mature including two conjoined oocytes. Both conjoined oocytes were normal-sized metaphase II (MII), surrounded by an individual zona pellucida (ZP), with the two ZP connected by a thin common isthmus of ZP and appeared to be fused (Fig. 1). The semen sample used for ICSI showed a sperm concentration of 17 million/ml, 30% motility with 5% forward progression, and 95% abnormal forms. ICSI was performed for the 5 mature oocytes, of which 3 fertilized and yielded 2PN zygotes, including two conjoined zygotes (Fig. 2a). On day 2 after ICSI, the two fertilized conjoined oocyte had cleaved to the four-cell stage (Fig. 1), and both reached the blastocyst stage on day 5, while the remaining separated embryo was blocked at cleavage stage and did not reach blastocyst stage.
Fig. 1.
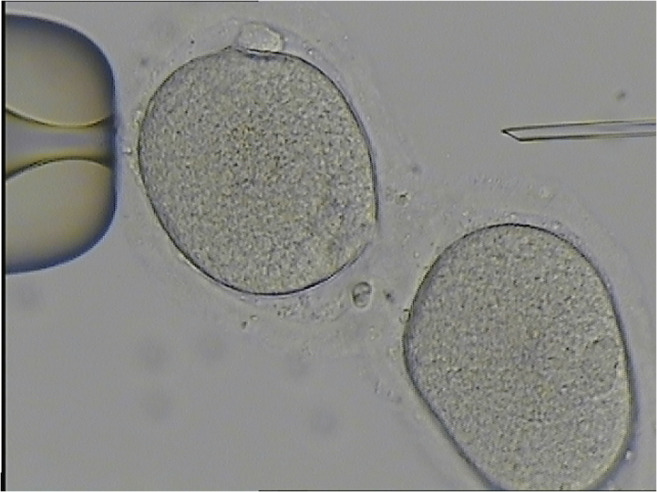
A pair of conjoined MII normal-sized oocytes arise from binovular complex
Fig. 2.
a Both conjoined oocytes were shown to be fertilized, having two pronuclei (2PN), b two good quality 4-cell embryos on day 2 after ICSI, c two good quality blastocysts (2AA) attached at the zona resulting from the conjoined oocyte
On day 5 and before the embryo transfer procedure, the patient was informed with all the details about the conjoined blastocysts developed from unfamiliar mature conjoined oocytes. We informed her about the possibility of pregnancy and deliver of healthy livebirth as previously reported in only two cases [13, 14]. The possibility of twin pregnancy was also discussed, although no current evidence supports this hypothesis.
Prior to embryo transfer, separation of both blastocysts was done using LASER with the aid of mechanical pipetting. Both blastocysts were graded as 2AA according to the classification of Gardner and Schoolcraft [15] and transferred on day 5 (111 h after ICSI). At time of transfer, the endometrial thickness measured 9.5 mm. The luteal phase was supported with intramuscular progesterone (100 mg/mL, Prontogest; IBSA), starting on the day of oocyte retrieval.
The β-hCG level was positive 15 days after embryo transfer (1457.4 mIU/mL). The patient was then referred back to her obstetrician, who performed transvaginal sonography at 7 weeks’ gestation. Examination revealed a normal clinical pregnancy with two gestational sacs with heartbeats. The woman gave birth to two healthy boys (2350 and 2420 g) in the mid of 38 week of gestation by cesarean section. Their Apgar scores at 1 and 5 min were 10–10 for both twins. Separate placentas were identified at delivery determined dizygosity. Written consent was obtained from the patient for anonymized data only to be used for the purposes of scientific discussion and publication.
Discussion
We report, to our knowledge, the first occurrence of DZ twin delivery resulted from transfer of a pair of conjoined blastocysts after ICSI of a pair of conjoined oocytes.
Since the inception of ART, handling gametes in vitro has shed light on irregularities in their morphology and development. The normal pathway for a follicle to develop should result in a haploid secondary oocyte of diploid oogonium following the first meiotic division [2]. Binovular follicles including a pair of conjoined oocytes within a common zona pellucida or their fusion in the zonal region constitute a notable exception [8, 16]. Conjoined oocytes have drawn attentions due to its possible role in DZ twining [7]. However, around two-thirds of binovular follicles result in discordant conjoined oocytes concerning the maturity [17], with at least one of them is germinal vesicle (GV) or atretic [8, 17, 18]. When both of the conjoined oocytes are mature, they are rarely both fertilized, with no reported DZ delivery thus far [7, 13].
There are very limited data available in the literature on clinical significance of conjoined oocytes in the IVF laboratory setting [14]. This may be due to the rarity of such event. Furthermore, it is possible that these oocytes would be classified as abnormal and discarded without attempts at fertilization and subsequent embryo culture [14, 19]. Moreover, some reports in the literature concluded that mature oocytes contained in conjoined-oocyte complexes should be considered as abnormal, because some showed limited developmental potential after fertilization [20]. However, the delivery of the first healthy livebirth from a genetically normal blastocyst obtained from conjoined oocytes provided the first evidence that such conjoined oocytes can be safely used to achieve viable pregnancies and livebirth [14]. Another successful pregnancy and livebirth was reported by Yano et al., 2017, in which single blastocyst was developed from fertilization of one mature oocyte from two mature conjoined oocytes [13]. Herein, our experience involved two blastocyst obtained from a pair conjoined oocytes, that resulted in DZ pregnancy ended with birth of two healthy babies. This further confirms that we should be extremely conservative in discarding any mature oocyte without sufficient data about its useless future to result in a healthy baby.
To our knowledge, this is the first reported case that a conjoined pair of mature MII oocytes from a single human follicle; both of them were fertilized and developed normally in culture up to blastocyst stage. Both conjoined blastocysts were transferred at day 5. Sonographic data confirmed that both of blastocysts implanted initially after embryo transfer, resulted in two viable gestational sacs and culminated in births. Thus, this shows clear evidence to support the idea that a pair of mature conjoined oocytes arising from binovular follicles may be a cause of dizygotic twinning.
In conclusion, this report provides exploratory evidence that dizygotic twins can be achieved from a conjoined oocyte. This also confirms that we should be extremely conservative in discarding any mature oocyte without sufficient data about its useless future to result in a healthy baby.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hall JG. Twinning. Lancet. 2003;362:735–743. doi: 10.1016/S0140-6736(03)14237-7. [DOI] [PubMed] [Google Scholar]
- 2.Hoekstra C, Zhao ZZ, Lambalk CB, Willemsen G, Martin NG, Boomsma DI, Montgomery GW. Dizygotic twinning. Hum Reprod Update. 2008;14(1):37–47. doi: 10.1093/humupd/dmm036. [DOI] [PubMed] [Google Scholar]
- 3.Bensdorp AJ, Hukkelhoven CW, van der Veen F, Mol BW, Lambalk CB, van Wely M. Dizygotic twin pregnancies after medically assisted reproduction and after natural conception: maternal and perinatal outcomes. Fertil Steril. 2016;106(2):371–377. doi: 10.1016/j.fertnstert.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Rafael Z, Mastroianni L, Jr, Kopf GS. In vitro fertilization and cleavage of a single egg from a binovular follicle containing two individual eggs surrounded by a single zona pellucida. Fertil Steril. 1987;47(4):707–709. doi: 10.1016/S0015-0282(16)59128-6. [DOI] [PubMed] [Google Scholar]
- 5.Fishel S, Kaufman MH, Jackson P, Webster J, Faratian B. Recovery of two human oocytes surrounded by a single zona pellucida. Fertil Steril. 1989;52(2):325–327. doi: 10.1016/S0015-0282(16)60863-4. [DOI] [PubMed] [Google Scholar]
- 6.Hartshorne GM, Blayney M, Dyson H, Elder K. In vitro fertilization and development of one of two human oocytes with fused zonae pellucidae: case report. Fertil Steril. 1990;54(5):947–949. doi: 10.1016/S0015-0282(16)53965-X. [DOI] [PubMed] [Google Scholar]
- 7.Zeilmaker GH, Alberda AT, van Gent I. Fertilization and cleavage of oocytes from a binovular human ovarian follicle: a possible cause of dizygotic twinning and chimerism. Fertil Steril. 1983;40(6):841–843. doi: 10.1016/S0015-0282(16)47490-X. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbusch B. The potential significance of binovular follicles and binucleate giant oocytes for the development of genetic abnormalities. J Genet. 2012;91(3):397–404. doi: 10.1007/s12041-012-0195-x. [DOI] [PubMed] [Google Scholar]
- 9.Reynaud K, Halter S, Tahir Z, Thoumire S, Chebrout M, Chastant-Maillard S. Polyovular follicles. Gynecol Obstet Fertil. 2010;38:395–397. doi: 10.1016/j.gyobfe.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Vicdan K, Işik AZ, Daðli HG, Kaba A, Kişnişçi H. Fertilization and development of a blastocyst-stage embryo after selective intracytoplasmic sperm injection of a mature oocyte from a binovular zona pellucida: a case report. J Assist Reprod Genet. 1999;16:355–357. doi: 10.1023/A:1020537812619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telfer E, Gosden RG. A quantitative cytological study of polyovular follicles in mammalian ovaries with particular reference to the domestic bitch (Canis familiaris) J Reprod Fertil. 1987;81:137–147. doi: 10.1530/jrf.0.0810137. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbusch B, Hancke K. Conjoined human oocytes observed during assisted reproduction: description of three cases and review of the literature. Romanian J Morphol Embryol. 2012;53(1):189–192. [PubMed] [Google Scholar]
- 13.Yano K, Hashida N, Kubo T, Ohashi I, Koizumi A, Kageura R, Furutani K, Yano C. Repeated collection of conjoined oocytes from a patient with polycystic ovary syndrome, resulting in one successful live birth from frozen thawed blastocyst transfer: a case report. J Assist Reprod Genet. 2017;34(11):1547–1552. doi: 10.1007/s10815-017-1012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummins L, Koch J, Kilani S. Live birth resulting from a conjoined oocyte confirmed as euploid using array CGH: a case report. Reprod BioMed Online. 2016;32(1):62–65. doi: 10.1016/j.rbmo.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 16.Fishel S, Kaufman MH, Jackson P, Webster J, Faratian B. Recovery of two humanoocytes surrounded by a single zona pellucida. Fertil Steril. 1989;52:325–327. doi: 10.1016/S0015-0282(16)60863-4. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka A, Nakamura H, Kumasawa K, Tsutsui T, Furuya K, Kim N, Koizumi K, Kimura T. A case report of conjoined oocytes with independent zona pellucida from polycystic ovary syndrome. J Gynaecol Obstet. 2016;4(5):25–29. [Google Scholar]
- 18.Ron-El R, Nachum H, Golan A, Herman A, Yigal S, Caspi E. Binovular human ovarian follicles associated with in vitro fertilization: incidence and outcome. Fertil Steril. 1990;54:869–872. doi: 10.1016/S0015-0282(16)53948-X. [DOI] [PubMed] [Google Scholar]
- 19.Turkalj B, Kotanidis L, Nikolettos N. Binovular complexes after ovarian stimulation. A report of four cases. Hippokratia. 2013;17(2):169–170. [PMC free article] [PubMed] [Google Scholar]
- 20.Asimakopoulos B, Kotanidis L, Nikolettos N. Binovular complexes after ovarian stimulation. A report of four cases. Hippokratia. 2013;17:169–170. [PMC free article] [PubMed] [Google Scholar]