Abstract
Immunoglobulin G (IgG) is a major antibody and functions as a hub linking specific antigen binding and recruitment of effector molecules typified by Fcγ receptors (FcγRs). These activities are associated primarily with interactions involving its Fab and Fc sites, respectively. An IgG molecule is characterized by a multiple domain modular structure with conserved N-glycosylation in Fc. The molecule displays significant freedom in internal motion on various spatiotemporal scales. The consequent conformational flexibility and plasticity of IgG glycoproteins are functionally significant and potentially important factors for design and engineering of antibodies with enhanced functionality. In this article, experimental and computational approaches are outlined for characterizing the conformational dynamics of IgG molecules in solution. In particular, the importance of integration of these approaches is highlighted, as illustrated by dynamic intramolecular interactions between the pair of N-glycans and their proximal amino acid residues in Fc. These interactions can critically affect effector functions mediated by human IgG1 and FcγRIII. Further improvements in individual biophysical techniques and their integration will advance understanding of dynamic behaviors of antibodies in physiological and pathological conditions. Such understanding will provide opportunities for engineering antibodies through controlling allosteric networks in IgG molecules.
Keywords: Immunoglobulin G, Antibody, Fcγ receptor, N-glycan, Molecular dynamics simulation, Solution scattering, X-ray crystallography, Nuclear magnetic resonance spectroscopy, Core fucosylation, Dynamic conformational ensemble
Introduction
Immunoglobulin G (IgG) is a glycoprotein composed of multiple homologous domains (the so-called Ig domains) and plays key roles as an immune system antibody (Chiu et al. 2019) (Fig. 1). This glycoprotein consists of two identical light chains, each divided into VL and CL domains, and two identical heavy chains, each containing VH, CH1, CH2, and CH3 domains. CH1 and CH2 domains are connected by a protease-susceptible hinge segment. Cleavage of this segment gives rise to two Fab fragments constituted by VL, VH, CL, and CH1 domains and one Fc fragment constituted by two CH2 and two CH3.
Fig. 1.
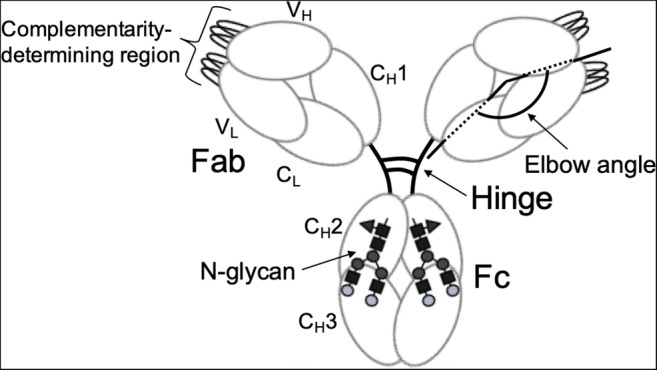
Schematic drawing of IgG. An IgG molecule is characterized by a multiple domain modular structure with conserved N-glycosylation in Fc and significant freedom for internal motion
Major functions of IgG are recognition of antigens on surfaces of invading viruses and bacteria and recruitment of effector molecules, such as complement component C1 and Fcγ receptors (FcγRs), for elimination of such pathogens. Thus, IgG serves as a hub that links these two functions.
VH and VL domains are structurally variable and are responsible for antigen recognition. The remaining domains are much less divergent but are classified into several isotypes. The constant region of the IgG heavy chain defines subclasses—IgG1–4 in humans. VH and VL domains each display three hypervariable loops that are directly involved in specific antigen binding and are thus often referred to as complementarity-determining regions (CDRs). Each CH2 domain of Fc homodimer possesses one conserved N-glycosylation site (Asn297) A biantennary complex-type oligosaccharide is expressed at this site, with microheterogeneity resulting from nonreducing terminal fucose (Fuc), galactose (Gal), bisecting N-acetylglucosamine (GlcNAc), and sialic acid residues (Yamaguchi et al. 2007). This N-glycosylation is essential for interactions with effector molecules, which are particularly affected by terminal structures of N-glycans.
Currently, IgGs are widely used for detection, quantification, and characterization of biological and pathological molecules and as biopharmaceuticals that target diseases, including cancers. A variety of engineered IgG antibodies and their derivatives have been developed and used for diagnostic and therapeutic purposes (Chiu et al. 2019). The structure of IgG is characterized by considerable conformational flexibility and plasticity, which are supposed to be of relevance to antigen binding and interactions with the effectors (Jay et al. 2018; Yang et al. 2017). An IgG molecule possesses hierarchical degrees of freedom in internal motion across various spatiotemporal scales. This conformational dynamic of IgG is critical for design and engineering of recombinant antibodies with enhanced functionality for interactions with antigens and effector molecules.
In this review, dynamic views of IgG structures are outlined, highlighting the importance of integration of experimental and computational approaches.
Experimental approaches for investigating IgG conformational dynamics
Early X-ray crystallographic studies of monoclonal IgGs and their light chains derived from multiple myeloma patients revealed their modular structures—Ig domains exhibiting longitudinal and transverse interactions within Fab portions (Schiffer et al. 1973; Edmundson et al. 1975). However, crystal structures provided no interpretable electron density for the Fc portion (Colman et al. 1976; Marquart et al. 1980). In contrast, crystallographic data of naturally occurring IgG mutants that lack hinge segments were able to visualize both Fab and Fc (Silverton et al. 1977; Rajan et al. 1983). These findings suggested that internal motion of IgG molecules is attributable to the flexible nature of the hinge region. Subsequently, crystal structures were reported for an Fc fragment cleaved from human IgG1 that displayed a pair of oligosaccharides packed between the two CH2 domains (Deisenhofer 1981). Three-dimensional structures of intact IgG molecule closely packed in the crystal lattice were eventually visualized in 1992 (Harris et al. 1992). Concurrently, crystallographic data were accumulated for Fab fragments complexed with their specific antigens (or haptens), followed by reports of crystal structures of Fc fragments bound to their physiological and pathological binding proteins (Padlan 1994; Yamaguchi et al. 2007).
These studies underscore the significant freedom of internal motion in Fab and Fc antibody fragments. Conformational changes in CDR loops in variable domains of Fab exhibited conformational differences between free and antigen-bound forms (Padlan 1994; Poljak 1975). In particular, the third CDR in the VH domain often exhibited conformational polymorphism even among crystal structures in the absence of antigen (Nakasako et al. 2005; Fernández-Quintero et al. 2018). This suggests that dynamic properties of antigen-binding loops for induced-fit and conformational selection mechanisms underlie antigen recognition. Crystallographic data also demonstrate orientation adjustment of VH and VL domains upon binding to antigen (Dunbar et al. 2013). Fab crystal structures show significant variation of the elbow angle, defined as the angle between pseudo-twofold axes for VL to VH and CL to CH1 (Stanfield et al. 2006). Cumulative crystallographic data for Fc also indicate variation in quaternary structures, which might depend on glycoforms and conformational transition of Fc on binding partners such as FcγRs (Yagi, Yanaka et al. 2018; Yamaguchi et al. 2007; Yanaka et al. 2019a). These conformational variations observed in crystal structures should be carefully interpreted because such structural snapshots are unavoidably influenced by crystal packing.
Solution structures of IgG molecules have been characterized using scattering and spectroscopic techniques. The former provides overall structure information of antibodies in solution, and the latter enables microscopic characterization of dynamic structures. Small-angle X-ray scattering (SAXS) has been used to delineate solution structures of IgG. Comparative SAXS analyses successfully revealed differences in orientation of Fab arms among mouse and human IgG subclasses, presumably dependent on their hinge structures (Gregory et al. 1987; Igarashi et al. 1990), as suggested by conventional electron microscopy (EM) and fluorescence depolarization measurements (Roux et al. 1997; Schneider et al. 1988; Tan et al. 1990; Oi et al. 1984). SAXS analysis also revealed mutational alteration of the quaternary structure of IgG-Fc. In contrast to SAXS, small-angle neutron scattering (SANS) can distinguish isotopic species, especially 1H and 2H by differences in neutron scattering length densities (Bernado et al. 2018). This ability enables observation of specific protein components in complexes by selective deuteration combined with a contrast matching. This method was used to examine spatial arrangements of selectively deuterated antigenic proteins bound to the Fab arms in different mouse IgG subclasses. In particular, distances between two antigen-binding sites were estimated (Sosnick et al. 1992). Further, a recent study demonstrated the capability of SANS to detect quaternary structure deformation of IgG1-Fc by interaction with 75% deuterated FcγRIIIb in a 2H2O solution (Yogo et al. 2017). Considering the partial dissociation of the low-affinity complex, the SANS data were successfully interpreted based on the previously reported crystal structure of the complex.
IgG structures in solution are depicted as dynamic conformational ensembles, which are not reproduced solely based on solution scattering observations. To interpret solution scattering results, possible conformational space occupied by target proteins must be defined (Bernado et al. 2018). This definition could use computational simulations based on structural data obtained from crystal structures. Recent advances in EM image analysis make it possible to visualize conformational spaces occupied by whole IgG molecules (Jay et al. 2018). The integration of the above methods is critical to providing dynamic views of IgG molecules in solution.
In contrast to solution scattering, nuclear magnetic resonance (NMR) spectroscopy provides information on antibody conformational dynamics over a broad range of timescales at atomic resolution (Arata et al. 1994; Barb 2017). Earlier NMR studies of an anti-DANSYL Fv fragment, a heterodimer of VH and VL domains, highlighted slow conformational dynamics, at a millisecond timescale, of the CDR3 loop, a primary binding site of the hapten (Odaka et al. 1992; Takahashi et al. 1994). A recent NMR study showed that the framework region of an anti-lysozyme Fv exhibited conformational fluctuation on a sub-microsecond timescale (Yanaka et al. 2017a). Moreover, a mutation at this site suppressed the conformational fluctuations, resulting in an increased affinity for the antigen. This finding suggests a potential strategy for engineering antibodies to improve antigen-binding properties through use of mutation at a region outside of the binding site.
Dynamic structures of IgG hinge regions have been investigated by a series of NMR studies, revealing a disulfide-linked rigid core (termed core hinge) flanked by extremely flexible segments (Matsunaga et al. 1991; Kim et al. 1994). Cleavage of the disulfide bridge renders the core hinge flexible and impairs interactions of IgG with effector proteins. NMR data also demonstrated that the hinge and the following Fc portions remain mobile even in immune complexes where Fab arms are cross-linked by multivalent antigens (Kim 1994). This result is consistent with suggestions from crystallographic studies. The CH1-proximal flexible segment (termed upper hinge) endows mobility to the Fab arm, and the flexible segment proximal to the CH2 domain (termed lower hinge) is involved in interaction with FcγRs.
Antibodies undergo much slower conformational transition at timescales of seconds or longer. These transitions can be detected by hydrogen-deuterium exchange experiments using NMR spectroscopy and, more recently, mass spectrometry. These techniques can offer useful probes for examining global unfolding of Ig domains, domain-domain interactions, and ligand binding of IgG molecules (Kawata et al. 1988; Shimba et al. 1995; Yogo et al. 2019; Zhang et al. 2011).
Stable isotope labeling of proteins is essential for a detailed NMR study of dynamic structures. Because glycoproteins are not expressed in bacterial systems, various eukaryotic vehicles have been developed for producing isotopically labeled IgG glycoproteins (Yanaka et al. 2018; Kato et al. 2018b; Kato et al. 2010; Yamaguchi et al. 2017).
High-speed atomic force microscopy (HS-AFM) offers a powerful emerging tool for observing dynamic behaviors of IgG. Several studies have been reported real-time observation of the interactions of IgG molecules with antigens and effector molecules on membrane-like surfaces (Yogo et al. 2019; Yanaka et al. 2019b; Strasser et al. 2019).
Computational tools for exploring IgG conformational spaces
Computational approaches exemplified by MD and Monte Carlo simulations offer powerful tools for providing ensembles of biomolecular structures (Hansmann et al. 1996). MD simulation has been applied to IgG and its fragments to describe physical movements in solution (Frank et al. 2014; Lee and Im 2017; Yanaka et al. 2017a; Yanaka et al. 2019a). MD simulations with all atoms of glycans, proteins, and surrounding waters are a straightforward way to obtain conformational ensembles. To sufficiently cover the conformation spaces, a longer-time simulation is necessary, which is conducted often by employing parallel computing for improving conformation sampling (Hansmann 2003). Despite high computational cost, conventional all-atom MD simulation is insufficient for adequately exploring conformational spaces, especially spaces occupied by large, complicated biomacromolecules (Lane et al. 2013).
Several methods have been proposed to enhance the efficiency of conformational sampling, including metadynamics and simulated annealing (Bernardi et al. 2015). One efficient method is replica-exchange molecular dynamics simulation (Sugita and Okamoto 1999). This method has been applied to explore conformational spaces of various oligosaccharides and glycans in complexes with of IgG1-Fc and FcγRIIIa (Yagi et al. 2018b; Yagi-Utsumi et al. 2017; Kato et al. 2018a). In the latter case, protein backbone conformations were mostly constrained, as those in the initial crystal structure in order to minimize calculation costs.
A bolder approach in molecular simulation is based on a coarse-grained modeling, in which proteins are represented by pseudo-atoms approximating whole amino acid residues to reduce the degrees of freedom and thereby enable much longer simulation in comparison with all-atom simulations (Ingólfsson et al. 2014). The coarse-grained approach was used for simulation of highly complex systems containing many proteins, as exemplified by intracellular molecular crowding environments (Feig et al. 2017). The approach should be useful for MD simulation of whole IgG molecules and their complexes, given that appropriate pseudo-atom sets can be established that approximate amino acid and sugar residues.
MD simulation results, especially results obtained by coarse-grained methods, heavily depend on calculation protocol. In addition, longer MD simulations generally generate cumulative errors in numerical integration. Such error can be minimized with proper choice of parameters and algorithms, but it cannot be eliminated. Therefore, appropriate experimental validation of simulation results is necessary. In MD simulations of oligosaccharides, NMR paramagnetic effects were successfully used for experimental validation (Kato and Yamaguchi 2015; Kato et al. 2018a).
Dynamic interplay of the protein and carbohydrate parts revealed by integrative experimental and computational approaches
The pair of N-glycans linked to the Fc portion of IgG critically affects effector functions through modulation of interactions with FcγRs and complement component C1q (Yamaguchi et al. 2007). These N-glycans are packed between the two CH2 domains and interact with domain inner surfaces. X-ray crystallographic studies indicate that core and Manα1–6 branches of glycans interact with inner surfaces of CH2 domains, leaving Manα1–3 branches projecting into the central space of Fc. Nonreducing terminal Gal residues at Manα1–6 branches make extensive contact with several amino acid residues. Gal residues on Manα1–3 branches are often invisible (or absent) in the crystal structures. NMR studies also revealed distinct mobilities of the two terminal Gal residues in solution (Yamaguchi et al. 1998; Barb and Prestegard 2011). Crystallographic data indicate that the intramolecular interaction network of N-glycans is rearranged on binding to FcγRs. Stable-isotope-assisted NMR revealed that truncation of outer carbohydrate moieties indirectly affected functional conformation of the FcγR-binding lower hinge segments (Yamaguchi et al. 2006; Kato et al. 2010).
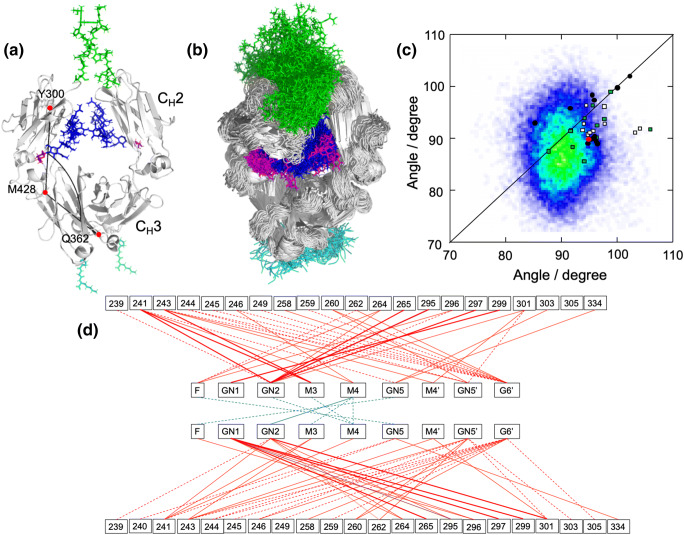
Recently, dynamic views of human IgG1-Fc were published after long-timescale all-atom MD simulations starting from crystal structures (Frank et al. 2014; Lee and Im 2017; Yanaka et al. 2019a). An extensive MD simulation for 3 μs in total, which was experimentally validated by SAXS (Yanaka et al. 2019a), indicated that dynamic conformational ensembles of Fc encompass a range of crystallographic snapshots previously reported for both free and complex forms (Fig. 2a, b, c). Notably, major Fc conformers in solution derived from the MD simulation exhibited almost symmetric and stouter quaternary structures, in contrast to crystal structures. In the MD-derived conformational ensemble of Fc, the N-glycan transiently makes contacts with amino acid residues located on the CH2 surface and with the other N-glycans, which are not observed in crystal structures (Fig. 2d). MD simulations also indicated that removal of either the N-glycans or the hinge segments from Fc resulted in expansion of the conformational space, with increases in populations of asymmetrically distorted quaternary conformations, which, in addition to the local conformational perturbations in the hinge region, may negatively affect the affinity of IgG-Fc for FcγRs due to conformational entropy loss. Thus, experimentally validated MD simulations suggest that the N-glycans restrict the motional freedom of CH2 and endow quaternary structure plasticity through multiple intramolecular interaction networks.
Fig. 2.
Dynamic view of IgG-Fc provided by experimentally validated computational approach. (a) The starting structure of SAXS-validated MD simulation based on the crystal structure of fucosyl IgG1-Fc (3AVE) supplemented with the hinge (green) and C-terminal (cyan) segments along with the terminal galactose residues (magenta) of the Manα1–6 branches. N-glycans are depicted in blue except for the terminal galactose. The intra-chain domain-orientation angle between CH2 and CH3 defined by Cα atoms of Y300, M428, and Q362 are shown in chain A. (b) The superposition of 256 structures extracted every 100 ns from the MD trajectory. The structures were visualized by PyMOL (https://www.pymol.org). (c) Distribution of intra-chain domain-orientation angles between CH2 and CH3 for ensemble models of IgG1-Fc and various crystal structures of IgG1-Fc. Angles between CH2 and CH3 domains of chain A and chain B are plotted on the x and y axes, respectively. Data depict results from ensemble models derived from MD simulations along with angles observed in crystal structures [circles for uncomplexed Fc (red, the starting structures used for the corresponding MD simulations; black, Fc with native N-glycans), rectangles for complexed Fc structures (white, complex with sFcγRs; green, complex with other ligands)]. (d) Pairs of contact residues found within 4 Å in the ensemble model derived from the MD simulation are connected by different types of line segments (red for carbohydrate-protein contact and cyan for carbohydrate-carbohydrate contact) according to incidence as follows: more than 24,000 pairs (thick solid line), 24,000 to 16,000 pairs (thin solid line), and 16,000 to 8000 pairs (dashed line). Adapted from reference (Yanaka et al. 2019a) with modification
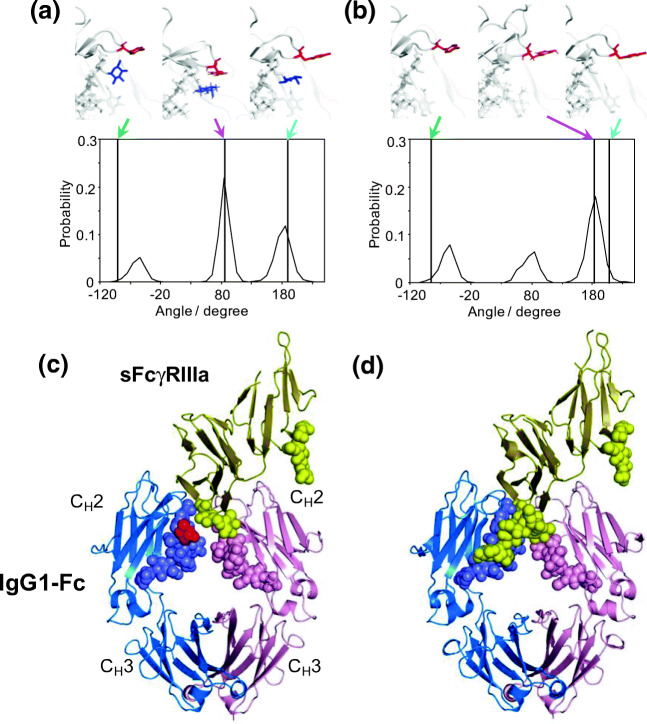
The core fucosylation of N-glycans of human IgG1-Fc is of biopharmaceutical interest because defucosylation increases its affinity for FcγRIIIa. The increase in affinity results in a dramatic enhancement of antibody-dependent cellular cytotoxicity activity (Isoda et al. 2015). Crystallographic and mutational studies highlight the importance of the Tyr296 side chain of IgG1-Fc. This residue is directly involved in interactions with FcγRIIIa and other FcγRs (Mizushima et al. 2011; Radaev et al. 2001; Kiyoshi et al. 2015). Long-timescale MD simulations showed that the largest populations of Tyr296 conformations are significantly different between fucosylated and defucosylated forms, and both considerably deviate from semi-outward conformations observed in crystal structures used as initial structures in the simulations (Yanaka et al. 2019a). The Tyr296 side chain mainly exhibits an inward conformation in contact with the core fucose (Fig. 3a). This contact was experimentally confirmed by observing NOE connectivity. In contrast, in the defucosylated form of IgG1-Fc, Tyr296 primarily exhibits an outward conformation that is favorable for FcγRIIIa binding (Fig. 3b).
Fig. 3.
Effects of core fucosylation of the Fc N-glycans on conformational dynamics of Tyr296 of IgG1-Fc and the N162 glycan of FcγR. Distributions of χ1 dihedral angles of Tyr296 in the ensemble models derived from MD simulations are plotted for (a) fucosylated IgG1-Fc and (b) nonfucosylated IgG1-Fc. The typical conformational snapshots of derived from the major conformational states (magenta arrows) in the simulation trajectory are shown along with the crystal structures used for building the starting models (green arrows; A, 3AVE; B, 2DTS) and those of sFcγRIIIa-bound Fc (cyan arrows; A, 5XJE; B, 3AY4). Crystal structures of (c) fucosylated and (d) nonfucosylated Fc complexed with bis-glycosylated soluble form of FcγRIIIa (sFcγRIIIa). Chains A and B of Fc are blue and pink, respectively; sFcγRIIIa is yellow. Carbohydrates are represented as spheres. Fuc is colored in red. In the complex of fucosylated Fc, the N162 glycans of sFcγRIIIa gave electron densities only for the reducing-terminal residues, in marked contrast to the observation of the same sFcγRIIIa in complex with nonfucosylated Fc, in which the N162 glycan is extensively involved in interactions with the Fc glycan. Adapted from reference (Yanaka et al. 2019a) and (Sakae et al. 2017) with the modification
Besides IgG-Fc, extracellular regions of FcγRs have several N-glycosylation sites (Yagi et al. 2018a). FcγRIIIa possesses five such sites: Asn38, Asn45, Asn74, Asn162, and Asn169. Among the N-glycans at these sites, the Asn45 and Asn162 glycans have negative and positive effects on IgG-binding affinity for FcγRIIIa (Shibata-Koyama et al. 2009; Cambay et al. 2020). Crystal structures of the complex formed between nonfucosylated IgG1-Fc and the extracellular region of FcγRIIIa with these two N-glycans indicate that interactions for the two glycoproteins are mediated not only through protein-protein interactions but also carbohydrate-carbohydrate interactions between the FcγRIIIa N162 glycan and one of the Fc N297 glycans (Mizushima et al. 2011; Ferrara et al. 2011) (Fig. 3c). In contrast, the N162 glycan of FcγRIIIa was largely invisible in crystal structures of its complex with fucosylated IgG1-Fc. This glycan is apparently not stabilized and remains mobile in the complex (Fig. 3d). REMD simulations based on these crystal structures allowed exploration of conformational dynamics of the four N-glycans were explored by extrapolating the missing information (Sakae et al. 2017). The Asn162 glycan is stabilized in solution mainly through interaction with the nonfucosylated form of Fc glycan. The glycan is, however, sterically hindered by the core fucose of Fc, rendering the Asn162 glycan more distal from Fc and more mobile.
Core fucosylation disrupts optimum intermolecular carbohydrate-carbohydrate interaction and restricts motional freedom of the Tyr296 side chain. This understanding explains how defucosylation increases the affinity of IgG1-Fc for FcγRIIIa and leads to improved efficacy of therapeutic antibodies. These studies highlight the importance of integration of experimental and computational approaches for elucidating molecular mechanisms underlying glycosylation-dependent effector function promoted by interactions between IgG1 and FcγRIIIa.
Future perspective
A variety of experimental and computational tools are now available for characterizing conformational dynamics of antibodies. To date, antibody functions have been assigned to individual parts in the molecule. Also, the atomic-level analyses based on NMR spectroscopy and MD simulation have been used to focus on specific parts of antibodies. Antibodies exert their multiple functions in a highly coordinated manner, as best exemplified by the interlocking of promotion of effector functions with antigen recognition (Yang et al. 2017). Recent HS-AFM data illustrate that IgG molecules bound to antigenic membranes are self-assembled into a hexameric ring and thereby recruit C1q (Yanaka et al. 2019b; Yogo et al. 2019). Moreover, Fab portions of IgG contribute to its interaction with FcγRIII (Yogo et al. 2019). Thus, a more integrated view of conformational dynamics of the entire antibody molecule is necessary for understanding putative allosteric mechanisms underlying antibody functions. Experimental and computational identification of correlations of local conformational dynamics will lead to elucidation of an intramolecular interaction network and interrelated allosteric pathways in the antibody molecule. Further, antibodies function in heterogeneous bloodstreams, characterized by molecular crowding and promiscuous intermolecular interactions (Yanaka et al. 2017b). Improvements in individual techniques and their integration will promote understanding of dynamic behaviors of antibodies under varying physiological and pathological conditions. This understanding will provide opportunities for engineering antibodies through control of allosteric networks of IgG molecules.
Acknowledgments
We thank Dr. Hirokazu Yagi (Nagoya City University) for the useful discussion.
Funding information
This work was supported in part by the JSPS Research Fellowship for Young Scientists (to R.Y.) and the grants-in-aid for Scientific Research (Grant Numbers, JP18K14892 and JP20K15981 to S.Y., JP19H01017 to K.K., JP19J15602 to R.Y.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arata Y, Kato K, Takahashi H, Shimada I. Nuclear-magnetic-resonance study of antibodies - a multinuclear approach. Method Enzymol. 1994;239:440–464. doi: 10.1016/s0076-6879(94)39017-7. [DOI] [PubMed] [Google Scholar]
- Barb AW. Quantifying carbohydrate motions through solution measurements: applications to immunoglobulin G Fc. In: Kato K, Peters T, editors. NMR in Glycoscience and Glycotechnology. Cambridge: RSC Publishing; 2017. [Google Scholar]
- Barb AW, Prestegard JH. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat Chem Biol. 2011;7:147–153. doi: 10.1038/nchembio.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernado P, Shimizu N, Zaccai G, Kamikubo H, Sugiyama M. Solution scattering approaches to dynamical ordering in biomolecular systems. Biochim Biophys Acta Gen Subj. 2018;1862:253–274. doi: 10.1016/j.bbagen.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Bernardi RC, Melo MCR, Schulten K. Enhanced sampling techniques in molecular dynamics simulations of biological systems. Biochim Biophys Acta Gen Subj. 2015;1850:872–877. doi: 10.1016/j.bbagen.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambay F, Forest-Nault C, Dumoulin L, Seguin A, Henry O, Durocher Y, De Crescenzo G. Glycosylation of Fcγ receptors influences their interaction with various IgG1 glycoforms. Mol Immunol. 2020;121:144–158. doi: 10.1016/j.molimm.2020.03.010. [DOI] [PubMed] [Google Scholar]
- Chiu ML, Goulet DR, Teplyakov A, Gilliland GL (2019) Antibody structure and function: the basis for engineering therapeutics. Antibodies (Basel) 8 [DOI] [PMC free article] [PubMed]
- Colman PM, Deisenhofer J, Huber R. Structure of the human antibody molecule Kol (immunoglobulin G1): an electron density map at 5 A resolution. J Mol Biol. 1976;100:257–278. doi: 10.1016/s0022-2836(76)80062-9. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-Å resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- Dunbar J, Fuchs A, Shi J, Deane CM. ABangle: characterising the VH-VL orientation in antibodies. Protein Eng Des Sel. 2013;26:611–620. doi: 10.1093/protein/gzt020. [DOI] [PubMed] [Google Scholar]
- Edmundson AB, Ely KR, Abola EE, Schiffer M, Panagiotopoulos N. Rotational allomerism and divergent evolution of domains in immunoglobulin light-chains. Biochemistry. 1975;14:3953–3961. [PubMed] [Google Scholar]
- Feig M, Yu I, Wang PH, Nawrocki G, Sugita Y. Crowding in cellular environments at an atomistic level from computer simulations. J Phys Chem B. 2017;121:8009–8025. doi: 10.1021/acs.jpcb.7b03570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Quintero ML, Loeffler JR, Kraml J, Kahler U, Kamenik AS, Liedl KR. Characterizing the diversity of the CDR-H3 loop conformational ensembles in relationship to antibody binding properties. Front Immunol. 2018;9:3065. doi: 10.3389/fimmu.2018.03065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umana P, Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Walker RC, Lanzilotta WN, Prestegard JH, Barb AW. Immunoglobulin G1 Fc domain motions: implications for Fc engineering. J Mol Biol. 2014;426:1799–1811. doi: 10.1016/j.jmb.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory L, Davis KG, Sheth B, Boyd J, Jefferis R, Nave C, Burton DR. The solution conformations of the subclasses of human IgG deduced from sedimentation and small angle X-ray scattering studies. Mol Immunol. 1987;24:821–829. doi: 10.1016/0161-5890(87)90184-2. [DOI] [PubMed] [Google Scholar]
- Hansmann UHE. New algorithms and the physics of proteins. Physica A. 2003;321:152–163. [Google Scholar]
- Hansmann UHE, Okamoto Y, Eisenmenger F. Molecular dynamics, Langevin and hybrid Monte Carlo simulations in a multicanonical ensemble. Chem Phys Lett. 1996;259:321–330. [Google Scholar]
- Harris LJ, Larson SB, Hasel KW, Day J, Greenwood A, McPherson A. The three-dimensional structure of an intact monoclonal antibody for canine lymphoma. Nature. 1992;360:369–372. doi: 10.1038/360369a0. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Sato M, Katsube Y, Takio K, Tanaka T, Nakanishi M, Arata Y. Structure of a mouse immunoglobulin G that lacks the entire CH1 domain: protein sequencing and small-angle X-ray scattering studies. Biochemistry. 1990;29:5727–5733. doi: 10.1021/bi00476a013. [DOI] [PubMed] [Google Scholar]
- Ingólfsson HI, Lopez CA, Uusitalo JJ, de Jong DH, Gopal SM, Periole X, Marrink SJ. The power of coarse graining in biomolecular simulations. Wiley Interdiscip Rev Comput Mol Sci. 2014;4:225–248. doi: 10.1002/wcms.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda Y, Yagi H, Satoh T, Shibata-Koyama M, Masuda K, Satoh M, Kato K, Iida S (2015) Importance of the side chain at position 296 of antibody Fc in interactions with FcγRIIIa and other Fcγ receptors. PLoS One 10 [DOI] [PMC free article] [PubMed]
- Jay JW, Bray B, Qi Y, Igbinigie E, Wu H, Li J, Ren G (2018) IgG antibody 3D structures and dynamics. Antibodies (Basel) 7 [DOI] [PMC free article] [PubMed]
- Kato K, Yamaguchi T. Paramagnetic NMR probes for characterization of the dynamic conformations and interactions of oligosaccharides. Glycoconj J. 2015;32:505–513. doi: 10.1007/s10719-015-9599-1. [DOI] [PubMed] [Google Scholar]
- Kato K, Yamaguchi Y, Arata Y. Stable-isotope-assisted NMR approaches to glycoproteins using immunoglobulin G as a model system. Prog Nucl Mag Res Sp. 2010;56:346–359. doi: 10.1016/j.pnmrs.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Kato, Koichi, Yagi, Hirokazu , and Yamaguchi, Takumi (2018a) NMR characterization of the dynamic conformations of oligosaccharides. In G.A. Webb (ed.), Modern Magnetic Resonance (Springer International Publishing)
- Kato K, Yanaka S, Yagi H (2018b) Technical basis for nuclear magnetic resonance approach for glycoproteins. In: Naito A, Asakura T, Shimada I, Takegoshi K, Yamamoto Y (eds) Experimental approaches of NMR spectroscopy-methodology and application to life science and materials science. Springer Nature, Singapore
- Kawata Y, Goto Y, Hamaguchi K, Hayashi F, Kobayashi Y, Kyogoku Y. Hydrogen-exchange kinetics of the indole NH proton of the buried tryptophan in the constant fragment of the immunoglobulin light chain. Biochemistry. 1988;27:346–350. doi: 10.1021/bi00401a052. [DOI] [PubMed] [Google Scholar]
- Kim H (1994) PhD thesis in Japanese
- Kim H, Matsunaga C, Yoshino A, Kato K, Arata Y. Dynamical structure of the hinge region of immunoglobulin G as studied by 13C nuclear magnetic resonance spectroscopy. J Mol Biol. 1994;236:300–309. doi: 10.1006/jmbi.1994.1136. [DOI] [PubMed] [Google Scholar]
- Kiyoshi M, Caaveiro JM, Kawai T, Tashiro S, Ide T, Asaoka Y, Hatayama K, Tsumoto K. Structural basis for binding of human IgG1 to its high-affinity human receptor FcγRI. Nat Commun. 2015;6:6866. doi: 10.1038/ncomms7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TJ, Shukla D, Beauchamp KA, Pande VS. To milliseconds and beyond: challenges in the simulation of protein folding. Curr Opin Struct Biol. 2013;23:58–65. doi: 10.1016/j.sbi.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Im W. Effects of N-glycan composition on structure and dynamics of IgG1 Fc and their implications for antibody engineering. Sci Rep. 2017;7:12659. doi: 10.1038/s41598-017-12830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart M, Deisenhofer J, Huber R, Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980;141:369–391. doi: 10.1016/0022-2836(80)90252-1. [DOI] [PubMed] [Google Scholar]
- Matsunaga C, Kato K, Arata Y. A 13C NMR study of the hinge region of a mouse monoclonal antibody. J Biomol NMR. 1991;1:379–390. doi: 10.1007/BF02192861. [DOI] [PubMed] [Google Scholar]
- Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M, Kato K. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes to cells : devoted to molecular & cellular mechanisms. 2011;16:1071–1080. doi: 10.1111/j.1365-2443.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasako M, Oka T, Mashumo M, Takahashi H, Shimada I, Yamaguchi Y, Kato K, Arata Y. Conformational dynamics of complementarity-determining region H3 of an anti-dansyl Fv fragment in the presence of its hapten. J Mol Biol. 2005;351:627–640. doi: 10.1016/j.jmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Odaka A, Kim JI, Takahashi H, Shimada I, Arata Y. Isotope-edited nuclear magnetic resonance study of Fv fragment of anti-dansyl mouse monoclonal antibody: recognition of the dansyl hapten. Biochemistry. 1992;31:10686–10691. doi: 10.1021/bi00159a007. [DOI] [PubMed] [Google Scholar]
- Oi VT, Vuong TM, Hardy R, Reidler J, Dangl J, Herzenberg LA, Stryer L. Correlation between segmental flexibility and effector function of antibodies. Nature. 1984;307:136–140. doi: 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- Padlan EA. Anatomy of the antibody molecule. Mol Immunol. 1994;31:169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Poljak RJ. X-ray diffraction studies of immunoglobulins. Adv Immunol. 1975;21:1–33. [PubMed] [Google Scholar]
- Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcγ receptor in complex with Fc. J Biol Chem. 2001;276:16469–16477. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- Rajan SS, Ely KR, Abola EE, Wood MK, Colman PM, Athay RJ, Edmundson AB. Three-dimensional structure of the Mcg IgG1 immunoglobulin. Mol Immunol. 1983;20:787–799. doi: 10.1016/0161-5890(83)90057-3. [DOI] [PubMed] [Google Scholar]
- Roux KH, Strelets L, Michaelsen TE. Flexibility of human IgG subclasses. J Immunol. 1997;159:3372–3382. [PubMed] [Google Scholar]
- Sakae Y, Satoh T, Yagi H, Yanaka S, Yamaguchi T, Isoda Y, Iida S, Okamoto Y, Kato K. Conformational effects of N-glycan core fucosylation of immunoglobulin G Fc region on its interaction with Fcγ receptor IIIa. Sci Rep. 2017;7:13780. doi: 10.1038/s41598-017-13845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M, Girling RL, Ely KR, Edmundson AB. Structure of a lambda-type Bence-Jones protein at 3.5-Å resolution. Biochemistry. 1973;12:4620–4631. doi: 10.1021/bi00747a013. [DOI] [PubMed] [Google Scholar]
- Schneider WP, Wensel TG, Stryer L, Oi VT. Genetically engineered immunoglobulins reveal structural features controlling segmental flexibility. Proc Natl Acad Sci U S A. 1988;85:2509–2513. doi: 10.1073/pnas.85.8.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata-Koyama M, Iida S, Okazaki A, Mori K, Kitajima-Miyama K, Saitou S, Kakita S, Kanda Y, Shitara K, Kato K, Satoh M. The N-linked oligosaccharide at FcγRIIIa Asn-45: an inhibitory element for high FcγRIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology. 2009;19:126–134. doi: 10.1093/glycob/cwn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimba N, Torigoe H, Takahashi H, Masuda K, Shimada I, Arata Y, Sarai A. Comparative thermodynamic analyses of the Fv, Fab* and Fab fragments of anti-dansyl mouse monoclonal antibody. FEBS Lett. 1995;360:247–250. doi: 10.1016/0014-5793(95)00113-n. [DOI] [PubMed] [Google Scholar]
- Silverton EW, Navia MA, Davies DR. Three-dimensional structure of an intact human immunoglobulin. Proc Natl Acad Sci U S A. 1977;74:5140–5144. doi: 10.1073/pnas.74.11.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnick TR, Benjamin DC, Novotny J, Seeger PA, Trewhella J. Distances between the antigen-binding sites of three murine antibody subclasses measured using neutron and X-ray scattering. Biochemistry. 1992;31:1779–1786. doi: 10.1021/bi00121a028. [DOI] [PubMed] [Google Scholar]
- Stanfield RL, Zemla A, Wilson IA, Rupp B. Antibody elbow angles are influenced by their light chain class. J Mol Biol. 2006;357:1566–1574. doi: 10.1016/j.jmb.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Strasser J, de Jong RN, Beurskens FJ, Wang GB, Heck AJR, Schuurman J, Parren PWHI, Hinterdorfer P, Preiner J. Unraveling the macromolecular pathways of IgG oligomerization and complement activation on antigenic surfaces. Nano Lett. 2019;19:4787–4796. doi: 10.1021/acs.nanolett.9b02220. [DOI] [PubMed] [Google Scholar]
- Sugita Y, Okamoto Y. Replica-exchange molecular dynamics method for protein folding. Chem Phys Lett. 1999;314:141–151. [Google Scholar]
- Takahashi H, Tamura H, Shimba N, Shimada I, Arata Y. Role of the domain-domain interaction in the construction of the antigen combining site. A comparative study by 1H-15N shift correlation NMR spectroscopy of the Fv and fab fragments of anti-dansyl mouse monoclonal antibody. J Mol Biol. 1994;243:494–503. doi: 10.1006/jmbi.1994.1675. [DOI] [PubMed] [Google Scholar]
- Tan LK, Shopes RJ, Oi VT, Morrison SL. Influence of the hinge region on complement activation, C1q binding, and segmental flexibility in chimeric human immunoglobulins. Proc Natl Acad Sci U S A. 1990;87:162–166. doi: 10.1073/pnas.87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Takakura D, Roumenina LT, Fridman WH, Sautes-Fridman C, Kawasaki N, Kato K (2018a) Site-specific N-glycosylation analysis of soluble Fcγ receptor IIIb in human serum. Sci Rep 8 [DOI] [PMC free article] [PubMed]
- Yagi H, Yanaka S, Kato K. Structure and dynamics of immunoglobulin G glycoproteins. Adv Exp Med Biol. 2018;1104:219–235. doi: 10.1007/978-981-13-2158-0_11. [DOI] [PubMed] [Google Scholar]
- Yagi-Utsumi M, Yamaguchi Y, Uekusa Y, Kato K. NMR characterization of the conformations, dynamics, and interactions of glycosphingolipids. In: Kato K, Peters T, editors. NMR in Glycoscience and Glycotechnology. Cambridge: RSC publishing; 2017. [Google Scholar]
- Yamaguchi Y, Kato K, Shindo M, Aoki S, Furusho K, Koga K, Takahashi N, Arata Y, Shimada I. Dynamics of the carbohydrate chains attached to the fc portion of immunoglobulin G as studied by NMR spectroscopy assisted by selective 13C labeling of the glycans. J Biomol NMR. 1998;12:385–394. doi: 10.1023/a:1008392229694. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K, Kato K. Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta. 2006;1760:693–700. doi: 10.1016/j.bbagen.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Takahashi N, Kato K. Antibody structures. Elsevier: Oxford; 2007. [Google Scholar]
- Yamaguchi Y, Yagi H, Kato K. Stable isotope labeling of glycoproteins for NMR study. In: Kato K, Peters T, editors. NMR in Glycoscience and Glycotechnology. Cambridge: RSC Publishing; 2017. [Google Scholar]
- Yanaka S, Moriwaki Y, Tsumoto K, Sugase K. Elucidation of potential sites for antibody engineering by fluctuation editing. Sci Rep. 2017;7:9597. doi: 10.1038/s41598-017-10246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaka S, Yamazaki T, Yogo R, Noda M, Uchiyama S, Yagi H, Kato K (2017b) NMR detection of semi-specific antibody interactions in serum environments. Molecules 22 [DOI] [PMC free article] [PubMed]
- Yanaka S, Yagi H, Yogo R, Yagi-Utsumi M, Kato K. Stable isotope labeling approaches for NMR characterization of glycoproteins using eukaryotic expression systems. J Biomol NMR. 2018;71:193–202. doi: 10.1007/s10858-018-0169-2. [DOI] [PubMed] [Google Scholar]
- Yanaka S, Yogo R, Inoue R, Sugiyama M, Itoh SG, Okumura H, Miyanoiri Y, Yagi H, Satoh T, Yamaguchi T, Kato K (2019a) Dynamic views of the fc region of immunoglobulin G provided by experimental and computational observations. Antibodies (Basel) 8 [DOI] [PMC free article] [PubMed]
- Yanaka S, Yogo R, Watanabe H, Taniguchi Y, Satoh T, Komura N, Ando H, Yagi H, Yuki N, Uchihashi T, Kato K (2019b) On-membrane dynamic interplay between anti-GM1 IgG antibodies and complement component C1q. Int J Mol Sci 21 [DOI] [PMC free article] [PubMed]
- Yang D, Kroe-Barrett R, Singh S, Roberts CJ, Laue TM. IgG cooperativity - is there allostery? Implications for antibody functions and therapeutic antibody development. mAbs. 2017;9:1231–1252. doi: 10.1080/19420862.2017.1367074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo R, Yanaka S, Yagi H, Martel A, Porcar L, Ueki Y, Inoue R, Sato N, Sugiyama M, Kato K. Characterization of conformational deformation-coupled interaction between immunoglobulin G1 Fc glycoprotein and a low-affinity Fcγ receptor by deuteration-assisted small-angle neutron scattering. Biochem Biophys Rep. 2017;12:1–4. doi: 10.1016/j.bbrep.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo R, Yamaguchi Y, Watanabe H, Yagi H, Satoh T, Nakanishi M, Onitsuka M, Omasa T, Shimada M, Maruno T, Torisu T, Watanabe S, Higo D, Uchihashi T, Yanaka S, Uchiyama S, Kato K. The Fab portion of immunoglobulin G contributes to its binding to Fcγ receptor III. Sci Rep. 2019;9:11957. doi: 10.1038/s41598-019-48323-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Willison LN, Tripathi P, Sathe SK, Roux KH, Emmett MR, Blakney GT, Zhang HM, Marshall AG. Epitope mapping of a 95 kDa antigen in complex with antibody by solution-phase amide backbone hydrogen/deuterium exchange monitored by Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem. 2011;83:7129–7136. doi: 10.1021/ac201501z. [DOI] [PMC free article] [PubMed] [Google Scholar]