Abstract
Purpose
To evaluate whether mtDNA content at the blastocyst stage differs between embryos derived from fresh or vitrified sibling oocytes.
Material and methods
A retrospective analysis was performed between March 2017 and September 2018, including 504 blastocysts from 94 couples undergoing preimplantation genetic testing for aneuploidies (PGT-A), using fresh oocytes together with previously vitrified oocytes. Trophectoderm biopsies were performed and subjected to next generation sequencing.
Results
On average, 1.8 ± 1.0 oocyte vitrification cycles were performed per patient. Between fresh and vitrified cycles, no difference was observed between the number of fertilized oocytes (5.3 ± 4.2 versus 5.5 ± 3.0). Blastulation rate on day 5 per fertilized oocyte was significantly higher in the fresh group (62% ± 29% versus 44% ± 31%; p < 0.001). For the 504 biopsied blastocysts, 294 fresh versus 210 vitrified, no significant differences were found in the euploid rate, 40.5% versus 38.6% (p = 0.667), and mtDNA content, 30.1 (± 10.6) versus 30.0 (± 12.5) (p = 0.871), respectively. Regardless of the origin of the oocytes, aneuploid blastocysts contained significantly higher mtDNA values compared with the euploid ones (31.4 versus 28.0; p = 0.001). Furthermore, top-quality blastocysts had a significantly lower mtDNA content compared with moderate and poor-quality blastocysts (p < 0.001) and blastocysts biopsied on day 5 showed significantly lower mtDNA content compared with day 6 or day 7 blastocysts (p < 0.001). However, when analyzing the blastocyst mtDNA content according to the ploidy state, no differences were found for blastocyst quality or day of biopsy between blastocysts originating from fresh or vitrified oocytes.
Conclusion
Oocyte vitrification does not affect the mtDNA content of trophectoderm biopsies.
Keywords: Oocyte vitrification, Blastocyst, mtDNA, PGT-A, NGS
Introduction
Oocyte vitrification from repeated ovarian stimulations is a well-established strategy to potentially increase the number of blastocysts available for preimplantation genetic testing for aneuploidies (PGT-A) and consequently increases the chances of obtaining a euploid embryo [1]. Oocyte vitrification yields similar fertilization and pregnancy rates compared with fresh oocytes in ICSI cycles [2] with no increased risk of aneuploidy [3]. However, lower cleavage rates on day 3 and usable blastocyst rates after oocyte vitrification have also been reported, suggesting that cryopreservation procedures might have an impact on the oocyte physiology and as such further preimplantation development [3].
There has been an increasing focus on research into mitochondrial features over the last few years, since they are organelles extremely active in embryogenesis [4]. Mitochondria contain one or more copies of their own circular double-stranded genome, the mitochondrial DNA (mtDNA), and is indicative for the number of mitochondria present in the oocyte [5]. The mature human oocyte is the richest cell in terms of mtDNA content, which is required to acquire competence for fertilization and for the embryo to reach the blastocyst stage [6–8]. Mitochondria are involved in the regulation of multiple critical cellular processes: apoptosis, amino acid synthesis, calcium homeostasis, and the generation of energy in the form of adenosine triphosphate (ATP) [9, 10]. For this reason, mitochondrial organelles are known for being the power houses of the cells [11]. They are extremely dynamic organelles that undergo cycles of fusion to exchange their content including mtDNA, and fission for mitochondrial biogenesis and segregation of inactive mitochondria by autophagy [12]. Their dynamics are crucial to maintain a proper mitochondrial function in many aspects of a cell’s life while dysfunction has been implicated in impaired embryonic developmental processes in mice [13, 14].
Differences in the embryo developmental competence may be attributed to variations in mtDNA content, considering it a potential marker of embryo viability [5]. Appropriate cytoplasmic distribution of mitochondria is important for specific cell functions during early cleavages [15]. However, osmotic forces during the vitrification procedure may cause a defect in mitochondrial function and distribution as previously seen in mouse, human, and bovine oocytes, compromising the developmental ability of the resulting embryos due to loss of cytoskeletal integrity [16–19]. Vitrification causes a temporal reduction of the mitochondrial membrane potential with a negative effect on intra-oocyte ATP levels as previously seen in discarded human MII oocytes [20, 21]. However, some studies revealed no impact of vitrification on developmental competence and ATP content after vitrification of in vitro matured mice oocytes [22].
Mitochondrial DNA (mtDNA) has some peculiarities compared with nuclear DNA (nDNA). Due to its location in the mitochondrion as well as its lack of histones, it is extremely vulnerable to the harmful effect of reactive oxygen species (ROS) and particularly prone to mutations that cause functional degradation [23] though it has been suggested that mtDNA mutations are mainly caused by mtDNA replication errors rather than by the oxidative damage, resulting in a different proportion of the mtDNA present within every cell (heteroplasmy) [24]. An increase in ROS levels in discarded mature human oocytes indicates a higher oxidative stage in oocytes after vitrification [25] leading to a significant decrease in mtDNA copy number, as previously described in vitrified mouse oocytes compared with fresh oocytes [25, 26].
Taking into consideration the potential deleterious effects of oocyte vitrification on the mtDNA content and subsequent embryonic development, the present study aimed to evaluate whether mtDNA content at the blastocyst stage differs between blastocysts derived from fresh and vitrified sibling oocytes.
Materials and methods
Patient population
This retrospective study was performed at IVIRMA Middle East Fertility Clinic, Abu Dhabi, between March 2017 and September 2018.
Cycles from patients for which intracytoplasmic sperm injection (ICSI) and preimplantation genetic testing for aneuploidies (PGT-A) by next generation sequencing (NGS) were performed using fresh autologous oocytes together with previously accumulated vitrified oocytes were included. In case of oocyte accumulation, the vitrified oocytes from multiple stimulation cycles were warmed in combination with a fresh oocyte retrieval. Patients underwent on average 1.8 stimulation cycles in which oocytes were vitrified. Two groups were defined and compared in this sibling oocyte study; blastocysts coming from fresh oocytes and blastocysts derived from vitrified oocytes, only if at least one blastocyst was biopsied from each group. Anti-Müllerian hormone (AMH), age, and body mass index (BMI) values for the female partners were recorded.
Only couples in whom the male partner had produced a fresh ejaculate on the day of ICSI were included in the study and patients with progesterone levels > 1.5 ng/ml at the time of final oocyte maturation were excluded since increased serum progesterone values during stimulation significantly influence mtDNA values [27].
Ovarian stimulation protocols
Ovarian stimulation was performed by standard protocols, either gonadotropin-releasing hormone (GnRH) agonist or GnRH antagonist protocols using rFSH (recombinant follicle stimulating hormone) or HP-HMG (highly purified human menopausal gonadotropin) as stimulation medication. The dosage of the stimulation medication was chosen according to the ovarian reserve parameters [28]. Trigger for final oocyte maturation was achieved by administration of either 5.000–10.000 IU of hCG, 0.3 mg of GnRH agonist (triptorelin), or dual trigger (hCG and GnRH-agonist) as soon as ≥ 3 follicles ≥ 17 mm were present.
Ovum pick-up, vitrification/warming, intracytoplasmic injection
Oocyte retrieval was performed 34–36 h post-trigger. Follicles were aspirated under ultrasound guidance and the cumulus oocyte complexes (COCs) were collected in a HEPES-buffered medium and cultured in either in Quinn’s Advantage Protein Plus for Fertilization (SAGE, CooperSurgical, Målov, Denmark) or Global Total for fertilization (CooperSurgical, Målov, Denmark) until denudation.
In case of oocyte vitrification, COCs were denuded and subsequently the mature (MII) oocytes were vitrified 37–38 h post-trigger using the Cryotop method (Kitazato, Biopharma) as described elsewhere [29]. Using a 170-μm pipette, MII oocytes were transferred from fertilization media to a 20-μL drop of basic solution (BS) at room temperature. Equilibration solution (ES) containing 7.5% (v/v) ethylene glycol (EG) and 7.5% (v/v) dimethyl sulphoxide (DMSO) was gradually added into the well to complete a volume of 300 μL. After completing 15 min in the ES solution, oocytes were transferred to 300 μL of vitrification solution (VS) composed of 15% (v/v) EG, 15% (v/v) DMSO, and 0.5 mol/L sucrose for 1 min; afterwards, they were rapidly placed onto a polypropylene strip of the Cryotop (Kitazato) in a volume of less than 0.1 μL and submerged into liquid nitrogen (LN2).
Warming procedure of the vitrified MII was performed as described by Kuwayama et al. [29]. The Cryotop was removed and placed into preheated (37 °C) thawing solution (TS) containing 1 mol/L sucrose for 1 min at 37 °C. The oocytes were then transferred to DS solution (0.5 mol/L sucrose) for 3 min at room temperature, followed by 5 min in washing solution (WS), and then 1 min in WS before the oocytes were placed in fertilization medium. Surviving oocytes were injected 3 h post-warming.
For the fresh oocytes, COCs were denuded 39 h post-trigger and ICSI was performed 1 h later [30].
Embryo culture and development
After ICSI, all inseminated oocytes were cultured either in Quinn’s Advantage Sequential media (SAGE, CooperSurgical, Måløv, Denmark) or single-step media (Global Total LP, CooperSurgical, Måløv, Denmark), overnight pre-equilibrated and maintained at the same incubation conditions 37 °C, 5% O2, 6% CO2, and 89% N2. Fertilization was assessed 17–20 h post-ICSI by the presence of two pronuclei. On day 3 of embryo development, culture medium was changed either to Quinn’s Advantage Protein Plus Blastocyst Medium (SAGE, CooperSurgical, Måløv, Denmark) or refreshed with Global total LP media (CooperSurgical, Måløv, Denmark).
Embryo quality (EQ) score on day 3 of embryo development was based on the number and symmetry of blastomeres, percentage of fragmentation, presence of vacuoles, granulation, and multinucleation. According to these parameters, embryos were assigned into 4 different EQ groups; EQ 1 (excellent), EQ 2 (good), EQ 3 (moderate), or EQ 4 (poor) as previously described [31] with a minor difference that embryos with > 20% of fragmentation were included in EQ 3 and not in EQ 2.
Only expanded blastocysts (Gardner expansion grade 3–6) [32] with a clear and differentiated inner cell mass (ICM) and trophectoderm (TE) cells were subjected to trophectoderm biopsy on day 5, day 6, or day 7 of embryo development. Blastocyst scoring system was based on the aspect, number, and integrity of ICM and TE following the Spanish Asociación para el estudio de la Biología de la Reproducción (ASEBIR) consensus [33]. Score A was given to a compacted ICM or TE made of many homogeneous cells that formed a tightly joined epithelium; score B to a loose aspect of the ICM or fewer TE cells that still formed a homogeneous epithelium; score C shows no sign of compaction in the ICM and very few TE cells and score D when ICM or TE cells showed signs of degeneration. Blastulation rate was defined as the number of cavitating blastocysts on day 5 per normally fertilized zygote.
Quinn’s Advantage Medium with HEPES (SAGE, CooperSurgical, Målov, Denmark) supplemented with HSA, (Vitrolife, Göteborg, Sweden) was used for the biopsy procedure. Three to five laser pulses on the zona pellucida (2.2 ms) along with mechanical “flicking” method were used to cut the trophectoderm cells inside the aspiration pipette; trophectoderm biopsies were washed and placed in 0.2-ml PCR tubes containing 2.5 μL PBS.
Ploidy status of blastocyst by NGS
A whole genome amplification (WGA) protocol was performed on all individual samples (PicoPlex technology by Rubicon Genomics, Inc.; Ann Arbor, MI, USA). After WGA, library preparation consisted of the incorporation of individual barcodes for the amplified DNA of each embryo. After isothermal amplification and enrichment, sequencing was performed in a 316 or 318 chip using the Personal Genome Machine sequencing (Life-Thermo Fisher, USA). Ion Reporter software, for sequencing analysis and data interpretation, was employed. The herein used NGS platform has been validated in previous studies [34, 35] and is commercially available.
Mitochondrial DNA copy number
Values of mitochondrial DNA were directly obtained from the software and were analyzed using the Igenomix algorithm for day 3 and day 5 biopsies. An optimized algorithm was applied using the output dataset obtained from the PGT-A analysis for the mtDNA content calculation. To calculate the relative mtDNA content, the number of reads after filtering mapping to the mitochondrial genome is divided by the number of reads mapping to the nuclear DNA (nDNA) [36]. This allows normalization of each batch and therefore reduces variability during NGS experiments as it makes the calculation independent of the number of cells obtained in each biopsy. Crucially, using nDNA values for normalization assumes that the composition of nDNA is equal across samples. Only embryos with informative results for the PGT-A and a mtDNA content below 1000 were analyzed in the study. This technique has been validated internally by Igenomix.
Statistical data
Continuous variables were presented as a mean (expressed as percentage in case of rates) together with standard deviations. Patient’s characteristics were compared using Student’s t test for paired samples between fresh and vitrified sibling oocytes. When more than two groups were compared, ANOVA test has been applied. Categorical variables related with the embryo scoring and chromosomal status of the blastocysts were compared using Fisher’s exact test in 2 × 2 contingency tables, providing the number of cases and the proportion in the sample (expressed as a percentage), along with the odds ratio. For of the remaining contingency tables, a chi-square test was performed.
To perform statistical inference, linear regression models were applied. In addition, the classical model was extended to a mixed-effect model to consider the intrapatient variability. Data analysis was performed using the statistical software R (version 3.5.0) and p < 0.05 was considered statistically significant.
Results
Patients were on average 38.7 ± 4.7 years old with a BMI of 25.9 ± 3.4 kg/m2 and AMH values of 1.4 ± 1.9 ng/ml. Oocytes were accumulated in on average 1.8 ± 1.0 ovarian stimulations to potentially increase the number of euploid blastocysts.
Outcome of fertilization and embryo development between fresh and vitrified sibling oocytes is shown in Table 1. There was a significant difference in the number of COCs (8.7 ± 6.6 versus 11.8 ± 6.0; p < 0.001) and number of MII oocytes (6.7 ± 5.0 versus 8.5 ± 4.0; p = 0.001) derived from fresh and vitrified oocytes. Due to a survival rate of 87.5% in the vitrified group, no difference was observed between the number of injected oocytes (6.6 ± 4.8 versus 7.4 ± 3.8; p = 0.097) and the number of normally fertilized oocytes (5.3 ± 4.2 versus 5.5 ± 3.0; p = 0.705); however, fertilization rate was significantly higher for fresh oocytes (81% ± 19% versus 75% ± 19%; p = 0.036).
Table 1.
Outcome of fertilization and embryo development between fresh and vitrified oocytes
| Fresh | Vitrified | p value | |
|---|---|---|---|
| Number of stimulations | 1.0 | 1.8 | |
| COCs | 8.7 ± 6.6 | 11.8 ± 6.0 | < 0.0001 |
| MII | 6.7 ± 5.0 | 8.5 ± 4.0 | 0.001 |
| MII injected | 6.6 ± 4.8 | 7.4 ± 3.8 (87.5% survival rate) | 0.097 |
| Fertilization rate | 81% | 75% | 0.036 |
| Normal fertilization | 5.3 ± 4.2 | 5.5 ± 3.0 | 0.642 |
| EQ 1 rate on day 3 | 338 (67.6%) | 275 (53%) | < 0.0001 |
| EQ 2 rate on day 3 | 72 (14.4%) | 84 (16.2%) | |
| EQ 3 rate on day 3 | 65 (13%) | 95 (18.3%) | |
| EQ 4 rate on day 3 | 25 (5.0%) | 65 (12.5%) | |
| EQ on day 5 A | 35 (11.9%) | 17 (8.1%) | 0.171 |
| EQ on day 5 B | 135 (45.9%) | 92 (43.8%) | |
| EQ on day 5 C | 113 (38.4%) | 97 (46.2%) | |
| EQ on day 5 D | 11 (3.7%) | 4 (1.9%) | |
| number of embryos cultured to D5 | 5.3 ± 4.1 | 5.3 ± 3.0 | 0.941 |
| number of blastocysts D5 | 3.1 ± 2.9 | 2.2 ± 1.8 | 0.005 |
| Blastulation rate | 62% ± 29 | 44% ± 31 | < 0.0001 |
Results are expressed as mean ± SD. SD, standard deviation. t test and chi-squared test for categorical data
COCs, cumulus oocytes complex; MII, mature oocyte; EQ, embryo quality
EQ on day 3: EQ 1—excellent; EQ 2—good; EQ 3—moderate; or EQ 4—poor
EQ on day 5: A—excellent; B—good; C—moderate; D—poor
When comparing the EQ between the fertilized sibling oocytes, significant differences between groups were noted. On day 3 of embryo development, there were differences seen in the proportion of EQ 1 and EQ 4 between fresh compared with the vitrified group (p < 0.0001). On day 5, no significant differences were observed in the EQ between blastocysts from fresh versus vitrified oocytes (p = 0.171). However, embryos coming from fresh oocytes had a higher blastulation rate on day 5 (62% ± 29% versus 44% ± 31%; p < 0.001) compared with their vitrified siblings. Furthermore, they had a higher capacity to develop into a usable blastocyst for trophectoderm biopsy (66% ± 26% versus 47% ± 26% p < 0.001). A significantly higher proportion of blastocysts biopsied on day 5 were coming from fresh oocytes in comparison with vitrified oocytes (63.8% versus 36.2%; p = 0.002).
For genetic testing, a total of 504 blastocysts were biopsied: 294 blastocysts from fresh and 210 blastocysts from vitrified oocytes. Day 5 TE biopsy yielded an average euploid rate of 47% versus 46% (p = 0.899), whereas on day 6 the euploid rate was 33% versus 34% (p = 0.886) between blastocyst originating from fresh and vitrified oocytes respectively (Table 2).
Table 2.
Aneuploid and euploid rate between blastocysts coming from fresh and vitrified oocytes stratified per biopsy day
| Aneuploid | Euploid | OR (95% CI) | p value | ||
|---|---|---|---|---|---|
| D5 | Fresh | 90 (53.3%) | 79 (46.7%) | 0.964 (0.56–1.64) | 0.899 |
| Vitrified | 52 (54.2%) | 44 (45.8%) | |||
| D6 | Fresh | 80 (67.2%) | 39 (32.8%) | 1.073 (0.59–1.96) | 0.886 |
| Vitrified | 65 (65.7%) | 34 (34.3%) | |||
| D7 | Fresh | 5 (83.3%) | 1 (16.7%) | 1.237 (0.075–78.54) | 1.000 |
| Vitrified | 12 (80.0%) | 3 (20.0%) |
Results are expressed as number of blastocysts (%). Fisher’s exact test, OR, odds ratio; 95% CI
D5, day 5 biopsy; D6, day 6 biopsy; D7, day 7 biopsy; n, number of blastocysts
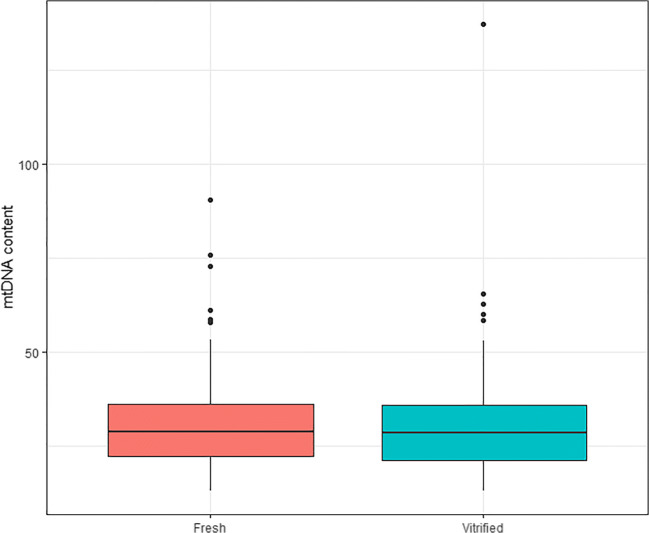
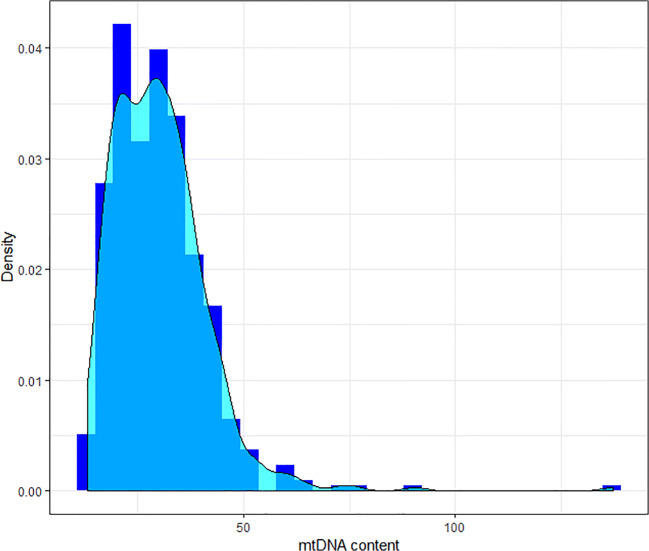
The mean mtDNA value for blastocysts from fresh oocytes was 30.1 (± 10.6) compared with 30.0 (± 12.6) for blastocysts from vitrified oocytes. These differences were not significantly different (p = 0.87), even when comparing intrapatient variability (p = 0.98) (Fig. 1). The mtDNA values followed a non-normal distribution (Fig. 2), resulting in a kurtosis of 19.5 and a positive skewness of 2.5. A mixed model was used to analyze patients’ characteristics that could potentially affect mtDNA, such as age of the patient, BMI, and AMH, but none of them were found to be associated with mtDNA content in trophectoderm cells.
Fig. 1.
mtDNA content after trophectoderm biopsy from fresh and vitrified oocytes. Box plot representing the mtDNA content of all 504 biopsied blastocysts according to the oocyte origin: 294 from fresh and 210 from vitrified oocytes
Fig. 2.
mtDNA content distribution. Distribution of mtDNA content in trophectoderm of human blastocysts, from fresh and vitrified oocytes, measured by next generation sequencing. The distribution shows a positive asymmetry and a long kurtosis
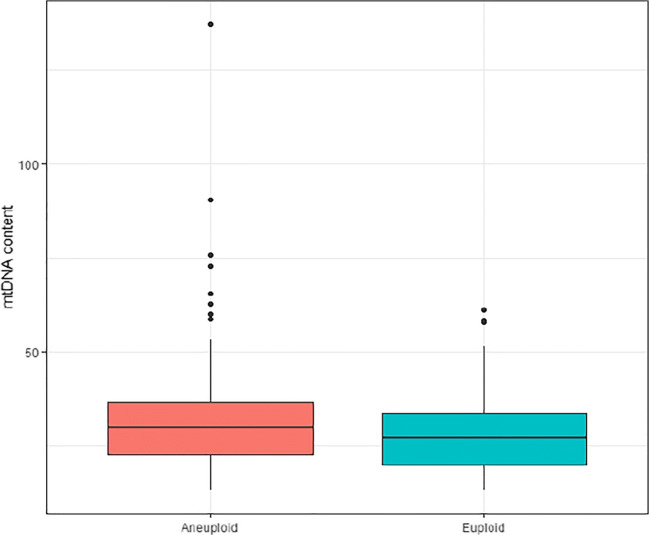
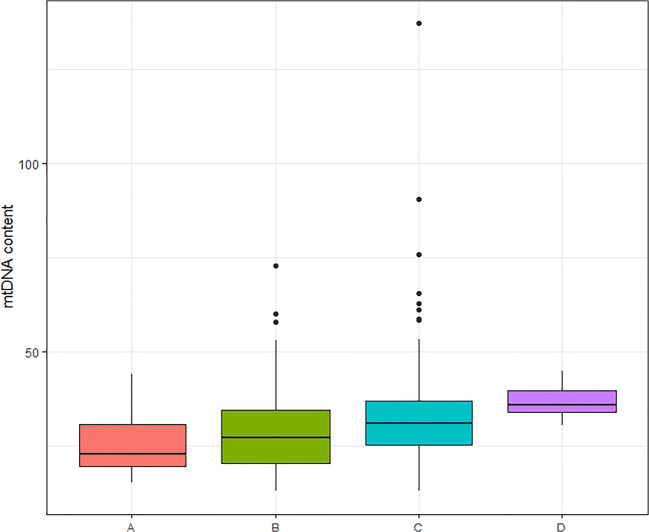
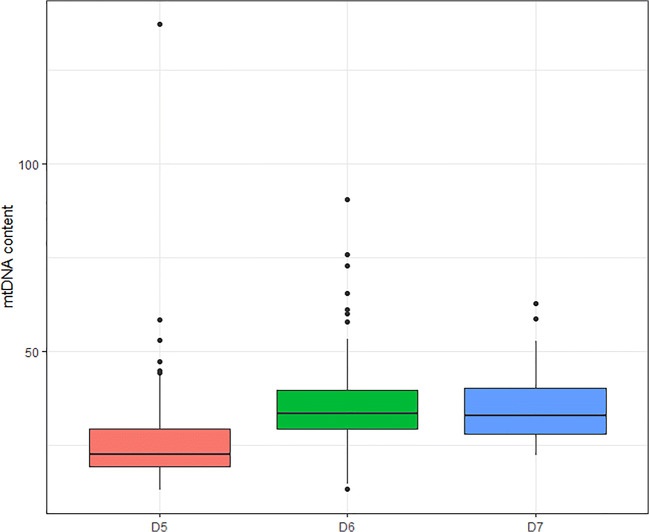
Irrespective of the oocyte origin, univariate linear analysis showed that aneuploidy and blastocyst quality affected the mtDNA content. Aneuploid blastocysts showed significantly higher mtDNA content compared with the euploid ones (31.4 versus 28.0; p < 0.001) (Fig. 3). ANOVA test revealed that there were statistical differences between mtDNA content and embryo quality. Blastocysts with a higher quality (score A) showed significantly lower mtDNA content in comparison with score B, C, or D (25.4, 28.2, 32.7, 36.8, respectively; p < 0.001) (Fig. 4) and were significantly more likely to be biopsied on day 5 (p < 0.001). Subset analysis was performed between mtDNA content and the day when the TE biopsy was performed (day 5, day 6, or day 7). In line with the previous findings, blastocysts biopsied on day 5 showed significantly lower mtDNA content compared with day 6 or day 7 blastocysts (25.4, 35.2, 36.2 respectively; p < 0.001) (Fig. 5).
Fig. 3.
mtDNA content after trophectoderm biopsy according to ploidy. Box plot corresponding to mtDNA content of 304 aneuploid and 200 euploid blastocysts (31.4 versus 28.0; p < 0.001)
Fig. 4.
mtDNA content and blastocyst quality. Box plot corresponding to mtDNA content according to the blastocyst quality for fresh and vitrified oocytes; A: top quality (n = 52), B: good quality (n = 227), C: moderate quality (n = 210), D: poor quality (n = 15) (25.4, 28.2, 32.7, 36.8, respectively; p < 0.001)
Fig. 5.
mtDNA content and day of the biopsy. Box plot corresponding to mtDNA content according to the day of the biopsy for fresh and vitrified oocytes: D5, day 5 (n = 265); D6, day 6 (n = 218); D7, day 7 (n = 21) (25.4, 35.2, 36.2 respectively; p < 0.001)
When stratifying the blastocyst quality and the day of biopsy according to the ploidy outcome, no differences were observed in the blastocyst mtDNA content between embryos from fresh and vitrified oocytes (Tables 3 and 4). Additionally, stratifying the blastocyst quality and the oocyte origin (fresh or vitrified) according to the euploidy state (euploid or aneuploid), no differences in mtDNA content were observed (data not shown).
Table 3.
mtDNA content and blastocyst quality, according to ploidy and oocyte origin
| Blastocyst quality | PGT-A | Oocyte origin | Mean | SD | 95% CI (mean) | n | p value |
|---|---|---|---|---|---|---|---|
| A | Aneuploid | Fresh | 26.8 | 9.8 | (18.5, 35.0) | 8 | 0.083 |
| Vitrified | 19.5 | 2.7 | (16.2, 22.9) | 5 | |||
| Euploid | Fresh | 27.1 | 7.7 | (24.1, 30.1) | 27 | 0.053 | |
| Vitrified | 23.0 | 4.8 | (20.0, 26.1) | 12 | |||
| B | Aneuploid | Fresh | 28.9 | 10.5 | (26.4, 31.3) | 74 | 0.709 |
| Vitrified | 28.2 | 9.8 | (25.5, 30.9) | 52 | |||
| Euploid | Fresh | 28.1 | 9.5 | (25.7, 30.5) | 61 | 0.693 | |
| Vitrified | 27.3 | 10.0 | (24.10, 30.5) | 40 | |||
| C | Aneuploid | Fresh | 33.7 | 12.1 | (12.1, 31.0) | 82 | 0.804 |
| Vitrified | 34.3 | 16.0 | (16.0, 30.5) | 70 | |||
| Euploid | Fresh | 29.2 | 9.8 | (25.6, 32.8) | 31 | 0.817 | |
| Vitrified | 29.8 | 10.3 | (25.7, 33.9) | 27 | |||
| D | Aneuploid | Fresh | 36.0 | 3.1 | (33.9, 38.0) | 11 | 0.854 |
| Vitrified | 37.6 | 10.1 | (− 53.0, 128.3) | 2 | |||
| Euploid | Fresh | - | - | - | - | - | |
| Vitrified | 40.6 | 0.4 | (36.6, 44.5) | 2 |
Mean mtDNA content and 95% CI of aneuploid and euploid blastocysts coming from fresh and vitrified oocytes according to the blastocyst quality
Blastocyst quality: A—excellent; B—good; C—moderate; D—poor
PGT-A, preimplantation genetic testing for aneuploidies; SD, standard deviation; CI, confidence interval; n, number of blastocysts
Table 4.
mtDNA content and day of the biopsy, according to ploidy and oocyte origin
| Biopsy day | PGT-A | Oocyte origin | Mean | SD | 95% CI (mean) | n | p value |
|---|---|---|---|---|---|---|---|
| D5 | Aneuploid | Fresh | 26.2 | 8.2 | (24.4, 27.9) | 90 | 0.928 |
| Vitrified | 25.9 | 17.2 | (21.1, 30.7) | 52 | |||
| Euploid | Fresh | 25.1 | 7.4 | (23.4, 26.7) | 79 | 0.360 | |
| Vitrified | 23.6 | 8.6 | (21.0, 26.3) | 44 | |||
| D6 | Aneuploid | Fresh | 36.9 | 11.4 | (34.4, 39.5) | 80 | 0.223 |
| Vitrified | 34.9 | 9.1 | (32.6, 37.1) | 65 | |||
| Euploid | Fresh | 34.4 | 9.4 | (31.4, 37.4) | 39 | 0.400 | |
| Vitrified | 32.6 | 8.9 | (29.5, 35.7) | 34 | |||
| D7 | Aneuploid | Fresh | 39.9 | 10.6 | (26.7, 53.1) | 5 | 0.460 |
| Vitrified | 35.3 | 12.6 | (27.3, 43.3) | 12 | |||
| Euploid | Fresh | 29.9 | - | - | 1 | - | |
| Vitrified | 35.6 | 8.6 | (14.4, 56.9) | 3 |
Mean mtDNA content and 95% CI of aneuploid and euploid blastocysts coming from fresh and vitrified oocytes according to the day of the biopsy
D5, day 5 biopsy; D6, day 6 biopsy; D7, day 7 biopsy
PGT-A, preimplantation genetic testing for aneuploidies; SD, standard deviation; CI, confidence interval; n, number of blastocysts
Discussion
The current retrospective study evaluated the effect of oocyte vitrification on the mtDNA content of TE cells. Based on the findings, it was clearly demonstrated that oocyte vitrification does not affect mtDNA content at the blastocyst stage. However, independent of the oocyte origin, mtDNA seems to be correlated with ploidy, blastocyst quality, and the day of the blastocyst biopsy.
Contrary to several studies that failed to demonstrate an impact of vitrification on fertilization, cleavage, and pregnancy outcomes in autologous IVF cycles when comparing fresh and vitrified oocytes [37–40], the present study demonstrates significant differences in fertilization rate and embryo development between fresh and vitrified sibling oocytes. Embryos from vitrified oocytes did not only show a slower embryo development on day 3, characterized by a lower number of blastomeres and a higher percentage of fragmentation, but also a lower blastulation rate.
In recent years, oocyte vitrification has become a standard clinical procedure since many significant advancements have been made to eliminate or reduce the possible chemical toxicity and osmotic deleterious effects which might physiologically impact the structure and genomic integrity of the human MII oocyte [41, 42]. Such physical stress can affect intracellular organelles like mitochondria, resulting in an impaired developmental competence of the resulting embryo. Indeed, an impaired blastulation capacity was observed in embryos originating from vitrified oocytes. Even though there were differences seen in the embryo division pattern in the present study, mtDNA content was not affected by vitrification. Consequently, we hypothesize that there is an impact of oocyte vitrification on embryo development that is not necessarily related to mtDNA content. In line with previous studies, oocyte vitrification did not increase the risk of aneuploidy and did not affect the blastocyst quality [1, 3].
Conflicting results have been reported when mtDNA content and ploidy at the blastocyst stage were correlated, due to discrepancies in the mtDNA assessment. The controversy began when Victor and colleagues (2017) applied a mathematical correction factor accounting for possible variations in the nDNA compositions among different sex and ploidy, resulting in a failure to discriminate ploidy based on mtDNA content alone [43].
Mitochondrial biogenesis is crucial during oogenesis to constitute a mitochondrial pool large enough to support early embryo development and further implantation [44]. As mitotic divisions start, the total amount of mitochondria in each cell will be diluted as they will be segregated between blastomeres, with no mtDNA replication up to the preimplantation stage [45]. Therefore, an expanded high-quality blastocyst with more TE cells is expected to have lower mtDNA content due to dilution of mitochondria with each cell division. Indeed, irrespective of the oocyte origin, the results of our study showed that a superior embryo grading, mainly based on trophectoderm quality, has been correlated with lower mtDNA content. However, when stratifying the blastocyst quality according to ploidy and according to oocyte origin, there was no correlation between mtDNA values in euploid embryos and blastocyst quality which is consistent with a previous study [46] but inconsistent with other in which higher mtDNA content in TE was an indicator of compromised blastocysts [47]. As for embryo quality, similar results were obtained when looking at aneuploidy alone; aneuploid blastocysts have a higher mtDNA content compared with euploid ones, as previously described by others [4, 27]. Again, after stratifying according to embryo quality, the difference in the mtDNA values between euploid and aneuploid blastocysts disappeared. This indicates that lower quality blastocysts are more likely to have an increased mtDNA content and are more likely to be aneuploid. This finding suggests an important role for the TE quality on the obtained mtDNA content.
Developmentally delayed embryos, which reached the blastocyst stage on day 6 or day 7 compared with day 5, showed higher mtDNA levels (25.4 versus 35.2 versus 36.2; p < 0.001). A possible explanation could be that slow nuclear DNA replication leads to a higher mtDNA/nDNA ratio which can be reflected in a lower blastocyst quality as the day of biopsy is postponed due to slower development [48]. However, euploid blastocysts biopsied on day 7 have shown the potential to implant if they are good quality [48]. As high-quality embryos are more likely to be biopsied on day 5, slower ones might be under metabolic stress causing an increase in mtDNA copy number [49]. However, when stratifying according to ploidy and the oocyte origin, our results showed no differences in the mtDNA content between blastocysts biopsied on day 5, day 6, or day 7.
Clinical outcomes regarding implantation and mtDNA content have been followed in order to understand whether elevated mtDNA content is associated with cellular stress. Some authors have postulated that an increased amount of mtDNA content in euploid embryos has been correlated with poor implantation rates (embryo viability) [4, 49] whereas others could not find any correlation between mtDNA content and implantation rates [43, 50]. Recently, healthy pregnancies and live births have been reported after transferring euploid blastocysts with highly elevated mtDNA levels; thus, elevated mtDNA content does not necessarily mean enhanced mitochondrial function or metabolic activity [50]. A link has been demonstrated between mutation on mitochondrial genome that may limit the energy production, and the increased mtDNA associated with reduced implantation [51]. Indeed, during implantation, the trophoblast mitochondria should undergo morphological and functional changes as they differentiate into syncytiotrophoblast of which the function is to secrete proteolytic enzymes that erode the endometrial epithelium, and hence, leads to implantation in the endometrium. However, the direct correlation between the TE mtDNA content and capacity to transform into a functional syncytiotrophoblast has not been explored so far.
Even though different culture media (Global Total LP and Sage) were used throughout the study, sibling oocytes were always cultured in one culture medium. Analysis of mtDNA content on sibling oocytes between Global Total LP and Sage is similar between the two reported culture media (unpublished data). One of the strengths of our study is the mtDNA content comparison on blastocysts developed from sibling oocytes, leading to a well-controlled investigation excluding patient-specific variables. Although oocytes, even from the same patient—due to inherent biological variability—may react differently upon exposure to hyperosmotic solutions [52], the same results were found between blastocyst mtDNA content in fresh and vitrified oocytes originating from the same patient. Applying a mixed model, no correlation of age, AMH, or BMI with mtDNA quantity in TE cell was observed even when analyzing intrapatient mtDNA content. However, as the sibling oocytes were obtained during different stimulation cycles, we cannot exclude the interstimulation effect. A limitation of the study is that we only included cycles in which blastocyst biopsy was performed on at least one blastocyst from fresh and one blastocyst from vitrified oocytes. If indeed the mtDNA content should reach a minimal threshold, impaired embryo development may be expected from oocytes below these values and as such will not be included in our analysis. The mtDNA obtained in this study is derived from TE cells while it is known that in different mammalian species, the number of mitochondria in the ICM is lower than in trophectoderm, which means that it is less metabolically active and has a reduced contribution to preimplantation embryogenesis compared with trophectoderm [5]. In the herein described study, mtDNA content was obtained by dividing the total number of reads of mtDNA by all nuclear DNA reads after low coverage NGS. An internally validated correction model was applied to calculate the mtDNA content. Although this is not the optimal technique to estimate the mtDNA content, variability attributed to the biopsy and whole genome amplification protocol was excluded since all the sibling TE samples were subjected to the same NGS test in the same analysis, so mtDNA content per sibling oocyte would be biased in the same direction.
To the best of our knowledge, this is the first study to evaluate in human fully competent sibling MII oocytes, the possible effect of oocyte vitrification on blastocyst mtDNA content. Our findings indicate that there is no impact of oocyte vitrification on blastocyst mtDNA content. Therefore, it seems that the delayed blastocyst formation is not related to a numerical alteration in mitochondria. It might not only be the copy number of mtDNA that counts but also a proper mitochondrial biogenesis, dynamics, and mitophagy—what is called mitochondrial turnover—to maintain the mtDNA and the function of active mitochondria. During the process of oocyte vitrification, function and distribution of mitochondria may be affected influencing the developmental potential of embryos. Therefore, further studies are required in order to better understand the developmental dissimilarities between fresh and vitrified sibling oocytes.
Acknowledgments
We would like to thank Mr. Victor Lozoya for his help with the statistical interpretation.
Compliance with ethical standards
Ethical approval
Approval for this study was obtained from the local Ethical Committee of IVIRMA Middle East Fertility Clinic, Abu Dhabi, UAE (Research Ethics Committee REFA029/2018).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ana Arnanz, Email: Ana.arnanz@ivirma.com.
Neelke De Munck, Email: Neelke.DeMunck@ivirma.com.
Aşina Bayram, Email: Asina.Bayram@ivirma.com.
Ahmed El-Damen, Email: Ahmed.damen@ivirma.com.
Andrea Abdalla, Email: Andrea.abdalla@ivirma.com.
Ibrahim ElKhatib, Email: Ibrahim.Elkhatib@ivirma.com.
Laura Melado, Email: Laura.melado@ivirma.com.
Barbara Lawrenz, Email: Barbara.Lawrenz@ivirma.com.
Human M. Fatemi, Email: Human.Fatemi@ivirma.com
References
- 1.Chamayou S, Sicali M, Alecci C, Ragolia C, Liprino A, Nibali D, Storaci G, Cardea A, Guglielmino A. The accumulation of vitrified oocytes is a strategy to increase the number of euploid available blastocysts for transfer after preimplantation genetic testing. J Assist Reprod Genet. 2017;34(4):479–486. doi: 10.1007/s10815-016-0868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Practice Committees of American Society for Reproductive Medicine: Society for Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43. [DOI] [PubMed]
- 3.Forman EJ, Li X, Ferry KM, Scott K, Treff NR, Scott RT. Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: a novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril. 2012;98(3):644–649. doi: 10.1016/j.fertnstert.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Fragouli E, Wells D. Mitochondrial DNA assessment to determine oocyte and embryo viability. Semin Reprod Med. 2015;33(06):401–409. doi: 10.1055/s-0035-1567821. [DOI] [PubMed] [Google Scholar]
- 5.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Reynier P, May-Panloup P, Chretien M-F, Morgan CJ, Jean M, Savagner F, Barriere P, Malthiery Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425–429. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- 7.Babayev E, Seli E. Oocyte mitochondrial function and reproduction. Current Opinion in Obstetrics and Gynecology. 2015;27(3):175–181. doi: 10.1097/GCO.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos TA, El Shourbagy S. St. John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85(3):584–591. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20(3):346–353. doi: 10.1016/j.semcdb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 11.May-Panloup P, Chretien M, Malthiery Y, Reynier P. Mitochondrial DNA in the oocyte and the developing embryo. In: Current Topics in Developmental Biology [Internet]. Elsevier; 4Y [cited 2019 Sep 3]. p. 51–83. Available from: https://linkinghub.elsevier.com/retrieve/pii/S007021530677003X [DOI] [PubMed]
- 12.Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212(4):379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto YI, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 15.Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization, ATP content and competence. Hum Reprod. 2000;15(12):2621–2633. doi: 10.1093/humrep/15.12.2621. [DOI] [PubMed] [Google Scholar]
- 16.Lei T, Guo N, Tan M, Li Y. Effect of mouse oocyte vitrification on mitochondrial membrane potential and distribution. J Huazhong Univ Sci Technol [Med Sci] 2014;34(1):99–102. doi: 10.1007/s11596-014-1238-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Liu T, Jin S-B, Tomilin N, Castro J, Shupliakov O, Lendahl U, Nister M. The novel conserved mitochondrial inner-membrane protein MTGM regulates mitochondrial morphology and cell proliferation. J Cell Sci. 2009;122(13):2252–2262. doi: 10.1242/jcs.038513. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X-M, Du W-H, Wang D, Hao H-S, Liu Y, Qin T, et al. Recovery of mitochondrial function and endogenous antioxidant systems in vitrified bovine oocytes during extended in vitro culture. Mol Reprod Dev. 2011;78(12):942–950. doi: 10.1002/mrd.21389. [DOI] [PubMed] [Google Scholar]
- 19.Gualtieri R, Mollo V, Barbato V, Fiorentino I, Iaccarino M, Talevi R. Ultrastructure and intracellular calcium response during activation in vitrified and slow-frozen human oocytes. Hum Reprod. 2011;26(9):2452–2460. doi: 10.1093/humrep/der210. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Han S, Liu W, Wang Y, Huang G. Effect of vitrification on mitochondrial membrane potential in human metaphase II oocytes. J Assist Reprod Genet. 2012;29(10):1045–1050. doi: 10.1007/s10815-012-9848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manipalviratn S, Tong Z-B, Stegmann B, Widra E, Carter J, DeCherney A. Effect of vitrification and thawing on human oocyte ATP concentration. Fertil Steril. 2011;95(5):1839–1841. doi: 10.1016/j.fertnstert.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Nazmara Z, Salehnia M, HosseinKhani S. Mitochondrial distribution and ATP content of vitrified, in vitro matured mouse oocytes. Avicenna J Med Biotechnol. 2014;6(4):210–217. [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenlaub-Ritter U, Wieczorek M, Lüke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;11(5):783–796. doi: 10.1016/j.mito.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Gaziev AI, Abdullaev S, Podlutsky A. Mitochondrial function and mitochondrial DNA maintenance with advancing age. Biogerontology. 2014;15(5):417–438. doi: 10.1007/s10522-014-9515-2. [DOI] [PubMed] [Google Scholar]
- 25.Nohales-Córcoles M, Sevillano-Almerich G, Di Emidio G, Tatone C, Cobo AC, Dumollard R, et al. Impact of vitrification on the mitochondrial activity and redox homeostasis of human oocyte. Hum Reprod. 2016;31(8):1850–1858. doi: 10.1093/humrep/dew130. [DOI] [PubMed] [Google Scholar]
- 26.Amoushahi M, Salehnia M, Mowla SJ. Vitrification of mouse MII oocyte decreases the mitochondrial DNA copy number, TFAM gene expression and mitochondrial enzyme activity. J Reprod Infertil. 2017;18(4):343–351. [PMC free article] [PubMed] [Google Scholar]
- 27.de los Santos MJ, Diez Juan A, Mifsud A, Mercader A, Meseguer M, Rubio C, et al. Variables associated with mitochondrial copy number in human blastocysts: what can we learn from trophectoderm biopsies? Fertil Steril. 2018;109(1):110–117. doi: 10.1016/j.fertnstert.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 28.La Marca A, Grisendi V, Giulini S, Argento C, Tirelli A, Dondi G, et al. Individualization of the FSH starting dose in IVF/ICSI cycles using the antral follicle count. J Ovarian Res. 2013;6(1):11. doi: 10.1186/1757-2215-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Van Steirteghem AC, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, et al. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 1993;8(7):1061–1066. doi: 10.1093/oxfordjournals.humrep.a138192. [DOI] [PubMed] [Google Scholar]
- 31.De Munck N, Santos-Ribeiro S, Mateizel I, Verheyen G. Reduced blastocyst formation in reduced culture volume. J Assist Reprod Genet. 2015;32(9):1365–1370. doi: 10.1007/s10815-015-0541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Cuevas Saiz I, Carme Pons Gatell M, Vargas MC, Delgado Mendive A, Rives Enedáguila N, Moragas Solanes M, et al. The embryology interest group: updating ASEBIR’s morphological scoring system for early embryos, morulae and blastocysts. Medicina Reproductiva y Embriología Clínica. 2018;5(1):42–54. [Google Scholar]
- 34.Wells D, Kaur K, Grifo J, Glassner M, Taylor JC, Fragouli E, Munne S. Clinical utilisation of a rapid low-pass whole genome sequencing technique for the diagnosis of aneuploidy in human embryos prior to implantation. J Med Genet. 2014;51(8):553–562. doi: 10.1136/jmedgenet-2014-102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kung A, Munné S, Bankowski B, Coates A, Wells D. Validation of next-generation sequencing for comprehensive chromosome screening of embryos. Reprod BioMed Online. 2015;31(6):760–769. doi: 10.1016/j.rbmo.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Phillips NR, Sprouse ML, Roby RK. Simultaneous quantification of mitochondrial DNA copy number and deletion ratio: a multiplex real-time PCR assay. Sci Rep. 2015;4(1):3887. doi: 10.1038/srep03887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunnala V, Schattman G. Oocyte vitrification for elective fertility preservation: the past, present, and future. Curr Opin Obstet Gynecol. 2017;29(1):59–63. doi: 10.1097/GCO.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 38.Ubaldi F, Anniballo R, Romano S, Baroni E, Albricci L, Colamaria S, Capalbo A, Sapienza F, Vajta G, Rienzi L. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum Reprod. 2010;25(5):1199–1205. doi: 10.1093/humrep/deq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almodin CG, Minguetti-Camara VC, Paixao CL, Pereira PC. Embryo development and gestation using fresh and vitrified oocytes. Hum Reprod. 2010;25(5):1192–1198. doi: 10.1093/humrep/deq042. [DOI] [PubMed] [Google Scholar]
- 40.Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, Colamaria S, Sapienza F, Ubaldi F. Embryo development of fresh “versus” vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25(1):66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopeika J, Thornhill A, Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update. 2015;21(2):209–227. doi: 10.1093/humupd/dmu063. [DOI] [PubMed] [Google Scholar]
- 42.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96(2):277–285. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 43.Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, et al. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertility and Sterility. 2017;107(1):34–42.e3. doi: 10.1016/j.fertnstert.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 44.May-Panloup P, Boucret L, Chao de la Barca J-M, Desquiret-Dumas V, Ferré-L’Hotellier V, Morinière C, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22(6):725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- 45.St. John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update. 2010;16(5):488–509. doi: 10.1093/humupd/dmq002. [DOI] [PubMed] [Google Scholar]
- 46.Klimczak AM, Pacheco LE, Lewis KE, Massahi N, Richards JP, Kearns WG, Saad AF, Crochet JR. Embryonal mitochondrial DNA: relationship to embryo quality and transfer outcomes. J Assist Reprod Genet. 2018;35(5):871–877. doi: 10.1007/s10815-018-1147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treff NR, Zhan Y, Tao X, Olcha M, Han M, Rajchel J, et al. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum Reprod. 2017;23:1–9. doi: 10.1093/humrep/dex034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho JR, Arrach N, Rhodes-Long K, Salem W, McGinnis LK, Chung K, et al. Blastulation timing is associated with differential mitochondrial content in euploid embryos. J Assist Reprod Genet. 2018;35(4):711–720. doi: 10.1007/s10815-018-1113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertility and Sterility. 2015;104(3):534–541.e1. doi: 10.1016/j.fertnstert.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Viotti M, Zouves C, Brake A, Tyndall J, Victor A, Griffin D, Barnes F. Blastocysts with disproportionally high mtDNA copy number can result in healthy babies. Reprod BioMed Online. 2019;38:e25–e26. [Google Scholar]
- 51.Lledo B, Ortiz JA, Morales R, García-Hernández E, Ten J, Bernabeu A, et al. Comprehensive mitochondrial DNA analysis and IVF outcome. Human Reproduction Open [Internet]. 2018 Sep 1 [cited 2019 Sep 3];2018(4). Available from: https://academic.oup.com/hropen/article/doi/10.1093/hropen/hoy023/5253765 [DOI] [PMC free article] [PubMed]
- 52.De Munck N, Vajta G. Safety and efficiency of oocyte vitrification. Cryobiology. 2017;78:119–127. doi: 10.1016/j.cryobiol.2017.07.009. [DOI] [PubMed] [Google Scholar]