Abstract
Recently, medical research has been shifting its focus to nanomedicine and nanotherapeutics in the pursuit of drug development research. Quantum dots (QDs) are a critical class of nanomaterials due to their unique properties, which include optical, electronic, and engineered biocompatibility in physiological environments. These properties have made QDs an attractive biomedical resource such that they have found application as both in vitro labeling and in vivo theranostic (therapy-diagnostic) agents. Considerable research has been conducted exploring the suitability of QDs in theranostic applications, but the cytotoxicity of QDs remains an obstacle. Several types of QDs have been investigated over the past decades, which may be suitable for use in biomedical applications if the barrier of cytotoxicity can be resolved. This review attempts to report and analyze the cytotoxicity of the major QDs along with relevant related aspects.
Keywords: Quantum dots, Cytotoxicity, Theranostic agents, Biocompatibility, Biomedical applications
Introduction
Quantum dots (QDs) or semiconductor nanocrystals are inorganic nanomaterials having dimensions in size range of 1–10 nm. They are composed of a semiconductor central core stabilized by a shell composed of inorganic salts (e.g., CdS, ZnS) (Mansur 2010). A semiconductor has an electron-filled region, the “valence band,” and an electron-deficient region, known as the “conduction band.” When photon energy (hν) equal to the bandgap energy irradiates the semiconductor, an electron is promoted from the valence to the conduction band. As a result, there will be a “hole” in the valence band due to the absence of the electron. This hole can be phenomenologically treated as a “particle” with a particular effective mass and a positive charge (Simon et al. 2010). QDs have unique optical properties, such as sharp and symmetric emission spectra and high fluorescence and photostability. In the past two decades, QD utilization has attracted significant attraction. There are several commercial areas for which QD utilization has been explored, such as biomedical applications. Most of these efforts so far have been devoted to tuning their semiconductor properties to develop smaller and more complex devices with better performance (Field et al. 2020).
The shell and core of QDs are both semiconductors. QD nanoparticles are generally found to be unstable and only slightly soluble in aqueous environments such as the cell cytosol (Hardman 2005). Also, water solubility can be enhanced through charged compounds covalently attached to the surface of QDs via the thiol group (Idowu et al. 2008). Molecules attached to the shell can be selected such that they are further able to conjugate to functional ligands or biomolecules (Mahmoudi et al. 2012; Hardman 2006).
Nanomedicine is a kind of medical intervention that takes place at the molecular scale. The main purposes of nanomedicine are in the treatment of disease and restoration and repair of function to damaged tissues such as the bone, muscle, or nerve (Juliano 2013). In both of these tasks, it is necessary to visualize both cell structures and the molecules involved in their metabolism. One solution for this challenge is to label them with a proper marker to make them easily observable. One of the most common labeling techniques in cell biology is fluorescence labeling (Koren et al. 2020). There are two main groups of existing fluorescent labels: organic dyes and inorganic nanocrystals. Organic dyes are the most exploited probes in cell biology. However, fast photobleaching and broad overlapping emission lines are drawbacks of the organic dyes. Their application area can be significantly affected due to these drawbacks, especially in long-term imaging and multicolor detection.
The unique properties of QDs, particularly their optical properties, have made them a promising choice to be used as fluorescent labels in analytical chemistry, cell biology, and medicine. There are some important differences between the common organic dyes and quantum dots as inorganic semiconductor dyes; for instance, the QD emission wavelength can be quickly and precisely tuned by adjusting the nanocrystal size, narrow symmetric emission spectra of QDs (which makes the simultaneous excitation of multiple semiconductor QDs possible) by only a single light source, less photobleaching in QD due to no excitation induced damage, and less exposure of the fluorescence center to solvent. Furthermore, the obtained images using semiconductor QDs often exhibit better contrast due to their high resistance to bleaching, which are among their most important advantages.
Conjugation between QD and biomolecules (such as proteins and enzymes) makes them applicable for use in a wide range of applications, such as nanomedicine (Mansur 2010), tracking proteins in living cell (Parak et al. 2002; Pathak et al. 2001), fluorescence labeling (Dwarakanath et al. 2004; Peppley et al. 1999), biosensors (Sapsford et al. 2006), deep-tissue imaging (Klostranec and Chan 2006), ex vivo/in situ live cell imaging, and in vivo targeting of cells, tissues, and tumors with monitoring by PET and MRI (Bera et al. 2010) and high-throughput screening.
Physiochemical properties of QDs
Achieving an understanding of the interfacial characteristics of QDs helps develop an understanding of how they interact with the different biological systems (Clift and Stone 2012). QD cores consist of elements from II–VI or III–V of the periodic table. QD cores are covered by a shell of semiconductor compounds. A semiconductor is a material that has an electrical conductivity lower than that of an electrically conductive material and higher than that of a non-conductive material. Examples of groups III–IV QDs include indium phosphide (InP), indium arsenide (InAs), gallium arsenate (GaAs), and gallium nitride (GaN) (Male et al. 2008). Examples of groups II–VI QDs include zinc sulfide (ZnS), zinc selenium (ZnSe), cadmium selenium (CdSe), and cadmium tellurium (CdTe) (Taniguchi et al. 2011). Some studies have also shown that higher atomic mass elemental combinations such as CdTe/CdSe or CdSe/ZnTe can also act as QDs.
The functionalization of the core-shell can give the desired bioactivity to QDs for application in biological systems (Hardman 2006). Many biomolecules, such as proteins, peptides, and lipids, can attach to the surface of the QD shell. As mentioned earlier, the thiol group capping through covalent linage was reported to be useful for enhancing water solubility to the QDs (Idowu et al. 2008). Also, polymer coatings (such as polyvinyl alcohol (PVA), polymethyl methacrylate (PMMA), and polylactide co glycolides (PLGA)) can be applied to the surface of QDs which makes the semiconductor QDs able to be targeted to specific organs within the body to diagnose, treat, or prevent disease (Wang et al. 2012). The most common polymer shell used, especially for nanomedicine purposes, is polyethylene glycol (PEG). Different methods such as electrostatic interactions, physical adsorption (physisorption), multivalent chelation, and covalent bonding can be used to functionalize the QD outer shell. Applying surface attachments can have a significant effect on the size of QDs (Clift and Stone 2012).
Several physical properties experience significant changes when the bulk material is in the form of nano-sized particles. For semiconductor nanoparticles, changing the particle size noticeably affects the bandgap. The average distance between the electron, which is photogenerated, and the hole is called the exciton Bohr radius (Zhang et al. 2014). As the particle size of the semiconductor approaches its exciton Bohr radius, the dependence of its optical and electrical properties on its physical dimensions becomes higher (Amelia et al. 2012). Because the QD particles are small, the generated electrons are confined to a smaller space than the natural space they would occupy in bulk semiconductors. This quantum confinement is the reason that the size of QD has a strong effect on the optical and electrical properties (Zhu et al. 2017). As the QD particle size decreases, there is a higher confinement degree, which results in higher bandgap energy.
As a consequence, the QD particle size tunes its bandgap energy and the emission wavelength. By adjusting the particle size, it is possible to prepare a QD for fluorescent emission from the UV into the IR spectra. Multiplexing of QD signals adds the possibility of imaging and tracking multiple molecular targets simultaneously. Furthermore, it creates promising opportunities in medical applications, since numerous genes and proteins are involved in many diseases. QD signals can be multiplexed as a result of broad and narrow absorption bands combination. Emission behavior of QDs can be tuned by their structural modifications which gives the possibility of fabricating materials for efficient light emitting diode applications (Ramalingam et al. 2019). Common organic dyes have wide emission bands, which significantly increase the complexity of detecting multiple signals.
Additionally, there are some essential fluorophores in biological tissues and fluids which produce a background signal. This background signal significantly decreases the detection ability and sensitivity of the probe. Biological fluorescence typically shows high background intensity in the blue-to-green spectral region. This is why most cell and tissue micrographs have a faint greenish color. QDs can minimize such auto-fluorescence since they can be tuned to emit in desired spectral regions.
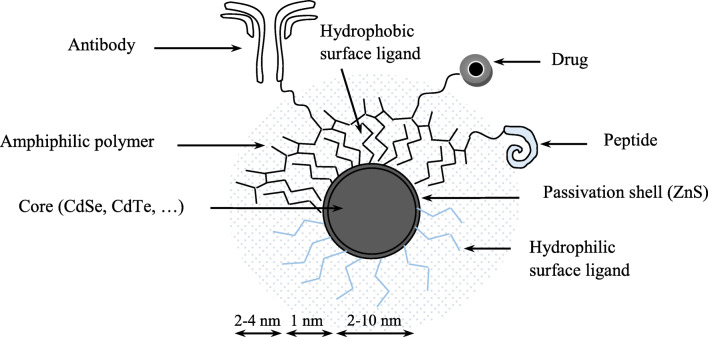
In summary, a variety of different surface modifications (surface-covered functional groups and biomolecules covering the surface of QDs) can be applied to the surface of QDs, which changes their physiochemical properties. Fig. 1 schematically represents the structure and different core regions of the quantum dot along with the common surface capping agents (Maysinger et al. 2007)
Fig. 1.
Structure of quantum dot with surface coating agents. Reprint with permission (Maysinger et al. 2007)
It is important to know that the physiochemical properties of QDs can be adjusted and tuned at their synthesis stage. Generally, it can be said that the physiochemical properties of QDs are considered to be defined by their core-shell conjugate constitution (Hoshino et al. 2004).
Mechanisms of QD cytotoxicity
Although QDs have received much attention and have entered into preclinical use, one key unresolved issue is their potential toxicity. It has been suggested that QD toxicity can be rationalized based on their physicochemical properties, such as core-shell materials, size, surface charge, ligands nature, and interaction with other present molecules in biological media (Oh et al. 2016). In other words, their toxicity may be due to either some inherent chemical feature or their nanoscale properties. Aspects related to inherent toxicity are mostly due to the elements contained with the QD core, such as cadmium and selenium, which exhibit significant toxicity to both cell cultures and live animals. Such studies have demonstrated toxicity at the supra-micromolar concentrations. Elemental toxicity is considerably dependent upon the accessibility of the core QD atoms to the surrounding solvent (Kirchner et al. 2005).
Regarding this point, cadmium atom toxicity is related to its relative permeability to oxygen and protons of conjugated groups. Oxygen can diffuse to the surface of the QD shell and trigger oxidation of the core atoms. Hydrogen ions can also cause protonation of the ligands and cause them to become detached from the QD surface (Derfus et al. 2004; Aldana et al. 2001). The biochemical mechanisms resulting in QD cytotoxicity are still controversial. Studies were reported which analyzed the effect of QDs on the liver and found that there is a direct correlation between Cd2+ release and cytotoxicity based on a mechanism involving inactivation of essential mitochondrial proteins through Cd-sulfhydryl group interactions (Derfus et al. 2004). It was demonstrated that the adsorption/accumulation of QDs on the cell surface could also impair cell function. Based on these observations, it was proposed that QD toxicity was a function of cell ingestion/uptake and not due to possible leaching of ions from the QDs into solution external to the cells (Parak et al. 2005). Another possible mechanism of QD toxicity is the generation of reactive oxygen species, such as free radicals and the creation of singlet oxygen (Zhou et al. 2017). Generation of such reactive oxygen species can cause irreversible damage to nucleic acids, enzymes, and cellular components such as mitochondria and both the plasma and nuclear membranes (Samia et al. 2003). Another study observed that CdSe and CdSe/ZnS QDs were able to generate free radicals (Choi et al. 2007). Another study postulated that surface oxidation of QDs leads to the generation and release of free cadmium ions, causing apoptosis (Derfus et al. 2004). It is also reported that cytotoxicity of QDs can be due to the type of molecules adsorbed to the surface of QDs in addition to the monocrystalline particle itself (Kirchner et al. 2005).
Decreasing the toxicity of QDs in biomedical applications
A major goal of QD research is its use in the development of biomedical applications. Among the various applications of QD in the biomedical field are the following: bioimaging, targeted drug delivery, and photodynamic therapy. The following factors determine the criteria of QDs suitable for employing in biomedical applications: (i) biocompatibility, (ii) cytotoxicity, and (iii) fluorescence behavior. Concern over QD cytotoxicity has been an important research topic over the last few decades, and various methods have been reported to reduce the toxicity of QD during their preparation. Silicon QDs are a family of well-studied QDs owing to their excellent biocompatibility and tunable physical and chemical properties, making them good candidates for theranostic applications (Sivasankarapillai et al. 2019). However, when we search through the literature focusing on the cytotoxic properties of all types of QDs, the diverse nature of the individual QD systems makes comparison difficult. This feature creates an attempt to generalize the toxicity of QDs, a nearly impossible task. In this section, we briefly discuss the cytotoxicity aspects of the most explored class of QDs available in the literature.
A significant observation was reported that CdTe QDs are highly cytotoxic due to the release of cadmium ions. The authors demonstrated that the presence of a ZnS outer-layer significantly improves the biocompatibility of QDs, with no observed cytotoxicity even at very high concentrations and long-time exposure in cells. However, it should be noted that the cytotoxicity of CdTe QDs cannot be solely attributed to the toxic effect of free Cd2+ ions through a systematic investigation on HEK293 cells (Su et al. 2010). This study demands further investigation of the specific properties of QDs responsible for the observed cytotoxicity of CdTe QDs. Another study investigated the cytotoxicity of a series of aqueous synthesized QDs such as CdTe, CdTe/CdS core-shell structured, and CdTe/CdS/ZnS core-shell-shell structured QDs. The authors suggested that released cadmium ions were responsible for the observed cytotoxicity of cadmium-based QDs (Chen et al. 2012). This study also provides additional features of QDs responsible for cytotoxicity using genome-wide gene expression profiling and subcellular localization of synthesized QDs with synchrotron-based scanning transmission X-ray microscopy (STXM).
Interesting work was reported in which L-cysteine (Cys) capped CdTe QDs were prepared in an aqueous medium. This study suggested that the capping agent reduced cytotoxicity upon the basis of experiments involving HeLa cancer cell lines (Kim et al. 2015). For cytotoxicity of CdSe/CdS QDs, it was paradoxically found that comparing toxicity based on particle concentrations was extremely difficult (Soenen et al. 2015). QDs possessing significant cytotoxicity have also been found to rapidly degrade under endosomal pH, resulting in leached Cd (II). Cytotoxicity of CdSe, CdTe, and InP based on four QD formulations involving (i) mercaptopropionic acid-modified CdSe/CdS/ZnS QDs (CdSe-MPA), (ii) PEGylated phospholipid encapsulated CdSe/CdS/ZnS QDs (CdSe-Phos), (iii) PEGylated phospholipid encapsulated InP/ZnS QDs (InP-Phos), and (iv) pluronic F127 encapsulated CdTe/ZnS QDs (CdTe-F127) was investigated. Interestingly, two cancer cells (gastric adenocarcinoma (BGC-823) and neuroblastoma (SH-SY5Y) showed different toxicity responses (Liu et al. 2015). This study gives valuable insight to the fact that the toxicity of QDs does not solely depend on a single factor but rather depends on a combination of elements from the particle formulations and extent of cellular uptake.
Meta-analysis is a valuable tool to apply data from the literature when dealing with a vast amount of scientific documentation. Literature is available, which shows meta-analysis investigation on the toxicity of Cd-based QDs using random forest regression models to analyze the data. The authors reported that the toxicity of QD is closely correlated with surface properties, including shell composition, ligand and surface modifications, QD diameter along with assay type, and exposure time to the biological environment (Oh et al. 2016). Also, aspects of the mechanism of cytotoxicity of Cd containing QDs were reviewed. Using CdTe/CdS 655 (QD 655), the authors showed that this QD elicited toxicity in vitro and in vivo by activating cell autophagy (Fan et al. 2016).
The effect of negatively charged CdTe QDs (− 21.63 ± 0.91 mV) on human umbilical vein endothelial cells (HUVECs) was reported. The authors said that both caveolae/raft- and clathrin-mediated endocytosis were involved in the endothelial uptake of CdTe QDs, and the QDs were transported to the endoplasmic reticulum (ER). The results indicated that the toxicity mechanism is initiated through stress response by upregulation of the ER stress markers GRP78/GRP94 and activation of protein kinase RNA-like ER kinase-eIF2α which activates the transcription factor 4 pathway. This study reported that all three ER stress-mediated apoptosis pathways were activated and that the ER was involved in the direct participation of CdTe QDs-caused apoptotic cell death in HUVECs (Yan et al. 2016). The cellular uptake of four CdSe/ZnS QDs (COOH CdSe/ZnS 525, COOH CdSe/ZnS 625, NH2 CdSe/ZnS 525, and NH2 CdSe/ZnS 625) and their ability to induce physiological responses in Phanerochaete chrysosporium (P. chrysosporium) was studied and reported (Hu et al., 2017). The authors showed that the four CdSe/ZnS QDs accumulated mostly in the hyphae and caused oxidative stress to P. chrysosporium in the tested concentration range (10–80 nM). Furthermore, this work provided evidence for the fact that cytotoxicity of these QDs was related to the physicochemical properties of the QDs, such as particle size and surface charges. Another exciting study reported nontoxic concentrations of CdSe/ZnS core/shell QDs vary between 4.13 and 12.7 nm/ml and identified the limit of CdSe/ZnS QD concentration at which they manifest themselves as reasonably safe and nontoxic agents for biological applications (Bozrova et al. 2018).
Graphene has generated much interest due to its unique electronic properties. Significant theoretical investigations on graphene quantum dots (GQDs) using molecular dynamics simulations were reported (Liang et al., 2016). At high GQD concentrations, the GQDs aggregated in water but disaggregated upon entering into the membrane interior. Moreover, high concentrations of GQDs could induce changes in the structural properties and fluidity of the lipid bilayer. On this basis, the authors speculated that QDs might affect cell signal transduction. On the other hand, the authors found that GQDs of relatively small size was not large enough to mechanically damage the lipid membrane and thus concluded that cytotoxicity of GQDs was size-specific and that small-sized QDs may be more appropriate for biomedical application.
Gradient-alloyed quantum dots (GA-QDs) are a novel class of QDs for biomedical imaging applications due to their improved fluorescent and luminescent properties over conventional QDs. Toxicity aspects of these compounds are of great importance to fully utilize their superior luminescent properties for clinical applications. Peynshaert and coworkers report on the relation between the surface coating of GA-QDs and their cytotoxicity. The authors carefully examined the toxicity of two identical gradient-alloyed QDs, differing only in their surface coatings, namely 3-mercaptopropionic (MPA) acid and polyethylene glycol (PEG) on HeLa cells. Both types have a gradient CdSexS1-x core surrounded by a ZnS shell. The authors observed that PEGylated QDs were significantly more toxic due to increased ROS production and lysosomal impairment, which further caused autophagy dysfunction (Peynshaert et al. 2017). Toxicity of halloysite nanotube stabilized CdS QDs on cell lines derived from human skin fibroblasts and prostate cancer cells was reported. The authors suggest that the immobilization of QD onto the surface of halloysite nanotubes may lower the cytotoxicity induced by released Cd (II) ions (Stavitskaya et al. 2018). Towards this point, the azine-mixed system, HNTs-azine-Cd0.7Zn0.3S, showed the lowest cytotoxicity due to the lowest release of Cd (II). Another study compared the cytotoxicity of CdTe QDs against the rate and extent of their degradation within the cell. The authors used a validated high-content screening approach, and QD degradation was monitored through the loss of fluorescence intensity (Manshian et al., 2017). This work established the strong dependence of the cytotoxicity of CdTe QD with its degradation. As mentioned, targeted drug delivery is a significant area of QD research since QDs can be used as both drug carrier vehicles and also for the bioimaging process for diagnostic purposes. Several works report on the cytotoxicity evaluation of drugs conjugated with QDs, especially anti-cancer drugs. Methotrexate (MTX) is a potent anticancer drug which is limited in use due to the development of drug resistance by malignant cells. An exciting work synthesized MTX-conjugated l-cysteine capped CdSe QDs (MTX-QD nano-conjugates) and evaluated their uptake and cytotoxicity in KB cells with/without resistance to MTX (Johari-Ahar et al. 2016). The authors observed that MTX-QD nanoconjugates efficiently internalized into the cancer cells, and induced markedly high cytotoxicity (IC50, 62 μg/mL) in the MTX-resistant KB cells as compared to the free MTX molecules (IC50,105.0 μg/mL), whereas these values were respectively about 3.1 and 3.6 ng/mL in the MTX-sensitive KB cells.
Graphene QDs (GQDs) are essential candidates for biological applications, and these aspects are recently reviewed and available in the literature (Li et al., 2019). An interesting genotoxicity analysis of N-doped GQDs was reported (Şenel et al., 2019). DNA binding analysis showed that N-doped GQDs interact with CT-DNA via both intercalation and electrostatic binding. The study of the DNA cleavage patterns showed that the N-doped GQDs cleaved DNA without any external agents and thus established significant genotoxicity. Also, siRNA loaded GQDs showed an excellent possibility to act as potential antitumor agents through the induction of DNA and mRNA breakage. Surface functional groups play an essential role in determining the toxicity of QDs as they act as the first point of contact between the compound and biological environment. Thus it is essential to analyze the influence of functional groups regarding their role in the toxicity of QDs. Also, the influence of functional groups on the toxicity of graphene QDs was investigated and reported. The authors selectively deposited either ketone carbonyl, carboxylic, or hydroxyl groups on GQDs and then compared the ROS generating ability of the different GQD derivatives (Zhou et al. 2017). This study reports that the ROS production ability of GQDs is closely related to the reduced state of the surface oxygen functional group. Removal of the oxygen functional groups on GQDs can increase the photostability and lower the photo-induced cytotoxicity.
A number of investigations have examined the change in toxicity of GQDs when in association with other metal ions like silver nanoparticles (Ag NPs). Literature suggests that the use of PEGylated silver nanoparticles decorated with graphene quantum dots (Ag-GQDs) for targeted delivery of doxorubicine (DOX) against HeLa and DU145 cancer cells was reported in vitro (Habiba et al. 2015). The authors used a photosensitizer to investigate the synergistic effect of chemo and photodynamic therapy in this system. The treatment of Ag-GQDs conjugated with doxorubicin under irradiation with a 425-nm lamp significantly increased the death in DU145 and HeLa cells. Interestingly, the toxicity of graphene oxide (GO) QDs is found to be rectified on the coating with other biomolecules like folic acid. Another study demonstrated the lack of cytotoxicity of folic acid-modified graphene oxide (GO) quantum dots using HaCaT cells (Goreham et al. 2018). The modified GO QDs were found to be non-toxic to macrophage cells even after prolonged exposure and high concentrations. This finding needs to be further investigated as it raises the possibility of implementing GQDs for biomedical applications by resolving their toxicity through the surface coating.
The toxicity of CuInS2 quantum dots (CIS QDs) was analyzed using Caenorhabditis elegans (C. elegans) as a model organism (Chen et al. 2015). The authors synthesized CIS QDs through the hydrothermal method and observed that QDs have no significant cytotoxicity in the organism and have excellent chemical stability. A similar work evaluated the cytotoxicity of CuInS2/ZnS QDs coated with polymeric shells and found them to have good hemo-compatibility and negligible cytotoxicity even after their penetration into cells (Speranskaya et al. 2016). This study reveals that cellular uptake is not necessarily the reason for cytotoxicity. The toxicity of ZnO QDs was found to be enhanced in the presence of Cu (II) ions along with the concomitant production of ROS species in Escherichia coli cells (Moussa et al. 2016).
Similarly, the cytotoxicity of InP/ZnS QDs having three different surface functional groups, NH2, COOH, OH, were evaluated and reported. The uptake efficiency of QDs, the cell apoptosis, and ROS generation was assessed on two different cell lines (human lung cancer cell HCC-15 and alveolar type II epithelial cell RLE-6TN). The authors observed that all the InP/ZnS QDs were able to enter the cells, with high uptake efficiency for InP/ZnS-COOH and InP/ZnS-NH2 exhibited at low concentrations of QD (23 nm/ml) (Chen et al. 2018). High doses of InP/ZnS QDs caused the cell viability to decrease, and InP/ZnS-COOH QDs and InP/ZnS-NH2 QDs appeared to be more toxic than InP/ZnS-OH QDs. Besides, all these InP/ZnS QDs promoted cell apoptosis and intracellular ROS generation after being co-cultured with cells.
To summarize this section, we emphasize the following points: Despite the many advantages shown by QDs, there are concerns regarding their cytotoxicity. It is difficult to provide a blanket evaluation of the toxicity of QDs because there are so many different categories according to their method of production, size, composition, charge, concentration, outer coating (capping material, functional groups), oxidative properties, photolytic conversion rate, and mechanical stability. All of these factors are determining factors in QD toxicity. Several studies have shown that QDs can cause damage to cells and produce significant DNA damage due to acute toxic effects. Evidence showed that if QDs were retained in cells or accumulated in the body for an extended period, their coatings might be degraded, yielding “naked” QDs can induce damage to the plasma membrane, mitochondrion, and nucleus, leading to cell death (Lovrić et al. 2005). Significant work was reported. The study of Clift et al. [13] that assessed the effects of a series of different surface-coated QDs on J774.A1 macrophage by cytotoxic examination (MTT assay and LDH release) showed that hydrophobic QDs caused a significant reduction in the cell metabolic activity (MTT assay) with subsequent release of LDH from J774.A1 macrophages (Clift and Stone 2012). It was also reported that QDs might induce cytotoxic effects in L929 fibroblasts at high exposure concentrations (Zhang et al. 2015a, b). The QDs were also found to cause oxidative stress, which led to DNA damage and subsequent apoptosis in liver cells. From a broader perspective, an assessment of QD blood compatibility showed that concentration of 29 ng/mL might serve as a threshold level for the types of QDs used in this study (also perhaps particular to their use in L929 fibroblast studies). Commercially available CdSe core/ZnS shell QDs of two different sizes (QD 565 and QD 655) and three different surface coatings (PEG, PEG-amines, and carboxylic acids) were used to test the hypothesis that QDs would be differentially taken up by the human epidermis (Ryman-Rasmussen et al. 2007). The authors concluded that grouping or classification of QDs about their potential toxicity based on size or other physicochemical properties alone would prove troublesome. They suggested that each QD type needs to be characterized individually to assess their potential toxicity. The findings in that work indicate that under certain conditions, QDs may affect environmental and human health, which needs to be individually determined for utilizing QDs for various applications.
Effect of QDs on environment and ecosystem
Particle size and surface area are important material characteristics from a toxicological perspective. As the size of a particle decreases, its surface area to volume ratio increases allowing a more significant proportion of its atoms or molecules to be displayed on the surface rather than the interior of the material. The change in the physicochemical and structural properties of engineered nanomaterial with a decrease in size could be responsible for a number of material interactions that could lead to toxicological effects. The very properties of nanoscale particles being exploited in certain applications (such as high surface reactivity and the ability to cross cell membranes) might also be responsible for their adverse health and environmental impacts. As a result, nanomaterials may present new health and environmental risks that have not been encountered before.
An increase in nanomaterial research will undoubtedly lead to the effective dumping of a lot of QDs into the environment, which may ultimately result in environmental toxicity (Zhang et al. 2012; Rocha et al. 2017). To date, there are no detailed studies on the mechanism of transport and biodegradation or association of QDs with biological materials that may eliminate nanomaterials. The presence of nanomaterials in the environment also affects the ecosystem. In a recent study, the toxicity of fullerene-C60 in two aquatic species, Daphnia and Pimephales, showed elevated lipid peroxidation (LPO) in the brain, significantly increased LPO in gill, and resulted in a significant increase in expression of genes related to the inflammatory response and metabolism. Processes that control transport and removal of NPs in water and wastewater have not yet been investigated to understand the fate of QDs. Studies on the effect of QDs on plants and microbes are also largely absent. The fate of nanomaterials in an aqueous environment is controlled by many biotic/abiotic processes such as solubility/dispersibility, interactions between the nanomaterials, and natural/anthropogenic chemicals in the ecosystem. Ecological risk assessment is essential to understand the environmental implications of nanomaterials. Before unknowingly dumping a large number of dangerous nanomaterials into the environment, we need to investigate the solubility and degradability of engineered NPs in soils and waters, to establish baseline information on their safety, toxicity, and the adaptation of soil and aquatic life.
Until now, there are different opinions about the toxicity of QDs. Thus, we list here the limited number of toxicity studies conducted at four levels of organism complexity (i) in amoeba (as a primary eukaryote), (ii) in plants, (iii) in animals, and (iv) in aquatic life (Valizadeh et al. 2012).
-
(i).
In amoeba: It has been determined that QD labeling had no detectable effect on cell growth and had no deleterious effects on cellular signaling and motility during the development of the Dictyostelium discoideum cells (Jaiswal et al. 2003).
-
(ii).
In-plant: The ratio of reduced glutathione levels (GSH) relative to the oxidized glutathione (GSSG) in plants suggests that QDs caused oxidative stress on the plant at this condition (Navarro et al. 2012).
-
(iii).
In animal: Yan et al. investigated the potential vascular endothelial toxicity of mercaptosuccinic acid (2-sulfanylbutanedioic acid)-capped QDs in vitro. Their results suggested that QDs could not only impair the mitochondria but also exert endothelial toxicity through activation of the mitochondrial death pathway and induction of endothelial apoptosis (Yan et al. 2011).
More recently, Chen et al. have studied the cytotoxicity of CdTe/CdS (core-shell) structured and also CdTe/CdS/ZnS (core-shell-shell) structured aqueous synthesized QDs, and their results suggest that the cytotoxicity of CdTe QDs not only comes from the release of Cd2+ ions but also intracellular distribution of QDs in cells and the associated nanoscale effects as discussed earlier (Chen et al. 2012).
-
(iv).
In aquatic ecosystems: zebrafish embryos provide an economical medium for screening the toxicity of QDs (Fako and Furgeson 2009). Assessment of nanotoxicity can be semi-quantified as sublethal toxicities (viz. survival of the embryo and the severity of phenotypic and gross morphological differences). This screening toolkit allows several parameters to be varied, including concentration, nanomaterial size, chemical composition, density, route of exposure, time of exposure, and the point of embryonic development at which the nanomaterial is administered. To semi-quantify these physicochemical metrics and associated-toxicity in the zebrafish model, a modified scoring spectrum was used based on the phenotypic changes of the zebrafish embryos, ranging from (normal phenotype) 1 (minor phenotypic changes), 2 (moderate alterations), 3 (severe embryo deformation), and 4 (embryo death) (Deng et al. 2018). Unlike traditional biochemical assays that explore specific molecular targets, the zebrafish model relies on the analysis of phenotypic changes. This method allows researchers to bypass several roadblocks commonly associated with current drug discovery efforts, which are based on in vitro biochemical screens followed by in vivo mammalian studies. The zebrafish model, therefore, potentially serves as a rapid and cost-effective method to conservatively assess the toxicity of novel pharmaceuticals, flagging those samples displaying toxicity for closer scrutiny and possible removal from continued drug development.
Most of the current literature on the toxicity of NPs comes from studies on mammalian cells, but it is essential to know their potentially harmful effects on the environment. Frequent detection of NPs in the aquatic environment reflects a rapidly growing number of engineered nanoparticles being used and their incomplete removal during passage through sewage treatment plants and relatively high persistence in water matrices (Farkas et al. 2011; Mühling et al. 2009). Particularly, hotspots of NPs could be present in hospital wastewater due to their ever more frequent use in medical applications for drug delivery.
Recent studies have confirmed that NPs are released into the environment and, in particular, the aquatic environment. For example, significant concentrations of nano-Ag can be released from AgNP-containing textiles during washing (Geranio et al. 2009). At the same time, contamination of sewage sludge with Ag and AgNPs has been detected (Kim et al. 2010), and 0.1 μg L−1 AgNPs have been identified in wastewater effluents (Mitrano et al. 2012). Zinc oxide nanoparticles (ZnO NPs) are also one of the most used NPs, and consequently, they are also significantly dispersed in the environment (Kahru and Dubourguier 2010).
To summarize this section: Environmental risk assessment is required to ensure the safety of nanomaterials and to protect the environment from unintentional adverse effects. In a regulatory context, this requires reliable and relevant environmental hazard data upon which predicted no-effect concentration (PNEC) values could be estimated. For nanomaterials, it is well-known that ecotoxicity testing is not straightforward and that the applicability of commonly used test guidelines and guidance can be questioned. Nanomaterials are known to behave very differently in ecotoxicity test systems compared to soluble chemicals, for which most guidelines were intended. This current lack of appropriate guidance implies that previous and current guideline-based hazard testing may not be suitable for testing of engineered nanomaterials (ENMs). It further entails that the data, upon which currently available PNEC values have been established, may not correctly reflect the actual ecotoxicity of these ENMs. This means that existing data from non-standard tests—or tests following modified test guidelines—in some cases may provide information on equal or higher reliability compared to strictly guideline-based tests. This would be the case if these modifications were applied to cater for nanomaterial properties and behavior in the test system. Such data should therefore not per se be considered less reliable as a basis for PNEC estimation.
One key area of research is the improvement of stability, safety, and efficacy of NPs through binding to peptides or peptidomimetics. Modifications of the surface of NPs with peptides will allow a reduction of their toxicity and enhancement of stability, while perhaps also determining an improvement of the properties of the peptides. NPs can serve as innovative drug delivery systems for antimicrobial peptides (AMPs) offering the possibility to target the delivery of AMPs to a specific site with controlled-release over time, thus minimizing side effects and increasing efficacy also due to NPs potential multi-valency (Vale et al. 2016; Galdiero et al. 2015). Inorganic nanomaterials have attracted significant attention since they display their antimicrobial activity, which may provide additive or synergistic effects when combined with AMPs (Tal et al. 2002).
Tables 1, 2, 3, and 4 summarize more results for toxicity associated with various QDs available in the literature.
Table 1.
Results for toxicity associated with graphene quantum dots
| QD | Model | Administration | QD concentration | Exposure duration | Toxicity | Ref |
|---|---|---|---|---|---|---|
| Cu2+ ion-labeled GQD | Mice, GC-1 spg (ts) (ATCC # CRL-2053) and TM3 (ATCC # CRL-1714) cell lines | Oral gavage and intravenous injection |
Oral: 0 (PBS), 60, 100, and 300 mg GQDs/kg mouse (500 μL per mouse) IV: 0 (PBS), 25, 75, and 150 mg GQDs/kg mouse (200 μL per mouse) |
24 h | High doses of GQDs administered via oral gavage or intravenous injection produce no discernible short- and long-term toxic effects on male reproductive ability and health of offspring | Zhang et al. 2019 |
| Nitrogen-doped graphene quantum dots (N-GQDs) | Zebrafish embryos | Feeding | 0, 25, 50, and 100 μg/mL | 72 h | N-GQDs could perturb the endogenous antioxidant enzyme system via transcriptional or posttranscriptional repression of antioxidant enzymes | Deng et al. 2019 |
| GQD | Rats | Intraperitoneal injection | 10 mg/kg/day | (i) From the day of immunization until the end of the experiment, (ii) during the first 7 days, (iii) from day 8 post-immunization until the end of the experiment, (iv) or from the day when first symptoms appeared until the end of the experiment | Neuroinflammation and alleviating immune-mediated CNS damage | Tosic et al. 2019 |
| GQDs | Zebrafish | Well dispersions of aqueous solutions of GQDs | 0–100 μg/mL | 7 days | Molecular regulatory networks are comprised of different signaling pathways triggered by GQDs | Deng et al. 2018 |
| GQD | Zebrafish | Direct exposure | 12.5–200 μg/m | 96 h | GQDs induced developmental nanotoxicity, which resulted in persistent effects on zebrafish larvae. Therefore, the exposure to high concentrations (> 50 μg/mL) of GQDs might constitute a developmental hazard to zebrafish | Wang et al. 2015a, b |
| N-GQDs | RBC | Hemolysis assay | 3.1, 6.2, 12.5, 25, 50, 100, 200 μg m/L | 3 h | Lots of echinocytes were observed unexpectedly which may be due to the incorporation of small N-GQDs into the lipid membrane | Wang et al. 2015a, b |
Table 2.
Results for toxicity associated with cadmium quantum dots
| QD | Model | Administration | QD concentration | Exposure duration | Toxicity | Ref |
|---|---|---|---|---|---|---|
| CdS-QDs | Soybean plants | Growth medium | 50–200 mg/L | 14 days | Peroxidases play the predominant role in quenching the oxidative stress caused by CdS-QD exposure. At the highest CdS-QD treatment (200 mg/L), root lignification allowed the plants to restrict Cd accumulation, except in QD-PVP, where lignification was reduced by 21% leading to higher Cd content in shoots | Majumdar et al. 2019 |
| CdSe and CdSe/ZnS | Escherichia coli (E. coli, represents a prokaryotic system) and Phanerochaete chrysosporium (P. chrysosporium, represents eukaryotic system) | In vitro and growth medium | 0, 10, 20, 50, and 80 nM | 48 h | Bioaccumulation amounts of CdSe QDs by E. coli and P. chrysosporium were larger than those of CdSe/ZnS QDs due to the smaller particle size and less negative surface charges of CdSe QDs. | Hu et al. 2019 |
| CdSe QD, CdSe/ZnS QD | Shewanella oneidensis MR-1 | In vitro | A total volume of 150 μL at varying concentrations | 15 min | QD interaction leads to membrane disruption, which is mechanical and depends on the QD concentration and their affinity to the liposome membranes | Williams et al. 2018 |
| CdSe/ ZnS | SPF grade female and male BABL/c mice | Subcutaneous injection | 5.0, 1.0, and 0.1 pmol/day/mouse | 14 days | QDs are found in the ovaries, but no changes are detected on the behavior and estrous cycle on the female mice. The mRNA downregulations of FSHr and LHr are observed, and the number of matured oocytes had shown a significant decrease when the QDs dosage was above 1.0 pmol/day along with a decrease in fertilization rate | Xu et al. 2016 |
| CdSe/ZnS | Human hepatic cell line L02 and 8-week-old male C57BL/6 mice |
In vitro (L02), IV injection (mice) |
20, 40, and 80 nM (L02) 10 nmol/kg body weight (mice) |
48 h (L02) 2 weeks (mice) |
CdSe/ZnS QDs conjugated with carboxyl groups induced hepatocyte pyroptosis, and liver inflammation and dysfunction. The in vitro and in vivo hepatic toxicity of QDs was mediated by QDs-induced NLRP3 activation which was attributed to Ca2+ mobilization and mtROS production after exposure to QDs | Lu et al. 2016 |
| CdSe/ZnS | TK6, BEAS-2B, and HFF-1 | In vitro | 0–20 nM | 24 h | Cytotoxicity and genotoxicity are strongly affected by a multitude of parameters including (1) differences in cell type potentially resulting in varying surface area contact with the exposed material, in addition to inherent cellular differences in internalizing NPs and ability to cope with an exogenous insult; (2) the nature of the QD surface chemistry; (3) the degree of QD agglomeration in the presence of varying amounts of serum proteins; (4) differences in cell culture media composition; and (5) time of exposure | Lin et al. 2015 |
| CdSe@MSA and CdSe(S)@MSA QDs | Escherichia coli | In vitro | 0–4000 nM | 100 min | The toxicity observed for CdSe QDs may be directly linked to •OH radicals produced | Kauffer et al. 2014 |
| TGA/TGH-CdTe | HeLa cells and Kunming mice | In vitro, intravenous injection (in vivo) |
In vitro: 45 mg m/L In vivo: 0.4 (low dose), 2.0 (representing medium dose) and 10.0 (representing high dose) mg kg1 |
24 h | PEG modification played an important role in reducing the toxicity of the QDs. PEG conjugated with the QD surface through chemical bonds and thus altered their surface state. Furthermore, PEG formed a fence-like structure on the QD surface, which could more effectively prevent Cd2+ release, which was induced by the diffusion effect from the QD surface to the solution | Du et al. 2019 |
| GSH-CdQDs and MPA-CdQDs | Lemna minor | Direct exposure | 0–15 mg/L | 168 h | GSH- and MPA-capped Cd-based QDs have similar toxicity for L. minor but are significantly less toxic than CdCl2 | Modlitbová et al. 2018 |
| CdS QDs | Yeast strains | Transcriptomic analysis | 0.25 mg/L | 24 h | In this case, yeast can be a good model to correlate genes with human orthologues in a cross-species comparison that might help to elucidate the response to toxic insults, like to CdS QDs, at a system level as well as to predict the mode of action of similar compounds in other species | Pasquali et al. 2017 |
|
Negatively charged: MPA-CdTe/ZnS QDs, MPA/MPO-CdTe/ZnS QDs, NAC-CdTe/ZnS QDs, GSH-CdTe/ZnS QDs |
E. coli | Optical density (OD) assays | 2 h | QDs decrease the growth rate of E. coli. The inhibition ratio of positive QDs is higher than that of negative QDs | Lai et al. 2017 | |
|
Positively charged: CA-CdTe QDs and CA-CdTe/ZnS QDs | ||||||
| CdTe | Liver mitochondria from female Wistar rats | Direct exposure | 100 nM | 60 min | Two kinds of QDs, coated with MPA and TGA respectively, could impair mitochondrial energy metabolism and affect mitochondrial lipid peroxidation | Xiang et al. 2017 |
| CdTe | Male BALB/c mice | IV injection | High dose of 2.0 nmol per mouse and a low dose of 0.2 nmol per mouse | 90 days | Bodyweight measurements demonstrated there was no overt toxicity for both dose at day 90 after exposure, but the high dose CdTe affected body weight up to 15 days after exposure | Lin et al. 2015 |
| CdTe | 8-week-old male mice | Intravenous administration | 4.125, 8.25, and 16.5 mg/kg | 4 weeks | Increased the level of lipid peroxides marker, MDA, in the liver | Zhang et al. 2015a, b |
| CdTe and CdTe@ZnS QDs | Caenorhabditis elegans | Foraging behavior assay | 0.001, 0.01, 0.1, and 1 g/L (CdTe) 0.1 and 1 g/L (CdTe@ZnS QDs) | Neurotoxicity of CdTe QDs at concentrations of 0.1–1 g/L on both the development and function of RMEs motor neurons in nematodes. Data demonstrate the impairment of foraging behavior after CdTe QDs exposure and imply the possible severe ecological risk of long-term exposure to low concentrations of CdTe QDs to environmental animals | Zhang et al. 2015a, b | |
| CdTe | Bombyx mori | IV injection | 0.08 nM and 0.32 nM | 48 h | Time and dose-dependent damage in the hematopoietic organ and hematocytes. With ROS might be one of the influencing mechanisms | Liu et al. 2014 |
| CdS | Mytilus galloprovincialis | In vitro | 0.001, 0.01, 0.1, 1, 10, 25, 50, and 100 mg Cd/L | 24 h | CdS QDs exposures decreased the cell viability of both hemocytes and gill cells. Main mechanisms of toxicity of CdS QDs in mussel hemocytes and gill cells involve ROS production and genotoxicity | Katsumiti et al. 2014 |
| Phospholipid micelle encapsulated CdSe/CdS/ZnS QDs | Kunming mice | IV injection | 0.81 mg (7.2 μmol)/kg | 14 days | QD exposure with a short buffering period before conception does not cause overt pregnancy complications or significant toxicity effects | Xiang et al. 2017 |
Table 3.
Results for toxicity associated with indium quantum dots
| QD | Model | Administration | QD concentration | Exposure duration | Toxicity | Ref |
|---|---|---|---|---|---|---|
| InPZnS QDs | Hydra vulgaris | Direct exposure | 70 nM | 72 h | Hydra is very susceptible to aquatic pollutants; these QDs may not represent a concrete risk for environmental health | Allocca et al. 2019 |
| Indium-based QDs (CFQD) | Female Lister Hooded rats | IV injection | 12.5 mg/kg and 50 mg/kg | 90 days | QDs mainly accumulated in the liver and spleen and were excreted from the body gradually and possess good biocompatibility | Yaghini et al. 2018 |
| InZnP and InZnPS QD | Human skin samples | In vitro | 6.25–200 nM (cytotoxicity and cell proliferation), 12.5–100 nM (oxidative stress) or 50 nM (comet assay, X-ray absorption spectroscopy, electron microscopy) | 24 h | Toxicity of pristine QDs was essentially non-toxic and the toxicity of aged QDs, which proved to be all toxic | Tarantini et al. 2019 |
Table 4.
Results for toxicity associated with other quantum dots
| QD | Model | Administration | QD concentration | Exposure duration | Toxicity | Ref |
|---|---|---|---|---|---|---|
| Ag2S QD | Chinese hamster lung fibroblast (V79) | In vitro | 5–2000 μg/mL | 24 h | The cytotoxic effects of DMSA/Ag2 S QDs may occur at high doses through the apoptotic pathways | Vardar et al. 2019 |
| PEGylated CuInS2/ZnS | Male BALB/c mice of 7 weeks old | IV injection | 100 μL | 90 days | No significant difference in body weight, no histopathological and no biochemical abnormalities | Zou et al. 2019 |
| CQDs | Zebrafish (Danio rerio), zooplankton (Daphnia magna), and phytoplankton (Scenedesmus obliquus) | Direct exposure | 0–200 mg/L |
96 h (zebrafish) 48 h (D. magna and S. obliquus) |
Results indicated trophic level-specific toxicity of CQDs, and the oxidative stress and pH alterations served as potential mechanisms underlying the toxicity of CQDs on S. obliquus, which may separately or jointly disturb the physiological processes in algal cells and affect growth | Yao et al. 2018 |
| PEG-coated Ag2Se | Male CD-1 (ICR) mice | IV injection | 8 μmol/kg body weight | 28 days | Ag2Se QDs-PEG only showed slight toxicity to the liver at day 28 post-exposure | Tang et al. 2016 |
Conclusion
The world of nanomaterials is extremely different from the world of bulk materials. Size-dependent properties make it nearly difficult to generalize when comparing the properties and behavior of different QD. There has been tremendous advancement in material science research after the discovery of QDs. Multiple factors make QDs useful for a wide range of purposes, and more applications are still in exploration. QD research has allowed the fabrication of new classes of QDs having tunable properties using easy preparation processes. Even though these new classes of QDs possess ever-better physical properties, aspects related to their cytotoxicity have held QD research back. Thus in order to address the challenges of QD research, it is essential to look deeply into what features make QDs toxic. In this review, we have made a detailed survey of recent works reported regarding the toxicity of QDs and summarized the cytotoxicity of QDs at the cellular, organism, and environmental levels. It is evident and worth mentioning that the toxicity of QDs depends on various factors and varies in a complex manner, which makes it difficult to generalize the aspects of toxicity. These factors include the nature of the biological environment, physiological parameters, nature of agent used for surface capping or surface functionalization, the extent of cellular uptake, and also on the nature of the QD employed. Thus there is urgency for novel analytical and predictive tools to provide a clearer understanding of the factors influencing QD toxicity.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aldana J, Wang YA, Peng X. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J Am Chem Soc. 2001;123:8844–8850. doi: 10.1021/ja016424q. [DOI] [PubMed] [Google Scholar]
- Allocca M, Mattera L, Bauduin A, Miedziak B, Moros M, De Trizio L, Tortiglione C. An integrated multilevel analysis profiling biosafety and toxicity induced by indium-and cadmium-based quantum dots in vivo. Environ Sci Technol. 2019;53:3938–3947. doi: 10.1021/acs.est.9b00373. [DOI] [PubMed] [Google Scholar]
- Amelia M, Lincheneau C, Silvi S, Credi A. Electrochemical properties of CdSe and CdTe quantum dots. Chem Soc Rev. 2012;41:5728–5743. doi: 10.1039/c2cs35117j. [DOI] [PubMed] [Google Scholar]
- Bera D, Qian L, Tseng TK, Holloway PH. Quantum dots and their multimodal applications: a review. Materials. 2010;3:2260–2345. [Google Scholar]
- Bozrova SV, Baryshnikova MA, Sokolova ZA, Nabiev IR, Sukhanova AV (2018) In vitro cytotoxicity of CdSe/ZnS quantum dots and their interaction with biological systems. KnE Energy:58–63
- Chen N, He Y, Su Y, Li X, Huang Q, Wang H, Fan C. The cytotoxicity of cadmium-based quantum dots. Biomaterials. 2012;33:1238–1244. doi: 10.1016/j.biomaterials.2011.10.070. [DOI] [PubMed] [Google Scholar]
- Chen CW, Wu DY, Chan YC, Lin CC, Chung PH, Hsiao M, Liu RS. Evaluations of the chemical stability and cytotoxicity of CuInS2 and CuInS2/ZnS core/shell quantum dots. J Phys Chem C. 2015;119:2852–2860. [Google Scholar]
- Chen T, Li L, Xu G, Wang X, Wang J, Chen Y, Lin G. Cytotoxicity of InP/ZnS quantum dots with different surface functional groups toward two lung-derived cell lines. Front Pharmacol. 2018;9:763. doi: 10.3389/fphar.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift MJ, Stone V. Quantum dots: an insight and perspective of their biological interaction and how this relates to their relevance for clinical use. Theranostics. 2012;2:668. doi: 10.7150/thno.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Jia PP, Zhang JH, Junaid M, Niu A, Ma YB, Pei DS. Transcriptomic response and perturbation of toxicity pathways in zebrafish larvae after exposure to graphene quantum dots (GQDs) J Hazard Mater. 2018;357:146–158. doi: 10.1016/j.jhazmat.2018.05.063. [DOI] [PubMed] [Google Scholar]
- Deng S, Fu A, Junaid M, Wang Y, Yin Q, Fu C, Pei DS. Nitrogen-doped graphene quantum dots (N-GQDs) perturb redox-sensitive system via the selective inhibition of antioxidant enzyme activities in zebrafish. Biomaterials. 2019;206:61–72. doi: 10.1016/j.biomaterials.2019.03.028. [DOI] [PubMed] [Google Scholar]
- Derfus AM, Chan WC, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Zhong Y, Dong J, Qian C, Sun S, Gao L, Yang D. The effect of PEG functionalization on the in vivo behavior and toxicity of CdTe quantum dots. RSC Adv. 2019;9:12218–12225. doi: 10.1039/c9ra00022d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwarakanath S, Bruno JG, Shastry A, Phillips T, John A, Kumar A, Stephenson LD. Quantum dot-antibody and aptamer conjugates shift fluorescence upon binding bacteria. Biochem Biophys Res Commun. 2004;325:739–743. doi: 10.1016/j.bbrc.2004.10.099. [DOI] [PubMed] [Google Scholar]
- Fako VE, Furgeson DY. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Adv Drug Deliv Rev. 2009;61:478–486. doi: 10.1016/j.addr.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Fan J, Sun Y, Wang S, Li Y, Zeng X, Cao Z, Gao H. Inhibition of autophagy overcomes the nanotoxicity elicited by cadmium-based quantum dots. Biomaterials. 2016;78:102–114. doi: 10.1016/j.biomaterials.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Farkas J, Christian P, Gallego-Urrea JA, Roos N, Hassellöv M, Tollefsen KE, Thomas KV. Uptake and effects of manufactured silver nanoparticles in rainbow trout (Oncorhynchus mykiss) gill cells. Aquat Toxicol. 2011;101:117–125. doi: 10.1016/j.aquatox.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Field LD, Chen YC, Delehanty JB (2020) Semiconductor quantum dots for visualization and sensing in neuronal cell systems. In: Basic Neurobiology Techniques 152:1–18
- Galdiero S, Falanga A, Berisio R, Grieco P, Morelli G, Galdiero M. Antimicrobial peptides as an opportunity against bacterial diseases. Curr Med Chem. 2015;22:1665–1677. doi: 10.2174/0929867322666150311145632. [DOI] [PubMed] [Google Scholar]
- Geranio L, Heuberger M, Nowack B. The behavior of silver nanotextiles during washing. Environ Sci Technol. 2009;43:8113–8118. doi: 10.1021/es9018332. [DOI] [PubMed] [Google Scholar]
- Goreham RV, Schroeder KL, Holmes A, Bradley SJ, Nann T. Demonstration of the lack of cytotoxicity of unmodified and folic acid modified graphene oxide quantum dots, and their application to fluorescence lifetime imaging of HaCaT cells. MicrochimicaActa. 2018;185:128. doi: 10.1007/s00604-018-2679-8. [DOI] [PubMed] [Google Scholar]
- Habiba K, Bracho-Rincon DP, Gonzalez-Feliciano JA, Villalobos-Santos JC, Makarov VI, Ortiz D, Morell G (2015) Synergistic antibacterial activity of PEGylated silver–graphene quantum dots nanocomposites. Applied Materials Today 1:80–87
- Hardman R. Atoxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Fujioka K, Oku T, Suga M, Sasaki YF, Ohta T, Yamamoto K. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4:2163–2169. [Google Scholar]
- Hu L, Wan J, Zeng G, Chen A, Chen G, Huang Z, Lai C. Comprehensive evaluation of the cytotoxicity of CdSe/ZnS quantum dots in Phanerochaete chrysosporium by cellular uptake and oxidative stress. Environ Sci Nano. 2017;4:2018–2029. [Google Scholar]
- Hu L, Zhong H, He Z. The cytotoxicities in prokaryote and eukaryote varied for CdSe and CdSe/ZnS quantum dots and differed from cadmium ions. Ecotoxicol Environ Saf. 2019;181:336–344. doi: 10.1016/j.ecoenv.2019.06.027. [DOI] [PubMed] [Google Scholar]
- Idowu M, Lamprecht E, Nyokong T. Interaction of water-soluble thiol capped CdTe quantum dots and bovine serum albumin. J Photochem Photobiol A Chem. 2008;198:7–12. [Google Scholar]
- Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- Johari-Ahar M, Barar J, AlizadehAM DS, Omidi Y, Rashidi MR. Methotrexate-conjugated quantum dots: synthesis, characterisation and cytotoxicity in drug resistant cancer cells. J Drug Target. 2016;24:120–133. doi: 10.3109/1061186X.2015.1058801. [DOI] [PubMed] [Google Scholar]
- Juliano R. Nanomedicine: is the wave cresting? Nat Rev Drug Discov. 2013;12:171–172. doi: 10.1038/nrd3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahru A, Dubourguier HC. From ecotoxicology to nanoecotoxicology. Toxicology. 2010;269:105–119. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Katsumiti A, Gilliland D, Arostegui I, Cajaraville MP. Cytotoxicity and cellular mechanisms involved in the toxicity of CdS quantum dots in hemocytes and gill cells of the mussel Mytilus galloprovincialis. Aquat Toxicol. 2014;153:39–52. doi: 10.1016/j.aquatox.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Kauffer FA, Merlin C, Balan L, Schneider R. Incidence of the core composition on the stability, the ROS production and the toxicity of CdSe quantum dots. J Hazard Mater. 2014;268:246–255. doi: 10.1016/j.jhazmat.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Kim J, Park Y, Yoon TH, Yoon CS, Choi K. Phototoxicity of CdSe/ZnSe quantum dots with surface coatings of 3-mercaptopropionic acid or tri-n-octylphosphine oxide/gum arabic in Daphnia magna under environmentally relevant UV-B light. Aquat Toxicol. 2010;97:116–124. doi: 10.1016/j.aquatox.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Kim J, Huy BT, Sakthivel K, Choi HJ, Joo WH, Shin SK, Lee YI. Highly fluorescent CdTe quantum dots with reduced cytotoxicity-a robust biomarker. Sens Bio-Sens Res. 2015;3:46–52. [Google Scholar]
- Kirchner C, Liedl T, Kudera S, Pellegrino T, Muñoz Javier A, Gaub HE, Parak WJ. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5:331–338. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- Klostranec JM, Chan WC. Quantum dots in biological and biomedical research: recent progress and present challenges. Adv Mater. 2006;18:1953–1964. [Google Scholar]
- Koren A, Lunder M, Molek P, Kopač P, Zahirović A, Gattinger P, Korošec P (2020) Fluorescent labeling of major honeybee allergens Api m 1 and Api m 2 with Quantum dots and the development of a multiplex basophil activation test. Allergy 00:1–4 [DOI] [PubMed]
- Lai L, Li SJ, Feng J, Mei P, Ren ZH, Chang YL, Liu Y. Effects of surface charges on the bactericide activity of CdTe/ZnS quantum dots: a cell membrane disruption perspective. Langmuir. 2017;33:2378–2386. doi: 10.1021/acs.langmuir.7b00173. [DOI] [PubMed] [Google Scholar]
- Li M, Chen T, Gooding JJ, Liu J. Review of carbon and graphene quantum dots for sensing. ACS Sensors. 2019;4:1732–1748. doi: 10.1021/acssensors.9b00514. [DOI] [PubMed] [Google Scholar]
- Liang L, Kong Z, Kang Z, Wang H, Zhang L, Shen JW. Theoretical evaluation on potential cytotoxicity of graphene quantum dots. ACS Biomater Sci Eng. 2016;2:1983–1991. doi: 10.1021/acsbiomaterials.6b00390. [DOI] [PubMed] [Google Scholar]
- Lin G, Ouyang Q, Hu R, Ding Z, Tian J, Yin F, Yong KT. In vivo toxicity assessment of non-cadmium quantum dots in BALB/c mice. Nanomedicine. 2015;11:341–350. doi: 10.1016/j.nano.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Liu T, Xing R, Zhou YF, Zhang J, Su YY, Zhang KQ, Xu SQ. Hematopoiesis toxicity induced by CdTe quantum dots determined in an invertebrate model organism. Biomaterials. 2014;35:2942–2951. doi: 10.1016/j.biomaterials.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Liu J, Hu R, Liu J, Zhang B, Wang Y, Liu X, Yong KT. Cytotoxicity assessment of functionalized CdSe, CdTe and InP quantum dots in two human cancer cell models. Mater Sci Eng. 2015;57:222–231. doi: 10.1016/j.msec.2015.07.044. [DOI] [PubMed] [Google Scholar]
- Lovrić J, Cho SJ, Winnik FM, Maysinger D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol. 2005;12:1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lu Y, Xu S, Chen H, He M, Deng Y, Cao Z, Gao P. CdSe/ZnS quantum dots induce hepatocyte pyroptosis and liver inflammation via NLRP3 inflammasome activation. Biomaterials. 2016;90:27–39. doi: 10.1016/j.biomaterials.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Hofmann H, Rothen-Rutishauser B, Petri-Fink A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem Rev. 2012;112:2323–2338. doi: 10.1021/cr2002596. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Pagano L, Wohlschlegel JA, Villani M, Zappettini A, White JC, Keller AA. Proteomic, gene and metabolite characterization reveal the uptake and toxicity mechanisms of cadmium sulfide quantum dots in soybean plants. Environ Sci Nano. 2019;6:3010–3026. [Google Scholar]
- Male KB, Lachance B, Hrapovic S, Sunahara G, Luong JH. Assessment of cytotoxicity of quantum dots and gold nanoparticles using cell-based impedance spectroscopy. Anal Chem. 2008;80(14):5487–5493. doi: 10.1021/ac8004555. [DOI] [PubMed] [Google Scholar]
- Manshian BB, Jiménez J, Himmelreich U, Soenen SJ. Personalized medicine and follow-up of therapeutic delivery through exploitation of quantum dot toxicity. Biomaterials. 2017;127:1–12. doi: 10.1016/j.biomaterials.2017.02.039. [DOI] [PubMed] [Google Scholar]
- Mansur HS. Quantum dots and nanocomposites. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:113–129. doi: 10.1002/wnan.78. [DOI] [PubMed] [Google Scholar]
- Maysinger D, Lovrić J, Eisenberg A, Savić R (2007) Fate of micelles and quantum dots in cells. Eur J Pharm Biopharm 65:270–281 [DOI] [PubMed]
- Mitrano DM, Lesher EK, Bednar A, Monserud J, Higgins CP, Ranville JF. Detecting nanoparticulate silver using single-particle inductively coupled plasma–mass spectrometry. Environ Toxicol Chem. 2012;31:115–121. doi: 10.1002/etc.719. [DOI] [PubMed] [Google Scholar]
- Modlitbová P, Novotný K, Pořízka P, Klus J, Lubal P, Zlámalová-Gargošová H, Kaiser J. Comparative investigation of toxicity and bioaccumulation of Cd-based quantum dots and Cd salt in freshwater plant Lemna minor L. Ecotoxicol Environ Saf. 2018;147:334–341. doi: 10.1016/j.ecoenv.2017.08.053. [DOI] [PubMed] [Google Scholar]
- Moussa H, Merlin C, Dezanet C, Balan L, Medjahdi G, Ben-Attia M, Schneider R. Trace amounts of Cu2+ ions influence ROS production and cytotoxicity of ZnO quantum dots. J Hazard Mater. 2016;304:532–542. doi: 10.1016/j.jhazmat.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Mühling M, Bradford A, Readman JW, Somerfield PJ, Handy RD. An investigation into the effects of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Mar Environ Res. 2009;68:278–283. doi: 10.1016/j.marenvres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Ramalingam G, Ragupathi C, Kaviyarasu K, Letsholathebe D, Mohamed SB, Magdalane CM, Maaza M (2019) Up-Scalable Synthesis ofSize-Controlled White-Green Emitting Behavior of Core/Shell (CdSe/ZnS) Quantum Dots for LED Applications. Journal of nanoscience andnanotechnology 19(7):4026–4032 [DOI] [PubMed]
- Navarro DA, Bisson MA, Aga DS. Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J Hazard Mater. 2012;211:427–435. doi: 10.1016/j.jhazmat.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Oh E, Liu R, Nel A, Gemill KB, Bilal M, Cohen Y, Medintz IL. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat Nanotechnol. 2016;11:479. doi: 10.1038/nnano.2015.338. [DOI] [PubMed] [Google Scholar]
- Parak WJ, Boudreau R, Le Gros M, Gerion D, Zanchet D, Micheel CM, Larabell C. Cell motility and metastatic potential studies based on quantum dot imaging of phagokinetic tracks. Adv Mater. 2002;14:882–885. [Google Scholar]
- Parak WJ, Pellegrino T, Plank C. Labelling of cells with quantum dots. Nanotechnology. 2005;16(2):R9. doi: 10.1088/0957-4484/16/2/R01. [DOI] [PubMed] [Google Scholar]
- Pasquali F, Agrimonti C, Pagano L, Zappettini A, Villani M, Marmiroli M, Marmiroli N. Nucleo-mitochondrial interaction of yeast in response to cadmium sulfide quantum dot exposure. J Hazard Mater. 2017;324:744–752. doi: 10.1016/j.jhazmat.2016.11.053. [DOI] [PubMed] [Google Scholar]
- Pathak S, Choi SK, Arnheim N, Thompson ME. Hydroxylated quantum dots as luminescent probes for in situ hybridization. J Am Chem Soc. 2001;123:4103–4104. doi: 10.1021/ja0058334. [DOI] [PubMed] [Google Scholar]
- Peppley BA, Amphlett JC, Kearns LM, Mann RF. Methanol–steam reforming on Cu/ZnO/Al2O3 catalysts. Part 2. A comprehensive kinetic model. Appl Catal A Gen. 1999;179:31–49. [Google Scholar]
- Peynshaert K, Soenen SJ, Manshian BB, Doak SH, Braeckmans K, De Smedt SC, Remaut K. Coating of quantum dots strongly defines their effect on lysosomal health and autophagy. Actabiomaterialia. 2017;48:195–205. doi: 10.1016/j.actbio.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Rocha TL, Mestre NC, Sabóia-Morais SMT, Bebianno MJ. Environmental behaviour and ecotoxicity of quantum dots at various trophic levels: a review. Environ Int. 2017;98:1–17. doi: 10.1016/j.envint.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J Investig Dermatol. 2007;127:143–153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- Samia ACS, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc. 2003;125:15736–15737. doi: 10.1021/ja0386905. [DOI] [PubMed] [Google Scholar]
- Sapsford KE, Pons T, Medintz IL, Mattoussi H. Biosensing with luminescent semiconductor quantum dots. Sensors. 2006;6:925–953. [Google Scholar]
- Şenel B, Demir N, Büyükköroğlu G, Yıldız M. Graphene quantum dots: synthesis, characterization, cell viability, genotoxicity for biomedical applications. Saudi Pharm J. 2019;27:846–858. doi: 10.1016/j.jsps.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Protasenko V, Lian C, Xing H, Jena D. Polarization-induced hole doping in wide–band-gap uniaxial semiconductor heterostructures. Science. 2010;327(5961):60–64. doi: 10.1126/science.1183226. [DOI] [PubMed] [Google Scholar]
- Sivasankarapillai VS, Jose J, Shanavas MS, Marathakam A, Uddin M, Mathew B. Silicon quantum dots: promising theranostic probes for the future. Curr Drug Targets. 2019;20:1255–1263. doi: 10.2174/1389450120666190405152315. [DOI] [PubMed] [Google Scholar]
- Soenen SJ, Abe S, Manshian BB, Aubert T, Hens Z, De Smedt SC, Braeckmans K. The effect of intracellular degradation on cytotoxicity and cell labeling efficacy of inorganic ligand-stabilized colloidal CdSe/CdS quantum dots. J Biomed Nanotechnol. 2015;11:631–643. doi: 10.1166/jbn.2015.1853. [DOI] [PubMed] [Google Scholar]
- Speranskaya ES, Sevrin C, De Saeger S, Hens Z, Goryacheva IY, Grandfils C. Synthesis of hydrophilic CuInS2/ZnS quantum dots with different polymeric shells and study of their cytotoxicity and hemocompatibility. ACS Appl Mater Interfaces. 2016;8:7613–7622. doi: 10.1021/acsami.5b11258. [DOI] [PubMed] [Google Scholar]
- Stavitskaya AV, Novikov AA, Kotelev MS, Kopitsyn DS, Rozhina EV, Ishmukhametov IR, Vinokurov VA. Fluorescence and cytotoxicity of cadmium sulfide quantum dots stabilized on clay nanotubes. Nanomaterials. 2018;8:391. doi: 10.3390/nano8060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Hu M, Fan C, He Y, Li Q, Li W, Huang Q. The cytotoxicity of CdTe quantum dots and the relative contributions from released cadmium ions and nanoparticle properties. Biomaterials. 2010;31:4829–4834. doi: 10.1016/j.biomaterials.2010.02.074. [DOI] [PubMed] [Google Scholar]
- Tal S, Guller V, Gurevich A, Levi S. Fever of unknown origin in the elderly. J Intern Med. 2002;252:295–304. doi: 10.1046/j.1365-2796.2002.01042.x. [DOI] [PubMed] [Google Scholar]
- Tang H, Yang ST, Yang YF, Ke DM, Liu JH, Chen X, Liu Y. Blood clearance, distribution, transformation, excretion, and toxicity of near-infrared quantum dots Ag2Se in mice. ACS Appl Mater Interfaces. 2016;8:17859–17869. doi: 10.1021/acsami.6b05057. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Green M, Rizvi SB, Seifalian A. The one-pot synthesis of core/shell/shell CdTe/CdSe/ZnSe quantum dots in aqueous media for in vivo deep tissue imaging. J Mater Chem. 2011;21(9):2877–2882. [Google Scholar]
- Tarantini A, Wegner KD, Dussert F, Sarret G, Beal D, Mattera L, Gallet B. Physicochemical alterations and toxicity of InP alloyed quantum dots aged in environmental conditions: a safer by design evaluation. NanoImpact. 2019;14:100168. [Google Scholar]
- Tosic J, Stanojevic Z, Vidicevic S, Isakovic A, Ciric D, Martinovic T, Todorovic-Markovic B. Graphene quantum dots inhibit T cell-mediated neuroinflammation in rats. Neuropharmacology. 2019;146:95–108. doi: 10.1016/j.neuropharm.2018.11.030. [DOI] [PubMed] [Google Scholar]
- Vale G, Mehennaoui K, Cambier S, Libralato G, Jomini S, Domingos RF. Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: a critical overview. Aquat Toxicol. 2016;170:162–174. doi: 10.1016/j.aquatox.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Akbarzadeh A, Davaran S. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett. 2012;7:480. doi: 10.1186/1556-276X-7-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardar DÖ, Aydin S, Hocaoglu I, Acar HY, Basaran N (2019) Aninvitro study on the cytotoxicity and genotoxicity of silver sulfide quantum dots coated with meso-2, 3-dimercaptosuccinic acid. Turk J Pharm Sci 16:282-291 [DOI] [PMC free article] [PubMed]
- Wang J, Han S, Ke D, Wang R (2012) Semiconductor quantum dots surface modification for potential cancer diagnostic and therapeutic applications. J Nanomater 2012:1–8
- Wang ZG, Rong ZHOU, Jiang D, Jing ES, Qian XU, Jing SI, Zhang H. Toxicity of graphene quantum dots in zebrafish embryo. Biomed Environ Sci. 2015;28:341–351. doi: 10.3967/bes2015.048. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhu S, Jiang X. Toxicity mechanism of graphene oxide and nitrogen-doped graphene quantum dots in RBCs revealed by surface-enhanced infrared absorption spectroscopy. Toxicol Res. 2015;4:885–894. [Google Scholar]
- Williams DN, Pramanik S, Brown RP, Zhi B, McIntire E, Hudson-Smith NV, Rosenzweig Z. Adverse interactions of luminescent semiconductor quantum dots with liposomes and Shewanella oneidensis. ACS Appl Nano Mater. 2018;1:4788–4800. doi: 10.1021/acsanm.8b01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Wu C, Zhang BR, Gao T, Zhao J, Ma L, Liu Y. The relationship between the length of surface ligand and effects of CdTe quantum dots on the physiological functions of isolated mitochondria. Chemosphere. 2017;184:1108–1116. doi: 10.1016/j.chemosphere.2017.06.091. [DOI] [PubMed] [Google Scholar]
- Xu G, Lin G, Lin S, Wu N, Deng Y, Feng G, Niu H. The reproductive toxicity of CdSe/ZnS quantum dots on the in vivo ovarian function and in vitro fertilization. Sci Rep. 2016;6:1–11. doi: 10.1038/srep37677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghini E, Turner H, Pilling A, Naasani I, MacRobert AJ. In vivo biodistribution and toxicology studies of cadmium-free indium-based quantum dot nanoparticles in a rat model. Nanomedicine. 2018;14:2644–2655. doi: 10.1016/j.nano.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Zhang Y, Xu K, Fu T, Qin H, Zheng X. An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicology. 2011;282:94–103. doi: 10.1016/j.tox.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Yan M, Zhang Y, Qin H, Liu K, Guo M, Ge Y, Zheng X. Cytotoxicity of CdTe quantum dots in human umbilical vein endothelial cells: the involvement of cellular uptake and induction of pro-apoptotic endoplasmic reticulum stress. Int J Nanomedicine. 2016;11:529. doi: 10.2147/IJN.S93591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Lv X, Zheng G, Chen Z, Jiang Y, Zhu X, Cai Z. Effects of carbon quantum dots on aquatic environments: comparison of toxicity to organisms at different trophic levels. Environ Sci Technol. 2018;52:14445–14451. doi: 10.1021/acs.est.8b04235. [DOI] [PubMed] [Google Scholar]
- Zhang S, Jiang Y, Chen CS, Spurgin J, Schwehr KA, Quigg A, Santschi PH. Aggregation, dissolution, and stability of quantum dots in marine environments: importance of extracellular polymeric substances. Environ Sci Technol. 2012;46:8764–8772. doi: 10.1021/es301000m. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Li C, Chen X, Wang Q. Controlled synthesis of Ag2S quantum dots and experimental determination of the exciton Bohr radius. J Phys Chem. 2014;118:4918–4923. [Google Scholar]
- Zhang T, Hu Y, Tang M, Kong L, Ying J, Wu T, Pu Y. Liver toxicity of cadmium telluride quantum dots (CdTe QDs) due to oxidative stress in vitro and in vivo. Int J Mol Sci. 2015;16:23279–23299. doi: 10.3390/ijms161023279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wang Y, Kong L, Xue Y, Tang M. Threshold dose of three types of quantum dots (QDs) induces oxidative stress triggers DNA damage and apoptosis in mouse fibroblast L929 cells. Int J Environ Res Public Health. 2015;12:13435–13454. doi: 10.3390/ijerph121013435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang Z, Wu Y, Fu K, Chen Y, Li W, Chu M. Systematic evaluation of graphene quantum dot toxicity to male mouse sexual behaviors, reproductive and offspring health. Biomaterials. 2019;194:215–232. doi: 10.1016/j.biomaterials.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Sun H, Wang F, Ren J, Qu X. How functional groups influence the ROS generation and cytotoxicity of graphene quantum dots. Chem Commun. 2017;53:10588–10591. doi: 10.1039/c7cc04831a. [DOI] [PubMed] [Google Scholar]
- Zhu S, Song Y, Wang J, Wan H, Zhang Y, Ning Y, Yang B. Photoluminescence mechanism in graphene quantum dots: quantum confinement effect and surface/edge state. Nano Today. 2017;13:10–14. [Google Scholar]
- Zou W, Lin G, Wang X, Chen Y, Yang Z, Chen T, Liu D. In vivo toxicity evaluation of PEGylated CuInS2/ZnS quantum dot in BALB/c mice. Front Pharmacol. 2019;10:437. doi: 10.3389/fphar.2019.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]