Keywords: autophagy, cargo receptors, ciliophagy, LC3, NBR1, primary cilia
Abstract
Reduced ciliary expression is reported in several tumors, including cholangiocarcinoma (CCA). We previously showed primary cilia have tumor suppressor characteristics, and HDAC6 is involved in ciliary loss. However, mechanisms of ciliary disassembly are unknown. Herein, we tested the hypothesis that HDAC6-dependent autophagy of primary cilia, i.e., ciliophagy, is the main mechanism driving ciliary disassembly in CCA. Using the cancer genome atlas database, human CCA cells, and a rat orthotopic CCA model, we assessed basal and HDAC6-regulated autophagy levels. The effects of RNA-silencing or pharmacological manipulations of ciliophagy on ciliary expression were assessed. Interactions of ciliary proteins with autophagy machinery was assessed by immunoprecipitations. Cell proliferation was assessed by MTS and IncuCyte. A CCA rat model was used to assess the effects of pharmacological inhibition of ciliophagy in vivo. Autophagy is increased in human CCA, as well as in a rat orthotopic CCA model and human CCA cell lines. Autophagic flux was decreased via inhibition of HDAC6, while it was increased by its overexpression. Inhibition of autophagy and HDAC6 restores cilia and decreases cell proliferation. LC3 interacts with HDAC6 and ciliary proteins, and the autophagy cargo receptor involved in targeting ciliary components to the autophagy machinery is primarily NBR1. Treatment with chloroquine, Ricolinostat (ACY-1215), or their combination decreased tumor growth in vivo. Mice that overexpress the autophagy transcription factor TFEB show a decrease of ciliary number. These results suggest that ciliary disassembly is mediated by HDAC6-regulated autophagy, i.e., ciliophagy. Inhibition of ciliophagy may decrease cholangiocarcinoma growth and warrant further investigations as a potential therapeutic approach.
NEW & NOTEWORTHY This work identifies novel targets against primary ciliary disassembly that can lead to new cholangiocarcinoma therapeutic strategies. Furthermore, ciliary loss has been described in different tumors, increasing the significance of our research.
INTRODUCTION
Hepatobiliary malignancies account for 13% of the annual cancer-related deaths worldwide and for 3% of the annual cancer-related deaths in the United States (1, 45). Cholangiocarcinoma (CCA) is a malignancy derived from cholangiocytes, the epithelial cells lining the biliary tree (32). CCA accounts for 15% to 20% of primary hepatobiliary malignancies (2). In the last four decades, intrahepatic CCA incidence rates in the United States have increased by 165% with 2,500 new cases diagnosed each year (1, 4, 40). Surgery is currently the only potentially curative therapeutic option for CCA (2).
Primary cilia are nonmotile microtubule-based cellular organelles present in nearly every cell (10, 12, 22, 34). The primary cilium is an antenna-like structure that senses the environment for various signals, which are transduced into intracellular pathways, generating a cellular response (10, 22, 31). Specifically, the Wingless and Int-1 (WNT), Hedgehog, mammalian target of rapamycin (mTOR), Hippo, transforming growth factor-β (TGF-β), NOTCH, and platelet-derived growth factor (PDGF) signaling pathways have all been shown to be regulated through ciliary-dependent mechanisms with diverse consequences on cell proliferation, size, differentiation, autophagy, apoptosis, and tumorigenesis (13, 23). Cholangiocyte cilia also act as mechanosensors, chemosensors, and osmosensors, detecting changes in bile flow, bile acids, nucleotides, exosomes, and osmolarity (10, 22, 31). The loss of primary cilia has been reported in different tumoral tissues, including cholangiocarcinoma, pancreas, breast cancer, renal cell carcinoma, melanoma, prostate, ovarian, and colon cancer (7, 8, 15, 41). When normal cholangiocytes are experimentally deciliated by gene silencing of molecules involved in ciliary assembly, such as IFT88, KIF3A, or CEP164, the induction of proliferation, anchorage-independent growth, and invasion were observed (16), suggesting that the primary cilium has a role as a tumor-suppressor organelle.
Histone deacetylase 6 protein (HDAC6) regulates the acetylation of nonhistone targets, such as α-tubulin and the microtubule-associated protein 1A/1B-light chain 3 (LC3) (30), which has a role in autophagosome maturation. Additionally, HDAC6 has the capacity to bind polyubiquitinated misfolded proteins (21, 24, 30). HDAC6 deacetylates the α-tubulin in the ciliary axoneme and promotes ciliary disassembly through microtubule destabilization (16, 21). We previously showed that HDAC6 is overexpressed in cholangiocarcinoma and decreases ciliary expression, favoring tumor growth (16), but the mechanisms behind ciliary disassembly remain unknown. Autophagy is a major intracellular degradation pathway by which cytoplasmic components, including organelles, are delivered to and degraded in lysosomes. In addition, autophagy is involved in many physiological and pathological processes, such as aging, infection, and cancer. Specifically, some reports suggest that autophagy is increased in CCA, but the mechanisms and consequences are unclear (20, 35, 43). Interestingly, HDAC6 may be related with the autophagy process. HDAC6 promotes autophagosome maturation, and it even was proposed as a link between autophagy and the ubiquitin proteasome system (11, 28, 29, 39). Therefore, we hypothesized that autophagy may be involved in the resorption of primary cilia in CCA- and HDAC6-dependent autophagy of cilia [i.e., ciliophagy (26)] may play an important role in the pathogenesis and progression of CCA.
MATERIALS AND METHODS
Cell lines and culture.
H69 (CVCL_8121) and NHC cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO) supplemented as listed in the Supplemental Materials and Methods (https://doi.org/10.6084/m9.figshare.11739783; all supplemental material may be found at this site.). The cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. HuCCT1 (CVCL_0324), KMCH, WITT, EGI1 (CVCL_1193), and rat CCA cell line, BDEneu (CVCL_AS29), were maintained in F-12 media supplemented as listed in the Supplemental Materials and Methods.
For ciliary expression, confluent cells were incubated overnight with opti-MEM (Gibco, Waltham, MA). Chloroquine (Sigma-Aldrich) was used at a concentration of 30 µM, hydroxychloroquine (Sigma-Aldrich) at a concentration of 30 µM, bafilomycin-A1 (Fisher Scientific, Portsmouth, NH) at a concentration of 1 or 20 µM, Ricolinostat (ACY-1215, kindly provided by Matthew Jarpe, Regenacy Pharmaceuticals, Waltham, MA) at a concentration of 10 µM and PYR41 (Sigma-Aldrich) of 1 µM.
Cancer genome atlas analysis.
The Cancer Genome Atlas (TCGA) available data sets on cholangiocarcinoma were downloaded from the National Cancer Institute Genomic Data Commons data portal (https://portal.gdc.cancer.gov/projects/TCGA-CHOL), as described in the Supplemental Materials and Methods.
Immunofluorescence.
Cells were seeded and grown on four-well chamber slides for 4 days. After the cells reached 100% confluence, they were incubated for 48 h in media and 24 h in opti-MEM (Gibco) with the respective treatments to induce ciliary expression. Liver samples from mice that overexpress the transcription factor EB (TFEB) in the liver (36) or the rat orthotopic model of CCA were fixed in 10% formaldehyde, dehydrated with sequential steps, and then paraffin embedded. The immunofluorescence was performed as mentioned in Supplemental Materials and Methods.
Immunoprecipitation.
We inhibited autophagic flux for 24 h with bafilomycin-A1 to avoid degradation of interacting proteins in H69 and HuCCT1 cells before the immunoprecipitation. Cells were washed with PBS, and IP Lysis/Wash buffer (Thermo Fisher Scientific, Rockford, IL) was used to obtain protein. The homogenate was centrifuged at 13,000 g for 10 min, and the supernatant was cleaned for unspecific interactions with 40 µL protein A/G plus-agarose beads (Santa Cruz Biotechnology). Ten microliters of anti-LC3B antibody (Sigma, AB_10959661) were used and then added 20 µL of A/G plus-agarose beads to pull down LC3 protein overnight. The negative reaction used the same reagents, except the specific antibody, and for the antibody Western blot line, only the specific antibody and no sample were loaded. After four washes with PBS, samples were mixed with loading buffer and used for the Western blot analysis protocol.
Western blot analysis.
Western blot analysis was performed as depicted in Supplemental Materials and Methods.
Proliferation.
A CellTiter 96 AQueous one solution cell proliferation assay (MTS) from Promega (Madison, WI) was used to evaluate cell proliferation, according to the manufacturer’s instructions. 2×103 cells were seeded into each well of a 96-well plate and cultured in 100 µL of media and then treated with ACY-1215, bafilomycin-A1, or the combination of the two for 48 h. Alternatively, cell proliferation was analyzed by live-cell imaging assays. A total of 5×103 cells in 200 μL of media were seeded in 96-well plates and incubated in a tissue culture incubator at 37°C and 5% CO2 with IncuCyte, respectively (Essen BioScience, Ann Arbor, MI). Collected data were analyzed with IncuCyte S3 software.
Nucleofection.
For siRNA transfection, cells were nucleofected, according to manufacturer’s instructions (Lonza, Switzerland). 7.5×105 H69 cells were nucleofected with 10 µL CALCOCO2, NBR1, OPTN, or SQSTM1 siRNA pools and cultured for 4 days before immunofluorescence. H69 and HuCCT1 cells were nucleofected with HDAC6-FLAG plasmid (HDAC6 FLAG was a gift from Eric Verdin, Addgene, no. 13823) (9) and selected with G418.
Lentivirus production.
The lentivirus shRNA NBR1 or shNT were produced as described in the Supplemental Materials and Methods.
Invasion.
Invasion assay was performed using Corning BioCoat Matrigel Invasion Chamber. Briefly, cells were suspended at 50,000 cells/well in their respective serum-free media with treatments into the upper chamber well coated with Matrigel. Media supplemented with 10% FBS was placed in the lower well of the chamber with the respective treatments. The cells were allowed to invade the Matrigel for 48 h at 37°C. The membranes were stained with crystal violet, cells from the upper surface of the chamber were removed, and wells were observed using an inverted microscope. The percentage of invasion was assessed by counting cells on four random fields at ×200 magnification.
Orthotopic model of cholangiocarcinoma.
All animal experimentation was performed according to the Association for Assessment and Accreditation of Laboratory Animal Care using protocols approved by the Institutional Animal Care and Use Committee. Briefly, and as previously described by Sirica et al. (46), in vivo cell transplantation was carried out in young adult Fisher 344 male rats (Envigo, Somerset, NJ), with initial mean body weights typically ranging between 200 and 225 g. BDEneu cells (kindly provided by Dr. Alphonse E. Sirica, Department of Pathology, Virginia Commonwealth University-Medical College of Virginia, Richmond, VA) were inoculated at 1 × 106 suspended in 0.1 ml PBS under the capsule of the left hepatic lobe after ligation of the left bile duct. After 6 days, rats were treated for 8 days with ACY-1215 (30 mg/kg), hydroxychloroquine (15 mg/kg), their combination, or saline solution. The procedure for intraperitoneal injection of ACY-1215, hydroxychloroquine, or saline solution followed the Canadian Council on Animal Care guidelines for acceptable injection volumes.
Statistical analysis.
Data were expressed as the means ± SE and statistically subjected to Student’s unpaired t test or Mann-Whitney U test. A level of P < 0.05, P < 0.01, P < 0.001 and P < 0.0001 were considered statistically significant.
RESULTS
Autophagy is increased in CCA.
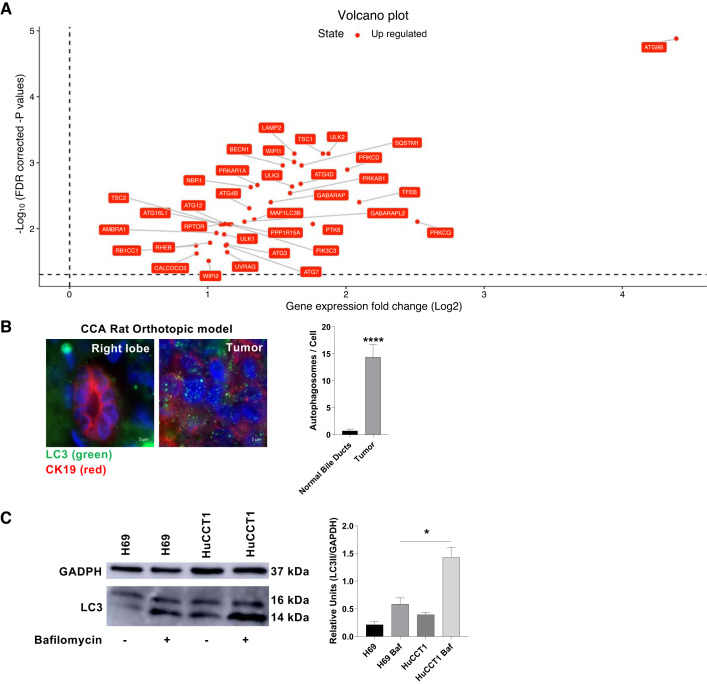
To assess the levels of autophagy in CCA, we selected 56 core autophagy genes (Supplemental Table S1) and verified whether they were differentially expressed by bioinformatic analysis on TCGA available data sets on cholangiocarcinoma. Our analysis shows that expression levels of 35 autophagy genes significantly increased in intrahepatic CCA tumor samples compared with normal controls (Fig. 1A and Supplemental Table S1), suggesting that autophagy is enhanced in CCA patients. Interestingly, among those genes we found LC3, and the autophagy cargo receptors NBR1 and CALCOCO2. We also quantified the number of autophagosomes on normal and tumor cholangiocytes from the CCA orthotopic rat model. The CK19+ cells showed a higher number of autophagosomes in the tumor than in normal bile ducts (Fig. 1B). Additionally, we assessed the autophagic flux in the human normal cholangiocytes H69 and CCA HuCCT1 cell lines. We compared the expression of the autophagy marker LC3 before and after treatment with the bafilomycin-A1, inhibitor of autophagosome-lysosome fusion. Consistently, the amount of LC3II protein increased in the tumoral cells when compared with the normal cells treated with bafilomycin-A1, confirming the increased autophagosome biogenesis in the CCA cells (Fig. 1C). The same results were confirmed in human normal cholangiocytes NHC and CCA KMCH cell lines (Supplemental Fig. S1).
Fig. 1.
Autophagy is increased in cholangiocarcinoma. A: gene expression analysis on autophagy genes from The Cancer Genome Atlas available data sets on cholangiocarcinoma. The expression volcano plot shows the fold change expression data of 35 autophagy genes in intrahepatic cholangiocarcinoma (CCA) samples compared with normal samples [x-axis threshold: 0 (logFC); P values threshold: 0.05 (false discovery rate, FDR)]. B: microtubule-associated protein 1A/1B-light chain 3 (LC3) levels on bile duct cells (stained in red with the epithelial marker CK19) in healthy liver tissue (right lobe) or a CCA tumor originated on a rat orthotopic model (n = 3 rats, Mann-Whitney U test, ****P < 0.0001, ×630). C: LC3 Western blot analysis for H69 and HuCCT1 cells treated or untreated with bafilomycin-A1 for 6 h. (20 µM) (n = 3 samples; Man-Whitney U test, *P < 0.05).
HDAC6 controls autophagy in CCA.
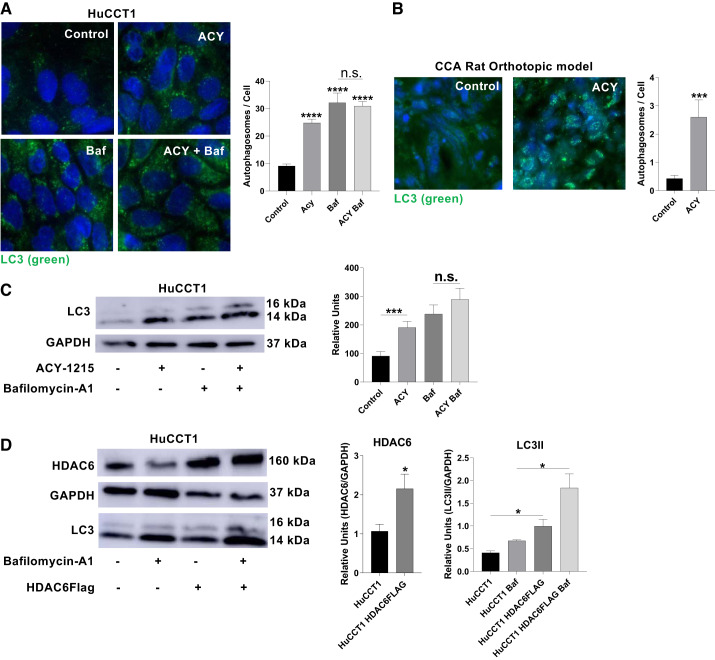
To assess the role of HDAC6 in autophagy in CCA, we performed immunofluorescence of HuCCT1 cells treated with a specific HDAC6 inhibitor, ACY-1215, with bafilomycin-A1, or their combination, and we found a similar pattern, i.e., an accumulation of LC3 puncta (Fig. 2A), suggesting that ACY-1215, as bafilomycin-A1, works as an autophagy flux inhibitor. Additionally, the CCA orthotopic model rats treated with ACY-1215 showed higher levels of LC3 vesicles compared with control, consistent with a blockade of autophagy flux (Fig. 2B). Therefore, we measured the effect of ACY-1215 on the autophagic flux in HuCCT1 cells by Western blot analysis. The use of the HDAC6 inhibitor resulted in an increase of LC3II and NBR1 protein levels compared with the control, but no significant changes were observed in the presence of the autophagy inhibitor bafilomycin-A1, suggesting that the inhibition of HDAC6 blocked autophagy flux (Fig. 2C and Supplemental Fig. S2). Taken together, these data suggest that HDAC6 inhibition affects HuCCT1 autophagy flux. On the other hand, HuCCT1 cells that overexpress HDAC6 (HDAC6-FLAG) show increased levels of LC3II, and the use of bafilomycin exacerbates this effect, compared with empty vector-transfected samples, showing that the increase of HDAC6 increases autophagosome biogenesis in CCA (Fig. 2D).
Fig. 2.
Ricolinostat (ACY-1215) inhibits autophagy flux in cholangiocytes. A: immunofluorescence for microtubule-associated protein 1A/1B-light chain 3 (LC3; green) of cells treated with bafilomycin-A1, ACY-1215, or combination for 4 h (n = 5 samples, Mann-Whitney U test, ****P < 0.0001, ×630). B: LC3 levels on the tumors treated with ACY-1215 or vehicle for 8 days on a cholangiocarcinoma (CCA) rat orthotopic model (n = 3 rats, unpaired t test, ***P < 0.001, ×630). C: HuCCT1 cells Western blot analysis showing effect on levels of LC3 by using the HDAC6 inhibitor ACY-1215 (10 µM) and/ or the autophagy flux inhibitor bafilomycin-A1 (20 µM) for 24 h (n = 6 samples, Mann-Whitney U test, ***P < 0.001). D: Western blot analysis of HDAC6 and LC3 levels of HuCCT1 with empty vector and HuCCT1 cells overexpressing HDAC6-Flag with or without bafilomycin-A1 (20 µM) (n = 3 samples, Mann-Whitney U test, *P < 0.05). n.s., Not significant.
Inhibition of HDAC6 and autophagy restores cilia and decreases cell proliferation.
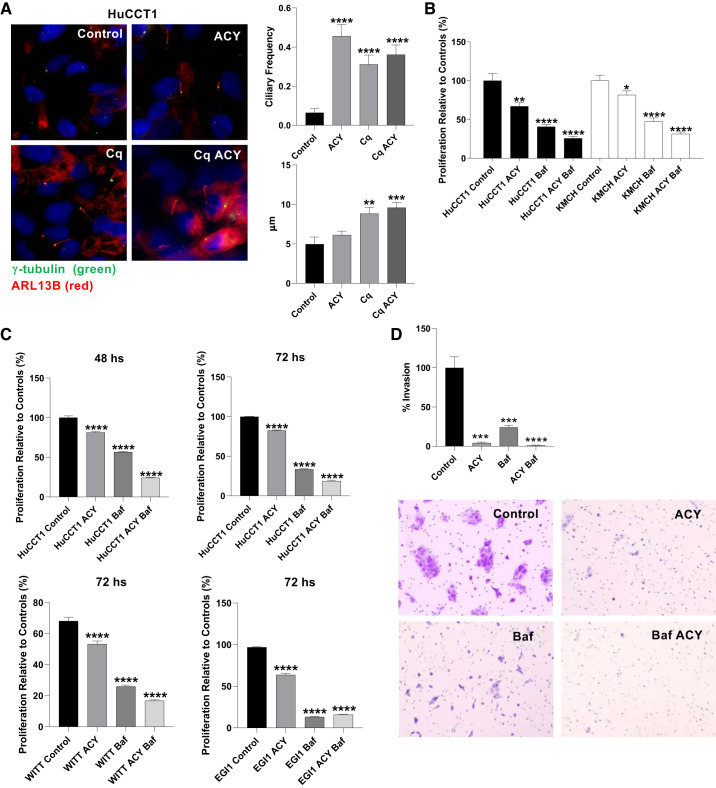
Previous work showed that experimental ciliary loss in cultured normal cholangiocytes induces a malignant phenotype, and HDAC6-mediated deacetylation of ciliary proteins has a negative impact on the stability of cilia (16). HuCCT1 cells treated with ACY-1215, the autophagy inhibitor chloroquine, or the combination of the two, showed an increase of ciliary expression, as shown by immunostaining for the ciliary marker ARL13B. Additionally, the cells treated with chloroquine, or the combination of chloroquine and ACY-1215 show an increased ciliary length (Fig. 3A). Rapamycin (Rap), an autophagy inducer, has no effect on ciliary length on HuCCT1 cells and do not revert the action of ACY-1215 (Supplemental Fig. S3A). Additionally, the autophagy inhibition through the downregulation of ATG5 using siRNA translates to an increase of ciliary length in H69 cells (Supplemental Fig. S3B). Because the increase of cilia is linked with a decrease of malignancy on tumor cell lines, we evaluated proliferation of HuCCT1 and KMCH cells after treatment with ACY-1215, bafilomycin-A1, or their combination and found decreased proliferation by MTS assay (Fig. 3B), and decreased proliferation of HuCCT1, KMCH, EGI1 and WITT cells by real-time cell imaging by IncuCyte after treatment with ACY-1215, bafilomycin-A1, or their combination (Fig. 3C). Invasion of HuCCT1 cells decreased after treatment with ACY-1215, bafilomycin-A1, or their combination (Fig. 3D). We propose that autophagy inhibition may also stimulate ciliogenesis in nonmalignant cholangiocytes. To test this hypothesis, autophagy was inhibited using chloroquine treatment in cycling H69 cells for 24 h. Despite the cells being cultured in complete medium, and thus cycling, chloroquine treatment had a strong effect on cilium formation, as 29% of treated cells had visible cilia, whereas only ~11% of cycling serum-stimulated control cholangiocytes were ciliated (Supplemental Fig. S3C), as expected for nonquiescent cells.
Fig. 3.
Inhibition of HDAC6 and autophagy restores cilia and decreases cell proliferation. A: immunofluorescence for ARL13B (red) and γ-tubulin (green) and quantification of frequency (cilia/basal body) and length of ciliated cells in HuCCT1 cells after 72 h of treatment with Ricolinostat (ACY-1215; 10 µM), chloroquine (Cq; 30 µM), or their combination. B: cell proliferation rates relative to their respective controls in cholangiocarcinoma (CCA), HuCCT1, and KMCH cell lines after treatment with the HDAC6 inhibitor ACY-1215 (10 µM) alone or in combination with the autophagy inhibitor bafilomycin-A1 (Baf; 20 µM) for 48 h using CellTiter 96 AQueous One cell proliferation assay (n = 16 observations, unpaired t test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. control). C: proliferation of HuCCT1 cells at 48 and 72 h, and EGI1 and WITT cells for 72 h relative to their respective controls after treatment with the HDAC6 inhibitor ACY-1215 (10 µM) alone or in combination with the autophagy inhibitor bafilomycin-A1 (Baf; 20 µM) using IncuCyte (n = 12 observations, Mann-Whitney U test, ****P < 0.0001). D: invasion of HuCCT1 cells at 48 relative to their respective controls after treatment with ACY-1215 (10 µM) alone or in combination with bafilomycin-A1 (Baf; 20 µM) (n = 8 samples; Mann-Whitney U test; ***P < 0.001, ****P < 0.0001).
Autophagy marker LC3 colocalizes with ciliary structures.
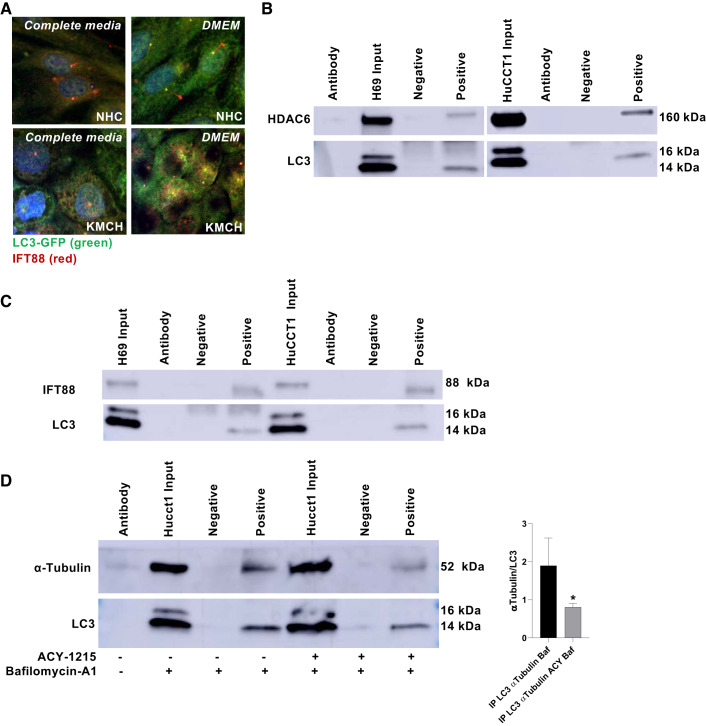
We assessed the intracellular localization of LC3 and IFT88, a ciliary marker, by confocal immunofluorescence, and found that this ciliary protein colocalized with autophagosomes in the CCA cell line KMCH. Interestingly, in normal ciliated cells LC3 localized also to the basal body of primary cilia (Fig. 4A). In an effort to understand the mechanisms that may explain how the inhibition of HDAC6 may restore cilia through autophagy, we tested whether HDAC6 and the autophagy protein LC3 interacted. When LC3 was immunoprecipitated in H69 and HuCCT1 cells, HDAC6 was present in the coimmunoprecipitate (Fig. 4B). Additionally, LC3 interacts with the ciliary protein IFT88 (Fig. 4C), consistent with the immunofluorescence showing its presence in the autophagosomes of tumoral cells (Fig. 4A). Furthermore, LC3 also immunoprecipitated with tubulin, the main component of the ciliary axoneme (Fig. 4D). Interestingly the levels of α-tubulin coimmunoprecipitated with LC3 decreased when cells were treated with the HDAC6 inhibitor, ACY-1215, suggesting that the inhibition of HDAC6 is decreasing the ability of LC3 to interact with α-tubulin.
Fig. 4.
Autophagy colocalizes with ciliary structures. A: immunofluorescence for microtubule-associated protein 1A/1B-light chain 3-green fluorescent protein (LC3-GFP; green) and IFT88 (red), a ciliary marker, of normal human cholangiocyte cells (NHC), and cholangiocarcinoma (CCA) KMCH cells with complete or serum-free DMEM media (nuclei in blue, ×1,000). B: coimmunoprecipitation of LC3 revealing for HDAC6. C: coimmunoprecipitation of LC3 revealing for IFT88. D: quantification of total tubulin relative to immunoprecipitated levels of LC3 from samples treated with bafilomycin-A1 or Ricolinostat (ACY-1215) + bafilomycin-A1 (1 Baf; negative: reaction without specific antibody; antibody: only the specific antibody was loaded on the Western blot line (n = 3 experiments; Mann-Whitney U test, *P < 0.05).
Autophagy cargo receptors NBR1 and CALCOCO2 are involved in HDAC6-induced ciliophagy.
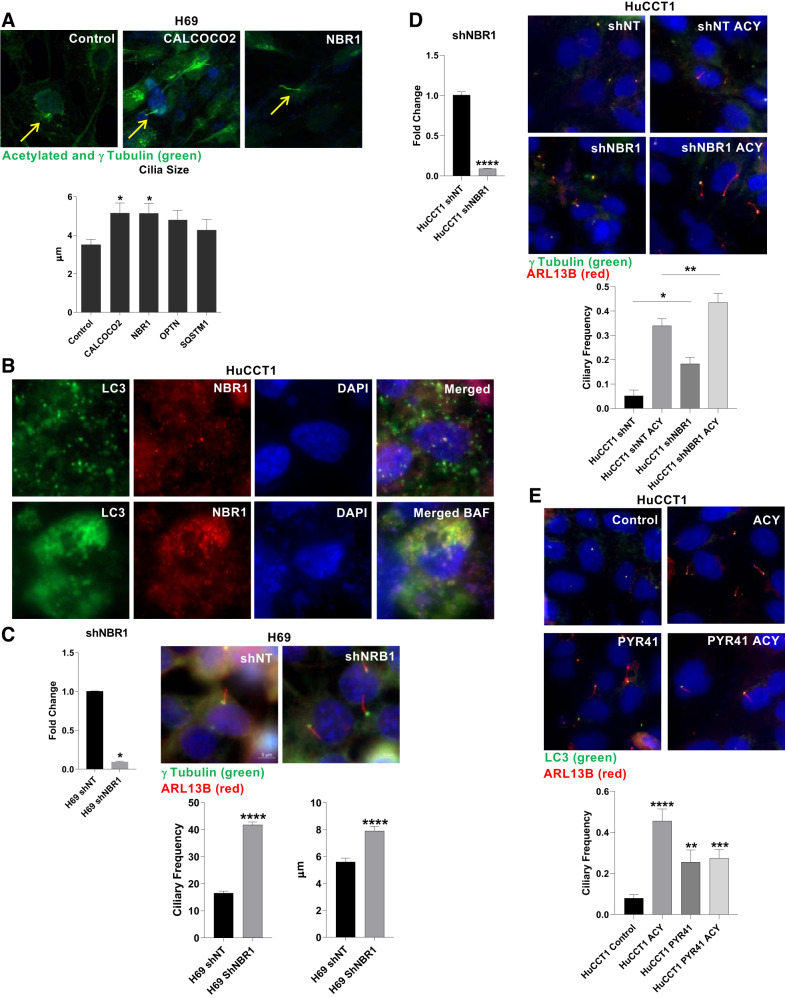
Autophagy cargo receptors are responsible for targeting autophagosomes to diverse cellular structures. To understand how autophagosomes are directed to the ciliary structure, we inhibited the expression of the autophagy cargo receptors CALCOCO2, NBR1, OPTN, and SQSTM1. The inhibition of the expression (Supplemental Fig. S3B) of CALCOCO2 and NBR1 using siRNA translated into an increase of ciliary length in H69 cells (Fig. 5A). NBR1 is reported to interact with LC3 (6), which we confirmed via coimmunofluorescence (Fig. 5B). We additionally generated a stable cell line using shRNA to NBR1 and found an increase on ciliary length and frequency in the normal cell line H69 (Fig. 5C). Furthermore, the ciliary frequency also increased in HuCCT1 cells with a downregulation of NBR1 by shRNA, an effect that is significantly increased by the HDAC6 inhibitor ACY-1215 (Fig. 5D). NBR1 is reported to operate in selective autophagy of ubiquitinated targets (25), and HDAC6 is reported to bind ubiquitinated proteins (24); therefore, we decided to study the impact of ubiquitination inhibition on ciliary disassembly. The use of the ubiquitination inhibitor PYR41 or its combination with ACY-1215 also translates to an increase of ciliary frequency (Fig. 5E).
Fig. 5.
NBR1 downregulation induces longer cilia. A: cilia size of normal H69 cells after inhibition of the mRNA expression of different autophagy cargo receptors with siRNA and representative pictures of ciliary immunofluorescence (n = 10 observations, Mann-Whitney U test, *P < 0.05, ×1,000), Acetylated and γ tubulin, green, DAPI, blue, arrows point cilia, P < 0.05 vs. controls, Mann Whitney U test). B: immunofluorescence of microtubule-associated protein 1A/1B-light chain 3 (LC3) and NBR1 with or without inhibition of autophagy using bafilomycin-A1 (1 µM) for 3 days (×630). C: quantitative PCR of shNBR1 knockdown quantification and immunofluorescence depicting ciliary length (µm) and ciliary frequency (number of cilia per field) on H69 cells (n = 40 observations, unpaired t test; ****P < 0.0001, ×630). D: qPCR of shNBR1 knockdown quantification, and immunofluorescence depicting ciliary frequency (cilia/basal body) per number of cells on HuCCT1 shNBR1 cells treated or not with Ricolinostat (ACY-1215; n = 13 observations; Mann-Whitney U test, *P < 0.05, **P < 0.01, ×630). E: representative pictures of an immunofluorescence depicting primary cilia of cells treated with ACY-1215, PYR41, or combination and quantification of ciliary frequency (cilia/basal body) per number of cells treated or not with ACY-1215 (n = 5 observations, Mann-Whitney U test, **P < 0.01, ***P < 0.001, ****P < 0.0001, ×630).
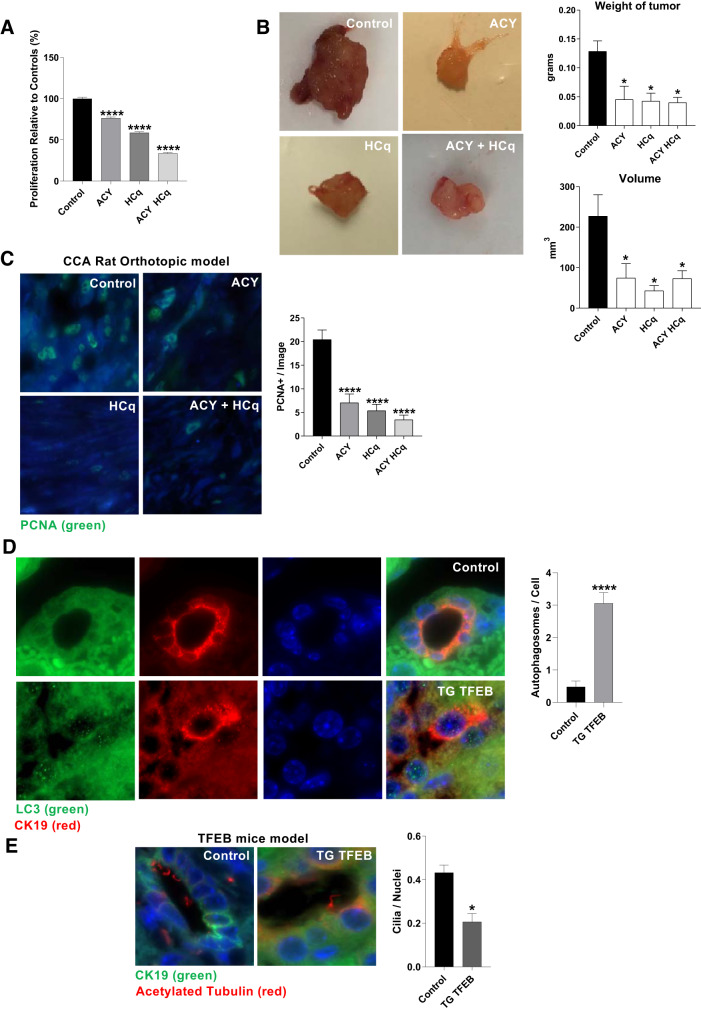
Ciliophagy inhibition reduces tumoral growth in vivo.
To evaluate the efficacy of ACY-1215 and hydroxychloroquine (HCq) as a therapeutic approach, we studied the effect of these drugs in vitro and in vivo using an orthotopic syngeneic rat model of CCA (46). The treatment of BDEneu cells with ACY-1215, HCq, and their combination reduces cell proliferation in vitro (Fig. 6A). Furthermore, the treatment of the animals with ACY-1215, HCq, or their combination resulted in decreased tumor size compared with the control rats injected with vehicle (Fig. 6B). Finally, we evaluated the levels of proliferating cell nuclear antigen (PCNA) on the different experimental group by immunofluorescence, and we found decreased PCNA-positive cells on the tumors of animals treated with HCq, ACY-1215, or their combination (Fig. 6C). To further support the notion that autophagy is involved in ciliary loss, we used a mouse model overexpressing the master regulator TFEB, a transcription factor that acts as a positive regulator of autophagy by promoting expression of genes involved in autophagy (44). Interestingly, these 3-mo-old mice show a cholangiocarcinoma-like phenotype (36), and we found increased levels of LC3 puncta (Fig. 6D), and lower levels of cilia on CK19+ cells compared with control normal mice (Fig. 6E).
Fig. 6.
HDAC6 and autophagy inhibition combination therapy decrease tumor weight and volume in an in vivo orthotopic model. A: proliferation of BDEneu cells treated by Ricolinostat (ACY-1215), hydroxychloroquine (HCq), or a combination of the two relative to controls (n = 12 observations, one-way ANOVA; ****P < 0.0001). B: representative pictures of the tumor, tumoral volume, and weight of rats that received a daily dose of HCq, ACY-1215, a combination of both drugs or vehicle on a cholangiocarcinoma (CCA) orthotopic model (n = 5 rats, Mann-Whitney U test, *P < 0.05). C: proliferating cell nuclear antigen (PCNA)-positive nuclei on tumor samples of rats treated with ACY-1215, HCq, combination of both drugs or vehicle (n = 3 rats, Mann-Whitney U test, ****P < 0.0001, ×630). D: immunofluorescence for microtubule-associated protein 1A/1B-light chain 3 (LC3) and CK19 and quantification of LC3 on CK19+ cells on mice that overexpress transcription factor EB (TFEB; n = 7 observations, Mann-Whitney U test, ****P < 0.0001, ×630). E: immunofluorescence for acetylated-α-tubulin and CK19 and quantification of the number of cilia per CK19+ cells on mice that overexpress TFEB (n = 5 mice, Mann-Whitney U test, *P < 0.05, ×630).
DISCUSSION
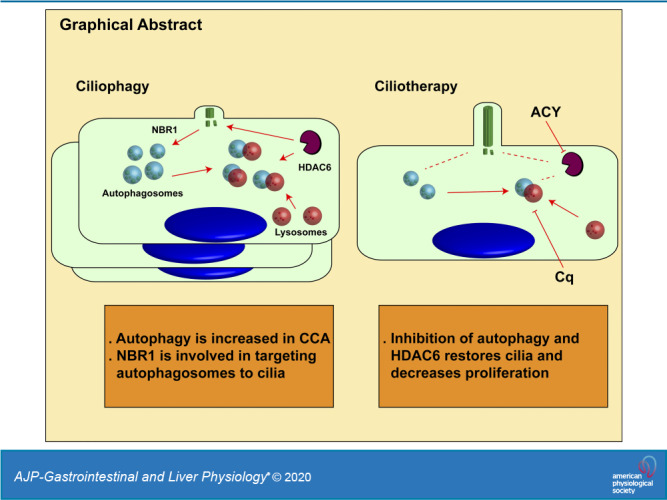
The key findings presented here relate to the novel HDAC6-dependent autophagy of cholangiocyte primary cilia, i.e., ciliophagy. We found that 1) autophagy is increased in human CCA, as well as in a rat orthotopic CCA model and human CCA cell lines, 2) HDAC6 inhibition decreases autophagic flux, 3) HDAC6 overexpression increases autophagy, 4) inhibition of autophagy and HDAC6 restores cilia and decreases tumoral cell proliferation, 5) LC3 interacts with HDAC6 and ciliary proteins, 6) the autophagy cargo receptor involved in targeting ciliary components to the autophagy machinery is primarily NBR1, 7) ubiquitination is involved in CCA cells ciliary loss, 8) ciliophagy inhibition by the combination of hydroxychloroquine and the HDAC6 inhibitor ACY-1215 decreased tumor growth in an CCA rat orthotopic model, and 9) liver-specific increase of autophagy in a mouse model decreased ciliary expression.
We previously showed that cholangiocarcinoma cells have a significant reduction in the expression of cilia, and experimentally deciliated normal cholangiocytes show a malignant-like phenotype. Furthermore, HDAC6 expression is increased in tumoral cells and linked to the deciliation process (16). Our results show a dysregulation of autophagy in cholangiocarcinoma. The role of autophagy in cancer is complex and depends on the type of tumor or context. Autophagy is dysregulated, for example, in pancreatic cancer, and its inhibition by chloroquine leads to tumor regression (51). Cancers that have activating mutations in Ras commonly have high levels of basal autophagy, and Ras inhibition leads to impaired cell growth (17). Consistently with our findings, the levels of LC3 are elevated in cholangiocarcinogenesis (43), and autophagy may be related with poor prognosis (35). The expression of autophagy-related proteins LC3, beclin-1, and p62 is increased since early stages of cholangiocarcinogenesis (43), suggesting a deregulated autophagy process that may be related with the development of cholangiocarcinoma. Samples from clinical intrahepatic cholangiocarcinoma present higher autophagic activity than normal cholangiocytes (19). Under starvation, cholangiocarcinoma cells CCKS1 and HuCCT1 show an increase of LC3 puncta and invasive activity, while the treatment with chloroquine attenuates the invasive phenotype (35). Additionally, autophagy is increased in polycystic liver disease cholangiocytes, which have a high proliferative profile (33).
In the present work, HDAC6 inhibition mimics autophagic flux inhibitors, and the overexpression of HDAC6 increases LC3 levels. Consistent with our results, it has been previously demonstrated that HDAC6 is involved in the activation of autophagy on glioblastoma cell lines (29). Since HDAC6 binds to ubiquitinated proteins, it was also suggested as a mediator of aggregation and autophagic clearance (47). Additionally, HDAC6 is reported to mediate LC3B-II deacetylation (30), control the fusion of autophagosomes to lysosomes (28), and interact with SQSTM1/p62 (50). Our results show that autophagy is also involved in cholangiocyte ciliary disassembly.
Defects on primary cilia on the cholangiociliopathy polycystic liver disease generate cysts and increased proliferation. In this scenario, the therapeutic use of tubastatin-A, tubacin, ACY-1215, hydroxychloroquine, or bafilomycin-A1 reduced cell proliferation in vitro, and the use of ACY-1215 or hydroxychloroquine reduced liver cysts in an animal model of the disease in vivo (14, 33). In the present work, the inhibition of autophagy and HDAC6 translates into an increase of ciliary expression and decreased proliferation in CCA cells. Increased autophagosome biogenesis is associated with defective ciliogenesis in primary Hürhle cell tumors (27). It has been reported that autophagic machinery colocalizes at the cilia, and autophagy-impaired Atg5−/− MEFs cells form cilia both longer and faster than wild-type MEFs (38). Consistently, autophagy mediated by the deacetylase HDAC6 proved to be an important process for motile cilia degradation on a model of chronic obstructive pulmonary disease. In this context, mice with autophagy-deficient machinery or mice that were injected with the HDAC6 inhibitor tubastatin-A, resist cilia shortening by cigarette smoke (5, 26). Additionally, tracheal epithelial cells derived from mice with a chromosome deletion of HDAC6 showed lower levels of autophagy (26). Xu et al. (48) showed that the use of a drug that induces autophagy, silibinin, shortened the length of primary cilia and increased the levels of HDAC6. Additionally, siRNA against LC3 enhanced the length of primary cilia, and siRNA against HDAC6 reduced autophagy levels and enhanced the length of primary cilia in confluent mouse embryo fibroblast 3T3-L1 cells (49). On the other hand, TFEB is a master transcription factor that enhances autophagy (44). We found that these mice, which present a CCA-like phenotype, show a disorganized proliferation of cholangiocytes and decreased number of primary cilia. Taken together, this supports our hypothesis that autophagy is involved in the process of ciliary disassembly.
According to Liu et al. (30), LC3B-II deacetylation is, in part, mediated by HDAC6, since the specific inhibitor tubacin partially decreased its acetylation levels. HDAC6 is involved in autophagic degradation during serum starvation. In this work, we show that after inhibition of autophagy using bafilomycin-A1, it is possible to assess the interaction between LC3B-II and HDAC6 though immunoprecipitation. Additionally, this is the first report that links autophagy cargo receptors (NBR1 and CALCOCO2) and ciliophagy. NBR1 is an autophagy cargo receptor that has a ubiquitin and LC3 binding domain and has been linked with degradation of ubiquitinated substrates (25). HDAC6 has also been associated with the degradation of ubiquitinated proteins (24). Our results show that the inhibition of ubiquitination increases ciliary frequency; therefore, we could speculate that HDAC6 deacetylation of ciliary proteins exposes a lysine for ubiquitination that then is recognized by NBR1 and targeted to the autophagy pathway by LC3, suggesting an interaction between HDAC6-induced deacetylation, ubiquitination, and autophagy for ciliary disassembly that warrants further studies.
We have observed a decrease in tumoral growth on rats with an orthotopic model of CCA when treated with ACY-1215, HCq, and the combination of the two. Tumors and cells derived from a model of intrahepatic cholangiocarcinoma from genetically engineered mice with somatic activation of KRASG12D and deletion of TP53, shows an increment of LC3 (37). Additionally, a treatment with chloroquine inhibits growth of the cholangiocarcinoma cells (20, 37). Autophagy inhibition has been linked with decreased tumoral growth in hepatocarcinoma when coadministered with chemotherapy (18), and with decreased pancreatic metastatic cancer stem cells with gemcitabine treatment through hedgehog signaling inhibition (17). Additionally, the use of chloroquine and hydroxychloroquine inhibits the growth of MCF-7 and MDA-MB-231 human breast cancer cells in vitro (42). HDAC6 inhibition using tubastatin-A or short hairpin RNA produced proliferation inhibition on CCA cells (16), and HDAC6 inhibition using ACY-1215 produced a cellular decrease of proliferation in esophageal squamous cell carcinoma (3).
These results show that HDAC6 is a key protein for ciliophagy, thus decreasing ciliary expression and increasing autophagy. Autophagosomes are led to the cilia, in part, through the action of the autophagy cargo receptor NBR1. When autophagy and HDAC6 are inhibited, there is decreased proliferation and increased cilia expression, showing the importance of this novel ciliotherapy. In summary, the present work provides evidence for the role of autophagy and HDAC6 on ciliary disassembly and tumoral growth. Furthermore, this study also suggests that the inhibition of ciliophagy could be an important therapeutic target for CCA.
GRANTS
This work was supported by National Institutes of Health Grant R01CA183764 (to S. A. Gradilone), the Randy Shaver Cancer Research and Community Fund Award (to S. A. Gradilone), the Chainbreaker GOpher a Cure award, provided by the Masonic Cancer Center University of Minnesota, The Hormel Foundation, and by Italian Association for Cancer Research (AIRC) Grant IG17711 (to B. Franco).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.P., A.M., N.P., A. Ballabio, B.F., and S.A.G. conceived and designed research; E.P., S.J., K.T., A. Biswas, M.M., S.H., and F.C. performed experiments; E.P., S.J., K.T., M.M., and S.H. analyzed data; E.P., K.T., M.M., S.H., and S.A.G. interpreted results of experiments; E.P. and M.M. prepared figures; E.P. drafted manuscript; E.P., S.R., M.M., and S.A.G. edited and revised manuscript; S.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Telethon Institute of Genetics and Medicine Bioinformatics Core.
REFERENCES
- 1.Ayala D, Blackstock AW. Effective treatment strategies for cholangiocarcinoma: the challenge remains. Gastrointest Cancer Res 2: 251–252, 2008. [PMC free article] [PubMed] [Google Scholar]
- 2.Blechacz B. Cholangiocarcinoma: current knowledge and new developments. Gut Liver 11: 13–26, 2017. doi: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao J, Lv W, Wang L, Xu J, Yuan P, Huang S, He Z, Hu J. Ricolinostat (ACY-1215) suppresses proliferation and promotes apoptosis in esophageal squamous cell carcinoma via miR-30d/PI3K/AKT/mTOR and ERK pathways. Cell Death Dis 9: 817, 2018. doi: 10.1038/s41419-018-0788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardinale V, Semeraro R, Torrice A, Gatto M, Napoli C, Bragazzi MC, Gentile R, Alvaro D. Intra-hepatic and extra-hepatic cholangiocarcinoma: new insight into epidemiology and risk factors. World J Gastrointest Oncol 2: 407–416, 2010. doi: 10.4251/wjgo.v2.i11.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloonan SM, Lam HC, Ryter SW, Choi AM. “Ciliophagy”: the consumption of cilia components by autophagy. Autophagy 10: 532–534, 2014. doi: 10.4161/auto.27641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Agostino C, Nogalska A, Cacciottolo M, King Engel K, Askanas V. Abnormalities of NBR1, a novel autophagy-associated protein, in muscle fibers of sporadic inclusion-body myositis. Acta Neuropathol 122: 627–636, 2011. doi: 10.1007/s00401-011-0874-3. [DOI] [PubMed] [Google Scholar]
- 7.Dere R, Perkins AL, Bawa-Khalfe T, Jonasch D, Walker CL. β-catenin links von Hippel-Lindau to aurora kinase A and loss of primary cilia in renal cell carcinoma. J Am Soc Nephrol 26: 553–564, 2015. doi: 10.1681/ASN.2013090984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egeberg DL, Lethan M, Manguso R, Schneider L, Awan A, Jørgensen TS, Byskov AG, Pedersen LB, Christensen ST. Primary cilia and aberrant cell signaling in epithelial ovarian cancer. Cilia 1: 15, 2012. doi: 10.1186/2046-2530-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischle W, Emiliani S, Hendzel MJ, Nagase T, Nomura N, Voelter W, Verdin E. A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1p. J Biol Chem 274: 11713–11720, 1999. doi: 10.1074/jbc.274.17.11713. [DOI] [PubMed] [Google Scholar]
- 10.Fry AM, Leaper MJ, Bayliss R. The primary cilium: guardian of organ development and homeostasis. Organogenesis 10: 62–68, 2014. doi: 10.4161/org.28910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galindo-Moreno M, Giráldez S, Sáez C, Japón MA, Tortolero M, Romero F. Both p62/SQSTM1-HDAC6-dependent autophagy and the aggresome pathway mediate CDK1 degradation in human breast cancer. Sci Rep 7: 10078, 2017. doi: 10.1038/s41598-017-10506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell 137: 32–45, 2009. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhardt C, Leu T, Lier JM, Rüther U. The cilia-regulated proteasome and its role in the development of ciliopathies and cancer. Cilia 5: 14, 2016. doi: 10.1186/s13630-016-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gradilone SA, Habringer S, Masyuk TV, Howard BN, Masyuk AI, Larusso NF. HDAC6 is overexpressed in cystic cholangiocytes and its inhibition reduces cystogenesis. Am J Pathol 184: 600–608, 2014. doi: 10.1016/j.ajpath.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gradilone SA, Pisarello MJL, LaRusso NF. Primary cilia in tumor biology: the primary cilium as a therapeutic target in cholangiocarcinoma. Curr Drug Targets 18: 958–963, 2017. doi: 10.2174/1389450116666150223162737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gradilone SA, Radtke BN, Bogert PS, Huang BQ, Gajdos GB, LaRusso NF. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res 73: 2259–2270, 2013. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, Coller HA, Dipaola RS, Gelinas C, Rabinowitz JD, White E. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev 25: 460–470, 2011. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo XL, Li D, Hu F, Song JR, Zhang SS, Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ, Wu MC, Wei LX. Targeting autophagy potentiates chemotherapy-induced apoptosis and proliferation inhibition in hepatocarcinoma cells. Cancer Lett 320: 171–179, 2012. doi: 10.1016/j.canlet.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Hou YJ, Dong LW, Tan YX, Yang GZ, Pan YF, Li Z, Tang L, Wang M, Wang Q, Wang HY. Inhibition of active autophagy induces apoptosis and increases chemosensitivity in cholangiocarcinoma. Lab Invest 91: 1146–1157, 2011. doi: 10.1038/labinvest.2011.97. [DOI] [PubMed] [Google Scholar]
- 20.Huang JL, Hezel AF. Autophagy in intra-hepatic cholangiocarcinoma. Autophagy 8: 1148–1149, 2012. doi: 10.4161/auto.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458, 2002. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 12: 222–234, 2011. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CA, Collis SJ. Ciliogenesis and the DNA damage response: a stressful relationship. Cilia 5: 19, 2016. doi: 10.1186/s13630-016-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115: 727–738, 2003. doi: 10.1016/S0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 25.Kirkin V, Lamark T, Sou YS, Bjørkøy G, Nunn JL, Bruun JA, Shvets E, McEwan DG, Clausen TH, Wild P, Bilusic I, Theurillat JP, Øvervatn A, Ishii T, Elazar Z, Komatsu M, Dikic I, Johansen T. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell 33: 505–516, 2009. doi: 10.1016/j.molcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, An CH, Shan B, Franks JM, Haley KJ, Owen CA, Tesfaigzi Y, Washko GR, Quackenbush J, Silverman EK, Rahman I, Kim HP, Mahmood A, Biswal SS, Ryter SW, Choi AM. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest 123: 5212–5230, 2013. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Yi S, Kang YE, Chang JY, Kim JT, Sul HJ, Kim JO, Kim JM, Kim J, Porcelli AM, Kim KS, Shong M. Defective ciliogenesis in thyroid hürthle cell tumors is associated with increased autophagy. Oncotarget 7: 79117–79130, 2016. doi: 10.18632/oncotarget.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, Pandey UB, Kaushik S, Tresse E, Lu J, Taylor JP, Cuervo AM, Yao TP. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J 29: 969–980, 2010. doi: 10.1038/emboj.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin TW, Chen MT, Lin LT, Huang PI, Lo WL, Yang YP, Lu KH, Chen YW, Chiou SH, Wu CW. TDP-43/HDAC6 axis promoted tumor progression and regulated nutrient deprivation-induced autophagy in glioblastoma. Oncotarget 8: 56612–56625, 2017. doi: 10.18632/oncotarget.17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu KP, Zhou D, Ouyang DY, Xu LH, Wang Y, Wang LX, Pan H, He XH. LC3B-II deacetylation by histone deacetylase 6 is involved in serum-starvation-induced autophagic degradation. Biochem Biophys Res Commun 441: 970–975, 2013. doi: 10.1016/j.bbrc.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Malicki JJ, Johnson CA. The cilium: cellular antenna and central processing unit. Trends Cell Biol 27: 126–140, 2017. doi: 10.1016/j.tcb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzioni M, Invernizzi P, Candelaresi C, Maggioni M, Saccomanno S, Selmi C, Rychlicki C, Agostinelli L, Cassani B, Miozzo M, Pasini S, Fava G, Alpini G, Benedetti A. Human cholangiocarcinoma development is associated with dysregulation of opioidergic modulation of cholangiocyte growth. Dig Liver Dis 41: 523–533, 2009. doi: 10.1016/j.dld.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masyuk AI, Masyuk TV, Lorenzo Pisarello MJ, Ding JF, Loarca L, Huang BQ, LaRusso NF. Cholangiocyte autophagy contributes to hepatic cystogenesis in polycystic liver disease and represents a potential therapeutic target. Hepatology 67: 1088–1108, 2018. doi: 10.1002/hep.29577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Res 66: 6463–6467, 2006. doi: 10.1158/0008-5472.CAN-06-0462. [DOI] [PubMed] [Google Scholar]
- 35.Nitta T, Sato Y, Ren XS, Harada K, Sasaki M, Hirano S, Nakanuma Y. Autophagy may promote carcinoma cell invasion and correlate with poor prognosis in cholangiocarcinoma. Int J Clin Exp Pathol 7: 4913–4921, 2014. [PMC free article] [PubMed] [Google Scholar]
- 36.Pastore N, Niculin NH, Herz J, Calcagní A, Klisch TJ, Brunetti L, Kim K, De Giorgi M, Hurley A, Carissimo A, Mutarelli M, Aleksieva N, D’Orsi L, Lagor WR, Moore DD, Settembre C, Finegold MJ, Forbes SJ, Ballabio A. TFEB regulates murine liver cell fate during development and regeneration. Nat Commun In press. doi: 10.1038/s41467-020-16300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Dell MR, Huang JL, Whitney-Miller CL, Deshpande V, Rothberg P, Grose V, Rossi RM, Zhu AX, Land H, Bardeesy N, Hezel AF. Kras(G12D) and p53 mutation cause primary intrahepatic cholangiocarcinoma. Cancer Res 72: 1557–1567, 2012. doi: 10.1158/0008-5472.CAN-11-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pampliega O, Orhon I, Patel B, Sridhar S, Díaz-Carretero A, Beau I, Codogno P, Satir BH, Satir P, Cuervo AM. Functional interaction between autophagy and ciliogenesis. Nature 502: 194–200, 2013. doi: 10.1038/nature12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447: 859–863, 2007. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 40.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 33: 1353–1357, 2001. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 41.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129: 1351–1363, 2007. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahim R, Strobl JS. Hydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cells. Anticancer Drugs 20: 736–745, 2009. doi: 10.1097/CAD.0b013e32832f4e50. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki M, Nitta T, Sato Y, Nakanuma Y. Autophagy may occur at an early stage of cholangiocarcinogenesis via biliary intraepithelial neoplasia. Hum Pathol 46: 202–209, 2015. doi: 10.1016/j.humpath.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Settembre C, Ballabio A. TFEB regulates autophagy: an integrated coordination of cellular degradation and recycling processes. Autophagy 7: 1379–1381, 2011. doi: 10.4161/auto.7.11.17166. [DOI] [PubMed] [Google Scholar]
- 45.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 24: 115–125, 2004. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 46.Sirica AE, Zhang Z, Lai GH, Asano T, Shen XN, Ward DJ, Mahatme A, Dewitt JL. A novel “patient-like” model of cholangiocarcinoma progression based on bile duct inoculation of tumorigenic rat cholangiocyte cell lines. Hepatology 47: 1178–1190, 2008. doi: 10.1002/hep.22088. [DOI] [PubMed] [Google Scholar]
- 47.Trüe O, Matthias P. Interplay between histone deacetylases and autophagy—from cancer therapy to neurodegeneration. Immunol Cell Biol 90: 78–84, 2012. doi: 10.1038/icb.2011.103. [DOI] [PubMed] [Google Scholar]
- 48.Xu Q, Liu W, Liu X, Liu W, Wang H, Yao G, Zang L, Hayashi T, Tashiro S, Onodera S, Ikejima T. Silibinin negatively contributes to primary cilia length via autophagy regulated by histone deacetylase 6 in confluent mouse embryo fibroblast 3T3-L1 cells. Mol Cell Biochem 420: 53–63, 2016. doi: 10.1007/s11010-016-2766-2. [DOI] [PubMed] [Google Scholar]
- 49.Xu Q, Liu W, Liu X, Otkur W, Hayashi T, Yamato M, Fujisaki H, Hattori S, Tashiro SI, Ikejima T. Type I collagen promotes primary cilia growth through down-regulating HDAC6-mediated autophagy in confluent mouse embryo fibroblast 3T3-L1 cells. J Biosci Bioeng 125: 8–14, 2018. doi: 10.1016/j.jbiosc.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Yan J, Seibenhener ML, Calderilla-Barbosa L, Diaz-Meco MT, Moscat J, Jiang J, Wooten MW, Wooten MC. SQSTM1/p62 interacts with HDAC6 and regulates deacetylase activity. PLoS One 8: e76016, 2013. doi: 10.1371/journal.pone.0076016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev 25: 717–729, 2011. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]