Abstract
Obesity is associated with several chronic comorbidities, one of which is type 2 diabetes mellitus (T2DM). The pathogenesis of obesity and T2DM is influenced by alterations in diet macronutrient composition, which regulate energy expenditure, metabolic function, glucose homeostasis, and pancreatic islet cell biology. Recent studies suggest that increased intake of dietary carbohydrates plays a previously underappreciated role in the promotion of obesity and consequent metabolic dysfunction. Thus, in this study, we utilized mouse models to test the hypothesis that dietary carbohydrates modulate energetic, metabolic, and islet adaptions to high-fat diets. To address this, we exposed C57BL/6J mice to 12 wk of 3 eucaloric high-fat diets (>60% calories from fat) with varying total carbohydrate (1–20%) and sucrose (0–20%) content. Our results show that severe restriction of dietary carbohydrates characteristic of ketogenic diets reduces body fat accumulation, enhances energy expenditure, and reduces prevailing glycemia and insulin resistance compared with carbohydrate-rich, high-fat diets. Moreover, severe restriction of dietary carbohydrates also results in functional, morphological, and molecular changes in pancreatic islets highlighted by restricted capacity for β-cell mass expansion and alterations in insulin secretory response. These studies support the hypothesis that low-carbohydrate/high-fat diets provide antiobesogenic benefits and suggest further evaluation of the effects of these diets on β-cell biology in humans.
Keywords: β-cell, high-fat diet, insulin, ketogenic diet, obesity
INTRODUCTION
Obesity is a major health problem currently facing society, attributed largely to its causative association with metabolic diseases such as type 2 diabetes mellitus (T2DM) (3). In particular, obesity promotes development of T2DM through induction of interrelated pathophysiological conditions that include, but are not limited to, the following: 1) suppression of energy expenditure (42), 2) ectopic lipid accumulation (32), 3) induction of insulin resistance (32), 4) glucose intolerance (2), and 5) pancreatic β-cell functional and morphological failure (43). Thus, understanding mechanistic underpinnings of obesity-mediated metabolic dysfunction may provide insights into prevention and treatment of T2DM.
The primary drivers of the obesity epidemic remain elusive. It is clear that genetic factors play an important role in the pathogenesis of obesity (13). However, genetic factors alone cannot account for a dramatic rise in obesity prevalence, suggesting that environmental and dietary influences likely play a contributory role (36). Alterations in diet macronutrient composition such as increased saturated fat content have been shown to promote adiposity and development of T2DM (36). In support of this premise, chronic exposure to saturated free fatty acids (i.e., lipotoxicity) recapitulates many pathophysiological aspects of obesity and T2DM, such as hepatic/skeletal muscle insulin resistance (31), adipose tissue dysfunction (36), and β-cell failure (30).
Conversely, evidence also suggests that increased intake of dietary carbohydrates and consequent hyperinsulinemia play a previously underappreciated role in the promotion of obesity and consequent metabolic dysfunction (18, 26). This view is supported by evidence that increased carbohydrate intake and/or constitutive hyperinsulinemia results in increased adipogenesis and detrimental ectopic lipid accumulation (4, 7, 21). Additionally, increased intake of refined sugars has additional deleterious effects on promotion of adipogenesis, insulin resistance, and β-cell dysfunction (35). Furthermore, evidence points to the potential efficacy of very low-carbohydrate diets, even in the context of high fat consumption of >70% of caloric intake (e.g., ketogenic diets), in decreasing adiposity and improving energy expenditure as well as enhancing the regulation of lipid and glucose homeostasis (11, 29).
Human trials have been employed to delineate metabolic effects of diets with varying macronutrient contents (e.g., high/low fat, carbohydrate, and protein) (9). Although these studies provide important information, there are limitations related to adherence, control groups, duration, etc. Furthermore, human trials are unable to address the role of macronutrient composition on the endocrine pancreas composition and/or specific molecular changes in pancreatic islets. Thus, in the current study we utilized preclinical mouse models to test the hypothesis that dietary carbohydrates modulate metabolic and islet adaptations to high-fat diets. Our findings indicate that severe restrictions of dietary carbohydrates (e.g., up to 90% energy from fat) characteristic of ketogenic diets attenuated deleterious effects of standard high-fat diets on body composition, energy expenditure, and reduced prevailing glycemia and insulin resistance. Notably, pancreatic islets of mice exposed to ketogenic diet displayed severely attenuated β-cell mass and replicative capacity. Transcriptome profiling demonstrated that ketogenic diet leads to reprogramming of the islet transcriptome by modulating expression of transcripts regulating cell cycle and conversely enhanced gene expression associated with the regulation of hormonal secretion.
RESEARCH DESIGN AND METHODS
Animals and study design.
All experimental procedures were approved by Mayo Clinic Institutional Animal Care and Use Committee. In total, 50 male and 40 female C57BL/6J mice were used for this study (Jackson laboratories, strain no. 0006641). All mice were housed at Mayo Clinic Animal Facility under standard 12-h light, 12-h dark cycle. Upon arrival from Jackson laboratories, all mice were fed standard chow diet (Harlan Laboratories, Indianapolis, IN) until the age of 10 wk, after which they were placed for 6 or 12 wk on either high-fat (HF: 60% fat, 20% protein, and 20% carbohydrates with 7% sucrose content); high-fat, high-sugar (HFHS: 60% fat, 20% protein, and 20% carbohydrates with 20% sucrose); high-fat ketogenic (HFKETO: 89% fat, 10% protein, and 1% carbohydrates with 0% sucrose); or low-fat control (LF: 10% fat, 20% protein, and 70% carbohydrates with 0% sucrose) diet. All solid diets were obtained from Research Diets, Inc. (for detailed diet composition, see Table 1).
Table 1.
Detailed nutritional composition of diets used in the current study
| Low Fat |
High Fat |
High Fat Ketogenic |
High Fat High Sugar |
|||||
|---|---|---|---|---|---|---|---|---|
| g% | kcal% | g% | kcal% | g% | kcal% | g% | kcal% | |
| Diet | ||||||||
| Protein | 19 | 20 | 26 | 20 | 17 | 10 | 26 | 20 |
| Carbohydrate | 67 | 70 | 26 | 20 | 2 | 1 | 26 | 20 |
| Fat | 4 | 10 | 35 | 60 | 66 | 89 | 35 | 60 |
| Total | 100 | 100 | 100 | 100 | ||||
| kcal/gm | 3.8 | 5.2 | 6.6 | 5.2 | ||||
| Ingredient | ||||||||
| Casein | 200 | 800 | 200 | 800 | 100 | 400 | 200 | 800 |
| l-cystine | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 |
| Corn starch | 550 | 2,200 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maltodextrin 10 | 150 | 600 | 125 | 500 | 0 | 0 | 0 | 0 |
| Sucrose | 0 | 0 | 68.8 | 275 | 0 | 0 | 193.8 | 775 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | 0 |
| Soybean oil | 25 | 225 | 25 | 225 | 25 | 225 | 25 | 225 |
| Lard | 20 | 180 | 245 | 2,205 | 175.5 | 1,580 | 145 | 1,305 |
| Cocoa butter | 0 | 0 | 0 | 0 | 200 | 1,800 | 100 | 900 |
| Mineral mix, S10026 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 |
| Dicalcium phosphate | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 |
| Calcium carbonate | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 |
| Potassium citrate, 1 H2O | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 | 0 | 0 |
| Vitamin mix, V1001 | 10 | 40 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 |
| FD&C yellow dye no. 5 | 0 | 0 | 0 | 0 | 0.025 | 0 | 0.025 | 0 |
| FD&C red dye no. 40 | 0.025 | 0 | 0 | 0 | 0.025 | 0 | 0 | 0 |
| FD&C blue dye no. 1 | 0.025 | 0 | 0.05 | 0 | 0 | 0 | 0.025 | 0 |
| Total | 1,055.55 | 4057 | 773.85 | 4,057 | 610.55 | 4,057 | 773.85 | 4,057 |
Monitoring of circadian activity and metabolic rate.
For analysis of circadian behavioral rhythms in activity and feeding, mice were housed individually in cages fitted with an optical beam sensor system (Promethion behavioral caging system, Sable Systems) and monitored for 20 days under standard 12-h light, 12-h dark cycle. Oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were monitored over a 24-h period using a comprehensive laboratory animal monitoring system equipped with photocells (CLAMS equipped with an Oxymax Open Circuit Calorimeter System; Columbus Instruments, Columbus, OH). V̇o2 and V̇co2 levels were used to calculate the respiratory exchange ratio (RER), and V̇o2 and RER values were used to determine the energy expenditure rate (kcal·kg−1·h−1). Body composition (total body lean and fat mass) was assessed at study end point by quantitative magnetic resonance (EchoMRI‐100).
Assessment of oral glucose tolerance, in vivo insulin secretion, and insulin tolerance.
To assess oral glucose tolerance, 6-h fasted mice received 1 g/kg body weight of 50% dextrose solution via oral gavage. Blood was subsequently sampled at 0, 15, 30, 60, and 90 min after dextrose injection into chilled microcentrifuge tubes and immediately centrifuged at 4°C for subsequent collection of plasma and assessment of insulin concentrations. To assess insulin tolerance, 6-h fasted mice received 0.75 mU/g body weight of insulin solution via intraperitoneal injection, and blood glucose was serially measured at 0, 15, 30, 60, and 90 min after insulin.
Pancreas immunohistochemistry and immunofluorescence analysis.
Mice were euthanized and the pancreas was quickly excised and weighed, then fixed in 4% paraformaldehyde overnight at 4°C and subsequently embedded in paraffin. Deparaffinized pancreatic sections were stained for insulin (guinea pig anti-insulin, 1:100; ab7842, Abcam, Cambridge, MA) in conjunction with secondary biotin-conjugated donkey anti-guinea pig antibody (1:100; Jackson ImmunoResearch, Westgrove, PA) and detected using a diaminobenzidine (DAB) peroxidase (HRP) substrate kit (SK-4100, Vector Laboratories, Burlingame, CA). Additionally, adjacent deparaffinized sections were coimmunostained by immunofluorescence for insulin and Ki-67 (BD PharMingen, San Jose, CA) for determination of β-cell replication. All slides were coverslipped with Vectashield-DAPI mounting medium (Vector Laboratories), stored in dark at 4°C, and analyzed within 1–3 days after staining. Slides were viewed, imaged, and analyzed using a Zeiss Axio Observer Z1 microscope (Carl Zeiss Microscopy, LLC) and ZenPro software (Carl Zeiss Microscopy).
Islet RNA extraction and microarray analysis.
Pancreatic islets were isolated using standard collagenase methodology, washed with PBS, and immediately transferred to RNeasy RLT Lysis Buffer (Qiagen, Valencia, CA) and stored at −80°C for subsequent RNA isolation. Total RNA was extracted using RNeasy Mini Kit (Qiagen), quantified by NanoDrop OneC (Thermo Scientific, Waltham, MA), and assessed for overall integrity using agarose gel electrophoresis. Microarray analysis was performed utilizing Agilent Whole Genome Expression Array platform according to manufacturer’s instructions (Mus musculus) (Arraystar Inc., Rockville, MD), as previously described in detail (27). A fold regulation cutoff set to 2 was used with statistical significance at P < 0.05, adjusted for multiple comparisons to identify up- or downregulated transcripts. Differentially expressed gene list was subjected to Gene ontology (GO) enrichment analysis using DAVID (14). GO terms with a false discovery rate <0.1 were considered to be significantly enriched. Differentially expressed gene list was also subjected to Gene Set Enrichment Analysis (University of California San Diego and Broad Institute).
Analytical methods.
Blood glucose was measured with FreeStyle Lite Blood Glucose measuring system (Abbott Laboratories, Abbott Park, IL). Insulin was measured using Mouse Ultrasensitive Insulin ELISA Kit (Alpco Diagnostics, Salem, NH). Ketone bodies were measured using Precision ketone strips (Abbott, Alameda, CA). HbA1c was measured using A1CNow strips (PTS Diagnostics, Whitestown, IN).
Statistical analysis and calculations.
Activity recordings were analyzed using ExpeData (Sable Systems, Las Vegas, NV) and ClockLab software (Actimetrics, Wilmette, IL). Homeostasis model assessment index (HOMA-IR) of insulin sensitivity was calculated as previously outlined and validated for the use in mice (19). Statistical analysis was performed using ANOVA with post hoc tests wherever appropriate (GraphPad Prism v.8.2, San Diego, CA). Data in graphs are presented as means ± SE and assumed statistically significant at P < 0.05.
RESULTS
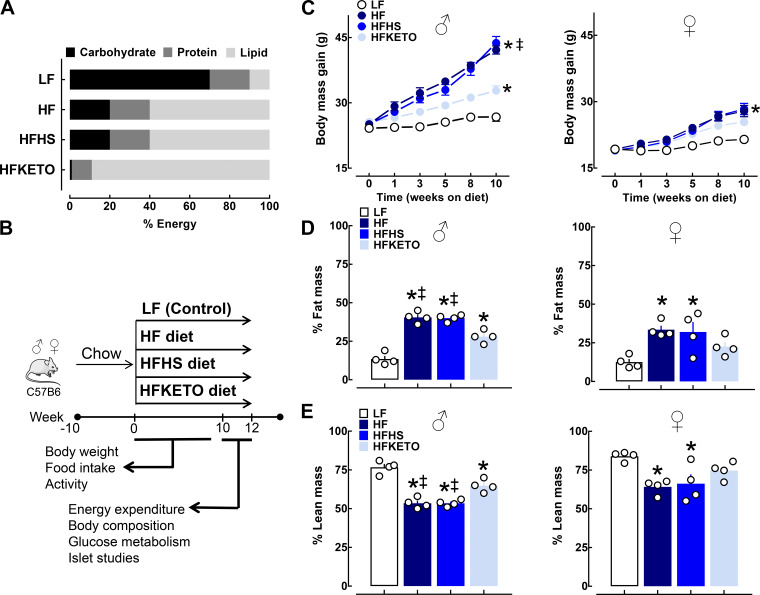
To address whether dietary carbohydrates modulate metabolic and islet adaptions to high-fat diets, we exposed C57BL/6J male/female mice to 12 wk of 4 custom-designed eucaloric diets: 1) control low-fat diet (LF), 2) high-fat diet (HF), 3) high-fat, high-sugar diet (HFHS), or 4) high-fat ketogenic diet (HFKETO) (Fig. 1 and Table 1). Exposure to all three high-fat diets induced a significant increase in body mass gain and fat mass and decreased lean body mass compared with LF controls (P < 0.05 for LF vs. all diets, Fig. 1, C–E). However, consistent with previous studies, the total body mass gain in response to high-fat diets was significantly attenuated in female versus male mice (~64%, Fig. 1C). Notably, mice maintained on HFKETO diet (male and female) demonstrated attenuated body mass (~30%) and fat mass gain (~40%) and, correspondingly, exhibited greater lean body mass (~17%) compared with HF and HFHS groups (P < 0.05 for HFKETO vs. HF and HFHS, Fig. 1, C–E).
Fig. 1.
Dietary carbohydrates modulate body mass gain and body composition in response to high-fat diets. A: macronutrient composition of the control and high-fat diets used in the study. B: schematic representation of the study design. Male and female C57BL/6J mice were studied for a period of 12 wk exposed to either high-fat (HF); high fat, high sugar (HFHS); high-fat ketogenic (HFKETO); or low-fat control diet (LF). C: body mass gain in male and female mice exposed for 12 wk to either LF (n = 8–10), HF (n = 8–10), HFHS (n = 7–10), or HFKETO (n = 7–10) diets. D and E: percentage body fat and lean body mass determined by quantitative magnetic resonance (EchoMRI‐100) in male and female mice exposed for 12 wk to either LF (n = 4), HF (n = 4), HFHS (n = 4), or HFKETO (n = 4) diets. Values are means ± SE;*P < 0.05 denotes statistical significance vs. LF and ‡P < 0.05 denotes significance vs. HFKETO.
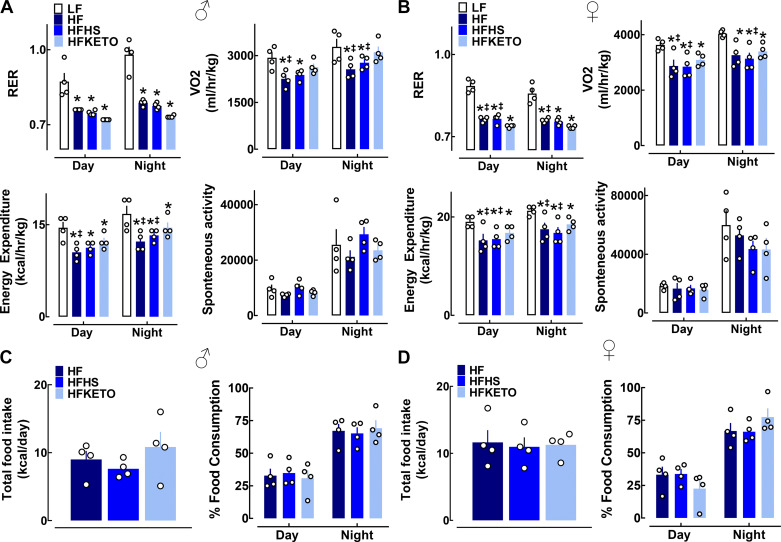
We next assessed whole body metabolic function and energy expenditure utilizing indirect calorimetry (Fig. 2, A and B). Respiratory exchange ratio (RER) of all HF-treated groups was significantly lower compared with LF, implicating an expected shift to preferential fat utilization both during the inactive (day) and active (night) circadian cycle (Fig. 2, A and B). Additionally, HF and HFHS-fed male and female mice demonstrated decreased (~30%) oxygen consumption (V̇o2) and consequent lower total energy expenditure compared with LF controls (P < 0.05, Fig. 2, A and B). Interestingly, HFKETO mice exhibited modest but significantly greater (~20%) V̇o2 and total energy expenditure compared with HF and HFHS male and female counterparts (Fig. 2, A and B). Importantly, there was no significant difference in total or diurnal patterns of food consumption (or caloric intake) among the three high-fat diet groups (Fig. 2, C and D).
Fig. 2.
Dietary carbohydrates modulate regulation of energy expenditure and metabolic function in response to high-fat diets. A and B: average measurements of respiratory exchange ratio (RER), oxygen consumption (V̇o2), metabolic rate, and the spontaneous activity monitored over a 24-h period in male and female mice exposed for 12 wk to low-fat control (LF); high-fat (HF); high-fat, high-sugar (HFHS); or high-fat ketogenic (HFKETO) diets. Calorimetric recordings were made using a comprehensive laboratory animal monitoring system (CLAMS). Values are means ± SE (n = 4 per group). *P < 0.05 denotes statistical significance vs. LF and ‡P < 0.05 denotes significance vs. HFKETO. C and D: average daily food intake and percentage of food consumption during the inactive (day) and active (night) circadian cycle measured in male (C) and female (D) mice exposed for 12 wk to HF, HFHS, or HFKETO diets. Values are means ± SE (n = 4 per group).
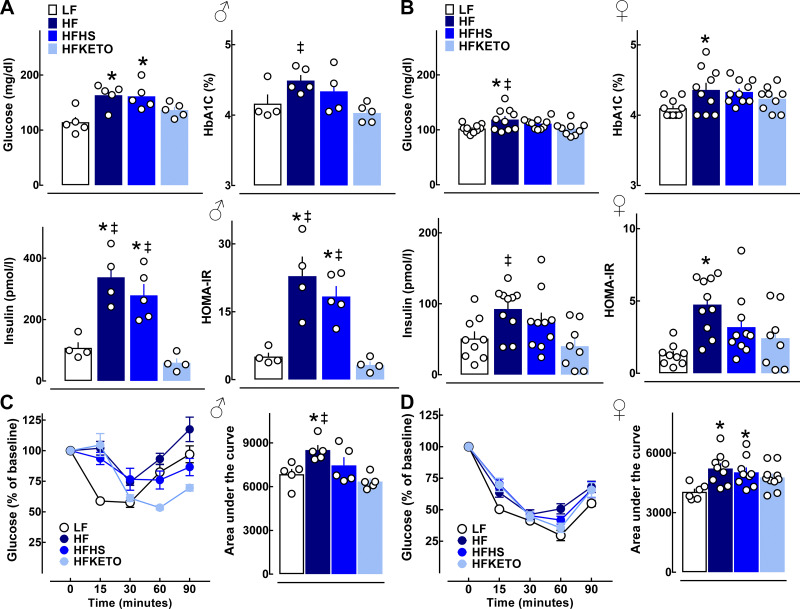
Exposure of male mice to either HF or HFHS diet resulted in the induction of fasting hyperglycemia, severe hyperinsulinemia (approximately threefold vs. LF), and a corresponding higher HOMA-IR index of insulin resistance (P < 0.05 for all variables vs. LF, Fig. 3A). Male mice on HFKETO diet displayed evidence of enhanced glycemic control characterized by lower prevailing glycemia, HbA1c, and robustly attenuated levels of fasting hyperinsulinemia and HOMA-IR (23 ± 4 vs. 3 ± 1, P < 0.05 for HOMA-IR, HF vs. HFKETO, Fig. 3A). Similar trends in regard to hyperinsulinemia/insulin resistance were detected with HF and HFHS in female mice, whereas exposure of female mice to HFKETO resulted in significantly attenuated glycemia (102 ± 4 vs. 119 ± 6 mg/dL, P < 0.05 for HFKETO vs. HF), hyperinsulinemia (40 ± 10 vs. 93 ± 10 pmol/l\L, P < 0.05 for HFKETO vs. HF), and HOMA-IR (2.4 ± 0.7 vs. 4.8 ± 0.5, P = 0.055 for HFKETO vs. HF) compared with HF (Fig. 3B). Consistently, HFKETO mice displayed increased insulin tolerance in response to an insulin bolus test (Fig. 3, C and D).
Fig. 3.
Dietary carbohydrates modulate blood metabolic parameters and insulin sensitivity in response to high-fat diets. A and B: fasted blood glucose, HbA1c, plasma insulin, and a corresponding index of insulin resistance (HOMA-IR) in male and female mice exposed for 12 wk to low-fat control (LF); high-fat (HF); high-fat, high-sugar (HFHS); or high-fat ketogenic (HFKETO) diets. C and D: insulin tolerance test with corresponding area under the curve after insulin bolus in male and female mice exposed to LF, HF, HFHS, or HFKETO diets. Values are means ± SE (n = 4–10 per group). *P < 0.05 denotes statistical significance vs. LF and ‡P < 0.05 denotes significance vs. HFKETO.
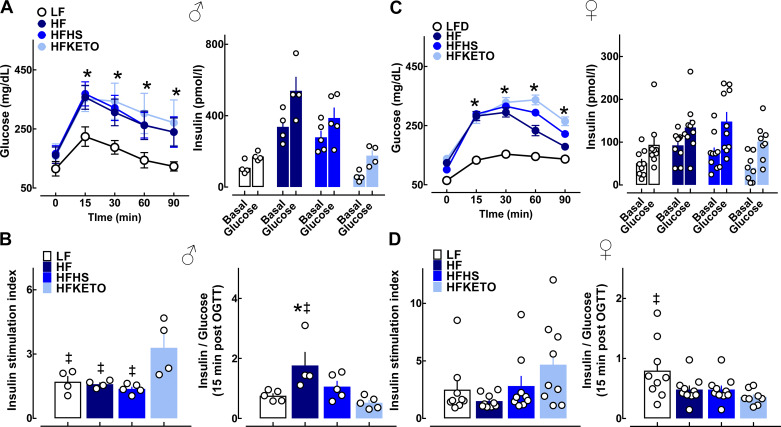
Next we performed oral glucose tolerance tests (OGTT) with concomitant measure of in vivo glucose-stimulated insulin response (Fig. 4). There was frank impairment of oral glucose tolerance with all three HF diets (in male and female mice) evident by an approximately twofold increase in glucose area under the curve (AUC) during OGTT in HF, HFHS, and HFKETO versus LF (P < 0.05, Fig. 4, A and C). To examine glucose-stimulated insulin secretion dynamics in vivo, we measured plasma insulin at baseline and 15 min after oral glucose challenge (Fig. 4, B–D). Although insulin stimulation index (assessed as fold change over baseline) was significantly diminished in HF and HFHS groups compared with HFKETO (1.4 ± 0.1 vs. 3.3 ± 0.7, P < 0.05 for HFHS vs. HFKETO, Fig. 4, B–D), the overall glucose-stimulated insulin secretory response was attenuated in HFKETO compared with LF and HF groups (P < 0.05 for HF vs. HFKETO in male mice, Fig. 4, B–D).
Fig. 4.
Dietary carbohydrates modulate in vivo oral glucose tolerance and β-cell function in response to high fat diets. A and C: mean blood glucose levels during oral glucose tolerance tests (OGTT) and plasma insulin concentrations measured at time 0 (basal) and 15 min after oral glucose bolus (glucose) in male and female mice exposed to low-fat control (LF); high-fat (HF); high-fat, high-sugar (HFHS); or high-fat ketogenic (HFKETO) diets. B and D: insulin stimulation index (expressed as 15-min insulin/0-min insulin) and insulin-to-glucose ratio calculated at 15-min post-OGTT administration in male and female mice exposed to LF, HF, HFHS, and HFKETO. Values are means ± SE (n = 4–10 per group). *P < 0.05 denotes statistical significance vs. LF and ‡P < 0.05 denotes significance vs. HFKETO.
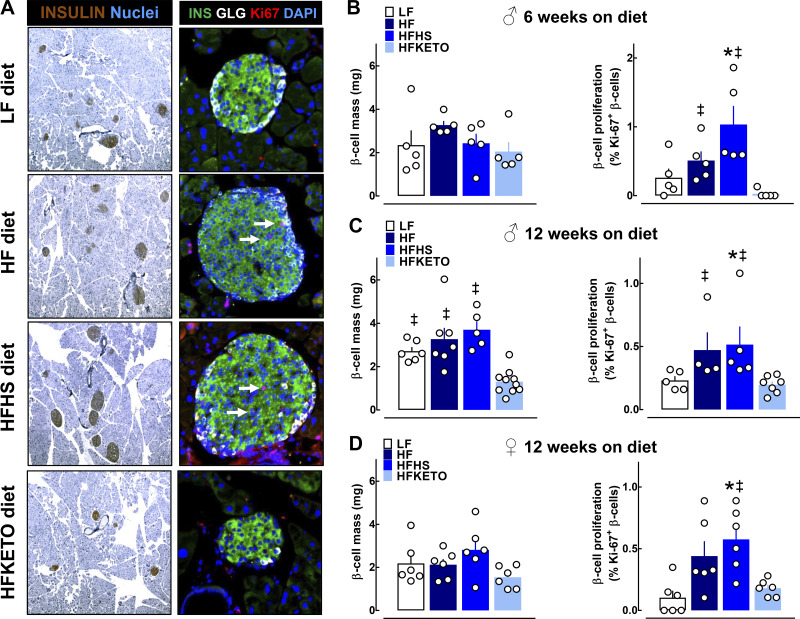
To address whether dietary carbohydrates modulate islet morphological adaption to high-fat diets, we next assessed HF-induced capacity for β-cell expansion and corresponding changes in the islet transcriptome (Figs. 5 and 6). As expected, mice exposed to HF and HFHS diets were characterized by a significant increase in β-cell turnover highlighted by ~2.5-fold increase in β-cell proliferation assessed as the percentage of β-cells stained for a common proliferation marker Ki-67 (P < 0.05 for HFHS vs. LF in males and females, Fig. 5, A–D). Interestingly, male mice exposed to ketogenic diet displayed restricted capacity for HF-induced β-cell proliferation/expansion resulting in an approximately threefold decline in β-cell mass compared with HF and HFHS following 12 wk on diet (1.3 ± 0.2 vs. 3.7 ± 0.4 mg, P < 0.05 for HFKETO vs. HFHS, Fig. 5C). This marked decline in β-cell mass in HFKETO mice appeared to be largely driven by decreased β-cell proliferation, which was evident at midpoint of our study (6 wk on diet) (P < 0.05 for HFKETO vs. HFHS and HF, Fig. 5B). Notably, β-cell mass in HFKETO female mice was not significantly different compared with HF and HFHS, confirming previously described sex differences in morphological islet adaption to HF diets (P > 0.05, Fig. 5D).
Fig. 5.
Dietary carbohydrates modulate β-cell mass and turnover in response to high-fat diets. A: representative examples of pancreatic sections and individual islets stained for insulin (brown or green), glucagon (white), proliferation marker Ki67 (red), and DAPI (blue) imaged at ×5 and ×20 [obtained from mice fed low-fat control (LF); high-fat (HF); high-fat, high-sugar (HFHS); or high-fat ketogenic (HFKETO)]. B–D: quantification of β-cell mass and proliferation in male and female mice exposed to LF, HF, HFHS, and HFKETO for 6 or 12 wk on respective diet. Values are means ± SE (n = 4–8); *P < 0.05 denotes statistical significance vs. LF and ‡P < 0.05 denotes significance vs. HFKETO.
Fig. 6.
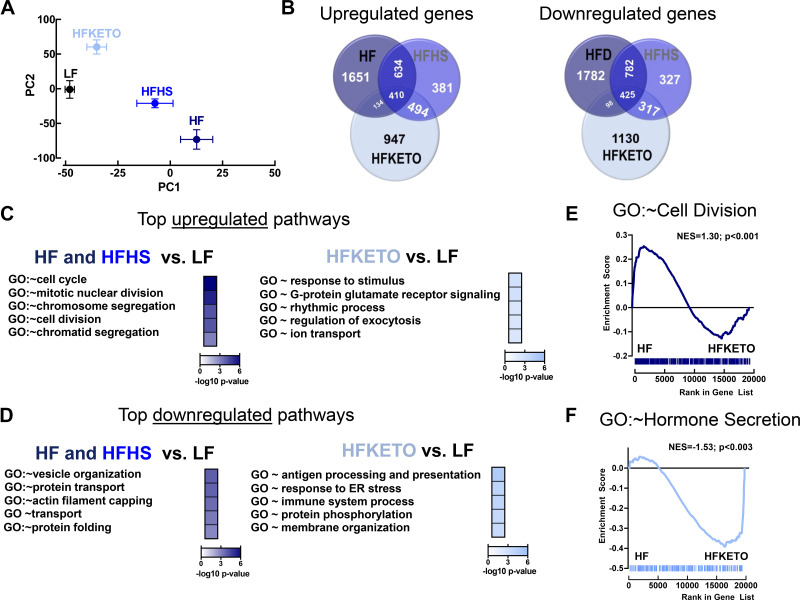
Dietary carbohydrates modulate islet transcriptome changes in response to high-fat diets. A: principle component analysis of islet whole genome array from mice exposed for 12 wk to either low-fat control (LF; n = 3), high-fat (HF; n = 2); high-fat, high-sugar (HFHS; n = 3); or high-fat ketogenic (HFKETO; n = 3) diets. PC1 indicates principle component 1 and PC2, principle component 2. B: analysis of differentially upregulated and downregulated genes in isolated islets of LF, HF, HFHS, and HFKETO mice. C and D: functional gene ontology (GO) enrichment analysis shows top 5 pathways significantly upregulated (or downregulated) in HF and HFHS or HFKETO vs. LF islets. E and F: gene-set enrichment analysis (GSEA) performed using preranked analysis of genes expressed in HF and HFKETO islets using genes annotated to either GO biological process “Cell Division” or GO biological process “Hormone Secretion.”
Next we performed microarray analysis of isolated islets from mice exposed to the three HF diets versus LF (Fig. 6). Principal component analysis (PCA) of all expressed genes showed similarities in the gene expression pattern for HF and HFHS islets and distinct gene signature in HFKETO group (Fig. 6A). Indeed, HFKETO islets were characterized by ~1,000 uniquely upregulated or downregulated (vs. LF) transcripts compared with HF and HFHS islets (Fig. 6B). Gene ontology (GO) pathway analysis of commonly upregulated genes in islets of HF and HFHS (twofold increase vs. LF, P < 0.05) showed significant enrichment for genes involved in the regulation of β-cell expansion/proliferation (e.g., GO:0007049 ~ cell cycle, GO:0007067 ~ mitotic nuclear division, and GO:0051301 ~ cell division) (Fig. 6C), whereas commonly downregulated genes in islets of HF and HFHS displayed enrichment for genes involved in the regulating protein processing and hormonal secretion (GO:0016050 ~ vesicle organization, GO:0015031 ~ protein transport, and GO:0006457 ~ protein folding) (Fig. 6D). In contrast, pathway analysis of genes upregulated in HFKETO (vs. LF) showed significant enrichment in transcripts regulating exocytosis and ion channel activation (e.g., GO:0050896 ~ response to stimulus, GO:0017157 ~ regulation of exocytosis, GO:0006811 ~ ion transport) and downregulation of genes mediating response to ER stress and immune activation (GO:0034976 ~ response to endoplasmic reticulum stress and GO:0002376 ~ immune system process) (Fig. 6D). Importantly, gene-set enrichment analysis (GSEA) confirmed that transcripts upregulated in islets of HF-fed mice were significantly enriched for genes regulating cell proliferation (e.g., GO: ~ cell division, P < 0.001, Fig. 6E) compared with HFKETO, whereas HFKETO islets were significantly enriched for genes regulating hormonal secretion compared with HF (e.g., GO: ~ hormone secretion, P < 0.003, Fig. 6E).
DISCUSSION
Diets high in saturated fats have long been associated with development of T2DM attributed in part to induction of key metabolic and islet defects (1, 2, 8, 10, 23, 30–33, 36, 42). Given the importance of diet macronutrient composition in the regulation of metabolic health, in the present study we sought to determine whether dietary carbohydrates modulate energetic, metabolic, and islet adaptions to high-saturated fat diets in male and female C57BL/6J mice. Our main findings indicate that a severe dietary restriction of carbohydrates (e.g., 90% kcal – fat, 1% carbohydrates, 0% kcal – sugar) characteristic of commonly used ketogenic diets significantly alters primary metabolic effects of high-fat diets on body composition, energy expenditure, and glucose metabolism. Moreover, islets of mice exposed to ketogenic diet demonstrate restricted capacity for β-cell expansion, attenuated expression of transcripts regulating cell cycle, and enhanced gene expression associated with hormonal secretion.
Previous studies explored temporal characterization of the metabolic and islet abnormalities associated with the exposure to 60% HF diet in C57BL/6J mice (1, 8, 10, 17, 23, 28, 33). Notably, skeletal muscle and hepatic insulin resistance, β-cell dysfunction, and consequent glucose intolerance develop in as little as 3–7 days after initiation of HF treatment. Induction of insulin resistance and glucose intolerance appears to coincide with increased body adiposity and ectopic fat accumulation in insulin-responsive organs (e.g., liver) leading to the downregulation of insulin signaling and consequent decrease in glucose disposal (1, 17). These effects on glucose tolerance and insulin sensitivity worsen with prolonged HF exposure attributed partly to tissue-specific inflammation and deleterious effects of constitutive hyperinsulinemia (1, 17). Similarly, impairments in glucose-stimulated insulin secretion have been observed within 1 wk of HF initiation and have been attributed to alterations in β-cell metabolic flux and mitochondrial function, whereas prolonged exposure to HF also leads to the induction of the unfolded protein response and compromised ER function (8, 10, 23, 28, 33, 39). Moreover, β-cell proliferation and β-cell mass also increase within the first week of HF, likely attributed to proproliferative signaling associated with hyperinsulinemia, episodic hyperglycemia and insulin resistance (23, 33, 34, 40).
The data presented in the current study are, for the most part, consistent with metabolic and islet changes previously reported with chronic exposure to 60% HF diet in C57BL/6J mice (23, 33). Interestingly, in our study nearly threefold increase in dietary sucrose characteristic of HFHS versus HF group (i.e., 7% in HF to 20% in HFHS) did not materialize in significant changes in body composition, energy expenditure, glucose tolerance, or islet functional and/or morphological parameters. Indeed, HF and HFHS islets displayed ~1,000 commonly upregulated and ~1,200 commonly downregulated transcripts enriched for pathways regulating key aspects of HF-induced islet adaptations (e.g., cell cycle regulation, cell division, protein folding, vesicle transport, etc). Exposure to HF or HFHS diet also demonstrated congruent effects in female mice. However, consistent with previous studies, the magnitude of metabolic and islet HF-mediated adaptations in female mice was attenuated compared with male mice (25). Although our study design precluded from examination of mechanisms regulating differential adaptation to HF diets in male versus female mice, previous studies have suggested the role for protective metabolic and islet effects of estrogen receptor signaling (20).
One of the key observations in our study was the clear metabolic improvements associated with HFKETO diet compared with standard HF and HFHS diets. These improvements manifested as enhanced energy expenditure (e.g., V̇o2) and attenuated increase in total body and fat mass, as well as an increase in lean body mass. These results are consistent with clinical studies in individuals maintained on ketogenic diets (11, 29). The mechanisms underlying these observations are complex but likely include alterations in the metabolic and endocrine milieu associated with a significant decline in carbohydrate utilization and reduction in insulin secretion resulting in enhanced fat mobilization, oxidation, and energy expenditure. Indeed, in our study mice maintained on HFKETO diet displayed substantially attenuated prevailing insulin levels (e.g., approximately sixfold decrease vs. HF and HFHS and approximately twofold vs. LF), which were reflected by significantly reduced pancreatic insulin expression/β-cell mass. Consistently, a recent series of studies examined metabolic effects of reducing insulin production/secretion in the context of HF diet using genetically mediated reduction in insulin gene dosage (21, 37, 38). These studies demonstrate that genetic reduction (i.e., via Ins1 and/or Ins2 gene deletion) of prevailing insulin levels leads to near full protection from the development of key metabolic abnormalities associated with HF-mediated obesity, including attenuated weight gain, reduction in body adiposity, and enhanced energy expenditure (21, 37). The fact that metabolic improvements associated with HFKETO appear to phenocopy some metabolic improvements observed in HF-fed Ins1/2-deficient mice suggests that decreased circulating insulin may be one of the mechanisms mediating antiobesogenic effects of HFKETO diet.
It is important to point out that our study was performed in young C57BL/6J mice known to be highly susceptible to HF diet-induced obesity, glucose intolerance, and insulin resistance (22). Thus, beneficial effects of ketogenic diet will likely be attenuated in mouse strains with inherent resistance to HF-induced insulin resistance and obesity, such as BALB/c strain (22). Additionally, metabolic consequences of HF diets also demonstrate age-dependent effects (5). For example, it was recently shown that aged mice (22 mo old) display a high degree of heterogeneity in regard to the weight gain and development of glucose intolerance/insulin resistance (5). Interestingly, in aged mice, weight gain was closely correlated with the degree of glucose intolerance/hyperinsulinemia, implicating a close relationship between these two parameters (5). This suggests that beneficial metabolic effects of HFKETO diet will be more pronounced in aged mice with the highest degree of weight gain and hyperinsulinemia. Indeed, reduction of insulin levels in aged mice (via partial Ins2 gene deletion) was shown to enhance glucose metabolism and extend life span (38).
Another notable observation from our study is that exposure to HFKETO diet prevented HF-induced β-cell expansion and proliferation. Indeed β-cell mass in HFKETO group was only ~30% of β-cell mass in HF and HFHS groups and was also significantly lower compared with LF control. Consistent with these observations, transcriptomic analysis revealed that top transcripts upregulated in HF and HFHS groups were enriched for the regulation of cell cycle and cell division and top downregulated genes were involved in the regulation of vesicular processes, protein transport, and folding. Interestingly, no such effect was observed in HFKETO islets, which showed upregulation in genetic pathways regulating exocytosis and ion channel activation and downregulation of pathways mediating the response to ER stress and inflammation. These data are consistent with recent findings of repressed transcriptional activation of pathways regulating glucose-stimulated insulin secretion and protein processing in actively replicating β-cells (16). The exact mechanisms underlying attenuated β-cell mass expansion in the HFKETO group are not completely understood but likely involve loss of insulin-mediated proproliferative effects due to decline in prevailing insulin concentrations observed with ketogenic diet (24). Furthermore, reductions in prevailing hyperglycemia due to restricted carbohydrate intake in HFKETO also likely contributed to attenuated β-cell mass and proliferation (34, 40).
In recent years, very low-carbohydrate/high-fat diets (i.e., ketogenic-based diets) have garnered a lot of attention as means to treat chronic metabolic conditions such as obesity and T2DM (9). The premise of these diets consists of severe reduction in carbohydrate intake along with corresponding increase in energy intake from dietary fats (i.e., >70%) to mimic fasting state and induce ketosis. Consequently, changes in the metabolic milieu are predicated to promote fat utilization, reduce body adiposity, and enhance energy expenditure. Moreover, recent experimental evidence also suggests that ketone bodies (e.g., β-hydroxybutyrate) may exert beneficial cellular effects such as reduction in cellular senescence and enhancement of the mitochondrial function (12, 41). In this regard, our data do provide some support for beneficial metabolic and islet effects of HFKETO versus HF and HFHS diets. However, there is limited evidence of long-term beneficial metabolic effects of ketogenic diets in obesity/T2DM (15). There are also concerns related to potential adverse metabolic and cardiovascular effects that require additional experimental attention (15). Notably, despite significant metabolic improvements, the HFKETO group displayed severe glucose intolerance, an observation consistent with previous human and animal studies (6, 29). This effect has been attributed to impaired insulin-mediated regulation of lipolysis, but based on our results it may also be due to diminished β-cell mass and decline in insulin secretory capacity and response.
In conclusion, our results demonstrate that severe restriction of dietary carbohydrates attenuates obesogenic metabolic effects of HF and HFHS diets in male and female mice. Chronic exposure to HFKETO diet also results in functional, morphological, and molecular changes in pancreatic islets highlighted by restricted capacity for β-cell mass expansion in response to HF diets. These studies support the hypothesis that low-carbohydrate/high-fat diets provide potential antiobesogenic benefits, but additional studies are needed to evaluate functional and morphological effects of these diets on the endocrine pancreas in humans.
GRANTS
We acknowledge funding support from the National Institutes of Health (2R01DK098468 to A.V.M.), (R01AG053832 to N.K.L.), and the Center for Regenerative Medicine (Mayo Clinic, Rochester, MN).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.V.M. conceived and designed research; T.K.H., W.S.L., M.R.B., and K.R. performed experiments; T.K.H., W.S.L., and M.R.B. analyzed data; W.S.L., N.K.L., and A.V.M. interpreted results of experiments; T.K.H., W.S.L., and M.R.B. prepared figures; A.V.M. drafted manuscript; W.S.L., N.K.L., K.R., and A.V.M. edited and revised manuscript; T.K.H., W.S.L., M.R.B., N.K.L., K.R., and A.V.M. approved final version of manuscript.
REFERENCES
- 1.Ahrén B, Pacini G. Insufficient islet compensation to insulin resistance vs. reduced glucose effectiveness in glucose-intolerant mice. Am J Physiol Endocrinol Metab 283: E738–E744, 2002. doi: 10.1152/ajpendo.00199.2002. [DOI] [PubMed] [Google Scholar]
- 2.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68: 1456–1467, 1981. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17: 961–969, 1994. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 4.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 61: 4–13, 2012. doi: 10.2337/db11-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Leon ER, Brinkman JA, Fenske RJ, Gregg T, Schmidt BA, Sherman DS, Cummings NE, Peter DC, Kimple ME, Lamming DW, Merrins MJ. Age-dependent protection of insulin secretion in diet induced obese mice. Sci Rep 8: 17814, 2018. doi: 10.1038/s41598-018-36289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellenbroek JH, van Dijck L, Töns HA, Rabelink TJ, Carlotti F, Ballieux BE, de Koning EJ. Long-term ketogenic diet causes glucose intolerance and reduced β- and α-cell mass but no weight loss in mice. Am J Physiol Endocrinol Metab 306: E552–E558, 2014. doi: 10.1152/ajpendo.00453.2013. [DOI] [PubMed] [Google Scholar]
- 7.Erion KA, Corkey BE. Hyperinsulinemia: a cause of obesity? Curr Obes Rep 6: 178–186, 2017. doi: 10.1007/s13679-017-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fex M, Nitert MD, Wierup N, Sundler F, Ling C, Mulder H. Enhanced mitochondrial metabolism may account for the adaptation to insulin resistance in islets from C57BL/6J mice fed a high-fat diet. Diabetologia 50: 74–83, 2007. doi: 10.1007/s00125-006-0464-4. [DOI] [PubMed] [Google Scholar]
- 9.Freire R. Scientific evidence of diets for weight loss: different macronutrient composition, intermittent fasting, and popular diets. Nutrition 69: 110549, 2020. doi: 10.1016/j.nut.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Gupta D, Jetton TL, LaRock K, Monga N, Satish B, Lausier J, Peshavaria M, Leahy JL. Temporal characterization of β cell-adaptive and -maladaptive mechanisms during chronic high-fat feeding in C57BL/6NTac mice. J Biol Chem 292: 12449–12459, 2017. doi: 10.1074/jbc.M117.781047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT, Ravussin E. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr 104: 324–333, 2016. doi: 10.3945/ajcn.116.133561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han YM, Bedarida T, Ding Y, Somba BK, Lu Q, Wang Q, Song P, Zou MH. β-Hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of Oct4. Mol Cell 71: 1064–1078.e5, 2018. doi: 10.1016/j.molcel.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henkin L, Bergman RN, Bowden DW, Ellsworth DL, Haffner SM, Langefeld CD, Mitchell BD, Norris JM, Rewers M, Saad MF, Stamm E, Wagenknecht LE, Rich SS. Genetic epidemiology of insulin resistance and visceral adiposity. The IRAS Family Study design and methods. Ann Epidemiol 13: 211–217, 2003. doi: 10.1016/S1047-2797(02)00412-X. [DOI] [PubMed] [Google Scholar]
- 14.Huang DW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC, Lempicki RA. The DAVID Gene Functional Classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol 8: R183, 2007. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi S, Ostfeld RJ, McMacken M. The ketogenic diet for obesity and diabetes-enthusiasm outpaces evidence. JAMA Intern Med 179: 1163–1164, 2019. doi: 10.1001/jamainternmed.2019.2633. [DOI] [PubMed] [Google Scholar]
- 16.Klochendler A, Caspi I, Corem N, Moran M, Friedlich O, Elgavish S, Nevo Y, Helman A, Glaser B, Eden A, Itzkovitz S, Dor Y. The genetic program of pancreatic β-cell replication in vivo. Diabetes 65: 2081–2093, 2016. doi: 10.2337/db16-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60: 2474–2483, 2011. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out”. JAMA Intern Med 178: 1098–1103, 2018. doi: 10.1001/jamainternmed.2018.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mather K. Surrogate measures of insulin resistance: of rats, mice, and men. Am J Physiol Endocrinol Metab 296: E398–E399, 2009. doi: 10.1152/ajpendo.90889.2008. [DOI] [PubMed] [Google Scholar]
- 20.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34: 309–338, 2013. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, Bamji SX, Clee SM, Johnson JD. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab 16: 723–737, 2012. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, Turner N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56: 1129–1139, 2013. doi: 10.1007/s00125-013-2846-8. [DOI] [PubMed] [Google Scholar]
- 23.Mosser RE, Maulis MF, Moullé VS, Dunn JC, Carboneau BA, Arasi K, Pappan K, Poitout V, Gannon M. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am J Physiol Endocrinol Metab 308: E573–E582, 2015. doi: 10.1152/ajpendo.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA 104: 8977–8982, 2007. doi: 10.1073/pnas.0608703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira RB, Maschio DA, Carvalho CP, Collares-Buzato CB. Influence of gender and time diet exposure on endocrine pancreas remodeling in response to high fat diet-induced metabolic disturbances in mice. Ann Anat 200: 88–97, 2015. doi: 10.1016/j.aanat.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Page MM, Johnson JD. Mild suppression of hyperinsulinemia to treat obesity and insulin resistance. Trends Endocrinol Metab 29: 389–399, 2018. doi: 10.1016/j.tem.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Rakshit K, Qian J, Ernst J, Matveyenko AV. Circadian variation of the pancreatic islet transcriptome. Physiol Genomics 48: 677–687, 2016. doi: 10.1152/physiolgenomics.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roat R, Rao V, Doliba NM, Matschinsky FM, Tobias JW, Garcia E, Ahima RS, Imai Y. Alterations of pancreatic islet structure, metabolism and gene expression in diet-induced obese C57BL/6J mice. PLoS One 9: e86815, 2014. doi: 10.1371/journal.pone.0086815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum M, Hall KD, Guo J, Ravussin E, Mayer LS, Reitman ML, Smith SR, Walsh BT, Leibel RL. Glucose and lipid homeostasis and inflammation in humans following an isocaloric ketogenic diet. Obesity (Silver Spring) 27: 971–981, 2019. doi: 10.1002/oby.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J Clin Endocrinol Metab 97: 3302–3309, 2012. doi: 10.1210/jc.2012-1428. [DOI] [PubMed] [Google Scholar]
- 31.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 148: 852–871, 2012. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 371: 1131–1141, 2014. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 33.Stamateris RE, Sharma RB, Hollern DA, Alonso LC. Adaptive β-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. Am J Physiol Endocrinol Metab 305: E149–E159, 2013. doi: 10.1152/ajpendo.00040.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamateris RE, Sharma RB, Kong Y, Ebrahimpour P, Panday D, Ranganath P, Zou B, Levitt H, Parambil NA, O’Donnell CP, García-Ocaña A, Alonso LC. Glucose induces mouse β-cell proliferation via IRS2, MTOR, and Cyclin D2 but not the insulin receptor. Diabetes 65: 981–995, 2016. doi: 10.2337/db15-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90: 23–46, 2010. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 36.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 93: 359–404, 2013. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 37.Templeman NM, Clee SM, Johnson JD. Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia 58: 2392–2402, 2015. doi: 10.1007/s00125-015-3676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Templeman NM, Flibotte S, Chik JHL, Sinha S, Lim GE, Foster LJ, Nislow C, Johnson JD. Reduced circulating insulin enhances insulin sensitivity in old mice and extends lifespan. Cell Reports 20: 451–463, 2017. doi: 10.1016/j.celrep.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 39.Teodoro-Morrison T, Schuiki I, Zhang L, Belsham DD, Volchuk A. GRP78 overproduction in pancreatic beta cells protects against high-fat-diet-induced diabetes in mice. Diabetologia 56: 1057–1067, 2013. doi: 10.1007/s00125-013-2855-7. [DOI] [PubMed] [Google Scholar]
- 40.Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T. Glucokinase and IRS-2 are required for compensatory β cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 117: 246–257, 2007. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-β-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112: 892–901, 2003. doi: 10.1172/JCI200318797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells JC, Siervo M. Obesity and energy balance: is the tail wagging the dog? Eur J Clin Nutr 65: 1173–1189, 2011. doi: 10.1038/ejcn.2011.132. [DOI] [PubMed] [Google Scholar]
- 43.Ye R, Onodera T, Scherer PE. Lipotoxicity and β Cell Maintenance in Obesity and Type 2 Diabetes. J Endocr Soc 3: 617–631, 2019. doi: 10.1210/js.2018-00372. [DOI] [PMC free article] [PubMed] [Google Scholar]