Abstract
Myokines, such as irisin, have been purported to exert physiological effects on skeletal muscle in an autocrine/paracrine fashion. In this study, we aimed to investigate the mechanistic role of in vivo fibronectin type III domain-containing 5 (Fndc5)/irisin upregulation in muscle. Overexpression (OE) of Fndc5 in rat hindlimb muscle was achieved by in vivo electrotransfer, i.e., bilateral injections of Fndc5 harboring vectors for OE rats (n = 8) and empty vector for control rats (n = 8). Seven days later, a bolus of D2O (7.2 mL/kg) was administered via oral gavage to quantify muscle protein synthesis. After an overnight fast, on day 9, 2-deoxy-d-glucose-6-phosphate (2-DG6P; 6 mg/kg) was provided during an intraperitoneal glucose tolerance test (2 g/kg) to assess glucose handling. Animals were euthanized, musculus tibialis cranialis muscles and subcutaneous fat (inguinal) were harvested, and metabolic and molecular effects were evaluated. Muscle Fndc5 mRNA increased with OE (~2-fold; P = 0.014), leading to increased circulating irisin (1.5 ± 0.9 to 3.5 ± 1.2 ng/mL; P = 0.049). OE had no effect on protein anabolism or mitochondrial biogenesis; however, muscle glycogen was increased, along with glycogen synthase 1 gene expression (P = 0.04 and 0.02, respectively). In addition to an increase in glycogen synthase activation in OE (P = 0.03), there was a tendency toward increased glucose transporter 4 protein (P = 0.09). However, glucose uptake (accumulation of 2-DG6P) was identical. Irisin elicited no endocrine effect on mitochondrial biogenesis or uncoupling proteins in white adipose tissue. Hindlimb overexpression led to physiological increases in Fndc5/irisin. However, our data indicate limited short-term impacts of irisin in relation to muscle anabolism, mitochondrial biogenesis, glucose uptake, or adipose remodeling.
Keywords: FNDC5, glucose metabolism, irisin, muscle, overexpression
INTRODUCTION
Skeletal muscle myokines such as brain-derived neurotrophic factor, IL-6, FGF2, and the more recently identified irisin are purported to exert their actions in an endocrine, paracrine, and autocrine fashion. Irisin, in particular, is a myokine that has received much attention. Irisin is a proteolytic cleavage product of 112 amino acids, with a molecular mass of 12 kDa, derived from its precursor, fibronectin type III domain-containing 5 (Fndc5; Refs. 21, 34). Fndc5 is a type I transmembrane glycoprotein consisting of 212 amino acids in humans and 209 in mice and rats. It is coded by the Fndc5 gene, composed of a signal peptide, 2 fibronectin type III domains, and a membrane-bound hydrophobic COOH terminus. The cleavage product of Fndc5-irisin has been associated with promoting weight loss via increased expression of uncoupling protein 1 (UCP1) in white adipose tissue and by increasing nonshivering thermogenesis and energy expenditure in subcutaneous adipose tissue (5). Moreover, upregulation of Fndc5 in muscle has been linked to the activation of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α; Refs. 5, 36), a regulator of mitochondrial biogenesis. This led to the notion that irisin may underlie certain health-promoting and adaptive responses to exercise.
Despite this, the role for irisin underlying the health benefits of exercise and the effect of exercise on Fndc5/irisin expression remains contentious. Huh et al. (19) showed that exercise training induced Fndc5 muscle gene expression and increased plasma irisin in humans, whereas a study following the impact of 1 yr of physical activity in obese children demonstrated increased irisin concentrations ~12% over baseline (4). In another study, increased circulating irisin was only transiently observed with high-intensity exercise training (80% of V̇o2max), 6 and 19 h after exercise, whereas low-intensity exercise (40% of V̇o2max) induced a fall in irisin (45). In other studies, 8 wk of endurance training elicited an increase in circulatory irisin in middle-aged and elderly participants but not younger (28). Similarly, Timmons et al. (44) showed an increase in Fndc5 gene expression only in elderly subjects undergoing a variety of exercise training regimens. In contrast, others have been unable to demonstrate an effect of exercise training on Fndc5/irisin (13, 23). Pekkala et al. (35) showed that neither acute or chronic endurance training nor combined endurance and resistance training enhanced Fndc5/irisin expression. Data from animal studies are also equivocal. In one study, 6 wk of free running enhanced Fndc5 expression in mice skeletal muscle (30), whereas another reported increased irisin levels in lean (27) and obese rats following 8 wk of swim training (27, 52); however, another resulted in elevated irisin acutely but not chronically (41). In contrast, 8 wk of free running failed to show increased Fndc5 expression, whereas endurance showed increased Fndc5 protein expression muscle without the expected increased irisin secretion (39), and even an acute reduction in Fndc5/irisin levels has been reported after exercise training (12). Despite these inconsistencies, Fndc5/irisin has been linked to the beneficial effects of exercise, including improving lipid profiles (27) and muscle strength (22). Irisin is also considered to be protective against weight gain, and several studies report a positive correlation to body mass index (BMI) or obesity (19, 25, 33, 43); however, other studies have observed either negative (9, 29) or no correlation (43) to BMI and obesity. Therefore, the regulation of Fndc5/irisin by exercise and its links to health outcomes, including obesity, remain contentious and unclear.
Whether Fndc5/irisin exerts its physiological effects in an endocrine or autocrine fashion (21, 34) in skeletal muscle is poorly defined. Nonetheless, in vitro, recombinant irisin treatment of muscle cells was shown to enhance mitochondrial content and oxidative metabolism (47), to induce growth regulatory genes such as IGF-1 (17), and to augment glucose uptake and glycogen synthesis (18). Moreover, in vivo treatment of mice with irisin resulted in increases in muscle mass and strength (38). At the molecular level, increased AMP-activated protein kinase (AMPK) phosphorylation with irisin treatment appeared to mediate enhanced glucose uptake (18), whereas protein kinase B (known as Akt) and extracellular signal-regulated protein kinase (ERK1/2) pathways were involved in the hypertrophic response, as was reduced atrogin-1 and muscle RING-finger protein-1 (MuRF1) expression (38). In contrast, systemic elevation of irisin after Fndc5 hepatic adenoviral induction failed to exert any effects on muscle metabolism (5). This raises questions as to the specificity of the effects of muscle Fndc5-derived irisin compared with other tissue-derived irisin products and recombinant irisin, i.e., which may not reflect muscle Fndc5 expression.
In this study, we investigated the potential autocrine role of Fndc5/irisin on skeletal muscle metabolism and the endocrine role on white adipose tissue using a novel approach. We developed an in vivo electroporation (IVE) technique to overexpress Fndc5 in dual rat hindlimbs to determine the impacts on endogenous irisin and resulting autocrine/endocrine responses in skeletal muscle and adipose tissue.
MATERIALS AND METHODS
IVE.
The transgenic electroporation procedure was performed as previously described (10). Briefly, musculus tibialis cranialis (TC) muscles were injected with six separate 50-μL aliquots of Fndc5 DNA prepared in an endotoxin-free sterile saline solution (Qiagen Maxi/Mega-Prep kits; Doncaster, Victoria, Australia). Injections were followed by 80 V/cm 100-ms pulses at 1 Hz sequentially via a tweezer electrode attached to an ECM 830 electroporator (BTX Technologies, Holliston, MA).
Experimental design and ethics.
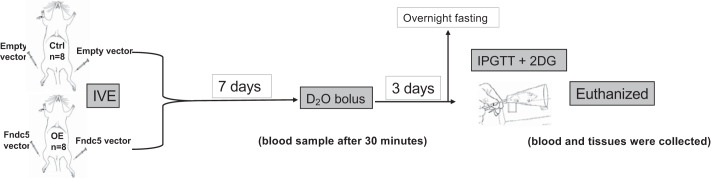
Seventeen Wistar male rats (280–340 g) were obtained from Charles River (Margate, United Kingdom) and maintained at 22 ± 0.5°C under a 12:12-h light-dark cycle on standard chow diet and left for 1 wk to acclimatize to their new environment (40). They were then randomized into two groups. The control group (Ctrl; n = 8) had bilateral IVE with an empty vector, whereas the overexpressed group (OE; n = 8) had bilateral IVE with Fndc5 vector. In addition, a single control rat was used to provide background enrichment measures for both blood and muscle alanine deuterium labeling before D2O tracer administration. IVE was performed under isoflurane anesthesia. Seven days after IVE, a bolus of D2O was administered by gavage (7.2 mL/kg) to both groups. Thirty minutes later, a blood sample was taken to determine peak body water deuterium enrichment. On day 9, all animals were fasted overnight. The following day, an intraperitoneal glucose tolerance test (IPGTT) was initiated (2 g/kg glucose), along with 2-deoxy-d-glucose (2-DG; 6 mg/kg) to assess muscle glucose uptake (40). Blood and muscle tissues were collected at the end of the procedure after euthanasia (Fig. 1). The transfection was performed at the Royal Veterinary College (RVC), University of London. The procedure was approved by the RVC ethics and welfare committee and was carried out under the United Kingdom Home Office license to comply with the Animals (Scientific Procedures) Act 1986.
Fig. 1.
Study schematic. 2DG, 2-deoxy-glucose (6 mg/kg); Ctrl, control group; D2O, deuterium (7.2 mL/kg); Fndc5, fibronectin type III domain-containing 5; IPGTT, intraperitoneal glucose tolerance test (2 g/kg); IVE, in vitro electroporation; OE, overexpressed group.
Glycogen content in skeletal muscles.
Glycogen was extracted and quantified as described previously (40). Briefly, TC (21.5–39.5 mg) were digested in 1 M KOH. Na2SO4 and ethanol were used to precipitate glycogen, with 0.3 mg/mL amyloglucosidase in 0.25 M acetate buffer (pH 4.75) used to digest the glycogen pellet overnight at 37°C. Samples were then incubated for 25 min at 37°C in phosphate buffer containing 0.5 mg/mL 4-aminoantipyrine, 1.6 U/mL peroxidase, and 10 U/mL glucose oxidase with a pH of 7.0. Glucose was quantified at 490 nm against a standard curve. Concentrations were normalized to muscle weight.
IPGTT.
Glucose and 2-DG were administered together (2 g/kg glucose and 6 mg/kg 2-DG; Amersham Biosciences) as an intraperitoneal injection into overnight-fasted rats. Blood from the tail vein was collected at 0, 15, 30, 60, and 90 min after injection. Blood glucose was measured directly after collection on an ACCU-CHEK Advantage meter (Roche Diagnostics, Castle Hill, New South Wales, Australia; Ref. 10).
Determination of glucose uptake using 2-deoxyglucose.
Briefly, 100 μL of plasma was aliquoted into fresh Eppendorf tubes with 10 μL of fluorodeoxyglucose as the internal standard and vortex-mixed. One milliliter of ice-cold absolute ethanol was then added to each tube, which were incubated in the refrigerator for 20 min and then spun for 2 min at 10,000 rpm. The supernatant was removed into test tubes and dried completely on a Techne block at 90°C for 10 min. One hundred microliters of oxime reagent (20 mg hydroxylamine HCl/mL pyridine) was then added to each tube, which were vortex-mixed and incubated at 85°C for 30 min on a Techne block. Samples were cooled for 5 min, and then 70 μL of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) was added, mixed, and incubated at 85°C for 30 min on a Techne block. Seventy microliters was transferred carefully into autosampler vials and capped. 2-DG was quantified by GC-MS (Ref. 8; ISQ; Thermo Fisher Scientific, Hemel Hempstead, United Kingdom) with reference to a standard curve of known concentration.
Muscle 2-deoxyglucose-6-phosphate accumulation.
Approximately 20 mg of powdered muscle tissue was homogenized three times with metallic beads for 2 min (30 Hz). During the first homogenization phase, 125 μL of double-distilled water (ddH2O) with 20 μL of internal standard [[13C]2-deoxyglucose-6-phosphate ([13C]2-DG6P)] was added and then centrifuged for 2 min at 15,000 g at 4°C. In the second phase, 375 μL of chilled methanol was added, followed by centrifugation for 10 min at 15,000 g at 4°C. The supernatant was then transferred to autosampler vials. Finally, the pellet was washed with 500 µL of ice-cold methanol, and all supernatants were combined before the next step. All samples were dried under nitrogen for 30 min at 50°C before a two-step derivatization. Seventy-five microliters of methoxyamine solution (20 mg/mL methoxyamine hydrochloride in pyridine) was added before samples were vortexed and then incubated for 60 min at 90°C. The mixture was cooled for 10 min, and then 75 μL of BSTFA was added before samples were vortexed and again incubated at 90°C for 60 min (11). Samples were transferred to autosampler vials ready for GC-MS analysis. A standard curve of known 2-DG6P concentration was prepared alongside each batch for quantitation.
RNA extraction and cDNA synthesis.
RNA was extracted from 5 to 10 mg of TC and fat tissue after homogenization with TRIzol solution (Life Technologies). RNA was suspended in 20-µL RNase-free water and then stored at −80°C. For cDNA synthesis, a total of 500 ng of RNA was reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies; Ref. 31).
Real-time quantitative PCR.
One microliter of cDNA was added to each well of 384-well optical plates (Life Technologies) after being diluted with RNase-free water (1:4). Each sample was run in triplicate with primers (Table 1) using SYBR Select Master Mix (Life Technologies) on a ViiA 7 Real-Time PCR System (Life Technologies; Ref. 31). All gene expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels.
Table 1.
Primer sequences
| Gene | Forward 5′–3′ | Reverse 5′–3′ |
|---|---|---|
| FNDC5 | CTTCATGTGGGCAGGTGTCAT | ATTGGGCTCGTTGTCCTTGAT |
| PGC-1α | CATGTGCAGCCAAGACTCTG | AAAGCTGTCTGTGTCCAGGT |
| TFAM | GACTTCTGCCCACTGAATGC | AAGCAAACGGCAGAACTCGT |
| NRF1 | GACCATCAGCAAAGCCGTGA | ACGTAAGCTCTGCCTGGTTG |
| UCP1 | GCTCCTCCACAAATAGCCCTG | CGGAAGTTGTCGAACTCACCA |
| UCP2 | AGCAGTTCTACACCAAGGGC | TGGAAGCGGACCTTTACCAC |
| UCP3 | CGCCTGGAACAGAACAAAGC | TAACAGTGCAGGGTTCCGTC |
| ATP5J2 | CGAATTTCTGCGGACAACAGG | CTGCAATACCACTGGGGGTG |
| ATP2A3 | TGCTGTTTTCTGGCACCAAT | CAAATTCATCCAGCTTGCGCT |
| MYOD | CTGCTCTGATGGCATGATGG | CTCCACTATGCTGGACAGGC |
| MYOG | GTGAATGCAACTCCCACAGC | CGAGCAAATGATCTCCTGGGT |
| MRF4 | TAAGGAAGGAGGAGCAAGCG | GGGAGTTTGCGTTCCTCTGA |
| IGF-1 | CAGAGCAGATAGAGCCTGCG | TGGGCAGGAATAATGAGGCA |
| MSTN | ACCATGCCTACCGAGTCTGA | ATCCACAGCTGGGCCTTTAC |
| FOLLISTATIN | GTGTGCCATGAAGGAAGCTG | TCCGAGATGGAGTTGCAAGA |
| REDD1 | GTCTAGTGCCCACCTTTCAGTT | GCTCGGAGCTGTAGAGTTTCTT |
| BCL-2 | TTCATCACAACCCACCTCTCTG | TGCAAGTCCCAACACTCGGG |
| BAX | CCAGGATCGAGCAGAGAGGAT | TGTTGTTGTCCAGTTCATCGC |
| ATG7 | CAGCCTGTTCATCCAAAGTTCTTG | CTGTGGTTGCTCAGACGGT |
| ATG5 | CAGAAGCTGTTCCGTCCTGT | CCGTGAATCATCACCTGGCT |
| CSTL | CTATCGCCACCAGAAGCACA | ACCACACTGGCCCTGATTCT |
| MURF-1 | CCAAGGACAGAAGACTGAACTGA | CTCCTGCTCCTGAGTGATCC |
| ATROGIN-1 | CCAAAACTCAGTATTTCCATCAG | GACTTTGCTATCAGCTCCAACA |
| CASPASE-3 | CGGACCTGTGGACCTGAAAA | CGGCCTCCACTGGTATCTTC |
| M-CALPAIN | TCGGCATCTATGAGGTCCCA | ATTCTTGTGGGGCTCGAAGG |
| GLUT4 | GGCCGGGACACTATACCCTAT | TCCCCATCTTCAGAGCCGAT |
| HK2 | TGGTTTCAAAGCGGTCGAACT | TCGAGTAGAGAAACCGAGGC |
| GYS1 | CCTGCTCAGAGTGAATGGCA | TTGGCTGTGTCCCATAGCTG |
| GAPDH | CTCTCTGCTCCTCCCTGTTC | CGATACGGCCAAATCCGTTC |
ATG5 and ATG7, autophagy-related genes; ATP2A3 and ATP5J2, ATP synthesis genes; BAX, B-cell lymphoma 2 (BCL-2)-associated X; FNDC5, fibronectin type III domain-containing 5; GLUT4, glucose transporter 4; GYS1, glycogen synthase 1; HK2, hexokinase 2; MRF4, myogenic regulatory factor 4; MURF-1, muscle RING-finger protein-1; MYOD, myogenic differentiation; MYOG, myogenin; NRF1, nuclear respiratory factor 1; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; REDD1, regulated in development and DNA damage response 1; TFAM, mitochondrial transcription factor A; UCP, uncoupling protein.
Immunoblotting.
Ten micrograms of protein was loaded onto a 12% polyacrylamide gel (Criterion XT Bis-Tris Precast Gels; Bio-Rad), the gel was run, and proteins were then transferred to polyvinylidene difluoride membrane (PVDF; Millipore). The membrane was incubated with primary antibody [anti-Fndc5 (ab174833), anti-PGC-1α (ab54481), oxidative phosphorylation (OXPHOS) cocktail (ab110413), and glucose transporter 4 (GLUT4; ab33780) from Abcam; cytochrome c, heat shock protein 60 (HSP60), succinate dehydrogenase enzyme A (SDHA), and voltage-dependent anion channel (VDAC) from mitochondrial sampler kit (cat. no. 5674), phosphorylated (phospho-) Akt (Ser473; cat. no. 9272), phospho-proline-rich Akt substrate of 40 kDa (PRAS40; Thr246; cat. no. 2640), phospho-mechanistic target of rapamycin (mTOR; Ser2448; cat. no. 2971), phosphorylated (P-) p70 S6 (Thr389; cat. no. 9234), phospho-4E-binding protein 1 (4E-BP1; Ser65/Thr70; cat. no. 945), phospho-AMPK (Thr172; cat. no. 2535), phospho-acetyl-CoA carboxylase (Ser79; cat. no. 3661), phosphatidylinositol 3-kinase (PI3K) class III (cat. no. 3358), beclin-1 (cat. no. 3495), light chain 3B (LC3B; cat. no. 2775), atrogin-1, MuRF1, phospho-AS160 Thr642 (cat. no. 8881), phospho-glycogen synthase kinase-3 (GSK3)-α/β (Ser21/9; cat. no. 9331), phospho-glycogen synthase (GS; Ser641; cat. no. 3891), and GS (cat. no. 3893) from Cell Signaling Technology] overnight with gentle agitation at 4°C and then washed free of excessive primary antibody and incubated with horseradish peroxidase-conjugated secondary antibody [1:2,000 in 2.5% (wt/vol) skimmed milk]. Chemiluminescence (Millipore) was detected using a ChemiDoc XRS, and band intensity was analyzed using Image Lab software. Target band intensity was normalized to membrane Coomassie bands. The membrane was washed free of chemiluminescence reagent with 3× Tris-buffered saline-Tween 20 before it was placed in Coomassie stain for 1 min and then rinsed (50:50 ddH2O-methanol), followed by ddH2O (3).
Citrate synthase activity.
The enzymatic activity of citrate synthase (CS) was estimated after incubating a mitochondrial suspension of TC with CS buffer, which contains reaction substrates, acetyl-CoA, and oxaloacetate. As described previously (2), 5–10 mg of frozen TC muscle was homogenized in ice-cold homogenization buffer (50 mM KH2PO4, 1 mM EDTA, and 1% Triton X-100) and centrifuged for 30 min at 22,000 g at 4°C. Twenty microliters of supernatant was then added to 300 μL of CS buffer in a 96-well plate, incubated for 3 min at 25°C, and then read at 412 nm. Delta optical density (sample OD minus blank OD) was calculated for each sample. Protein concentration was measured using the Pierce BCA Protein Assay.
Muscle protein, DNA, and RNA concentrations.
To determine muscle alkali-soluble protein (ASP), DNA, and RNA concentrations, ~15 mg of wet TC muscle was homogenized in 1 mL of 0.2 M perchloric acid (PCA) and centrifuged at 4°C at 11,000 rpm for 8 min. The supernatant was discarded, and the pellet was washed twice with 1 mL of 0.2 M PCA, vortexed, and centrifuged at 4°C at 11,000 rpm for 8 min. The protein pellet was dissolved in 800 μL of 0.3 M NaOH at 37°C for 30 min. ASP was then quantified by spectrophotometry at 280 nm (NanoDrop Lite; Thermo Fisher Scientific). The protein was precipitated with 400 μL of 1 M PCA before being centrifuged at 4°C at 5,000 rpm for 5 min, and the supernatant, containing RNA, was quantified by spectrophotometry. The pellet was resuspended in 1 mL of 2 M PCA, incubated for 60 min on a hot block at 70°C, vortex-mixed, and centrifuged at 5,000 rpm for 5 min, and the DNA in supernatant was quantified (7).
Determination of muscle protein synthesis: plasma enrichment of D2O.
The enrichment of body water was measured as previously described (49). Briefly, 2 μL of 10 M NaOH and 1 μL of acetone were added to 50 μL of plasma and incubated for 24 h at room temperature to exchange deuterium from the water with hydrogen positions on the acetone under high pH. Acetone was extracted into 200 μL of n-heptane, and then 0.5 μL of heptane was injected into the GC-MS. D2O enrichment was calculated from the known standard curve run alongside the plasma samples.
Isolation, hydrolysis, and derivatization of muscle protein fractions.
Thirty to fifty milligrams of TC muscle was homogenized in 10 μL/µg ice-cold homogenization buffer (Tris·HCl, pH 7.4, 50 mM NaF, 10 mM β-glycerophosphate disodium, 1 mM EDTA, 1 mM EGTA, and 1 mM activated Na3VO; Sigma-Aldrich) with cOmplete Protease Inhibitor Cocktail Tablets (Roche Diagnostics, West Sussex, United Kingdom). The supernatant was collected from homogenates after 10 min of rotating followed by 5-min centrifugation at 13,000 g at 4°C. The pellet was further homogenized in 500 μL of mitochondrial extraction buffer (pH 7.5, 20 mM MOPS, 110 mM KCl, and 1 mM EGTA) and centrifuged at 1,000 g at 4°C for 5 min to isolate mitochondrial fraction, which was pelleted by spinning at 11,000 g at 4°C for 15 min. The myofibrillar pellet was separated from insoluble collagen by centrifugation and suspended in 0.3 M NaOH. One molar PCA was used to precipitate myofibrillar proteins. Sarcoplasmic proteins were precipitated from the main homogenate in 1 M PCA and separated by centrifugation. The insoluble collagen was washed with 0.3 M NaOH followed by 70% ethanol and isolated by centrifugation. To release protein-bound amino acids (AAs) from the myofibrillar and sarcoplasmic protein fractions, acid hydrolysis was used (i.e., overnight-incubated in 0.1 M HCl in Dowex H+ resin slurry) before elution from the resin with 2 M NH4OH and evaporated to dryness. AAs were then derivatized as their N-methoxycarbonyl methyl esters. Dried samples were resuspended in 60 μL of distilled water and 32 μL of methanol, and following vortex, 10 μL of pyridine and 8 μL of methyl chloroformate were added. Samples were then vortexed for 30 s and incubated at room temperature for 5 min to react. One hundred microliters of chloroform was used to extract the newly formed N-methoxycarbonyl methyl esters AAs, and a molecular sieve was added for ~20 s to remove any remaining water by size exclusion before being transported to new, clean autosampler vials. Incorporation of deuterium into protein-bound alanine was determined by TSQ GS-MS (Thermo Fisher Scientific) alongside a standard curve of known l-alanine-2,3,3,3-d4 enrichment to validate the measurement accuracy of the instrument (49).
Fractional synthetic rate of protein.
Muscle protein synthesis (MPS) in the myofibrillar and sarcoplasmic fractions were determined from the incorporation of deuterium-labeled alanine into protein, using the enrichment of body water, which was corrected for the mean number of deuterium moieties incorporated per alanine as the precursor. The standard equation used is:
where FSR is fractional synthesis rate, APEAla is deuterium enrichment of protein-bound alanine, APEP is mean precursor enrichment over the time period, and t is the time between the D2O injection and muscle harvesting (49).
Adenosine triphosphate, phosphocreatine, and creatine assay.
As described previously (48), 25 mg of freeze-dried TC muscle was powdered, and connective tissue was removed. Samples were prepared for assay by extraction into PCA. ATP levels were measured by adding hexokinase along with reaction substrates followed by creatine kinase (CK) enzyme to measure phosphocreatine (PCr) concentration. For creatine measurement, pyruvate kinase (PK) was added before CK. Absorption was read before and after the enzyme reaction at 340 nm, and water was used as a blank.
Statistical analysis.
The data are quoted as means ± SD. Comparisons between treated and control groups were made using either unpaired Student’s t test or two-way ANOVA as appropriate. The analyses were conducted using GraphPad Prism 7 software. A P value <0.05 was considered significant.
RESULTS
Fndc5 gene and protein expression in TC muscle and plasma irisin concentration.
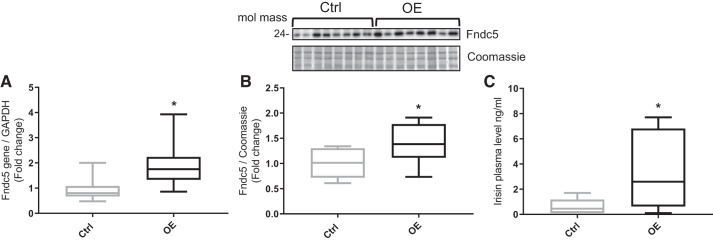
The expression of Fndc5 gene was determined in TC muscles from both groups to determine the success and specificity of the electroporation procedure. A significant increase in Fndc5 expression (2-fold; P = 0.014; Fig. 2A) was observed in the TC muscle of OE animals. Additionally, Fndc5 protein (~24 kDa) was significantly increased (1.4-fold; P = 0.036; Fig. 2B) in the TC muscle of OE animals. Finally, plasma irisin concentration was augmented in OE animals (3.5 ± 1.2 ng/mL) compared with control animals (1.5 ± 0.9 ng/mL; P = 0.049; Fig. 2C).
Fig. 2.
Fibronectin type III domain-containing 5 (Fndc5) expression and irisin levels in control (Ctrl; n = 8) and Fndc5 overexpression (OE; n = 8) animals. A: Fndc5 mRNA in tibialis cranialis (TC) muscle (P = 0.014). B: Fndc5 protein expression in TC (P = 0.036). C: plasma irisin concentration (P = 0.049). Values are means ± SD. Statistical analysis was via unpaired t test. *P < 0.05 vs. Ctrl. Molecular mass is in kilodaltons.
Measurement of glycogen content and factors regulating glycogen levels in muscle.
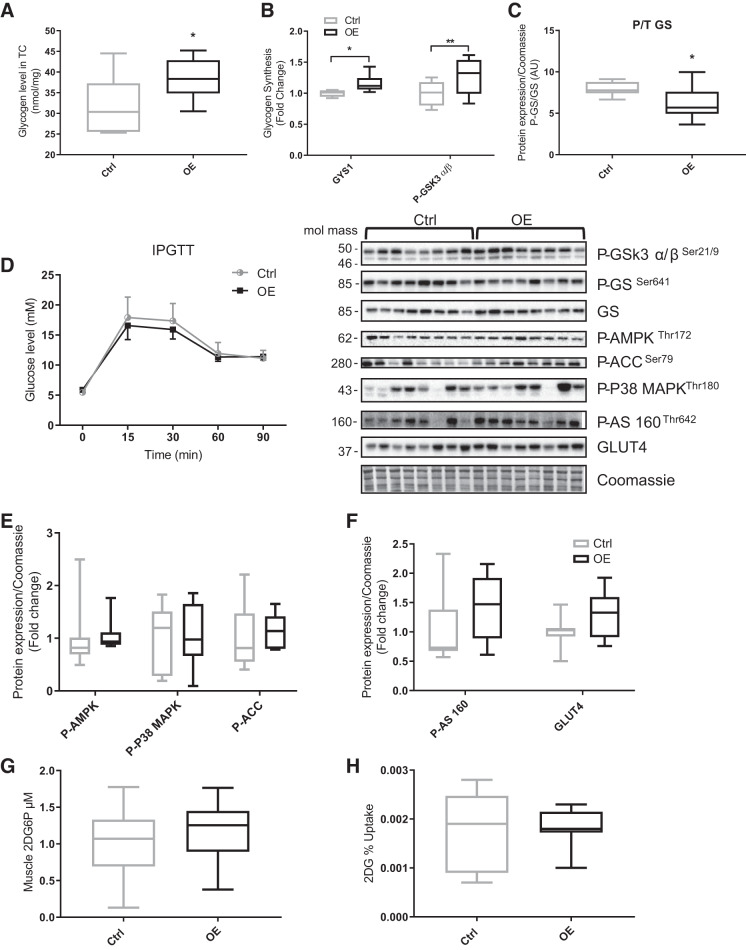
Glycogen content in TC muscle was significantly increased in OE animals (OE 38.5 ± 1.8 vs. Ctrl 31.7 ± 2.4 nmol/mg wet wt; P = 0.04; Fig. 3A), which showed a significant correlation with Fndc5 protein expression (r = 0.5, P = 0.05). The gene expression of glycogen synthase 1 (GYS1) was significantly elevated (20%; P = 0.007) in OE TC muscle, as was the phosphorylation status of GSK3 Ser21/19 protein (30%; P = 0.039; Fig. 3B). The GS activity, estimated by dividing the phospho-GS protein expression by total GS protein expression, was decreased in OE animals (by ~30%; P = 0.039; Fig. 3C). Finally, GYS1 (r = 0.57, P = 0.01), phospho-GSK3 (r = 0.6, P = 0.01), and phospho-/total GS (r = 0.7, P = 0.002) all correlated with Fndc5 gene expression levels.
Fig. 3.
Glycogen content and glucose uptake in control (Ctrl; n = 8) and fibronectin type III domain-containing 5 overexpression (OE; n = 8) animals. A: tibialis cranialis (TC) muscle glycogen content (P = 0.04). B: glycogen synthase 1 (GYS1) gene expression (P = 0.03) and phosphorylated glycogen synthase kinase-3α/β (P-GSK3 α/β) Ser21/9 protein (P = 0.007). C: glycogen synthase (GS) activation (i.e., P-GS divided by GS protein expression; P = 0.048). D: intraperitoneal glucose tolerance test (IPGTT). E: phosphorylated 5-AMP-activated protein kinase (P-AMPK) Thr172, P-P38 MAPK Thr180, and P-acetyl-CoA carboxylase (P-ACC) Ser79. F: P-AS160 Thr642 and glucose transporter 4 (GLUT4) protein expression. G: TC muscle 2-deoxy-d-glucose-6-phosphate (2DG6P). H: 2-deoxy-d-glucose (2DG) fractional uptake (i.e., 2DG6P divided by area under the curve of 2DG plasma). Values are means ± SD. Statistical analysis was via unpaired t test. *P < 0.05, **P < 0.01 vs. Ctrl. Molecular mass is in kilodaltons. AU, arbitrary units; T, total.
Glucose uptake and markers of increased glucose uptake in muscle.
Following an IPGTT, the temporal profile (area under the curve) of plasma glucose was not significantly different between Ctrl and OE animals (Fig. 3D). Circulating plasma 2-DG following intraperitoneal injection was not different between the groups, nor was the accumulation of 2-DG6P in TC muscle (Fig. 3G). Calculation of fractional extraction was also identical in both groups, suggesting glucose uptake was not different (Fig. 3H). In support of this, there was no difference in the gene expression of GLUT4 or hexokinase 2. Finally, no significant change was seen in GLUT4 protein expression or in its upregulators (Fig. 3F).
Mitochondrial biogenesis in TC muscle.
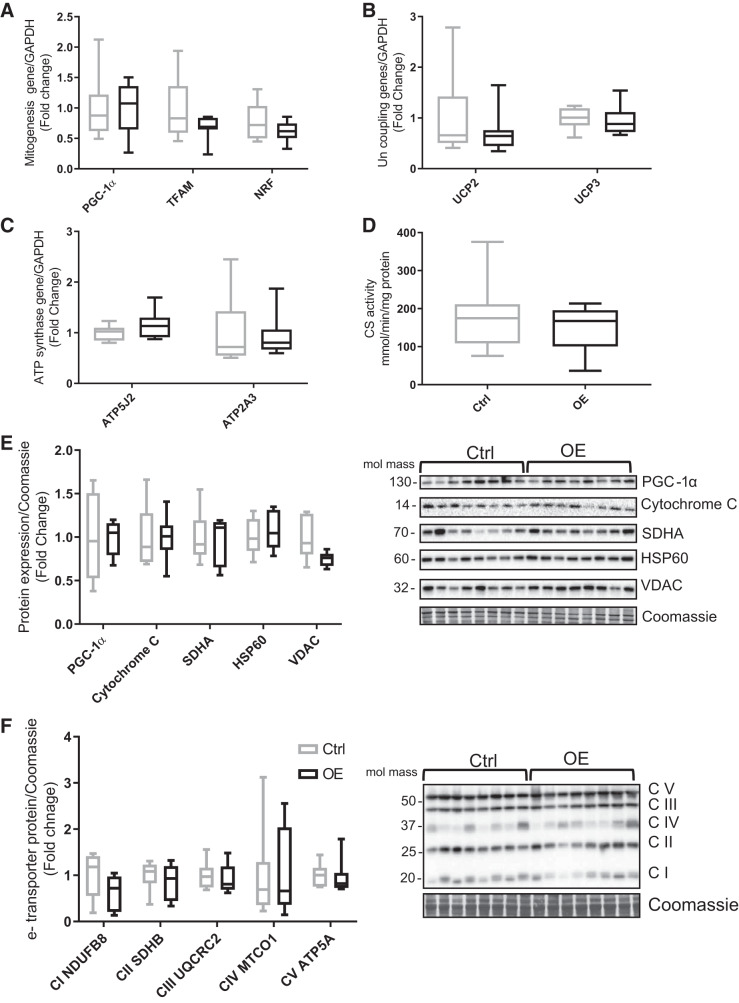
Fndc5 overexpression had no significant effect on the gene expression of PGC-1α nor its downstream regulatory genes, nuclear respiratory factor (NRF) and mitochondrial transcription factor A (TFAM; Fig. 4A). There were also no significant changes in muscle UCP2 and UCP3 nor ATP synthesis genes (ATP5J2 and ATP2A3; Fig. 4, B and C). The expression of PGC-1α protein and mitochondrial protein markers such as cytochrome c, SDHA, HSP60, and VDAC were not different between animals (Fig. 4E). In addition, the electron transport chain complexes (I–IV) were also found to be unaffected by Fndc5 overexpression (Fig. 4F). Similarly, there was no significant difference in mitochondrial density, assessed by CS activity (Fig. 4D).
Fig. 4.
Markers of mitochondrial biogenesis and density in tibialis cranialis muscle. A: mitochondrial biogenesis regulatory genes: peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), mitochondrial transcription factor A (TFAM), and nuclear respiratory factors (NRF). B: gene expression of uncoupling proteins 2 and 3 (UCP2/3). C: ATP synthase genes ATP5J2 and ATP2A3. D: mitochondrial density represented by citrate synthase (CS) activity. E: protein levels of mitochondrial biogenesis markers: PGC-1α, cytochrome c, succinate dehydrogenase enzyme A (SDHA), heat shock protein 60 (HSP60), and voltage-dependent anion channel (VDAC). F: electron (e-) transporter complexes (C) of mitochondria: CI NADH dehydrogenase [ubiquinone] 1β subunit 8 (NDUFB8), CII succinate dehydrogenase subunit B (SDHB), CIII ubiquinone cytochrome c reductase complex (UQCRC2), CIV mitochondria encoded cytochrome c oxidase (MTCO1), and CV mitochondria membrane ATP synthase 5A (ATP5A). Values are means ± SD. Statistical analysis was via unpaired t test. No statistically significant differences were observed. Molecular mass is in kilodaltons. Ctrl, control group; OE, overexpressed group.
ATP and PCr level on TC muscle.
No significant differences in ATP or PCr were observed between the two groups, even when normalizing to total creatine, nor in the ratio of PCr to ATP (Table 2).
Table 2.
ATP, phosphocreatine, and total creatine levels in tibialis cranialis muscle
| Ctrl | OE | Units | P Value | |
|---|---|---|---|---|
| TCr | 97.5 ± 8.1 | 102.2 ± 15.7 | mM/mg dry muscle wt | 0.49 |
| ATP | 19.9 ± 3.6 | 18.7 ± 4.8 | mM/mg dry muscle wt | 0.59 |
| PCr | 32.9 ± 10.1 | 32.2 ± 17.4 | mM/mg dry muscle wt | 0.91 |
| ATP-to-TCr ratio | 0.20 ± 0.03 | 0.18 ± 0.02 | Arbitrary unit | 0.19 |
| PCr-to-TCr ratio | 0.33 ± 0.1 | 0.30 ± 0.1 | Arbitrary unit | 0.57 |
| PCr-to-ATP ratio | 1.68 ± 0.5 | 1.62 ± 0.7 | Arbitrary unit | 0.87 |
Values are means ± SD. Statistical analysis was via unpaired t test. No statistically significant differences were observed. Ctrl, control group; OE, overexpressed group; PCr, phosphocreatine; TCr, total creatine.
Rates of integrated myofibrillar and sarcoplasmic protein synthesis and protein, RNA, and DNA content in TC muscle.
Integrated protein synthesis of myofibrillar (15.5 ± 2.0%/day in Ctrl, 14.4 ± 1.7%/day in OE) and sarcoplasmic (15.7 ± 1.4%/day in Ctrl, 13.9 ± 1.4%/day in OE) fractions from TC muscle was not different between the groups (Fig. 5B). In line with this, no differences in alkali-soluble protein or nucleic acid content, ASP (87.7 ± 4.9 in Ctrl, 84.2 ± 5.4 in OE), DNA (2.6 ± 0.1 in Ctrl, 2.5 ± 0.1 in OE), and RNA (15.02 ± 0.2 in Ctrl, 15.4 ± 0.2 in OE), all in µg/mg wet wt, were observed. In addition, there were no differences in cell size, estimated from the ratio of total ASP to DNA (33.8 ± 1.31 in Ctrl, 33.5 ± 2.0 in OE), ribosomal capacity from RNA to DNA (0.76 ± 0.04 in Ctrl, 0.75 ± 0.05 in OE), or measures of ribosomal efficiency from ratio of RNA to ASP (22.9 ± 1.8 in Ctrl, 23.3 ± 2.5 in OE).
Fig. 5.
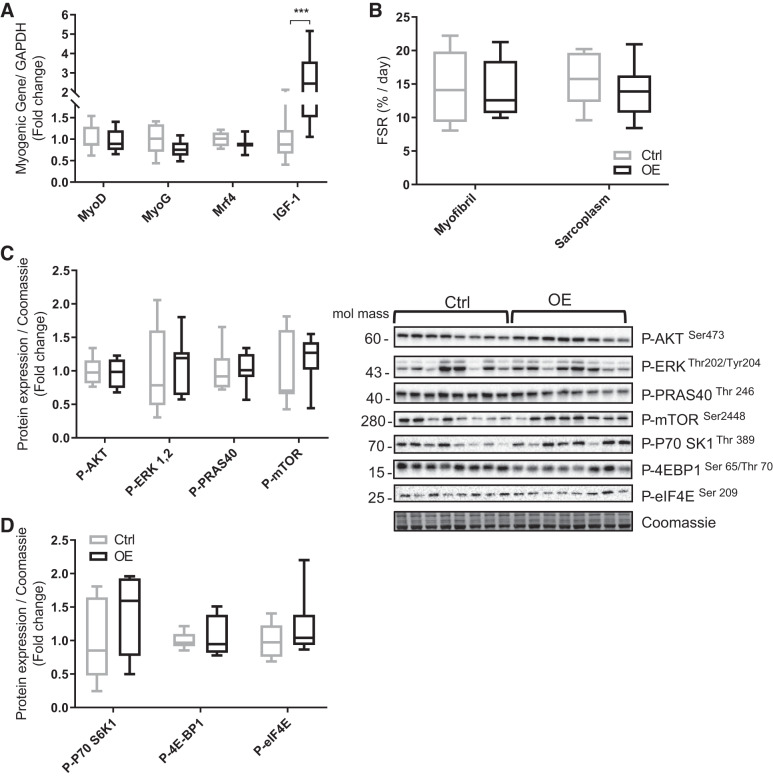
A: gene expression of muscle regulatory growth factors and insulin-like growth factor-1 (IGF-1; P = 0.0002) in tibialis cranialis muscle. B: fractional synthesis rate (FSR) in myofibrillar and sarcoplasmic muscle fractions. C and D: protein expression of proteins involved in anabolic signaling: phosphorylated (P-) protein kinase B (Akt) Ser473, P-ERK Thr202/Tyr204, P-proline-rich Akt substrate of 40 kDa (P-PRAS40 Thr246), P-mechanistic target of rapamycin (P-mTOR Ser2448), P-ribosomal protein S6 kinase 1 (P-P70 S6K1 Thr389), P-4E-binding protein 1 (P-4EBP1 Ser65/Thr70), and P-eukaryotic initiation factor 4E (P-eIF4E Ser209). Values are means ± SD. Statistical analysis was via unpaired t test. ***P < 0.001 vs. control group (Ctrl). Molecular mass is in kilodaltons. MyoG, myogenin; OE, overexpressed group.
Markers of myogenesis and anabolic cell signaling.
The gene expression of myogenic regulatory factors such as myogenic differentiation (MyoD), myogenin (MyoG), and myogenic regulatory factor 4 (Mrf4) were not significantly altered by Fndc5 OE, whereas we observed a significant increase (2.6-fold change) in IGF-1 in the OE animals (P = 0.0002; Fig. 5A). In addition, no significant differences were detected in the Akt-mTOR pathway or their downstream signaling proteins, e.g., PRAS40, phosphorylated ribosomal protein S6 kinase 1 (p70 S6K1), 4E-BP1, and eukaryotic initiation factor 4E (Fig. 5, C and D).
Markers of muscle protein breakdown.
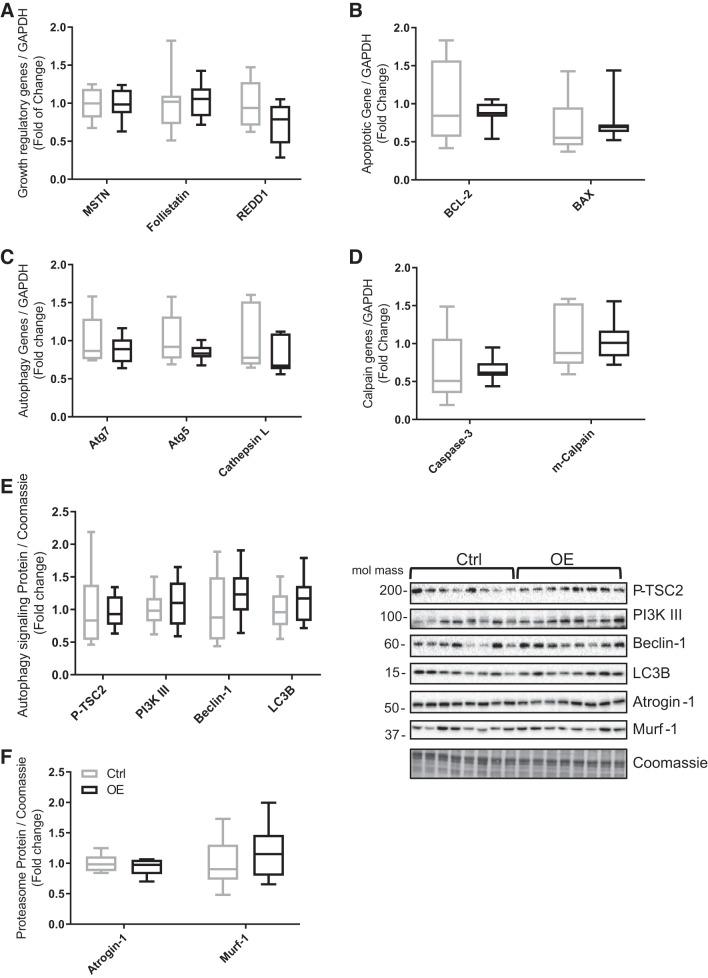
Fndc5 overexpression had limited impact on markers of muscle catabolism. Gene expression of negative regulators of muscle mass such as myostatin, follistatin, and regulated in development and DNA damage response 1, apoptotic factors, e.g., B-cell lymphoma 2 (BCL-2), BCL-2-associated X (BAX) proapoptotic factor, or autophagy factors such as Atg5 and Atg7 and calpain genes like cysteine-aspartic acid protease (caspase-3) and m-calpain was not different between control and OE muscle (Fig. 6, A–D). Finally, the expression of proteins involved in muscle protein catabolism were not different between animals. There were no changes in the phosphorylation of proteins in the autophagy signaling pathway, i.e., tumor suppressor 2, or protein expression of beclin-1, LC3B, atrogin-1, and MuRF1 (Fig. 6, E and F).
Fig. 6.
Gene expression levels in tibialis cranialis muscle of the following. A: negative growth regulatory factors: myostatin (MSTN), follistatin, and regulated in development and DNA damage response 1 (REDD1). B: apoptotic factors: B-cell lymphoma 2 (BCL-2) apoptosis suppressor and BCL-2-associated X (BAX) proapoptotic factor. C: autophagy-related genes: Atg7, Atg5, and cathepsin L. D: calpain factors: cysteine-aspartic acid protease (caspase-3) and m-calpain. E and F: protein expression of autophagy signaling pathway components: phosphorylated tuberin or tumor suppressor 2 (P-TSC2), phosphatidylinositol 3-kinase class III (PI3K III), beclin-1, autophagy marker light chain 3 (LC3B), atrogin-1, and muscle RING-finger protein-1 (Murf-1). Values are means ± SD. Statistical analysis was via unpaired t test. No statistically significant differences were observed. Molecular mass is in kilodaltons. Ctrl, control group; OE, overexpressed group.
Markers of mitochondrial biogenesis and fat browning in subcutaneous fat.
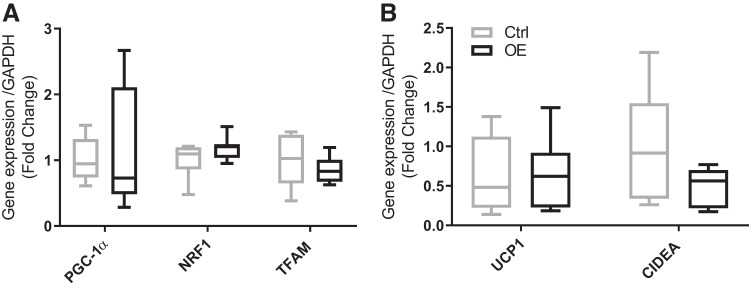
There were no significant differences observed in genes involved in mitochondrial biogenesis and markers of fat browning, e.g., PGC-1α, NRF, or TFAM. In addition, there were no significant differences in UCP1 or cell death activator (CIDEA; Fig. 7).
Fig. 7.
Markers of mitochondrial biogenesis in subcutaneous fat. A: peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), nuclear respiratory factors (NRF1), and mitochondrial transcription factor A (TFAM). B: uncoupling protein 1 (UCP1) and cell death activator A (CIDEA). Values are means ± SD. Statistical analysis was via unpaired t test. No statistically significant differences were observed. Ctrl, control group; OE, overexpressed group.
DISCUSSION
Our model of overexpression increased Fndc5 gene expression (~2-fold) to a similar extent reported in humans (5, 32, 35) and mice (5) following an exercise intervention, i.e., a physiologically relevant increase. For example, 8 wk of treadmill exercise (1) increased Fndc5 expression in mouse skeletal muscle ~1.6-fold. In addition, circulating plasma irisin (3.5 ng/mL) in the OE group was similar to concentrations measured (by mass spectrometry) following an exercise intervention (3.6–4.3 ng/mL; Ref. 20). Although others have reported irisin concentrations ranging from 0.01 to 2,000 ng/mL in human (36) and 6 to 1,900 ng/mL in rats (27, 41, 46, 53), these are likely the result of technical issues surrounding previously used ELISA kits (36) and perhaps the timing of the irisin measurement (41). In sum, our model achieved physiologically relevant increases in Fndc5/irisin, justifying our aims to investigate underlying changes in muscle and adipose tissue.
We report a significant increase in glycogen content (~20%) in skeletal muscle 10 days following Fndc5 OE. Treatment with recombinant irisin has been shown to increase glycogen storage in livers of diabetic mice (26). Glycogen content is regulated by glycogen synthesis or glycogenolysis (37). The expression of GYS1 indicates an increase in glycogen synthesis in our OE model, as in Huh et al. (18). Despite the complexity of the glycogen synthesis signaling pathway, GS is the central regulator and is deactivated by phosphorylation at Ser641. Therefore, a decrease in the ratio of phospho-GS to GS suggests GS is activated, as observed in our model, and also with irisin treatment in human hepatocellular carcinoma cells (26). GSK3α/β downregulates GS activation; however, phosphorylation at Ser21/9 (phospho-GSK3α/β) inhibits this effect (24), which falls in line with our observations and also those of Liu et al. (26), thereby supporting the notion of an increase in GS activity in our OE model (or with irisin treatment). The correlations between glycogen content and regulatory factors involved in the synthesis of muscle glycogen (GYS1, phospho-GSK3, and GS activity) with Fndc5 expression further strengthen the notion of a role for Fndc5 in glycogen storage regulation. However, we observed no sign of insulin (Akt/PI3K) signaling as Liu et al. (26) reported. Crucially, the accumulation of 2-DG6P was also identical in both groups, indicating that glucose uptake was similar during the IPGTT in both groups, at least during the 90-min IPGTT, suggesting increased glucose uptake was not responsible for the increased TC muscle glycogen content. Similarly, systemic glucose disposal/handling was also similar in both groups, in line with other studies where an improvement in systemic glucose disposal has been observed only in diabetic animals with both irisin recombinant treatment and Fndc5 viral induction overexpression (5, 26, 50). This may indicate that Fndc5/irisin (within the physiological range) has no or at least limited impacts on muscle glucose uptake. Nonetheless, it cannot be discounted that increases in glycogen may point to some effects in relation to substrate metabolism.
Our next area of investigation is related to mitochondrial biogenesis; it was previously reported that recombinant irisin treatment (5 nM) of C2C12 cells induced expression of PGC-1α, NRF, TFAM, and UCP3 after 24-h increasing mitochondrial content (47). In contrast, irisin treatment (10 and 50 nM) in human primary skeletal muscle cells did not impact PGC-1α expression (17). Similarly, in our study, we observed no difference in PGC-1α or any of its downstream targets, with no difference in CS activity (mitochondrial content proxy; Ref. 16) or electron transport chain complexes. Our data reveal that local Fndc5 overexpression in skeletal muscle, as in our model, or systemically as in Boström et al. (5), with an increase in circulating irisin level, has no impact on muscle mitochondrial biogenesis using in vivo models of Fndc5 induction.
Another area purported to be under Fndc5/irisin’s regulation is the control of muscle mass. For instance, irisin, was reported to increase muscle weight, strength, and cross-sectional area in mice (38). On a molecular level, irisin treatment of primary human skeletal muscle cells was shown to increase mRNA expression of IGF-1, a growth factor that stimulates cellular proliferation and hypertrophy (15) while decreasing mRNA expression of myostatin, a negative regulator of muscle growth (17). However, despite our observation of increased IGF-1 mRNA with Fndc5 OE, there was no effect on myostatin nor on other growth myogenic regulatory factors, e.g., MyoD and myogenin. The lack of an irisin effect on MyoD and myogenin was also reported in vitro, where irisin treatment increased myotube size but not the expression of myogenic factors (38). Similarly, our end points of MPS and muscle protein and nucleic acid content do not infer a role for Fndc5/irisin; integrated MPS, measured over the 10 days following electroporation, was identical in control and OE TC muscle with similar ratios of protein to DNA, RNA to protein, and RNA to DNA between groups. Despite reports of irisin treatment having an effect on both ERK1/2 (17, 38) and Akt (38) signaling, we did not observe this. Similarly, although supraphysiological recombinant irisin (1,000 ng/mL) treatment for 48 h decreased expression of muscle protein breakdown-related factors atrogin-1 and MuRF1 in C2C12 cells (38), the physiological levels of Fndc5/irisin expression, used in this study, had limited impact on markers of muscle proteolysis. Overall, these data do not support the notion of anabolic properties of Fndc5/irisin when induced at physiological concentrations.
Finally, given the proposed endocrine function of irisin in browning white adipose tissue (WAT), we investigated the effects of muscle hindlimb OE of Fndc5 and increases in circulating irisin in relation to WAT biomarkers of browning. Boström et al. (5) reported that increased circulating irisin after adenoviral injection of Fndc5 gene enhanced UCP1 and CIDEA in subcutaneous adipose tissue with significant effects also seen on PGC-1α; we saw no evidence of this in our model. Increases in UCP1 were also reported in another in vivo study with evidence of enhancing lipolysis (51); again our data do not support this. However, both studies used systemic induction of Fndc5, which may raise irisin levels beyond those found physiologically.
Nevertheless, given the relatively small number, there is the possibility that natural variation in gene and protein expression in outbred animals (14) may have led to a randomization artifact, which may have impacted on our data.
In conclusion, dual hindlimb OE of Fndc5 in muscle led to an increase in circulating irisin (similar to that after exercise training), and we saw no evidence of effects on mitochondrial biogenesis or protein anabolism. Moderate effects noted on glycogen content could not be explained by acute insulin-stimulated glucose uptake. In addition, short-term induction of plasma irisin (~10 days) had no significant impact on browning biomarkers in WAT. Our data collectively indicate that short-term physiological increases in Fndc5/irisin are insufficient to induce gross metabolic effects, but this does not preclude a role for irisin in combination with other exercise factors/myokines or in relation to more prolonged OE approaches.
GRANTS
This research was supported by the Medical Research Council Versus Arthritis Centre for Musculoskeletal Ageing Research (Grants MR/P021220/1 and MR/R502364/1) and National Institute for Health Research Nottingham Biomedical Research Centre. W. Farrash was supported by a government PhD studentship grant of Umm Al-Qura University, Makkah, Saudi Arabia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.E.P., D.J.W., K.S., M.C., and P.J.A. conceived and designed research; W.F., M.B., H.C., J.C., D.J.W., D.C.-T., P.L.G., and M.C. performed experiments; W.F., M.B., H.C., J.C., D.J.W., D.C.-T., M.C., and P.J.A. analyzed data; W.F. and M.C. interpreted results of experiments; W.F. prepared figures; W.F. drafted manuscript; B.E.P., K.S., and P.J.A. edited and revised manuscript; W.F., M.B., H.C., B.E.P., J.C., D.J.W., D.C.-T., P.L.G., K.S., M.C., and P.J.A. approved final version of manuscript.
REFERENCES
- 1.Abedpoor N, Taghian F, Ghaedi K, Niktab I, Safaeinejad Z, Rabiee F, Tanhaei S, Nasr-Esfahani MH. PPARγ/Pgc-1α-Fndc5 pathway up-regulation in gastrocnemius and heart muscle of exercised, branched chain amino acid diet fed mice. Nutr Metab (Lond) 15: 59, 2018. doi: 10.1186/s12986-018-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker DJ, Constantin-Teodosiu D, Jones SW, Timmons JA, Greenhaff PL. Chronic treatment with the β2-adrenoceptor agonist prodrug BRL-47672 impairs rat skeletal muscle function by inducing a comprehensive shift to a faster muscle phenotype. J Pharmacol Exp Ther 319: 439–446, 2006. doi: 10.1124/jpet.106.107045. [DOI] [PubMed] [Google Scholar]
- 3.Beltran Valls MR, Wilkinson DJ, Narici MV, Smith K, Phillips BE, Caporossi D, Atherton PJ. Protein carbonylation and heat shock proteins in human skeletal muscle: relationships to age and sarcopenia. J Gerontol A Biol Sci Med Sci 70: 174–181, 2015. doi: 10.1093/gerona/glu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blüher S, Panagiotou G, Petroff D, Markert J, Wagner A, Klemm T, Filippaios A, Keller A, Mantzoros CS. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity (Silver Spring) 22: 1701–1708, 2014. doi: 10.1002/oby.20739. [DOI] [PubMed] [Google Scholar]
- 5.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468, 2012. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594: 7399–7417, 2016. doi: 10.1113/JP272857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chee C, Shannon CE, Burns A, Selby AL, Wilkinson D, Smith K, Greenhaff PL, Stephens FB. Relative contribution of intramyocellular lipid to whole-body fat oxidation is reduced with age but subsarcolemmal lipid accumulation and insulin resistance are only associated with overweight individuals. Diabetes 65: 840–850, 2016. doi: 10.2337/db15-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi YK, Kim MK, Bae KH, Seo HA, Jeong JY, Lee WK, Kim JG, Lee IK, Park KG. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract 100: 96–101, 2013. doi: 10.1016/j.diabres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW. Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol 21: 215–228, 2007. doi: 10.1210/me.2006-0154. [DOI] [PubMed] [Google Scholar]
- 11.Cuthbertson DJ, Babraj JA, Mustard KJ, Towler MC, Green KA, Wackerhage H, Leese GP, Baar K, Thomason-Hughes M, Sutherland C, Hardie DG, Rennie MJ. 5-Aminoimidazole-4-carboxamide 1-β-d-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes 56: 2078–2084, 2007. doi: 10.2337/db06-1716. [DOI] [PubMed] [Google Scholar]
- 12.Czarkowska-Paczek B, Zendzian-Piotrowska M, Gala K, Sobol M, Paczek L. One session of exercise or endurance training does not influence serum levels of irisin in rats. J Physiol Pharmacol 65: 449–454, 2014. [PubMed] [Google Scholar]
- 13.Ellefsen S, Vikmoen O, Slettaløkken G, Whist JE, Nygaard H, Hollan I, Rauk I, Vegge G, Strand TA, Raastad T, Rønnestad BR. Irisin and FNDC5: effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur J Appl Physiol 114: 1875–1888, 2014. doi: 10.1007/s00421-014-2922-x. [DOI] [PubMed] [Google Scholar]
- 14.Festing MF. Warning: the use of heterogeneous mice may seriously damage your research. Neurobiol Aging 20: 237–244, 1999. doi: 10.1016/s0197-4580(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 15.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17: 481–517, 1996. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 16.Galgani JE, Johannsen NM, Bajpeyi S, Costford SR, Zhang Z, Gupta AK, Ravussin E. Role of skeletal muscle mitochondrial density on exercise-stimulated lipid oxidation. Obesity (Silver Spring) 20: 1387–1393, 2012. doi: 10.1038/oby.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes 38: 1538–1544, 2014. doi: 10.1038/ijo.2014.42. [DOI] [PubMed] [Google Scholar]
- 18.Huh JY, Mougios V, Kabasakalis A, Fatouros I, Siopi A, Douroudos II, Filippaios A, Panagiotou G, Park KH, Mantzoros CS. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab 99: E2154–E2161, 2014. doi: 10.1210/jc.2014-1437. [DOI] [PubMed] [Google Scholar]
- 19.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61: 1725–1738, 2012. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab 22: 734–740, 2015. doi: 10.1016/j.cmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 15: 2748–2750, 2001. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, So B, Choi M, Kang D, Song W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp Gerontol 70: 11–17, 2015. doi: 10.1016/j.exger.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, Srbecky M, Imrich R, Kyselovicova O, Belan V, Jelok I, Wolfrum C, Klimes I, Krssak M, Zemkova E, Gasperikova D, Ukropec J, Ukropcova B. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol 592: 1091–1107, 2014. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Kim MS. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract 77, Suppl 1: S49–S57, 2007. doi: 10.1016/j.diabres.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Liu JJ, Wong MD, Toy WC, Tan CS, Liu S, Ng XW, Tavintharan S, Sum CF, Lim SC. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications 27: 365–369, 2013. doi: 10.1016/j.jdiacomp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, Chen Q, Li YH, Wang JJ, Kang YM, Zhu GQ. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci (Lond) 129: 839–850, 2015. doi: 10.1042/CS20150009. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Li H, Shen SW, Shen ZH, Xu M, Yang CJ, Li F, Feng YB, Yun JT, Wang L, Qi HJ. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids Health Dis 15: 93, 2016. doi: 10.1186/s12944-016-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto-Mikami E, Sato K, Kurihara T, Hasegawa N, Fujie S, Fujita S, Sanada K, Hamaoka T, Tabata I, Iemitsu M. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS One 10: e0120354, 2015. doi: 10.1371/journal.pone.0120354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, Ricart W, Fernández-Real JM. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab 98: E769–E778, 2013. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 30.Morton TL, Galior K, McGrath C, Wu X, Uzer G, Uzer GB, Sen B, Xie Z, Tyson D, Rubin J, Styner M. Exercise increases and browns muscle lipid in high-fat diet-fed mice. Front Endocrinol (Lausanne) 7: 80, 2016. doi: 10.3389/fendo.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakhuda A, Josse AR, Gburcik V, Crossland H, Raymond F, Metairon S, Good L, Atherton PJ, Phillips SM, Timmons JA. Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss. Am J Clin Nutr 104: 557–565, 2016. doi: 10.3945/ajcn.116.132563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 281: 739–749, 2014. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 33.Pardo M, Crujeiras AB, Amil M, Aguera Z, Jiménez-Murcia S, Baños R, Botella C, de la Torre R, Estivill X, Fagundo AB, Fernández-Real JM, Fernández-García JC, Fruhbeck G, Gómez-Ambrosi J, Rodríguez R, Tinahones FJ, Fernández-Aranda F, Casanueva FF. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int J Endocrinol 2014: 857270, 2014. doi: 10.1155/2014/857270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedersen BK. Muscles and their myokines. J Exp Biol 214: 337–346, 2011. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 35.Pekkala S, Wiklund PK, Hulmi JJ, Ahtiainen JP, Horttanainen M, Pöllänen E, Mäkelä KA, Kainulainen H, Häkkinen K, Nyman K, Alén M, Herzig KH, Cheng S. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol 591: 5393–5400, 2013. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perakakis N, Triantafyllou GA, Fernández-Real JM, Huh JY, Park KH, Seufert J, Mantzoros CS. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol 13: 324–337, 2017. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prats C, Gómez-Cabello A, Hansen AV. Intracellular compartmentalization of skeletal muscle glycogen metabolism and insulin signalling. Exp Physiol 96: 385–390, 2011. doi: 10.1113/expphysiol.2010.052860. [DOI] [PubMed] [Google Scholar]
- 38.Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, Sharma M, Kambadur R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun 8: 1104, 2017. doi: 10.1038/s41467-017-01131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocha-Rodrigues S, Rodríguez A, Gouveia AM, Gonçalves IO, Becerril S, Ramírez B, Beleza J, Frühbeck G, Ascensão A, Magalhães J. Effects of physical exercise on myokines expression and brown adipose-like phenotype modulation in rats fed a high-fat diet. Life Sci 165: 100–108, 2016. doi: 10.1016/j.lfs.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Roustit MM, Vaughan JM, Jamieson PM, Cleasby ME. Urocortin 3 activates AMPK and AKT pathways and enhances glucose disposal in rat skeletal muscle. J Endocrinol 223: 143–154, 2014. doi: 10.1530/JOE-14-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samy DM, Ismail CA, Nassra RA. Circulating irisin concentrations in rat models of thyroid dysfunction – effect of exercise. Metabolism 64: 804–813, 2015. doi: 10.1016/j.metabol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, Klapp BF. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity – correlation with body mass index. Peptides 39: 125–130, 2013. doi: 10.1016/j.peptides.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature 488: E9–E10, 2012. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- 45.Tsuchiya Y, Ando D, Goto K, Kiuchi M, Yamakita M, Koyama K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J Exp Med 233: 135–140, 2014. doi: 10.1620/tjem.233.135. [DOI] [PubMed] [Google Scholar]
- 46.Varela-Rodríguez BM, Pena-Bello L, Juiz-Valiña P, Vidal-Bretal B, Cordido F, Sangiao-Alvarellos S. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci Rep 6: 29898, 2016. doi: 10.1038/srep29898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaughan RA, Gannon NP, Barberena MA, Garcia-Smith R, Bisoffi M, Mermier CM, Conn CA, Trujillo KA. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes Metab 16: 711–718, 2014. doi: 10.1111/dom.12268. [DOI] [PubMed] [Google Scholar]
- 48.Wibom R, Hultman E, Johansson M, Matherei K, Constantin-Teodosiu D, Schantz PG. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J Appl Physiol (1985) 73: 2004–2010, 1992. doi: 10.1152/jappl.1992.73.5.2004. [DOI] [PubMed] [Google Scholar]
- 49.Wilkinson DJ, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WK, Szewczyk NJ, Greenhaff PL, Atherton PJ, Smith K. A validation of the application of D2O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab 306: E571–E579, 2014. doi: 10.1152/ajpendo.00650.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xin C, Liu J, Zhang J, Zhu D, Wang H, Xiong L, Lee Y, Ye J, Lian K, Xu C, Zhang L, Wang Q, Liu Y, Tao L. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes 40: 443–451, 2016. doi: 10.1038/ijo.2015.199. [DOI] [PubMed] [Google Scholar]
- 51.Xiong XQ, Chen D, Sun HJ, Ding L, Wang JJ, Chen Q, Li YH, Zhou YB, Han Y, Zhang F, Gao XY, Kang YM, Zhu GQ. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta 1852: 1867–1875, 2015. doi: 10.1016/j.bbadis.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 52.Yang XQ, Yuan H, Li J, Fan JJ, Jia SH, Kou XJ, Chen N. Swimming intervention mitigates HFD-induced obesity of rats through PGC-1α-irisin pathway. Eur Rev Med Pharmacol Sci 20: 2123–2130, 2016. [PubMed] [Google Scholar]
- 53.Yang Z, Chen X, Chen Y, Zhao Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int J Clin Exp Pathol 8: 6490–6497, 2015. [PMC free article] [PubMed] [Google Scholar]