Abstract
Circadian rhythms are endogenous and entrainable daily patterns of physiology and behavior. Molecular mechanisms underlie circadian rhythms, characterized by an ~24-h pattern of gene expression of core clock genes. Although it has long been known that breathing exhibits circadian rhythms, little is known concerning clock gene expression in any element of the neuromuscular system controlling breathing. Furthermore, we know little concerning gene expression necessary for specific respiratory functions, such as phrenic motor plasticity. Thus, we tested the hypotheses that transcripts for clock genes (Bmal1, Clock, Per1, and Per2) and molecules necessary for phrenic motor plasticity (Htr2a, Htr2b, Bdnf, and Ntrk2) oscillate in regions critical for phrenic/diaphragm motor function via RT-PCR. Tissues were collected from male Sprague-Dawley rats entrained to a 12-h light-dark cycle at 4 zeitgeber times (ZT; n = 8 rats/group): ZT5, ZT11, ZT17, and ZT23; ZT0 = lights on. Here, we demonstrate that 1) circadian clock genes (Bmal1, Clock, Per1, and Per2) oscillate in regions critical for phrenic/diaphragm function, including the caudal medulla, ventral C3–C5 cervical spinal cord, and diaphragm; 2) the clock protein BMAL1 is localized within CtB-labeled phrenic motor neurons; 3) genes necessary for intermittent hypoxia-induced phrenic/diaphragm motor plasticity (Htr2b and Bdnf) oscillate in the caudal medulla and ventral C3–C5 spinal cord; and 4) there is higher intensity of immunofluorescent BDNF protein within phrenic motor neurons at ZT23 compared with ZT11 (n = 11 rats/group). These results suggest local circadian clocks exist in the phrenic motor system and confirm the potential for local circadian regulation of neuroplasticity and other elements of the neural network controlling breathing.
Keywords: BDNF, circadian rhythm, control of breathing, motor neuron, plasticity, serotonin, spinal cord
INTRODUCTION
Circadian rhythms are biological cycles driven by an endogenous oscillator with a period of approximately 1 day (5, 31). Circadian rhythms emerge from self-sustaining oscillations of autoregulatory transcription-translation feedback loops of circadian clock genes (Bmal1, Clock, Per1, Per2, Cry1, Cry2, Npas2, Per3, Nr1d1, and Nr1d2) known as the “molecular clock” (30, 68). Circadian rhythms were originally thought to be driven strictly by a small subpopulation of neurons in the hypothalamus, the suprachiasmatic nucleus (SCN), because surgical SCN removal undermines daily rhythms in motor behavior (e.g., wheel running) (19, 62). Advanced genetic models led to the discovery of endogenous 24-h clocks in peripheral tissues that oscillate independent of the SCN (74). Moreover, although central and peripheral circadian oscillators utilize the same autoregulatory gene network (3, 47), entrainment cues (zeitgebers) and regulated physiological functions (clock outputs) are tissue specific. Despite its functional importance for life, rhythmic clock gene transcription has not been identified in any element of the neuromuscular system controlling breathing.
Physiological measures of respiratory function, including pulmonary ventilation, tidal volume, and frequency, follow circadian patterns independent of sleep state and activity level (49, 58, 59, 61, 63). Circadian clock genes oscillate in respiratory-related tissues, including the lungs (65) and airways (9), suggesting a role for clock genes in lung biology. However, little is known concerning clock gene expression in the respiratory neural networks and muscles responsible for breathing. Clock gene regulation of the respiratory control network is of considerable importance since it may help match breathing to circadian changes in metabolic rate (2, 55, 56). Thus we hypothesized that expression of core clock genes (Bmal1, Clock, Per1, and Per2) exhibits a daily rhythm in regions of interest to the neural control of breathing, including the caudal medulla, the ventral portion of the C3–C5 spinal cord associated with the phrenic motor nucleus, and the diaphragm muscle.
In addition to baseline breathing, the capacity for the neural network controlling breathing to express neuroplasticity may also exhibit a daily rhythm. The circadian clock regulates many forms of neuroplasticity including hippocampal long-term potentiation (13) and sensorimotor long-term sensitization in the gastropod mollusk Aplysia (40). The most widely studied model of respiratory neuroplasticity is long-term facilitation of phrenic motor output (pLTF) following acute intermittent hypoxia (AIH) (18, 45, 46). Brief episodes of moderate hypoxia ( = 35–45 mmHg) initiate serotonin-dependent neuroplasticity in the phrenic motor circuit, manifested as a sustained increase in the activity of spinal phrenic motor neurons and diaphragm muscle (6, 26, 32, 70). AIH-induced pLTF requires spinal serotonin receptor activation (8, 67), new synthesis of brain-derived neurotrophic factor (BDNF; Ref. 7), and activation of the high-affinity BDNF receptor TrkB (16). Since circadian rhythms regulate neuroplasticity in other neural systems (24, 28, 36), and cyclic gene regulation contributes to this effect (40), we tested the hypothesis that mRNAs for molecules necessary for AIH-induced pLTF (serotonin receptors, BDNF, and TrkB) exhibit daily rhythms in the caudal medulla and ventral C3–C5 spinal cord.
Daily rhythms in AIH-induced respiratory motor plasticity are of considerable interest since 1) AIH is emerging as a promising therapeutic modality to increase respiratory motor output in situations where breathing is compromised (15, 29, 43) and 2) bidirectional interactions between the circadian clock and hypoxia have been reported (1, 2, 20, 41, 50, 73), although these interactions are far from resolved. Notably, exposure to chronic/sustained hypoxia elicits time-of-day-dependent and tissue-specific transcriptional responses (41), can induce tissue-specific phase shifts (41), and dampens the daily rhythm in ventilation (50), whereas moderate levels of hypoxia may be beneficial and have been reported to accelerate the adaptation time to a new lighting schedule (1).
The goals of this study were to the test the hypotheses that 1) mRNAs for clock genes (Bmal1, Clock, Per1, and Per2) exhibit a daily rhythm across the phrenic motor circuit (caudal medulla, ventral C3–C5 spinal cord, and diaphragm muscle); 2) mRNAs for relevant serotonin receptors (5-HT2A and 5-HT2B), BDNF, and TrkB exhibit a daily rhythm in the caudal medulla and ventral C3–C5 spinal cord; 3) BMAL1 clock protein expression within phrenic motor neurons changes across the light-dark cycle; and 4) BDNF protein expression within phrenic motor neurons changes across the light-dark cycle.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (300–383 g) maintained under a 12-h light/dark cycle [zeitgeber time (ZT): ZT0 = lights on (6 AM); ZT12 lights off (6 PM)] for 14 days. Rats were provided access to food and water ad libitum. One group (n = 32) was used for RNA analysis, and another (n = 26) for protein analysis. All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee (IACUC no. 201808817).
Gene expression sample collection.
After a 14-day entrainment period, rats were euthanized at 6-h intervals: ZT5 (11:00 AM), ZT11 (5:00 PM), ZT17 (11:00 PM), and ZT23 (5:00 AM); each n = 8). During the dark phase (ZT17 and ZT23), tissues were collected under dim red light to minimize light cues. Rats were euthanized under isoflurane anesthesia (4%) via intracardiac perfusion of phosphate-buffered saline with a peristaltic pump (Masterflex, Cole-Parmer). Diaphragm, caudal medulla, and C3–C5 cervical spinal segments were rapidly removed and immersed in ice-cold, sterile saline. Each C3–C5 spinal cord sample was transferred to a freezing microtome (−22.5°C) and cut horizontally at the level of the central canal using a double edge blade. Ventral portions of spinal segments, caudal medulla, and midcostal portion of the diaphragm muscle were placed in RNAlater stabilization solution (Thermo Fisher Scientific) and stored at 4°C until RNA isolation. Sample wet weights were as follows: ventral C3–C5 spinal cord [mean (M) = 45.16 mg, standard deviation (SD) = 7.78 mg]; caudal medulla (M = 52.78 mg, SD = 7.80 mg) and diaphragm muscle (M = 49.80 mg, SD = 12.50 mg).
RNA isolation and quantification procedures.
Samples were homogenized in 800 µl TRIzol Reagent (Invitrogen) using Bead Mill 4 Homogenizer (Thermo Fisher Scientific). Nucleic acid solubilization, phase separation, and RNA extraction were performed according to manufacturer’s instructions. The product was cleaned by ethanol precipitation, transferred to RNeasy spin columns (RNeasy kit, Qiagen), and purified. RNA concentration was quantified via spectrophotometry (λ = 260 nm; NanoDrop model 2000C, Thermo Fisher Scientific). RNA purity was estimated by the absorbance ratio A260/A280 (all samples had a ratio from 1.8 to 2.0). To ensure RNA integrity, two samples from each region were selected randomly and analyzed with a bioanalyzer; RNA integrity number (RIN) values for all samples were between 8.8 and 9.1.
Real-time reverse transcription-polymerase chain reaction.
First-strand complementary DNA (cDNA) was synthesized from 2.5 µg of total RNA using random primers in SuperScript VILO cDNA Synthesis Kit (Invitrogen). The resulting cDNAs were diluted to 4 ng/µL and used as templates in real-time quantitative polymerase chain reactions (PCRs; QuantStudio3; Applied Biosystems). TaqMan oligonucleotide primers and probe sets (TaqMan Gene Expression Assays; Applied Biosystems) used for PCR are listed in Table 1. Twenty microliter reactions were prepared. Reaction mixtures consisted of 5 µL cDNA (4 ng/µL), 10 µL TaqMan Fast Advanced Master Mix (Applied Biosystems), 4 µL DEPC-treated RNAse-free water, and 1 µL of the corresponding TaqMan gene expression assay. All reactions were performed in duplicate. Cycling conditions were as follows: uracil-N-glycosylase (UNG) incubation at 50°C for 2 min, AmpliTaq Fast DNA polymerase activation at 95°C for 2 min, followed by 40 cycles of 95°C for 1 s (denaturation) and 60°C for 20 s (annealing and extension). Negative control reactions with no template were generated for each gene on each plate to control for reagent contamination; no detectable amplification signal was observed in nontemplate control wells. Threshold cycle number (CT) values across plates were exported from ExpressionSuite Software (Thermo Fisher Scientific) for analysis using MATLAB R2019b (Mathworks, Natick, MA). The expression of clock genes and plasticity genes was quantified using β-actin as the reference gene in caudal medulla and ventral C3–C5 spinal cord samples. Since β-actin was reported to exhibit circadian rhythmicity in mouse skeletal muscle in the CIRCAdb database (circadb.hogeneschlab.org/mouse) (52), skeletal muscle alpha(α)-actin was used as the reference gene in diaphragm muscle samples. Relative quantification was performed using the ΔΔCT method (39). ΔCT values were calculated as CT(gene of interest) − CT(reference gene). The relative steady-state mRNA levels are presented in fold change (2−ΔΔCT) relative to ZT5 (ZT5 set to 1) to plot data across the light-dark cycle (ZT0–ZT24).
Table 1.
TaqMan oligonucleotide primers and probe sets used for PCR
| Gene Name | Protein Encoded | TaqMan Assay ID | Amplicon Length |
|---|---|---|---|
| Actb | B-Actin | Rn00667869_m1 | 91 |
| Acta1 | Actin alpha 1 | Rn00570060_g1 | 71 |
| Arntl | BMAL1 | Rn00577590_m1 | 137 |
| Clock | CLOCK | Rn00573120_m1 | 112 |
| Per1 | Per1 | Rn01325256_m1 | 62 |
| Per2 | Per2 | Rn01427704_m1 | 100 |
| Htr2a | 5-HT2A | Rn01468302_m1 | 77 |
| Htr2b | 5-HT2B | Rn00691836_m1 | 81 |
| Bdnf | BDNF | Rn01484924_m1 | 106 |
| Ntrk2 | TrkB | Rn01441749_m1 | 73 |
TaqMan Gene Expression assays (primer and probe sets) used to detect clock gene transcripts, neuroplasticity-related gene transcripts and reference gene transcripts.
Gene expression analysis and statistical procedures.
The homogeneity of variance assumption was met for each gene and time point using Levene’s test. A one-way ANOVA was conducted on ΔCT values between time points to determine a statistically significant effect of time. Since ANOVAs alone do not provide information regarding periodicity (54), circadian rhythmicity of clock genes and plasticity genes was evaluated using the single cosinor method (51, 54) using custom MATLAB code adapted from MATLAB Central File Exchange (14). Relative RNA level was fit to the cosine curve with a 24-h period using a least squares method. A zero-amplitude test was conducted with a null hypothesis that no rhythm is observed. Rejecting this null hypothesis (the zero-amplitude assumption) indicates that the fitted curve generated by the model is a better estimate of the data than a straight line with zero slope. Rhythm detection was considered statistically significant at the P < 0.05 level. In genes that exhibited significant rhythmicity, we determined estimates of rhythm parameters including: mesor (midpoint; midline statistic of rhythm), amplitude (1/2 the difference between the minimum and maximum of the function), and acrophase (the time of the peak in fitted cosine function). Data were analyzed using custom scripts in MATLAB R2019b.
Retrograde labeling of phrenic motor neurons.
Phrenic motor neurons were labeled retrogradely via intrapleural injections of the nonpathogenic β-subunit of cholera toxin in 26 rats, each 3–4 mo of age (CtB; Calbiochem 227039) (42). Before injection, rats were anesthetized with 2.5% isoflurane in 100% O2. Adequate anesthesia was confirmed by the absence of a toe pinch response. CtB was injected bilaterally (15-µL injections bilaterally; 30 µL total per animal 2 µg/µL in sterile H2O) using a sterile 50-µL Hamilton syringe with sterile custom made 9.52-mm blunt tip needles into the fifth intercostal pleural space at a depth of ~6 mm. After the injections were administered, rats recovered from anesthesia and showed no signs of distress or respiratory compromise.
Protein expression sample collection.
Fourteen days after CtB injection, rats were euthanized at two time points: ZT23 and ZT11 (n = 13/time point). ZT23 corresponds to the end of the active phase and 1 h before the onset of the rest phase. ZT11 corresponds to the end of the rest phase and 1 h before the onset of the active phase. During the dark phase (ZT23), tissues were collected under dim red light to minimize light cues. Rats were euthanized under isoflurane anesthesia (4%) by intracardiac perfusion with 100 mL 0.1 M phosphate-buffered saline (PBS; pH 7.4), followed by 400 mL paraformaldehyde fixation (4% weight/volume in 0.1 M PBS, pH 7.4) via peristaltic pump (Masterflex Pump, Cole-Parmer). The brain and spinal cord were dissected and postfixed for 24 h in a 50-mL conical tube filled with paraformaldehyde (4% weight/volume in 0.1 M PBS, pH 7.4). Tissues were cryoprotected in 20% sucrose until they sank, followed by 30% sucrose solution until sinking (~1 wk). C3–C5 segments were isolated and embedded using M1 embedding matrix (Thermo Fisher Scientific) and cut in 40-µm transverse sections using a cryostat maintained at −20°C. Sectioned tissues were placed sequentially in 96-well plates filled with antifreeze (30% glycerol + 30% ethylene glycol in 0.1 M PBS) and stored at −20°C until immunofluorescent staining was performed.
Immunofluorescence of phrenic motor neurons.
We investigated intracellular BMAL1 and BDNF protein distribution in phrenic motor neurons via immunofluorescence; antibody details and product numbers are listed in Table 2. A systematic random sampling approach was used, in which every 18th section (~350 µm between sections) from the mid C3 to mid C5 region was selected for staining to obtain eight representative sections of the phrenic motor pool from each animal. All tissues from both time points (26 rats, 208 sections) were stained in a single batch to avoid batch effects and enable comparisons across groups. Double staining was used to label either the clock protein BMAL1 or the plasticity protein BDNF and CtB to label phrenic motor neurons. Tissues were first washed in 0.1 M PBS followed by 30-min incubation in heat-induced epitope retrieval (HIER; TissuePro Technology, Gainesville FL) at 85°C to improve detection in fixed tissues. Tissues were then washed in 0.1M PBS + 0.2% Triton followed by a 1-h incubation in blocking agent (5% normal donkey serum (GeneTex, CTX30972) and 0.25% bovine serum albumin (BSA; Jackson ImmunoResearch 001-000-162) in 0.1 M PBS + 0.2% Triton to block nonspecific sites. Sections were incubated overnight at 4°C with the following primary antibodies: BMAL1 (Cell Signaling) or BDNF (Alomone) and CtB (Millipore). The next day, sections were washed in 0.1 M PBS + 0.2% Triton and incubated at room temperature in the dark for 2 h with the following secondary antibodies: donkey anti-rabbit Alexafluor Plus 488 (Invitrogen) to label BMAL1 or BDNF and donkey anti-goat Alexafluor Plus 647 (Invitrogen) to label CtB. Following secondary antibody incubation, sections were washed in 0. 1M PBS and mounted on charged slides (Shandon Superfrost Plus, Thermo Fisher Scientific 6776214) using Vectashield Hardset Mounting medium (Vector laboratories, H-1400). Four rats were excluded from analysis due to failed CtB injections (n = 2 rats) or inadequate fixation (n = 2 rats).
Table 2.
Antibodies used for immunofluorescence studies
| Type | Reactivity (Isotype) | Host | Conjugate | Dilution | Manufacturer | Product Number |
|---|---|---|---|---|---|---|
| Primary/polyclonal | Anti-CtB (IgG) | Goat | – | 1:1,000 | Millipore, Sigma | 227040 |
| Primary/monoclonal | Anti-BMAL1 (IgG) | Rabbit | – | 1:1,000 | Cell Signaling | D2L7G |
| Primary/polyclonal | Anti-BDNF (IgG) | Rabbit | – | 1:750 | Alomone | ANT-010 |
| Secondary/polyclonal | Anti-Goat (IgG) (H+L) | Donkey | AlexaFlour Plus-647 | 1:1,000 | Invitrogen | A32849 |
| Secondary/polyclonal | Anti-Rabbit (IgG) (H+L) | Donkey | AlexaFlour Plus-488 | 1:1,000 | Invitrogen | A32790 |
BMAL1, BDNF, and CtB antibody validation.
Dilution series (5 concentrations) were performed to determine optimal dilutions for immunofluorescence. The anti-BMAL1 is a monoclonal antibody that binds residues surrounding Gly552 of human BMAL1 protein. Primary controls (+primary antibody/−secondary antibody) and secondary controls (−primary antibody/+secondary antibody) showed no positive labeling. The absence of staining has also been shown in kidney specific BMAL1 knockout mice (Dr. Michelle Gumz, Department of Medicine in the College of Medicine, University of Florida personal communication). The anti-BDNF antibody is a polyclonal antibody directed against an epitope of the human BDNF protein. This antibody does not cross react with other neurotrophins and has been validated in knockout animals (64, 69). Primary controls (+primary antibody/−secondary antibody) and secondary controls (−primary antibody/+secondary antibody) showed no positive labeling. The antibody used to detect CtB recognizes the β-subunit of cholera toxin; this protein is not expressed in rats without retrograde labeling as confirmed by the absence of staining in spinal tissue from a rat that did not receive CtB injections. Primary controls (+primary antibody/−secondary antibody) and secondary controls (−primary antibody/+secondary antibody) showed no positive labeling.
Image acquisition and analysis.
All immunofluorescent images were acquired using a digital microscope (Keyence, Osaka Japan) with a ×20 objective (Keyence, 972032). BMAL1 immunolabelling was detected using a GFP filter (Keyence, OP-87763) and an exposure time of 1/15 s. CtB immunolabelling was detected using a Cy5 filter (Keyence, OP-87766) and an exposure time of 1/5 s. BDNF immunolabelling was detected using a GFP filter (Keyence, OP-87763) and an exposure time of 1/120 s. To enhance resolution, high-precision optical sectioning was implemented. This method projects a grating onto the sample and reconstructs a final image from a set of images acquired at different grating positions to enhance resolution (34). The illumination pattern sectioning parameter was set to 16 across all fluorescence filters. After acquisition, CtB-positive phrenic motor neurons were outlined manually and semiquantitation and analysis was performed using a custom written MATLAB code by a blinded investigator. Within-rat averaging of immunofluorescence intensity was calculated before averaging within group (M = 60 neurons SD = 29 neurons). Data for each group were summarized as means ± SD. An independent samples t test was used to compare BDNF protein and BMAL1 protein between group ZT11 (end of rest phase; 1 h before lights off) and ZT23 (end of active phase; 1 h before lights on). Representative images for figures were selected from animals closest to the mean intensity level for each group.
RESULTS
Rhythmic expression of clock genes.
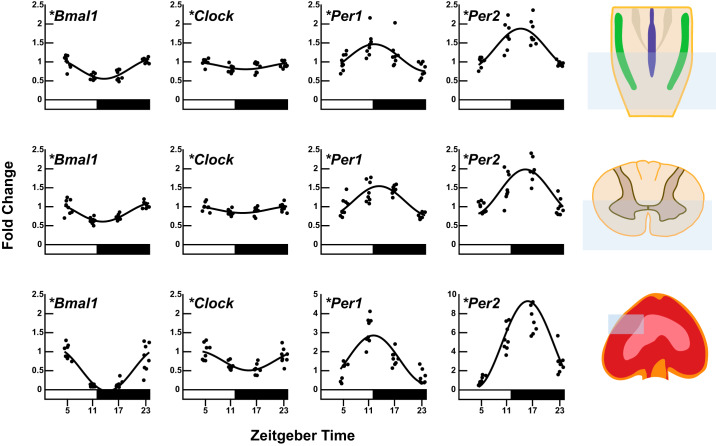
A statistically significant effect of time and significant rhythm was detected in steady-state mRNA level of the core clock genes Bmal1, Clock, Per1, and Per2 in the caudal medulla, ventral C3–C5 spinal cord, and diaphragm muscle (PANOVA < 0.05, PCosinor < 0.05; Fig. 1). Gene expression of both Bmal1 and Clock peaked during the rest phase in all three regions, 1–2.5 h after lights on. Gene expression of Per1 and Per2 peaked during the active phase in all three regions. Gene expression of Per1 peaked within the first 1.5 h after lights off and gene expression of Per2 peaked 2–4 h after lights off. Per2 amplitude was the largest, and Clock was the smallest amplitude of clock genes in every region tested. Estimates of rhythm parameters for clock gene expression and P values from ANOVAs and cosinor analysis are listed in Table 3. We compared our findings to results reported in the Circadian Expression Profiles Database CircaDB (circadb.hogeneschlab.org/mouse) (52). The CIRCAdb database has much higher resolution (every 2 h) across a 48-h period of constant darkness. Despite limited resolution in our data set, the patterns of Bmal1, Clock, Per1, and Per2 expression from the caudal medulla are consistent with the mouse brainstem data reported in CIRCAdb. Furthermore, our diaphragm clock gene results are consistent with mouse skeletal muscle. Thus our measures qualitatively fit the curves publicly available in the database. Ventral C3–C5 spinal cord clock gene expression (which has never been reported) is consistent with other neural tissues (e.g., mouse brainstem). In our data set, the diaphragm consistently had larger amplitudes in clock gene expression than the neural tissues. The CIRCAdb database indicates that clock gene amplitudes are larger for skeletal muscle than any brain region included in the data set. Therefore, diaphragm amplitudes being larger than either neural sample analyzed here are consistent with previous reports. Our data serve as an initial step for future research exploring the functional implications of differences in neural versus muscle clock gene amplitude.
Fig. 1.
Daily expression profiles of circadian clock genes (Bmal1, Clock, Per1, and Per2) in caudal medulla, ventral C3–C5 cervical spinal cord, and diaphragm homogenates (n = 8 samples/time point). Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed to determine mRNA levels of Bmal1, Clock, Per1, and Per2. Caudal medulla and ventral C3–C5 spinal cord mRNA levels were normalized to the β-actin. Diaphragm mRNA levels were normalized to skeletal muscle α-actin. The black line shows the corresponding fitted cosine function. The photoperiod is represented by the white and black bars, representing the light/rest phase and dark/active phase respectively. Time in hours is denoted by ZT because light is a zeitgeber. ZT0 = lights on (rest onset) in the entrained cycle and ZT12 = lights off (active onset). *Significance of daily rhythmicity (P < 0.05) determined from the zero-amplitude test.
Table 3.
Estimates of rhythm parameters for core clock genes in ventral C3–C5 spinal cord, caudal medulla, and diaphragm muscle homogenates calculated using cosinor analysis
| Gene/Region | Mesor | Amplitude (95% CI) | Acrophase (ZT; h:min) | Cosinor P Value | ANOVA P Value |
|---|---|---|---|---|---|
| Bmal1 | |||||
| Ventral C3–C5 spinal cord | 0.86 | 0.25 (0.17, 0.32) | 1:14 | P = 5.57E−9* | P = 2.38E−8* |
| Medulla | 0.83 | 0.27 (0.20, 0.35) | 1:34 | P = 3.61E−10* | P = 3.20E−8* |
| Diaphragm | 0.53 | 0.56 (0.43, 0.70) | 2:19 | P = 1.73E−11* | P = 3.42E−12* |
| Clock | |||||
| Ventral C3–C5 spinal cord | 0.93 | 0.09 (0.02, 0.15) | 1:41 | P = 0.004* | P = 0.013* |
| Medulla | 0.90 | 0.09 (0.02, 0.16) | 2:23 | P = 0.004* | P = 0.006* |
| Diaphragm | 0.76 | 0.26 (0.14, 0.37) | 3:08 | P = 5.51E−6* | P = 1.38E−5* |
| Per1 | |||||
| Ventral C3–C5 spinal cord | 1.14 | 0.40 (0.27, 0.53) | 13:21 | P = 1.87E−8* | P = 4.04E−8* |
| Medulla | 1.11 | 0.36 (0.18, 0.53) | 12:13 | P = 3.06E−5* | P = 1.19E−4* |
| Diaphragm | 1.59 | 1.26 (0.90, 1.63) | 12:04 | P = 1.59E−9* | P = 1.20E−7* |
| Per2 | |||||
| Ventral C3–C5 spinal cord | 1.38 | 0.60 (0.38, 0.81) | 15:14 | P = 1.08E−7* | P = 1.12E−7* |
| Medulla | 1.35 | 0.53 (0.37, 0.68) | 14:10 | P = 1.19E−9* | P = 2.44E−9* |
| Diaphragm | 4.76 | 4.55 (2.41, 6.69) | 15:46 | P = 1.64E−5* | P = 4.18E−11* |
Numerical values for mesor, amplitude, acrophase, and significance of the rhythm (cosinor P value; H0: amplitude = 0) are presented. Additionally, a one-way ANOVA was conducted to determine an overall effect of time (ANOVA P value; H0: all time point means are equal). Mesor is the midline estimating statistic of rhythm in fold change relative to ZT5. Amplitude is half the value of the range of oscillation in fold change. Acrophase is the predicted time of peak mRNA expression (h:min). Time in hours is denoted by ZT because light is a zeitgeber. ZT0 = lights on (rest onset) in the entrained cycle, and ZT12 = lights off (active onset); CI, confidence interval.
Significance of daily rhythmicity (P < 0.05).
Rhythmic expression of plasticity genes.
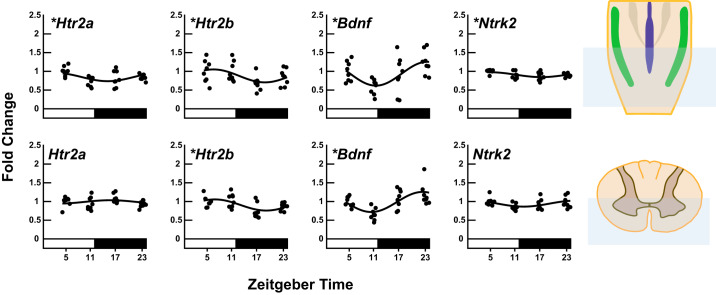
Since we are interested in understanding mechanisms of neuroplasticity in the phrenic motor system, we investigated mRNA levels of genes known to be involved in phrenic long term facilitation (pLTF): serotonin receptor 2a (Htr2a), serotonin receptor 2b (Htr2b), brain-derived neurotrophic factor (Bdnf), and the high-affinity BDNF receptor tropomyosin receptor kinase b (NTrk2) (Fig. 2) in the caudal medulla (containing medullary respiratory network neurons) and the ventral C3–C5 spinal cord (containing phrenic motor neurons which innervate the diaphragm). A statistically significant effect of time (PANOVA < 0.05) and significant rhythm (PCosinor < 0.05) was detected in steady-state mRNA level of Bdnf, Htr2a, Htr2b and NTrk2 transcripts in the caudal medulla (PANOVA < 0.05, PCosinor < 0.05). In our primary region of interest, the ventral C3–C5 spinal cord, a statistically significant effect of time and significant rhythm was detected in steady-state mRNA level of Htr2b and Bdnf (PANOVA < 0.05, PCosinor < 0.05) with similar estimates in peak expression time. Bdnf had the largest amplitude {0.27 95% confidence interval (CI) [0.12, 0.41]} with estimated time of peak expression late in the active phase (~ZT23), whereas steady-state mRNA levels of Htr2b peaked during the rest phase (~ZT7) but with a smaller amplitude compared with Bdnf (0.15 95% CI [0.04, 0.25]). Estimates of rhythm parameters for plasticity gene expression, P values from ANOVAs, and P values from cosinor analysis are listed in Table 4.
Fig. 2.
Daily expression profiles of genes involved in phrenic long-term facilitation elicited by acute intermittent hypoxia (Htr2a, Htr2b, Bdnf, and Ntrk2) in caudal medulla and ventral C3–C5 cervical spinal cord homogenates (n = 8 samples/time point). Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed to determine mRNA levels of Htr2a, Htr2b, Bdnf, and Ntrk2. Caudal medulla and ventral C3–C5 spinal cord mRNA levels were normalized to the β-actin. The black line shows the corresponding fitted cosine function. The photoperiod is represented by the white and black bars, representing the light/rest phase and dark/active phase, respectively. Time in hours is denoted by ZT because light is a zeitgeber. ZT0 = lights on (rest onset) in the entrained cycle and ZT12 = lights off (active onset). *Significance of daily rhythmicity (P < 0.05) determined from the zero-amplitude test.
Table 4.
Estimates of rhythm parameters for genes necessary for phrenic long-term facilitation in caudal medulla and ventral C3–C5 spinal cord homogenates calculated using cosinor analysis
| Gene/Region | Mesor | Amplitude (CI) | Acrophase (ZT; h:min) |
Cosinor P Value |
ANOVA P Value |
|---|---|---|---|---|---|
| Htr2a | |||||
| Ventral C3–C5 spinal cord | 0.99 | 0.04 (0.04, 0.13) | 15:38 | P = 0.419 | P = 0.122 |
| Medulla | 0.85 | 0.11 (0.01, 0.21) | 3:01 | P = 0.025* | P = 0.011* |
| Htr2b | |||||
| Ventral C3–C5 spinal cord | 0.91 | 0.15 (0.04, 0.25) | 7:03 | P = 0.003* | P = 0.003* |
| Medulla | 0.88 | 0.17 (0.01, 0.33) | 6:44 | P = 0.027* | P = 0.048* |
| Bdnf | |||||
| Ventral C3–C5 spinal cord | 0.99 | 0.27 (0.12, 0.41) | 22:26 | P = 1.14E−4* | P = 5.36E−5* |
| Medulla | 0.94 | 0.32 (0.10, 0.53) | 23:31 | P = 0.002* | P = 0.016* |
| Ntrk2 | |||||
| Ventral C3–C5 spinal cord | 0.94 | 0.08 (0.00, 0.16) | 1:01 | P = 0.050 | P = 0.074 |
| Medulla | 0.91 | 0.06 (0.01, 0.12) | 5:11 | P = 0.013* | P = 0.018* |
Numerical values for mesor, amplitude, acrophase, and significance of the rhythm (cosinor P value; H0: amplitude = 0) are presented. Additionally, a one-way ANOVA was conducted to determine an overall effect of time (ANOVA P value; H0: all time point means are equal). Mesor is the midline estimating statistic of rhythm in fold change relative to ZT5. Amplitude is half the value of the range of oscillation in fold change. Acrophase is the predicted time of peak mRNA expression (h:min). Time in hours is denoted by ZT because light is a zeitgeber. ZT0 = lights on (rest onset) in the entrained cycle, and ZT12 = lights off (active onset); CI, confidence interval.
Significance of daily rhythmicity (P < 0.05).
Circadian clock protein BMAL1 is expressed in phrenic motor neurons.
Immunofluorescent staining revealed that BMAL1 protein is expressed within phrenic motor neurons (Fig. 3A). We did not detect a difference in Bmal1 protein intensity within phrenic motor neurons between ZT23 (end of the active phase; 1 h before lights on) and ZT11 (end of the rest phase; 1 h before lights off; P = 0.09; Fig. 3B). While we found no statistical difference in BMAL1 protein expression at these two time points, our experiments do not rule out the possibility of rhythmic BMAL1 protein expression. Future studies with enhanced time resolution and specificity are needed to determine if BMAL1 oscillates within phrenic motor neurons. BMAL1 protein was also expressed in nearby cells. We determined that some of these cells were putative astrocytes [glial fibrillary acidic protein (GFAP) positive] and putative microglia (CD11b positive; data not shown). However, since the predominant site of intermittent hypoxia-induced neuroplasticity is phrenic motor neurons per se (16, 17), we restricted focus of the present study to these cells.
Fig. 3.
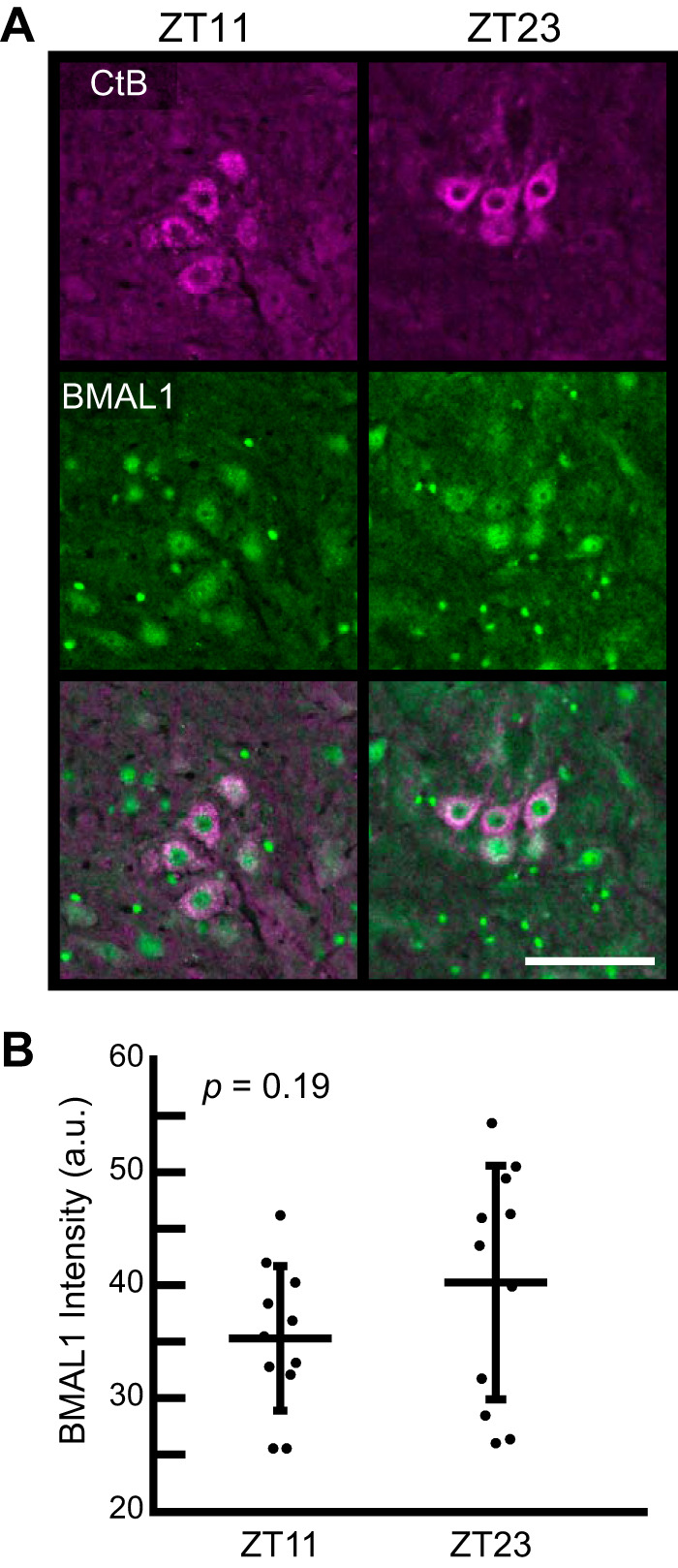
Immunofluorescence (A) and semiquantification (B) of BMAL1 expression within phrenic motor neurons retrogradely labeled with cholera toxin beta (CtB) in the ventral C3–C5 spinal cord from rats harvested at the end of the rest phase [zeitgeber time (ZT)11; 5 PM] compared with the end of the active phase (ZT23; 5 AM); a.u., arbitrary units. Scale bar = 100 µm. Values represent means ± SD of n = 11 rats/group) by Student’s t test. P = 0.19 (nonsignificant difference).
BDNF protein intensity is higher at the end of the active versus rest phase.
Immunofluorescent staining revealed that BDNF protein is expressed within phrenic motor neurons (Fig. 4A). We detected a significant BDNF fluorescence intensity difference between ZT23 (end of the active phase; 1 h before lights on) and ZT11 (end of the rest phase; 1 h before lights off; P = 0.02; Fig. 4B). Higher levels of BDNF within phrenic motor neurons during the active phase may have important implications for phrenic motor plasticity. However, the qualitative nature of immunofluorescence assessment is limited. Future studies using assays with higher quantitative capabilities and increased time resolution are needed to determine whether BDNF protein levels oscillate across the 24-h circadian cycle.
Fig. 4.
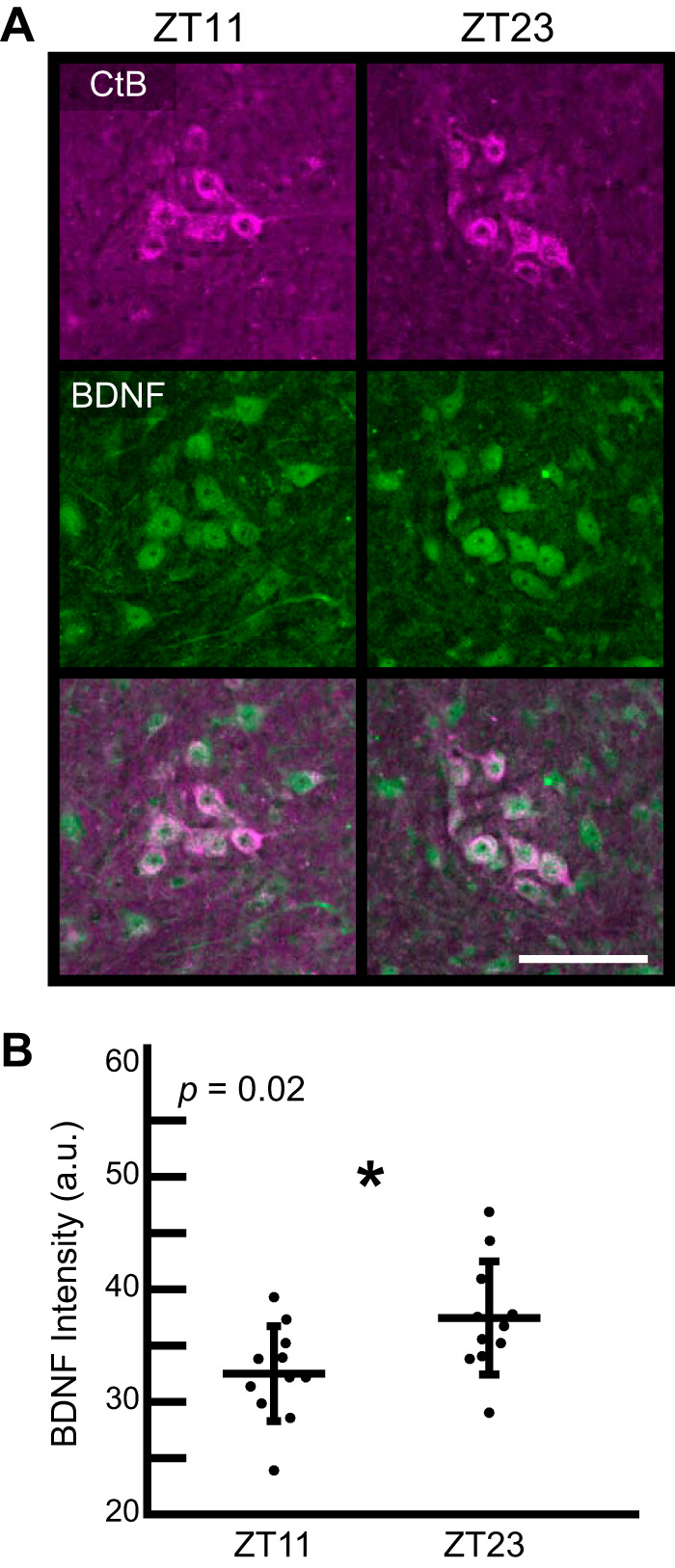
Immunofluorescence (A) and semiquantification (B) of BDNF protein expression within phrenic motor neurons retrogradely labeled with cholera toxin beta (CtB) in the ventral C3–C5 spinal cord from rats harvested at the end of the rest phase (ZT11; 5 PM) compared with the end of the active phase (ZT23; 5 AM). BDNF, green; CtB, magenta; a.u., arbitrary units. Scale bar = 100 µm. Values represent means ± SD of n = 11 rats/group). *P < 0.05, Student’s t test.
DISCUSSION
We demonstrate that the circadian clock genes Bmal1, Clock, Per1, and Per2 are expressed rhythmically in key elements of the phrenic motor system: the caudal medulla, the ventral C3–C5 spinal cord, and diaphragm muscle. Furthermore, we confirm that clock protein BMAL1 is expressed in identified phrenic motor neurons. Since the molecular clock mechanism is highly complex (68), we focused on four components of the clock core as an initial step. Future research is needed to determine patterns of distinct core clock genes and genes reflecting accessory loops (i.e., Nr1d1, Nr1d2, Rora, Rorb, Per3, Cry1, Cry2, and NPas2) before building a complete model of circadian regulation in the phrenic motor system. Additionally, the relative contribution of each region to circadian timekeeping and clock-regulated respiratory functions remain to be elucidated.
To our knowledge, this is the first demonstration of daily rhythms in clock gene expression in the ventral spinal cord (any segment) and localization of clock proteins in any identified spinal motor neuron population. However, circadian clocks have been identified in spinal microglia (25) and astrocytes (48). Clock transcripts were also recently localized to hypoglossal motor neurons (33), a cranial motor neuron pool that plays a distinct role in the control of breathing: upper airway regulation. Differences in clock genes were associated with changes in lingual and nuchal electromyography between the rest and active phase, leading these authors to hypothesize that motor neuron excitability is regulated by the motor neuron clock across the circadian cycle (33). Future studies are needed to determine if the circadian clock regulates inter- versus intracellular integration of neural information across respiratory centers and, ultimately, breathing behaviors.
Circadian clock modulation of breathing could occur through 1) indirect influences of the SCN via hormonal/neurochemical factors; 2) influences of (mono or poly) synaptic pathways from the central clock to the neural network controlling breathing; and/or 3) local circadian clocks within respiratory neurons and muscles. Here, we found that clock genes express daily rhythms in the caudal medulla, ventral C3–C5 spinal cord, and diaphragm muscle, with coordinated peak expression times. Whereas a robust functional circadian clock has been identified in skeletal muscle (4, 21, 35, 57), our findings highlight a previously overlooked possibility: that the circadian clock of the spinal cord may coordinate circadian timing between the central nervous system and muscles.
Our observation that expression of molecules involved in phrenic motor plasticity (Bdnf and Htr2b) oscillate with time of day suggests the possibility of circadian regulation of neuroplasticity in the phrenic motor circuit. For example, acute intermittent hypoxia may be most effective at eliciting phrenic motor plasticity at times with the greatest capacity for serotonin-induced BDNF synthesis. Serotonergic raphe neurons exhibit endogenous rhythms in vitro (71), and serotonin release exhibits prominent circadian rhythms, peaking during the active phase (38). 5-HT2a receptor mRNA and protein levels in the hypoglossal motor nucleus are higher at the onset of the active phase (ZT11–12) versus rest phase onset (ZT1–2) (72), demonstrating that time of day may regulate serotonin-dependent mechanisms in other motor neuron pools relevant to breathing. Furthermore, AIH effects on upper airway muscles and diaphragm pump muscles may have unique temporal effects.
Daily rhythms in molecules necessary for AIH-induced plasticity in animal models may help explain time of day effects reported for AIH-induced plasticity in humans, particularly in people living with sleep apnea (22, 27, 66). Specifically, ventilatory LTF was higher when acute intermittent hypoxia was administered in the evening (6–7 PM) versus morning (7–8 AM) in people living with sleep apnea (27). Furthermore, AIH administration at night (10 PM to 1 AM) reduces the therapeutic pressure required to maintain upper airway patency versus the pressure required following AIH administration in the morning (6 AM to 9 AM) (23). These data provide the first evidence in humans that a chronotherapeutic approach to AIH may be beneficial for this emerging therapeutic.
We suggest that changes in molecules involved in phrenic motor plasticity may coordinate circadian phase with serotonergic regulation of motor neuron function across the 24-h cycle, including serotonin-dependent modulation and/or plasticity (46). Consequently, changes in serotonin receptor expression and BDNF levels across the day suggest that intermittent hypoxia will be most potent at eliciting phrenic motor plasticity at certain times of circadian cycle, although this possibility remains to be tested. Importantly, diurnal rhythms/time of day differences may or may not be driven by the circadian clock. Additional studies with enhanced specificity (e.g., specific motor neuron pools/ distinct neural regions), enhanced time resolution, and measurement under constant conditions are needed to create a comprehensive model of circadian rhythms and respiratory neuroplasticity and identify specific genes/proteins contributing to time of day modulation of respiratory motor plasticity.
Perspectives and Significance
Although we did not uncouple intrinsic cellular clocks from zeitgebers (e.g., light/dark) in this initial study, we confirm the potential for local circadian regulation in regions of interest to respiratory motor control, specifically the phrenic/diaphragm motor system. We postulate that circadian clock gene oscillations in respiratory motor neurons may 1) synchronize neuronal activity to changing metabolic demand and 2) promote adaptation (i.e., plasticity) to an environment that changes throughout the day. By understanding these daily rhythms, we may be able to optimize the therapeutic potential of AIH-induced neuroplasticity. Timing of intervention (e.g., therapeutic acute intermittent hypoxia) may influence respiratory or nonrespiratory motor function in people living with spinal cord injury, ALS, or other severe neuromuscular disorders (29, 44). Understanding how responses to stimuli/interventions vary with time of day and endogenous periodicities within respiratory motor neurons could also have important implications for optimization of the therapeutic AIH protocol. Clock genes in the neural network controlling breathing may play key roles in a wide range of disorders of respiratory control, including, but not limited to, sleep apnea (12, 43), sudden infant death syndrome (SIDS) (11), respiratory depression/failure during seizures (i.e., SUDEP) (53, 60), nocturnal anxiety and panic disorders (37), asthma (10), and chronic airway disease (65).
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-HL-69064, R01-HL-148030, R01-AR-066082, and 1R01-DK-109570-01A1 and the McKnight Brain Institute Grant BSCIRTF. M. Kelly was supported by the BREATHE Training Program Grant T32 HL134621. M. Sunshine was supported by NIH Grant F31-HL-145831.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.N.K., X.Z., M.L.G., K.A.E., and G.S.M. conceived and designed research; M.N.K., D.N.S., and A.R. performed experiments; M.N.K., D.N.S., M.D.S., A.R., and X.Z. analyzed data; M.N.K., M.D.S., X.Z., M.L.G., K.A.E., and G.S.M. interpreted results of experiments; M.N.K. and M.D.S. prepared figures; M.N.K. drafted manuscript; M.N.K., D.N.S., M.D.S., A.R., X.Z., M.L.G., K.A.E., and G.S.M. edited and revised manuscript; M.N.K., D.N.S., M.D.S., A.R., X.Z., M.L.G., K.A.E., and G.S.M. approved final version of manuscript.
REFERENCES
- 1.Adamovich Y, Ladeuix B, Golik M, Koeners MP, Asher G. Rhythmic oxygen levels reset circadian clocks through HIF1α. Cell Metab 25: 93–101, 2017. doi: 10.1016/j.cmet.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Adamovich Y, Ladeuix B, Sobel J, Manella G, Neufeld-Cohen A, Assadi MH, Golik M, Kuperman Y, Tarasiuk A, Koeners MP, Asher G. Oxygen and carbon dioxide rhythms are circadian clock controlled and differentially directed by behavioral signals. Cell Metab 29: 1092–1103.e3, 2019. doi: 10.1016/j.cmet.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74: 246–260, 2012. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA 107: 19090–19095, 2010. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aschoff J. Circadian timing. Ann NY Acad Sci 423: 442–468, 1984. doi: 10.1111/j.1749-6632.1984.tb23452.x. [DOI] [PubMed] [Google Scholar]
- 6.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 7.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 8.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bando H, Nishio T, van der Horst GT, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. J Neurosci 27: 4359–4365, 2007. doi: 10.1523/JNEUROSCI.4131-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes PJ. Circadian variation in airway function. Am J Med 79, 6A: 5–9, 1985. doi: 10.1016/0002-9343(85)90080-4. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan GF. Impaired CO2-induced arousal in SIDS and SUDEP. Trends Neurosci 42: 242–250, 2019. doi: 10.1016/j.tins.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchanan GF. Timing, sleep, and respiration in health and disease. Prog Mol Biol Transl Sci 119: 191–219, 2013. doi: 10.1016/B978-0-12-396971-2.00008-7. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms 20: 225–236, 2005. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheart Cosinor Analysis. Natick, MA: MATLAB Central File Exchange, 2008. https://www.mathworks.com/matlabcentral/fileexchange/20329-cosinor-analysis [16 December 2019]. [Google Scholar]
- 15.Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29: 39–48, 2014. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale EA, Fields DP, Devinney MJ, Mitchell GS. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Exp Neurol 287: 130–136, 2017. doi: 10.1016/j.expneurol.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS. Phrenic long-term facilitation requires PKCθ activity within phrenic motor neurons. J Neurosci 35: 8107–8117, 2015. doi: 10.1523/JNEUROSCI.5086-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eastman CI, Mistlberger RE, Rechtschaffen A. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol Behav 32: 357–368, 1984. doi: 10.1016/0031-9384(84)90248-8. [DOI] [PubMed] [Google Scholar]
- 20.Egg M, Köblitz L, Hirayama J, Schwerte T, Folterbauer C, Kurz A, Fiechtner B, Möst M, Salvenmoser W, Sassone-Corsi P, Pelster B. Linking oxygen to time: the bidirectional interaction between the hypoxic signaling pathway and the circadian clock. Chronobiol Int 30: 510–529, 2013. doi: 10.3109/07420528.2012.754447. [DOI] [PubMed] [Google Scholar]
- 21.Ehlen JC, Brager AJ, Baggs J, Pinckney L, Gray CL, DeBruyne JP, Esser KA, Takahashi JS, Paul KN. Bmal1 function in skeletal muscle regulates sleep. eLife 6: e26557, 2017. doi: 10.7554/eLife.26557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Chami M, Shaheen D, Ivers B, Syed Z, Badr MS, Lin HS, Mateika JH. Time of day affects the frequency and duration of breathing events and the critical closing pressure during NREM sleep in participants with sleep apnea. J Appl Physiol (1985) 119: 617–626, 2015. doi: 10.1152/japplphysiol.00346.2015. [DOI] [PubMed] [Google Scholar]
- 23.El-Chami M, Sudan S, Lin HS, Mateika JH. Exposure to intermittent hypoxia and sustained hypercapnia reduces therapeutic CPAP in participants with obstructive sleep apnea. J Appl Physiol (1985) 123: 993–1002, 2017. doi: 10.1152/japplphysiol.00204.2017. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez RI, Lyons LC, Levenson J, Khabour O, Eskin A. Circadian modulation of long-term sensitization in Aplysia. Proc Natl Acad Sci USA 100: 14415–14420, 2003. doi: 10.1073/pnas.2336172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun 45: 171–179, 2015. doi: 10.1016/j.bbi.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol 121: 135–146, 2000. doi: 10.1016/S0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- 27.Gerst DG III, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T, Anthouard MN, Mateika JH. The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J Appl Physiol (1985) 110: 15–28, 2011. doi: 10.1152/japplphysiol.00524.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerstner JR, Lyons LC, Wright KP Jr, Loh DH, Rawashdeh O, Eckel-Mahan KL, Roman GW. Cycling behavior and memory formation. J Neurosci 29: 12824–12830, 2009. doi: 10.1523/JNEUROSCI.3353-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119: 1455–1465, 2015. doi: 10.1152/japplphysiol.00235.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafson CL, Partch CL. Emerging models for the molecular basis of mammalian circadian timing. Biochemistry 54: 134–149, 2015. doi: 10.1021/bi500731f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halberg F, Cornélissen G, Katinas G, Syutkina EV, Sothern RB, Zaslavskaya R, Halberg F, Watanabe Y, Schwartzkopff O, Otsuka K, Tarquini R, Frederico P, Siggelova J. Transdisciplinary unifying implications of circadian findings in the 1950s. J Circadian Rhythms 1: 2, 2003. doi: 10.1186/1740-3391-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- 33.Herr KB, Mann GL, Kubin L. Modulation of motoneuronal activity with sleep-wake states and motoneuronal gene expression vary with circadian rest-activity cycle. Front Integr Nuerosci 12: 32, 2018. doi: 10.3389/fnint.2018.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirvonen LM, Wicker K, Mandula O, Heintzmann R. Structured illumination microscopy of a living cell. Eur Biophys J 38: 807–812, 2009. doi: 10.1007/s00249-009-0501-6. [DOI] [PubMed] [Google Scholar]
- 35.Hodge BA, Wen Y, Riley LA, Zhang X, England JH, Harfmann BD, Schroder EA, Esser KA. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle 5: 17, 2015. doi: 10.1186/s13395-015-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyer R, Wang TA, Gillette MU. Circadian gating of neuronal functionality: a basis for iterative metaplasticity. Front Syst Neurosci 8: 164, 2014. doi: 10.3389/fnsys.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levitan MN, Nardi AE. Nocturnal panic attacks: clinical features and respiratory connections. Expert Rev Neurother 9: 245–254, 2009. doi: 10.1586/14737175.9.2.245. [DOI] [PubMed] [Google Scholar]
- 38.Liu T, Borjigin J. Relationship between nocturnal serotonin surge and melatonin onset in rodent pineal gland. J Circadian Rhythms 4: 12, 2006. doi: 10.1186/1740-3391-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Lyons LC. Critical role of the circadian clock in memory formation: lessons from Aplysia. Front Mol Neurosci 4: 52, 2011. doi: 10.3389/fnmol.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manella G, Aviram R, Bolshette N, Muvkadi S, Golik M, Smith DF, Asher G. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc Natl Acad Sci USA 117: 779–786, 2020. doi: 10.1073/pnas.1914112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 182: 244–249, 2009. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mateika JH, El-Chami M, Shaheen D, Ivers B. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J Appl Physiol (1985) 118: 520–532, 2015. doi: 10.1152/japplphysiol.00564.2014. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell GS. Respiratory Plasticity Following Intermittent Hypoxia: a Guide for Novel Therapeutic Approaches to Ventilatory Control Disorders? Boston, MA: Springer, 2007, p. 291–311. [Google Scholar]
- 45.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90: 2466–2475, 2001. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol (1985) 94: 358–374, 2003. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 47.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35: 445–462, 2012. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morioka N, Sugimoto T, Tokuhara M, Nakamura Y, Abe H, Hisaoka K, Dohi T, Nakata Y. Spinal astrocytes contribute to the circadian oscillation of glutamine synthase, cyclooxygenase-1 and clock genes in the lumbar spinal cord of mice. Neurochem Int 60: 817–826, 2012. doi: 10.1016/j.neuint.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Mortola JP. Correlations between the circadian patterns of body temperature, metabolism and breathing in rats. Respir Physiol Neurobiol 155: 137–146, 2007. doi: 10.1016/j.resp.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Mortola JP. Hypoxia and circadian patterns. Respir Physiol Neurobiol 158: 274–279, 2007. doi: 10.1016/j.resp.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia 6: 305–323, 1979. [PubMed] [Google Scholar]
- 52.Pizarro A, Hayer K, Lahens NF, Hogenesch JB. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res 41: D1009–D1013, 2012. doi: 10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purnell BS, Thijs RD, Buchanan GF. Dead in the night: sleep-wake and time-of-day influences on sudden unexpected death in epilepsy. Front Neurol 9: 1079, 2018. doi: 10.3389/fneur.2018.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 38: 275–325, 2007. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol 20: 227–241, 2019. doi: 10.1038/s41580-018-0096-9. [DOI] [PubMed] [Google Scholar]
- 56.Ribas-Latre A, Eckel-Mahan K. Interdependence of nutrient metabolism and the circadian clock system: Importance for metabolic health. Mol Metab 5: 133–152, 2016. doi: 10.1016/j.molmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroder EA, Harfmann BD, Zhang X, Srikuea R, England JH, Hodge BA, Wen Y, Riley LA, Yu Q, Christie A, Smith JD, Seward T, Wolf Horrell EM, Mula J, Peterson CA, Butterfield TA, Esser KA. Intrinsic muscle clock is necessary for musculoskeletal health. J Physiol 593: 5387–5404, 2015. doi: 10.1113/JP271436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seifert EL, Knowles J, Mortola JP. Continuous circadian measurements of ventilation in behaving adult rats. Respir Physiol 120: 179–183, 2000. doi: 10.1016/S0034-5687(00)00108-0. [DOI] [PubMed] [Google Scholar]
- 59.Seifert EL, Mortola JP. The circadian pattern of breathing in conscious adult rats. Respir Physiol 129: 297–305, 2002. doi: 10.1016/S0034-5687(01)00316-4. [DOI] [PubMed] [Google Scholar]
- 60.Spencer DC, Sun FT, Brown SN, Jobst BC, Fountain NB, Wong VS, Mirro EA, Quigg M. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia 57: 1495–1502, 2016. doi: 10.1111/epi.13455. [DOI] [PubMed] [Google Scholar]
- 61.Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol 526: 683–694, 2000. doi: 10.1111/j.1469-7793.2000.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69: 1583–1586, 1972. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephenson R, Liao KS, Hamrahi H, Horner RL. Circadian rhythms and sleep have additive effects on respiration in the rat. J Physiol 536: 225–235, 2001. doi: 10.1111/j.1469-7793.2001.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun C, Krimm R, Hill DL. Maintenance of mouse gustatory terminal field organization is dependent on BDNF at adulthood. J Neurosci 38: 6873–6887, 2018. doi: 10.1523/JNEUROSCI.0802-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sundar IK, Yao H, Sellix MT, Rahman I. Circadian molecular clock in lung pathophysiology. Am J Physiol Lung Cell Mol Physiol 309: L1056–L1075, 2015. doi: 10.1152/ajplung.00152.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Syed Z, Lin HS, Mateika JH. The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long-term facilitation. J Appl Physiol (1985) 114: 52–65, 2013. doi: 10.1152/japplphysiol.00985.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tadjalli A, Mitchell GS. Cervical spinal 5-HT2A and 5-HT2B receptors are both necessary for moderate acute intermittent hypoxia-induced phrenic long-term facilitation. J Appl Physiol (1985) 127: 432–443, 2019. doi: 10.1152/japplphysiol.01113.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18: 164–179, 2017. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry 15: 80–92, 2010. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terada J, Mitchell GS. Diaphragm long-term facilitation following acute intermittent hypoxia during wakefulness and sleep. J Appl Physiol (1985) 110: 1299–1310, 2011. doi: 10.1152/japplphysiol.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trulson ME, Trulson VM. Activity of nucleus raphe pallidus neurons across the sleep-waking cycle in freely moving cats. Brain Res 237: 232–237, 1982. doi: 10.1016/0006-8993(82)90572-8. [DOI] [PubMed] [Google Scholar]
- 72.Volgin DV, Stettner GM, Kubin L. Circadian dependence of receptors that mediate wake-related excitatory drive to hypoglossal motoneurons. Respir Physiol Neurobiol 188: 301–307, 2013. doi: 10.1016/j.resp.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Y, Tang D, Liu N, Xiong W, Huang H, Li Y, Ma Z, Zhao H, Chen P, Qi X, Zhang EE. Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab 25: 73–85, 2017. doi: 10.1016/j.cmet.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]