Abstract
There are examples of physiological conditions under which thirst is inappropriately exaggerated, and the mechanisms for these paradoxical ingestive behaviors remain unknown. We are interested in thirst mechanisms across the female life cycle and have identified a novel mechanism through which ingestive behavior may be activated. We discovered a previously unrecognized endogenous hypothalamic peptide, phoenixin (PNX), identified physiologically relevant actions of the peptide in brain and pituitary gland to control reproductive hormone secretion in female rodents, and in the process identified the previously orphaned G protein-coupled receptor Gpr173 to be a potential receptor for the peptide. Labeled PNX binding distribution in brain parallels areas known to be important in ingestive behaviors as well in areas where gonadal steroids feedback to control estrous cyclicity (Stein LM, Tullock CW, Mathews SK, Garcia-Galiano D, Elias CF, Samson WK, Yosten GLC, Am J Physiol Regul Integr Comp Physiol 311: R489–R496, 2016). We have demonstrated upregulation of Gpr173 during puberty, fluctuations across the estrous cycle, and, importantly, upregulation during the last third of gestation. It is during this hypervolemic, hyponatremic state that both vasopressin secretion and thirst are inappropriately elevated in humans. Here, we show that central administration of PNX stimulated water drinking in both males and females under ad libitum conditions, increased water drinking after overnight fluid deprivation, and increased both water and 1.5% NaCl ingestion under fed and hydrated conditions. Importantly, losartan pretreatment blocked the effect of PNX on water drinking, and knockdown of Gpr173 by use of short interfering RNA constructs significantly attenuated water drinking in response to overnight fluid deprivation. These actions, together with the stimulatory action of PNX on vasopressin secretion, suggest that this recently discovered neuropeptide may impact the recruitment of critically important neural circuits through which ingestive behaviors and endocrine mechanisms that maintain fluid and electrolyte homeostasis are regulated.
Keywords: angiotensin II, fluid and electrolyte homeostasis, phoenixin, salt appetite, thirst
INTRODUCTION
Using a bioinformatic approach coupled with traditional peptide purification and sequencing techniques in 2013, we (21) identified a novel hypothalamic peptide that we named phoenixin (PNX). Multiple posttranslational products are derived from the prohormone, most notably the COOH-terminally amidated 14- and 20-amino acid forms. In our hands, the 14- and the 20-amino acid forms exert the same bioactivity (21). We have focused our studies on phoenixin-20 amide (PNX-20), because it is the more abundant form found in rodents and in particular the hypothalamus (21). Labeled PNX-20 binding was most abundant in anterior pituitary gland and ovary, with specific binding also present in hypothalamus. In that initial discovery paper (21), we demonstrated that PNX-20 exerted pharmacological actions in hypothalamus and pituitary gland to control reproductive hormone secretion and ovarian function.
We then employed our patented “Deductive Reasoning Strategy” (20) to identify a potential receptor for PNX-20 to be the previously orphaned G protein-coupled receptor Gpr173 (17) and demonstrated that knockdown of receptor message with small interfering (si)RNA constructs prevented the pharmacological actions of PNX-20 in vitro and, importantly, in vivo. Furthermore, estrous cyclicity was interrupted by siRNA-induced knockdown of either the message for Gpr173 or that for PNX-20, establishing the physiological relevance of this putative ligand-receptor pair (17, 21). During our work, we localized PNX-like immunoreactivity and labeled PNX-20 binding to diencephalic and mesencephalic sites known to be important in not only reproductive function but also fluid and electrolyte homeostasis. In particular, endogenous peptide and labeled PNX binding localized to the paraventricular and supraoptic nuclei of the hypothalamus suggesting to us that PNX-20 might control neurohypophysial hormone secretion. Indeed, PNX-20 pharmacologically stimulated vasopressin (AVP) but not oxytocin (OT) release in vitro and in vivo, and the in vivo action was prevented by compromise of Gpr173 mRNA levels in hypothalamus (4).
Thus, in addition to the effects of the peptide on reproductive hormone secretion, actions related to the control of fluid and electrolyte homeostasis were established. Peptide binding in rostral hypothalamic sites related to salt and water ingestion (10) was also observed in our initial studies (17); thus, we hypothesized that PNX-20 might exert actions related to fluid intake that complemented those on AVP secretion. We detail herein experiments that demonstrated significant actions of PNX-20 on water and saline drinking as well as changes in PNX-20 and Gpr173 expression that occur across the female rat life cycle. Our studies led us to hypothesize that PNX interacting with Gpr173 might be important for the changes in fluid and electrolyte homeostasis that occur across the male and female life cycles, in particular being responsible for the paradoxically increased thirst and AVP secretion that occur during the hypervolemia and hyponatremia of third trimester pregnancy. The studies detailed here lay the groundwork for future work directly addressing that hypothesis.
METHODS
Animal Procedures
All procedures were approved by the Animal Care and Use Committee of Saint Louis University and complied with National Institutes of Health guidelines for animal welfare. Male and female Sprague-Dawley rats (200–225 g) were obtained from Envigo (Indianapolis, IN) and housed in cages of three until surgical procedures were conducted, after which they were housed individually. Rats were maintained on a 12:12-h light-dark (lights on at 0600) cycle at 23°C with ad libitum access to tap water and standard laboratory chow unless otherwise indicated. Prepubertal female rats (Envigo) were housed in groups of three and euthanized on 1 of 3 days: 2 days before the day of expected vaginal opening (23 days postweaning in our colony), on the day of vaginal opening, or 2 days following vaginal opening. Normal estrous cyclicity was verified by vaginal smears taken between 0800 and 1000 daily for at least two consecutive 4- to 5-day cycles. For breeding purposes, cycling female rats were housed with males (both Envigo) on the days of proestrus (defined as day 1) and estrus and thereafter housed individually. These animals were euthanized by decapitation 7, 14, and 20 days later, and pregnancy was verified by the presence of fetuses. Ovariectomized female rats (200–225 g) were purchased from Charles River (Worcester, MA) 7 days after surgery and housed locally until minimally 10 days after ovariectomy.
Surgery
An indwelling stainless steel cannula (23 gauge, 17 mm) was implanted into the right lateral cerebroventricle by use of a stereotaxic apparatus, as previously described (13). All animals were given a mixture of ketamine (60 mg/mL; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (8 mg/mL; TransquiVed, VedCo, Saint Joseph, MO) anesthetic intraperitoneally at 0.1 mL/100 g body wt with subcutaneous buprenorphine SR-LABORATORY analgesia (1.0 mg/mL, 0.1 mL/100 g body wt; ZooPharm, Laramie, WY).
Drinking Protocols
Graduated bottles were employed to measure water and/or 1.5% NaCl intakes visually, as previously described (9, 14, 18). All test substances were administered via indwelling right lateral cerebroventricular cannulas in a volume of 2 μL in sterile 0.9% NaCl.
Experiment 1.
Male rats were moved to a quiet testing location immediately after lights on, body weights were recorded, and then the rats were allowed to rest undisturbed for at least 2 h. Thirty minutes before experimentation, food and water bottles were removed. Injections (vehicle: 2 μL of sterile 0.9% NaCl; 1.0 or 3.0 nmol PNX-20 in vehicle) were made via the indwelling cerebroventricular cannulas, and 10 min later, water bottles were returned to the cages. Cumulative water intakes were measured 5, 15, 30, 45, 60 90, 120, 180, and 240 min later. Food was returned after the final sampling, and body weights and overnight water intakes were measured the following morning.
Experiment 2.
Vaginal smears confirmed stage of estrous cycle on the morning of experimentation. Rats were weighed and then moved to a quiet testing room, and the procedure described in experiment 1 followed, with the exception that only one dose of PNX-20 (3.0 nmol) was employed. Stage of estrous cycle was verified once again the following day when body weights and overnight water consumption were recorded.
Experiment 3.
Water bottles were removed from male rats before lights out (food remained present). After the food was weighed and removed on the following morning, rats were moved to a quiet testing room and left undisturbed for at least 2 h. Vehicle or vehicle containing 3.0 nmol PNX-20 was injected intracerebroventricularly (icv), and rats were left undisturbed for 10 min, after which water bottles were returned and sampling was completed as described in experiment 1.
Experiment 4.
In vivo compromise of Gpr173 expression was examined. Male rats, implanted with lateral cerebroventricular cannulas, were administered 2 μg of siRNA (121 pmol) targeting either enhanced green fluorescent protein (eGFP) or Gpr173 in 2 μL of saline on the afternoons of 2 consecutive days. Food remained available, but water was removed overnight after the second injections, and testing was conducted the following morning. Rats were weighed, moved to a quiet testing room, and left undisturbed for at least 2 h after removal of the food. Water bottles were replaced without arousing the rats, and monitoring of intakes was conducted as described above for 120 min only. After the 120-min sample was collected, rats were rapidly decapitated, and hypothalami were harvested for determination of Gpr173 expression as described below in Tissue Collections.
Experiment 5.
Male rats were prepared for experimentation as in experiment 1. There were four groups. Group 1 received an intracerebroventricular injection of vehicle and then 20 min later a second injection of vehicle was administered. Group 2 received an intracerebroventricular injection of the angiotensin type 1 receptor blocker losartan (5 µg in 2 μL of vehicle) and then vehicle 20 min later. Group 3 received vehicle and then 20 min later another injection of vehicle containing 3.0 nmol PNX-20 was administered. Group 4 received losartan (as in group 2) and then 3.0 nmol of PNX-20 in the second injection. Ten minutes later, water bottles were returned to the cages, and sampling was conducted as in experiment 1.
Experiment 6.
For examining water and saline drinking, male rats were habituated to the presence of water bottles and bottles containing 1.5% NaCl (saline) for 4 days before experimentation. On the day of experimentation, rats were weighed and moved to a quiet testing room. Food and drinking bottles were removed, and animals were left undisturbed for at least 2 h, after which either vehicle or vehicle containing 3.0 nmol PNX-20 was injected, and then the drinking bottles were only returned 10 min later. Sampling was conducted as in experiment 1.
Experiment 7.
Prepubertal female rats were housed in groups and euthanized by decapitation on 1 of 3 days: 2 days before the day of expected vaginal opening (23 days postweaning in our colony), on the day of vaginal opening, or 2 days following vaginal opening. Tissues were rapidly removed and prepared for RNA extraction.
Experiment 8A.
Female rats were euthanized by decapitation between 0900 and 1000 on each of the 4 days of the estrous cycle (verified by the presence of 2 consecutive 4- to 5-day cycles including on the day of tissue collection), and hypothalami were removed for RNA extraction.
Experiment 8B.
Hypothalami were harvested from female rats on the morning of diestrus and from ovariectomized rats between 0900 and 1000.
Experiment 9.
Diestrous female rats or timed pregnant females (days 7, 14, or 21 after mating) were euthanized by decapitation between 0900 and 1000. Trunk blood was collected for the determination of plasma levels of AVP, OT, or PNX. Hypothalami were harvested, divided into right and left halves, and prepared for either peptide or RNA extraction.
Peptides, Receptor Antagonist, and siRNA Constructs
PNX-20 (catalog no. 079-03) and angiotensin II (catalog no. 02-12) were purchased from Phoenix Pharmaceuticals (Burlingame, CA), solubilized in 0.001 N acetic acid, and divided into 0.01-mg aliquots that were subsequently dried in a vacuum desiccator and stored at −20°C. Aliquots were solubilized and diluted in sterile 0.9% NaCl to a dose of either 1.0 or 3.0 µg in 2 μL of saline. The angiotensin type 1 receptor blocker losartan potassium (catalog no. 61188) was obtained from Sigma-Aldrich (St. Louis, MO) and diluted in sterile 0.9% saline at a dose of 5 μg in 2 μL. siRNA constructs were designed by and purchased from Integrated DNA Technologies (Coralville, IA) targeting either Gpr173 or eGFP, as previously reported (16).
cDNA Synthesis and Quantitative RT-PCR
RNA was isolated from tissues by use of a PureLink RNA isolation kit (Life Technologies–Fisher Scientific), according to the manufacturer’s instructions. First-strand complementary DNA (cDNA) was synthesized using iScript reverse transcriptase followed by real-time PCR using iQ SYBR Green Master Mix and a Bio-Rad CFX96 Real-Time System (all Bio-Rad, Hercules, CA). The following Gpr173 primers were designed with PrimerQuest software developed by Integrated DNA Technologies (Coralville, IA): HPRT-1 (hypoxanthine-guanine phosphoribosyltransferase): forward, 5′-AGTCCCAGTGTCGTGATTAGTGAT-3′ and reverse 5′-CTCGAGCAAGTCTTTCAG TCCTGT3′; and Gpr173: forward 5′-CTGGCGAGTGTTTGTGAAAG-3′ and reverse 5′-TCTTGAGGTCCTTGTTAAGCA-3′. Primer specificity was confirmed using PrimerBlast (National Center for Biotechnology Information, Bethesda, MD). The sequences of primers for PNX have been reported previously (17). Changes in mRNA expression were calculated using the ΔΔCT method (15), and data were normalized to the housekeeping gene HPRT-1 (NM_012583.2).
Radioimmunoassays
The PNX-20 radioimmunoassay (catalog no. 079-03) was purchased from Phoenix Pharmaceuticals and employed according to the manufacturer’s instructions. Tissues were homogenized by sonication in 0.2 N acetic acid on ice and extracts centrifuged at 6,000 g for 5 min at 7°C. An aliquot of the supernatant was saved for determination of protein content (Bradford assay), and the remainder was dried in a rotary evaporator before reconstitution in assay buffer. AVP and OT contents were determined by radioimmunoassays developed by us as previously described (4, 11, 12).
Tissue Collections
To avoid the effects of prior anesthesia or restraint on tissue peptide of mRNA contents, rats were stunned and rapidly decapitated. Ovarian tissue was separated from the isthmus of the uterus and from adjacent fat tissue. The anterior pituitary gland was harvested after removal of the posterior pituitary. When whole hypothalamus was collected, the tissue was visually isolated by cuts just rostral to the optic chiasm, bilaterally along the hypothalamic sulcus, and at the posterior aspect of the mammillary bodies. Dorsally the dissection was completed at the level of the thalamic-hypothalamic interface. When smaller portions of the hypothalamus were required, the arcuate nucleus was dissected from the base of the hypothalamus using fine scissors, and the hypothalamus was divided into a rostral section, including the lamina terminalis, organum vaculosum of the lamina terminalis, the median preoptic area, and the subfornical organ. The medial portion of the hypothalamus included the supraoptic, suprachiasmatic, and paraventricular nuclei and portions of the anterior, lateral, and ventromedial hypothalamic nuclei. When collected, the brain stem tissue contained a coronal section from the level of the rostral to the caudal limits of the nucleus tractus solitarius, including the area postrema and the dorsal vagal complex.
Statistical Analyses
When results between two groups were compared for significance, a t test was employed. Differences among multiple groups were determined by analysis of variance with Tukey’s multiple comparison testing. Pearson’s correlation was employed when changes in peptide content across pregnancies were compared. Differences in mRNA expression between siRNA-treated groups were determined using the Mann-Whitney U test. A finding of P < 0.05 was considered significant. SPSS software was employed.
RESULTS
Experiment 1
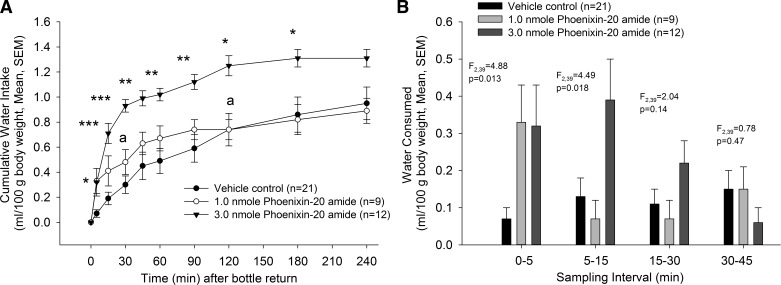
PNX-20 administration stimulated ad libitum water drinking in a dose-related fashion (Fig. 1). The effect was already observed 5 min after bottles were returned to the cages for both doses of the peptide compared with animals administered saline vehicle (both P < 0.05 vs. vehicle). Whereas no further significance was attained when comparing cumulative water intake between the 1.0 nmol PNX-20-dosed animals and controls at any further time point, animals in the 3.0 nmol treatment group continued to display significantly elevated water drinking until 4 h after bottles were returned to the cages. Significant differences were observed in water drinking between the 1.0 and the 3.0 nmol PNX-20-treated animals at 30 min (P < 0.01) and 120 min (P < 0.05) after water bottles were made available. When displayed as water consumed per sampling interval, the cumulative intake differences can be explained by the rapid effect of the peptide on water drinking (Fig. 1B). The amount of water consumed expressed in milliliters were as follows: 5-min sample, Control 0.2 ± 0.1 (mean ± SE), 1.0 nmol PNX 1.0 ± 0.3, 3.0 nmol PNX 0.8 ± 0.3, F2,39 = 5.13, P = 0.011; 30-min sample, Control 0.8 ± 0.2. 1.0 nmol PNX 1.4 ± 0.3, 3.0 nmol PNX 2.4 ± 0.1, F2,39 = 15.8, P < 0.001.
Fig. 1.
A: phoenixin-20 amide administered into the lateral cerebroventricle stimulated water drinking in male rats. Group sizes are indicated in parentheses. Data analyzed by ANOVA and Tukey’s test for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001 vs. controls; aP < 0.05 vs. intake in animals receiving the 3.0-nmol dose. B: same data as in A, expressed as amounts consumed during each time interval. Data analyzed by ANOVA.
Experiment 2
The stimulatory effect of PNX-20 also was observed in cycling female rats (Fig. 2) both on days when ovarian steroid levels would have been the lowest (diestrus) (16) and on estrus after exposure to ovulatory levels of estrogen and progesterone (n = 7–8) during the previous 12–18 h. Again, as in males, the stimulatory effect of 3.0 nmol PNX-20 was already present 5 min after water became available. In females, water consumption following PNX-20 administration remained significantly elevated over that observed in control animals for 90 min on both days (diestrus or estrus). There were no significant differences in water intakes between controls on diestrus or estrus or the amounts of water consumed following PNX-20 administration on diestrus or estrus. As in males, the effect of the peptide was observed at the initial 5-min sampling period, and this increase was responsible for the cumulative differences observed at later sampling points (Fig. 2B). The amount of water consumed (expressed in milliliters) was the following: 5-min sample, Estrus Control 0.1 ± 0.1 (mean ± SE), Estrus 3.0 nmol PNX 1.4 ± 0.2, Diestrus Control 0.3 ± 0.2, Diestrus 3.0 nmol PNX 1.9 ± 0.3, F3,27 = 19.38, P < 0.001; 30-min sample, Estrus Control 1.0 ± 0.3, Estrus 3.0 nmol PNX 2.1 ± 0.1, Diestrus Control 1.0 ± 0.3, Diestrus 3.0 nmol PNX 2.6 ± 0.3, F3,27 = 9.13, P < 0.001.
Fig. 2.
A: phoenixin-20 amide administration into the lateral cerebroventricle stimulated water drinking in cycling female rats. Group sizes are indicated in parentheses. Data analyzed by ANOVA and Tukey’s test for multiple comparisons. *P < 0.05 at these sampling times vs. intakes in animals administered vehicle. B: same data as in A, expressed as amounts consumed during each time interval. Data analyzed by ANOVA.
Experiment 3
In male rats denied water but not food overnight, lateral intracerebroventricular administration of 3.0 nmol PNX-20 (n = 10) resulted in significantly greater water intake than in vehicle-treated controls (n = 11), but the effect was present only at the earliest time points: 5 min (P < 0.05) and 15 min (P < 0.01) after water was made available (Fig. 3A). Intake that occurred during the initial 5-min period was the main contributing factor to the cumulative differences at the 15-min sampling point (Fig. 3B).
Fig. 3.
A: phoenixin-20 amide administration into the lateral cerebroventricle stimulated water drinking in male rats deprived of water overnight. Numbers in parentheses indicate group sizes. Differences between treatment groups determined by Student’s t test. *P < 0.05, **P < 0.01. B: same data as in A, expressed as amount consumed during each time intervals. Data analyze by t test.
Experiment 4
To determine whether the pharmacological effect of PNX-20 on water drinking following overnight water restriction was physiologically relevant, male rats were treated for 2 days with either the siRNA construct targeting Gpr173 expression (n = 9) or the control construct targeting eGFP expression (n = 7). Water was removed from the cages before lights out on the second day of siRNA administration. The following day, food was removed from the cages, and animals were weighed before being moved to a quiet testing room where they remained undisturbed. Water bottles were returned the following morning 3 h after lights on without being aroused by further injections or manipulations. Although animals in both groups slowly responded to the water availability, significantly less water was consumed in the Gpr173-compromised rats (90-min time point, P < 0.05; 120-min time point, P < 0.01; Fig. 4). Animals were euthanized by decapitation immediately after the 120-min sampling period, and hypothalami were removed for determination of Gpr173 mRNA levels. Compared with noninjected control rats, rats treated with eGFP siRNA exhibited a nonsignificant, 6% increase in Gpr173 mRNA expression, whereas a 45% decrease in Gpr173 mRNA levels was observed in rats treated with Gpr173 siRNA (P = 0.037 vs. eGFP siRNA-treated controls, as determined by Student’s t test).
Fig. 4.
Cumulative water drinking following overnight water deprivation in male rats pretreated with siRNA targeting either G protein-coupled receptor 173 (Gpr173) or enhanced green fluorescent protein (eGFP) expression. Numbers in parentheses indicate group sizes. Differences between treatment groups were determined by Student’s t test with significance levels indicated.
Experiment 5
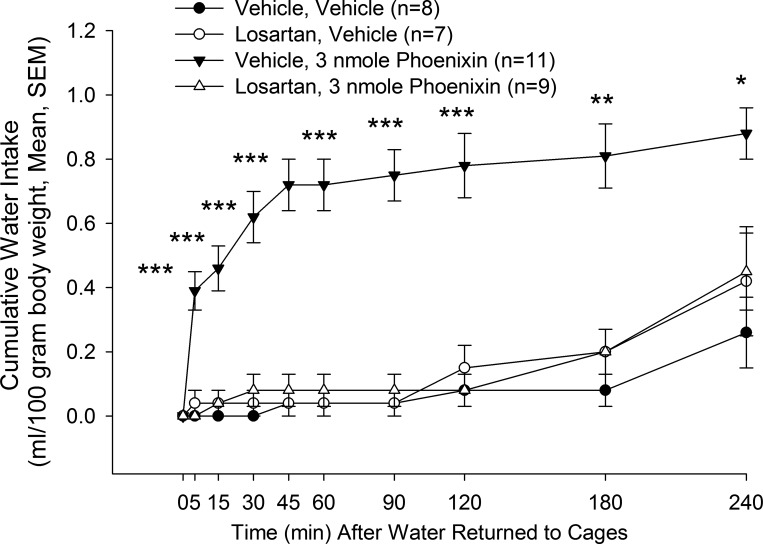
To examine a possible mechanism for the dipsogenic action of PNX-20, male rats were allowed ad libitum access to food and water until just before central administration of angiotensin type 1 receptor blocker losartan or vehicle control followed 20 min later by lateral intracerebroventricular injection of 3.0 nmol PNX-20 or vehicle control. Water bottles, but not food, were replaced 10 min after the second injection. As in a previous experiment, lateral intracerebroventricular administration of 3.0 nmol PNX-20 in vehicle-pretreated male rats (n = 11) resulted in a rapid increase in water drinking (Fig. 5). There were no significant differences in water intakes among three of the groups: losartan pretreated and vehicle injected (n = 7), losartan pretreated and PNX-20 administered (n = 9), and animals administered vehicle twice (n = 8). Thus, while PNX-20 administration significantly increased water drinking by itself, losartan pretreatment completely abrogated that effect (P < 0.001 at all of the time points up until 180 min, P < 0.01, P < 0.05 at 240 min).
Fig. 5.
Pretreatment with angiotensin type 1a receptor antagonist losartan blocked the action of phoenixin-20 amide (PNX-20) to stimulate water drinking in male rats. Numbers in parentheses indicate group sizes. Data analyzed by ANOVA and Tukey’s test for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001; water intake following PNX-20 administration vs. intakes in animals administered losartan and vehicle, losartan and PNX-20, or vehicle only.
Experiment 6
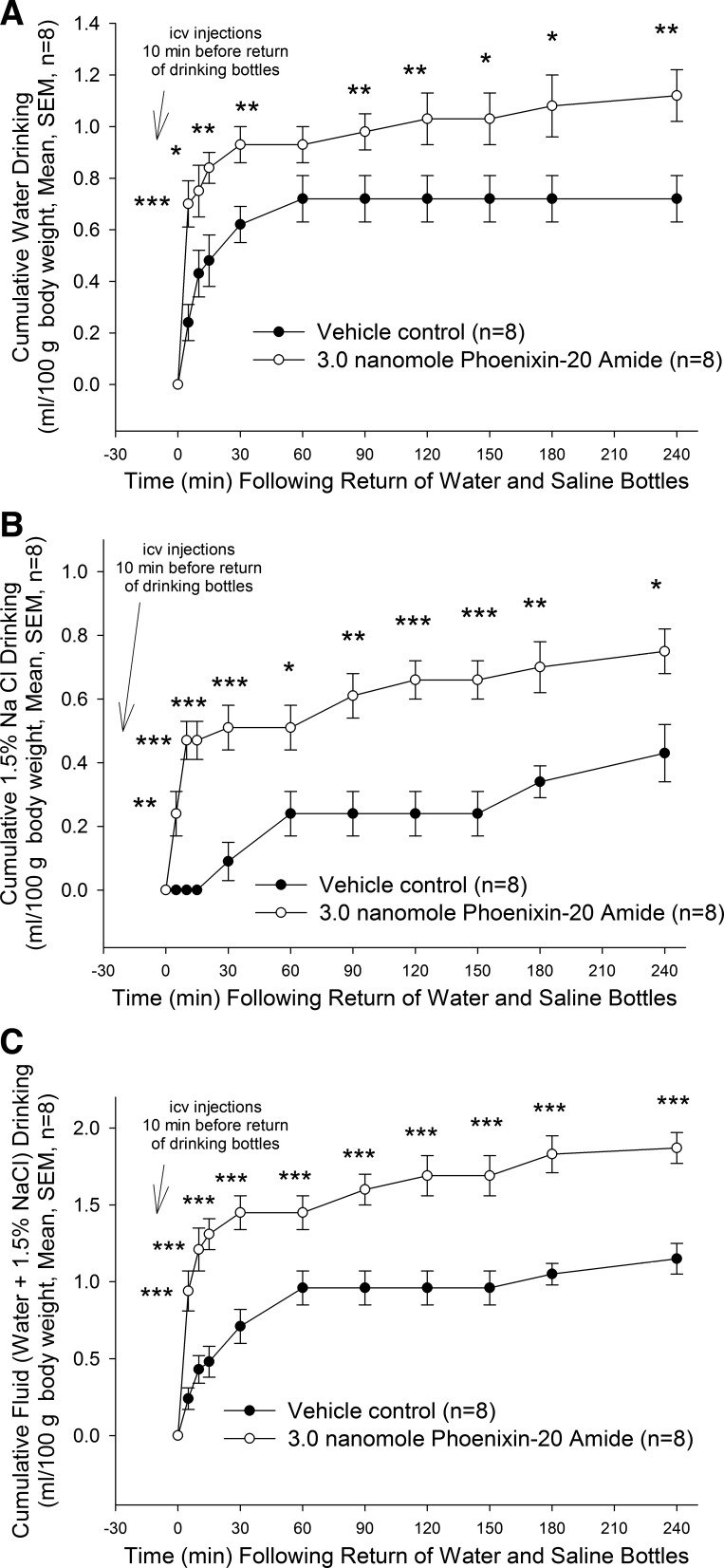
PNX-like immunoreactivity and labeled PNX binding were both localized to hypothalamic areas known to be important in the control of salt ingestion, suggesting a role for the peptide in sodium appetite (17, 21). To address this possibility, male rats were habituated to the availability of both tap water and a saline solution (1.5% sodium chloride) for 4 days. On the fifth day, drinking bottles and food were removed 30 min before lateral cerebroventricular administration of 3.0 nmol PNX-20 or vehicle. Drinking bottles were replaced 10 min after injections, and then ad libitum intake of water and saline solutions were monitored for 4 h (no food present during the observation period). A rapid and significant stimulation of both water and saline drinking was observed compared with vehicle-injected controls. As in previous experiments that examined only the action of PNX-20 on ad libitum water drinking, the peptide stimulated a rapid and significant increase in water intake already at 5 min upon bottle reintroduction (P < 0.001). The increased water intake remained significant throughout the experiment, with the exception of intakes at the 60-min sampling (Fig. 6A).
Fig. 6.
Phoenixin-20 amide administration into the lateral cerebroventricle stimulated water (A), saline (B), and total fluid intake (C) in male rats. Numbers in parentheses indicate group sizes. Differences between treatment groups were determined by Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle-treated controls.
Saline drinking in response to 3.0 nmol PNX-20 increased rapidly, attaining significance already at 5 min (P < 0.01) and continuing for the remainder of the sampling period (Fig. 6B). When combined fluid intakes were examined, animals administered 3.0 nmol PNX-20 consumed significantly more fluid than controls (Fig. 6C) at all time points (P < 0.001).
Experiment 7
To determine whether endogenous PNX-20 or Gpr173 might participate in changes in fluid homeostasis across the female life cycle, we examined the expression of the peptide in hypothalamus and the receptor in hypothalamic tissues, pituitary gland, and ovary during the pubertal transition. Whereas no significant changes in PNX mRNA levels were observed (data not shown) on the 3 days studied (2 days before expected vaginal opening, the day of vaginal opening, and 2 days following vaginal opening), significant elevations in Gpr173 mRNA levels were observed in all tissues after the pubertal event compared with those present before or on the day of vaginal opening (Fig. 7).
Fig. 7.
G protein-coupled receptor 173 (Gpr173) expression in hypothalamic tissue [arcuate nucleus, anteroventral paraventricular area (AVPV), hypothalamus at the level of the paraventricular area (PVN)], anterior pituitary gland, and ovary during the pubertal transition. Numbers in parentheses indicate groups sizes. Data analyzed by ANOVA and Tukey’s test for multiple comparisons. ***P < 0.001 vs. levels present before and during time of vaginal opening.
Experiment 8
We then asked whether hormone status affected the expression of PNX. When compared with PNX mRNA levels on diestrus day 2 (Fig. 8B), significantly lower PNX mRNA levels were observed in the hypothalamic extracts from ovariectomized rats (P < 0.01). On the other hand, hypothalamic Gpr173 mRNA levels were highest on diestrus day 2 (Fig. 8A) and dropped to the lowest levels on estrus (P < 0.05 diestrus day 2 vs. estrus).
Fig. 8.
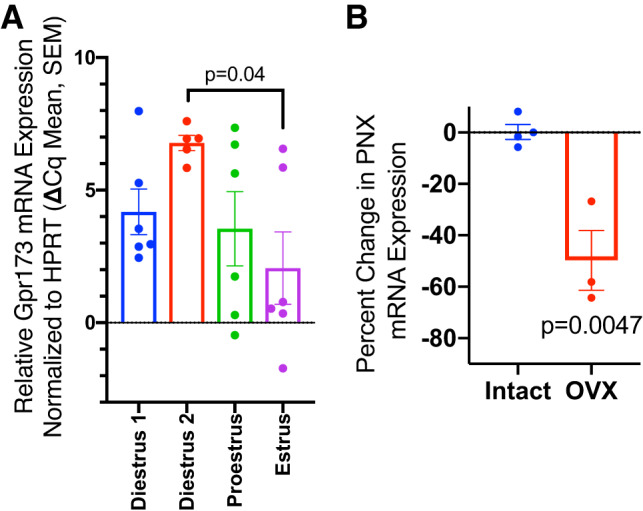
A: hypothalamic G protein-coupled receptor 173 (Gpr173) expression across the estrous cycle (Diestrus 1, n = 6; Diestrus 2, n = 5; Proestrus, n = 6; Estrus, n = 6). Mann-Whitney U test, significant difference indicated. B: relative phoenixin (PNX) expression in hypothalamus in ovariectomized (OVX) rats vs. intact, diestrous animals (Diestrus, n = 4, OVX, n = 3); Mann-Whitney U test, significant difference indicated.
Experiment 9
We then monitored PNX and Gpr173 levels during pregnancy and observed an increase in PNX-like immunoreactivity in hypothalamic extracts harvested from pregnant animals compared with that present in nonpregnant, diestrous rats, which progressed (Fig. 9A), attaining significance at the end of pregnancy (P < 0.05 vs. diestrous females). A parallel increase in AVP immunoreactivity (Fig. 9B), but not OT immunoreactivity, was observed in hypothalamic extracts. Across-pregnancy increases in AVP content correlated significantly (P < 0.01) with changes in PNX levels (Fig. 9C). No significant differences across the sampling intervals were observed in PNX-like immunoreactivity in either anterior pituitary gland or brain stem extracts (data not shown). Hypothalamic Gpr173 mRNA levels similarly increased across pregnancy, again attaining significance (P < 0.05) by day 20 of pregnancy compared with levels present in diestrous females (Fig. 9D).
Fig. 9.
Hypothalamic levels of phoenixin (PNX; A) and vasopressin (AVP; B) across pregnancy compared with levels present during diestrus (Diestrus, n = 6; day 7, n = 10; day 14, n = 8; day 20, n = 9). Data analyzed by ANOVA and Tukey’s test for multiple comparisons. ANOVA values and probabilities compared with levels present in diestrus are indicated. C: correlation of mean hypothalamic AVP and oxytocin (OT) levels with those of PNX on diestrus and days 7, 14, and 20 of pregnancy (Pearson’s test). D: percent change in hypothalamic G protein-coupled receptor 173 (Gpr173) expression relative to levels present in diestrus across pregnancy. Mann-Whitney U test, probabilities indicated vs. diestrus.
DISCUSSION
A bioinformatics approach allowed us to identify a previously unrecognized gene sequence, conserved across species, that encoded the novel peptide hormone PNX (21). The hypothalamus contained the most abundant PNX immunoreactivity of all tissues examined, and, in our initial studies, specific binding of labeled PNX peptide was highest in the anterior pituitary gland and ovary, with specific binding also present in hypothalamus. We previously demonstrated that PNX acts in both hypothalamus and pituitary gland to augment luteinizing hormone (LH) release in female rats (17, 21). We then employed our patented “Deductive Reasoning Strategy” (20) to identify the orphan G protein-coupled receptor Gpr173 as a potential PNX receptor and demonstrated that the actions of the peptide on reproductive hormone secretion could be blocked by compromise of the receptor in hypothalamus or the pituitary gland. During our characterization of the hypothalamic and extrahypothalamic sites that contained PNX-like immunoreactivity and labeled PNX binding, we observed signal for both peptide and binding in the supraoptic and paraventricular nuclei, as well as in rostral hypothalamic areas known to be important in the control of fluid and electrolyte homeostasis (10). Indeed, we (4) have demonstrated that PNX depolarized magnocellular neurons of the paraventricular nucleus in slice preparations, activated c-Fos expression in supraoptic nucleus neurons in vivo, and stimulated the release of AVP but not OT both in vitro and in vivo. This led us to examine the possibility that PNX contributes to the central nervous system control of fluid and electrolyte homeostasis not only by affecting AVP release but also by interacting with neural centers controlling thirst and sodium appetite. In addition, since changes in fluid and electrolyte homeostasis prevail across the female life cycle, we sought to determine whether PNX and Gpr173 levels are similarly altered by reproductive status.
PNX-20 significantly increased water drinking in both male and female rats. In females the effect was present both on the morning of diestrus day 2, when circulating levels of ovarian steroids over the prior 12–18 h would have been low (but hypothalamic Gpr173 mRNA levels were the highest) and on the morning of estrus after exposure to elevated estrogen and progesterone levels (16) during the previous 12–18 h (when hypothalamic Gpr173 mRNA levels were the lowest). We did not conduct a full dose response in females, and thus we may be underestimating the sensitivity of females to the peptide compared with males. Indeed, in both diestrous and estrous females, the magnitude of the 5-min responses to 3.0 nmol PNX (almost 1.0 mL/100 g body wt) exceeded that in males (~0.4 mL/100 g body wt).
PNX-20 not only stimulated ad libitum water drinking but also transiently increased water drinking in male rats subjected to overnight water deprivation. The physiological relevance of endogenous PNX in the control of water drinking is strongly suggested by our observation that compromise of hypothalamic Gpr173 expression significantly reduced compensatory water intake following fluid restriction. The potential mechanism of PNX’s action was revealed when animals were pretreated with the angiotensin type 1 receptor blocker losartan before PNX administration. Using a dose of losartan reported by others (19) and verified by us in a parallel study (data not shown) to block the dipsogenic action of centrally administered angiotensin II, we observed that the dipsogenic action of PNX-20 was completely abrogated by the angiotensin receptor blocker. The possible link between PNX and the central angiotensin system merits further investigation.
It is important to point out that all in vivo drinking experiments reported here were conducted in the absence of food. This was intentional to avoid the possible contribution of prandial water drinking to our protocols (5–7, 22). Indeed, this also was important when examining the effect of PNX on saline drinking, since solutes generated postprandially might affect saline ingestion behavior before or following absorption from the gastrointestinal tract. Under these conditions, a robust stimulation of 1.5% NaCl solution was observed. Also, it should be pointed out that these experiments were designed to examine treatment effects; thus, any interaction with time cannot be determined. Because of the relatively rapid action of PNX in both males and females, future studies will have to employ a more exacting analysis including intake patterns and lick microstructure. Time and treatment effect interactions can best be determined under those conditions.
Hormone response elements have been reported present upstream of the promotor sequences for both PNX and Gpr173 (http://epd.epfl.ch//index.php). When examining the possible effects of ovarian steroids on PNX message levels in hypothalamus, we observed that PNX mRNA levels were significantly lower in the hypothalami of ovariectomized animals than in cycling females on diestrus. This raised the possibility that ovarian steroids regulate the expression of PNX and that the expression of either PNX itself or Gpr173 might change depending on hormone status. Indeed, this is the case. Across the estrous cycle, hypothalamic Gpr173 mRNA levels fluctuated, with the highest present on diestrus day 2 and the lowest on estrus. Furthermore, the steroid surges that accompany the pubertal event may control receptor expression in a variety of PNX-responsive tissues. Importantly, we have identified significant increases in PNX and Gpr173 mRNA levels as pregnancy progresses, suggesting a possible role for the peptide and receptor in the development of the hypervolemia (1–3) and thirst (2, 8) experienced during pregnancy.
Perspectives and Significance
In addition to our novel descriptions of PNX and its interaction with the orphan G protein coupled receptor Gpr173 in vivo and in vitro, we now propose that the reproductive actions of the peptide may be associated with its effects on fluid retention, via stimulation of AVP release, and water and solute ingestion, resulting in a coordination of the two events at the hypothalamic level. It will be important to develop additional approaches to establish the physiological relevance of the actions on fluid and electrolyte homeostasis across the female life cycle. Our knowledge of the distribution of Gpr173 expression in brain is currently limited to our previously published labeled PNX binding (17), because we have not yet found a commercially available Gpr173 with sufficient specificity to convincingly demonstrate selectivity. In the absence of a truly specific antibody, we have turned in current studies to the use of the RNAscope approach to identify cell-specific Gpr173 expression and are attempting to identify the chemical and genetic profile of those cells. In addition, we recognize the importance of seeking funding to commission the development of a transgenic model with which to extend these initial studies, and we are pursuing that alternative now.
GRANTS
This study was supported by the Saint Louis University President’s Research Fund and by National Institutes of Health Grants HL-121456 and DK-118340.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.H., G.L.C.Y., and W.K.S. conceived and designed research; C.J.H., G.A.-P., L.M.S., G.L.C.Y., and W.K.S. performed experiments; C.J.H., G.A.-P., G.L.C.Y., and W.K.S. analyzed data; C.J.H., G.A.-P., G.L.C.Y., and W.K.S. interpreted results of experiments; C.J.H., G.L.C.Y., and W.K.S. prepared figures; C.J.H. and W.K.S. drafted manuscript; C.J.H., G.A.-P., L.M.S., G.L.C.Y., and W.K.S. edited and revised manuscript; C.J.H., G.A.-P., L.M.S., and G.L.C.Y. approved final version of manuscript.
REFERENCES
- 1.Brunton PJ, Arunachalam S, Russel JA. Control of neurohypophysial hormone secretion, blood osmolality and volume in pregnancy. J Physiol Pharmacol 59, Suppl 8: 27–45, 2008. [PubMed] [Google Scholar]
- 2.Davison JM, Gilmore EA, Dürr J, Robertson GL, Lindheimer MD. Altered osmotic thresholds for vasopressin secretion and thirst in human pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 246: F105–F109, 1984. doi: 10.1152/ajprenal.1984.246.1.F105. [DOI] [PubMed] [Google Scholar]
- 3.Davison JM, Shiells EA, Philips PR, Lindheimer MD. Influence of humoral and volume factors on altered osmoregulation of normal human pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 258: F900–F907, 1990. doi: 10.1152/ajprenal.1990.258.4.F900. [DOI] [PubMed] [Google Scholar]
- 4.Gasparini S, Stein LM, Loewen SP, Haddock CJ, Soo J, Ferguson AV, Kolar GR, Yosten GLC, Samson WK. Novel regulator of vasopressin secretion: phoenixin. Am J Physiol Regul Integr Comp Physiol 314: R623–R628, 2018. doi: 10.1152/ajpregu.00426.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kissileff HR. Food-associated drinking in the rat. J Comp Physiol Psychol 67: 284–300, 1969. doi: 10.1037/h0026773. [DOI] [PubMed] [Google Scholar]
- 6.Kissileff HR. Oropharyngeal control of prandial drinking. J Comp Physiol Psychol 67: 309–319, 1969. doi: 10.1037/h0026774. [DOI] [PubMed] [Google Scholar]
- 7.Kraly FS. Eating provides important physiological signals for satiety and drinking. Physiol Behav 82: 49–52, 2004. doi: 10.1016/j.physbeh.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Lindheimer MD, Davison JM. Osmoregulation, the secretion of arginine vasopressin and its metabolism during pregnancy. Eur J Endocrinol 132: 133–143, 1995. doi: 10.1530/eje.0.1320133. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Yosten GL, Ji H, Zhang D, Zheng W, Speth RC, Samson WK, Sandberg K. Selective inhibition of angiotensin receptor signaling through Erk1/2 pathway by a novel peptide. Am J Physiol Regul Integr Comp Physiol 306: R619–R626, 2014. doi: 10.1152/ajpregu.00562.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clin Exp Pharmacol Physiol 23, Suppl 3: S99–S104, 1996. doi: 10.1111/j.1440-1681.1996.tb02821.x. [DOI] [PubMed] [Google Scholar]
- 11.Samson WK. Atrial natriuretic factor inhibits dehydration and hemorrhage-induced vasopressin release. Neuroendocrinology 40: 277–279, 1985. doi: 10.1159/000124085. [DOI] [PubMed] [Google Scholar]
- 12.Samson WK, McDonald JK, Lumpkin MD. Naloxone-induced dissociation of oxytocin and prolactin releases. Neuroendocrinology 40: 68–71, 1985. doi: 10.1159/000124053. [DOI] [PubMed] [Google Scholar]
- 13.Samson WK, Murphy TC, Resch ZT. Central mechanisms for the hypertensive effects of preproadrenomedullin-derived peptides in conscious rats. Am J Physiol Regul Integr Comp Physiol 274: R1505–R1509, 1998. doi: 10.1152/ajpregu.1998.274.5.R1505. [DOI] [PubMed] [Google Scholar]
- 14.Samson WK, White MM, Price C, Ferguson AV. Obestatin acts in brain to inhibit thirst. Am J Physiol Regul Integr Comp Physiol 292: R637–R643, 2007. doi: 10.1152/ajpregu.00395.2006. [DOI] [PubMed] [Google Scholar]
- 15.Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, Chen C. Real-time PCR quantification of precursor and mature microRNA. Methods 44: 31–38, 2008. doi: 10.1016/j.ymeth.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96: 219–226, 1975. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 17.Stein LM, Tullock CW, Mathews SK, Garcia-Galiano D, Elias CF, Samson WK, Yosten GLC. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol Regul Integr Comp Physiol 311: R489–R496, 2016. doi: 10.1152/ajpregu.00191.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein LM, Yosten GL, Samson WK. Adropin acts in brain to inhibit water drinking: potential interaction with the orphan G protein-coupled receptor GPR19. Am J Physiol Regul Integr Comp Physiol 310: R476–R480, 2016. doi: 10.1152/ajpregu.00511.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vento PJ, Daniels D. Repeated administration of angiotensin II reduces its dipsogenic effect without affecting saline intake. Exp Physiol 95: 736–745, 2010. doi: 10.1113/expphysiol.2010.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yosten GLC, Kolar GR, Redlinger LJ, Samson WK. Evidence for an interaction between proinsulin C-peptide and GPR146. J Endocrinol 218: B1–B8, 2013. doi: 10.1530/JOE-13-0203. [DOI] [PubMed] [Google Scholar]
- 21.Yosten GLC, Lyu RM, Hsueh AJ, Avsian-Kretchmer O, Chang JK, Tullock CW, Dun SL, Dun N, Samson WK. A novel reproductive peptide, phoenixin. J Neuroendocrinol 25: 206–215, 2013. doi: 10.1111/j.1365-2826.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol 288: R1450–R1467, 2005. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]