Abstract
T-helper (TH)17s, IL-17, and cytolytic natural killer cells (cNKs) are increased in preeclampsia and contribute to the hypertension, inflammation, and fetal growth restriction that occurs in response to placental ischemia in the reduced uterine perfusion pressure (RUPP) rat model of preeclampsia. As IL-17 stimulates NK cytotoxicity in vitro, we tested the hypothesis that IL-17 inhibition in RUPP rats would decrease cNK activation as a mechanism to improve maternal and fetal outcomes. On gestation day (GD) 14, rats undergoing RUPP received a miniosmotic pump infusing IL-17RC (100 pg/day), a soluble IL-17 receptor (RUPP + IL-17RC). On GD19, mean arterial pressure (MAP) was measured in normal pregnant (NP), RUPP, and RUPP + IL-17RC rats (n = 10–12/group), animals were euthanized, and blood and tissues were collected for analysis. MAP was 30% higher in RUPP compared with NP (P < 0.0001) and was 12% lower in RUPP + IL-17RC (P = 0.0007 vs. RUPP). Placental cytolytic NK cells were 132% higher in RUPP than in NP (P = 0.04 vs. NP) and were normalized in RUPP + IL-17RC (P = 0.03 vs. RUPP). Placental levels of TNF-α, a cNK-secreted cytokine, and macrophage inflammatory protein-3α (MIP-3α), a cNK chemokine, were higher in RUPP vs. NP and lower after IL-17 blockade. Placental VEGF was lower in RUPP vs. NP and was normalized in RUPP + IL-17RC. In vitro cytolytic activity of RUPP placental NKs was higher compared with NP and was blunted in RUPP + IL-17RC NKs. Finally, both fetal weight and placental weight were lower in RUPP compared with NP, and were improved in RUPP + IL-17RC. These data identify IL-17 as a mediator of cNK activation in response to placental ischemia during pregnancy.
Keywords: hypertension, IL-17, natural killer cells, placental ischemia, preeclampsia
INTRODUCTION
Preeclampsia (PE) is a severe pregnancy disorder characterized by new onset hypertension after the 20th week of gestation with fetal growth restriction, proteinuria, thrombocytopenia, liver dysfunction, pulmonary edema, or cerebral disturbances (10, 43, 92). Despite being a significant contributor to maternal and fetal mortality, fetal growth restriction, and preterm births, the pathophysiology of PE remains poorly understood (43, 47, 96). The initiating event is believed to be insufficient invasion of the myometrium and endometrium by cytotrophoblasts that results in impaired remodeling of maternal vessels and placental ischemia (34, 69, 109). Placental factors such as soluble fms tyrosine kinase-1 (sFlt-1), soluble endoglin (sEng), angiotensin II type 1 receptor auto antibodies (AT1AA), and inflammatory cytokines are secreted in response to the ischemic conditions. This results in maternal endothelial dysfunction and contributes to the pathology of the disease (49, 55, 73, 102).
Several distinct immunological imbalances have been observed in preeclamptic women compared with women with normal pregnancies (74, 77). While normal pregnancy is characterized by a predominance of T-helper (TH)2 cells and their associated cytokines interleukin (IL)-10 and IL-4, both TH1 cells and their associated cytokines IL-6, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) are significantly increased in the circulation of PE patients (23, 75, 76). Additionally, it has been recognized that PE women have an increased proinflammatory TH17 cell to T-regulatory (Treg) cell ratio compared with their normal pregnant counterparts (21, 26, 29, 44). TH17s mainly secrete IL-17, and this cytokine is significantly increased in the serum of PE women (22, 64).
More recent research has focused on the imbalance of natural killer (NK) cell subsets observed in PE. Normally, decidual NK (dNK) cells have little cytotoxic activity and play a supportive role during early pregnancy before decreasing to low numbers during the third trimester (18, 41, 45, 63). However, several studies have identified that women with PE have a significantly higher population of NK cells in the decidua and placenta at term compared with normal pregnant women (4, 18). Furthermore, NK cells of PE women demonstrate enhanced cytolytic activation (cNK) with increased secretion of IFN-γ and TNF-α; however, the cause of this activation remains unclear (17, 30, 68, 76, 79). Our current focus is to better understand the stimuli for cNK cell activation and how these cells contribute to PE pathophysiology and progression.
The reduced uterine perfusion pressure (RUPP) rat is an established and well-characterized model of placental ischemia that mimics many of the characteristics of human preeclampsia, including increases in mean arterial pressure (MAP), sEng, sFlt-1, oxidative stress, AT1-AA, renal vasoconstriction, and inflammation as well as decreased glomerular filtration rate (GFR) and fetal growth restriction (7, 8, 32, 36–38, 53, 54, 81, 85, 100). In the RUPP model, placental ischemia is induced by surgical placement of clips during late pregnancy and therefore cannot be used to investigate early mechanisms leading to the development of placental ischemia, However, this model has been extensively used by our laboratory and others to examine immunological and pathophysiological mechanisms that contribute to PE progression during late pregnancy (9, 13, 24, 31, 56, 78, 95, 101, 103, 104). Similar to what is observed in preeclamptic women, we have previously reported an increase in TH17 cells and cytolytic NK cells in RUPP rats. In a separate study, we showed that adoptive transfer of RUPP TH17 cells into normal pregnant rats resulted in a PE-like phenotype and increased cNK cell activation (84). Furthermore, we recently published that chronic infusion of IL-17 into pregnant rats stimulated increased cNK activation, cytolytic proteins, endothelial dysfunction, oxidative stress, and inflammatory cytokines (93). Therefore, we hypothesize that inhibiting IL-17 in placental ischemic rats will decrease cNK cell activation and cytotoxic activity as a mechanism to improve maternal and fetal outcomes. To test this hypothesis, we infused 100 pg/day of soluble IL-17 receptor C (IL-17RC) into RUPP rats, a dose that we have previously used to inhibit IL-17 signaling and improve maternal and fetal outcomes (19). We then evaluated circulating and placental NK cell profiles, levels of inflammatory and cytolytic proteins, and in vitro NK cell cytolytic activity. We also evaluated placental oxidative stress, maternal MAP, placental weight, and fetal outcomes.
MATERIALS AND METHODS
Twelve- to thirteen-week-old, timed pregnant Sprague-Dawley rats purchased from Envigo RMS (Indianapolis, IN) were used in this study. The animals were delivered to the Center for Comparative Research at the University of Mississippi Medical Center on day 10 or 11 of their gestation and weighed ~250–260 g on delivery. The animals were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle and maintained on Teklad 8640 diet (Envigo). The rats were group housed until surgery was performed, and rats were randomly assigned to experimental groups. All experimental procedures conducted in this study were in accordance with the National Institute of Health guidelines for the use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Reduction in uterine perfusion pressure.
On gestation day (GD) 14, a subset of timed pregnant Sprague-Dawley rats, underwent the RUPP surgery under isoflurane anesthesia delivered by an anesthesia apparatus (Ohio Medical Products, Madison, WI). Briefly, a midline incision was made, and a constrictive silver clip (0.203 mm) was placed on the abdominal aorta superior to the iliac bifurcation. To prevent compensatory blood flow via the ovarian arteries, restrictive silver clips (0.100 mm) were applied to the bilateral uterine arcades at the ovarian end. The animals received carprofen immediately following surgery and 24 h postsurgery (5 mg/kg) to control for postoperative pain. Rats were excluded when the procedure resulted in total reabsorption of all fetuses.
IL-17RC infusion.
A subset of rats undergoing the RUPP procedure also received a miniosmotic pump (Alzet model 2002, Alzet Scientific, Cupertino, CA) that infused 100 pg/day of recombinant mouse IL-17 receptor C (IL-17RC) (R&D Systems Minneapolis, MN) into the intraperitoneal cavity from GD14–19. Murine IL-17RC has 87% homology and 86% identity to rat IL-17RC indicating high biological similarity to the naturally occurring rat protein. Furthermore, several in vitro and in vivo studies have shown that soluble IL-17RC is a potent inhibitor of IL-17 signaling by binding to and neutralizing both IL-17A and IL-17F (12, 51, 105). The dose administered in this study was determined based on binding ability of the soluble receptor to IL-17 A-F, as performed by the manufacturer. Furthermore, our laboratory has previously used this dose to inhibit circulating and placental TH17 cell populations. Three groups were examined in this study: normal pregnant (NP), RUPP, and RUPP + IL-17RC (n = 10–12 rats/group).
Measurement of mean arterial pressure in conscious rats.
Under isoflurane anesthesia, 0.58-mm inner diameter × 0.99-mm outer diameter vinyl catheter tubing (Scientific Commodities, Lake Havasu City, AZ) was implanted into the carotid arteries and tunneled to the back of the neck on GD18 for the measurement of mean arterial pressure. On GD19, rats were placed in individual restrainers and conscious mean arterial pressure (MAP) was monitored with a pressure transducer (Powerlab, AD Instruments, Colorado Springs, CO). The MAP was recorded for 30 min following a 30-min stabilization period.
Sample collection.
After MAP measurement, the animals were anesthetized for blood and tissue collection. Tissues collected from the placentation site consisted of the decidua, junctional, and labyrinth zones. Total litter size as well as the number of live pups were recorded. Placental and fetal weights were recorded for each dam and averaged. Randomly selected placenta tissues were snap frozen in liquid nitrogen and stored at −80°C until analyses.
Determination of circulating and placental NK and TH17 cell populations using flow cytometry.
Single-cell suspensions of leukocytes were prepared as previously described by our group (93). Briefly, one placenta from each rat was homogenized and filtered through a 70-μm cell strainer and resuspended in 10 mL of Roswell Park Memorial Institute medium (RPMI) (10% FBS). Whole blood was collected in an EDTA tube and diluted with 5 mL of RPMI. Peripheral blood mononuclear cells (PBMCs) and placental lymphocytes were isolated by centrifugation on a cushion of Ficoll-Isopaque (Lymphoprep, Accurate Chemical & Scientific, Westbury, NY) according to manufacturer instructions. Single-cell suspensions (1 × 106 cells) were stained for flow cytometry after blocking with 10% goat and mouse serum. Antibodies used for flow cytometry were as follows: VioGreen-conjugated anti-CD3 (Miltenyi Biotec, Auburn, CA), anti-ANK61 antibody (Abcam, ab36392), anti-mouse IgG fluorescein isothiocyanate (FITC) (Abcam, ab97239), anti-ANK44 (Abcam, ab36388), anti-mouse IgG AlexaFluor 405 (Abcam, ab175663), FITC-conjugated anti-CD4 antibody (Miltenyi), phycoerythrin (PE)-conjugated anti-CD25 (BD PharMingen, Clone OX-39), and peridinin-chlorophyll-protein (PerCP)-conjugated anti-RORγt (R&D Systems, Clone 600380). Flow cytometry was performed on the Miltenyi MACSQuant Analyzer 10 (San Diego, CA) and analyzed using FlowLogic software (Innovai, Sydney, Australia). Lymphocytes were gated in the forward and side scatter plot. After doublet exclusion, additional gates were set using fluorescence minus one (FMO) controls. CD3−ANK 61+ cells comprised total NK cells, and CD3−ANK61+ANK44+ cells comprised the activated NK cell population. CD4+CD25−RORγ+ cells comprised the TH17 cell population. Results are expressed as percentage of cells in the gated lymphocyte population.
Natural killer cell isolation.
On GD19, two placentas were collected from each group and single cell suspensions of lymphocytes were isolated as previously described (93). Anti-CD3 and anti-CD161 antibodies (BD Biosciences San Jose, CA) were biotinylated using the DSB-X protein labeling kit (Life Technologies, Grand Island, NY), according to the manufacturer’s instructions. The isolated lymphocytes were incubated with the biotin-labeled anti-CD3 and the CD3+ population was isolated using FlowComp Dynabeads Flexi Kit (Invitrogen, Oslo, Norway) according to the manufacturer’s instructions. The supernatant containing the CD3− population of lymphocytes was then incubated with biotin labeled anti-CD161 antibody. This solution was incubated again with FlowComp Dynabeads and the CD3−CD161− supernatant was removed. The CD3− CD161+ cells bound to the FlowComp Dynabeads were detached using release buffer, and the biotin-labeled antibodies were separated according to the manufacturer’s protocol before culture and expansion. The collected cells were resuspended in NK cell media: RPMI, 10% FBS, 1% penicillin-streptomycin (Pen/Strep), and 2 ng/mL recombinant rat IL-2 (R&D Systems, Minneapolis, MN), seeded at 3 × 105 cells/well in a 6-well plate, and allowed to expand for 48 h.
Assessment of natural killer cell cytotoxicity.
Cytotoxic activity of isolated NK cells was assessed using the Cytotox 96 Non-Radioactive Cytotoxicity Assay Kit (Promega, San Luis Obispo, CA) according to the manufacturer’s instructions. YAC1 cells (ATCC, Manassas, VA,) served as target cells. The assay was performed in quadruplicate using an NK cell to target cell ratio of 50:1 with a 5-h incubation time. Percent cytotoxicity was calculated using the formula provided in the manufacturer’s instructions. The results are expressed as fold change in cytotoxic activity.
Placental reactive oxygen species measurement.
Superoxide production in the placenta was measured using the lucigenin technique, as previously described by our laboratory (93). Briefly, placentas chosen at random from NP, RUPP, and RUPP + IL-17RC rats were snap frozen in liquid nitrogen immediately after collection and stored at −80°C until further processing. Placentas were homogenized using the Bio-Rad Cell Lysis Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. The tissue lysate was incubated with lucigenin (Sigma-Aldrich, St. Louis, MO) at a concentration of 5 μM. The samples were allowed to equilibrate for 15 min in the dark, and the luminescence was measured for 10 s with a BioTek Plate Reader (BioTek, Winooski, VT). Luminescence was recorded as relative light units per minute (RLU/min). An assay blank containing lucigenin with no homogenate was subtracted from the reading before transformation of the data. Each sample was run in triplicate, and the average was used for data transformation. The protein concentration was measured using a protein assay with BSA standards (Pierce, Rockford, IL). All data were normalized to protein concentration and are expressed as RLU per minute per mg protein.
Measurement of circulating and placental inflammatory mediators and cytolytic proteins.
Circulating and placental levels of IL-6, IL-12, IL-17, TNF-α, IFN-γ, macrophage inflammatory protein-3α (MIP-3α), and vascular endothelial growth factor (VEGF) were measured using the Bio-Plex Pro Rat Cytokine Immunoassay Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Plasma sFlt-1 levels were measured via commercial ELISA (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. Circulating and placental levels of cytolytic proteins perforin, granzyme A, granzyme B, and granzyme K were measured using commercial ELISA kits (MyBioSource, San Diego, CA) according to the manufacturer’s instructions. All sample analyses were performed in duplicate. Protein concentration of the placental homogenates was measured using a protein assay with BSA standards. All placental data were normalized to protein (expressed as pg/mg).
Statistical analysis.
All of the data are expressed as means ± SD. Statistical analyses were performed using one-way ANOVA with multiple comparisons followed by Tukey’s post hoc correction via GraphPad Prism 8 software. P < 0.05 was considered statistically significant.
RESULTS
Effects of IL-17 inhibition on IL-17 and TH17 populations.
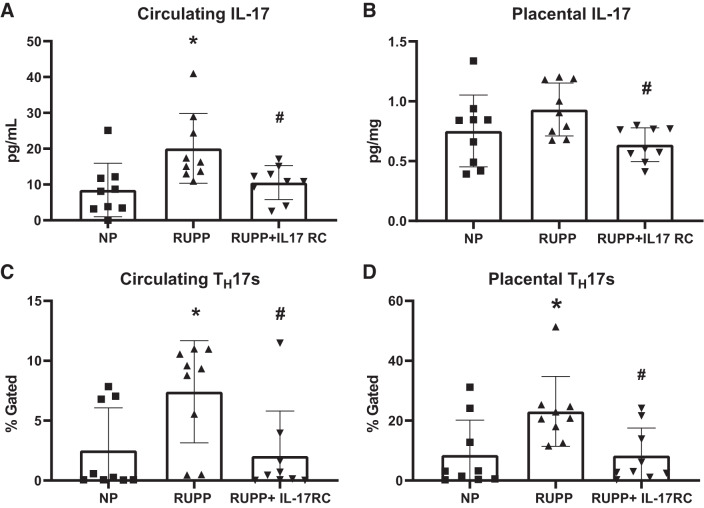
Circulating IL-17 was higher in RUPP at 20 ± 10 pg/mL compared with 8 ± 7 pg/mL in NP to (P = 0.01 vs. NP), and this was normalized to 11 ± 5 pg/mL in RUPP + IL-17RC (P = 0.03 vs. RUPP; Fig. 1A). Placental IL-17 was 0.75 ± 0.30 pg/mg in NP and 0.93 ± 0.22 pg/mL in RUPP (P = 0.24 vs. NP); however, the levels were lower in RUPP + IL-17RC with a mean of 0.64 ± 0.14 pg/mL (P = 0.0311 vs. RUPP; Fig. 1B).
Fig. 1.
Chronic inhibition of IL-17 in reduced uterine perfusion pressure (RUPP) rats normalizes circulating and placental IL-17 and T-helper (TH)17 cell populations. A subset of pregnant rats undergoing the RUPP procedure also received a miniosmotic pump infusing 100 pg/day of a soluble IL-17 receptor (IL-17RC) into the intraperitoneal cavity from gestational days (GD) 14–19. On GD19, blood and placentas were collected, processed, and analyzed via ELISA and flow cytometry to obtain levels of circulating (A) and placental (B) IL-17 and percentages of circulating (C) and placental (D) TH17 cells. Normal pregnant (NP): n = 9 rats; RUPP: n = 9 rats RUPP + IL-17RC: n = 9 rats. All data are expressed as means ± SD. Statistical analysis was performed using one-way ANOVA with multiple comparisons followed by Tukey’s post hoc correction. *P < 0.05 vs. NP; #P < 0.05 vs. RUPP.
Consistent with our previous observations, circulating TH17s were 194% higher in RUPP compared with NP, (P = 0.03 vs. NP), while treatment with IL-17RC normalized circulating TH17s in the RUPP + IL-17RC group (P = 0.02 vs. RUPP; Fig. 1C). Placental TH17s were 170% higher in RUPP rats compared with NP (P = 0.02 vs. NP) and were normalized in RUPP + IL-17RC (P = 0.02 vs. RUPP; Fig. 1D). The gating strategy for TH17 analyses is shown in Supplemental Fig. S1B (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.11916666).
Effect of IL-17 inhibition on maternal MAP and fetal outcomes.
MAP was 30% higher in RUPP compared with NP (P < 0.0001 vs. NP), and RUPP + IL-17RC was 12% lower than RUPP (P = 0.0007 vs. RUPP) although it remained higher than NP (P = 0.012 vs. NP; Fig. 2). We also observed improved fetal outcomes following chronic infusion of IL-17RC, and these data are summarized in Table 1. Fetal weight was 12% lower in RUPP compared with NP (P = 0.0002 vs. NP) and was normalized in RUPP + IL-17RC (P = 0.035 vs. RUPP). Similarly, placental weight was 22% lower in RUPP compared with NP (P = 0.0005 vs. NP) and was normalized in RUPP recipients of IL-17RC (P = 0.01 vs. RUPP). Total litter size was unchanged between all groups, but the number of live pups at the time of euthanization was 47% lower in RUPP compared with NP (P < 0.0001 vs. NP) and was 42% higher in RUPP + IL-17RC compared with RUPP (P = 0.02 vs. RUPP).
Fig. 2.
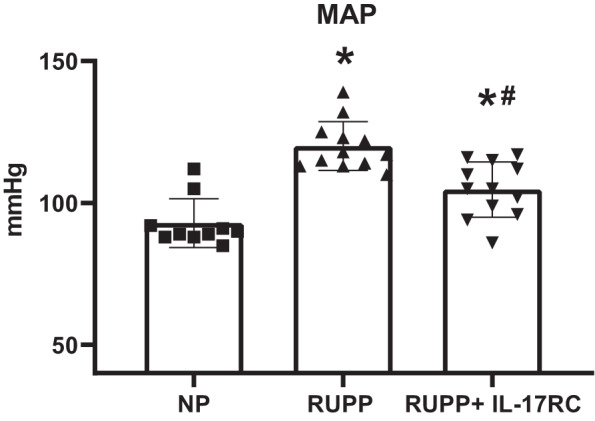
Chronic inhibition of IL-17 in reduced uterine perfusion pressure (RUPP) rats improved maternal mean arterial pressure (MAP). A subset of pregnant rats undergoing the RUPP procedure also received a miniosmotic pump infusing 100 pg/day of a soluble IL-17 receptor (IL-17RC) into the intraperitoneal cavity from gestational days (GD) 14–19. On GD19, conscious MAP was measured via carotid catheters. Normal pregnant (NP): n = 10 rats; RUPP: n = 12 rats; RUPP + IL-17RC: n = 12 rats. All data are expressed as means ± SD. Statistical analysis was performed using one-way ANOVA with multiple comparisons followed by Tukey’s post hoc correction. *P < 0.05 vs. NP; #P < 0.05 vs. RUPP.
Table 1.
Effects of chronic IL-17 inhibition on litter size and fetal and placental weight in pregnant rats
| NP | RUPP | RUPP IL-17RC | |
|---|---|---|---|
| Total litter size | 15 ± 2 | 14 ± 2 | 14 ± 1 |
| Total live pups | 14.5 ± 2 | 7.8 ± 3* | 11 ± 3*# |
| Fetal weight, g | 2.4 ± 0.04 | 2.1 ± 0.04* | 2.3 ± 0.05# |
| Placental weight, g | 0.55 ± 0.01 | 0.43 ± 0.04* | 0.52 ± 0.03# |
All data are expressed as means ± SD. A subset of pregnant rats undergoing the reduced uterine perfusion pressure (RUPP) procedure also received a miniosmotic pump infusing 100 pg/day of a soluble IL-17 receptor (IL-17RC) into the intraperitoneal cavity from gestational days (GD) 14–19. After rats were euthanized on GD19, the average litter size, live pups, fetal weight, and placental weight were calculated for all groups. Normal pregnant (NP): n = 8–12 rats; RUPP: n = 7–12 rats; RUPP + IL-17RC: n = 7–12 rats. Statistical analysis was performed using one-way ANOVA with multiple comparisons followed by Tukey’s post hoc correction.
P < 0.05 vs. NP,
P < 0.05 vs. RUPP.
Effects of IL-17 inhibition on circulating and placental NK cells.
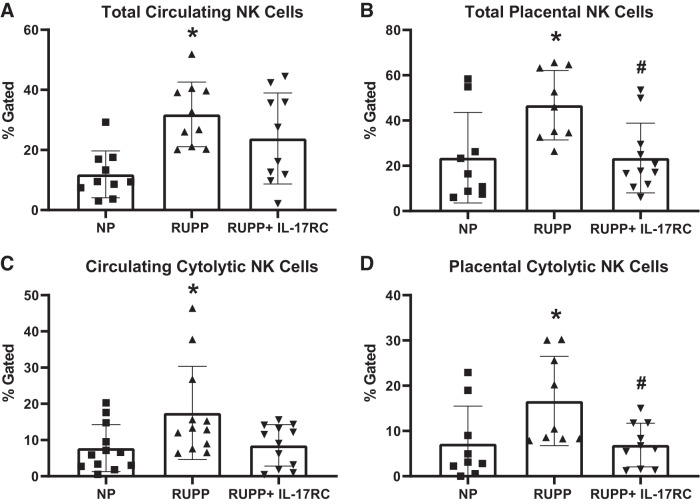
Total circulating NK cells were 168% higher in RUPP rats compared with NP (P = 0.001 vs. NP), and IL-17RC infusion did not reduce this (P = 0.29 vs. RUPP; Fig. 3A). Circulating cytolytic NK were 124% higher in RUPP than in NP rats (P = 0.03 vs. NP) and trended toward a decrease in RUPP + IL-17RC (P = 0.052 vs. RUPP; Fig. 3B). Total placental NK cells were 98% higher in RUPP than in NP (P = 0.02 vs. NP), and this was normalized in RUPP + IL-17RC (P = 0.01 vs. RUPP; Fig. 3C). Similarly, placental cytolytic NK cells were 132% higher in RUPP than in NP (P = 0.04 vs. NP) and were normalized in RUPPs receiving IL-17RC (P = 0.03 vs. RUPP; Fig. 3D). The gating strategy for NK analyses is shown in Supplemental Fig. S1A.
Fig. 3.
Chronic inhibition of IL-17 in reduced uterine perfusion pressure (RUPP) rats results in reduced natural killer (NK) cell activation. A subset of pregnant rats undergoing the RUPP procedure also received a miniosmotic pump infusing 100 pg/day of a soluble IL-17 receptor (IL-17RC) into the intraperitoneal cavity from gestational days (GD) 14–19. On GD19, blood and placentas were collected, processed, and analyzed via flow cytometry to obtain percentages of circulating total (A) and placental total (B) NK cells as well as circulating (C) and placental (D) cytolytic NK cells. Normal pregnant (NP): n = 9–12 rats RUPP: n = 9–12 rats; RUPP + IL-17RC: n = 10–12 rats. All data are expressed as means ± SD. Statistical analysis was performed using one-way ANOVA with multiple comparisons followed by Tukey’s post hoc correction. *P < 0.05 vs. NP; #P < 0.05 vs. RUPP.
NK cytotoxicity and placental oxidative stress following IL-17 inhibition.
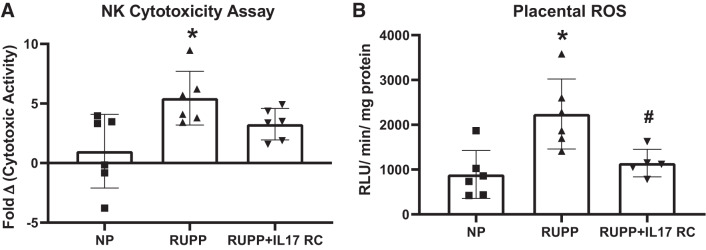
The cytolytic activity of RUPP placental NK cells was 5.5-fold higher than NP controls (P = 0.01), while the cytolytic activity of the NK cells isolated from RUPP + IL-17RC group was 3.3-fold higher than NP placental NK cells (P = 0.25 vs. NP; P = 0.26 vs. RUPP; Fig. 4A). We also found that placental ROS was higher in RUPP at 2,240 ± 781 RLU·min−1·mg−1 compared with 890 ± 534 RLU·min−1·mg−1 in NP (P = 0.004 vs. NP) and infusion of IL-17RC in RUPP rats lowered the levels to 1145 ± 306 RLU·min−1·mg−1 (P = 0.02 vs. RUPP; Fig. 4B).
Fig. 4.
Chronic inhibition of IL-17 in reduced uterine perfusion pressure (RUPP) rats blunts natural killer (NK) cytotoxicity and normalizes placental oxidative stress. A subset of pregnant rats undergoing the RUPP procedure also received a miniosmotic pump infusing 100 pg/day of a soluble IL-17 receptor (IL-17RC) into the intraperitoneal cavity from gestational days (GD) 14–19. A: the cytotoxic activity of isolated placental NK cells was measured using a cytotoxicity assay based on lactate dehydrogenase (LDH) release. The results are expressed as fold change in cytotoxic activity. B: superoxide production from placental homogenates was analyzed using the lucigenin assay. ROS, reactive oxygen species. The results are expressed as relative light units (RLUs)·min−1·mg−1. Normal pregnant (NP): n = 6 rats; RUPP: n = 6 rats; RUPP + IL-17RC: n = 5–6 rats. All data are expressed as means ± SD. Statistical analysis was performed using one-way ANOVA with multiple comparisons followed by Tukey’s post hoc correction. *P < 0.05 vs. NP; #P < 0.05 vs. RUPP.
Cytokines and cytotoxic proteins after IL-17 inhibition.
All cytokine and cytolytic protein data are summarized in Table 2. Placental IL-6 was 12 ± 7 pg/mg in NP and 19 ± 11 pg/mg in RUPP (P = 0.17 vs. NP) and was lower at 8 ± 3 pg/mg in RUPP + IL-17RC (P = 0.02 vs. RUPP). Placental IL-12 was higher in RUPP at 45 ± 32 pg/mg compared with 12 ± 14 pg/mg in NP (P = 0.003 vs. NP), and IL-17RC infusion lowered placental IL-12 to 11 ± 14 pg/mg (P = 0.003 vs. RUPP). Likewise, placental levels of TNF-α, a cNK-associated cytokine, were higher at 46 ± 21 pg/mg in RUPP compared with 29 ± 10 pg/mg in NP (P = 0.03 vs. NP), and the levels were normalized to 29 ± 14 pg/mg in RUPP + IL-17RC (P = 0.04 vs. RUPP). Placental IFN-γ was unchanged between all groups with values of 5 ± 5 pg/mg, 9 ± 2.5 pg/mg, and 7 ± 2 pg/mg for NP, RUPP, and RUPP + IL-17RC, respectively. MIP-3α, a cytolytic NK chemokine, was higher in RUPP at 1.44 ± 0.51 pg/mg compared with 0.84 ± 0.40 pg/mg in NP (P = 0.009 vs. NP), and this was normalized to 0.76 ± 0.20 pg/mg in RUPP + IL-17RC (P = 0.003 vs. RUPP). Levels of granzymes A, B, and K did not differ between the groups. Placental perforin was higher at 138 ± 22 pg/mL in RUPP compared with 90 ± 18 pg/mg in NP (P = 0.005 vs. NP), but this remained at 130 ± 18 pg/mg following IL-17RC infusion.
Table 2.
Effects of chronic IL-17 inhibition on circulating and placental cytokines and cytolytic proteins in pregnant rats
| NP | RUPP | RUPP + IL-17RC | |
|---|---|---|---|
| Placental, pg/mg | |||
| IL-6 | 12 ± 7 | 19 ± 11 | 8 ± 3# |
| IL-12 | 12 ± 14 | 45 ± 32* | 11 ± 14# |
| TNF-α | 29 ± 10 | 46 ± 21* | 29 ± 14# |
| IFN-γ | 5 ± 5 | 9 ± 2.5 | 7 ± 2 |
| MIP-3α | 0.84 ± 0.40 | 1.44 ± 0.51* | 0.76 ± 0.20# |
| Granzyme A | 104 ± 23 | 136 ± 18 | 126 ± 29 |
| Granzyme B | 24 ± 7 | 28 ± 14 | 29 ± 5 |
| Granzyme K | 1,844 ± 1,048 | 2,187 ± 1,030 | 1,681 ± 625 |
| Perforin | 90 ± 18 | 138 ± 22* | 130 ± 18 |
| Circulating, pg/mL | |||
| IL-6 | 14 ± 21 | 181 ± 101* | 68 ± 44# |
| IL-12 | 303 ± 204 | 577 ± 179* | 335 ± 163# |
| TNF-α | 7 ± 11 | 65 ± 89* | 7 ± 13# |
| IFN-γ | 7 ± 6.5 | 35 ± 28* | 13 ± 8# |
| MIP-3α | 8 ± 3 | 11 ± 6 | 7 ± 3 |
| Granzyme A | 66 ± 91 | 102 ± 69 | 84 ± 56 |
| Granzyme B | 97 ± 50 | 306 ± 208* | 194 ± 48 |
| Granzyme K | 42 ± 33 | 67 ± 17 | 52 ± 10 |
| Perforin | 181 ± 25 | 180 ± 37 | 161 ± 16 |
All data are expressed as means ± SD. A subset of pregnant rats undergoing the reduced uterine perfusion pressure (RUPP) procedure also received a miniosmotic pump infusing 100 pg/day of a soluble IL-17 receptor (IL-17RC) into the intraperitoneal cavity from gestational days (GD) 14–19. Levels of cytokines were measured in blood and placental homogenates of each group using the Bio-Plex Pro Rat Cytokine Immunoassay Kit. Cytolytic granzymes and perforin were measured using commercial ELISAs. Placental data are normalized to total protein. MIP-3α, macrophage inflammatory protein-3α. Normal pregnant (NP): n = 5–12 rats; RUPP: n = 5–12 rats; RUPP + IL-17RC: n = 5–12 rats. Statistical analyses were performed using one-way ANOVA.
P < 0.05 vs. NP;
P < 0.0 vs. RUPP.
Circulating IL-6 was higher at 181 ± 101 pg/mL in RUPP compared with 14 ± 21 pg/mL in NP (P = 0.0001 vs. NP) and was lower in RUPP + IL-17RC with a mean of 68 ± 44 pg/mL (P = 0.006 vs. RUPP). Similarly, circulating IL-12 was higher in RUPP at 577 ± 179 pg/mL compared with 303 ± 204 pg/mL in NP (P = 0.01 vs. NP) and was lower at 335 ± 163 pg/mL in RUPP + IL-17RC (P = 0.03 vs. RUPP). Circulating TNF-α was also higher in RUPP at 65 ± 89 pg/mL compared with 7 ± 11 pg/mL in NP (P = 0.047 vs. NP), and IL-17RC infusion normalized these levels to 7 ± 13 pg/mL (P = 0.046 vs. RUPP). We also observed that circulating IFN-γ was higher at 35 ± 28 pg/mL in RUPP compared with 7 ± 6.5 pg/mL in NP (P = 0.0074 vs. NP), and this was lower in RUPP + IL-17RC with a mean of 13 ± 8 pg/mL (P = 0.037 vs. RUPP). There were no significant differences in plasma MIP-3α between the groups. Circulating granzyme B was higher at 306 ± 208 pg/mL in RUPP compared with 97 ± 50 pg/mL in NP (P = 0.03 vs. NP), and it remained elevated at 194 ± 48 pg/mL in RUPP + IL-17RC (P = 0.31 vs. RUPP). There were no differences in the circulating levels of perforin, granzyme A, or granzyme K between the three groups.
Angiogenic factors following IL-17 inhibition.
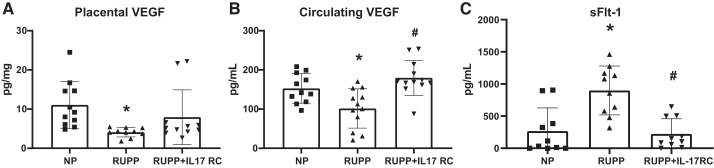
VEGF, a proangiogenic growth factor, was lower in the placentas of RUPP rats at 4 ± 1 pg/mg compared with 11 ± 6 pg/mg in NP (P = 0.02 vs. NP) and was 8 ± 7 pg/mg in RUPP + IL-17RC (P = 0.26 vs. RUPP; Fig. 5A). Circulating VEGF was also lower in RUPP at 102 ± 50 pg/mL compared with 153 ± 38 pg/mL in NP (P = 0.03 vs. NP), and this was higher in RUPP + IL-17RC with a value of 169 ± 58 pg/mL (P = 0.0004 vs. RUPP; Fig. 5B). s-Flt1 was higher in RUPP at 899 ± 380 pg/mL compared with 267 ± 361 pg/mL in NP (P = 0.0006 vs. NP), and the levels were lower in RUPP + IL-17RC with a value of 224 ± 235 pg/mL (P = 0.0003 vs. RUPP; Fig. 5C).
Fig. 5.
Chronic inhibition of IL-17 in reduced uterine perfusion pressure (RUPP) rats increased circulating VEGF and decreased soluble fms tyrosine kinase-1 (sFlt-1). A subset of pregnant rats undergoing the RUPP procedure also received a miniosmotic pump infusing 100 pg/day of a soluble IL-17 receptor (IL-17RC) into the intraperitoneal cavity from gestational days (GD) 14–19. Placental VEGF (A), circulating VEGF (B), and circulating s-Flt1 (C) measured via ELISA are shown. Normal pregnant (NP): n = 10–11 rats; RUPP: n = 10–12 rats; RUPP + IL-17RC: n = 10–12 rats. All data are expressed as means ± SD. Statistical analysis was performed using one-way ANOVA with multiple comparisons followed by Tukey’s post hoc correction. *P < 0.05 vs. NP; #P < 0.05 vs. RUPP.
DISCUSSION
In the current study, we tested the hypothesis that IL-17 inhibition would decrease cNK cell activation and cytotoxic activity as a mechanism to improve maternal and fetal outcomes in placental ischemic rats. We demonstrated that IL-17 inhibition prevented placental ischemia-induced increases in circulating and placental NK cells as well as NK cell activation and cytotoxic activity. We also observed that RUPP + IL-17RC rats displayed decreased circulating and placental cytokine levels, decreased TH17 cells, and decreased placental oxidative stress. Finally, inhibition of IL-17 signaling resulted in a significant decrease in maternal MAP and an increase in both fetal and placental weight. The results of the current study combined with our previous work demonstrate a direct role for IL-17 to mediate NK cytotoxic activation in response to placental ischemia during pregnancy.
Several clinical studies have shown that preeclamptic women display an increased TH17 cell profile and increased levels of IL-17, the main cytokine produced by these cells (21, 26, 44). Although we did not observe a change in circulating IL-17 levels between RUPP and NP rats, there were more circulating and placental TH17 cells in RUPP rats, which is consistent with our previous results (19). Serum IL-17 has been observed to be increased in hypertensive patients compared with normotensive controls (59, 107), and investigations using animal models have shown a direct role for IL-17 in hypertension. Several mechanisms have been proposed to explain how IL-17 promotes hypertension. A 2010 study by Madhur et al. showed that the development of ANG II-induced hypertension was not sustained in IL-17-deficient mice, and they also observed decreased vascular ROS production in these mice compared with the control mice (59). Other studies have shown that IL-17 infusion into mice results in increased blood pressure, with increased renal immune cell infiltration (66), and endothelial dysfunction (65) being potential explanations for the resultant hypertension. While these mechanisms continue to be explored in the development of hypertension, the mechanism by which IL-17 contributes to preeclampsia has not yet been identified. We propose that cNK activation is one potential mechanism, and the results obtained during this study in the RUPP rat model suggest that this is a mechanism in PE pathophysiology that warrants further investigation.
NK cells are innate lymphoid cells that have the ability to kill targeted cells without prior sensitization, and they employ a balance of activating and inhibitory signals to control their activity (20, 67, 99). While human NK cells normally make up 5–10% of peripheral leukocytes, they can make up 70% of decidual leukocytes during pregnancy (20, 28, 35, 63, 83). Despite containing similar levels of cytolytic enzymes, dNK cells display very little cytotoxic activity and instead secrete chemokines, proangiogenic factors, and immunomodulatory cytokines (28, 41, 42, 45, 63, 83). However, it has been observed in women with pregnancy disorders such as recurrent spontaneous abortion, placental insufficiency, and PE that these normally benign dNK cells shift toward a more cytotoxic phenotype characterized by increased production of TNF-α and IFN-γ as well as increased cytolytic activity (23, 30, 52, 74). Yet, the stimulus of this shift is not well understood. We postulate that IL-17 is a major stimulus to activate NK cells in the RUPP model and PE women. Several studies have linked IL-17 to NK activation in the context of cancer and fungal immunity. A 2009 study by Kryczek et al. (50) found that IL-17-deficient mice displayed increased tumor growth as well as decreased NK cell cytotoxicity against YAC1 cells. A 2014 study by Bär et al. (15) found that mice lacking the IL-17 receptor demonstrated a decrease in functional NK cells and had increased susceptibility to systemic candidiasis. Additionally, Al Omar et al. demonstrated that IL-17 increased human NK proliferation and perforin production in vitro (6). Our group has recently demonstrated that IL-17 infusion into NP rats resulted in increased circulating and placental NK cell activation and cytotoxicity (93), and in this study we observed a significant decrease in total NK cells and NK activation in RUPP rats following IL-17 inhibition. We also observed that the placental ischemia in RUPP rats caused an increase in NK cytotoxicity that was blunted in response to IL-17RC infusion. This decrease in cytotoxicity likely contributes to the decreased maternal MAP and the increased fetal and placental weight observed in RUPP + IL-17RC rats.
Circulating and placental TNF-α as well as circulating IFN-γ was higher in RUPP rats, and this was normalized by chronic IL-17RC infusion. These results are consistent with the reported increase in TNF-α and IFN-y seen in women with preeclampsia and other pregnancy disorders (14, 71, 82, 94). It is important to note that although these cytokines are produced by activated NK cells (91), they can also be produced by TH1 cells (89). However, a study by Borzychowski et al. (17) showed that there was no change in the TH1/TH2 ratio in preeclamptic women, but the NK cells of these women did reflect a shift toward a type 1 phenotype. This suggests that NK cells are the major source of these cytokines in PE women. This has also been reflected in the RUPP rat, as we have previously shown NK cell depletion normalized circulating levels of TNF-α and IFN-y (25). The reduced level of these cytokines observed after IL-17RC treatment is likely a mechanism responsible for the improvements in maternal blood pressure and fetal outcomes observed in this study. TNF-α has been linked to hypertension in humans and increased MAP in animal models with TNF-α inhibition reducing blood pressure in RA patients (108) and animal models of hypertension (40, 108). Furthermore, TNF-α has been shown to be associated with increased blood pressure, endothelial dysfunction, and poor pregnancy outcomes in animal models (87). Similarly, increased levels of IFN-γ have been observed in preeclamptic women (71), and excessive amounts have been shown to cause spontaneous abortion in animal models (57) although the mechanisms involved are not yet fully understood.
The angiogenic imbalance between s-Flt1 and VEGF has also been identified as a significant contributor to PE (48). Increased levels of sFlt-1 and decreased levels of circulating VEGF have been observed in PE women and animal models when compared with their normal pregnant counterparts (1, 48, 58, 61). This is also reflected in our data with lower circulating and placental VEGF as well as higher s-Flt-1 levels seen in RUPP rats. NK cells are typically a major source of VEGF during pregnancy (45) but upon activation, VEGF secretion decreases (18, 30, 41). In the current study, we found that IL-17 inhibition normalized sFlt-1 and partially restored circulating levels of VEGF in RUPP recipients. This is another mechanism that may contribute to the improved maternal and fetal outcomes as VEGF supplementation has been shown to ameliorate PE pathophysiology in animal models and is being considered as a potential therapeutic option for PE women (2, 16, 39, 48, 86). Together, these data indicate that the improvement in maternal blood pressure and fetal growth following IL-17RC infusion can be partially attributed to the decrease in cNK cell activation and the resultant reduction in inflammatory cytokines and increase in VEGF levels.
The reduction of cNK activation observed in the RUPP + IL-17RC rats may have been the result of both direct and indirect actions of IL-17. We observed that IL-12 is increased in both the placenta and circulation of RUPP rats and is decreased following IL-17 inhibition. IL-17 can stimulate the production of IL-12, which is also known to cause NK cell activation (80, 106). Thus this represents another mechanism by which IL-17 may activate NK cells in PE. MIP-3α is a chemoattractant for activated NK cells (5) and is also stimulated by IL-17 (33). We observed that placental levels of this chemokine are significantly increased in RUPP rats and are normalized following IL-17 inhibition. The increased placental levels of this chemokine may explain why placental NK cells are significantly increased in RUPP rats late into pregnancy. In normal human pregnancy, dNK cells normally decrease in number until they are nearly absent at term (18, 45). Studies of NK cells in the rat uterus report dNK cells in the metrial triangle during normal pregnancy (70, 88, 90). However, conflicting results are reported regarding NK cell numbers during mid-to-late gestation. A study by Ain et al. reported a significant reduction in the presence of dNK cells in the placentation site at gestation day 18 (3), but Tessier et al. reported no decreases in dNK cells in the placentation site up through gestational day 18.5 (90). Importantly, Fonseca, et al. (27) reported an increased population of dNK cells present in reabsorption sites of NP rats. The authors concluded that dNK cells played a significant role in adverse outcomes of pregnancy (27), in addition to other studies that conclude dNK cells have important functions in the later stages of gestation (90). These observations support our current theory that increased cNK cells in the placentation site play a role in the pathophysiology in response to placental ischemia. While the precise origin of dNK cells is unknown, it is theorized that they are recruited from the circulating NK population into the decidua, where they become differentiated (18, 35, 45). Elevated MIP-3α levels in the placenta could result in increased recruitment of peripheral cNK cells into the placenta, leading to a significant increase in the placental cNK cell population during late pregnancy observed in our current study.
Another important factor in PE pathophysiology is increased ROS and oxidative stress. Several studies have shown that ROS are increased in preeclamptic women, and similar results were found in this study and previous studies using RUPP rats (11, 62, 73, 98). IL-17 is known to activate neutrophils and increase ROS production, which may contribute to the increased ROS observed in RUPPs and the decrease seen after IL-17 inhibition. Additionally, a 2017 study found that ROS can induce NK cell proliferation (84); thus IL-17 induced ROS may indirectly lead to NK activation. However, in our previous study examining TH17 adoptive transfer and the resultant NK activation, scavenging ROS with the antioxidant Tempol did not change cNK activation in the TH17 recipients (84). This suggests that in RUPP rats, ROS is not the stimulus of NK activation, but rather the ROS is a by-product of increased NK activation. Several studies have shown that NK-mediated apoptosis also contributes to oxidative stress (46, 60). The decreased NK cytotoxic activity that we observed following IL-17RC may also contribute to the reduction in placental oxidative stress in RUPP + IL-17RC rats. This is further supported by studies by Vaka et al. (97) in which they examined the role of placental ischemia-stimulated NK cells in mitochondrial dysfunction and reported that RUPP rats display a significant reduction in state 3 respiration and complex I and IV activity as well as a significant increase in mitochondrial ROS production. They also found that NK cell depletion in RUPP rats significantly improved state 3 respiration and decreased mitochondrial ROS generation. These data combined with the data from the current study indicate that cNKs are a significant source of ROS in PE and that preventing cNK activation through IL-17 inhibition is an effective strategy for reducing placental oxidative stress and improving PE pathophysiology.
Although chronic administration of IL-17RC resulted in a significant reduction in cNKs and inflammatory cytokines, there are some limitations to the current study. Administration of IL-17RC not only reduced cNK activation, it also diminished TH17 cell proliferation. Thus the resulting improvements in maternal MAP, fetal outcomes, and inflammatory cytokines cannot be specifically attributed to the reduction in cNK cells only. However, the results of this study considered with our previous findings of IL-17-induced cNK activation (93) provide strong evidence that TH17 cells induce cNK activation primarily though IL-17 signaling. Additionally, while we have identified an inducer of cNKs in RUPP rats, the direct role of cNK cells in the phenotype of placental ischemic rats was not examined in this study. This will be the subject of future investigations in our laboratory using direct NK cell adoptive transfer methods.
Perspectives and Significance
The results of this study suggest that IL-17 is the mediator by which TH17s promote cNK activation in PE. By identifying this stimulus, novel therapeutics can be developed that inhibit NK activation while also retaining their beneficial roles in pregnancy maintenance and immune defense. The data obtained suggest that targeting IL-17 signaling may be effective in ameliorating several known contributing factors of PE and could improve both maternal and fetal outcomes.
GRANTS
This work was funded by National Institutes of Health Grants T32-HL-105324 (to O. K. Travis), R01-DK-109133 (to J. M. Williams), and R00-HL-130456 and P20-GM-104357 (to D. C. Cornelius).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.C.C. conceived and designed research; O.K.T., D.W., C.H.B., C.G., W.T., C.S., M.G., J.P.L., J.M.W., and D.C.C. performed experiments; O.K.T., D.W., C.H.B., W.T., J.P.L., and D.C.C. analyzed data; O.K.T., D.W., and D.C.C. interpreted results of experiments; O.K.T. prepared figures; O.K.T. drafted manuscript; O.K.T., D.W., C.H.B., J.M.W., and D.C.C. edited and revised manuscript; O.K.T., D.W., C.H.B., C.G., W.T., C.S., M.G., J.P.L., J.M.W., and D.C.C. approved final version of manuscript.
REFERENCES
- 1.Aggarwal PK, Chandel N, Jain V, Jha V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens 26: 236–241, 2012. doi: 10.1038/jhh.2011.29. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A, Singh J, Khan Y, Seshan SV, Girardi G. A new mouse model to explore therapies for preeclampsia. PLoS One 5: e13663, 2010. doi: 10.1371/journal.pone.0013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol 260: 176–190, 2003. doi: 10.1016/S0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 4.Akhlaq M, Nagi AH, Yousaf AW. Placental morphology in pre-eclampsia and eclampsia and the likely role of NK cells. Indian J Pathol Microbiol 55: 17–21, 2012. doi: 10.4103/0377-4929.94848. [DOI] [PubMed] [Google Scholar]
- 5.Al-Aoukaty A, Rolstad B, Giaid A, Maghazachi AA. MIP-3alpha, MIP-3beta and fractalkine induce the locomotion and the mobilization of intracellular calcium, and activate the heterotrimeric G proteins in human natural killer cells. Immunology 95: 618–624, 1998. doi: 10.1046/j.1365-2567.1998.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Omar S, Flanagan BF, Almehmadi M, Christmas SE. The effects of IL-17 upon human natural killer cells. Cytokine 62: 123–130, 2013. doi: 10.1016/j.cyto.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 8.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 9.Amaral LM, Faulkner JL, Elfarra J, Cornelius DC, Cunningham MW, Ibrahim T, Vaka VR, McKenzie J, LaMarca B. Continued investigation into 17-OHPC: results from the preclinical rupp rat model of preeclampsia. Hypertension 70: 1250–1255, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci 19: 1496, 2018. doi: 10.3390/ijms19051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardeljan D, Wang Y, Park S, Shen D, Chu XK, Yu CR, Abu-Asab M, Tuo J, Eberhart CG, Olsen TW, Mullins RF, White G, Wadsworth S, Scaria A, Chan CC. Interleukin-17 retinotoxicity is prevented by gene transfer of a soluble interleukin-17 receptor acting as a cytokine blocker: implications for age-related macular degeneration. PLoS One 9: e95900, 2014. doi: 10.1371/journal.pone.0095900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakrania BA, Hall ME, Shahul S, Granger JP. The reduced uterine perfusion pressure (RUPP) rat model of preeclampsia exhibits impaired systolic function and global longitudinal strain during pregnancy. Pregnancy Hypertens 18: 169–172, 2019. doi: 10.1016/j.preghy.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee S, Smallwood A, Moorhead J, Chambers AE, Papageorghiou A, Campbell S, Nicolaides K. Placental expression of interferon-gamma (IFN-gamma) and its receptor IFN-gamma R2 fail to switch from early hypoxic to late normotensive development in preeclampsia. J Clin Endocrinol Metab 90: 944–952, 2005. doi: 10.1210/jc.2004-1113. [DOI] [PubMed] [Google Scholar]
- 15.Bär E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 40: 117–127, 2014. doi: 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves AC, Gröne HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med 14: 1857–1867, 2010. doi: 10.1111/j.1582-4934.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borzychowski AM, Croy BA, Chan WL, Redman CW, Sargent IL. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur J Immunol 35: 3054–3063, 2005. doi: 10.1002/eji.200425929. [DOI] [PubMed] [Google Scholar]
- 18.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol 54: 281–294, 2010. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- 19.Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, Wallukat G, Dechend R, LaMarca B. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 62: 1068–1073, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cristiani CM, Palella E, Sottile R, Tallerico R, Garofalo C, Carbone E. Human NK cell subsets in pregnancy and disease: toward a new biological complexity. Front Immunol 7: 656, 2016. doi: 10.3389/fimmu.2016.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, Oleszczuk J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol 93: 75–81, 2012. doi: 10.1016/j.jri.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Darmochwal-Kolarz D, Michalak M, Kolarz B, Przegalinska-Kalamucka M, Bojarska-Junak A, Sliwa D, Oleszczuk J. The role of interleukin-17, interleukin-23, and transforming growth factor-beta in pregnancy complicated by placental insufficiency. BioMed Res Int 2017: 1, 2017. doi: 10.1155/2017/6904325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darmochwal-Kolarz D, Rolinski J, Leszczynska-Goarzelak B, Oleszczuk J. The expressions of intracellular cytokines in the lymphocytes of preeclamptic patients. Am J Reprod Immunol 48: 381–386, 2002. doi: 10.1034/j.1600-0897.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 24.Ding L, Bai C, Liu Y. Interleukin-6 contributes to myocardial damage in pregnant rats with reduced uterine perfusion pressure. Braz J Med Biol Res 51: e6921, 2018. doi: 10.1590/1414-431x20186921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elfarra J, Amaral LM, McCalmon M, Scott JD, Cunningham MW Jr, Gnam A, Ibrahim T, LaMarca B, Cornelius DC. Natural killer cells mediate pathophysiology in response to reduced uterine perfusion pressure. Clin Sci (Lond) 131: 2753–2762, 2017. doi: 10.1042/CS20171118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 148: 13–21, 2016. doi: 10.1111/imm.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonseca BM, Almada M, Costa MA, Teixeira NA, Correia-da-Silva G. Rat spontaneous foetal resorption: altered α2-macroglobulin levels and uNK cell number. Histochem Cell Biol 142: 693–701, 2014. doi: 10.1007/s00418-014-1252-8. [DOI] [PubMed] [Google Scholar]
- 28.Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, Wei H. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci USA 110: E231–E240, 2013. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu B, Tian Z, Wei H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell Mol Immunol 11: 564–570, 2014. doi: 10.1038/cmi.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukui A, Yokota M, Funamizu A, Nakamua R, Fukuhara R, Yamada K, Kimura H, Fukuyama A, Kamoi M, Tanaka K, Mizunuma H. Changes of NK cells in preeclampsia. Am J Reprod Immunol 67: 278–286, 2012. doi: 10.1111/j.1600-0897.2012.01120.x. [DOI] [PubMed] [Google Scholar]
- 31.Fushima T, Sekimoto A, Minato T, Ito T, Oe Y, Kisu K, Sato E, Funamoto K, Hayase T, Kimura Y, Ito S, Sato H, Takahashi N. Reduced uterine perfusion pressure (RUPP) model of preeclampsia in mice. PLoS One 11: e0155426, 2016. doi: 10.1371/journal.pone.0155426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension 48: 711–716, 2006. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 33.Gaffen SL. An overview of IL-17 function and signaling. Cytokine 43: 402–407, 2008. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gathiram P, Moodley J. Pre-eclampsia: its pathogenesis and pathophysiolgy. Cardiovasc J Afr 27: 71–78, 2016. doi: 10.5830/CVJA-2016-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaynor LM, Colucci F. Uterine natural killer cells: functional distinctions and influence on pregnancy in humans and mice. Front Immunol 8: 467, 2017. doi: 10.3389/fimmu.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57: 941–948, 2011. doi: 10.1161/HYPERTENSIONAHA.111.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 53: 399–403, 2009. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55: 380–385, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12: 1065–1074, 2006. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 42.Higuma-Myojo S, Sasaki Y, Miyazaki S, Sakai M, Siozaki A, Miwa N, Saito S. Cytokine profile of natural killer cells in early human pregnancy. Am J Reprod Immunol 54: 21–29, 2005. doi: 10.1111/j.1600-0897.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 43.Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol 2: 543–549, 2007. doi: 10.2215/CJN.03761106. [DOI] [PubMed] [Google Scholar]
- 44.Hosseini A, Dolati S, Hashemi V, Abdollahpour-Alitappeh M, Yousefi M. Regulatory T and T helper 17 cells: Their roles in preeclampsia. J Cell Physiol 233: 6561–6573, 2018. doi: 10.1002/jcp.26604. [DOI] [PubMed] [Google Scholar]
- 45.Jabrane-Ferrat N, Siewiera J. The up side of decidual natural killer cells: new developments in immunology of pregnancy. Immunology 141: 490–497, 2014. doi: 10.1111/imm.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacquemin G, Margiotta D, Kasahara A, Bassoy EY, Walch M, Thiery J, Lieberman J, Martinvalet D. Granzyme B-induced mitochondrial ROS are required for apoptosis. Cell Death Differ 22: 862–874, 2015. doi: 10.1038/cdd.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev 71, Suppl 1: S18–S25, 2013. doi: 10.1111/nure.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jha V, Aggarwal PK, Chandel N, Jain V. Angiogenic balance and diagnosis of pre-eclampsia: selecting the right VEGF receptor. J Hum Hypertens 26: 205–206, 2012. doi: 10.1038/jhh.2011.89. [DOI] [PubMed] [Google Scholar]
- 49.Keelan JA, Mitchell MD. Placental cytokines and preeclampsia. Front Biosci 12: 2706–2727, 2007. doi: 10.2741/2266. [DOI] [PubMed] [Google Scholar]
- 50.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 114: 357–359, 2009. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol 179: 5462–5473, 2007. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwak-Kim JY, Gilman-Sachs A, Kim CE. T helper 1 and 2 immune responses in relationship to pregnancy, nonpregnancy, recurrent spontaneous abortions and infertility of repeated implantation failures. Chem Immunol Allergy 88: 64–79, 2005. doi: 10.1159/000087821. [DOI] [PubMed] [Google Scholar]
- 53.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 52: 1168–1172, 2008. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 55.Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol 94: 247–257, 2013. doi: 10.1189/jlb.1112603. [DOI] [PubMed] [Google Scholar]
- 56.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 303: H1–H8, 2012. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li ZY, Chao HH, Liu HY, Song ZH, Li LL, Zhang YJ, Yang Y, Peng JP. IFN-γ induces aberrant CD49b+ NK cell recruitment through regulating CX3CL1: a novel mechanism by which IFN-γ provokes pregnancy failure. Cell Death Dis 5: e1512, 2014. doi: 10.1038/cddis.2014.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luttun A, Carmeliet P. Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest 111: 600–602, 2003. doi: 10.1172/JCI18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinvalet D, Zhu P, Lieberman J. Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity 22: 355–370, 2005. doi: 10.1016/j.immuni.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarthy C, Kenny LC. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci Rep 6: 32683, 2016. doi: 10.1038/srep32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest 124: 1872–1879, 2014. doi: 10.1172/JCI68107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molvarec A, Czegle I, Szijártó J, Rigó J Jr. Increased circulating interleukin-17 levels in preeclampsia. J Reprod Immunol 112: 53–57, 2015. doi: 10.1016/j.jri.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 97: 696–704, 2013. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orejudo M, Rodrigues-Diez RR, Rodrigues-Diez R, Garcia-Redondo A, Santos-Sánchez L, Rández-Garbayo J, Cannata-Ortiz P, Ramos AM, Ortiz A, Selgas R, Mezzano S, Lavoz C, Ruiz-Ortega M. Interleukin 17A participates in renal inflammation associated to experimental and human hypertension. Front Pharmacol 10: 1015, 2019. doi: 10.3389/fphar.2019.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 89: 216–224, 2011. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 68.Perussia B. The cytokine profile of resting and activated NK cells. Methods 9: 370–378, 1996. doi: 10.1006/meth.1996.0042. [DOI] [PubMed] [Google Scholar]
- 69.Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol 11: 1102–1113, 2016. doi: 10.2215/CJN.12081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Picut CA, Swanson CL, Parker RF, Scully KL, Parker GA. The metrial gland in the rat and its similarities to granular cell tumors. Toxicol Pathol 37: 474–480, 2009. doi: 10.1177/0192623309335632. [DOI] [PubMed] [Google Scholar]
- 71.Pinheiro MB, Martins-Filho OA, Mota AP, Alpoim PN, Godoi LC, Silveira AC, Teixeira-Carvalho A, Gomes KB, Dusse LM. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine 62: 165–173, 2013. doi: 10.1016/j.cyto.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 73.Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem 8: 204–226, 2010. doi: 10.2174/187152510792481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 179: 80–86, 1998. doi: 10.1016/S0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 75.Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol 59: 161–173, 2003. doi: 10.1016/S0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 76.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol 117: 550–555, 1999. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med 28: 192–209, 2007. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 78.Santiago-Font JA, Amaral LM, Faulkner J, Ibrahim T, Vaka VR, Cunningham MW, LaMarca B. Serelaxin improves the pathophysiology of placental ischemia in the reduced uterine perfusion pressure rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 311: R1158–R1163, 2016. doi: 10.1152/ajpregu.00192.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sargent IL, Borzychowski AM, Redman CW. NK cells and pre-eclampsia. J Reprod Immunol 76: 40–44, 2007. doi: 10.1016/j.jri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 80.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol 154: 5320–5330, 1995. [PubMed] [Google Scholar]
- 81.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serin ÝS, Özçelik B, Bapbu∂ M, Kýlýç H, Okur D, Erez R. Predictive value of tumor necrosis factor alpha (TNF-alpha) in preeclampsia. Eur J Obstet Gynecol Reprod Biol 100: 143–145, 2002. doi: 10.1016/S0301-2115(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 83.Sharma S. Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol 58: 219–229, 2014. doi: 10.1387/ijdb.140109ss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shields CA, McCalmon M, Ibrahim T, White DL, Williams JM, LaMarca B, Cornelius DC. Placental ischemia-stimulated T-helper 17 cells induce preeclampsia-associated cytolytic natural killer cells during pregnancy. Am J Physiol Regul Integr Comp Physiol 315: R336–R343, 2018. doi: 10.1152/ajpregu.00061.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol 293: H2080–H2084, 2007. doi: 10.1152/ajpheart.00667.2007. [DOI] [PubMed] [Google Scholar]
- 86.Siddiqui AH, Irani RA, Zhang Y, Dai Y, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Recombinant vascular endothelial growth factor 121 attenuates autoantibody-induced features of pre-eclampsia in pregnant mice. Am J Hypertens 24: 606–612, 2011. doi: 10.1038/ajh.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Small HY, Nosalski R, Morgan H, Beattie E, Guzik TJ, Graham D, Delles C. Role of tumor necrosis factor-alpha and natural killer cells in uterine artery function and pregnancy outcome in the stroke-prone spontaneously hypertensive rat. Hypertension 68: 1298–1307, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soares MJ, Chakraborty D, Kubota K, Renaud SJ, Rumi MA. Adaptive mechanisms controlling uterine spiral artery remodeling during the establishment of pregnancy. Int J Dev Biol 58: 247–259, 2014. doi: 10.1387/ijdb.140083ms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol 166: 232–240, 2001. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- 90.Tessier DR, Raha S, Holloway AC, Yockell-Lelièvre J, Tayade C, Gruslin A. Characterization of immune cells and cytokine localization in the rat utero-placental unit mid- to late gestation. J Reprod Immunol 110: 89–101, 2015. doi: 10.1016/j.jri.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Thum MY, Abdalla HI, Bhaskaran S, Harden EL, Ford B, Sumar N, Shehata H, Bansal A. The relationship of systemic TNF-alpha and IFN-gamma with IVF treatment outcome and peripheral blood NK cells. Am J Reprod Immunol 57: 210–217, 2007. doi: 10.1111/j.1600-0897.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 92.Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens 4: 97–104, 2014. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Travis OK, White D, Pierce WA, Ge Y, Stubbs CY, Spradley FT, Williams JM, Cornelius DC. Chronic infusion of interleukin-17 promotes hypertension, activation of cytolytic natural killer cells, and vascular dysfunction in pregnant rats. Physiol Rep 7: e14038, 2019. doi: 10.14814/phy2.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Udenze I, Amadi C, Awolola N, Makwe CC. The role of cytokines as inflammatory mediators in preeclampsia. Pan Afr Med J 20: 219, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ushida T, Kotani T, Tsuda H, Imai K, Nakano T, Hirako S, Ito Y, Li H, Mano Y, Wang J, Miki R, Yamamoto E, Iwase A, Bando YK, Hirayama M, Ohno K, Toyokuni S, Kikkawa F. Molecular hydrogen ameliorates several characteristics of preeclampsia in the reduced uterine perfusion pressure (RUPP) rat model. Free Radic Biol Med 101: 524–533, 2016. doi: 10.1016/j.freeradbiomed.2016.10.491. [DOI] [PubMed] [Google Scholar]
- 96.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 7: 467–474, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vaka VR, McMaster KM, Cornelius DC, Ibrahim T, Jayaram A, Usry N, Cunningham MW Jr, Amaral LM, LaMarca B. Natural killer cells contribute to mitochondrial dysfunction in response to placental ischemia in reduced uterine perfusion pressure rats. Am J Physiol Regul Integr Comp Physiol 316: R441–R447, 2019. doi: 10.1152/ajpregu.00279.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vishnyakova PA, Volodina MA, Tarasova NV, Marey MV, Kan NE, Khodzhaeva ZS, Vysokikh MY, Sukhikh GT. Alterations in antioxidant system, mitochondrial biogenesis and autophagy in preeclamptic myometrium. BBA Clin 8: 35–42, 2017. doi: 10.1016/j.bbacli.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 9: 503–510, 2008. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 100.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walsh SK, English FA, Johns EJ, Kenny LC. Plasma-mediated vascular dysfunction in the reduced uterine perfusion pressure model of preeclampsia: a microvascular characterization. Hypertension 54: 345–351, 2009. doi: 10.1161/HYPERTENSIONAHA.109.132191. [DOI] [PubMed] [Google Scholar]
- 102.Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 24: 147–158, 2009. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 103.Wat JM, Baczyk D, Kingdom JC. The antithrombin binding regions of heparin mediate fetal growth and reduced placental damage in the rupp model of preeclampsia. Biol Reprod ioaa006, 2020. doi: 10.1093/biolre/ioaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williamson RD, McCarthy FP, Manna S, Groarke E, Kell DB, Kenny LC, McCarthy CM. L-(+)-ergothioneine significantly improves the clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 75: 561–568, 2020. doi: 10.1161/HYPERTENSIONAHA.119.13929. [DOI] [PubMed] [Google Scholar]
- 105.Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, Tomkinson KN, Fitz LJ, Wolfman NM, Collins M, Dunussi-Joannopoulos K, Chatterjee-Kishore M, Carreno BM. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol 181: 2799–2805, 2008. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 106.Wu HP, Shih CC, Chu CM, Huang CY, Hua CC, Liu YC, Chuang DY. Effect of interleukin-17 on in vitro cytokine production in healthy controls and patients with severe sepsis. J Formos Med Assoc 114: 1250–1257, 2015. doi: 10.1016/j.jfma.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 107.Yao W, Sun Y, Wang X, Niu K. Elevated serum level of interleukin 17 in a population with prehypertension. J Clin Hypertens (Greenwich) 17: 770–774, 2015. doi: 10.1111/jch.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, Shoda T, Takai S, Makino S, Hanafusa T. Infliximab, a TNF-α inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens 28: 165–169, 2014. doi: 10.1038/jhh.2013.80. [DOI] [PubMed] [Google Scholar]
- 109.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol 5: 173–192, 2010. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]