Abstract
The aim of this study was to determine the effects of endothelin-1 (ET-1)-generated endothelial microvesicles (EMVs) on endothelial cell inflammation, apoptosis, and endothelial nitric oxide synthase (eNOS). Human umbilical vein endothelial cells (HUVECs) were treated with ET-1 for 24 h. EMVs released into the supernatant from cells treated with ET-1 or vehicle were isolated and quantified. EMV release was higher (P < 0.05) in cells treated with ET-1 compared with control (95 ± 15 vs. 54 ± 5 EMV/µL). Fresh HUVECs were then treated with either ET-1, ET-1-induced EMVs, or control EMVs for 24 h. ET-1-generated EMVs induced significantly higher release of IL-6 (181.0 ± 16.0 vs. 132.1 ± 8.1 pg/mL) and IL-8 (303.4 ± 37.4 vs. 211.8 ± 10.0 pg/mL), as well as greater total NF-κB p65 (76.0 ± 7.6 vs. 57.1 ± 2.1 AU) and active NF-κB p65 (Ser-536) (11.6 ± 0.9 vs. 6.8 ± 1.0 AU) expression than control EMVs. There were no significant differences in expression of caspase-9 (230.1 ± 24.3 vs. 243.6 ± 22.3 AU), caspase-3 (271.9 ± 22.7 vs. 265.1 ± 30.5 AU), and active caspase-3 (4.4 ± 0.4 vs. 4.3 ± 0.1 AU) in cells treated with ET-1-EMVs versus control EMVs. Total eNOS (108.4 ± 11.4 vs. 158.8 ± 1.6 AU) and activated eNOS (4.7 ± 0.5 vs. 9.6 ± 1.4 AU) were significantly lower in endothelial cells treated with ET-1-generated EMVs compared with control EMVs. The effects of ET-1-generated EMVs on cellular markers and mediators of endothelial inflammation, as well as eNOS function, was comparable to the effects of ET-1. In summary, ET-1 induces an EMV phenotype that adversely affects endothelial cell function. ET-1-generated EMVs may contribute to the atherogenic effect of ET-1.
NEW & NOTEWORTHY Endothelin-1 (ET-1) is a potent vasoconstrictor peptide released by the endothelium that contributes to the regulation of vascular tone. Overexpression of ET-1 has been implicated in the etiology of atherosclerotic vascular disease. Endothelial cell-derived microvesicles (EMVs) play a pivotal role in vascular health and disease. Their functional phenotype is largely dictated by the stimulus for release. EMVs released in response to various pathological conditions have been shown to elicit deleterious vascular effects. In the present study, we determined, in vitro, the effect of ET-1 on EMV release from endothelial cells and the effects of ET-1-generated EMVs on endothelial cell inflammation, apoptosis, and endothelial nitric oxide synthase (eNOS). ET-1 induced a marked increase in EMV release. ET-1-generated EMVs significantly increased endothelial cell inflammation and reduced eNOS protein expression and activation. Moreover, the endothelial effects of ET-1-derived EMVs were similar to the direct effects of ET-1. ET-1-generated EMVs may contribute to the proatherogenic profile of ET-1.
Keywords: endothelial cells, endothelin-1, eNOS, inflammation, microvesicles
INTRODUCTION
Endothelin (ET)-1 is a potent vasoconstrictor peptide produced and released primarily by endothelial cells (61). In addition to its vasoregulatory properties, overexpression of ET-1 is associated with the development and progression of atherosclerotic vascular disease and is generally considered to be an atherogenic peptide (4, 36, 38, 45). Indeed, ET-1 stimulates the migration and proliferation of vascular smooth muscle cells (34); is a strong chemoattractant for circulating monocytes (19); activates macrophages (19); promotes fibrous tissue formation (8); induces chemotaxis and replication of fibroblasts (44); inhibits endothelial nitric oxide synthase (eNOS) and, in turn, nitric oxide (NO) production (47); and induces vascular smooth muscle inflammation (5). Moreover, increased tissue ET-1 levels are evident in early and advanced stages of coronary artery disease and correlate with the severity of disease (30, 31, 36). In fact, in patients with chronic kidney disease, ET-1 tissue immunoreactivity is the main predicting factor of atherosclerosis progression (41). Although ET-1-mediated endothelial cell dysfunction likely contributes to the peptide’s proatherogenic effects (31, 36), the direct effects of ET-1 on endothelial cell function are not fully understood. For example, it is unknown whether ET-1 affects endothelial cell microvesicle release. Endothelial cell-derived microvesicles (EMVs) are increasingly recognized to play a pivotal role in vascular health and disease (63). Because their functional phenotype is largely dictated by the stimulus or condition for their release, their functional phenotype can be either vascular protective or destructive (22, 63).
EMVs are small (0.1–1.0 µm in diameter), anucleate vesicles shed constitutively by the endothelium (13, 29). Clinical interest in EMVs has expanded, as it has become clear that these microvesicles are involved in the pathogenesis of atherosclerosis (22). Under physiological conditions, EMVs can aid in cell-to-cell communication, activate repair or defense mechanisms, and/or stimulate immune responses (1). Under pathological conditions, however, EMVs are released in greater number, and their functional phenotype is more likely to evoke and perpetuate pathological cellular effects and responses (12, 50). For example, circulating EMVs have been shown to correlate positively with the extent and severity of coronary stenosis at angiography and independently predict future cardiovascular events in adults with a high risk of coronary artery disease, as well as predict acute allograft rejection in heart transplant patients (12, 51). In addition, EMVs released in response to various pathological conditions have been shown to elicit deleterious vascular effects (23, 24).
The experimental aims of this study were to determine 1) whether ET-1 stimulates EMV release from endothelial cells in vitro and 2) the effects of ET-1-generated EMVs on endothelial cell inflammation, apoptosis, and eNOS. We hypothesized that ET-1 increases EMV release, and ET-1-generated EMVs negatively affect endothelial cell inflammation, apoptosis, and eNOS. Moreover, we hypothesized that the endothelial cell effects of ET-1-generated EMVs would mimic the direct effects of ET-1.
METHODS
Cell culture, ET-1 treatment, and EMV isolation.
Human umbilical vein endothelial cells (HUVECs) were obtained from Life Technologies (Thermo Fisher Scientific, Waltham, MA). Cells were cultured in endothelial growth media (EBM-2 BulletKit) (Lonza, Basel, Switzerland) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin under standard cell culture conditions (37°C and 5% CO2). Growth media were replaced at standard intervals, 24 h after initial culture, and every 48 h thereafter. Confluence was assessed by light microscopy. Upon reaching 80–90% confluence, cells were serially passaged or harvested for experimentation on passages 3–5. Cells were seeded into tissue culture flasks (Falcon, Corning, NY) and cultured with media in the absence or presence of ET-1 (200 pM) (Clinalfa, AG) for 24 h (n = 5 experimental units). After 24 h, the supernatant and cells from each condition were collected for EMV characterization and enumeration.
EMV characterization and enumeration.
The concentration of EMVs in the collected supernatant was determined as previously described (2). Briefly, the supernatant from each condition (untreated control and ET-1-treated cells) was centrifuged at 13,000 g at room temperature for 2 min to pellet cellular debris. After centrifugation, 100 µL of the cell-free supernatant was transferred to TruCount tubes (BD Biosciences, Franklin Lakes, NJ), and samples were incubated with CD144-phycoerythrin (VE-cadherin) antibody for 20 min in the dark at room temperature. Thereafter, samples were fixed with 83 μL of 2% formaldehyde (ChemCruz Biochemicals, Santa Cruz, CA) and diluted with 500 µL of PBS. All samples were analyzed using a FACS Aria I flow cytometer (BD Biosciences). EMV size threshold was established using Megamix-Plus SSC calibrator beads (Biocytex, Marseille, France), and only events >0.16 µm and <1 µm in size were counted (40). The concentration of EMVs from control and ET-1-treated cells were determined using the formula: [(number of events in region containing EMVs/number of events in absolute count bead region) × (total number of beads per test/total volume of sample)] (25).
EMV-treated cells.
In a separate set of experiments (n = 5), HUVECs were cultured as described above and seeded into six-well tissue culture plates. Supernatant containing EMVs collected previously from the control and ET-1-treated cells were centrifuged at 20,500 g for 30 min at 4°C to pellet EMVs (25). The pelleted EMVs were resuspended in EBM-2 at a concentration of 1.0 × 107 EMV/mL. The number of EMVs isolated from control (untreated) and ET-1-treated cells was determined by flow cytometry as described above. Cells were treated with EMVs at a ratio of 2:1 EMVs to cells and were equal between conditions. HUVECs were incubated with either control EMVs or ET-1-generated EMVs for 24 h. Thereafter, the supernatant and cells were harvested for analysis.
Intracellular protein expression.
The expression of the principal proinflammatory transcription factor, NF-κB was measured to assess the inflammatory status of treated cells (32, 59). To determine the proapoptotic susceptibility of treated cells caspase-9, a critical upstream activator of the apoptosis cascade, and the terminator of the apoptosis cascade, caspase-3, were measured (60). Through the production of NO, eNOS plays a critical role in maintaining endothelial health and function. Higher levels of eNOS activation is associated with greater NO production and endothelial health (47, 58). Protein expression was determined using the Wes system (ProteinSimple, Santa Clara, CA), as previously described by our laboratory (21). Briefly, untreated cells, ET-1-treated cells and the EMV-treated cells were washed in ice-cold PBS and lysed in ice-cold RIPA buffer containing protease and phosphatase inhibitors (Thermo Fisher Scientific). Cell lysates were incubated on ice for 10 min and then sonicated for 20 s (four 5-s cycles spaced by 90 s between each cycle). Following sonication, samples were incubated on ice for an additional 15 min and then centrifuged at 13,000 g at 4°C for 10 min, and the supernatant from each sample was collected. Protein concentration was determined using the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA). Protein expression was measured by capillary electrophoresis immunoassay (Wes, ProteinSimple, Santa Clara, CA). Briefly, 2–3 ng of cell lysate was combined with a provided sample master mix (ProteinSimple) consisting of 1× sample buffer, 1× fluorescent molecular weight markers, and 40 mM DTT. Samples were vortexed and heated at 95°C for 5 min before combining with blocking solution, primary antibodies, horseradish peroxidase-conjugated secondary antibody, chemiluminescent substrate, and separation and stacking matrices for automated electrophoresis (375 V for 25 min) and immunodetection using the Wes system (ProteinSimple). Protein expression was quantified as peak area at the molecular weight for the corresponding protein of interest normalized to total protein (total protein detection, no. DM-TP01, ProteinSimple) in the sample. Rabbit primary antibodies against NF-κB p65 (D14E12), phospho-NF-κB p65 (Ser-536) (93H1), caspase-3 (8G10), activated caspase-3 (Asp-175) (5A1E), caspase-9 (9502S) (diluted 1:50, 1:25, 1:25, 1:25, and 1:25, respectively) (Cell Signaling Technologies, Danvers, MA); eNOS (PA1-037) and phospho-eNOS (Ser-1177) (PA5-35879) (diluted 1:25 and 1:50, respectively) (Thermo Fisher Scientific, Waltham, MA) were used. Initial titrations were performed to optimize antibody and total protein concentration for each protein.
Intracellular miRNA expression.
Intracellular expression of anti-inflammatory microRNAs, miR-146a and miR-181b, as well as eNOS-regulating miR-126 was determined by RT-PCR, as previously described (9, 21, 35, 59). After treatment with ET-1, ET-1-derived EMVs, and control EMVs, 1.0 × 105 cells were harvested, and total cellular RNA was isolated using the miRVANA RNA isolation kit (Exiqon, Vedbaek, Denmark), and RNA concentration was determined using a Nanodrop Lite (Thermo Fisher Scientific). A total of 250 ng of RNA was reverse transcribed using the miScript II reverse transcription kit (Qiagen, Hilden, Germany). RT-PCR was performed using the Bio-Rad CFX96 RT-PCR platform with the miScript SYBR green PCR kit (Qiagen, Hilden, Germany) and specific primers miR-146a, miR-181b, miR-126, and U6 (Qiagen, Hilden, Germany). All samples were assayed in duplicate. miRNA expression was quantified using the comparative Ct method and normalized to U6. The relative expression of each transcript was calculated as the 2−ΔΔCt where 2−(Ct[miR]−Ct[RNU6]) and presented as fold change.
Cytokine release and nitric oxide production.
To confirm the proinflammatory status of endothelial cells, the release of two key proinflammatory cytokines, IL-6 and IL-8, was measured. Concentration of interleukin (IL)-6 and IL-8 was determined in the supernatant from each condition by chemiluminescent ELISA (R&D Systems, Minneapolis, MN). To assess NO production, total nitrite in the supernatant was determined using the total nitric oxide and nitrate/nitrite parameter assay kit (R&D Systems). Intra-assay coefficient of variation for the media-based ELISAs were <10% for each assay.
Statistical analysis.
Differences between treatment and control conditions for the two separate experiments, ET-1 EMV vs. control EMV and ET-1 vs. control, were determined by two-tailed, unpaired Student’s t-test. Total EMV release was compared between cells treated with ET-1 or vehicle control. Markers of endothelial integrity were also compared between ET-1 EMV-treated cells and control EMV-treated cells, or ET-1 and vehicle-treated cells. Statistical comparison was not performed between cells treated with ET-1 or vehicle and ET-1 EMVs and control EMVs. Data are reported as means ± SE for five independent HUVEC experiments for both the ET-1 EMV experiments and the ET-1 experiments. Statistical significance was set a priori at P < 0.05.
RESULTS
EMV release.
The number of EMVs generated from HUVECs treated with ET-1 (95 ± 15 EMV/μL) was significantly higher (~75%) compared with EMVs released under control culture conditions (control: 54 ± 5 EMV/μL).
EMV effect on endothelial cell inflammation.
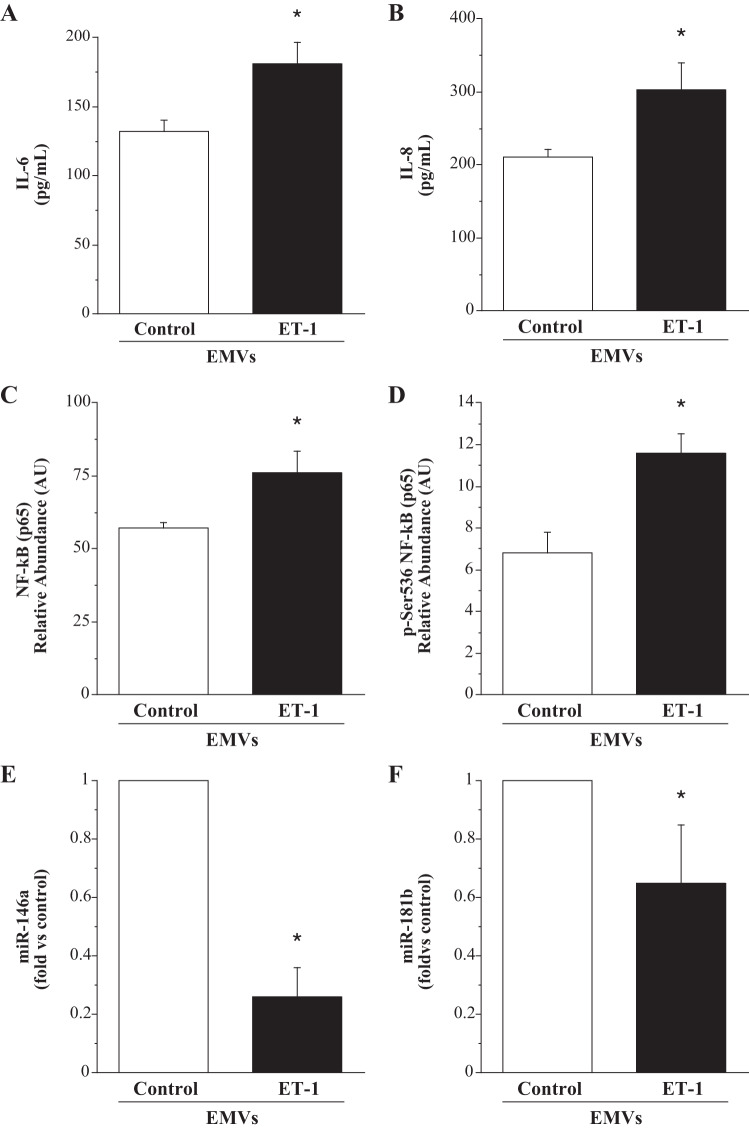
The effect of ET-1-derived EMVs on endothelial inflammatory markers is shown in Fig. 1. ET-1 EMVs induced significantly higher IL-6 (181.0 ± 16.0 vs. 132.1 ± 8.1 pg/mL) and IL-8 (303.4 ± 37.4 vs. 211.8 ± 10.0 pg/mL) release compared with control EMVs. Concordantly, cell expression of total NF-κB p65 (76.0 ± 7.6 vs. 57.1 ± 2.1 AU) and active (phospho-) NF-κB p65 (Ser-536) (11.6 ± 0.9 vs. 6.8 ± 1.0 AU) was significantly higher (~30% and ~70%; P < 0.05), respectively, in the HUVECs treated with ET-1 EMVs. In addition, expression of both miR-146a (0.3 ± 0.1 fold vs. control) and miR-181b (0.7 ± 0.2 fold vs. control) were significantly lower in the HUVECs treated with ET-1 EMVs. The activation of NF-κB is inhibited by miR-146a through the blockade of upstream signaling molecules; miR-181b inhibits NF-κB activity by suppressing translocation through nuclear pores (9, 35, 59).
Fig. 1.
Endothelial cell release of IL-6 (A) and IL-8 (B) and intracellular expression of total NF-κB p65 (C), phosphorylated-NF-κB p65(Ser-536) (D), miR-146a (E), and miR-181b (F) in response to treatment with EMVs. Values are presented as means ± SE. AU indicates arbitrary unit. *P < 0.05 vs. untreated control.
ET-1 effects on endothelial cell inflammation.
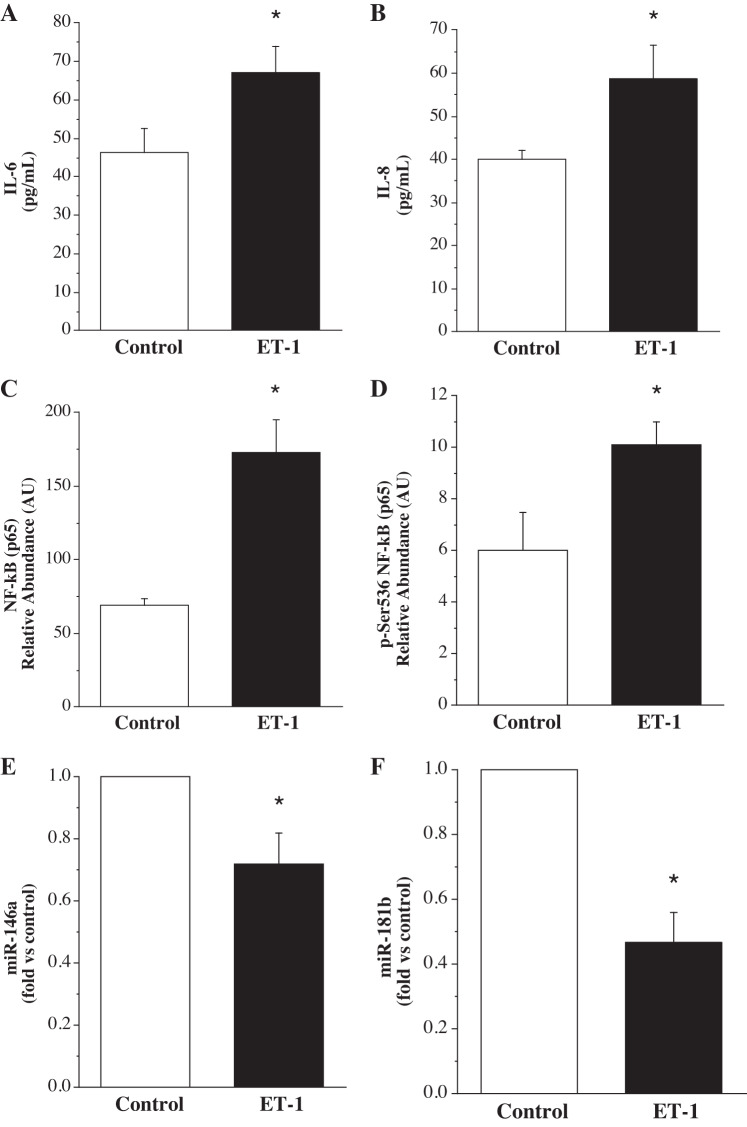
HUVECs treated with ET-1 released greater (P < 0.05) IL-6 (67.1 ± 6.7 vs. 46.3 ± 6.2 pg/mL) and IL-8 (58.8 ± 7.6 vs. 40.1 ± 2.0 pg/mL) compared with HUVECs not treated with ET-1. Cellular expression of total NF-κB p65 (172.8 ± 22.5 vs. 69.1 ± 4.8 AU) and phospho-NF-κB p65 (10.1 ± 0.9 vs. 6.0 ± 1.5 AU) was also significantly higher (~150% and ~70%, respectively) in the ET-1-treated cells. Additionally, ET-1 markedly reduced (P < 0.05) miR-146a (0.7 ± 0.1 fold vs. control) and miR-181b (0.5 ± 0.1 fold vs. control) expression (Fig. 2).
Fig. 2.
Endothelial cell release of IL-6 (A) and IL-8 (B) and intracellular expression of total NF-κB p65 (C), phosphorylated-NF-κB p65(Ser-536) (D), miR-146a (E), and miR-181b (F) in response to treatment with ET-1. Values are presented as means ± SE. AU indicates arbitrary unit. *P < 0.05 vs. untreated control.
EMV effect on endothelial apoptosis.
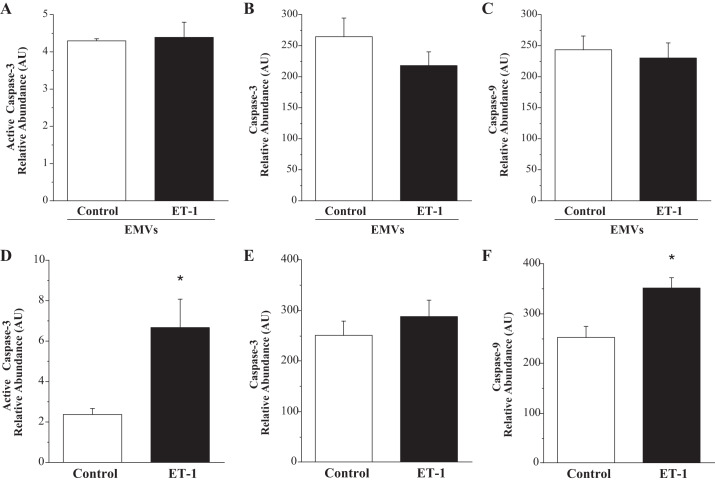
The effect of ET-1-generated EMVs and ET-1 on endothelial cell expression of caspase-3 and caspase-9 is shown in Fig. 3. There were no significant differences in cellular caspase-9 (230.1 ± 24.3 vs. 243.6 ± 22.3 AU), caspase-3 (271.9 ± 22.7 vs. 265.1 ± 30.5 AU), and active caspase-3 (4.4 ± 0.4 vs. 4.3 ± 0.1 AU) levels in HUVECs treated with ET-1 EMVs compared with control EMVs.
Fig. 3.
Endothelial cell expression of total caspase-3, active caspase-3, and caspase-9 in response to treatment with ET-1-generated EMVs (A, B, and C) and ET-1 (D, E, and F). Values are presented as means ± SE. AU indicates arbitrary unit. *P < 0.05 vs. respective control.
ET-1 effect on endothelial apoptosis.
In response to ET-1, active caspase-3 (6.7 ± 1.5 vs. 2.4 ± 0.3 AU) and caspase-9 (351.7 ± 20.9 vs. 253.3 ± 21.2 AU) expression was significantly higher than untreated cells (Fig. 3, D and F). There was no significant difference in total caspase-3 expression between ET-1-treated (287.8 ± 32.8 AU) and untreated (250.7 ± 28.7 AU) cells.
EMV effect on endothelial nitric oxide synthase and NO production.
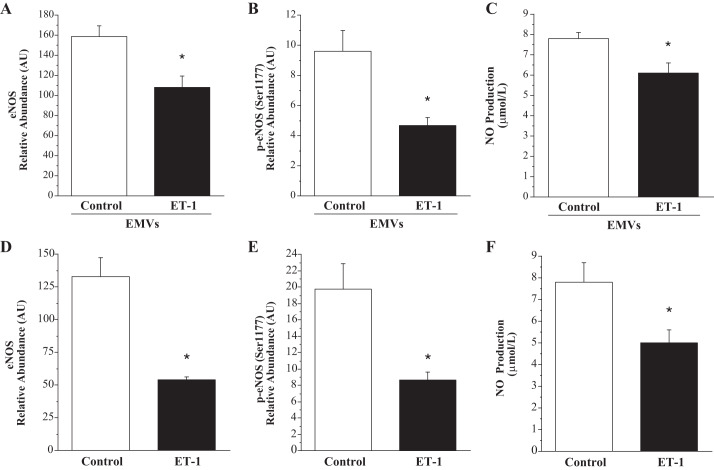
The effect of ET-1-generated EMVs and ET-1 on endothelial cell expression of eNOS and NO production is shown in Fig. 4. Expression of total eNOS (108.4 ± 11.4 vs. 158.8 ± 1.6 AU) and p-eNOS (4.7 ± 0.5 vs. 9.6 ± 1.4 AU) were lower (~30% and ~50%, respectively; P < 0.05) in HUVECs treated with ET-1 EMVs compared with control EMVs. When p-eNOS was normalized to total eNOS, p-eNOS was not different in the cells treated with ET-1-derived EMVs (0.05 ± 0.01 vs. 0.06 ± 0.01 AU). NO production was significantly lower from cells treated with ET-1-derived EMVs (6.1 ± 0.5 vs 7.8 ± 0.03 µmol/L). In addition, expression of miR-126 (0.3 ± 0.1 fold vs. control) was significantly lower in the HUVECs treated with ET-1 EMVs.
Fig. 4.
Endothelial cell expression of total eNOS, phosphorylated eNOS (Ser-1177), and NO production in response to treatment with ET-1-generated EMVs (A, B, and C) and ET-1 (D, E, and F). Values are presented as means ± SE. AU indicates arbitrary unit. *P < 0.05 vs. respective control.
ET-1 effect on endothelial nitric oxide synthase and NO production.
In ET-1-treated HUVECs, total eNOS (54.3 ± 1.9 vs. 133.0 ± 14.7 AU) and p-eNOS (8.7 ± 0.9 vs. 19.8 ± 3.1 AU) in the cells was lower compared with control cells. When p-eNOS was normalized to total eNOS, there was no significant difference between the ET-1-treated and control cells (0.16 ± 0.01 vs. 0.15 ± 0.03 AU). NO production was significantly lower from cells treated with ET-1 compared with control (5.0 ± 0.9 vs. 7.8 ± 0.6 µmol/L) (Fig. 4). In addition, expression of miR-126 (0.6 ± 0.1 fold vs. control) was significantly lower in the HUVECs treated with ET-1.
DISCUSSION
The novel finding of the present study is that ET-1-generated EMVs increase endothelial cell inflammation and lower eNOS protein expression and NO production. Moreover, the effect of ET-1-derived EMVs on cellular markers and mediators of endothelial inflammation, as well as eNOS function was comparable to the effects of ET-1 per se. To our knowledge, this is the first study to determine the effects of ET-1-generated EMVs on endothelial cell function. ET-1-related EMVs may amplify the atherogenic properties of ET-1.
There is increasing evidence that EMVs released in response to pathologic stimuli or conditions are key protagonists in the development and progression of atherosclerotic cardiovascular disease (CVD) and related thrombotic events (18, 22, 63). Clinically, circulating concentrations of EMVs are elevated in several disease states, and the magnitude of elevation is commensurate with the severity of disease. As such, EMVs are considered to be sensitive biomarkers of endothelial dysfunction and indicators of CVD severity and risk of adverse events (22, 55, 63). The functional phenotype of EMVs is highly dependent upon the stimulus driving their production and release. For example, both in vitro (2) and clinical studies (16, 28) have shown that hyperglycemic conditions result in an increase in EMV release and, perhaps more importantly, a pathogenic EMV phenotype that induces similar endothelial dysfunction to that of high glucose per se. Increased ET-1 synthesis and release are associated with a greater propensity of endothelial dysfunction, CVD risk, and disease (4, 36, 38, 45). In the present study, consistent with the findings of Jung et al. (26), we demonstrate that ET-1 stimulates EMV release. Endothelial cells treated with ET-1 released significantly more (~45%) EMVs than untreated cells. These in vitro data are consistent with the clinical observation that circulating EMVs are elevated in conditions associated with increased ET-1 production such as hypertension (46, 64), atherosclerosis (7), acute coronary syndromes (3), heart failure (42), and ischemic stroke (54). The mechanisms underlying ET-1-induced release of EMVs is unclear. Endothelial cell activation and increased intracellular Ca2+, two primary effects of ET-1 on endothelial cells (19, 62), are likely causative triggers (29). Cell activation results in a ubiquitous spike in p38 activity. Increased p38 activity coupled with elevations in intracellular Ca2+ promote cytoskeletal rearrangement leading to membrane budding, cleavage, and shedding of extracellular vesicles (48, 52). Independent of the mechanisms underlying their release, it is the stimulus for release that ultimately dictates the functional phenotype of EMVs and their associated vascular effects.
Endothelial cell inflammation is a major initiating event in atherosclerosis (49). Increased release of IL-6 and IL-8 is a hallmark feature of a proinflammatory endothelial cell and, in turn, an atherogenic prone endothelial phenotype (39). In the present study, ET-1-derived EMVs induced significantly greater endothelial cell release of both IL-6 and IL-8 compared with control EMVs. Furthermore, consistent with the cytokine response, cellular NF-κB expression and activation were also markedly higher in the cells treated with ET-1-generated EMVs. NF-κB is the primary transcription factor involved in regulating IL-6 and IL-8 expression and release (32, 59). Thus, the increase in total and activated NF-κB is likely a causal mechanism for enhanced IL-6 and IL-8 release in response to ET-1 EMVs. Additionally, intracellular expression of miR-146a and miR-181b—microRNAs that are directly involved in the regulation of NF-κB activation—was significantly lower in cells treated with ET-1 EMVs, providing potential mechanistic insight for the observed increase in activated NF-κB. Indeed, miR-146a targets adaptor proteins, such as IL-1 receptor-associated kinase 1 and tumor necrosis factor receptor-associated factor 6 (35) that limit NF-κB signaling and activation (9). miR-181b suppresses importin-α3, which reduces nuclear translocation of NF-κB, resulting in diminished gene transcription (35, 59). ET-1 has been shown to increase IL-6 from HUVECS (57), and it has been shown to increase both IL-6 and IL-8 from vascular smooth muscle cells through NF-κB activation (5). The results of the present study are consistent with these findings and demonstrate that the effect of ET-1-generated EMVs on the various indicators and mediators of inflammation was quantitatively comparable to the direct effects of ET-1. Collectively, these findings demonstrate the imposing effects of ET-1-generated EMVs on endothelial cell inflammatory processes. Moreover, our findings suggest that the proinflammatory effects of ET-1 may be perpetuated by ET-1-related EMVs.
Enhanced apoptotic susceptibility increases the propensity for endothelial dysfunction, atherogenesis, thrombosis, and plaque instability (10, 15, 27). Apoptosis is a highly preserved, highly regulated cellular process to protect against aberrant cell proliferation and/or death (60). Unlike cell death through necrosis, apoptosis occurs independent of, and does not result in, cellular inflammation (17). The activation of a caspase family of cysteine proteases, most notably caspase-3, triggers the execution phase of apoptosis, leading to the destabilization and destruction of the cell (11). Increased active levels of caspase-3 heightens the rate of cellular apoptosis and risk of atherosclerosis (10, 15, 20). In contrast to endothelial cell inflammation, ET-1-generated EMVs do not appear to induce a proapoptotic endothelial phenotype. Cellular expression of total caspase-3 and active caspase-3 was not significantly altered by ET-1 EMVs. Concordantly, caspase-9, an initiating caspase involved in the activation of caspase-3, was also not affected by ET-1-related EMVs. Interestingly, the effects of ET-1-generated EMVs on caspase-3 and caspase-9 were discordant with the effect of ET-1 on these caspases. Endothelial cells treated with ET-1 demonstrated significantly higher active caspase-3 and caspase-9 expression compared with control cells. Thus, although ET-1 increases the apoptotic susceptibility of endothelial cells, potentially contributing to its atherogenic effects, this affect is not propagated by ET-1-generated EMVs.
A major proatherogenic effect of ET-1 on endothelial cells is the peptide’s ability to reduce NO production by downregulating the expression and activity of eNOS (47). In a seminal study, Sud and Black (58) showed that ET-1 stimulates protein kinase Cδ (PKCδ) activity, resulting in activation of STAT3 which, in turn, blunts eNOS transcription, signaling and, ultimately, NO production. In addition, the oxidative effects of ET-1 on endothelial cells also leads to uncoupling of eNOS and reduced NO bioavailability (33). Consistent with previous studies (4, 56), we demonstrate that both eNOS expression and activation were significantly lower in endothelial cells treated with ET-1. Moreover, miR-126 expression was also lower in response to ET-1, providing additional mechanistic insight regarding the detrimental effect of ET-1 on eNOS and NO production. miR-126 safeguards the activity of the phosphatidylinositol 3-kinase/protein kinase B/eNOS signaling pathway critical for eNOS activation (65). Reduced intracellular miR-126 has been directly linked to depressed eNOS function and a loss of NO production (21, 37). The results of the present study significantly extend these findings by demonstrating that the unfavorable effects of ET-1 on eNOS and miR-126 expression are also conferred by ET-1-generated EMVs. Similar to ET-1, ET-1-generated EMVs induced significant reductions in both eNOS expression and activation, as well as miR-126 levels. The similar reduction in intracellular miR-126 observed in response to both ET-1 and ET-1-generated EMVs suggests, at least, one common mechanism for altering eNOS activation shared by ET-1 and ET-1-related EMVs. Future studies are needed to determine whether ET-1-generated EMVs also affect PKC activity and STAT3 activation in a similar manner to ET-1. In addition, the potential synergistic effects of ET-1 and ET-1-generated EMVs on eNOS is unknown. The negative effects of ET-1-induced EMVs on eNOS expression and NO production, as well as miR-126, may be an important factor contributing to the marked endothelial vasodilator dysfunction and enhanced susceptibility to atherosclerosis in conditions associated with increased ET-1 production.
There are a few experimental considerations regarding the present study that deserve mention. First, given the in vitro nature of this study, we cannot make definitive statements regarding ET-1, ET-1-generated EMVs, and clinical risk. However, the markers and mediators of endothelial cell function studied have been shown to have strong causative links with the development of atherosclerosis and its clinical sequela (4, 49). Second, we were unable to perform inferential statistics directly comparing the endothelial effects of ET-1 with ET-1-generated EMVs because the respective treatments were not carried out on the same endothelial cell experimental units. Third, the use of HUVECs rather than an artery-derived cell line may cause concern that the expression of proteins and the function of venous endothelial cells in culture may not be representative of arterial endothelial cells and, in turn, limit connection to arterial atherosclerotic disease. However, the expression of key proteins involved in the regulation of vascular endothelial function and disease risk (such as NF-κB and eNOS) has been shown to be remarkably analogous between endothelial cells acquired from an artery or vein (53). Moreover, venous and arterial cells exhibit comparable, reproducible responses to a myriad of physiological and pathological stimuli, both in vitro and in vivo (6, 14, 43).
In conclusion, the results of this study demonstrate that ET-1 induces an increase in EMV release and an EMV phenotype that elicits similar deleterious effects on endothelial cell function to that produced by ET-1. Indeed, the known adverse endothelial effects of ET-1 on NF-κB-mediated inflammation and eNOS expression and activation were also observed by the EMVs released in response to ET-1. It is highly plausible that ET-1-generated EMVs add to the proatherogenic profile of ET-1. From a clinical perspective, the beneficial vascular effects of targeting ET-1 bioavailability and system activity may extend beyond mitigating the direct effects of ET-1 on endothelial function and involve the attenuation of the release of ET-1-associated EMVs that can serve as second messengers in perpetuating ET-1-related vascular dysfunction.
GRANTS
This study was supported, in part, by National Institutes of Health Grants HL-077450 and HL-107715.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.D. conceived and designed research; L.M.B., V.P.G., M.V.L., K.A.S., A.G., N.M.D., J.J.G., and J.G.H. performed experiments; L.M.B., V.P.G., M.V.L., K.A.S., A.G., N.M.D., J.J.G., J.G.H., and C.A.D. analyzed data; L.M.B., V.P.G., M.V.L., J.J.G., J.G.H., and C.A.D. interpreted results of experiments; L.M.B., V.P.G., K.A.S., J.J.G., and J.G.H. prepared figures; C.A.D. drafted manuscript; J.J.G., J.G.H., and C.A.D. edited and revised manuscript; L.M.B., V.P.G., M.V.L., K.A.S., A.G., N.M.D., J.J.G., J.G.H., and C.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the staff at the University of Colorado Anschutz Medical Campus ACI/ID Flow Core for their technical assistance.
REFERENCES
- 1.Alexandru N, Costa A, Constantin A, Cochior D, Georgescu A. Microparticles: from biogenesis to biomarkers and diagnostic tools in cardiovascular disease. Curr Stem Cell Res Ther 12: 89–102, 2017. doi: 10.2174/1574888X11666151203224058. [DOI] [PubMed] [Google Scholar]
- 2.Bammert TD, Hijmans JG, Reiakvam WR, Levy MV, Brewster LM, Goldthwaite ZA, Greiner JJ, Stockelman KA, DeSouza CA. High glucose derived endothelial microparticles increase active caspase-3 and reduce microRNA-Let-7a expression in endothelial cells. Biochem Biophys Res Commun 493: 1026–1029, 2017. doi: 10.1016/j.bbrc.2017.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, de Marchena E, Ahn YS. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J 145: 962–970, 2003. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 4.Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 76: 8–18, 2007. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Browatzki M, Schmidt J, Kübler W, Kranzhöfer R. Endothelin-1 induces interleukin-6 release via activation of the transcription factor NF-kappaB in human vascular smooth muscle cells. Basic Res Cardiol 95: 98–105, 2000. doi: 10.1007/s003950050170. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Gong Y, Liu L, Zhou Y, Fang X, Zhang C, Li Y, Li J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: a review. J Appl Toxicol 37: 1359–1369, 2017. doi: 10.1002/jat.3470. [DOI] [PubMed] [Google Scholar]
- 7.Carracedo J, Alique M, Ramírez-Carracedo R, Bodega G, Ramírez R. Endothelial extracellular vesicles produced by senescent cells: pathophysiological role in the cardiovascular disease associated with all types of diabetes mellitus. Curr Vasc Pharmacol 17: 447–454, 2019. doi: 10.2174/1570161116666180820115726. [DOI] [PubMed] [Google Scholar]
- 8.Chang W, Lajko M, Fawzi AA. Endothelin-1 is associated with fibrosis in proliferative diabetic retinopathy membranes. PLoS One 13: e0191285, 2018. doi: 10.1371/journal.pone.0191285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med 5: 1017–1034, 2013. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol 33: 1673–1690, 2001. doi: 10.1006/jmcc.2001.1419. [DOI] [PubMed] [Google Scholar]
- 11.Cohen GM. Caspases: the executioners of apoptosis. Biochem J 326: 1–16, 1997. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis AM, Edelberg J, Jonas R, Rogers WT, Moore JS, Syed W, Mohler ER III. Endothelial microparticles: sophisticated vesicles modulating vascular function. Vasc Med 18: 204–214, 2013. doi: 10.1177/1358863X13499773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 31: 27–33, 2011. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 14.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 15.Durand E, Scoazec A, Lafont A, Boddaert J, Al Hajzen A, Addad F, Mirshahi M, Desnos M, Tedgui A, Mallat Z. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: a clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation 109: 2503–2506, 2004. doi: 10.1161/01.CIR.0000130172.62481.90. [DOI] [PubMed] [Google Scholar]
- 16.Feng B, Chen Y, Luo Y, Chen M, Li X, Ni Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis 208: 264–269, 2010. doi: 10.1016/j.atherosclerosis.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Haanen C, Vermes I. Apoptosis and inflammation. Mediators Inflamm 4: 5–15, 1995. doi: 10.1155/S0962935195000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafiane A, Daskalopoulou SS. Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease. Metabolism 85: 213–222, 2018. doi: 10.1016/j.metabol.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Haller H, Schaberg T, Lindschau C, Lode H, Distler A. Endothelin increases [Ca2+]i, protein phosphorylation, and O2·− production in human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 261: L478–L484, 1991. doi: 10.1152/ajplung.1991.261.6.L478. [DOI] [PubMed] [Google Scholar]
- 20.Han DK, Haudenschild CC, Hong MK, Tinkle BT, Leon MB, Liau G. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am J Pathol 147: 267–277, 1995. [PMC free article] [PubMed] [Google Scholar]
- 21.Hijmans JG, Stockelman KA, Garcia V, Levy MV, Brewster LM, Bammert TD, Greiner JJ, Stauffer BL, Connick E, DeSouza CA. Circulating microparticles aAre elevated in treated HIV -1 infection and are deleterious to endothelial cell function. J Am Heart Assoc 8: e011134, 2019. doi: 10.1161/JAHA.118.011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen F, Li Q, Pfeifer A, Werner N. Endothelial- and immune cell-derived extracellular vesicles in the regulation of cardiovascular health and disease. JACC Basic Transl Sci 2: 790–807, 2017. doi: 10.1016/j.jacbts.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen F, Rohwer K, Vasa-Nicotera M, Mellert F, Grube E, Nickenig G, Werner N, Sinning JM. CD-144 positive endothelial microparticles are increased in patients with systemic inflammatory response syndrome after TAVI. Int J Cardiol 204: 172–174, 2016. doi: 10.1016/j.ijcard.2015.11.179. [DOI] [PubMed] [Google Scholar]
- 24.Jansen F, Wang H, Przybilla D, Franklin BS, Dolf A, Pfeifer P, Schmitz T, Flender A, Endl E, Nickenig G, Werner N. Vascular endothelial microparticles-incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc Diabetol 15: 49, 2016. doi: 10.1186/s12933-016-0367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, Becher MU, Abu Hussein N, Kebschull M, Bedorf J, Franklin BS, Latz E, Nickenig G, Werner N. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol 32: 1925–1935, 2012. doi: 10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- 26.Jung C, Lichtenauer M, Wernly B, Franz M, Goebel B, Rafnsson A, Figulla HR, Pernow J. Effect of endothelin-1 and endothelin receptor blockade on the release of microparticles. Eur J Clin Invest 46: 707–713, 2016. doi: 10.1111/eci.12652. [DOI] [PubMed] [Google Scholar]
- 27.Kavurma MM, Bhindi R, Lowe HC, Chesterman C, Khachigian LM. Vessel wall apoptosis and atherosclerotic plaque instability. J Thromb Haemost 3: 465–472, 2005. doi: 10.1111/j.1538-7836.2005.01120.x. [DOI] [PubMed] [Google Scholar]
- 28.Koga H, Sugiyama S, Kugiyama K, Watanabe K, Fukushima H, Tanaka T, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 45: 1622–1630, 2005. doi: 10.1016/j.jacc.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 29.Koganti S, Eleftheriou D, Brogan PA, Kotecha T, Hong Y, Rakhit RD. Microparticles and their role in coronary artery disease. Int J Cardiol 230: 339–345, 2017. doi: 10.1016/j.ijcard.2016.12.108. [DOI] [PubMed] [Google Scholar]
- 30.Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC Jr. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med 325: 997–1001, 1991. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- 31.Lerman A, Holmes DR Jr, Bell MR, Garratt KN, Nishimura RA, Burnett JC Jr. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation 92: 2426–2431, 1995. doi: 10.1161/01.cir.92.9.2426. [DOI] [PubMed] [Google Scholar]
- 32.Liang Y, Li X, Zhang X, Li Z, Wang L, Sun Y, Liu Z, Ma X. Elevated levels of plasma TNF-α are associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-κB and p38 mitogen-activated protein kinase in endothelial cells. Shock 41: 275–281, 2014. doi: 10.1097/SHK.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 33.Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther 315: 1058–1064, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Lüscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 102: 2434–2440, 2000. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 35.Ma S, Tian XY, Zhang Y, Mu C, Shen H, Bismuth J, Pownall HJ, Huang Y, Wong WT. E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci Rep 6: 22910, 2016. doi: 10.1038/srep22910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew V, Hasdai D, Lerman A. The role of endothelin in coronary atherosclerosis. Mayo Clin Proc 71: 769–777, 1996. doi: 10.1016/S0025-6196(11)64842-8. [DOI] [PubMed] [Google Scholar]
- 37.Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J Mol Cell Cardiol 53: 64–72, 2012. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol 61: 391–415, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Mudaliar H, Pollock C, Ma J, Wu H, Chadban S, Panchapakesan U. The role of TLR2 and 4-mediated inflammatory pathways in endothelial cells exposed to high glucose. PLoS One 9: e108844, 2014. doi: 10.1371/journal.pone.0108844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen MH, Beck-Nielsen H, Andersen MN, Handberg A. A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. J Extracell Vesicles 3: 3, 2014. doi: 10.3402/jev.v3.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noshad H, Argani H, Nezami N, Ghojazadeh M, Zomorrodi A, Bohlouli A, Bonyadi MR, Fakhrjou A, Ghorbanihaghjo A, Gharedaghi A, Javadzadegan H, Sadreddini S. Arterial atherosclerosis in patients with chronic kidney disease and its relationship with serum and tissue endothelin-1. Iran J Kidney Dis 3: 203–209, 2009. [Erratum in Iran J Kidney Dis 4: 88, 2010.] [PubMed] [Google Scholar]
- 42.Nozaki T, Sugiyama S, Sugamura K, Ohba K, Matsuzawa Y, Konishi M, Matsubara J, Akiyama E, Sumida H, Matsui K, Jinnouchi H, Ogawa H. Prognostic value of endothelial microparticles in patients with heart failure. Eur J Heart Fail 12: 1223–1228, 2010. doi: 10.1093/eurjhf/hfq145. [DOI] [PubMed] [Google Scholar]
- 43.Onat D, Brillon D, Colombo PC, Schmidt AM. Human vascular endothelial cells: a model system for studying vascular inflammation in diabetes and atherosclerosis. Curr Diab Rep 11: 193–202, 2011. doi: 10.1007/s11892-011-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peacock AJ, Dawes KE, Shock A, Gray AJ, Reeves JT, Laurent GJ. Endothelin-1 and endothelin-3 induce chemotaxis and replication of pulmonary artery fibroblasts. Am J Respir Cell Mol Biol 7: 492–499, 1992. doi: 10.1165/ajrcmb/7.5.492. [DOI] [PubMed] [Google Scholar]
- 45.Pernow J, Shemyakin A, Böhm F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci 91: 507–516, 2012. doi: 10.1016/j.lfs.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 46.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 41: 211–217, 2003. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 47.Ramzy D, Rao V, Tumiati LC, Xu N, Sheshgiri R, Miriuka S, Delgado DH, Ross HJ. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKC-dependent pathway. Circulation 114, Suppl: I319–I326, 2006. doi: 10.1161/CIRCULATIONAHA.105.001503. [DOI] [PubMed] [Google Scholar]
- 48.Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, Tedgui A, Boulanger CM. Microparticles, vascular function, and atherothrombosis. Circ Res 109: 593–606, 2011. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- 49.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999. doi: 10.1160/TH16-03-0176. [DOI] [PubMed] [Google Scholar]
- 50.Santilli F, Marchisio M, Lanuti P, Boccatonda A, Miscia S, Davì G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb Haemost 116: 220–234, 2016. doi: 10.1160/TH16-03-0176. [DOI] [PubMed] [Google Scholar]
- 51.Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med 379: 958–966, 2018. doi: 10.1056/NEJMra1704286. [DOI] [PubMed] [Google Scholar]
- 52.Shu Z, Tan J, Miao Y, Zhang Q. The role of microvesicles containing microRNAs in vascular endothelial dysfunction. J Cell Mol Med 23: 7933–7945, 2019. doi: 10.1111/jcmm.14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res 47: 1–8, 2010. doi: 10.1159/000231715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost 4: 1296–1302, 2006. doi: 10.1111/j.1538-7836.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- 55.Sinning JM, Losch J, Walenta K, Böhm M, Nickenig G, Werner N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J 32: 2034–2041, 2011. doi: 10.1093/eurheartj/ehq478. [DOI] [PubMed] [Google Scholar]
- 56.Song R, Chou YI, Kong J, Li J, Pan B, Cui M, Zhou E, Zhang Y, Zheng L. Association of endothelial microparticle with NO, eNOS, ET-1, and fractional flow reserve in patients with coronary intermediate lesions. Biomarkers 20: 429–435, 2015. doi: 10.3109/1354750X.2015.1094140. [DOI] [PubMed] [Google Scholar]
- 57.Stankova J, D’Orléans-Juste P, Rola-Pleszczynski M. ET-1 induces IL-6 gene expression in human umbilical vein endothelial cells: synergistic effect of IL-1. Am J Physiol Cell Physiol 271: C1073–C1078, 1996. doi: 10.1152/ajpcell.1996.271.4.C1073. [DOI] [PubMed] [Google Scholar]
- 58.Sud N, Black SM. Endothelin-1 impairs nitric oxide signaling in endothelial cells through a protein kinase Cdelta-dependent activation of STAT3 and decreased endothelial nitric oxide synthase expression. DNA Cell Biol 28: 543–553, 2009. doi: 10.1089/dna.2009.0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM, Feinberg MW; MICU Registry . MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest 122: 1973–1990, 2012. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 267: 1456–1462, 1995. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 61.Thorin E, Webb DJ. Endothelium-derived endothelin-1. Pflugers Arch 459: 951–958, 2010. doi: 10.1007/s00424-009-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tykocki NR, Watts SW. The interdependence of endothelin-1 and calcium: a review. Clin Sci (Lond) 119: 361–372, 2010. doi: 10.1042/CS20100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vítková V, Živný J, Janota J. Endothelial cell-derived microvesicles: potential mediators and biomarkers of pathologic processes. Biomark Med 12: 161–175, 2018. doi: 10.2217/bmm-2017-0182. [DOI] [PubMed] [Google Scholar]
- 64.Wang JM, Su C, Wang Y, Huang YJ, Yang Z, Chen L, Wu F, Xu SY, Tao J. Elevated circulating endothelial microparticles and brachial-ankle pulse wave velocity in well-controlled hypertensive patients. J Hum Hypertens 23: 307–315, 2009. doi: 10.1038/jhh.2008.137. [DOI] [PubMed] [Google Scholar]
- 65.Yang HH, Chen Y, Gao CY, Cui ZT, Yao JM. Protective effects of microRNA-126 on human cardiac microvascular endothelial cells against hypoxia/reoxygenation-induced injury and inflammatory response by activating PI3K/Akt/eNOS signaling pathway. Cell Physiol Biochem 42: 506–518, 2017. doi: 10.1159/000477597. [DOI] [PubMed] [Google Scholar]