Abstract
Histamine mediates vasodilation during inflammatory and immune responses, as well as following endurance exercise. During exercise, intramuscular histamine concentration increases, and its production, appears related to exercise intensity and duration. However, whether histamine contributes to exercise hyperemia and promotes exercise blood flow in an intensity- or duration-dependent pattern is unknown. The purpose of this study was to compare leg blood flow across a range of exercise intensities, before and after prolonged exercise, with and without histamine-receptor antagonism. It was hypothesized that combined oral histamine H1/H2-receptor antagonism would decrease leg blood flow, and the effect would be greater at higher intensities and following prolonged exercise. Sixteen (7F, 9M) volunteers performed single-leg knee-extension exercise after consuming either placebo or combined histamine H1/H2-receptor antagonists (Blockade). Exercise consisted of two graded protocols at 20, 40, 60, and 80% of peak power, separated by 60 min of knee-extension exercise at 60% of peak power. Femoral artery blood flow was measured by ultrasonography. Femoral artery blood flow increased with exercise intensity up to 2,660 ± 97 mL/min at 80% of peak power during Placebo (P < 0.05). Blood flow was further elevated with Blockade to 2,836 ± 124 mL/min (P < 0.05) at 80% peak power (9.1 ± 4.8% higher than placebo). These patterns were not affected by prolonged exercise (P = 0.13). On average, femoral blood flow during prolonged exercise was 12.7 ± 2.8% higher with Blockade vs. Placebo (P < 0.05). Contrary to the hypothesis, these results suggest that histamine receptor antagonism during exercise, regardless of intensity or duration, increases leg blood flow measured by ultrasonography.
NEW & NOTEWORTHY Leg blood flow during exercise was increased by taking antihistamines, which block the receptors for histamine, a molecule often associated with inflammatory and immune responses. The elevated blood flow occurred over exercise intensities ranging from 20 to 80% of peak capacity and during exercise of 60 min duration. These results suggest that exercise-induced elevations in histamine concentrations are involved in novel, poorly understood, and perhaps complex ways in the exercise response.
Keywords: antihistamine, endurance exercise, histamine, regional blood flow
INTRODUCTION
Histamine, an important component of immune and inflammatory responses, is produced and released within the affected tissue, eliciting an increase in blood flow by its vasodilatory actions on arterioles and an increase in vascular permeability by its actions on capillaries (8, 29, 51). Beyond immune and inflammatory responses, endogenously produced histamine is associated with increased skin blood flow during heat stress (59), elevated limb blood flow in reactive hyperemia (4, 15, 19), elevated limb blood flow during sustained postexercise vasodilation (32, 33), and increased tissue blood flow during tumor growth (40). In all these situations, consumption of histamine H1- and/or H2-receptor antagonists have attenuated the elevations in blood flow (15, 19, 32, 33, 48, 57, 59). Histamine primarily mediates an increase in blood flow by the activation of histamine H1 and H2 receptors on endothelial cells and vascular smooth muscle, respectively, resulting in an arterial vasodilation (32, 33). Recent evidence indicates that histamine concentrations increase within the skeletal muscle interstitial fluid during exercise (45); however, no known study has explored the role of histamine in contributing to the rise in blood flow to skeletal muscle during exercise.
If histamine contributes to exercise hyperemia, it may be dependent on the duration or intensity of muscle contractions. Some evidence suggests that the enzymatic activity of skeletal muscle tissue histidine decarboxylase (the enzyme that produces histamine) is positively correlated with exercise duration (5, 39). Along these lines, blocking histamine receptors does not appear to affect performance outcomes of exercise tests that last seconds (single muscle contractions) to a few minutes in duration (17, 36, 37), but it does appear to decrease the ability to perform tasks that are 15 min to hours long in duration (17, 39, 60). The short-duration exercise tests were of maximal intensity and relied primarily on anaerobic metabolic pathways, while those that were of longer duration were performed at submaximal intensities. Submaximal intensity exercise requires adequate skeletal muscle blood flow for oxygen and nutrient delivery and metabolite removal, integral in staving off many factors implicated in fatigue (e.g., hydrogen ion buildup, reduced oxygen content, intramuscular glycogen depletion) and maintaining exercise output. Thus, it is possible that skeletal muscle histaminergic pathways contribute to the normal rise in skeletal muscle blood flow during repeated contractions typical of endurance exercises.
Therefore, the purpose of this experiment was to examine the influence of histamine in the regulation of skeletal muscle blood flow during exercise. As the contribution of the histamine pathway to hyperemia may be exercise intensity- and/or duration-dependent, exercising limb blood flow was quantified at multiple exercise intensities before and after an extended bout of exercise. Specifically, femoral blood flow was measured in subjects who performed knee-extension exercise at 20, 40, 60, and 80% of peak power output before and after 60 min of sustained moderate-intensity exercise (60% of peak power) following consumption of oral antihistamine medications (fexofenadine and ranitidine) or placebo. Prior to the study, it was hypothesized that blocking histamine’s interaction with H1 and H2 receptors would decrease femoral artery muscle blood flow at high exercise intensities, and the effect would be greater following a bout of endurance exercise.
METHODS
Subjects
This study was approved by the Institutional Review Board of the University of Utah and the Salt Lake City Veterans Affairs Medical Center. Each volunteer gave written and informed consent before participation, and the study conformed to the principles of the Declaration of Helsinki. No subjects were using over-the-counter or prescription medications at the time of the study, with the exception of oral contraceptives. Female participants were studied during the early follicular phase of their menstrual cycle or during the placebo phase of their oral contraceptive to minimize any potential cardiovascular effects of sex-specific hormones (35).
Screening Visit
An initial screening visit was completed to expose the subjects to all of the testing procedures and to obtain demographic and anthropometric information (age, height, and weight). Subjects also completed a single-leg, knee-extension test to determine peak power output.
Experimental Design
This study was completed using a balanced, double-blind, placebo-controlled design. Subjects performed experiments after a 4-h fast and abstained from caffeine, alcohol, and strenuous exercise for 24 h. All testing was performed in a temperate environment (21–23°C, 13–46% relative humidity) at the Veterans Affairs Salt Lake City Geriatric, Research, Education, and Clinical Center in the Utah Vascular Research Laboratory (altitude ~1,400 m).
Testing Visits
Subjects completed two testing visits that were performed at least 7 days apart (range: 7–37 days) and at the same time of day to reduce the potential for circadian rhythms to influence skeletal muscle blood flow (11). Figure 1 depicts the timeline for the testing visits. Initially, 60 min before the start of single-leg knee-extension exercise, subjects ingested either placebo pills (placebo) or combined histamine H1- and H2-receptor antagonist pills (Blockade) with ~90 mL of water. Subjects were then seated on the ergometer for the remainder of the visit. After 60 min of seated rest, baseline measures of femoral blood flow, blood pressure, heart rate, cardiac output, oxygen consumption (V̇o2), muscle pH, muscle tissue oxygenation index, and a rating of perceived exertion were measured.
Fig. 1.
Study time line. After ingestion of placebo or blockade (Rx), subjects performed 3-min stages of knee extension at 20, 40, 60, and 80% of peak power. Subjects next performed a 60-min bout of sustained moderate-intensity (60% of peak power) knee-extension exercise. Finally, subjects repeated the 3-min stages of knee extension at 20, 40, 60, and 80% of peak power. All exercise was performed at a rate of 60 contractions per minute. The 60-min bout of sustained exercise was separated from the graded stages of exercise by 20 min of seated rest. Each open arrow (⇓) represents a time point where blood flow, blood pressure, heart rate, and cardiac output were measured. Solid arrows indicate points where V̇o2, ventilation, muscle tissue oxygenation index, tissue pH, and rating of perceived exertion were measured.
Femoral blood flow was measured during 3-min stages of single-leg knee-extension exercise at 20, 40, 60, and 80% peak power. Femoral blood flow was then measured every 10 min during a sustained bout (60 min) of moderate-intensity exercise (60% of peak power). Following the sustained bout of exercise, femoral blood flow was again measured during 3-min stages of exercise at 20, 40, 60, and 80% of peak power. Exercise was continued at each exercise intensity for 3 min, and blood flow was recorded in the final 1–2 min. Each increase in workload (20, 40, 60, and 80%) was separated by 2 min of seated resting recovery (Fig. 1). A 20-min seated rest period was provided before and following the sustained bout of exercise to avoid subject fatigue. Additionally, blood pressure, cardiac output, V̇o2, muscle pH, and muscle oxygen saturation were measured with each increase in exercise intensity. V̇o2, muscle pH, and muscle tissue oxygenation index were measured for 3 min at 10, 30, and 50 min during the sustained bout.
Measurements
Single-leg knee-extension exercise and peak test.
Subjects were seated on an adjustable chair with a cycle ergometer (Monark 828E, Vansbro, Sweden) positioned behind the chair. Resistance was created by varying friction applied to a flywheel that was turned upon knee extension of the subject’s dominant leg via a rigid link from the ergometer crank arm and a boot worn by the subject (3). For the peak power test, workload was increased 5 to 10 W every 2 min, based on the subject’s rating of perceived exertion until the subject reached volitional fatigue. This peak test was used to establish individual workload assignments for exercise during the testing visits. As stated above, during the two test visits, subjects exercised for 3 min at 20, 40, 60, and 80% of peak power and rested for 2 min between each workload. For the sustained bout, subjects exercised at 60% of peak power for 60 min. In all phases, subjects were required to maintain a leg extension rate of 60 per min.
Histamine H1 and H2-receptor antagonism.
Oral administration of 540 mg of fexofenadine, a selective H1-receptor antagonist, reaches peak plasma concentrations within 1 h and has a 12-h half-life (46). Oral administration of 300 mg ranitidine, a selective H2-receptor antagonist, reaches peak plasma concentration within 2 h and has a 3-h half-life (22). This dosage of histamine-receptor antagonists results in more than 90% inhibition of histamine H1 and H2 receptors lasting for 6 h after administration (22). Fexofenadine and ranitidine are not thought to cross the blood-brain barrier or to have sedative effects (22, 46). Importantly, it has been shown that histamine H1 and H2 receptor antagonism does not alter blood flow, heart rate, blood pressure, or smooth muscle tone at rest (16, 18, 32, 33, 44). The placebos were manufactured by the University of Utah pharmacy and contained the inactive ingredients of the fexofenadine and ranitidine tablets.
Central hemodynamics.
Heart rate was monitored using a three-lead electrocardiogram, and arterial pressure was measured on the left brachial artery using an automated auscultometric sphygmomanometer (Tango+, SunTech Medical, Raleigh, NC). Mean arterial pressure was calculated as diastolic pressure plus 1/3 pulse pressure (systolic pressure − diastolic pressure) and was reported in mmHg. Cardiac output was estimated noninvasively from a finger photoplethysmography-derived arterial pressure waveform (Finometer, Finapres Medical Systems BV, Amsterdam, The Netherlands) using the “Modelflow method,” which has been shown to approximate changes in cardiac output with exercise (56). This method relies on the standardized model of aortic compliance to estimate stroke volume, which is multiplied by heart rate to provide an estimate of cardiac output that correlates well (r values of 0.91–0.98) when compared with echocardiography (56). Waveform signals were recorded via a data acquisition device (Medwave Vasotrac APM250A; Biopac, Goleta, CA). We did not rely on the Finometer for arterial pressure values reported in this study (26).
Femoral blood flow and vascular conductance.
Blood velocity and vessel diameter were measured from the common femoral artery in the exercising leg, 2–3 cm proximal of the bifurcation of the superficial and deep branches of the femoral artery using Doppler ultrasonography. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area, where the best spatial resolution was achieved. Velocity measurements were made at 5 MHz with a linear array transducer (Logiq e9, GE Medical Systems, Milwaukee, WI). Care was taken to avoid aliasing the blood velocity spectra by using scale adjustments, especially during exercise. All blood velocity measures were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to the vessel size and was centered within the vessel based on real-time ultrasound visualization. Femoral blood velocity was determined using the on-board software of the ultrasound system (Logic e9; GE Medical Systems, Milwaukee, WI). Femoral blood flow (mL/min) was calculated by multiplying the mean blood velocity over 60 s by the cross-sectional area of the common femoral artery during resting baseline measures:
The common femoral artery diameter during rest was used in the calculation of exercise blood flow, as earlier studies have indicated that there is a low coefficient of variation (CV ~1.5%) in repeated diameter measurements of the common femoral artery between systole and diastole, from rest to exercise, and at increasing exercise intensities (41, 42, 53). Leg vascular conductance was calculated as blood flow / mean arterial pressure and expressed in mL·min−1·mmHg−1.
Skeletal muscle pH and muscle tissue oxygenation index.
Intramuscular microvascular blood pH and tissue oxygenation of the vastus lateralis muscle were estimated using near-infrared spectroscopy (Reflectance Medical Inc., Oximeter 1100, Westborough, MA). Near-infrared spectroscopy is commonly used to estimate capillary oxygen saturation in the muscle, as oxygenated and deoxygenated hemoglobin absorb light at 800 nm, and deoxygenated hemoglobin absorbs light at 760 nm, the difference between the two can give an indication of the oxygenation state of the tissue (31). Additionally, shifts in the near-infrared spectra in the 725–880-nm wavelength range are correlated with changes in hydrogen ion concentrations (55). This method of correlated spectra with changes in muscle pH has been validated against invasive intramuscular probes during exercise (55). The sensor was placed one-third of the distance between the knee joint and the greater trochanter, and the sensor was held in place by a mounting pad that conformed to the shape of the thigh and prevented sensor movement on the skin during exercise. The sensor was further held in place with black self-adherent wrap (Coban, 3M, Maplewood, MN), which also limited ambient luminosity.
Whole body metabolism.
Whole body oxygen uptake (V̇o2) and carbon dioxide production (V̇co2) were measured during single-leg, knee-extension exercise using a mixing chamber system (TrueOne 2400, Parvo-Medics, Sandy, UT). Respiratory exchange ratio was calculated as V̇co2/V̇o2.
Rating of perceived exertion.
A subjective rating of exertion was documented on a 1–10 scale (9) during the last minute of each graded exercise stage, as well as 10, 30, and 50 min into the sustained bout of exercise.
Statistical analysis.
Statistical inferences were drawn from two or three-way repeated-measures ANOVAs with a priori contrasts. We also modeled exercise variables using a stepwise regression, which was run with SAS Proc GLMSELECT (SAS version 9.2; SAS Institute, Cary, NC). This approach allowed examination of linear relationships across time or exercise intensity and tested whether or not the relationships differed between drug condition or pre- and post-sustained exercise. Independent variables remained in the model if a minimal P value threshold were met (P < 0.15). For all tests, significance was set at P < 0.05. All data are presented as means ± SE, except for data characterizing the subjects, which are presented as means ± SD (Table 1).
Table 1.
Subject characteristics
| Characteristic | Value |
|---|---|
| n | 16 (7F, 9M) |
| Age, yr | 25 ± 5 |
| Height, cm | 174.9 ± 8.1 |
| Weight, kg | 73.9 ± 12.7 |
| Body mass index, kg/m2 | 24.1 ± 3.3 |
| Knee-extension peak power, W | 50 ± 10 |
| 60% Workload, W | 30 ± 5 |
Values are expressed as means ± SD. F, female; M, male.
RESULTS
Subject Characteristics
Sixteen (7 female, 9 male) healthy, nonsmoking individuals volunteered for the present study. Subject demographic and anthropometric characteristics obtained from the screening visit, including age, height, weight, body mass index, knee-extension peak power, and 60% workload are presented in Table 1.
Two subjects were excluded from blood flow and femoral vascular conductance analysis due to large differences in resting femoral artery diameter (0.07 and 0.08 cm, ~10%) between the Placebo and Blockade trials, suggesting that a different anatomical location may have been insonated on each visit. For comparison, there was a range from 0.00- to 0.02-cm difference in femoral artery diameter for the remaining 14 subjects that were included in the analysis. Therefore, only 14 subjects were included in the analysis of femoral blood flow and femoral vascular conductance, so we could be confident that blood velocities were being recorded at similar positions of the common femoral artery.
Ramped Exercise Response Pre- and Post-Sustained Exercise
Subject’s workloads at 20, 40, 60, and 80% of peak power output corresponded to 10 ± 2, 20 ± 4, 29 ± 6, and 39 ± 9 W (means ± SD).
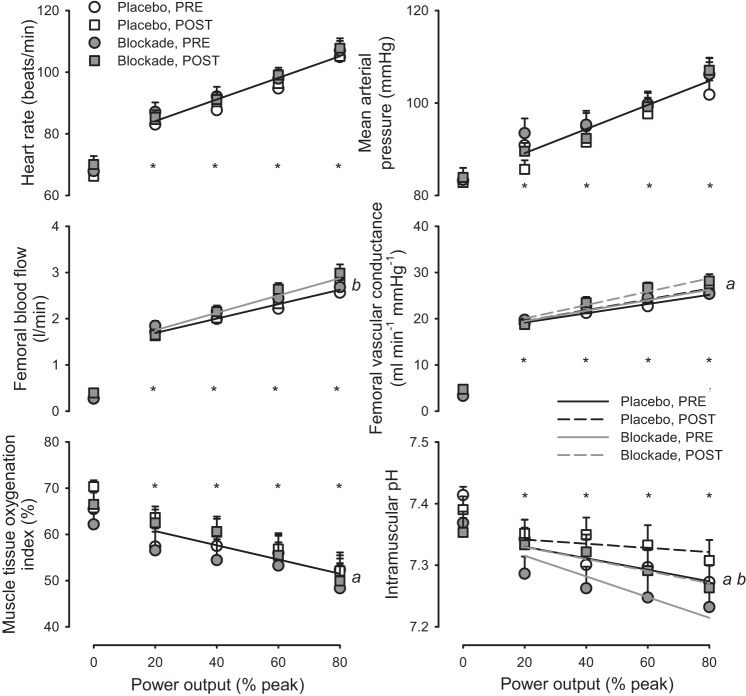
As shown in Fig. 2, heart rate, mean arterial pressure, femoral blood flow, and femoral vascular conductance increased with increasing workloads, while muscle tissue oxygenation and intramuscular pH decreased with increasing workloads (all P < 0.05). The rise of heart rate, mean arterial pressure, and femoral blood flow during increasing workloads were not different pre- compared with post-sustained exercise (all P > 0.05). Femoral vascular conductance, muscle tissue oxygenation, intramuscular pH (Fig. 2), and respiratory exchange ratio (RER, Fig. 3) were higher post- compared with pre-sustained exercise (all P < 0.05).
Fig. 2.
Hemodynamic and local muscle responses to graded exercise. Heart rate, mean arterial pressure, femoral blood flow, femoral vascular conductance, muscle tissue oxygenation index, and intramuscular pH in response to increasing power output before (PRE; circles) and after (POST; squares) 60 min of sustained moderate-intensity exercise with either Placebo (open symbols) or histamine receptor antagonism (Blockade, solid symbols). *P < 0.05, exercise vs. resting at specified power output. a denotes P < 0.05 main effect for Post vs. Pre, and b denotes P < 0.05, main effect for Blockade vs. Placebo. Solitary regression lines for a variable indicate the absence of differences between Placebo and Blockade and PRE and POST. Multiple regression lines indicate models differed P < 0.05 across experimental conditions. n = 16 except for blood flow and conductance, where n = 14.
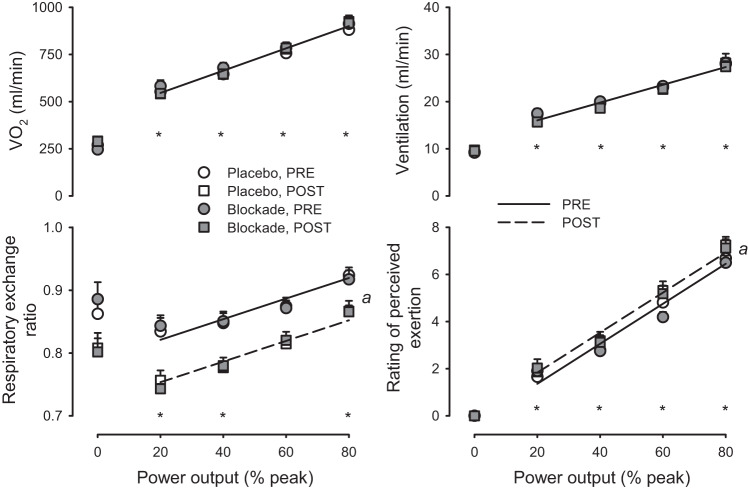
Fig. 3.
Whole body metabolic and perception of effort responses to graded exercise. Oxygen uptake (V̇o2), ventilation, respiratory exchange ratio, and rating of perceived exertion in response to increasing power output before (PRE; circles) and after (POST; squares) 60-min sustained moderate-intensity exercise with either placebo (open symbols) or histamine receptor antagonism (blockade, solid symbols). *P < 0.05, exercise vs. resting at specified power output; a denotes P < 0.05, main effect for Post vs. Pre. Solitary regression lines for a variable indicate the absence of differences between Placebo and Blockade and PRE and POST. Multiple regression lines indicate models differed P < 0.05 across experimental conditions. n = 16.
Additionally, Modelflow estimates of cardiac output increased from rest (5.7 ± 0.2 L/min) through each increase in workload (20%: 7.6 ± 0.3, 40%: 8.0 ± 0.3, 60%: 8.9 ± 0.3, and 80%: 9.3 ± 0.3 L/min; P < 0.05) but were not different pre- to post-sustained exercise (P > 0.05).
Blockade resulted in an elevated femoral blood flow at all exercise intensities in both pre- and post-sustained exercise compared with Placebo (P < 0.05). Blood flow was elevated 6.4 ± 5.2, 10.6 ± 5.8, 15.3 ± 5.2, and 9.1 ± 4.8% at 20, 40, 60, and 80% of peak power, respectively, with Blockade compared with Placebo. In addition, blockade resulted in a lower intramuscular pH during both pre- and post-sustained exercise compared with Placebo (P < 0.05). Blockade had no effect on heart rate, mean arterial pressure, femoral vascular conductance, and tissue oxygenation pre- and post-sustained exercise compared with Placebo (all P > 0.05). Furthermore, blockade had no effect on cardiac output (Placebo: 5.6 ± 0.4, 7.4 ± 0.4, 7.8 ± 0.5, and 8.7 ± 0.5, 9.4 ± 0.5 L/min; Blockade: 5.8 ± 0.3, 7.8 ± 0.4, 8.2 ± 0.5, 9.0 ± 0.4, and 9.7 ± 0.4 L/min, at 20, 40, 60, and 80% of peak power, respectively; P = 0.25).
As shown in Fig. 3, V̇o2, ventilation, RER, and rating of perceived exertion (RPE) increased with increasing workloads (all P < 0.05). The rise of V̇o2 and ventilation was not different pre- compared with post-sustained exercise (both P > 0.05). RPE was lower post- compared with pre-sustained exercise (P < 0.05). Blockade had no effect on V̇o2, ventilation, RER, or RPE compared with Placebo (all P > 0.05).
Sustained Moderate-Intensity Exercise
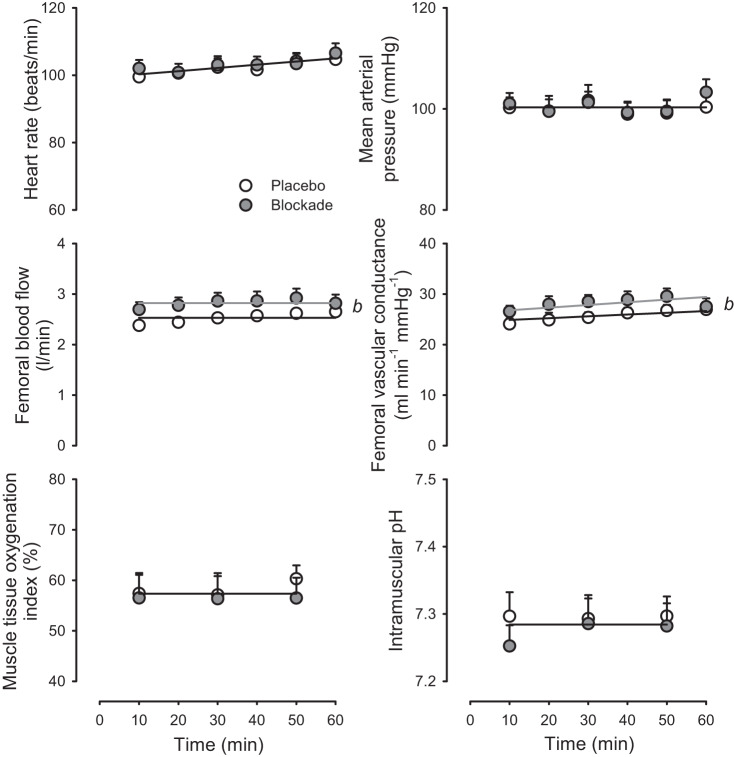
Subjects maintained 30 ± 5 W (means ± SD) for the 60-min duration of sustained moderate-intensity exercise.
As shown in Fig. 4, heart rate, mean arterial pressure, muscle tissue oxygenation, and intramuscular pH were stable throughout exercise and were not different between Blockade and Placebo (P > 0.05). While femoral blood flow and femoral vascular conductance were stable throughout exercise, femoral blood flow was 12.7 ± 2.8% greater, and vascular conductance was 16.2 ± 3.5% greater in Blockade compared with Placebo (P < 0.05).
Fig. 4.
Hemodynamic and local muscle responses to sustained moderate-intensity exercise. Heart rate, mean arterial pressure, femoral blood flow, femoral vascular conductance, muscle tissue oxygenation index, and intramuscular pH in response to 60 min of sustained moderate-intensity exercise with either Placebo (open symbols) or histamine receptor antagonism (Blockade, solid symbols). b denotes P < 0.05, main effect for blockade vs. placebo. Solitary regression lines for a variable indicate the absence of differences between placebo and blockade. Multiple regression lines indicate models differed P < 0.05 across experimental conditions. n = 16 except for blood flow and conductance, where n = 14.
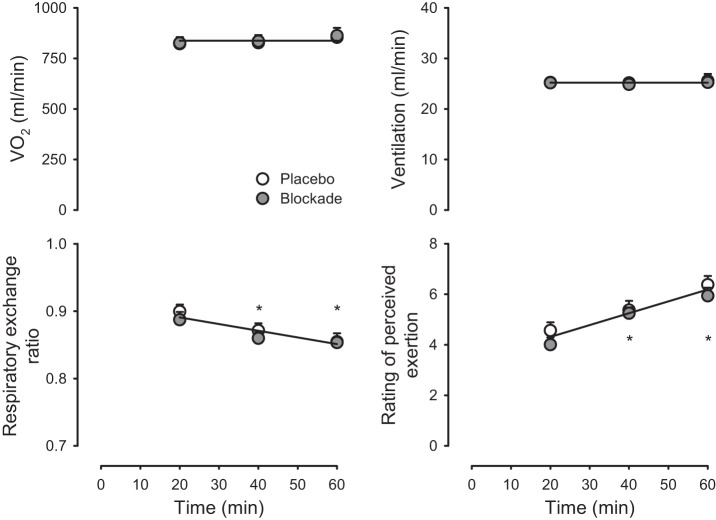
As shown in Fig. 5, V̇o2 and ventilation were stable throughout exercise and were not different between Placebo and Blockade (P > 0.05). Respiratory exchange ratio decreased with time throughout exercise (P < 0.05) but was similar between Placebo and Blockade (P > 0.05). Rating of perceived exertion increased with time throughout exercise (P < 0.05) but was similar between Placebo and Blockade (P > 0.05).
Fig. 5.
Whole body metabolic and perception of effort responses to sustained moderate-intensity exercise. Oxygen uptake (V̇o2), ventilation, respiratory exchange ratio, and rating of perceived exertion in response to 60 min of sustained moderate-intensity exercise with either placebo (open symbols) or histamine receptor antagonism (blockade, solid symbols). *P < 0.05, exercise vs. resting at specified power output. Solitary regression lines for a variable indicate the absence of differences between placebo and blockade. n = 16.
DISCUSSION
The main finding of the present study was that histamine H1 and H2 receptor blockade resulted in an elevation of exercising limb blood flow. The elevated blood flow occurred acutely with increasing exercise intensities, during a sustained moderate-intensity exercise bout, and again with increasing exercise intensities after the sustained bout. These findings are opposite of the a priori hypothesis that interrupting histamine’s interactions with H1 and H2 receptors would reduce the exercise hyperemic response. They are also counter to the broader current understanding of histamine’s ability to elicit vasodilation via H1 and H2 receptors, studied under circumstances apart from exercise.
Leg Blood Flow During Increasing Exercise Intensities
Over the past three decades, a large number of studies across several research programs have documented that femoral blood flow increases linearly with intensity during single-leg knee-extension exercise (6, 10, 24, 58). Like previous investigations, the present results show within the Placebo condition, exercising limb blood flow increases linearly with increasing exercise intensities from 20 to 80% of single-leg, knee-extension peak power (Fig. 2), a well-characterized model of dynamic small muscle-mass exercise. The elevation in blood flow occurred concomitantly with linear increases in heart rate, mean arterial pressure, femoral vascular conductance (Fig. 2), oxygen consumption, ventilation, respiratory exchange ratio, and rating of perceived exertion, while muscle tissue oxygenation index and pH decreased (Fig. 3).
Skeletal Muscle Histamine and Its Hypothesized Influence on Exercise Hyperemia
During exercise, skeletal muscle histamine concentration increases due to mast cell degranulation and increased production from histidine decarboxylase (HDC) (45). The stimuli to cause the degranulation and increase HDC production are unknown, but in situ and in vitro studies have shown stimuli associated with exercise, such as hypoxia (27), increased temperature (49, 51), vibration (34), hyperosmolality (34), and decreased pH (49) can all act to increase histamine release or production. As exercise intensity and duration increase, the positive allosteric influences of these factors should lead to an increased skeletal muscle histamine concentration. In fact, microdialysis examination of intramuscular histamine concentrations show a 3–4-fold increase with 60 min of activity (45). These studies indicate that multiple exercise-related pathways may contribute to the rise of histamine and suggest that it has an important function in the skeletal muscle response to endurance exercise; however, the nature of that function is unknown.
On the basis of the known vasodilatory actions of histamine in inflammatory and immune responses, following tissue damage, in reactive hyperemia, and during sustained postexercise vasodilation (13, 19, 29, 32, 33, 51, 52), it was hypothesized that increases in intramuscular histamine would aid in local vasodilation and contribute to exercise hyperemia. We expected that antagonism of histamine H1 and H2 receptors would have differential effects on limb blood flow across exercise intensity and duration based on the following tenets: 1) exercise hyperemia is governed primarily by mechanical factors at light workloads, while vasodilator substances are more important for increasing blood flow at higher intensities (43), 2) vasodilatory substances, such as prostaglandins and histamine, increase with exercise intensity (10, 58), and 3) a positive correlation between exercise duration and histidine decarboxylase mRNA transcription and enzymatic activity (5, 20, 39).
Effect of Combined Histamine H1 and H2 Receptor Antagonism on Leg Blood Flow
It has been shown that histamine H1 and H2 receptor antagonism does not alter leg blood flow at rest (16, 18, 32, 33, 44), indicating that histamine is not a likely modulator of vascular resistance during resting conditions.
Contrary to the hypothesis, blocking histamine receptors did not result in a reduced femoral blood flow at any exercise intensity or after an extended exercise bout (Fig. 2). Indeed, antagonism of histamine H1 and H2 receptors resulted in an increase in exercising limb blood flow that was similar in magnitude from 20 (6.4 ± 5.2%) to 80% (9.1 ± 4.8%) of peak power output. The elevated blood flow was also observed during sustained moderate-intensity exercise (12.7 ± 2.8%) (Fig. 4), and across multiple exercise intensities following a bout of sustained exercise (Fig. 2). The elevation of blood flow occurred without any significant change in heart rate, mean arterial pressure (Fig. 2), oxygen consumption, ventilation, respiratory exchange ratio, or perceived exertion between Placebo and Blockade conditions (Fig. 3). It is unknown how blocking histamine H1 and H2 receptors elevated femoral blood flow, but the indirect measures of the skeletal muscle microenvironment may indicate a disconnect between microcirculatory perfusion of skeletal muscle and femoral artery blood flow or perhaps more likely, some other disruption of microvascular function beyond changes in perfusion.
Histamine is an intrinsic regulator of the microvasculature and initiates the contraction of endothelial and pericyte cells on the venule side of the capillary, widening intercellular gaps, resulting in increased capillary permeability (30, 50). It could be that the rise in skeletal muscle histamine concentration promotes interstitial homeostasis by increasing the exchange of substances across the skeletal muscle capillary bed, as exercise intensity increases (50). It is possible that antagonism of H1 and H2 receptors reduced the permeability of the capillaries, such that hydrogen ion transport/diffusion out of the tissue was more restricted, resulting in the lowered tissue pH that was observed with blockade (Fig. 2). Alternatively, blockade may have modified skeletal muscle metabolism by limiting nutrient delivery and shifted the balance of ATP production toward glycolysis of intramuscular glycogen stores, resulting in elevated hydrogen ion production. Thus, it is possible that metabolic disruption or augmented level of vasodilators may underlie the greater blood flow observed with histamine receptor antagonism. Future studies that focus on capillary permeability during exercise may be necessary to understand the physiological role of histamine in the response to exercise.
Leg Blood Flow During Dynamic Small Muscle-Mass Exercise
Beyond the new insight regarding the role of histamine in the regulation of exercising muscle blood flow, the present study also provided several unique observations regarding the mechanics of exercise hyperemia during dynamic, small muscle-mass exercise. Most examinations of femoral blood flow are at fixed workloads for 3 to 10 min in duration and involve short rest periods before changes in work load or administering an intervention (1, 2, 6, 10, 14, 24, 43), precluding the ability to assess the constancy of exercising limb blood flow over extended exercise bouts. In fact, to our knowledge, this is the first study to systematically evaluate femoral blood flow during sustained exercise that lasted longer than 25 min in duration. Schrage et al. (53) reported in five individuals (time controls in a larger study) the blood flow response to 25 min of exercise, and Rådegran (42) provided data on two individuals who exercised for 60 min. Both reports used single-leg knee-extension exercise at 19–20 W (equivalent to our 40% peak power and light intensity) and found low coefficients of variation (CV ~9 to 14%) in blood flow over multiple time points (42, 53). We expand this by providing statistically robust results at a more relevant moderate intensity. We also note that in the present study, exercising limb blood flows during the 60 min of sustained exercise at 60% of peak power were similar to those obtained during 60% of peak in the ramped exercise and were maintained throughout the sustained bout. Associated with the stable and reproducible blood flow level were steady-state heart rate, mean arterial pressure, tissue oxygen content, intramuscular pH, V̇o2, and ventilation. Thus, the current results support the notion that 60% of peak power represents a stable hemodynamic and metabolic state below critical power and that steady-state values can be achieved within 3 min at this exercise intensity, given the preceding lower-intensity warm-up. We also note that as exercise continued, the RER decreased and RPE increased (Fig. 5). This suggests shifting substrate utilization throughout exercise, as has been shown for whole body exercise (12).
Another unique aspect of the current study was the repetition of the progressive stages of exercise following the bout of sustained moderate-intensity exercise. We observed that elevations in femoral blood flow during graded exercise are similar in the Placebo condition, analogous to the prior observation that exercise hyperemia in this model is not affected by repeated trials that are separated by 120 min of sedentary rest (6, 58). Although previous studies have identified a faster rise at exercise onset (25) and shorter time to reach steady state (21) in femoral blood flow following short exercise bouts, we did not specifically look at the first minute of each exercise stage when such an effect might be evident. We did observe a subtle elevation in the vascular conductance response to graded exercise following the sustained exercise bout, which may be associated with an attenuated drop in intramuscular pH, suggesting some modification of the vascular response to exercise that resulted in altered oxygen and nutrient delivery or metabolite removal (Fig. 2). The mechanism for this subtle additional vasodilation in unclear.
Perspective
During exercise, skeletal muscle arterioles dilate and blood flow rises in proportion to the metabolic demands of the tissue (28). The vasodilation occurs primarily through locally derived vasodilator release by or near the active musculature and modulation of neural vasoconstriction by some of those same vasodilators (28). The primary mediator leading to vasodilation is unknown, and the current thinking is that there is a redundancy in their formation and synergy in their actions. As evidence, blocking the actions of one (58), two (10, 53), or even three (38) of these known vasodilators has produced negligible reductions in skeletal muscle blood flow during acute exercise (<10 min duration) in healthy individuals. Further, the majority of studies have investigated the influence of vasodilators on exercise hyperemia at the start of exercise (10, 14, 24, 43), but the factors regulating steady-state exercise blood flow may be different from those initiating exercise hyperemia (54). Although the present study does not provide clear evidence of a role of histamine in exercise hyperemia, it suggests that histamine may modify the local milieu surrounding active skeletal muscle in unique ways that have not been appreciated. On the surface, the elevated blood flow generated during exercise with combined H1 and H2 receptor antagonism might be viewed as beneficial. However, it is possible that pharmacologically interrupting the tightly regulated matching of blood flow and metabolism may actually result in an increased oxygen delivery and consumption, as we have previously reported (7). Furthermore, considering the expected reduction in pH within the exercising skeletal muscle, and in light of the deleterious impact of H1 and H2 receptor antagonism on cycling performance during time trials after sustained moderate-intensity exercise (17), it is highly likely that histamine generates a net beneficial response within the exercising skeletal muscle microcirculation. Further, these benefits are likely compromised when histamine H1 and H2 receptors are blocked, such that homeostasis is disrupted and a larger blood flow is necessary to compensate for the loss of histamine’s influence.
Conclusions
Blocking the receptors for histamine, a molecule often associated with inflammatory and immune responses, resulted in elevated skeletal muscle blood flow during exercise. This elevation in blood flow occurred over exercise intensities ranging from 20 to 80% of peak capacity and during exercise of 60-min duration. These results suggest that exercise-induced elevations in histamine concentrations are involved in the rather complex regulation of blood flow during exercise.
GRANTS
This research was supported, in part, by National Institutes of Health (NIH) Grant HL-115027, the Eugene and Clarissa Evonuk Memorial Graduate Fellowship, NIH Grant HL-118313, the Veterans Affairs Rehabilitation Research and Development Career Development (IK2RX001215), the American Heart Association (14SDG18850039), and Department of Veterans Affairs (I01RX001311).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.R.E., J.D.T., D.W.W., and J.R.H. conceived and designed research; M.R.E., S.M.R., D.T.L.S., and J.D.T. performed experiments; M.R.E., S.M.R., D.T.L.S., and J.R.H. analyzed data; M.R.E., J.D.T., D.W.W., and J.R.H. interpreted results of experiments; M.R.E. and J.R.H. prepared figures; M.R.E. and J.R.H. drafted manuscript; M.R.E., S.M.R., D.T.L.S., J.D.T., D.W.W., and J.R.H. edited and revised manuscript; M.R.E., S.M.R., D.T.L.S., J.D.T., D.W.W., and J.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to thank the volunteers who participated.
REFERENCES
- 1.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MAH, Jonathan Groot H, Walter Wray D, Stehlik J, Richardson RS. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174: 368–375, 2014. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 59: 1647–1653, 1985. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- 4.Anrep GV, Barsoum GS, Salama S, Souidan Z. Liberation of histamine during reactive hyperaemia and muscle contraction in man. J Physiol 103: 297–305, 1944. doi: 10.1113/jphysiol.1944.sp004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayada K, Watanabe M, Endo Y. Elevation of histidine decarboxylase activity in skeletal muscles and stomach in mice by stress and exercise. Am J Physiol Regul Integr Comp Physiol 279: R2042–R2047, 2000. doi: 10.1152/ajpregu.2000.279.6.R2042. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Endothelin-A-mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci 70: 554–565, 2015. doi: 10.1093/gerona/glu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett-O’Keefe Z, Kaplon RE, Halliwill JR. Sustained postexercise vasodilatation and histamine receptor activation following small muscle-mass exercise in humans. Exp Physiol 98: 268–277, 2013. doi: 10.1113/expphysiol.2012.066605. [DOI] [PubMed] [Google Scholar]
- 8.Beer DJ, Matloff SM, Rocklin RE. The influence of histamine on immune and inflammatory responses. Adv Immunol 35: 209–268, 1984. doi: 10.1016/S0065-2776(08)60577-5. [DOI] [PubMed] [Google Scholar]
- 9.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2: 92–98, 1970. [PubMed] [Google Scholar]
- 10.Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543: 691–698, 2002. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Brito LC, Rezende RA, da Silva Junior ND, Tinucci T, Casarini DE, Cipolla-Neto J, Forjaz CLM. Post-exercise hypotension and its mechanisms differ after morning and evening exercise: a randomized crossover study. PLoS One 10: e0132458, 2015. doi: 10.1371/journal.pone.0132458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyle EF, Hagberg JM, Hurley BF, Martin WH, Ehsani AA, Holloszy JO. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J Appl Physiol 55: 230–235, 1983. doi: 10.1152/jappl.1983.55.1.230. [DOI] [PubMed] [Google Scholar]
- 13.Daniel A, Honig CR. Does histamine influence vasodilation caused by prolonged arterial occlusion or heavy exercise? J Pharmacol Exp Ther 215: 533–538, 1980. [PubMed] [Google Scholar]
- 14.DeLorey DS, Shaw CN, Shoemaker JK, Kowalchuk JM, Paterson DH. The effect of hypoxia on pulmonary O2 uptake, leg blood flow and muscle deoxygenation during single-leg knee-extension exercise. Exp Physiol 89: 293–302, 2004. doi: 10.1113/expphysiol.2003.026864. [DOI] [PubMed] [Google Scholar]
- 15.Duff F, Patterson GC, Whelan RF. The effect of intra-arterial antihistamines on the hyperaemia following temporary arrest of the circulation in the human forearm. Clin Sci 14: 267–273, 1955. [PubMed] [Google Scholar]
- 16.Ely MR, Romero SA, Sieck DC, Mangum JE, Luttrell MJ, Halliwill JR. A single dose of histamine-receptor antagonists before downhill running alters markers of muscle damage and delayed-onset muscle soreness. J Appl Physiol (1985) 122: 631–641, 2017. doi: 10.1152/japplphysiol.00518.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ely MR, Sieck DC, Mangum JE, Larson EA, Brito LC, Minson CT, Halliwill JR. Histamine-receptor antagonists slow 10-km cycling performance in competitive cyclists. Med Sci Sports Exerc 51: 1487–1497, 2019. doi: 10.1249/MSS.0000000000001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emhoff C-AW, Barrett-O’Keefe Z, Padgett RC, Hawn JA, Halliwill JR. Histamine-receptor blockade reduces blood flow but not muscle glucose uptake during postexercise recovery in humans. Exp Physiol 96: 664–673, 2011. doi: 10.1113/expphysiol.2010.056150. [DOI] [PubMed] [Google Scholar]
- 19.Emmelin K, Emmelin N. Histamine and reactive hyperaemia. Acta Physiol Scand 14: 16–18, 1947. doi: 10.1111/j.1748-1716.1947.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 20.Endo Y, Tabata T, Kuroda H, Tadano T, Matsushima K, Watanabe M. Induction of histidine decarboxylase in skeletal muscle in mice by electrical stimulation, prolonged walking and interleukin-1. J Physiol 509: 587–598, 1998. doi: 10.1111/j.1469-7793.1998.587bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuba Y, Ohe Y, Miura A, Kitano A, Endo M, Sato H, Miyachi M, Koga S, Fukuda O. Dissociation between the time courses of femoral artery blood flow and pulmonary V̇o2 during repeated bouts of heavy knee extension exercise in humans Exp Physiol 89: 243–253, 2004. doi: 10.1113/expphysiol.2003.026609. [DOI] [PubMed] [Google Scholar]
- 22.Garg DC, Eshelman FN, Weidler DJ. Pharmacokinetics of ranitidine following oral administration with ascending doses and with multiple-fixed doses. J Clin Pharmacol 25: 437–443, 1985. doi: 10.1002/j.1552-4604.1985.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 24.González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. J Physiol 572: 295–305, 2002. [Erratum in Circ Res 92: 61e, 2003.] doi: 10.1161/01.RES.0000044939.73286.E2. [DOI] [PubMed] [Google Scholar]
- 25.Hughson RL, Schijvens H, Burrows S, Devitt D, Betik AC, Hopman MTE. Blood flow and metabolic control at the onset of heavy exercise. Int J Sport Health Sci 1: 9–18, 2003. doi: 10.5432/ijshs.1.9. [DOI] [Google Scholar]
- 26.Imholz BPM, van Montfrans GA, Settels JJ, van der Hoeven GM, Karemaker JM, Wieling W. Continuous non-invasive blood pressure monitoring: reliability of Finapres device during the Valsalva manoeuvre. Cardiovasc Res 22: 390–397, 1988. doi: 10.1093/cvr/22.6.390. [DOI] [PubMed] [Google Scholar]
- 27.Jeong HJ, Moon PD, Kim SJ, Seo JU, Kang TH, Kim JJ, Kang IC, Um JY, Kim HM, Hong SH. Activation of hypoxia-inducible factor-1 regulates human histidine decarboxylase expression. Cell Mol Life Sci 66: 1309–1319, 2009. doi: 10.1007/s00018-009-9001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol 583: 855–860, 2007. doi: 10.1113/jphysiol.2007.135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majno G, Palade GE, Schoefl GI. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol 11: 607–626, 1961. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majno G, Shea SM, Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol 42: 647–672, 1969. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol (1985) 77: 2740–2747, 1994. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 32.McCord JL, Beasley JM, Halliwill JR. H2-receptor-mediated vasodilation contributes to postexercise hypotension. J Appl Physiol (1985) 100: 67–75, 2006. doi: 10.1152/japplphysiol.00959.2005. [DOI] [PubMed] [Google Scholar]
- 33.McCord JL, Halliwill JR. H1 and H2 receptors mediate postexercise hyperemia in sedentary and endurance exercise-trained men and women. J Appl Physiol (1985) 101: 1693–1701, 2006. doi: 10.1152/japplphysiol.00441.2006. [DOI] [PubMed] [Google Scholar]
- 34.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 77: 1033–1079, 1997. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 35.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. doi: 10.1161/01.CIR.101.8.862. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery LC, Deuster PA. Acute antihistamine ingestion does not affect muscle strength and endurance. Med Sci Sports Exerc 23: 1016–1019, 1991. doi: 10.1249/00005768-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery LC, Deuster PA. Ingestion of an antihistamine does not affect exercise performance. Med Sci Sports Exerc 24: 383–388, 1992. doi: 10.1249/00005768-199203000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Mortensen SP, González-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niijima-Yaoita F, Tsuchiya M, Ohtsu H, Yanai K, Sugawara S, Endo Y, Tadano T. Roles of histamine in exercise-induced fatigue: favouring endurance and protecting against exhaustion. Biol Pharm Bull 35: 91–97, 2012. doi: 10.1248/bpb.35.91. [DOI] [PubMed] [Google Scholar]
- 40.Panula P, Lintunen M, Karlstedt K. Histamine in brain development and tumors. Semin Cancer Biol 10: 11–14, 2000. doi: 10.1006/scbi.2000.0302. [DOI] [PubMed] [Google Scholar]
- 41.Paterson ND, Kowalchuk JM, Paterson DH. Effects of prior heavy-intensity exercise during single-leg knee extension on V̇o2 kinetics and limb blood flow. J Appl Physiol (1985) 99: 1462–1470, 2005. doi: 10.1152/japplphysiol.00173.2005. [DOI] [PubMed] [Google Scholar]
- 42.Rådegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol (1985) 83: 1383–1388, 1997. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- 43.Rådegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- 44.Romero SA, Hocker AD, Mangum JE, Luttrell MJ, Turnbull DW, Struck AJ, Ely MR, Sieck DC, Dreyer HC, Halliwill JR. Evidence of a broad histamine footprint on the human exercise transcriptome. J Physiol 594: 5009–5023, 2016. doi: 10.1113/JP272177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero SA, McCord JL, Ely MR, Sieck DC, Buck TM, Luttrell MJ, MacLean DA, Halliwill JR. Mast cell degranulation and de novo histamine formation contribute to sustained postexercise vasodilation in humans. J Appl Physiol (1985) 122: 603–610, 2017. doi: 10.1152/japplphysiol.00633.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell T, Stoltz M, Weir S. Pharmacokinetics, pharmacodynamics, and tolerance of single- and multiple-dose fexofenadine hydrochloride in healthy male volunteers. Clin Pharmacol Ther 64: 612–621, 1998. doi: 10.1016/S0009-9236(98)90052-2. [DOI] [PubMed] [Google Scholar]
- 48.Sandilands EA, Crowe J, Cuthbert H, Jenkins PJ, Johnston NR, Eddleston M, Bateman DN, Webb DJ. Histamine-induced vasodilatation in the human forearm vasculature. Br J Clin Pharmacol 76: 699–707, 2013. doi: 10.1111/bcp.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savany A, Cronenberger L. Properties of histidine decarboxylase from rat gastric mucosa. Eur J Biochem 123: 593–599, 1982. doi: 10.1111/j.1432-1033.1982.tb06574.x. [DOI] [PubMed] [Google Scholar]
- 50.Schayer RW. Evidence that induced histamine is an intrinsic regulator of the microcirculatory system. Am J Physiol 202: 66–72, 1962. doi: 10.1152/ajplegacy.1962.202.1.66. [DOI] [PubMed] [Google Scholar]
- 51.Schayer RW. Histamine and hyperæmia of muscular exercise. Nature 201: 195, 1964. doi: 10.1038/201195a0. [DOI] [PubMed] [Google Scholar]
- 52.Schayer RW. Histamine and microcirculation. Life Sci 15: 391–401, 1974. doi: 10.1016/0024-3205(74)90338-5. [DOI] [PubMed] [Google Scholar]
- 53.Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, Curry TB, Joyner MJ. Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol (1985) 109: 768–777, 2010. doi: 10.1152/japplphysiol.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shepherd JT. Circulation to skeletal muscle (Compr Physiol 2011, Suppl. 8). Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow Bethesda, MD: Am. Physiol. Soc., p. 319–370 (First published in print 1983). doi: 10.1002/cphy.cp020311. [DOI] [Google Scholar]
- 55.Soller BR, Yang Y, Lee SMC, Wilson C, Hagan RD. Noninvasive determination of exercise-induced hydrogen ion threshold through direct optical measurement. J Appl Physiol (1985) 104: 837–844, 2008. doi: 10.1152/japplphysiol.00849.2007. [DOI] [PubMed] [Google Scholar]
- 56.Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179: 361–366, 2003. doi: 10.1046/j.0001-6772.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 57.Weigert G, Zawinka C, Resch H, Schmetterer L, Garhöfer G. Intravenous administration of diphenhydramine reduces histamine-induced vasodilator effects in the retina and choroid. Invest Ophthalmol Vis Sci 47: 1096–1100, 2006. doi: 10.1167/iovs.05-1174. [DOI] [PubMed] [Google Scholar]
- 58.Wilson JR, Kapoor SC. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am J Physiol Heart Circ Physiol 265: H171–H175, 1993. doi: 10.1152/ajpheart.1993.265.1.H171. [DOI] [PubMed] [Google Scholar]
- 59.Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoneda H, Niijima-Yaoita F, Tsuchiya M, Kumamoto H, Watanbe M, Ohtsu H, Yanai K, Tadano T, Sasaki K, Sugawara S, Endo Y. Roles played by histamine in strenuous or prolonged masseter muscle activity in mice. Clin Exp Pharmacol Physiol 40: 848–855, 2013. doi: 10.1111/1440-1681.12167. [DOI] [PubMed] [Google Scholar]