Abstract
Synapse-associated protein 97 (SAP97) is a scaffolding protein crucial for the functional expression of several cardiac ion channels and therefore proper cardiac excitability. Alterations in the functional expression of SAP97 can modify the ionic currents underlying the cardiac action potential and consequently confer susceptibility for arrhythmogenesis. In this study, we generated a murine model for inducible, cardiac-targeted Sap97 ablation to investigate arrhythmia susceptibility and the underlying molecular mechanisms. Furthermore, we sought to identify human SAP97 (DLG1) variants that were associated with inherited arrhythmogenic disease. The murine model of cardiac-specific Sap97 ablation demonstrated several ECG abnormalities, pronounced action potential prolongation subject to high incidence of arrhythmogenic afterdepolarizations and notable alterations in the activity of the main cardiac ion channels. However, no DLG1 mutations were found in 40 unrelated cases of genetically elusive long QT syndrome (LQTS). Instead, we provide the first evidence implicating a gain of function in human DLG1 mutation resulting in an increase in Kv4.3 current (Ito) as a novel, potentially pathogenic substrate for Brugada syndrome (BrS). In conclusion, DLG1 joins a growing list of genes encoding ion channel interacting proteins (ChIPs) identified as potential channelopathy-susceptibility genes because of their ability to regulate the trafficking, targeting, and modulation of ion channels that are critical for the generation and propagation of the cardiac electrical impulse. Dysfunction in these critical components of cardiac excitability can potentially result in fatal cardiac disease.
NEW & NOTEWORTHY The gene encoding SAP97 (DLG1) joins a growing list of genes encoding ion channel-interacting proteins (ChIPs) identified as potential channelopathy-susceptibility genes because of their ability to regulate the trafficking, targeting, and modulation of ion channels that are critical for the generation and propagation of the cardiac electrical impulse. In this study we provide the first data supporting DLG1-encoded SAP97’s candidacy as a minor Brugada syndrome susceptibility gene.
Keywords: arrhythmia, ion channels, Sap97
INTRODUCTION
Heart disease resulting in lethal ventricular arrhythmias and sudden cardiac death (SCD) is the leading cause of mortality in developed countries (18). The majority of these cases are caused by coronary artery disease and heart failure, yet a significant proportion of cases occurs in the absence of any discernible structural disease, and may stem from inherited channelopathies. In several instances, the arrhythmias result from abnormalities in the genes that encode the primary cardiac channel protein (6). There is increasing evidence that a growing proportion of disease-causing mutations occur in non-ion channel proteins that serve as regulatory partners (channel-interacting proteins, ChIPs) to the channel proteins (7, 17, 25, 37). Dysfunction in either pore-forming α-subunits or the ChIPs can disrupt the delicate balance of ionic currents that underlie the cardiac action potential (AP), potentially resulting in life-threatening arrhythmias. In the past two decades, alterations in genes encoding or regulating ion channel function underlie several forms of arrhythmogenic disease of which long QT syndrome (LQTS), catecholaminergic polymorphic ventricular tachycardia (CPVT), Brugada syndrome (BrS), and short QT syndrome (SQTS) constitute the majority (6). However, a significant percentage of clinically suspected channelopathies remain genetically elusive. While 17 LQTS-susceptibility genes have been identified that account for 75–80% of LQTS, in BrS, 20 susceptibility genes have been identified that encode primary and/or auxiliary subunits of several cardiac ion channels. However, these genes account for only 25–30% of all BrS cases, with only SCN5A playing a major role (9). There is increasing evidence that some of the susceptibility genes are elusive cases because the mutations reside in genes encoding ChIPs, such as proteins for structure or scaffolding/subcellular targeting (14, 32).
Membrane-associated guanylate kinases (MAGUKs) contain several conserved sites of protein-protein interaction domains. Most notably, MAGUKs contain varying numbers of postsynaptic density protein-95, discs-large, and zona occludens-1 (PDZ)-binding domains that interact with sequences commonly found at the COOH-terminus of many cardiac ion channels (33). The canonical MAGUK is synapse-associated protein 97 (SAP97), which is encoded by disks large homolog-1 gene (DLG1). SAP97 and its family members are expressed in a wide variety of tissues and are involved in protein trafficking, β-adrenergic receptor recycling, and in the functional regulation of ion channels (12–14, 23, 24, 28, 36). Nevertheless, SAP97 is the only member of the SAP family with ubiquitous expression in the myocardium (21, 22). Previous in vitro studies demonstrated that SAP97 interacts with and regulates cardiac ion channels in heterologous, as well as in, native cell systems (12, 14, 24, 33, 36). In this study, a multidisciplinary approach of in vivo, in vitro, and in silico experiments have been conducted to understand the role of SAP97 in the myocardium. Specifically, we used a murine model of inducible, cardiac-specific ablation of Sap97, a BrS-associated SAP97 human mutation expressed in human-induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs), as well as computer simulations to examine the role of this archetypical MAGUK in arrhythmogenesis.
METHODS
Generation of inducible, cardiac-specific Sap97-knockout mice.
Two mouse strains were obtained from the Jackson Laboratory (Bar Harbor, ME) and crossbred in our animal housing facilities for the proposed study. The first strain (Tg(Myh6-cre/Esr1*)1Jmk) has the α-MHC-MerCreMer transgene, in which the mouse Myh6 promoter directs the expression of tamoxifen-inducible Cre recombinase. The α-MHC-MerCreMer transgene was designed with the mouse Myh6 promoter (myosin, heavy polypeptide 6, cardiac muscle, α; α-MHC) upstream of the MerCreMer protein. The MerCreMer double-fusion protein has a Cre recombinase cDNA sequence flanked on each end with a mutated murine estrogen receptor (Mer) ligand binding domain (amino acids 281–599, G525R), thus rendering Cre expression tamoxifen inducible, yet estrogen insensitive. The α-MHC-MerCreMer mice from line 1 were backcrossed to mice with a (B6 × 129/Sv)F1 genetic background for 15 generations before arrival at The Jackson Laboratory repository (Stock No. 005650). Upon arrival, some mice were backcrossed to C57BL/6J inbred mice (Stock No. 000664) for several generations to generate this congenic strain (Stock No. 005657). As of July 2011, the colony has been backcrossed onto C57BL/6J for at least nine generations. The second mouse strain (B6;129-Dlg1tm1Rlh/J) has locus of X-over P1 (LoxP) sites on either side of an exon encoding portions of both the PDZ1 and PDZ2 domains of Sap97. Briefly, an FRT-flanked neomycin resistance cassette and a loxP site were introduced to the intron following the second exon of the PDZ2 domain. A second LoxP site was placed in an upstream intron, between two exons encoding the PDZ1 domain. The targeting construct was electroporated into (129X1/SvJ × 129S1/Sv) F1-Kitl+-derived R1 embryonic stem (ES) cells. The neomycin resistance cassette was excised through a cross with a β-actin (ACTB) promoter FLP strain on a C57BL/6 background, leaving a single floxed exon encoding portions of both the PDZ1 and PDZ2 domains. This strain was backcrossed once to C57BL/6 by the donating laboratory. Mice were genotyped (at approximately ages 2–4 wk) and were studied between ages 2–8 mo. Control and test mice were injected with tamoxifen (20 mg/kg ip), dissolved in peanut oil (vehicle) at 4–8 wk of age over a 5-day period.
Electrocardiography.
Surface ECGs were carried out in anesthetized mice (2.0% isoflurane) placed on a heated downdraft table. Data acquisition began once the animal’s body temperature stabilized at 37°C. Data were acquired using PONEMAH software (version 5) and a DSI 7700 digital signal conditioner (Data Sciences International). Standard electrophysiological characterization was performed.
Isolation of adult murine cardiac myocytes.
Murine cardiac myocytes were isolated using the standard Langendorff technique. Briefly, mice (2–4 mo old) were injected with 1 mL heparin (100 IU/mL ip) 20 min before heart excision. Animals were anesthetized with a mixture of ketamine (116 mg/kg ip) and acepromazine (11 mg/kg ip). The heart was removed quickly from the chest and retrogradely perfused through the aorta at a constant flow (4 mL/min) at 37°C for 4 min with a Ca2+-free buffer containing (in mmol/L) 113 NaCl, 4.7 KCl, 1.2 MgSO4, 0.6 Na2HPO4, 0.6 KH2PO4, 10 KHCO3, 12 NaHCO3, 10 HEPES, 10 2.3-butanedione monoxime (BDM, Sigma), 30 taurine, and 5.5 glucose. All solutions were filtered (0.2-µm filter) and equilibrated with 100% O2 for at least 20 min before use. Enzymatic digestion was initiated by adding collagenase type II (773.4 U/mL; Worthington), trypsin (0.14 mg/mL), and CaCl2 (12.5 µmol/L) to the perfusion solution. After 6–8 min of digestion, the ventricles were removed and single cells isolated by physical separation. Cells were used for electrophysiological recording within 8 h after isolation.
Immunohisto-(cyto-)chemistry.
Isolated cells were fixed and permeabilized in 100% ethanol at −20°C. Cells were blocked in 3% bovine serum albumin and 1% fetal goat serum in PBS. Cells were stained with primary antibody overnight in blocking solution. Cells were stained with secondary antibodies in blocking solution for >1 h at room temperature. Secondary antibodies included Alexa-conjugated donkey anti-mouse 488 and 568 and donkey anti-rabbit 488 and 568. Cells were imaged on an LSM 780 confocal microscope (Carl Zeiss). Myocytes were imaged using identical confocal settings between genotypes. At least 20 myocytes were examined for each staining protocol.
Lateral membrane vs. intercalated disk analysis of NaV1.5 density in wild-type and Sap97 knockout mice.
Image analysis was performed with FIJI-ImageJ (https://imagej.net/Citing). Images of immunofluorescence against NaV1.5 and N-cadherin were transformed into eight-bit images and, after threshold adjustment, were subsequently used for quantification of cardiomyocytes area and integrated channel density (immunostaining against NaV1.5). Additionally, images were submitted to analysis with the plot profile tool to determine the integrated density of NaV1.5 on the lateral membrane and intercalated disk (identified with the staining against N-cadherin). Data were presented as a ratio of NaV1.5 expression on intercalated disk versus the lateral membrane. Comparisons between groups were performed with Student’s t-test (GraphPad Prism).
Western blot analysis.
Proteins were separated on 4–12% Novex Tris-glycine polyacrylamide gels (Invitrogen) and electrophoretically transferred to nitrocellulose membrane for 1 h at 400 mA. Membranes were blocked in 10% nonfat dry milk-PBS for 4 h at room temperature and then incubated overnight at 4°C with the primary antibody. After being washed three times with 0.05% Tween 20-PBS, the membranes were incubated with horseradish peroxidase-labeled secondary antibody for 4 h at room temperature, washed, and immunoreactive bands visualized using enhanced chemiluminescence (Pierce). The intensity of each immunoreactive band was quantified using densitometry (Bio-Rad FluorS imager and the Quantity One software package), and the values were normalized to actin levels. The normalized values for each protein species were compared between groups.
Electrophysiology in adult murine ventricular myocytes.
Whole cell voltage- and current-clamp recordings were carried out in isolated adult mouse cardiac myocytes and hiPSC-CMs, using standard patch-clamp techniques. Recordings were done using the Axopatch 200B or Axoclamp 700B amplifiers (Molecular Devices, Sunnyvale, CA). Sodium currents (INa) were recorded at room temperature (20–22°C) with pipette resistances of <2.8 MΩ when filled with pipette filling solution containing (in mM) 5 NaCl, 135 CsF (135), 10 EGTA, 5 MgATP, and 5 HEPES at pH 7.2. The extracellular bathing solution contained (in mM) 5 NaCl, 1 MgCl2, 1.8 CaCl2, and 0.1 CdCl2. To assess the INa density, cells were held at −160 mV and stepped to various test potentials from −100 to 30 mV in 5-mV increments, with 200-ms duration pulses and 2,800-ms interpulse intervals. Peak INa was assessed by an algorithm that calculates peak inward signal. As a result, any outward INa returns a zero value. Voltage-dependent activation of INa was assessed by generating conductance-voltage relationships (m-infinity curves) and fitting the data with a standard Boltzmann function (Origin 8.1, Northampton, MA). Voltage-dependence of inactivation was assessed by holding the cells from −140 to −40 mV for 300 ms, followed by a 30-ms test pulse to −40 mV; interpulse interval was 2,700 ms. Recovery from inactivation was studied by holding cells at −160 mV and applying two 20-ms test pulses (S1, S2) to −45 mV, separated by increasing increments of 1 ms to a maximum S1-S2 interval of 50 ms. The S1-S1 interval was kept constant at 2,000 ms.
The transient outward K+ current (Ito) recordings were conducted at room temperature. The bath solution contained (in mM) 136 NaCl, 4 KCl, 1.8 CaCl2, 2 MgCl2, 10 HEPES, 0.03 tetrodotoxin, and 0.005 nifedipine, adjusted to pH 7.4 with NaOH. Recording pipettes contained (in mM) 135 KCl, 1 MgCl2, 10 EGTA, 10 HEPES, and 5 glucose, adjusted to pH 7.2 with KOH. Ito was recorded using a step protocol with a holding potential of −70 mV and stepping from −40 to +60 mV in 10-mV increments of 5 s at each potential, every 20 s. Ito was measured as the difference between the peak current and the current at the end of the 5-s pulse.
Action potentials were elicited using square wave pulses (30–50 pA amplitude, 10–30-ms duration), generated by a DS8000 digital stimulator (World Precision Instruments, Sarasota, FL), and recorded at 37°C with pipette solution containing (in mM) 1 MgCl2, 1 EGTA, 150 KCl, 5 HEPES, 5 phosphocreatine, 4.46 K2ATP, 2 b-hydroxybutyric acid, adjusted to pH 7.2 with KOH, and extracellular solution containing (in mM) 148 NaCl, 0.4 NaH2PO4, 1 MgCl2, 5.5 glucose, 5.4 KCl, 1 CaCl2, 15 HEPES, and 1 EGTA, adjusted to pH 7.2 with NaOH. Appropriate corrections for access resistance compensation, as well as for leak and capacitive current subtractions, were made.
Electrophysiology in HEK293 cells and iPSC-CMs.
Experiments in HEK293 and iPSC-CMs were carried out using standard cell culture and electrophysiological techniques as previously published from the laboratory. Briefly, HEK293 cells stably expressing inwardly rectifying (Kir2.1) channels (26), or NaV1.5/β1b (a generous gift from L. Isom laboratory, University of Michigan) were cultured for cotransfection with SAP97 wild-type (WT) or and SAP97-M827T plasmids. Transfection procedures were as previously published (38). Voltage-clamp protocols for Kir2.1 and NaV1.5/β1b were as previously described (3, 24, 26, 36). For sodium current recordings, external [Na+] was reduced to 50 mM. All voltage-clamp recordings were conducted at room temperature. Vials of iCell human cardiac myocytes were obtained from Cellular Dynamics International (Madison, WI). Adenoviral-mediated infection was used for WT and mutant Sap97 under cell culture conditions. Solutions and electrophysiological recording protocols were as previously described (16, 35). Inward rectifier current (IK1) was recorded with pipette solution containing (in mM) 148 KCl (148), 1 MgCl2, 5 EGTA, 5 HEPES, 2 creatine, 5 ATP, and 5 phosphocreatine, adjusted to pH 7.2 with KOH, and extracellular solution containing (in mM) 148 NaCl, 0.04 NaH2PO4, 1 MgCl2, 5.5 glucose, 5.4 KCl, 1 CaCl2, and 15 HEPES, adjusted to pH 7.4 with NaOH. Activation of IK1 was elicited by a step protocol using 400-ms steps ranging from −120 to +20 mV in 10-mV increments with a holding potential of −50 mV and with 2 s between successive steps. Nifedipine (5 μM) was added to block ICa,L channels and the Ca2+-sensitive ICl. BaCl2 (1 mM) was used to isolate IK1 from other background currents.
Human study sample of genetically elusive LQTS and BrS.
The study population consisted of 40 unrelated patients with clinically robust but genetically elusive LQTS who were referred to the Windland Smith Rice Sudden Death Genomics Laboratory at Mayo Clinic (Rochester, MN) and, separately, 38 unrelated patients with clinically diagnosed BrS who were referred for research-based genetic testing to either the Molecular Cardiology Laboratory of the University of Pavia, Italy (n = 29), or to Mayo Clinic (n = 9). The diagnosis of BrS was made using strict guidelines according to the criteria of the Consensus Conference Document (2). All 38 patients with BrS included in this study remained genotype negative after comprehensive genetic testing for mutations involving 12 known BrS-susceptibility genes [SCN5A, GPD1L, CACNA1C, CACNB2, SCN1B (including the alternatively spliced exon 3A; SCN1Bb), SCN3B, KCNE3, KCNJ8, KCND3, CACNA2D1, MOG1 and HCN4] (9). This study was approved by both the Mayo Foundation Institutional Review Board and the Medical Ethical Committee of Fondazione IRCCS Policlinico San Matteo (Pavia, Italy). Informed consent was obtained for all patients.
DLG1 mutational analysis.
Comprehensive open reading frame/splice site mutational analysis of all amino acid coding exons and intron borders of DLG1 was performed using polymerase chain reaction (PCR), denaturing high-performance liquid chromatography (DHPLC), and DNA sequencing as previously described (1). PCR primer sequences and PCR/DHPLC conditions are available upon request.
To be considered as a putative pathogenic mutation, any DLG1 variant had to be 1) nonsynonymous and 2) absent among at least 900 ethnically matched controls obtained from the European Collection of Cell Cultures (HPA Culture Collections, UK); the Human Genetic Cell Repository, sponsored by the National Institute of General Medical Sciences; the Coriell Institute for Medical Research (Camden, New Jersey); and from the Blood Transfusional Centre in IRCCS Policlinico San Matteo of Pavia (Italy) and with a minor allele frequency (MAF) < 0.00005 (1/20,000 alleles) in the Exome Aggregation Consortium (ExAC, n = 60,706 unrelated individuals, www.exac.broadinstitute.org). The NCBI GenBank accession number NM _001204386.1 was used as the reference sequence, and mutations were annotated using the single-letter nomenclature whereby M827T, for example, denotes a nonsynonymous variant producing a missense amino acid change involving the substitution of methionine (M) by a threonine (T) at position 827 of the protein sequence.
Construction of expression vectors and mutagenesis.
The DLG1 human cDNA clone in pCMV6-XL4 was obtained from Origene Technologies (SC125478; accession number: NM_004087.1). DNA sequence confirmation revealed that this cDNA actually matched the accession number: NM_001204386.1 (SAP97 isoform containing the I3 domain but not the I1A domain (15) and harbored a deletion of the amino acid glutamine (Q) at the start of the I3 domain. PCR-based site directed mutagenesis (Stratagene Quickchange XL Site-Directed Mutagenesis Kit) using the forward primer, 5′-ATTCTAAAACGAGAGATAAAGGGCAGTCATTCAATGACAAGCGTAAAAA-3′, and reverse primer, 5′-TTTTTACGCTTGTCATTGAATGACTGCCCTTTATCTCTCGTTTTAGAAT-3′, which were used to insert the deleted glutamine at the beginning of the I3 domain. This corrected DLG1 cDNA was subcloned into pIRES2-DsRed2 (Clontech, Mountain View, CA) to produce pIRES2-DLG1WT-DsRed2. PCR-based, site-directed mutagenesis was used to engineer the M827T mutation into pIRES2-DLG1WT-DsRed2 producing pIRES2-DLG1M827T-DsRed2. These plasmids express either WT or mutant SAP97 and DsRed2 as a bicistronic mRNA, allowing SAP97-expressing cells to be selected for electrophysiological studies. A plasmid encoding wild-type human KCND3 (Kv4.3) and GFP on a bicistronic mRNA, pIreGFP-KCND3WT, was used for electrophysiological studies. All vector sequences were confirmed by direct DNA sequencing.
Cell culture, transfection procedures and electrophysiology in CHO cells.
Chinese hamster ovary (CHO) cells were cultured in F-12 medium (Gibco, Invitrogen, Carlsbad, CA) and supplemented with 10% fetal bovine serum (FBS). All cells were plated in T25 flasks and stored in a 5% CO2 incubator at 37°C for 24 h. Heterologous expression of Kv4.3 and SAP97 was accomplished by cotransfecting 0.5 μg of pIreGFP-KCND3WT with 2.0 μg pIRES2-DsRed2 empty vector or pIRES2-DLG1WT-DsRed2 (SAP97-WT) or pIRES2-DLG1M827T-DsRed2 (SAP97-M827T) using 7 μl of Lipofectamine transfection reagent (Invitrogen, Carlsbad, CA) in Gibco OPTI-MEM media (Invitrogen, Carlsbad, CA). Cells exhibiting both green fluorescence and red fluorescence after 48 h, posttransfection, were selected for electrophysiological experiments. Generation of constructs for transfection and adenoviruses constructs were created as previously described (7).
Standard whole cell patch-clamp technique was used to measure Kv4.3 current coexpressed with empty vector or SAP97-WT or SAP97-M827T at room temperature (22–24°C) with the use of an Axopatch 200B amplifier, Digidata 1440A and pClamp version 10.2 software (Axon Instruments, Foster City, CA). The extracellular (bath) solution contained (in mM) 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, and 10 HEPES, adjusted to pH 7.4 with NaOH. The pipette solution contained (in mM) 110 KCl, 31 KOH, 10 EDTA, 5.17 CaCl2, 1.42 MgCl2, 4 MgATP, and 10 HEPES, adjusted to pH 7.2 with KOH, following established protocols (10). Microelectrodes were pulled on a P-97 puller (Sutter Instruments, Novato, CA) and fire polished to a final resistance of 2–3 MΩ. Series resistance was compensated by 80–85%. Currents were filtered at 5 kHz and digitized at 10 kHz. The voltage dependence of activation and steady-state inactivation were determined using voltage-clamp protocols described in the figure legends and insets. Data were analyzed using Clampfit (Axon Instruments), Excel (Microsoft, Redmond, WA), and fitted with Origin 8 (OriginLab Corporation, Northampton, MA).
The voltage-dependent inactivation curve was fitted with a Boltzmann function: I/Imax = f−1, where V1/2 and k are the half-maximal voltage of inactivation and the slope factor respectively. Inactivation time constants for each voltage step were determined by fitting a monoexponential function to current decay. Total charge as a function of voltage was obtained by measuring the area under the curve of each voltage step.
Optical mapping in human iPSC-CM monolayers.
iCell cardiomyocytes were obtained from Cellular Dynamics International (CDI, Madison, WI). Cryopreserved iCell cardiomyocytes were thawed, plated at a density of 50,000 cells per well in 96 well plates, and cultured in two-dimensional monolayer format as described previously (19). Adenoviral gene transfer was monitored by continuous time-lapse imaging to obtain GFP expression data (IncuCyte Zoom, Essen Bioscience, Ann Arbor, MI). Monolayers were given 1 wk to form following the thaw and then treated with adenovirus for gene transfer. Optical mapping was done 1 wk after adenoviral gene transfer. Thus, at the time of electrophysiological recording, the iCell cardiomyocytes were cultured for a period of 2 wk. Calcium impulse propagation or calcium transients were measured in rhod2-loaded monolayers, imaging of calcium impulse was done using a high-speed CCD camera (Little Joe, 200 fps) and LED based illumination essentially as before (4, 19). In a subset of monolayers, the action potential propagation was also measured using di-4-ANBDQPQ with simultaneous monitoring of calcium flux using rhod2 with rapid excitation switching as described before (20). Optical mapping data were analyzed using custom software (Scroll) as previously described (5)
Optical mapping in Sap97 WT and knockout mice.
Epicardial optical mapping experiments were conducted in isolated, Langendorff-perfused hearts WT and Sap97-knockout (KO) mice. Optical-mapping experiments were conducted as previously described in our laboratories (3). Volume-conducted ECG was recorded, and activation and phase maps were then generated.
Simulated ventricular epicardial action potential.
Both right ventricular (RV) and left ventricular (LV) epicardial action potentials (APs) were simulated using a Luo-Rudy II (LRII) AP model, modified to include the Ito as previously described (7, 12). The maximal conductance of the calcium current (ICa,L) was decreased by 30% to compensate for the larger driving force during the AP notch. The maximal conductance of the Ito expressed in the presence of the SAP97-WT was set to 1.3 mS/μF in the RV epicardium and to 0.5 mS/μF in the LV epicardium. In the presence of the SAP97-M827T, the maximal Ito conductance was increased by 70% to 2.2 mS/μF in RV and to 0.85 mS/μF in LV. An inactivation time constant of 10.1 ms was used for WT (11), and 11.0 ms for Ito expressed with SAP97-M827T, reflecting a 10% increase. With these values for the maximal conductance and the inactivation time constants, the SAP97-M827T mutation resulted in the increase of the total charge carried by Ito by 90% and the shift of the steady-state inactivation of Ito by 0.5 mV toward more positive potentials. All modeled parameters agreed with experimentally derived values.
Statistical analyses.
Data are presented as means ± SE. Differences between groups of experiments were determined using appropriate statistical tests including unpaired Student’s t-test, nonparametric test, or ANOVA as appropriate for each set of data. Shapiro-Wilk test was used to determine normality of data distribution. Unpaired t-tests were used where indicated for the whole cell recordings. Western Blot data were analyzed by using two-way analysis of variance. One-way ANOVA was used to determine significant differences between three or more different groups. If significance was indicated, the Bonferroni post hoc test was used to detect the level of the significant differences. The level of statistical significance was set to P < 0.05.
RESULTS
Cardiac-specific Dlg1 (Sap97) knockout murine model.
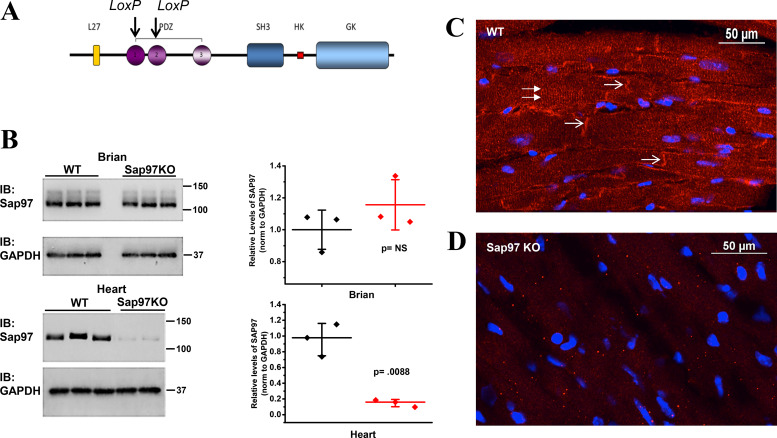
To examine the in vivo role of Sap97 expression, we generated an inducible cardiac-specific murine model for targeted Dlg1 gene ablation. Two mouse strains were obtained from the Jackson Laboratory and crossbred. The first carried the α-MHC-MerCreMer transgene, in which the mouse Myh6 promoter directs the expression of tamoxifen-inducible Cre recombinase and the second has loxP sites flanking exons encoding portions of both the PDZ1 and PDZ2 domains of Sap97 (Fig. 1A). Sap97 control (WT) (fl+/+:cre−/−) and Sap97-KO (fl+/+:cre+/−) animals were injected with tamoxifen (20 mg/kg ip) at 8 wk of age over a 5-day period. Two weeks after the final tamoxifen injection, brain and heart tissue lysates were collected from both control and KO animals and probed for Sap97 protein. While expression was unaltered in the brain, Sap97 levels in the heart were decreased significantly (83%; P = 0.0088), confirming cardiac specificity of the knockdown (Fig. 1B). Immunofluorescent (IF) microscopy confirmed the lower levels of expression of Sap97 illustrated by a loss of immunoreactive signal throughout the myocardium and most importantly a distinct lack of expression at the intercalated disk (Fig. 1, C and D; white arrows).
Fig. 1.
Characterization of synapse-associated protein 97-knockout (Sap97-KO) murine model. A: schematic representation of the archetypical Sap97. Locus of X-over P1 (LoxP) sites are indicated by black arrows. B: Western blot analysis of brain (top) and heart (bottom) lysates from control and Sap97-KO mice. Quantification of the Western blots shows significant protein knockdown in the hearts of Sap97-KO animals (N = 3,3 and P = 0.0088; t-test); horizontal bars represent means; vertical bars, SD. C and D: immunolocalization of Sap97 in the ventricle of wild-type (WT) and Sap97-KO mice (in red). KO animals show a dramatic loss of immunoreactive signal both within the intracellular space (double arrows) and at the intercalated disk (thick arrows). PDZ, postsynaptic density protein-95, discs-large, and zona occludens-1; SH3, SRC homology-3 domain; HK, Hook domain; GK, guanylate kinase domain; IB, immunoblot; NS, not significant.
Sap97 disrupts the subcellular organization of Kir2.1.
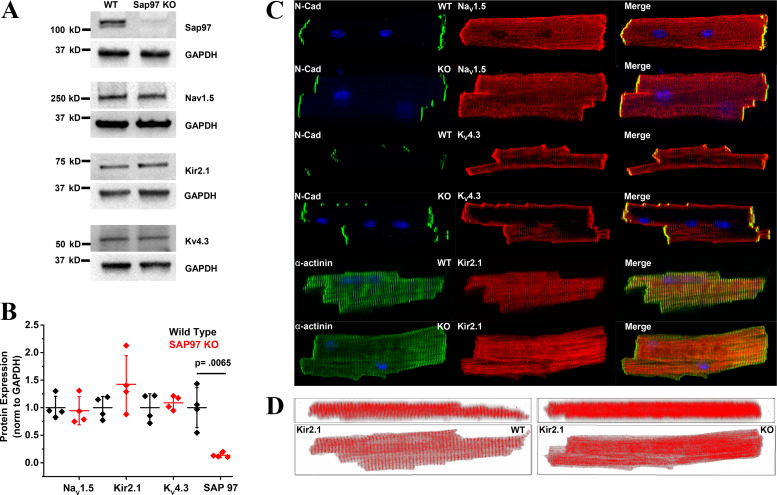
To examine the effects of Dlg1 knockdown on the expression of the major cardiac ion channels, adult mouse ventricular myocytes were isolated from WT and KO animals and probed for the primary cardiac voltage-gated sodium channel NaV1.5, the classical inwardly rectifying potassium channel Kir 2.1, and the fast-transient outward potassium channel Kv4.3. As shown previously, Sap97 protein levels were significantly lower (Fig. 2, A and B). Surprisingly, and in contrast to previously published in vitro studies (14, 36), whole cell protein levels of the main cardiac ion channels were unchanged (Fig. 2, A and B, Supplemental Fig. S6). Supplementary data are contained in the section for Online Supplementary Materials (https://doi.org/10.6084/m9.figshare.10262747). IF analyses corroborated the results of Western blot analysis for NaV1.5 and Kv4.3, indicating that protein expression and subcellular localization appeared unaffected. In addition, we also examined whether subpopulations of NaV1.5 were affected differentially by quantifying the relative expression of NaV1.5 at the lateral membrane versus intercalated disk in ventricular myocytes isolated from WT and Sap97-KO mice (similar to images shown in Fig. 2C). We did not discover any significant difference between cell area, NaV1.5 channel density, and subcellular distribution of NaV1.5 in Sap97-KO versus WT cells (Supplemental Fig. S1). Furthermore, epicardial optical-mapping experiments conducted in WT and Sap97-KO hearts revealed similar anisotropic tissue properties confirming that the ratio metric distribution of NaV1.5 was most likely preserved (Supplemental Fig. S2). In contrast, confocal images of Kir2.1 in Sap97-KO cardiac myocytes showed gross protein mislocalization with the Kir2.1 protein appearing much less organized compared with WT cells (Fig. 2C, bottom). Two-dimensional rendering of z-stack images clearly showed a striated pattern of staining, reminiscent of t tubules, for Kir2.1 in WT cardiac myocytes that is lost in the Sap97-KO cells (Fig. 2D).
Fig. 2.
Knockdown of synapse-associated protein 97 (Sap97) alters subcellular distribution of Kir2.1. A: Western blots of Sap97, NaV1.5, KV4.3, and Kir2.1 in heart lysates from wild-type (WT) and Sap 97-knockout (KO) animals (N = 4,4). B: quantification of the Western blots demonstrates that downregulation of Sap97 (*P = 0.0065) in Sap 97-KO animals does not alter protein profiles of the major cardiac ion channels. Horizontal bars represent means; vertical bars, SD. C: confocal images of ventricular myocytes isolated from adult WT and Sap 97-KO mice. NaV1.5 and Kv4.3 protein retain their subcellular distribution. Subcellular organization of Kir2.1 protein is significantly disrupted in KO cells. D: confocal z-stacks represented in two dimensions illustrate that the striated organization of Kir2.1 is lost in Sap97-KO cells. N-cadherin (N-Cad) is used as a marker for intercalated disk and α-actinin for sarcomeric structure.
Sap97-KO cells display prolonged AP duration and altered ion channel function.
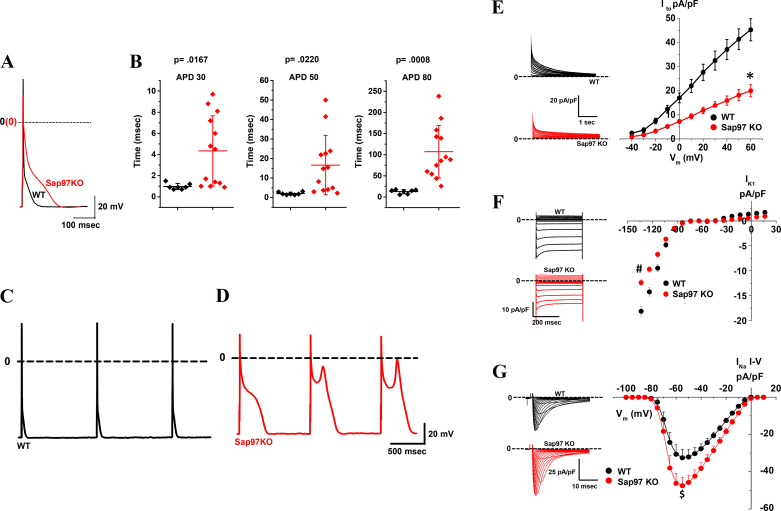
To examine the functional effects of Sap97 knockdown, the whole cell patch-clamp method was used to elicit action potentials and record ionic currents. Action potential duration (APD) at 30, 50, and 80% repolarization were significantly longer in Sap97-KO cells compared with WT cells (Fig. 3, A and B). While arrhythmogenic early afterdepolarizations (EADs) were never observed in WT cells (Fig. 3C), stimulation at 1 HZ frequently elicited EADs in KO cells (Fig. 3D). Voltage-clamp experiments were carried out to examine the effects of Sap97 ablation on ionic currents. Consistent with Sap-97 ablation-induced effects on APD, there were significant changes in membrane K+ and Na+ currents (Fig. 3, E–G). There was an ~54 and 20% decrease, respectively, for Ito (Fig. 3E, peak outward at +60 mV) and IK1 (Fig. 3F, peak inward at −120 mV). Specifically, Ito and IK1 density (at +60 and −120 mV, respectively) was 43.68 ± 3.91 and −16.38 ± 1.14 pA/pF in WT myocytes and 19.96 ± 2.48 and −13.25 ± 0.76 pA/pF in Sap97-KO cells. Surprisingly, there was an upregulation of INa in KO mice (Fig. 3G), with no significant effects on voltage-dependent inactivation and time-dependent recovery from inactivation (Supplemental Fig. S4, D and E). These results demonstrate the fundamental role that Sap97 plays in orchestrating the functional expression of the major cardiac ion channels and determining the shape of the cardiac AP.
Fig. 3.
Reduced synapse-associated protein 97 (Sap97) expression lengthens the cardiac action potential (AP), reduces K+ currents, and enhances Na+ current (INa) A and B: action potentials recorded at 1 Hz in isolated Sap97-knockout (KO) mouse ventricular myocytes (red) demonstrate significantly longer AP duration (APD) 30,50 and 90% repolarization [N = 3, n = 7 wild-type (WT); N = 3, n = 13 Sap97-KO]. Horizontal bars represent means; vertical bars, SD. C and D: Sap97-KO cells frequently demonstrated early afterdepolarizations absent in WT cells. E–G: current-voltage (I-V) relationships for inward rectifier (IK1,), transient outward K+ (Ito,), and the fast INa currents. Peak inward IK1 (at test voltage −120 mV) and peak outward Ito (at test voltage +60 mV) were reduced 20 and 54%, respectively (in pA/pF; IK1, −16.38 ± 1.14 WT, 13.25 ± 0.76 Sap97-KO; N = 3,3; n = 7,9. *P = 0.036; t-test; Ito, 43.68 ± 3.91 WT, 19.96 ± 2.48 Sap97-KO; N = 3,5; n = 10,20; #P = 0.027; t-test). Peak INa (at a test voltage −55mV) was upregulated ~47% (in pA/pF; −32.52 ± 4.60 WT, −47.54 ± 4.47 Sap97-KO; N = 3,4; n = 10,10; $P = 0.0193; t-test). Vm, membrane potential.
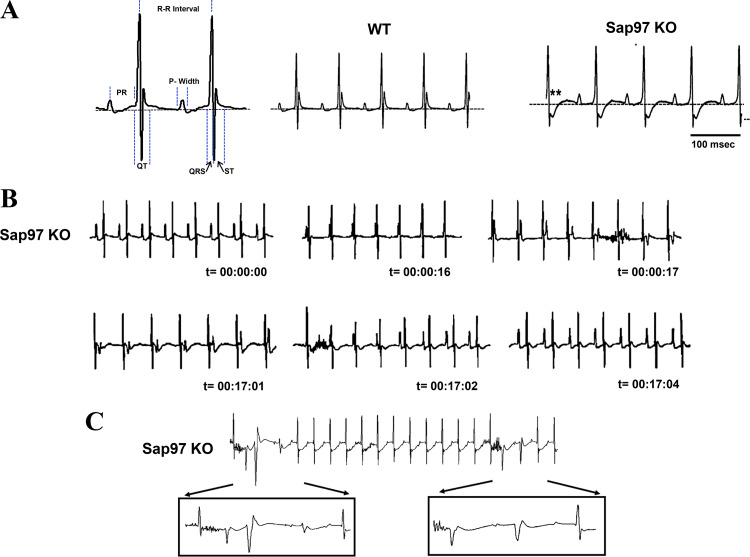
Electrocardiographic abnormalities in Sap97-KO mice.
Electrocardiographic (ECG) records demonstrate significant abnormalities in KO mice. Analysis of ECG parameters in KO animals acquired in sinus rhythm (SR) revealed a prolongation of the ST and QT intervals consistent with an ECG waveform displaying delayed repolarization. The end of repolarization was determined to correspond to the QRS returning to a predetermined isoelectric point corresponding to the initiation point of the QRS complex. (Fig. 4, left, dotted line). Other ECG parameters remained unchanged (Fig. 4, middle and right; Table 1). KO animals also presented several arrhythmogenic phenotypes. Figure 4B displays an example of atrioventricular (AV) dissociation recorded over a period of ~18 min. Excerpts from a longer ECG sequence show a recording in a KO animal that proceeds from SR into complete AV dissociation as the p wave moves independently from the QRS complex. After ~16 min of recording, the activation patterns spontaneously revert back to SR. Another common-observed arrhythmia was frequent runs of premature ventricular contractions (PVCs) as shown in Fig. 4C and magnified in the lower panels. These PVCs are likely initiated by the EADs shown in Fig. 3D. Consistent with the cellular data, the animal data provide the first critical evidence that proper Sap97 expression is essential for maintaining proper impulse initiation and propagation in the heart. To examine possible physical remodeling, echocardiography was performed on control and KO animals to determine if Sap97 ablation resulted in any significant structural remodeling. Results were unremarkable, indicating that Sap97-KO hearts were physically normal (data not shown).
Fig. 4.
Electrocardiographic analysis of synapse-associated protein 97 (Sap97) mice indicates repolarization abnormalities and spontaneous arrhythmias in Sap97-knockout (KO) mice. A: schematic representing details for analysis of ECG waveforms. Note typical changes (asterisks) in ST of KO animals displaying profound delayed repolarization effects. Surface ECGs performed under anesthesia (2% isoflurane) revealed several arrhythmias in Sap97-KO animals. B: example of atrioventricular dissociation, p wave moves independently of the QRS complex. C: short runs of premature ventricular contractions were frequently observed. Neither of these phenotypes were observed in wild-type (WT) animals (N = 7 Sap97KO; N = 6 WT).
Table 1.
ECG parameters
| ECG | n | RR, ms | ST, ms | QRS, ms | PR, ms | QT, ms | P Width, ms |
|---|---|---|---|---|---|---|---|
| WT | 13 | 108.7 ± 2.80 | 15.5 ± 2.22 | 8.7 ± 0.26 | 41.7 ± 1.13 | 23.9 ± 2.19 | 11.4 ± 0.55 |
| Sap97-KO | 13 | 112.7 ± 4.19 | 34.8 ± 3.61 | 9.4 ± 0.35 | 41.3 ± 1.21 | 43.7 ± 3.46 | 13.0 ± 0.72 |
| P values | 0.5 | 0.0001* | 0.2 | 0.8 | 0.00001* | 0.14 |
Values are means ± SE; n, number of mice. WT, wild-type; Sap97-KO, synapse-associated protein 97-knockout.
Significance.
A missense mutation in DLG1 (SAP97) is associated with human arrhythmia.
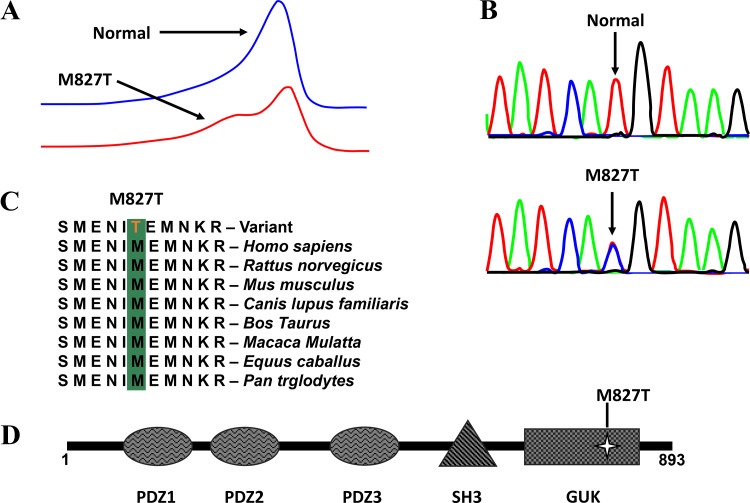
Despite the prolonged AP phenotype of the Sap97-KO mouse, no pathogenic mutations in DLG1 were identified among the 40 genetically elusive LQTS cases. Instead, comprehensive mutational analysis of DLG1-encoded SAP97 identified a novel, potentially pathogenic mutation found in 1 of 38 (2.6%) BrS1–12 genotype-negative/phenotype-positive patients (Fig. 5A). The identified DLG1 mutation c.2480 T>C (g.196786760 A>G) resulted in a M827T missense amino acid substitution at a residue localizing to the guanylate kinase-like (GUK) domain of the protein (Fig. 5, B and D). The nucleotide affected occurs at the very last nucleotide position of exon 23 of the protein and could possibly result in abnormal splicing; however, results from three in silico splicing prediction tools [NNSPLICE 0.9 version (http://www.fruitfly.org/seq_tools/splice.html), ESEfinder (http://krainer01.cshl.edu/cgi-bin/tools/ESE3/esefinder.cgi?process=home), and Human Splicing Finder (http://www.umd.be/HSF3/index.html)] revealed that this nucleotide substitution is not predicted to alter splicing. Additionally, this mutation involved a variant that is completely conserved among 100 vertebrates and has high and maximum PhyloP and PhastCons scores, respectively (Fig. 5C). The M827T variant is present with a MAF of 0.0000165 (2/121042 alleles) in the ExAC browser but absent in all other publicly available exome databases and was absent in over 900 ethnically matched, internal control individuals.
Fig. 5.
Identification of disks large homolog-1 gene (DLG1)-associated mutation in a genotype-negative clinically diagnosed Brugada syndrome cohort. A: denaturing high-performance liquid chromatography profiles (normal, blue trace, and abnormal, red trace). B: DNA sequence chromatograms showing a T→C nucleotide change at position 2480 of DLG1 resulting in a methionine (M) to threonine (T) substitution at position 827 (M827T) vs. normal. C: sequence conservation across species for M827T in DLG1. D: the linear topology of synapse-associated protein 97 (SAP97) with mutation localization. PDZ, postsynaptic density protein-95, discs-large, and zona occludens-1; SH3, SRC homology-3 domain; GUK, guanylate kinase.
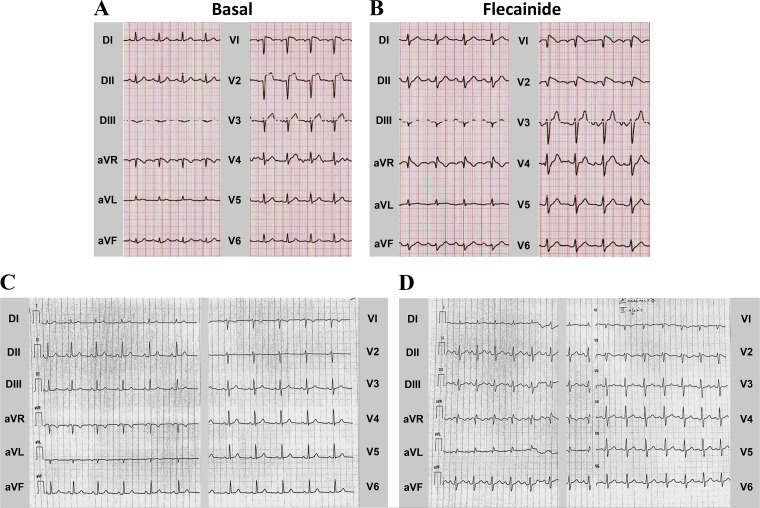
The SAP97-M827T index case was a 60-yr-old man who exhibited an abnormal ECG pattern consistent with BrS during hypokalemia after an episode of vomiting and diarrhea (Fig. 6A). After normalization of electrolytes and resolution of his acute illness, he subsequently underwent a flecainide challenge that induced a diagnostic type 1 Brugada ECG pattern (Fig. 6B). His personal history had no record of syncope or cardiac events; however, he did have a positive family history of SCD (maternal uncle died suddenly at the age of 35 yr; DNA not available). Subsequently, his daughter was clinically evaluated after a syncopal event of probable vaso-vagal nature and a subsequent episode of palpitation and dizziness during fever. Her basal ECG (Fig. 6C) is not diagnostic for Brugada Syndrome, but she had a positive flecainide test (Fig. 6D). Molecular screening identified the same SAP97 mutation detected in the father, supporting its pathogenic role.
Fig. 6.
Electrocardiographic phenotype. A: representative basal ECG in 69-yr-old man suffering from hypokalemia. B: flecainide challenge during 12-lead ECG unmasked the typical type 1 coved Brugada syndrome (BrS) ECG pattern in the M827T-positive BrS index case. C: basal ECG of his daughter carrying the M827T variant. D: flecanide test was similarly positive for BrS. D, bipolar limb leads; aVR, aVL, and aVF, unipolar limb leads; V, unipolar chest leads.
SAP97-M827T expressed in hiPSC-CMs shortens APD and calcium transients.
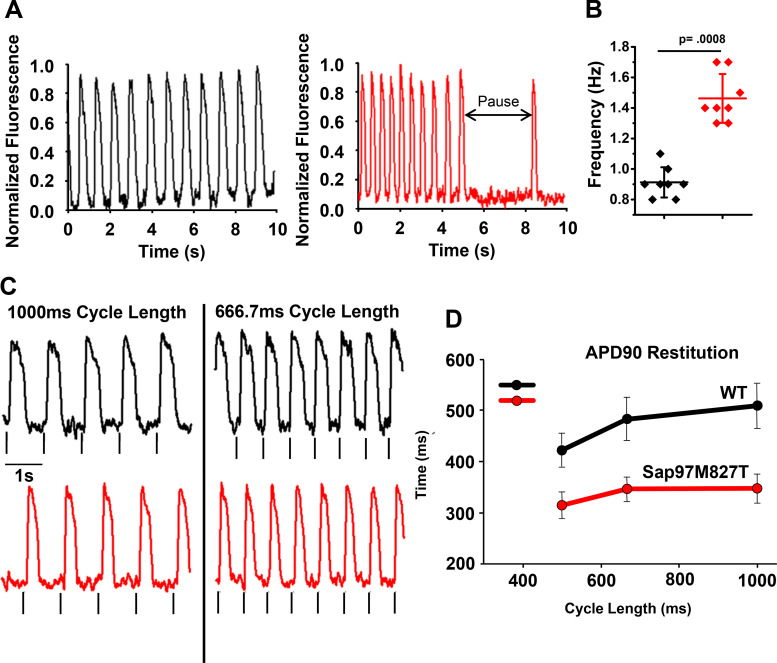
The generation of iPSCs has opened up new avenues to examine potential disease-causing mutations in more relevant cellular environments than those provided by heterologous-expression cell systems, such as HEK293 and CHO cells. As such, we obtained commercially available hiPSC-CMs (Cellular Dynamics International, Madison, WI) and used adenoviral transfections to express either SAP97-WT or SAP97-M827T in hiPSC-CM monolayers to determine the effects on electrical propagation. Adenoviral constructs were made carrying the sequences for WT or SAP97-M827T protein tagged with GFP. Monolayers of hiPSC-CMs were infected with the adenoviral constructs with high efficiency as shown in Supplemental Fig. S3, A–D. iCell cardiac myocytes were plated, and following transfection, cells were stimulated and action potentials and calcium transients were monitored using membrane potential and calcium dyes (FluoVolt and rhod2). Monolayers expressing adSAP97-M827T had significantly faster spontaneous activation rates (with occasional long pauses) than adSAP97-WT monolayers (Fig. 7, A and B) and averaged, respectively, 1.46 ± 0.16 and 0.91 ± 0.10 Hz (n = 8 per group; *P < 0.001). The monolayers also had faster conduction velocities, shorter calcium-transient duration and APD80, relative to the WT counterparts (data not shown). APD90 restitution plot (Fig. 7, C and D) at different pacing frequencies indicates significant APD shortening induced by expression of adSAP97-M827T (P < 0.001). Furthermore, adSAP97-WT monolayers were more sensitive to E-4031 (IKr blocker) than those expressing adSAP97-M827T, suggesting these monolayers had excess repolarization reserve (Supplemental Fig. S5).
Fig. 7.
Electrical activity in spontaneously beating in induced pluripotent stem cell-derived cardiac myocytes monolayers. Action potentials were monitored using the membrane potential dye, FluoVolt. A: optical recordings of spontaneous hiPSC-CM monolayer activations captured over a 10-s duration. Black traces on the left are from monolayers transduced with ad-synapse-associated protein 97 (adSAP97) wild-type (WT); red traces on the right are from monolayers transduced with advSAP97M827T. Mutant SAP97 monolayers exhibited faster spontaneous activation rate with occurrences of irregular pauses. B: monolayers expressing adSAP97M827T had significantly faster spontaneous activation rate (1.46 ± 0.16 vs. 0.91 ± 0.10 Hz; n = 8 per group; P < 0.001. Horizontal bars represent means; vertical bars, SD. C: representative traces of electrical activity during pacing (field stimulation). Black traces, advWTSAP97; red traces, advSAP97M827T. Gray vertical lines under each trace indicate pacing events. D: plot of action potential duration at 90% repolarization (APD90) restitution in advSAP97WT and advSAP97M827T expressing monolayers showing significant APD shortening in monolayers expressing advSAP97M827T (P < 0.001 at each cycle length; means ± SD).
SAP97-M827T mutation results in Kv4.3 gain of function.
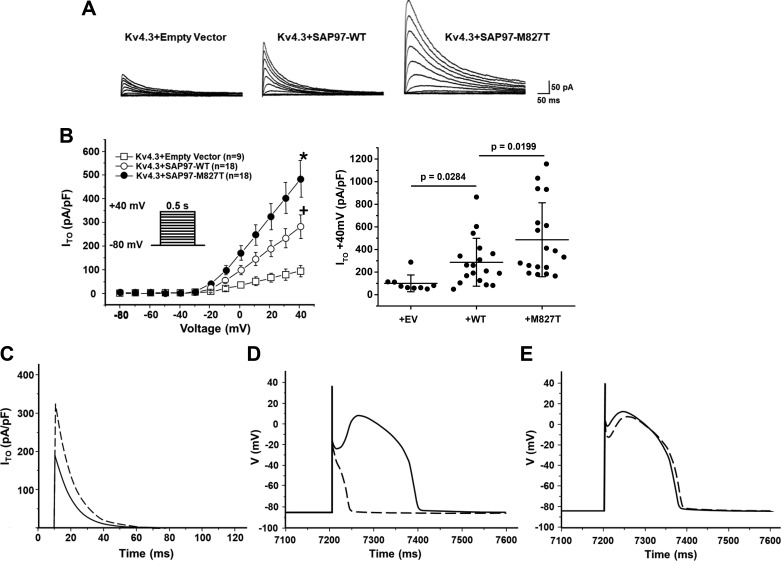
In vitro expression studies were conducted to examine the functional consequences of the SAP97-M827T mutation on the Ito. Figure 8A shows the representative tracings of Kv4.3 coexpression with empty vector, SAP97-WT, or SAP97-M827T in CHO cells. Coexpression of SAP97 (WT) significantly increased Kv4.3 peak current density at +40 mV, nearly threefold compared with Kv4.3 alone (286.8 ± 49.9 pA/pF vs. 100.1 ± 24.6 pA/pF, Fig. 8B). More importantly, coexpression with mutant SAP97-M827T further increased Kv4.3 peak current density at +40 mV by an additional 70.4% to 488.6 ± 78.0 pA/pF (Fig. 8B), confirming that the SAP97-M827T mutation results in a Kv4.3 gain of function. We next used a modified Luo-Rudy II AP model, used to simulate the electrophysiological consequences of the SAP97-M827T mutation. Simulated Ito traces during step depolarization for 120 ms to +40 mV from a holding potential of −90 mV illustrate that the expression of the SAP97-M827T mutant increases the peak current by ~70% with no significant difference in the decay time (Fig. 8C). With the use of values of the simulated peak Ito and inactivation constants, the mutation conferred a 90% increase in total charge for SAP97-M827T relative to WT. This computer simulation was congruent with our experimentally obtained value of a 95.5% increase in total charge from our heterologous expression studies. To assess whether this magnitude of Ito upregulation by the SAP97-M827T mutant would affect the AP morphology and provide an arrhythmogenic substrate, we simulated both RV and LV APs using Ito expressed in the presence of SAP97-WT and SAP97-M827T incorporated in the modified Luo-Rudy II AP model. Multiple cycles of simulation at the basic cycle length of 800 ms showed a marked loss of the “spike and dome” morphology in the simulated RV epicardial AP using Ito in the presence of SAP97-M827T compared with SAP97-WT (Fig. 8D). Interestingly, simulations of the LV epicardial AP, which incorporates a smaller maximal Ito conductance, showed only a minor accentuation of the action potential notch (Fig. 8E).
Fig. 8.
Ionic basis of synapse-associated protein 97 (SAP97)-M827T effects on the cardiac action potential (AP). A: SAP97-wild-type (WT) and SAP97-M827T coexpression increased Kv4.3 current in heterologous system. Representative whole cell recordings of Kv4.3 alone, coexpressed with SAP97-WT or SAP97-M827T in Chinese hamster ovary cells. B: current-voltage relationship for Kv4.3 control (n = 9), coexpression with SAP97-WT (n = 18), or SAP97-M827T (n = 18). All values represent means ± SE. Whisker plot (right) shows peak current density at +40 mV for Kv4.3 alone (n = 9), coexpressed with SAP97-WT (n = 18) or SAP97-M827T (n = 18). *P = 0.0199, Kv4.3+SAP97-M827T vs. Kv4.3+SAP97-WT; +P = 0.0284, Kv4.3+SAP97-WT vs. Kv4.3 + empty vector. Horizontal bars represent means; vertical bars, SD. C–E: simulated effects of SAP97-M827T on the right (RV) and left (LV) ventricular epicardial APs. C: Simulated transient outward K+ (Ito) traces during step depolarization for 120 ms to +40 mV from a holding potential of −90 mV. Solid line, WT; dashed line, SAP97-M827T. D: RV epicardial APs simulated using Ito expressed in the presence of WT and M827T-SAP97 incorporated into a modified Luo-Rudy II AP model. Basic cycle length (BCL) = 800 ms (75 beats/min). Solid line, WT; dashed line, SAP97-M827T. The 10th AP in the equilibration chain is displayed here; the AP shape does not change in subsequent cycles. E: simulated LV epicardial APs using a modified Luo-Rudy II AP model with Ito expressed in the presence of WT and SAP97-M827T. BCL = 800 ms (75 beats/min). Solid line, WT; dashed line, SAP97-M827T. Only the 10th AP in the equilibration chain is displayed here, the AP shape does not change in subsequent cycles.
In addition, hiPSC-CMs were transiently transfected with either SAP97-WT or SAP97-M827T to determine the effects on Kv4.3-mediated current density (Supplemental Fig. S3A). As expected, expression of SAP97 significantly increased Ito density in hiPSC-CMs, 6-fold for advSAP97-WT and 11-fold for advSAP97-M827T. These results are consistent with those obtained in CHO cells (Fig. 8, A and B) and with those demonstrated in AP simulations (Fig. 8, C–E). Furthermore, to assess if the SAP97-M827T had any collateral effects on additional ion channel targets, SAP-97 WT and SAP97-M827T were coexpressed with either NaV1.5 or Kir2.1 in human embryonic kidney (HEK) cells. Whole cell patch-clamp recordings of INa and IK1 failed to demonstrate any significant differences in current density in the presence of the SAP-M827T variant (Supplemental Fig. S4, B and C). In summary, expression of the variant (SAP97-M827T) in iPSC-CMs results in a cellular phenotype that favors early repolarization mechanisms that have been proposed to serve as the underlying substrate that favors the pathogenesis of BrS.
DISCUSSION
Abnormalities in the biogenesis of ion channels have long been linked to arrhythmogenic diseases (8). For several decades, the genetic basis of these channelopathies has been identified as alterations (7) in the primary genetic code of the ion channels themselves. More recent investigations have identified an expanding class of arrhythmia susceptibility genes that encode ChIPs capable of regulating and modulating ion channel expression, subcellular distribution, and function (7, 17, 25, 37). These proteins coassemble with the primary ion channels forming macromolecular complexes essential for the normal initiation and propagation of the cardiac impulse. Despite an abundance of in vitro data demonstrating the critical role of ChiP SAP97 in orchestrating cardiac excitability, there has not been any evidence linking alterations in SAP97 with human arrhythmogenic disease. Here, we present the first evidence that a mutation in SAP97 is associated with a human arrhythmogenic disease.
To better elucidate the in vivo role of SAP97 in cardiac excitability, we generated a murine line for inducible, cardiac-specific ablation of Sap97. Sap97 knockdown in ventricular myocytes resulted in a downregulation of both Kv4.3 and Kir2.1 repolarizing potassium currents. We also present the first in vivo data demonstrating that Sap97 knockdown results in a severe disruption of Kir2.1 localization. In contrast to in vitro data and other published results, NaV1.5-mediated currents in Sap97-KO animals were upregulated while protein expression and localization remained unchanged (14, 24). These alterations resulted in a marked prolongation of the APD that was prone to arrhythmogenic EADs. These cellular phenotypes translated into electrocardiographic abnormalities that manifested as ST- and QT-interval prolongation with a propensity for arrhythmogenic events. Importantly, we show for the first time that a human mutation in SAP97 (SAP97-M827T) may confer susceptibility for a human arrhythmogenic disease. Furthermore, expression of this SAP97 variant in human cardiomyocytes derived from iPSCs allowed us to verify the arrhythmogenic phenotype. These results strongly suggest that DLG1 may represent a new, albeit minor, susceptibility gene for inherited arrhythmia.
In accordance with the data presented here, previous independent investigations have demonstrated a clear role for SAP97 in the regulation and expression of voltage-gated potassium channels. Specifically, previous data from our laboratory demonstrated that shRNA knockdown of Sap97 in adult ventricular myocytes resulted in lowered levels of protein and surface expression of several Kir2.x isoforms (38). Furthermore, work from the Hatem laboratory has demonstrated an association between Sap97 and Kv4.3, where shRNA-mediated knockdown of Sap97 in atrial myocytes results in decreased Ito density (12). With a constitutive cardiac-specific (αMHC Cre) SAP97-KO mouse model, the Abriel laboratory demonstrated reductions in whole cell IK1 and Ito in vivo (14). Likewise, we demonstrate significant reductions in IK1 and Ito density (Fig. 3, E and F), leading to significant prolongation of the ventricular APD (Fig. 3). A significant finding from our investigation is the demonstration, for the first time, that APD prolongations lead to the development of proarrhythmic EADs in isolated ventricular myocytes. We also demonstrate that gross mislocalization of Kir2.1 protein in Sap97-KO ventricular myocytes, revealing the mechanism underlying the downregulation of the Kir2.1-mediated potassium current. Together, these data substantiate a critical role for SAP97 in the regulation of the cardiac potassium channels Kir2.1 and Kv4.3 in vivo.
In vitro experiments have demonstrated a role for SAP97 in the functional expression and localization of NaV1.5, the dominant voltage-gated sodium channel in cardiomyocytes. Previous reports from the Abriel group demonstrated that shRNA-mediated knockdown of SAP97 in HEK293 cells led to lower levels of NaV1.5 surface expression, leading to decreased INa density (30). Similarly, work from our group demonstrated that shRNA knockdown of Sap97 in adult rat ventricular myocytes also caused significant decreases in INa densities compared with scrambled shRNA controls. In contrast to in vitro findings, a recent study revealed that cardiac-specific silencing of Sap97 in a separate murine model did not alter functional INa in isolated cardiomyocytes (14). In contrast to both findings, INa in our model was significantly upregulated. Collectively, these data illustrate the extreme ambiguity that can arise when comparing data gathered from in vitro systems to experiments conducted using in vivo animal models that have compensatory mechanisms unavailable to cultured cells. The differences between the two mouse models could be a consequence of the manner of Sap97 gene ablation. The former is a constitutive cardiac-specific (αMHC Cre) Sap97-KO mouse model in which programmed ablation begins in the early postnatal period. Our model is under the control of a tamoxifen inducible promoter, and mice were approximately 3 mo old when gene ablation was induced. Nonetheless, the preponderance of experimental data would strongly suggest that proper functional expression of INa is regulated by the presence of Sap97.
Despite a significant degree of divergence regarding the specific effects Sap97 knockdown has on the cardiac ion channels, the arrhythmogenic potential of Sap97 knockdown is clear. Sap97-KO mice manifest longer QT intervals in the ECG waveforms that are consistent with the alterations observed in the underlying ionic currents. These effects result in prolonged APs with a propensity for EADs, the cellular correlate for arrhythmias. Sap97-KO animals also demonstrate spontaneous ventricular and atrial arrhythmias.
The phenotype, observed in our Sap97-KO animal, suggests that the DLG1 might be a good candidate gene for LQTS. This in fact was also suggested in Gilet et al. (14). At least among the 40 genetically elusive LQTS cases that were examined, no DLG1 mutations resulting in SAP97 loss of function were identified. However, the phenotype arising from a KO mouse model characterized by the complete absence of a targeted protein can often contrast with the clinical correlate in which the genetic anomaly is often caused by a single missense mutation or small deletion allowing the expression of the protein to remain intact yet cause significant dysfunction. This in fact is well illustrated in KO models of SCN5A, the gene encoding the primary cardiac voltage-gated sodium channel NaV1.5. While embryonically lethal in the homozygous condition, SCN5a+/− animals demonstrate conduction abnormalities and a loss of function phenotype consistent with BrS (29). Nonetheless, both loss- and gain-of-function mutations have been described for SCN5A associating with a myriad of inherited arrhythmogenic disease including BrS, LQT3, atrial fibrillation (AF), and sick sinus syndrome (29, 34). Furthermore, there have been described instances where a single mutation can result in a disease with overlapping phenotypes (31). In light of the multitude of roles that SAP97 plays in driving the functional expression, targeting and regulation of many of the integral components of cardiac excitability, it is critical to cast a wide net and explore DLG1's candidacy as a possible susceptibility gene for various types of genetically undefined arrhythmias. These observations provide significant rationale for reexamining inherited arrhythmias in genotype-negative patients for possible genetic alterations in the DLG1 gene.
To our knowledge, this study is the first to provide evidence that human DLG1 (SAP97) variants can create a substrate for arrhythmogenic syndromes. We identified a novel putative pathogenic SAP97 mutation (M827T) in 1 of 38 (2.6%) BrS1–12 genotype-negative/phenotype-positive patients. A flecainide challenge induced the diagnostic type 1 Brugada ECG pattern in a father and daughter who have a positive family history of SCD. In vitro data from CHO, HEK, and hIPSCs cells demonstrate coexpression of M827T results in an enhancement of Ito that results in a predicted shortening of the AP consistent with an early repolarization mechanism underlying the pathogenesis of BrS. Adenoviral expression of this variant in iPSC-CMs results in shortened APs and CaTD in agreement with the AP simulations and consistent with the mechanisms underlying a BrS phenotype.
Interestingly, the SAP97-M827T variant appears to act as a gain-of-function mutation, resulting in the upregulation of Ito in contrast with the data gathered from Sap97-KO mice, in which Ito was downregulated. These results underscore the extreme care that must be taken when attempting to correlate the expected consequence of human variants with experimental KO models. In consideration of the numerous cellular interactions in which SAP97 participates, it can be expected that mutations in the primary gene can and will result in several complex phenotypes with broad implications in the pathogenesis of inherited arrhythmias. In this study, we provide the first evidence that alterations in SAP97 expression can underlie a human arrhythmogenic syndrome. We expect that future investigations will identify new variants in SAP97 for other genetically elusive BrS cases. In addition, based on the Sap97-KO mouse’s clear prolonged QT phenotype, DLG1 should be analyzed in other cohorts of genetically elusive LQTS since we only had 40 such cases to interrogate.
In summary, we provide the first data supporting DLG1-encoded SAP97’s candidacy as a minor BrS susceptibility gene. Indeed, the DLG1 (SAP97) gene has joined a growing list of genes encoding ion channel-interacting proteins identified as potential channelopathy candidate genes because of their ability to regulate the trafficking, targeting, and modulation of membrane ion channels that are critical for the generation and propagation of the cardiac electrical impulse. Dysfunction in any of these critical components of cardiac excitability can potentially result in fatal cardiac disease. Advances in genetic testing and advances in molecular biology allow us the opportunity to efficiently identify these new candidate genes and potentially determine the molecular mechanisms underlying the arrhythmogenic disease. Expanding our knowledge regarding these genotype-phenotype correlations will enable us to better assess risk and provide appropriate therapeutic avenues to best serve patients burdened with arrhythmogenic disease and identify and protect affected family members.
Study limitations.
The LQT arrhythmia phenotype expressed in our Sap97-KO mouse contrasts with the BrS phenotype found in the patient harboring the M827T mutation. Based on the mutation’s associated phenotype (BrS), now a knock-in mouse model of SAP97-M827T, rather than our original Sap97-KO, would serve to better correlate with our clinical phenotype. Furthermore, it is important to recognize the underlying ionic basis of the mouse cardiac action potential is significantly different from that of the human and therefore acknowledge that the effects of SAP97 deficiency may manifest differently in regard to the human AP (27). Importantly, Ito is the principal repolarizing current in mouse ventricular myocytes. In comparison, in humans, Ito contributes primarily to phase 1 repolarization without exerting a significant influence on phase 3 or final repolarization. Thus, a reduction of Ito as a consequence of a loss-of-function mutation in DLG1 may not cause a long QT phenotype in human, but rather may serve to protect against the development of BrS. As such, despite the long QT phenotype in the Sap97-KO mouse, DLG1 may not be a biologically attractive candidate gene for LQTS in humans. However, since we examined only 40 genetically elusive LQTS cases, it may be somewhat premature, at this point, to make such a conclusion.
Often the most significant information we acquire from studying a KO model is to define the potential targets susceptible to dysregulation of a targeted protein. Therefore, we must acknowledge that although the gain-of-function effects in Ito that we describe for the SAP97-M827T variant are consistent with an early repolarization phenotype ascribed to BrS, we cannot discount effects on the other components of cardiac cell excitability susceptible to SAP-97 dysregulation.
Finally, we must acknowledge that electrocardiographic examinations in the presence of volatile anesthetics can alter cardiac activity and mask certain arrhythmia phenotypes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-124319-01A1 and American Heart Association Grant AHA 14GRNT19710006 (to J. M. Anumonwo). Part of this work was supported by a grant from the State of Michigan Economic Development Fund (UM MTRAC for Life Sciences, to T. J. Herron).
DISCLOSURES
M. J. Ackerman is a consultant for Boston Scientific, Gilead Sciences, Medtronic, and St. Jude Medical. M. J. Ackerman and Mayo Clinic also receive sales-based royalties from Transgenomic for their FAMILION-LQTS and FAMILION-CPVT genetic tests. However, none of these entities were involved in these studies. The other authors have declared that no conflicts of interest exist.
AUTHOR CONTRIBUTIONS
H.M., C.A.M., T.J.H., D.J.T., A.M.D.R., D.Y., L.C., V.V.N., S.C., M.T., M.-C.K., F.D., C.A., P.J.S., M.J.A., and J.M.A. conceived and designed research; H.M., C.A.M., T.J.H., M.M., D.J.T., R.O., B.R., G.G.-S., M.L.M., V.V.N., and J.M.A. performed experiments; H.M., C.A.M., T.J.H., M.M., D.J.T., R.O., G.G.-S., M.L.M., V.V.N., and J.M.A. analyzed data; H.M., C.A.M., T.J.H., M.M., D.J.T., R.O., and J.M.A. interpreted results of experiments; H.M., C.A.M., T.J.H., and J.M.A. prepared figures; H.M. and J.M.A. drafted manuscript; H.M., P.J.M., and J.M.A. edited and revised manuscript; H.M., C.A.M., T.J.H., M.M., D.J.T., R.O., B.R., G.G.-S., A.M.D.R., D.Y., L.C., V.V.N., S.C., M.T., M.-C.K., F.D., C.A., P.J.M., P.J.S., M.J.A., and J.M.A. approved final version of manuscript.
REFERENCES
- 1.Ackerman MJ, Tester DJ, Jones GS, Will ML, Burrow CR, Curran ME. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc 78: 1479–1487, 2003. doi: 10.4065/78.12.1479. [DOI] [PubMed] [Google Scholar]
- 2.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada syndrome: report of the second consensus conference. Heart Rhythm 2: 429–440, 2005. doi: 10.1016/j.hrthm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Bao Y, Willis BC, Frasier CR, Lopez-Santiago LF, Lin X, Ramos-Mondragón R, Auerbach DS, Chen C, Wang Z, Anumonwo J, Valdivia HH, Delmar M, Jalife J, Isom LL. Scn2b deletion in mice results in ventricular and atrial arrhythmias. Circ Arrhythm Electrophysiol 9: e003923, 2016. doi: 10.1161/CIRCEP.116.003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bizy A, Guerrero-Serna G, Hu B, Ponce-Balbuena D, Willis BC, Zarzoso M, Ramirez RJ, Sener MF, Mundada LV, Klos M, Devaney EJ, Vikstrom KL, Herron TJ, Jalife J. Myosin light chain 2-based selection of human iPSC-derived early ventricular cardiac myocytes. Stem Cell Res (Amst) 11: 1335–1347, 2013. doi: 10.1016/j.scr.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell K, Calvo CJ, Mironov S, Herron T, Berenfeld O, Jalife J. Spatial gradients in action potential duration created by regional magnetofection of hERG are a substrate for wavebreak and turbulent propagation in cardiomyocyte monolayers. J Physiol 590: 6363–6379, 2012. doi: 10.1113/jphysiol.2012.238758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campuzano O, Sarquella-Brugada G, Brugada R, Brugada J. Genetics of channelopathies associated with sudden cardiac death. Glob Cardiol Sci Pract 2015: 39, 2015. doi: 10.5339/gcsp.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci USA 104: 20990–20995, 2007. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O’Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature 392: 293–296, 1998. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 9.Crotti L, Marcou CA, Tester DJ, Castelletti S, Giudicessi JR, Torchio M, Medeiros-Domingo A, Simone S, Will ML, Dagradi F, Schwartz PJ, Ackerman MJ. Spectrum and prevalence of mutations involving BrS1- through BrS12-susceptibility genes in a cohort of unrelated patients referred for Brugada syndrome genetic testing: implications for genetic testing. J Am Coll Cardiol 60: 1410–1418, 2012. doi: 10.1016/j.jacc.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delpón E, Cordeiro JM, Núñez L, Thomsen PE, Guerchicoff A, Pollevick GD, Wu Y, Kanters JK, Larsen CT, Hofman-Bang J, Burashnikov E, Christiansen M, Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol 1: 209–218, 2008. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumaine R, Towbin JA, Brugada P, Vatta M, Nesterenko DV, Nesterenko VV, Brugada J, Brugada R, Antzelevitch C. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res 85: 803–809, 1999. doi: 10.1161/01.RES.85.9.803. [DOI] [PubMed] [Google Scholar]
- 12.El-Haou S, Balse E, Neyroud N, Dilanian G, Gavillet B, Abriel H, Coulombe A, Jeromin A, Hatem SN. Kv4 potassium channels form a tripartite complex with the anchoring protein SAP97 and CaMKII in cardiac myocytes. Circ Res 104: 758–769, 2009. doi: 10.1161/CIRCRESAHA.108.191007. [DOI] [PubMed] [Google Scholar]
- 13.Fourie C, Li D, Montgomery JM. The anchoring protein SAP97 influences the trafficking and localisation of multiple membrane channels. Biochim Biophys Acta 1838: 589–594, 2014. doi: 10.1016/j.bbamem.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Gillet L, Rougier JS, Shy D, Sonntag S, Mougenot N, Essers M, Shmerling D, Balse E, Hatem SN, Abriel H. Cardiac-specific ablation of synapse-associated protein SAP97 in mice decreases potassium currents but not sodium current. Heart Rhythm 12: 181–192, 2015. doi: 10.1016/j.hrthm.2014.09.057. [DOI] [PubMed] [Google Scholar]
- 15.Godreau D, Vranckx R, Maguy A, Goyenvalle C, Hatem SN. Different isoforms of synapse-associated protein, SAP97, are expressed in the heart and have distinct effects on the voltage-gated K+ channel Kv1.5. J Biol Chem 278: 47046–47052, 2003. doi: 10.1074/jbc.M308463200. [DOI] [PubMed] [Google Scholar]
- 16.Herron TJ, Rocha AM, Campbell KF, Ponce-Balbuena D, Willis BC, Guerrero-Serna G, Liu Q, Klos M, Musa H, Zarzoso M, Bizy A, Furness J, Anumonwo J, Mironov S, Jalife J. Extracellular matrix-mediated maturation of human pluripotent stem cell-derived cardiac monolayer structure and electrophysiological function. Circ Arrhythm Electrophysiol 9: e003638, 2016. doi: 10.1161/CIRCEP.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa T, Sato A, Marcou CA, Tester DJ, Ackerman MJ, Crotti L, Schwartz PJ, On YK, Park JE, Nakamura K, Hiraoka M, Nakazawa K, Sakurada H, Arimura T, Makita N, Kimura A. A novel disease gene for Brugada syndrome: sarcolemmal membrane-associated protein gene mutations impair intracellular trafficking of hNav1.5. Circ Arrhythm Electrophysiol 5: 1098–1107, 2012. doi: 10.1161/CIRCEP.111.969972. [DOI] [PubMed] [Google Scholar]
- 18.Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al-Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol 57: 794–801, 2011. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee P, Klos M, Bollensdorff C, Hou L, Ewart P, Kamp TJ, Zhang J, Bizy A, Guerrero-Serna G, Kohl P, Jalife J, Herron TJ. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ Res 110: 1556–1563, 2012. doi: 10.1161/CIRCRESAHA.111.262535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee P, Wang K, Woods CE, Yan P, Kohl P, Ewart P, Loew LM, Terrar DA, Bollensdorff C. Cardiac electrophysiological imaging systems scalable for high-throughput drug testing. Pflugers Arch 464: 645–656, 2012. doi: 10.1007/s00424-012-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR 3rd, Vandenberg CA. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J Biol Chem 279: 22331–22346, 2004. doi: 10.1074/jbc.M400285200. [DOI] [PubMed] [Google Scholar]
- 22.Leonoudakis D, Conti LR, Radeke CM, McGuire LM, Vandenberg CA. A multiprotein trafficking complex composed of SAP97, CASK, Veli, and Mint1 is associated with inward rectifier Kir2 potassium channels. J Biol Chem 279: 19051–19063, 2004. doi: 10.1074/jbc.M400284200. [DOI] [PubMed] [Google Scholar]
- 23.Marcello E, Gardoni F, Mauceri D, Romorini S, Jeromin A, Epis R, Borroni B, Cattabeni F, Sala C, Padovani A, Di Luca M. Synapse-associated protein-97 mediates alpha-secretase ADAM10 trafficking and promotes its activity. J Neurosci 27: 1682–1691, 2007. doi: 10.1523/JNEUROSCI.3439-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milstein ML, Musa H, Balbuena DP, Anumonwo JM, Auerbach DS, Furspan PB, Hou L, Hu B, Schumacher SM, Vaidyanathan R, Martens JR, Jalife J. Dynamic reciprocity of sodium and potassium channel expression in a macromolecular complex controls cardiac excitability and arrhythmia. Proc Natl Acad Sci USA 109: E2134–E2143, 2012. doi: 10.1073/pnas.1109370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogné K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421: 634–639, 2003. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz V, Vaidyanathan R, Tolkacheva EG, Dhamoon AS, Taffet SM, Anumonwo JM. Kir2.3 isoform confers pH sensitivity to heteromeric Kir2.1/Kir2.3 channels in HEK293 cells. Heart Rhythm 4: 487–496, 2007. doi: 10.1016/j.hrthm.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 85: 1205–1253, 2005. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 28.Nooh MM, Naren AP, Kim SJ, Xiang YK, Bahouth SW. SAP97 controls the trafficking and resensitization of the beta-1-adrenergic receptor through its PDZ2 and I3 domains. PLoS One 8: e63379, 2013. doi: 10.1371/journal.pone.0063379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci USA 99: 6210–6215, 2002. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res 108: 294–304, 2011. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 31.Remme CA, Wilde AA, Bezzina CR. Cardiac sodium channel overlap syndromes: different faces of SCN5A mutations. Trends Cardiovasc Med 18: 78–87, 2008. doi: 10.1016/j.tcm.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Riuró H, Campuzano O, Arbelo E, Iglesias A, Batlle M, Pérez-Villa F, Brugada J, Pérez GJ, Scornik FS, Brugada R. A missense mutation in the sodium channel β1b subunit reveals SCN1B as a susceptibility gene underlying long QT syndrome. Heart Rhythm 11: 1202–1209, 2014. doi: 10.1016/j.hrthm.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 33.Shy D, Gillet L, Ogrodnik J, Albesa M, Verkerk AO, Wolswinkel R, Rougier JS, Barc J, Essers MC, Syam N, Marsman RF, van Mil AM, Rotman S, Redon R, Bezzina CR, Remme CA, Abriel H. PDZ domain-binding motif regulates cardiomyocyte compartment-specific NaV1.5 channel expression and function. Circulation 130: 147–160, 2014. doi: 10.1161/CIRCULATIONAHA.113.007852. [DOI] [PubMed] [Google Scholar]
- 34.Tan HL, Bezzina CR, Smits JP, Verkerk AO, Wilde AA. Genetic control of sodium channel function. Cardiovasc Res 57: 961–973, 2003. doi: 10.1016/S0008-6363(02)00714-9. [DOI] [PubMed] [Google Scholar]
- 35.Vaidyanathan R, O'Connell RP, Deo M, Milstein ML, Furspan P, Herron TJ, Pandit SV, Musa H, Berenfeld O, Jalife J, Anumonwo JM. The ionic bases of the action potential in isolated mouse cardiac purkinje cell. Heart Rhythm 10: 80–87, 2012. doi: 10.1016/j.hrthm.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaidyanathan R, Taffet SM, Vikstrom KL, Anumonwo JM. Regulation of cardiac inward rectifier potassium current (I(K1)) by synapse-associated protein-97. J Biol Chem 285: 28000–28009, 2010. doi: 10.1074/jbc.M110.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 114: 2104–2112, 2006. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 38.Vikstrom KL, Vaidyanathan R, Levinsohn S, O’Connell RP, Qian Y, Crye M, Mills JH, Anumonwo JM. SAP97 regulates Kir2.3 channels by multiple mechanisms. Am J Physiol Heart Circ Physiol 297: H1387–H1397, 2009. doi: 10.1152/ajpheart.00638.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]