Abstract
Protein kinases play an integral role in cardiac development, function, and disease. Recent experimental and clinical data have implied that protein kinases belonging to a family of atypical α-protein kinases, including α-protein kinase 2 (ALPK2), are important for regulating cardiac development and maintaining function via regulation of WNT signaling. A recent study in zebrafish reported that loss of ALPK2 leads to severe cardiac defects; however, the relevance of ALPK2 has not been studied in a mammalian animal model. To assess the role of ALPK2 in the mammalian heart, we generated two independent global Alpk2-knockout (Alpk2-gKO) mouse lines, using CRISPR/Cas9 technology. We performed physiological and biochemical analyses of Alpk2-gKO mice to determine the functional, morphological, and molecular consequences of Alpk2 deletion at the organismal level. We found that Alpk2-gKO mice exhibited normal cardiac function and morphology up to one year of age. Moreover, we did not observe altered WNT signaling in neonatal Alpk2-gKO mouse hearts. In conclusion, Alpk2 is dispensable for cardiac development and function in the murine model. Our results suggest that Alpk2 is a rapidly evolving gene that lost its essential cardiac functions in mammals.
NEW & NOTEWORTHY Several studies indicated the importance of ALPK2 for cardiac function and development. A recent study in zebrafish report that loss of ALPK2 leads to severe cardiac defects. In contrast, murine Alpk2-gKO models developed in this work display no overt cardiac phenotype. Our results suggest ALPK2, as a rapidly evolving gene, lost its essential cardiac functions in mammals.
INTRODUCTION
Protein kinases orchestrate a wide range of cellular processes and play integral roles in cardiac development, function, and disease, making them attractive therapeutic targets (4, 5, 8, 15, 29, 32, 33). The vast majority of human protein kinases are grouped into a eukaryotic protein kinase (ePK) superfamily as they share a common catalytic domain fold and mechanism. However, there is a small section of the human kinome, classified as atypical protein kinases, that lack sequence similarity relative to the ePK superfamily. The α-kinase family are atypical kinases that are relatively new evolutionarily as they are found first in metazoans and in green plants (25). Six α-protein kinases have been identified in the human kinome: eukaryotic elongation factor-2 kinase, ALPK1 (lymphocyte α-kinase, LAK), ALPK2 (heart α-kinase, HAK), and ALPK3 (muscle α-kinase, MAK), initially named after the origin of the cDNA library used for clone isolation (24). ALPK1/2/3 are 200–240-kDa large proteins consisting of a NH2-terminal Ig domain, largely unstructured central region, and COOH-terminal kinase domain with preceding Ig domain (24). The two remaining family members, TRPM6 (transient receptor potential cation channel, subfamily M, member 6) and TRPM7 (transient receptor potential cation channel, subfamily M, member 7) are unique two-domain proteins consisting of a TRP cation channel as well as the α-kinase catalytic domain (20).
Accumulating experimental and clinical data have revealed that the α-kinase family plays important roles in cardiovascular physiology. For example, TRPM6 and TRPM7 have been shown to modulate cardiac fibrosis, inflammation, and hypertension (16, 23). Recessive truncating mutations in ALPK3 were shown to be associated with severe early onset dilated cardiomyopathy that progressed to hypertrophic cardiomyopathy (HCM) or HCM with left ventricular noncompaction (1, 2, 6, 14). Experimentally, induced pluripotent stem cell-derived cardiomyocytes from patients with ALPK3 cardiomyopathy demonstrated structural and functional abnormalities, and gene-trap-based Alpk3 knockout mice developed cardiomyopathy (21, 31). Moreover, ALPK3 has been identified as a key molecule in promoting cardiomyocyte differentiation of P19CL6 cells (13).
However, significantly less is known about the cardiac role of ALPK2. A recent study reported that ALPK2 promotes cardiogenesis in zebrafish and human pluripotent stem cells, acting as a negative regulator of WNT signaling (12). Moreover, research in colorectal cancer cells demonstrated that ALPK2 can act as an apoptosis suppressor (36). To further investigate the cardiovascular functions of ALPK2 in mammals, we generated two independent global Alpk2-knockout (Alpk2-gKO) mouse models. Surprisingly, ALPK2-deficient mice display normal cardiac morphology and function up to one year of age. On a molecular level, no changes in the WNT pathway were detected in the hearts of Alpk2-knockout mice. Using these models, we demonstrate that ALPK2 is dispensable for murine cardiac development and function.
METHODS
Generation of Alpk2 global knockout.
Mice were generated using CRISPR/Cas9 technology as previously described (17). Genotyping of Alpk2-gKO mice was performed by PCR analysis using mouse-tail extracts and primers: for Alpk2Δ17, forward: 5′-CTTTAATCTTTTTTTTAATCTTCAGGTCAGC-3, reverse: 5′-CACGAGAGATGCAACAAATGAATATAC-3, and for Alpk2Δ31, forward 5′- CTCCCGAGCACTCCGGAAATAT-3′, reverse 5′- TAAGAAGCAAACAGATGATAACCGTG-3′. WNT indicator mice (11) were obtained from The Jackson Laboratory and genotyped according the provided protocol. All animal procedures were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
Echocardiography.
Echocardiography was performed as previously described (9, 37). Briefly, measurements were performed under isoflurane anesthesia (5% for induction and 0.5% for maintenance) using Fujifilm VisualSonics SonoSite Vevo 2100 ultrasound system with a 32- to 55-MHz linear transducer. The following echocardiographic parameters were measured from left ventricular (LV) M-mode tracing: heart rate (HR), LV internal dimensions at the end of diastole and systole (LVIDd and LVIDs, respectively), end-diastolic interventricular septum thickness (IVSd), and LV posterior wall thickness (LVPWd). Fractional shortening was used as an indicator of systolic cardiac function. In the present study, serial echocardiographic measurements were performed for the Alpk2Δ17 line, using 5–12 animals of both sexes per genotype (Alpk2Δ17/Δ17 and Alpk2+/+) at 2, 5, 7, and 12 mo of age. For the Alpk2Δ31 line, 4–12 animals per genotype Alpk2Δ31/Δ31, Alpk2Δ17/+, and Alpk2+/+ of both sexes were studied at 11 mo of age.
Quantitative real-time PCR.
Total RNA was isolated from the mouse ventricular tissue using TRIzol reagent (ThermoFisher Scientific, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was synthesized using Moloney murine leukemia virus reverse-transcriptase (Bio-Rad, Hercules, CA). Primer sequences for cardiac stress and fibrosis markers used for quantitative real-time PCR analysis were the same as in our previous manuscript (38). RT-PCR primers for Bax (forward: 5′-TGGAGCTGCAGAGGATGATT-3′, reverse: 5′-TCTTGGATCCAGACAAGCAGC-3′), Bcl2 (forward: 5′-GGATAACGGAGGCTGGGATGC-3′, reverse: 5′-ACTTGTGGCCCAGGTATGC-3′), Alpk1 (forward: 5′-TAGGATATCTGACACTCCCTCAGC-3′, reverse: 5′-TGACAACAAATGGTGGTCAAACTCC-3′), and Alpk3 (forward: 5′-CCAGAGGCTAGCTTATCAAGC-3′, reverse: 5′- GTCATCCTTGTACCAGGTCAC-3′) were designed using Primer-BLAST (35). RT-PCR was performed using SsoFast EvaGreen Real-Time PCR Master Mix (Bio-Rad, Hercules, CA) in 96-well, low-profile PCR plates in a Bio-Rad CFX96 thermocycler.
Quantification of WNT signaling.
Hearts were harvested from postnatal day 1 animals, fixed in 4% PFA in PBS overnight at +4°C, cryoprotected in sucrose, and embedded in Tissue-Tek OCT (Sakura Finetek, Torrance, CA) compound for cryotome sectioning. Sections were counterstained with DAPI and mounted in ProLong Diamond Antifade. Sections were imaged on the Leica SP8 confocal system. The endogenous green fluorescent protein (GFP) signal was used to detect WNT positive nuclei; DAPI signal was used as a general nuclear marker. Wild-type animals were used as controls for endogenous autofluorescence in the GFP channel. Obtained images were processed using Fiji (26), and WNT positive nuclei were counted using an automated pipeline developed in Cellprofiler (19). Nuclei (782–2011) were evaluated per animal from 3 to 4 independent fields using 2–4 animals per genotype.
Protein isolation and Western blot analysis.
Total protein lysates from ventricular tissue were made in urea-thiourea-SDS lysis buffer as previously described (10). Protein concentration was measured using RC DC Protein Assay (Bio-Rad, Hercules, CA). Protein (20 μg) lysate was separated using SDS-PAGE in 10-well Bolt 4–12% Bis-Tris Plus gels, and proteins were transferred onto PVDF membrane using wet-blotting technique for 1 h at constant 100 V. Membranes were blocked using SuperBlock T20 (TBS) blocking buffer (Thermo Fisher) for 1 h at room temperature. Cleaved caspase-3 (Asp175) antibody (Cat. No. 9661, Cell Signaling, Danvers, MA) and GAPDH antibody (sc-32233, Santa Cruz, CA) were used. Membranes were incubated with primary antibodies overnight and rocked at 4°C. Membranes were washed three times for 5 min with Tris-buffered saline + 0.2% Tween-20 and incubated with the appropriate secondary HRP-conjugated antibody (Dako, Santa Clara, CA). After incubation with secondary antibodies, membranes were washed in the manner described above and detected using SuperSignal West Pico PLUS chemiluminescent substrate (Thermo Fisher) or Western Bright ECL HRP substrate (Advansta, San Jose, CA) with the ChemiDoc Imaging System (Bio-Rad, Hercules, CA). Blots were quantified using Image Laboratory software (Bio-Rad, Hercules, CA)
Statistics.
Statistical analysis was performed using SPSS 26 (IBM), and graphs were prepared using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA). Distribution of mendelian ratios was evaluated using a χ2 goodness-of-fit test. Differences in echocardiographic parameters and expression of cardiac markers were assessed using multivariate analysis of variance (MANOVA). The effect of genotype on WNT positive nuclei numbers was evaluated using a negative binomial regression.
RESULTS
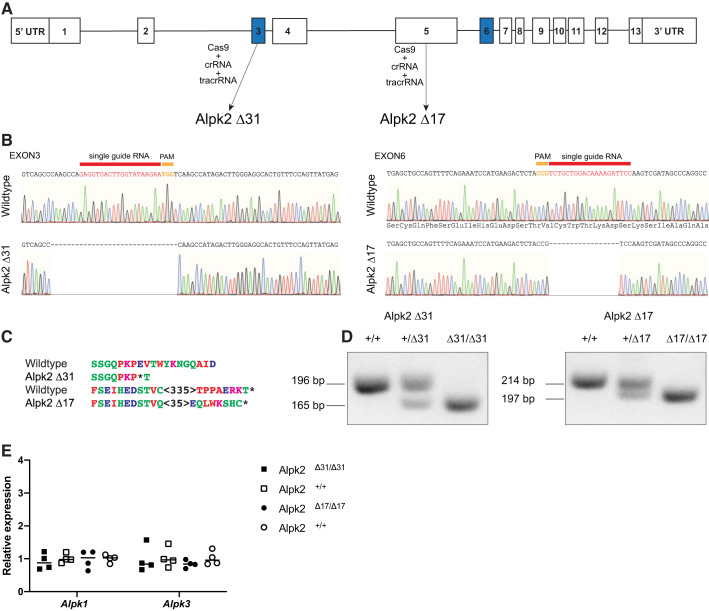
To investigate the function of ALPK2 in a mammalian system, we generated two independent global Alpk2-gKO mouse lines using cloning free CRISPR-Cas9 methodology (17). We designed crRNAs targeting either exon 3 of Alpk2, which contains the start codon, or exon 6, which encodes part of Ig-like domain (Fig. 1A). KO mice were generated by directly injecting the appropriate Cas9 protein:crRNA:tracrRNA complex into mouse zygotes. We identified on-target CRISPR/Cas9 events using a T7 endonuclease 1 (T7E1) assay and characterized alterations by subcloning and sequencing indel carrying founder animals (Fig. 1B). We obtained a 31-bp deletion (Alpk2Δ31) when exon 3 was targeted and 17-bp deletion (Alpk2Δ17) in exon 6. These deletions generated premature stop codons immediately at the targeting site for Alpk2Δ31 and 35-bp downstream of the targeting site for Alpk2Δ17 (Fig. 1C). RT-PCR using primers designed to bind exons flanking the crRNA targeting site confirmed generated deletions and did not detect possible alternative splicing that could have resulted in the expression of a functional ALPK2 isoform (Fig. 1D). To exclude the possibility that the other α-protein kinases are compensating in the Alpk2-KO hearts, we assessed the transcript levels of Alpk1 and Alpk3 in the generated mouse models (Fig. 1D). Based on these results, we did not observe compensation by the other α-protein kinases at transcript level in either model.
Fig. 1.
Generation and characterization of α-protein kinase 2-global knockout (Alpk2-gKO) mouse lines. A: targeting strategy. B: sequencing result of flanking regions surrounding the crRNA targeting for generated mouse lines. C: outcome of generated deletions at the protein level. D: results of RT-PCR reaction over the targeted region confirms deletion and do not indicate alternative splicing events. E: quantitative RT-PCR analysis of other α-protein kinase transcript levels indicate no compensation at the transcript level in Alpk2-KO animals. Data are normalized to corresponding GAPDH levels, and KO groups are expressed as fold changes relative to wild-type (+/+) control. Four homozygous knockout (Δ/Δ) and 4 littermate wild-type (+/+) control animals for each line were used to assess levels of α-protein kinases. No statistically significant effect of either the Alpk2Δ31 [F(1, 6) = 2.079, P = 0.199, Wilks' Λ = 0.743, partial η2 = 0.257] or Alpk2Δ17 [F(2, 5) = 1.001, P = 0.431, Wilks' Λ = 0.741 partial η2 = 0.286] genotype was observed on Alpk1 or Alpk3 transcripts. PAM, protospacer adjacent motif; bp, base pair.
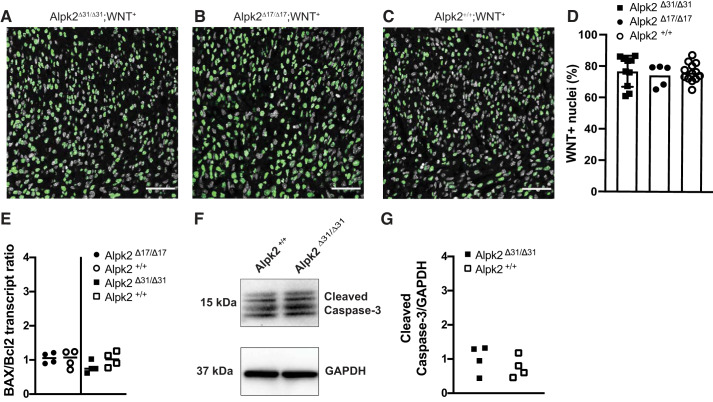
We hypothesized that our murine model would recapitulate the early cardiac phenotype reported in the recent zebrafish Alpk2-KO study, which arose immediately after heart tube formation, resulting in lack of blood circulation (12). Thus, we expected the Alpk2-gKO animals would die during embryonic development. To test this hypothesis, we assessed the genotypes of progeny from heterozygous × heterozygous cross of both the Alpk2Δ17 and Alpk2Δ31 lines (Table 1). The χ2 goodness-of-fit test indicated that the genotype frequencies at weaning age did not statistically differ from expected Mendelian ratios (for Alpk2Δ31, χ2 (2) = 0.864, P = 0.674; Alpk2Δ17, χ2 (2) = 3.24, P = 0.228), indicative of the absence of embryonic lethality in both strains. Previous research proposed that ALPK2 acts as an inhibitor of WNT signaling in developing cardiomyocytes based on experiments in induced human pluripotent stem cell-derived cardiomyocytes and zebrafish models (12). To assess the status of WNT signaling in the heart, we crossed both Alpk2-gKO lines with a WNT indicator line. This WNT reporter mouse line carries histone H2B-GFP fusion under control of six copies of a TCF/Lef responsive element and an hsp68 minimal promoter, reporting Wnt/β-catenin signaling activity with single cell resolution (11). We assessed the numbers of WNT reporter positive nuclei in hearts at postnatal day 1 (Fig. 2). We did not detect changes in numbers of WNT positive nuclei (Fig. 2, A–C). On average, 75% of nuclei were WNT positive in both studied Alpk2-gKO lines and WT animal hearts (Fig. 2D), and thus there was no statistically significant effect of genotype on numbers of WNT positive nuclei.
Table 1.
Genotypes of progeny from both Alpk2Δ31 and Alpk2Δ17 lines
| Observed N | Expected, % | Observed, % | |
|---|---|---|---|
| ALPK2Δ31/+ × ALPK2Δ31/+ | |||
| ALPK2Δ31/Δ31 | 17 | 25.0 | 28.8 |
| ALPK2Δ31/+ | 30 | 50.0 | 50.8 |
| ALPK2+/+ | 12 | 25.0 | 20.3 |
| Total | 59 | 100 | |
| ALPK2Δ17/+ × ALPK2Δ17/+ | |||
| ALPK2Δ17/Δ17 | 14 | 25.0 | 28.0 |
| ALPK2Δ17/+ | 19 | 50.0 | 38.0 |
| ALPK2+/+ | 17 | 25.0 | 34.0 |
| Total | 50 | 100 |
ALPK2, α-protein kinase 2.
Fig. 2.
WNT signaling is not altered in P1 hearts of α-protein kinase 2-global knockout (Alpk2-gKO) mice. A–C: sections of P1 mouse hearts. Nuclei are in gray (DAPI), WNT positive nuclei are in green. Scale bar = 50 μm. No difference in WNT positive nuclei fraction is seen between Alpk2+/+;WNT+(A), Alpk2 Δ17/Δ17;WNT+(B), and Alpk2 Δ31/Δ31;WNT+(C) animal hearts. D: quantification of WNT+ nuclei. Each point represents data from a single microscopy field. Homozygous Alpk2-KO (Δ/Δ) and wild-type (+/+) control animals heterozygous for the WNT reporter were used to study effect of Alpk2 on WNT signaling. Animal genotype had no effect on these numbers of WNT positive nuclei (Wald χ2 (2) = 0.232, P = 0.89). E: quantitative RT-PCR analysis of the BAX-to-Bcl2 ratio (Bax/Bcl2), a marker of tissue apoptotic propensity. Four homozygous knockout (Δ/Δ) and 4 littermate wild-type (+/+) control animals for each studied line were used to assess Bax/Bcl2 ratio. Bax/Bcl2 transcript ratio was not different between Alpk2Δ31 (P = 0.143)- or Alpk2Δ17 (P = 0.859)-KO animals compared with littermate wild-type controls.
Based on a previous study which indicated that ALPK2 acts as an apoptosis regulator, we assessed apoptotic markers in Alpk2-KO mice hearts at the transcript and protein level (Fig. 2, E–G). We did not observe changes in the Bax-to-Bcl2 transcript ratio, which we used as an indicator of apoptotic propensity (1a, 3, 18) between Alpk2-KO and WT controls (Fig. 2E). Similarly, at the protein level, we did not observe differences in the levels of cleaved caspase-3 between WT and Alpk2-KO animals (Fig. 2G).
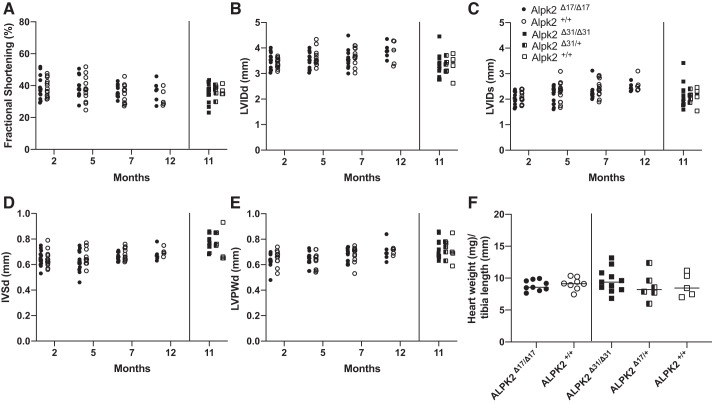
Because we did not observe any embryonic lethality in either Alpk3-gKO line, we hypothesized that Alpk2-gKO animals might exhibit a cardiac phenotype postnatally. To analyze the effect of Alpk2 deletion on cardiac function, we performed serial echocardiographic measurements on Alpk2-gKO animals over the course of 12 mo. Across all time points analyzed, neither of the Alpk2-gKO lines exhibited cardiac dysfunction (Fig. 3A–D). When analyzing the effect of the gKO genotypes in both lines alone or in time-dependent manner, we did not observe a statistically significant effect on any of the echocardiographic parameters. Heart weight-to-tibia length ratios (Fig. 3F) were also not statistically different in either of the Alpk2-gKO lines relative to controls. Genotype also had no effect on any of the measured parameters with regard to animal sex (P = 0.389 for females, and P = 0.216 for males). Taken together, our data indicate that global loss of ALPK2 in the murine model does not cause functional or morphological cardiac abnormalities up to 12 mo of age.
Fig. 3.
α-Protein kinase 2-global knockout (Alpk2-gKO) mice display normal cardiac function and morphology. A–E: echocardiographic analysis showing fractional shortening (A), left ventricular internal diameter during diastole (LVIDd; B), left ventricular internal diameter during systole (LVIDs; C), interventricular septum diameter at diastole (IVSd; D), and left ventricular posterior wall thickness during diastole (LVPWd; E). Animals (n = 4–12) of both sexes were measured per timepoint. Alpk2Δ17 genotype alone [F(4, 75) = 1.129, P = 0.35, Wilks' Λ = 0.943, partial η2 = 0.057] or over time [F(12, 198.723) = 0.462, P = 0.935, Wilks' Λ = 0.93, partial η2 = 0.024], genotype of Alpk2Δ31 had no effect either [F(8, 30) = 0.904, P = 0.526, Wilks' Λ = 0.649, partial η2 = 0.194] on echocardiographic parameters. Heart weight-to-tibia length ratio (F) is not changed for both studied Alpk2-gKO mouse lines at 12 mo of age. Alpk2Δ31 [F(2, 18) = 0.682, P = 0.518, partial η2 = 0.07] and Alpk2Δ17 [F(1, 15) = 0.444, P = 0.515, partial η2 = 0.029]. For Alpk2Δ17, homozygous knockouts (Δ/Δ) were compared with littermate wild-type animals (+/+). For Alpk2Δ31, 3 genotypes were compared: homozygous knockouts (Δ/Δ), heterozyogus knockouts (Δ/+), and wild-type (+/+) animals.
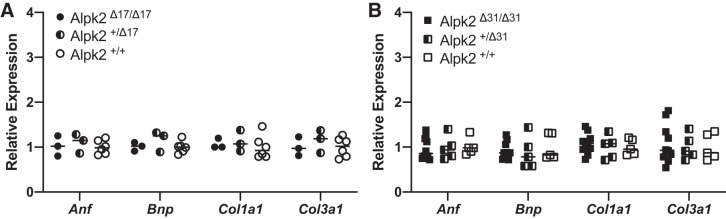
In agreement with our physiological measurements, we did not observe any changes in the expression levels of the cardiac stress markers atrial natriuretic factor (Anf) and B-type natriuretic peptide (Bnp) or in the fibrosis markers collagen-α1 type I (Coll1a1) and type III (Coll3a1) in hearts from either line (Fig. 4).
Fig. 4.
Quantitative RT-PCR analysis of cardiac stress markers atrial natriuretic factor (Anf) and B-type natriuretic peptide (Bnp) and fibrotic markers collagen-α1 type I (Coll3a1), collagen-α1 type III in Alpk2Δ17 mouse line at 2 mo of age, and α-protein kinase 2 (Alpk2)Δ31 line animals 12 mo of age. Data are normalized to corresponding Gapdh levels, and knockout groups are expressed as fold changes relative to wild-type (+/+) control. No statistically significant effect of Alpk2Δ31 [F(8, 28) = 0.154, P = 0.995, Wilks' Λ = 0.917, partial η2 = 0.042] and Alpk2Δ17 [F(12, 13.52) = 0.203, P = 0.996, Wilks' Λ = 0.645 partial η2 = 0.136] genotype on levels of cardiac stress and fibrosis markers. Three genotypes were compared for each line: homozygous knockouts (Δ/Δ), heterozygous knockouts (Δ/+), and wild-type (+/+) animals.
Taken together, we demonstrated that global deletion of Alpk2 in mice does not result in embryonic lethality, altered cardiac WNT signaling in the developing heart, or postnatal cardiac dysfunction.
DISCUSSION
A recent study using induced human pluripotent stem cell-derived cardiomyocytes and zebrafish reported that the atypical α-kinase, ALPK2, promotes cardiogenesis by suppressing WNT signaling (12). However, the role of ALPK2 in cardiogenesis had not been studied using an in vivo mammalian model. To address this need, we generated two global Alpk2-KO mouse lines. In contrast to the previous report, we did not observe cardiogenesis defects or postnatal differences in cardiac function in animals up to 12 mo of age. Our results indicate that ALPK2 is not essential for normal cardiac development or function at basal conditions.
Despite shared similarities between the zebrafish and mouse cardiovascular systems, these are evolutionary distant organisms, which mean scientific findings may not be always directly translatable. In this particular case, the observed discrepancy between the two models may be due to evolutionary differences. Novel genes, such as Alpk2, are known to have higher evolutionary rates, rapidly evolving indispensable roles in fundamental biological processes (7, 25, 34). Indeed, alignment (27) of representative ALPK2 protein sequences retrieved from the UniProt database (30) reveals low sequence identity between zebrafish ALPK2 and mouse ALPK2 (26.4%) compared with, for example, sarcomeric protein titin (57.8%). Moreover, no homolog of ALPK1 has been found in zebrafish. Interestingly, the sequence of zebrafish ALPK2 protein is equally similar to murine ALPK3 (19.5%) or zebrafish ALPK3 (20.2%). These observations suggest that differences between zebrafish and murine ALPK2 are substantial enough to result in different cardiac functions of this protein. It is compelling to speculate that zebrafish ALPK2 protein still retains important cardiovascular functions, that are distributed in mammals across the essential-for-the-heart ALPK3 (1, 2, 6, 14, 21, 31) and dispensable ALPK2 and ALPK1.
Noteworthy, ALPK2-knockout or -knockdown in cardiac progenitor cells generated using WNT agonist CHIR99021 from human induced pluripotent stem cells resulted in overactivation of WNT signaling (12). This finding suggests that ALPK2 might act in cellulo as an antagonist of WNT signaling in specific contexts. Similar to this observation, our data indicate that ALPK2 does not affect the apoptotic propensity of cardiac tissue in murine models. Speculatively, ALPK2 might regulate apoptosis in specific pathophysiological conditions, such a colorectal cancer (36).
In summary, our results indicate that Alpk2 is dispensable for murine cardiac development and function. However, further studies are needed to address the cardiac role of ALPK2 under WNT pathway-activating conditions such as cardiac remodeling during heart failure (28). The models generated here will be useful for future studies focused on addressing the requirements for ALPK2 in other organ systems as well.
GRANTS
J.C. is funded by grants from the National Heart, Lung, and Blood Institute and holds an American Heart Association endowed chair in cardiovascular research. C.T. is funded by Institutional Research and Academic Career Development Award National Institute of General Medical Sciences Grant K12-GM068524-17. Microscopy was performed at University of California, San Diego, School of Medicine Microscopy Core funded by Grant P30-NS047101.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.B., W.F., S.H., and J.C. conceived and designed research; J.B., W.F., M.D.Y., S.H., L.Z., C.T., Y.G., and S.S. performed experiments; J.B., Y.G., and S.S. analyzed data; J.B., W.F., M.D.Y., S.H., and C.T. interpreted results of experiments; J.B., W.F., and S.H. prepared figures; J.B. drafted manuscript; J.B., W.F., M.D.Y., S.H., C.T., S.S., and J.C. edited and revised manuscript; J.B., W.F., M.D.Y., S.H., L.Z., C.T., Y.G., S.S., and J.C. approved final version of manuscript.
REFERENCES
- 1.Al Senaidi K, Joshi N, Al-Nabhani M, Al-Kasbi G, Al Farqani A, Al-Thihli K, Al-Maawali A. Phenotypic spectrum of ALPK3-related cardiomyopathy. Am J Med Genet A 179: 1235–1240, 2019. [DOI] [PubMed] [Google Scholar]
- 1a.Aliparasti MR, Alipour MR, Almasi S, Feizi H. Ghrelin adiministration Increases the BAx/Bcl-2 gene expression ratio in the heart of hypoxic rats. Adv Pharm Bull 2015: 195–199, 2015. doi: 10.15171/apb.2015.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almomani R, Verhagen JM, Herkert JC, Brosens E, van Spaendonck-Zwarts KY, Asimaki A, van der Zwaag PA, Frohn-Mulder IM, Bertoli-Avella AM, Boven LG, van Slegtenhorst MA, van der Smagt JJ, van IJcken WF, Timmer B, van Stuijvenberg M, Verdijk RM, Saffitz JE, du Plessis FA, Michels M, Hofstra RM, Sinke RJ, van Tintelen JP, Wessels MW, Jongbloed JD, van de Laar IM. Biallelic truncating mutations in ALPK3 cause severe pediatric cardiomyopathy. J Am Coll Cardiol 67: 515–525, 2016. doi: 10.1016/j.jacc.2015.10.093. [DOI] [PubMed] [Google Scholar]
- 3.Bogomolovas J, Brohm K, Čelutkienė J, Balčiūnaitė G, Bironaitė D, Bukelskienė V, Daunoravičus D, Witt CC, Fielitz J, Grabauskienė V, Labeit S. Induction of Ankrd1 in dilated cardiomyopathy correlates with the heart failure progression. BioMed Res Int 2015: 273936, 2015. doi: 10.1155/2015/273936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogomolovas J, Gasch A, Simkovic F, Rigden DJ, Labeit S, Mayans O. Titin kinase is an inactive pseudokinase scaffold that supports MuRF1 recruitment to the sarcomeric M-line. Open Biol 4: 140041, 2014. doi: 10.1098/rsob.140041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol 16: 443–452, 2006. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Çağlayan AO, Sezer RG, Kaymakçalan H, Ulgen E, Yavuz T, Baranoski JF, Bozaykut A, Harmanci AS, Yalcin Y, Youngblood MW, Yasuno K, Bilgüvar K, Gunel M. ALPK3 gene mutation in a patient with congenital cardiomyopathy and dysmorphic features. Cold Spring Harb Mol Case Stud 3: 3, 2017. doi: 10.1101/mcs.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Krinsky BH, Long M. New genes as drivers of phenotypic evolution. Nat Rev Genet 14: 645–660, 2013. doi: 10.1038/nrg3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen P. Protein kinases–the major drug targets of the twenty-first century? Nat Rev Drug Discov 1: 309–315, 2002. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 9.Fang X, Bogomolovas J, Wu T, Zhang W, Liu C, Veevers J, Stroud MJ, Zhang Z, Ma X, Mu Y, Lao DH, Dalton ND, Gu Y, Wang C, Wang M, Liang Y, Lange S, Ouyang K, Peterson KL, Evans SM, Chen J. Loss-of-function mutations in co-chaperone BAG3 destabilize small HSPs and cause cardiomyopathy. J Clin Invest 127: 3189–3200, 2017. doi: 10.1172/JCI94310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang X, Bogomolovas J, Zhou PS, Mu Y, Ma X, Chen Z, Zhang L, Zhu M, Veevers J, Ouyang K, Chen J. P209L mutation in Bag3 does not cause cardiomyopathy in mice. Am J Physiol Heart Circ Physiol 316: H392–H399, 2019. doi: 10.1152/ajpheart.00714.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse. BMC Dev Biol 10: 121, 2010. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofsteen P, Robitaille AM, Strash N, Palpant N, Moon RT, Pabon L, Murry CE. ALPK2 promotes cardiogenesis in zebrafish and human pluripotent stem cells. iScience 2: 88–100, 2018. doi: 10.1016/j.isci.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosoda T, Monzen K, Hiroi Y, Oka T, Takimoto E, Yazaki Y, Nagai R, Komuro I. A novel myocyte-specific gene Midori promotes the differentiation of P19CL6 cells into cardiomyocytes. J Biol Chem 276: 35978–35989, 2001. doi: 10.1074/jbc.M100485200. [DOI] [PubMed] [Google Scholar]
- 14.Jaouadi H, Kraoua L, Chaker L, Atkinson A, Delague V, Levy N, Benkhalifa R, Mrad R, Abdelhak S, Zaffran S. Novel ALPK3 mutation in a Tunisian patient with pediatric cardiomyopathy and facio-thoraco-skeletal features. J Hum Genet 63: 1077–1082, 2018. doi: 10.1038/s10038-018-0492-1. [DOI] [PubMed] [Google Scholar]
- 15.Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edström L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science 308: 1599–1603, 2005. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Li M, Yi X, Guo F, Zhou Y, Chen S, Wu X. TRPM7 channels mediate the functional changes in cardiac fibroblasts induced by angiotensin II. Int J Mol Med 39: 1291–1298, 2017. doi: 10.3892/ijmm.2017.2943. [DOI] [PubMed] [Google Scholar]
- 17.Ma X, Chen C, Veevers J, Zhou X, Ross RS, Feng W, Chen J. CRISPR/Cas9-mediated gene manipulation to create single-amino-acid-substituted and floxed mice with a cloning-free method. Sci Rep 7: 42244, 2017. doi: 10.1038/srep42244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayorga M, Bahi N, Ballester M, Comella JX, Sanchis D. Bcl-2 is a key factor for cardiac fibroblast resistance to programmed cell death. J Biol Chem 279: 34882–34889, 2004. doi: 10.1074/jbc.M404616200. [DOI] [PubMed] [Google Scholar]
- 19.McQuin C, Goodman A, Chernyshev V, Kamentsky L, Cimini BA, Karhohs KW, Doan M, Ding L, Rafelski SM, Thirstrup D, Wiegraebe W, Singh S, Becker T, Caicedo JC, Carpenter AE. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol 16: e2005970, 2018. doi: 10.1371/journal.pbio.2005970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middelbeek J, Clark K, Venselaar H, Huynen MA, van Leeuwen FN. The alpha-kinase family: an exceptional branch on the protein kinase tree. Cell Mol Life Sci 67: 875–890, 2010. doi: 10.1007/s00018-009-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phelan DG, Anderson DJ, Howden SE, Wong RC, Hickey PF, Pope K, Wilson GR, Pébay A, Davis AM, Petrou S, Elefanty AG, Stanley EG, James PA, Macciocca I, Bahlo M, Cheung MM, Amor DJ, Elliott DA, Lockhart PJ. ALPK3-deficient cardiomyocytes generated from patient-derived induced pluripotent stem cells and mutant human embryonic stem cells display abnormal calcium handling and establish that ALPK3 deficiency underlies familial cardiomyopathy. Eur Heart J 37: 2586–2590, 2016. doi: 10.1093/eurheartj/ehw160. [DOI] [PubMed] [Google Scholar]
- 23.Rios FJ, Zou ZG, Harvey AP, Harvey KY, Nosalski R, Anyfanti P, Camargo LL, Lacchini S, Ryazanov AG, Ryazanova L, McGrath S, Guzik TJ, Goodyear CS, Montezano AC, Touyz RM. Chanzyme TRPM7 protects against cardiovascular inflammation and fibrosis. Cardiovasc Res 116: 721–735, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryazanov AG, Pavur KS, Dorovkov MV. Alpha-kinases: a new class of protein kinases with a novel catalytic domain. Curr Biol 9: R43–R45, 1999. doi: 10.1016/S0960-9822(99)80006-2. [DOI] [PubMed] [Google Scholar]
- 25.Scheeff ED, Bourne PE. Structural evolution of the protein kinase-like superfamily. PLOS Comput Biol 1: e49, 2005. doi: 10.1371/journal.pcbi.0010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539, 2011. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stylianidis V, Hermans KC, Blankesteijn WM. Wnt signaling in cardiac remodeling and heart failure. Handb Exp Pharmacol 243: 371–393, 2017. doi: 10.1007/164_2016_56. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, Terashima T, Takada S, Yamamura H, Akira S, Minami Y. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells 5: 71–78, 2000. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 30.UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47, D1: D506–D515, 2019. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Sligtenhorst I, Ding ZM, Shi ZZ, Read RW, Hansen G, Vogel P. Cardiomyopathy in α-kinase 3 (ALPK3)-deficient mice. Vet Pathol 49: 131–141, 2012. doi: 10.1177/0300985811402841. [DOI] [PubMed] [Google Scholar]
- 32.Ware JS, Cook SA. Role of titin in cardiomyopathy: from DNA variants to patient stratification. Nat Rev Cardiol 15: 241–252, 2018. doi: 10.1038/nrcardio.2017.190. [DOI] [PubMed] [Google Scholar]
- 33.Weinert S, Bergmann N, Luo X, Erdmann B, Gotthardt M. M line-deficient titin causes cardiac lethality through impaired maturation of the sarcomere. J Cell Biol 173: 559–570, 2006. doi: 10.1083/jcb.200601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf YI, Novichkov PS, Karev GP, Koonin EV, Lipman DJ. The universal distribution of evolutionary rates of genes and distinct characteristics of eukaryotic genes of different apparent ages. Proc Natl Acad Sci USA 106: 7273–7280, 2009. doi: 10.1073/pnas.0901808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13: 134, 2012. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida Y, Tsunoda T, Doi K, Fujimoto T, Tanaka Y, Ota T, Ogawa M, Matsuzaki H, Kuroki M, Iwasaki A, Shirasawa S. ALPK2 is crucial for luminal apoptosis and DNA repair-related gene expression in a three-dimensional colonic-crypt model. Anticancer Res 32: 2301–2308, 2012. [PubMed] [Google Scholar]
- 37.Zhang Z, Stroud MJ, Zhang J, Fang X, Ouyang K, Kimura K, Mu Y, Dalton ND, Gu Y, Bradford WH, Peterson KL, Cheng H, Zhou X, Chen J. Normalization of Naxos plakoglobin levels restores cardiac function in mice. J Clin Invest 125: 1708–1712, 2015. doi: 10.1172/JCI80335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Chen Z, Zhang L, Zhu M, Tan C, Zhou X, Evans SM, Fang X, Feng W, Chen J. Loss of Filamin C Is Catastrophic for Heart Function. Circulation 141: 869–871, 2020. doi: 10.1161/CIRCULATIONAHA.119.044061. [DOI] [PMC free article] [PubMed] [Google Scholar]