Abstract
Type 2 diabetic (T2DM) coronary resistance microvessels (CRMs) undergo inward hypertrophic remodeling associated with reduced stiffness and reduced coronary blood flow in both mice and pig models. Since reduced stiffness does not appear to be due to functional changes in the extracellular matrix, this study tested the hypothesis that decreased CRM stiffness in T2DM is due to reduced vascular smooth muscle cell (VSMC) stiffness, which impacts the traction force generated by VSMCs. Atomic force microscopy (AFM) and traction force microscopy (TFM) were conducted on primary low-passage CRM VSMCs from normal Db/db and T2DM db/db mice in addition to low-passage normal and T2DM deidentified human coronary VSMCs. Elastic modulus was reduced in T2DM mouse and human coronary VSMCs compared with normal (mouse: Db/db 6.84 ± 0.34 kPa vs. db/db 4.70 ± 0.19 kPa, P < 0.0001; human: normal 3.59 ± 0.38 kPa vs. T2DM 2.61 ± 0.35 kPa, P = 0.05). Both mouse and human T2DM coronary microvascular VSMCs were less adhesive to fibronectin compared with normal. T2DM db/db coronary VSMCs generated enhanced traction force by TFM (control 692 ± 67 Pa vs. db/db 1,507 ± 207 Pa; P < 0.01). Immunoblot analysis showed that T2DM human coronary VSMCs expressed reduced β1-integrin and elevated β3-integrin (control 1.00 ± 0.06 vs. T2DM 0.62 ± 0.14, P < 0.05 and control 1.00 ± 0.49 vs. T2DM 3.39 ± 1.05, P = 0.06, respectively). These data show that T2DM coronary VSMCs are less stiff and less adhesive to fibronectin but are able to generate enhanced force, corroborating previously published computational findings that decreasing cellular stiffness increases the cells’ ability to generate higher traction force.
NEW & NOTEWORTHY We show here that a potential causative factor for reduced diabetic coronary microvascular stiffness is the direct reduction in coronary vascular smooth muscle cell stiffness. These cells were also able to generate enhanced traction force, validating previously published computational models. Collectively, these data show that smooth muscle cell stiffness can be a contributor to overall tissue stiffness in the coronary microcirculation, and this may be a novel area of interest for therapeutic targets.
Keywords: atomic force microscopy, coronary microcirculation, diabetes, stiffness, traction force microscopy
INTRODUCTION
The American Heart Association classifies type 2 diabetes mellitus (T2DM) as a cardiovascular disease in part because two-thirds of diabetes-related deaths are directly due to heart disease (15). Furthermore, myocardial infarction can be up to four times more likely to occur in diabetic patients compared with nondiabetic patients (36). Coronary artery disease (CAD) in diabetic patients has also been linked to atherosclerosis and endothelial dysfunction (37). It is well established in T2DM experimental models and patients that both micro- and macrovasculopathies occur because of changes in vessel structure and function, which underlie many diabetic cardiovascular complications, including retinopathy, nephropathy, CAD, myocardial infarction, and stroke (7).
Previously, our group demonstrated that inward hypertrophic remodeling of coronary resistance microvessels (CRMs) was an early contributor to CAD and was associated with reduced coronary flow in both the db/db mouse model of T2DM and a porcine model of metabolic syndrome (MetS) (23, 39). Indeed, our laboratory has previously reported (2, 23) that diabetic CRMs are less stiff than normal CRMs at a time that appears to precede the onset of macrovascular disease; however, the decrease in T2DM coronary microvascular stiffness does not appear to be grossly due to changes in the extracellular matrix (ECM). These intriguing data may suggest that vascular smooth muscle cell (VSMC) stiffness may be a novel contributor to CRM stiffness in T2DM.
Adult VSMCs function as contractile cells that produce and organize the surrounding ECM under normal conditions. This net balance of contractile versus synthetic VSMC function is altered in cardiovascular diseases, and shifting this balance toward a more proliferative and/or synthetic VSMC phenotype often leads to aberrant vascular remodeling. In resistance microvessels, the contractile role of VSMCs is arguably more important than in larger arteries, as VSMCs in resistance microvessels work to regulate blood flow to tissues through changes in vascular tone. Previous studies have reported enhanced myogenic tone in coronary microvessels of T2DM (5, 33, 39). The Ghadiali laboratory has previously shown in computational analyses that decreased cell stiffness can lead to enhanced cellular traction forces (44). Collectively, given that CRM stiffness is not likely due to alterations in the ECM (2), we hypothesized that decreased CRM stiffness in T2DM is due to reduced VSMC stiffness. Secondarily, we also aimed to ascertain whether reduced VSMC stiffness enhanced traction forces of the T2DM CRM cell as predicted in computational models.
METHODS
Materials and reagents.
All reagents for solutions, unless otherwise specified, were purchased from Fisher Scientific (Waltham, MA). Antibodies for β1- and β3-integrins were purchased from Abcam (Cambridge, MA). Deidentified normal and T2DM human primary coronary VSMCs were obtained from both Lonza (Morristown, NJ) and ATCC (Manassas, VA).
Animals.
Male T2DM homozygous db/db and control nondiabetic heterozygous Db/db mice were obtained from The Jackson Laboratories. The db/db mice are leptin receptor deficient and develop obesity, hyperglycemia, insulin resistance, and dyslipidemia by 4–8 wk of age. They were housed under a 12-h:12-h light-dark cycle at 22°C and 60% humidity and were allowed ad libitum access to standard low-fat laboratory chow and water. All experiments were conducted at 16 wk of age. This study was conducted in accordance with National Institutes of Health guidelines, and it was approved by the Institutional Animal Care and Use Committee at Nationwide Children’s Hospital.
Blood glucose measurements.
Mice were fasted for 8 h during the light cycle, and blood was drawn from the tail vein. Glucose levels were determined with the AlphaTrak glucometer (Abbott Laboratories, Alameda, CA).
Mouse coronary VSMC isolation.
Normal and diabetic mouse CRM VSMCs were isolated as previously described by us (19). In brief, hearts were removed from anesthetized mice (3% isoflurane until lack of response to toe pinch) and gently perfused via retrograde Langendorff with digestion solution containing ~300 U/mL of collagenase type II (Worthington), 0.1 mg/mL soybean trypsin inhibitor, and 1 M CaCl2. Every 15 min, the perfusates were collected, centrifuged, resuspended in growth medium, and placed in a 37°C incubator. After perfusate collections were completed for a total of 90 min of digestion, all of the resuspended cells were placed in the same tube, pelleted by centrifugation, resuspended in plating medium, and plated on 35-mm tissue culture dishes. Diabetic cells were cultured in high-glucose (25 mM) DMEM, whereas nondiabetic cells were cultured in normal-glucose (15 mM) DMEM. All experiments were performed in passage 2 or less coronary microvascular VSMCs. To obtain sufficient numbers of cells for experiments, cells from n = 2 mice were pooled into one 35-mm dish for each biological replicate.
Mouse aortic VSMC isolation.
VSMCs from the same 16-wk-old Db/db heterozygous mice and db/db homozygous mice as used for coronary VSMC isolations were isolated from the thoracic aorta by collagenase digestion and cultured as described previously (28). Diabetic cells were cultured in high-glucose (25 mM) DMEM, whereas nondiabetic cells were cultured in normal-glucose (15 mM) DMEM. All experiments were performed in passage 2 or less aortic VSMCs.
Atomic force microscopy.
For mouse VSMC experiments, cells were isolated from 16-wk-old Db/db and db/db mice as described above and passage 1 cells were used for all experiments (n = 6–11 from n = 12–22 mice per group for all groups). For human VSMC experiments, passages 5–6 coronary VSMCs were used for all experiments (n = 4 or 5 from n = 4 or 5 individuals for all groups). With an Asylum MFP-3D-Infinity-BIO atomic force microscope (AFM) with probes (Bruker MLCT) coated with 0.5 mg/mL fibronectin (FN; Gibco), a nano-indentation protocol (10, 17) was used to measure elastic modulus, an indicator of cellular stiffness, and adhesion to FN. The following parameters were set for each experiment: force distance was set to 1.6 µm, sample rate was set to 625 Hz, and set point was set to 0.3 V. For each experiment, we collected 25–40 curves per cell and 8–12 cells per dish. These data were averaged for each biological replicate. The AFM data were collected and analyzed with IGOR Pro software (WaveMetrics). Elastic modulus was calculated with the Oliver–Pharr method (1), a modified Hertz model.
In separate experiments, we also treated normal and diabetic mouse VSMCs with both high and normal glucose (25 mM DMEM vs. 15 mM DMEM, respectively) to determine whether glucose concentrations alone impact coronary VSMC stiffness. Coronary VSMCs were isolated from 16-wk-old Db/db and db/db mice as described above; however, cells were isolated from four mice at a time. To have sufficient cells to test the effects of glucose concentrations on stiffness, and so that the same populations of cells were exposed to both conditions, these cells were pooled and then split into two 35-mm dishes: one dish was exposed to high glucose, and the other dish was exposed to low glucose. Passage 1 cells were used for all experiments (n = 4 from n = 8 mice for all groups), and AFM experiments were performed as described above.
Traction force microscopy.
Primary normal or diabetic coronary VSMCs (n = 4 from n = 8 mice per group) were isolated from normal or type 2 diabetic db/db mice and were evaluated by traction force microscopy (TFM). Cells were cultured in high-glucose (25 mM) or normal-glucose (15 mM) DMEM (diabetic or normal cells, respectively) on polyacrylamide gels containing red fluorescent microspheres/beads and grown overnight. Beads were imaged before and after trypsinization, bead displacements were determined with a particle tracking algorithm, and the data were analyzed with COMSOL finite-element software.
Cell contractility was measured with TFM as described previously (44). Briefly, polyacrylamide gels were prepared by mixing 8% acrylamide and 0.04% bis-acrylamide (Bio-Rad, Hercules, CA) with 0.05% ammonium persulfate and 0.05% N,N,N′,N′-tetramethylethylenediamine to polymerize on 22 mm × 22 mm glass coverslips. The gels contained 0.01% 0.5-µm red fluorescent carboxylate-modified microspheres (Invitrogen, Carlsbad, CA) for tracking displacement changes. The surface of the gel was activated by using Sulfo-SANPAH (Thermo Scientific, Rockford, IL) exposed under a 254-nm UV lamp for 5 min twice. Activated surface was coated with 200 μg/mL type I bovine collagen (Advanced BioMatrix, San Diego, CA) for 30 min at room temperature. For each gel, 1 × 104 coronary VSMCs were seeded on the surface and were grown overnight. Phase-contrast images were taken of individually isolated cells (n = 8 per dish) on the gel to obtain boundaries of the cells. Fluorescence images of the bead within the gels were taken before cell detachment to obtain displacement changes. MATLAB (MathWorks, Natick, MA) image registration was used to align the beads and calculate displacement by correlation-based particle image velocimetry. COMSOL Multiphysics (Burlington, MA), a 3D finite-element software, was used to compute traction stresses, Ti(r), by setting the displacement values as the boundary conditions on cell surface. Maximum traction force, Tmax = max [Ti(r)], average force, and contractile moment, Mnet, were analyzed as previously described (44). Thirty to thirty-two cell images from n = 4 each of primary diabetic coronary microvascular VSMCs and controls were used for analyses.
RNA isolation and qPCR.
Normal and T2DM mouse CRM VSMCs were grown in 35-mm tissue culture plastic and grown to 80% confluence before total RNA was isolated with RiboZol RNA extraction reagent from AMRESCO and purified with an RNeasy Mini Kit from Qiagen. The RNA was then quantified with a nanodrop, reverse transcription PCR was performed to make CDNA, and qPCR was completed for specific targets.
Western blot analysis.
In separate experiments, human coronary VSMCs were washed twice with ice-cold PBS and then lysed with 90 µL of ice-cold lysate buffer (modified Hunter buffer freshly supplemented with 0.5 mM PMSF, 10 μg/mL aprotinin, 1 mM Na3VO4). Lysates were sonicated at 4°C for 10 s and then spun at 15,000 rpm at 4°C for 15 min. Lysates were then moved to a new microcentrifuge tube and frozen at −80°C until analysis. Protein was quantified according to the specified protocol with the BCA kit (Pierce). Ten micrograms of VSMC protein lysates (n = 4 to 5 per group) was separated on 8–12% SDS-PAGE gels that were transferred to PVDF membranes for 1 h at 4°C. The membranes were then blocked in 5% milk in PBS with 0.5% Tween 20 (PBST) at room temperature for 1 h. β1- and β3-integrin antibodies (Abcam) were diluted in 2.5% milk with PBST and incubated overnight at 4°C. Membranes were washed three times in PBST and then incubated in the appropriate horseradish peroxidase (HRP) secondary antibody for 1 h at room temperature. Again, membranes were washed three times in PBST and then imaged and analyzed with Bio-Rad Image Lab. β1- and β3-integrins were quantified on the same immunoblot; thus the same β-actin control bands were used for both as loading controls.
Statistical analysis.
All data are expressed as means ± SE, with a probability of P < 0.05 used to denote statistical significance with GraphPad Prism 8 (GraphPad Software, La Jolla, CA). A power calculation (90% power, α < 0.05), based on mean differences in coronary VSMC stiffness, indicated that n = 3 mice were needed per group. Student’s t test was performed on the stiffness, adhesion, PCR, and Western blot experiments. TFM data were analyzed with a Mann–Whitney nonparametric test.
RESULTS
Baseline mouse and human characteristics.
T2DM mouse and human data are presented in Table 1.
Table 1.
Mouse and human characteristics
| Normal/Control | Diabetic | P Value | |
|---|---|---|---|
| Mouse | |||
| Body weight, g | 32.5 ± 2.0 | 47.48 ± 3.7 | <0.0001 |
| Fasting blood glucose, mg/dL | 133.8 ± 18.8 | 616.6 ± 76.9 | <0.0001 |
| Human | |||
| Average age [age range], yr | 43.8 [30–57] | 53.5 [45–60] | 0.2703 |
| Sex, male/female | 2/3 | 2/2 |
Values are means ± SD [ranges].
Mouse VSMC stiffness.
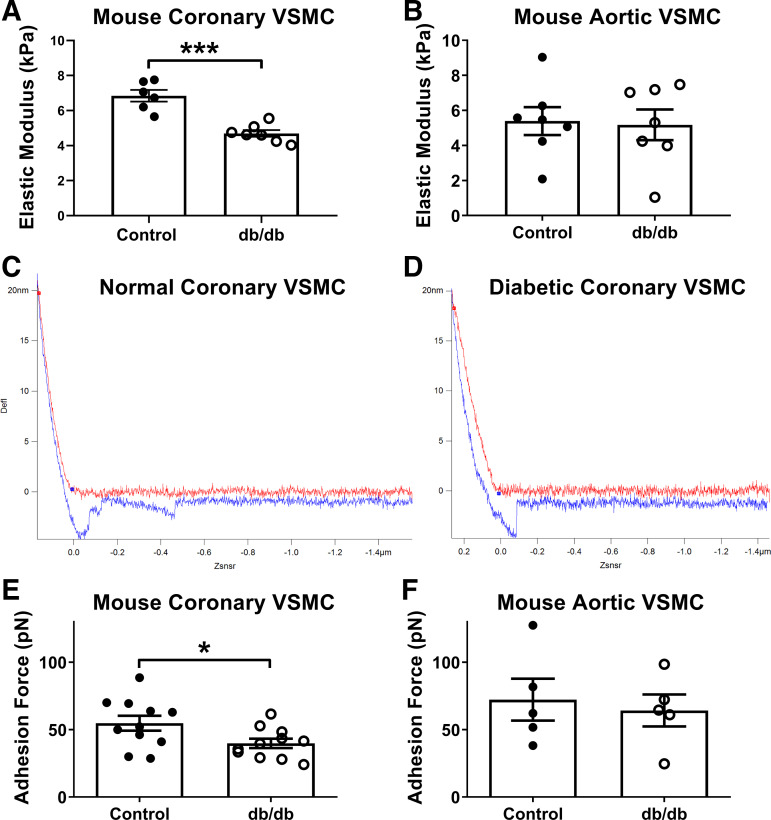
As shown in Fig. 1A, elastic modulus, a measurement of cellular stiffness, was reduced in coronary VSMCs from diabetic db/db mice compared with cells from control mice (Db/db 6.84 ± 0.34 kPa vs. db/db 4.70 ± 0.19 kPa, P < 0.0001). For comparison, there were no significant differences in elastic modulus between control and diabetic aortic VSMCs (Fig. 1B; Db/db 5.39 ± 0.79 kPa vs. db/db 5.18 ± 0.87 kPa, P = 0.861). Representative force curves are depicted in Fig. 1, C and D.
Fig. 1.
Diabetic mouse coronary vascular smooth muscle cells (VSMCs) are less stiff and less adhesive. A: elastic modulus, a measurement of cellular stiffness, was reduced in diabetic coronary VSMCs compared with normal. B: by comparison, there were no significant differences in elastic modulus between normal and diabetic aortic VSMCs. C: representative normal coronary VSMC force curve. D: representative diabetic coronary VSMC force curve. In C and D, red curve represents approach and blue curve represents retraction of the probe; therefore, x-axis is distance and y-axis is laser deflection. E: adhesive forces were reduced in diabetic coronary VSMCs compared with normal. F: by comparison, there were no significant differences in adhesive forces between normal and diabetic aortic VSMCs. n = 5–11 per group; *P < 0.05, ***P < 0.0001 vs. control. Statistical significance was assessed by unpaired Student’s t test.
To determine the potential impact of medium glucose levels on VSMC stiffness, we exposed coronary VSMCs from control Db/db mice to high-glucose medium (HG) and low-glucose medium (LG). The concentration of glucose in the medium did not affect the control VSMC stiffness (LG 6.64 ± 0.63 kPa vs. HG 6.21 ± 1.61 kPa, P = 0.71). We also exposed coronary VSMCs from db/db diabetic mice to both LG and HG conditions. The concentration of glucose in the medium did not affect the diabetic VSMC stiffness (LG 4.57 ± 0.39 kPa vs. HG: 4.14 ± 0.9 kPa, P = 0.158).
Mouse VSMC adhesion.
Adhesive forces to the ECM protein fibronectin were reduced in coronary VSMCs from db/db mice compared with cells from control mice (Fig. 1E; Db/db 54.78 ± 5.49 pN vs. db/db 39.8 ± 3.43 pN, P = 0.03). By comparison, there were no significant differences in adhesion between normal and diabetic aortic VSMCs (Fig. 1F).
We also assessed the role of glucose in coronary VSMC adhesion and determined that glucose concentration did not alter normal VSMC adhesion (LG 50.76 ± 17.45 pN vs. HG 39.51 ± 8.50 pN, P = 0.24), nor did it affect diabetic VSMC adhesion (LG 44.04 ± 10.91 pN vs. HG 43.72 ± 9.05 pN, P = 0.92)
Coronary VSMC traction force microscopy and topography.
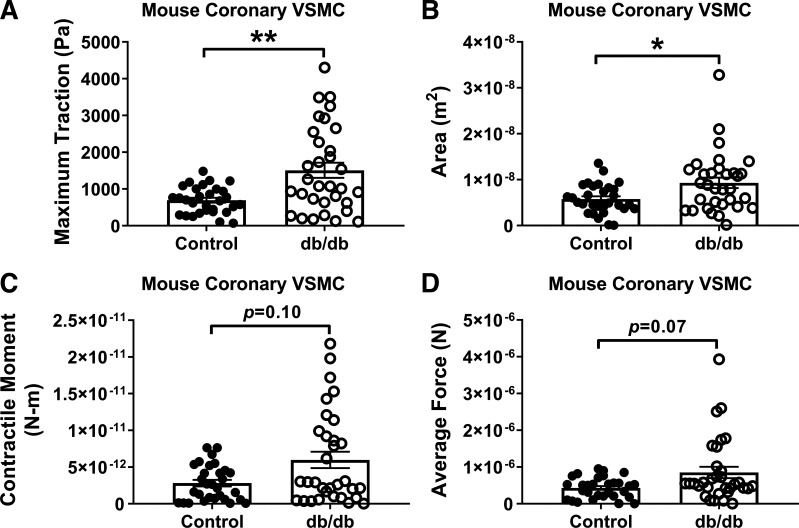
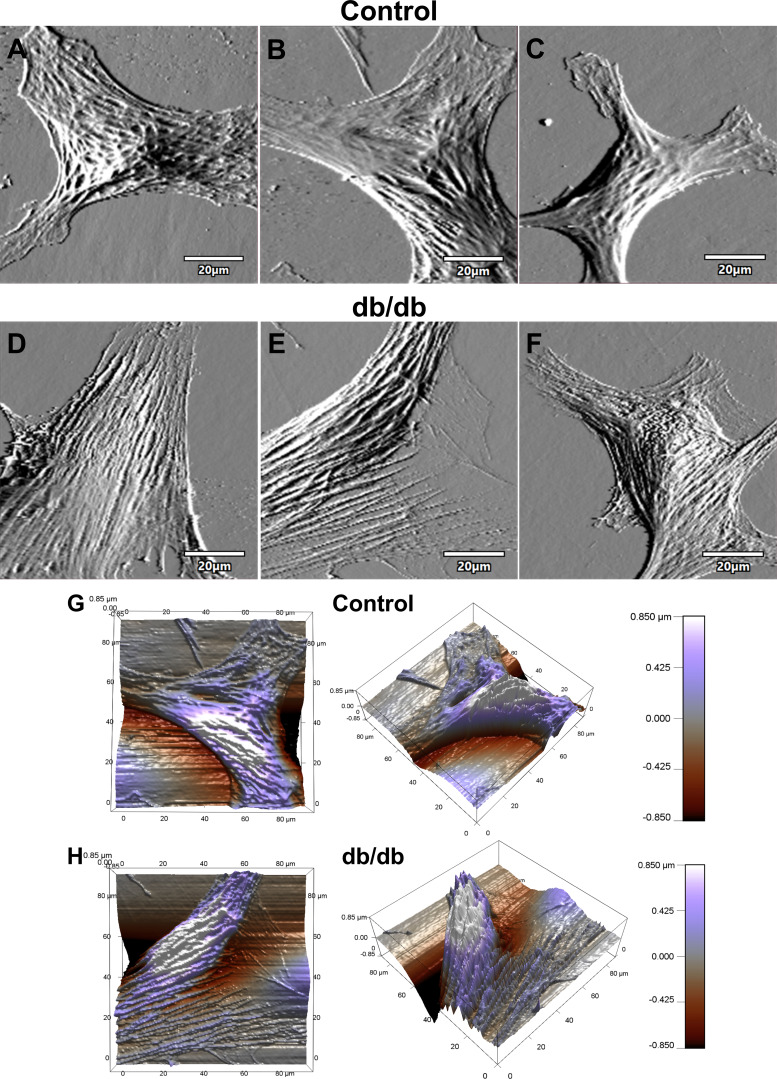
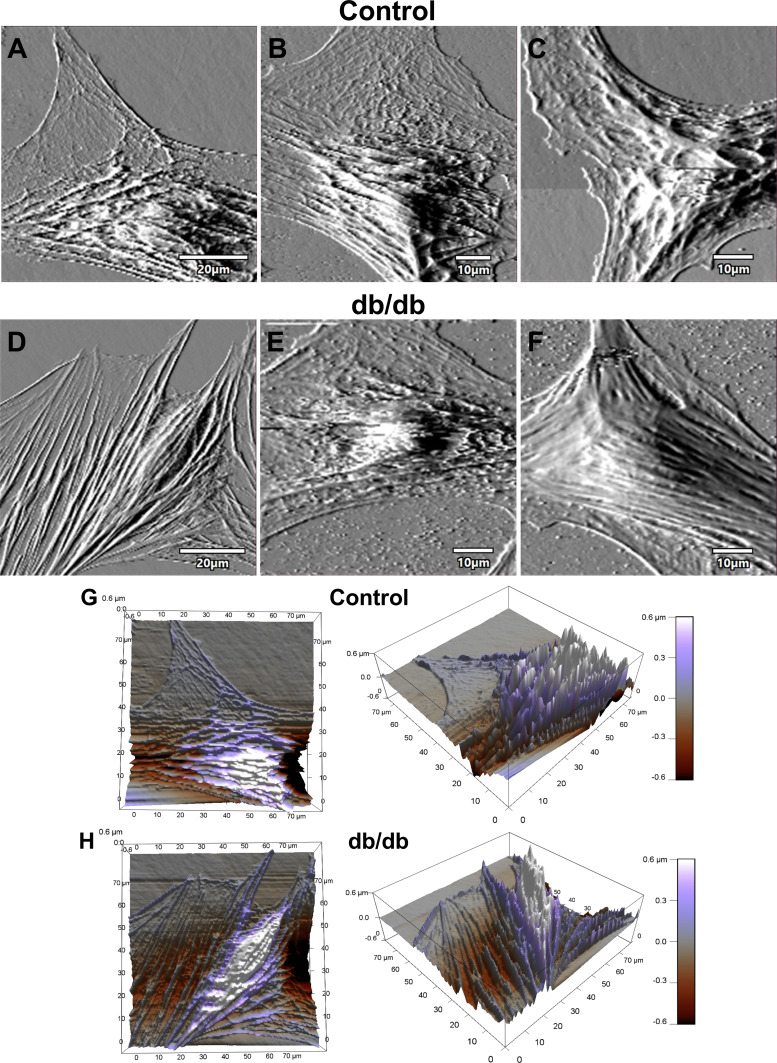
The mean maximum traction stress in coronary VSMCs from T2DM db/db mice was significantly larger than in controls (Fig. 2A; db/db 1,507 ± 207 Pa vs. control 692 ± 67 Pa, P < 0.01). The median spread area in diabetic coronary VSMCs was also significantly larger than in controls (Fig. 2B; db/db 9.3 × 10−9 ± 1.1 × 10−9 m2 vs. control 5.8 × 10−9 ± 0.6 × 10−9 m2, P = 0.03). The increases in average force and contractile moments of coronary VSMCs did not achieve statistical significance (Fig. 2, C and D). Atomic force microscopy was performed on normal and diabetic coronary VSMCs, and representative contact-mode images are shown in Fig. 3. The topographic images illustrate the overall cell shape, and these images support the differences in median spread area between normal and diabetic coronary VSMCs collected by traction force microscopy (Fig. 2B).
Fig. 2.
Traction force is increased in diabetic mouse coronary vascular smooth muscle cells (VSMCs). Traction force microscopy data revealed increased traction (A) and spread area (B) displayed by diabetic coronary VSMCs, and there were statistical trends for increased contractile moment (C) and average force (D). Area, spread area of the cells; average force, stress integrated over the spread area; contractile moment, measure of how polarized in one direction contractile forces are; maximum traction, contractile force generated by the cells. n = 30–32 cells from n = 4 cell isolations per group; *P < 0.05, **P < 0.01 vs. control. Data were analyzed with a Mann–Whitney nonparametric test.
Fig. 3.
Coronary vascular smooth muscle cell (VSMC) topography. A–F: representative contact-mode images of coronary VSMCs from control and diabetic mice: deflection images of normal control coronary VSMC (A–C) and diabetic coronary VSMC (D–F). G and H: representative 3-dimensional z-sensor images of normal control VSMC (G) and diabetic VSMC (H).
Aortic VSMC traction force microscopy and topography.
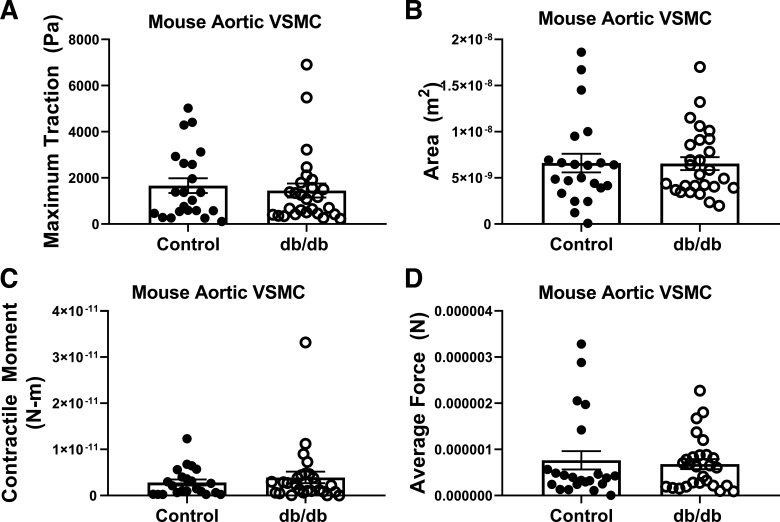
We did not observe any differences in intrinsic traction, force, contractile moment, or spread area between normal and diabetic aortic VSMCs (Fig. 4). Representative contact-mode images for normal and diabetic aortic VSMCs are depicted in Fig. 5.
Fig. 4.
Traction force is unaltered in diabetic mouse aortic vascular smooth muscle cells (VSMCs). In contrast to coronary VSMCs, aortic VSMCs from normal and type 2 diabetes mellitus mice displayed similar traction force behavior as evidenced by the lack of differences in maximum traction (A), spread area (B), contractile moment (C), or average force (D). n = 30–32 cells from n = 4 cell isolations per group; P = not significant vs. control. Data were analyzed with a Mann–Whitney nonparametric test.
Fig. 5.
Aortic vascular smooth muscle cell (VSMC) topography. A–F: representative contact-mode images of aortic VSMCs from normal and diabetic mice: deflection images of normal aortic VSMC (A–C) and diabetic aortic VSMC (D–F). G and H: representative 3-dimensional z-sensor images of normal VSMC (G) and diabetic VSMC (H).
Human coronary VSMC stiffness and adhesion.
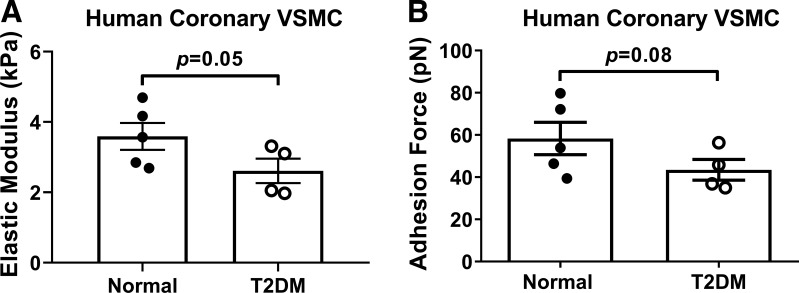
To confirm that our observation of reduced VSMC stiffness was not limited to a T2DM small animal model, we also undertook experiments in primary coronary VSMCs isolated from normal and T2DM humans. Similar to mice, we observed a reduction in elastic modulus and adhesion to fibronectin in diabetic coronary VSMCs compared with normal (Fig. 6A; elastic modulus: normal 3.59 ± 0.38 kPa vs. T2DM 2.61 ± 0.35 kPa, P = 0.05), although the reduction in adhesion force did not achieve statistical significance (Fig. 6B; normal 58.3 ± 7.65 pN vs. T2DM 43.45 ± 4.89 pN, P = 0.08).
Fig. 6.
Stiffness and adhesion forces are reduced in diabetic human coronary vascular smooth muscle cells (VSMCs). A: elastic modulus, a measurement of cellular stiffness, was reduced in diabetic coronary VSMCs compared with normal (P = 0.05). B: there was a trend for reduced adhesive forces in diabetic coronary VSMCs compared with normal (P = 0.08). n = 4 or 5 per group. Statistical significance was assessed by unpaired Student’s t test.
β-Integrins are differentially expressed in T2DM coronary VSMCs.
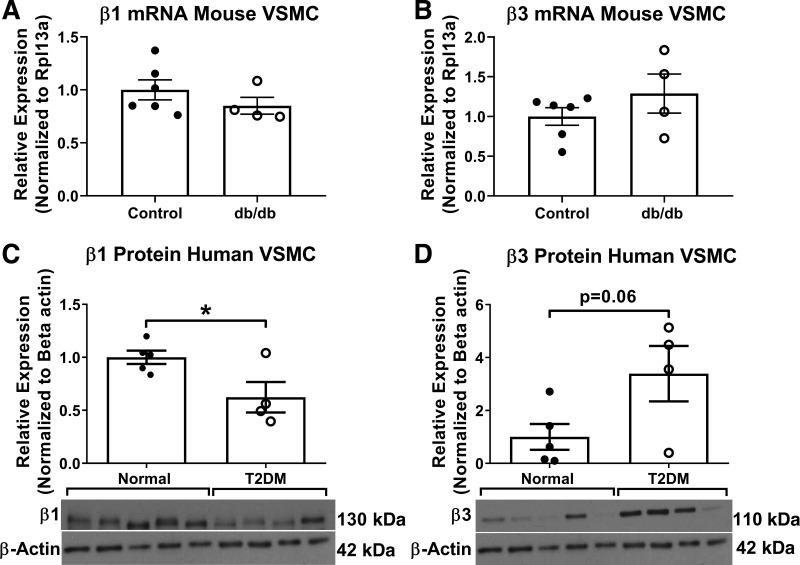
Next, we assessed β-integrin expression in T2DM mouse and human coronary VSMCs. We did not observe any differences in β1- or β3-integrin mRNA expression in coronary VSMCs isolated from Db/db and db/db mice (Fig. 7, A and B). Human coronary VSMC β1-integrin protein was significantly decreased whereas β3 was increased, although this increase did not reach significance (Fig. 7, C and D; normal 1.00 ± 0.06 vs. T2DM 0.62 ± 0.14, P = 0.04 and normal 1.00 ± 0.49 vs. T2DM 3.39 ± 1.05, P = 0.06, respectively).
Fig. 7.
β1-Integrin and β3-integrin expression are reduced and augmented, respectively, in type 2 diabetes mellitus. A and B: PCR analysis revealed that mouse coronary vascular smooth muscle cells (VSMCs) tend to express less β1-integrin (A) and elevated β3-integrin (B) compared with controls; however, this change does not reach statistical significance. C and D: immunoblot analysis of human coronary VSMC lysates confirmed the reduction in β1-integrin (C) and the increase in β3-integrin (D) protein levels compared with normal. n = 4–6 per group; *P < 0.05 vs. control. Statistical significance was assessed by unpaired Student’s t test.
DISCUSSION
T2DM is a well-known contributor to cardiovascular impairments and complications. Our laboratory has previously demonstrated (23, 31, 39) that T2DM leads to premature cardiovascular impairments including coronary microvessel inward remodeling associated with decreased stiffness and reduced coronary blood flow; we have recently (2) reported that reduced diabetic CRM stiffness does not appear to be due to gross changes in the ECM. In this study, we aimed to test the hypothesis that the decreased diabetic CRM stiffness is due to reduced VSMC stiffness and that a less stiff T2DM coronary VSMC could generate enhanced traction forces. To address this hypothesis, we used state-of-the-art atomic force microscopy and traction force microscopy. We showed here that diabetic coronary VSMCs have decreased stiffness, decreased adhesion to fibronectin, and increased traction force properties that, together, are mechanistically insightful in determining the underlying causes of altered mechanical and contractile properties of the intact diabetic CRM. Given previous computational data from Zielinski et al. (44) that demonstrated that less stiff cells had increased maximum traction force, it is intriguing to speculate that since diabetic cells are less stiff, their internal resistance to contraction is lessened, thereby allowing for enhanced traction force.
It is well established that T2DM increases macrovessel stiffness; however, our laboratory has previously reported in both the db/db mouse model and the Ossabaw MetS pig model that coronary resistance microvessels are less stiff (2, 23, 39). Several previous studies have reported that changes in ECM accompany changes in macrovessel stiffness (30, 35, 42). Indeed, we previously showed that changes in ECM also occur in T2DM coronary microvessels as they express elevated elastin mRNA (23, 39). We further reported that the ECM does not appear to be a primary cause of coronary microvascular stiffness in the db/db mouse model (2). Large conduit arteries (macrovessels) typically express more total ECM within the vessel wall to accommodate the higher pulse pressure created by the cardiac cycle. Therefore, the changes in ECM content and perhaps organization of macrovessels may contribute more to large vessel stiffness than VSMCs themselves, although, in the setting of hypertension VSMCs are more stiff concomitantly with accumulated ECM in a stiffer aorta (14, 46) and thus have been reported to play a role in large vessel stiffness. We contend that in microvessels, the contribution of cellular stiffness to whole tissue stiffness may be even more significant. To further investigate the contribution of coronary VSMC stiffness, we used atomic force microscopy to determine the elastic modulus of coronary VSMCs from control and db/db mice and from normal and T2DM humans. In both mice (Fig. 1) and humans (Fig. 6), we observed a decrease in elastic modulus in the diabetic coronary VSMCs. Our finding of a less stiff coronary resistance microvessel, combined with our present observations of reduced CRM VSMC stiffness, reflects different behavior reported to date in larger conduit blood vessels. In addition to changes in ECM in larger vessels, many studies have reported an increase in aortic VSMC and endothelial cell stiffness in western diet-fed animal models, and in many cases these were associated with increased VSMC stiffness (3, 11, 21, 29). Interestingly, Benech et al. (6) recently demonstrated that diabetes increases the stiffness of cardiomyocytes that may contribute to high diastolic left ventricular stiffness that is observed in T2DM patients. Our data in the coronary microcirculation, combined with other data in VSMCs from larger arteries and cardiomyocytes, suggest that cell stiffness and vascular wall stiffness are linked (32). This implication is further supported by our observed lack of difference in stiffness between normal and diabetic aortic VSMC, which agrees with our unpublished data that show similar passive stiffness and ECM content in normal and db/db aorta (“macrovessel”) from young adult (16 wk old) mice. We believe our time point is too early to observe the aortic ECM changes others have reported; however, our data implicate that the microvascular changes precede the aortic changes.
Under normal conditions, adult VSMCs function as contractile cells that produce and organize the surrounding ECM. In resistance microvessels, the contractile properties of VSMCs are arguably more important than in larger conduit arteries, as VSMCs of resistance microvessels work to regulate vascular resistance and blood flow related to organ perfusion. Myogenic tone is a form of pressure-induced VSMC constriction in the resistance vessels that is important for regulation of blood flow. There are conflicting reports in the literature as to how diabetes impacts myogenic tone. Lagaud et al. (26) reported increased myogenic tone in mesenteric arteries of db/db mice. It has also been reported that in both obese Zucker rats and streptozotocin-induced type 1 diabetic rats, myogenic tone was increased (12, 40). Increased myogenic tone was also observed in cerebral arteries from streptozotocin-induced type 1 diabetic rats (45). In a previous study, coronary myogenic tone trended upward but did not reach statistical significance in db/db mice (33). However, Kold-Petersen et al. (24) reported that isolated cerebral and coronary resistance arteries from Goto-Kakizaki (GK) T2DM rats have impaired myogenic tone. In a separate study, under passive conditions, arterioles from streptozotocin-induced type 1 diabetic rats appeared to be stiffer compared with similar arterioles from control animals, but under active conditions, i.e., in the presence of extracellular Ca2+, arterioles from the diabetic group showed impaired myogenic reactivity (16). To further highlight the fundamental differences in function and tissue specificity of VSMC contractility in microvessels compared with macrovessels, we report here no significant differences in traction force properties between control and diabetic aortic VSMCs (Fig. 4). The deleterious structural changes in CRMs that we previously reported were accompanied with a decrease in coronary blood flow (CBF) and coronary flow reserve (CFR) (23, 39). Collectively, data from our laboratory and many others suggest that reduced CBF in T2DM may be due to a combination of CRM remodeling, enhanced VSMC tone, and impaired function. It is intriguing to speculate that our observation of increased traction force (Fig. 2) and reduced cell stiffness (Fig. 1) in T2DM coronary VSMCs could be consistent with previous observations that the diabetic coronary microcirculation can express enhanced myogenic tone. Indeed, finite-element computational analyses have shown that reduced cell stiffness increases the apparent traction force generated by a cell, and our experimental data are in conceptual agreement with this notion. Decreased cell stiffness may provide less resistance to contraction and therefore allow for enhanced traction force. Moreover, the strength of integrin binding to the ECM at focal adhesions is known to alter the amount of force transmitted to the TFM substrate. Here, we report that diabetic coronary VSMCs are less adhesive to fibronectin yet display enhanced traction force. This may suggest that the enhanced traction force is not mediated by fibronectin per se but that other ECM substrates may contribute. How and whether stiffness modulates CBF and how the enhanced traction force and focal adhesion proteins specifically relate to myogenic tone are areas of further exploration.
In an attempt to elucidate possible mechanisms underlying the changes in CRM VSMC mechanical properties, we investigated integrins. Integrins are α- and β-heterodimeric plasma membrane-spanning receptors that function as key mechanosensors to detect mechanical signals and transduce the signal into cellular biochemical signals to alter cell behavior including stiffness, adhesion, and traction force (4, 13, 20, 27, 34, 41). The most predominant β receptors in VSMCs are β1 and β3. β1-Integrin, along with various corresponding α-integrins, binds to fibrillar and nonfibrillar collagens, fibronectin, and other ECM components (25). Typically, β3 dimerizes with αV and binds to fibronectin and other glycoproteins rather than the fibrillar collagens (25). Several studies have linked β1-integrin to increased traction force in various cell types, and blocking β1-integrin function reduces cellular traction force (4, 13, 27); however, no studies to date have related β1-integrin to traction force in coronary VSMCs. Therefore, we wanted to examine how diabetes impacts coronary VSMC β1 expression. Surprisingly, we observed a significant decrease in β1-integrin protein expression in diabetic coronary VSMCs (Fig. 7). A few studies have connected β1-integrin to cellular stiffness. Qiu et al. (34) showed that aortic VSMC stiffness increased with age, which may be due to increased β1-integrin expression. Moreover, β1-integrin induces focal adhesion formation and supports a force-dependent stiffening response (41). Interestingly, we demonstrate here a decrease in stiffness and an enhanced traction force in diabetic coronary VSMCs that have reduced β1-integrin expression (Figs. 1, 2, 6, and 7). It is well established that β1-integrin contributes to ECM adhesion in various cell types (20), including aortic VSMCs (8, 9), iliac VSMCs (22), and atrial VSMCs (43). We speculate that the reduced β1-integrin expression may be contributing to the decreased diabetic coronary VSMC stiffness and reduced adhesion to fibronectin.
In conclusion, these data demonstrate that primary diabetic coronary microvascular VSMCs are less stiff, display a larger spread area, and can retain enhanced traction forces compared with normal. We have previously reported that intact coronary microvessels are less stiff in diabetic models (2, 18, 23, 38, 39), which does not appear to be due to functional alterations in the ECM (2). The present study suggests that in the setting of diabetes coronary microvascular VSMC stiffness may be a novel contributor to overall tissue stiffness.
GRANTS
This work was supported by the National Institutes of Health Grant R00-HL-116769, R21-EB-026518, and S10-OD-023438 (to A.J.T.) and The Abigail Wexner Research Institute at Nationwide Children’s Hospital (to A.J.T.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.M., Y.C., Z.S., S.N.G., G.A.M., and A.J.T. conceived and designed research; P.M., Y.C., Z.S., S.N.G., and G.A.M. performed experiments; P.M., Y.C., Z.S., S.N.G., G.A.M., and A.J.T. analyzed data; P.M., Y.C., Z.S., S.N.G., G.A.M., and A.J.T. interpreted results of experiments; P.M. and A.J.T. prepared figures; P.M. and A.J.T. drafted manuscript; P.M., Y.C., Z.S., S.N.G., G.A.M., and A.J.T. edited and revised manuscript; P.M., Y.C., Z.S., S.N.G., G.A.M., and A.J.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Vasudha Shukla for help with traction force microscopy experiments.
REFERENCES
- 1.Akhtar R, Schwarzer N, Sherratt MJ, Watson RE, Graham HK, Trafford AW, Mummery PM, Derby B. Nanoindentation of histological specimens: mapping the elastic properties of soft tissues. J Mater Res 24: 638–646, 2009. doi: 10.1557/jmr.2009.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anghelescu M, Tonniges JR, Calomeni E, Shamhart PE, Agarwal G, Gooch KJ, Trask AJ. vascular mechanics in decellularized aortas and coronary resistance microvessels in type 2 diabetic db/db mice. Ann Biomed Eng 43: 2760–2770, 2015. doi: 10.1007/s10439-015-1333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aroor AR, Das NA, Carpenter AJ, Habibi J, Jia G, Ramirez-Perez FI, Martinez-Lemus L, Manrique-Acevedo CM, Hayden MR, Duta C, Nistala R, Mayoux E, Padilla J, Chandrasekar B, DeMarco VG. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol 17: 108, 2018. doi: 10.1186/s12933-018-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian L, Lo CM, Sham JS, Yip KP. Remanent cell traction force in renal vascular smooth muscle cells induced by integrin-mediated mechanotransduction. Am J Physiol Cell Physiol 304: C382–C391, 2013. doi: 10.1152/ajpcell.00234.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes 57: 1629–1637, 2008. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benech JC, Benech N, Zambrana AI, Rauschert I, Bervejillo V, Oddone N, Damián JP. Diabetes increases stiffness of live cardiomyocytes measured by atomic force microscopy nanoindentation. Am J Physiol Cell Physiol 307: C910–C919, 2014. doi: 10.1152/ajpcell.00192.2013. [DOI] [PubMed] [Google Scholar]
- 7.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ; American Heart Association; American Diabetes Association . Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 115: 114–126, 2007. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 8.Clyman RI, McDonald KA, Kramer RH. Integrin receptors on aortic smooth muscle cells mediate adhesion to fibronectin, laminin, and collagen. Circ Res 67: 175–186, 1990. doi: 10.1161/01.RES.67.1.175. [DOI] [PubMed] [Google Scholar]
- 9.Clyman RI, Turner DC, Kramer RH. An alpha1/beta1-like integrin receptor on rat aortic smooth muscle cells mediates adhesion to laminin and collagen types I and IV. Arteriosclerosis 10: 402–409, 1990. doi: 10.1161/01.ATV.10.3.402. [DOI] [PubMed] [Google Scholar]
- 10.Costa KD. Single-cell elastography: probing for disease with the atomic force microscope. Dis Markers 19: 139–154, 2004. doi: 10.1155/2004/482680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension 66: 99–107, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol 283: H2160–H2168, 2002. doi: 10.1152/ajpheart.00379.2002. [DOI] [PubMed] [Google Scholar]
- 13.Gershlak JR, Black LD 3rd. Beta1 integrin binding plays a role in the constant traction force generation in response to varying stiffness for cells grown on mature cardiac extracellular matrix. Exp Cell Res 330: 311–324, 2015. doi: 10.1016/j.yexcr.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Greenwald SE. Ageing of the conduit arteries. J Pathol 211: 157–172, 2007. doi: 10.1002/path.2101. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 100: 1134–1146, 1999. doi: 10.1161/01.CIR.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 16.Hill MA, Ege EA. Active and passive mechanical properties of isolated arterioles from STZ-induced diabetic rats. Effect of aminoguanidine treatment. Diabetes 43: 1450–1456, 1994. doi: 10.2337/diab.43.12.1450. [DOI] [PubMed] [Google Scholar]
- 17.Hong Z, Reeves KJ, Sun Z, Li Z, Brown NJ, Meininger GA. Vascular smooth muscle cell stiffness and adhesion to collagen I modified by vasoactive agonists. PLoS One 10: e0119533, 2015. doi: 10.1371/journal.pone.0119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husarek KE, Katz PS, Trask AJ, Galantowicz ML, Cismowski MJ, Lucchesi PA. The angiotensin receptor blocker losartan reduces coronary arteriole remodeling in type 2 diabetic mice. Vascul Pharmacol 76: 28–36, 2016. doi: 10.1016/j.vph.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husarek KE, Zhang X, McCallinhart PE, Lucchesi PA, Trask AJ. Isolation of murine coronary vascular smooth muscle cells. J Vis Exp 111: e53983, 2016. doi: 10.3791/53983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 21.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 118: 935–943, 2016. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kappert K, Schmidt G, Doerr G, Wollert-Wulf B, Fleck E, Graf K. Angiotensin II and PDGF-BB stimulate beta1-integrin-mediated adhesion and spreading in human VSMCs. Hypertension 35: 255–261, 2000. doi: 10.1161/01.HYP.35.1.255. [DOI] [PubMed] [Google Scholar]
- 23.Katz PS, Trask AJ, Souza-Smith FM, Hutchinson KR, Galantowicz ML, Lord KC, Stewart JA Jr, Cismowski MJ, Varner KJ, Lucchesi PA. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol 106: 1123–1134, 2011. doi: 10.1007/s00395-011-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kold-Petersen H, Brøndum E, Nilsson H, Flyvbjerg A, Aalkjaer C. Impaired myogenic tone in isolated cerebral and coronary resistance arteries from the Goto-Kakizaki rat model of type 2 diabetes. J Vasc Res 49: 267–278, 2012. doi: 10.1159/000335487. [DOI] [PubMed] [Google Scholar]
- 25.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev 97: 1555–1617, 2017. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 26.Lagaud GJ, Masih-Khan E, Kai S, van Breemen C, Dubé GP. Influence of type II diabetes on arterial tone and endothelial function in murine mesenteric resistance arteries. J Vasc Res 38: 578–589, 2001. doi: 10.1159/000051094. [DOI] [PubMed] [Google Scholar]
- 27.Lin GL, Cohen DM, Desai RA, Breckenridge MT, Gao L, Humphries MJ, Chen CS. Activation of beta1 but not beta3 integrin increases cell traction forces. FEBS Lett 587: 763–769, 2013. doi: 10.1016/j.febslet.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucchesi PA, Bell JM, Willis LS, Byron KL, Corson MA, Berk BC. Ca2+-dependent mitogen-activated protein kinase activation in spontaneously hypertensive rat vascular smooth muscle defines a hypertensive signal transduction phenotype. Circ Res 78: 962–970, 1996. doi: 10.1161/01.RES.78.6.962. [DOI] [PubMed] [Google Scholar]
- 29.Manrique C, Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Martinez-Lemus LA, Ramirez-Perez FI, Klein T, Meininger GA, DeMarco VG. Dipeptidyl peptidase-4 inhibition with linagliptin prevents western diet-induced vascular abnormalities in female mice. Cardiovasc Diabetol 15: 94, 2016. doi: 10.1186/s12933-016-0414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsh SA, Dell’Italia LJ, Chatham JC. Interaction of diet and diabetes on cardiovascular function in rats. Am J Physiol Heart Circ Physiol 296: H282–H292, 2009. doi: 10.1152/ajpheart.00421.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCallinhart PE, Sunyecz IL, Trask AJ. Coronary microvascular remodeling in type 2 diabetes: synonymous with early aging? Front Physiol 9: 1463, 2018. doi: 10.3389/fphys.2018.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meininger GA. The central importance of the cytoskeleton for increased cell stiffness in cardiovascular disease. Focus on “Diabetes increases stiffness of live cardiomyocytes measured by atomic force microscopy nanoindentation”. Am J Physiol Cell Physiol 307: C908–C909, 2014. doi: 10.1152/ajpcell.00279.2014. [DOI] [PubMed] [Google Scholar]
- 33.Moien-Afshari F, Ghosh S, Elmi S, Khazaei M, Rahman MM, Sallam N, Laher I. Exercise restores coronary vascular function independent of myogenic tone or hyperglycemic status in db/db mice. Am J Physiol Heart Circ Physiol 295: H1470–H1480, 2008. doi: 10.1152/ajpheart.00016.2008. [DOI] [PubMed] [Google Scholar]
- 34.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res 107: 615–619, 2010. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy GK. AGE-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res 68: 132–142, 2004. doi: 10.1016/j.mvr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw A, Doherty MK, Mutch NJ, MacRury SM, Megson IL. Endothelial cell oxidative stress in diabetes: a key driver of cardiovascular complications? Biochem Soc Trans 42: 928–933, 2014. doi: 10.1042/BST20140113. [DOI] [PubMed] [Google Scholar]
- 38.Trask AJ, Delbin MA, Katz PS, Zanesco A, Lucchesi PA. Differential coronary resistance microvessel remodeling between type 1 and type 2 diabetic mice: impact of exercise training. Vascul Pharmacol 57: 187–193, 2012. doi: 10.1016/j.vph.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trask AJ, Katz PS, Kelly AP, Galantowicz ML, Cismowski MJ, West TA, Neeb ZP, Berwick ZC, Goodwill AG, Alloosh M, Tune JD, Sturek M, Lucchesi PA. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J Appl Physiol (1985) 113: 1128–1140, 2012. doi: 10.1152/japplphysiol.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ungvari Z, Pacher P, Kecskemeti V, Papp G, Szollár L, Koller A. Increased myogenic tone in skeletal muscle arterioles of diabetic rats. Possible role of increased activity of smooth muscle Ca2+ channels and protein kinase C. Cardiovasc Res 43: 1018–1028, 1999. doi: 10.1016/S0008-6363(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 41.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124–1127, 1993. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 42.Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A, Lévy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA 95: 4630–4634, 1998. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto K, Yamamoto M. Cell adhesion receptors for native and denatured type I collagens and fibronectin in rabbit arterial smooth muscle cells in culture. Exp Cell Res 214: 258–263, 1994. doi: 10.1006/excr.1994.1256. [DOI] [PubMed] [Google Scholar]
- 44.Zielinski R, Mihai C, Kniss D, Ghadiali SN. Finite element analysis of traction force microscopy: influence of cell mechanics, adhesion, and morphology. J Biomech Eng 135: 071009, 2013. doi: 10.1115/1.4024467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res 81: 996–1004, 1997. doi: 10.1161/01.RES.81.6.996. [DOI] [PubMed] [Google Scholar]
- 46.Zulliger MA, Fridez P, Hayashi K, Stergiopulos N. A strain energy function for arteries accounting for wall composition and structure. J Biomech 37: 989–1000, 2004. doi: 10.1016/j.jbiomech.2003.11.026. [DOI] [PubMed] [Google Scholar]