Abstract
Recently, we reported that obese Dahl salt-sensitive (SS) leptin receptor mutant (SSLepRmutant) rats display progressive renal injury. The present study demonstrated that the early development of renal injury in the SSLepRmutant strain is associated with an increase in the renal infiltration of macrophages compared with lean SS rats. We also examined whether depletion of macrophages with clodronate would reduce the early progression of renal injury in the SSLepRmutant strain. Four-week-old SS and SSLepRmutant rats were treated with either vehicle (PBS) or clodronate (50 mg/kg ip, 2 times/wk) for 4 wk. While the administration of clodronate did not reduce renal macrophage infiltration in SS rats, clodronate decreased macrophages in the kidneys of SSLepRmutant rats by >50%. Interestingly, clodronate significantly reduced plasma glucose, insulin, and triglyceride levels and markedly improved glucose tolerance in SSLepRmutant rats. Treatment with clodronate had no effect on the progression of proteinuria or renal histopathology in SS rats. In the SSLepRmutant strain, proteinuria was markedly reduced during the first 2 wk of treatment (159 ± 32 vs. 303 ± 52 mg/day, respectively). However, after 4 wk of treatment, the effect of clodronate was no longer observed in the SSLepRmutant strain (346 ± 195 vs. 399 ± 50 mg/day, respectively). The kidneys from SSLepRmutant rats displayed glomerular injury with increased mesangial expansion and renal fibrosis versus SS rats. Treatment with clodronate significantly decreased glomerular injury and renal fibrosis in the SSLepRmutant strain. Overall, these data indicate that the depletion of macrophages improves metabolic disease and slows the early progression of renal injury in SSLepRmutant rats.
Keywords: clodronate, macrophages, obesity, renal disease, Dahl salt-sensitive leptin receptor mutant strain, salt-sensitive rat
INTRODUCTION
Obesity has become a growing epidemic worldwide and is considered a major risk factor for developing other cardiovascular diseases, including type 2 diabetes, hypertension, and metabolic syndrome, which all contribute to the development of renal disease (12). While the increasing trend of obesity has been observed in adults, the prevalence of childhood obesity has increased dramatically over the last few decades as well (11, 24). Recent studies have also suggested that childhood obesity is associated with increased microalbuminuria, a risk factor of renal injury (3). Moreover, obesity in the absence of hyperglycemia or hypertension has been found to predict the development of chronic kidney disease later in life in adults (10, 23). These data suggest that renal dysfunction may start long before the appearance of hyperglycemia or hypertension in patients with obesity. Recently, we reported that, before puberty, the Dahl salt-sensitive (SS) leptin receptor mutant (SSLepRmutant) strain exhibits progressive proteinuria and glomerular injury as early as 6 wk of age independent of hyperglycemia and elevations in arterial pressure (23). Additionally, this early progression of renal injury is associated with renal hyperfiltration (22). However, the mechanisms that may be involved in the early progression of renal injury in the SSLepRmutant strain remain unknown.
Over the last decade, immune mechanisms have been recognized to play important roles in the development of various cardiovascular diseases, including renal injury, in humans and animals (20, 28). Patients suffering from diabetes- and/or hypertension-induced renal disease have some degree of infiltration of macrophages and T lymphocytes (T cells) into the kidney (20, 34, 35). Macrophages have been demonstrated to be critically involved in the pathogenesis of renal injury, repair, and fibrosis in different experimental models of renal disease (1, 4). Recently, Fehrenbach et al. (8) observed that the development of hypertension-induced renal injury in SS rats is associated with the renal infiltration of macrophages. Moreover, previous studies have demonstrated that inhibition of renal macrophage infiltration reduces arterial pressure and prevents glomerular injury and fibrosis in various animal models of nephropathy (14, 16, 32). However, to our knowledge, the impact of macrophage depletion during renal injury associated with obesity in the absence of hyperglycemia and elevations in arterial pressure has not been studied. Therefore, the present study examined the impact of macrophage depletion with clodronate on the early progression of renal injury associated with obesity in the SSLepRmutant strain.
METHODS
General
Experiments were performed on 97 male and female wild-type Dahl SS (SSWT) and SSLepRmutant rats at 4–8 wk of age. Genotyping was performed by the Molecular and Genomics Facility at the University of Mississippi Medical Center. Rats had free access to food and water throughout the study. Rats were fed a 1% NaCl diet (TD8640, Harlan Laboratories, Madison, WI) to minimize the development of hypertension. Rats were housed in the Laboratory Animal Facility at the University of Mississippi Medical Center, which was approved by the American Association for the Accreditation of Laboratory Animal Care, and all protocols were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Protocols
Protocol 1: comparison of renal macrophage infiltration between male and female SWT and SSLepRmutant rats.
At 8 wk of age, male and female SWT and SSLepRmutant rats were anesthetized with 2% isoflurane, and the abdominal cavity was opened to perfuse both kidneys by placing a clamp above the right renal artery. Next, a 21-gauge needle was inserted into the abdominal aorta below both renal arteries, in which saline was injected to perfuse the kidneys. Once the kidneys visibly appeared pale, they were collected and rinsed with 1× PBS at 4°C several times. The kidneys were minced in RPMI-1640 containing 0.1% collagenase and 10 µg/ml DNAse I and were later grounded to make a homogenous solution using a tissue homogenizer. Next, the homogenized solution was filtered through a 100-µm filter and incubated for 30 min at 37°C. The kidney homogenate was then filtered through a 70-µm cell strainer and washed with FACS washing buffer (1× Dulbecco’s PBS without Ca2+ and Mg2+ with 2% FBS and 2 mM EDTA) at 4°C and centrifuged at 4°C for 10 min at 300 g. The pellet was resuspended in 5 mL washing buffer and filtered through a 40-µm cell strainer. After centrifugation at 300 g for 5 min, the pellet was resuspended in 3 mL of 30% Percoll (Sigma, Life Science, prepared in RPMI-1640) at room temperature, layered over 3 mL of 70% Percoll (Sigma, Life Science, prepared in 1× PBS), and centrifuged at 25°C at 1,200 rpm with no breaks for 30 min. The mononuclear cell layer resting above the 70% Percoll was removed, washed with FACS washing buffer, and centrifuged at 4°C at 1,600 rpm for 10 min. The pellet was resuspended in 1 mL FACS buffer, and cells were counted on an Automated Cell Counter and Image Cytometer (Nexcelom Bioscience). Single cell suspensions (1 × 106 cells) were stained for flow cytometry without exclusion of dead cells. Antibodies used for flow cytometry were as follows: recombinant phycoerythrin anti-rat CD68 (Miltenyi Biotec, Auburn, CA). Flow cytometry was performed on the Miltenyi MACSQuant Analyzer 10 (Miltenyi Biotec, Auburn, CA), and data were analyzed using FlowLogic software (Innovai, Sydney, NSW, Australia). Lymphocytes were gated in the side scatter and forward scatter plot. After doublet exclusion, the CD68+ gate was set using an unstained control. CD68+ cells were identified as macrophages.
Protocol 2: comparison of the time course of changes in renal macrophage infiltration in SSWT and SSLepRmutant rats.
Infiltration of macrophages was measured in the kidneys by flow cytometry in male and female SSWT and SSLepRmutant rats at 4 and 8 wk of age. The isolation and measurement of renal macrophage infiltration via flow cytometry were performed as described above in protocol 1. Since we did not observe any differences in the infiltration of macrophages in the kidneys between male and female SSWT and SSLepRmutant rats, we decided to combine male and female rats in each strain together for the rest of the study.
Protocol 3: effects of treatment with clodronate on the early development of renal injury in SSWT and SSLepRmutant rats.
Experiments were performed on 4-wk-old SSWT and SSLepRmutant rats. Rats were weighed and placed in metabolic cages for an overnight urine collection to determine proteinuria using the Bradford method (Bio-Rad Laboratories, Hercules, CA), and a blood sample was collected from the tail vein for the measurement of blood glucose levels (glucometer from Bayer HealthCare, Mishawaka, IN). After the collection of baseline data, SSWT and SSLepRmutant rats were randomly separated into the following four groups: SSWT rats treated with vehicle (PBS-lipsomes), 2) SSLepRmutant rats treated with vehicle (PBS-lipsomes), 3) SSWT rats treated with liposomal clodronate (50 mg/kg ip, 2 times/wk) for 4 wk, and 4) SSLepRmutant rats treated with liposomal clodronate (50 mg/kg ip, 2 times/wk) for 4 wk. Every 2 wk, rats were placed in metabolic cages and proteinuria and blood glucose levels were measured at each time period. During the last week of the study, rats were subjected to an intraperitoneal glucose tolerance test (IPGTT). During the performance of the IPGTT, rats were fasted overnight, and, on the next day, a baseline of blood glucose was taken. Rats were then injected with a solution containing glucose at a dose of 2 g/kg (ip), and blood samples (5–10 μL) were collected from the tail vein at 15, 30, 60, 90, and 120 min to measure glucose. At the end of the study, rats were placed under anesthesia, and a catheter was inserted in the femoral artery for the measurement of mean arterial pressure (MAP). After a 24-h recovery period, catheters were connected to pressure transducers (MLT0699, ADInstruments, Colorado Springs, CO) coupled to a computerized PowerLab data-acquisition system (ADInstruments). MAP was recorded continuously for 30 min after a 30‐min equilibration period. After arterial pressure measurements, a final blood sample was taken from the abdominal aorta to measure plasma cholesterol (Cayman Chemical, Ann Arbor, MI), triglyceride (Cayman Chemical), and insulin (Mercodia Rat Insulin ELISA, Uppsala, Sweden) concentrations, and kidneys were collected as previously described above. Both kidneys were weighed. The right kidney was cut in half; one half was fixed in 10% buffered formalin solution for histology, and the other half was snapped frozen in liquid nitrogen and stored at −80°C. Renal cytokines were measured using the Bio-Plex Pro Rat Cytokine 23-Plex Assay Reagent Kit on a Bio-Rad Bioplex 200 System according to the manufacturer’s protocol (Bio-Rad Laboratories), in which we only reported the cytokines that were either significantly different between the SSWT and SSLepRmutant strains or with clodronate treatment. The left kidney was used to measure the infiltration of macrophages by flow cytometry analysis as previously described above.
Renal Histopathology
Paraffin kidney sections were prepared from half of the kidneys collected from SSWT and SSLepRmutant rats at each time point. Kidney sections were cut into 3-µm sections and stained with periodic acid-Schiff and Masson’s trichrome. Thirty glomeruli per periodic acid-Schiff-stained section were scored in a blinded fashion to determine glomerular injury by scoring in a blinded fashion on a scale of 0–4 with 0 representing a normal glomerulus, 1 representing a 25% loss, 2 representing a 50% loss, 3 representing a 75% loss, and 4 representing a >75% loss of capillaries in the tuft (23, 30). To determine the degree of renal fibrosis, 10 representative images per section from each animal were captured using a Nikon Eclipse 55i microscope equipped with a Nikon DS-Fi1 color camera (Nikon, Melville, NY). We analyzed for the percentage of the image stained blue (primarily collagen) by identifying which animal had the most collagen and thresholded for the blue staining in the Masson's trichrome-stained sections using NIS-Elements D 3.0 software (23, 30). Next, we used those same thresholding parameters for the blue staining for each image per rat in the study to measure renal fibrosis.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA). The significance of the difference in mean values for a single time point was determined by one-way ANOVA followed by a Tukey’s multiple-comparisons test. Temporal changes in renal and cardiovascular parameters were compared between and within strains using two-way ANOVA followed by a Holm-Sidak test. P values of <0.05 were considered significantly different. Data are presented as means ± SE.
RESULTS
Protocol 1: Measurement of Renal Macrophage Infiltration in SSWT and SSLepRmutant Rats
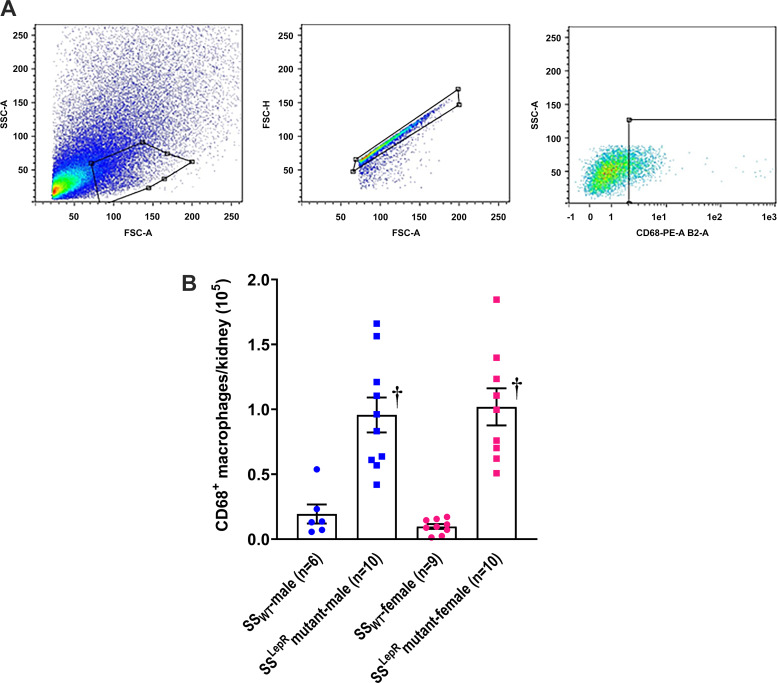
The flow cytometry gating strategy and sex differences in renal macrophage infiltration in SSWT and SSLepRmutant rats at 8 wk of age are shown in Fig. 1. After gating for the general cell population using side scatter and forward scatter, those that were singlets and CD68+ cells were used to gate for macrophages (Fig. 1A). We did not observe any sex differences in the infiltration of macrophages in both strains (Fig. 1B). However, renal macrophage infiltration was more than three-fold higher in male and female SSLepRmutant rats compared with values measured in male and female SSWT rats. Furthermore, the difference in macrophage infiltration between the two strains was not due to an increase in kidney weight in the SSLepRmutant strain. Kidney weights between lean SSWT and obese SSLepRmutant rats were not significantly different (2.41 ± 0.07 and 2.48 ± 0.05 g, respectively).
Fig. 1.
Measurement of renal macrophage infiltration. A and B: flow cytometry gating strategy (A) and sex differences in renal macrophage infiltration (B) in wild-type Dahl salt-sensitive (SSWT) rats and obese Dahl salt-sensitive leptin receptor mutant (SSLepRmutant) rats at 8 wk of age. Numbers in parentheses indicate the number of rats studied per group. Values are means ± SE. The significance of difference in mean values for a single time point was determined by one-way ANOVA followed by a Tukey’s multiple-comparisons test. P values of <0.05 were considered significantly different. †Significant difference from the corresponding value in SSWT rats within the same sex. SSC, side scatter; FSC, forward scatter; PE, phycoerythrin.
Protocol 2: Time-Course Changes in Renal Macrophage Infiltration in SSWT and SSLepRmutant Rats
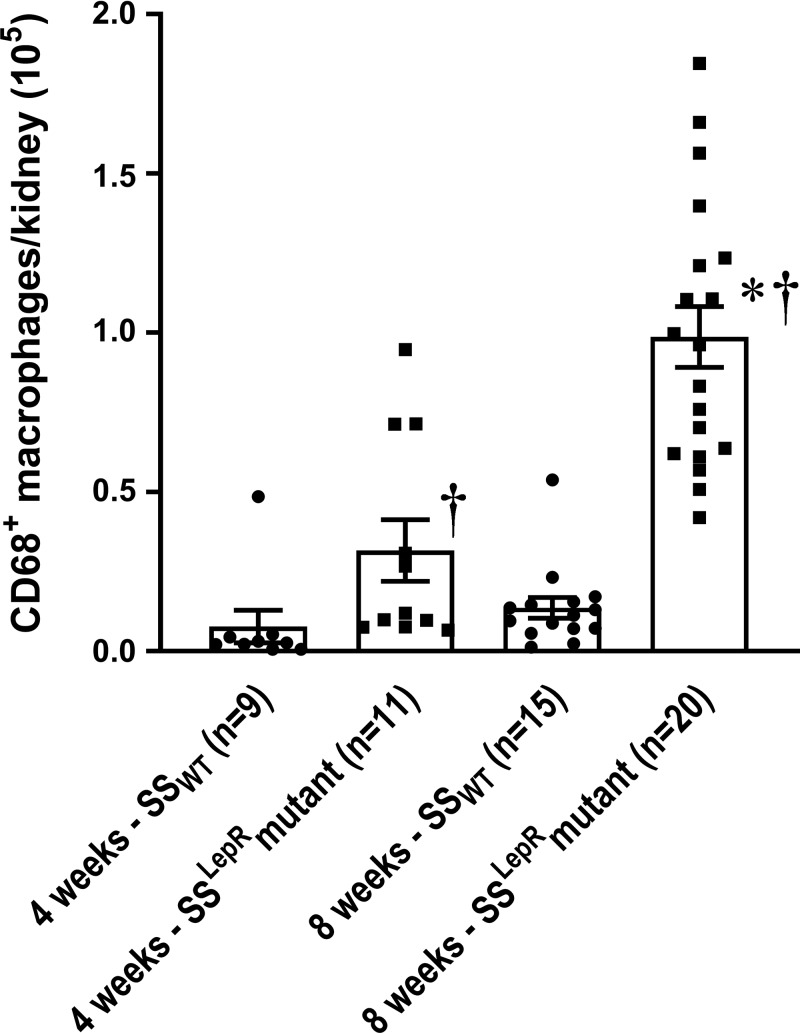
The time-course changes in renal macrophage infiltration in SSWT and SSLepRmutant rats are shown in Fig. 2. Renal macrophage infiltration was more than threefold higher in SSLepRmutant rats compared with values measured in SSWT rats at 4 wk of age, which was further increased by 8 wk of age.
Fig. 2.
Comparison of time-course changes in renal macrophage infiltration in wild-type Dahl salt-sensitive (SSWT) rats and obese Dahl salt-sensitive leptin receptor mutant (SSLepRmutant) rats. Numbers in parentheses indicate the number of rats studied per group. Values are means ± SE. The significance of difference in mean values for a single time point was determined by one-way ANOVA followed by a Tukey’s multiple-comparisons test. P values of <0.05 were considered significantly different. *Significant difference from the corresponding value within the same strain at baseline; †significant difference from the corresponding value in SSWT rats within the same weeks of age.
Protocol 3: Effects of Clodronate on Renal Macrophage Infiltration and the Early Progression of Renal Injury in SSWT and SSLepRmutant Rats
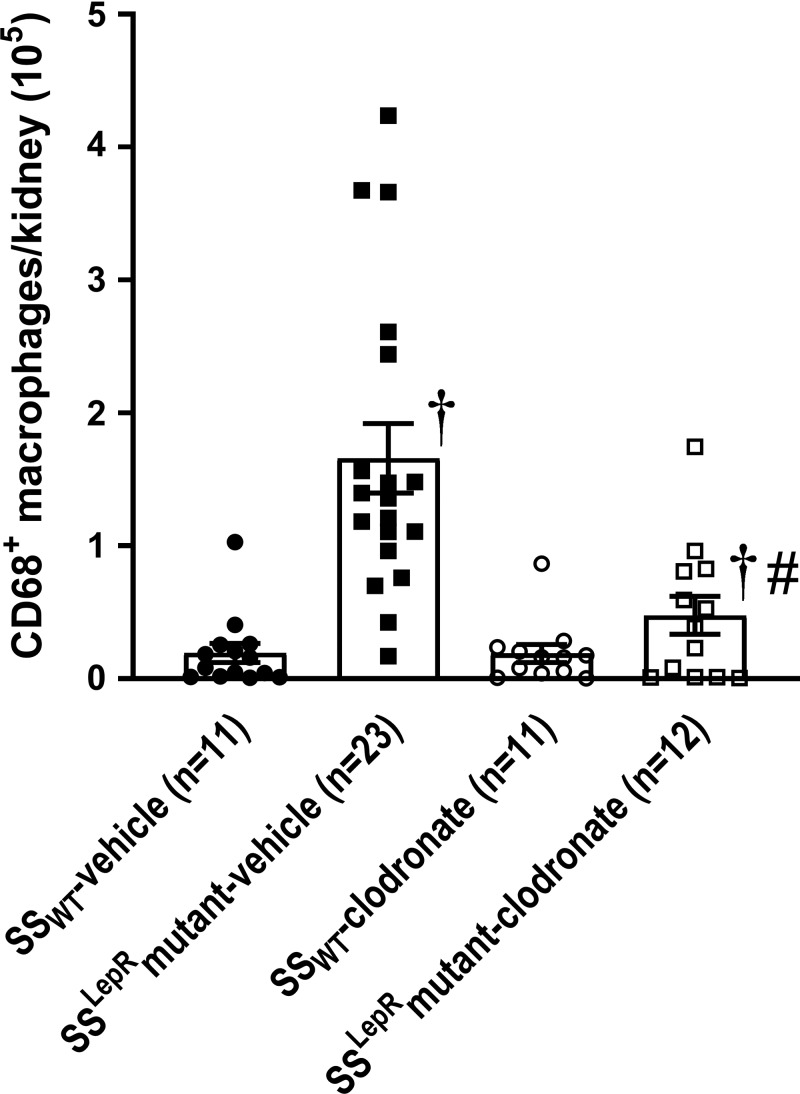
The effects of chronic treatment of clodronate on renal macrophage infiltration in SSWT and SSLepRmutant strains are shown in Fig. 3. Similar to protocol 1, we observed a significant increase in macrophage infiltration in the kidneys of SSLepRmutant rats compared with values measured in SSWT rats. While chronic treatment with clodronate did not decrease renal macrophage infiltration in SSWT rats, it markedly reduced the infiltration of macrophages by >60% in SSLepRmutant rats without affecting kidney weight (2.58 ± 0.14 and 2.26 ± 0.14 g, respectively).
Fig. 3.
Efficacy of clodronate treatment on renal macrophage infiltration in wild-type Dahl salt-sensitive (SSWT) rats and obese Dahl salt-sensitive leptin receptor mutant (SSLepRmutant) rats. Numbers in parentheses indicate the number of rats studied per group. Values are means ± SE. The significance of difference in mean values for a single time point was determined by one-way ANOVA followed by a Tukey’s multiple-comparisons test. P values of <0.05 were considered significantly different. †Significant difference from the corresponding value in SSWT rats within the same treatment; #significant difference from the corresponding value in vehicle-treated rats within the same strain.
Measurement of Metabolic Parameters
The effects of chronic clodronate treatment on metabolic parameters, including body weight and glucose tolerance (IPGTT), and the levels of glucose, insulin, cholesterol, and triglycerides in SSWT and SSLepRmutant rats are shown in Fig. 4. We observed a significant increase in body weight in SSLepRmutant rats compared with SSWT rats, and body weight remained higher throughout the study (Fig. 4A). Treatment with clodronate decreased body weight in SSWT rats but not in SSLepRmutant rats. Blood glucose levels were similar between SSWT and SSLepRmutant rats during the entire study (Fig. 4B). After 4 wk of clodronate treatment, blood glucose levels were only reduced in the SSLepRmutant strain. As shown in Fig. 4C, SSLepRmutant rats displayed impaired glucose tolerance versus their control counterparts, SSWT rats, and chronic treatment with clodronate significantly improved glucose tolerance in SSLepRmutant rats. Insulin levels were significantly higher in SSLepRmutant rats compared with SSWT rats (9.9 ± 0.4 vs. 0.8 ± 0.3 ng/dL, respectively; Fig. 4D). Treatment with clodronate markedly decreased insulin levels in SSLepRmutant rats (3.9 ± 1.2 ng/dL) but not in SSWT rats (0.5 ± 0.1 ng/dL). Total cholesterol levels were significantly higher in SSLepRmutant rats compared with values measured in SSWT rats (208 ± 15 vs. 89 ± 5 mg/dL, respectively), and clodronate treatment did not have an effect in either strain (Fig. 4E). Plasma triglyceride levels were more than six-fold higher in SSLepRmutant rats compared with SSWT rats, and treatment with clodronate prevented the increase in triglyceride levels only in SSLepRmutant rats (Fig. 4F).
Fig. 4.
A−F: comparison of metabolic parameters in wild-type Dahl salt-sensitive (SSWT) rats and obese Dahl salt-sensitive leptin receptor mutant (SSLepRmutant) rats treated clodronate: body weight (A), blood glucose (B), glucose tolerance (C), plasma insulin (D), plasma cholesterol (E), and plasma triglyceride (F). Numbers in parentheses indicate the number of rats studied per group. Values are means ± SE. Temporal changes in renal and cardiovascular parameters were compared between and within strains using two-way ANOVA followed by a Holm-Sidak test. The significance of difference in mean values for a single time point was determined by one-way ANOVA followed by a Tukey’s multiple-comparisons test. P values of <0.05 were considered significantly different. *Significant difference from the corresponding value within the same strain at baseline; †significant difference from the corresponding value in SSWT rats within the same treatment; #significant difference from the corresponding value in vehicle-treated rats within the same strain.
Measurement of MAP and Time-Course Changes in Proteinuria
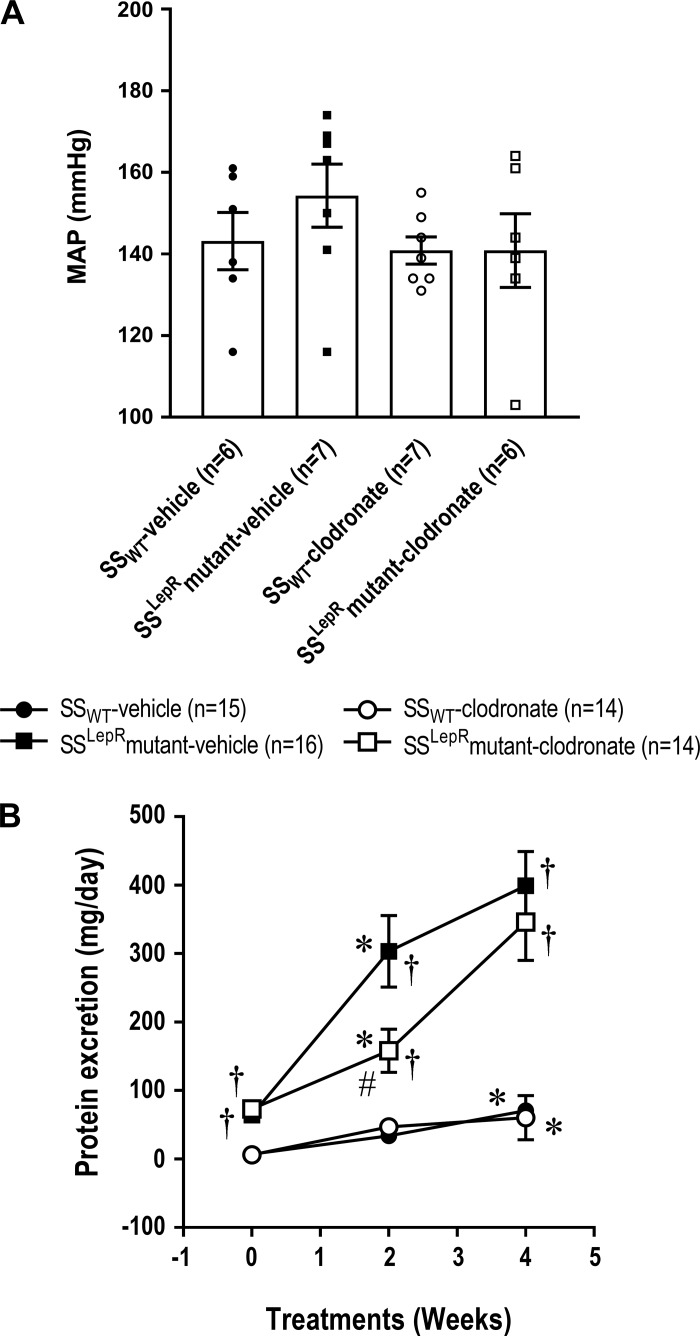
Measurement of MAP and time-course changes in proteinuria in SSWT and SSLepRmutant rats are shown in Fig. 5. We did not observe any differences in MAP between both strains, and treatment with clodronate did not have any effect on MAP in either strain (Fig. 5A). Proteinuria was markedly reduced in the SSLepRmutant strain during the first 2 wk of treatment (159 ± 32 vs. 303 ± 52 mg/day, respectively; Fig. 5B). However, after 4 wk of treatment, the effect of clodronate was no longer observed in the SSLepRmutant strain (346 ± 195 vs. 399 ± 50 mg/day, respectively).
Fig. 5.
Effects of clodronate treatment on mean arterial pressure (MAP; A) and proteinuria (B) in wild-type Dahl salt-sensitive (SSWT) rats and obese Dahl salt-sensitive leptin receptor mutant (SSLepRmutant) rats. Numbers in parentheses indicate the number of rats studied per group. Values are means ± SE. Temporal changes in renal and cardiovascular parameters were compared between and within strains using two-way ANOVA followed by a Holm-Sidak test. The significance of difference in mean values for a single time point was determined by one-way ANOVA followed by a Tukey’s multiple-comparisons test. P values of <0.05 were considered significantly different. *Significant difference from the corresponding value within the same strain at baseline; †significant difference from the corresponding value in SSWT rats within the same treatment; #significant difference from the corresponding value in vehicle-treated rats within the same strain.
Assessment of Renal Histopathology
Representative images and a corresponding analysis of the renal pathology in SSWT and SSLepRmutant rats treated with clodronate are shown in Fig. 6. The kidneys from SSLepRmutant rats exhibited increased mesangial expansion and severe glomerulosclerosis compared with their SSWT littermates (Fig. 6A), and treatment with clodronate significantly decreased glomerular injury in SSLepRmutant rats (Fig. 6C). Increased renal fibrosis (percent blue staining) was detected in SSLepRmutant rats versus SSWT rats (Fig. 6, B and D). Chronic treatment with clodronate reduced renal fibrosis in the SSLepRmutant strain by 15%.
Fig. 6.
A and B: representative images of renal histopathology: periodic acid-Schiff staining (A) and Masson’s trichrome staining (B). C and D: effects of clodronate treatment on glomerular injury (C) and renal fibrosis (percent blue staining; D) in wild-type Dahl salt-sensitive (SSWT) rats and obese Dahl salt-sensitive leptin receptor mutant (SSLepRmutant) rats. For glomerular injury, the numbers in parentheses indicate the number of glomeruli/rats studied per group; for renal fibrosis, the numbers in parentheses indicate either the number of images/rats studied per group. Values are means ± SE. The significance of difference in mean values for a single time point was determined by one-way ANOVA followed by a Tukey’s multiple-comparisons test. P values of <0.05 were considered significantly different. †Significant difference from the corresponding value in SSWT rats within the same treatment; #significant difference from the corresponding value in vehicle-treated rats within the same strain.
Measurement of Renal Cytokines
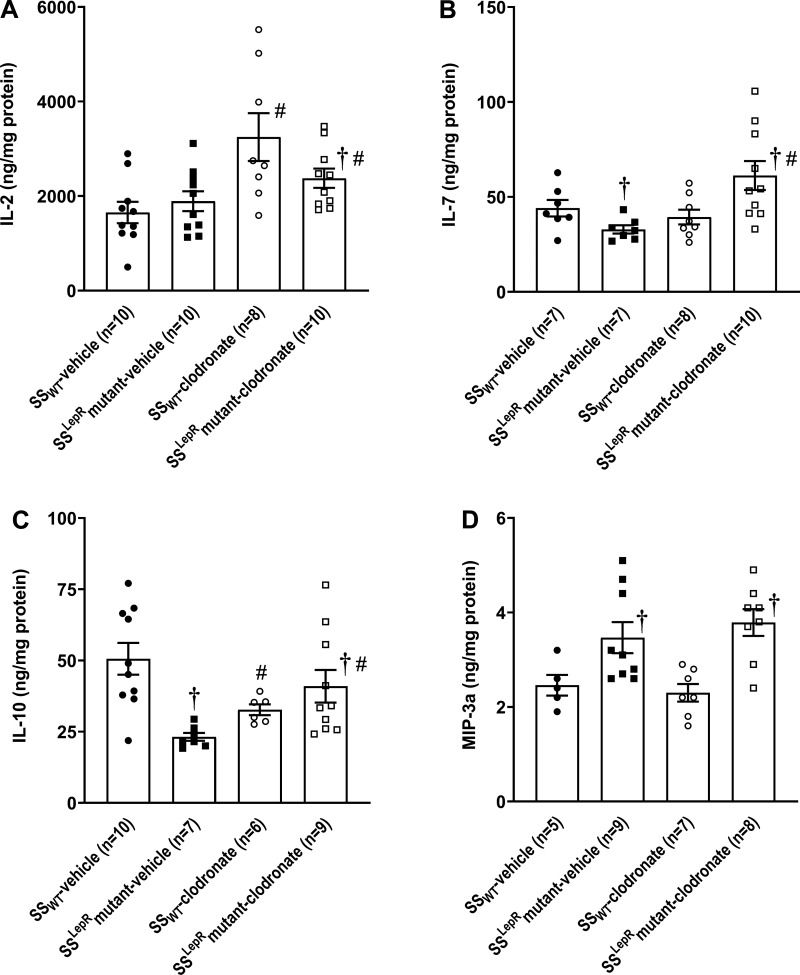
The effects of clodronate on renal cytokine levels in SSWT and SSLepRmutant rats are shown in Fig. 7. We did not observe any differences in renal IL-2 levels between SSWT and SSLepRmutant rats, and treatment with clodronate significantly increased IL-2 in the kidneys of both strains (Fig. 7A). IL-7 was decreased in SSLepRmutant rats compared with SSWT rats. While chronic treatment with clodronate had no effect on renal IL-7 levels in SSWT rats, IL-7 levels were significantly increased in SSLepRmutant rats (Fig. 7B). Similar to IL-7, renal IL-10 levels were decreased by 50% in SSLepRmutant rats versus SSWT rats, and clodronate markedly increased and significantly reduced the renal levels of IL-10 in SSLepRmutant and SSWT rats, respectively (Fig. 7C). Renal macrophage inflammatory peptide-3a (MIP-3a) levels were significantly higher in SSLepRmutant rats compared with their SSWT counterparts, and treatment with clodronate had no effect on renal MIP-3a in both strains (Fig. 7D).
Fig. 7.
A−D: effects of clodronate treatment on renal cytokine levels in wild-type Dahl salt-sensitive (SSWT) rats and Dahl salt-sensitive leptin receptor mutant (SSLepRmutant) strain: IL-2 (A), IL-7 (B), IL-10 (C), and macrophage inflammatory peptide-3a (MIP-3a; D). Numbers in parentheses indicate the number of rats studied per group. Values are means ± SE. The significance of difference in mean values for a single time point was determined by one-way ANOVA followed by a Tukey’s multiple-comparisons test. P values of <0.05 were considered significantly different. †Significant difference from the corresponding value in SSWT rats within the same treatment; #significant difference from the corresponding value in vehicle-treated rats within the same strain.
DISCUSSION
In the present study, we did not observe any sex differences in the renal infiltration of macrophages in both strains, but the development of proteinuria and progression of renal injury were associated with increased macrophage infiltration in the kidneys in the obese SSLepRmutant rats versus their lean SSWT counterparts. Importantly, this finding was seen as early as 4 wk of age, before puberty, and independent of hyperglycemia and elevations in arterial pressure. Since macrophages play an important role in the development of renal injury and fibrosis, we also examined whether depletion of macrophages with clodronate would prevent the early progression of renal disease in SSLepRmutant rats. The administration of clodronate successfully decreased macrophages by >50% in the kidneys of SSLepRmutant rats while not having an effect in SSWT rats. Interestingly, treatment with clodronate improved metabolic disease in SSLepRmutant rats by increasing insulin sensitivity and reducing plasma triglyceride levels. In the SSLepRmutant strain, proteinuria was markedly reduced during the first 2 wk of treatment, but this effect was not observed after 4 wk of treatment. The kidneys from SSLepRmutant rats displayed glomerular injury with increased mesangial expansion and renal fibrosis versus SS rats. Treatment with clodronate had a small but significant decrease in glomerular injury and renal fibrosis in the SSLepRmutant strain. Additionally, clodronate significantly reduced the proinflammatory cytokine IL-2 and increased anti-inflammatory cytokines (IL-7 and IL-10) in the kidneys from SSLepRmutant rats. Overall, these data indicate that the depletion of macrophages improves metabolic disease and slows the early progression of renal injury in SSLepRmutant rats.
One of the most intriguing findings from the present study was that clodronate improved insulin resistance and decreased dyslipidemia in the absence of body weight loss in SSLepRmutant rats. While the mechanism by which macrophage depletion improves metabolic disease in the SSLepRmutant strain is unknown, one potential mechanism is that clodronate systematically depletes macrophages from the body (i.e., adipose tissue, spleen, blood, etc.) and reduces chronic inflammation often observed during obesity. This may lead to an increase in insulin sensitivity and glucose utilization in peripheral tissues and a decrease in triglyceride levels seen in the SSLepRmutant strain. In support of this hypothesis, previous studies have demonstrated that chronic treatment with clodronate decreased the expression of genes involved in inflammation (9) and carbohydrate and lipid metabolism (2) in obese mice fed a high-fat diet. Interestingly, the improvement in insulin resistance did not affect body weight in SSLepRmutant rats. In support of this finding, Feng et al. (9) observed similar results, in which clodronate treatment did not reduce body weight in high-fat diet-fed obese mice. These data indicate that macrophages play an important role in the early development of insulin resistance during obesity and that further studies are needed to examine the potential mechanisms that may be involved in this process.
The immune system has been studied extensively in the pathogenesis of hypertension (21, 33). Clinical and preclinical studies have demonstrated an increased renal infiltration of immune cells in the setting of hypertension (13, 19), and adoptive transfer of immune cells from hypertensive animals to normotensive animals leads to the development of hypertension (25). Furthermore, modulation of immune cell activation and function can reduce elevations in arterial pressure and renal injury (26). When investigating the involvement of macrophages in the development of hypertension, there have been controversial results. Huang et al. (14) reported that clodronate administration for 4 wk significantly reduced salt-sensitive hypertension and renal disease in SS rats. Similarly, Thang et al. (32) reported that macrophage depletion decreased arterial pressure induced by deoxycorticosterone acetate and salt (32). However, Fehrenbach et al. (8) recently observed that clodronate augmented the rise in arterial pressure and development of renal injury in SS rats fed a high-salt diet. Interestingly, in the present study, the depletion of macrophages with clodronate had no effect on arterial pressure in either lean SSWT or obese SSLepRmutant rats. There are two potential reasons as to why we did not observe any changes in arterial pressure in response to clodronate treatment: 1) the animals in the present study were not fed a high-salt diet and 2) the animals used in this study were young (4–8 wk of age). Overall, these data indicate that macrophages may play either a preventive or augmented role in the development of salt-sensitive hypertension, and further studies are needed to better understand their function in arterial pressure regulation.
One of the most interesting findings from the present study was that chronic treatment with clodronate markedly reduced proteinuria during the first 2 wk in the SSLepRmutant strain, in which this effect was no longer observed after 4 wk of treatment. There are a few possible reasons for this finding. One reason is that obese SSLepRmutant rats experience renal hyperfiltration during this same time period (6 wk of age) (22). Elevations in glomerular filtration rate have been shown to contribute to the development of renal injury and infiltration of immune cells other than macrophages. Evans et al. (7) found that raising renal perfusion pressure to one kidney via servocontrol increased macrophages and T cell infiltration and renal injury in SS rats fed a high-salt diet. Another potential reason is that clodronate depletes the entire macrophage population, including M1 (proinflammatory) and M2 (anti-inflammatory). The early treatment with clodronate may be more selective for depleting M1 macrophages versus M2 macrophages, which would account for the early prevention of proteinuria in the SSLepRmutant strain during the first 2 wk of treatment. However, the possible depletion of M2 macrophages after 4 wk of treatment may produce an undesirable outcome such as increased protein excretion. Another reason for this diverse effect on proteinuria could be attributed to the effect of clodronate on proinflammatory (IL-2 and MIP-3a) and anti-inflammatory (IL-7 and IL-10) cytokines. Treatment with clodronate increased IL-2 and did not lower MIP-3a in the kidneys from the SSLepRmutant strain, in which both cytokines play a significant role in the growth, maturity, and recruitment of T cells (27, 29). We observed that clodronate significantly increased the renal levels of IL-7 and IL-10, which participate in T cell differentiation and homeostasis (6) and inhibit the activity of other immune cells [i.e., macrophages and natural killer and T helper 1 cells (5), respectively]. However, how these cytokines influence the preventive or augmented effect on the progression of proteinuria remains to be determined. Future studies are needed to assess the effects of renal hyperfiltration, macrophage polarization, and proinflammatory/anti-inflammatory cytokines on the progression of renal disease in the obese SSLepRmutant strain.
In the present study, we observed that depletion of macrophages with clodronate reduced renal inflammation, glomerular injury, and renal fibrosis in the SSLepRmutant strain. These results are in complete agreement with previous reports demonstrating that depletion of macrophages is beneficial for renal disease. Chronic treatment with clodronate significantly decreased tubular apoptosis and renal fibrosis along with reduced gene expression of TNF-α and transforming growth factor-β in rodent models of unilateral ureter obstruction (16, 31). Fernandez et al. (18) reported that depletion of macrophages reduced interstitial fibrosis in aldosterone-treated Wistar rats fed a high-salt diet. Similar results were observed in renal ischemia-reperfusion injury, in which clodronate reduced renal inflammation and tubulointerstitial fibrosis (15, 17). However, to our knowledge, the present study is the first study to demonstrate that chronic treatment with clodronate is beneficial in treating renal disease associated with obesity.
The overall goal of the present study was to determine whether the depletion of macrophages would prevent the early development of renal injury in the obese SSLepRmutant strain. While the results support the main goal, there were some limitations in the study that should be noted. One major limitation in the present study is the measurement of arterial pressure by the chronic carotid catheter method, which was performed on the day after surgery, rather than using radiotelemetry. This could be an additional reason as to why we did not observe an effect of clodronate treatment on arterial pressure. The reason why we did not choose to use radiotelemetry was that the rats are too small at 4 wk of age to place the catheter in the femoral artery, and our survival rate is minimal when placing the radiotelemetry transmitter in the abdominal aorta. Another limitation is that we did not examine the influence of clodronate on the renal infiltration of other immune cells (i.e., T cells and B cells). Fehrenbach et al. (8) demonstrated that chronic treatment with clodronate-liposomes increased renal T cell and B cell infiltration and progression of albuminuria in SS rats fed a high-salt diet, which could be contributing to the enhanced protein excretion after 4 wk of treatment in SSLepRmutant rats in the present study. However, we do not believe this is occurring in the present study because we did not observe any differences in proteinuria in lean SS rats treated with clodronate. An additional limitation of this study is that we did not evaluate the effect of clodronate on glomerular filtration rate within the first 2 wk of treatment since clodronate increased insulin sensitivity and slowed the early progression of proteinuria in the SSLepRmutant strain. Moreover, the impact of depleting macrophages on renal function has primarily been examined in older animals (8). Thus, the present study may be the first study to examine the effects of clodronate or depletion of macrophages on the development of renal disease in young animals.
Perspectives and Significance
Overall, the present study has shown that the development and progression of renal injury in the SSLepRmutant strain are associated with an increase in macrophages and that chronic treatment with clodronate reduced renal macrophage infiltration and inhibited metabolic disease by increasing insulin sensitivity and decreasing dyslipidemia in SSLepRmutant rats. Moreover, the depletion of macrophages slowed the progression of proteinuria and decreased glomerular injury and renal fibrosis in SSLepRmutant rats by possibly increasing anti-inflammatory cytokines. Therefore, these results indicate that macrophages play an important role in the early development and progression of renal disease associated with obesity before puberty, but further studies are needed to determine the potential mechanisms involved in renal injury secondary to macrophage activation.
GRANTS
This work was financially supported by National Institutes of Health Grants DK-109133 (to J. M. Williams) and HL-130456 (to D. C. Cornelius). The work performed through the Molecular and Genomics Facility of the University of Mississippi Medical Center was supported, in part, by funds from the National Institutes of Health, including Mississippi INBRE Grant P20-GM-103476, Obesity, Cardiorenal and Metabolic Diseases-COBRE Grant P20-GM-104357, and Mississippi Center of Excellence in Perinatal Research-COBRE Grant P20-GM-121334.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.P. and J.M.W. conceived and designed research; B.P., T.D.J., D.C.C., and J.M.W. performed experiments; B.P., D.C.C., and J.M.W. analyzed data; B.P., C.A.S., D.C.C., and J.M.W. interpreted results of experiments; B.P. and J.M.W. prepared figures; B.P., D.C.C., and J.M.W. drafted manuscript; B.P., A.K.B., U.S.E., T.D.J., D.C.C., and J.M.W. edited and revised manuscript; B.P., C.A.S., A.K.B., U.S.E., T.D.J., D.C.C., and J.M.W. approved final version of manuscript.
REFERENCES
- 1.Adamiec-Mroczek J, Oficjalska-Młyńczak J. Assessment of selected adhesion molecule and proinflammatory cytokine levels in the vitreous body of patients with type 2 diabetes−role of the inflammatory-immune process in the pathogenesis of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 246: 1665–1670, 2008. doi: 10.1007/s00417-008-0868-6. [DOI] [PubMed] [Google Scholar]
- 2.Bu L, Gao M, Qu S, Liu D. Intraperitoneal injection of clodronate liposomes eliminates visceral adipose macrophages and blocks high-fat diet-induced weight gain and development of insulin resistance. AAPS J 15: 1001–1011, 2013. doi: 10.1208/s12248-013-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgert TS, Dziura J, Yeckel C, Taksali SE, Weiss R, Tamborlane W, Caprio S. Microalbuminuria in pediatric obesity: prevalence and relation to other cardiovascular risk factors. Int J Obes 30: 273–280, 2006. doi: 10.1038/sj.ijo.0803136. [DOI] [PubMed] [Google Scholar]
- 4.Cao Q, Wang Y, Harris DC. Macrophage heterogeneity, phenotypes, and roles in renal fibrosis. Kidney Int Suppl 4: 16–19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diefenhardt P, Nosko A, Kluger MA, Richter JV, Wegscheid C, Kobayashi Y, Tiegs G, Huber S, Flavell RA, Stahl RAK, Steinmetz OM. IL-10 receptor signaling empowers regulatory T cells to control Th17 responses and protect from GN. J Am Soc Nephrol 29: 1825–1837, 2018. doi: 10.1681/ASN.2017091044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ElKassar N, Gress RE. An overview of IL-7 biology and its use in immunotherapy. J Immunotoxicol 7: 1–7, 2010. doi: 10.3109/15476910903453296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 70: 543–551, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, Mattson DL. Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am J Physiol Renal Physiol 317: F361–F374, 2019. doi: 10.1152/ajprenal.00096.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, Yang Z, Xu H. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS One 6: e24358, 2011. doi: 10.1371/journal.pone.0024358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 46: 871–880, 2005. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Hales CM, Carroll MD, Fryar CD, Ogden CL. NCHS Data Brief: Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. https://www.cdc.gov/nchs/data/databriefs/db288.pdf [19 May 2020]. [PubMed]
- 12.Haslam DW, James WP. Obesity. Lancet 366: 1197–1209, 2005. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 13.Heptinstall RH. Renal biopsies in hypertension. Br Heart J 16: 133–141, 1954. doi: 10.1136/hrt.16.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L, Wang A, Hao Y, Li W, Liu C, Yang Z, Zheng F, Zhou MS. Macrophage depletion lowered blood pressure and attenuated hypertensive renal injury and fibrosis. Front Physiol 9: 473, 2018. doi: 10.3389/fphys.2018.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006. doi: 10.1093/ndt/gfk047. [DOI] [PubMed] [Google Scholar]
- 16.Kitamoto K, Machida Y, Uchida J, Izumi Y, Shiota M, Nakao T, Iwao H, Yukimura T, Nakatani T, Miura K. Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci 111: 285–292, 2009. doi: 10.1254/jphs.09227FP. [DOI] [PubMed] [Google Scholar]
- 17.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 23: 842–852, 2007. doi: 10.1093/ndt/gfm694. [DOI] [PubMed] [Google Scholar]
- 18.Martín-Fernández B, Rubio-Navarro A, Cortegano I, Ballesteros S, Alía M, Cannata-Ortiz P, Olivares-Álvaro E, Egido J, de Andrés B, Gaspar ML, de Las Heras N, Lahera V, Moreno JA. Aldosterone induces renal fibrosis and inflammatory M1-macrophage subtype via mineralocorticoid receptor in rats. PLoS One 11: e0145946, 2016. doi: 10.1371/journal.pone.0145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattson DL. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol 15: 290–300, 2019. doi: 10.1038/s41581-019-0121-z. [DOI] [PubMed] [Google Scholar]
- 20.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 307: F499–F508, 2014. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPherson KC, Shields CA, Poudel B, Johnson AC, Taylor L, Stubbs C, Nichols A, Cornelius DC, Garrett MR, Williams JM. Altered renal hemodynamics is associated with glomerular lipid accumulation in obese Dahl salt-sensitive leptin receptor mutant rats. Am J Physiol Renal Physiol 318: F911–F921, 2020. doi: 10.1152/ajprenal.00438.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPherson KC, Taylor L, Johnson AC, Didion SP, Geurts AM, Garrett MR, Williams JM. Early development of podocyte injury independently of hyperglycemia and elevations in arterial pressure in nondiabetic obese Dahl SS leptin receptor mutant rats. Am J Physiol Renal Physiol 311: F793–F804, 2016. doi: 10.1152/ajprenal.00590.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 315: 2292–2299, 2016. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol Microbiol Scand [C] 88C: 1–6, 1980. doi: 10.1111/j.1699-0463.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 27.Ross SH, Cantrell DA. Signaling and function of interleukin-2 in T lymphocytes. Annu Rev Immunol 36: 411–433, 2018. doi: 10.1146/annurev-immunol-042617-053352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan MJ. An update on immune system activation in the pathogenesis of hypertension. Hypertension 62: 226–230, 2013. doi: 10.1161/HYPERTENSIONAHA.113.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev 14: 409–426, 2003. doi: 10.1016/S1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 30.Spires D, Poudel B, Shields CA, Pennington A, Fizer B, Taylor L, McPherson KC, Cornelius DC, Williams JM. Prevention of the progression of renal injury in diabetic rodent models with preexisting renal disease with chronic endothelin A receptor blockade. Am J Physiol Renal Physiol 315: F977–F985, 2018. doi: 10.1152/ajprenal.00182.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung SA, Jo SK, Cho WY, Won NH, Kim HK. Reduction of renal fibrosis as a result of liposome encapsulated clodronate induced macrophage depletion after unilateral ureteral obstruction in rats. Nephron, Exp Nephrol 105: e1–e9, 2006. doi: 10.1159/000096859. [DOI] [PubMed] [Google Scholar]
- 32.Thang LV, Demel SL, Crawford R, Kaminski NE, Swain GM, Van Rooijen N, Galligan JJ. Macrophage depletion lowers blood pressure and restores sympathetic nerve α2-adrenergic receptor function in mesenteric arteries of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 309: H1186–H1197, 2015. doi: 10.1152/ajpheart.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune mechanisms in arterial hypertension. J Am Soc Nephrol 27: 677–686, 2016. doi: 10.1681/ASN.2015050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You H, Gao T, Cooper TK, Brian Reeves W, Awad AS. Macrophages directly mediate diabetic renal injury. Am J Physiol Renal Physiol 305: F1719–F1727, 2013. doi: 10.1152/ajprenal.00141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Z, Zheng F. Immune cells and inflammation in diabetic nephropathy. J Diabetes Res 2016: 1, 2016. doi: 10.1155/2016/1841690. [DOI] [PMC free article] [PubMed] [Google Scholar]