Abstract
Acute kidney injury (AKI) due to cisplatin is a significant problem that limits its use as an effective chemotherapeutic agent. T cell receptor+CD4−CD8− double negative (DN) T cells constitute the major T cell population in the human and mouse kidney, express programmed cell death protein (PD)-1, and protect from ischemic AKI. However, the pathophysiological roles of DN T cells in cisplatin-induced AKI is unknown. In this study, wild-type mice were treated with cisplatin (30 mg/kg) or vehicle, and the effects on kidney DN T cell numbers and function were measured. In vitro experiments evaluated effects of kidney DN T cells on cisplatin-induced apoptosis and PD ligand 1 (PD-L1) in renal epithelial cells. Adoptive transfer experiments assessed the therapeutic potential of DN T cells during cisplatin-induced AKI. Our results show that kidney DN T cell population increased at 24 h and declined by 72 h after cisplatin treatment. Cisplatin treatment increased kidney DN T cell proliferation, apoptosis, CD69, and IL-10 expression, whereas CD62L, CD44, IL-17A, interferon-γ, and TNF-α were downregulated. Cisplatin treatment decreased both PD-1 and natural killer 1.1 subsets of kidney DN T cells with a pronounced effect on the PD-1 subset. In vitro kidney DN T cell coculture decreased cisplatin-induced apoptosis in kidney proximal tubular epithelial cells, increased Bcl-2, and decreased cleaved caspase 3 expression. Cisplatin-induced expression of PD ligand 1 was reduced in proximal tubular epithelial cells cocultured with DN T cells. Adoptive transfer of DN T cells attenuated kidney dysfunction and structural damage from cisplatin-induced AKI. These results demonstrate that kidney DN T cells respond rapidly and play a protective role during cisplatin-induced AKI.
Keywords: acute kidney injury, cisplatin, double negative T cells, kidney epithelial cells, T cell receptor
INTRODUCTION
Cisplatin-induced acute kidney injury (AKI) is a significant clinical problem that limits its chemotherapeutic use against multiple solid tumors including ovarian, head and neck, and testicular cell cancers (9). The pathophysiological mechanisms of cisplatin-induced AKI includes increased tubular injury, vascular injury, apoptosis, oxidative stress, and inflammation (12, 23, 24, 29). Increasing data support the modulatory role of T cells in cisplatin-induced AKI. In this context, CD4+, CD8+ T cells, CD4+CD25+FoxP3+ regulatory T cells, natural killer (NK) T cells, and γδ T cells have been shown to modulate cisplatin-induced AKI by either worsening tissue injury or promoting repair (7, 14, 16, 19).
Recent work has identified αβ T cell receptor (TCR)+CD4−CD8−, CD1d tetramer negative [double negative (DN)] T cells, which constitute a significant proportion of the T cell population in the mouse and human kidney and play important immunoregulatory roles to protect from ischemic AKI (1, 21). Moreover, kidney DN T cells are nonclassical major histocompatibility complex class I restricted and have distinct programmed cell death (PD)-1+ and NK1.1+ subsets (25). However, the pathophysiological role of DN T cells in cisplatin-induced AKI is not known.
In the present study, we tested the hypothesis that DN T cells serve as anti-inflammatory regulatory cells during cisplatin-induced AKI. Our results show that the kidney DN T cell population expands rapidly after cisplatin administration, with increased proliferation and activation compared with CD4+ and CD8+ T cells. Cisplatin treatment had a particularly marked effect on the PD-1 subset, which is important in the context of immune checkpoint inhibitor use for cancer treatment. Cisplatin treatment in vivo was also found to affect proliferation, apoptosis, and activation of kidney DN T cells. In vitro experiments demonstrated a direct protective effect of DN T cells from cisplatin-induced proximal tubular epithelial cell (PTEC) apoptosis, apoptotic proteins, and PD ligand-1 (PD-L1) expression. In vivo adoptive transfer experiments demonstrated a tissue and functional protective role of DN T cells in the prevention of cisplatin-induced AKI.
METHODS
Animals.
Male C57BL/6J wild-type (WT) mice (8−10 wk old) were purchased from Jackson Laboratory and housed under specific pathogen-free conditions at the Animal Facility of Johns Hopkins University. All experiments were performed using protocols approved by the Animal Care and Use Committee of Johns Hopkins University.
Cisplatin-induced AKI model.
Cisplatin (cis-diammineplatinum II dichloride, Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline at a concentration of 1 mg/mL. Mice were given intraperitoneal injection with a single dose of cisplatin (30 mg/kg body wt). This dose of cisplatin was derived from a pilot study that resulted in minimum mortality with a significant decline in kidney function in this strain of mice. Blood samples were obtained from mice before (0 h) and at 24, 48, and 72 h after cisplatin injection. Serum creatinine was measured as a marker of renal dysfunction by a Cobas Mira Plus automated analyzer (Roche, Nurley, NJ) using the Creatinine reagent set (Pointe Scientific, Canton, MI).
Histological examination.
Formalin-fixed and paraffin-embedded sections of mouse kidney tissues were cut and stained with hematoxylin and eosin. Renal tubular injury was assessed and scored by a pathologist blinded to the experimental groups for semiquantitative assessment of tubular injury.
Isolation of lymphocytes from the kidney.
Mononuclear cells from kidneys were isolated based on an established protocol (2, 20). Briefly, mice were anesthetized with intraperitoneal ketamine (130 mg/kg) and xylazine (7 mg/kg) either exsanguinated or perfused with 50 mL of ice-cold PBS through the left ventricle after a median thoracotomy to clear the kidney of leukocytes in the circulation. Both kidneys were minced and incubated in collagenase D (2 mg/mL, Sigma-Aldrich) solution for 30 min at 37°C. Single cell suspensions of kidney digestions were obtained by mechanical disruption of tissues using 70-μm strainers (BD Bioscience), and kidney mononuclear cells were isolated using Percoll density gradient centrifugation as previously described elsewhere (1, 2, 20, 21). The numbers of viable lymphocytes in each sample were determined using trypan blue exclusion dye under a light microscope. The absolute cell number was calculated by dividing the number in a cell subset with the total number of cells acquired on the flow cytometer and multiplying with the number of cells counted with the hemocytometer.
Antibodies and reagents.
Fluorochrome-conjugated anti-mouse monoclonal antibodies including anti-CD45 (30-F11) APC-Cy7/BUV395, anti-TCRβ (H57-597) FITC/BV421, anti-CD8α (53 ± 6.7) PerCP-Cy5.5/APC-fire750, anti-CD4 (RM4-5) APC/FITC/Pacific blue, anti-CD69 (H1.2F3) PE, anti-CD62L (MEL-14) FITC, anti-CD44 (IM7) PE, annexin V-FITC, anti-bromodeoxyuridine (BrdU) FITC, anti-NK1.1 (PK136) PE, anti-PD-1 (J43) BV421, anti-interferon (IFN)-γ (XMG1.2) PE, anti-TNF-α (MP6-XT22) PE, anti-IL-10 (JES5-16E3) APC, anti-IL-17A (eBio17B7) APC, anti-PD-L1(MIH7) PE, anti-epithelial cell adhesion molecule (G8.8) APC, and biotin-conjugated anti-CD16/32 (2.4G2) were purchased from BD Biosciences, eBioscience, or BioLegend and used at 1:100 dilution. Rabbit monoclonal antibodies against PD-L1 (no. 13684, Cell Signaling, Danvers, MA), cleaved caspase-3 (Asp175; no. 9661, Cell Signaling), Bcl-2 (D17C4; no. 3498, Cell Signaling), and β-actin (13E5; no. 4970, Cell Signaling) were used. Secondary antibodies included antibodies conjugated to Alexa Flour 488 (Abcam, Cambridge, UK), Alexa Flour 647 (Abcam), and Alexa Fluor 594 (Abcam) for horseradish peroxidase-conjugated anti-rabbit antibodies (Sigma-Aldrich) for chemiluminescence immunoblot analysis.
Flow cytometry analysis.
Flow cytometric analysis was performed using standard methods. Briefly, cells were resuspended in FACS buffer (PBS, 2% FBS, and 0.1% sodium azide) and preincubated with anti-CD16/CD32 antibodies for 10 min to minimize nonspecific binding through Fc receptors. Cells were stained by incubation with the appropriate cocktails of fluorochrome-conjugated monoclonal antibodies for 30 min at 4°C, washed, and resuspended in FACS buffer. Samples were acquired using a BD LSR II flow cytometer. Data were analyzed using FlowJo V10 software (Treestar Software).
Intracellular cytokine staining.
Single cell suspensions were prepared and stimulated with 5 ng/mL phorbol 12-myristate 13-acetate and 500 ng/mL ionomycin (Sigma-Aldrich) for 4 h at 37°C in a 5% CO2 humidified atmosphere incubator in the presence of Golgi Plug (BD Biosciences). Surface staining of stimulated cells was performed with anti-CD45, anti-CD8, anti-CD4, and anti-TCRβ monoclonal antibodies for 30 min at 4°C. Cells were then permeabilized with perm/wash solution followed by an additional 30 min of incubation with fluorochrome-conjugated IL-10, IL-17A, IFN-γ, or TNF-α monoclonal antibodies and analyzed by a LSR II flow cytometer.
Annexin V apoptosis assay.
Apoptosis was assessed using the Annexin V-FITC apoptosis kit according to the manufacturer’s instructions (BD PharMingen). Cells were stained for surface markers (CD45, TCR, CD4, and CD8) for 30 min at 4°C, washed twice with PBS, resuspended in annexin V binding buffer with anti-annexin monoclonal antibody for 15 min in dark at room temperature, acquired by a LSRII flow cytometer, and analyzed by FlowJo.
BrdU proliferation assay.
To assess proliferation in kidney purified T cells, mice were injected intraperitoneally with 1 mg BrdU in PBS twice within a 24-h period followed by analysis of different T cell subsets for BrdU incorporation using the BrdU flow kit protocol (BD PharMingen) following the manufacturer’s instructions.
In vitro coculture of PTECs and DN T cells.
The Boston University mouse proximal tubular (BUMPT-306) epithelial cell line was used for coculture experiments (3, 17, 30). Cells were cultured in DMEM with 10% FBS and 100 U/mL penicillin and streptomycin. BUMPT cells (5 × 104 cells) were treated with 20 μM cisplatin for 24 h before the coculture. DN T cells and CD4+ T cells were isolated from kidneys, and CD4+CD25+ T cells were isolated from spleens of WT mice using a FACS Aria flow sorter for coculture experiments. Cells were stimulated with plate-bound anti-CD3 (10 µg/mL) and anti-CD28 (10 µg/mL) for 24 h. Cisplatin-treated BUMPT cells were cocultured with either activated DN T cells or CD4+ T cells or CD4+CD25+ T cells at a 5:1 ratio for 24 h. Cells were harvested, counted, stained using the appropriate cocktails of fluorochrome-conjugated monoclonal antibodies. The percentage of each subpopulation was determined using FlowJo.
Western blot analysis.
Cell protein lysates for immunoblot analysis were prepared in urea sample buffer [8 M deionized urea, 0.5% SDS, 30 mM Tris (pH 6.8), 5% glycerol, and 5% β-mercaptoethanol] after whole cell lysates were collected from cultured cells. Samples were sheared using progressively finger-gauged needles (221/2, 251/2, and 261/2) and subjected to a Bradford assay and processed. Samples were loaded at 10 μg protein/lane for immunoblot analysis. Samples were separated using either 10% or 12.5% SDS-PAGE and transferred to 0.45-µm nitrocellulose membranes (Bio-Rad, Hercules, CA) via a transblot turbo transfer system (Bio-Rad). Blots were blocked in 5% milk in 10 mL (wt/vol) 10 mL Tris-buffered saline-Tween 20 for >1 h at room temperature (25°C) and subsequently incubated overnight at 4°C in the appropriate antibodies. Secondary antibodies were applied for 1 h at room temperature (25°C). Blots were developed using either ECL Select developing solution (GE Healthcare, Pittsburgh, PA) or SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Waltham, MA) and imaged using a FluorChem Q system (ProteinSimple, San Jose, CA). Signal quantification for Western blots were conducted using ImageJ software. All densitometry quantifications were calculated by dividing the band intensity for the target of interest by the loading control and then averaging all biological replicates across the same time point.
Adoptive transfer of DN T cells.
DN T cells were isolated from the lymph nodes or spleen of gld mice as previously described (20), and 1~1.5 × 106 flow-sorted DN T cells were transferred into 8-wk-old C57BL/6 male mice (n = 9) 24 h before cisplatin administration (intraveously). Age-matched mice in control groups were injected with PBS or CD4 T cells. Serum creatinine was measured by taking blood from a mouse tail vein before (0 h) and at 24, 48, and 72 h after cisplatin injection. Kidneys were harvested for histological examination at 72 h.
Statistical analysis.
Data were collected from at least three independent experiments and are expressed as means ± SE; n indicates the number of animals per group. Comparisons between multiple groups were performed by a one-way or two-way ANOVA test followed by Tukey’s or Sidak’s multiple-comparison tests. Statistical analysis was performed using Prism 7 (GraphPad Software), and significance was determined as P < 0.05.
RESULTS
Kidney DN T cells rapidly expand after cisplatin treatment.
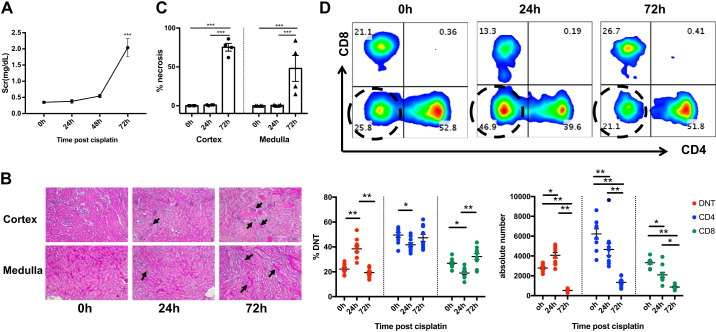
Cisplatin (30 mg/kg) resulted in a worsening of kidney function with a significant (P < 0.01) increase in serum creatinine at 72 h (Fig. 1A). Histological evaluation of kidney sections showed extensive renal tubular necrosis in both cortical and medullary regions at 72 h (Fig. 1, B and C). To evaluate the effects of cisplatin treatment on the kidney DN T cell population during AKI, kidney mononuclear cells were isolated at different time points and analyzed by flow cytometry. We observed a significant (P < 0.01) expansion, both in frequency and absolute numbers, of the kidney DN T cell population 24 h after cisplatin administration followed by a decline by 72 h (P < 0.01). The frequency of CD4+ and CD8+ T cells was initially reduced at 24 h, which returned to baseline levels by 72 h (Fig. 1D). However, there was a consistent decline in absolute numbers of CD4+ and CD8+ T cells at 24 and 72 h. We also found a significant reduction in total CD45+ and TCR+ cells (Supplemental Fig. S1 in the Supplemental Material, available online at https://doi.org/10.6084/m9.figshare.11704365.v1), which could be a possible explanation for the reduced absolute numbers of CD4+, CD8+, and DN T cells observed at 72 h after cisplatin administration.
Fig. 1.
Cisplatin treatment results in a significant expansion of kidney double negative (DN) T cell population. A: cisplatin (30 mg/kg) treatment resulted in a significant increase in serum creatinine (SCr) at 72 h. B: representative kidney sections stained with hematoxylin and eosin showing extensive renal tubular necrosis in the cortex and medulla 72 h after cisplatin treatment. Arrows indicate glomeruli per high-power field in the cortex (top) and injured tubules in medullary regions (bottom). C: graph showing the cumulative percentage of necrotic tubules in each group. D: flow cytometric analysis (top) of kidney mononuclear cells at the indicated time points after cisplatin treatment showing significant DN T cell expansion, both in frequency (bottom left graph; %DNT) and absolute numbers (bottom right graph) at 24 h, followed by a decline at 72 h. Data are presented as means ± SE from at least 5 independent experiments (n = 5 mice). *P < 0.05; **P < 0.01; ***P < 0.001.
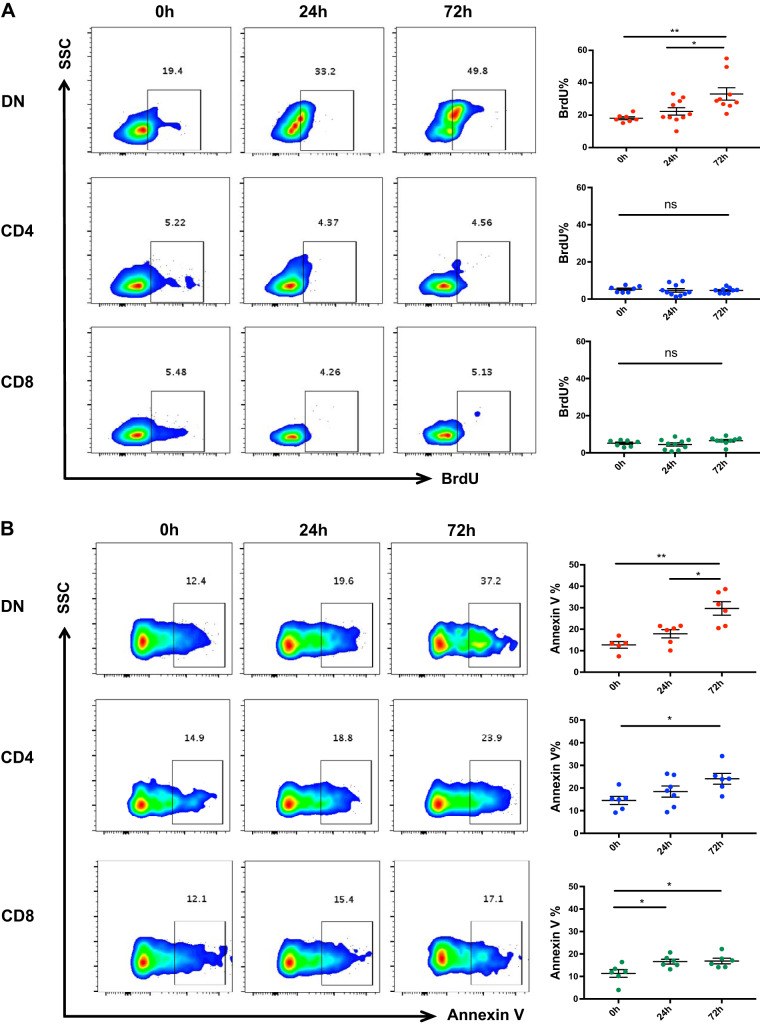
Cisplatin increases kidney DN T cell proliferation and apoptosis.
To determine the underlying mechanism of rapid DN T cell expansion following cisplatin administration in vivo, we assessed DN T cell proliferation and apoptosis using BrdU and annexin V assays, respectively. We observed significant (P < 0.05) DN T cell proliferation both in terms of frequency and absolute number after cisplatin administration. There was no effect on CD4+ or CD8+ T cell proliferation after cisplatin (Fig. 2A). We observed increased apoptosis in DN, CD4+, and CD8+ T cells over time that peaked at 72 h (Fig. 2B).
Fig. 2.
Cisplatin treatment increases proliferation and apoptosis of kidney double negative (DN) T cells. A: flow cytometric assessment of kidney mononuclear cells at different time points after cisplatin treatment using bromodeoxyuridine (BrdU) showed significant DN T cell proliferation at 72 h compared with 0- and 24-h time points. There was no effect on either CD4+ or CD8+ T cell proliferation after cisplatin treatment. B: cisplatin treatment also increased the frequency of apoptotic DN, CD4+, and CD8+ T cells over time that peaked at 72 h as assessed by annexin V assay. Data are presented as means ± SE from at least 5 independent experiments (n = 5 mice). *P < 0.05; **P < 0.01. SSC, side scatter; ns, not significant.
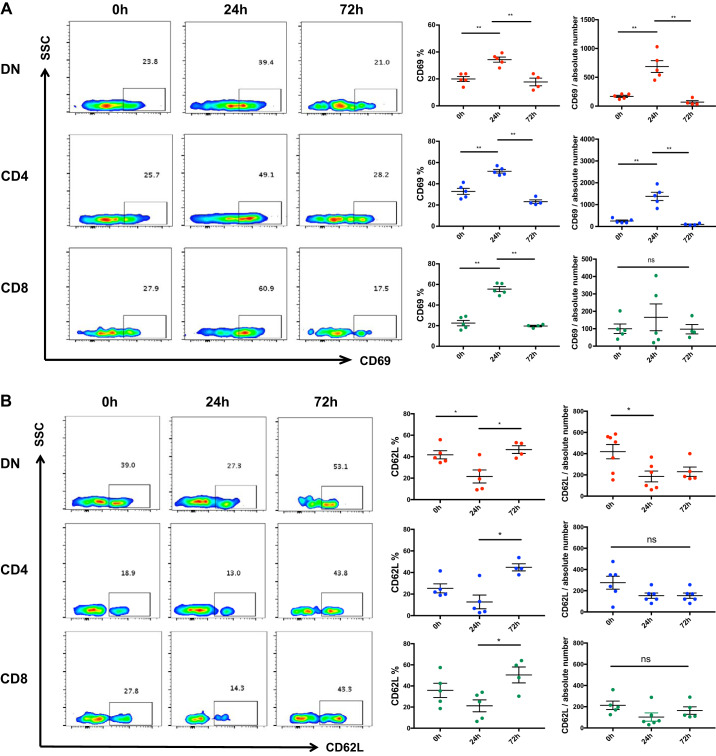
Kidney DN T cells become activated in response to cisplatin.
To study the effect of cisplatin on kidney DN T cell activation, we assessed the expression of T cell activation markers CD69 and CD62L at baseline and after cisplatin treatment. CD69 expression increased significantly (P < 0.05) in kidney DN, CD4+, and CD8+ cells 24 h after cisplatin administration and subsequently returned to basal level by 72 h (Fig. 3A). Concurrently, CD62L expression decreased significantly (P = 0.03) in DN T cells at 24 h but was comparable at 72 h compared with the saline-treated control (0 h) group (Fig. 3B). There was no statistically significant difference in CD62L expression on CD4+ or CD8+ cells at either time points.
Fig. 3.
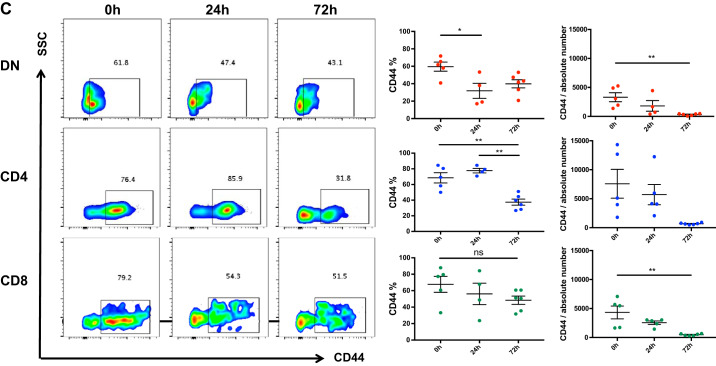
Cisplatin treatment activates kidney double negative (DN) T cells. A: the effect of cisplatin treatment on kidney DN T cell activation was assessed by flow cytometric quantification of T cell activation markers CD69 and CD62L. Cisplatin treatment resulted in significant increase in CD69 expression in kidney DN, CD4+, and CD8+ cells at 24 h that subsequently returned to the basal level by 72 h. B: there was a significant decline in CD62L expression in kidney DN T cells at 24 h, but was comparable at 72 h, compared with the saline treated control (0 h) group. The frequency of CD62L showed a decreasing trend at 24 h with subsequent restoration at 72 h. There was no change in the absolute number of CD62L-positive CD4+ or CD8+ cells. C: expression of homing receptor CD44 on kidney DN T cells after cisplatin was also assessed. The frequency and absolute number of CD44-expressing DN T cells significantly deceased 24 h after cisplatin administration compared with controls. There was no change in the frequency of CD44-expressing CD4+ and CD8+ cells compared with the control group, but the absolute numbers of CD4+ and CD8+ cells expressing CD44 was significantly reduced, especially at 72 h. Data are presented as means ± SE from at least 5 independent experiments (n = 5 mice). *P < 0.05; **P < 0.01. SSC, side scatter; ns, not significant.
Expression of homing receptor CD44 on kidney DN T cells after cisplatin was also measured. We observed significantly reduced CD44 expression on kidney DN T cells after cisplatin administration compared with controls (Fig. 3C). There was no significant change in the frequency of CD44-expressing CD4+ and CD8+ cells compared with the control group, but the absolute numbers of CD4+ and CD8+ cells expressing CD44 were significantly reduced, especially at 72 h.
Kidney DN T cells produce IL-10 in response to cisplatin.
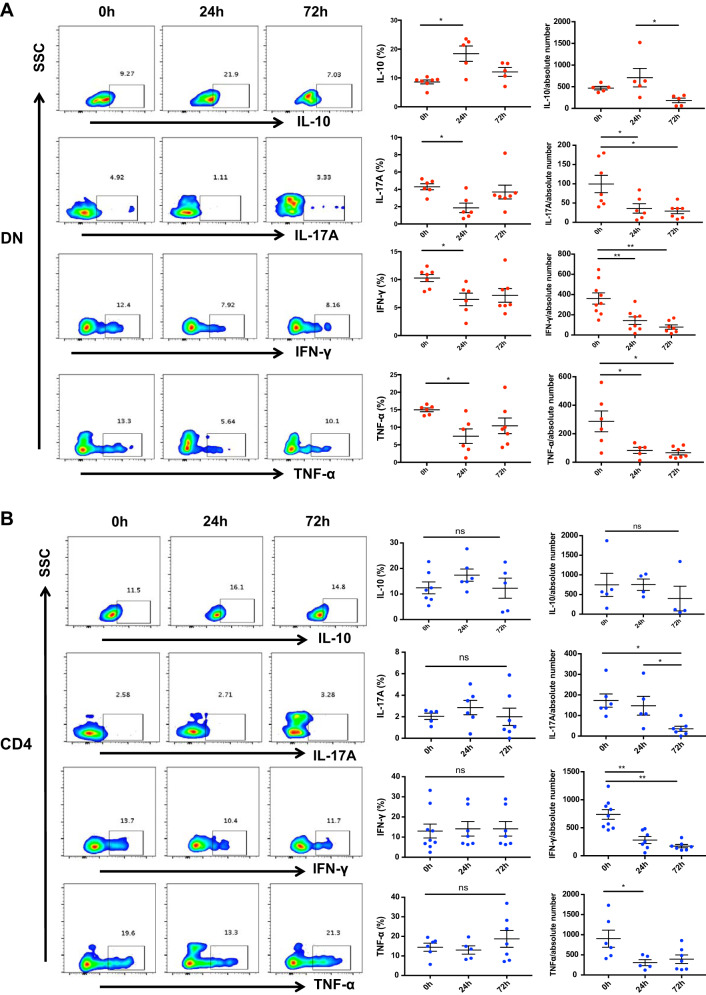
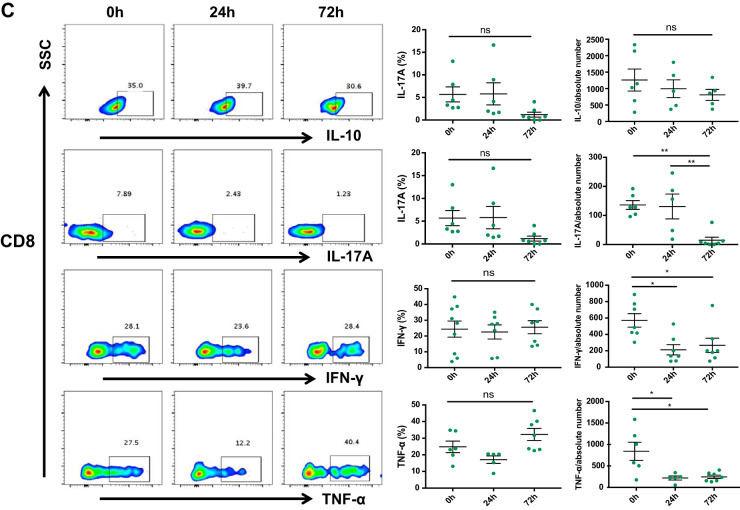
To explore whether cisplatin induces changes in kidney DN T cell cytokines, we assessed the proinflammatory cytokines IFN-γ, IL-17A, and TNF-α and the anti-inflammatory cytokine IL-10. We found a significant (P < 0.01) increase in IL-10 and decrease (P < 0.05) in IFN-γ, TNF-α, and IL-17A at 24 h (Fig. 4A). There was no difference in the frequency of CD4+ and CD8+ T cell cytokines studied; however, the absolute numbers of proinflammatory cytokine (IFN-γ, TNF-α, and IL-17A)-producing CD4+ and CD8+ T cells were significantly reduced after cisplatin treatment (Fig. 4, B and C).
Fig. 4.
Cisplatin treatment alters inflammatory cytokine production by kidney double negative (DN) T cells. A: we measured proinflammatory cytokines interferon (IFN)-γ, IL-17A, and TNF-α and anti-inflammatory cytokine IL-10 to explore cisplatin effects on kidney DN T cell effector function. Cisplatin treatment resulted in a significant increase in IL-10 and decrease in IFN-γ, TNF-α, and IL-17A at 24 h. B: there was no difference in the percentages of CD4+ and CD8+ T cell cytokine profiles between cisplatin-treated and control groups at any time point studied. C: the absolute numbers of CD4+ and CD8+ T cells producing proinflammatory cytokines (IFN-γ, TNF-α, and IL-17A) were, however, significantly reduced after cisplatin treatment. Data are presented as means ± SE from at least 5 independent experiments (n = 5 mice). *P < 0.05; **P < 0.01. SSC, side scatter; ns, not significant.
Cisplatin affects both PD-1+ and NK1.1+ DN T subsets in the kidney.
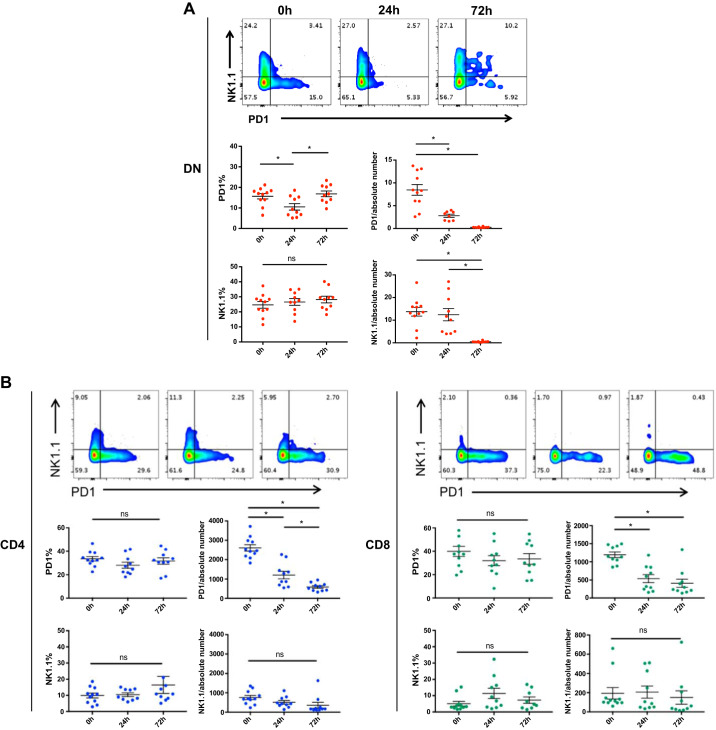
The existence of two distinct subsets of DN T cells based on their expression of PD-1 and NK1.1 has recently been found in mouse and human kidneys (25). Furthermore, the PD-1+ DN T cell subset was found to be the major responder during ischemia-reperfusion-induced AKI. We therefore examined the kidney DN T cell subset response to cisplatin. Cisplatin treatment did not affect the frequency of the NK1.1+ subset of DN, CD4+, or CD8+ T cells; however, the absolute number of NK1.1+ DN T cells decreased significantly at 24 and 72 h after cisplatin treatment (Fig. 5A). In addition, there was a significant decline in the frequency and absolute number of the PD-1+ DN T cell subset. There was no change in the frequency of PD-1+CD4+ and PD-1+CD8+ T cells; however, the absolute numbers of PD-1+CD4+ and PD-1+CD8+ T cells were significantly decreased (Fig. 5B). We found an increase in PD-1+NK1.1+ DN T cell frequency at 72 h.
Fig. 5.
Cisplatin affects both programmed cell death (PD)-1+ and natural killer (NK)1.1+ subsets of kidney double negative (DN) T cells. A: to understand the effect of cisplatin treatment on PD-1+ and NK1.1+ subsets of kidney DN T cells, we isolated kidney mononuclear cells from cisplatin-treated and vehicle-treated mice and assessed the frequency of different subsets with flow cytometry. The frequency and absolute numbers of the PD-1+ DN T cell subset significantly declined after cisplatin treatment. There was no change in the frequency of PD-1+CD4+ and PD-1+CD8+ T cells; however, the absolute numbers were significantly decreased. Cisplatin treatment did not affect the frequency of the NK1.1+ subset in DN, CD4+, or CD8+ T cells; however, the absolute number of NK1.1+ DN T cells decreased significantly at 24 and 72 h after cisplatin. The frequency of dual PD-1+NK1.1+ DN T cells increased at 72 h after cisplatin treatment. Data are presented as means ± SE from at least 5 independent experiments (n = 5 mice). *P < 0.05; **P < 0.01.
Kidney DN T cells protect PTECs from cisplatin induced-apoptosis.
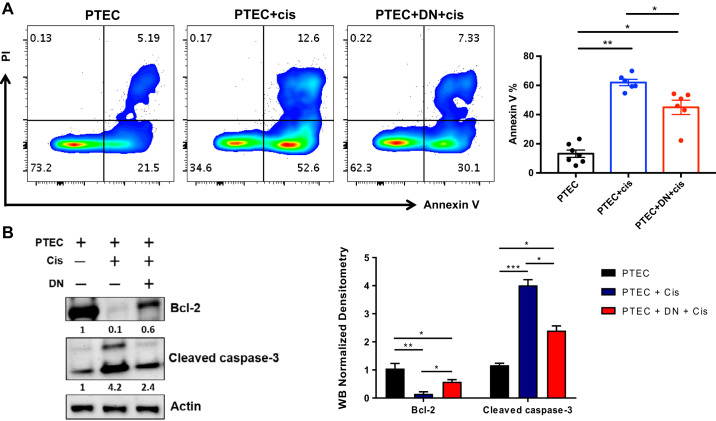
To explore functions of kidney DN T cells in maintaining kidney homeostasis and role in tissue protection, we cocultured flow-sorted kidney DN T cells with the PTEC cell line BUMPT. Flow-sorted DN T cells from WT mouse kidneys were activated by anti-CD3/CD28 antibodies for 24 h before being cocultured with mouse epithelial cells for an additional 24 h. Subsequently, cells were treated with a single dose of cisplatin (20 μM) and harvested after 72 h. Flow cytometric analysis showed a significant reduction in annexin V+ apoptotic PTECs cocultured with DN T cells compared with epithelial cells cultured alone (Fig. 6A). To identify the molecular pathways involved in the antiapoptotic effects of DN T cells on tubular epithelial cells, we investigated the expression of apoptosis signaling-related proteins in PTECs cultured with or without DN T cells after cisplatin treatment. Western blot analysis revealed that when PTECs were cocultured with DN T cells, levels of antiapoptotic protein Bcl-2 increased, whereas levels of proapoptotic cleaved caspase-3 were significantly decreased (Fig. 6B). These data support the hypothesis that DN T cells alleviate cisplatin-induced epithelial cytotoxicity by modulating the apoptotic pathway.
Fig. 6.
Double negative (DN) T cells protect proximal tubular epithelial cells from cisplatin (Cis)-induced apoptosis. To study the role of kidney DN T cells in kidney homeostasis and tissue protection, we cocultured DN T cells from wild-type mouse kidneys with proximal tubular epithelial cells (PTECs). Flow-sorted DN T cells were activated with anti-CD3/CD28 antibodies for 24 h before being cocultured with PTECs for 24 h. PTECs alone or in coculture were then treated with cisplatin (20 μM) and harvested after 72 h. A, left: representative flow cytometric plots showing a significant reduction in annexin V+ apoptotic PTECs cocultured with DN T cells compared with PTECs cultured alone. A, right: graph representing cumulative flow data from at least six different wells. B, left: Western blot analysis of PTECs alone or cocultured with DN T cells showing increased levels of Bcl-2, whereas the level of cleaved caspase-3 was significantly decreased. B, right: quantitative analysis of Western blots. Data are presented as means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001.
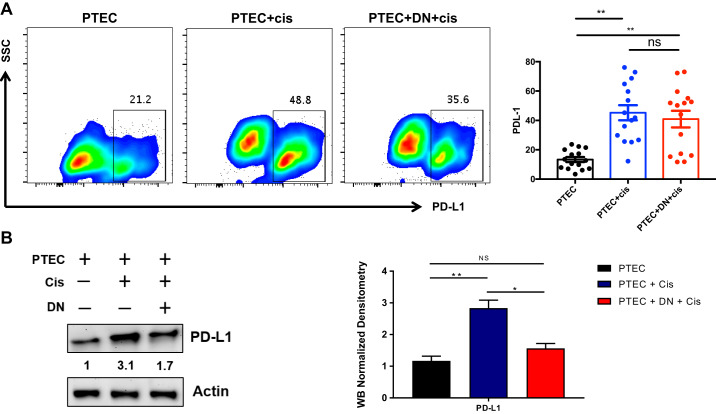
DN T cells suppress cisplatin-induced PD-L1 expression on kidney PTECs.
Since we found a more consistent alteration of the PD-1+ subset of kidney DN T cells after cisplatin injection in vivo, we next explored the expression of its ligand, PD-L1, on kidney epithelial cells. We observed a significant increase in PD-L1 expression in PTECs after cisplatin treatment for 72 h. Furthermore, analysis of cocultured PTECs with DN T cells demonstrated a trend toward reducing PD-L1 levels compared with PTECs cultured alone using FACs analysis (Fig. 7A). To evaluate if there was indeed a reduction in PD-L1 levels after DN T cell coculture, we directly quantified PD-L1 in PTECs using Western blot analysis. We observed a significant reduction in PD-L1 protein in PTECs in response to cisplatin treatment when PTECs were cocultured with DN T cell (Fig. 7B).
Fig. 7.
Double negative (DN) T cells reduce cisplatin (Cis)-induced programmed cell death ligand-1 (PD-L1) expression in proximal tubular epithelial cells (PTECs). A: we assessed the effect of DN T cell coculture on PTECs and quantified the expression of PD-L1. There was an increase in PD-L1 expression in PTECs after cisplatin treatment at 72 h. PTECs cocultured with DN T cells showed a trend toward reducing PD-L1 levels compared with PTECs cultured alone, but it was not statistically significant. B: to further confirm whether there was a reduction in PD-L1 levels after coculture, we directly quantified PD-L1 in PTECs using Western blot analysis. We observed a significant reduction in PD-L1 protein in PTECs in response to cisplatin treatment when PTECs were cocultured with DN T cells. Data are presented as means ± SE. *P < 0.05; **P < 0.01.
Adoptive transfer of DN T cells improves cisplatin-induced kidney injury.
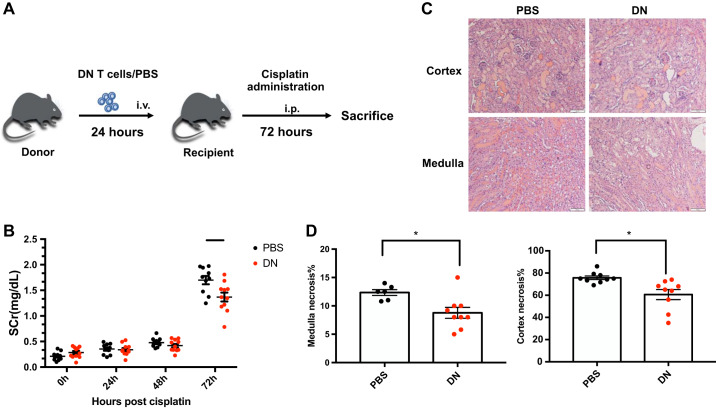
Given that kidney DN T cells suppressed apoptosis of PTECs after cisplatin treatment in vitro and our prior data demonstrated a protective role of DN T cells during ischemic AKI, we investigated whether prophylactic administration of DN T cells protected the kidney from cisplatin cytotoxicity in vivo. DN T cells used for adoptive transfer were isolated from the spleen and lymph nodes of gld mice as they harbor large numbers of DN T cells required for infusion studies. Previous studies have demonstrated that DN T cells from gld mice and WT mice show similar regulatory functions and ability to suppress conventional T cells (11, 22). We have also confirmed that adoptively transferred DN T cells (intraperitoneally or intravenously) can be detected in kidneys within 24 h (unpublished observations). In this experiment, we adoptively transferred (intravenously) DN T cells (1~1.5 × 106 cells) into 8- to 10-wk-old WT mice 24 h before cisplatin injection. Control mice received either CD4+ T cells or PBS. Mice received a single cisplatin injection (30 mg/kg body wt ip) and were assessed for structural (72 h) and functional changes (baseline and 24, 48, and 72 h) in the kidney (Fig. 8A). Our results showed that mice that received DN T cells demonstrated a significant decline in serum creatinine at 72 h compared with those that received either PBS (Fig. 8B) or CD4+ T cells (Supplemental Fig. S2). Histological examination of kidney tissue showed that mice that received DN T cells had reduced injury after cisplatin injection in both the medulla and cortex compared with PBS-treated mice (Fig. 8, C and D).
Fig. 8.
Adoptive transfer of double negative (DN) T cells improves cisplatin-induced acute kidney injury. A: DN T cells used for adoptive transfer were isolated from the spleen and lymph nodes of gld mice. We adoptively transferred 1~1.5 × 106 DN T cells into 8- to 10-wk-old wild-type mice. Control mice were treated with 100 μL PBS. Mice were administered cisplatin (30 mg/kg) 24 h after DN T cell transfer or PBS treatment and evaluated until 72 h postinjection. B: mice that received DN T cells had significantly reduced serum creatinine (SCr) at 72 h compared with those that received PBS. C: representative kidney sections from PBS-treated and DN T cell-transferred mice stained with hematoxylin and eosin showing necrotic tubules in the cortex and medulla. D: graphical representation of histological data showing that mice that received DN T cells had a reduced number of necrotic tubules after cisplatin injection in both the medulla and cortex compared with PBS-treated mice. Data are presented as means ± SE. *P < 0.05.
DISCUSSION
The pathophysiology of cisplatin-induced AKI involves many different molecular and cellular mechanisms including increased oxidative stress, inflammation, and apoptosis (12, 15, 29). There is a rapidly changing landscape of cancer chemotherapy with the success of immune checkpoint inhibitors that makes the immune responses to cisplatin even more clinically relevant (5, 13). We present data demonstrating a rapid increase in kidney DN T cells after cisplatin administration, accompanied by increased activation, apoptosis, expression of anti-inflammatory cytokine IL-10 and immune checkpoint molecule PD-1, and alterations in homing receptor CD44. This was in contrast to changes in kidney CD4+ and CD8+ T cells. We then demonstrated that cisplatin-induced apoptosis and PD-L1 expression in renal PTECs was reduced by coculture with DN T cells. Possible mechanisms for this include observed changes in caspase and IL-10 production by DN T cells. Adoptive transfer of DN T cells isolated from gld mice into cisplatin-treated mice significantly improved renal function and tubular injury, similar to previous observations in an ischemia-reperfusion model, and suggests important relevance for AKI prevention.
The kinetics of DN T cell proliferation in response to cisplatin treatment was distinct compared with conventional CD4+ and CD8+ T cells. We observed an early increase in the DN T cell population (24 h) followed by a rapid decline (72 h). Furthermore, the decline in DN T cell coincided with reduced kidney function at 72 h. The change in the DN T cell population in response to cisplatin was similar to our previous observations in ischemia-reperfusion model (21), indicating that kidney DN T cells respond quickly following an initial insult. Although both proliferation and apoptosis of DN T cells increased after cisplatin treatment, the dynamic relationships between the two processes could be responsible for the changes in DN T cell numbers observed at different time points after cisplatin treatment. Furthermore, high CD69 expression accompanied by low CD62L and CD44 expression at the 24-h time point indicated an early activation of DN T cells in response to cisplatin. In addition, we also found DN T cell upregulation of IL-10 expression at the 24-h time point in our cisplatin-induced AKI model. IL-10 is a potent immunoregulatory cytokine (26) that has been shown to ameliorate renal tissue injury (21) and also regulates various tumors (18, 28). We have previously demonstrated increased IL-10 expression by kidney DN T cells to be protective following ischemic AKI (21). However, further investigations are required to determine whether DN T cell-specific IL-10 is required for tissue protection during cisplatin-induced AKI and to explore its therapeutic potential. Based on recent findings that kidney DN T cells are composed of two subsets, PD-1 and NK1.1 (25), we examined these two subsets in cisplatin-induced AKI. We found that the PD-1 subset of DN T cells decreased rapidly following cisplatin treatment. This was different from our previous observations in ischemic AKI, where we found significant DN T cell expansion; thus, the cause of AKI alters DN T cell responses (25). Besides the PD-1 and NK1.1 subsets of DN T cells, it is likely that there are other currently undefined subsets that change during AKI. Our in vitro study showed a significant increase of its ligand, PD-L1, and proapoptotic markers (cleaved caspase-3) in PTECs after cisplatin treatment. Importantly, coculture of PTECs with DN T cells significantly reduced cisplatin-induced apoptosis, accompanied by an increase in antiapoptotic protein Bcl-2, as well as reduced PD-L1 expression by PTECs. The decrease in PD-L1 expression was clearly quantified by Western blot analysis, though a trend for this was seen by surface flow cytometric analysis. This could be a potential mechanism through which DN T cells protect kidney tissue from cisplatin-induced nephrotoxicity. Although recent exploitation of immune checkpoint therapy targeting PD-1 has resulted in a significant improvement in treating multiple cancers (5, 6, 27, 31), there has also been incidence of acute tubulointerstitial nephritis and immune complex-mediated glomerulonephritis reported in humans (4, 8, 10). Therefore, future studies will be required to better understand this PTEC-DN T cell relationship.
There were a number of limitations of our study. Our focus during the adoptive transfer experiments was pretreatment of cisplatin nephrotoxicity. We did not study the role of DN T cells in the recovery phase, which is also potentially important. We observed a decrease of both PD-1+ and NK1.1+ subsets but expansion of total DN T cells−and we have not studied what the other subsets could be. Furthermore, due to current limitations in harvesting sufficient numbers of DN T cells from kidneys in WT mice for adoptive transfer, we used DN T cells from gld mice, which are similar but not identical to WT mice. We also studied normal mice, not mice with cancers, and DN T cells could alter cancer growth. Nevertheless, our present findings provide novel information on DN T responses and their role in cisplatin-induced AKI. Furthermore, the interactions between cisplatin and DN T cell PD-1 could be important for patients who have received both traditional chemotherapy and novel immune checkpoint inhibitors for cancer.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-111209 and R01-DK-104662) and by Dr. Werner Jackstädt Foundation Scholarship S 134-10.117 (to J. T. Kurzhagen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.G., S.N., A.R.A.H., and H.R. conceived and designed research; J.G., J.H., M.S., and S.A.L. performed experiments; J.G., E.L.B., L.J.A., M.S., and S.A.L. analyzed data; J.G., S.N., M.S., J.T.K., A.R.A.H., and H.R. interpreted results of experiments; J.G. and J.H. prepared figures; J.G., S.N., A.R.A.H., and H.R. drafted manuscript; H.R. edited and revised manuscript; J.G., S.N., J.H., E.L.B., L.J.A., M.S., S.A.L., J.T.K., A.R.A.H., and H.R. approved final version of manuscript.
REFERENCES
- 1.Ascon DB, Ascon M, Satpute S, Lopez-Briones S, Racusen L, Colvin RB, Soloski MJ, Rabb H. Normal mouse kidneys contain activated and CD3+CD4−CD8− double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol 84: 1400–1409, 2008. doi: 10.1189/jlb.0907651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol 177: 3380–3387, 2006. doi: 10.4049/jimmunol.177.5.3380. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt K, Zhou L, Mi QS, Huang S, She JX, Dong Z. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol Med 16: 409–416, 2010. doi: 10.2119/molmed.2010.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottlaender L, Breton AL, de Laforcade L, Dijoud F, Thomas L, Dalle S. Acute interstitial nephritis after sequential ipilumumab - nivolumab therapy of metastatic melanoma. J Immunother Cancer 5: 57, 2017. doi: 10.1186/s40425-017-0261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28: 3167–3175, 2010. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465, 2012. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan AJ, Alikhan MA, Odobasic D, Gan PY, Khouri MB, Steinmetz OM, Mansell AS, Kitching AR, Holdsworth SR, Summers SA. Innate IL-17A-producing leukocytes promote acute kidney injury via inflammasome and Toll-like receptor activation. Am J Pathol 184: 1411–1418, 2014. doi: 10.1016/j.ajpath.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740: 364–378, 2014. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escandon J, Peacock S, Trabolsi A, Thomas DB, Layka A, Lutzky J. Interstitial nephritis in melanoma patients secondary to PD-1 checkpoint inhibitor. J Immunother Cancer 5: 3, 2017. doi: 10.1186/s40425-016-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamad AR, Mohamood AS, Trujillo CJ, Huang CT, Yuan E, Schneck JP. B220+ double-negative T cells suppress polyclonal T cell activation by a Fas-independent mechanism that involves inhibition of IL-2 production. J Immunol 171: 2421–2426, 2003. doi: 10.4049/jimmunol.171.5.2421. [DOI] [PubMed] [Google Scholar]
- 12.Iwakura T, Zhao Z, Marschner JA, Devarapu SK, Yasuda H, Anders HJ. Dipeptidyl peptidase-4 inhibitor teneligliptin accelerates recovery from cisplatin-induced acute kidney injury by attenuating inflammation and promoting tubular regeneration. Nephrol Dial Transplant 34: 1669–1680, 2019. doi: 10.1093/ndt/gfy397. [DOI] [PubMed] [Google Scholar]
- 13.Jaworska K, Ratajczak J, Huang L, Whalen K, Yang M, Stevens BK, Kinsey GR. Both PD-1 ligands protect the kidney from ischemia reperfusion injury. J Immunol 194: 325–333, 2015. doi: 10.4049/jimmunol.1400497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HR, Lee MK, Park AJ, Park ES, Kim DS, Ahn J, Kim J, Kim SH, Oh DJ. Reduction of natural killer and natural killer T cells is not protective in cisplatin-induced acute renal failure in mice. Nephrology (Carlton) 16: 545–551, 2011. doi: 10.1111/j.1440-1797.2011.01473.x. [DOI] [PubMed] [Google Scholar]
- 15.Landau SI, Guo X, Velazquez H, Torres R, Olson E, Garcia-Milian R, Moeckel GW, Desir GV, Safirstein R. Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int 95: 797–814, 2019. doi: 10.1016/j.kint.2018.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Nho D, Chung HS, Lee H, Shin MK, Kim SH, Bae H. CD4+CD25+ regulatory T cells attenuate cisplatin-induced nephrotoxicity in mice. Kidney Int 78: 1100–1109, 2010. doi: 10.1038/ki.2010.139. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Liu Z, Tang C, Cai J, Dong Z. FGF21 is induced in cisplatin nephrotoxicity to protect against kidney tubular cell injury. FASEB J 32: 3423–3433, 2018. doi: 10.1096/fj.201701316R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Ma Y, Xu Y. Follicular regulatory T cells infiltrated the ovarian carcinoma and resulted in CD8 T cell dysfunction dependent on IL-10 pathway. Int Immunopharmacol 68: 81–87, 2019. doi: 10.1016/j.intimp.2018.12.051. [DOI] [PubMed] [Google Scholar]
- 19.Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, Rabb H. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol 17: 765–774, 2006. doi: 10.1681/ASN.2005010102. [DOI] [PubMed] [Google Scholar]
- 20.Martina MN, Bandapalle S, Rabb H, Hamad AR. Isolation of double negative αβ T cells from the kidney. J Vis Exp 2014. doi: 10.3791/51192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martina MN, Noel S, Saxena A, Bandapalle S, Majithia R, Jie C, Arend LJ, Allaf ME, Rabb H, Hamad AR. Double-negative αβ T cells are early responders to AKI and are found in human kidney. J Am Soc Nephrol 27: 1113–1123, 2016. doi: 10.1681/ASN.2014121214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohamood AS, Bargatze D, Xiao Z, Jie C, Yagita H, Ruben D, Watson J, Chakravarti S, Schneck JP, Hamad AR. Fas-mediated apoptosis regulates the composition of peripheral alphabeta T cell repertoire by constitutively purging out double negative T cells. PLoS One 3: e3465, 2008. doi: 10.1371/journal.pone.0003465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest 124: 2355–2363, 2014. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okusa MD, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative XIII Workgroup . Therapeutic targets of human AKI: harmonizing human and animal AKI. J Am Soc Nephrol 27: 44–48, 2016. doi: 10.1681/ASN.2015030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadasivam M, Noel S, Lee SA, Gong J, Allaf ME, Pierorazio P, Rabb H, Hamad ARA. Activation and proliferation of PD-1+ kidney double-negative T cells is dependent on nonclassical MHC proteins and IL-2. J Am Soc Nephrol 30: 277–292, 2019. doi: 10.1681/ASN.2018080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 74: 27–34, 2015. doi: 10.1016/j.cyto.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt LH, Kümmel A, Görlich D, Mohr M, Bröckling S, Mikesch JH, Grünewald I, Marra A, Schultheis AM, Wardelmann E, Müller-Tidow C, Spieker T, Schliemann C, Berdel WE, Wiewrodt R, Hartmann W. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS One 10: e0136023, 2015. doi: 10.1371/journal.pone.0136023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vahl JM, Friedrich J, Mittler S, Trump S, Heim L, Kachler K, Balabko L, Fuhrich N, Geppert CI, Trufa DI, Sopel N, Rieker R, Sirbu H, Finotto S. Interleukin-10-regulated tumour tolerance in non-small cell lung cancer. Br J Cancer 117: 1644–1655, 2017. doi: 10.1038/bjc.2017.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, Arsenijevic N. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci 26: 25, 2019. doi: 10.1186/s12929-019-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Q, Su J, Dong G, Zhang M, Huo Y, Dong Z. Glycolysis inhibitors suppress renal interstitial fibrosis via divergent effects on fibroblasts and tubular cells. Am J Physiol Renal Physiol 316: F1162–F1172, 2019. doi: 10.1152/ajprenal.00422.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Ma Y, Lv C, Huang M, Li M, Dong B, Liu X, An G, Zhang W, Zhang J, Zhang L, Zhang S, Yang Y. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci 107: 1563–1571, 2016. doi: 10.1111/cas.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]