Abstract
In ANG II-dependent hypertension, ANG II activates ANG II type 1 receptors (AT1Rs), elevating blood pressure and increasing renal afferent arteriolar resistance (AAR). The increased arterial pressure augments interstitial ATP concentrations activating purinergic P2X receptors (P2XRs) also increasing AAR. Interestingly, P2X1R and P2X7R inhibition reduces AAR to the normal range, raising the conundrum regarding the apparent disappearance of AT1R influence. To evaluate the interactions between P2XRs and AT1Rs in mediating the increased AAR elicited by chronic ANG II infusions, experiments using the isolated blood perfused juxtamedullary nephron preparation allowed visualization of afferent arteriolar diameters (AAD). Normotensive and ANG II-infused hypertensive rats showed AAD responses to increases in renal perfusion pressure from 100 to 140 mmHg by decreasing AAD by 26 ± 10% and 19 ± 4%. Superfusion with the inhibitor P2X1Ri (NF4490; 1 μM) increased AAD. In normotensive kidneys, superfusion with ANG II (1 nM) decreased AAD by 16 ± 4% and decreased further by 19 ± 5% with an increase in renal perfusion pressure. Treatment with P2X1Ri increased AAD by 30 ± 6% to values higher than those at 100 mmHg plus ANG II. In hypertensive kidneys, the inhibitor AT1Ri (SML1394; 1 μM) increased AAD by 10 ± 7%. In contrast, treatment with P2X1Ri increased AAD by 21 ± 14%; combination with P2X1Ri plus P2X7Ri (A438079; 1 μM) increased AAD further by 25 ± 8%. The results indicate that P2X1R, P2X7R, and AT1R actions converge at receptor or postreceptor signaling pathways, but P2XR exerts a dominant influence abrogating the actions of AT1Rs on AAR in ANG II-dependent hypertension.
Keywords: angiotensin II, angiotensin II type 1 receptors, hypertension, P2X receptors, renal afferent arteriole
INTRODUCTION
In ANG II-induced hypertension, ANG II activates ANG II type 1 receptors (AT1Rs) inducing systemic hypertension and vasoconstriction (3, 50). Chronic activation of AT1Rs elicits renal vasoconstriction and contributes to renal injury (16, 42). In particular, chronic ANG II infusions elicit sustained renal afferent arteriolar vasoconstriction as supported by the increased intrarenal levels of ANG II (46) and vasodilator responses to AT1R blockade (47). However, purinergic P2X receptors (P2XRs) also exert substantial afferent arteriolar vasoconstriction in ANG II-induced hypertension, as evidenced by augmented levels of renal interstitial ATP (14) and renal microvascular responses to P2XR blockade (12).
Under normal and hypertensive conditions, there is a direct association between renal perfusion pressure (RPP) and renal interstitial ATP (14, 36, 37, 39) derived from sheer stress-induced release of ATP as well as release from macula densa cells stimulated by tubuloglomerular feedback signals (1). Further support is provided by a study that used an ATP-sensitive biosensor and reported that increases in arterial pressure increase cortical ATP levels (39). Studies using the juxtamedullary nephron preparation have demonstrated that interstitial ATP activates P2X1Rs and elicits afferent arteriolar vasoconstriction contributing to the autoregulatory response to increases in perfusion pressure (20). Activation of P2XRs by increased concentrations of ATP has been implicated in the genesis and maintenance of salt-sensitive hypertension (14, 26), DOCA-salt-induced hypertension (27), and ANG II-induced hypertension (14). Interestingly, in a micropuncture study in ANG II-dependent hypertension, P2X1R or P2X7R blockade returned renal afferent arterial resistance to near-normal values even though blood pressure remained elevated, indicating the persistence of elevated systemic ANG II concentrations (12). These results provide basic evidence implicating a complex interaction between P2X1R, P2X7R, and AT1R in regulating renal afferent arterial resistance and raise the conundrum of how P2XR and AT1R can both be responsible for the increased afferent arteriolar vascular resistance existing in hypertensive conditions.
A possible explanation that resolves the conundrum is that P2XR and AT1R share common receptor or postreceptor signaling mechanisms, which converge to elicit renal vasoconstriction in ANG II-induced hypertension. Accordingly, we posited that P2XR and AT1R share pos-receptor signaling pathways but that P2XRs are dominant in the control of afferent arteriolar resistance. Because in in vivo studies AT1R inhibition markedly reduces arterial pressure, which reduces interstitial ATP levels, the in vitro juxtamedullary nephron preparation was selected to allow maintenance of perfusion pressure at hypertensive levels as well as direct visualization of afferent arteriolar responses to AT1R, P2X1R, and P2X7R inhibition either individually or combined.
MATERIALS AND METHODS
The experimental protocol was approved by Tulane University Institutional Animal Care and Use Committee. Sprague-Dawley male rats weighing 220–250 g (Charles River Laboratories) were housed in metabolic cages and maintained in a temperature-controlled room regulated on a 12:12-h light-dark cycle with free access to water and standard rat chow.
Induction of Hypertension
ANG II was infused into male Sprague-Dawley rats (220–250 g, Sigma, St. Louis, MO) via subcutaneous osmotic minipumps (Alzet model 2002, Durect, Cupertino, CA) implanted while the rats were under isoflurane anesthesia. A minipump delivered ANG II at a rate of 80 ng/min. Experiments were performed 13–14 days after the initiation of ANG II infusion. Hypertensive rats prepared in this manner have been shown to have increased renal interstitial ATP and ANG II tissue levels (14, 31, 46).
Measurement of Systolic Blood Pressure
Systolic blood pressure in awake rats was measured using tail-cuff plethysmography. In ANG II-infused rats, systolic blood pressure increased significantly throughout the duration of 13–14 days from 111 ± 5 to 183 ± 6 mmHg (n = 25). In normotensive rats, the range of systolic blood pressure was 101–127 mmHg (x̄ = 109 ± 7 mmHg, n = 15).
Juxtamedullary Nephron Preparation
Afferent arteriolar diameters (AAD) were assessed in vitro using the isolated blood-perfused juxtamedullary nephron technique combined with videomicroscopy, as previously described (11, 41). Each experiment used one male Sprague-Dawley rat (Charles River Laboratories) weighing 300–380 g serving as the kidney donor as well as the blood donor. Rats were anesthetized with pentobarbital sodium (50 mg/kg ip), and a cannula was inserted in the left carotid artery for blood collection. Donor blood was collected in a heparinized (500 units) syringe via the carotid arterial cannula and centrifuged to separate the plasma and cellular fractions. The buffy coat was removed and discarded. After sequential passage of the plasma through 5-µm and 0.22-µm filters (Gelman Sciences, Ann Arbor, MI), erythrocytes were added to achieve a hematocrit of 33%. This reconstituted blood was passed through a 5-µm nylon mesh and thereafter stirred continuously in a closed reservoir that was pressurized with a 95% O2-5% CO2 gas mixture. The right kidney was perfused through a cannula inserted in the superior mesenteric artery and advanced into the right renal artery. The perfusate was Tyrode solution (pH 7.4) containing 5.1% BSA and a mixture of l-amino acids. The kidney was excised and sectioned longitudinally, retaining the papilla intact with the perfused dorsal two-thirds of the organ. The papilla was reflected to expose the pelvic mucosa and tissue covering the inner cortical surface. Overlying tissue was removed to expose the tubules, glomeruli, and related vasculature of the juxtamedullary nephrons. The arterial supply of the exposed microvasculature was isolated by ligating the larger branches of the renal artery. After the dissection was completed, the Tyrode perfusate was replaced with the reconstituted blood. Perfusion pressure was monitored by a pressure catheter centered in the tip of the perfusion cannula. RPP was regulated by adjusting the rate of gas inflow into the blood reservoir and set at 100 mmHg. The inner cortical surface of the kidney was continuously superfused with warmed (37°C) Tyrode solution containing 1% BSA. The tissue was transilluminated on the fixed stage of a microscope (Nikon) equipped with a water immersion objective (40). Video images of the microvessels were transferred by a Newvicon camera (model NC-67M, Dage-MTI, Michigan City, IN) through an image enhancer (model MFJ-1452, MFJ Enterprises, Starkville, MS) to a video monitor (Conrac Display Systems, Covina, CA), and single afferent arterioles were visualized. The video signal was recorded on DVD for later analysis. AADs were measured at 5-min intervals using a calibrated digital image-shearing monitor (Instrumentation for Physiology and Medicine, San Diego, CA).
Experimental Protocols
A single afferent arteriole close to its glomerulus that showed robust blood flow was selected for study. After a 10-min equilibration period, an experimental protocol was initiated, consisting of three to five periods of a 5-min duration. For each treatment, doses of the inhibitor compounds were selected on the basis of pilot experiments, and the following doses were used in the study: 1) 1 µM AT1 receptor inhibitor (SML1394, olmesartan, Sigma), 2) 1 µM P2X1R inhibitor (NF449, Calbiochem), and 3) 1 µM P2X7R inhibitor (A438079, Calbiochem).
Autoregulatory Responsiveness
Initially, the autoregulatory responses were assessed by measurement of AAD at a control RPP of 100 mmHg and following an acute increase in RPP to 140 mmHg. AADs were measured in kidneys from normotensive (n = 15) and hypertensive (n = 25) rats.
Experiments on Kidneys From Normotensive Rats
Effects of AT1R, P2X1R, and P2X7R blockade on AAD at increased RPP of 140 mmHg.
AAD was measured at a RPP of 100 mmHg and at increased RPP to 140 mmHg, which was maintained. To evaluate the influence of AT1R and P2XR, AAD was determined before and during treatment with each selective blocker by superfusion of solutions containing 1 µM SML1394 (n = 5) or 1 µM NF449 (n = 5) or 1 µM A438079 (n = 5).
Blockade of ATP action on afferent arterioles with P2X1R and P2X7R blockers.
Because ATP is the endogenous ligand for P2XRs, these experiments were designed to determine the efficacy of P2XR blockers in reversing the vasoconstrictor action of 5 µM ATP (Sigma). AAD was measured at a RPP of 100 mmHg before and during treatment with ATP alone and with superimposed P2X1R blockade with 1 µM NF449 (n = 4) or P2X7R blockade with 1 µM A438079 (n = 4) and then with combined 1 µM NF449 plus 1 µM A438079 in each group.
Effect of AT1R and P2X1R blockade on AAD during treatment with ANG II at a RPP of 100 mmHg followed by an increase in RPP to 140 mmHg.
The effect of ANG II on AAD was assessed using ANG II (1.0 nM) with RPP maintained at 100 mmHg (n = 7). After a 5-min control period, RPP was increased to 140 mmHg with continuous superfusion with ANG II, and AAD was determined. The P2X1R blocker (NF449) was then superfused for 5 min followed by the addition of the AT1R blocker (SML1394) to determine the residual effect of ANG II on AAD.
Experiments on Kidneys From Hypertensive Rats
Effect of AT1R, P2X1R, and P2X7R blockade on afferent arterioles maintained at increased RPP in hypertensive rat kidneys.
These experiments were designed to determine the role of the AT1R on afferent arterioles (n = 5) of chronic ANG II-infused hypertensive rats, which have increased renal interstitial ATP as well as elevated ANG II levels. AAD was measured at perfusion pressures of 100 and 140 mmHg. With RPP maintained at 140 mmHg, the tissue was superfused with 1 µM SML1394, and AAD was determined. The superfusate was changed to 1 µM NF449, and AAD was determined. In addition, AAD responses to combined treatment with NF449 plus A439079 were determined.
Effect of AT1R, P2X7R, and P2X1R plus P2X7R blockade on AAD at increased RPP.
These experiments were designed to evaluate the effects of AT1R blockade on afferent arterioles (n = 5). While RPP was held at 140 mmHg, the superfusion solution containing 1 µM SML1394 was superfused, and the change in ADD was determined. Subsequently, the superfusate was changed to 1 µM A439079, and AAD was determined. Finally, arteriolar responses to combined treatment with NF449 plus A439079 were determined.
Effect of P2X1R and AT1R blockade on AAD at increased RPP.
This series was designed to determine the effects of P2X1R blockade to vasodilate afferent arterioles at elevated RPP followed by additional AT1R inhibitor (n = 5). RPP was increased from 100 to 140 mmHg; the arteriolar response to 1 µM NF449 was then determined. After 5 min, during the continued presence of NF449 effect, the superfusion solution with 1 µM SML1394 was added, and the additional change in AAD was determined.
Effect of P2X7R and AT1R blockade on AAD at increased RPP.
These experiments were performed to determine the effects of P2X7R blockade to vasodilate afferent arterioles (n = 5). RPP was increased from 100 to 140 mmHg. The change in AAD following superfusion with 1 µM A438079 was determined. After 5 min with continued treatment with A438079 effect, 1 µM SML1394 was added, and AAD was determined.
Effects of P2X1R plus P2X7R and AT1R blockade on AAD following increases in RPP to 140 mmHg.
These experiments were performed to determine the effects of combined treatment with P2X1R plus P2X7R blockade on AAD with superimposed AT1R blockade (n = 5). Following the increase in RPP from 100 to 140 mmHg, the arteriolar response to 1 µM NF449 plus 1 µM A438079 was determined. After 5 min in the continued presence of NF449 plus A438079, the effect of superfusion with 1 µM SML1394 was determined.
Statistical Analysis
Data were evaluated using Prism software (Graph Pad, San Diego, CA). Results were evaluated using one-way ANOVA with repeated measures followed by the Newman-Keuls multiple-range test. P values of <0.05 were considered statistically significant. All values are expressed as means ± SD.
RESULTS
Systolic Blood Pressure Measured in Awake Rats During the Development of Hypertension
In ANG II-infused rats, systolic blood pressure increased progressively throughout the duration of 13–14 days from 111 ± 5 to 183 ± 6 mmHg (n = 25). In normotensive rats, the range of systolic blood pressure was 101–127 mmHg with an average of 109 ± 7 mmHg (n = 15).
Autoregulatory Capability in Kidneys From Normotensive and Hypertensive Rats
Changes in AAD were measured at the control RPP of 100 mmHg and in response to increases in RPP, which elicited pressure-mediated autoregulatory reductions in AAD. Under control conditions of 100 mmHg, AAD from normotensive rats averaged 12.9 ± 1.2 μm and decreased by 26 ± 10% (9.5 ± 1.6 μm, n = 15, P < 0.05) when RPP was increased to 140 mmHg. In kidneys from ANG II-infused hypertensive rats, the increase in RPP from 100 to 140 mmHg decreased AAD by 19 ± 4% (14.9 ± 0.1 vs. 12 ± 0.2 μm, n = 25, P < 0.05), suggesting reduced autoregulatory capability in AAD from hypertensive rats, as has been previously reported (19).
Experiments in Kidneys From Normotensive Rats
AAD responses to AT1R, P2X1R, and P2X7R inhibition at increased RPP.
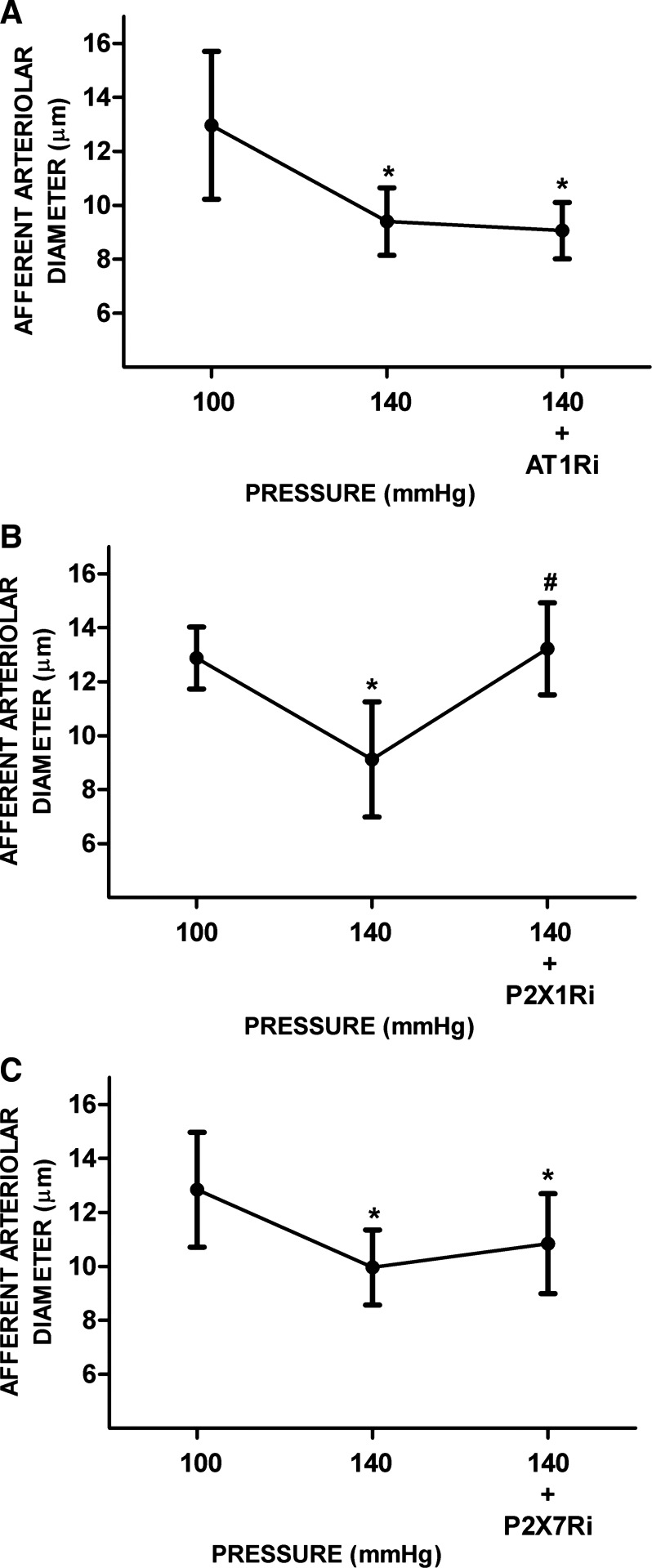
In separate experiments, we determined the effects of AT1R, P2X1R, and P2X7R inhibition on AAD after pressure-mediated autoregulatory responses. In this series, increasing RPP from 100 to 140 mmHg decreased AAD from 12.9 ± 2 to 9.4 ± 1.6 μm. Treatment with AT1R inhibitor alone did not alter AAD (9.4 ± 1.3 vs. 9 ± 1.1 μm, n = 5; Fig. 1A). Upon treatment with P2X1R inhibitor, AAD returned to a value similar to that at a RPP of 100 mmHg (from 9.1 ± 2.1 to 13.2 ± 1.7 μm, n = 5, P < 0.05; Fig. 1B). Treatment with P2X7R inhibitor did not vasodilate AAD as much (from 10 ± 1.4 to 10.8 ± 1.9 μm, n = 5, P < 0.05; Fig. 1C), indicating a minor role of P2X7R in the augmented preglomerular resistance in normal kidneys, consistent with a study that showed a lower abundance of P2X7R protein in kidneys from normotensive rats (12).
Fig. 1.
Responses of afferent arterioles to ANG II type 1 receptor (AT1R), purinergic P2X1 receptor (P2X1R), and purinergic P2X7 receptor (P2X7R) inhibition under renal perfusion pressure (RPP) at 140 mmHg in kidneys from normotensive rats. A−C: afferent arteriolar responses to AT1R inhibitor (AT1Ri; A), P2X1R inhibitor (P2X1Ri; B), and P2X7R inhibitor (P2X7Ri; C). Data are expressed as means ± SD; n = 5 per group. *P < 0.05 vs. RPP at 100 mmHg; #P < 0.05 vs. RPP at 140 mmHg.
AAD responses to ATP followed by P2X1R and P2X7R blockade.
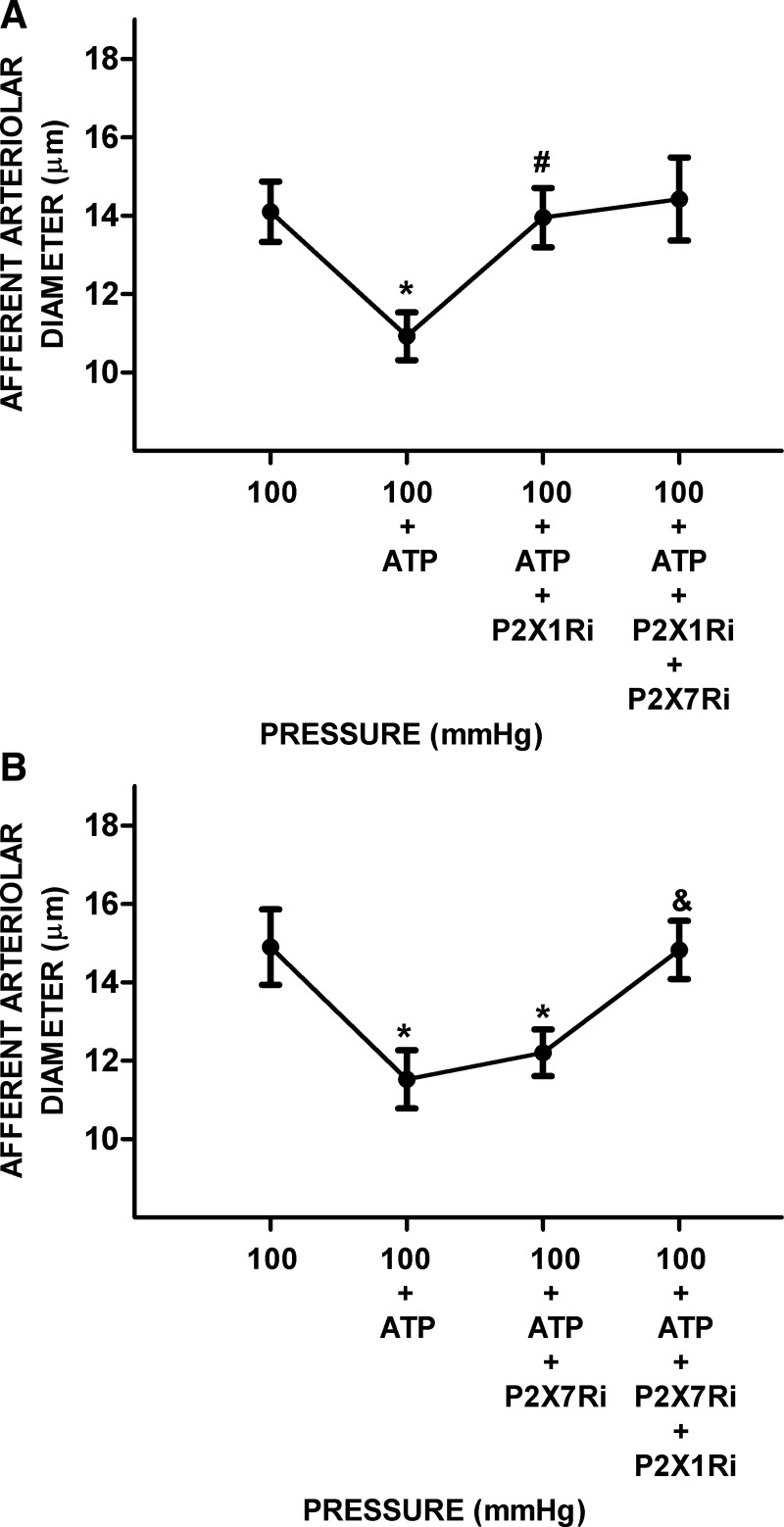
The effects of 5 µM ATP on AAD at control normotensive pressure showed a rapid initial vasoconstriction from a control of 14.5 ± 0.9 μm to 11.2 ± 0.7 μm (n = 8, P < 0.05). P2X1R inhibition restored AAD from 10.9 ± 0.6 to 14.0 ± 0.8 μm (P < 0.05). Further treatment with combined P2X1R plus P2X7R inhibitors did not cause further vasodilation (Fig. 2A). In separate experiments, P2X7R inhibitors did not vasodilate AAD significantly (12.2 ± 0.6 vs. 11.5 ± 0.7 μm). However, combined treatment with P2X1R plus P2X7R inhibitor returned AAD to values similar to control (14.8 ± 0.7 μm; Fig. 2B).
Fig. 2.
Afferent arteriolar responses to ATP alone followed by ATP plus inhibition of P2X1 receptor (P2X1R) or the combination of P2X1R + P2X7 receptor (P2X7R) inhibition in kidneys from normotensive rats at baseline pressure. After steady-state diameters were established, ATP (5 µmol/L) was superfused over the blood vessels. A: afferent arteriolar responses to ATP followed by ATP plus P2X1R inhibitor (P2X1Ri) and ATP plus P2X1Ri and P2X7R inhibitor (P2X7Ri). B: the order of the inhibitors was changed, and afferent arteriolar responses to ATP and ATP plus P2X7Ri were measured followed by ATP under P2X1Ri + P2X7Ri (n = 4 per group). Data are expressed as means ± SD. *P < 0.05 vs. renal perfusion pressure (RPP) at 100 mmHg; #P < 0.05 vs. RPP at 100 mmHg + ATP; &P < 0.05 vs. RPP at 100 mmHg + ATP + P2X7Ri.
Effects of P2X1R and AT1R inhibition in the presence of elevated ANG II and increased RPP.
To simulate conditions in kidneys from hypertensive rats, we tested the acute effects of P2X1R inhibition in the presence of elevated ANG II and increased RPP. ANG II (1 nM) caused significant decreases in AAD by 16 ± 4% (11.9 ± 1.2 vs. 10.0 ± 1.4 μm); an increase in RPP caused further decreases in AAD by 19 ± 5% to 8.1 ± 0.7 μm (n = 7, P < 0.05). Treatment with P2X1R inhibitor significantly increased AAD by 30 ± 6% to values greater than the value observed at 100 mmHg plus ANG II (10.5 ± 1.1 μm) and similar to the baseline value at 100 mmHg. In the presence of P2X1R inhibition, further treatment with AT1R inhibitor increased AAD only slightly more by 11 ± 7% (11.6 ± 1.1 μm), bringing it closer to the baseline value at 100 mmHg (11.9 ± 1.2 μm; Fig. 3).
Fig. 3.
Acute effect of ANG II infusion in afferent arterioles under high renal perfusion pressure (RPP) followed by P2X1 receptor (P2X1R) or ANG II type 1 receptor (AT1R) inhibition in normotensive rats. The afferent arteriolar response to ANG II (1 nmol/L) superfusion of the vessel at 100 mmHg was followed by a step increase in RPP to reach 140 mmHg. Superfusion of the P2X1R inhibitor (P2X1Ri) was then followed by the AT1R inhibitor (AT1Ri; n = 7). Data are expressed as means ± SD. *P < 0.05 vs. RPP at 100 mmHg; #P < 0.05 vs. RPP at 100 mmHg + ANG II; &P < 0.05 vs. RPP at 140 mmHg + ANG II.
Experiments in Kidneys From Hypertensive Rats
Interactions between P2XR and AT1R on AAD at elevated RPP.
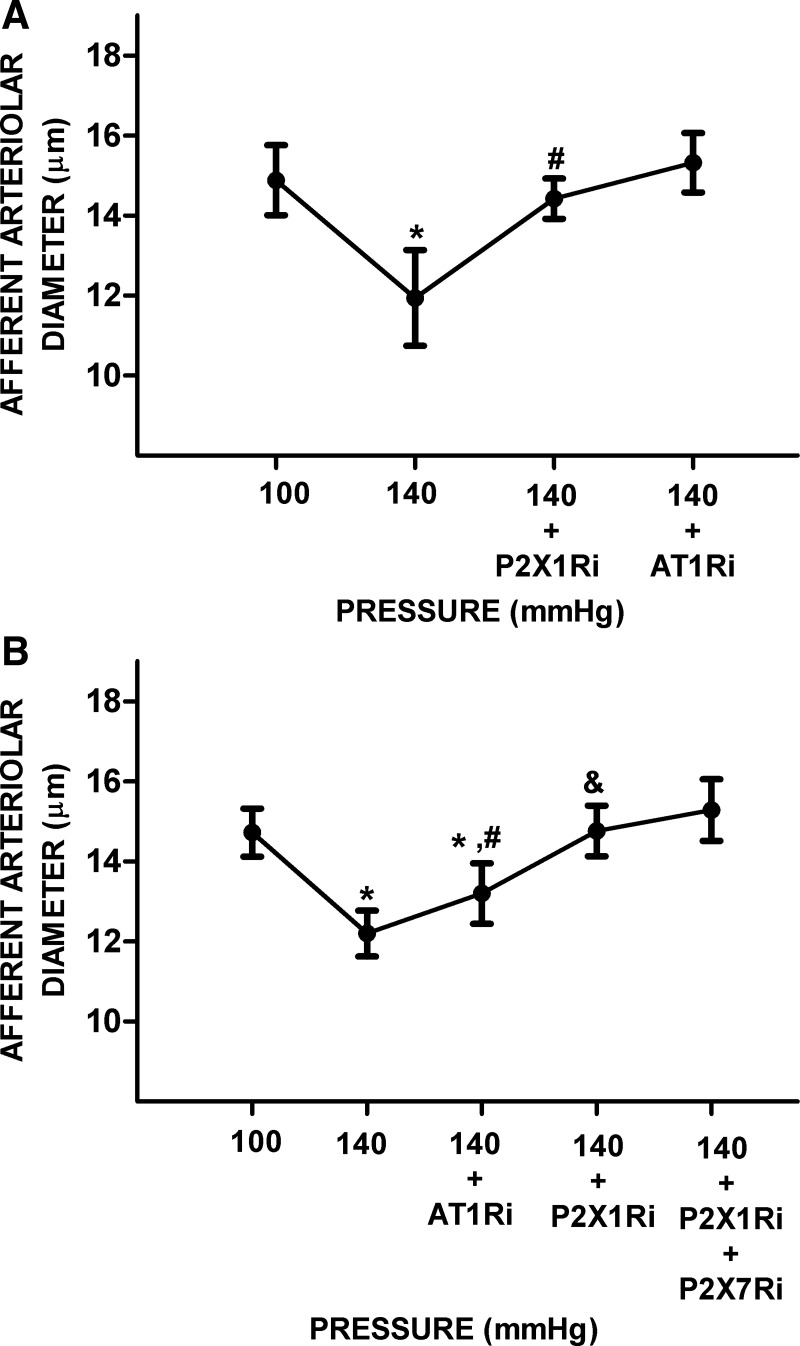
ANG II-infused hypertensive rats have increased interstitial ATP and ANG II tissue levels, indicating that, under these conditions, it is possible that both P2XR and AT1R regulate AAD. In this series, we determined the interactions between P2X1R, P2X7R, and AT1R on AAD at the increased perfusion pressure. An increase in RPP from 100 to 140 mmHg significantly decreased AAD by 20 ± 5% (14.9 ± 0.9 vs. 11.9 ± 1.2 μm, P < 0.05). Treatment with P2X1R inhibitor significantly increased AAD by 20 ± 14% (14.4 ± 0.5 μm, P < 0.05) to values similar to those observed at a RPP of 100 mmHg; AAD increased slightly to 15.3 ± 0.7 μm upon treatment with the AT1R blocker (Fig. 4A). In separate experiments, initial treatment with the AT1R inhibitor increased AAD by 8 ± 5% (13.2 ± 0.8 vs. 12.2 ± 0.6 μm, P < 0.05). Superimposed treatment with P2X1R inhibitor significantly increased AAD by 12 ± 5% (14.8 ± 0.6 μm, P < 0.05) to values similar to those at a RPP of 100 mmHg (14.7 ± 0.6 μm). Further treatment with P2X1R plus P2X7R inhibitors elicited further increases in AAD to 15.3 ± 0.8 μm, which were similar to baseline values (Fig. 4B).
Fig. 4.
Effect of ANG II type 1 receptor (AT1R) and P2X1 (P2X1R) and P2X7 receptor (P2X7R) blockade on afferent arteriolar diameter under high renal perfusion pressure (RPP) in kidneys from hypertensive rats. A: afferent arteriolar responses to increases in RPP from 100 to 140 mmHg were tested followed by P2X1R inhibitor (P2X1Ri) and then AT1R inhibitor (AT1Ri). B: the order of the inhibitors was reversed. First, afferent arteriolar responses to increases in RPP were followed by AT1Ri and P2X1R blockade (P2X1Ri); then, the combination of P2X1R plus P2X7R inhibitor (P2X1Ri) was tested. Data are expressed as means ± SD; n = 5 per group. *P < 0.05 vs. RPP at 100 mmHg; #P < 0.05 vs. RPP at 140 mmHg; &P < 0.05 vs. RPP at 140 mmHg + AT1Ri.
Effects of AT1R, P2X7R, and P2X1R plus P2X7R blockade on AAD at elevated RPP.
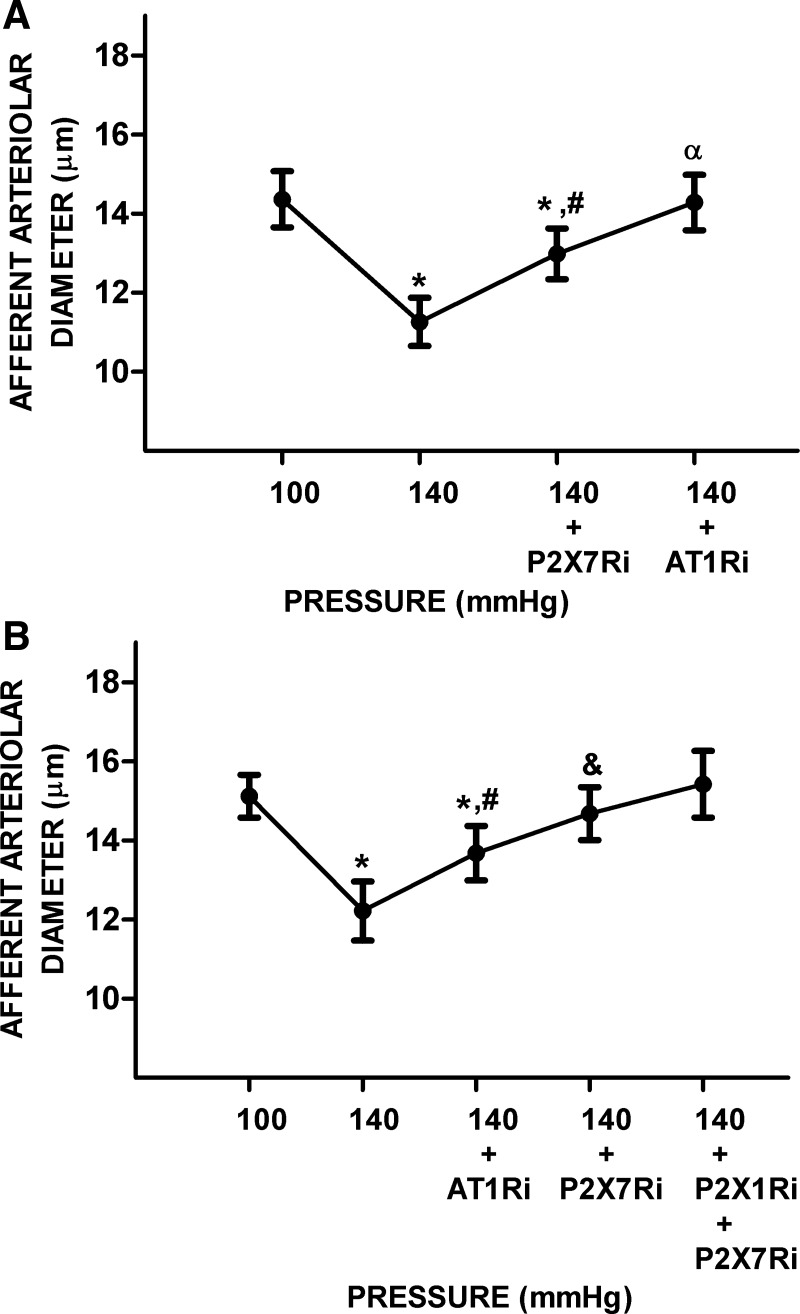
In this series, increasing RPP from 100 to 140 mmHg decreased diameters by 22 ± 1% (14.4 ± 0.7 vs. 11.3 ± 0.6 μm, P < 0.05). Treatment with the P2X7R inhibitor significantly increased AAD by 15 ± 3% (13.0 ± 0.6 μm, P < 0.05). AAD increased further by 10 ± 1% (14.3 ± 0.7 μm, P < 0.05) by treatment with the AT1R inhibitor (Fig. 5A). In separate experiments, initial treatment with the AT1R blocker increased AAD by 12 ± 8% (13.7 ± 0.7 vs. 12.2 ± 0.7 μm, P < 0.05). Further treatment with P2X7R inhibitor significantly increased AAD by 7 ± 5% (14.7 ± 0.7 μm, P < 0.05) to a value similar to that at a RPP of 100 mmHg (15.1 ± 0.5 μm). Additional treatment with P2X1R plus P2X7R inhibitors fully restored AAD to 15.4 ± 0.8 μm (Fig. 5B).
Fig. 5.
Effects of P2X7 receptor (P2X7R) or ANG II type 1 receptor (AT1R) inhibition alone and P2X1 receptor (P2X1R) plus P2X7R blockade on the afferent arteriolar response to an increase in renal perfusion pressure (RPP) in hypertensive rats. A: afferent arteriolar responses to increases in RPP were followed by P2X7R inhibitor (P2X7Ri) and AT1R inhibitor (AT1Ri). B: afferent arteriolar responses to increases in RPP followed by AT1Ri and P2X7R inhibitor (P2X7Ri) and the combination of P2X1Ri plus P2X7Ri. Data are expressed as means ± SD; n = 5 per group. *P < 0.05 vs. RPP at 100 mmHg; #P < 0.05 vs. RPP at 140 mmHg; &P < 0.05 vs. RPP at 140 mmHg + AT1Ri; αP < 0.05 vs. RPP at 140 mmHg + P2X7Ri.
Effect of P2X1R plus P2X7R and AT1R blockade on AAD at elevated RPP.
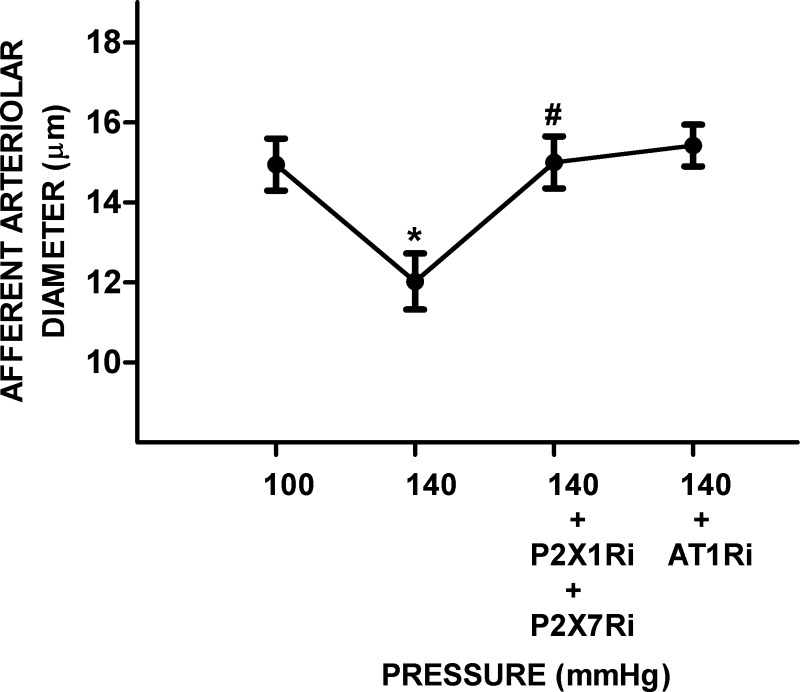
In this series, increasing RPP from 100 to 140 mmHg significantly decreased diameters by 19 ± 5% (14.9 ± 0.7 vs. 12 ± 0.7 μm, P < 0.05). Treatment with P2X1R plus P2X7R inhibitors significantly increased AAD by 25 ± 8% (15 ± 0.7 μm, P < 0.05) to a value not significantly different from the value at a RPP of 100 mmHg. Further treatment with AT1R inhibitor after treatment with the P2X1R inhibitor plus P2X7R inhibitor did not significantly modify AAD (15.4 ± 0.5 μm, not significant; Fig. 6).
Fig. 6.
Effect of P2X7 receptor (P2X7R), P2X1 receptor (P2X1R), and ANG II type 1 receptor (AT1R) blockade on the afferent arteriolar response to increased renal perfusion pressure (RPP) in hypertensive rats. Afferent arteriolar responses to increases in RPP (n = 5) were followed by treatment with P2X1R inhibitor (P2X1Ri) plus P2X7R inhibitor (P2X7Ri) and after the addition of AT1R inhibitor (AT1Ri) alone. Data are expressed as means ± SD. *P < 0.05 vs. RPP at 100 mmHg; #P < 0.05 vs. RPP at 140 mmHg.
DISCUSSION
Under inappropriate stimulation of the renin-angiotensin system, the increased circulating and tissue ANG II concentrations acting on AT1Rs increase systemic vascular resistance. The accompanying progressive increase in renal vascular resistance is due predominantly to the afferent and efferent arteriolar vasoconstriction mediated primarily by AT1R activation due to the increased ANG II concentrations (34). During the early stages of ANG II-dependent hypertension, renal arterioles are highly responsive to angiotensin-converting enzyme inhibition and angiotensin receptor blockers, indicating the predominant influence of the augmented ANG II levels activating AT1Rs (34). The increased renal vascular resistance is mediated by both the direct effect of AT1R actions on renal vascular smooth muscle as well as augmentation of the sensitivity of the tubuloglomerular feedback mechanism (34). There is strong evidence based on cross-transplantation studies that ANG II causes hypertension through direct actions on kidney AT1Rs (6). Also, ANG II-dependent responses of the renal circulation were almost abolished in AT1R knockout mice (6). In particular, afferent arteriolar reactivity to ANG II is enhanced in early stages of angiotensin-dependent hypertension (17).
Furthermore, in response to increases in RPP, intrarenal paracrine factors, including autacoids of the purinergic system, become activated. Increases in arterial pressure result in progressive increases in interstitial levels of ATP, which acts on P2XRs and P2Y receptors (P2YRs). Functional evidence for P2XR and P2YRs to the overall regulation of renal afferent arterioles and autoregulatory behavior has been provided in several studies (18, 22, 38). P2Y1R and P2Y2R are also expressed in afferent arterioles, whereas P2Y1Rs are expressed only in efferent arterioles (44). P2YR stimulation by low concentrations of selective agonists such as UTP or ATP-γ-S elicited vasodilation, but vasoconstriction occurred at higher concentrations (5, 9). However, the role of P2YRs in autoregulation and tubuloglomerular feedback responses remains uncertain (18, 22, 38). On the other hand, P2XRs, particularly P2X1R, elicit marked afferent arteriole vasoconstriction potentially compounding the ANG II-mediated effects.
During acute and transient increases in arterial pressure, the increases in ATP released by endothelial cells in response to increases in shear stress and by macula densa cells in response to tubuloglomerular feedback mechanism-mediated signals provide the protective regulation of afferent arteriolar resistance to maintain glomerular pressure and glomerular filtration rate. The dialysis method has shown sustained increases in interstitial ATP concentrations at increased arterial pressure (25, 37). A more recent study has demonstrated increases in interstitial ATP using a real-time electrochemical detection method that provides continuous measurement of interstitial ATP (39). Thus, this mechanism provides a rapid critical autoregulatory protective function preventing the increases in blood pressure from disrupting the kidney’s homeostatic function or causing glomerular and microvascular injury by excessive intrarenal pressures.
Arterial pressure-induced increases in intrarenal ATP levels are sustained in ANG II-dependent hypertension and presumably continue to provide the protective function that is served in acute situations (14). Thus, in ANG II-dependent hypertension, two powerful vasoconstrictor systems are each extending their influence on the renal vasculature, but whether their interactions are additive, overlapping, or synergistic remains unclear. If additive, the kidney’s homeostatic function would be threatened by devastating excessive vasoconstriction, which does not occur. Yet, when treated with angiotensin receptor blockers, the renal vascular resistance decreases toward normal levels in association with decreases in arterial pressure, thus maintaining or even increasing renal blood flow and glomerular filtration rate (4). This response suggests that AT1R activity is dominant, but the contribution of the decreased renal vascular resistance associated with the reductions in interstitial ATP caused by the decreases in arterial pressure is difficult to determine. In contrast, administration of P2XR inhibitors counteracts the activation of P2XRs in the kidney without causing associated changes in arterial pressure (12, 14). Indeed, P2XR blockade chronically or acutely administered to ANG II-induced hypertensive rats does not reduce the elevated arterial pressure but elicits profound changes in segmental renal vascular resistance (12). In a series of micropuncture studies, which allowed evaluation of glomerular dynamics in response to nonselective (13) or selective (12) P2XR inhibition, the results suggested a marked dominance of P2XRs on segmental vascular resistance. The combination of P2X1R and P2X7R completely normalized afferent arteriolar resistance in ANG II-infused hypertensive rats and increased single nephron plasma flow and filtration rate as well as glomerular pressure to values above normal because of the maintained arterial pressure. Under these conditions of maintained arterial pressure, it appeared that the P2XR effect was clearly dominant in regulating renal vascular resistance, thus raising the conundrum of how both angiotensin via AT1R and ATP via P2XR could be exerting the dominant effect to regulate renal vascular resistance in hypertension. To address this conundrum, it was necessary to study the actions of blockade of AT1R and P2XR, either alone or in combination, at hypertensive perfusion pressures, which could be maintained independent of the changes in renal vascular resistance. To achieve this requirement and to view directly the responses of the afferent arterioles, we used the in vitro blood perfused juxtamedullary nephron preparation. Importantly, the kidney preparation was perfused with its own blood so that kidneys from hypertensive rats were perfused with blood having the composition as affected by the 2 wk of ANG II perfusion.
We first evaluated the responses in kidneys from normotensive rats to determine the relative interactions between AT1R and P2XR. There are no pure selective antagonists for P2XRs. However, there are some antagonists with higher selectivity for certain receptors, such as NF449 for P2X1R and A438079 for P2X7R. These antagonists have a higher affinity for their receptors (7, 28, 29, 35). In normotensive rats studied at normotensive pressures, ATP elicited a robust afferent constriction, which was blocked almost completely by the P2X1R inhibitor, whereas there was no significant effect of P2X7R inhibition. These results are consistent with data indicating that renal P2X7R abundance is very low in normotensive rats (12). In response to an increase in RPP from 100 to 140 mmHg, kidneys from normotensive rats responded with a robust afferent arteriolar constriction that was mitigated primarily by the P2X1R inhibitor without contribution of the P2X7R inhibitor. Furthermore, AT1R blockade did not modify AAD significantly. These data are consistent with previous findings that the vasoconstriction caused by α,β-methylene ATP, a potent P2X1R agonist, was blocked by NF279 (20, 23). Additionally, P2X1R knockout mice displayed an impaired pressure-mediated autoregulatory response (21). Blockade of AT1Rs did not modify the response of AAD to the increase in RPP to 140 mmHg. These data are consistent with the findings that autoregulatory responses are not mediated by AT1Rs (25) but are strongly dependent on P2X1R activation, indicating that P2X1R serves as an important mediator of vascular resistance responses to increases in RPP. They are also consistent with the localization of P2X1R in the smooth muscle of afferent arterioles from normal rats (12, 44, 45). In contrast, no changes were observed with superimposed P2X7R inhibition, which is consistent with the low abundance of P2X7R in normal arterioles (31, 45).
To further study the interaction between AT1R and P2XR in kidneys from control rats, we simulated “in vivo” conditions by superfusing ANG II over the tissues and clearly demonstrated ANG II-mediated constriction. The addition of ANG II at baseline RPP caused a 10% decrease in AAD. Even in the presence of ANG II, an increase in RPP to 140 mmHg caused a 19% further decrease in AAD. P2X1R blockade returned AAD to a value between those seen at baseline RPP and with ANG II superfusion. With the addition of the AT1R inhibitor, AAD returned to baseline values. These results indicate a predominant P2X1R effect on the autoregulatory component along with a maintained influence of AT1Rs in the regulation of AAD during combined elevation in arterial pressure and acute treatment with ANG II.
To evaluate transformation effects in chronic ANG II-dependent hypertension, afferent arteriolar responses in kidneys from ANG II-infused hypertensive rats demonstrated greater interactions between the effects of AT1R and P2X1R. Blockade of P2X1R at high RPP mitigated the AT1R-indued constriction in renal afferent arterioles. These findings are consistent with increased expression of P2X1R in this model of hypertension (12).
In afferent arterioles from hypertensive rats, specific P2X1R or P2X7R blockade returned renal AAD to near-normal values, and only very slight effects of AT1R blockade were observed. When the AT1R blocker was used first, there was a small but distinctive increase in AAD, which indicates a small residual effect of AT1R. Addition of the P2X1R and P2X7R inhibitors restored AAD to control levels. The ability of P2X7R inhibitor to significantly augment AAD can be attributed to a higher expression of P2X7R in afferent arterioles of hypertensive rats (12).
Collectively, these results provide functional evidence that renal P2X1R and P2X7R exert dominant roles in the regulation of AAD in ANG II-infused hypertension and abrogate the AT1R influence on AAD. Understanding how these two receptors interact during the signaling process is crucial to understand the control of renal afferent arteriolar resistance under hypertension.
The intracellular mechanisms by which P2XR and AT1R interact will need to be studied further. AT1R is a G protein-coupled receptor, and P2XRs are ligand-gated ion channels. Both P2XR and AT1R mediate vasoconstriction via an increase in cytosolic Ca2+, but the intracellular mechanisms are different. Stimulation of AT1R activates Gαq/11 protein, which induces the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) and production of inositol 1,4,5-trisphosphate and diacylglycerol by activation of phospholipase C-β. Inositol 1,4,5-trisphosphate then induces intracellular Ca2+ mobilization from the endoplasmic reticulum to the cytosol. In contrast, activation of ligand-gated ion channels causes a remarkable influx of Ca2+ from the extracellular compartment (8), which leads to further increases in Ca2+ release from intracellular stores. This concert of regulation of Ca2+ signaling pathways plays a key role in controlling G protein-couple receptor- and ligand-gated ion channel-mediated renal arteriolar vasoconstriction.
PIP2, as a cofactor, may also be a critical regulatory factor for the P2X1R and P2X7R signaling pathway. It has been shown that the P2XR intracellular domain, containing a dual cluster of basic amino acids, enables PIP2 binding (2), which strongly potentiates the activities of P2X1R and P2X7R. It has been suggested that protein-PIP2 interaction induces a conformational change in the transmembrane segment (TM2) linking to the proximal COOH-terminal domain, which can influence channel opening activity. This evidence suggests that AT1R shares an important signaling pathway with P2X1R and P2X7R involving PIP2. Among the downstream components involved in the AT1R signaling pathway, AT1R is not limited to Gαq/11 protein but also increases Rho kinase activity via Gl2/l3. During ANG II treatment, Rho kinase inhibition in ANG II-infused rats led to greater vasodilation compared with control rats (32, 49). These data suggest that ANG II-activated AT1R-mediated activation of the Rho kinase pathway contributes to the increased afferent arteriolar resistance. Interestingly, the P2X1 receptor agonist α,β-methylene ATP reduced AAD, but this response was eliminated during Rho kinase inhibition (24, 34). These data demonstrate that Rho kinase activation is an important part of the intracellular signaling mechanisms that connect P2XR activation with vasoconstriction. Thus, it is possible that Rho kinase contributes to AT1R and P2XR signaling interactions (15).
Besides posttranslational interactions that mediate downstream signaling pathways, it is known that G protein-coupled receptors can interact directly with ligand-gated ion channels, leading to a functional and reciprocal modulation of each receptor type (30, 48). P2XRs are composed of three subunits, and the COOH-terminal tail of P2XR subunits interacts directly with the main intracellular loop of other membrane receptors. One noticeable feature of P2X7R is that it has a long COOH-terminal tail (43), a structural factor that facilitates physical interaction between receptors. The new insights of receptor interaction showed that AT1R can form complexes with other receptors (10, 13, 33, 40). However, a physical interaction between AT1R and P2XR has not been reported, but it would be interesting to investigate the possible physical interactions between P2X1R or P2X7R and AT1R in the control of renal afferent arteriolar resistance.
In conclusion, this work provides functional evidence that renal P2X1R and P2X7R exert dominant roles in the regulation of AAD in kidneys from ANG II-dependent hypertensive rats during elevation in arterial pressure and abrogate most of the AT1R influence on AAD.
Understanding how these two receptors interact during the signaling process is key to unlocking how renal afferent arteriolar resistance is controlled during hypertension. Resolving the mechanisms will facilitate our ability to design therapeutic interventions that will prevent the decline of kidney function early in the progression of hypertension.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant CoBRE P30-GM-103337 and by the Lavin-Bernick endowment at Tulane (to L. G. Navar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K., M.F., and L.G.N. conceived and designed research; S.K. performed experiments; S.K. analyzed data; S.K., W.S., M.F., and L.G.N. interpreted results of experiments; S.K. prepared figures; S.K., W.S., M.F., and L.G.N. drafted manuscript; S.K. and L.G.N. edited and revised manuscript; S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
Parts of this work have been presented in abstract form at the Experimental Biology Meeting 2018, the High Blood Pressure Council of the American Heart Association 2018, the Southern Regional Meeting for Clinical Investigation 2019, and the High Blood Pressure Council of the American Heart Association 2019.
REFERENCES
- 1.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322–4327, 2003. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernier LP, Ase AR, Séguéla P. Post-translational regulation of P2X receptor channels: modulation by phospholipids. Front Cell Neurosci 7: 226, 2013. doi: 10.3389/fncel.2013.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blantz RC, Konnen KS, Tucker BJ. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest 57: 419–434, 1976. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cervenka L, Wang CT, Navar LG. Effects of acute AT1 receptor blockade by candesartan on arterial pressure and renal function in rats. Am J Physiol Renal Physiol 274: F940–F945, 1998. doi: 10.1152/ajprenal.1998.274.5.F940. [DOI] [PubMed] [Google Scholar]
- 5.Churchill PC, Ellis VR. Pharmacological characterization of the renovascular P2 purinergic receptors. J Pharmacol Exp Ther 265: 334–338, 1993. [PubMed] [Google Scholar]
- 6.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol 157: 1203–1214, 2009. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan TM, Khakh BS. Contribution of calcium ions to P2X channel responses. J Neurosci 24: 3413–3420, 2004. doi: 10.1523/JNEUROSCI.5429-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltze M, Ullrich B. Characterization of vascular P2 purinoceptors in the rat isolated perfused kidney. Eur J Pharmacol 306: 139–152, 1996. doi: 10.1016/0014-2999(96)00244-0. [DOI] [PubMed] [Google Scholar]
- 10.Fellner SK, Arendshorst WJ. Angiotensin II-stimulated Ca2+ entry mechanisms in afferent arterioles: role of transient receptor potential canonical channels and reverse Na+/Ca2+ exchange. Am J Physiol Renal Physiol 294: F212–F219, 2008. doi: 10.1152/ajprenal.00244.2007. [DOI] [PubMed] [Google Scholar]
- 11.Feng MG, Prieto MC, Navar LG. Nebivolol-induced vasodilation of renal afferent arterioles involves β3-adrenergic receptor and nitric oxide synthase activation. Am J Physiol Renal Physiol 303: F775–F782, 2012. doi: 10.1152/ajprenal.00233.2012. [DOI] [PubMed] [Google Scholar]
- 12.Franco M, Bautista-Pérez R, Cano-Martínez A, Pacheco U, Santamaría J, Del Valle Mondragón L, Pérez-Méndez O, Navar LG. Physiopathological implications of P2X1 and P2X7 receptors in regulation of glomerular hemodynamics in angiotensin II-induced hypertension. Am J Physiol Renal Physiol 313: F9–F19, 2017. doi: 10.1152/ajprenal.00663.2016. [DOI] [PubMed] [Google Scholar]
- 13.Franco M, Bautista R, Tapia E, Soto V, Santamaría J, Osorio H, Pacheco U, Sánchez-Lozada LG, Kobori H, Navar LG. Contribution of renal purinergic receptors to renal vasoconstriction in angiotensin II-induced hypertensive rats. Am J Physiol Renal Physiol 300: F1301–F1309, 2011. doi: 10.1152/ajprenal.00367.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol 294: F161–F169, 2008. doi: 10.1152/ajprenal.00281.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan Z, Baty JJ, Zhang S, Remedies CE, Inscho EW. Rho kinase inhibitors reduce voltage-dependent Ca2+ channel signaling in aortic and renal microvascular smooth muscle cells. Am J Physiol Renal Physiol 317: F1132–F1141, 2019. doi: 10.1152/ajprenal.00212.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol Renal Physiol 273: F170–F177, 1997. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 17.Imig JD. Afferent arteriolar reactivity to angiotensin II is enhanced during the early phase of angiotensin II hypertension. Am J Hypertens 13: 810–818, 2000. doi: 10.1016/S0895-7061(00)00264-8. [DOI] [PubMed] [Google Scholar]
- 18.Inscho EW, Cook AK. P2 receptor-mediated afferent arteriolar vasoconstriction during calcium blockade. Am J Physiol Renal Physiol 282: F245–F255, 2002. doi: 10.1152/ajprenal.0038.2001. [DOI] [PubMed] [Google Scholar]
- 19.Inscho EW, Cook AK, Clarke A, Zhang S, Guan Z. P2X1 receptor-mediated vasoconstriction of afferent arterioles in angiotensin II-infused hypertensive rats fed a high-salt diet. Hypertension 57: 780–787, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand 181: 445–453, 2004. doi: 10.1111/j.1365-201X.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- 22.Inscho EW, Cook AK, Mui V, Miller J. Direct assessment of renal microvascular responses to P2-purinoceptor agonists. Am J Physiol 274: F718–F727, 1998. doi: 10.1152/ajprenal.1998.274.4.F718. [DOI] [PubMed] [Google Scholar]
- 23.Inscho EW, Cook AK, Navar LG. Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am J Physiol Renal Physiol 271: F1077–F1085, 1996. doi: 10.1152/ajprenal.1996.271.5.F1077. [DOI] [PubMed] [Google Scholar]
- 24.Inscho EW, Cook AK, Webb RC, Jin LM. Rho-kinase inhibition reduces pressure-mediated autoregulatory adjustments in afferent arteriolar diameter. Am J Physiol Renal Physiol 296: F590–F597, 2009. doi: 10.1152/ajprenal.90703.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol 10, Suppl 11: S178–S183, 1999. [PubMed] [Google Scholar]
- 26.Ji X, Naito Y, Hirokawa G, Weng H, Hiura Y, Takahashi R, Iwai N. P2X(7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens Res 35: 173–179, 2012. doi: 10.1038/hr.2011.153. [DOI] [PubMed] [Google Scholar]
- 27.Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N. P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol 303: F1207–F1215, 2012. doi: 10.1152/ajprenal.00051.2012. [DOI] [PubMed] [Google Scholar]
- 28.Kassack MU, Braun K, Ganso M, Ullmann H, Nickel P, Böing B, Müller G, Lambrecht G. Structure-activity relationships of analogues of NF449 confirm NF449 as the most potent and selective known P2X1 receptor antagonist. Eur J Med Chem 39: 345–357, 2004. doi: 10.1016/j.ejmech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Lambertucci C, Dal Ben D, Buccioni M, Marucci G, Thomas A, Volpini R. Medicinal chemistry of P2X receptors: agonists and orthosteric antagonists. Curr Med Chem 22: 915–928, 2015. doi: 10.2174/0929867321666141215093513. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Wong AH, Liu F. Ligand-gated ion channel interacting proteins and their role in neuroprotection. Front Cell Neurosci 8: 125, 2014. doi: 10.3389/fncel.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menzies RI, Howarth AR, Unwin RJ, Tam FW, Mullins JJ, Bailey MA. Inhibition of the purinergic P2X7 receptor improves renal perfusion in angiotensin-II-infused rats. Kidney Int 88: 1079–1087, 2015. doi: 10.1038/ki.2015.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyata K, Satou R, Shao W, Prieto MC, Urushihara M, Kobori H, Navar LG. ROCK/NF-κB axis-dependent augmentation of angiotensinogen by angiotensin II in primary-cultured preglomerular vascular smooth muscle cells. Am J Physiol Renal Physiol 306: F608–F618, 2014. doi: 10.1152/ajprenal.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogi M, Iwai M, Horiuchi M. New insights into the regulation of angiotensin receptors. Curr Opin Nephrol Hypertens 18: 138–143, 2009. doi: 10.1097/MNH.0b013e328324f5fa. [DOI] [PubMed] [Google Scholar]
- 34.Navar LG. Intrarenal renin-angiotensin system in regulation of glomerular function. Curr Opin Nephrol Hypertens 23: 38–45, 2014. doi: 10.1097/01.mnh.0000436544.86508.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT, Donnelly-Roberts DL, Niforatos W, Honore P, Jarvis MF, Faltynek CR, Carroll WA. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem 49: 3659–3666, 2006. doi: 10.1021/jm051202e. [DOI] [PubMed] [Google Scholar]
- 36.Nishiyama A, Jackson KE, Majid DS, Rahman M, Navar LG. Renal interstitial fluid ATP responses to arterial pressure and tubuloglomerular feedback activation during calcium channel blockade. Am J Physiol Heart Circ Physiol 290: H772–H777, 2006. doi: 10.1152/ajpheart.00242.2005. [DOI] [PubMed] [Google Scholar]
- 37.Nishiyama A, Majid DS, Taher KA, Miyatake A, Navar LG. Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ Res 86: 656–662, 2000. doi: 10.1161/01.RES.86.6.656. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama A, Rahman M, Inscho EW. Role of interstitial ATP and adenosine in the regulation of renal hemodynamics and microvascular function. Hypertens Res 27: 791–804, 2004. doi: 10.1291/hypres.27.791. [DOI] [PubMed] [Google Scholar]
- 39.Palygin O, Evans LC, Cowley AW Jr, Staruschenko A. Acute in vivo analysis of ATP release in rat kidneys in response to changes of renal perfusion pressure. J Am Heart Assoc 6: e006658, 2017. doi: 10.1161/JAHA.117.006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozenfeld R, Gupta A, Gagnidze K, Lim MP, Gomes I, Lee-Ramos D, Nieto N, Devi LA. AT1R-CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J 30: 2350–2363, 2011. doi: 10.1038/emboj.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao W, Rosales CB, Gonzalez C, Prieto MC, Navar LG. Effects of serelaxin on renal microcirculation in rats under control and high-angiotensin environments. Am J Physiol Renal Physiol 314: F70–F80, 2018. doi: 10.1152/ajprenal.00201.2017. [DOI] [PubMed] [Google Scholar]
- 42.Sparks MA, Stegbauer J, Chen D, Gomez JA, Griffiths RC, Azad HA, Herrera M, Gurley SB, Coffman TM. Vascular type 1A angiotensin II receptors control BP by regulating renal blood flow and urinary sodium excretion. J Am Soc Nephrol 26: 2953–2962, 2015. doi: 10.1681/ASN.2014080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toulmé E, Martinez A, Boué-Grabot E. Neurotransmitter receptor complexes in the brain: biochemical characterization and functional analysis of receptor-receptor interactions. Basic methods in protein purification and analysis. Hal Arch 978–14775550–5-7, 2013. [Google Scholar]
- 44.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175: 105–117, 2003. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- 45.Vonend O, Turner CM, Chan CM, Loesch A, Dell’Anna GC, Srai KS, Burnstock G, Unwin RJ. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int 66: 157–166, 2004. doi: 10.1111/j.1523-1755.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang CT, Navar LG, Mitchell KD. Proximal tubular fluid angiotensin II levels in angiotensin II-induced hypertensive rats. J Hypertens 21: 353–360, 2003. doi: 10.1097/00004872-200302000-00027. [DOI] [PubMed] [Google Scholar]
- 47.Wang CT, Zou LX, Navar LG. Renal responses to AT1 blockade in angiotensin II-induced hypertensive rats. J Am Soc Nephrol 8: 535–542, 1997. [PubMed] [Google Scholar]
- 48.Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci 124: 3477–3483, 2011. doi: 10.1242/jcs.087221. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto K, Imamura H, Ando J. Shear stress augments mitochondrial ATP generation that triggers ATP release and Ca2+ signaling in vascular endothelial cells. Am J Physiol Heart Circ Physiol 315: H1477–H1485, 2018. doi: 10.1152/ajpheart.00204.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou LX, Imig JD, von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension 28: 669–677, 1996. doi: 10.1161/01.HYP.28.4.669. [DOI] [PubMed] [Google Scholar]