Abstract
Activation of immunological pathways and disturbances of extracellular matrix (ECM) dynamics are important contributors to the pathogenesis of chronic kidney diseases. Glomerular mesangial cells (MCs) are critical for homeostasis of glomerular ECM dynamics. Interleukin-6 (IL-6) can act as a pro/anti-inflammatory agent relative to cell types and conditions. This study investigated whether IL-6 influences ECM protein production by MCs and the regulatory pathways involved. Experiments were carried out in cultured human MCs (HMCs) and in mice. We found that overexpression of IL-6 and its receptor decreased the abundance of fibronectin and collagen type IV in MCs. ELISA and immunoblot analysis demonstrated that thapsigargin [an activator of store-operated Ca2+ entry (SOCE)], but not the endoplasmic reticulum stress inducer tunicamycin, significantly increased IL-6 content. This thapsigargin effect was abolished by GSK-7975A, a selective inhibitor of SOCE, and by silencing Orai1 (the channel protein mediating SOCE). Furthermore, inhibition of NF-κB pharmacologically and genetically significantly reduced SOCE-induced IL-6 production. Thapsigargin also stimulated nuclear translocation of the p65 subunit of NF-κB. Moreover, MCs overexpressing IL-6 and its receptor in HMCs increased the content of the glucagon-like peptide-1 receptor (GLP-1R), and IL-6 inhibition of fibronectin was attenuated by the GLP-1R antagonist exendin 9–39. In agreement with the HMC data, specific knockdown of Orai1 in MCs using the targeted nanoparticle delivery system in mice significantly reduced glomerular GLP-1R levels. Taken together, our results suggest a novel SOCE/NF-κB/IL-6/GLP-1R signaling pathway that inhibits ECM protein production by MCs.
Keywords: extracellular matrix, glucagon-like peptide-1 receptor, interleukin-6, mesangial cells, nuclear factor-κB, Orai1, store-operated calcium entry
INTRODUCTION
Chronic kidney disease (CKD), also termed as a “disease multiplier,” not only leads to end-stage renal failure but also increases mortality due to other causes like cardiovascular events (13, 27, 54). CKD, including diabetic nephropathy (DN), often involves disturbances in extracellular matrix (ECM) protein dynamics at the early stages, which eventually progress to increased production of fibrotic proteins leading to end-stage renal disease (38, 44, 55). ECM dynamics are very complex and influenced by a variety of biological and pathological factors (19, 42). Thus, understanding the molecular pathways regulating ECM dynamics under physiological and pathological conditions is important to develop new therapeutic strategies for CKD, the current ones not being very effective (2).
Glomerular mesangial cells (MCs) play a major role in glomerular ECM formation, and the production of ECM proteins by MCs is exaggerated in CKDs (1, 44, 52, 55). Inflammation and activation of the immune system are critical to CKD pathogenesis and progression (41, 62). In response to pathological stimuli, renal cells produce cytokines/chemokines that recruit circulating immune cells, further accentuating expression of various cytokines (17, 28). Interleukin-6 (IL-6) is known as a pleotropic cytokine that can act as an anti- or proinflammatory agent depending on the cell types, receptors activated, downstream signaling pathways, and stimuli (7, 33, 43, 46). IL-6 is associated with pathophysiology of CKDs, including DN. However, the exact role of IL-6 in different types of kidney cells and the regulatory pathways involved have not yet been defined, particularly in MCs, the major source of cytokine/chemokine in many inflammatory kidney diseases (60). The present study aimed to investigate if IL-6 modulates ECM protein production by MCs and, if so, to define the upstream and downstream regulatory mechanisms involved.
MATERIALS AND METHODS
Materials
Expression plasmids.
The expression plasmid for the human IL-6/soluble IL-6 receptor (sIL-6R) complex (pCDM8-H-IL-6), which overexpresses human IL-6 protein fused to human sIL-6R, was obtained from Dr. Stefan Rose-John (Department of Biochemistry, Christian-Albrechts-University, Kiel, Germany).
siRNAs.
siRNA against human Orai1 (Gene Accession No. NM_032790, sense: 5′-UGGAACUGUCGGUCAGUCUUAUGGC-3′) and Cy3-labeled siRNA against mouse Orai1 (Gene Accession No. NM_175423, sense: 5′-/5Cy3/GGGUUGCUCAUCGUCUUUAGUGC-3′) were purchased from Integrated DNA Technologies (Chicago, IL). Scrambled control siRNA (ON-TARGET plus non-targeting control siRNA#1, catalog no. D-001810-01-20, target sequence: 5′-UGGUUUACAUGUCGACUAA-3′) was purchased from GE Healthcare Dharmacon (Lafayette, CO). NF-κB p65 siRNA against human p65 was purchased from Cell Signaling Technology (catalog no. 6261, Danvers, MA).
Antibodies.
Anti-IL-6 antibody was purchased from Proteintech (catalog no. 21865-1-AP, Rosemont, IL). Anti-collagen type IV (catalog no. ab135802) and anti-TATA box-binding protein (TBP) antibodies (catalog no. ab818) were purchased from Abcam (Cambridge, MA). Antibodies against fibronectin (catalog no. F3648) and Orai1(catalog no. O8264) were from Sigma-Aldrich (St. Louis, MO). Anti- glucagon-like peptide (GLP)-1 receptor (GLP-1R) antibody (catalog no. 97308) was from Novus Biologicals (Littleton, CO). Anti-p65 antibody (catalog no. 622601) was purchased from BioLegend (San Diego, CA). Anti-GAPDH antibody (catalog no. MAB374) was purchased from Millipore-Sigma (Burlington, MA), and anti-α-tubulin antibody was purchased from Santa Cruz Biotechnologies (catalog no. sc-5286, Dallas, TX). Secondary antibodies for immunoblot analysis, including goat anti-mouse Ig-horseradish peroxidase (catalog no. 31430) and goat anti-rabbit Ig-horseradish peroxidase (catalog no. 31460) were purchased from Invitrogen (Rockford, IL). The secondary antibody used for immunohistochemistry (anti-rabbit poly-horseradish peroxidase IHC reagent) was purchased from General Bioscience (catalog no. IHC-2291).
Chemicals.
Thapsigargin (TG; catalog no. 586005), tunicamycin (catalog no. 504570), and 2-aminoethyl diphenylborinate (2-APB; catalog no. D9754) were purchased from Calbiochem-Millipore-Sigma. Helenaline (catalog no. 50-148-9989) and exendin 9–39 (catalog no. AAJ66126EXD) were purchased from Fisher Scientific. GSK-7975A was kindly donated by GlaxoSmithKline (Brentford, UK). Peroxidase substrate solution (DAB peroxidase substrate kit SK-4100) was purchased from Vector Laboratories (Burlingame, CA).
Cell Culture
Human MCs (HMCs) were purchased from Sciencell Research Laboratories (catalog no. 4200, Carlsbad, CA). HMCs were cultured in a 75-cm2 flask with complete HMC media containing normal glucose (NG; 5.6 mM) DMEM (GIBCO, Carlsbad, CA) supplemented with 25 mM HEPES, 4 mM glutamine, 1.0 mM sodium pyruvate, 0.1 mM nonessential aminoacids, 100 U/mL penicillin, 100 μg/mL streptomycin, and 15% FBS. At ~80% confluence, cells were split into 60-mm culture plates or 12-well plates for various treatments as specified in the figures. Cells were growth arrested using serum-deprived media overnight. Culture media were replaced with fresh media at 2-day intervals. HMCs were used between subpassages 4 and 9 only.
Human proximal tubular epithelial cells (HPTECs) were also purchased from Sciencell Research Laboratories (catalog no. 4100). HPTECs were cultured in complete EpiCM media (ScienCell Research Laboratories) that was constituted by adding 5 mL Epi cell growth supplement, 10 mL FBS (2%), and 5 mL penicillin-streptomycin to the basal EpiCM media. Upon reaching ~80% confluence, cells were split into 60-mm dishes for transfection experiments. HPTECs were used between subpassages 4 and 9.
Transient Transfection of HMCs and HPTECs
To knock down the target proteins, HMCs were transiently transfected with siRNA against human Orai1 or human p65 or scrambled control siRNA (50 nM) using Dharmafect2 transfection reagent (Thermo Scientific, Rockford, IL) in serum-free DMEM following the protocol provided by the manufacturer. Media were changed to 15% FBS-DMEM after 6 h. Cells were harvested for Western blot analysis 72 h after transfection. Human IL-6/sIL-6R expression plasmids (0.5 µg/mL) transfection into HMCs and HPTECs was carried out using Lipofectamine LTX reagents (Invitrogen-BRL, Carlsbad, CA) in their respective serum-free media following the protocols provided by the manufacturer. Cells treated with transfection reagent alone served as the control. Media were changed with complete HMC media and complete EpiCM media after 6 h. Cells were collected 48 h after transfection for Western blot analysis.
Enzyme-Linked Immunosorbent Assay
Concentrations of IL-6 in media were determined by a solid-phase sandwich ELISA using the DuoSet ELISA Development kit for human IL-6 (catalog no. DY206-05, R&D System, Minneapolis, MN) to determine the abundance of IL-6 in supernatant media. Briefly, HMCs were plated in 12-well plates as 5 × 104 cells/well. After attaining confluency, cells were serum deprived until the end of the experiments. Cells were treated with 1 µM TG (SOCE activator) in the presence or absence of 10 µM GSK-7975A (SOCE inhibitor) 15 h before sample collection. Supernatant media were collected 24 h later, centrifuged at 1,500 rpm for 10 min at 4°C, and stored at −80°C until use. All samples were assayed using the protocol provided by the manufacturer. Optical density was determined at 450 nm in a microplate reader. The standard curve was obtained using the Sigma plot software version 11.
Immunoblot Analysis
Immunoblots were performed as described in our previous publication (11). Briefly, whole cell lysates or nuclear and cytosolic extracts were fractionated by 10% SDS-PAGE, transferred to PVDF membranes, and probed with primary antibodies. Bound antibodies were visualized with Super Signal West Femto or Pico Luminol/Enhancer Solution (catalog nos. 34095 and 34087, Thermo Scientific). The specific protein bands were visualized and captured using the AlphaEase FC Imaging System (Alpha Innotech, San Leandro, CA). The integrated density value (IDV) of each target band was measured by circumscribing the band with a rectangle using AlphaEase FC software with autobackground subtraction. Amounts of target proteins, except nuclear proteins, were quantified by normalization of their band IDVs to those of tubulin or GAPDH bands on the same blot. The contents of nuclear p65 proteins were normalized to TBP.
Preparation of Nuclear Extracts
Preparation of nuclear extracts from HMCs was performed using NE-PER nuclear and cytoplasmic extraction reagents (catalog no. 78833, Thermo Scientific) following the manufacturer’s protocol. Extracts were stored at −80°C until use.
Animals
All procedures involving mice were approved by the University of North Texas Health Science Center Institutional Animal Care and Use Committee. Six male C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). All mice used in this study were between 2 and 4 mo of age. Animals were maintained at the animal facility of the University of North Texas Health Science Center vivarium in accordance with local and National Institutes of Health guidelines.
In Vivo siRNA Delivery Into MCs of Mice
A targeted nanoparticle (NP)/siRNA in vivo delivery system was used in mice to deliver siRNAs against mouse Orai1 (siOrai1) selectively into MCs. The compositions and features of the NPs, the formulation of the NPs with siRNA, and the protocols of in vivo delivery of the NPs/siRNA complex have been described in our previous publications (10, 67, 68, 72). Mice were randomly divided into control and Orai1 knocked down groups (3 mice in each group). NPs containing siOrai1 (NP/siOrai1) were given through tail vein injection at a dose of 10 mg/kg siRNA in a volume of 100 µL to the mice in the Orai1 knocked down group. Mice in the control group were given NPs containing scrambled siRNA (NP/scrambled siRNA) through the same route with the same injection volume and dose. siRNA was injected on days 1 and 3, and mice were euthanized on day 5. Mice were euthanized by intraperitoneal injection of an overdose of anesthetic (100 mg/kg ketamine + 10 mg/kg xylazine) and then perfused with PBS to wash out the blood completely. After removal of the right kidney for biochemical assay, mice were further perfused with 4% paraformaldehyde. The left kidney was then removed, fixed in 4% paraformaldehyde overnight, dehydrated, embedded in paraffin, and cut into 4-µm-thick sections (Cryostat 2800 Frigocut-E, Leica Instruments).
Immunohistochemistry
After rehydration, kidney sections were immersed in 10 mM citrate buffer and heated to 60°C for 10 min to retrieve antigens. Sections were blocked by 5% goat serum for 30 min at room temperature and then incubated with anti-GLP-1R rabbit antibody at 1:200 in a humidified chamber overnight at 4°C. Sections were incubated with anti-rabbit poly horseradish peroxidase IHC reagent at room temperature for 1 h followed by incubation with peroxidase substrate solution. The slides were then dipped in hematoxylin solution for 30 s, dehydrated in an incubator at 60°C for 30 min, and coverslipped with mounting media. Sections were examined under an Olympus microscope (BX41) and an Olympus DP70 digital camera with DP manager software (version 2.2.1). Images were converted to 16-bit format and uniformly adjusted for brightness and contrast using ImageJ (version 1.50b, National Institutes of Health). Semiquantification of glomerular staining was conducted by a blinded observer using the ImageJ program following previously described instructions (53).
Statistical Analyses
Data are reported as means ± SE. Single-factor ANOVA plus Newman-Keuls post hoc analysis and an unpaired t test were used to analyze the differences among multiple groups and between two groups, respectively, except as indicated in figures. P < 0.05 was considered statistically significant. Statistical analyses were performed using SigmaStat (Jandel Scientific, San Rafael, CA).
RESULTS
Overexpression of IL-6 in HMCs Suppressed ECM Protein Production by HMCs
To determine the effect of IL-6 on ECM protein production by HMCs, we transfected HMCs with expression plasmid pCDM8-H-IL-6, which expresses the fusion protein for the IL-6 and sIL-6R complex (22). The IL-6/sIL-6R complex then associates with the glycoprotein 130 unit on HMCs to activate downstream IL-6 trans-signaling pathways. The fibronectin band at the size of 220 kDa and collagen type IV protein at the size of 160 kDa were analyzed by immunoblot analysis. Overexpression of the IL-6/sIL-6R compex in HMCs significantly decreased the protein abundance of fibronectin (Fig. 1, A and C) and collagen type IV (Fig. 1, B and D) compared with untransfected cells and cells transfected with transfection reagent only (control). To further determine if this effect of IL-6 was MC specific, we repeated the experiments in HPTECs. Contrary to the results in HMCs, overexpression of the IL-6/sIL-6R complex (i.e., upregulating IL-6 signaling) significantly increased fibronectin abundance in HPTECs (Fig. 1, E and F). Overexpression of the IL-6/sIL-6R complex by transfection with pCDM8-H-IL-6 plasmids was verified by immunoblot (Fig. 1, G and H). These results suggest that IL-6 effects in MCs are distinct from its effects in other kidney cell types and that IL-6 signaling in MCs is an inhibitory mechanism for ECM production.
Fig. 1.
Effects of interleukin-6 (IL-6) on extracellular matrix (ECM) protein production in cultured human mesangial cells (HMCs) and human proximal tubular epithelial cells (HPTECs). A and B: representative immunoblots showing fibronectin (220-kDa band) and collagen type IV (Col IV; 160-kDa band) content, respectively, in HMCs with and without overexpression of IL-6/soluble IL-6 receptor (sIL-6R). HMCs were either untransfected (UT) or treated with transfection reagents only (Reag; serving as control) or with pCDM8-H-IL-6 plasmid (pIL6). Cells were harvested 48 h after transfection. GAPDH was used as the loading control. L, protein ladder. C and D: summary data from experiments shown in A and B with n = 9 and n = 4, respectively. **P < 0.01 vs. UT and Reag. n indicates the number of independent experiments. E: representative immunoblots showing fibronectin abundance in HPTECs with and without overexpression of IL-6/sIL-6R. Cells were treated as described above for HMCs (A). α-Tubulin was used as the loading control. F: summary data from the experiment shown in E. n = 6. **P < 0.01 vs. UT and Reag. n indicates the number of independent experiments. G and H: representative immunoblots showing the expression of transfected IL-6 (between 60 and 80 kDa) and endogenous IL-6 bands (24 kDa) in HMCs and HPTECs with and without pCDM8-H-IL-6 expression plasmids, respectively. α-Tubulin was used as the loading control.
SOCE Increased Secretion and Production of IL-6 in HMCs
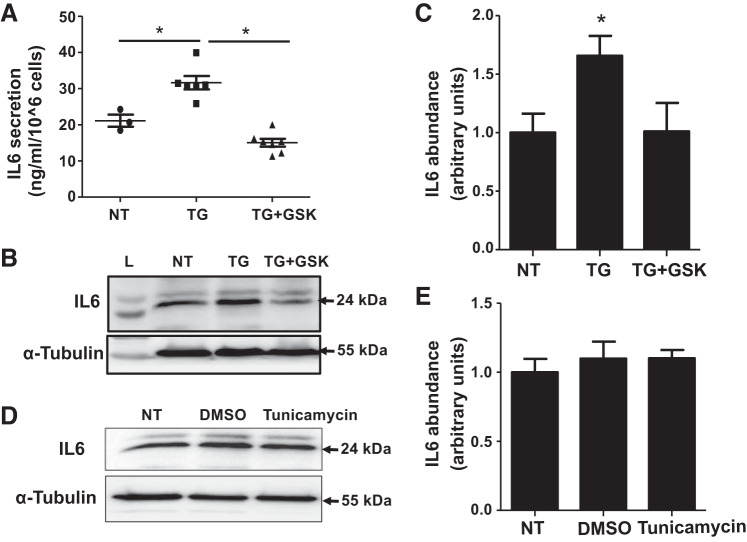
We have previously reported that SOCE suppressed production of ECM proteins including fibronectin and collagen type IV by MCs (68). To determine if the IL-6 signaling pathway mediates the SOCE effect, we examined the influence of SOCE manipulation on IL-6 secretion and production by HMCs. IL-6 secretion was evaluated by measuring IL-6 concentrations in the culture media of HMCs with or without TG (activation of SOCE) in the presence or absence of GSK-7975A (inhibition of SOCE). Compared with untreated control HMCs, TG treatment (1 µM for 15 h) significantly increased secretion of IL-6 and GSK-7975A (10 µM) abrogated the TG response (Fig. 2A).
Fig. 2.
Store-operated Ca2+ entry increased secretion and production of interleukin-6 (IL-6) protein by human mesangial cells (HMCs). A: ELISA showing IL-6 concentrations in HMC culture media. HMCs were growth arrested with serum-free DMEM overnight. One group had no treatment (NT), and the other two groups were treated with thapsigargin (TG; 1 μM) with or without GSK-7975A (GSK; 10 μM) for 15 h. Supernatants of the culture media were collected at the end of treatment. *P < 0.05. B: representative immunoblots showing the effect of Store-operated Ca2+ entry on IL-6 protein content in cultured HMCs. Serum-deprived HMCs had no treatment (NT) or were treated with TG (1 μM) with or without GSK (10 μM) for 15 h. α-Tubulin was used as the loading control. C: summary data from the experiment shown in B. *P < 0.05 vs. NT and TG + GSK. n = 5, where n indicates the number of independent experiments. D: representative immunoblots showing the effect of tunicamycin on IL-6 protein content in cultured HMCs. Serum-deprived HMCs had no treatment (NT) or were treated with DMSO (1:1,000) or tunicamycin (10 μM) for 15 h. α-Tubulin was used as the loading control. E: summary data from the experiment shown in B. n = 5, where n indicates the number of independent experiments.
Similarly, Western blot analysis showed a significant increase in the abundance of IL-6 protein in cells treated with TG (1 µM for 15 h), which was also prevented by GSK-7975A (10 µM; Fig. 2, B and C). Because TG is a known endoplasmic reticulum (ER) stress inducer (30, 49), we carried out additional experiments to examine if ER stress contributed to the TG-induced response. To this end, HMCs were treated with tunicamycin, which induces ER stress through a mechanism distinct from that of TG (26, 49, 71). As shown in Fig. 2, D and E, tunicamycin treatment (10 µM for 15 h) did not alter the abundance of IL-6 in HMCs compared with untreated cells, suggesting that the TG-induced increases of IL-6 secretion and production by MCs were attributable to SOCE activation, but not ER stress. Taken together, these results provide evidence that SOCE stimulates both secretion and production of IL-6 by MCs.
Knockdown of Orai1 Decreased IL-6 Content in HMCs
Orai1 protein is the pore-forming unit of the channel mediating SOCE (20, 51). If SOCE stimulated production of IL-6 protein, as shown above, silencing Orai1 and thus downregulating SOCE would be expected to reduce IL-6 content. This speculation was tested by transfecting HMCs with siRNA against human Orai1. Indeed, IL-6 content fell in HMCs transfected with Orai1 siRNA (Fig. 3). These data are consistent with the data presented above (Fig. 2) and further suggest a positive regulation of IL-6 production by SOCE in MCs.
Fig. 3.

Knockdown of Orai1 decreased interleukin-6 (IL-6) content in human mesangial cells (HMCs). A: representative immunoblots showing the effect of knockdown of Orai1 on IL-6 protein abundance in different groups of HMCs. HMCs were either untransfected (UT) or transfected with scrambled (Scr) or Orai1 siRNA (siOrai1). Cells were harvested 3 days after transfection. α-Tubulin was used as the loading control. B: summary data from experiments shown in A. *P < 0.05 vs. UT and Scr. n = 6, where n indicates the number of independent experiments.
Inhibition of NF-𝜅B Blunted SOCE-Stimulated IL-6 Production in HMCs
NF-κB is a major transcription factor for many immune-mediated processes. We next examined if NF-κB was involved in SOCE-induced IL-6 production. We suppressed NF-κB signaling pharmacologically with helenaline (NF-κB activation inhibitor) and biologically by silencing p65 (a key component of NF-κB) using the siRNA approach. As shown in Fig. 4, A and B, helenaline (1 µM) significantly blunted the TG-induced increase in IL-6 protein abundance in HMCs. In agreement with these results, p65 siRNA but not scrambled siRNA (control) completely abrogated the TG-induced increase in IL-6 in HMCs (Fig. 4, C and D).
Fig. 4.
Downregulation of the NF-κB pathway attenuated store-operated Ca2+ entry-induced interleukin-6 (IL-6) protein production in human mesangial cells (HMCs). A: representative immunoblots showing IL-6 protein abundance in HMCs after pharmacological inhibition of NF-κB. HMCs were given either no treatment (NT) or treated with thapsigargin (TG; 1 μM) in the presence or absence of helenaline (1 μM) for 15 h. α-Tubulin was used as the loading control. B: summary data showing IL-6 protein abundance in the different groups. *P < 0.05 vs. NT and TG + helenaline. n = 6, where n indicates the number of independent experiments. C: representative immunoblots showing protein levels of IL-6 and the p65 subunit of NF-κB in HMCs in the different groups. HMCs were either untreated (UT) or treated with DMSO (1:1,000) or TG (1 μM), or transfected with scrambled siRNA (Scr), scrambled siRNA with TG (Scr + TG), or p65 siRNA with TG (si-p65 + TG). DMSO and TG were added 15 h before cells were harvested. Cells were harvested 72 h after transfection. α-Tubulin was used as the loading control. D: summary data showing IL-6 protein abundance in the different groups. **P < 0.01 vs. UT and DMSO; ##P < 0.01 vs. Scr and si-p65 + TG. n = 5, where n indicates the number of independent experiments.
SOCE-Stimulated Nuclear Translocation of NF-𝜅B
After activation, NF-κB is translocated to the nucleus to modulate the transcriptional activity of its target genes (3). If NF-κB mediates SOCE-stimulated IL-6 production, manipulation of SOCE should alter the nuclear translocation of NF-κB. This speculation was tested by analyzing the dynamic distribution of the NF-κB p65 subunit in the cytosolic compartment and nucleus in response to SOCE activation. As shown in Fig. 5, TG treatment significantly increased p65 content in nuclear fractions, and this increase was significantly attenuated by 2-APB, an inhibitor of SOCE channels. However, the cytosolic extracts did not show any significant treatment effects on p65 content (Fig. 5). These results, combined with the results shown in Fig. 4, suggest that the NF-κB pathway mediated SOCE-stimulated IL-6 production in HMCs.
Fig. 5.

Store-operated Ca2+ entry increased NF-κB nuclear translocation in human mesangial cells (HMCs). A: representative immunoblots showing p65 protein abundance in nuclear and cytosolic fractions of HMCs. HMCs were either not treated (NT) or treated with DMSO (1:1,000) and thapsigargin (TG; 1 μM) with or without 2-aminoethyl diphenylborinate (2-APB; 50 μM) for 2 h. Anti-TATA box-binding protein (TBP) was the loading control for nuclear proteins; α-tubulin was used as the loading control for cytosolic proteins. B: summary data from the experiment shown in A. *P < 0.05 vs. TG; **P < 0.01 vs. UT and DMSO for nuclear proteins. n = 6, where n indicates the number of independent experiments.
GLP-1-Mediated IL6 Suppression of ECM Protein Production in HMCs
GLP-1 has multiple physiological actions, including stimulation of insulin secretion and regulation of glucose metabolism (8). GLP-1 is mainly produced by intestinal L cells, and its receptor, GLP-1R, is found on many target cell types, including glomerular MCs (9, 39). GLP-1R signaling was found to be protective in kidneys of diabetic animals (16). Recently, we reported that GLP-1R signaling suppressed ECM protein production in MCs (29). To further explore the possibility that this novel renoprotective pathway mediated IL-6 inhibition of ECM protein production by MCs, we conducted immunoblot analysis and examined GLP-1R contents in response to upregulation of IL-6 signaling. As shown in Fig. 6, A and B, overexpression of the IL-6/sIL-6R complex by transfecting HMCs with pCDM8-H-IL-6 plasmids (upregulating IL-6 signaling) significantly increased GLP-1R contents. To further interrogate the involvement of GLP-1R in IL-6 signaling, we treated HMCs that had been transfected with pCDM8-H-IL-6 plasmids with exendin 9–39, a GLP-1R antagonist. Immunoblot analysis was conducted to examine fibronectin protein abundance in response to the treatments. Same as the data shown in Fig. 1, overexpression of the IL-6/sIL-6R complex again reduced fibronectin protein abundance. However, this effect was significantly blunted, albeit not completely abolished, by inhibition of GLP-1R with exendin 9–39 (Fig. 6, C and D). These results suggest that GLP-1R signaling at least partially mediates IL-6-inhibited fibronectin production by HMCs.
Fig. 6.
The glucagon-like peptide-1 receptor (GLP-1R) pathway was involved in interleukin-6 (IL-6) suppression of fibronectin production in human mesangial cells (HMCs). A: representative immunoblots showing GLP-1R protein abundance in HMCs either untransfected (UT) or treated with transfection reagents only (Reag) or transfected with pCDM8-H-IL-6 plasmid (pIL6). Cells were harvested 48 h after transfection. α-Tubulin was used as the loading control. B: summary data from the experiments in A. *P < 0.05 vs. UT and Reag. n = 6, where n indicates the number of independent experiments. C: representative immunoblots showing fibronectin and GLP-1R protein abundance in HMCs that were either untransfected (UT), treated with transfection reagents only (Reag), or transfected with pCDM8-H-IL-6 plasmid (pIL6) with or without the addition of the GLP-1R antagonist exendin 9–39 (Exn9–39; 1 µM). Exendin 9–39 was added 24 h after transfection. Cells were harvested 48 h after transfection. α-Tubulin was used as the loading control. D: summary data from the experiments shown in C. *P < 0.05 vs. pIL6 and Reag; **P < 0.01 vs. UT and Reag. n = 6, where n indicates the number of independent experiments.
In Vivo Knockdown of Orai1 in MCs Decreased Glomerular GLP-1R Content in Mice
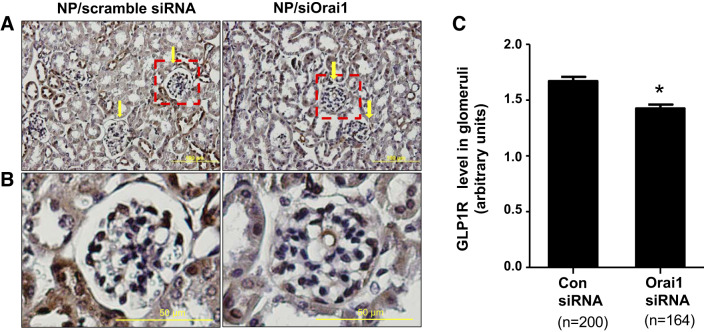
We have shown that SOCE promoted IL-6 protein production (Figs. 2 and 3). We also showed that GLP-1R signaling was a downstream mediator of IL-6 for its inhibition of fibronectin protein production (Fig. 6). To further confirm the SOCE/IL-6/GLP-1R cascade in vitro, we next knocked down Orai1 in MCs in mice using the established targeted NP-siRNA delivery system (68, 72). Our previous study demonstrated that these NP/siRNA complexes were distributed predominantly in glomeruli with limited distribution in surrounding tubules (72). The NP/siOrai1 complexes used in that study highly colocalized with integrin-8 (MC marker) but were not colocalized with synaptopodin (marker for podocytes), suggesting that the NP/siRNA complexes were selectively delivered to MCs (10). Orai1 silencing was verified by a marked decrease in Orai1 protein content in the renal cortex of mice treated with NP/siOrai1 (68). Using this established technique, we treated mice with either NP/scrambled siRNA (control) or NP/siOrai1 and examined GLP-1R content by immunohistochemistry in kidney sections. In mice treated with NP/scrambled siRNA, both glomeruli and tubules showed overt GLP-1R staining (brown color). However, in mice treated with NP/siOrai1, glomerular GLP-1R staining was significantly reduced while GLP-1R staining in the tubules changed very little (Fig. 7). These immunohistochemical data further support the proposed role of GLP-1R as a downstream effector of the SOCE pathway.
Fig. 7.
In vivo knockdown of Orai1 in mesangial cells decreased glucagon-like peptide-1 receptor (GLP-1R) content in glomeruli in mice. A: representative images of immunohistochemical staining of GLP-1R in kidneys of mice treated with nanoparticle (NP)/scrambled siRNA or NP/siRNA for Orai1 (siOrai1). GLP-1R staining is indicated by the brown color, whereas nuclei are blue. Arrows indicate glomeruli. Original magnification: ×200. B: enlarged images of the region indicated by the red dashed boxes in A. C: integrated density of GLP-1R staining averaged from 3 NP/scrambled siRNA- and 3 NP/siOrai1-treated mice. Numbers in parentheses under each bar represent the number of glomeruli analyzed from 5 sections/kidney. *P < 0.05 compared with NP/scrambled siRNA. Con, control.
DISCUSSION
The pathogenesis of CKDs is multifactorial. The role of activated immune processes and inflammation has been recently recognized in the development of CKDs, including DN (41, 47, 62). A potent regulator of immune and inflammatory responses (48), IL-6 also participates in cell growth and differentiation, hematopoiesis, and metabolism. IL-6 is a well-documented proinflammatory mediator in encephalitis, colitis, arthritis, and other inflammatory disorders (23, 57, 69). On the other hand, IL-6R blockade can exacerbate certain inflammatory conditions, e.g., inflammatory eye disease, psoriasis, and, in the kidney, crescentic immune complex glomerulonephritis (45, 65, 66). Therefore, IL-6 can play both proinflammatory and anti-inflammatory roles, although the molecular mechanisms for the double roles of IL-6 are not clear. Specifically, the role of IL-6 in renal inflammation needs to be delineated. Although data have indicated its clear proinflammatory role in lupus nephritis, IL-6 has been found to be protective in nephrotoxic nephritis (43). Lei et al. (37) found that both classical and trans-signaling of IL-6 in podocytes was detrimental in a high glucose environment, whereas Kurvari et al. (35) reported that IL-6 from podocytes acted as an anti-inflammatory agent for glomerular endothelial cells. Clearly, the role of IL-6 varies in the same organ possibly depending on the specific pathological stimulus, the cells affected, the molecular signaling pathways activated, and the interplays with other cytokines and immune factors.
This study focused on glomerular MCs since these cells not only produce various cytokines/chemokines but are also a major source of glomerular ECM proteins (1, 34, 44, 55, 60). Although IL-6 contributes to the function and growth of MCs in an autocrine fashion (12), IL-6’s function and regulation in these cells are not well known. MCs do not have the membrane receptor for IL-6 and depend on sIL-6R to activate IL-6 signaling. In this study, we genetically upregulated IL-6 signaling in MCs using pCDM8-H-IL-6 plasmids, which overexpress human IL-6 protein fused to human sIL-6R. This approach assures that there is no influence of any inhibitory factors that might be simultaneously secreted by MCs that might hamper the signaling. The results indicated that IL-6 signaling negatively regulated fibronectin and collagen type IV production by MCs. This inhibitory effect of IL-6 is cell type specific because overexpression of IL-6/sIL-6R increased fibronectin protein abundance in HPTECs. These distinct effects of IL-6 in different type of kidney cells exemplify the specificity of IL6’s pro- versus anti-inflammatory character. The finding that IL-6 inhibition on ECM protein production by MCs suggest that IL-6 signaling in MCs may be a renoprotective mechanism to counteract ECM expansion, a pathological hallmark of many kidney diseases.
A new finding in this study is that SOCE stimulated IL-6 protein production and secretion in MCs and thus is assumed to upregulate the IL-6 signaling pathway. To our knowledge, this is the first report that IL-6 signaling can be regulated by SOCE. As we discussed above, IL-6 function is cell context dependent. From the present study, it is unclear if the SOCE/IL-6 regulatory mechanism is MC specific or a general mechanism operating in multiple cell types. Another significance of this finding is that we identified a new downstream pathway of SOCE. Multifunctional SOCE involves arrays of signaling pathways (10, 40, 61, 67, 70). The present findings add IL-6 to the roster of signaling pathways impacted by SOCE. Because SOCE is the major Ca2+ signaling pathway in immune cells (58, 64) and IL-6 is an important proinflammatory/anti-inflammatory agent, our findings may contribute new insights on the pathogenesis of a broad spectrum of inflammatory diseases.
We previously demonstrated that SOCE inhibited ECM protein production by MCs through inhibition of Smad1 and Smad3 pathways (10, 67, 68). The present study showed that IL-6 reduced fibronectin and collagen type IV production and that SOCE upregulated the IL-6 pathway. Therefore, activation of IL-6 signaling may also be one of mechanism mediating SOCE inhibitory effects on ECM protein production in MCs. However, it is not clear whether these downstream pathways of SOCE are independent of one another (in parallel) or share a common pathway (in serial) from the present study. It would be interesting to further study whether these downstream pathways have cross-talk in the future.
It is interesting to note that GLP-1R signaling at least partially mediated IL-6 suppression of ECM protein production. GLP-1 is secreted by intestinal L cells and plays multiple physiological functions apart from insulin secretion and regulation of glucose metabolism (15, 18, 36, 59). GLP-1R agonists have recently been used for patients with type 2 diabetes to improve glycemic control (4, 21). GLP-1R is expressed in kidney cells, including MCs (6, 9, 39, 56). Increased GLP-1R signaling in the kidneys of diabetic animals delayed the progression of DN and ameliorated renal inflammation and injury (16, 24, 32, 50). In the present study, GLP-1R antagonist attenuated, but did not entirely reverse, the inhibitory effect of IL-6 on fibronectin production, suggesting that in addition to GLP-1R pathways, one or more additional mechanisms contribute to the IL-6 effects. For instance, IL-6 may directly inhibit the downstream molecules of the profibrotic pathways like transforming growth factor-β signaling involving Smad3 and Smad1, as reported by a previous study (31). Alternatively, IL-6 can directly suppress fibrotic factors like connective tissue growth factor, which has been demonstrated by several previous studies (25, 39, 63). Furthermore, IL-6 may regulate the JAK-STAT pathway. When this pathway is activated, STAT is phosphorylated and then translocated to the nucleus to promote STAT-dependent gene expression. The matrix metalloproteinase (MMP) family of proteases, such as MMP-1, MMP-3, and MMP-9, are known STAT target genes (5, 14). Via JAK-STAT signaling, IL-6 could conceivably activate these MMPs via the JAK-STAT pathway, leading to increased degradation of ECM proteins (14). Nevertheless, further study is needed to identify the additional mechanisms mediating IL-6 inhibition on ECM protein production by MCs.
In summary, we identified a novel SOCE/NF-κB/IL6/GLP-1R signaling pathway in MCs (Fig. 8). This pathway may protect against kidney diseases by inhibiting MC-derived ECM protein deposition. Because overproduction of ECM proteins and mesangial expansion are major features of many kidney diseases, the present findings may foster the development of new treatments for patients with kidney diseases.
Fig. 8.
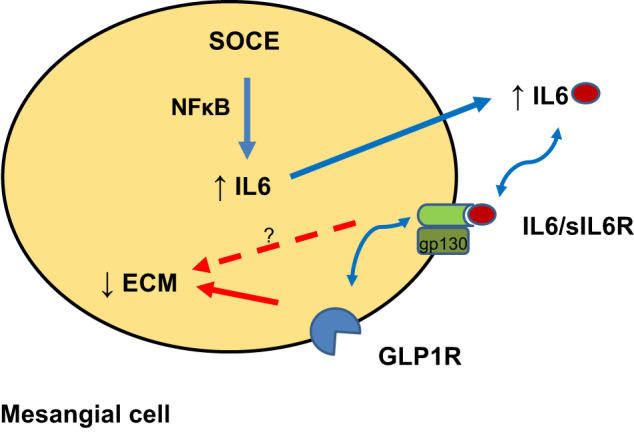
Store-operated Ca2+ entry (SOCE)-induced, interleukin-6 (IL-6)-glucagon-like peptide-1 receptor (GLP-1R)-mediated suppression of extracellular matrix (ECM) deposition by mesangial cells. The simplified summary diagram depicts the pathway mobilized by activation of IL-6 signaling by Orai1-mediated SOCE in mesangial cells and the effect of IL-6 on ECM proteins. Blue arrows indicate promotion of the pathway, the red arrow indicates inhibition of the pathway, and the red dashed arrow indicates possible alternative mechanisms. sIL-6R, soluble IL-6 receptor; gp130, glycoprotein 130.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5-RO1-DK-079968-01 (to R. Ma) and by American Heart Association Southwestern Affiliate Postdoctoral Fellowship 20POST35210685 (to S. Chaudhari).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.C. and R.M. conceived and designed research; S.C. performed experiments; S.C. and R.M. analyzed data; S.C., P.Y.S., and R.M. interpreted results of experiments; S.C. and R.M. prepared figures; S.C. drafted manuscript; P.Y.S., Y.T., M.E.D., R.T.M., and R.M. edited and revised manuscript; S.C., P.Y.S., Y.T., M.E.D., R.T.M., and R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank GlaxoSmithKline (Brentford, UK) for providing GSK-7975A compound and Dr. S. Rose-John (Christian-Albrechts-University, Kiel, Germany) for providing the expression plasmid pCDM8-H-IL-6.
REFERENCES
- 1.Abboud HE. Mesangial cell biology. Exp Cell Res 318: 979–985, 2012. doi: 10.1016/j.yexcr.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin AS., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol 14: 649–681, 1996. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab 11, Suppl 3: 26–34, 2009. doi: 10.1111/j.1463-1326.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter RL, Lo HW. STAT3 target genes relevant to human cancers. Cancers (Basel) 6: 897–925, 2014. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol 297: F1647–F1655, 2009. doi: 10.1152/ajprenal.00082.2009. [DOI] [PubMed] [Google Scholar]
- 7.Cash H, Relle M, Menke J, Brochhausen C, Jones SA, Topley N, Galle PR, Schwarting A. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol 37: 60–70, 2010. doi: 10.3899/jrheum.090194. [DOI] [PubMed] [Google Scholar]
- 8.Ceccarelli E, Guarino EG, Merlotti D, Patti A, Gennari L, Nuti R, Dotta F. Beyond glycemic control in diabetes mellitus: effects of incretin-based therapies on bone metabolism. Front Endocrinol (Lausanne) 4: 73, 2013. doi: 10.3389/fendo.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JT, Liang YJ, Hsu CY, Chen CY, Chen PJ, Yang YF, Chen YL, Pei D, Chang JB, Leu JG. Glucagon-like peptide receptor agonists attenuate advanced glycation end products-induced inflammation in rat mesangial cells. BMC Pharmacol Toxicol 18: 67, 2017. doi: 10.1186/s40360-017-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhari S, Li W, Wang Y, Jiang H, Ma Y, Davis ME, Zuckerman JE, Ma R. Store-operated calcium entry suppressed the TGF-β1/Smad3 signaling pathway in glomerular mesangial cells. Am J Physiol Renal Physiol 313: F729–F739, 2017. doi: 10.1152/ajprenal.00483.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhari S, Wu P, Wang Y, Ding Y, Yuan J, Begg M, Ma R. High glucose and diabetes enhanced store-operated Ca2+ entry and increased expression of its signaling proteins in mesangial cells. Am J Physiol Renal Physiol 306: F1069–F1080, 2014. doi: 10.1152/ajprenal.00463.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman DL, Ruef C. Interleukin-6: an autocrine regulator of mesangial cell growth. Kidney Int 41: 604–606, 1992. doi: 10.1038/ki.1992.91. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 14.Cutler SJ, Doecke JD, Ghazawi I, Yang J, Griffiths LR, Spring KJ, Ralph SJ, Mellick AS. Novel STAT binding elements mediate IL-6 regulation of MMP-1 and MMP-3. Sci Rep 7: 8526, 2017. doi: 10.1038/s41598-017-08581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest 93: 2263–2266, 1994. doi: 10.1172/JCI117225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dieter BP, Alicic RZ, Tuttle KR. GLP-1 receptor agonists in diabetic kidney disease: from the patient-side to the bench-side. Am J Physiol Renal Physiol 315: F1519–F1525, 2018. doi: 10.1152/ajprenal.00211.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. Inflammatory cytokines in diabetic nephropathy. J Diabetes Res 2015: 948417, 2015. doi: 10.1155/2015/948417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9: 1173–1179, 2003. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 19.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 20.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 21.Fineman MS, Mace KF, Diamant M, Darsow T, Cirincione BB, Booker Porter TK, Kinninger LA, Trautmann ME. Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab 14: 546–554, 2012. doi: 10.1111/j.1463-1326.2012.01561.x. [DOI] [PubMed] [Google Scholar]
- 22.Fischer M, Goldschmitt J, Peschel C, Brakenhoff JP, Kallen KJ, Wollmer A, Grötzinger J, Rose-John S. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol 15: 142–145, 1997. doi: 10.1038/nbt0297-142. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto M, Serada S, Mihara M, Uchiyama Y, Yoshida H, Koike N, Ohsugi Y, Nishikawa T, Ripley B, Kimura A, Kishimoto T, Naka T. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum 58: 3710–3719, 2008. doi: 10.1002/art.24126. [DOI] [PubMed] [Google Scholar]
- 24.Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ, Seino Y, Yamada Y. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 85: 579–589, 2014. doi: 10.1038/ki.2013.427. [DOI] [PubMed] [Google Scholar]
- 25.Gressner OA, Peredniene I, Gressner AM. Connective tissue growth factor reacts as an IL-6/STAT3-regulated hepatic negative acute phase protein. World J Gastroenterol 17: 151–163, 2011. doi: 10.3748/wjg.v17.i2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa K, Nakajima S, Hiramatsu N, Okamura M, Huang T, Saito Y, Tagawa Y, Tamai M, Takahashi S, Yao J, Kitamura M. ER stress depresses NF-κB activation in mesangial cells through preferential induction of C/EBP β. J Am Soc Nephrol 21: 73–81, 2010. doi: 10.1681/ASN.2009040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 572–586, 2011. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 28.Hickey FB, Martin F. Diabetic kidney disease and immune modulation. Curr Opin Pharmacol 13: 602–612, 2013. doi: 10.1016/j.coph.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Ma R, Lin T, Chaudhari S, Shotorbani PY, Yang L, Wu P. Glucagon-like peptide-1 receptor pathway inhibits extracellular matrix production by mesangial cells through store-operated Ca2+ channel. Exp Biol Med (Maywood) 244: 1193–1201, 2019. doi: 10.1177/1535370219876531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inagi R, Kumagai T, Nishi H, Kawakami T, Miyata T, Fujita T, Nangaku M. Preconditioning with endoplasmic reticulum stress ameliorates mesangioproliferative glomerulonephritis. J Am Soc Nephrol 19: 915–922, 2008. doi: 10.1681/ASN.2007070745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue-Mochita M, Inoue T, Kojima S, Futakuchi A, Fujimoto T, Sato-Ohira S, Tsutsumi U, Tanihara H. Interleukin-6-mediated trans-signaling inhibits transforming growth factor-β signaling in trabecular meshwork cells. J Biol Chem 293: 10975–10984, 2018. doi: 10.1074/jbc.RA118.003298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibashi Y, Nishino Y, Matsui T, Takeuchi M, Yamagishi S. Glucagon-like peptide-1 suppresses advanced glycation end product-induced monocyte chemoattractant protein-1 expression in mesangial cells by reducing advanced glycation end product receptor level. Metabolism 60: 1271–1277, 2011. doi: 10.1016/j.metabol.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Karkar AM, Smith J, Tam FW, Pusey CD, Rees AJ. Abrogation of glomerular injury in nephrotoxic nephritis by continuous infusion of interleukin-6. Kidney Int 52: 1313–1320, 1997. doi: 10.1038/ki.1997.456. [DOI] [PubMed] [Google Scholar]
- 34.Kashgarian M, Sterzel RB. The pathobiology of the mesangium. Kidney Int 41: 524–529, 1992. doi: 10.1038/ki.1992.74. [DOI] [PubMed] [Google Scholar]
- 35.Kuravi SJ, McGettrick HM, Satchell SC, Saleem MA, Harper L, Williams JM, Rainger GE, Savage CO. Podocytes regulate neutrophil recruitment by glomerular endothelial cells via IL-6-mediated crosstalk. J Immunol 193: 234–243, 2014. doi: 10.4049/jimmunol.1300229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson H, Holst JJ, Ahrén B. Glucagon-like peptide-1 reduces hepatic glucose production indirectly through insulin and glucagon in humans. Acta Physiol Scand 160: 413–422, 1997. doi: 10.1046/j.1365-201X.1997.00161.x. [DOI] [PubMed] [Google Scholar]
- 37.Lei CT, Su H, Ye C, Tang H, Gao P, Wan C, He FF, Wang YM, Zhang C. The classic signalling and trans-signalling of interleukin-6 are both injurious in podocyte under high glucose exposure. J Cell Mol Med 22: 251–260, 2018. doi: 10.1111/jcmm.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levey AS, Coresh J. Chronic kidney disease. Lancet 379: 165–180, 2012. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Cui M, Wei Y, Kong X, Tang L, Xu D. Inhibition of the expression of TGF-β1 and CTGF in human mesangial cells by exendin-4, a glucagon-like peptide-1 receptor agonist. Cell Physiol Biochem 30: 749–757, 2012. doi: 10.1159/000341454. [DOI] [PubMed] [Google Scholar]
- 40.Liang SJ, Zeng DY, Mai XY, Shang JY, Wu QQ, Yuan JN, Yu BX, Zhou P, Zhang FR, Liu YY, Lv XF, Liu J, Ou JS, Qian JS, Zhou JG. Inhibition of Orai1 store-operated calcium channel prevents foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol 36: 618–628, 2016. doi: 10.1161/ATVBAHA.116.307344. [DOI] [PubMed] [Google Scholar]
- 41.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm 2012: 146154, 2012. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 43.Luig M, Kluger MA, Goerke B, Meyer M, Nosko A, Yan I, Scheller J, Mittrücker H-W, Rose-John S, Stahl RA, Panzer U, Steinmetz OM. Inflammation-induced IL-6 functions as a natural brake on macrophages and limits GN. J Am Soc Nephrol 26: 1597–1607, 2015. doi: 10.1681/ASN.2014060620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol 14: 1358–1373, 2003. doi: 10.1097/01.ASN.0000065640.77499.D7. [DOI] [PubMed] [Google Scholar]
- 45.Matsuo Y, Mizoguchi F, Kohsaka H, Ito E, Eishi Y, Miyasaka N. Tocilizumab-induced immune complex glomerulonephritis in a patient with rheumatoid arthritis. Rheumatology (Oxford) 52: 1341–1343, 2013. doi: 10.1093/rheumatology/kes403. [DOI] [PubMed] [Google Scholar]
- 46.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol 112: 397–402, 1998. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7: 327–340, 2011. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 48.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev 22: 83–89, 2011. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol 490: 71–92, 2011. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park CW, Kim HW, Ko SH, Lim JH, Ryu GR, Chung HW, Han SW, Shin SJ, Bang BK, Breyer MD, Chang YS. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol 18: 1227–1238, 2007. doi: 10.1681/ASN.2006070778. [DOI] [PubMed] [Google Scholar]
- 51.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233, 2006. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 52.Pyram R, Kansara A, Banerji MA, Loney-Hutchinson L. Chronic kidney disease and diabetes. Maturitas 71: 94–103, 2012. doi: 10.1016/j.maturitas.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Rangan GK, Tesch GH. Quantification of renal pathology by image analysis (Methods in Renal Research). Nephrology (Carlton) 12: 553–558, 2007. doi: 10.1111/j.1440-1797.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 54.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung JT, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine CE, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Kurella Tamura M, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, Shahinian V. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 73, Suppl 1: A7–A8, 2019. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16, Suppl 1: S30–S33, 2005. doi: 10.1681/ASN.2004110970. [DOI] [PubMed] [Google Scholar]
- 56.Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept 141: 120–128, 2007. doi: 10.1016/j.regpep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 57.Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, Yoshida H, Nishikawa T, Terabe F, Ohkawara T, Takahashi T, Ripley B, Kimura A, Kishimoto T, Naka T. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 105: 9041–9046, 2008. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw PJ, Feske S. Physiological and pathophysiological functions of SOCE in the immune system. Front Biosci (Elite Ed) E4: 2253–2268, 2012. doi: 10.2741/e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skov J, Dejgaard A, Frøkiær J, Holst JJ, Jonassen T, Rittig S, Christiansen JS. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab 98: E664–E671, 2013. doi: 10.1210/jc.2012-3855. [DOI] [PubMed] [Google Scholar]
- 60.Sterzel RB, Schulze-Lohoff E, Marx M. Cytokines and mesangial cells. Kidney Int Suppl 39: S26–S31, 1993. [PubMed] [Google Scholar]
- 61.Targos B, Barańska J, Pomorski P. Store-operated calcium entry in physiology and pathology of mammalian cells. Acta Biochim Pol 52: 397–409, 2005. doi: 10.18388/abp.2005_3452. [DOI] [PubMed] [Google Scholar]
- 62.Tesch GH. Diabetic nephropathy−is this an immune disorder? Clin Sci (Lond) 131: 2183–2199, 2017. doi: 10.1042/CS20160636. [DOI] [PubMed] [Google Scholar]
- 63.Toda N, Mukoyama M, Yanagita M, Yokoi H. CTGF in kidney fibrosis and glomerulonephritis. Inflamm Regen 38: 14, 2018. doi: 10.1186/s41232-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol 10: 21–27, 2009. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wendling D, Letho-Gyselinck H, Guillot X, Prati C. Psoriasis onset with tocilizumab treatment for rheumatoid arthritis. J Rheumatol 39: 657, 2012. doi: 10.3899/jrheum.111166. [DOI] [PubMed] [Google Scholar]
- 66.Wendling D, Paccou J, Berthelot JM, Flipo R-M, Guillaume-Czitrom S, Prati C, Dernis E, Direz G, Ferrazzi V, Ristori JM; CRI . New onset of uveitis during anti-tumor necrosis factor treatment for rheumatic diseases. Semin Arthritis Rheum 41: 503–510, 2011. doi: 10.1016/j.semarthrit.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Wu P, Ren Y, Ma Y, Wang Y, Jiang H, Chaudhari S, Davis ME, Zuckerman JE, Ma R. Negative regulation of Smad1 pathway and collagen IV expression by store-operated Ca2+ entry in glomerular mesangial cells. Am J Physiol Renal Physiol 312: F1090–F1100, 2017. doi: 10.1152/ajprenal.00642.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu P, Wang Y, Davis ME, Zuckerman JE, Chaudhari S, Begg M, Ma R. Store-operated Ca2+ channels in mesangial cells inhibit matrix protein expression. J Am Soc Nephrol 26: 2691–2702, 2015. doi: 10.1681/ASN.2014090853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol 164: 4878–4882, 2000. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 70.Yang J, Yu J, Li D, Yu S, Ke J, Wang L, Wang Y, Qiu Y, Gao X, Zhang J, Huang L. Store-operated calcium entry-activated autophagy protects EPC proliferation via the CAMKK2-MTOR pathway in ox-LDL exposure. Autophagy 13: 82–98, 2017. doi: 10.1080/15548627.2016.1245261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Y, Zhang L, Liu Q, Tang L, Sun H, Guo H. Endoplasmic reticulum stress preconditioning antagonizes low-density lipoprotein-induced inflammation in human mesangial cells through upregulation of XBP1 and suppression of the IRE1α/IKK/NF-κB pathway. Mol Med Rep 11: 2048–2054, 2015. doi: 10.3892/mmr.2014.2960. [DOI] [PubMed] [Google Scholar]
- 72.Zuckerman JE, Gale A, Wu P, Ma R, Davis ME. siRNA delivery to the glomerular mesangium using polycationic cyclodextrin nanoparticles containing siRNA. Nucleic Acid Ther 25: 53–64, 2015. doi: 10.1089/nat.2014.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]