Abstract
The descending corticofugal fibers originate from the auditory cortex and exert control on the periphery via the olivocochlear efferents. Medial efferents are thought to enhance the discriminability of transient sounds in background noise. In addition, the observation of deleterious long-term effects of efferent sectioning on the response properties of auditory nerve fibers in neonatal cats supports an efferent-mediated control of normal development. However, the role of the efferent system in human hearing remains unclear. The objective of the present study was to test the hypothesis that the medial efferents are involved in the development of frequency discrimination in noise. The hypothesis was examined with a combined behavioral and physiological approach. Frequency discrimination in noise and efferent inhibition were measured in 5- to 12-yr-old children (n = 127) and young adults (n = 37). Medial efferent strength was noninvasively assayed with a rigorous otoacoustic emission protocol. Results revealed an age-mediated relationship between efferent inhibition and frequency discrimination in noise. Efferent inhibition strongly predicted frequency discrimination in noise for younger children (5–9 yr). However, for older children (>9 yr) and adults, efferent inhibition was not related to frequency discrimination in noise. These findings support the role of efferents in the development of hearing-in-noise in humans; specifically, younger children compared with older children and adults are relatively more dependent on efferent inhibition for extracting relevant cues in noise. Additionally, the present findings caution against postulating an oversimplified relationship between efferent inhibition and measures of auditory perception in humans.
NEW & NOTEWORTHY Despite several decades of research, the functional role of medial olivocochlear efferents in humans remains controversial and is thought to be insignificant. Here it is shown that medial efferent inhibition strongly predicts frequency discrimination in noise for younger children but not for older children and adults. Young children are relatively more dependent on the efferent system for listening-in-noise. This study highlights the role of the efferent system in hearing-in-noise during childhood development.
Keywords: auditory development, efferent inhibition, frequency discrimination, medial olivocochlear efferents, otoacoustic emissions
INTRODUCTION
The significance of the efferent system in hearing is a fundamental question in sensory neuroscience. The descending efferent neural pathway shapes the representation of sounds in the ascending auditory system. Descending projections from the auditory cortex reach into the cochlea through olivocochlear neurons in the superior olivary complex (Xiao and Suga 2002). The medial part of the olivocochlear efferents (hereafter, efferents) contains thick myelinated fibers that innervate outer hair cells via cholinergic synapses. Numerous studies in humans and animals have shown that efferent stimulation alters cochlear physiological responses to sounds (for review, see Guinan 2006; Lopez-Poveda 2018). However, what role these efferent effects play in human hearing remains unclear.
Among several putative functions of the efferent system, the antimasking hypothesis has received considerable attention (e.g., Smith and Keil 2015). The antimasking model posits that the efferent system enhances the discriminability of transient signals in a noisy background. This conjecture is based on the observation that in a continuously noisy environment efferent stimulation enhances auditory nerve responses to transient sounds by partially restoring the auditory nerve dynamic range (Kawase et al. 1993; Nieder and Nieder 1970; Winslow and Sachs 1987). The efferent antimasking model has been expanded by animal experiments with severed efferents. For example, rhesus monkeys with sectioned efferents showed impaired vowel discrimination ability in noise (Dewson 1968). Similar results were reported in cats for intensity discrimination of high-frequency tones in noise (May and McQuone 1995). However, the evidence for a role of the efferent system in auditory perception in humans remains controversial (see Lopez-Poveda 2018 for review).
The present study explores another kind of antimasking benefit for the sound-evoked brain stem activity (medial efferent inhibition) in humans during childhood development. The efferent control of masking is particularly important when the immature auditory perception is challenged with background noise and thereby may facilitate auditory perceptual development. Such a hypothesis emerges from the observation of descending control of auditory maturation in animal models with little or no efferent innervation in the cochlea (Lauer and May 2011; Walsh et al. 1998). Walsh et al. (1998) reported long-term effects of efferent sectioning on sensitivity, frequency selectivity, and spontaneous discharge rate of auditory nerve fibers in neonatal cats. Likewise, Lauer and May (2011) found a decreased ability to process rapid acoustic events in α9-knockout mice (lacking efferents) reared in noise from birth relative to wild-type control mice. They suggested that the regulation of auditory nerve activity by the medial efferents preserves normal postnatal development when the immature auditory system is exposed to noise. However, to date, surprisingly little is known about how the development of auditory perception in children is influenced by the efferents that enhance the encoding of transient signals in background noise.
Examination of the efferent antimasking mechanism with regard to frequency discrimination offers several novel and important perspectives. Frequency discrimination is a fundamental ability to detect a change in the frequency of a pure tone and is critical for processing and perception of complex sounds, including speech and music. Frequency discrimination is determined by temporal (phase locking) mechanisms at low frequencies (<4 kHz) and by place-based mechanisms at higher frequencies (see Oxenham 2018 for review). Efferent stimulation is known to modulate cochlear gain and the cochlear response associated with spatial irregularity (see Guinan 2018 for review). The efferent-induced reduction of cochlear amplifier gain could enhance the masked auditory discrimination by decompressing the dynamic range of cochlear responses to transient sounds. Also, the alterations in the cochlear irregularity profile could potentially affect the ability to use the phase-locking cues (Otsuka et al. 2014). Cats with sectioned efferent connections to the cochlea performed more poorly on perceptual tasks involving vowel formant (frequency) discrimination in noise, whereas the perception in quiet was unchanged (Hienz et al. 1998). In humans, Scharf et al. (1997) showed that frequency discrimination was not affected in vestibular neurotomy patients after the surgical removal of efferents. This finding is not surprising, as the performance in quiet is not expected to receive a benefit from the efferent activity. To date, only one study has reported equivocal data on pure-tone frequency discrimination in noise from an adult vestibular neurectomy patient (Scharf et al. 1994), leaving open the possibility that efferent input facilitates frequency discrimination in noise. Poor frequency discrimination in children is associated with oral language abilities (McArthur and Bishop 2004; Mengler et al. 2005), dyslexia (Hämäläinen et al. 2013; Schulte-Körne and Bruder 2010), and reading difficulties (Ahissar et al. 2000). Frequency discrimination in children matures between 8 and 12 yr of age (Buss et al. 2014; Moore et al. 2010, 2011; Sutcliffe and Bishop 2005). This prolonged maturation is considered to reflect central (Bishop et al. 2011) and/or nonsensory (Buss et al. 2014; Moore et al. 2008) mechanisms.
The objective of the present study was to investigate the influence of the medial efferent system on the development of hearing-in-noise in children (5–12 yr). The specific aim was to examine the relationship between efferent inhibition and frequency discrimination in noise and evaluate this relationship as a function of age. Based on a previous study in cats (Hienz et al. 1998), it was hypothesized that individuals with stronger efferent inhibition would perform better in a frequency discrimination task in noise compared with individuals with weaker efferent inhibition. Importantly, this relationship between efferent inhibition and frequency discrimination in noise will be different for mature (adult) and developing (children) auditory systems. With weaker efferent inhibition controlling cochlear responses and potentially shaping brain stem activity, the developing auditory system may be inefficient for signal discrimination in noise. In contrast, such weaker efferent modulation may not affect auditory perception in a mature system because of the availability of other robust (central) mechanisms.
METHODS
Experimental design.
Measurements of otoacoustic emission (OAE)-based efferent inhibition and frequency discrimination (FD) thresholds were obtained for young adults and children in a cross-sectional design. The OAE measurement goal was to estimate efferent effects at 1,000 Hz. For behavioral measurements, FD thresholds at 1,000 Hz were measured in quiet and noise conditions. The test ear for the FD task was the same as the probe ear for efferent measurements. The order of behavioral and physiological measurements was counterbalanced across participants. The test session for each participant lasted from 2 to 3 h. Study protocols were approved by the New Mexico State University Institutional Review Board. Adult subjects and parents or guardians of child subjects provided written informed consent.
Participants.
A total of 127 children (5–12 yr) and 37 young adults (19–35 yr) participated in the present study. Children were stratified into seven groups according to age: 5, 6, 7, 8, 9, 10, and 11–12 yr. Inclusion in the study required that participants were within clinically accepted normal limits on pure-tone audiometry (≤15 dB HL at octave frequencies from 250 to 8,000 Hz) and tympanometry (middle ear admittance ≥ 0.3 with middle ear pressure between 50 and −100 daPa). An additional criterion for children included no evidence of a developmental disorder. None of the participants had a prior psychoacoustic listening experience. All procedures were completed in a double-walled sound-attenuating booth.
Otoacoustic emissions.
Reflection-type OAEs were used for assaying efferent inhibition of cochlear responses with contralateral broadband noise (BBN). Click-evoked (CEOAEs) and stimulus-frequency (SFOAEs) OAEs, recorded at low levels, arise by linear coherent reflections in the cochlea and represent reflection-type OAEs (Shera 2004; Shera and Guinan 1999). Besides, they are free from short-delay components for the stimulus parameters used in this study (Lewis and Goodman 2015; Sisto et al. 2013) and do not elicit probe-induced efferent or middle ear muscle reflexes (MEMRs). Therefore, reflection-type OAEs are suitable for estimating efferent effects (Guinan et al. 2003). At low and moderate stimulus levels, click-evoked and stimulus-frequency OAEs yield equivalent results (i.e., nearly identical input/output transfer functions) in humans (Kalluri and Shera 2007), including similar efferent effects in adults (Francis and Guinan 2010; Marshall et al. 2014) and children (Mishra et al. 2018).
OAE-based efferent inhibition measurement procedures applied here are based on robust methods detailed in our recent work (Mishra et al. 2018; Mishra and Biswal 2019; Mishra and Dinger 2016). The measurement goal was to estimate efferent effects at 1,000 Hz with one-half-octave intervals (841–1,190 Hz) with either click-evoked or stimulus-frequency OAEs. The 1,000 Hz region typically has a high signal-to-noise ratio (SNR) (Mishra and Abdala 2015; Mishra and Talmadge 2018) and yields robust efferent effects (Abdala et al. 2013; Goodman et al. 2013; Mishra and Dinger 2016). In all OAE procedures, the contralateral BBN elicitor (100–10,000 Hz) was presented at 60 dB SPL with an ER-2 insert transducer (Etymotic Research, Elk Grove Village, IL). Participants were instructed to remain as calm and quiet as they could during the recording procedures. Children watched age-appropriate closed-caption videos throughout the recording duration.
CEOAEs were recorded from 73 participants (21 adults) with the Mimosa HearID system (Mimosa Acoustics, Champaign, IL) with an ER-10C probe assembly (Etymotic Research, Elk Grove Village, IL) similar to our past work (Mishra et al. 2018; Mishra and Dinger 2016). An in-the-ear calibration method, i.e., a voltage correction, was applied to achieve the target stimulus level in the ear canal for each participant. Clicks (83.3 μs) at 60 dB pSPL were presented at a rate of 45 Hz with a linear recording paradigm (Kemp et al. 1986). Recordings were made for 2,000 clicks in eight interleaved blocks: four with and four without BBN elicitor. The interblock interval was 10 s. An onset delay of 2 s was set for recordings in the BBN elicitor block to sufficiently allow for efferent time constants (Backus and Guinan 2006). The artifact or noise rejection level was adjusted between 45 and 49 dB SPL for avoiding instantaneous noise during the measurement to achieve good recordings. A block was accepted if rejections were fewer than 50. Responses were time-windowed between 2 and 20 ms. Time-frequency analysis using Stockwell transform was applied to extract OAE magnitude and delay in the 1,000 Hz region for computation of efferent inhibition (Mishra and Biswal 2016; Mishra and Dinger 2016).
Stimulus-frequency OAEs were measured in 75 participants (13 adults) with a suppressor technique, similar to a previous study (Mishra and Talmadge 2018). Stimulus generation and recording of signals were controlled with a customized version of the RecordAppX software (Talmadge et al. 1999) interfaced with a MOTU 828x audio interface (MOTU, Cambridge, MA). An ER-10B+ probe microphone system that included two ER-2 insert earphones (Etymotic Research, Elk Grove Village, IL) was inserted into the ear canal for recording OAEs. A depth-compensated calibration was applied for each participant (Lee et al. 2012; Mishra and Abdala 2015). The probe and suppressor levels were fixed at 40 and 60 dB SPL, respectively. The ratio between the probe and suppressor frequencies was 1.1, with the suppressor frequency greater than the probe frequency. Stimuli were swept at a rate of 0.188 octaves/s. The phase was inverted for every other use of the suppressor. For the elicitor condition, the BBN was interleaved for the probe sweep only, for instance, probe without BBN, probe with BBN, and probe + suppressor. The BBN elicitor was activated 2 s before the onset of sweeps, with a time interval of 3 s between the presentation of two consecutive sweeps. OAEs were estimated from 8 probe and 8 probe-plus-suppressor sweep tones with the least-squares-fit (LSF) method (Long et al. 2008). Briefly, the LSF filter models both probe and suppressor sweeps and estimates OAEs to minimize the sum of the squared error between the model and the response. The noise floor was estimated from the average pairwise sweep difference obtained by subtraction of the probe from the probe + suppressor runs. Separate estimates of the magnitude, phase, and noise floor were obtained with and without BBN elicitor (Mishra et al. 2018).
A signal-to-noise ratio criterion of 9 dB was applied for estimating efferent effects. The vector difference in complex pressures between OAEs with and without BBN elicitor utilized both magnitude and phase information. The difference was normalized by the baseline OAEs for computing the efferent inhibition in percentage (∆OAEs) for the analysis frequency range (841–1,190 Hz), with 1,000 Hz as the center frequency (Mishra et al. 2018).
All stimuli, including the elicitor, used in OAE recordings were low enough in level not to elicit MEMRs. However, to confirm the absence of MEMRs, a stimulus-level shift test for clicks and a group-delay test for swept tones were applied. Shift in click levels, on a linear scale, of <1.4% due to the elicitor was regarded as the lack of MEMRs (Mishra and Dinger 2016). For swept tones, the phase slope of the shift in the compound tone due to the elicitor > 4 ms was considered a sign of no MEMRs (Mishra and Biswal 2019).
Frequency discrimination.
FD testing was implemented as an animated computer game using the System for Testing Auditory Responses (Barry et al. 2010; Chilekwa et al. 2009). The selection of specific parameters and procedure was guided by the goal of obtaining reliable threshold estimates in the shortest possible time in order to minimize noncompliance among children and was based on relevant FD work in children (Halliday et al. 2008; Moore et al. 2008, 2010, 2011). Stimuli were 200-ms tones, gated with 10-ms raised-cosine ramps, presented at 70 dB SPL with an interstimulus interval of 400 ms with HDA 200 supra-aural headphones (Sennheiser, Germany). The task employed an adaptive, three-interval, three-alternative (odd-ball) forced-choice paradigm. Each trial contained two intervals of a standard tone (1,000 Hz) and a third randomly determined interval with a target tone higher in frequency. The frequency of the target tone (odd-ball) varied adaptively between trials following a staircase procedure. To reduce the test duration, the initial target tone was 50% higher in frequency (∆F = 50% or F = 1,500 Hz), and the ∆F was halved following a 1-down, 1-up rule until the first error or reversal. The staircase then used a 3-down, 1-up rule, with ∆F multiplied or divided by a factor of √2. Trials continued until three reversals were obtained in the second phase. The threshold estimate for a given run was computed as the geometric mean of ∆F in the last two reversals. Measurements were repeated to ensure the reliability of the threshold estimate. FD threshold, expressed as a percentage of the standard frequency (∆F%), was defined as the mean of threshold estimates obtained from two consecutive runs. If three reversals were not achieved with 25 trials in a given run or there was a discrepancy of >10% between two runs (Moore et al. 2011), a third run was administered. In the case of a third run, the threshold was the mean of the two closest estimates. FD thresholds were obtained in two conditions: in quiet and in the presence of ipsilateral, continuous broadband noise at 10-dB SNR. Preliminary experiments showed that this SNR caused threshold elevation relative to the quiet condition without increasing noncompliance, associated with task difficulty due to the introduction of noise, among young children. However, it is plausible that the discrimination task at 10-dB SNR was easier for adults. To address this task difficulty issue, FD thresholds at an additional SNR condition of 6 dB were measured in a subset of adults (n = 12).
The FD test protocol was presented via a child-friendly computer game similar to that used in a previous study (Moore et al. 2008). In the computer screen, a cartoon character corresponded to each stimulus interval, and each character corresponded to one of the three buttons on a response box. Participants were allowed unlimited time to respond by indicating the cartoon associated with the target tone. Visual feedback was provided for all correct responses, and an indicator showed the progress for a given run.
A familiarization procedure before the testing included a presentation of easy-to-discriminate stimuli (1,000 vs. 1,500 Hz). A participant was expected to discriminate at least three out of five trials. This practice ensured that participants understood the test paradigm (the odd-ball and forced-choice formats). Familiarization was further facilitated by providing a practice version of the actual run in quiet and noise. In the noise condition before discrimination practice testing, the detection of the target tone was confirmed in every participant by a yes-no procedure. During the practice phase, the tester provided additional clarifications and instructions as needed.
Statistical analyses.
Statistical analyses were performed with SPSS Statistics (version 25; IBM Corp., Armonk, NY). Data exclusion criteria included noncompliance, low SNR in OAEs, and incorrect responses in the familiarization (easy-to-discriminate stimuli) stage. Exclusions were based on individual tests rather than the entire data set from a participant. In addition, outliers were defined as data points outside the ±1.5 interquartile range. FD threshold data were log-transformed for all statistical analyses. Logarithmic units reflect the underlying perceptual (ratio) scale in FD tasks and tend to equalize variance in the data (Micheyl et al. 2006). Efferent inhibition data were pooled together from CEOAE and SFOAE recordings for maximizing the sample size.
Repeated-measures analysis of variance (ANOVA) was applied to test for the statistical significance of condition (quiet vs. noise), age group, sex, ear (right vs. left), and/or OAEs (click-evoked vs. stimulus-frequency) on mean FD thresholds. It was predicted that FD thresholds would be lower (better) in the quiet compared with the noise condition and the thresholds would improve (lower) with age. The effects of OAEs (click-evoked vs. stimulus-frequency) and other demographic variables (age group, sex, and ear) on the mean efferent inhibition were tested with univariate, fixed-factors ANOVA. For significant ANOVA results, post hoc analysis with appropriate corrections for multiple comparisons was planned. Corrections for multiple comparisons were made with a false discovery rate method (Benjamini and Yekutieli 2001). To test the central hypothesis that FD thresholds in noise are a function of multiple factors and, more specifically, whether age moderates the relationship between efferent inhibition and FD thresholds, a hierarchical multiple regression analysis was conducted. Multiple variables such as efferent inhibition, age, sex, ear, and click-evoked/stimulus-frequency OAEs were input into the regression model. Categorical variables were coded as dummy variables, and the reference category was adults. For the moderation analysis (Rose et al. 2004), to avoid potentially problematic high multicollinearity with the interaction term, efferent inhibition data were centered, and an interaction term between efferent inhibition and age group (∆OAEs × age group) was created (Aiken and West 1991). In addition, Pearson product-moment correlation coefficients and bias-corrected and accelerated 95% confidence intervals from bootstrapping (1,000 samples) were computed for examining relationships between efferent inhibition and FD thresholds in noise separately for each age group. To further confirm the correlation analysis, participants were categorized into two groups based on median values (DeCoster et al. 2011): stronger and weaker efferent inhibition. The two groups were defined separately for adults and children. One-way multivariate analysis of covariance (MANCOVA) with the median-split groups as the fixed factor and age (in logs) as the covariate was conducted to examine whether the frequency discrimination thresholds were different for children with stronger and weaker efferent inhibition. An effect was considered as statistically significant when the null hypothesis could be rejected with 95% confidence (P < 0.05). For brevity, detailed results are presented for significant findings only.
RESULTS
The majority of participants (86%) completed the study protocol. Data inclusion rate was similar to other large-scale behavioral studies [Moore et al. 2010 (92%); Moore et al. 2011 (74%)]. Table 1 presents the distribution of data inclusions from participants by age group. As expected, data inclusions were relatively lower for younger children (5–7 yr; 76%). Data exclusions for OAEs were mainly due to low SNRs, whereas noncompliance and ceiling performance were primary reasons for exclusions for the FD task.
Table 1.
Data inclusions by age group
| Adults | Children, yr |
|||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 10 | 11–12 | ||
| n | 37 | 22 | 18 | 15 | 19 | 19 | 17 | 17 |
| Female | 25 | 11 | 5 | 7 | 10 | 12 | 9 | 6 |
| Right ear | 22 | 13 | 10 | 8 | 6 | 9 | 8 | 11 |
| Completed | 33 | 18 | 12 | 12 | 18 | 17 | 15 | 16 |
Efferent inhibition.
Figure 1 shows efferent inhibition for various age groups. Mean efferent inhibition was 33% for adults and varied between 30% and 41% across age groups for children. Previous studies reported similar mean values for adults (29–37%; Backus and Guinan 2007; Marshall et al. 2014) as well as for 5- to 10-yr-old children (29–30%; Mishra et al. 2018). ANOVA showed no significant main or interaction effects of OAEs (click-evoked vs. stimulus-frequency), age group, sex, and ear on mean ∆OAEs (P > 0.05). Reflection OAEs appear to yield similar efferent inhibition at the group level regardless of stimuli (clicks or tones), consistent with past work in adults (Francis and Guinan 2010; Marshall et al. 2014) and children (Mishra et al. 2018). This finding extends the equivalency between click-evoked and stimulus-frequency OAEs in humans (Kalluri and Shera 2007) in a new way. The lack of an effect of age group on efferent inhibition is not surprising considering that efferent inhibition measured at 1,000 Hz is generally considered to be mature in full-term neonates (Abdala et al. 2013). Likewise, the lack of sex and ear laterality effects in efferent inhibition is also reported (Stuart and Kerls 2018; but see Khalfa et al. 1997).
Fig. 1.
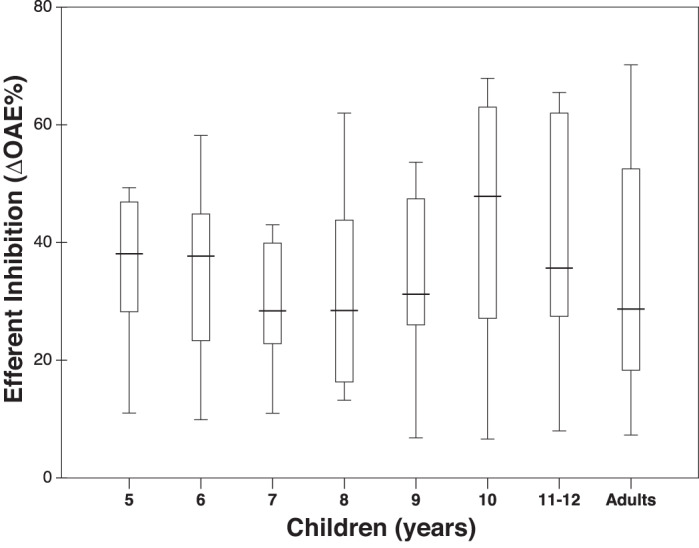
Box-whisker plots showing efferent inhibition expressed as % of the baseline otoacoustic emissions (∆OAEs) for adults and children. Horizontal lines indicate the median, boxes span from the 25th to the 75th percentile, and whiskers show the maximum and minimum values.
Frequency discrimination in noise.
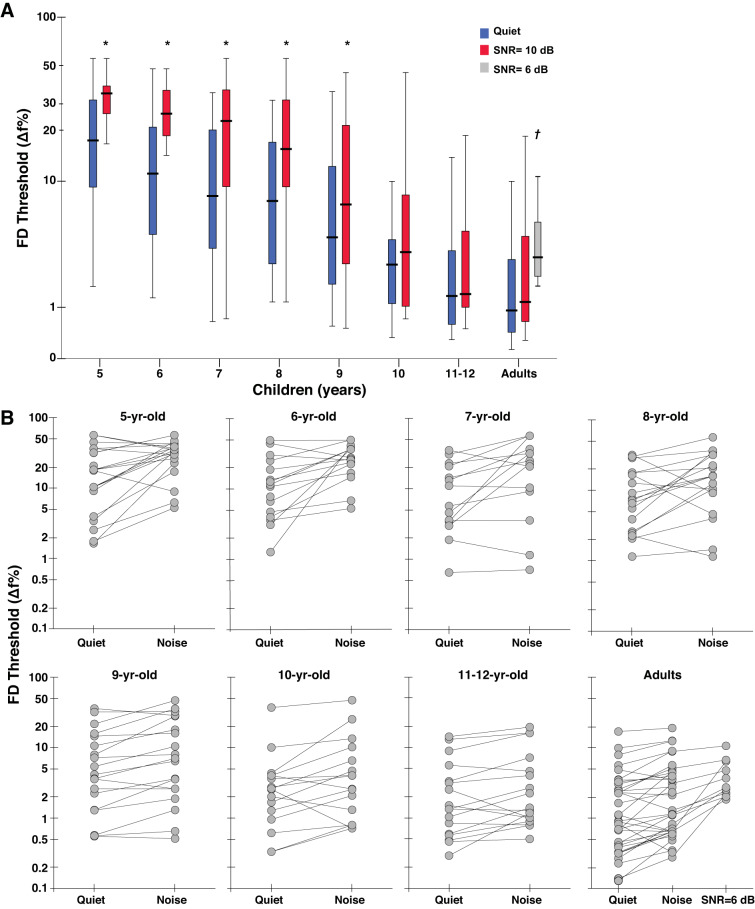
Frequency discrimination thresholds, expressed as ∆F%, in quiet and noise for various age groups are plotted in Fig. 2. FD thresholds consistently improved with age, from ∼17% at 5 yr of age to ∼1.3% in 11- to 12-yr-old children (0.9% in adults), similar to previous reports on the development of frequency discrimination in children (Buss et al. 2017; Halliday et al. 2008; Moore et al. 2008, 2010, 2011). In the original units (∆F%), FD thresholds in noise were higher compared with the quiet condition by a factor of 1.3–3 depending on the age group. Although a direct comparison between studies is problematic because of the differences in stimulus parameters, median FD thresholds in noise (1.2% and 3.2% in 10- and 6-dB SNRs, respectively) for adults were slightly lower compared with thresholds (1.6–2.2% for ∼12- to 15-dB SNRs) reported in previous studies (Cardozo 1974; Dye and Hafter 1980; Henning 1967).
Fig. 2.
A: Box-whisker plots showing frequency discrimination (FD) thresholds represented as % of the standard frequency (1,000 Hz); y-axis is (safe1) log scaled. Horizontal lines indicate the median, boxes span from the 25th to the 75th percentile, and whiskers show the maximum and minimum values. *Significantly higher thresholds compared with adults (P < 0.001); †significant difference between the 6-dB and 10-dB signal-to-noise ratio (SNR) conditions for adults (P < 0.01). Note: thresholds were measured in 2 SNR conditions for adults. B: individual FD thresholds in quiet and noise (SNR = 10 dB) are plotted for children and adults; FD thresholds for an additional SNR condition (6 dB) are shown for a subset of adults (n = 12). y-Axis is log-scaled. Lines connect FD thresholds across conditions for each participant.
All statistical comparisons between children and adults involving the noise condition were made for the 10-dB SNR condition; data from children were collected only for this SNR. ANOVA showed a significant main effect of test condition and age group on mean FD thresholds (log transformed). No other main (sex, ear, and OAEs) or interaction effects were statistically significant (P > 0.05). FD thresholds worsened with noise (F1,96 = 39.61; P < 0.001) but improved with age (F1,7 = 11.42; P < 0.001). The lack of interaction effects suggests that the rates of development of pure-tone frequency discrimination in quiet and noise are similar. In adults, the mean threshold in the 6-dB SNR condition was significantly higher than the 10-dB SNR condition (t10 = 4.35; P = 0.001). Post hoc comparisons showed that FD thresholds in noise were significantly lower for adults compared with 5- through 9-yr-old children (P < 0.001). Although FD thresholds in noise for 10-yr-olds were higher compared with adults, this did not reach statistical significance (P = 0.57). In contrast, thresholds for 11- to 12-yr-olds were similar to adults (P = 0.34). Significant across-group comparisons with reference to adults are labeled in Fig. 2.
Relationship between efferent inhibition and frequency discrimination in noise.
Multiple linear regression revealed that only age accounted for a significant portion of the variance in FD thresholds in quiet (R2 = 0.40; F8,134 = 11.25; P < 0.001). Efferent inhibition and categorical variables (sex, ear, OAEs) did not contribute significantly to the regression model.
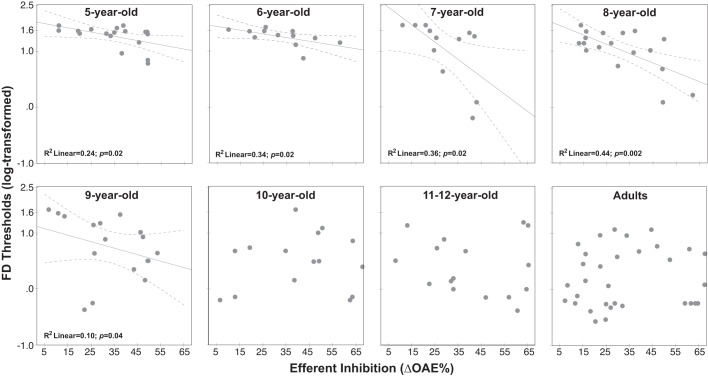
The relationships between efferent inhibition and FD thresholds in noise for various age groups are shown in bivariate scatterplots in Fig. 3. Figure 4 shows correlation coefficients between ∆OAEs and FD thresholds, separately for each age group. It appears that the efferent inhibition is inversely associated with FD thresholds in noise—smaller thresholds are associated with stronger (higher) efferent inhibition—for 5- through 9-yr-olds; however, no relationship exists for other age groups, including adults for the 6-dB SNR condition. Hierarchical multiple regression modeling showed that ∆OAEs accounted for a small but significant amount of variance in FD thresholds in noise (R2 = 0.06; F1,139 = 8.49; P = 0.004). Adding age group to the model accounted for significantly more variance than just ∆OAEs (∆R2 = 0.46; ∆F7,132 = 18.08; P < 0.004). Furthermore, including an interaction term (∆OAEs × age group) significantly improved the model prediction compared with the model with efferent inhibition and age group (∆R2 = 0.06; F7,125 = 2.35; P = 0.03), suggesting that there is potentially significant moderation between efferent inhibition and age group on FD thresholds in noise. The interaction terms were significant particularly for 7- and 8-yr-old children (7 yr: B = −0.04, P = 0.01; 8 yr: B = −0.02, P = 0.02). Overall, efferent inhibition, age group, and their interaction accounted for a significant proportion of the variance in FD thresholds in noise (adjusted R2 = 0.52). The adjusted R2 is a modified version of R2 that considers the number of predictors in the model and is always lower than the R2. Importantly, the analysis showed that age moderates the relationship between efferent inhibition and frequency discrimination in noise.
Fig. 3.
Frequency discrimination (FD) thresholds in noise (log transformed) for individual listeners plotted as a function of efferent inhibition [∆otoacoustic emissions (OAEs)] for each age group, separately; y-axis is (safe) log scaled. Lines (solid) indicate the linear association between efferent inhibition and FD thresholds in noise and are inserted, along with R2 linear and P values, only for age groups showing significant relationships. Dashed lines show 95% confidence intervals of the mean.
Fig. 4.
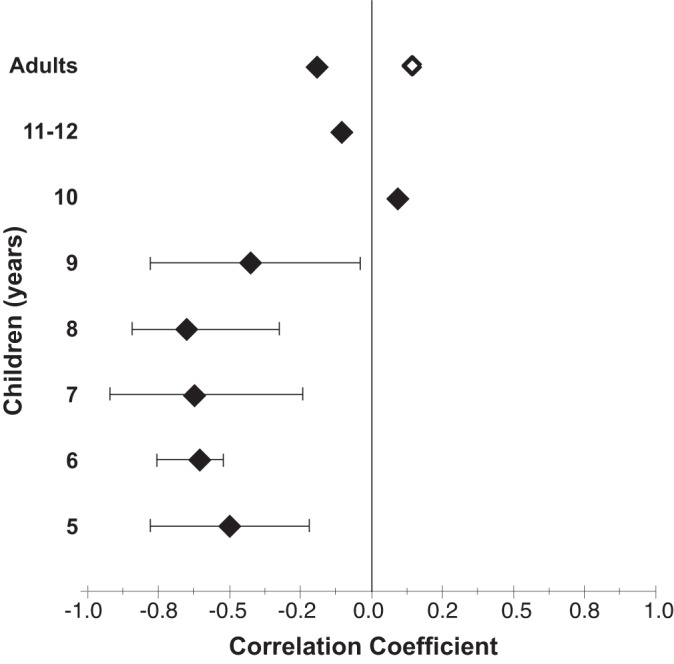
Correlation coefficients (filled symbols) showing relationships between efferent inhibition and frequency discrimination thresholds in noise for each age group; the open symbol for adults shows the coefficient for the 6-dB signal-to-noise ratio condition. Horizontal error bars show bias-corrected and accelerated 95% confidence intervals computed from bootstrapping (1,000 samples) for age groups with significant correlation coefficients.
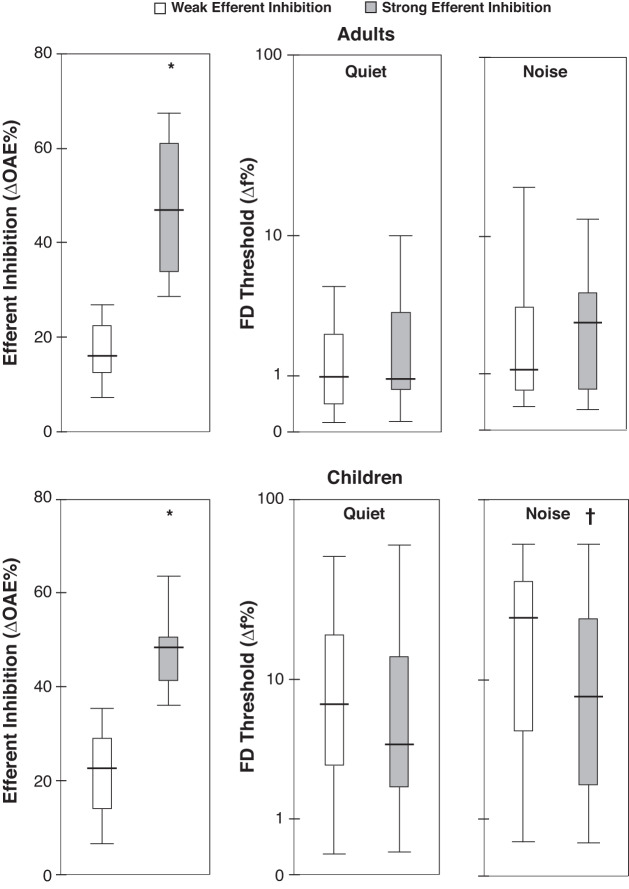
Efferent inhibition and FD thresholds for the median-split groups (stronger and weaker efferent inhibition) are shown in Fig. 5; note that age was not a covariate for plotting this figure. For children, MANCOVA revealed a statistically significant difference between the stronger and weaker efferent inhibition groups on the combined FD thresholds after controlling for age (F2,116 = 6.03, P = 0.003, Wilks' Λ = 0. 91). Pairwise comparisons with false discovery rate adjustments showed a significant difference for FD thresholds in noise (P = 0.007) but not for FD thresholds in quiet (P = 0.28). Children with stronger efferent inhibition (∆OAEs ≥ 35.71%; mean = 48.72%) had significantly lower FD thresholds in noise but not in quiet relative to children with weaker inhibition (mean = 21.94%) after controlling for age. For adults, ANOVA showed no significant main effect of median-split groups (median ∆OAEs = 27.85%) on FD thresholds (P > 0.05).
Fig. 5.
Box-whisker plots showing efferent inhibition and frequency discrimination (FD) thresholds for stronger and weaker efferent inhibition groups for adults (top) and children (bottom); y-axis is (safe) log scaled for FD thresholds. Horizontal lines indicate the median, boxes span from the 25th to the 75th percentile, and whiskers show the maximum and minimum values. Significant differences between the median-split groups are indicated at top (*P < 0.001, †P = 0.004).
DISCUSSION
Principal findings.
The present study investigated the functional role of medial olivocochlear efferents in human auditory development, specifically the development of frequency discrimination in noise. Pure-tone frequency discrimination in noise follows a protracted period of development, similar to that in quiet, and remains immature at least until 9 yr of age (Fig. 2). As expected, no relationship between efferent inhibition and frequency discrimination in quiet was found for any group. The relationship between efferent inhibition and frequency discrimination in noise is not static but is determined by the age of the listener. In younger children (5–9 yr), listeners with stronger (higher) efferent inhibition were associated with better performance in noise (i.e., lower FD thresholds). In contrast, no relationship was found between efferent inhibition and frequency discrimination in noise for older children (≥10 yr) and adults. In addition, the strength of efferent inhibition (stronger vs. weaker) had a significant effect on frequency discrimination in noise for children but not in quiet or for adults. The extent of threshold elevation in noise relative to the quiet condition was not statistically different for various age groups. However, to address the potential issue of task difficulty (i.e., the discrimination task at 10-dB SNR is easier for adults), the 6-dB SNR condition was additionally tested for adults. However, the 6-dB SNR condition did not alter the lack of a relationship between efferent inhibition and FD thresholds in noise for adults. Importantly, the relationship between efferent inhibition and frequency discrimination in noise was evident only for children whose behavioral performance was immature (≤9 yr). These findings provide evidence for a potential role of olivocochlear efferents in the development of human hearing-in-noise. They extend the results of previous studies using animal models that support the importance of efferent control in auditory development (Lauer and May 2011; Walsh et al. 1998) in a new way. In addition, the present findings suggest that poor frequency discrimination in noise in children may not be entirely due to nonauditory or cognitive factors.
Some methodological considerations.
Methodological difficulties make it hard to measure medial efferent activity and investigate the efferent neural bases of perceptual processes in humans. Some issues are considered for accurate interpretation of findings. For the perceptual test, both tone and the noise were in the same ear; as a result, the noise (60 dB SPL) likely elicited the ipsilateral efferent pathway. In contrast, the contralateral efferent pathway was stimulated for the OAE test. Although ipsilateral and contralateral BBN elicit similar efferent activity with equivalent timescales (Backus and Guinan 2006; Boothalingam et al. 2016; Guinan et al. 2003; Lilaonitkul and Guinan 2009; Mishra and Biswal 2019), it is currently unknown whether contralateral efferent inhibition can be substituted for ipsilateral measurement. Nevertheless, it is commonly assumed that the innervation for medial efferents that respond to ipsilateral and contralateral stimulation is similar (Lopez-Poveda 2018; but see Brown 2014). All things considered, this ipsi-contra difference cannot explain the observed differential perceptual benefit of efferents for adults and children.
Another difficulty is related to the MEMR evoked by the BBN elicitor used for OAE-based efferent measurements. The potential contribution of MEMR to the SFOAE measurements was carefully eliminated with a sensitive group-delay test (see methods). However, the click-based method for examining potential MEMRs in CEOAEs is not a flawless approach. For example, when the MEMR is active the impedance changes can cause an increase in the ear canal reflectance in the lower frequencies (<1,000 Hz) and minimal changes for the higher frequencies (Feeney et al. 2003). Boothalingam and Purcell (2015) suggested that the stimulus level variations associated with the decrease and increase in reflectance across frequency may cancel out and could lead to a null difference in the composite click despite the presence of MEMR. Thus, the null result (≤1.4% difference) can be interpreted as a sign of true “no MEMR” or a false negative MEMR. The case for a false negative MEMR in the present CEOAE data cannot be entirely ruled out, but the likelihood is very low because the stimulus pressure changes associated with the MEMR across the frequency (below and above 1,000 Hz) need to be equal and out of phase for a null difference in the composite click (and a false negative) to occur.
An additional consideration is regarding the correlational approach for relating efferent inhibition with FD thresholds (Fig. 4). It is well known that a statistical relationship between two variables may not necessarily imply a causal link. However, the physiological mechanisms predict for a perceptual benefit of efferent inhibition for auditory discrimination in noise (Kawase et al. 1993). Specifically, the efferent feedback can increase the SNR when a signal is masked by noise, thereby enhancing the encoding of signals in noise (Tomchik and Lu 2006). Accordingly, the observed correlations are most likely the consequences of an underlying relationship between the medial efferent activity and the behavioral ability to discriminate tones in background noise. Age correlated with FD thresholds in quiet and in noise but did not correlate with efferent inhibition. Another crucial aspect of the results is that efferent inhibition was associated with FD thresholds in noise but not in quiet. This suggests that efferent inhibition is related to the detrimental effects of the noise rather than with the baseline discrimination performance in the quiet. These condition-specific findings and similar outcomes from the regression modeling, as well as the median-split analyses (Fig. 5), provide further evidence that the correlation results are not just coincidental findings.
Mechanisms for the influence of age on the efferent antimasking function.
The efferent feedback is thought to be unimportant for hearing in quiet environments. Consistent with this notion, no relationship between efferent inhibition and FD thresholds in quiet was found for any age group. Scharf et al. (1997) in vestibular neurectomy patients and Delano et al. (2007) in chinchillas also did not find evidence for a role of efferents in an auditory frequency discrimination task in quiet. In quiet, the temporal cues—the pattern of phase locking of the auditory nerve discharges—are thought to provide the basis for frequency discrimination ability at 1,000 Hz (Moore and Ernst 2012; Siebert 1970). In addition to the temporal cues, rate-place codes (i.e., cues representing the spectral features in terms of the distribution of average discharge rates across fibers with different characteristic frequencies) preserved by the low-spontaneous rate fibers may be effective in the presence of masking noise (Costalupes 1985; Schmiedt et al. 1996; Young and Barta 1986). This is because the low-spontaneous rate fibers have higher thresholds and wider dynamic ranges than the high-spontaneous rate fibers that tend to saturate in noise (e.g., Huet et al. 2016). Since efferent-induced changes in cochlear amplification affect all spontaneous rate classifications (Kawase et al. 1993), the coding capabilities of the low-spontaneous rate fibers may be strengthened in noise. The efferent system represents a mechanism for preserving the rate-place codes and overcoming saturation effects in the presence of background noise (Sinnott and Brown 1993).
It is important to note that there is a considerable and ongoing debate on the upper frequency limit for the use of temporal cues, with no fixed demarcation for the transition from a temporal to a rate-place code and that not all listeners use the same cues (Micheyl et al. 2012; Verschooten et al. 2019). Furthermore, although frequency discrimination at low frequencies depends on the temporal code, the contribution of rate-place codes associated with cochlear gain and spatial irregularity cannot be eliminated (Otsuka et al. 2014). Such complex issues impacted the selection of frequency for the FD task. For example, Moore and Ernst (2012) suggested that frequency discrimination may depend on temporal cues up to 10 kHz. Efferent inhibition is neither robust nor very reliable at high frequencies (>4 kHz) for both adults and children (Mishra and Abdala 2015; Mishra and Dinger 2016). In light of these controversies, the following discussion offers important interpretations that may not be straightforward and is applicable for frequencies that share similar perceptual mechanisms as 1,000 Hz.
The influence of age on the relationship between efferent inhibition and FD thresholds in noise suggests that the efferent antimasking function worked differently for the perception of pure-tone frequency in noise for listeners with mature (≥9 yr) and immature performance. Two interpretations are derived. First, the relative usage of the temporal and rate-place codes for frequency discrimination in noise varied between adults and children. Adults and older children with mature performance likely used temporal cues, whereas younger children (≤9 yr) additionally relied on rate-place cues (affected by efferent action), presumably because of their inability to effectively use temporal cues in the presence of noise. Evidence supports that the ability to use temporal codes is slow to develop in young children (Thompson et al. 1999). Additionally, converging data suggest that adults may rely on temporal fine structure cues to a greater extent than infants for auditory discrimination in noise, albeit for consonants (Cabrera and Werner 2017). This interpretation may be tempered in the future as the codes for pitch perception in the presence of noise for children are clearly identified. Second, the efferent system provided similar cochlear amplifier gain reduction for all age groups, as shown by efferent inhibition (Fig. 1); however, only the younger children used this antimasking mechanism for discrimination of pure tones in noise. For adults and older children, this efferent inhibitory activity may not have been a determinant factor to discriminate, as listeners may have relied on other (central) mechanisms. Since central neural mechanisms are under development in young children (see, e.g., Ponton et al., 2000, 2002), it is plausible that these listeners are more dependent on the antimasking mechanism provided by the efferent feedback for auditory perception in noise.
Medial olivocochlear efferents and development of hearing-in-noise.
The antimasking mechanism (Guinan 2006) is thought to provide the link between the efferent system and auditory perception in noise for normal-hearing adults (Andéol et al. 2011; de Boer and Thornton 2008; Mertes et al. 2018; Mishra and Lutman 2014) and adults with vestibular neurectomy—compromised efferents (Giraud et al. 1997; Zeng et al. 2000). However, some studies either failed to show the influence of efferents on auditory perception or found that efferent inhibition is negatively related to the performance in certain auditory tasks (de Boer et al. 2012; Garinis et al. 2011; Scharf et al. 1997; Wagner et al. 2008). The lack of a relationship for adults in the present study is not consistent with the findings in cats with sectioned efferents, showing deficits in vowel formant discrimination in high levels of background noise (Hienz et al. 1998), potentially because of species- and/or stimulus-related differences. A recent study showed that the medial efferent system is less robust in humans compared with cats (Liberman and Liberman 2019). In all likelihood, the relationship between efferent inhibition and hearing-in-noise appears to depend on the type of the psychoacoustic task for human adults. For example, efferent inhibition is related to masked thresholds in broadband noise and random-frequency maskers but not in a fixed-frequency tonal masker condition (Garinis et al. 2011).
More importantly, the present findings indicate that differences in efferent inhibition can explain, in part, the observed variability in frequency discrimination performance in noise for young children. This suggests that efferents are important for the ability to discriminate in noise, particularly when the behavioral performance is immature and developing. In other words, the present work provides evidence that efferent feedback enhances frequency discrimination in noise during development; however, once the auditory perception in noise is mature, listeners do not rely on the efferent feedback. This parallels with the observation that cochlear deefferentation does not change the sensitivity or tuning characteristics of auditory nerve fibers in adult cats (Liberman 1990) but alters the development of auditory nerve fiber properties in neonatal cats (Walsh et al. 1998). In humans, a previous study showed that the efferent inhibition interacts with sound localization-in-noise differently for children relative to adults, for reasons unclear to the authors (Boothalingam et al. 2016). The question then arises why the efferent system plays different roles during and after the auditory perceptual development. Work by Lauer and May (2011) sheds some light on this. The ability of the efferents to enhance auditory signals in background noise appears advantageous from the perspective of activity-dependent development of auditory perception. The efferent feedback may shape the sound-evoked activity (in the brain stem and/or central auditory processing) during development by preserving the response to the information-bearing elements of relevant sounds and reducing the noise-driven activity. Once the auditory system is mature—the auditory processing is already adult shaped—the efferent feedback is no longer used by the higher auditory centers for auditory perception. Part of this proposition is broadly similar to the finding that the efferent feedback protects the central auditory system during critical stages of activity-dependent development (Lauer and May 2011). The present findings indirectly demonstrate the importance of the efferent system in the development of hearing-in-noise in humans and provide a new kind of evidence in support of the antimasking function of the efferent system in humans.
Implications.
The present study provides the first evidence for the role of medial efferents in human auditory development, specifically the development of hearing-in-noise. The findings have important implications for investigating and understanding the efferent function in humans. First, considering how the relationship between efferent inhibition and frequency discrimination in noise varied across age groups (Figs. 3 and 4), oversimplified relationships between efferent inhibition and behavioral performance in adults, as is typically sought in many studies, may not exist for several psychoacoustic and speech-in-noise tasks. Second, even though efferent-induced gain reduction can benefit hearing-in-noise, listeners may not necessarily utilize this antimasking mechanism, as was observed for adults. This is similar to a previous suggestion for speech-in-noise perception (Mishra and Lutman 2014). Third, whatever role the antimasking mechanism plays in hearing-in-noise likely changes with development. In other words, the antimasking function of medial efferents in human hearing is not static across the life span. Fourth, if efferent inhibition is weak or low, young children may show poor performance in noise, whereas adults may be unaffected. Efferent inhibition may predict speech-in-noise recognition for children, as several aspects of speech perception follow a prolonged developmental trajectory (Eisenberg et al. 2000; Goldsworthy and Markle 2019). Finally, restoration of efferent action through signal processing (Lopez-Poveda et al. 2016, 2017, 2020) may provide a greater benefit to children compared with adults, as children are relatively more dependent on this mechanism for hearing-in-noise.
GRANTS
This work was supported by a grant from the National Institutes of Health National Institute on Deafness and other Communication Disorders (R03 DC-014573).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
S.K.M. conceived and designed research; performed experiments; analyzed data; interpreted results of experiments; prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
Several students from the New Mexico State University Children’s Auditory Research Lab contributed to data collection, and Dr. Milan Biswal provided scripts for OAE analysis. Dr. Hansapani Rodrigo, The University of Texas Rio Grande Valley, is thanked for statistical consultation.
Footnotes
The safe log scale uses a modified function to handle 0. The safe log formula is sign(x) × log[1 + abs(x)]. This was used only to represent the 0 in the y-axis. All other log transformations were performed with the standard base 10 procedure.
REFERENCES
- Abdala C, Mishra S, Garinis A. Maturation of the human medial efferent reflex revisited. J Acoust Soc Am 133: 938–950, 2013. doi: 10.1121/1.4773265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar M, Protopapas A, Reid M, Merzenich MM. Auditory processing parallels reading abilities in adults. Proc Natl Acad Sci USA 97: 6832–6837, 2000. doi: 10.1073/pnas.97.12.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage, 1991. [Google Scholar]
- Andéol G, Guillaume A, Micheyl C, Savel S, Pellieux L, Moulin A. Auditory efferents facilitate sound localization in noise in humans. J Neurosci 31: 6759–6763, 2011. doi: 10.1523/JNEUROSCI.0248-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus BC, Guinan JJ Jr. Time-course of the human medial olivocochlear reflex. J Acoust Soc Am 119: 2889–2904, 2006. doi: 10.1121/1.2169918. [DOI] [PubMed] [Google Scholar]
- Backus BC, Guinan JJ Jr. Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions. J Assoc Res Otolaryngol 8: 484–496, 2007. doi: 10.1007/s10162-007-0100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JG, Ferguson MA, Moore DR. Making sense of listening: the IMAP test battery. J Vis Exp 44: e2139, 2010. doi: 10.3791/2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 29: 1165–1188, 2001. [Google Scholar]
- Bishop DV, Hardiman MJ, Barry JG. Is auditory discrimination mature by middle childhood? A study using time-frequency analysis of mismatch responses from 7 years to adulthood. Dev Sci 14: 402–416, 2011. doi: 10.1111/j.1467-7687.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothalingam S, Macpherson E, Allan C, Allen P, Purcell D. Localization-in-noise and binaural medial olivocochlear functioning in children and young adults. J Acoust Soc Am 139: 247–262, 2016. doi: 10.1121/1.4939708. [DOI] [PubMed] [Google Scholar]
- Boothalingam S, Purcell DW. Influence of the stimulus presentation rate on medial olivocochlear system assays. J Acoust Soc Am 137: 724–732, 2015. [Erratum in J Acoust Soc Am 137: 2987, 2015.] doi: 10.1121/1.4906250. [DOI] [PubMed] [Google Scholar]
- Brown MC. Single-unit labeling of medial olivocochlear neurons: the cochlear frequency map for efferent axons. J Neurophysiol 111: 2177–2186, 2014. doi: 10.1152/jn.00045.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Flaherty MM, Leibold LJ. Development of frequency discrimination at 250 Hz is similar for tone and /ba/ stimuli. J Acoust Soc Am 142: EL150–EL154, 2017. doi: 10.1121/1.4994687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Taylor CN, Leibold LJ. Factors affecting sensitivity to frequency change in school-age children and adults. J Speech Lang Hear Res 57: 1972–1982, 2014. doi: 10.1044/2014_JSLHR-H-13-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera L, Werner L. Infants’ and adults’ use of temporal cues in consonant discrimination. Ear Hear 38: 497–506, 2017. doi: 10.1097/AUD.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo BL. Some notes on frequency discrimination and masking. Acustica 31: 330–336, 1974. [Google Scholar]
- Chilekwa V, Folkard T, Hind S, Ferguson M, Moore D. STAR: a software platform for testing hearing in children (Abstract). Int J Audiol 48: 503, 2009. [Google Scholar]
- Costalupes JA. Representation of tones in noise in the responses of auditory nerve fibers in cats. I. Comparison with detection thresholds. J Neurosci 5: 3261–3269, 1985. doi: 10.1523/JNEUROSCI.05-12-03261.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Thornton AR. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J Neurosci 28: 4929–4937, 2008. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Thornton ARD, Krumbholz K. What is the role of the medial olivocochlear system in speech-in-noise processing? J Neurophysiol 107: 1301–1312, 2012. doi: 10.1152/jn.00222.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoster J, Gallucci M, Iselin AM. Best practices for using median splits, artificial categorization, and their continuous alternatives. J Exp Psychopathol 2: 197–209, 2011. doi: 10.5127/jep.008310. [DOI] [Google Scholar]
- Delano PH, Elgueda D, Hamame CM, Robles L. Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J Neurosci 27: 4146–4153, 2007. doi: 10.1523/JNEUROSCI.3702-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson JH., 3rd Efferent olivocochlear bundle: some relationships to stimulus discrimination in noise. J Neurophysiol 31: 122–130, 1968. doi: 10.1152/jn.1968.31.1.122. [DOI] [PubMed] [Google Scholar]
- Dye RH Jr, Hafter ER. Just-noticeable differences of frequency for masked tones. J Acoust Soc Am 67: 1746–1753, 1980. doi: 10.1121/1.384301. [DOI] [PubMed] [Google Scholar]
- Eisenberg LS, Shannon RV, Schaefer Martinez A, Wygonski J, Boothroyd A. Speech recognition with reduced spectral cues as a function of age. J Acoust Soc Am 107: 2704–2710, 2000. doi: 10.1121/1.428656. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH, Marryott LP. Contralateral acoustic reflex thresholds for tonal activators using wideband energy reflectance and admittance. J Speech Lang Hear Res 46: 128–136, 2003. doi: 10.1044/1092-4388(2003/010). [DOI] [PubMed] [Google Scholar]
- Francis NA, Guinan JJ Jr. Acoustic stimulation of human medial olivocochlear efferents reduces stimulus-frequency and click-evoked otoacoustic emission delays: implications for cochlear filter bandwidths. Hear Res 267: 36–45, 2010. doi: 10.1016/j.heares.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinis A, Werner L, Abdala C. The relationship between MOC reflex and masked threshold. Hear Res 282: 128–137, 2011. doi: 10.1016/j.heares.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Garnier S, Micheyl C, Lina G, Chays A, Chéry-Croze S. Auditory efferents involved in speech-in-noise intelligibility. Neuroreport 8: 1779–1783, 1997. doi: 10.1097/00001756-199705060-00042. [DOI] [PubMed] [Google Scholar]
- Goldsworthy RL, Markle KL. Pediatric hearing loss and speech recognition in quiet and in different types of background noise. J Speech Lang Hear Res 62: 758–767, 2019. doi: 10.1044/2018_JSLHR-H-17-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SS, Mertes IB, Lewis JD, Weissbeck DK. Medial olivocochlear-induced transient-evoked otoacoustic emission amplitude shifts in individual subjects. J Assoc Res Otolaryngol 14: 829–842, 2013. doi: 10.1007/s10162-013-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27: 589–607, 2006. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: their action, effects, measurement and uses, and the impact of the new conception of cochlear mechanical responses. Hear Res 362: 38–47, 2018. doi: 10.1016/j.heares.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ Jr, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol 4: 521–540, 2003. doi: 10.1007/s10162-002-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday LF, Taylor JL, Edmondson-Jones AM, Moore DR. Frequency discrimination learning in children. J Acoust Soc Am 123: 4393–4402, 2008. doi: 10.1121/1.2890749. [DOI] [PubMed] [Google Scholar]
- Hämäläinen JA, Salminen HK, Leppänen PH. Basic auditory processing deficits in dyslexia: systematic review of the behavioral and event-related potential/field evidence. J Learn Disabil 46: 413–427, 2013. doi: 10.1177/0022219411436213. [DOI] [PubMed] [Google Scholar]
- Henning GB. Frequency discrimination in noise. J Acoust Soc Am 41: 774–777, 1967. doi: 10.1121/1.1910405. [DOI] [PubMed] [Google Scholar]
- Hienz RD, Stiles P, May BJ. Effects of bilateral olivocochlear lesions on vowel formant discrimination in cats. Hear Res 116: 10–20, 1998. doi: 10.1016/S0378-5955(97)00197-4. [DOI] [PubMed] [Google Scholar]
- Huet A, Batrel C, Tang Y, Desmadryl G, Wang J, Puel JL, Bourien J. Sound coding in the auditory nerve of gerbils. Hear Res 338: 32–39, 2016. doi: 10.1016/j.heares.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Near equivalence of human click-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am 121: 2097–2110, 2007. doi: 10.1121/1.2435981. [DOI] [PubMed] [Google Scholar]
- Kawase T, Delgutte B, Liberman MC. Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J Neurophysiol 70: 2533–2549, 1993. doi: 10.1152/jn.1993.70.6.2533. [DOI] [PubMed] [Google Scholar]
- Kemp DT, Bray P, Alexander L, Brown AM. Acoustic emission cochleography—practical aspects. Scand Audiol Suppl 25: 71–95, 1986. [PubMed] [Google Scholar]
- Khalfa S, Morlet T, Micheyl C, Morgon A, Collet L. Evidence of peripheral hearing asymmetry in humans: clinical implications. Acta Otolaryngol 117: 192–196, 1997. doi: 10.3109/00016489709117767. [DOI] [PubMed] [Google Scholar]
- Lauer AM, May BJ. The medial olivocochlear system attenuates the developmental impact of early noise exposure. J Assoc Res Otolaryngol 12: 329–343, 2011. doi: 10.1007/s10162-011-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Dhar S, Abel R, Banakis R, Grolley E, Lee J, Zecker S, Siegel J. Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear 33: 315–329, 2012. doi: 10.1097/AUD.0b013e31823d7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Goodman SS. Basal contributions to short-latency transient-evoked otoacoustic emission components. J Assoc Res Otolaryngol 16: 29–45, 2015. doi: 10.1007/s10162-014-0493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Liberman MC. Cochlear efferent innervation is sparse in humans and decreases with age. J Neurosci 39: 9560–9569, 2019. doi: 10.1523/JNEUROSCI.3004-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Effects of chronic cochlear de-efferentation on auditory-nerve response. Hear Res 49: 209–223, 1990. doi: 10.1016/0378-5955(90)90105-X. [DOI] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ Jr. Human medial olivocochlear reflex: effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J Assoc Res Otolaryngol 10: 459–470, 2009. doi: 10.1007/s10162-009-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GR, Talmadge CL, Lee J. Measuring distortion product otoacoustic emissions using continuously sweeping primaries. J Acoust Soc Am 124: 1613–1626, 2008. doi: 10.1121/1.2949505. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA. Olivocochlear efferents in animals and humans: From anatomy to clinical relevance. Front Neurol 9: 197, 2018. doi: 10.3389/fneur.2018.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Eustaquio-Martín A, Fumero MJ, Gorospe JM, Polo López R, Gutiérrez Revilla MA, Schatzer R, Nopp P, Stohl JS. Speech-in-noise recognition with more realistic implementations of a binaural cochlear-implant sound coding strategy inspired by the medial olivocochlear reflex. Ear Hear, 2020. doi: 10.1097/AUD.0000000000000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Eustaquio-Martín A, Stohl JS, Wolford RD, Schatzer R, Gorospe JM, Ruiz SS, Benito F, Wilson BS. Intelligibility in speech maskers with a binaural cochlear implant sound coding strategy inspired by the contralateral medial olivocochlear reflex. Hear Res 348: 134–137, 2017. doi: 10.1016/j.heares.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Eustaquio-Martín A, Stohl JS, Wolford RD, Schatzer R, Wilson BS. A binaural cochlear implant sound coding strategy inspired by the contralateral medial olivocochlear reflex. Ear Hear 37: e138–e148, 2016. doi: 10.1097/AUD.0000000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Lapsley Miller JA, Guinan JJ, Shera CA, Reed CM, Perez ZD, Delhorne LA, Boege P. Otoacoustic-emission-based medial-olivocochlear reflex assays for humans. J Acoust Soc Am 136: 2697–2713, 2014. doi: 10.1121/1.4896745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BJ, McQuone SJ. Effects of bilateral olivocochlear lesions on pure-tone intensity discrimination in cats. Audit Neurosci 1: 385–400, 1995. [PMC free article] [PubMed] [Google Scholar]
- McArthur GM, Bishop DV. Frequency discrimination deficits in people with specific language impairment: reliability, validity, and linguistic correlates. J Speech Lang Hear Res 47: 527–541, 2004. doi: 10.1044/1092-4388(2004/041). [DOI] [PubMed] [Google Scholar]
- Mengler ED, Hogben JH, Michie P, Bishop DV. Poor frequency discrimination is related to oral language disorder in children: a psychoacoustic study. Dyslexia 11: 155–173, 2005. doi: 10.1002/dys.302. [DOI] [PubMed] [Google Scholar]
- Mertes IB, Wilbanks EC, Leek MR. Olivocochlear efferent activity is associated with the slope of the psychometric function of speech recognition in noise. Ear Hear 39: 583–593, 2018. doi: 10.1097/AUD.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheyl C, Delhommeau K, Perrot X, Oxenham AJ. Influence of musical and psychoacoustical training on pitch discrimination. Hear Res 219: 36–47, 2006. doi: 10.1016/j.heares.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Xiao L, Oxenham AJ. Characterizing the dependence of pure-tone frequency difference limens on frequency, duration, and level. Hear Res 292: 1–13, 2012. doi: 10.1016/j.heares.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Abdala C. Stability of the medial olivocochlear reflex as measured by distortion product otoacoustic emissions. J Speech Lang Hear Res 58: 122–134, 2015. doi: 10.1044/2014_JSLHR-H-14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Biswal M. Time-frequency decomposition of click evoked otoacoustic emissions in children. Hear Res 335: 161–178, 2016. doi: 10.1016/j.heares.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Biswal M. Neural encoding of amplitude modulations in the human efferent system. J Assoc Res Otolaryngol 20: 383–393, 2019. doi: 10.1007/s10162-019-00720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Biswal M, Amatya A. Efferent-induced alterations in distortion and reflection otoacoustic emissions in children. J Acoust Soc Am 143: 640–644, 2018. doi: 10.1121/1.5022793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Dinger Z. Influence of medial olivocochlear efferents on the sharpness of cochlear tuning estimates in children. J Acoust Soc Am 140: 1060–1071, 2016. doi: 10.1121/1.4960550. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Lutman ME. Top-down influences of the medial olivocochlear efferent system in speech perception in noise. PLoS One 9: e85756, 2014. doi: 10.1371/journal.pone.0085756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Talmadge CL. Sweep-tone evoked stimulus frequency otoacoustic emissions in humans: development of a noise-rejection algorithm and normative features. Hear Res 358: 42–49, 2018. doi: 10.1016/j.heares.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Moore BC, Ernst SM. Frequency difference limens at high frequencies: evidence for a transition from a temporal to a place code. J Acoust Soc Am 132: 1542–1547, 2012. doi: 10.1121/1.4739444. [DOI] [PubMed] [Google Scholar]
- Moore DR, Cowan JA, Riley A, Edmondson-Jones AM, Ferguson MA. Development of auditory processing in 6- to 11-yr-old children. Ear Hear 32: 269–285, 2011. doi: 10.1097/AUD.0b013e318201c468. [DOI] [PubMed] [Google Scholar]
- Moore DR, Ferguson MA, Edmondson-Jones AM, Ratib S, Riley A. Nature of auditory processing disorder in children. Pediatrics 126: e382–e390, 2010. doi: 10.1542/peds.2009-2826. [DOI] [PubMed] [Google Scholar]
- Moore DR, Ferguson MA, Halliday LF, Riley A. Frequency discrimination in children: perception, learning and attention. Hear Res 238: 147–154, 2008. doi: 10.1016/j.heares.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Nieder P, Nieder I. Stimulation of efferent olivocochlear bundle causes release from low level masking. Nature 227: 184–185, 1970. doi: 10.1038/227184a0. [DOI] [PubMed] [Google Scholar]
- Otsuka S, Furukawa S, Yamagishi S, Hirota K, Kashino M. Interindividual variation of sensitivity to frequency modulation: its relation with click-evoked and distortion product otoacoustic emissions. J Assoc Res Otolaryngol 15: 175–186, 2014. doi: 10.1007/s10162-013-0439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham AJ. How we hear: the perception and neural coding of sound. Annu Rev Psychol 69: 27–50, 2018. doi: 10.1146/annurev-psych-122216-011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton C, Eggermont JJ, Khosla D, Kwong B, Don M. Maturation of human central auditory system activity: separating auditory evoked potentials by dipole source modeling. Clin Neurophysiol 113: 407–420, 2002. doi: 10.1016/S1388-2457(01)00733-7. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol 111: 220–236, 2000. doi: 10.1016/S1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Rose BM, Holmbeck GN, Coakley RM, Franks EA. Mediator and moderator effects in developmental and behavioral pediatric research. J Dev Behav Pediatr 25: 58–67, 2004. doi: 10.1097/00004703-200402000-00013. [DOI] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Chays A. On the role of the olivocochlear bundle in hearing: 16 case studies. Hear Res 103: 101–122, 1997. doi: 10.1016/S0378-5955(96)00168-2. [DOI] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Collet L, Ulmer E, Chays A. On the role of the olivocochlear bundle in hearing: a case study. Hear Res 75: 11–26, 1994. doi: 10.1016/0378-5955(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol 76: 2799–2803, 1996. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G, Bruder J. Clinical neurophysiology of visual and auditory processing in dyslexia: a review. Clin Neurophysiol 121: 1794–1809, 2010. doi: 10.1016/j.clinph.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Shera CA. Mechanisms of mammalian otoacoustic emission and their implications for the clinical utility of otoacoustic emissions. Ear Hear 25: 86–97, 2004. doi: 10.1097/01.AUD.0000121200.90211.83. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ Jr. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am 105: 782–798, 1999. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Siebert WM. Frequency discrimination in the auditory system: place or periodicity mechanisms? Proc IEEE 58: 723–730, 1970. doi: 10.1109/PROC.1970.7727. [DOI] [Google Scholar]
- Sinnott JM, Brown CH. Effects of varying signal and noise levels on pure-tone frequency discrimination in humans and monkeys. J Acoust Soc Am 93: 1535–1540, 1993. doi: 10.1121/1.406811. [DOI] [PubMed] [Google Scholar]
- Sisto R, Sanjust F, Moleti A. Input/output functions of different-latency components of transient-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am 133: 2240–2253, 2013. doi: 10.1121/1.4794382. [DOI] [PubMed] [Google Scholar]
- Smith DW, Keil A. The biological role of the medial olivocochlear efferents in hearing: separating evolved function from exaptation. Front Syst Neurosci 9: 12, 2015. doi: 10.3389/fnsys.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart A, Kerls AN. Does contralateral inhibition of transient evoked otoacoustic emissions suggest sex or ear laterality effects? Am J Audiol 27: 272–282, 2018. doi: 10.1044/2018_AJA-17-0106. [DOI] [PubMed] [Google Scholar]
- Sutcliffe P, Bishop D. Psychophysical design influences frequency discrimination performance in young children. J Exp Child Psychol 91: 249–270, 2005. doi: 10.1016/j.jecp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Talmadge CL, Long GR, Tubis A, Dhar S. Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions. J Acoust Soc Am 105: 275–292, 1999. doi: 10.1121/1.424584. [DOI] [PubMed] [Google Scholar]
- Thompson NC, Cranford JL, Hoyer E. Brief-tone frequency discrimination by children. J Speech Lang Hear Res 42: 1061–1068, 1999. doi: 10.1044/jslhr.4205.1061. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Lu Z. Modulation of auditory signal-to-noise ratios by efferent stimulation. J Neurophysiol 95: 3562–3570, 2006. doi: 10.1152/jn.00063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschooten E, Shamma S, Oxenham AJ, Moore BC, Joris PX, Heinz MG, Plack CJ. The upper frequency limit for the use of phase locking to code temporal fine structure in humans: a compilation of viewpoints. Hear Res 377: 109–121, 2019. doi: 10.1016/j.heares.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Frey K, Heppelmann G, Plontke SK, Zenner HP. Speech-in-noise intelligibility does not correlate with efferent olivocochlear reflex in humans with normal hearing. Acta Otolaryngol 128: 53–60, 2008. doi: 10.1080/00016480701361954. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, McFadden SL, Liberman MC. Long-term effects of sectioning the olivocochlear bundle in neonatal cats. J Neurosci 18: 3859–3869, 1998. doi: 10.1523/JNEUROSCI.18-10-03859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol 57: 1002–1021, 1987. doi: 10.1152/jn.1987.57.4.1002. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nat Neurosci 5: 57–63, 2002. doi: 10.1038/nn786. [DOI] [PubMed] [Google Scholar]
- Young ED, Barta PE. Rate responses of auditory nerve fibers to tones in noise near masked threshold. J Acoust Soc Am 79: 426–442, 1986. doi: 10.1121/1.393530. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Martino KM, Linthicum FH, Soli SD. Auditory perception in vestibular neurectomy subjects. Hear Res 142: 102–112, 2000. doi: 10.1016/S0378-5955(00)00011-3. [DOI] [PubMed] [Google Scholar]