Abstract
The menstrual cycle in humans and its analogous cycle in rodents, the estrous cycle, modulate brain function and behavior. Both cycles are characterized by the cyclical fluctuation of ovarian hormones including estrogens such as estradiol. Estradiol induces cycle- and sex-dependent differences in the phenotype and incidence of many behaviors, including those related to reward and motivation. The nucleus accumbens core (AcbC), a limbic and premotor system nexus region, directly regulates these behaviors. We previously showed that the estrous cycle modulates intrinsic excitability and excitatory synapse properties of medium spiny neurons (MSNs) in the AcbC. The identity of the underlying hormone mechanism is unknown, with estradiol being a prime candidate. The present study tests the hypothesis that estradiol induces estrous cycle-relevant differences in MSN electrophysiology. To accomplish this goal, a time- and dose-dependent estradiol replacement paradigm designed to simulate the rise of circulating estradiol levels across the estrous cycle was employed in ovariectomized adult female rats as well as a vehicle control group. Estradiol replacement decreased MSN excitability by modulating properties such as resting membrane potential, input resistance in both the linear and rectified ranges, and rheobase compared with vehicle-treated females. These differences in MSN excitability mimic those previously described regarding estrous cycle effects on MSN electrophysiology. Excitatory synapse properties were not modulated in response to this estradiol replacement paradigm. These data are the first to demonstrate that an estrous cycle-relevant estradiol exposure modulates MSN electrophysiology, providing evidence of the fundamental neuroendocrine mechanisms regulating the AcbC.
NEW & NOTEWORTHY The present study shows, for the first time, that an estrous cycle-relevant estradiol exposure modulates nucleus accumbens neuron excitability. This evidence provides insight into the neuroendocrine mechanisms by which estradiol cyclically alters neuron properties during the estrous cycle. Overall, these data emphasize the significant influence of hormone action in the brain and especially individual neuron physiology.
Keywords: estradiol, estrous cycle, excitability, nucleus accumbens, sex differences
INTRODUCTION
Female humans and rodents of reproductive age exhibit cyclical fluctuations of ovarian sex steroid hormones including progesterone and estrogens. In humans, cyclical changes in the production of ovarian hormones such as progesterone and estradiol occur over the ~28-day menstrual cycle, in which circulating levels of estradiol peak during the follicular phase (Sherman and Korenman 1975). In rodents, including rats, fluctuations in ovarian hormones occur over the ~4- to 5-day estrous cycle. In rats, circulating levels of estradiol gradually rise during the diestrus phase, reach peak levels during the proestrus phase, and then quickly decrease, with select persisting effects (Erskine 1989; Hubscher et al. 2005; Westwood 2008). Progesterone levels similarly peak during the proestrus phase. This combination of estrogen and progesterone action is necessary for the expression of full mating behaviors. In this study, we focus on the role of estrogens such as estradiol. Accumulating evidence suggests that, in both humans and rodents, fluctuations in ovarian estrogen production influence behaviors related to motivation, reward, and reinforcement and related disorders such as anxiety, depression, and addiction (Becker et al. 2001; Becker and Hu 2008; Evans and Foltin 2006; Lebron-Milad and Milad 2012; Milad et al. 2009; Tonn Eisinger et al. 2018a; Yoest et al. 2018). The underlying neural substrates mediating these behaviors are likewise susceptible to ovarian estrogen fluctuations. Indeed, neural physiology in the striatal regions implicated in these behaviors and disorders, including the caudate-putamen and nucleus accumbens core (AcbC), differs across the estrous cycle (Alonso-Caraballo and Ferrario 2019; Proaño et al. 2018; Willett et al. 2019b). Previously, we showed that the estrous cycle robustly modulates the intrinsic excitability and excitatory synapse properties of female rat medium spiny neurons (MSNs) in the AcbC (Proaño et al. 2018), a critical nexus region between the limbic and premotor systems that mediates the cognitive processing of reward and reinforcement, among other functions (Floresco 2015; Francis and Lobo 2017). These changes in MSN electrophysiological properties varied with both circulating estradiol and progesterone levels (Proaño et al. 2020). These physiological findings support the differential expression of AcbC-mediated behaviors and disorders across the estrous cycle in rodents and the menstrual cycle in humans.
Accumulating evidence has firmly established estradiol as a potent modulator of AcbC function (Meitzen et al. 2018; Yoest et al. 2018). The output neurons of the AcbC, medium spiny neurons (MSNs), in adulthood express membrane-associated estrogen receptors α, β, and G protein-coupled estrogen receptor 1 (GPER-1) (Almey et al. 2015; Lorsch et al. 2018). MSNs integrate a host of inputs, including dopamine from the ventral tegmental area and glutamatergic and GABAergic projections from cortex, amygdala, thalamus, and hippocampus (Deroche et al. 2020; Kalivas and Nakamura 1999; Salgado and Kaplitt 2015). These neurotransmitters are sensitive to estradiol in a sex-, temporal- and developmental stage-dependent manner (Becker 1999; Becker et al. 2012; Calipari et al. 2017; Cao et al. 2016, 2018a; Forlano and Woolley 2010; Krentzel et al. 2019, 2020; Meisel and Mullins 2006; Mermelstein et al. 1996; Perry et al. 2013; Tonn Eisinger et al. 2018a). Estradiol also modifies MSN transcription factor phosphorylation and dendritic spine structure by activating metabotropic glutamate receptor 5 (mGluR5) signaling (Grove-Strawser et al. 2010; Mermelstein et al. 1996; Peterson et al. 2015; Staffend et al. 2011). However, estradiol’s actions on the electrophysiological properties of MSNs, especially within the context of a natural variable such as the estrous cycle, are not well defined. This is a critical knowledge gap given the aforementioned differential expression of MSN electrophysiological properties across the estrous cycle (Proaño et al. 2018), which along with changes in neurotransmitter systems could potentially underlie the reported sex and estrous cycle differences in AcbC-mediated behaviors and disorders. In the present study, we address this gap by testing the hypothesis that estradiol injections designed to simulate circulating estradiol levels during the estrous cycle modulate AcbC MSN electrophysiological properties. To accomplish this, we administered sequential doses of estradiol or vehicle to ovariectomized (OVX) female rats and then performed whole cell patch-clamp recordings of AcbC MSNs to assess both intrinsic and excitatory synapse properties.
METHODS
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee at North Carolina State University. Female Sprague-Dawley CD IGS rats were born from 10 timed-pregnant dams purchased from Charles River Laboratories. Rats were housed with their littermates and dam until weaning at postnatal day (P)21. After weaning, females were group housed until they were bilaterally gonadectomized at P60 ± 2 as previously described (Krentzel et al. 2020). Littermates were randomly assigned to each group. Upon gonadectomy, animals were individually housed to facilitate vehicle or estradiol injection administration, which began at P74 ± 2: vehicle control (n = 11 females; n = 25 neurons; 2.3 neurons were recorded on average from each female) and estradiol (n = 15 females; n = 26 neurons; 1.7 neurons were recorded on average from each female). The injection paradigm is documented in Fig. 1. Age at recording ranged from P73 to P85 and was matched between the groups (mean ± SE; vehicle control P78 ± 1 and estradiol injected P79 ± 1). All animals were housed in a temperature- and light-controlled room (23°C, 40% humidity, 12:12-h light-dark cycle) at the Biological Resource Facility of North Carolina State University. All cages were polysulfone bisphenol A (BPA) free and were filled with bedding manufactured from virgin hardwood chips (Beta Chip; NEPCO, Warrensburg, NY) to avoid the presence of endocrine disruptors in corncob bedding (Mani et al. 2005; Markaverich et al. 2002; Villalon Landeros et al. 2012). Soy protein-free rodent chow (2020X; Teklad, Madison, WI) and glass bottle-provided water were available ad libitum.
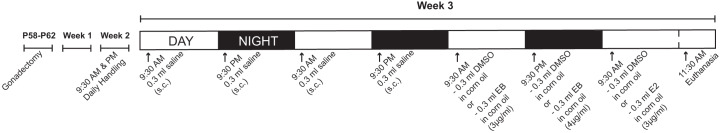
Fig. 1.
Estradiol replacement protocol. Two weeks after gonadectomy, female rats received a series of subcutaneous injections of estradiol benzoate (EB) and 17β-estradiol (E2) to simulate temporal changes in physiologically relevant circulating levels of estradiol during the estrous cycle. Animals were euthanized during the replacement protocol equivalent of the proestrus phase. Protocol was originally developed and validated by Scharfman and colleagues (Scharfman et al. 2007). P, postnatal day.
Estradiol injection protocol.
One week after gonadectomy, female rats were handled (picked up, cradled, and stroked) twice daily until day of euthanasia. Two weeks after gonadectomy, animals experienced a 2-day injection acclimatization period via injections of 0.3 mL of normal saline (Sigma) twice daily at 9:30 AM and 9:30 PM. Estradiol or vehicle injections began after the 2-day injection acclimatization period. Estradiol injection protocols were from a previously published study (Scharfman et al. 2007) and mimic the changing temporal circulating levels of estradiol during the diestrus and proestrus phases of the rat estrous cycle. Stock solutions of estradiol benzoate (EB) and 17β-estradiol (E2) (collectively referred to as estradiol in this report) were made by diluting a concentrated stock solution (1 mg of EB or E2 in 1 mL of DMSO) in corn oil (Sigma). Stocks were stored in the dark at room temperature. Injection volume (0.3 mL) was kept constant by using stock solutions that contained differing concentrations of EB and E2 (3 µg/mL for a 3 µg/kg dose and 4 µg/mL for a 4 µg/kg dose). Vehicle solutions were prepared in the same manner with DMSO and corn oil. Injections were administered subcutaneously in the back with a 1-mL syringe and a 26-gauge needle. Three micrograms per milliliter of EB or vehicle was injected at 9:30 AM on day 1, 4 µg/mL of EB or vehicle was injected at 9:30 PM on day 1, and 3 µg/mL of E2 or vehicle was injected at 9:30 AM on day 2. Two hours (11:30 AM) after the last injection, animals were processed for acute brain slice preparation (Fig. 1).
Acute brain slice preparation.
Brain slices containing the nucleus accumbens core were prepared as previously described (Proaño et al. 2018). Animals were deeply anesthetized with isoflurane gas before decapitation. The brain was rapidly extracted into ice-cold oxygenated sucrose artificial cerebrospinal fluid containing (in mM) 75 sucrose, 1.25 NaH2PO4, 3 MgCl2, 0.5 CaCl2, 2.4 Na pyruvate, and 1.3 ascorbic acid from Sigma-Aldrich (St. Louis, MO) and 75 NaCl, 25 NaHCO3, 15 dextrose, and 2 KCl from Fisher (Pittsburgh, PA). The osmolarity of the sucrose ACSF was 295–305 mosM, and the pH was between 7.2 and 7.4. Coronal brain slices (300 μm) were prepared with a vibratome and then incubated in regular ACSF containing (in mM) 126 NaCl, 26 NaHCO3, 10 dextrose, 3 KCl, 1.25 NaH2PO4, 1 MgCl2, and 2 CaCl2 (295–305 mosM, pH 7.2–7.4) for 30 min at 30–35°C and then for at least 30 min at room temperature (22–23°C). Slices were stored submerged in room temperature oxygenated ACSF for up to 6 h after sectioning in a large-volume bath holder.
Electrophysiological recordings.
Slices rested for at least 1 h after sectioning. They were then placed in a Zeiss Axioscope equipped with infrared-differential interference contrast (IR-DIC) optics, a Dage IR-1000 video camera, and ×10 and ×40 lenses with optical zoom and superfused with oxygenated ACSF heated to ~29.0°C. Whole cell patch-clamp recordings were used to record the electrical properties of MSNs in the nucleus accumbens core (Fig. 2). MSN density and nucleus accumbens core volume do not differ by sex (Meitzen et al. 2011; Wong et al. 2016), and neurons were recorded from both hemispheres. Glass electrodes contained (in mM) 115 K d-gluconate, 8 NaCl, 2 EGTA, 2 MgCl2, 2 MgATP, 0.3 NaGTP, and 10 phosphocreatine from Sigma-Aldrich and 10 HEPES from Fisher (285 mosM, pH 7.2–7.4). Signals were amplified, filtered (2 kHz), and digitized (10 kHz) with a MultiClamp 700B amplifier attached to a Digidata 1550 system and a personal computer using pCLAMP 10.7 software. Membrane potentials were corrected for a calculated liquid junction potential of 13.5 mV. As previously described (Dorris et al. 2015), recordings were first made in current clamp to assess neuronal electrophysiological properties. MSNs were identified by medium-sized somas, the presence of a slow-ramping subthreshold depolarization in response to low-magnitude positive current injections, a hyperpolarized resting potential more negative than −65 mV, inward rectification, and prominent spike afterhyperpolarization (Belleau and Warren 2000, O’Donnell and Grace 1993). Only one MSN was recorded from a single brain slice. In a subset of recordings, oxygenated ACSF containing both the GABAA receptor antagonist picrotoxin (PTX, 150 μM; Fisher) and the voltage-gated sodium channel blocker tetrodotoxin (TTX, 1 μM; Abcam Biochemicals) was applied to the bath to abolish inhibitory postsynaptic current events and action potentials, respectively. Once depolarizing current injection no longer generated an action potential, MSNs were voltage clamped at −70 mV, and miniature excitatory postsynaptic currents (mEPSCs) were recorded for at least 5 min. These settings enable recordings from almost exclusively AMPA glutamate receptors (Nowak et al. 1984) and were confirmed by our laboratory in a previous study (Proaño et al. 2018). Input/series resistance was monitored per minute, and cells were excluded if resistance changed >25%.
Fig. 2.
Location of nucleus accumbens (Acb) core in whole cell patch-clamped medium spiny neurons from estradiol- and vehicle-treated female rats. AC, anterior commissure; LV, lateral ventricle.
Data recording and analysis.
Intrinsic electrophysiological properties and action potential characteristics were analyzed with pCLAMP 10.7. After break-in, the resting membrane potential was first allowed to stabilize ~1–2 min, as previously described (Mu et al. 2010). After stabilization, resting membrane potential was assessed in the absence of injected current. At least two series of depolarizing and hyperpolarizing current injections were applied to elicit basic neurophysiological properties. All properties measured followed definitions previously adopted by our laboratory (Cao et al. 2016; Dorris et al. 2015; Proaño et al. 2018; Willett et al. 2016, 2019b), which were based on those of Perkel and colleagues (Farries et al. 2005; Farries and Perkel 2000, 2002; Meitzen et al. 2009). For each neuron, measurements were made of at least two action potentials generated from minimal current injections. These measurements were then averaged to generate the reported action potential measurement for that neuron. For action potential measurements, only the first generated action potential was used unless more action potentials were required to meet the standard three action potentials per neuron. Action potential threshold was defined as the first point of sustained positive acceleration of voltage (δ2V/δt2) that was also 3 times the SD of membrane noise before the detected threshold (Baufreton et al. 2005). The delay to first action potential is the average in milliseconds of the time from the initial deflection generated by the current step function to the action potential threshold of the first spike. Action potential width at half peak is the width of the action potential halfway between action potential peak and threshold in milliseconds. The action potential amplitude is the change in millivolts between action potential threshold and peak. Afterhyperpolarization peak amplitude is the difference in millivolts between action potential threshold and the most hyperpolarized voltage point after action potential peak. Afterhyperpolarization time to peak amplitude is the time measured in milliseconds between the action potential threshold voltage point on the descending phase of the action potential and the afterhyperpolarization peak amplitude. Rheobase, measured in nanoamps, is the lowest amplitude of injected positive current needed to produce an initial action potential. The slope of the linear range of the evoked action potential firing rate-to-positive injected current curve (FI slope) was calculated from the first current stimulus that evoked an action potential to the first current stimulus that generated an evoked firing rate that persisted for at least two consecutive current stimuli. Input resistance in the linear, nonrectified, range was calculated from the steady-state membrane potential in response to 0.02-nA hyperpolarizing pulses. Rectified-range input resistance, inward rectification, and percent inward rectification were calculated as described previously, with rectified-range input resistance measured using the most hyperpolarizing current injected into the MSN (Belleau and Warren 2000). Inward rectification is the input resistance of the 0.02-nA step minus the rectified-range input resistance. Percent inward rectification is defined as rectified-range input resistance/input resistance × 100. The time constant of the membrane was calculated by fitting a single exponential curve to the membrane potential change in response to 0.02-nA hyperpolarizing pulses. Possible differences in hyperpolarization-induced “sag” were assessed with the “sag index” (Farries et al. 2005). Briefly, the sag index is defined as the difference between the minimum voltage measured during the largest hyperpolarizing current pulse and the steady-state voltage deflection of that pulse, divided by the steady-state voltage deflection. A cell with no sag would exhibit a sag index of 0, whereas a cell whose maximum voltage deflection is twice that of the steady-state deflection would exhibit a sag index of 1. Cells with considerable sag typically have an index of 0.1. Frequency, amplitude, and decay of mEPSCs were analyzed off-line with Mini Analysis (Synaptosoft, http://www.synaptosoft.com/MiniAnalysis). mEPSC threshold was set at a minimum value of 5 pA, and accurate event detection was validated by visual inspection. mEPSC frequency was defined as the number of detected mEPSC events per second (Hz). mEPSC amplitude was calculated as the difference between the averaged baseline 10 ms before initial mEPSC rise and peak amplitude. mEPSC decay was calculated as the time required for peak mEPSC amplitude to return to baseline.
Statistics.
Data were analyzed as appropriate with two-tailed t tests and Mann–Whitney U tests, as determined by a D’Agostino and Pearson normality test, or with linear regressions and analyses of covariance (ANCOVAs) (GraphPad Prism 8). P values < 0.05 were considered a priori as significant and P values < 0.10 as a trend. Data are presented as means ± SE.
RESULTS
Estradiol increases action potential rheobase and hyperpolarizes resting membrane potential.
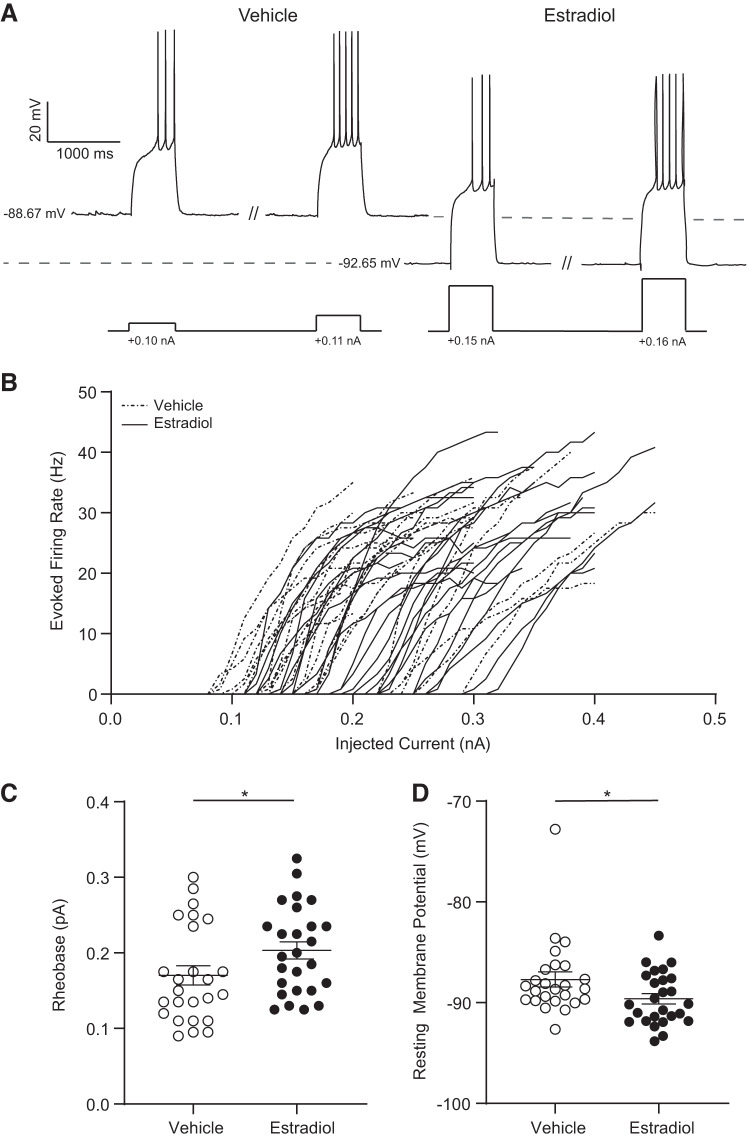
To test the hypothesis that action potential generation properties are influenced by estradiol, we injected a series of positive-current injections into MSNs from estradiol- and vehicle-treated animals and analyzed the initiation and number of evoked action potentials (Fig. 3A). Complete statistical information as well as documentation of all assessed electrophysiological properties is in Table 1. Regarding action potential initiation, the minimum amount of depolarizing current necessary to initiate an action potential significantly differed between estradiol- and vehicle-treated groups (Fig. 3B). Rheobase was increased in MSNs recorded from estradiol-treated animals compared with vehicle-treated animals (Fig. 3C). Changes in rheobase are usually complementary to changes in action potential threshold, resting membrane potential, and/or input resistance. There were no changes in action potential threshold (Table 1). Resting membrane potential was hyperpolarized in MSNs recorded from estradiol-treated animals (Fig. 3D). This hyperpolarization is consistent with an increased rheobase value. This finding suggests that more excitatory current is required for action potential initiation in MSNs from estradiol-treated females. Overall, these results indicate that estradiol decreases excitability in adult female AcbC MSNs.
Fig. 3.
Action potential properties in medium spiny neurons (MSNs) recorded from estradiol- and vehicle-treated female rats. A: voltage response of MSNs recorded from estradiol- and vehicle-treated animals to a series of depolarizing current injections; // denotes x-axis breaks. B: action potential firing rates evoked by depolarizing current injections. C: rheobase. D: resting membrane potential. Horizontal line superimposed upon scatterplots in C and D indicates the mean. Complete statistics are described in Table 1. *P < 0.05.
Table 1.
Nucleus accumbens core medium spiny neuron electrophysiological properties
| Property | Vehicle | Estradiol | Statistics (U/t, P) |
|---|---|---|---|
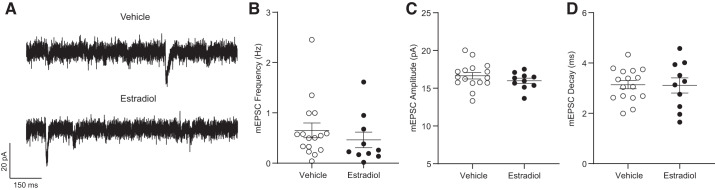
| mEPSC frequency, Hz | 0.7 ± 0.1 (16) | 0.5 ± 0.2 (10) | 55.500, 0.205 |
| mEPSC amplitude, pA | 16.7 ± 0.4 (16) | 16.0 ± 0.4 (10) | 1.107, 0.279 |
| mEPSC decay, ms | 3.1 ± 0.2 (16) | 3.1 ± 0.3 (10) | 0.101, 0.920 |
| Resting membrane potential, mV | −87.7 ± 0.8 (25) | −89.6 ± 0.5 (26) | 216, 0.040 |
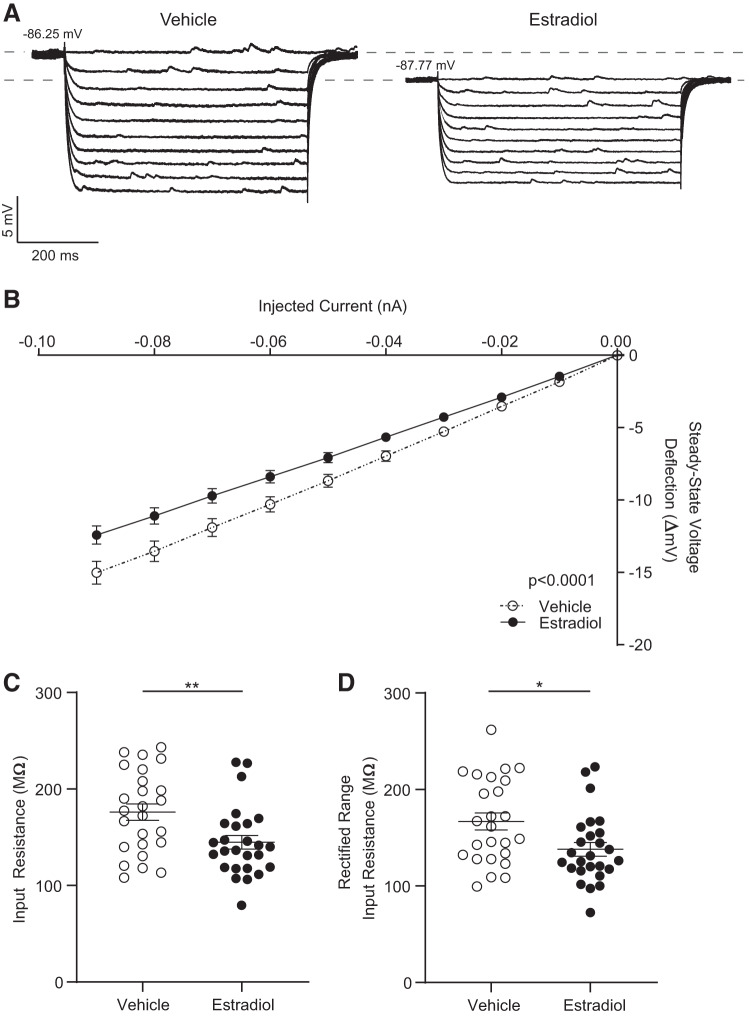
| Input resistance, MΩ | 176.1 ± 8.5 (25) | 144.8 ± 7.1 (26) | 185, 0.008 |
| Rectified-range input resistance, MΩ | 167.0 ± 8.8 (25) | 138.1 ± 7.1 (26) | 2.574, 0.013 |
| Inward rectification, MΩ | 9.1 ± 2.7 (25) | 6.8 ± 1.1 (26) | 0.819, 0.417 |
| % Inward rectification | 94.7 ± 1.4 (25) | 95.1 ± 0.8 (26) | 294, 0.569 |
| Sag index (unitless) | −0.006 ± 0.002 (25) | −0.000 ± 0.003 (26) | 1.412, 0.164 |
| Time constant of membrane, ms | 9.2 ± 0.9 (25) | 8.2 ± 0.5 (26) | 292, 0.544 |
| Capacitance, pF | 55.5 ± 6.9 (25) | 59.5 ± 4.1 (26) | 250, 0.162 |
| Rheobase, pA | 0.17 ± 0.01 (25) | 0.20 ± 0.01 (26) | 220.5, 0.049 |
| Delay to first AP, ms | 450.7 ± 18.0 (22) | 489.5 ± 12.8 (23) | 1.770, 0.084 |
| AP threshold, mV | −42.0 ± 1.5 (25) | −39.3 ± 1.9 (26) | 276, 0.364 |
| AP amplitude, mV | 35.1 ± 1.5 (25) | 36.7 ± 2.0 (26) | 323, 0.978 |
| AP width at half-peak amplitude, ms | 2.5 ± 0.1 (25) | 2.4 ± 0.1 (26) | 0.653, 0.517 |
| AHP peak amplitude, mV | −7.3 ± 0.4 (25) | −6.5 ± 0.4 (26) | 1.419, 0.162 |
| AHP time to peak, ms | 29.7 ± 1.7 (25) | 29.2 ± 2.0 (26) | 0.172, 0.864 |
| FI slope, Hz/nA | 298.1 ± 15.0 (25) | 304.5 ± 11.9 (26) | 283, 0.435 |
Values are mean ± SE excitatory synapse and intrinsic excitability properties recorded from nucleus accumbens core medium spiny neurons from adult female rats treated with either vehicle or estradiol. Numbers in parentheses indicate the number of neurons in each group (experimental n). Bold font indicates statistical significance; italic font indicates a statistical trend. AHP, afterhyperpolarization; AP, action potential; FI, evoked firing rate-to-positive current curve; mEPSC, miniature excitatory postsynaptic current.
Estradiol decreases input resistance in both the linear and rectified ranges.
The increase in rheobase in MSNs recorded from estradiol-treated animals may also be driven by a decrease in input resistance. To investigate input resistance in the linear and rectified ranges, we injected a series of hyperpolarizing current injections in MSNs from estradiol- and vehicle-treated animals (Fig. 4A). The steady-state voltage deflection evoked by injected hyperpolarizing current curve (IV curve) (Fig. 4B) showed that MSNs recorded from estradiol-treated females displayed a decreased voltage deflection in response to higher-magnitude hyperpolarizing current injections compared with MSNs recorded from vehicle-treated females. A linear regression analysis revealed that the slopes of the steady-state voltage deflections evoked by injected hyperpolarizing current curves between estradiol- and vehicle-treated animals are significantly different (P < 0.0001). We further evaluated this difference by quantifying input resistance in both the linear and rectified ranges. Input resistance in the linear range was decreased in MSNs from estradiol-treated animals compared with MSNs from vehicle-treated animals (Fig. 4C). Input resistance in the rectified range was also decreased in MSNs from estradiol-treated animals (Fig. 4D). We also assessed whether this difference in the rectified range was due to an estradiol action on MSN inward rectification itself. No differences were detected in inward rectification or percent inward rectification (Table 1), indicating that the difference in rectified-range input resistance is not due to changes in rectification per se. The estradiol-induced decrease in input resistance in both the linear and rectified ranges is consistent with the estradiol-induced increase in rheobase, indicating decreased excitability in MSNs recorded from estradiol-treated females.
Fig. 4.
Input resistance properties from medium spiny neurons (MSNs) recorded from estradiol- and vehicle-treated female rats. A: voltage response of MSNs recorded from estradiol- and vehicle-treated animals to a series of hyperpolarizing current injections. B: the injected current-to-steady-stage voltage deflection curve (IV curve). C: input resistance in the linear range. D: input resistance in the rectified range. Horizontal line superimposed upon scatterplots in C and D indicates the mean. Complete statistics are described in Table 1. *P < 0.05, **P < 0.01.
Individual action potential properties do not differ between estradiol- and vehicle-treated groups.
We also tested whether individual action potential properties differed between estradiol- and vehicle-treated groups. MSNs from estradiol-treated females trended toward a longer delay to first action potential in comparison to MSNs from vehicle-treated animals (Table 1; P = 0.084). This delay is a canonical aspect of MSNs and reflects the underlying slow-inactivating A current (Nisenbaum et al. 1994). No differences were detected in action potential threshold, action potential width at half-peak amplitude, action potential amplitude, action potential afterhyperpolarization time to peak amplitude, or action potential afterhyperpolarization peak amplitude (Table 1). These findings indicate that individual action potential properties do not robustly differ between MSNs recorded from estradiol- and vehicle-treated animals.
mEPSC properties do not differ between estradiol- and vehicle-treated groups.
mEPSC properties differ between estrous cycle phases in adult female rat AcbC (Proaño et al. 2018). To test whether this estradiol replacement paradigm influenced mEPSC properties, we voltage clamped MSNs from estradiol- and vehicle-treated animals to −70 mV and recorded mEPSCs while exposing MSNs to 1 µM TTX and 150 µM PTX to block sodium-dependent action potentials and GABAA receptors, respectively (Fig. 5A). We assessed mEPSC frequency, amplitude, and decay. There were no differences in mEPSC frequency (Fig. 5B), mEPSC amplitude (Fig. 5C), or mEPSC decay (Fig. 5D). These findings indicate that mEPSC properties do not differ between MSNs recorded from estradiol- and vehicle-treated animals.
Fig. 5.
Miniature excitatory postsynaptic current (mEPSC) properties of medium spiny neurons recorded from estradiol- and vehicle-treated female rats. A: representative examples of mEPSCs. B: mEPSC frequency. C: mEPSC amplitude. D: mEPSC decay. Horizontal line superimposed upon scatterplots in B–D indicates the mean. Complete statistics are described in Table 1.
DISCUSSION
The findings presented here demonstrate that AcbC MSN membrane excitability is decreased by estrous cycle-relevant doses of estradiol in female rats. Specifically, estradiol induced an increase in rheobase while concomitantly hyperpolarizing resting membrane potential and decreasing input resistance in both the linear and rectified ranges. The collective impact of these changes is to profoundly decrease overall MSN excitability. These findings recapitulated the changes observed in MSN excitability during the appropriate phases of the estrous cycle that likewise feature high estradiol levels or impact: proestrus and estrus, respectively (Proaño et al. 2018). Together, these findings are the first to demonstrate a causal role for estrous cycle-relevant doses of estradiol in regulating AcbC MSN excitability.
This newly demonstrated causal link between estradiol and MSN electrophysiology builds on a line of research dating back to at least the early 1980s, if discussion is focused specifically on MSN electrophysiological properties. In vivo spontaneous firing rates and dopamine sensitivity of caudate-putamen MSNs changed when ovariectomized (OVX) female rats were administered estradiol or when ovary-intact females were in the high-estradiol-impact phases of the estrous cycle (Arnauld et al. 1981; Tansey et al. 1983). Later, Mermelstein and colleagues (Mermelstein et al. 1996) demonstrated that estradiol rapidly decreased L-type calcium currents in dissociated female but not male rat caudate-putamen MSNs. This specific finding directly foreshadows the present work, although decreases in L-type calcium channel currents are more typically associated with intracellular signaling rather than resting membrane potential hyperpolarization. The first indication of a sex difference in nucleus accumbens MSN electrophysiological properties came from Wissman and colleagues (Wissman et al. 2011), who showed that mEPSC frequency was increased in gonad-intact female compared with male rats. Our laboratory then built upon these findings by showing that rat MSNs in both the AcbC and caudate-putamen exhibit development- and estrous cycle-dependent sex differences in intrinsic excitability and excitatory synapse properties (Cao et al. 2016, 2018b; Dorris et al. 2015; Proaño et al. 2018; Willett et al. 2019b). Mice MSNs in general exhibit less robust sex differences than rat MSNs in both the AcbC and caudate-putamen (Cao et al. 2018a; Willett et al. 2019a). Regarding the rat AcbC, during the prepubertal period female rat MSNs exhibit increased mEPSC frequency but no differences in intrinsic excitability compared with male MSNs (Cao et al. 2016). This increased mEPSC frequency in female prepubertal MSNs is abolished by masculinizing/feminizing doses of estradiol or testosterone during the perinatal critical period. In adult gonad-intact female rats, AcbC MSNs show changes in both membrane excitability and excitatory synapse properties across estrous cycle phases, which generates sex differences compared with male MSNs. Regarding membrane excitability, MSNs recorded in early proestrus (proestrus AM) and estrus showed hyperpolarized resting membrane potentials accompanied by decreases in input resistance and increases in rheobase (Proaño et al. 2018). These electrophysiological differences are abolished when females are ovariectomized. The present study employed estradiol doses that mimicked circulating estradiol levels experienced during the estrous cycle and featured MSNs recorded during the equivalent of proestrus. This manipulation induced differences in membrane excitability that match what was previously observed in MSNs recorded from females in proestrus and estrus phases. As in proestrus, the MSNs recorded from estradiol-exposed females from the present study exhibited hyperpolarized resting membrane potential, decreased input resistance in both the linear and rectified ranges, and increased rheobase, indicating an overall decrease in membrane excitability.
Surprisingly, estrous cycle-relevant doses of estradiol did not induce differences in mEPSC excitatory synapse properties. This finding does not mimic the previous study of estrous cycling females, given that excitatory synapse properties such as mEPSC frequency, amplitude, and decay robustly differed across the estrous cycle. Specifically, mEPSC frequency is significantly increased during proestrus AM and estrus compared with diestrus and males (Proaño et al. 2018), consistent with previous work in proestrus-phase females and males (Wissman et al. 2011). Relevant to this finding, sex differences in excitatory synapse number and associated markers have been identified between proestrus-phase females and males (Forlano and Woolley 2010; Wissman et al. 2011, 2012). The lack of an effect on excitatory synapse properties in the present study is of especial interest given estradiol’s rapid regulation of excitatory synapse mEPSC frequency and amplitude in female but not male AcbC (Krentzel et al. 2019). Also relevant are anatomical experiments that have demonstrated estradiol-induced decreases in dendritic spine density in AcbC MSNs in female rats and hamsters (Peterson et al. 2015; Staffend et al. 2011). Several not necessarily mutually exclusive possibilities may explain why no differences in mEPSC properties were detected in response to the estradiol doses employed in this study. First, we note that no exogenous estradiol replacement paradigm in an ovariectomized female perfectly matches the natural in vivo conditions; however, great care was taken in the development of this paradigm to mimic the temporally cycling circulating estradiol concentrations across the estrous cycle (Scharfman et al. 2007). Second, estradiol action on MSN excitatory synapse properties could be solely regulated by a rapid action of estradiol, which would be consistent with the results of Krentzel, Mermelstein, Becker, and others (Hu et al. 2006; Krentzel et al. 2019; Song et al. 2019). A rapid action of estradiol may not be detected by the present study, given that animals were euthanized 2 h after the last estradiol injection. This time of euthanasia was chosen to more accurately mimic proestrus-phase estradiol levels and to match the previously validated protocol (Scharfman et al. 2007). A third possibility is that there are temporal differences in the impact of estradiol on electrophysiological properties, especially between the rapid (seconds to minutes) and nonrapid (hours to days) timescales, and in response to repeated doses of estradiol, as has been demonstrated in other systems (Zakon 1998). This conjecture is consistent with previous work showing that AcbC-mediated behaviors are sensitive to estrogen priming (Becker and Rudick 1999; Krentzel et al. 2020). Fourth, it is always possible that a secondary factor may be present. One example could be stress and anxiety. Stress may sex-specifically influence glutamatergic synapse and estrogen receptor action in the nucleus accumbens core, as has been demonstrated in the nucleus accumbens shell (Brancato et al. 2017; Hodes et al. 2015; Lorsch et al. 2018). Although we have attempted to mitigate the impact of stress by performing extensive handling and saline injection habituation before experimentation, formally this possibility cannot be ruled out.
Fifth, other hormones also cycle, most prominently progesterone, and progesterone alone and in combination with estradiol likely regulates glutamatergic synapse properties or other electrophysiological properties. We favor this possibility as a highly likely scenario. Indeed, circulating progesterone but not estradiol levels correlate with AcbC MSN mEPSC frequency and amplitude (Proaño et al. 2020). Circulating levels of estradiol and progesterone together correlate with AcbC MSN mEPSC amplitude, resting membrane potential, and input resistance. There is evidence for progesterone receptors in rodent AcbC (Dluzen and Ramirez 1989; Ke and Ramirez 1990; Piechota et al. 2017), and progesterone is known to modulate AcbC-relevant behaviors and disorders (Becker 1999). A salient future experiment will be to perform exogenous progesterone supplementation and test whether this manipulation modulates MSN electrophysiology. Please see Proaño et al. (2020) for further discussion of the potential role of progesterone in modulating AcbC function.
Other potential factors of note include differences in estrogen sensitivity and/or sex differences between MSN subtypes (Cao et al. 2018a), although the robust nature of MSN estrogen sensitivity and sex differences may argue against this particular limitation. Nevertheless, an important future experiment will be to determine which MSN subtypes are estrogen sensitive, using techniques other than the transgenic mice that show minimal or no sexual differentiation in MSNs, at least during the prepubertal period (Cao et al. 2018a; Willett et al. 2019a). These future experiments are all the more relevant given that MSN subtypes can differentially express dopamine receptors, including but not limited to the D1 and D2 dopamine receptors. AcbC MSN D1 and D2 dopamine receptors have been documented to underlie rewarding versus aversive behaviors, among many other functions (Calipari et al. 2016). This is relevant to estrogen action, as the expression of D1 and D2 dopamine receptors can be influenced by estradiol exposure (Chavez et al. 2010; Le Saux et al. 2006; Morissette et al. 2008). Estradiol administration has been documented to reverse an ovariectomy-induced decrease in D2 receptor expression (Le Saux et al. 2006). Female rats have been reported to exhibit greater expression of the D1-D2 heterodimer complex, a protein receptor complex known to induce depression-like and anxiety-like behaviors that could ultimately play a role in modulating MSN electrophysiological properties (Hasbi et al. 2020; Shen et al. 2015). Thus, there are likely complex interactions between estradiol, dopamine, glutamate, and MSN electrophysiological properties.
Although this discussion has focused upon ovarian-sourced estradiol action in the AcbC, de novo synthesis of this hormone by the enzyme aromatase may also be implicated in mediating electrophysiological properties in this brain region. There is some evidence that aromatase is active in the nucleus accumbens, although this area of research is relatively understudied (Krentzel and Meitzen 2018; Yoest et al. 2018). Aromatase presence in the nucleus accumbens was detected in mice pretreated with vorozole, an aromatase inhibitor known to increase the intensity of aromatase immunoreactivity in quail brains, as well as in mice enhanced with green fluorescent protein (EGFP) that activated upon transcription of the Cyp19A1 aromatase-encoding gene (Foidart et al. 1995; Stanić et al. 2014). In addition, changes in nucleus accumbens gene expression accompany sex-specific suppression of spontaneous physical activity in aromatase-knockout mice (Shay et al. 2020). Functionally, in the caudate-putamen of male rats at least one type of long-term potentiation in MSNs is mediated by aromatase-induced synthesis of estradiol, although females and the nucleus accumbens were not included in the study (Tozzi et al. 2015). In female but not male nucleus accumbens core, estradiol rapidly regulates mEPSC frequency (Krentzel et al. 2019). Building upon this evidence, we speculate that locally synthesized estradiol rapidly regulates the sexually differentiated MSN glutamatergic synapse properties, in concert with progesterone (Proaño et al. 2020), whereas slower ovarian-sourced estradiol action may modulate MSN membrane excitability. This speculation is similar to aspects of estrogen action on CA1 hippocampal dendritic spine density and synaptic plasticity function, which feature influences of both ovarian-sourced and locally sourced estradiol action (Grassi et al. 2011; Kretz et al. 2004; Prange-Kiel et al. 2008; Woolley and McEwen 1993). Future studies can examine the contribution of aromatase-induced estradiol synthesis in modulating AcbC excitatory synapse properties independently or in concert with ovarian-sourced estradiol and progesterone across the estrous cycle.
Given that we have established that estradiol induces profound differences in MSN electrophysiological properties, future research can also focus on elucidating the relevant receptor mechanisms. The adult AcbC exclusively expresses membrane-associated estrogen receptors (mERs) α, β, and GPER-1 (Almey et al. 2015). Although GPER-1 is a G protein-coupled receptor, membrane-associated ERα and ERβ are not. Instead, membrane-associated ERα and ERβ are palmitoylated and interact with caveolin proteins to couple with group I and group II metabotropic glutamate receptors (mGluRs) in the neuronal membrane to induce rapid hormone effects (Grove-Strawser et al. 2010; Meitzen et al. 2013, 2019; Peterson et al. 2015, 2016; Tonn Eisinger et al. 2018b). In addition to rapid hormone effects, ERα and ERβ coupled with mGluRs can also activate second messenger signaling and long-term changes to neuron function (Meitzen and Mermelstein 2011). For example, the reported decrease in dendritic spine density in adult OVX rat AcbC upon estradiol administration is mediated by ERα and group I mGluR5 coupling as well as by mGluR-mediated endocannabinoid release and activation of CB1 receptors (Peterson et al. 2015, 2016). In addition, AcbC-mediated behaviors and disorders such as anxiety and drug addiction have also shown estradiol sensitivity by mGluR5 activation (Martinez et al. 2014, 2016; Miller et al. 2020; Pomierny-Chamiolo et al. 2014, 2017). Thus, the differences in membrane excitability induced by estradiol could be mediated by membrane-associated ERα and ERβ (possibly via mGluR activation) and/or GPER-1. Finally, although our working model posits that estradiol acts on receptors in the AcbC itself to induce changes in MSN excitability, it is also possible that estradiol also acts on receptors in afferent brain regions, which then transsynaptically signal to AcbC MSNs. To conclude, we believe that the findings of this study provide a strong foundation upon which to pursue these future avenues of investigation.
GRANTS
This work was supported by the following funding sources: National Institutes of Health (NIH) Grants R01 MH-109471 (J. Meitzen) and P30 ES-025128 (Center for Human Health and the Environment).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.B.P. and J.M. conceived and designed research; S.B.P. performed experiments; S.B.P. and J.M. analyzed data; S.B.P. and J.M. interpreted results of experiments; S.B.P. and J.M. prepared figures; S.B.P. and J.M. drafted manuscript; S.B.P. and J.M. edited and revised manuscript; S.B.P. and J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We especially thank Dr. Helen Scharfman for assisting with the estradiol replacement protocol and Dr. Amanda Krentzel for training. We also thank David Dorris, Gina Kim, and Lanie Kimble for preparing solutions.
REFERENCES
- Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 74: 125–138, 2015. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Caraballo Y, Ferrario CR. Effects of the estrous cycle and ovarian hormones on cue-triggered motivation and intrinsic excitability of medium spiny neurons in the nucleus accumbens core of female rats. Horm Behav 116: 104583, 2019. doi: 10.1016/j.yhbeh.2019.104583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnauld E, Dufy B, Pestre M, Vincent JD. Effects of estrogens on the responses of caudate neurons to microiontophoretically applied dopamine. Neurosci Lett 21: 325–331, 1981. doi: 10.1016/0304-3940(81)90225-1. [DOI] [PubMed] [Google Scholar]
- Baufreton J, Atherton JF, Surmeier DJ, Bevan MD. Enhancement of excitatory synaptic integration by GABAergic inhibition in the subthalamic nucleus. J Neurosci 25: 8505–8517, 2005. doi: 10.1523/JNEUROSCI.1163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64: 803–812, 1999. doi: 10.1016/S0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol 29: 36–47, 2008. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann NY Acad Sci 937: 172–187, 2001. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ 3: 14, 2012. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav 64: 53–57, 1999. doi: 10.1016/S0091-3057(99)00091-X. [DOI] [PubMed] [Google Scholar]
- Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol 84: 2204–2216, 2000. doi: 10.1152/jn.2000.84.5.2204. [DOI] [PubMed] [Google Scholar]
- Brancato A, Bregman D, Ahn HF, Pfau ML, Menard C, Cannizzaro C, Russo SJ, Hodes GE. Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience 350: 180–189, 2017. doi: 10.1016/j.neuroscience.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci USA 113: 2726–2731, 2016. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han MH, Nestler EJ. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8: 13877, 2017. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dorris DM, Meitzen J. Neonatal masculinization blocks increased excitatory synaptic input in female rat nucleus accumbens core. Endocrinology 157: 3181–3196, 2016. doi: 10.1210/en.2016-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dorris DM, Meitzen J. Electrophysiological properties of medium spiny neurons in the nucleus accumbens core of prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice. J Neurophysiol 120: 1712–1727, 2018a. doi: 10.1152/jn.00257.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Willett JA, Dorris DM, Meitzen J. Sex Differences in medium spiny neuron excitability and glutamatergic synaptic input: heterogeneity across striatal regions and evidence for estradiol-dependent sexual differentiation. Front Endocrinol (Lausanne) 9: 173, 2018b. doi: 10.3389/fendo.2018.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res 1321: 51–59, 2010. doi: 10.1016/j.brainres.2009.12.093. [DOI] [PubMed] [Google Scholar]
- Deroche MA, Lassalle O, Castell L, Valjent E, Manzoni OJ. Cell-type- and endocannabinoid-specific synapse connectivity in the adult nucleus accumbens core. J Neurosci 40: 1028–1041, 2020. doi: 10.1523/JNEUROSCI.1100-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD. Progesterone effects upon dopamine release from the corpus striatum of female rats. II. Evidence for a membrane site of action and the role of albumin. Brain Res 476: 338–344, 1989. doi: 10.1016/0006-8993(89)91255-9. [DOI] [PubMed] [Google Scholar]
- Dorris DM, Cao J, Willett JA, Hauser CA, Meitzen J. Intrinsic excitability varies by sex in prepubertal striatal medium spiny neurons. J Neurophysiol 113: 720–729, 2015. doi: 10.1152/jn.00687.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav 23: 473–502, 1989. doi: 10.1016/0018-506X(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology 31: 659–674, 2006. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Farries MA, Meitzen J, Perkel DJ. Electrophysiological properties of neurons in the basal ganglia of the domestic chick: conservation and divergence in the evolution of the avian basal ganglia. J Neurophysiol 94: 454–467, 2005. doi: 10.1152/jn.00539.2004. [DOI] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. Electrophysiological properties of avian basal ganglia neurons recorded in vitro. J Neurophysiol 84: 2502–2513, 2000. doi: 10.1152/jn.2000.84.5.2502. [DOI] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci 22: 3776–3787, 2002. doi: 10.1523/JNEUROSCI.22-09-03776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66: 25–52, 2015. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Foidart A, Harada N, Balthazart J. Aromatase-immunoreactive cells are present in mouse brain areas that are known to express high levels of aromatase activity. Cell Tissue Res 280: 561–574, 1995. doi: 10.1007/BF00318360. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Woolley CS. Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 518: 1330–1348, 2010. doi: 10.1002/cne.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Lobo MK. Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol Psychiatry 81: 645–653, 2017. doi: 10.1016/j.biopsych.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi S, Tozzi A, Costa C, Tantucci M, Colcelli E, Scarduzio M, Calabresi P, Pettorossi VE. Neural 17β-estradiol facilitates long-term potentiation in the hippocampal CA1 region. Neuroscience 192: 67–73, 2011. doi: 10.1016/j.neuroscience.2011.06.078. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170: 1045–1055, 2010. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Nguyen T, Rahal H, Manduca JD, Miksys S, Tyndale RF, Madras BK, Perreault ML, George SR. Sex difference in dopamine D1-D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol Sex Differ 11: 8, 2020. doi: 10.1186/s13293-020-00285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Ménard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci 35: 16362–16376, 2015. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse 59: 122–124, 2006. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem 80: 79–87, 2005. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol 9: 223–227, 1999. doi: 10.1016/S0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Ke FC, Ramirez VD. Binding of progesterone to nerve cell membranes of rat brain using progesterone conjugated to 125I-bovine serum albumin as a ligand. J Neurochem 54: 467–472, 1990. doi: 10.1111/j.1471-4159.1990.tb01895.x. [DOI] [PubMed] [Google Scholar]
- Krentzel AA, Barrett LR, Meitzen J. Estradiol rapidly modulates excitatory synapse properties in a sex- and region-specific manner in rat nucleus accumbens core and caudate-putamen. J Neurophysiol 122: 1213–1225, 2019. doi: 10.1152/jn.00264.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Meitzen J. Biological sex, estradiol and striatal medium spiny neuron physiology: a mini-review. Front Cell Neurosci 12: 492, 2018. doi: 10.3389/fncel.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Proaño S, Patisaul HB, Meitzen J. Temporal and bidirectional influences of estradiol on voluntary wheel running in adult female and male rats. Horm Behav 120: 104694, 2020. doi: 10.1016/j.yhbeh.2020.104694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci 24: 5913–5921, 2004. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux M, Morissette M, Di Paolo T. ERbeta mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology 50: 451–457, 2006. doi: 10.1016/j.neuropharm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord 2: 3, 2012. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch ZS, Loh YE, Purushothaman I, Walker DM, Parise EM, Salery M, Cahill ME, Hodes GE, Pfau ML, Kronman H, Hamilton PJ, Issler O, Labonté B, Symonds AE, Zucker M, Zhang TY, Meaney MJ, Russo SJ, Shen L, Bagot RC, Nestler EJ. Estrogen receptor α drives pro-resilient transcription in mouse models of depression. Nat Commun 9: 1116, 2018. doi: 10.1038/s41467-018-03567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Reyna AM, Alejandro MA, Crowley J, Markaverich BM. Disruption of male sexual behavior in rats by tetrahydrofurandiols (THF-diols). Steroids 70: 750–754, 2005. doi: 10.1016/j.steroids.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, Velez-Trippe C, Murchison C, O’Malley B, Faith R. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect 110: 169–177, 2002. doi: 10.1289/ehp.02110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, Foster Olive M, Carroll ME, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine self-administration in female rats requires activation of mGluR5. eNeuro 3: ENEURO.0140-16.2016, 2016. doi: 10.1523/ENEURO.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res 271: 39–42, 2014. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Mullins AJ. Sexual experience in female rodents: cellular mechanisms and functional consequences. Brain Res 1126: 56–65, 2006. doi: 10.1016/j.brainres.2006.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Britson KA, Tuomela K, Mermelstein PG. The expression of select genes necessary for membrane-associated estrogen receptor signaling differ by sex in adult rat hippocampus. Steroids 142: 21–27, 2019. doi: 10.1016/j.steroids.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology 154: 4293–4304, 2013. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Meisel RL, Mermelstein PG. Sex differences and the effects of estradiol on striatal function. Curr Opin Behav Sci 23: 42–48, 2018. doi: 10.1016/j.cobeha.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat 42: 236–241, 2011. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Pflepsen KR, Stern CM, Meisel RL, Mermelstein PG. Measurements of neuron soma size and density in rat dorsal striatum, nucleus accumbens core and nucleus accumbens shell: differences between striatal region and brain hemisphere, but not sex. Neurosci Lett 487: 177–181, 2011. doi: 10.1016/j.neulet.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Weaver AL, Brenowitz EA, Perkel DJ. Plastic and stable electrophysiological properties of adult avian forebrain song-control neurons across changing breeding conditions. J Neurosci 29: 6558–6567, 2009. doi: 10.1523/JNEUROSCI.5571-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16: 595–604, 1996. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164: 887–895, 2009. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CK, Krentzel AA, Patisaul HB, Meitzen J. Metabotropic glutamate receptor subtype 5 (mGlu5) is necessary for estradiol mitigation of light-induced anxiety behavior in female rats. Physiol Behav 214: 112770, 2020. doi: 10.1016/j.physbeh.2019.112770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol 108: 327–338, 2008. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, Schlüter OM, Dong Y. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci 30: 3689–3699, 2010. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Xu ZC, Wilson CJ. Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. J Neurophysiol 71: 1174–1189, 1994. doi: 10.1152/jn.1994.71.3.1174. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307: 462–465, 1984. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse 13: 135–160, 1993. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Behav 64: 573–578, 2013. doi: 10.1016/j.yhbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Martinez LA, Meisel RL, Mermelstein PG. Estradiol impacts the endocannabinoid system in female rats to influence behavioral and structural responses to cocaine. Neuropharmacology 110: 118–124, 2016. doi: 10.1016/j.neuropharm.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct 220: 2415–2422, 2015. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechota M, Korostynski M, Golda S, Ficek J, Jantas D, Barbara Z, Przewlocki R. Transcriptional signatures of steroid hormones in the striatal neurons and astrocytes. BMC Neurosci 18: 37, 2017. doi: 10.1186/s12868-017-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L, Miszkiel J, Frankowska M, Bystrowska B, Filip M. Cocaine self-administration, extinction training and drug-induced relapse change metabotropic glutamate mGlu5 receptors expression: evidence from radioligand binding and immunohistochemistry assays. Brain Res 1655: 66–76, 2017. doi: 10.1016/j.brainres.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L, Rup K, Pomierny B, Niedzielska E, Kalivas PW, Filip M. Metabotropic glutamatergic receptors and their ligands in drug addiction. Pharmacol Ther 142: 281–305, 2014. doi: 10.1016/j.pharmthera.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J Cell Biol 180: 417–426, 2008. doi: 10.1083/jcb.200707043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proaño SB, Krentzel AA, Meitzen J. Differential and synergistic roles of 17β-estradiol and progesterone in modulating adult female rat nucleus accumbens core medium spiny neuron electrophysiology. J Neurophysiol, 2020. doi: 10.1152/jn.00157.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proaño SB, Morris HJ, Kunz LM, Dorris DM, Meitzen J. Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J Neurophysiol 120: 1356–1373, 2018. doi: 10.1152/jn.00263.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado S, Kaplitt MG. The nucleus accumbens: a comprehensive review. Stereotact Funct Neurosurg 93: 75–93, 2015. doi: 10.1159/000368279. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci 26: 2595–2612, 2007. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay DA, Welly RJ, Givan SA, Bivens N, Kanaley J, Marshall BL, Lubahn DB, Rosenfeld CS, Vieira-Potter VJ. Changes in nucleus accumbens gene expression accompany sex-specific suppression of spontaneous physical activity in aromatase knockout mice. Horm Behav 121: 104719, 2020. doi: 10.1016/j.yhbeh.2020.104719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MY, Perreault ML, Bambico FR, Jones-Tabah J, Cheung M, Fan T, Nobrega JN, George SR. Rapid anti-depressant and anxiolytic actions following dopamine D1-D2 receptor heteromer inactivation. Eur Neuropsychopharmacol 25: 2437–2448, 2015. doi: 10.1016/j.euroneuro.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest 55: 699–706, 1975. doi: 10.1172/JCI107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Yang H, Peckham EM, Becker JB. Estradiol-induced potentiation of dopamine release in dorsal striatum following amphetamine administration requires estradiol receptors and mGlu5. eNeuro 6: ENEURO.0446–18.2019, 2019. doi: 10.1523/ENEURO.0446-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct 215: 187–194, 2011. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanić D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β, and androgen receptors. PLoS One 9: e90451, 2014. doi: 10.1371/journal.pone.0090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey EM, Arbuthnott GW, Fink G, Whale D. Oestradiol-17 beta increases the firing rate of antidromically identified neurones of the rat neostriatum. Neuroendocrinology 37: 106–110, 1983. doi: 10.1159/000123527. [DOI] [PubMed] [Google Scholar]
- Tonn Eisinger KR, Larson EB, Boulware MI, Thomas MJ, Mermelstein PG. Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids 133: 53–59, 2018a. doi: 10.1016/j.steroids.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonn Eisinger KR, Woolfrey KM, Swanson SP, Schnell SA, Meitzen J, Dell’Acqua M, Mermelstein PG. Palmitoylation of caveolin-1 is regulated by the same DHHC acyltransferases that modify steroid hormone receptors. J Biol Chem 293: 15901–15911, 2018b. doi: 10.1074/jbc.RA118.004167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Tantucci M, Durante V, Quiroga-Varela A, Giampà C, Di Mauro M, Mazzocchetti P, Costa C, Di Filippo M, Grassi S, Pettorossi VE, Calabresi P. Endogenous 17β-estradiol is required for activity-dependent long-term potentiation in the striatum: interaction with the dopaminergic system. Front Cell Neurosci 9: 192, 2015. doi: 10.3389/fncel.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon Landeros R, Morisseau C, Yoo HJ, Fu SH, Hammock BD, Trainor BC. Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-α expression in the brain. Endocrinology 153: 949–953, 2012. doi: 10.1210/en.2011-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood FR. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol 36: 375–384, 2008. doi: 10.1177/0192623308315665. [DOI] [PubMed] [Google Scholar]
- Willett JA, Cao J, Dorris DM, Johnson AG, Ginnari LA, Meitzen J. Electrophysiological properties of medium spiny neuron subtypes in the caudate-putamen of prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice. eNeuro 6: ENEURO.0016–19.2019, 2019a. doi: 10.1523/ENEURO.0016-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JA, Cao J, Johnson A, Patel OH, Dorris DM, Meitzen J. The estrous cycle modulates rat caudate-putamen medium spiny neuron physiology. Eur J Neurosci 2019: ejn.14506, 2019b. doi: 10.1111/ejn.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JA, Will T, Hauser CA, Dorris DM, Cao J, Meitzen J. No evidence for sex differences in the electrophysiological properties and excitatory synaptic input onto nucleus accumbens shell medium spiny neurons. eNeuro 3: ENEURO.0147–15.2016, 2016. doi: 10.1523/ENEURO.0147-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, May RM, Woolley CS. Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct 217: 181–190, 2012. doi: 10.1007/s00429-011-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology 61: 217–227, 2011. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JE, Cao J, Dorris DM, Meitzen J. Genetic sex and the volumes of the caudate-putamen, nucleus accumbens core and shell: original data and a review. Brain Struct Funct 221: 4257–4267, 2016. doi: 10.1007/s00429-015-1158-9. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336: 293–306, 1993. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Quigley JA, Becker JB. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm Behav 104: 119–129, 2018. doi: 10.1016/j.yhbeh.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakon HH. The effects of steroid hormones on electrical activity of excitable cells. Trends Neurosci 21: 202–207, 1998. doi: 10.1016/S0166-2236(97)01209-5. [DOI] [PubMed] [Google Scholar]