Abstract
Diabetes mellitus type 2, a chronic metabolic disease, has globally increased in incidence and prevalence throughout the lifespan due to the rise in obesity and sedentary lifestyle. The end-organ cardiovascular and cerebrovascular effects of diabetes mellitus result in significant morbidity and mortality that increases with age. Thus, it is crucial to fully understand how molecular mechanisms are influenced by diabetes mellitus and may influence the development of end-organ complications. Circulating factors are known to play important physiological and pathological roles in diabetes. Recent data have implicated extracellular vesicles (EVs) as being circulating mediators in type 2 diabetes. These small lipid-bound vesicles are released by cells into the circulation and can carry functional cargo, including lipids, proteins, and nucleic acids, to neighboring cells or between tissues. In this review, we will summarize the current evidence for EVs as promising diagnostic and prognostic factors in diabetes, the mechanisms that drive EV alterations with diabetes, and the role EVs play in the pathology associated with diabetes.
Keywords: exosomes, EV, insulin resistance, microparticles, microvesicles, obesity, type 2 diabetes mellitus
INTRODUCTION
More than five decades ago, platelet particles and platelet dust were discovered in human plasma (11, 87). Now, as we embark decades after these initial discoveries, we are beginning to understand the nature of these vesicles, termed extracellular vesicles (EVs), and the roles that EVs play in normal physiology and disease. One area of interest that has sparked particular attention is the role that EVs play in type 2 diabetes. This review will focus on EVs in the context of type 2 diabetes in humans, but some of the landmark findings in mice will also briefly be discussed.
DIABETES MELLITUS TYPE 2
Diabetes mellitus type 2 (T2DM) is a commonly occurring group of metabolic disorders in adults whose incidence and prevalence is increasing in parallel to the epidemic of obesity (10a, 35a, 95). The increased prevalence of obesity among sedentary children and adolescents contributes to the increased incidence of T2DM in these groups (13). T2DM is considered a major global public health problem, as the burden of this disease has risen to epidemic levels. Worldwide, diabetes mellitus is the ninth major cause of death (95). In 2019, the International Diabetes Federation (IDF) estimated that there are 463 million adults with diabetes mellitus (35a). T2DM encompasses the majority (90–95%) of these reported cases. The IDF forecasts that there will be a 51% increase in the number of adults with diabetes over the next 26 years (35a). In the United States, it is estimated that 9.4% (∼30 million) of the population have diabetes (10a). The prevalence of diabetes is higher in racial minorities and individuals with a lower educational level (10a). Diabetes mellitus increases the risk for complications due to hypertension, end-stage renal disease, stroke, myocardial infarction, and lower-extremity amputation (31). Given the prevalence of diabetes mellitus and its impact on health worldwide, it is critical to identify novel potential effective biomarkers or therapeutic modalities. Therefore, in this review, we will focus on the current, promising role for EVs in type 2 diabetes.
T2DM is characterized by insulin resistance, impaired insulin secretion, or both. Multiple organs and tissues contribute to elevated glucose levels in individuals with T2DM. In some cases, these organs may secrete or be the recipient of EVs that carry signaling molecules related to the diabetes mellitus disease process. The pancreas, which releases insulin and glucagon, is central to glucose homeostasis in the body. Other organs are also critical. The liver regulates blood glucose levels by releasing glucose in response to glucagon and by taking up glucose in response to insulin, which is cleared through the liver after its secretion into the abdominal portal vein (58). Approximately 50% of secreted insulin is cleared by the liver; this clearance rate is influenced by ancestry among individuals without diabetes. Insulin hepatic clearance is lower in African American adults and children, which results in peripheral hyperinsulinemia (5). While the liver is the primary site of insulin clearance, the remaining systemic insulin is cleared in the kidney by both glomerular filtration and proximal tubular reabsorption and degradation by lysosomes. Insulin acts on other major target tissues, including skeletal muscle. The adrenals also play a role in glucose homeostasis via epinephrine secretion, which raises blood pressure and triggers release of glucagon, leading to increased blood glucose levels. Perhaps most significantly from the perspective of EV secretion, adipose tissue is a metabolically active endocrine organ that influences glucose levels and metabolism, insulin resistance, and insulin signaling by the presence of circulating pro-inflammatory factors, including high sensitivity C-reactive protein, TNFα, cytokines, adipokines, and free fatty acids (29). This is particularly true for visceral adipose tissue. Although peripheral insulin resistance is an important element in T2DM, central insulin resistance also contributes to regulating glucose metabolism. The brain plays an important role in regulating energy homeostasis and glucose metabolism; dysregulation of the highly coordinated signaling between the brain and peripheral metabolic organs is a known contributor to type 2 diabetes (68). Within the brain, there are insulin receptors as well as insulin receptor substrates (47). Therefore, multiple organs and tissues play a role in T2DM, and recent evidence suggests that EVs are important mediators for interorgan communication in T2DM.
WHAT ARE EVs?
EVs is a broad term that encompasses vesicles derived from several mechanisms (86). There are three main subtypes of EVs that have been described. Exosomes are intraluminal vesicles, which are released when multivesicular bodies (MVB) fuse with the plasma membrane (Fig. 1) (33, 40, 66). Microvesicles (also referred to as ectosomes, microparticles) are released into the extracellular environment through budding and pinching off of the plasma membrane (12, 91). As membranes are blebbing during apoptosis, apoptotic bodies are released (Fig. 1) (12, 91). For these subtypes, EVs are classified based on their biogenesis. Different types of EVs have also been classified based on size, structure, external or internal molecular cargo, cell of origin, and initiating function (32). Many of these subtypes overlap in size, cargo, and other characteristics (32, 48). Thus far, it has been difficult to discriminate between different classes of EVs given the current isolation techniques. Ongoing efforts in the field are working to establish better and more precise purification methods and also standardization of analytics for EVs (77). Therefore, the collective term EVs is an appropriate term of use (86). In this review, we will in some cases use terms that have been identified in the primary literature for consistency.
Fig. 1.
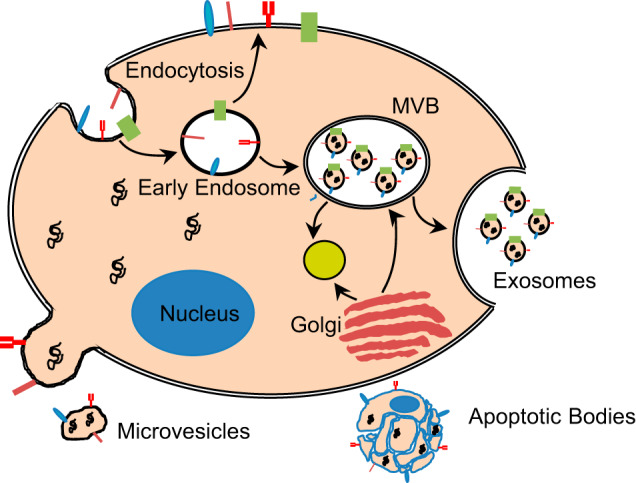
Biogenesis and sites of origin of three main subtypes of extracellular vesicles (EVs). Exosomes are intraluminal vesicles that are released when multivesicular bodies (MVB) fuse with the plasma membrane. Microvesicles are released through budding and pinching off of the plasma membrane and apoptotic bodies are formed during membrane blebbing during apoptosis.
EVs are small nano-sized vesicles encapsulated in a lipid bilayer (Fig. 2). Most, if not all, cells secrete EVs into the extracellular space. Thus far, the main thrust of research has been characterizing EVs in tissue culture cells or tissue explants from mice grown in vitro, but characterizing EVs from human biofluids has gained considerable recent attention. This focus has been embarked upon since EVs have been found in a variety of bodily fluids, including plasma, serum, and urine (8, 44, 63). Their location in these easily accessible body fluids makes them attractive for the use as biomarkers. This concept of a “liquid biopsy” can aid in providing noninvasive diagnostic and prognostic information about various diseases and also to monitor response to various treatments (61).
Fig. 2.
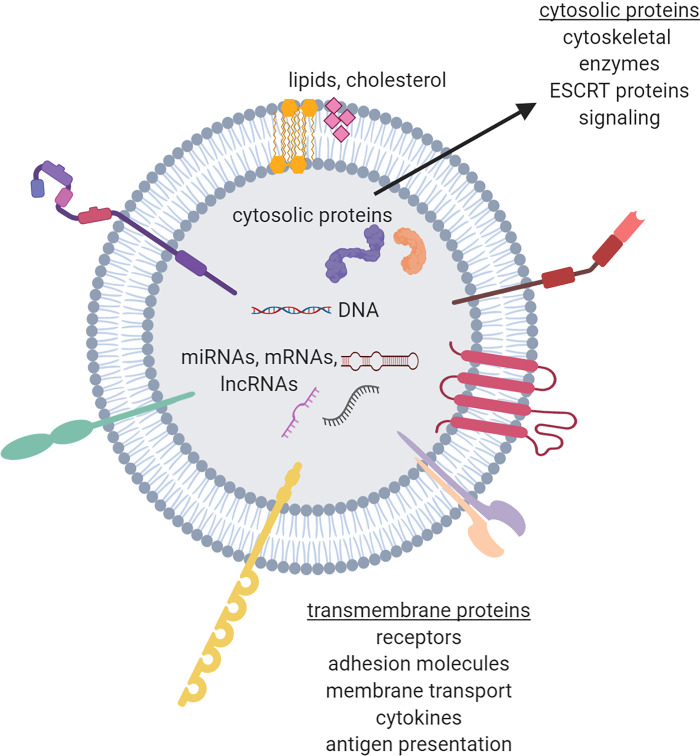
Extracellular vesicles carry cargo. This cargo includes both transmembrane and cytosolic proteins, RNAs [e.g., microRNAs, mRNA, long noncoding RNAs (lncRNAs)], DNA, and lipids. Examples of these types of cargo are indicated. ESCRT, endosomal sorting complex required for transport.
EV FUNCTIONS
EVs contain molecular cargo, including RNAs (mRNA, microRNAs, and long noncoding RNAs), DNA, proteins, and lipids (Fig. 2) (30, 41, 46, 76, 91). EVs from different cell types appear to carry some cell type-specific molecules but also contain cargo that overlaps (30, 46, 48, 54). The capacity to carry cellular information makes EVs attractive as biomarkers, as they may be indicators of cellular or tissue dysfunction or disease. In addition, the potential to transfer these molecules to target cells makes them also of interest due to the ability to affect cellular function (67, 81). Cell-cell communication via EVs can be utilized both locally to neighboring cells and also distally to various tissues and organs. This makes EVs of important interest for various diseases, including metabolic diseases like type 2 diabetes, that have systemic effects with multiple end-organ complications.
EVs have been reported to also play cell-intrinsic roles. These include trafficking and removal of cellular material. Although in many cases this may be the normal recycling/removal of cellular material, it is thought that in some cases EVs offer a way for cells to eliminate cellular waste. Evidence for this includes the discovery that EVs contain pathogenic proteins that are higher in individuals with Alzheimer’s disease (24). In many of these examples, it is still unknown whether these EVs are simply carriers of cellular waste or propagators of disease. It is has been well established in the cancer field that EVs promote cancer progression by facilitating cross talk between tumor cells and the surrounding stromal environment, tumor vasculature, and immune systems (43, 60, 97).
The ability of EVs to elicit functional changes in target cells has sparked immense interest. We are only beginning to understand whether some of the reported functions are cell type specific or cell context specific. Nevertheless, EVs were initially described as important mediators in eliciting immune responses, but now their functions have been broadened to include, but are not limited to, responding to and eliciting both pro- and anti-inflammatory processes, tissue repair, angiogenesis, developmental processes, and stem cell differentiation (7, 91). EVs have been described to play roles in a plethora of conditions and diseases spanning from pregnancy to cancer, which have been extensively reviewed elsewhere (6, 37, 43, 80, 91). EVs are coated with surface receptor proteins that interact with the extracellular matrix and the cell surface. The repertoire of receptors coating the EVs to an extent is determined by the cell of origin (34). EVs are thought to elicit functional effects through either binding through these protein-protein-mediated interactions and initiating signaling cascades within the target cell or through uptake or endocytosis by the target cell. It appears that the mechanism of uptake may be cell type dependent, and a variety of paths have been described, including both clathrin-mediated and non-clathrin-mediated mechanisms (reviewed in Refs. 1 and 27). Given their role as intercellular communicators, there has been recent interest in EVs in the context of diabetes mellitus. Other reviews have focused on the role of EVs in obesity (45), complications from diabetes (96), metabolic syndrome (38, 53), cardiovascular risk and diabetes (62), and therapeutic potential in type 2 diabetes (88).
EVMPs AND DIABETES MELLITUS
Once it was determined that EVs serve as communicators of disease-specific messages, several groups identified a role for EVs in diabetes mellitus. Early studies examining the role of EVs in diabetes focused on larger EVs that are isolated from plasma and then phenotyped using cell-specific antibodies via flow cytometry. Given the limitations of traditional flow cytometry, these investigators were isolating larger EVs, which they referred to as microparticles, or clusters of EVs. Many of these original papers did not indicate size parameters used for gating strategies or whether reference beads were used as size controls. However, some studies did indicate size as an upward parameter of detection that was typically between 1 μm and 2 μm. Here, we will keep this original terminology but abbreviate as EVMP, which is consistent with recent publications referring to this specific population of EVs (53).
Abrams et al. (2) combined platelet-specific markers and flow cytometry to enable the identification of platelet-derived microparticles (PMP; EVPMP) from human blood. Several studies showed that EVPMPs were higher in diabetic individuals compared with controls (56, 57, 72). In these studies, treatment of diabetic individuals with anti-platelet drugs cilostazol (n = 19) or sarpogrelate hydrochloride (n = 19) significantly reduced the number of circulating EVPMPs when compared with before treatment (57, 72). Sabatier et al. (69) compared EVMPs derived from endothelial cells (EMPs; EVEMP) with EVPMPs and also used total annexin V-positive blood cell microparticles (total MPs; TMPs; EVTMP). This study also compared control individuals to those with either type 1 (n = 24) or type 2 (n = 52) diabetes (69). If combining both the type 1 and type2 DM, significantly higher levels of EVEMPs, EVTMP, and procoagulant TMP activity were observed (69). However, if the data are broken down to compare T2DM individuals with their appropriate age-matched controls, only EVTMPs were found to be significantly higher. These initial studies indicated that levels of larger EVMPs may be elevated in individuals with diabetes mellitus.
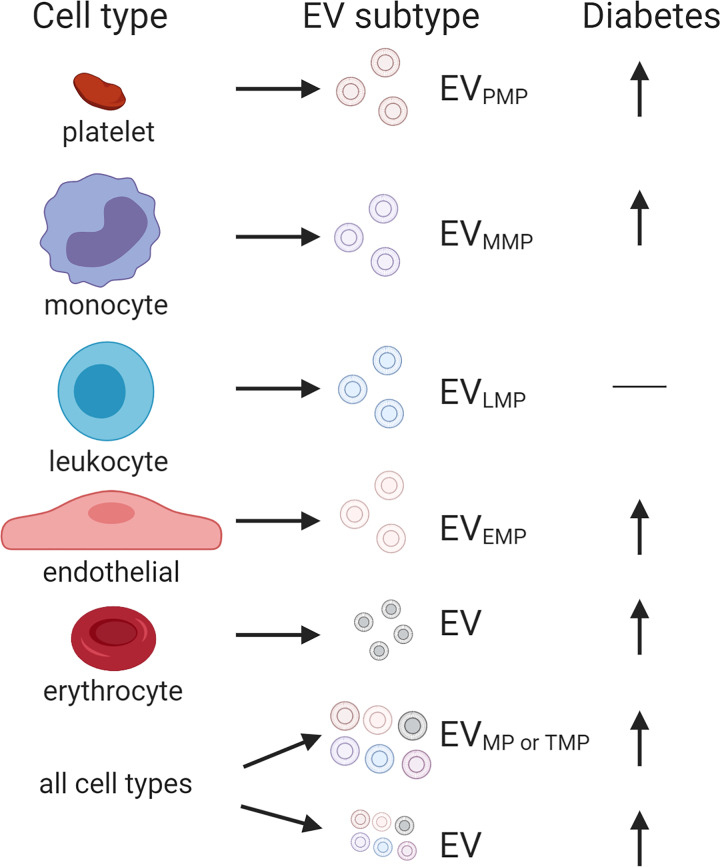
Indeed, after this initial work, there have been many studies that have examined EVMPs in the context of diabetes mellitus (Fig. 3). A recent meta-analysis of studies conducted through 2015 included 48 studies for systemic review, and this number was then parsed to 34 for quantitative study for the meta-analysis (52). Using data from the 34 studies, the authors found that circulating EVTMP, EVPMPs, monocyte-derived microparticles (MMPs; EVMMPs), and EVEMPs were significantly higher in individuals with type 2 diabetes mellitus compared with controls (Fig. 3) (52). Leukocyte-derived MPs (LMPs; EVLMPs) were not significantly different between T2DM patients and controls. It should be noted that for EVLMPs, only four studies met their criteria for inclusion (n = 128 T2DM; n = 148 controls), which is substantially less than the studies that have examined other EVMP subtypes. Perhaps more studies are warranted to establish whether there are differences in EVLMPs in diabetic individuals compared with controls. It is also important to note that most studies examined (18/34, so 53%) were from one group in Japan with Japanese study participants, highlighting the need for studies of ethnically and racially diverse populations. In addition, it will be interesting to examine in future studies whether EVs derived from other cell types are also altered with diabetes mellitus. Nevertheless, this meta-analysis shows that levels of larger EVMP are higher in individuals with T2DM (Fig. 3). This accumulating evidence indicates that the levels of circulating larger EVMPs could be used as a biomarker for T2DM. Specifically, total EVMPs and also EVMPs derived from monocytes, platelets, and endothelial cells show promise as biomarkers. It should be noted that recent advances in high-sensitivity flow cytometry have enabled detection of single EVs down to 40 nm (78), which will enable future in-depth characterization of subtypes of smaller EVs in diabetes mellitus.
Fig. 3.
Differences in cell type-derived extracellular vesicles (EVs) with diabetes mellitus. Schematic representation of cell-specific EVs that have been examined in the circulation of individuals with or without type 2 diabetes mellitus. Microparticles (MP) derived from the following cell types are abbreviated as follows: platelet-derived EVPMPs; monocyte-derived EVMMP; endothelial cells EVEMP; total annexin V-positive EVs (total MPs; EVMP or EVTMP). EVs denote EVs isolated using ultracentrifugation or precipitation methods. The arrow indicates that the levels of most EV types increase with diabetes mellitus.
EVs AND DIABETES MELLITUS
Most of the initial work focused on characterizing large circulating EVMPs used flow cytometry. Recently, the field has focused on characterizing smaller EVs that can also be isolated from body fluids (most notably plasma/serum for diabetes studies). Here the EVs range in size from ∼30–400 nm. Using common isolation techniques, including precipitation methods and ultracentrifugation, a typical size distribution of EVs isolated from plasma/serum are within this size range with an approximate peak ∼150 nm, as measured using nanoparticle tracking analysis (NTA). Electron microscopy images show similar size distributions of these EVs, although there are documented size variations between NTA and electron microscopy (18).
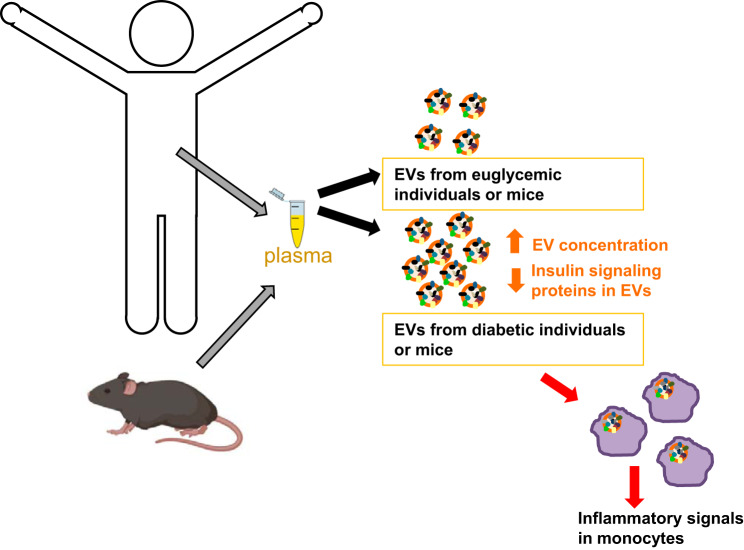
Using these EV isolation techniques, we recently reported that individuals with diabetes mellitus had significantly higher levels of circulating EVs compared with euglycemic controls (Fig. 3 and 4) (26). To determine whether the development of diabetes mellitus over a 5-yr time period affected EV concentration, we analyzed a longitudinal cohort of individuals that were euglycemic or prediabetic and developed diabetes mellitus. Individuals that developed diabetes had elevated levels of EVs compared with euglycemic controls (26). In a cell marker-specific assay using intact EVs, it was found that erythrocyte-derived EVs were significantly higher in individuals with diabetes mellitus compared with euglycemic controls (Fig. 3) (26). There was a trend toward higher levels of leukocyte- and platelet-derived EVs in individuals with diabetes, but the differences were not significant. Future studies lie in further defining cell type-specific differences in EVs in diabetes mellitus. This is an area of emerging interest but has proven to be difficult to address in large cohorts. These issues include but are not limited to assaying EVs from limited amounts of serum/plasma and technical issues, including the high viscosity of serum/plasma, the small size of EVs, and contaminants, including lipoproteins, that potentially coprecipitate during EV isolation (77). Furthermore, one of the most important limitations is the heterogeneity of EVs both within and between cell types. This heterogeneity presents a challenge to identify the right markers to monitor and to accurately quantify a cell-specific EV population. Therefore, additional work and reproducibility in larger cohorts is needed to consider EV levels as biomarkers for diabetes mellitus. However, these challenges do not diminish the potential for EVs to be an informative biomarker of this disease.
Fig. 4.
Extracellular vesicles (EVs) in diabetes mellitus. Higher levels of plasma EVs are found in individuals with diabetes compared with euglycemic controls. These EVs contain cargo that can induce inflammatory signals in monocytes.
FACTORS INFLUENCING EVs IN DIABETES MELLITUS TYPE 2
Insulin Resistance
EVs in the context of diabetes mellitus may be altered due to several mechanisms. In humans, insulin resistance can be measured quantitatively through homeostatic model assessment (HOMA) of insulin resistance (HOMA-IR). We found that HOMA-IR was significantly associated with EV concentration (26). In agreement with this finding, the number of EVs isolated from omental adipose tissue (OAT-EVs)-derived adipose tissue explants ex vivo also correlated positively with HOMA-IR (50). This data is also strengthened by our finding that insulin resistance in vitro increases EV secretion (26). We also found that EV concentration was also positively associated with HOMA of β-cell function, indicating that EVs may also be indicators of β-cell function (26). Exposure of endothelial cells in vitro to high glucose can also alter EVEMP content and induce changes in cellular function (reviewed in Ref. 53). In addition, EVs from pancreatic cancer cells and human adipose tissue explants can alter insulin signaling in muscle and liver cells (51, 83). Therefore, one mechanism for higher EV concentration in type 2 diabetes may be through insulin resistance. It should also be noted that in vitro studies indicate that EVs may also impair insulin signaling through EV-associated calpain 2 cleavage of the insulin receptor in hepatocellular carcinoma cells (92). Thus, EVs in some cases may also modulate insulin signaling.
Body Composition and Inflammation
Obesity has also been shown to influence EV characteristics (45). For example, body mass index (BMI) correlates with EV concentration in pregnant women (20) and in an aging cohort (19). Furthermore, significantly higher levels of EVMP [measured EV subtypes included TMP, PMP, P-selection+, EMP, LMP, and tissue factor (TF)+] were found in obese individuals compared with normal weight controls (9). In addition, procoagulant activity measured as clotting time of the EVMPs was significantly shorter for obese individuals compared with controls, indicating a higher procoagulant activity of EVs from obese individuals (9). EVMP, EVPMP and EVEMP were all elevated with obesity in a cohort of women with or without metabolic syndrome (74). After a 1-yr follow-up period, the obese individuals who lost weight through diet/exercise or through bariatric surgery had no significant change in either EVPMP or EVEMP levels compared with baseline levels (74). This contrasts with a previous report that showed that weight loss reduced EVPMP levels (55). These conflicting results may be due to differences in the initial BMI classifications (BMI = 41, morbid obesity; and BMI = 27, overweight) or their race (Japanese vs. European) or the presence of metabolic syndrome. Perhaps it is possible to conclude that levels of some types of EVMPs are elevated with obesity; however, future studies are warranted to examine the effects of different BMI categories on EVMPs and the influence of weight reduction on plasma EVMPs levels.
Recent data also suggests that the content of EVs may be altered with obesity. For example, EVs derived from adipose tissue taken from obese and lean individuals contained different microRNAs (miRNAs) (23). Subsequently, this group isolated circulating adipose-derived EVs using antibodies against fatty acid binding protein 4 (FABP4). They found that African American females pre- and post-bariatric surgery had different miRNA profiles from adipose-derived EVs (35). miRNAs are small noncoding RNAs that regulate gene expression through binding and targeting specific mRNAs for degradation, translational repression, or, in some cases, stabilization (4). Because one miRNA can target a plethora of mRNAs, pathway analysis can be performed on the mRNAs known or predicted to be targeted by the miRNA. In this study, the differentially regulated miRNAs targeted mRNAs involved in a number of cellular signaling pathways, but most importantly, the insulin signaling pathway. Furthermore, miRNA levels correlated with changes in HOMA (35). Although not the focus of this review, changes in EVs and miRNAs comparing obese and lean mice have been described (10, 45). Most notably, adipose tissue macrophage-derived exosomes (termed ATM-EXOs) isolated from obese mice confer insulin resistance and glucose intolerance when injected in lean mice (92). Furthermore, improvement of insulin resistance was observed when ATM-EXOs from lean mice were injected into obese mice (92). This study also points to miRNA content, specifically miR-155, as being important for conveying these signals. Another study observed similar effects on insulin resistance and glucose tolerance in mice using plasma EVs. This study found several miRNAs that may mediate these effects, including miR-122, miR-192, miR-27a-3p, and miR-27b-3p (10). Similar results were also obtained when adipose-derived stem cell EVs were injected into obese mice, although in this case it was thought to be protein mediated through EV-associated STAT3 (94). These data suggest that the miRNA and protein content in EVs may relay adipose tissue signals when injected in mice and indicate that these strategies may hold promise for future therapies to combat obesity.
The chronic low-grade systemic inflammation associated with obesity may be maintained or promulgated through EVs that may also convey systemic signals through modulating inflammatory signals. Deng et al. (16) isolated EVs from adipose tissue of leptin-deficient ob/ob mice and then injected these EVs into C57BL/6j wild-type male mice fed a high-fat diet for 3 mo. EVs obtained from the ob/ob mice induced circulating levels of TNFα and IL-6 and increased monocyte activation compared with EVs from wild-type mice (Fig. 4) (16). EVs from ob/ob mice also increased macrophage tissue infiltration and impaired insulin signaling (16). Similar results examining differences in EVs isolated from obese and lean mice have also been described (53, 92).
Although these studies were conducted in mice, it led to further investigation as focused on whether EVs may mediate inflammatory signals in humans with diabetes mellitus. Consistent with this idea, treatment of human monocytes with EVs from diabetic individuals induced inflammatory signals compared with monocytes treated with EVs from euglycemic individuals (Fig. 4) (26). EVs isolated from the circulation of pregnant women induced IL-6, IL-8, and TNFα levels in endothelial cells, and this effect was more pronounced in EVs from obese women compared with overweight or lean women (20). In addition, EVs from adipose tissue contain inflammatory cytokines, in particular those termed adipokines, and induce differentiation and secretion of inflammatory cytokines in monocytes (50). Cytokines have been found to be encapsulated in EVs, but this association varies with different cell types and context (25).
Intriguingly, both in mouse circulation and in freshly isolated human peripheral blood mononuclear cells (PBMCs), monocytes are the primary cell type that internalizes circulating EVs (16, 19, 26). However, B cells are also a cell type that internalizes circulating EVs to a lesser extent, and both B cells and monocytes have been shown to preferentially internalize EVs from individuals with diabetes mellitus (26). These data point to EVs as being important mediators of inflammation in diabetes mellitus but also suggest that more studies should be conducted to confirm this effect.
Diet, Drugs, and Exercise
Data in humans suggest that EV levels and subtypes can be modulated by drugs, diet, and also exercise. The oral hypoglycemic agent pioglitazone hydrochloride reduced EVEMP levels compared with metformin for newly diagnosed diabetic patients (21). Diabetic individuals treated with antiplatelet therapies decreased EVPMP levels (57, 72). Consumption of a diet rich in oats reduced fibrinogen-positive EVPMP and CD11b-positive monocyte microparticles (EVMMP) (93).
In addition, higher EV concentration was observed in skeletal muscle explants from C57Bl/6 mice fed a standard diet enriched with 20% palm oil (HP) compared with skeletal muscle from control standard diet-fed mice (3). Treatment of muscle cells in vitro with these skeletal muscle-derived EVs from HP-fed mice reduced markers of muscle cell differentiation and induced cell proliferation. Data from this model indicated that damaging lipids can be transferred via EVs (3). Furthermore, EVs derived from skeletal muscle can be taken up by the pancreas and can affect β-cell function in islet explants (36). Exercise, which is well known to provide health benefits, induces EV secretion, alters proteomic content, and may also help mediate the beneficial effects of exercise systemically (28, 85). These data indicate that the pool of circulating EVs may be altered in both a beneficial and detrimental manner, depending on the physiological and pathophysiological context.
EV PROTEIN CARGO IN T2DM
In addition to miRNAs, EVs contain other cargo, including cytosolic and membrane-bound proteins (Fig. 2). Few studies have examined EV protein cargo in the context of human diabetes mellitus. Insulin-signaling proteins, including phospho-p70S6K, phospho-S6RP, phospho-GSK3β, phospho-Akt, phospho-insulin receptor (IR), phospho-IRS1 (Ser312), tyr-phospho-IRS1, phospho-IGF-1R, leptin receptor, and FGF21, are present in circulating EVs, and the levels of these proteins are influenced by diabetes mellitus (26). Specifically, EV levels of leptin receptor and phospho-IR were decreased in individuals with diabetes mellitus. In addition, several insulin-signaling proteins are also associated with HOMA-B or HOMA-IR levels, indicating that circulating EVs may be indicators of cellular β-cell function or insulin resistance (26).
In the SMART study, investigators analyzed EVs in the context of metabolic complications in a cohort of patients with cardiovascular disease (49). They particularly focused on four different cardiovascular disease (CVD)-associated EV markers. The development of diabetes was found be associated with EV protein levels of CD14. EV-associated serpin G1 was associated with the presence of type 2 diabetes at study baseline (49). Higher EV-cystatin C levels were associated with elevated high-sensitivity C-reactive protein (hsCRP) and lower HDL cholesterol. Higher EV cystatin C levels were associated with a higher prevalence of metabolic syndrome. High EV-CD14 levels connoted a 16% lower risk for the development of type 2 diabetes (49).
In addition, recent evidence pointed to endothelial cell-derived (CD31+) EVs isolated from serum as having a role in apoptosis resistance in vascular smooth muscle cells (VSMCs) (79). It was shown that these effects on VSMCs are due to platelet-derived growth factor-BB (PDGF-BB) expression on the CD31+EVs Therefore, this study identifies a mechanism by which EVs from diabetic individuals may contribute to vascular dysfunction (79).
These studies highlight that insulin-signaling proteins, CVD-associated proteins, and growth factors present on plasma EVs may mediate intercellular communication between tissues in diabetes mellitus. Examining EV protein cargo from human biofluids in diabetes has been limited by the small sample volumes and the need for more material for robust proteomics. As technologies advance, especially related to proteomics and high-sensitivity flow cytometry, more in-depth characterization of EV protein cargo will allow us to determine whether protein cargo can be utilized as biomarkers of diabetes mellitus and its complications.
URINARY EVs AS BIOMARKERS FOR DIABETIC NEPHROPATHY
EVs can also be isolated from urine. Recent research has focused on identifying whether urinary EVs (uEVs) and their associated cargo can be utilized as biomarkers of diabetes-associated diabetic nephropathy (DN). This debilitating complication of diabetes mellitus is a common form of chronic kidney disease and can lead to end-stage renal disease requiring dialysis or renal transplantation. Many researchers are pursuing avenues that might lead to identifying noninvasive biomarkers of early stage DN and indicators of progressive renal dysfunction, which may help in delaying or preventing the progression of renal disease. Isolation of EVs from urine has its own challenges due to the large amounts of proteins in urine (71, 82). Therefore, specific methodological considerations should be taken to avoid contaminants (71, 82).
There are very few studies that have focused on analyzing uEVs in the context of human type 2 diabetic nephropathy. Of these studies, the majority have focused on identifying uEV-miRNA profiles that differ between controls, type 2 diabetics, and type 2 diabetics with nephropathy (15, 39, 64, 89). Differential levels of many EV-associated miRNAs were found comparing diabetics with or without nephropathy. However, very few miRNAs overlap between the studies. miR-15a-5p and miR-15b-5p, two members of the highly conserved miR-15 family (84), were found to be lower in diabetic individuals with renal disease in two different studies (64, 89). Two additional studies have reported that uEV levels of miR-192 were higher in diabetics individuals with renal disease compared with individuals with diabetes (14, 39). In the study by De et al. (14), higher concentrations of uEVs were found in diabetics with renal disease compared with diabetic individuals. uEV concentration was also higher in diabetic individuals compared with controls, which is similar to what is observed in plasma. uEV concentration was also negatively correlated with estimated glomular filtration rate (eGFR) (14). This contrasts with another report that found that uEV concentration was lower in diabetics with renal failure compared with those with normal renal function (42). Similar to De et al. (14), this study found a negative correlation eGFR (42). In addition to miRNAs, mRNAs have also been assessed. Higher levels of the mRNA encoding uromodulin (UMOD) were reported in uEVs from individuals with diabetic nephropathy compared with diabetic individuals with normal renal function (90).
Protein cargo may also be different in uEVs from individuals with diabetic nephropathy. Higher levels of the glycoprotein C-megalin in uEVs increase with the progression of diabetic nephropathy (14, 39). C-megalin is an endocytic receptor that plays a role in the uptake of potentially pathogenic substances into proximal tubule epithelial cells. In cultured immortalized rat proximal tubule cells and a mouse model of kidney injury, these authors found that kidney injury increased uEV secretion and C-megalin levels, suggesting that C-megalin in uEVs may be a promising biomarker for the progression of diabetic nephropathy. Elf3 has also been implicated as a marker of kidney injury and was reported to be expressed on uEVs from patients with diabetic nephropathy but not on uEVs from controls or individuals with minimal change nephrotic syndrome (70). Other proteins in human uEVs have also been implicated to differentiate between those diabetic individuals with renal disease, including CD133 (17) and mixed lineage leukemia 3 (MLL3), α1-microglobulin/bikunin precursor (AMBP), and voltage-dependent anion channel 1 (VDAC1) (98).
There is certainly promise in the utilization of uEVs for biomarkers of diabetic nephropathy. The ability to monitor the progression of renal dysfunction holds enormous promise, yet there remain significant challenges at this point, including the standardization of methods for isolation, the reproducibility between studies, and the need for repetition in larger-cohort studies.
EVs AS THERAPEUTICS FOR DIABETES MELLITUS
Although many studies point to EVs as mediators of the negative consequences of diabetes mellitus, EVs have also been shown to induce beneficial effects. Recent attention has focused on utilizing EVs for therapeutics or delivery vehicles for therapeutics due to their low immunogenicity, stability in the circulation, ability to infiltrate tissues, and their native lipid bilayer which allows for fusion with the plasma membrane and delivery of specific cargo (59). For example, EVs from adipose-derived stem cells can induce anti-inflammatory cytokine production in macrophages that helps to improve metabolic homeostasis in mice (94). These and other studies suggest that EVs, especially those derived from stem cells, may have potential to improve glucose tolerance and insulin sensitivity in diabetic individuals. Studies in animal models have used EVs for the treatment of diabetes mellitus and complications from diabetes, including diabetic wounds, erectile dysfunction, diabetic nephropathy, diabetic cardiomyopathy, and cognitive impairment (reviewed in Ref. 88). Therefore, there is immense interest in utilizing EVs for therapeutics for diabetes and its complications, but more extensive research is needed before EVs can be used clinically for treatment.
CONCLUSIONS/FUTURE PROSPECTS
There is growing interest in utilizing EVs as diagnostic and prognostic factors in many different diseases, including type 2 diabetes. Accumulating evidence indicates that EVs play important roles in insulin signaling and resistance and also in the pathogenesis and complications of diabetes. However, there remain challenges that must be overcome before exploiting EVs therapeutically in the clinic. EV-based clinical studies should be further characterized in diverse cohorts, taking into consideration age, sex, and race. More in-depth characterization of EV cargo is needed and should be replicated in additional studies. This point is in fact hindered by technical issues in profiling proteins, lipids, and nucleic acids from the small amounts of starting material in biofluids collected from most human cohorts. Other technical challenges facing the field include interpreting results from different isolation procedures (here comparing FACS studies with other EV preparations) and identifying cell type-specific smaller EV populations. Another challenge is lipoprotein contamination in EV preparations from plasma. Many isolation techniques also coprecipitate lipoproteins, and enumeration of EVs may also overestimate particle numbers by also counting coprecipitated lipoproteins (73, 77). Therefore, technological developments for both enumeration and purification would be beneficial to the field.
Although not discussed in depth in this review, EVs are also being purposed as therapeutic agents to deliver specific cargo or drugs to treat disease. This avenue may be exploited for the therapeutic treatment of type 2 diabetes, but at this stage the data are far too scarce to make this transition. However, promising data have shown that EVs isolated from human mesenchymal stem cells improve insulin sensitivity and glucose levels in diabetic rats (75). In fact, several animal models have shown beneficial effects of EVs as therapies for type 2 diabetes and its associated complications (88).
In summary, existing data suggest an important role for EVs in diabetes mellitus. Future work lies in further characterization of how EVs mediate cross talk between organ systems in diabetes. Gaining additional information has the potential to guide biomarker development, new interventions, and therapeutic strategies for the treatment and management of diabetes.
GRANTS
This study was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Project AG-000989.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.N.H. prepared figures; N.N.H. drafted manuscript; N.N.H. and M.K.E. edited and revised manuscript; N.N.H. and M.K.E. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jennifer F. O’Connell for critical reading of the manuscript. Some figures were created using BioRender.com.
REFERENCES
- 1.Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36: 301–312, 2016. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams CS, Ellison N, Budzynski AZ, Shattil SJ. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood 75: 128–138, 1990. doi: 10.1182/blood.V75.1.128.128. [DOI] [PubMed] [Google Scholar]
- 3.Aswad H, Forterre A, Wiklander OPB, Vial G, Danty-Berger E, Jalabert A, Lamazière A, Meugnier E, Pesenti S, Ott C, Chikh K, El-Andaloussi S, Vidal H, Lefai E, Rieusset J, Rome S. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 57: 2155–2164, 2014. doi: 10.1007/s00125-014-3337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman RN, Piccinini F, Kabir M, Kolka CM, Ader M. Hypothesis: role of reduced hepatic insulin clearance in the pathogenesis of type 2 diabetes. Diabetes 68: 1709–1716, 2019. [Erratum in Diabetes 68: 2350, 2019.] doi: 10.2337/db19-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 14: 259–272, 2017. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 7.Buzas EI, György B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol 10: 356–364, 2014. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 8.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol 17: 879–887, 2005. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 9.Campello E, Zabeo E, Radu CM, Spiezia L, Gavasso S, Fadin M, Woodhams B, Vettor R, Simioni P. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thromb Haemost 113: 85–96, 2015. doi: 10.1160/TH14-02-0156. [DOI] [PubMed] [Google Scholar]
- 10.Castaño C, Kalko S, Novials A, Párrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci USA 115: 12158–12163, 2018. doi: 10.1073/pnas.1808855115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Centers for Disease Control and Prevention National Diabetes Statistics Report (Online). https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
- 11.Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem 166: 189–197, 1946. [PubMed] [Google Scholar]
- 12.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 13.D’Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care 34, Suppl 2: S161–S165, 2011. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De S, Kuwahara S, Hosojima M, Ishikawa T, Kaseda R, Sarkar P, Yoshioka Y, Kabasawa H, Iida T, Goto S, Toba K, Higuchi Y, Suzuki Y, Hara M, Kurosawa H, Narita I, Hirayama Y, Ochiya T, Saito A. Exocytosis-mediated urinary full-length megalin excretion is linked with the pathogenesis of diabetic nephropathy. Diabetes 66: 1391–1404, 2017. doi: 10.2337/db16-1031. [DOI] [PubMed] [Google Scholar]
- 15.Delić D, Eisele C, Schmid R, Baum P, Wiech F, Gerl M, Zimdahl H, Pullen SS, Urquhart R. Urinary exosomal miRNA signature in type II diabetic nephropathy patients. PLoS One 11: e0150154, 2016. doi: 10.1371/journal.pone.0150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, Wang J, Xiang X, Zhang S, Zhuang X, Shah SV, Sun D, Michalek S, Grizzle WE, Garvey T, Mobley J, Zhang HG. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 58: 2498–2505, 2009. doi: 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimuccio V, Peruzzi L, Brizzi MF, Cocchi E, Fop F, Boido A, Gili M, Gallo S, Biancone L, Camussi G, Bussolati B. Acute and chronic glomerular damage is associated with reduced CD133 expression in urinary extracellular vesicles. Am J Physiol Renal Physiol 318: F486–F495, 2020. doi: 10.1152/ajprenal.00404.02019. [DOI] [PubMed] [Google Scholar]
- 18.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJP, Hole P, Carr B, Redman CWG, Harris AL, Dobson PJ, Harrison P, Sargent IL. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine (Lond) 7: 780–788, 2011. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eitan E, Green J, Bodogai M, Mode NA, Bæk R, Jørgensen MM, Freeman DW, Witwer KW, Zonderman AB, Biragyn A, Mattson MP, Noren Hooten N, Evans MK. Age-related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci Rep 7: 1342, 2017. doi: 10.1038/s41598-017-01386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elfeky O, Longo S, Lai A, Rice GE, Salomon C. Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta 50: 60–69, 2017. doi: 10.1016/j.placenta.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Esposito K, Maiorino MI, Di Palo C, Gicchino M, Petrizzo M, Bellastella G, Saccomanno F, Giugliano D. Effects of pioglitazone versus metformin on circulating endothelial microparticles and progenitor cells in patients with newly diagnosed type 2 diabetes–a randomized controlled trial. Diabetes Obes Metab 13: 439–445, 2011. doi: 10.1111/j.1463-1326.2011.01367.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrante SC, Nadler EP, Pillai DK, Hubal MJ, Wang Z, Wang JM, Gordish-Dressman H, Koeck E, Sevilla S, Wiles AA, Freishtat RJ. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res 77: 447–454, 2015. doi: 10.1038/pr.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement 11: 600–607.e1, 2015. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L. A system of cytokines encapsulated in extracellular vesicles. Sci Rep 8: 8973, 2018. doi: 10.1038/s41598-018-27190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman DW, Noren Hooten N, Eitan E, Green J, Mode NA, Bodogai M, Zhang Y, Lehrmann E, Zonderman AB, Biragyn A, Egan J, Becker KG, Mattson MP, Ejiogu N, Evans MK. Altered extracellular vesicle concentration, cargo and function in diabetes mellitus. Diabetes 67: 2377–2388, 2018. doi: 10.2337/db17-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.French KC, Antonyak MA, Cerione RA. Extracellular vesicle docking at the cellular port: extracellular vesicle binding and uptake. Semin Cell Dev Biol 67: 48–55, 2017. doi: 10.1016/j.semcdb.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frühbeis C, Helmig S, Tug S, Simon P, Krämer-Albers EM. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles 4: 28239, 2015. doi: 10.3402/jev.v4.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio Metabolism Study. Diabetes 66: 815–822, 2017. doi: 10.2337/db16-1167. [DOI] [PubMed] [Google Scholar]
- 30.Greening DW, Xu R, Gopal SK, Rai A, Simpson RJ. Proteomic insights into extracellular vesicle biology—defining exosomes and shed microvesicles. Expert Rev Proteomics 14: 69–95, 2017. doi: 10.1080/14789450.2017.1260450. [DOI] [PubMed] [Google Scholar]
- 31.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 370: 1514–1523, 2014. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 32.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68: 2667–2688, 2011. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97: 329–339, 1983. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335, 2015. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubal MJ, Nadler EP, Ferrante SC, Barberio MD, Suh J-H, Wang J, Dohm GL, Pories WJ, Mietus-Snyder M, Freishtat RJ. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring) 25: 102–110, 2017. doi: 10.1002/oby.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.International Diabetes Federation IDF Diabetes Atlas, 9th ed http://www.diabetesatlas.org [March 2020].
- 36.Jalabert A, Vial G, Guay C, Wiklander OPB, Nordin JZ, Aswad H, Forterre A, Meugnier E, Pesenti S, Regazzi R, Danty-Berger E, Ducreux S, Vidal H, El-Andaloussi S, Rieusset J, Rome S. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia 59: 1049–1058, 2016. doi: 10.1007/s00125-016-3882-y. [DOI] [PubMed] [Google Scholar]
- 37.Janas AM, Sapoń K, Janas T, Stowell MH, Janas T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim Biophys Acta 1858: 1139–1151, 2016. doi: 10.1016/j.bbamem.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Javeed N. Shedding perspective on extracellular vesicle biology in diabetes and associated metabolic syndromes. Endocrinology 160: 399–408, 2019. doi: 10.1210/en.2018-01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia Y, Guan M, Zheng Z, Zhang Q, Tang C, Xu W, Xiao Z, Wang L, Xue Y. miRNAs in urine extracellular vesicles as predictors of early-stage diabetic nephropathy. J Diabetes Res 2016: 1–10, 2016. doi: 10.1155/2016/7932765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262: 9412–9420, 1987. [PubMed] [Google Scholar]
- 41.Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 289: 3869–3875, 2014. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamińska A, Platt M, Kasprzyk J, Kuśnierz-Cabala B, Gala-Błądzińska A, Woźnicka O, Jany BR, Krok F, Piekoszewski W, Kuźniewski M, Stępień EŁ. Urinary extracellular vesicles: potential biomarkers of renal function in diabetic patients. J Diabetes Res 2016: 1–12, 2016. doi: 10.1155/2016/5741518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanada M, Bachmann MH, Contag CH. Signaling by extracellular vesicles advances cancer hallmarks. Trends Cancer 2: 84–94, 2016. doi: 10.1016/j.trecan.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med 9: 86, 2011. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim A, Shah AS, Nakamura T. Extracellular vesicles: a potential novel regulator of obesity and its associated complications. Children (Basel) 5: 152, 2018. doi: 10.3390/children5110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA 8: e1413, 2017. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koch L, Wunderlich FT, Seibler J, Könner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Brüning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest 118: 2132–2147, 2008. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113: E968–E977, 2016. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kranendonk ME, de Kleijn DP, Kalkhoven E, Kanhai DA, Uiterwaal CS, van der Graaf Y, Pasterkamp G, Visseren FL; SMART Study Group . Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc Diabetol 13: 37, 2014. doi: 10.1186/1475-2840-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kranendonk ME, Visseren FL, van Balkom BW, Nolte-’t Hoen EN, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MH, Kalkhoven E. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring) 22: 1296–1308, 2014. doi: 10.1002/oby.20679. [DOI] [PubMed] [Google Scholar]
- 51.Kranendonk MEG, Visseren FLJ, van Herwaarden JA, Nolte-’t Hoen ENM, de Jager W, Wauben MHM, Kalkhoven E. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring) 22: 2216–2223, 2014. doi: 10.1002/oby.20847. [DOI] [PubMed] [Google Scholar]
- 52.Li S, Wei J, Zhang C, Li X, Meng W, Mo X, Zhang Q, Liu Q, Ren K, Du R, Tian H, Li J. Cell-derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell Physiol Biochem 39: 2439–2450, 2016. doi: 10.1159/000452512. [DOI] [PubMed] [Google Scholar]
- 53.Martínez MC, Andriantsitohaina R. Extracellular vesicles in metabolic syndrome. Circ Res 120: 1674–1686, 2017. doi: 10.1161/CIRCRESAHA.117.309419. [DOI] [PubMed] [Google Scholar]
- 54.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21: 9–17, 2019. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 55.Murakami T, Horigome H, Tanaka K, Nakata Y, Ohkawara K, Katayama Y, Matsui A. Impact of weight reduction on production of platelet-derived microparticles and fibrinolytic parameters in obesity. Thromb Res 119: 45–53, 2007. doi: 10.1016/j.thromres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Nomura S, Shouzu A, Omoto S, Hayakawa T, Kagawa H, Nishikawa M, Inada M, Fujimura Y, Ikeda Y, Fukuhara S. Effect of cilostazol on soluble adhesion molecules and platelet-derived microparticles in patients with diabetes. Thromb Haemost 80: 388–392, 1998. doi: 10.1055/s-0037-1615217. [DOI] [PubMed] [Google Scholar]
- 57.Omoto S, Nomura S, Shouzu A, Hayakawa T, Shimizu H, Miyake Y, Yonemoto T, Nishikawa M, Fukuhara S, Inada M. Significance of platelet-derived microparticles and activated platelets in diabetic nephropathy. Nephron 81: 271–277, 1999. doi: 10.1159/000045292. [DOI] [PubMed] [Google Scholar]
- 58.Patel BM, Goyal RK. Liver and insulin resistance: new wine in old bottle!!! Eur J Pharmacol 862: 172657, 2019. doi: 10.1016/j.ejphar.2019.172657. [DOI] [PubMed] [Google Scholar]
- 59.Patel DB, Santoro M, Born LJ, Fisher JP, Jay SM. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv 36: 2051–2059, 2018. doi: 10.1016/j.biotechadv.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18: 883–891, 2012. [Erratum in Nat Med 22: 1502, 2016.] doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perakis S, Speicher MR. Emerging concepts in liquid biopsies. BMC Med 15: 75, 2017. doi: 10.1186/s12916-017-0840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pernomian L, Moreira JD, Gomes MS. In the view of endothelial microparticles: novel perspectives for diagnostic and pharmacological management of cardiovascular risk during diabetes distress. J Diabetes Res 2018: 1–7, 2018. doi: 10.1155/2018/9685205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373, 2004. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prabu P, Rome S, Sathishkumar C, Gastebois C, Meugnier E, Mohan V, Balasubramanyam M. MicroRNAs from urinary extracellular vesicles are non-invasive early biomarkers of diabetic nephropathy in type 2 diabetes patients with the ‘Asian Indian phenotype’. Diabetes Metab 45: 276–285, 2019. doi: 10.1016/j.diabet.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183: 1161–1172, 1996. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20: 847–856, 2006. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 68.Roh E, Song DK, Kim M-S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med 48: e216, 2016. doi: 10.1038/emm.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG, Arnoux D, Charpiot P, Freyssinet JM, Oliver C, Sampol J, Dignat-George F. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes 51: 2840–2845, 2002. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 70.Sakurai A, Ono H, Ochi A, Matsuura M, Yoshimoto S, Kishi S, Murakami T, Tominaga T, Nagai K, Abe H, Doi T. Involvement of Elf3 on Smad3 activation-dependent injuries in podocytes and excretion of urinary exosome in diabetic nephropathy. PLoS One 14: e0216788, 2019. doi: 10.1371/journal.pone.0216788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salih M, Zietse R, Hoorn EJ. Urinary extracellular vesicles and the kidney: biomarkers and beyond. Am J Physiol Renal Physiol 306: F1251–F1259, 2014. doi: 10.1152/ajprenal.00128.2014. [DOI] [PubMed] [Google Scholar]
- 72.Shouzu A, Nomura S, Hayakawa T, Omoto S, Shimizu H, Miyake Y, Yonemoto T, Fukuhara S, Iwasaka T, Nishikawa M, Inada M. Effect of sarpogrelate hydrochloride on platelet-derived microparticles and various soluble adhesion molecules in diabetes mellitus. Clin Appl Thromb Hemost 6: 139–143, 2000. doi: 10.1177/107602960000600304. [DOI] [PubMed] [Google Scholar]
- 73.Simonsen JB. What are we looking at? Extracellular vesicles, lipoproteins, or both? Circ Res 121: 920–922, 2017. doi: 10.1161/CIRCRESAHA.117.311767. [DOI] [PubMed] [Google Scholar]
- 74.Stepanian A, Bourguignat L, Hennou S, Coupaye M, Hajage D, Salomon L, Alessi M-C, Msika S, de Prost D. Microparticle increase in severe obesity: not related to metabolic syndrome and unchanged after massive weight loss. Obesity (Silver Spring) 21: 2236–2243, 2013. doi: 10.1002/oby.20365. [DOI] [PubMed] [Google Scholar]
- 75.Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Wu P, Shi Y, Mao F, Yan Y, Xu W, Qian H. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano 12: 7613–7628, 2018. doi: 10.1021/acsnano.7b07643. [DOI] [PubMed] [Google Scholar]
- 76.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 24: 766–769, 2014. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach J-M, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman MLD, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DRF, Caruso S, Chamley LW, Chang Y-T, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TAP, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DCI, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG-E, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ II, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers E-M, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee M-S, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li ITS, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SLN, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen ENM, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BCH, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IKH, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KMA, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PRM, Silva AM, Skowronek A, Snyder OL II, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BWM, van der Grein SG, Van Deun J, van Herwijnen MJC, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MHM, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian Y, Ma L, Gong M, Su G, Zhu S, Zhang W, Wang S, Li Z, Chen C, Li L, Wu L, Yan X. Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano 12: 671–680, 2018. doi: 10.1021/acsnano.7b07782. [DOI] [PubMed] [Google Scholar]
- 79.Togliatto G, Dentelli P, Rosso A, Lombardo G, Gili M, Gallo S, Gai C, Solini A, Camussi G, Brizzi MF. PDGF-BB carried by endothelial cell-derived extracellular vesicles reduces vascular smooth muscle cell apoptosis in diabetes. Diabetes 67: 704–716, 2018. doi: 10.2337/db17-0371. [DOI] [PubMed] [Google Scholar]
- 80.Turpin D, Truchetet ME, Faustin B, Augusto JF, Contin-Bordes C, Brisson A, Blanco P, Duffau P. Role of extracellular vesicles in autoimmune diseases. Autoimmun Rev 15: 174–183, 2016. doi: 10.1016/j.autrev.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 82.Wang D, Sun W. Urinary extracellular microvesicles: isolation methods and prospects for urinary proteome. Proteomics 14: 1922–1932, 2014. doi: 10.1002/pmic.201300371. [DOI] [PubMed] [Google Scholar]
- 83.Wang L, Zhang B, Zheng W, Kang M, Chen Q, Qin W, Li C, Zhang Y, Shao Y, Wu Y. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci Rep 7: 5384, 2017. doi: 10.1038/s41598-017-05541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang S, Zhu W, Xu J, Guo Y, Yan J, Meng L, Jiang C, Lu S. Interpreting the MicroRNA-15/107 family: interaction identification by combining network based and experiment supported approach. BMC Med Genet 20: 96, 2019. doi: 10.1186/s12881-019-0824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JFP, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27: 237–251.e4, 2018. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles 8: 1648167, 2019. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 13: 269–288, 1967. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 88.Xiao Y, Zheng L, Zou X, Wang J, Zhong J, Zhong T. Extracellular vesicles in type 2 diabetes mellitus: key roles in pathogenesis, complications, and therapy. J Extracell Vesicles 8: 1625677, 2019. doi: 10.1080/20013078.2019.1625677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie Y, Jia Y, Cuihua X, Hu F, Xue M, Xue Y. Urinary exosomal microRNA profiling in incipient type 2 diabetic kidney disease. J Diabetes Res 2017: 1–10, 2017. doi: 10.1155/2017/6978984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamamoto CM, Murakami T, Oakes ML, Mitsuhashi M, Kelly C, Henry RR, Sharma K. Uromodulin mRNA from urinary extracellular vesicles correlate to kidney function decline in type 2 diabetes mellitus. Am J Nephrol 47: 283–291, 2018. doi: 10.1159/000489129. [DOI] [PubMed] [Google Scholar]
- 91.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066, 2015. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 171: 372–384.e12, 2017. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 93.Zhang X, McGeoch SC, Megson IL, MacRury SM, Johnstone AM, Abraham P, Pearson DWM, de Roos B, Holtrop G, O’Kennedy N, Lobley GE. Oat-enriched diet reduces inflammatory status assessed by circulating cell-derived microparticle concentrations in type 2 diabetes. Mol Nutr Food Res 58: 1322–1332, 2014. doi: 10.1002/mnfr.201300820. [DOI] [PubMed] [Google Scholar]
- 94.Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 67: 235–247, 2018. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 95.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14: 88–98, 2018. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 96.Zhou F, Huang L, Qu SL, Chao R, Yang C, Jiang ZS, Zhang C. The emerging roles of extracellular vesicles in diabetes and diabetic complications. Clin Chim Acta 497: 130–136, 2019. doi: 10.1016/j.cca.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 97.Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SIJ, Wurdinger T, Pegtel DM, van Rheenen J. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161: 1046–1057, 2015. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zubiri I, Posada-Ayala M, Sanz-Maroto A, Calvo E, Martin-Lorenzo M, Gonzalez-Calero L, de la Cuesta F, Lopez JA, Fernandez-Fernandez B, Ortiz A, Vivanco F, Alvarez-Llamas G. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J Proteomics 96: 92–102, 2014. doi: 10.1016/j.jprot.2013.10.037. [DOI] [PubMed] [Google Scholar]