Abstract
The transport of electrolytes and fluid by the intestinal epithelium is critical in health to maintain appropriate levels of fluidity of the intestinal contents. The transport mechanisms that underlie this physiological process are also subject to derangement in various digestive disease states, such as diarrheal illnesses. This article summarizes the 2019 Hans Ussing Lecture of the Epithelial Transport Group of the American Physiological Society and discusses some pathways by which intestinal transport is dysregulated, particularly in the setting of infection with the diarrheal pathogen, Salmonella, and in patients treated with small-molecule inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor (EGFr-TKI). The burdensome diarrhea in patients infected with Salmonella may be attributable to decreased expression of the chloride-bicarbonate exchanger downregulated in adenoma (DRA) that participates in electroneutral NaCl absorption. This outcome is possibly secondary to increased epithelial proliferation and/or decreased epithelial differentiation that occurs following infection. Conversely, the diarrheal side effects of cancer treatment with EGFr-TKI may be related to the known ability of EGFr-associated signaling to reduce calcium-dependent chloride secretion. Overall, the findings described may suggest targets for therapeutic intervention in a variety of diarrheal disease states.
Keywords: chloride secretion, epidermal growth factor receptor, infectious diarrhea, sodium absorption, Ussing chamber
BACKGROUND
Dr. Hans H. Ussing (1911–2000), has been called the “founder of epithelial physiology” (27). Certainly, his classic paper describing an apparatus that could be used to study electrogenic ion transport across an epithelium (in his case, frog skin) was entirely groundbreaking and has spawned many hundreds, if not thousands, of papers that base their conclusions on the eponymous “Ussing chamber” (43). Ussing and his coworkers (42) supplied much of our early conceptual understanding of how ions and water are transported across biological membranes, including epithelia. Using radioactive isotopic tracers, he showed experimentally that a significant proportion of sodium transport was active in nature, dependent on membrane potential, and subject to regulation by hormones (19, 41). Perhaps surprisingly to many modern transport physiologists, these ideas were quite controversial at the time! He also defined the relative contributions of paracellular and transcellular transport pathways to overall epithelial transport, introduced the concepts of unidirectional fluxes, flux ratios, and solvent drag, and established the Na+ recirculation theory to explain isotonic secretion [the topic of his very last publication (26)].
Simultaneously elegant and precise, the Ussing chamber immediately fascinated me when I came to the University of California (UC) San Diego in 1985 to take up a position as a research faculty member in the laboratory of Dr. Kiertisin Dharmsathaphorn. Kiertisin, a gastroenterologist, was convinced that Ussing chambers could be adapted to study transport phenomena in intestinal epithelial cell lines, a highly original and ambitious goal for a young faculty member at that time. In fact, shortly before I joined his group, he succeeded in designing an Ussing chamber that could accommodate an epithelial monolayer grown on a home-made permeable support (10). The opportunity that this afforded to identify transport mechanisms that were integral to the epithelium itself, as well as associated signaling mechanisms, quickly became my passion, too, not least because of the immediate gratification one obtained from experiments of this type. Together with Kiertisin and other colleagues, I initially studied how immune mediators (and especially those released by mast cells) could activate chloride secretion across intestinal epithelial cell lines, a putative mechanism in inflammatory diarrhea (1–3, 45). We also addressed the relative roles of calcium and protein kinase C in regulating the overall process of chloride secretion (20, 21). Following Kiertisin’s untimely death in 1990, I inherited his custom chambers and used them for many years, until we had the opportunity to move into the modern age with a computer-driven system from Physiologic Instruments (http://www.physiologicinstruments.com/Default.asp, founded by a fellow Ussing chamber aficionado and former colleague at UC San Diego, Steve Thompson). Because the Ussing chamber has been so integral to the majority of the papers I have published in my career, it was certainly a special honor to be invited by the Epithelial Transport Group of the American Physiological Society to deliver the Hans Ussing lecture in 2019. This article provides a brief summary of my presentation.
INTRODUCTION
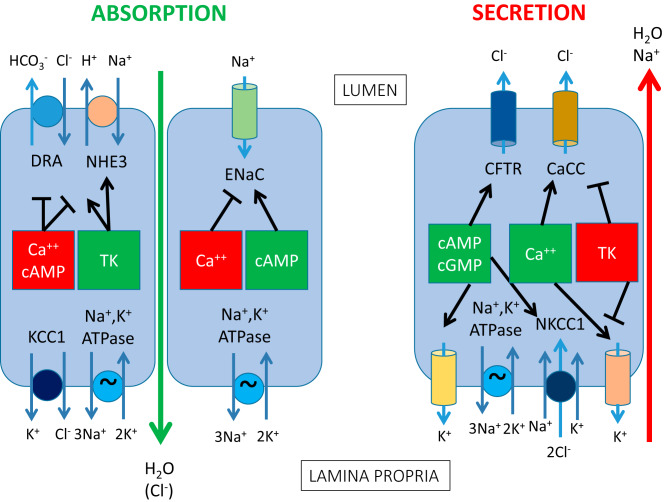
It is now well-established that the intestinal epithelium participates in active electrolyte transport to subserve its physiological functions in digestion and absorption (4, 24). It is important to note that both secretory and absorptive pathways are involved, thereby controlling the fluidity of the intestinal contents on a minute-to-minute basis during the assimilation of a meal (Fig. 1). Secretion is driven predominantly by the active secretion of chloride ions, whereas the absorptive vector is largely provided by the uptake of digested nutrients in tandem with electrolytes when the meal is present and sodium ions with or without the accompanying active transport of chloride ions in the periods between meals (24). In health, while secretion is ongoing throughout the length of the gut, the absorptive vector vastly predominates such that the 8 to 9 liters of fluid secreted into the gut or ingested orally on a daily basis is reclaimed, typically leaving only around 200 mL to be lost to the stool (5). It is evident, therefore, that these processes are tightly regulated to avoid excessive fluid loss and accompanying dehydration.
Fig. 1.
Major pathways for electrolyte absorption and secretion across epithelial cells in the mammalian intestine. The left-hand side of the figure shows absorptive pathways, with the electroneutral NaCl absorptive mechanism depicted in the left-most cell present throughout the small intestine and colon, whereas the electrogenic sodium absorptive mechanism in the adjacent cell is present only in the distal colon. The right-hand side of the figure shows the electrogenic chloride secretory mechanism that is also present throughout the small intestine and colon. The central boxes depict intracellular signals that either inhibit (red) or activate specific membrane transport proteins, with their targets depicted by arrows. Note that many of the factors that inhibit absorption trigger secretion and vice versa. For both absorptive and secretory mechanisms, active electrolyte transport results in accompanying paracellular transport of water as well as (in the case of electrogenic transport mechanisms) an appropriate counterion. CaCC, calcium-activated chloride channel; CFTR, cystic fibrosis transmembrane conductance regulator; DRA, downregulated in adenoma; ENaC, epithelial sodium channel; KCC1, potassium-2 chloride co-transporter-1; NHE3, sodium/hydrogen exchanger-3; NKCC1, sodium-potassium-2 chloride cotransporter-1; TK, tyrosine kinase (e.g., epidermal growth factor receptor).
In fact, alterations in the processes that regulate intestinal electrolyte transport are known to be involved in diarrheal diseases of varying etiologies. The classical example is cholera, where the primary toxin elaborated by Vibrio cholerae causes a large and irreversible increase in epithelial cAMP, resulting in a correspondingly profound active transcellular secretion of chloride ions as well as the loss of accompanying water and sodium ions paracellularly (13). It is this link of epithelial transport to disease that has guided my own research program for more than 30 years. Indeed, diarrheal diseases remain a scourge of humanity, especially in developing countries where sanitation cannot be assured. In this article, I will discuss our efforts to define the mechanisms that underpin disease caused by the most burdensome bacterial diarrheal pathogens, nontyphoidal Salmonella spp (16). However, even in developed countries with excellent infrastructure, food-borne diarrheal diseases such as those caused by Salmonella remain an issue, especially in vulnerable populations, and diarrhea also may occur as an undesirable side effect of treatments for other conditions (8). Therefore, I will additionally address our recent work that has explored mechanisms that may account for the diarrheal side effects of tyrosine kinase inhibitors directed at the receptor for epidermal growth factor (EGFr-TKI) used in the treatment of non-small cell lung cancer as well as other tumors (36).
MECHANISMS OF DIARRHEAL DISEASE IN THE SETTING OF SALMONELLA INFECTION
Nontyphoidal Salmonella infections are a leading cause of food-borne death worldwide and exert a particularly high economic burden. Developed countries like the United States are certainly not immune to these threats, with the Centers for Disease Control (CDC) reporting that these infections cause about 1.35 million illnesses, 26,500 hospitalizations, and almost 500 deaths each year in America. The CDC website reveals that multi-state outbreaks occur almost monthly, or even more frequently in some years (https://www.cdc.gov/salmonella/outbreaks.html), and that antibiotic resistance is increasing. In the United States alone, estimates in 2010 placed the economic burden of Salmonella infections at between $2.65 and $14.6 billion per year inclusive of direct healthcare costs, lost productivity, and the cost of premature death, with the range in the estimates reflecting whether or not costs for pain and suffering and functional disability are included (http://www.cidrap.umn.edu/news-perspective/2010/05/usda-estimates-e-coli-salmonella-costs-31-billion). Doubtless, costs have increased still further in the ensuing decade. Moreover, neither estimate includes costs borne by governments or the food industry.
However, unlike enterotoxigenic diarrheal diseases, the pathogenesis of diarrhea in the setting of Salmonella infection (or indeed infection with other invasive pathogens) was rather poorly understood. In part, this may have been due to the lack of a tractable small animal model of the disease, since most laboratory strains of mice rapidly succumb to a systemic disease resembling typhoid fever when infected orally with nontyphoidal Salmonella rather than the human finding of diarrhea. Evidence suggested that the failure of mice to contain the disease was related to the fact that most laboratory strains express a mutant form of the Nramp transporter (SLC11A1), which is important to control intracellular infection in macrophages (34). Therefore, our work in this area was greatly facilitated by the finding of our infectious disease collaborators, Josh Fierer and Don Guiney, that wild-type but not invasion-deficient strains of S. typhimurium caused diarrhea (measured as an increase in stool water) in mice that had been engineered to be congenic for the wild-type form of Nramp and which were pretreated with the antibiotic, kanamycin (46). We hypothesized that the diarrhea occurring in these mice was due to alterations in the ion transport function of affected gut segments. Samples of proximal and distal colon from these mice were mounted in Ussing chambers, which somewhat surprisingly revealed that both basal and forskolin-stimulated short circuit current were reduced in infected animals, without an effect on calcium-dependent chloride secretion stimulated by carbachol (29). Thus, there was no evidence for active chloride secretion, of the type seen in cholera, as a diarrheal mechanism. So we undertook an effort to catalog the expression and localization of the major transport proteins in infected mice both to explain the Ussing chamber findings and to further elucidate the likely diarrheal mechanism.
In fact, while CFTR expression was unaffected by infection at both the mRNA and protein level, confocal microscopy revealed that in mice infected with wild-type but not invasion-deficient Salmonella strains, CFTR no longer co-localized with the apical marker, villin, in crypt epithelial cells and instead was located intracellularly (29). This likely accounts for the reduction in forskolin-stimulated current in infected tissues mounted in Ussing chambers. However, it obviously cannot explain diarrheal symptoms. Similarly, ENaC expression was significantly reduced by infection in surface epithelial cells of the distal colon, perhaps also contributing to the reduction in basal and forskolin-stimulated current in this segment (29). However, electrogenic sodium absorption is likely a salvage mechanism that plays a quantitatively small role in overall fluid balance, and thus a deficit in this mechanism would be unlikely alone to result in diarrhea. Despite the Ussing chamber findings, therefore, we were prompted to examine other transporters, including those involved in electroneutral transport mechanisms, as contributors to the diarrheal phenotype in infected mice.
In fact, expression of the chloride-bicarbonate exchanger, downregulated in adenoma (DRA), was profoundly suppressed in the proximal colon of infected mice (29). DRA participates in electroneutral NaCl absorption via its paired activity with the sodium-hydrogen exchanger NHE3 (Fig. 1), although interestingly, expression of this latter transporter was unaffected by infection. Nevertheless, since both transporters must work in tandem, the loss of either should suppress the capacity for NaCl and thus fluid reabsorption, as also illustrated by congenital chloride diarrhea, a rare condition arising from loss-of-function mutations in DRA (17). Interestingly, others have now reported that DRA expression and/or activity is reduced in the setting of infection with other intestinal pathogens, such as Citrobacter rodentium and enteropathogenic E. coli, with or without concomitant loss of NHE3 activity (7, 15, 25). We conclude that suppression of DRA activity may be a common diarrheal mechanism in a variety of settings and perhaps also a therapeutic target.
Therefore, we sought to define the mechanism whereby DRA expression is downregulated in the setting of infection with nontyphoidal Salmonella. Initial studies in mice showed that wild-type infection was accompanied by evidence of increased epithelial turnover and crypt hyperplasia (29). Furthermore, in contrast to long-standing dogma, neither this crypt hyperplasia as well as diarrheal symptoms nor the downregulation of DRA and ENaC required the gut neutrophil infiltration normally triggered by infection, since all of these effects persisted following infection of mice lacking the receptor for IL-8 despite an abolished neutrophil response (30). Therefore, we concluded that an epithelial-intrinsic mechanism contributes to disease, and perhaps there was an alteration in epithelial maturity that resulted in the failure to express DRA.
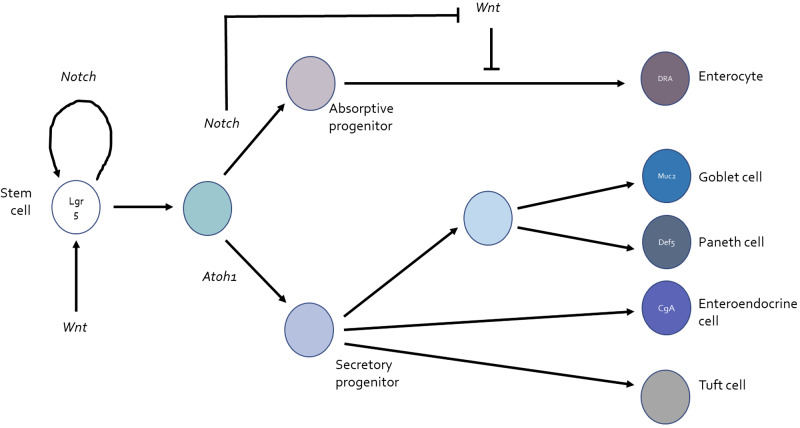
At this stage, we believed that our mouse model was not ideal for further exploration of epithelial differentiation events that might be impacted by infection, not least because the timing of epithelial infection following the oral administration of bacteria could not carefully be controlled. Cell lines likewise were not an option because of their failure to differentiate into the full panoply of epithelial lineages present in the native intestine. Depending on the precise balance of regulatory factors in the environment, including, importantly, Notch and Wnt, stem cells in the gut differentiate into an appropriate census of five lineages: absorptive enterocytes and the so-called secretory lineages of goblet, Paneth, enteroendocrine, and tuft cells (Fig. 2) (38, 44). Fortunately, the burgeoning science of enteroid culture, as well as methods to convert these three-dimensional mini-guts into two-dimensional monolayers amenable to apical infection, allowed us to begin to address whether infection with Salmonella not only can influence epithelial proliferation but can also alter the balance of differentiation to the absorptive cells that normally express DRA (18, 28, 47).
Fig. 2.
Differentiation of intestinal epithelial cells to absorptive and secretory lineages and effects of Notch and Wnt. Lgr5-positive pluripotent stem cells cycle in the crypt base under the influence of both Notch and Wnt. If levels of Notch signaling are high, committed progeny are directed towards differentiation along the absorptive pathway, ultimately resulting in mature enterocytes that express (among other markers) the chloride-bicarbonate exchanger downregulated in adenoma (DRA). Notch also suppresses the effect of Wnt that otherwise downregulates absorptive differentiation. On the other hand, when Notch signaling is relatively less active, absorptive differentiation is suppressed by unopposed Wnt activity, and differentiation to the various secretory lineages directed by Atoh1 expression, goblet cells expressing mucin 2 (Muc2), Paneth cells expressing defensing 5 (Def5), enteroendocrine cells expressing chromogranin A (CgA) and tuft cells, is promoted (44).
In fact, using enteroid-derived monolayers (EDM) generated from murine colonic crypts (in work currently in preparation for submission), we have shown that these models not only recapitulated the loss of DRA expression following infection that we had observed in vivo but also displayed increased expression of the secretory lineage transcription factor Atoh1 and the goblet cell marker Muc2. We hypothesized that infection might alter the balance of factors specifying absorptive versus secretory differentiation, and indeed, infection reduced levels of the Notch intracellular domain in EDM as well as the Notch target Hes1 without altering either expression or phosphorylation of the Wnt target β-catenin. Finally, the effect of infection on DRA expression could be reproduced by a Notch inhibitor.
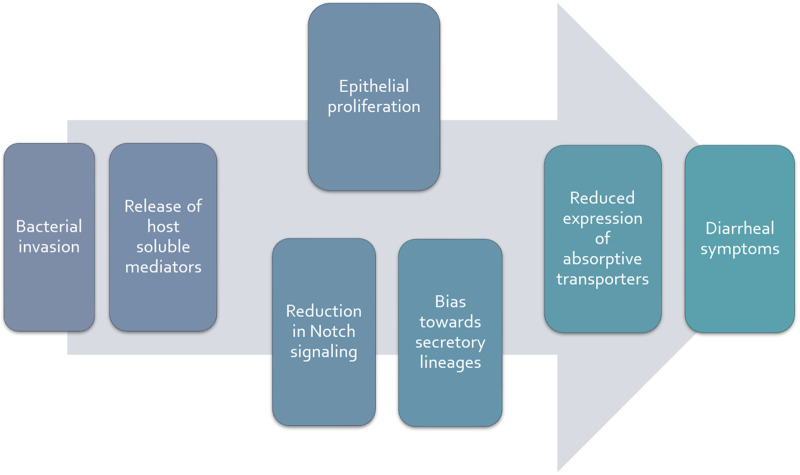
Our work in this area is ongoing, but our data thus far lead us to conclude that Salmonella infection does indeed impact absorptive capacity in a manner that is independent of inflammatory cells and their mediators and perhaps is accounted for by a switch in the balance between absorptive and secretory differentiation secondary to reduced Notch signaling (Fig. 3). While a disease mechanism that rests on changes in epithelial differentiation would be expected to develop more slowly than a direct effect of the bacteria on transporter function, in fact this corresponds well to the kinetics of diarrheal disease in our mouse model, where measurable increases in stool water do not begin for around 2 days after oral infection. This significantly exceeds the estimate of six to eight hours of total gastrointestinal transit time in mice and implies that the mechanism of diarrhea requires time to develop once the target epithelial cells are infected (35). Overall, nevertheless, diarrheal disease in the setting of this common infection is attributable to defects in epithelial transport. Strategies for increasing the absorptive capacity of the gut after infection may be of benefit and are the subject of our ongoing work. Not only is absorption compromised, moreover, but the paradoxical relative lack of active crypt chloride secretion might compromise the sterility of the stem cell niche and/or be permissive for bacterial invasion.
Fig. 3.
Working hypothesis for the promotion of diarrheal symptoms following infection of intestinal epithelial cells with nontyphoidal Salmonella spp. Bacterial invasion results in the release of soluble mediators from host epithelial cells that act in a paracrine fashion to stimulate epithelial proliferation (with accompanying immaturity of absorptive enterocytes) and/or reduced Notch signaling, promoting an overabundance of secretory epithelial lineages. The net effect is reduced expression of absorptive transporters such as the chloride-bicarbonate exchanger, downregulated in adenoma (DRA). In turn, there is a reduction of electroneutral NaCl absorption, and fluid accumulates in the intestinal lumen beyond the reserve capacity of the colon to reabsorb it (particularly when expression of the epithelial sodium channel, ENaC, in the distal colon is also suppressed), resulting in diarrhea.
REGULATION OF EPITHELIAL CHLORIDE SECRETION BY EGFR
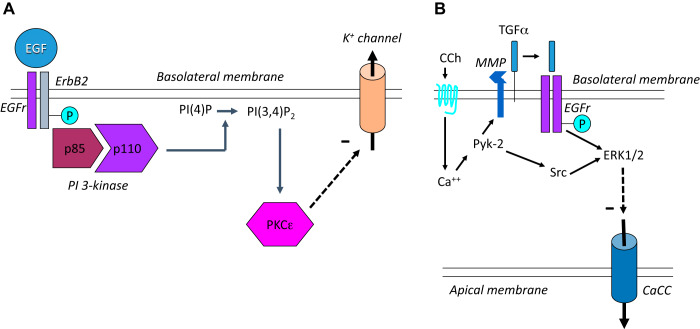
The second half of my presentation in the 2019 Ussing Lecture focused on our work that identified EGFr as a key intrinsic regulator of calcium-dependent chloride secretion and our more recent realization that this may well be relevant for the unfortunate propensity of anti-cancer drugs that target EGFr to cause diarrhea as a side effect. Several years ago, we published a series of papers showing that not only activation of EGFr by its cognate ligands but also transactivation of the receptor following activation of G protein-coupled receptors (GPCR) such as that for carbachol could limit the extent of calcium-dependent chloride secretion, albeit via different signaling mechanisms and with distinct membrane transporters as targets of the inhibitory effect (Fig. 4) (6, 22, 23, 39, 40). Indeed, we speculated that the known transience of calcium-dependent secretory responses, compared with the sustained effects produced by secretagogues acting via cAMP, might in fact reflect the existence of these inhibitory mechanisms that limit the extent of secretion. Short, self-limited, secretory responses could be useful in particular physiological circumstances, such as the reflex described by Cooke and coworkers where mechanical deformation of the epithelium triggers serotonin-induced chloride secretion to lubricate the passage of a food bolus (14). Therefore, the secretory response could be restricted just to the precise moment it is needed, rather than risking dehydration. Similarly, the ability of EGF to restrict chloride secretion, without itself acting as an agonist of the process, could be of value in the setting of epithelial injury (accompanied by upregulation of growth factors, including EGF) to redirect cellular resources to the processes of restitution and repair rather than the energetically costly chloride secretory mechanism.
Fig. 4.
Regulation of epithelial chloride secretion by the epidermal growth factor receptor (EGFr). A: the direct effect of EGF on chloride secretion involves phosphorylation and activation of EGFr-ErbB2 homodimers, binding of the p85 regulatory and p110 catalytic subunits of phosphatidylinositol (PI) 3-kinase, generation of PI(3, 4)P2 from membrane PI(4)P, and recruitment of the ε-isoform of protein kinase C (PKC) that in turn exerts a negative effect (dashed arrow) on basolateral potassium channels. B: the muscarinic agonist carbachol (CCh) can also reduce chloride secretion by transactivating EGFr downstream of the soluble tyrosine kinases Src and Pyk-2, release of membrane-bound transforming growth factor-α by a matrix metalloproteinase (MMP), and activation of the extracellular-regulated kinase (ERK) 1 and 2 isoforms of mitogen-activated protein kinases. However, in this case, ErbB2 is not involved, and the eventual target of the inhibitory mechanism is an apical calcium-activated chloride channel (CaCC). For further details, see text.
We showed, using human colonic T84 cells as a model [another innovation of Dharmsathaphorn and colleagues (10)], that EGF acts on its basolateral receptor to recruit the heterodimeric phosphatidylinositol 3-kinase (PI3K) to its substrates in the plasma membrane, secondarily activating protein kinase C-ε and inhibiting the function of a basolateral potassium conductance needed to sustain the driving force for apical chloride exit in response to stimuli that elevate intracellular calcium (Fig. 4A) (6, 9, 39, 40). On the other hand, when EGFr was transactivated by GPCR ligands such as carbachol, while the ensuing secretory response and those to subsequent calcium-dependent stimuli were reduced in an EGFr-dependent fashion, the mechanism was distinct. This pathway involved calcium-dependent activation of the soluble tyrosine kinases Pyk-2 and Src, matrix metalloproteinase-mediated cleavage of the membrane-bound form of the alternate EGFr ligand, transforming growth factor-α (TGF-α), recruitment of mitogen-activated protein kinases rather than PI3K downstream of EGFr, and inhibition of an apical chloride conductance rather than a basolateral potassium channel (Fig. 4B) (6, 32, 33). Our studies also begged the question of how the epithelium can distinguish between the activation of EGFr by EGF versus TGF-α released in response to GPCR agonists such as carbachol. In fact, while the responses to exogenous EGF and TGF-α were qualitatively similar, carbachol appears concomitantly to recruit protein tyrosine phosphatases (PTP), including PTP1B, that trim specific phosphotyrosines from particular residues on the intracellular tail of EGFr, dictating alternate signaling outcomes (31).
Most recently, with the advent of small-molecule EGFr-TKIs for the treatment of small-cell lung cancer, we were prompted to ask whether our previous findings related to the role of EGFr in regulating chloride secretion might account for the ability of these drugs to cause severe diarrheal side effects (11, 36). In fact, diarrhea occurs frequently with these powerful drugs and may be not only dose-limiting but may even require cessation of treatment entirely even in the face of a tumor response. There are now three classes of EGFr-TKI. First-generation drugs are reversible inhibitors of EGFr alone, whereas second-generation EGFr-TKIs irreversibly inhibit not only EGFr but also other ErbB family members (37). Finally, third-generation EGFr-TKIs appear to be specific for inhibition of the kinase activity only of mutated forms of EGFr (37). In general, second-generation agents such as afatinib have a much greater propensity to cause diarrhea than first-generation agents such as erlotinib or third-generation drugs such as osimertinib (36). In work recently submitted for publication, we asked whether this variable incidence of diarrheal side effects might be related to the ability of EGFr-TKIs to influence chloride secretion (Kim, Quach, Das, and Barrett, unpublished observations).
In fact, the second-generation EGFr-TKI afatinib not only reversed the inhibitory effect of EGF on chloride secretion evoked by carbachol but also potentiated the secretory response to carbachol alone (Table 1). Conversely, the first-generation EGFr-TKI erlotinib reversed the inhibitory effect of EGF on chloride secretory responses but did not significantly alter secretion in response to carbachol alone. Finally, osimertinib neither reversed the effect of EGF nor influenced the secretory response to carbachol alone. In summary, the effects of these agents on chloride secretion largely correlate with their propensity to induce clinically meaningful diarrhea (Table 1). Others have recently reached broadly similar conclusions (12). Overall, we are hopeful that studies of this type may inform the design of effective anti-tumor agents whose application will not be limited by side effects.
Table 1.
Effect of selected EGFr-directed tyrosine kinase inhibitors on calcium-dependent chloride secretion and diarrhea
| Class of EGFr-TKI | First Generation | Second Generation | Third Generation |
|---|---|---|---|
| Example | Erlotinib | Afatinib | Osimertinib |
| Target | EGFr | ErbB family of receptors | Mutant EGFr |
| Incidence of all grades of diarrhea/grade 3 or 4 diarrhea* | 18–68%/1–12% | 87–95%/5–17% | 41%/1% |
| Reverse inhibitory effect of EGF on CCh-induced chloride secretion? | Yes | Yes | No |
| Potentiate CCh-induced chloride secretion alone? | No | Yes | No |
CCh, carbachol; EGFr, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
From Rugo et al. (36).
CONCLUSIONS
Hopefully, with the two examples I delineated in my presentation, I have conveyed that intestinal homeostasis requires the precise control of epithelial transport mechanisms (which also implies a need to regulate barrier function). This control is achieved via the actions of both exogenous and endogenous factors that dynamically modulate the physiological properties of epithelial cells, and such control mechanisms, including those that impact epithelial turnover and differentiation (thereby secondarily modifying transport functions), can be subverted in various pathological states. The overarching goal of our research has been to unravel these regulatory mechanisms in health and disease with the understanding that this might reveal novel mechanisms and/or targets that could be exploited to ameliorate diarrheal diseases, which remain a major scourge worldwide.
In closing, I would like to note that I have thoroughly enjoyed my life encompassing studies of “all things Ussing chambers.” This is in large part due to the many wonderful colleagues whom I have had the honor to work with in producing the research described here as well as other studies from my group. Indeed, it was a special pleasure to have been introduced for my lecture by former trainee and now independent investigator Stephen Keely and to have celebrated the event in Orlando, FL, not only with Stephen, but also with two other former trainees who have since forged their own paths, Declan McCole and Melanie Gareau. While I have found fulfillment in our collective scientific outputs and their potential for understanding and treating diarrheal diseases, I consider that my far more important legacy will be to have contributed, at least in some small way, to the career development of Stephen, Declan, and Melanie and the many others who have worked with me who are listed below. In this regard, I also acknowledge the enormous contributions of my own mentors, not only the aforementioned Kiertisin Dharmsathaphorn, but also my thesis supervisor Fred Pearce of University College London, and my post-doc mentor, Dean Metcalfe at the NIH. All of them modeled the mentorship ideal that I have aspired to.
GRANTS
The studies described herein have been supported by grants to the author from the National Institutes of Health and an unrestricted grant from the Estratest Settlement fund, as well as fellowship and career development support to members of my laboratory from the National Institutes of Health, Crohn’s and Colitis Foundation, and the Korea Institute of Radiological and Medical Sciences (funded by the Ministry of Science and ICT, Republic of Korea).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
K.E.B. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
I am grateful to my collaborators Joshua Fierer, Donald Guiney, Sharon Okamoto, and Soumita Das (University of California, San Diego), Robert Coffey (Vanderbilt University), and Mark Donowitz and Nicholas Zachos (Johns Hopkins University) for assistance with some of the studies described and to my colleagues at University of California San Diego, Mamata Sivagnanam and Soumita Das, for intellectual input to the direction of several of these projects. Most importantly, the work I have outlined involved major contributions from the following current and former members of my laboratory (listed alphabetically): Lone Bertelsen, Melanie Gareau, Elaine Hanson, Rashini Jayaratne, Stephen Keely, Younjoo Kim, Rachel Klinkenburg, Beomjae Lee, Ronald Marchelletta, Declan McCole, Andrew Quach, Colin Reardon, Elise Roel, Jane Smitham, Cheryl Stork, Jorge Uribe, Anouk van Berkel, and Roos Visser.
REFERENCES
- 1.Barrett KE. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol 265: C859–C868, 1993. doi: 10.1152/ajpcell.1993.265.4.C859. [DOI] [PubMed] [Google Scholar]
- 2.Barrett KE, Cohn JA, Huott PA, Wasserman SI, Dharmsathaphorn K. Immune-related intestinal chloride secretion. II. Effect of adenosine on T84 cell line. Am J Physiol 258: C902–C912, 1990. doi: 10.1152/ajpcell.1990.258.5.C902. [DOI] [PubMed] [Google Scholar]
- 3.Barrett KE, Huott PA, Shah SS, Dharmsathaphorn K, Wasserman SI. Differing effects of apical and basolateral adenosine on colonic epithelial cell line T84. Am J Physiol 256: C197–C203, 1989. doi: 10.1152/ajpcell.1989.256.1.C197. [DOI] [PubMed] [Google Scholar]
- 4.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572, 2000. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 5.Barrett KE, Keely SJ. Integrative physiology and pathophysiology of intestinal electrolyte transport. In: Physiology of the Gastrointestinal Tract (4th ed.), edited by Johnson LR. San Diego, CA: Academic, 2006, vol 1 and 2, p. 1931–1951. [Google Scholar]
- 6.Barrett KE, Smitham J, Traynor-Kaplan A, Uribe JM. Inhibition of Ca2+-dependent Cl− secretion in T84 cells: membrane target(s) of inhibition is agonist specific. Am J Physol Cell Physiol 274: C958–C965, 1998. doi: 10.1152/ajpcell.1998.274.4.C958. [DOI] [PubMed] [Google Scholar]
- 7.Borenshtein D, Schlieper KA, Rickman BH, Chapman JM, Schweinfest CW, Fox JG, Schauer DB. Decreased expression of colonic Slc26a3 and carbonic anhydrase iv as a cause of fatal infectious diarrhea in mice. Infect Immun 77: 3639–3650, 2009. doi: 10.1128/IAI.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzby JC, Roberts T. The economics of enteric infections: human foodborne disease costs. Gastroenterology 136: 1851–1862, 2009. doi: 10.1053/j.gastro.2009.01.074. [DOI] [PubMed] [Google Scholar]
- 9.Chow JY, Uribe JM, Barrett KE. A role for protein kinase C-epsilon in the inhibitory effect of epidermal growth factor on calcium-stimulated chloride secretion in human colonic epithelial cells. J Biol Chem 275: 21169–21176, 2000. doi: 10.1074/jbc.M002160200. [DOI] [PubMed] [Google Scholar]
- 10.Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol 246: G204–G208, 1984. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 11.Ding PN, Lord SJ, Gebski V, Links M, Bray V, Gralla RJ, Yang JC, Lee CK. Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: a meta-analysis of clinical trials of Gefitinib, Erlotinib, and Afatinib in advanced EGFR-mutated non-small cell lung cancer. J Thorac Oncol 12: 633–643, 2017. doi: 10.1016/j.jtho.2016.11.2236. [DOI] [PubMed] [Google Scholar]
- 12.Duan T, Cil O, Thiagarajah JR, Verkman AS. Intestinal epithelial potassium channels and CFTR chloride channels activated in ErbB tyrosine kinase inhibitor diarrhea. JCI Insight 4: e126444, 2019. doi: 10.1172/jci.insight.126444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field M. Field M: intestinal secretion: effect of cyclic AMP and its role in cholera. N Engl J Med 284: 1137–1144, 1971. doi: 10.1056/NEJM197105202842008. [DOI] [PubMed] [Google Scholar]
- 14.Frieling T, Wood JD, Cooke HJ. Submucosal reflexes: distension-evoked ion transport in the guinea pig distal colon. Am J Physiol 263: G91–G96, 1992. doi: 10.1152/ajpgi.1992.263.1.G91. [DOI] [PubMed] [Google Scholar]
- 15.Gujral T, Kumar A, Priyamvada S, Saksena S, Gill RK, Hodges K, Alrefai WA, Hecht GA, Dudeja PK. Mechanisms of DRA recycling in intestinal epithelial cells: effect of enteropathogenic E. coli. Am J Physiol Cell Physiol 309: C835–C846, 2015. doi: 10.1152/ajpcell.00107.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, de Silva NR, Gargouri N, Speybroeck N, Cawthorne A, Mathers C, Stein C, Angulo FJ, Devleesschauwer B; World Health Organization Foodborne Disease Burden Epidemiology Reference Group . World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med 12: e1001923, 2015. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14: 316–319, 1996. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 18.In JG, Foulke-Abel J, Estes MK, Zachos NC, Kovbasnjuk O, Donowitz M. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nat Rev Gastroenterol Hepatol 13: 633–642, 2016. doi: 10.1038/nrgastro.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnsen VK, Ussing HH. The influence of the corticotropic hormone from ox on the active salt uptake in the axolotl. Acta Physiol Scand 17: 38–43, 1949. doi: 10.1111/j.1748-1716.1949.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 20.Kachintorn U, Vajanaphanich M, Traynor-Kaplan AE, Dharmsathaphorn K, Barrett KE. Activation by calcium alone of chloride secretion in T84 epithelial cells. Br J Pharmacol 109: 510–517, 1993. doi: 10.1111/j.1476-5381.1993.tb13599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kachintorn U, Vongkovit P, Vajanaphanich M, Dinh S, Barrett KE, Dharmsathaphorn K. Dual effects of a phorbol ester on calcium-dependent chloride secretion by T84 epithelial cells. Am J Physiol 262: C15–C22, 1992. doi: 10.1152/ajpcell.1992.262.1.C15. [DOI] [PubMed] [Google Scholar]
- 22.Keely SJ, Barrett KE. ErbB2 and ErbB3 receptors mediate inhibition of calcium-dependent chloride secretion in colonic epithelial cells. J Biol Chem 274: 33449–33454, 1999. doi: 10.1074/jbc.274.47.33449. [DOI] [PubMed] [Google Scholar]
- 23.Keely SJ, Calandrella SO, Barrett KE. Carbachol-stimulated transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T(84) cells is mediated by intracellular Ca2+, PYK-2, and p60(src). J Biol Chem 275: 12619–12625, 2000. doi: 10.1074/jbc.275.17.12619. [DOI] [PubMed] [Google Scholar]
- 24.Kiela PR, Ghishan FK. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol 30: 145–159, 2016. doi: 10.1016/j.bpg.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar A, Anbazhagan AN, Coffing H, Chatterjee I, Priyamvada S, Gujral T, Saksena S, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. Am J Physiol Gastrointest Liver Physiol 311: G817–G826, 2016. doi: 10.1152/ajpgi.00173.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen EH, Nedergaard S, Ussing HH. Role of lateral intercellular space and sodium recirculation for isotonic transport in leaky epithelia. Rev Physiol Biochem Pharmacol 141: 153–212, 2000. doi: 10.1007/BFb0119579. [DOI] [PubMed] [Google Scholar]
- 27.Lindemann B. Hans Ussing, experiments and models. J Membr Biol 184: 203–210, 2001. doi: 10.1007/s00232-001-0103-4. [DOI] [PubMed] [Google Scholar]
- 28.Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA, Sato T, Shroyer NF. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol 3: 217–240, 2013. doi: 10.1002/9780470942390.mo130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchelletta RR, Gareau MG, McCole DF, Okamoto S, Roel E, Klinkenberg R, Guiney DG, Fierer J, Barrett KE. Altered expression and localization of ion transporters contribute to diarrhea in mice with Salmonella-induced enteritis. Gastroenterology 145: 1358–1368.e4, 2013. doi: 10.1053/j.gastro.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchelletta RR, Gareau MG, Okamoto S, Guiney DG, Barrett KE, Fierer J. Salmonella-induced diarrhea occurs in the absence of IL-8 receptor (CXCR2)-dependent neutrophilic inflammation. J Infect Dis 212: 128–136, 2015. doi: 10.1093/infdis/jiu829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCole DF, Bunz M, Barrett KE. Protein tyrosine phosphatase 1b governs differential epidermal growth factor receptor phosphorylation and signaling induced by EGFR vs. GPCR ligands (Abstract). Gastroenterology 132: A221, 2007. [Google Scholar]
- 32.McCole DF, Keely SJ, Coffey RJ, Barrett KE. Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-alpha. J Biol Chem 277: 42603–42612, 2002. doi: 10.1074/jbc.M206487200. [DOI] [PubMed] [Google Scholar]
- 33.McCole DF, Truong A, Bunz M, Barrett KE. Consequences of direct versus indirect activation of epidermal growth factor receptor in intestinal epithelial cells are dictated by protein-tyrosine phosphatase 1B. J Biol Chem 282: 13303–13315, 2007. doi: 10.1074/jbc.M700424200. [DOI] [PubMed] [Google Scholar]
- 34.Nairz M, Fritsche G, Crouch MLV, Barton HC, Fang FC, Weiss G. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol 11: 1365–1381, 2009. doi: 10.1111/j.1462-5822.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padmanabhan P, Grosse J, Asad ABMA, Radda GK, Golay X. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res 3: 60, 2013. doi: 10.1186/2191-219X-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rugo HS, Di Palma JA, Tripathy D, Bryce R, Moran S, Olek E, Bosserman L. The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea. Breast Cancer Res Treat 175: 5–15, 2019. doi: 10.1007/s10549-018-05102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah R, Lester JF. Tyrosine kinase inhibitors for the treatment of EGFR mutation-positive non-small-cell lung cancer: a clash of the generations. Clin Lung Cancer S1525-7304(19)30346-8, 2019. doi: 10.1016/j.cllc.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Tian H, Biehs B, Chiu C, Siebel CW, Wu Y, Costa M, de Sauvage FJ, Klein OD. Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep 11: 33–42, 2015. doi: 10.1016/j.celrep.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uribe JM, Gelbmann CM, Traynor-Kaplan AE, Barrett KE. Epidermal growth factor inhibits Ca2+-dependent Cl- transport in T84 human colonic epithelial cells. Am J Physiol 271: C914–C922, 1996. doi: 10.1152/ajpcell.1996.271.3.C914. [DOI] [PubMed] [Google Scholar]
- 40.Uribe JM, Keely SJ, Traynor-Kaplan AE, Barrett KE. Phosphatidylinositol 3-kinase mediates the inhibitory effect of epidermal growth factor on calcium-dependent chloride secretion. J Biol Chem 271: 26588–26595, 1996. doi: 10.1074/jbc.271.43.26588. [DOI] [PubMed] [Google Scholar]
- 41.Ussing HH. The active ion transport through the isolated frog skin in the light of tracer studies. Acta Physiol Scand 17: 1–37, 1949. doi: 10.1111/j.1748-1716.1949.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 42.Ussing HH, Erlij D, Lassen U. Transport pathways in biological membranes. Annu Rev Physiol 36: 17–49, 1974. doi: 10.1146/annurev.ph.36.030174.000313. [DOI] [PubMed] [Google Scholar]
- 43.Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand 23: 110–127, 1951. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 44.VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139: 488–497, 2012. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wasserman SI, Barrett KE, Huott PA, Beuerlein G, Kagnoff MF, Dharmsathaphorn K. Immune-related intestinal Cl− secretion. I. Effect of histamine on the T84 cell line. Am J Physiol 254: C53–C62, 1988. doi: 10.1152/ajpcell.1988.254.1.C53. [DOI] [PubMed] [Google Scholar]
- 46.Woo H, Okamoto S, Guiney D, Gunn JS, Fierer J. A model of Salmonella colitis with features of diarrhea in SLC11A1 wild-type mice. PLoS One 3: e1603, 2008. doi: 10.1371/journal.pone.0001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin Y, Zhou D. Organoid and enteroid modeling of Salmonella infection. Front Cell Infect Microbiol 8: 102, 2018. [Erratum in Front Cell Infect Microbiol 8: 257, 2018.] doi: 10.3389/fcimb.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]