Abstract
Cellular communication network (CCN) proteins are matricellular proteins that coordinate signaling among extracellular matrix, secreted proteins, and cell surface receptors. Their specific in vivo function is context-dependent, but they play profound roles in pathological conditions, such as fibrosis and cancers. Anti-CCN therapies are in clinical consideration. Only recently, however, has the function of these complex molecules begun to emerge. This review summarizes and interprets our current knowledge regarding these fascinating molecules and provides experimental evidence for their utility as therapeutic targets.
Keywords: cancer, CCN proteins, fibrosis, microenvironment, matricellular proteins
INTRODUCTION
Matricellular proteins are dynamically expressed and are secreted into the extracellular microenvironment, notably the extracellular matrix (ECM). They do not play a stable, structural role in the ECM but regulate cell function by interacting with ECM structural proteins and cell-surface receptors, proteases, hormones, and other bioeffector molecules, and may sequester and modulate activities of specific growth factors (13).
Members of the cellular communication network (CCN) family of matricellular proteins, which include CCN1–6, were first identified in the late 1980s and early-to-mid 1990s (66, 92, 95, 96). Initially, each protein was named based on what type of screen was used to initially identify each protein. In some cases, the name of the protein was based on its structure, such as cysteine-rich 61 (cyr61), or what induced the expression of each protein, such as wnt-inducible secreted protein 1–3 (WISP1–3) or where is was expressed, such as hypertrophic chondrocyte-specific gene product (Hcs24) or nephroblastoma-overexpressed (nov) or by its purported activity, such as connective tissue growth factor (CTGF) or ecogenin (15, 96, 112). In some cases, most notably for connective tissue growth factor, the original names were subsequently found to be misleading, generating much confusion and misunderstanding in the literature and with the lay public. Of course, at the time of their original discovery, it was not recognized that each protein was a member of a related family. This fact was first determined by Bork (10). All CCN proteins share a common four-module domain structure, except CCN5, which lacks a COOH-terminal heparin-binding domain.
On the basis of efforts by many laboratories and, notably, by the International CCN Society, as of 2018, HUGO (a committee of the Human Genome Organisation) now recognizes officially that, in order, Cyr61, CTGF, nov, and WISP1–3 should be renamed CCN1–CCN6 (97). In all cases, people who were the discoverers or pioneers in the early study of each protein had to give up emotional attachments to largely misleading, and consequently nonheuristically useful, names. The story of how we came to rename these proteins has been superbly outlined elsewhere (96, 97) and is beyond the scope of this review.
Since their initial discovery, CCN proteins have been recognized as being of potential developmental and pathological importance due to the observation that their expression is highly spatiotemporally regulated (66). They possess signal peptides and are secreted through the Golgi into the cellular microenvironment during development and under conditions of tissue remodeling and repair. They are also potently dysregulated in pathological conditions in which fibroblasts are activated, such as in fibrotic disease and cancers. That CCN2 (also known as CTGF) was potently induced when transforming growth factor (TGFβ), but not a myriad of other cytokines, was applied to fibroblasts, which led to an initial hypothesis that CCN2 could be a mediator of the profibrotic effects of TGFβ (30). We now know that this initial hypothesis was considerably oversimplistic. However, this hypothesis led to the formation in 1994 of a company, FibroGen, that, initially, was focused on developing anti-CTGF strategies to treat fibrosis. Notably and incredibly, no thanks in part to a reluctance among some to recognize CTGF not as a growth factor but as a matricellular protein, as of the summer of 2019, that is, 25 years later, the anti-CCN2 antibody (FG-3019, pamrevlumab) is now in Phase III trials for both idiopathic pulmonary fibrosis (IPF) and pancreatic cancer (https://www.fibrogen.com/pamrevlumab/). Intriguingly, the actual mechanism underlying the pathological roles and mechanisms of CCN2, and, indeed, the remaining CCN proteins, has remained largely elusive.
In this article, I focus specifically on what we know to be the mechanistic roles of CCN proteins in fibrosis and cancers, and why, mechanistically, these proteins may be appropriate therapeutic targets.
CCN GENE REGULATION
The mechanisms and factors that regulate CCN gene expression have been expertly covered elsewhere, and the reader is referred to these reviews (18, 66). In summary, CCN proteins are regulated by precisely the same pathways that one would expect, should CCN proteins be regulated by and promote a fibrotic and oncogenic microenvironment. As discussed above, since the mid-1990s, CCN2 has been known to be highly induced in fibroblasts by the potent profibrotic cytokine TGFβ (31, 36, 42) and to be highly overexpressed in all fibrotic conditions, including scleroderma, idiopathic pulmonary fibrosis, diabetic nephropathy, atherosclerosis, macular degeneration, gingival hyperplasia, and liver fibrosis (46, 66, 80). Moreover, CCN proteins are generally overexpressed in tumor cells and in stroma, notably in pancreatic and breast cancers and melanoma (4, 66, 125).
In fibroblasts, TGFβ-induced CCN2 gene expression does not require de novo protein synthesis and involves not only the canonical activin-like kinase (ALK5)/Smad pathway, but also the noncanonical mechanotransduction/adhesion pathway, mediated by focal adhesion kinase (FAK)/TGFβ-activated kinase (TAK1)/extracellular signal-regulated kinases (ERK)/yes-activated protein (YAP)/TEA domain family member (TEAD)/Ets1 (30, 32, 36, 57, 67, 68, 91, 119). These results are particularly interesting, as cell attachment to extracellular matrix is obligatory for the appearance of myofibroblasts and the initiation and perpetuation of fibrosis (60, 113). Similarly, TGFβ induces CCN1 by ALK5/FAK/TAK1/ERK/YAP (91, 114). Indeed, the literature considers CCN1 and CCN2 to be prototypical YAP/transcriptional coactivator with PDZ-binding motif (TAZ) targets, and to be particularly responsive to both mechanotransduction and by the ability of actin networks (18). As might be expected, the induction of CCN proteins by mechanical strain is linked to the activation of latent TGFβ (32). Crucially, CCN1 and CCN2 are also induced by hypoxia, and specifically by hypoxia-inducible factor 1-α (HIF1-α; 35, 58, 119, 126).
The noncanonical proadhesive signaling pathway, but not ALK5/Smads, is important for the constitutive upregulation of CCN2 in pathological conditions, including scleroderma (22, 36, 37, 108, 124). That Smad3 was not involved in the hyperactivity of the CCN2 promoter in SSc fibroblasts led to the initial conclusion in 2001 that autocrine TGFβ signaling was insufficient to perpetuate the fibrotic phenotype of SSc dermal fibroblasts (36). Indeed, an activated adhesive pathway is a fundamental characteristic of SSc fibroblasts, and may be linked to a decreased expression of the adhesive signaling repressor phosphatase and tensin homolog (PTEN) (88, 89).
Although the regulation of the other CCN family members has been less studied, it is known that CCN3 is reciprocally regulated to both CCN1 and CCN2 in a yin/yang fashion (103, 104). For example, in fibroblasts, TGFβ suppresses CCN3 mRNA in a fashion that involves ALK5/FAK/ERK, but not YAP/TAK1 (91). That CCN3 and CCN2/CCN1 are reciprocally regulated may have therapeutic implications (see below).
The important consideration to remember is that CCN protein expression does not reflect a particular pathology per se, but of whether or not the individual system examined is being subjected to increased loading/mechanical strain or to hypoxia. This feature is important to consider when interpreting published observations (see below).
CCNs AS MATRICELLULAR PROTEINS
Probably the most helpful progress in the last 20 yr has been the recognition that CCNs are matricellular proteins (17). Many years have been expended uncovering specific CCN receptors, under the assumption that, at least, CTGF was a growth factor. We now know that this assumption is in error. An excellent review focusing on historical attempts to uncover CCN family receptors has been published elsewhere, and the reader is referred to that article (62). Indeed, perhaps the strongest evidence that CCN proteins are properly considered as matricellular proteins has arisen due to the attempts to find a receptor. That CCNs are secreted into the ECM, together with the discovery that CCN proteins bind directly to integrins, the receptors mediating ECM signaling, initially led to the proposal that CCNs are similar to and may be considered as matricellular proteins (63). The integrins through which CCNs signal vary widely depending on the situation, and include: αvβ3, αvβ5, α5β1, α6β1, αIIbβ3, αMβ2, and αDβ2. CCN2 can also interact with heparan sulfate proteoglycans (HSPGs) such as syndecan 4. Similarly, CCNs can bind ECM components such as fibronectin. As such, in vitro, CCNs act as proadhesive molecules; in fact, adhesion assays are probably the only robust, universally agreed-upon in vitro assays for assessing CCN activity, at least of full-length CCN proteins.
FAK plays a critical role downstream of integrin engagement, and mediates CCN activities (6, 20, 53, 115). Similarly, CCN1, and likely other CCN proteins such as CCN2, activates YAP via integrin αvβ3 (90). As discussed above, Ccn1/2 expression is also dependent on FAK activation and YAP (28, 108, 109), consistent with a hypothesis that an autocrine proadhesive signaling loop operating via FAK/YAP promotes Ccn1/2 expression and function. In this regard, it is important to remember the point, described above, that it is now understood among experts within the fibrotic field that an autocrine proadhesive signaling loop operating via integrin-mediated FAK activation is both necessary and sufficient to perpetuate the fibrotic phenotype (60, 71, 113).
Integrin receptor binding domains decorate CCN molecules. Thus, it is important to remember that CCN activities mediated by one integrin binding domain are likely to be distinct from those mediated by a different integrin binding domain; for example, an amino acid mutation (D125A) at the CCN1 binding site for αvβ3/αvβ5 that abolishes CCN1 binding to integrins αvβ3 and αvβ5 and impairs αvβ3/αvβ5-dependent functions does not affect other CCN1-dependent functions mediated by α6β1 integrin (21). Evaluating the biological significance of these individual interactions requires assessment in vivo, and, testing each individual interaction, although informative, may not necessarily give an accurate picture of what the in vivo role of the entire CCN protein may be at a particular juncture [see section CCN1 (cyr61)].
CCNs also interact with receptors other that integrins; for example, CCN2 signals through TrkA (26, 122), and binds both LRP1 (51, 107) and the mannose 6 phosphate receptor (7). The in vivo role of these receptors in mediating CCN activity is unclear.
These observations are further complicated by recent findings, supported by earlier functional data, that, to be active, CCN proteins may have to be cleaved into COOH-terminal heparin-binding fragments (49). Indeed, truncated and full-length CCN2 and CCN3 isoforms have been observed (16, 92). These issues might be particularly important in the case of cancer and fibrosis, which are characterized by the overproduction of many different kinds of proteases. The dysregulation of proteolysis would increase the relative ratio of truncated over full-length isoforms, therefore causing abnormal signaling. Of course, the receptors responsible for signaling by fragments might differ than those that have been detected using full-length material. Indeed, it is not a novel idea that CCN fragments may have different activities than full-length protein (16, 92–94).
The important consideration is that these proteins are not growth factors, making it understandable how it has been essentially impossible to establish robust cell-based assays (other than those examining cell adhesion). To date, the effects of CCN proteins have been only effectively studied in vivo. Nonetheless, it is probable that, in the future, the generation of 3D culture models could prove invaluable.
CCN2 (CTGF)
I will start with the most studied member of the CCN family, CCN2. Initial studies largely focused on the correlation of high CCN2 expression with clinical outcome, that is, as a potential surrogate marker of disease (66, 104). The initial observation that CCN2 was induced by TGFβ was extremely misleading, as CCN2 is not exactly a downstream mediator of TGFβ’s effects, but rather, when initially present, acts as cofactor to amplify TGFβ action, through adhesive/ERK pathways. This was first shown, elegantly, by Takehara and subsequently by other groups (52, 82, 83, 109). This notion is supported by the fact that fibroblast-specific expression of CCN2 is utterly dispensable for normal, cutaneous tissue repair in mice, including the formation of myofibroblasts in response to injury (76). Thus, it is somewhat frustrating that people still have the erroneous impression that CCN2 is a downstream mediator of TGFβ, as no expert in the CCN field has made that claim, nor is it based on any experimental observations.
Because of the misconceptions outlined above, it has awaited the development of key reagents, notably genetic knockout animals and antagonistic antibodies (FG-3019) to demonstrate conclusively in vivo activities of CCN2 and, hence, to validate CCN2 as a bona fide therapeutic target. The anti-CCN2 antibody FG-3019, which may act as a clearance antibody, is a high-affinity humanized monoclonal antibody (14). In models of pancreatic cancer, this antibody has been shown since 2006 to be effective at blocking metastasis in models of pancreatic cancer and also in enhancing the response to chemotherapy, without affecting the efficacy of drug delivery (25, 85). This antibody also blocks melanoma metastasis, impedes ovarian cancer progression, and prolongs survival in response to chemotherapy in acute lymphoblastic leukemia (64).
FG-3019 also successfully protects against animal models of idiopathic pulmonary fibrosis (IPF), including radiation-induced fibrosis (106). Although FG-3019 was tested in human diabetic patients (1), it appears that Phase I/II trials have not been initiated or reported on, possibly due to lack of efficacy because of possible issues with lack of antibody penetration into target tissues in diabetic patients. However, FG-3019 has had a successful Phase II trial for IPF (65, 102). In terms of pancreatic cancer, the results of a Phase I/II trial were published in an OMICS journal that is unfortunately no longer accessible online. Nonetheless, Phase III trials have been initiated for IPF (Zephyrus, March 2023) and pancreatic cancer (Lapis, December 2023), and a Phase II trial is ongoing for muscular dystrophy, with an expected completion date of April 2021.
Genetic analyses using animal models have shown that CCN2 expression by fibroblasts is required for skin fibrosis. and the skin and lung fibrosis caused by loss of PTEN (i.e., by the activation of adhesive signaling/Akt), and liver fibrosis (74, 75, 89, 98). CCN2 is required for the formation of myofibroblasts in fibrotic, but, as mentioned above, not in tissue repair, models (74, 76). In fibrosis, myofibroblasts are derived from “your favorite” progenitor cells, including resident fibroblasts that acquire a progenitor cell-like phenotype, that stably differentiate into myofibroblasts and are, therefore, positive for progenitor cell markers such as NG2, platelet derived growth factor receptor (PDGFRβ), or Sox2 (sex-determining region Y-box 2) (23, 56, 73, 100, 116). This population does not appear to be a significant contributor to tissue repair (50, 76, 117). Although synthetic (collagen-expressing) fibroblasts clearly play an important role in both cutaneous tissue repair and fibrosis, only in fibrotic/cancer models do synthetic fibroblasts (33, 71, 72, 74) generate stable myofibroblasts, likely through a progenitor cell-like intermediate (117). CCN2 is not required for the formation of the preponderance of myofibroblasts during tissue repair but is required for the differentiation of the progenitor cell intermediate to the myofibroblasts that occur in fibrotic/cancer models (39, 74, 76, 116). This latter activity appears to occur in response to activated mechanotransduction pathways.
In cancer models, CCN2 expression by CAFs is also required for metastasis, including the formation of new blood vessels via vasculogenic mimicry, apparently because CCN2 is required for the expression of both periostin and integrin α11 (ITGA11) (40, 41). In human melanoma patients, CCN2 expression correlates with a highly fibrotic subset of ITGA11/COL1A1/FAP-expressing CAFs that is correlated with poor clinical outcome (117). CCN2 expression is independent of BRAF mutational status, suggesting that CCN2 may be a target for patients resistant to BRAF inhibitors (40). As might be anticipated, in human melanoma patients, CCN2 expression correlated with the development of a fibrotic tumor stroma (40).
Collectively, these data suggest that the role of CCN2 in vivo is to promote the differentiation of progenitor cells to a myofibroblast phenotype, and that the presence of these CCN2-dependent myofibroblasts correlates with a poor clinical prognosis. Thus, CCN2 would appear to be a bona fide therapeutic target.
CCN1 (cyr61)
CCN1 is the second most studied CCN family member. The available clinical data also suggest that CCN1 is also a target for both fibrosis and for cancer (4, 5, 59, 99), although the data are somewhat confusing. It should be pointed out that perceived divergences in the published data may be explained because, sometimes, the effects of endogenous CCN1 are examined, and, at other times, the effects of exogenously added or overexpressed CCN1 are evaluated. Overexpression of CCN1, and, indeed, of all CCN proteins, causes an unfolded protein response, a stress-generated response in the endoplasmic reticulum, and cellular death (11). Also, observed effects may differ based on the molecular basis of why CCN proteins are being expressed. As discussed above, generally when endogenous CCN proteins are induced, it is due to mechanical loading or hypoxia; if triggers other than mechanical loading or hypoxia are examined, it is entirely conceivable that different results may be obtained.
I will start with data consistent with the notion that targeting CCN1 may be a useful therapeutic strategy in both cancers and fibrosis. As discussed above, CCN1, like CCN2, is induced by both TGFβ and hypoxia. CCN1 promotes angiogenesis and tumor growth in vivo (2) and promotes models of breast cancer by promoting tumor cell extravasation and protecting from anoikis (38). Similarly, CCN1 promotes progression of pancreatic tumors and metastasis by promoting neoangiogenesis, via hedgehog signaling, and also resistance to chemotherapy (4, 5, 34, 77, 78). The data involving the role of CCN1 in melanoma are fairly limited; however, one study reported, as expected, that hypoxia and ECM stiffness promoted CCN1 expression in endothelial cells, and that knockout of Ccn1 in endothelial cells inhibited the binding of melanoma cancer cells to blood vessels (99). This phenomenon, a critical step in the transit of cancer cells through the vasculature during metastasis (99), is consistent with the notion that CCN1 promotes metastasis in melanoma.
The data regarding the role of CCN1 in tissue repair and fibrosis are somewhat contradictory. CCN1 expression by fibroblasts contributes to bleomycin-induced skin fibrosis; loss of CCN1 expression by fibroblasts results in a disorganized, nonlinear collagen fiber network, suggesting that CCN1 may act as a molecular chaperone to promote collagen organization (99). Moreover, in humans, CCN1 is overexpressed in acute lung injury; in mice, adenoviral-mediated overexpression of CCN1 in lung promoted inflammation, causing fibrosis and the expression of profibrotic proteins (29, 59). Adenoviral-based overexpression of CCN1 by in portal myofibroblasts in liver, however, causes cellular senescence that suppressed fibrogenesis; as pointed out above, this appears to be because this causes an unfolded protein stress response (11, 12).
One thing to remember is that CCN proteins, including CCN1 and CCN2, readily generate reactive oxygen species (ROS) (19, 47). Of course, low levels of ROS act to promote signaling, whereas high levels of ROS act to promote stress (81). For example, ROS, generated by NOX4, works as a signaling molecule downstream of integrin β1 to promote profibrotic gene expression in healthy fibroblasts and also mediates the fibrotic phenotype of scleroderma fibroblasts and animal models of scleroderma (70, 84, 110, 123). Conversely, ROS is also a key component of a stress response, for example, that generated in the endoplasmic reticulum during an unfolded protein response. This latter response can be cytotoxic. Thus, low levels of CCN1 may promote signaling via ROS but much higher amounts of CCN1, which may or may not be physiologically relevant, may cause cell mortality and, hence, indirectly due to causing (myo)fibroblast death, limit excessive collagen deposition in fibrotic models (11, 12, 48).
Of course, the physiological role of CCN1 entirely depends on which integrin is engaged; CCN1 promotes cell proliferation, survival, and angiogenesis via integrin αvβ3, but it promotes apoptosis and senescence through integrin α6β1 and heparan sulfate proteoglycans, as well as a sustained level of ROS (61). Thus, mutation of a single integrin α6β1 binding domain in CCN1 results in a senescence-defective CCN1 protein and enhances fibrotic responses to wounding (45). Conversely, CCN1 induces a proinflammatory M1-like genetic program in macrophages and promotes adhesion of activated macrophages via integrin αMβ2 and HSPGs (3). Thus, the observed results may differ depending on which integrin is being engaged by CCN1 and the relative affinity of CCN1 to each integrin.
In summation, CCN1 and CCN2 may generate distinct but overlapping activities and depending on the context, both appear to contribute to fibrosis and cancers. These observations suggest that careful modulation of the pathological effects of both proteins might be useful therapeutically to treat these conditions.
CCN3 (nov)
CCN3 is somewhat less studied that CCN1, but is equally confusing. CCN3, or nov, was initially discovered as an integration site for the MAV retrovirus in avian nephroblastomas (44). This gene was designated nov because it was highly expressed in all nephroblastomas, compared with adult kidney where its expression was low, yet detectable. In this initial report, CCN3 was observed to inhibit growth of chicken embryonic fibroblasts, and thus provided the first evidence that the CCN family contains negative regulators (44, 92). The COOH-terminal module of CCN3 was sufficient to induce a strong (80%) cellular growth inhibition, due to the ability of CCN3 to act as a brake on the cell cycle, resulting in an accumulation of CCN3-treated cells in the S-phase of the cell cycle (9). Thus emerged the notion of coregulation by CCN proteins where CCN3 may act as a counter-regulator interfering with a pathogenic process caused by another CCN protein (44, 91, 92, 103, 104).
The role of CCN3 in cancers appears to be stage-specific and complex. For a detailed essay, the reader is referred to Ref. 125. To explain what CCN3 may be doing in cancers, I will, for simplicity, focus on melanoma. CCN3 expression is initially high. Generally, the primary tumors and skin metastases appear to lose CCN3 expression; this loss appears to permit growth and invasion (27, 118). Conversely, CCN3 expression appears to be significantly higher in distant, visceral metastases, and to permit the establishment of tumor cells in their new location (118). Consistent with this notion, CCN3, when exogenously added to 1205Lu melanoma cells, decreased cell invasion and the transcription and activation of matrix metalloproteinase-2 and -9 (27). Conversely, the high levels of CCN3 at distal visceral sites are correlated with the ability of CCN3 to promote melanoma cells’ adhesive ability and integrin expression (118), which would permit cells to embed in their new location. Collectively, these data are consistent with the idea that loss of CCN3 permits the development of a fibrotic microenvironment, surrounding the initial tumor site, which is required for cancer cells to metastasize.
Indeed, the available data indicated that loss of CCN3 expression is permissive for fibrosis. In anti-Thy1-induced glomerulonephritis, CCN1 and CCN2 expression was increased, whereas CCN3 expression was decreased. Glomerular cell proliferation was positively correlated with CCN1 and CCN2 expression, but negatively correlated with CCN3 expression (121). In the mesangial cell culture model of diabetic renal fibrosis, CCN3 and CCN2 work in a yin/yang-like manner (105). When applied to mesangial cells, TGFβ induced CCN2, but suppressed CCN3 expression. Conversely, this ability was blocked in the presence of recombinant human CCN3 (rhCCN3) (105). Similarly, CCN3 blocks TGFβ-induced CCN1 and CCN2 mRNA expression in human dermal fibroblasts (91). Finally, exogenously added CCN3 blocked the fibrosis seen in the BTBR ob/ob mouse, and, more specifically, protected against, and even reversed, the podocyte loss and the establishment of glomerular hypertrophy in this model (103).
Generally speaking, CCN3 expression is high, and CCN1 and CCN2 expression low, during tissue homeostasis. During pathologies or in disease models, CCN1 and CCN2 expression increases, whereas CCN3 expression decreases. These data have led to the hypothesis that adding exogenous CCN3, by restoring the CCN1/2:CCN3 ratio to that found in healthy tissue, could be used as an antifibrotic therapy (103–105). Precisely why CCN3 behaves antagonistically to CCN1/CCN2 is unclear; it may act as a dominant negative by blocking CCN1/2 binding to the cell surface by binding to the same integrins engaged by CCN1/2, thus preventing CCN1/2 from bringing proteins binding CCN1/2 to the cell surface.
CCN4–6 (WISP1–3)
There is relatively little known about these three proteins. For a review on the role of CCN4 in cancers, the reader is referred elsewhere (86). Briefly, CCN4 appears to stimulate aggressive behaviors of various cancers, except in lung cancer and prostate cancer cells, where CCN4 does the opposite. In terms of fibrosis, CCN4 inhibition by a CCN4 monoclonal antibody in vivo significantly attenuated CCl4-induced liver injury and the progression of liver fibrosis (69). Similar results are also seen in a mouse model of pulmonary fibrosis; neutralizing monoclonal antibodies specific for CCN4 reduced the expression of genes characteristic of fibrosis and markedly attenuated lung fibrosis, by decreasing collagen deposition and improving lung function and survival (55). Recently, it was shown that CCN4 is a novel maker of obesity in diabetic patients (54).
CCN5, a ~29–35 kDa protein that lacks the heparin-binding carboxy-terminal domain, acts as a dominant negative molecule. CCN5 may be a novel therapeutic agent to treat cardiac hypertrophy and heart failure. CCN5 can reverse established cardiac fibrosis by inhibiting the generation of and enhancing apoptosis of myofibroblasts in the myocardium (43). CCN5 is also a tumor suppressor gene in pancreatic cancer. CCN5 protein is overexpressed in normal and preneoplastic cells, but it is mostly undetected or minimally detected in various pancreatic and breast cancer cell lines and tissue samples (4). Functional studies demonstrate that the exposure of pancreatic cancer cells to CCN5 recombinant protein reverses epithelial/mesenchymal transition (24). Whether CCN5 acts by soaking up proteins binding CCN1/CCN2 or whether CCN5 binds cell surface receptors without engaging additional receptors, such as heparan sulfate-containing proteoglycans, which bind the carboxy-terminal domain of CCN proteins is unclear.
The role of CCN6 is largely unknown; but Ccn6fl/fl;MMTV-Cre mice developed invasive mammary carcinomas with histopathological features and gene signatures resembling human spindle cell metaplastic breast carcinoma. Ccn6fl/fl;MMTV-Cre mammary tumors were highly aggressive and metastasized in 46% of the cases. CCN6 loss, due to mutations or other mechanisms, results in deregulated BMP4 and IGF signaling, with increased p-38/TAK1 activation, and activation AKT1 (79).
CONCLUSION
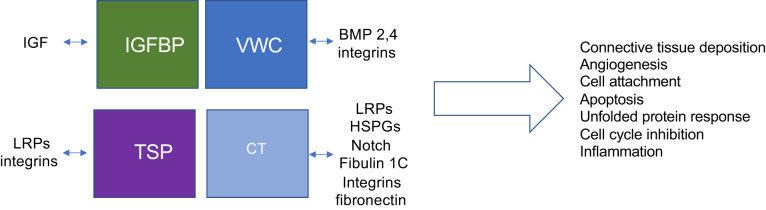
The available evidence strongly supports the idea that coordinated, collective action of CCN proteins and their partners controls fibrosis and cancer progression. The different actions that CCN proteins mediate vary widely, depending on the proteins and receptors they interact with, and the resultant downstream signaling pathways they stimulate or suppress. The outcome, therefore, also depends on the bioavailablity of the various partners, on the affinity of each protein for the particular partner, and the domains of each CCN molecule that are physically present in the microenvironment (Fig. 1). They truly form a cellular communication network that integrates signals that are occurring at a particular point in time. Indeed, stage-specific therapeutic intervention targeting CCN proteins is warranted.
Fig. 1.
Combinatorial interactions dictate cellular communication network (CCN) function. Each domain of CCN proteins interacts with different ligands. What CCN domains are present in the microenvironment depends on which CCN molecules are being synthesized [for example, CCN5 lacks the heparin-binding carboxy-terminal (CT) domain], protease activity, and differential splicing. The affinity of CCN molecules for each ligand is likely to vary. Thus, the overall biological effect of CCN proteins can vary widely depending on bioavailability of CCN proteins, their modules/fragments, and their interacting partners. CCN proteins act as central mediators of mechanotransduction. BMP, bone morphogenetic protein; HSPG, heparan sulfate proteoglycans; LRP, lipoprotein receptor-related protein; TSP, thrombospondin-1; VWC, von Willebrand factor C.
GRANTS
This work was supported in part by Arthritis Society Grant SOG-17-0039, Canadian Institutes of Health Research Grant MOP-77603, and Natural Sciences and Engineering Research Council of Canada Grant RGPIN-2016-04756.
DISCLOSURES
AL is a shareholder (less than 5% or $500,000) in FibroGen.
AUTHOR CONTRIBUTIONS
A.L. drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Adler SG, Schwartz S, Williams ME, Arauz-Pacheco C, Bolton WK, Lee T, Li D, Neff TB, Urquilla PR, Sewell KL. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin J Am Soc Nephrol 5: 1420–1428, 2010. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA 95: 6355–6360, 1998. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol 184: 3223–3232, 2010. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee SK, Maity G, Haque I, Ghosh A, Sarkar S, Gupta V, Campbell DR, Von Hoff D, Banerjee S. Human pancreatic cancer progression: an anarchy among CCN-siblings. J Cell Commun Signal 10: 207–216, 2016. doi: 10.1007/s12079-016-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee S, Ghosh A, VonHoff DD, Banerjee SK. Cyr61/CCN1 targets for chemosensitization in pancreatic cancer. Oncotarget 10: 3579–3580, 2019. doi: 10.18632/oncotarget.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batmunkh R, Nishioka Y, Aono Y, Azuma M, Kinoshita K, Kishi J, Makino H, Kishi M, Takezaki A, Sone S. CCN6 as a profibrotic mediator that stimulates the proliferation of lung fibroblasts via the integrin β1/focal adhesion kinase pathway. J Med Invest 58: 188–196, 2011. doi: 10.2152/jmi.58.188. [DOI] [PubMed] [Google Scholar]
- 7.Blalock TD, Gibson DJ, Duncan MR, Tuli SS, Grotendorst GR, Schultz GS. A connective tissue growth factor signaling receptor in corneal fibroblasts. Invest Ophthalmol Vis Sci 53: 3387–3394, 2012. doi: 10.1167/iovs.12-9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleau AM, Planque N, Perbal B. CCN proteins and cancer: two to tango. Front Biosci 10: 998–1009, 2005. doi: 10.2741/1594. [DOI] [PubMed] [Google Scholar]
- 9.Bleau AM, Planque N, Lazar N, Zambelli D, Ori A, Quan T, Fisher G, Scotlandi K, Perbal B. Antiproliferative activity of CCN3: involvement of the C-terminal module and post-translational regulation. J Cell Biochem 101: 1475–1491, 2007. doi: 10.1002/jcb.21262. [DOI] [PubMed] [Google Scholar]
- 10.Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett 327: 125–130, 1993. doi: 10.1016/0014-5793(93)80155-N. [DOI] [PubMed] [Google Scholar]
- 11.Borkham-Kamphorst E, Steffen BT, Van de Leur E, Tihaa L, Haas U, Woitok MM, Meurer SK, Weiskirchen R. Adenoviral CCN gene transfers induce in vitro and in vivo endoplasmic reticulum stress and unfolded protein response. Biochim Biophys Acta 1863: 2604–2612, 2016. doi: 10.1016/j.bbamcr.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Borkham-Kamphorst E, Steffen BT, van de Leur E, Haas U, Weiskirchen R. Portal myofibroblasts are sensitive to CCN-mediated endoplasmic reticulum stress-related apoptosis with potential to attenuate biliary fibrogenesis. Cell Signal 51: 72–85, 2018. doi: 10.1016/j.cellsig.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal 3: 163–165, 2009. doi: 10.1007/s12079-009-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner MC, Krzyzanski W, Chou JZ, Signore PE, Fung CK, Guzman D, Li D, Zhang W, Olsen DR, Nguyen VT, Koo CW, Sternlicht MD, Lipson KE. FG-3019, a human monoclonal antibody recognizing connective tissue growth factor, is subject to target-mediated drug disposition. Pharm Res 33: 1833–1849, 2016. doi: 10.1007/s11095-016-1918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brigstock DR. The CCN family: a new stimulus package. J Endocrinol 178: 169–175, 2003. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 16.Brigstock DR, Steffen CL, Kim GY, Vegunta RK, Diehl JR, Harding PA. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem 272: 20275–20282, 1997. doi: 10.1074/jbc.272.32.20275. [DOI] [PubMed] [Google Scholar]
- 17.Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeger H. Proposal for a unified CCN nomenclature. Mol Pathol 56: 127–128, 2003. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaqour B. Caught between a “Rho” and a hard place: are CCN1/CYR61 and CCN2/CTGF the arbiters of microvascular stiffness? J Cell Commun Signal In press. doi: 10.1007/s12079-019-00529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CC, Young JL, Monzon RI, Chen N, Todorović V, Lau LF. Cytotoxicity of TNFα is regulated by integrin-mediated matrix signaling. EMBO J 26: 1257–1267, 2007. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CY, Su CM, Huang YL, Tsai CH, Fuh LJ, Tang CH. CCN1 induces oncostatin M production in osteoblasts via integrin-dependent signal pathways. PLoS One 9: e106632, 2014. doi: 10.1371/journal.pone.0106632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N, Leu S-J, Todorovic V, Lam SC-T, Lau LF. Identification of a novel integrin αvβ3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J Biol Chem 279: 44166–44176, 2004. doi: 10.1074/jbc.M406813200. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Shi-wen X, Eastwood M, Black CM, Denton CP, Leask A, Abraham DJ. Contribution of activin receptor-like kinase 5 (transforming growth factor β receptor type I) signaling to the fibrotic phenotype of scleroderma fibroblasts. Arthritis Rheum 54: 1309–1316, 2006. doi: 10.1002/art.21725. [DOI] [PubMed] [Google Scholar]
- 23.Contreras O, Rossi FM, Brandan E. Adherent muscle connective tissue fibroblasts are phenotypically and biochemically equivalent to stromal fibro/adipogenic progenitors. Matrix Biology Plus 2: 100006, 2019. doi: 10.1016/j.mbplus.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhar G, Mehta S, Banerjee S, Gardner A, McCarty BM, Mathur SC, Campbell DR, Kambhampati S, Banerjee SK. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett 254: 63–70, 2007. doi: 10.1016/j.canlet.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Dornhöfer N, Spong S, Bennewith K, Salim A, Klaus S, Kambham N, Wong C, Kaper F, Sutphin P, Nacamuli R, Höckel M, Le Q, Longaker M, Yang G, Koong A, Giaccia A. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res 66: 5816–5827, 2006. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- 26.Fragiadaki M, Hill N, Hewitt R, Bou-Gharios G, Cook T, Tam FW, Domin J, Mason RM. Hyperglycemia causes renal cell damage via CCN2-induced activation of the TrkA receptor: implications for diabetic nephropathy. Diabetes 61: 2280–2288, 2012. doi: 10.2337/db11-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukunaga-Kalabis M, Martinez G, Telson SM, Liu ZJ, Balint K, Juhasz I, Elder DE, Perbal B, Herlyn M. Downregulation of CCN3 expression as a potential mechanism for melanoma progression. Oncogene 27: 2552–2560, 2008. doi: 10.1038/sj.onc.1210896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graness A, Cicha I, Goppelt-Struebe M. Contribution of Src-FAK signaling to the induction of connective tissue growth factor in renal fibroblasts. Kidney Int 69: 1341–1349, 2006. doi: 10.1038/sj.ki.5000296. [DOI] [PubMed] [Google Scholar]
- 29.Grazioli S, Gil S, An D, Kajikawa O, Farnand AW, Hanson JF, Birkland T, Chen P, Duffield J, Schnapp LM, Altemeier WA, Matute-Bello G. CYR61 (CCN1) overexpression induces lung injury in mice. Am J Physiol Lung Cell Mol Physiol 308: L759–L765, 2015. doi: 10.1152/ajplung.00190.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-β action on fibroblasts. Cytokine Growth Factor Rev 8: 171–179, 1997. doi: 10.1016/S1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 31.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor β response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ 7: 469–480, 1996. [PubMed] [Google Scholar]
- 32.Guo F, Carter DE, Leask A. Mechanical tension increases CCN2/CTGF expression and proliferation in gingival fibroblasts via a TGFβ-dependent mechanism. PLoS One 6: e19756, 2011. doi: 10.1371/journal.pone.0019756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo F, Hutchenreuther J, Carter DE, Leask A. TAK1 is required for dermal wound healing and homeostasis. J Invest Dermatol 133: 1646–1654, 2013. doi: 10.1038/jid.2013.28. [DOI] [PubMed] [Google Scholar]
- 34.Haque I, De A, Majumder M, Mehta S, McGregor D, Banerjee SK, Van Veldhuizen P, Banerjee S. The matricellular protein CCN1/Cyr61 is a critical regulator of Sonic Hedgehog in pancreatic carcinogenesis. J Biol Chem 287: 38569–38579, 2012. doi: 10.1074/jbc.M112.389064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol 287: F1223–F1232, 2004. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 36.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem 276: 10594–10601, 2001. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- 37.Holmes A, Abraham DJ, Chen Y, Denton C, Shi-wen X, Black CM, Leask A. Constitutive connective tissue growth factor expression in scleroderma fibroblasts is dependent on Sp1. J Biol Chem 278: 41728–41733, 2003. doi: 10.1074/jbc.M305019200. [DOI] [PubMed] [Google Scholar]
- 38.Huang YT, Lan Q, Lorusso G, Duffey N, Rüegg C. The matricellular protein CYR61 promotes breast cancer lung metastasis by facilitating tumor cell extravasation and suppressing anoikis. Oncotarget 8: 9200–9215, 2017. doi: 10.18632/oncotarget.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchenreuther J, Leask A. A tale of two origins: do myofibroblasts originate from different sources in wound healing and fibrosis? Cell Tissue Res 365: 507–509, 2016. doi: 10.1007/s00441-016-2419-5. [DOI] [PubMed] [Google Scholar]
- 40.Hutchenreuther J, Vincent KM, Carter DE, Postovit LM, Leask A. CCN2 expression by tumor stroma is required for melanoma metastasis. J Invest Dermatol 135: 2805–2813, 2015. doi: 10.1038/jid.2015.279. [DOI] [PubMed] [Google Scholar]
- 41.Hutchenreuther J, Vincent K, Norley C, Racanelli M, Gruber SB, Johnson TM, Fullen DR, Raskin L, Perbal B, Holdsworth DW, Postovit LM, Leask A. Activation of cancer-associated fibroblasts is required for tumor neovascularization in a murine model of melanoma. Matrix Biol 74: 52–61, 2018. doi: 10.1016/j.matbio.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 4: 637–645, 1993. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong D, Lee MA, Li Y, Yang DK, Kho C, Oh JG, Hong G, Lee A, Song MH, LaRocca TJ, Chen J, Liang L, Mitsuyama S, D’Escamard V, Kovacic JC, Kwak TH, Hajjar RJ, Park WJ. Matricellular protein CCN5 reverses established cardiac fibrosis. J Am Coll Cardiol 67: 1556–1568, 2016. [Erratum in J Am Coll Cardiol 67: 2809, 2016.] doi: 10.1016/j.jacc.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J, Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol 12: 10–21, 1992. doi: 10.1128/MCB.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685, 2010. [Erratum in Nat Cell Biol 12: 1249, 2010.] doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 10: 945–963, 2011. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jun JI, Lau LF. CCN2 induces cellular senescence in fibroblasts. J Cell Commun Signal 11: 15–23, 2017. doi: 10.1007/s12079-016-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juric V, Chen CC, Lau LF. TNFα-induced apoptosis enabled by CCN1/CYR61: pathways of reactive oxygen species generation and cytochrome c release. PLoS One 7: e31303, 2012. doi: 10.1371/journal.pone.0031303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaasbøll OJ, Gadicherla AK, Wang JH, Monsen VT, Hagelin EMV, Dong MQ, Attramadal H. Connective tissue growth factor (CCN2) is a matricellular preproprotein controlled by proteolytic activation. J Biol Chem 293: 17953–17970, 2018. doi: 10.1074/jbc.RA118.004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapoor M, Liu S, Huh K, Parapuram S, Kennedy L, Leask A. Connective tissue growth factor promoter activity in normal and wounded skin. Fibrogenesis Tissue Repair 1: 3, 2008. doi: 10.1186/1755-1536-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawata K, Kubota S, Eguchi T, Aoyama E, Moritani NH, Kondo S, Nishida T, Takigawa M. Role of LRP1 in transport of CCN2 protein in chondrocytes. J Cell Sci 125: 2965–2972, 2012. doi: 10.1242/jcs.101956. [DOI] [PubMed] [Google Scholar]
- 52.Khankan R, Oliver N, He S, Ryan SJ, Hinton DR. Regulation of fibronectin-EDA through CTGF domain-specific interactions with TGFβ2 and its receptor TGFβRII. Invest Ophthalmol Vis Sci 52: 5068–5078, 2011. doi: 10.1167/iovs.11-7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiwanuka E, Andersson L, Caterson EJ, Junker JP, Gerdin B, Eriksson E. CCN2 promotes keratinocyte adhesion and migration via integrin α5β1. Exp Cell Res 319: 2938–2946, 2013. doi: 10.1016/j.yexcr.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 54.Klimontov VV, Bulumbaeva DM, Fazullina ON, Lykov AP, Bgatova NP, Orlov NB, Konenkov VI, Pfeiffer AFH, Pivovarova-Ramich O, Rudovich N. Circulating Wnt1-inducible signaling pathway protein-1 (WISP-1/CCN4) is a novel biomarker of adiposity in subjects with type 2 diabetes. J Cell Commun Signal November 28, 2019. doi: 10.1007/s12079-019-00536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Günther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119: 772–787, 2009. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kramann R, DiRocco DP, Humphreys BD. Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J Pathol 231: 273–289, 2013. doi: 10.1002/path.4253. [DOI] [PubMed] [Google Scholar]
- 57.Kuk H, Hutchenreuther J, Murphy-Marshman H, Carter D, Leask A. 5Z-7-oxozeanol inhibits the effects of TGFβ1 on human gingival fibroblasts. PLoS One 10: e0123689, 2015. doi: 10.1371/journal.pone.0123689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kunz M, Moeller S, Koczan D, Lorenz P, Wenger RH, Glocker MO, Thiesen HJ, Gross G, Ibrahim SM. Mechanisms of hypoxic gene regulation of angiogenesis factor Cyr61 in melanoma cells. J Biol Chem 278: 45651–45660, 2003. doi: 10.1074/jbc.M301373200. [DOI] [PubMed] [Google Scholar]
- 59.Kurundkar AR, Kurundkar D, Rangarajan S, Locy ML, Zhou Y, Liu RM, Zmijewski J, Thannickal VJ. The matricellular protein CCN1 enhances TGF-β1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury. FASEB J 30: 2135–2150, 2016. doi: 10.1096/fj.201500173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lagares D, Busnadiego O, García-Fernández RA, Kapoor M, Liu S, Carter DE, Abraham D, Shi-Wen X, Carreira P, Fontaine BA, Shea BS, Tager AM, Leask A, Lamas S, Rodríguez-Pascual F. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum 64: 1653–1664, 2012. doi: 10.1002/art.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci 68: 3149–3163, 2011. doi: 10.1007/s00018-011-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal 10: 121–127, 2016. doi: 10.1007/s12079-016-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res 248: 44–57, 1999. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 64.Leask A. A centralized communication network: recent insights into the role of the cancer associated fibroblast in the development of drug resistance in tumors. Semin Cell Dev Biol. In press. doi: 10.1016/j.semcdb.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 65.Leask A. Breathe, breathe in the air: the anti-CCN2 antibody pamrevlumab (FG-3019) completes a successful phase II clinical trial for idiopathic pulmonary fibrosis. J Cell Commun Signal 13: 441–442, 2019. doi: 10.1007/s12079-019-00542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci 119: 4803–4810, 2006. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 67.Leask A, Sa S, Holmes A, Shiwen X, Black CM, Abraham DJ. The control of ccn2 (ctgf) gene expression in normal and scleroderma fibroblasts. Mol Pathol 54: 180–183, 2001. doi: 10.1136/mp.54.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-β2 in fibroblasts. J Biol Chem 278: 13008–13015, 2003. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Chen Y, Ye W, Tao X, Zhu J, Wu S, Lou L. Blockade of CCN4 attenuates CCl4-induced liver fibrosis. Arch Med Sci 11: 647–653, 2015. doi: 10.5114/aoms.2015.52371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu S, Leask A. Integrin β1 is required for dermal homeostasis. J Invest Dermatol 133: 899–906, 2013. doi: 10.1038/jid.2012.438. [DOI] [PubMed] [Google Scholar]
- 71.Liu S, Kapoor M, Denton CP, Abraham DJ, Leask A. Loss of β1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum 60: 2817–2821, 2009. doi: 10.1002/art.24801. [DOI] [PubMed] [Google Scholar]
- 72.Liu S, Kapoor M, Leask A. Rac1 expression by fibroblasts is required for tissue repair in vivo. Am J Pathol 174: 1847–1856, 2009. doi: 10.2353/ajpath.2009.080779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu S, Taghavi R, Leask A. Connective tissue growth factor is induced in bleomycin-induced skin scleroderma. J Cell Commun Signal 4: 25–30, 2010. doi: 10.1007/s12079-009-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu S, Shi-wen X, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum 63: 239–246, 2011. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- 75.Liu S, Parapuram SK, Leask A. Fibrosis caused by loss of PTEN expression in mouse fibroblasts is crucially dependent on CCN2. Arthritis Rheum 65: 2940–2944, 2013. doi: 10.1002/art.38121. [DOI] [PubMed] [Google Scholar]
- 76.Liu S, Thompson K, Leask A. CCN2 expression by fibroblasts is not required for cutaneous tissue repair. Wound Repair Regen 22: 119–124, 2014. doi: 10.1111/wrr.12131. [DOI] [PubMed] [Google Scholar]
- 77.Maity G, Mehta S, Haque I, Dhar K, Sarkar S, Banerjee SK, Banerjee S. Pancreatic tumor cell secreted CCN1/Cyr61 promotes endothelial cell migration and aberrant neovascularization. Sci Rep 4: 4995, 2015. doi: 10.1038/srep04995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maity G, Haque I, Ghosh A, Dhar G, Gupta V, Sarkar S, Azeem I, McGregor D, Choudhary A, Campbell DR, Kambhampati S, Banerjee SK, Banerjee S. The MAZ transcription factor is a downstream target of the oncoprotein Cyr61/CCN1 and promotes pancreatic cancer cell invasion via CRAF-ERK signaling. J Biol Chem 293: 4334–4349, 2018. doi: 10.1074/jbc.RA117.000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin EE, Huang W, Anwar T, Arellano-Garcia C, Burman B, Guan JL, Gonzalez ME, Kleer CG. MMTV-cre;Ccn6 knockout mice develop tumors recapitulating human metaplastic breast carcinomas. Oncogene 36: 2275–2285, 2017. doi: 10.1038/onc.2016.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mason RM. Fell-Muir lecture: Connective tissue growth factor (CCN2)—a pernicious and pleiotropic player in the development of kidney fibrosis. Int J Exp Pathol 94: 1–16, 2013. doi: 10.1111/j.1365-2613.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol 80: 50–64, 2018. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 82.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-β in persistent fibrosis: a mouse fibrosis model. J Cell Physiol 181: 153–159, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 83.Mori Y, Hinchcliff M, Wu M, Warner-Blankenship M, Lyons KM, Varga J. Connective tissue growth factor/CCN2-null mouse embryonic fibroblasts retain intact transforming growth factor-β responsiveness. Exp Cell Res 314: 1094–1104, 2008. doi: 10.1016/j.yexcr.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy-Marshman H, Quesnel K, Shi-Wen X, Barnfield R, Kelly J, Peidl A, Stratton RJ, Leask A. Antioxidants and NOX1/NOX4 inhibition blocks TGFβ1-induced CCN2 and α-SMA expression in dermal and gingival fibroblasts. PLoS One 12: e0186740, 2017. doi: 10.1371/journal.pone.0186740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci USA 110: 12325–12330, 2013. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nivison MP, Meier KE. The role of CCN4/WISP-1 in the cancerous phenotype. Cancer Manag Res 10: 2893–2903, 2018. doi: 10.2147/CMAR.S133915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ono M, Masaki A, Maeda A, Kilts TM, Hara ES, Komori T, Pham H, Kuboki T, Young MF. CCN4/WISP1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts via α5β1 and TNFα. Matrix Biol 68-69: 533–546, 2018. doi: 10.1016/j.matbio.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parapuram SK, Shi-wen X, Elliott C, Welch ID, Jones H, Baron M, Denton CP, Abraham DJ, Leask A. Loss of PTEN expression by dermal fibroblasts causes skin fibrosis. J Invest Dermatol 131: 1996–2003, 2011. doi: 10.1038/jid.2011.156. [DOI] [PubMed] [Google Scholar]
- 89.Parapuram SK, Thompson K, Tsang M, Hutchenreuther J, Bekking C, Liu S, Leask A. Loss of PTEN expression by mouse fibroblasts results in lung fibrosis through a CCN2-dependent mechanism. Matrix Biol 43: 35–41, 2015. doi: 10.1016/j.matbio.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 90.Park MH, Kim AK, Manandhar S, Oh SY, Jang GH, Kang L, Lee DW, Hyeon DY, Lee SH, Lee HE, Huh TL, Suh SH, Hwang D, Byun K, Park HC, Lee YM. CCN1 interlinks integrin and hippo pathway to autoregulate tip cell activity. eLife 8: August 20, 2019. doi: 10.7554/eLife.46012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peidl A, Perbal B, Leask A. Yin/Yang expression of CCN family members: transforming growth factor β1, via ALK5/FAK/MEK, induces CCN1 and CCN2, yet suppresses CCN3, expression in human dermal fibroblasts. PLoS One 14: e0218178, 2019. doi: 10.1371/journal.pone.0218178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol 54: 57–79, 2001. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perbal B. New insight into CCN3 interactions–nuclear CCN3 : fact or fantasy? Cell Commun Signal 4: 6, 2006. doi: 10.1186/1478-811X-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perbal B. Alternative splicing of CCN mRNAs .... it has been upon us. J Cell Commun Signal 3: 153–157, 2009. doi: 10.1007/s12079-009-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perbal B. The concept of the CCN protein family revisited: a centralized coordination network. J Cell Commun Signal 12: 3–12, 2018. doi: 10.1007/s12079-018-0455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perbal A, Perbal B. The CCN family of proteins: a 25th anniversary picture. J Cell Commun Signal 10: 177–190, 2016. doi: 10.1007/s12079-016-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perbal B, Tweedie S, Bruford E. The official unified nomenclature adopted by the HGNC calls for the use of the acronyms, CCN1-6, and discontinuation in the use of CYR61, CTGF, NOV and WISP 1-3 respectively. J Cell Commun Signal 12: 625–629, 2018. [Erratum in J Cell Commun Signal 13: 435, 2019.] doi: 10.1007/s12079-018-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pi L, Robinson PM, Jorgensen M, Oh SH, Brown AR, Weinreb PH, Trinh TL, Yianni P, Liu C, Leask A, Violette SM, Scott EW, Schultz GS, Petersen BE. Connective tissue growth factor and integrin αvβ6: a new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology 61: 678–691, 2015. doi: 10.1002/hep.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quesnel K, Shi-wen X, Hutchenreuther J, Xiao Z, Liu S, Peidl A, Naskar D, Siquiera WL. O’Gorman DB, Hinz B, Stratton RJ, Leask A. CCN1 expression by fibroblasts is required for bleomycin-induced skin fibrosis. Matrix Biol Plus 3: 100009, 2019. doi: 10.1016/j.mbplus.2019.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rajkumar VS, Sundberg C, Abraham DJ, Rubin K, Black CM. Activation of microvascular pericytes in autoimmune Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum 42: 930–941, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 101.Reid SE, Kay EJ, Neilson LJ, Henze AT, Serneels J, McGhee EJ, Dhayade S, Nixon C, Mackey JB, Santi A, Swaminathan K, Athineos D, Papalazarou V, Patella F, Román-Fernández Á, ElMaghloob Y, Hernandez-Fernaud JR, Adams RH, Ismail S, Bryant DM, Salmeron-Sanchez M, Machesky LM, Carlin LM, Blyth K, Mazzone M, Zanivan S. Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J 36: 2373–2389, 2017. doi: 10.15252/embj.201694912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richeldi L, Fernández Pérez ER, Costabel U, Albera C, Lederer DJ, Flaherty KR, Ettinger N, Perez R, Scholand MB, Goldin J, Peony Yu KH, Neff T, Porter S, Zhong M, Gorina E, Kouchakji E, Raghu G. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 8: 25–33, 2020. doi: 10.1016/S2213-2600(19)30262-0. [DOI] [PubMed] [Google Scholar]
- 103.Riser BL, Najmabadi F, Garchow K, Barnes JL, Peterson DR, Sukowski EJ. Treatment with the matricellular protein CCN3 blocks and/or reverses fibrosis development in obesity with diabetic nephropathy. Am J Pathol 184: 2908–2921, 2014. doi: 10.1016/j.ajpath.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 104.Riser BL, Barnes JL, Varani J. Balanced regulation of the CCN family of matricellular proteins: a novel approach to the prevention and treatment of fibrosis and cancer. J Cell Commun Signal 9: 327–339, 2015. doi: 10.1007/s12079-015-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riser BL, Najmabadi F, Perbal B, Peterson DR, Rambow JA, Riser ML, Sukowski E, Yeger H, Riser SC. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathol 174: 1725–1734, 2009. doi: 10.2353/ajpath.2009.080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sakai N, Nakamura M, Lipson KE, Miyake T, Kamikawa Y, Sagara A, Shinozaki Y, Kitajima S, Toyama T, Hara A, Iwata Y, Shimizu M, Furuichi K, Kaneko S, Tager AM, Wada T. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci Rep 7: 5392, 2017. doi: 10.1038/s41598-017-05624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR III, Carmichael DF. The low density lipoprotein receptor-related protein/α2-macroglobulin receptor is a receptor for connective tissue growth factor. J Biol Chem 276: 40659–40667, 2001. doi: 10.1074/jbc.M105180200. [DOI] [PubMed] [Google Scholar]
- 108.Shi-Wen X, Rodríguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, Dashwood MR, du Bois RM, Denton CP, Black CM, Abraham DJ, Leask A. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol 26: 5518–5527, 2006. doi: 10.1128/MCB.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi-wen X, Stanton LA, Kennedy L, Pala D, Chen Y, Howat SL, Renzoni EA, Carter DE, Bou-Gharios G, Stratton RJ, Pearson JD, Beier F, Lyons KM, Black CM, Abraham DJ, Leask A. CCN2 is necessary for adhesive responses to transforming growth factor-β1 in embryonic fibroblasts. J Biol Chem 281: 10715–10726, 2006. doi: 10.1074/jbc.M511343200. [DOI] [PubMed] [Google Scholar]
- 110.Shi-wen X, Thompson K, Khan K, Liu S, Murphy-Marshman H, Baron M, Denton CP, Leask A, Abraham DJ. Focal adhesion kinase and reactive oxygen species contribute to the persistent fibrotic phenotype of lesional scleroderma fibroblasts. Rheumatology (Oxford) 51: 2146–2154, 2012. doi: 10.1093/rheumatology/kes234. [DOI] [PubMed] [Google Scholar]
- 111.Subramaniam MM, Lazar N, Navarro S, Perbal B, Llombart-Bosch A. Expression of CCN3 protein in human Wilms’ tumors: immunohistochemical detection of CCN3 variants using domain-specific antibodies. Virchows Arch 452: 33–39, 2008. doi: 10.1007/s00428-007-0523-3. [DOI] [PubMed] [Google Scholar]
- 112.Takigawa M. An early history of CCN2/CTGF research: the road to CCN2 via hcs24, ctgf, ecogenin, and regenerin. J Cell Commun Signal 12: 253–264, 2018. doi: 10.1007/s12079-017-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-β1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 278: 12384–12389, 2003. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 114.Thompson K, Murphy-Marshman H, Leask A. ALK5 inhibition blocks TGFβ-induced CCN1 expression in human foreskin fibroblasts. J Cell Commun Signal 8: 59–63, 2014. doi: 10.1007/s12079-014-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Todorovic V, Chen CC, Hay N, Lau LF. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J Cell Biol 171: 559–568, 2005. doi: 10.1083/jcb.200504015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tsang M, Leask A. CCN2 is required for recruitment of Sox2-expressing cells during cutaneous tissue repair. J Cell Commun Signal 9: 341–346, 2015. doi: 10.1007/s12079-014-0245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsang M, Quesnel K, Vincent K, Hutchenreuther J, Postovit LM, Leask A. Insights into fibroblast plasticity: cellular communication network 2 is required for activation of cancer-associated fibroblasts in a murine model of melanoma. Am J Pathol 190: 206–221, 2020. doi: 10.1016/j.ajpath.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 118.Vallacchi V, Daniotti M, Ratti F, Di Stasi D, Deho P, De Filippo A, Tragni G, Balsari A, Carbone A, Rivoltini L, Parmiani G, Lazar N, Perbal B, Rodolfo M. CCN3/nephroblastoma overexpressed matricellular protein regulates integrin expression, adhesion, and dissemination in melanoma. Cancer Res 68: 715–723, 2008. doi: 10.1158/0008-5472.CAN-07-2103. [DOI] [PubMed] [Google Scholar]
- 119.Valle-Tenney R, Rebolledo DL, Lipson KE, Brandan E. Role of hypoxia in skeletal muscle fibrosis: synergism between hypoxia and TGF-β signaling upregulates CCN2/CTGF expression specifically in muscle fibers. Matrix Biol In press. [DOI] [PubMed] [Google Scholar]
- 120.Van Beek JP, Kennedy L, Rockel JS, Bernier SM, Leask A. The induction of CCN2 by TGFβ1 involves Ets-1. Arthritis Res Ther 8: R36, 2006. doi: 10.1186/ar1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Roeyen CR, Eitner F, Scholl T, Boor P, Kunter U, Planque N, Gröne HJ, Bleau AM, Perbal B, Ostendorf T, Floege J. CCN3 is a novel endogenous PDGF-regulated inhibitor of glomerular cell proliferation. Kidney Int 73: 86–94, 2008. doi: 10.1038/sj.ki.5002584. [DOI] [PubMed] [Google Scholar]
- 122.Wahab NA, Weston BS, Mason RM. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J Am Soc Nephrol 16: 340–351, 2005. doi: 10.1681/ASN.2003100905. [DOI] [PubMed] [Google Scholar]
- 123.Wermuth PJ, Mendoza FA, Jimenez SA. Abrogation of transforming growth factor-β-induced tissue fibrosis in mice with a global genetic deletion of Nox4. Lab Invest 99: 470–482, 2019. doi: 10.1038/s41374-018-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu SW, Liu S, Eastwood M, Sonnylal S, Denton CP, Abraham DJ, Leask A. Rac inhibition reverses the phenotype of fibrotic fibroblasts. PLoS One 4: e7438, 2009. doi: 10.1371/journal.pone.0007438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yeger H, Perbal B. CCN family of proteins: critical modulators of the tumor cell microenvironment. J Cell Commun Signal 10: 229–240, 2016. doi: 10.1007/s12079-016-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.You JJ, Yang CM, Chen MS, Yang CH. Regulation of Cyr61/CCN1 expression by hypoxia through cooperation of c-Jun/AP-1 and HIF-1α in retinal vascular endothelial cells. Exp Eye Res 91: 825–836, 2010. doi: 10.1016/j.exer.2010.10.006. [DOI] [PubMed] [Google Scholar]