Abstract
Tetraspanin-2A (Tsp2A) is an integral membrane protein of smooth septate junctions in Drosophila melanogaster. To elucidate its structural and functional roles in Malpighian tubules, we used the c42-GAL4/UAS system to selectively knock down Tsp2A in principal cells of the tubule. Tsp2A localizes to smooth septate junctions (sSJ) in Malpighian tubules in a complex shared with partner proteins Snakeskin (Ssk), Mesh, and Discs large (Dlg). Knockdown of Tsp2A led to the intracellular retention of Tsp2A, Ssk, Mesh, and Dlg, gaps and widening spaces in remaining sSJ, and tumorous and cystic tubules. Elevated protein levels together with diminished V-type H+-ATPase activity in Tsp2A knockdown tubules are consistent with cell proliferation and reduced transport activity. Indeed, Malpighian tubules isolated from Tsp2A knockdown flies failed to secrete fluid in vitro. The absence of significant transepithelial voltages and resistances manifests an extremely leaky epithelium that allows secreted solutes and water to leak back to the peritubular side. The tubular failure to excrete fluid leads to extracellular volume expansion in the fly and to death within the first week of adult life. Expression of the c42-GAL4 driver begins in Malpighian tubules in the late embryo and progresses upstream to distal tubules in third instar larvae, which can explain why larvae survive Tsp2A knockdown and adults do not. Uncontrolled cell proliferation upon Tsp2A knockdown confirms the role of Tsp2A as tumor suppressor in addition to its role in sSJ structure and transepithelial transport.
Keywords: drosokinin, Drosophila, paracellular barrier, smooth septate junctions, tetraspanin, tumors and cysts
INTRODUCTION
Septate junctions are the intercellular junctions of epithelia in arthropods (39). Like tight junctions in epithelia of vertebrates, septate junctions define the barrier and permselectivity properties of the paracellular pathway, i.e., the route between the cells of epithelia and endothelia (12, 69). In addition to their role in paracellular transport, septate junctions are involved in 1) adhesion of epithelial sheets and tubes (4, 29, 43), 2) signaling to the nucleus (44, 81), 3) defense against pathogens (3, 23), 4) the development of epithelia (28), and 5) the replacement of damaged epithelial cells (55, 86). In Drosophila, septate junctions were first identified in salivary glands as a belt-like ring surrounding epithelial/endothelial cells near the apical surface (80). Two morphological variants of septate junctions have been described in insects. Pleated septate junctions (pSJ), usually found in tissues derived from ectoderm, appear as undulating rows in oblique lanthanum-treated sections prepared for electron microscopy. Smooth septate junctions (sSJ) are usually found in tissues derived from endoderm. They appear as parallel rows under the electron microscope (49, 75). More than 20 proteins are associated with pSJs, including Sinuous, Megatrachea, and Kune-kune, which are Drosophila homologs of vertebrate claudins, namely those proteins that define the barrier and permselectivity properties of vertebrate epithelia and endothelia (6, 40, 59, 85). The recent identification of the first proteins that are uniquely associated with smooth septate junctions, Snakeskin (Ssk), Mesh, and Tetraspanin 2A (Tsp2A), indicates that the differences between pSJs and sSJs are also molecular and not just ultrastructural (40, 41, 88). Moreover, the genetic manipulation of each of the first three sSJ proteins has strikingly similar structural and functional consequences for the Drosophila midgut, suggesting the requirement of a Ssk/Mesh/Tsp2A complex for 1) the assembly of sSJs at the paracellular pathway in apicolateral regions, and 2) the barrier function of the paracellular pathway.
Smooth septate junctions are also present in Malpighian tubules of Drosophila, and the sSJ protein Mesh has recently been shown necessary for normal tubule structure and function (42). In view of our good understanding of the mechanism and regulation of epithelial transport in this renal epithelium, we examined the effect of knocking down Tsp2A on the structure and function of Malpighian tubules. Tsp2A was knocked down in specifically principal cells of Malpighian tubules, leaving the expression of Tsp2A in stellate cells and in other epithelia of the fly intact. The knockdown of Tsp2A in principal cells of Malpighian tubules was lethal to adult flies within 1 wk of eclosion for at least three reasons: 1) disrupted septate junctions and tubule organization, 2) an extremely leaky tubule unable to excrete fluid for lack of epithelial barriers, and 3) Malpighian tubules with tumor-like and cyst-like structures. These tubular defects apparently lead to renal failure, generalized edema, collapse of extracellular fluid homeostasis, and death of young adult flies.
MATERIALS AND METHODS
Fly stocks.
The following fly stocks were obtained from the Bloomington Stock Center (BDSC, Bloomington, IN): BL 5905 [Research Resource Identifiers (RRID):BDSC 5905, white1118], BL 5428 (RRID:BDSC 5428, UAS-eGFP) and BL 57349 (RRID:BDSC 57349, act5C-GAL4), and BL 6658 (BRID:BSC 658, UAS-2xEGFP). The v101081-line (UAS-Tsp2A-RNAi) was obtained from the Vienna Drosophila Resource Center (VDRC, Vienna, Austria) and the 11415-R2 line (UAS-Tsp2A RNAi) from the National Institute of Genetics (NIG-Fly, Mishima, Shizuoka, Japan). The c42-GAL4 driver line was obtained from Julian Dow (University of Glasgow, Scotland, UK). Flies were reared under standard conditions at 22°C on cornmeal agar and a 12:12-light-dark cycle. The UAS-GAL4 system (27, 62) was used for RNAi-mediated Tsp2A knockdown. Crosses were reared and maintained at 18°C or 27°C, respectively, to utilize the temperature sensitivity of the UAS-GAL4 system (60).
The abdominal volume of flies was estimated from optical measurements of the long and short axes of the abdomen and by considering the abdomen a prolate spheroid, i.e., abdominal volume = (4/3π)ab2, where a and b are the radii of the major and minor axes, respectively.
Driver specificity.
The spatial and temporal expression of c42-GAL4 was verified by crossing the respective line to UAS-eGFP (Fig. 1). In the fluid-secreting main segment of the Malpighian tubule, c42-GAL4 drives expression in principal cells to the exclusion of stellate cells (62), see also Fig. 1, G–I.
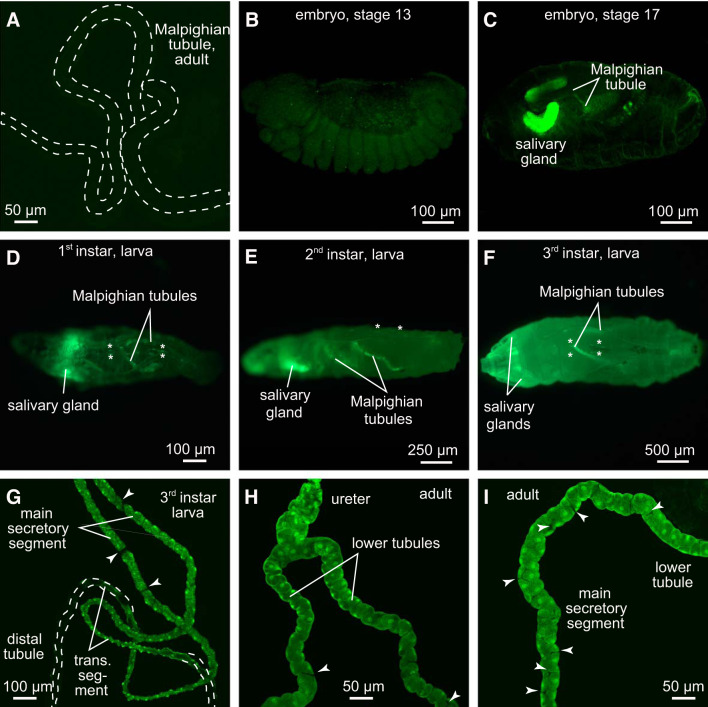
Fig. 1.
Verification of c42-GAL4 activity in Malpighian tubules of female Drosophila melanogaster. A: control stain (w;+/+;c42-GAL4/+) yields Malpighian tubules of adult Drosophila melanogaster lacking enhanced green fluorescent protein (eGFP). B–I: eGFP expression in w;+/+;c42-GAL4/UAS-eGFP. B: stage 13 embryo;no distinct eGFP expression is visible. C: stage 17 embryo;first eGFP expression becomes apparent in Malpighian tubules and salivary glands. D–F: c42-GAL4 activity in 1st, 2nd, and 3rd larval instars, respectively. Additional eGFP expression is visible in salivary gland cells and in pericardial cells (asterisks). G: tubules isolated from 3rd instar larvae. H and I: adult Malpighian tubules. Arrowheads in G–I point to stellate cells that do not express eGFP.
Quantitative RT-PCR.
We used 1) the Tsp2A-RNAi lines v101081 (VDRC) and 11415-R2 (NIG-Fly) to assess the general knockdown efficiency of the two lines, and 2) the ubiquitously active act5C-GAL4 driver to induce RNAi hairpin expression. Total-RNA isolated from 1-day-old adult males (RNeasy Mini Kit, Qiagen, Hilden, Germany) was treated with DNase I (Invitrogen, Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions and used as a template for cDNA synthesis (Luna Script Reverse Transcriptase, New England Biolabs, Ipswich, MA). We performed qRT-PCR according to standard protocols using “ORA qPCR Green ROX L Mix” (highQu, Kraichtal, Germany) and an iCycler iQ Real-Time PCR System (Bio-Rad, Munich, Germany). Primer pairs were designed with “QuantPrime” (2) applying the presettings to consider only regions containing at least one intron and to accept splice variant hits. Data were evaluated as described in Ref. 66. rp49 was used as the reference gene. Primer pairs used were 5′-cacaaatggcgcaagcccaag-3′ (rp49 forward), 5′-cattttttaactaaaagtccg-3′ (rp49 reverse); 5′-acgagaatcgacccaagcaagc-3′ (Tsp2A forward), and 5′-atcatccaggccacgatgttgaag-3′ (Tsp2A reverse). At least three biological replicates, consisting of three technical replicates each, were performed.
Malpighian tubules.
Malpighian tubules were isolated for morphological and physiological studies from adult female flies not older than 5 days. After the fly was anesthetized on ice for 10 min, the fly was submerged in basic Ringer solution on ice and the anterior pair of Malpighian tubules was isolated using forceps Drummond #5. Care was taken not to handle or touch the tubules. Typically, the pair of anterior Malpighian tubules together with the ureter and a piece of gut was removed from the fly and immediately prepared for light microscopy, immunostaining, and electron microscopy or used for measurements of 1) fluid secretion rates by the method of Ramsay (61) modified by us (10, 63), 2) transepithelial voltage and resistance by the method of Beyenbach (9), and 3) V-ATPase activity by the method of Tiburcy et al. (76). When fluid secretion rate was not measured directly as in electrophysiological studies, the downstream movement of luminal precipitates, calcium oxalate, xanthines, and uric acid (20, 24, 45, 58) indicates the active transepithelial secretion of fluid.
Ringer solutions.
The basic Ringer solution (BRS) contained the following (in mM): 117.5 NaCl, 20 KCl, 2 CaCl2, 8.5 MgCl2, 10.2 NaHCO3, 15 HEPES, 4.3 NaH2PO4, and 20 glucose. After adjustment of the pH to 7, the average [H+] was 9.44 × 10−8 ± 7.45 × 10−9 in 15 solutions, ~pH 7.03. The average osmotic pressure of BRS was 329.4 ± 9.9 mosmol/kgH2O (n = 15). Phosphate buffer saline (PBS) consisted of the following (in mM): 137 NaCl, 2.7 KCl, 8 Na2HPO4, and 1.5 KH2PO4 adjusted to pH 7.4. The diuretic hormone drosokinin (JPT Peptides, Berlin) was used at a concentration of 1 µM.
Electrophysiology.
Stable microelectrode impalements of epithelial cells and tubule lumen require the immobilization of Malpighian tubules so that the tubule does not roll during attempts to impale epithelial cells or the tubule lumen with microelectrodes. Poly-lysine (Sigma-Aldrich, St. Louis, MO) was used toward that end (56) by coating glass coverslips on the bottom of the perfusion bath with 9 short puffs of 0.1 mM poly-lysine from a vaporizer. The poly-lysine was allowed to dry at room temperature after each three puffs. The poly-lysine coverslips were then stored in the refrigerator until installing them to form the bottom of the perfusion bath. Although the tubules stick to the poly-lysine glass surface they can still be straightened along the axis of the microelectrode impalement by pulling on the small piece of gut still attached to the ureter of the tubule pair. The bath volume was 150 µL. Inflow and outflow lines allowed the change of the perfusion bath. The perfusion bath was mounted under a Leica Stereoscope MZ95 for viewing the isolated Malpighian tubule at magnifications ranging from ×16 to ×150. Microelectrodes were pulled with a Sutter P-97 Flaming/Brown microelectrode puller and filled with 3 M KCl. The electrical resistance for both current-injection and voltage electrodes was on average 45.0 ± 1.9 MΩ (n = 82). The methods for measuring membrane and transepithelial voltages and resistances in isolated Malpighian tubules have been described recently (9). In view of the geometric inconsistency of the tubule lumen in Malpighian tubules of Tsp2A knockdown flies, measurements of transepithelial resistance were not possible. Instead, the input resistances between the voltage and current electrodes (at constant distance ~600 μm apart) were compared, first with both electrodes in the peritubular bath and then with both electrodes in the tubule lumen or cyst.
Immunohistochemistry.
In the Osnabrück laboratory, isolated Malpighian tubules were first transferred to PBS and then fixed in 4% PFA (paraformaldehyde) for 60 min. The tubules were then washed three times with PBS and then permeabilized with 0.1% Triton X-100 for 60 min and then blocked with Rotiblock (Carl Roth, Karlsruhe, Germany) for 45 min. Rabbit anti-Tsp2A (302AP, 1:1,000) provided by the Furuse laboratory (40) and a Cy2-conjugated secondary antibody (Dianova, 1:200) were used for the immunolocalization of Tsp2A shown in Fig. 5. The primary antibodies were incubated overnight at 4°C. Images were acquired with a Zeiss LSM 5 Pascal and the Zen Blue software (Zeiss, Jena, Germany). Stained tissues were embedded in Fluoromount-G DAPI (Thermo Fisher Scientific, Waltham, MA). Images were processed with Affinity Photo (Serif Europe Ltd., Nottingham, England, UK).
Fig. 5.
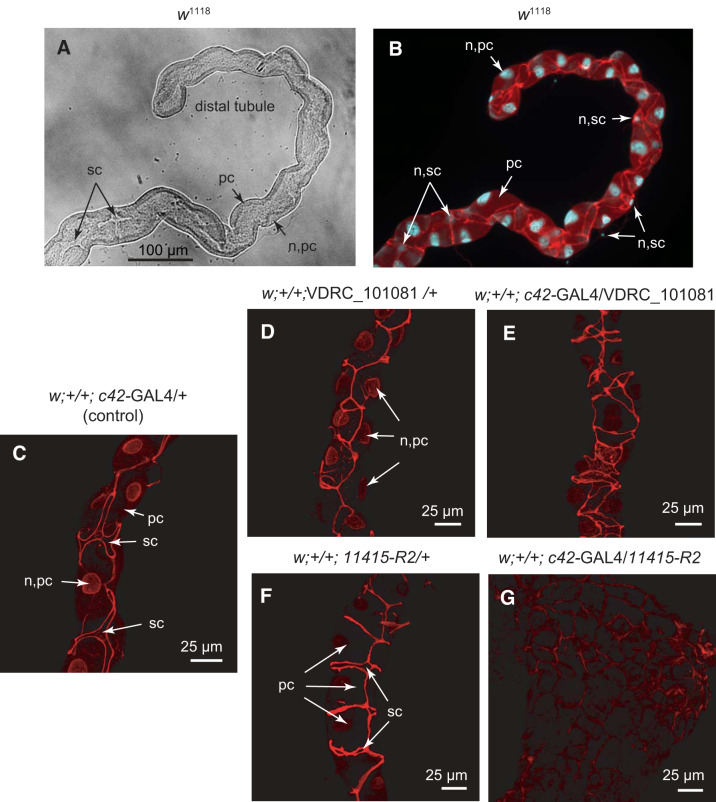
Localization of Tsp2A in whole mounts of the anterior Malpighian tubule of female Drosophila melanogaster. A: light microscopy of the distal tubule of a wild-type fly (w1118). B: immunolocalization of Tsp2A (red) between principal cells (pc) and between principal and stellate cells (sc) of the distal anterior tubule of a wild-type fly. Cell nuclei (n) are counterstained with DAPI (blue). C: c42-GAL4 driver control for both moderate and strong knockdown of Tsp2A. D and E: weak Tsp2A knockdown, UAS VDRC_101081 control (D) and weak knockdown of Tsp2A in principal cells (E). F and G: strong Tsp2A knockdown, UAS 11415-R2 control (F), and strong knockdown of Tsp2A in principal cells (G).
In the Okazaki laboratory of Furuse, adult flies (3–4 days old) were dissected in Hanks’ balanced salt solution and the Malpighian tubules were fixed with 4% PFA in PBS/0.2% Tween-20 for 30 min. The fixed specimens were washed three times with PBS/0.4% Triton X-100 and blocked with 5% skim milk in PBS/0.2% Tween-20. Thereafter, the samples were incubated with primary antibodies at 4°C overnight, washed three times with PBS/0.2% Tween-20, and incubated with secondary antibodies for 3 h. After another three washes, the samples were mounted in Fluoro-KEEPER (12593-64; Nakalai Tesque, Kyoto, Japan). Images were acquired with a confocal microscope (model TCSSPE; Leica Microsystems, Wetzlar, Germany) using its accompanying software and HC PLAN Apochromat ×20 NA 0.7 and HCX PL objective lenses (Leica Microsystems). Images were processed with Adobe Photoshop software (Adobe Systems Incorporated, San Jose, CA).
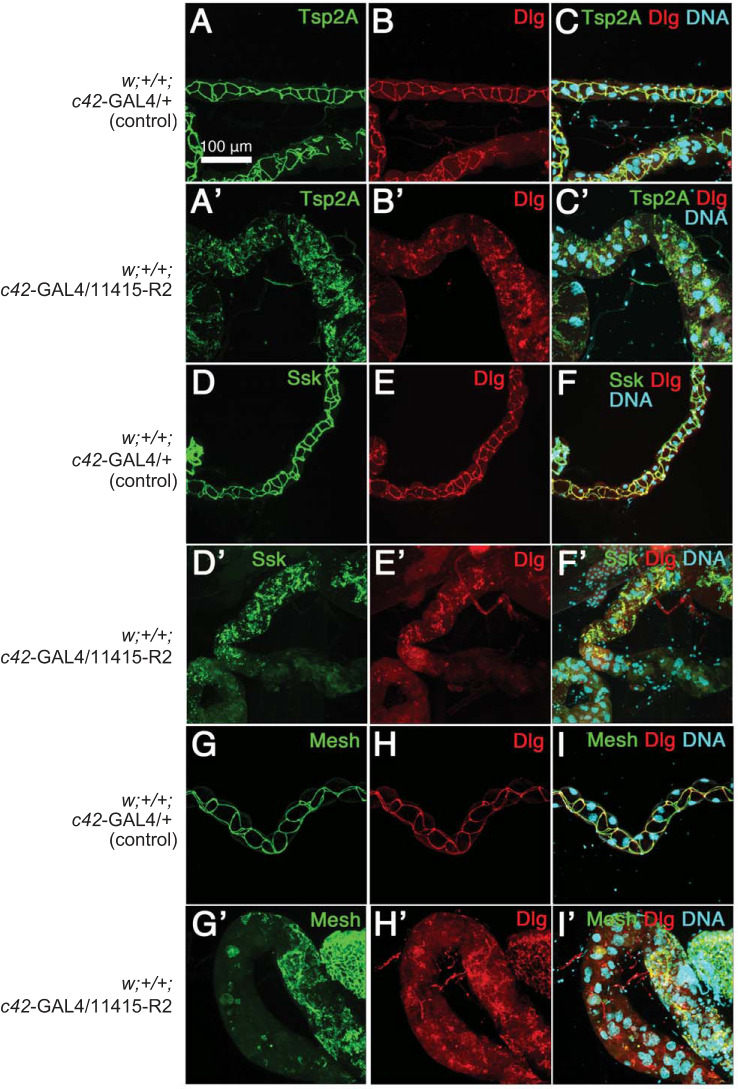
The following antibodies were used for the localization studies shown in Fig. 6: rabbit anti-Mesh (955-1; 1:1,000) (41), rabbit anti-Tsp2A (302AP, 1:200) (40), rabbit anti-Ssk (6981-1; 1:1,000) (88), and mouse anti-Dlg (4F3, Developmental Studies Hybridoma Bank, Iowa City, Iowa, 1:50). Alexa Fluor 488-conjugated (ThermoFisher, Waltham, MA), and Cy3- and Cy5-conjugated (Jackson ImmunoResearch Laboratories, West Grove, PA) secondary antibodies were used at 1:400. Alexa Fluor 488-conjugated (A21206, ThermoFisher) secondary antibody was used at 1:400. Nuclei were stained with propidium iodide (Nakarai tesque; 0.1 mg/mL). The specificity of the applied anti-Tsp2A antibodies was verified previously (40) and confirmed in this study by observing strong reduction in signal intensity as a result of Tsp2A knockdown, compared with controls (see Fig. 5, F and G).
Fig. 6.
Effect of strong Tsp2A knockdown (UAS line 11415-R2) on the localization of Tsp2A and Dlg (A–C’), Ssk and Dlg (D–F’), and Mesh and Dlg (G–I’) in the main secretory segment of anterior Malpighian tubules of adult female Drosophila melanogaster. DNA revealed by propidium iodide staining identifies cell nuclei. Flies were reared at 27°C. An affinity purified antibody against Tsp2A was used.
Protein content and activity of the V-type H+-ATPase.
The protein content of Malpighian tubules and the activity of the V-type H+-ATPase was determined as described previously (76) with the following modifications. The frozen tubules were homogenized on ice with a micro pestle in 100 µL of lysis buffer [5 mM Na-HEPES, pH 7.1, 20 mM NaCl, 2 mM EGTA, 10 mM β-mercaptoethanol, and Protease Inhibitor Cocktail Set I (Calbiochem, Merck KGaA, Darmstadt, Germany)] and then centrifuged (6 × 106 g min, 4°C). To remove endogenous phosphate, the crude membrane pellet was washed once more with 100 µL lysis buffer and centrifuged again. The final crude membrane pellet was resuspended in lysis buffer without protease inhibitor to yield a concentration of 0.4 Malpighian tubules/µL. V-ATPase activity assays were performed in duplicates, using an equivalent of 16 Malpighian tubules in 160 µL of a solution consisting of 50 mM Tris-MOPS, pH 8.1, 3.75 mM MgCl2, 0.1 mM sodium orthovanadate, 20 mM KCl, 0.5 mM NaN3, 5 mM Tris·HCl, 2.5 mM β-mercaptoethanol, 1.5 mM di-Tris-ATP, and 6.25% DMSO, with or without 3 µM bafilomycin A1. Samples were incubated at 30°C for 20 min and the reaction was stopped by freezing the samples in liquid nitrogen. To assess ATPase activity we measured inorganic phosphate as described previously (79).
Transmission electron microscopy.
Malpighian tubules of 6- to 8-day-old adult female flies of 1) control (w;+/+;c42-GAL4/+), 2) moderate Tsp2A knockdown (w;+/+;c42-GAL4/VDRC_101081), and 3) strong Tsp2A knockdown (w;+/+;c42-GAL4/11415R2) were isolated as described above, transferred to PBS, and prepared for electron microscopy using the method of Tepass and Hartenstein (75). The Malpighian tubules were transferred from PBS to fixative containing 2% glutaraldehyde, 1% osmium tetroxide and 50 mM cacodylate buffer (pH 7.0) for 2 h at 4°C. Thereafter, the fixed tubules were washed several times in 100 mM cacodylate buffer (pH 7.0) and postfixed in 1% osmium tetroxide for 1 h, washed with 100 mM cacodylate buffer, and subsequently dehydrated on ice in the graded ethanol series 30%, 50%, 70%, twice in 95%, and twice in 100%. The fixed tubules were incubated twice in acetone for 15 min each and then overnight in a 1:1 mixture of Epon 812 and acetone at room temperature. Thereafter, the tubules were transferred to fresh Epon for 4 to 6 h at room temperature and transferred to fresh Epon and left overnight. The tubules were then transferred to molds with fresh Epon, oriented, and polymerized at 60°C for 48 h. Ultrathin sections (70 nm) were cut using a Leica EM UC7 Microtome, mounted on single hole grids coated with 3% formvar, dissolved in chloroform, and then stained with 2% uranyl acetate for 30 min, and stained in lead citrate for 20 min. The grids were locked into transmission electron microscopes from Zeiss, Model TEM 902 A or Model TEM LEO 912 Omega and viewed at either 80 kV or 120 kV.
Statistical treatment of the data.
Data are presented as means ± SE and analyzed using 1) ANOVA with α adjusted for multiple comparisons where appropriate, and 2) the two-tailed Student’s t test for the significant difference of unpaired samples.
RESULTS
Verification of driver specificity.
To attribute phenotypic effects of Tsp2A knockdown exclusively to Malpighian tubules, we first confirmed the specificity of the applied c42-GAL4 driver by crossing corresponding animals to UAS-eGFP (BL 6658, BDSC). Figure 1 illustrates the expression of c42-GAL4 driven green fluorescent protein (GFP) during the development of the fly. The control cross for this series of experiments (w;+/+;c42-GAL4/+) yields Malpighian tubules in adult flies that lack GFP (Fig. 1A). The early developmental stages up to stage 13 did not reveal GFP signal (Fig. 1B). Embryos expressed eGFP in Malpighian tubules only in very late stages, 16–17, of development (Fig. 1C). In addition to Malpighian tubules, late stage embryos displayed eGFP expression in salivary glands (Fig. 1C), confirming the “leaky” expression of c42-GAL4 previously reported by Gautam et al. (27). The expression of eGFP continued to increase in Malpighian tubules in 1st instar larvae, where pericardial cells also expressed eGFP (Fig. 1D, asterisks). c42-GAL4 expression in salivary gland, Malpighian tubules and pericardial cells persisted through 2nd and 3rd instar larval stages into early adults (Fig. 1, E–I). In tubules of 3rd instar larvae, activity of the c42-GAL4 driver had advanced from the ureter to the transitional tubule (Fig. 1G). Next to validating eGFP expression in Malpighian tubules, Fig. 1 confirms that the c42-GAL4 driver targets principal cells selectively (62). Stellate cells did not express eGFP (Fig. 1, G–I, arrowheads).
Selection of Tsp2A RNAi lines.
To evaluate the general knockdown efficiencies of the two Tsp2A-specific RNAi lines, we utilized the ubiquitous act5C-GAL4 driver to induce RNAi hairpin expression in flies reared at 25°C (Fig. 2). Quantitative PCR revealed significantly reduced Tsp2A transcripts in the corresponding progeny of UAS lines VDRC_101081 (P < 0.037) and 11415-R2 lines (P < 0.020), compared with control act5C-GAL4 x w1118 (Fig. 2). The knockdown efficiency by the RNAi line 11415-R2 was significantly stronger (P < 0.014) than that by VDRC_101081. Accordingly, we consider from here on knockdown by VDRC_101081 as moderate and by 11415-R2 as strong.
Fig. 2.
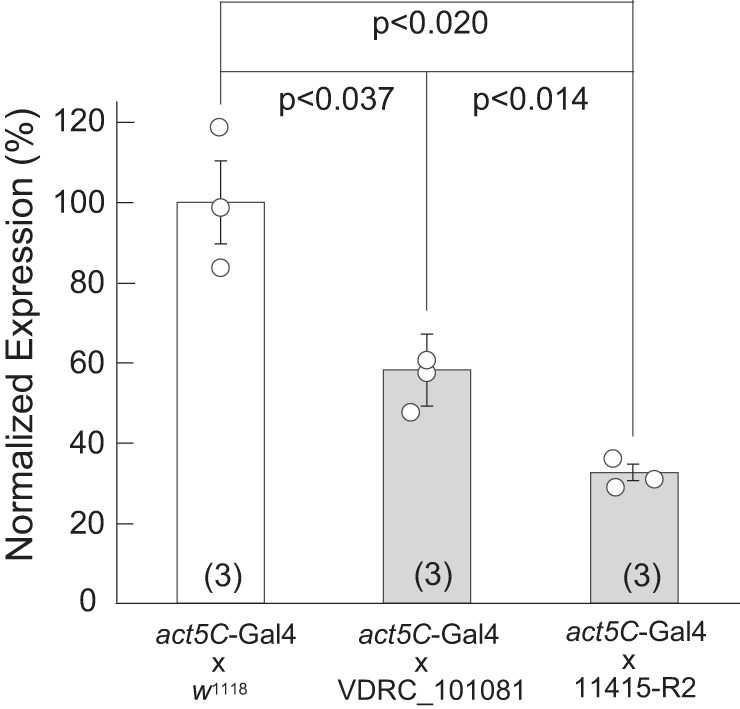
Quantitative PCR of act5C-GAL4-driven knockdown of Tsp2A. Expression of Tsp2A in the cross act5C-GAL4 × w1118 serves as control (100% Tsp2A expression). The RNAi lines VDRC_101081 and 11415-R2 induced, respectively, moderate and strong knockdown of Tsp2A. Female flies of Drosophila melanogaster were maintained at 25°C. Statistical significant difference was evaluated with the Student’s t test. Data are means ± SE of 3 biological replicates.
Effect of strong Tsp2A knockdown on the morphology of Malpighian tubules.
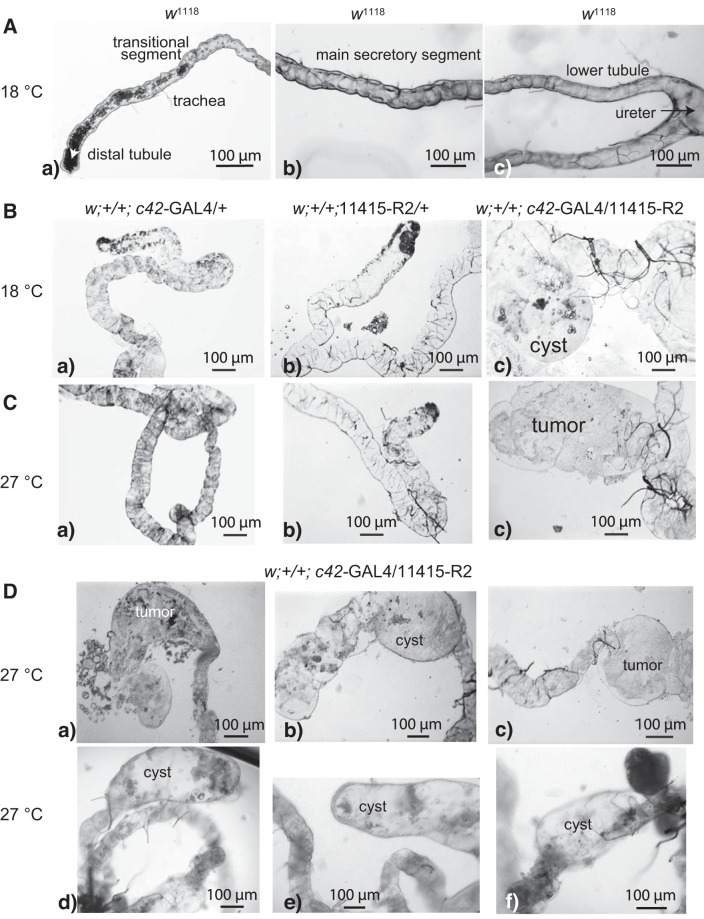
The effects of strong Tsp2A knockdown on Malpighian tubules were obvious to the unaided eye (Fig. 3). Abnormalities were numerous and varied. Knockdown tubules (w;+/+;c42-GAL4/11415-R2) were more fragile during dissection and easily damaged compared with all control tubules (w1118). Tubule length was usually shorter than that of control tubules. Most obvious was the growth of tumors and cysts (Fig. 3, B–D). Tumors appeared as clumps of cells (Fig. 3, Cc, Da, and Dc). In contrast, cysts were typically thin-walled, transparent and filled with fluid (Fig. 3, Bc, Db, and Dd–Df). When tubular structures were present, they were usually thicker than control tubules. The tubule lumen was collapsed with rare exception. Trachea associated with Malpighian tubules appeared more numerous around cysts compared with control tubules. The tubule abnormalities were observed in flies reared at 18°C and 27°C (Fig. 3). Although we occasionally observed morphological changes in c42-GAL4 controls and less so in UAS controls, tubules isolated from these flies secreted fluid normally in vitro and shared similar voltages and resistances with those of wild-type flies (vide infra). Moreover, c42-GAL4 and UAS control flies did not suffer extracellular volume expansion and death (vide infra).
Fig. 3.
The effect of strong Tsp2A knockdown (w;+/+;c42-GAL4/11415-R2) on anterior Malpighian tubules of female Drosophila melanogaster. A: anterior tubules, w1118 control, with precipitates in the lumen of the distal and transitional tubule. B and C: tubules of c42-GAL4 driver control (w;+/+;c42-GAL4/+), UAS control (w;+/+;11415-R2/+), and Tsp2A knockdown in flies reared at the indicated temperatures. D: tumors and cysts in tubules from Tsp2A knockdown flies reared at 27°C.
Effect of moderate and strong Tsp2A knockdown on the ultrastructure of Malpighian tubules.
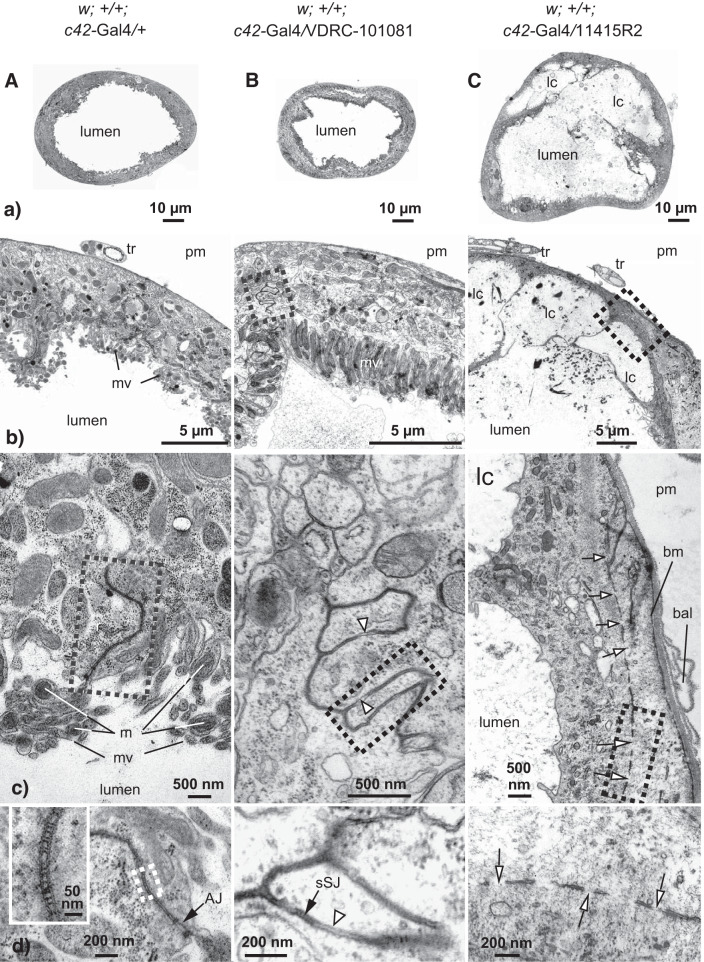
Figure 4A illustrates increasing EM magnifications of the main secretory segment of the anterior Malpighian tubule of Drosophila melanogaster from the control cross w;+/+;c42-GAL4+. The rounded appearance of the tubule in cross section reflects the transepithelial secretion of fluid into the tubule lumen and the build-up of luminal hydrostatic pressure that 1) drives secreted fluid downstream, and 2) presses principal cells against the basement membrane of the tubule (Fig. 4Aa). A closer look at the tubule wall reveals basal and apical domains of epithelial cells with the characteristic microvilli on the luminal side and trachea on the basal side (Fig. 4Ab). Figure 4Ac focuses on the paracellular apicolateral region of two principal cells. Microvilli of the brush border contain mitochondria (Fig. 4Ac) that energize transepithelial solute secretion by fueling the V-type H+-ATPase with ATP (8, 13). The narrow, electron dense structure of ~5-µm length extending from the tubule lumen into the paracellular space includes the smooth septate junction, sSJ (Fig. 4Ac). The cutout enlargement of this region in Fig. 4Ad reveals the location of the sSJ basal to the adherens junction (zonula adherens), typical of invertebrate epithelia of ecto- and mesodermal origin (19). The ×8,5000 magnification of the sSJ in the window of Fig. 4Ad reveals the typical ladder-like septate junction of invertebrates. The molecular components that form the rungs of the ladder are unknown, though Mesh has the necessary dimension for bridging the paracellular gap between adjacent epithelial cells (39, 41).
Fig. 4.
Effect of moderate and strong knockdown of Tsp2A on the ultrastructure of the main secretory segment of the adult Malpighian tubule of female Drosophila melanogaster. Transmission electron microscopy of the driver control (A: w;+/+;c42-GAL4/+); moderate Tsp2A knockdown (B; w;+/+;c42-GAL4/VDRC_101081); and strong Tsp2A knockdown (C; w;+/+;c42-GAL4/11415R2). AJ, adherens junction; bal, basal lamina; bm, basement membrane; lc, luminal compartment; m, mitochondria; mv, microvilli; pm, peritubular medium; sSJ, smooth septate junction; tr, trachea. Open arrows point to gaps in septate junctions; open arrowheads point to apposed cell membranes. Note the location of the AJ on the apical side of the sSJ (Ad).
Figure 4B illustrates progressive EM enlargements of the effect of moderate Tsp2A knockdown. While tubules maintained their general morphology, they were less rounded and appeared more flaccid than c42-GAL4 control tubules, consistent with reduced fluid secretion (vide infra) and reduced luminal hydrostatic pressure (Fig. 4, A and Ba). Magnification of the tubule wall shows that principal cells appear normally polarized with apical and basal membrane domains (Fig. 4Bb). The cutout of the paracellular region enlarged in Fig. 4Bc reveals unusual contours of junctions that apparently stem from interdigitating principal cells (see also Fig. 5E). The junctions include ladder-like sSJ and irregular apposed cell membranes can be found (Fig. 4, Bc and Bd).
The strong knockdown of Tsp2A elicited profound changes in tubule ultrastructure (Fig. 4C). The tubules were much larger and more fragile than those from control and moderate knockdown flies (Fig. 4, A, B, and Ca). Segments along the tubule revealed multiple luminal compartments, tumor-like growths and cysts (see Fig. 3C). Cysts were most common in distal regions of the tubule, while tumors exhibited no preferred location. Where tubular segments occurred, cross sections revealed a thin-walled, distorted tubule consistent with its flaccid and fragile nature (Fig. 4Ca). Most striking is the appearance of luminal compartments of variable size and shape in basal regions of the tubule (Fig. 4, Ca–Cc). Thin cells with stunted microvilli partially enclose luminal compartments that open to the main lumen (Fig. 4, Cb and Cc). Cells along luminal compartments often revealed long intercellular junctions (Fig. 4Cc). Running parallel to basal and apical cell membranes, the long junctions reflect the disorientation of epithelial cells. Junctions could completely surround luminal compartments in some locations; at other locations, junctions were much reduced in length with very few or no septa. Invariably, junctions revealed many gaps (Fig. 4, Cc and Cd). Where septa could be identified on rare occasion, they were next to gaps (Fig. 4, Cc and Cd). Next to disoriented and abnormal septate junctions, strong Tsp2A knockdown led to a greatly reduced basal labyrinth. The basal lamina (extracellular matrix part of the basement membrane) often detached from the basement membrane (Fig. 4Cc). The basement membrane appeared unstable and loosely associated with the basal cell membrane. Cell nuclei were misshaped and nuclear envelopes were disorganized compared with control tubules. At apical regions, cell membranes offered microvilli without mitochondria in cells with few cell organelles (data not shown). Principal and stellate cells could not be clearly differentiated based on morphology.
Immunolocalization of the septate junctional protein Tsp2A in Malpighian tubules.
We used anti-Tsp2A antibodies to localize Tsp2A in Malpighian tubules of adult female flies and DAPI to counterstain nuclei. The control (w1118) tubule illustrated in Fig. 5, A and B, exhibits a wide lumen consistent with transepithelial fluid secretion and tubular flow that has washed out luminal precipitates. The antibody against Tsp2A reveals the belt-like outline of the septate junction in the apicolateral region between 1) large principal cells, and 2) principal cells and small stellate cells (Fig. 5, B and C). The DAPI stain reveals 1) large polyploid nuclei in principal cells (25) butting against the basolateral membrane, and 2) small nuclei in stellate cells.
Figure 5, C–G, illustrates the effect of the moderate and strong knockdown of Tsp2A mediated by the UAS lines VDRC_101081 and 11415-R2, respectively. The control crosses (Fig. 5, C, D, and F) reveal the characteristic location of Tsp2A in belts surrounding principal cells and stellate cells. In the case of principal cells, Tsp2A outlines the ~2 µm belt of sSJ near the apical border forming a trapezoid ribbon along the length of the tubule (Figs. 5D and 6). In contrast, Tsp2A outlines the lateral borders of stellate cells that typically are less than 5 µm thick (Fig. 5F). The moderate knockdown of Tsp2A by the UAS line VDRC_101081 presents a disorganized pattern of sSJs and an abnormal arrangement of epithelial cells (Fig. 5E, see also Fig. 4, Ab–Cb and Ac–Cc). In contrast, the strong knockdown of Tsp2A by the UAS line 11415R-2 leaves only faint traces of Tsp2A in a mass of cells (Fig. 5G), consistent with tumor-like growth (Figs. 3 and 4).
The effect of strong Tsp2A knockdown on the localization of Tsp2A, Ssk, Mesh, and Discs large.
As in midgut epithelial cells (38, 40), Tsp2A, Ssk, and Mesh associate with the septate junction in the paracellular pathway between the epithelial cells of control tubules (Fig. 6, A, D, and G). In addition, Discs large (Dlg) associates with septate junctions in the control tubules (Fig. 6, B, E, and H). Moreover, overlay images indicate the co-localization of Dlg with each of the three septate junctional proteins: Tsp2A (Fig. 6C), Ssk (Fig. 6F), and Mesh (Fig. 6I). Thus, the number of junctional proteins co-localizing in sSJs of Malpighian tubules increases from three to four. The strong knockdown of Tsp2A reduces not only the localization of Tsp2A at septate junctions (Fig. 6A’) but also Dlg (Fig. 6, B’, E’, and H’), Ssk (Fig. 6D’), and Mesh (Fig. 6G’). Moreover, the largely intracellular localization of Tsp2A, Ssk, Mesh, and Dlg indicates their failure to move to paracellular cell membranes for the assembly of sSJs. Overlay images of Dlg with each of Tsp2A, Ssk and Mesh document the association of the four proteins at sSJs under control conditions (Fig. 6, C, F, and I) and inside cells under conditions of Tsp2A knockdown (Fig. 6, C’, F’, and I’). As in Fig. 3, the outer diameter of Tsp2A knockdown tubules is significantly larger (Fig. 6, A’–I’) compared with control tubules (Fig. 6, A–I), consistent with hyperplasia further supported by the apparent increase in cell nuclei (Fig. 6, C’, F’, and I’).
Effect of strong Tsp2A knockdown on fluid secretion in isolated Malpighian tubules.
Experiments were designed to test the effects of genotype and the diuretic drosokinin (1 µM) on the rate of transepithelial fluid secretion in isolated anterior Malpighian tubules of Drosophila melanogaster at two different rearing temperatures (Fig. 7). At 18°C (Fig. 7A), in which GAL4 is less active than at 27°C, there was a significant effect of genotype (P = 0.0343) on control fluid secretion in two-way ANOVA, but in Sidak’s multiple comparison testing, there was no significant reduction in control rates of fluid secretion in Tsp2A knockdown tubules compared with wild-type flies (w1118) (P = 0.22). There were also no significant effects on fluid secretion in the control genotypes (P = 0.9692 for w;+/+;c42-GAL4/+ compared with w1118, and P = 0.9651 for w;+/+;11415-R2/+ compared with w1118). In contrast, there was a significant effect of drosokinin (P < 0.0001) and a statistically significant increase in fluid secretion in response to drosokinin in all genotypes (Fig. 7A). There was no interaction between drosokinin effect and genotype (P = 0.8794).
Fig. 7.
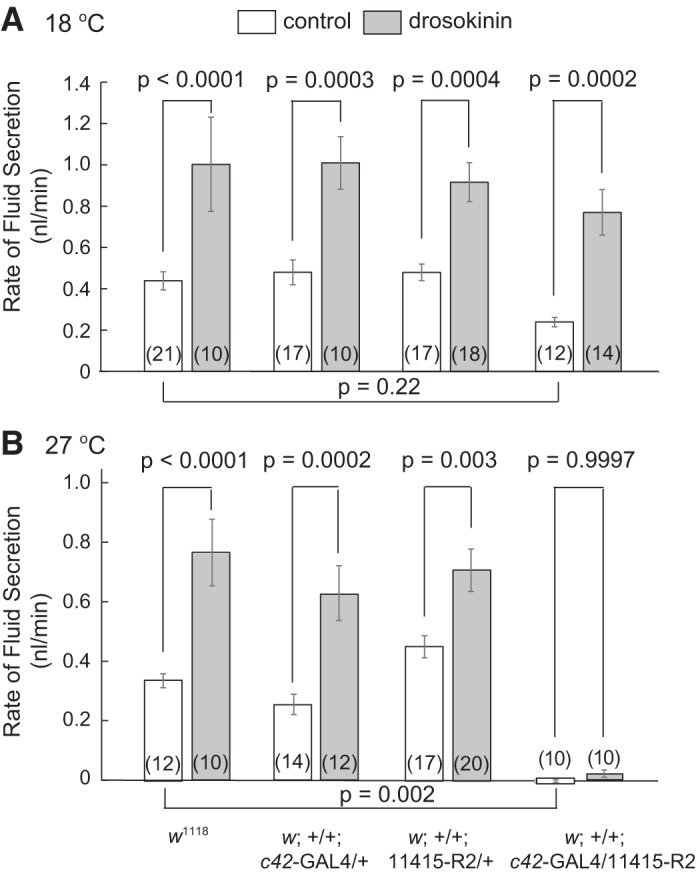
Effect of strong Tsp2A knockdown (w;+/+;c42-GAL4/11415-R2) on rates of transepithelial fluid secretion in Malpighian tubules isolated from female Drosophila melanogaster reared at 18°C (A) and 27°C (B). Isolated tubules were bathed in BRS and studied in the absence and presence of the diuretic hormone drosokinin (10−6M). Results were analyzed by two-way ANOVA and Sidak’s multiple comparisons test. The driver control is w;+/+;c42-GAL4/+ and the UAS control is w;+/+;11415-R2/+. Data are means ± SE (number of tubules).
At 27°C, the effects of drosokinin (P < 0.0001), genotype (P < 0.0001), and interaction (P = 0.0173) were all statistically significant by two-way ANOVA (Fig. 7B). The GAL4 and UAS control genotypes were not significantly different from w1118 (P = 0.7087 and 0.3701, respectively). Drosokinin significantly increased rates of fluid secretion in the three control genotypes: 1) w1118, 2) w;+/+;c42-GAL4/+, and 3) w;+/+;11415-R2/+. In contrast, Tsp2A knockdown tubules had a striking lack of fluid secretion (P = 0.002 compared with w1118) and failed to respond to drosokinin (P = 0.9997). In summary, at 18°C, Tsp2A knockdown failed to significantly reduce spontaneous rates of fluid secretion, and drosokinin significantly increased fluid secretion rates in all four genotypes. At 27°C tubules from Tsp2A knockdown flies did not secrete fluid under control conditions and did not respond to drosokinin.
In separate experiments we examined the effect of moderate Tsp2A knockdown on spontaneous rates of fluid secretion. At 27°C, tubules from RNAi control flies (w;+/+;VDRC_101081/+) secreted fluid at a rate of 0.39 ± 0.05 nl/min (n = 15) while those from Tsp2A knockdown flies (w;+/+;c42-GAL4/VDCR_101081) secreted fluid at a significantly (P < 0.032) lower rate, 0.27 ± 0.03 nl/min (n = 18).
Effect of strong Tsp2A knockdown on tubule electrophysiology.
As shown in Fig. 8, control (w1118) tubules had an apical membrane voltage (Va) of 70.9 ± 3.8 mV (n = 11), a transepithelial voltage (Vt) of 31.6 ± 4.0 mV (n = 11), and a basolateral membrane voltage (Vbl) of −39.3 ± 3.8 (n = 11). In tubules of w;+/+;c42-GAL4/+ control flies, Va was 67.0 ± 5.3 mV (n = 9), Vt was 26.1 ± 4.5 mv (n = 9), and Vbl was −40.9 ± 2.7 (n = 9). In tubules of w;+/+;11415-R2/+ control flies, Va was 70.7 ± 7.2 (n = 6), Vt was 30.1 ± 5.1 (n = 7), and Vbl was −41.7 ± 2.4 (n = 6) (Fig. 8). ANOVA revealed no significant difference between the three control genotypes for Va and Vbl (Va, P = 0.8283; Vbl, P = 0.8784). Likewise, Vt was not significantly different in the three control genotypes (vide infra).
Fig. 8.
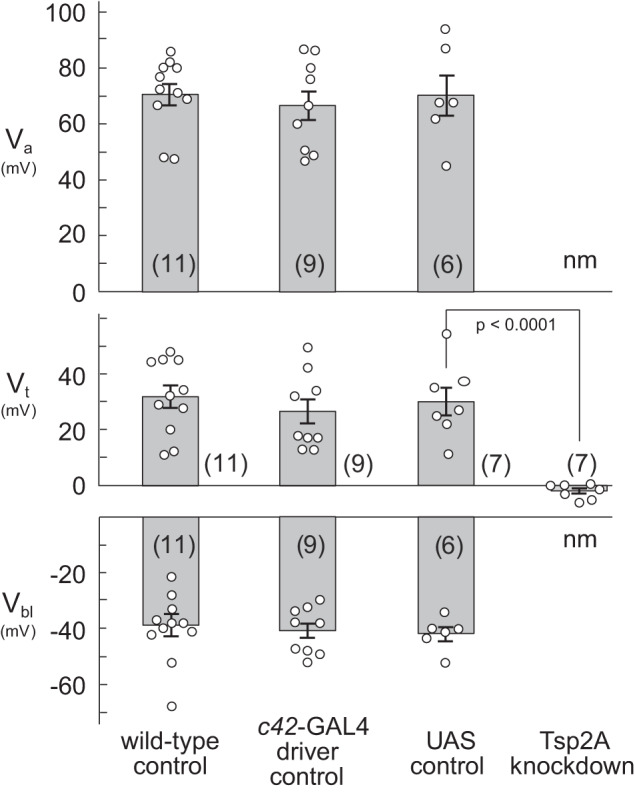
Effect of strong Tsp2A knockdown on apical (Va) and basolateral (Vbl) membrane voltages of principal cells and on transepithelial (Vt) voltage of anterior Malpighian tubules of female Drosophila melanogaster. Membrane voltages in tubules and cysts of strong Tsp2A knock down flies could not be measured (nm). Wild-type (w1118) flies were reared at 22°C; all other flies were reared at 27°C. The c42-GAL4 driver control is w;+/+;c42-GAL4/+. The UAS control is w;+/+;11415-R2/+. The Tsp2A knockdown is w;+/+;c42-GAL4/11415-R2/+. Statistical significance was evaluated with ANOVA followed by Dunnett’s multiple comparison test for transepithelial voltage. Data are means ± SE (number of tubules).
Cysts and tumor-like structures induced by strong Tsp2A knockdown did not allow the measurement of intracellular voltages. However, it was possible to measure transepithelial voltage and resistance in cysts and in some main secretory segments of flies raised at 27°C. Vt was not significantly different in cysts and the lumen of tubules (P = 0.7250, unpaired t test). For tubule lumens, Vt averaged −2.0 ± 1.0 mV (n = 7). In one-way ANOVA (P < 0.0001), there were no significant differences in Vt between the w;+/+;c42-GAL4/+ and w;+/+;11415-R2/+ tubules compared with w1118 control (P = 0.5516 and 0.9851, Dunnett’s multiple comparison test). However, there was a significant difference in Vt of Tsp2A knockdown tubules compared with the w1118 control (P < 0.0001, Dunnett’s multiple comparison test) (Fig. 8). Moreover, the 95% confidence of the Vt in cysts (−4.50 mV, 0.50 mV) and tubule lumen (−5.54 mV, 0.39 mV) includes 0 mV. Thus tubules from the Tsp2A knockdown flies had no significant transepithelial voltage. Tubules and cysts also lacked significant transepithelial resistance. The input resistance of cysts (~17.3 KΩ) was not significantly different from the input resistance measured between the current and voltage electrodes in BRS, indicating no measurable transepithelial resistance in cysts.
In flies reared at 18°C, cysts appeared less frequently than in tubules from flies reared at 27°C. Vt in the tubule lumen was −3.7 ± 1.9 mV (n = 7) and not significantly different from −2.3 ± 3.1 mV (n = 7) in cysts. The 95% confidence of the Vt in the tubule lumen (−8.41 mV, 0.99 mV) and in cysts (−9.91 mV, 5.33 mV) included 0 mV. Again, the input resistance of cysts (~16.9 KΩ) was not significantly different from the input resistance between the two microelectrodes in BRS. Thus strong knockdown of Tsp2A causes tubules to lose their transepithelial barrier in flies reared at 18°C and 27°C.
Effect of strong Tsp2A knockdown on tubule membrane protein content and V-type H+-ATPase activity.
Malpighian tubules from female wild-type flies (w1118) reared at room temperature (22°C) had an average membrane protein content of 0.47 ± 0.06 µg/fly (n = 5) (Fig. 9A). The protein content of tubules from flies reared at 27°C was similar in c42-GAL4 driver and UAS controls: 0.45 ± 0.06 µg/fly (n = 5) and 0.44 ± 0.04 µg/fly (n = 6), respectively. In contrast, protein content significantly (P < 0.0001) increased to 1.24 ± 0.04 µg/fly (n = 4) in tubules from Tsp2A knockdown flies reared at 27°C (Fig. 9). The increase is consistent with the hyperplasia observed in tubules upon strong Tsp2A knockdown (Figs. 3, 4, and 6).
Fig. 9.
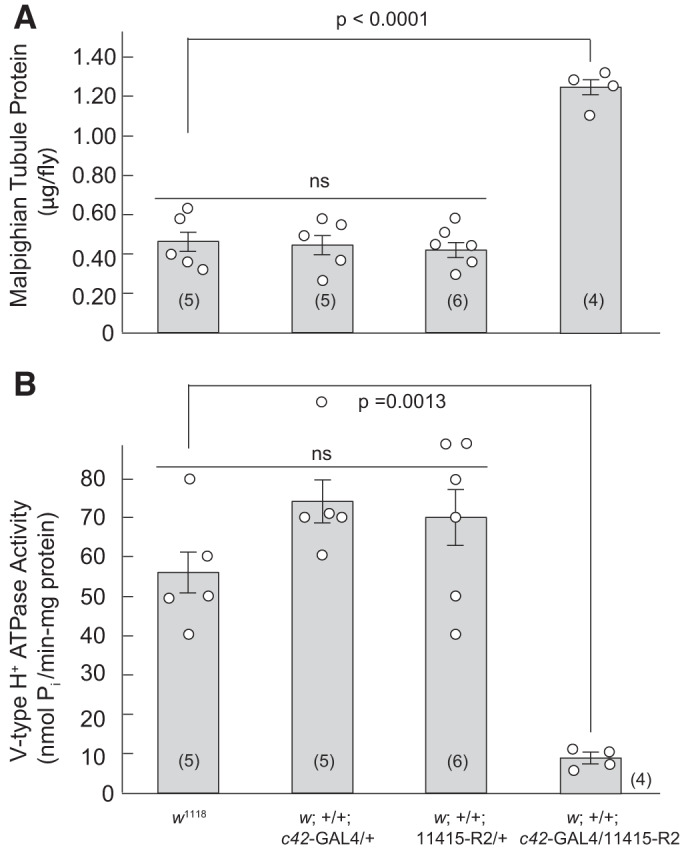
The effect of Tsp2A knockdown on the protein content and the activity of the V-type H+-ATPase in Malpighian tubules of female Drosophila melanogaster. A: protein content normalized to four tubules per fly. In Dunnett’s multiple comparison testing, there were no significant differences (ns) between the c42-GAL4 (P = 0.9922) and UAS (P = 0.9312) controls compared with w1118. In contrast, protein content is significantly higher (P < 0.0001) in Tsp2A knockdown tubules compared with w1118 tubules. B: V-type H+-ATPase activity normalized to mg protein. In Dunnett’s multiple comparison testing, there were no significant differences between the c42-GAL4 (P = 0.2160) and UAS (P = 0.3618) controls compared with w1118. In contrast, V-type H+-ATPase activity is significantly (P = 0.0013) lower in Tsp2A knockdown tubules compared with w1118 tubules. Control w1118 flies were reared at 22°C, all other genotypes were reared at 27°C. Statistical significance was evaluated with one-way ANOVA. Data are mean ± SE (number of measurements).
Malpighian tubules from female wild-type (w1118) flies reared at room temperature (22°C) had an average V-type H+-ATPase activity of 56.5 ± 5.5 nmol Pi·min−1·mg protein−1 (n = 5) (Fig. 9B). V-type H+-ATPase in c42-GAL4 and UAS controls was 74.4 ± 5.6 nmol Pi·min−1·mg protein−1 (n = 5) and 70.0 ± 7.7 nmol Pi·min−1·mg protein−1 (n = 6), respectively, and was significantly decreased to 9.1 ± 1.4 nmol Pi·min−1·mg protein−1 (n = 4) in tubules from Tsp2A knockdown flies (Fig. 9B), consistent with hyperplasia observed in the microscopic and immunological studies (Figs. 3, 4, and 6).
Effect of strong Tsp2A knockdown on extracellular fluid volume in adult flies.
The life span of Drosophila melanogaster is influenced by sex, diet, stress and ambient temperature (53). Adult flies usually live between 40 and 50 days. In the present study, flies submitted to strong Tsp2A knockdown died with rare exception within 1 wk of eclosion. Upon eclosion, the abdominal volume of control GAL4 and UAS flies increased slightly during the first 4 days of adult life and then levelled out (Fig. 10B). In contrast, the abdominal volume of Tsp2A knockdown flies nearly doubled on day 3 and increased threefold on day 6 after eclosion (Fig. 10B). The increase in abdominal volume likely reflects the retention of extracellular fluid because 1) extracellular fluid seeped spontaneously from the exoskeleton of the abdomen in Tsp2A knockdown flies before they died, and 2) puncture of the swollen abdomen of cold-anesthetized Tsp2A flies yielded the sudden exudate of extracellular fluid much larger in volume than that of control flies. Although fluid retention was quantified by measuring abdominal volume, all body parts were volume expanded, indicating generalized edema.
Fig. 10.
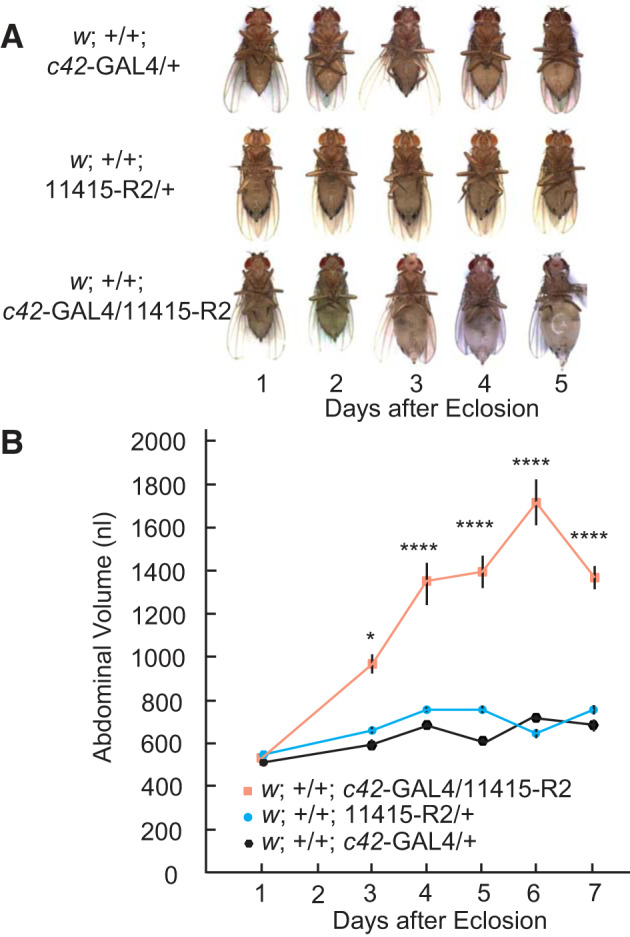
The effect of Tsp2A knockdown on fluid retention in female Drosophila melanogaster reared at 27°C. w;+/+;c42-GAL4/+ and w;+/+;11415-R2/+ serve, respectively, as driver and UAS controls for the strong knockdown of Tsp2A, w;+/+;c42-GAL4/11415-R2. A: generalized edema beginning on day 2 after eclosion in Tsp2A knockdown flies and ending with death 2–4 days later. B: abdominal volume as a function of time after eclosion of Drosophila melanogaster. Data were analyzed by two-way ANOVA, with significant effects of genotype (P < 0.0001), days after eclosion (P < 0.0001) and interaction (P < 0.0001). In Dunnett’s multiple comparison test, there were no significant differences between w;+/+;11415-R2/+ and w;+/+;c42-GAL4/+ controls at any day. Significant differences were observed between the Tsp2A knockdown (w;+/+;c42-GAL4/11415-R2) and the driver control (w;+/+;c42-GAL4/+) from days 2 to 7 as indicated: * P < 0.05 and ****P < 0.0001. Data are means ± SE of 11 to 22 flies.
DISCUSSION
Tetraspanins.
The tetraspanins form a subset of the transmembrane 4 superfamily (TM4SF) of proteins that are highly conserved in eukaryotic organisms from plants to mammals (16). They are present in virtually every cell, and some cells have up to 100,000 copies of protein (14, 33). Integral membrane proteins of small size (20–50 kDa), the tetraspanins first drew attention as “clusters of differentiation” proteins (CD9, CD81, CD151, etc.) before they were found to share the sequence of tetraspanins. Protruding only for a short distance (3–5 nm) from membranes, tetraspanins are suited better for lateral than linear associations with each other and with other proteins. However, binding laterally to membrane proteins that extend into the extracellular space, tetraspanins may have enabled contact with other cells as the first step toward multicellular organisms (35, 92). Today, tetraspanins are known to associate with integrins, cadherins, Rac, matrix-metalloproteases, EGF receptors and immunoglobulins, thereby serving scaffolding, cell adhesion, signal transduction, intracellular signaling, cell motility and migration, cell proliferation and differentiation, cell-cell fusion, endocytic trafficking, host-parasite interactions, metastasis, and viral infection (7, 15, 17, 32–34, 46, 51, 52, 83, 89, 91).
Tetraspanins are also found at paracellular junctions of epithelia (1, 18, 37, 65). The tetraspanins Tspan3, CO-029, CD9, CD15, CD81, and CD181 associate with claudins in tight junctions of vertebrates (30, 40, 46, 47, 50). In invertebrates, Izumi was first to find tetraspanins in septate junctions (40). In particular, he and coworkers found Tsp2A to localize to smooth septate junctions in the midgut and Malpighian tubules of Drosophila. The present study confirmed the location of Tsp2A in Malpighian tubules by localizing this protein in a well-defined band surrounding epithelial cells near the tubule lumen of Malpighian tubules of Drosophila (Figs. 5 and 6). Sharing this band with Ssk and Mesh identifies Tsp2A as a protein of the smooth septate junction, as in Drosophila midgut epithelial cells (40–42, 88). With length along the Malpighian tubule, the band of Tsp2A, Ssk, or Mesh forms a trapezoid ribbon (Figs. 5 and 6) dominated by principal cells that comprise 80% of tubule cells (67). The present study shows further that Dlg, the membrane-associated guanylate kinase (MAGUK) required for the formation of both septate and tricellular junctions and for epithelial polarization in Drosophila (40, 64, 81), is part of the trapezoid ribbon in Malpighian tubules. It suggests that Ssk, Mesh, Tsp2A, and Dlg form a complex at sSJs in Malpighian tubules and perhaps also in the midgut of Drosophila (39–41, 88). Although Malpighian tubules and midgut utilize a single-layered epithelium for mediating transepithelial transport, they differ in the relative location of SJs and adherens junctions (AJs). In the midgut, sSJs are apical to AJs as in vertebrate tight junctions (19, 80), while enigmatically the reverse is true for Malpighian tubules (Fig. 4Ad), even though Malpighian tubules and midgut share the same canonical polarization factors (26, 82).
Structural and functional consequences of Tsp2A knockdown.
The trapezoid ribbon of Tsp2A, Ssk, Mesh, and Dlg no longer forms upon the developmental knockdown of just one of these proteins, Tsp2A (Fig. 6). The four septate junctional proteins remain largely in the cytoplasm of principal cells in close association, consistent with the need for the full complement of sSJ proteins before they can move to their usual paracellular location near the apical membrane (Fig. 6). The few sSJs that can still be observed in Tsp2A knockdown tubules now spot the paracellular pathway for long distances with frequent gaps, wide gaps between the cell membranes of adjacent cells, and the loss of rungs in the ladder-like sSJ architecture (Fig. 4). Junctions appearing at several places along the apical/basal axis indicate the diffuse distribution of sSJs along the paracellular pathway. Principal cells are also compromised. At basal surfaces, the basal lamina is unraveling consistent with the loss of structural support indicated by the increase in tubule diameter and hyperplasia (Figs. 3, 4, and 6). At the apical membrane, microvilli can be entirely absent; those present are shorter, less dense, and often devoid of mitochondria compared with control tubules (Fig. 3 and 4). Thus the effects of knocking down Tsp2A extend beyond sSJs to the breakdown of the structural features of basolateral and apical plasma membranes in principal cells.
The physiological consequences of the structural defects are severe. Malpighian tubules isolated from Tsp2A knockdown flies reared at 27°C failed to secrete fluid during their short life span less than a week (Figs. 7B and 10). For one reason, apical microvilli, the sites that generate the driving force for transepithelial electrolyte secretion (8, 11, 13), are far less developed in Tsp2A knockdown tubules than in control tubules (Fig. 4) consistent with the diminished V-type H+-ATPase activity and the absence of significant lumen-positive transepithelial voltages in Tsp2A knockdown tubules (Figs. 8 and 9). For another reason, the paracellular pathway has completely lost its barrier property in view of transepithelial input resistances that are not significantly different from the input resistances measured between the two measuring electrodes in Ringer solution. In the absence of paracellular barriers, the little solute and water still secreted into the tubule lumen via transcellular transport likely leak back to the peritubular bath (or hemolymph) via the paracellular pathway instead of flowing downstream for excretion. The tubular failure to excrete fluid is expected to disrupt extracellular fluid homeostasis as solute and water accumulate in the extracellular fluid compartment. The volume expansion leads to severe generalized edema and to death in the first week of adult life (Fig. 10).
In a recent study we found that Tsp2A, Ssk, and Dlg also remain largely in principal cells upon the developmental knockdown of mesh (42). Furthermore, mesh knockdown tubules fail to secrete fluid, and the flies gain extracellular fluid volume, become edematous, and die in early adulthood, as in the knockdown of Tsp2A. Similar effects of the knockdown of Tsp2A and mesh suggest that the full complement of septate junctional proteins consisting of Mesh, Tsp2A, Ssk, Dlg (and possibly other proteins) must be expressed before the sSJ can form in apicolateral regions in Malpighian tubules. The full complement of septate junctional proteins is indeed required for the formation sSJs in the midgut. There, the three proteins, Tsp2A, Mesh, and Ssk, are mutually dependent for their proper localization at the sSJ (40, 41).
Molecular mechanisms triggered by the knockdown of Tsp2A.
The molecular mechanisms that bring about the structural and functional defects caused by the knockdown of Tsp2A were beyond the aims of the present study. Nevertheless, studies by others are relevant. Malpighian tubules are formed in the embryonic stage and become functional in the second half of the first day of development, responding in part to the 100-fold increase in uric acid by secreting this xanthine into the tubule lumen (68, 74). From then on, the tubules do not undergo metamorphosis (22, 67, 71). Instead, they grow during the larval stages by increasing cell size associated with the endoreplication of DNA (21), and they acquire renal stem cells as late as the first day of pupation (67, 73). Accordingly, the development of tumors and cysts upon the knockdown of Tsp2A must take place late in the development of the fly, which may explain why larvae survive the knockdown of Tsp2A or mesh (or other sSJ proteins) but not adults (42).
Renal stem cells (RSCs) were first identified in Malpighian tubules as “tiny cells” in the ureter and lower tubule of adult flies but not larvae (67). RSCs are thought to be multipotent, capable of generating all types of Malpighian tubule cells under autocrine regulation of JAK-STAT signaling, which stimulates RSCs to form renal blasts (RBs) that migrate and differentiate to stellate cells and principal cells in the upper tubule (67, 77). Thus RSC activity can be expected to replace Tsp2A-deficient principal cells from the pupal stage on. Alas, the knockdown of Tsp2A may cause RSCs to produce Tsp2A-deficient principal cells, and failing to replace defective principal cells with normal cells, RSCs may remain active. Unstopped cell proliferation may then produce the hypertrophy, tumor- and cyst-like structures observed in the present study (Figs. 3–6). Support for this hypothetical scenario in Malpighian tubules comes from the recent study of the role of sSJs in cell proliferation in the midgut of Drosophila (38). Here, enterocytes deficient of Tsp2A, Ssk, or Mesh exhibit defective sSJs, epithelial barrier dysfunction, abnormal enterocytes, and increased cell proliferation and intestinal hypertrophy, which the flies do not survive for more than 10 days. Thus the phenotype produced by knocking down Tsp2A in the intestine is similar to that of knocking down Tsp2A in Malpighian tubules. Moreover, the proliferation of intestinal stem cells (ISCs) was activated by Ras-MAP kinase and by the cytokines Unpaired2 (Upd2) and Unpaired3 (Upd3); the latter are known to activate JAK-STAT pathway involved in regulating RSC activity in Malpighian tubules (67, 77). Similar molecular mechanisms in the intestine and Malpighian tubules suggest that septate junctional proteins and sSJs are critically involved in the regulation of stem cell proliferation in both tissues (5, 38) in as much as midgut intestinal stem cells (ISCs) arise from the same pool of midgut progenitor as RSCs in Malpighian tubules (67, 73, 77, 87).
Xu et al. have recently uncovered the critical role of Tsp2A in regulating stem cell proliferation in the Drosophila midgut via Hippo signaling (86). From fly to mammals, the Hippo pathway modulates cell proliferation, differentiation, and migration in developing tissues and limits growth in adults (57). First identified in Drosophila in 2003, the Hippo pathway has now grown to a signaling network of more than 40 players (31, 57, 70, 78, 84). One critical player is the transcription cofactor and oncogene Yorkie (Yki), which has been associated with Yki tumors and excessive body fluid retention described as “bloating syndrome” in Drosophila (36, 48). The Hippo pathway is regulated by components at or near cell junctions such as tight, septate, and adherens junctions (72, 86, 90). Transitions of components of the pathway [atypical protein kinase C (aPKC); Warts (Wts); and Hippo kinase (Hpo)] between the membrane and cytoplasm are thought to activate/inactivate the Hippo pathway while the shuttling of Yki between the cytoplasm and the nucleus is thought to regulate transcription and cell proliferation and apoptosis. As proposed by Xu et al. (86), damage to the Drosophila midgut increases Yki activity and stimulates ISC proliferation. As the wound heals, Tsp2A undergoes internalization to facilitate the endocytic degradation of aPKC. Reduced aPKC activity allows Hpo to dimerize at the membrane, setting off a series of phosphorylations that ends with phosphorylated Yki and its cytoplasmic restriction, which stops ISC proliferation. Indeed, defects in Tsp2A expression or defective Tsp2A-sSJ assembly cause excessive aPKC-Yki-JAK-STAT activity and make the midgut epithelium highly proliferative, like a wound that cannot heal (86). Whether Hippo signaling is similarly activated in Tsp2A knockdown tubules will be of interest for future studies.
Hippo signaling is not the sole regulator of cell proliferation. The laboratory of Davies has recently discovered a signaling pathway involving the transcription factor GATAe capable of producing tumor-like structures in Malpighian tubules of Drosophila (55). In the case of RSCs, GATAe is required for the migration of midgut progenitor stems cells to the ureter and lower tubule where they become RSCs in the early pupa. In the case of stellate cells, GATAe controls their cell number. GATAe knockdown reduced to less than half the number of stellate cells in the tubule while significantly reducing the diuretic response of tubules to drosokinin. In the case of principal cells, GATAe behaves like a tumor suppressor because GATAe knockdown induced uncontrolled cell proliferation leading to tumors in tubules. Moreover, GATAe knockdown in specifically principal cells produces the same phenotype as the knockdown of Tsp2A including malformed Malpighian tubules, disrupted sSJs, loss of the Dlg ribbon along Malpighian tubules, impaired transepithelial fluid secretion, extracellular fluid retention, bloated abdomens, uncontrolled proliferation of RSCs, and significantly reduced survival time of adult flies (55). The striking similarities of the effects of GATAe knockdown and Tsp2A knockdown suggest that Hippo signaling may be involved. Septate junctional proteins were not pursued in the study by Martinez-Coralles et al. (55). Thus it remains to be seen if Tsp2A and Hippo signaling are part of GATAe induced tumor formation.
The above hypothetical scenarios are based on the view that RSCs in the ureter and lower tubule give rise to new epithelial cells that migrate upstream to take their place as principal cells and stellate cells (55, 67). However, recent studies by Wang and Spradling find no evidence for cell migration in Malpighian tubules of Drosophila and no evidence for long distance signaling to RSCs (77). Moreover, only unipotent RSCs were found in the lower tubule and ureter where ~70% of all cells are RSCs (77). In adult tubules they remain quiescent and respond only to damage or loss of principal cells not further than 10 cells away from RSCs. The genetic ablation of cells in or injury to the upper tubule did not increase RSC activity, and lineage tracing studies indicated that RSC daughter cells do not migrate toward upper tubules. Since there is no evidence for stem cells in the upper Malpighian tubule, tubule segments here (main secretory segment, transitional segment and distal tubule) resemble tissues like adult Drosophila hindgut and epidermis, which utilize RSC-independent mechanisms to cope with cell loss, namely wound-induced polyploidization (54). Wound-induced polyploidization (WIP) is the process of replacing cell mass by endoreplication instead of mitotic proliferation. For example, a puncture wound in the adult epidermis first closes with a melanized scab formed by cross linking oxidized phenols mediated by hemolymph enzymes and blood cells (54). Thereafter, epithelial cells near the wound fuse to form a large syncytium containing 87 nuclei on average. Rac GTPase activity is needed for syncytium formation, and Hippo signaling and Yki modulate polyploidization and cell fusion. Large cells produced by polyploidization or cell fusion are thought better able than diploid cells to mechanically stabilize wounds (54).
The above discussion presented three plausible molecular mechanisms that produce the hyperplasia induced by the developmental knockdown of Tsp2A. Hippo signaling appears to be involved whether epithelia repair themselves by mechanisms of mitotic stem cell proliferation or endocyclic polyploidization. Regardless of the mechanism for replacing Tsp2A-deficient principal cells in Malpighian tubules, the present study confirms the critical role of Tsp2A and the sSJ in maintaining the structure and function of Malpighian tubules after their development in the embryo.
GRANTS
The authors acknowledge the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-110358 (A.R.R. and S.J.) and the DFG (German Research Foundation) for enabling this work under the auspices of Priority Research Award SFB 944: Physiology and Dynamics of Cellular Microcompartments (to A. P. and H.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.W.B., M.F., H.M., A.R.R., and A.P. conceived and designed research; K.W.B., F.S., L.F.B., F.T., Y.I., and H.M. performed experiments; K.W.B., F.S., L.F.B., F.T., M.F., Y.I., H.M., A.R.R., and A.P. analyzed data; K.W.B., F.S., L.F.B., F.T., M.F., Y.I., H.M., S.J., and A.R.R. interpreted results of experiments; K.W.B., F.S., L.F.B., F.T., Y.I., H.M., and A.R.R. prepared figures; K.W.B. and A.R.R. drafted manuscript; K.W.B., F.S., L.F.B., F.T., M.F., Y.I., H.M., S.J., A.R.R., and A.P. edited and revised manuscript; K.W.B., F.S., L.F.B., F.T., M.F., Y.I., H.M., S.J., A.R.R., and A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank E. Cordes for expert support in immunohistochemistry and C. Wang (Carnegie Institution for Science) and C. Xu (Harvard Medical School) for fruitful discussions.
REFERENCES
- 1.Abe M, Sugiura T, Takahashi M, Ishii K, Shimoda M, Shirasuna K. A novel function of CD82/KAI-1 on E-cadherin-mediated homophilic cellular adhesion of cancer cells. Cancer Lett 266: 163–170, 2008. doi: 10.1016/j.canlet.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 2.Arvidsson S, Kwasniewski M, Riaño-Pachón DM, Mueller-Roeber B. QuantPrime—a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinformatics 9: 465, 2008. doi: 10.1186/1471-2105-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bätz T, Förster D, Luschnig S. The transmembrane protein Macroglobulin complement-related is essential for septate junction formation and epithelial barrier function in Drosophila. Development 141: 899–908, 2014. doi: 10.1242/dev.102160. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 87: 1059–1068, 1996. doi: 10.1016/S0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- 5.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol 338: 28–37, 2010. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 6.Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell 5: 611–620, 2003. doi: 10.1016/S1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 7.Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 114: 4143–4151, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Beyenbach KW. Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol Sci 16: 145–151, 2001. doi: 10.1152/physiologyonline.2001.16.4.145. [DOI] [PubMed] [Google Scholar]
- 9.Beyenbach KW. Voltages and resistances of the anterior Malpighian tubule of Drosophila melanogaster. J Exp Biol 222: jeb.201574, 2019. doi: 10.1242/jeb.201574. [DOI] [PubMed] [Google Scholar]
- 10.Beyenbach KW, Dantzler WH. Comparative kidney tubule sources, isolation, perfusion, and function. Methods Enzymol 191: 167–226, 1990. doi: 10.1016/0076-6879(90)91014-W. [DOI] [PubMed] [Google Scholar]
- 11.Beyenbach KW, Pannabecker TL, Nagel W. Central role of the apical membrane H+-ATPase in electrogenesis and epithelial transport in Malpighian tubules. J Exp Biol 203: 1459–1468, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Beyenbach KW, Piermarini PM. Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti. Acta Physiol (Oxf) 202: 387–407, 2011. doi: 10.1111/j.1748-1716.2010.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol 209: 577–589, 2006. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- 14.Boucheix C, Duc GH, Jasmin C, Rubinstein E. Tetraspanins and malignancy. Expert Rev Mol Med 3: 1–17, 2001. doi: 10.1017/S1462399401002381. [DOI] [PubMed] [Google Scholar]
- 15.Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci 58: 1189–1205, 2001. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charrin S, Jouannet S, Boucheix C, Rubinstein E. Tetraspanins at a glance. J Cell Sci 127: 3641–3648, 2014. doi: 10.1242/jcs.154906. [DOI] [PubMed] [Google Scholar]
- 17.Charrin S, le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J 420: 133–154, 2009. doi: 10.1042/BJ20082422. [DOI] [PubMed] [Google Scholar]
- 18.Chattopadhyay N, Wang Z, Ashman LK, Brady-Kalnay SM, Kreidberg JA. α3β1 integrin–CD151, a component of the cadherin-catenin complex, regulates PTPμ expression and cell-cell adhesion. J Cell Biol 163: 1351–1362, 2003. doi: 10.1083/jcb.200306067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Sayadian AC, Lowe N, Lovegrove HE, St Johnston D. An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol 16: e3000041, 2018. doi: 10.1371/journal.pbio.3000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi T, Kim MS, Lang S, Bose N, Kahn A, Flechner L, Blaschko SD, Zee T, Muteliefu G, Bond N, Kolipinski M, Fakra SC, Mandel N, Miller J, Ramanathan A, Killilea DW, Brückner K, Kapahi P, Stoller ML. A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS One 10: e0124150, 2015. doi: 10.1371/journal.pone.0124150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denholm B. Shaping up for action: the path to physiological maturation in the renal tubules of Drosophila. Organogenesis 9: 40–54, 2013. doi: 10.4161/org.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denholm B, Sudarsan V, Pasalodos-Sanchez S, Artero R, Lawrence P, Maddrell S, Baylies M, Skaer H. Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr Biol 13: 1052–1057, 2003. doi: 10.1016/S0960-9822(03)00375-0. [DOI] [PubMed] [Google Scholar]
- 23.Dostálová A, Rommelaere S, Poidevin M, Lemaitre B. Thioester-containing proteins regulate the Toll pathway and play a role in Drosophila defence against microbial pathogens and parasitoid wasps. BMC Biol 15: 79, 2017. doi: 10.1186/s12915-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dow JA, Romero MF. Drosophila provides rapid modeling of renal development, function, and disease. Am J Physiol Renal Physiol 299: F1237–F1244, 2010. doi: 10.1152/ajprenal.00521.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dow JA, Davies SA. The Drosophila melanogaster Malpighian tubule. Adv Insect Physiol 28: 1–83, 2001. doi: 10.1016/S0065-2806(01)28008-4. [DOI] [Google Scholar]
- 26.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 17: 375–412, 1963. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautam NK, Verma P, Tapadia MG. Ecdysone regulates morphogenesis and function of Malpighian tubules in Drosophila melanogaster through EcR-B2 isoform. Dev Biol 398: 163–176, 2015. doi: 10.1016/j.ydbio.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Hall S, Ward RE IV. Septate junction proteins play essential roles in morphogenesis throughout embryonic development in Drosophila. G3 (Bethesda) 6: 2375–2384, 2016. doi: 10.1534/g3.116.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harden N, Wang SJ, Krieger C. Making the connection–shared molecular machinery and evolutionary links underlie the formation and plasticity of occluding junctions and synapses. J Cell Sci 129: 3067–3076, 2016. doi: 10.1242/jcs.186627. [DOI] [PubMed] [Google Scholar]
- 30.Harris HJ, Farquhar MJ, Mee CJ, Davis C, Reynolds GM, Jennings A, Hu K, Yuan F, Deng H, Hubscher SG, Han JH, Balfe P, McKeating JA. CD81 and claudin 1 coreceptor association: role in hepatitis C virus entry. J Virol 82: 5007–5020, 2008. doi: 10.1128/JVI.02286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467, 2003. doi: 10.1016/S0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 32.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 6: 801–811, 2005. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 33.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol 19: 397–422, 2003. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- 34.Homsi Y, Schloetel JG, Scheffer KD, Schmidt TH, Destainville N, Florin L, Lang T. The extracellular δ-domain is essential for the formation of CD81 tetraspanin webs. Biophys J 107: 100–113, 2014. doi: 10.1016/j.bpj.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang S, Yuan S, Dong M, Su J, Yu C, Shen Y, Xie X, Yu Y, Yu X, Chen S, Zhang S, Pontarotti P, Xu A. The phylogenetic analysis of tetraspanins projects the evolution of cell-cell interactions from unicellular to multicellular organisms. Genomics 86: 674–684, 2005. doi: 10.1016/j.ygeno.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Shi L, Cao J, He F, Li R, Zhang Y, Miao S, Jin L, Qu J, Li Z, Lin X. The sterile 20-like kinase tao controls tissue homeostasis by regulating the hippo pathway in Drosophila adult midgut. J Genet Genomics 41: 429–438, 2014. doi: 10.1016/j.jgg.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Ishibashi T, Ding L, Ikenaka K, Inoue Y, Miyado K, Mekada E, Baba H. Tetraspanin protein CD9 is a novel paranodal component regulating paranodal junctional formation. J Neurosci 24: 96–102, 2004. doi: 10.1523/JNEUROSCI.1484-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumi Y, Furuse K, Furuse M. Septate junctions regulate gut homeostasis through regulation of stem cell proliferation and enterocyte behavior in Drosophila. J Cell Sci 132: jcs232108, 2019. doi: 10.1242/jcs.232108. [DOI] [PubMed] [Google Scholar]
- 39.Izumi Y, Furuse M. Molecular organization and function of invertebrate occluding junctions. Semin Cell Dev Biol 36: 186–193, 2014. doi: 10.1016/j.semcdb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Izumi Y, Motoishi M, Furuse K, Furuse M. A tetraspanin regulates septate junction formation in Drosophila midgut. J Cell Sci 129: 1155–1164, 2016. doi: 10.1242/jcs.180448. [DOI] [PubMed] [Google Scholar]
- 41.Izumi Y, Yanagihashi Y, Furuse M. A novel protein complex, Mesh-Ssk, is required for septate junction formation in the Drosophila midgut. J Cell Sci 125: 4923–4933, 2012. doi: 10.1242/jcs.112243. [DOI] [PubMed] [Google Scholar]
- 42.Jonusaite S, Beyenbach KW, Meyer H, Paululat A, Izumi Y, Furuse M, Rodan AR. The septate junction protein Mesh is required for epithelial morphogenesis, ion transport, and paracellular permeability in the Drosophila Malpighian tubule. Am J Physiol Cell Physiol 318: C675–C694, 2020. doi: 10.1152/ajpcell.00492.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonusaite S, Donini A, Kelly SP. Occluding junctions of invertebrate epithelia. J Comp Physiol B 186: 17–43, 2016. doi: 10.1007/s00360-015-0937-1. [DOI] [PubMed] [Google Scholar]
- 44.Khadilkar RJ, Tanentzapf G. Septate junction components control Drosophila hematopoiesis through the Hippo pathway. Development 146: dev166819, 2019. doi: 10.1242/dev.166819. [DOI] [PubMed] [Google Scholar]
- 45.Knauf F, Preisig PA. Drosophila: a fruitful model for calcium oxalate nephrolithiasis? Kidney Int 80: 327–329, 2011. doi: 10.1038/ki.2011.166. [DOI] [PubMed] [Google Scholar]
- 46.Kovalenko OV, Metcalf DG, DeGrado WF, Hemler ME. Structural organization and interactions of transmembrane domains in tetraspanin proteins. BMC Struct Biol 5: 11, 2005. doi: 10.1186/1472-6807-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovalenko OV, Yang XH, Hemler ME. A novel cysteine cross-linking method reveals a direct association between claudin-1 and tetraspanin CD9. Mol Cell Proteomics 6: 1855–1867, 2007. doi: 10.1074/mcp.M700183-MCP200. [DOI] [PubMed] [Google Scholar]
- 48.Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell 33: 36–46, 2015. doi: 10.1016/j.devcel.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lane NJ, Dallai R, Martinucci G, Burighel P. Electron microscopic structure and evolution of epithelial junctions. In: Molecular Mechanisms of Epithelial Cell Junctions: From Development to Disease, edited by Citi S. Austin, TX: R.G. Landes, 1994, p. 23–43. [Google Scholar]
- 50.Lazo PA. Functional implications of tetraspanin proteins in cancer biology. Cancer Sci 98: 1666–1677, 2007. doi: 10.1111/j.1349-7006.2007.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levy S, Shoham T. Protein-protein interactions in the tetraspanin web. Physiology (Bethesda) 20: 218–224, 2005. doi: 10.1152/physiol.00015.2005. [DOI] [PubMed] [Google Scholar]
- 52.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol 5: 136–148, 2005. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 53.Linford NJ, Bilgir C, Ro J, Pletcher SD. Measurement of lifespan in Drosophila melanogaster. J Vis Exp 71: e50068, 2013. doi: 10.3791/50068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Losick VP, Fox DT, Spradling AC. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol 23: 2224–2232, 2013. doi: 10.1016/j.cub.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martínez-Corrales G, Cabrero P, Dow JA, Terhzaz S, Davies SA. Novel roles for GATAe in growth, maintenance and proliferation of cell populations in the Drosophila renal tubule. Development 146: dev178087, 2019. doi: 10.1242/dev.178087. [DOI] [PubMed] [Google Scholar]
- 56.Mazia D, Schatten G, Sale W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol 66: 198–200, 1975. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev 30: 1–17, 2016. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller J, Chi T, Kapahi P, Kahn AJ, Kim MS, Hirata T, Romero MF, Dow JA, Stoller ML. Drosophila melanogaster as an emerging translational model of human nephrolithiasis. J Urol 190: 1648–1656, 2013. doi: 10.1016/j.juro.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson KS, Furuse M, Beitel GJ. The Drosophila Claudin Kune-kune is required for septate junction organization and tracheal tube size control. Genetics 185: 831–839, 2010. doi: 10.1534/genetics.110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perrimon N, Ni JQ, Perkins L. In vivo RNAi: today and tomorrow. Cold Spring Harb Perspect Biol 2: a003640, 2010. doi: 10.1101/cshperspect.a003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramsay JA. Active transport of potassium by the Malpighian tubules of insects. J Exp Biol 30: 358–369, 1953. [Google Scholar]
- 62.Rosay P, Davies SA, Yu Y, Sözen MA, Kaiser K, Dow JA. Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci 110: 1683–1692, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Schellinger JN, Rodan AR. Use of the Ramsay assay to measure fluid secretion and ion flux rates in the Drosophila melanogaster Malpighian tubule. J Vis Exp 105: e53144, 2015. doi: 10.3791/53144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schulte J, Charish K, Que J, Ravn S, MacKinnon C, Auld VJ. Gliotactin and Discs large form a protein complex at the tricellular junction of polarized epithelial cells in Drosophila. J Cell Sci 119: 4391–4401, 2006. doi: 10.1242/jcs.03208. [DOI] [PubMed] [Google Scholar]
- 65.Shigeta M, Sanzen N, Ozawa M, Gu J, Hasegawa H, Sekiguchi K. CD151 regulates epithelial cell-cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. J Cell Biol 163: 165–176, 2003. doi: 10.1083/jcb.200301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon P. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19: 1439–1440, 2003. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- 67.Singh SR, Liu W, Hou SX. The adult Drosophila Malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell 1: 191–203, 2007. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skaer HB. Development of the alimentary canal. In: The Development of Drosophila, edited by Bate CM, Martinez Arias A. Long Island, NY: Cold Spring Harbor, 1993, p. 941–1012. [Google Scholar]
- 69.Skaer HB, Maddrell SH, Harrison JB. The permeability properties of septate junctions in Malpighian tubules of Rhodnius. J Cell Sci 88: 251–265, 1987. [DOI] [PubMed] [Google Scholar]
- 70.Snigdha K, Gangwani KS, Lapalikar GV, Singh A, Kango-Singh M. Hippo signaling in cancer: lessons from Drosophila models. Front Cell Dev Biol 7: 85, 2019. doi: 10.3389/fcell.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sözen MA, Armstrong JD, Yang M, Kaiser K, Dow JA. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci USA 94: 5207–5212, 1997. doi: 10.1073/pnas.94.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun S, Irvine KD. Cellular organization and cytoskeletal regulation of the Hippo signaling network. Trends Cell Biol 26: 694–704, 2016. doi: 10.1016/j.tcb.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takashima S, Paul M, Aghajanian P, Younossi-Hartenstein A, Hartenstein V. Migration of Drosophila intestinal stem cells across organ boundaries. Development 140: 1903–1911, 2013. doi: 10.1242/dev.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tennessen JM, Bertagnolli NM, Evans J, Sieber MH, Cox J, Thummel CS. Coordinated metabolic transitions during Drosophila embryogenesis and the onset of aerobic glycolysis. G3 (Bethesda) 4: 839–850, 2014. doi: 10.1534/g3.114.010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol 161: 563–596, 1994. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- 76.Tiburcy F, Beyenbach KW, Wieczorek H. Protein kinase A-dependent and -independent activation of the V-ATPase in Malpighian tubules of Aedes aegypti. J Exp Biol 216: 881–891, 2013. doi: 10.1242/jeb.078360. [DOI] [PubMed] [Google Scholar]
- 77.Wang C, Spradling AC. An abundant quiescent stem cell population in Drosophila Malpighian tubules protects principal cells from kidney stones. eLife 9: e54096, 2020. doi: 10.7554/eLife.54096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watt KI, Harvey KF, Gregorevic P. Regulation of tissue growth by the mammalian Hippo signaling pathway. Front Physiol 8: 942, 2017. doi: 10.3389/fphys.2017.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wieczorek H, Cioffi M, Klein U, Harvey WR, Schweikl H, Wolfersberger MG. Isolation of goblet cell apical membrane from tobacco hornworm midgut and purification of its vacuolar-type ATPase. Methods Enzymol 192: 608–616, 1990. doi: 10.1016/0076-6879(90)92098-X. [DOI] [PubMed] [Google Scholar]
- 80.Wiener J, Spiro D, Loewenstein WR. Studies on an epithelial (gland) cell junction. II. Surface structure. J Cell Biol 22: 587–598, 1964. doi: 10.1083/jcb.22.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol 134: 1469–1482, 1996. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woods DF, Wu JW, Bryant PJ. Localization of proteins to the apico-lateral junctions of Drosophila epithelia. Dev Genet 20: 111–118, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 83.Wright MD, Moseley GW, van Spriel AB. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens 64: 533–542, 2004. doi: 10.1111/j.1399-0039.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- 84.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456, 2003. doi: 10.1016/S0092-8674(03)00549-X. [DOI] [PubMed] [Google Scholar]
- 85.Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol 164: 313–323, 2004. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu C, Tang HW, Hung RJ, Hu Y, Ni X, Housden BE, Perrimon N. The septate junction protein Tsp2A restricts intestinal stem cell activity via endocytic regulation of aPKC and Hippo signaling. Cell Reports 26: 670–688.e6, 2019. doi: 10.1016/j.celrep.2018.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu K, Liu X, Wang Y, Wong C, Song Y. Temporospatial induction of homeodomain gene cut dictates natural lineage reprogramming. eLife 7: e33934, 2018. doi: 10.7554/eLife.33934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yanagihashi Y, Usui T, Izumi Y, Yonemura S, Sumida M, Tsukita S, Uemura T, Furuse M. Snakeskin, a membrane protein associated with smooth septate junctions, is required for intestinal barrier function in Drosophila. J Cell Sci 125: 1980–1990, 2012. doi: 10.1242/jcs.096800. [DOI] [PubMed] [Google Scholar]
- 89.Yáñez-Mó M, Barreiro O, Gordon-Alonso M, Sala-Valdés M, Sánchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 19: 434–446, 2009. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 90.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154: 1342–1355, 2013. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang XA, Huang C. Tetraspanins and cell membrane tubular structures. Cell Mol Life Sci 69: 2843–2852, 2012. doi: 10.1007/s00018-012-0954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmerman B, Kelly B, McMillan BJ, Seegar TC, Dror RO, Kruse AC, Blacklow SC. Crystal structure of a full-length human tetraspanin reveals a cholesterol-binding pocket. Cell 167: 1041–1051.e11, 2016. doi: 10.1016/j.cell.2016.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]