Abstract
Intraflagellar transport (IFT) is an evolutionarily conserved mechanism that is indispensable for the formation and maintenance of cilia and flagella; however, the implications and functions of IFT81 remain unknown. In this study, we disrupted IFT81 expression in male germ cells starting from the spermatocyte stage. As a result, homozygous mutant males were completely infertile and displayed abnormal sperm parameters. In addition to oligozoospermia, spermatozoa presented dysmorphic and nonfunctional flagella. Histological examination of testes from homozygous mutant mice revealed abnormal spermiogenesis associated with sloughing of germ cells and the presence of numerous multinucleated giant germ cells (symblasts) in the lumen of seminiferous tubules and epididymis. Moreover, only few elongated spermatids and spermatozoa were seen in analyzed cross sections. Transmission electron microscopy showed a complete disorganization of the axoneme and para-axonemal structures such as the mitochondrial sheath, fibrous sheath, and outer dense fibers. In addition, numerous vesicles that contain unassembled microtubules were observed within developing spermatids. Acrosome structure analysis showed normal appearance, thus excluding a crucial role of IFT81 in acrosome biogenesis. These observations showed that IFT81 is an important member of the IFT process during spermatogenesis and that its absence is associated with abnormal flagellum formation leading to male infertility. The expression levels of several IFT components in testes, including IFT20, IFT25, IFT27, IFT57, IFT74, and IFT88, but not IFT140, were significantly reduced in homozygous mutant mice. Overall, our study demonstrates that IFT81 plays an essential role during spermatogenesis by modulating the assembly and elongation of the sperm flagella.
Keywords: ciliogenesis, germ cell, IFT81, male infertility
INTRODUCTION
Cilia are common cell surface organelles that are observed on virtually all eukaryotic cells at some point during development and differentiation (12, 16, 48). In the biogenesis and maintenance of cilia, intraflagellar transport (IFT), defined as a bidirectional transport system composed of IFT protein complexes, is indispensable (18, 25). There are two major IFT protein complexes, IFT-A and IFT-B. The two complexes are separable using sucrose centrifugation, as complex B sediments more slowly. Subunits of the IFT complexes have been named according to their molecular weights. IFT-A contains six subunits including IFT43, IFT122A, IFT122B, IFT139, IFT140, and IFT144. Compared with IFT-A, IFT-B contains 10 more modules, IFT81 included (27, 37). Considering the pivotal role of both complexes in mediating the contact between cargo proteins and motors, any mutation in any IFT gene may cause disruption in ciliogenesis and/or sperm flagellum biogenesis (32, 36, 45, 47, 51, 63–65).
Mammalian IFT81, originally called carnitine deficiency-associated gene expressed in ventricle 1 (CDV-1), was cloned from the mouse ventricle in an effort to search for genes associated with carnitine-deficient juvenile visceral steatosis disease (34). CDV-1-related gene was cloned later and is highly expressed in the mouse testis (42). As a component of IFT-B, IFT81 forms a tubulin-binding module that specifically mediates the transport of tubulin within the cilium with IFT74 (3, 26). It binds tubulin via its calponin-homology-like region. Knockdown of IFT81 and rescue experiments with point mutants showed that tubulin binding by IFT81 was required for ciliogenesis in human cells (5, 26). The spectrum of IFT81-related disease expression included nephronophthisis, retinal dystrophy, cerebellar atrophy, and polydactyly (8–10, 43, 52, 55). Also, Hauer et al. (13) and Pettersson et al. (44) reported that intragenic duplications in IFT81 were associated with skeletal dysplasias. In Caenorhabditis elegans, loss-of-function Ift81 mutants showed an unusual behavioral property and small body size (24); in addition, IFT81 and IFT74 act coordinately in IFT in C. elegans’ sensory cilia (24).
Based on the above, the importance of normal physiological status of IFT81 for IFT and ciliogenesis is needless to say. However, given the fact that the research on IFT81 is relatively insufficient so far, not much is known about its role in mammalian sperm flagella formation. Particularly, our laboratory (51) discovered that IFT74, its binding partner, is essential for spermatogenesis and sperm formation. It is not known whether IFT81 possesses the same function. To help fill the void, in the present study, we generated the floxed Ift81 mice and disrupted Ift81 expression in male germ cells using conditional knockout (KO) strategy. Depending on the model, we observed the relationship between Ift81 gene disruption and spermiogenesis. Since IFT proteins usually associate with other proteins to form a complex to conduct their physiological effects, the expression levels of several other IFT proteins were also examined. To assess the potential effects on formation of the sperm tail, specific attention was given to the expression of components of the sperm flagellum, including the protein expression of sperm-associated antigen 16L (SPAG16L), outer dense fiber protein 2 (ODF2), and A-kinase anchor protein 4 (AKAP4), which are components of sperm tail’s axonemal central apparatus (64a, 66), outer dense fibers (17, 50), and fibrous sheath (35, 60), respectively. A role for IFT81 in spermiogenesis was uncovered, and its contribution to male fertility was demonstrated.
MATERIALS AND METHODS
Generation of Ift81 mutant mice.
All animal experiments were approved by Wayne State University’s ethics committee (protocol: IACUC-18-02-0534) in accordance with federal and local ethical terms regarding the use of nonprimate vertebrates in scientific research, and the mice were euthanized by CO2 inhalation followed by cervical dislocation. Stra8-iCre mice were obtained from Jackson (JAX; stock no. 008208; Ref. 46), and Ift81flox/flox mice were generated at the Center for Mouse Genome Modification at University of Connecticut. Briefly, the original Ift81tm1a (EUCOMM)Wtsi mouse line was obtained as cryopreserved embryos from the International Mouse Phenotyping Consortium (IMPC; https://www.mousephenotype.org/). The cryopreserved embryos were thawed according to instruction provided by IMPC and then transferred into pseudopregnant CD1 females. F1 pups were then mated with Rosa26-Flpe mice (JAX stock no. 003946), which have been backcrossed with C57BL/6 mice for over 30 generations to remove the LacZ/Neo cassette to generate the final Ift81 floxed mice. Two- to three-month-old Stra8-iCre males were crossed with 2- to 3-mo-old Ift81flox/flox females to obtain Stra8-iCre; Ift81flox/+ mice. The 2- to 3-mo-old Stra8-iCre; Ift81flox/+ males were crossed back with 2- to 3-mo-old Ift81flox/flox females again, the Stra8-iCre; Ift81flox/flox were considered to be the homozygous mutant mice (KO), and Stra8-iCre; Ift81flox/+ mice were used as controls. All subsequent breeding was maintained in C57BL/6 background. To genotype these mice, the following primer pairs were used: IFT Lox5F, 5′-CCTCTCTCACCAGCAGTCAGGCAC-3′, and IFT Lox5R, 5′-GGAGCATCCAAGAAGCTGTGCTG-3′, will amplify a fragment of 356 bp for wild type and 516 bp for the floxed allele; and IFT Lox5F + IFT Lox3R, 5′-CTGCAGGCCTAGCTCAGCTGC-3′, for a fragment of 381 bp specific for the KO allele.
Quantitative PCR.
Total tissue RNA was isolated using TRIzol reagent (QIAGEN), and cDNA was synthesized using a first-strand cDNA SensiFAST cDNA Synthesis Kit (Bioline). To compare Ift81 mRNA expression levels in different mouse tissues, quantitative PCR (qPCR) was conducted using the following primer pair designed with GenScript tools: forward, 5′-GAGGAGATGCCAGAGCAGAC-3′, and reverse, 5′-CACTTGGCCACCTCCAGTTTT-3′. 18S rRNA level was used to normalize expression level of target genes (53).
Assessment of fertility and fecundity.
To test fertility, we paired ≥6-wk-old Stra8-iCre; Ift81flox/Δ (KO) and control males with mature wild-type females for ≥2 mo and recorded the number of mice achieving a pregnancy and the litter size from each mating set or pregnancy. Mating behavior was also observed, and the females were checked for the presence of vaginal plugs. Eight control mice and five KO mice were analyzed in this study.
Spermatozoa counting and morphology.
Sperm were collected from the cauda epididymis and fixed in 2% formaldehyde solution for 10 min at room temperature. Then, under a light microscope, sperm were counted using a hemocytometer chamber, as previously reported (64a). Sperm morphology was also examined. Eight control mice and five KO mice were analyzed in this study.
Spermatozoa motility assay.
Sperm motility was evaluated under noncapacitation condition in a noncapacitating medium containing the following compounds: NaCl (10 mM), KCl (4.4 mM), KH2PO4 (1.2 mM), MgSO4 (1.2 mM), glucose (5.4 mM), pyruvic acid (0.8 mM), lactic acid (2.4 mM), and HEPES acid (20 mM; Ref. 38). Briefly, epididymal spermatozoa were squeezed out of the cauda epididymidis and placed in warm noncapacitating medium. Ten minutes after sperm were collected, sperm motility was observed using a Nikon TE200E inverted microscope on a prewarmed slide with Sanyo color charge-coupled device, Hi-Resolution camera (VCC-3972) and Pinnacle Studio HD (version 14.0) software. For each sperm sample, eight fields were analyzed. Individual spermatozoa were tracked using NIH ImageJ (Bethesda, MD) and the plugin MTrackJ. Sperm motility was calculated as curvilinear velocity (VCL), which is equivalent to the curvilinear distance (DCL) that is traveled by each individual spermatozoa in 1 s (VCL = DCL/t). Eight control mice and five KO mice were analyzed in this study.
Histology and immunofluorescence/immunochemistry.
Testes and epididymides of three control and three KO adult mice were fixed in 4% formaldehyde solution for ≥24 h and then paraffin embedded, and 5-μm sections were placed on glass slides. Histology and immunofluorescence (IF) staining were performed by using routine procedures. The anti-IFT81 antibody (cat. no. 11744-1-AP: Proteintech, Rosemont, IL) was used at a 1:200 dilution. The control slides were incubated with normal rabbit IgG. To display IFT81, Cy3 AffiniPure F(ab')2 fragment donkey anti-rabbit IgG (H+L) (cat. no. 711-166-152; 1:500; Jackson ImmunoResearch, West Grove, PA) and DyLight 488 horse anti-mouse IgG antibody (cat. no. DI-2488; 1:500; Vector, Burlingame, CA) were used as secondary antibodies for some slides. Then, the slides were sealed with 4′,6′-diamidino-2-phenylindole (H-1200; Vector). Images were captured using confocal laser-scanning microscopy (Leica TCS SP2 AOBS; Wetzlar, Germany). For immunohistochemistry, the sections were stained using the avidin-biotin technique (VECTASTAIN Elite kit; Vector) and visualized with diaminobenzidine (Sigma-Aldrich, St. Louis, MO).
TUNEL staining analysis.
The paraffin-embedded testis sections were heated at 60°C for 1 h. Then, the samples were deparaffinized with xylene twice and washed with 100% ethanol twice. After rehydrating with 70% ethanol for 5 min and 35% ethanol for 5 min, the slides were incubated with 3% H2O2 in methanol at room temperature for 20 min. Afterward, the sections were incubated and labeled following the manufacturer’s instructions for the In Situ Cell Death Detection Kit (cat. no. 11684795910; Roche).
Isolation of germ cells and immunofluorescence analysis.
Germ cells were isolated as described previously (29). Testes from three 2- to 4-mo-old control and three age-matched KO adult mice were dissected in a petri dish with 5 mL of DMEM containing 0.5 mg/mL collagenase IV and 1.0 μg/mL DNase I (Sigma-Aldrich) and incubated for 30 min at 32°C with gentle stirring. Released spermatogenic cells were pelleted by centrifugation (5 min at 1,000 rpm, 4°C). After being washed with PBS, the cells were fixed with 5 mL of 4% paraformaldehyde containing 0.1 M sucrose at room temperature. The dispersed, mixed testicular cells were washed three times with PBS. Afterward, the cells were resuspended in 2 mL of warm PBS, and 50 μL of cell suspension was loaded to the slide and allowed to air-dry. Cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) for 5 min at 37°C, washed with PBS three times, and blocked with 10% goat serum in PBS for 1 h. Then, cells were washed three times with PBS and incubated overnight with the indicated primary antibodies. After an extensive wash with PBS, the cells were incubated with Cy3-conjugated anti-rabbit IgG secondary antibody for 1 h. The slides were washed with PBS and mounted in VectaMount with DAPI (Vector) and sealed with nail polish. Images were captured by confocal laser-scanning microscopy as before. The primary and secondary antibodies were the same as those used for IF on tissue sections.
Western blot analysis.
Expression of the indicated proteins was examined by Western blotting using 3- to 5-mo-old control and three age-matched KO adult mice. Routine procedure was performed as described previously. Briefly, testes were homogenized by ice-cold radioimmunoprecipitation assay buffer [50 mM Tris·HCl (pH 8.0), 170 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 1 mM dithiothreitol, and protease inhibitors (cOmplete Mini; Roche Diagnostics, Indianapolis, IN)] containing 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.05% SDS. After centrifugation at 12,000 rpm for 10 min, the supernatant was collected. Protein concentration was determined by Lowry assay. Denatured proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes. Nonspecific sites were blocked with 5% nonfat dry milk in 0.5% Tween 20 in TBS for 1 h at room temperature, and the membranes were incubated overnight with indicated antibodies. After being washed three times with TBS, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Amersham, Pittsburgh, PA) for 2 h at room temperature. After being washed another three times in TBS, the membranes were visualized by less sensitive SuperSignal West Pico Chemiluminescent Substrate (cat. no. 34080) or the higher-sensitivity Femto Maximum Sensitivity Substrate (cat. no. 34095) kits purchased from Pierce/Thermo Fisher Scientific (Waltham, MA). Three control mice and three KO mice were analyzed in this study. The specific antibodies are as follows: anti-IFT81 (as above); IFT25 (cat. no. 15732-1-AP; Proteintech); IFT74 (cat. no. AAS27620e; Antibody Verify, Las Vegas, NV); IFT20, IFT27, IFT57, IFT88, and IFT140 from Dr. Pazour’s laboratory (20, 21, 40); anti-SPAG16L generated by Z. Zhang’s laboratory; anti-AKAP4 (1:4,000; Dr. George Gerton at University of Pennsylvania); anti-ODF2 (1:800; 12058-1-AP; Proteintech); and anti-β-actin (1:2,000; cat. no. 4967; Cell Signaling Technology, Danvers, MA). Horseradish peroxidase-labeled anti-rabbit (NA934V; 1:2,000) and anti-mouse (NA931V; 1:2,000) were obtained from Amersham (Pittsburgh, PA).
Transmission electron microscopy.
For transmission electron microscopy analysis, testes from two control and two KO adult male mice were extracted, and their tunicae albugineae were removed before being fixed for 24 h at 4°C, with 0.1 M HEPES, 4 mM CaCl2, 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA), and 2% formaldehyde (Electron Microscopy Sciences), pH adjusted to 7.4. The fixative was replaced four times during the 1st 2 h (every 30 min). Samples were then washed with buffer and postfixed with 1% osmium tetroxide (OsO4) for 45–60 min at 4°C. After being washed with 0.1 M HEPES (pH 7.2) containing 4 mM CaCl2, samples were incubated in 1% uranyl acetate for 60 min at 4°C before being dehydrated through graded series of ethanol and infiltrated gradually with epoxy (FLUKA) and then polymerized for 72 h at 60°C. Ultrathin sections of the samples were cut with an ultramicrotome (Leica), and the sections were poststained with 5% uranyl acetate and 0.4% lead citrate before being observed with an electron microscope at 80 kV (JEOL 1200EX). Images were acquired with a digital camera (Veleta; Olympus Soft Imaging Solutions), and morphometric analysis was performed with iTEM software (Olympus).
Statistical analysis.
All results are given as means ± standard deviation. Data analysis was performed using SPSS software (version 17.0; SPSS, Chicago, IL). Statistical significance was determined by the Student’s t test. P values <0.05 were considered to be statistically significant.
RESULTS
Qualitative and quantitative analysis of Ift81 mRNA and protein and spatial and temporal distribution of IFT81 in testis and isolated germ cells.
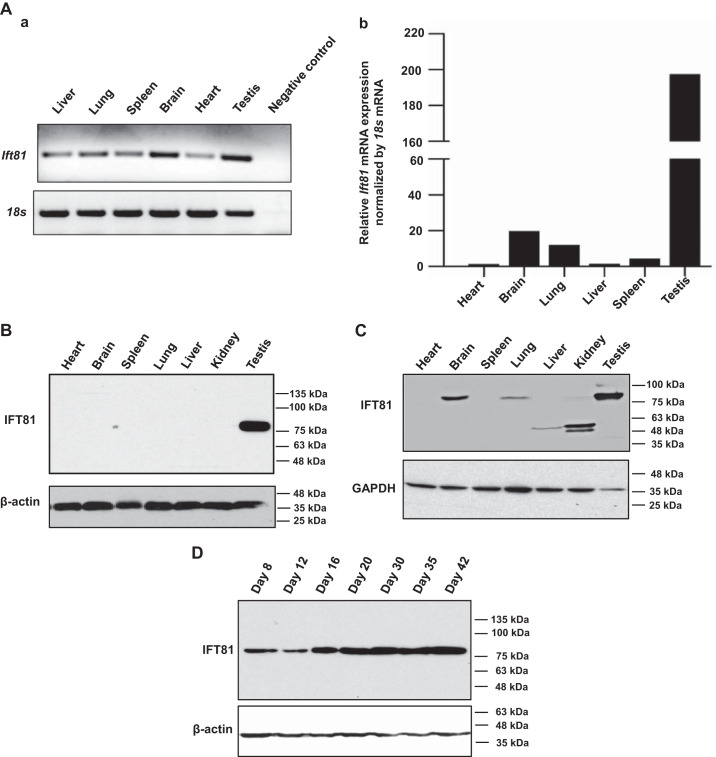
Ift81 mRNA tissue distribution was first examined by RT-PCR. It was found that the message was present in all tissues examined (Fig. 1Aa). Ift81 mRNA expression level in multiple mouse tissues was further examined by qPCR. It was found that Ift81 mRNA was less abundant in the heart but highly abundant in the testis (Fig. 1Ab). The protein expression level of IFT81 in several tissues, such as in the heart, brain, spleen, lung, liver, kidney, and testis, was examined by Western blot analysis. As shown in Fig. 1B, IFT81 protein was detected only in the testis when the less sensitive Pico system was used. However, when the more sensitive Femto system was used, full-length IFT81 protein was also detected in brain, lung, and kidney (Fig. 1C). In kidney, only trace amount of the full-length IFT81 was detected, but the antibody also detected two smaller proteins (about 48 and 50 kDa). Even though full-length IFT81 was not detected in liver, the 50-kDa protein was present (Fig. 1C). We next examined the dynamic changes of testicular IFT81 expression during germ cell development. Expression of IFT81 protein was first detected at postnatal day (PND) 8, its level increased dramatically from PND 16 to 42, and the level appeared to be stable during this period (Fig. 1D).
Fig. 1.
Mouse intraflagellar transport 81 (IFT81) was highly expressed in the testis and developmentally regulated during spermatogenesis. A: analysis of relative Ift81 mRNA expression in adult mouse tissues by RT-PCR (a) and quantitative PCR (b). Ift81 mRNA expression levels were normalized by 18S rRNA. Notice that the specific Ift81 mRNA was detected in all tissues examined, and the signal was the strongest in the testis. B: analysis of mouse IFT81 protein expression by Western blot using the less sensitive Pico system. Notice that IFT81 was only detected in testes. C: analysis of mouse IFT81 protein expression in multiple tissues by Western blot using the more sensitive Femto system. Notice that besides in the testis, the full-length IFT81 was also expressed in brain, lung, and kidney. The antibody also cross-reacted with a 50-kDa protein in liver and 2 smaller proteins (48 and 50 kDa) in kidney. Fifty micrograms of total protein was loaded in all tissues except testis. Only 10 μg of total testicular protein was loaded. D: IFT81 expression during the 1st wave of spermatogenesis. A representative Western blot result from 3 independent experiments shows that IFT81 protein was expressed from day 8 after birth, and the level was significantly increased from day 16 after birth.
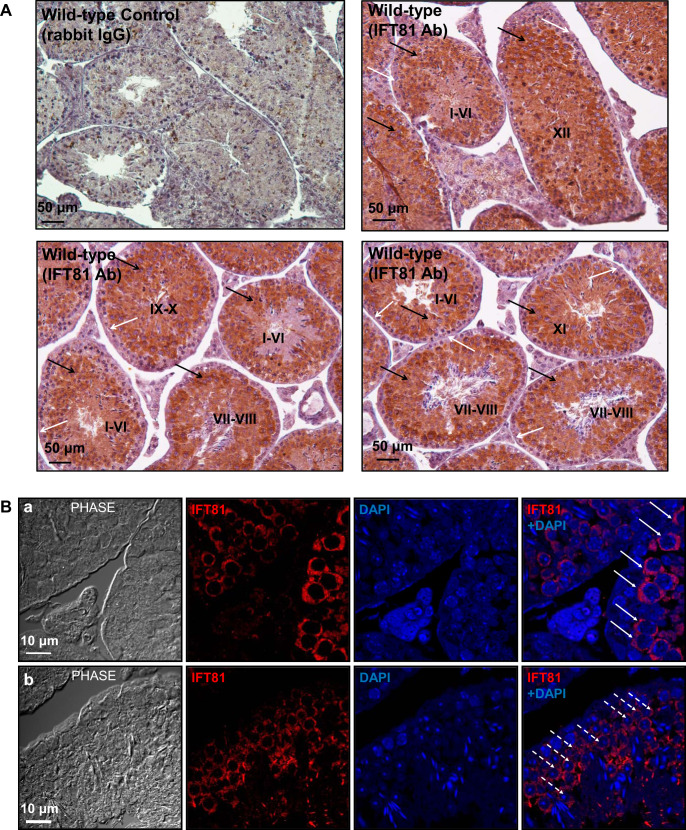
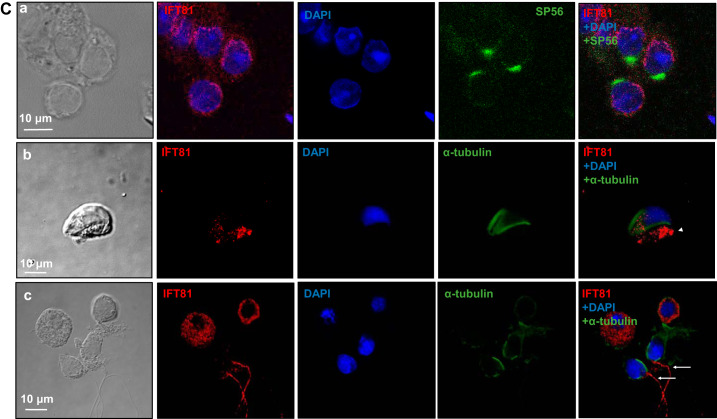
Immunochemistry/immunofluorescence staining was performed on testis sections and isolated germ cells to study the expression pattern of IFT81 during spermatogenesis and cellular and subcellular localization of IFT81 in germ cells. Immunochemistry on testis sections revealed that IFT81 protein was expressed in all stages in the seminiferous tubules as evaluated by a published staging method (Ref. 15; Fig. 2A), with a strong signal being detected in pachytene spermatocytes and round spermatids but not the cells located near the basement membrane of the seminiferous tubules, where spermatogonia and preleptotene spermatocytes were located (Fig. 2A and Supplemental Fig. S1; all supplemental material is available at https://doi.org/10.6084/m9.figshare.9978881). Localization of IFT81 was further examined by immunofluorescence staining. In testis sections, immunofluorescence staining showed that IFT81 was present in the cytoplasm of pachytene spermatocytes and round spermatids (Fig. 2B, a and b). In isolated germ cells, IFT81 was also found to be present in the cytoplasm of round spermatids (Fig. 2Ca), which was consistent with the results obtained from the analysis of testicular sections. Moreover, subcellular localization of IFT81 within elongating spermatids was examined after double staining using an anti-α-tubulin antibody, a marker for the manchette in addition to the anti-IFT81 antibody. This staining revealed that the IFT81 signal was present as clusters in the cytoplasm but did not coincide with α-tubulin (Fig. 2Cb). IFT81 was also present in the developing sperm tails (Fig. 2Cc).
Fig. 2.
Expression pattern of intraflagellar transport 81 (IFT81) in the seminiferous tubules and its localization in male germ cells of wild-type mice. A: examination of IFT81 expression in testis by immunochemistry staining. IFT81 was expressed in all stages (I–XII) of seminiferous tubules. The protein was not present in the cells near the basement of the seminiferous tubules (white arrows). Strong signal was detected in spermatocytes and round spermatids (black arrows). B: immunofluorescence staining of IFT81 in seminiferous tubules of wild-type mice. IFT81 was present in the cytoplasm of spermatocytes (arrows in a) and round spermatids (dashed arrows in b). C: localization of IFT81 in the isolated germ cells of wild-type mice. a: IFT81 was present as cytoplasm in round spermatids. SP56, an acrosome marker, was double stained. b: The cells were double stained with a manchette marker (α-tubulin). IFT81 (arrowhead pointing to red) was not colocalized with α-tubulin. c: IFT81 was present in the developing tails (white arrows). Ab, antibody.
Homozygous mutant adult Ift81 male mice were infertile.
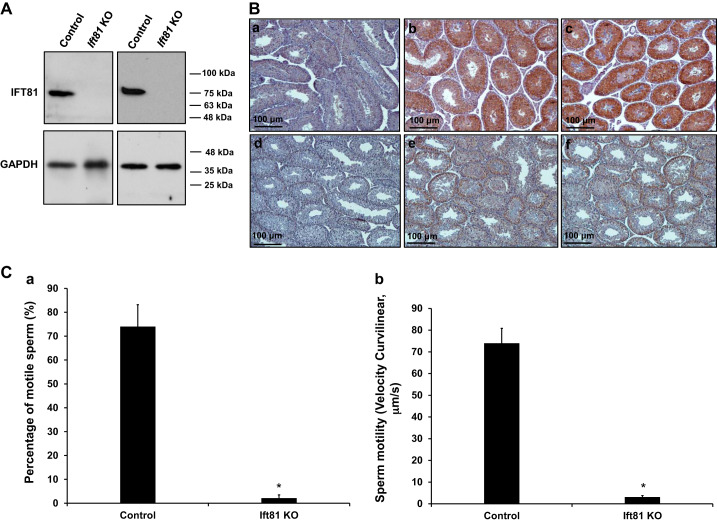
To study the function of IFT81 in male germ cell development and male fertility, we bred the floxed Ift81 mice with Stra8-iCre transgenic mice. Homozygous mice were identified by genotyping using tail snips (Supplemental Fig. S2). To determine whether the IFT81 protein would be expressed in the mutant mice, we conducted Western blotting assays on adult mice. As shown in Fig. 3A, compared with the control group, IFT81 protein was barely observed in the testis of Ift81 conditional KO mice. Immunohistochemistry was also conducted on testes from the control and conditional Ift81 KO mice. Specific strong IFT81 signal was detected in spermatocytes and round spermatids of control mice; however, only trace signal was found in the Ift81 conditional KO mice, most likely due to incomplete disruption of the gene using conditional KO strategy. The trace amount of IFT81 was not detected by Western blot analysis (Fig. 3B, Supplemental Fig. S3). All mutant mice survived to adulthood with no gross abnormalities, and body size was comparable with that of the control mice with different ages. To test fertility, 3- to 4-mo-old Ift81 mutant males and controls were bred with 3- to 4-mo-old wild-type females. After 2 mo of breeding, all eight females in control group went into labor, and the litter sizes were totally normal. Nevertheless, none of the five Ift81 mutant males tested produced pups (Table 1), even though they exhibited normal sexual behavior and vaginal plugs could be found in the paired females.
Fig. 3.
Disruption of intraflagellar transport 81 (Ift81) in male germ cells resulted in reduced sperm count and motility. A: Western blot analysis of testicular IFT81 protein expression in adult control and conditional Ift81 knockout (KO) mice. Notice that IFT81 protein was absent in the KO mice. B: low-magnification examination of IFT81 expression in the testes of control and conditional Ift81 KO mice by immunohistochemistry. Notice that specific strong IFT81 signal was observed in the control mice (b and c); however, only trace signal was seen in the conditional Ift81 KO mice (e and f). a And d are negative control (no IFT81 antibody was used) for a control mouse and a KO mouse. C: sperm motility of control (n = 8) and conditional Ift81 KO mice (n = 5). Notice that both percentage of motile sperm (a) and motility (b) were significantly reduced in KO mice. *P < 0.05.
Table 1.
Homozygous Ift81 knockout males were infertile
| Genotype | Fertility | Litter Size | Testis/Body Wt, mg/g | Sperm Counts, 104/mL |
|---|---|---|---|---|
| Control, n = 8 | 8/8 | 6.40 ± 0.76 | 7.40 ± 0.45 | 450.13 ± 42.34 |
| KO, n = 5 | 0/5 | 0 | 6.95 ± 0.72 | 15.21 ± 1.95 |
Values are means ± SD. To test fertility, 3- to 4-mo-old control and conditional intraflagellar transport 81 (Ift81) knockout (KO) mice were bred to 3- to 4-mo-old wild-type females for ≥2 mo. Litter size was recorded for each mating.
Abnormal sperm morphology and significantly reduced sperm number and motility in conditional Ift81 KO mice.
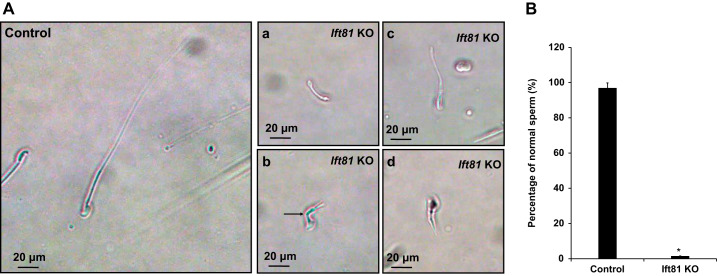
To determine sperm morphology, number, and motility of control and Ift81 mutant mice, sperm were collected from the cauda epididymidis and suspended in nutrient solution with the same dilution. Under light microscopy, sperm density was observed to be markedly decreased in the mutant mice compared with that of the control mice (Supplemental Fig. S4). Few sperm were motile, and motility of the few motile sperm was significantly reduced (Fig. 3C, Supplemental Videos A and B). Sperm morphology was examined under high magnification. Sperm from control mice were well developed, but the few sperm present from the mutant mice had shortened tails, and some appeared to be kinked (Fig. 4).
Fig. 4.
Abnormal morphology of mature sperm in the conditional intraflagellar transport 81 (Ift81) knockout (KO) mice. A: examination of epididymal sperm under high magnification of light microscope. Left: a representative image of epididymal sperm with normal morphology from a control mouse. The sperm had a normally shaped head and a long, smooth tail. Right, a–d: representative images of epididymal sperm from conditional Ift81 KO mice. All sperm had short tails. The arrow in b points to a sperm with a kinked tail. B: statistical analysis of abnormal sperm of the control and conditional Ift81 KO mice. More than 97% sperm were morphologically normal in the control mice (n = 8); however, only ~2% sperm appeared to be normal in the KO mice (n = 5). *P < 0.05.
Impaired spermiogenesis in the conditional Ift81 KO mice.
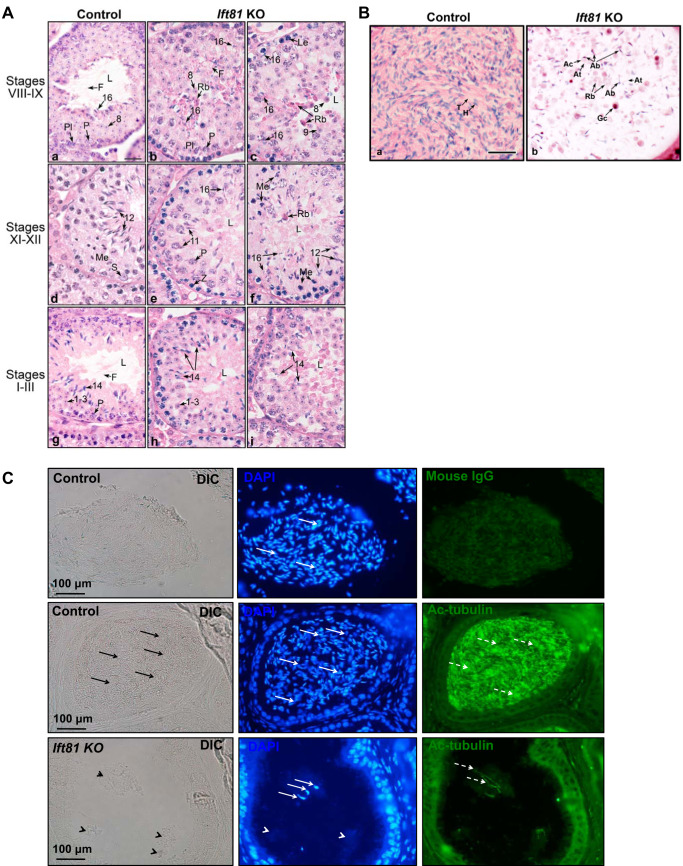
To understand the mechanism underlying the sperm defect observed in conditional Ift81 KO mice, testis and epididymis were examined. There was no significant difference in testis-to-body weight ratio between the control and mutant mice (Table 1). Histology of these testes was further examined. Control mice showed normal testicular histology throughout the stages in the cycle of the seminiferous epithelium, with normal organization of the elongating spermatids into discrete bundles (Fig. 5A, a, d, and g). However, in the conditional Ift81 KO mice, the abnormalities began in the elongating spermatids, after step 9 (Fig. 5A, b, c, e, f, h, and i). Round spermatids were normal in the KO testis but appeared to be sloughing into the lumen in stages VIII–XI along with many of the residual bodies, which are typically phagocytized by Sertoli cells. Spermatid flagella are very short and difficult to observe in the tubule lumens. In stages XI and XII, steps 11 and 12 elongating spermatids began to display gross abnormalities associated with the head formation, appearing to have a bulbous and abnormally shaped nucleus. The abnormal heads in elongating spermatids and lack of tail formation continued to be observed into the subsequent stages I–VIII, but there appeared to be fewer spermatids in the epithelium, as they were disorganized, losing their bundle appearance and sloughing into the lumen or being phagocytized by the Sertoli cells. In some stages, spermatid cytoplasm, possibly the entire elongating cell, was seen sloughing into the lumen. Also, evidence of failure of spermiation was seen with the heads of thin, highly condensed step 16 spermatids pulled deep into the epithelium. There was no increase in apoptotic cells in the seminiferous tubules of conditional Ift81 KO mice (Supplemental Fig. S5).
Fig. 5.
Abnormal spermiogenesis in the conditional intraflagellar transport 81 (Ift81) knockout (KO) mice. A: testis histology from control (a, d, and g) and conditional Ift81 KO mice (b, c, e, f, h, and i), showing stages of spermatogenesis that identified the observed pathological changes. 1–3 and 8, round spermatids; 11, 12, 14, and 16, elongating spermatids; F, flagellum; L, lumen; Le, leptotene spermatocyte; Me, meiotic division; P, pachytene spermatocyte; Pl, preleptotene spermatocyte; Rb, residual body; S, Sertoli cell; Z, zygotene spermatocytes. Bar = 20 μm for all images. a: Control stage VIII, with step 16 spermatids being released into the lumen, where sperm flagella were seen flowing from the spermatid heads. Round step 8 spermatids and pachytene and preleptotene spermatocytes were seen beneath the mature spermatids. b and c: Ift81 KO in stage VIII, showing abnormal step 16 spermatids with attached excess cytoplasm or residual bodies that stained dark red. Some step 16 heads were seen being phagocytosed, whereas others appeared to be released into the lumen. Spermatid flagella were very short and difficult to find in the lumen. d: Control stage XII, showing pachytene spermatocytes in meiotic division and numerous step 12 elongating spermatids lining the lumen with highly condensed nuclei. e and f: Ift81 KO in stages XI and XII, showing a greatly reduced number of steps 11 and 12 spermatids that had abnormally shaped heads and lack of tails. The spermatids had lost their bundle organization and appeared disorganized, with cytoplasm sloughing into the lumen. Evidence of failure of spermiation was seen with the heads of thin, highly condensed step 16 spermatids within the epithelium. g: Control stages I–III, showing step 14 elongating spermatids lining the seminiferous epithelium with their long flagella extending into the lumen. Round step 1–3 spermatids were seen between the heads of step 14 spermatids and the layer of pachytene spermatocytes. h and i: Ift81 KO in stages I–III, showing step 14 elongating spermatids with abnormally shaped heads. Excess cytoplasm extended into the lumen, but there was little evidence of flagellar formation. Some of the spermatids appeared to be sloughed into the lumen with the excess cytoplasm as round bodies. B: histology of the cauda epididymis from control and Ift81 KO mice showing the luminal contents. Bar = 25 μm for A and B. a: Control sample showing highly concentrated sperm in the lumen, with alignment of normal sperm heads (H) and tails (T). b: Ift81 KO cauda epididymis showing a very low concentration of sperm in the lumen. The few sperm present had abnormal heads (Ab) and abnormally short tails (At), with attached cytoplasm (Ac). Sloughed residual bodies (Rb) were numerous along with some round germ cells (Gc). C: examination of epididymis of control and conditional Ift81 KO mice by immunofluorescence staining. The sections were stained with an antiacetylated tubulin antibody that labeled sperm tails. DAPI was used to stain the nuclei. Top: negative control. No antiacetylated tubulin antibody was used, but the nuclei were stained blue (white arrows). Middle: the section from a control mouse was stained with an antiacetylated tubulin antibody. The lumen was full of sperm (black arrows), and the nuclei were stained in blue (white arrows), with positive acetylated tubulin staining in the tails (dashed white arrows). Bottom: conditional Ift81 KO: some clusters were found in the lumen (black arrowheads); only few were positive for DAPI (white arrows). The white arrowheads point to the DAPI-negative clusters, indicating residual bodies. Acetylated tubulin positive staining was limited (dashed arrows), indicating few sperm tails in the lumen. DIC, differential interference contrast.
In the control cauda epididymidis, the lumen was filled with normal sperm having aligned heads and tails. In contrast, the Ift81 KO epididymis showed a lumen filled with large cytoplasmic bodies that were likely residual bodies and sloughed spermatids and rare sperm with abnormal heads and short or absent tails. To further characterize the clusters in the lumen of epididymis from the conditional Ift81 KO mice, immunofluorescence staining was conducted using an antiacetylated tubulin antibody that labels sperm tails. The lumen in the control mice was full of well-developed sperm as indicated by acetylated tubulin signal almost in the entire lumen. However, only few acetylated tubulin staining was detected in the conditional Ift81 KO mice, indicating few sperm in the lumen. Remarkably, most clusters in the lumen were DAPI negative, indicating that they had no nuclei. These clusters might be residual bodies (Fig. 5C).
Ultrastructural changes in the seminiferous epithelium of the conditional Ift81 KO mice.
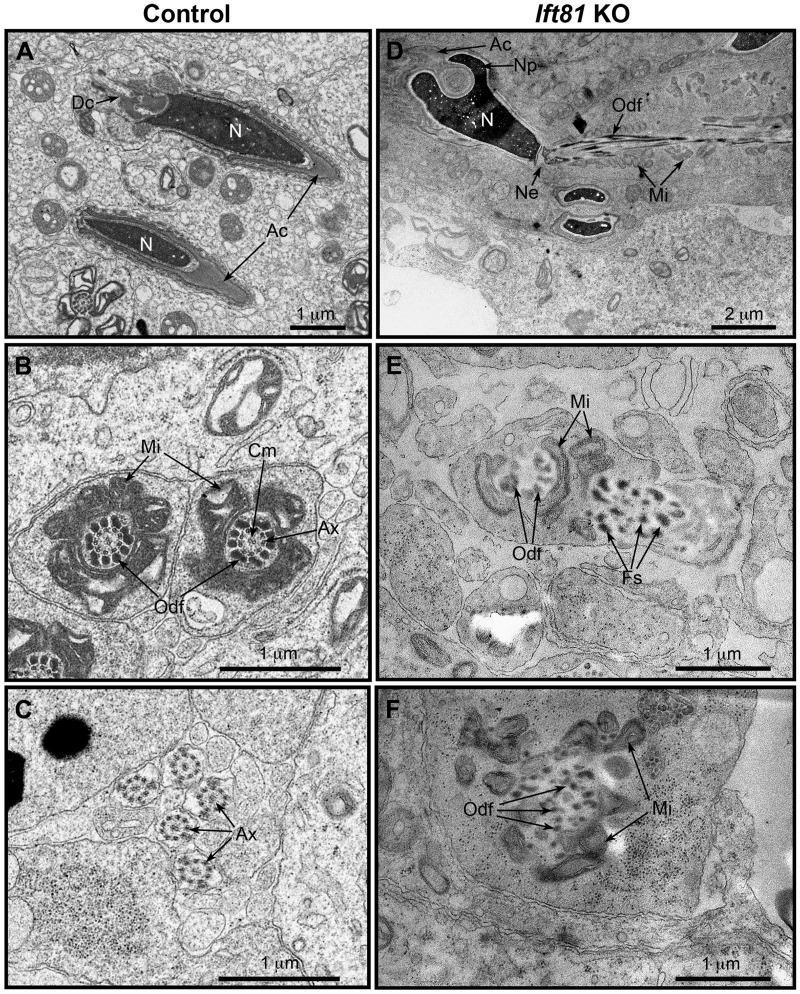
The major testicular lesion produced by the conditional knockout of Ift81 in the germ cells was identified by light microscopy as beginning in the early elongating spermatids. With electron microscopy, ultrastructure of the elongating spermatids in conditional Ift81 KO mice was compared with those from control mice (Fig. 6). Control samples showed the presence of normal elongating spermatids in the seminiferous epithelium, with condensing nuclei and an outer acrosomal cap. Control cross sections of the forming tail midpieces showed normal axonemal complexes that were surrounded by outer dense fibers, followed by a well-organized layer of mitochondria. In the flagellum, cross sections of the axonemal microtubules were clearly observed. In contrast, the seminiferous epithelium of Ift81 KO mice contained elongating spermatids with abnormal heads. The KO nuclei were condensed but showed abnormal shapes, with extensions of nucleopods (22) that were surrounded by the acrosome. In areas of nucleopod formation, the acrosome, although present, showed abnormal folding at the indented region of the nucleus. The KO spermatid tail was abnormal in both the midpiece and flagellum. The tail appeared to attempt to form, but without an axonemal complex of microtubules in the core. There was an abnormal alignment of the outer dense fibers and mitochondria around the absent axoneme, and at times there appeared to be abnormal aggregates of fibrous sheath components in areas bulging away from the core.
Fig. 6.
Conditional loss of intraflagellar transport 81 (IFT81) disturbed late-phase spermiogenesis and prevented correct flagellum assembly and elongation. Ultrastructural analysis of developing testicular germ cells by transmission electron microscopy was performed. A: control Ift81+/+ mouse showing normal components of elongating spermatids in the seminiferous epithelium. The nucleus (N) was condensed and elongated with an intact acrosomal cap (Ac). Dc, distal centriole. B: control Ift81+/+, showing cross sections of well-formed tail midpieces, with a normal axonemal complex (Ax), surrounded by the outer dense fibers (Odf) and then the layer of mitochondria (Mi). Cm, central microtubule. C: control Ift81+/+, showing normal flagellar cross sections. Ax, axonemal microtubules. D: Ift81 KO mouse showing an elongating spermatid with an abnormal head. The condensed nucleus had abnormal extensions of nucleopods (Np) that were surrounded by an acrosome (Ac) showing abnormal folding at the indented region of the nucleus. The tail had an abnormal alignment of the outer dense fibers (Odf) and mitochondria (Mi). Ne, neck region. E: Ift81 KO showing a cross section of an abnormally forming tail midpiece. Abnormal outer dense fibers (Odf) were seen partially surrounding the space where microtubules of the axonemal complex were missing. Components of the fibrous sheath (Fs) were displaced to 1 side and surrounded by an extension of the spermatid plasmalemma. F: Ift81 KO cross section of an abnormal midpiece that was forming with misplaced outer dense fibers (Odf) and mitochondria (Mi). Microtubules of the axoneme were lacking.
IFT81 regulates other IFT protein levels and some flagellar proteins.
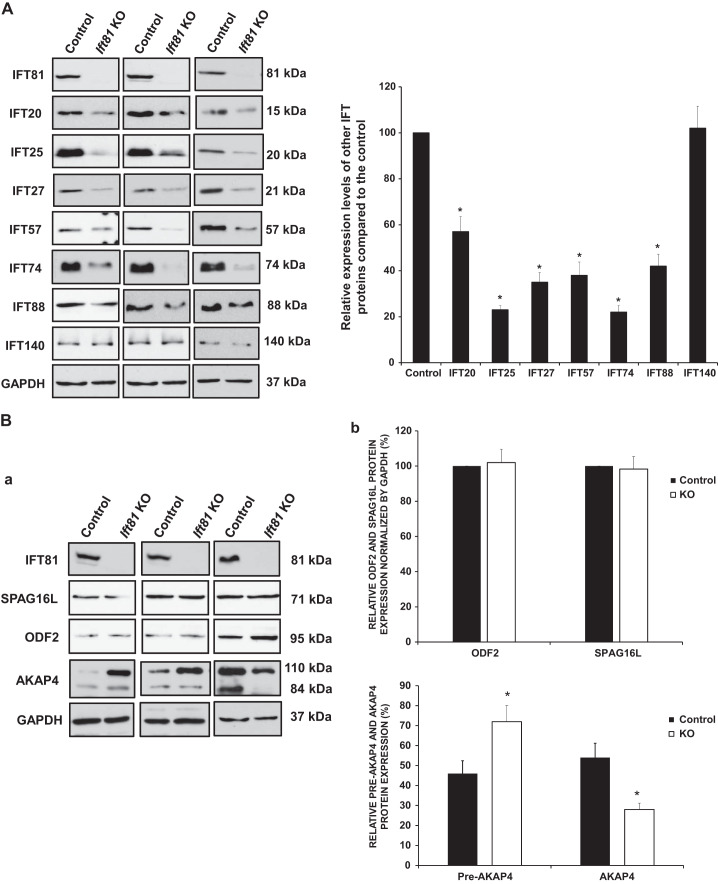
To investigate how the loss of IFT81 affects other IFT, we detected Western blot levels of select IFT proteins in the testis of control and conditional Ift81 KO mice. Most IFT components, such as IFT20, IFT25, IFT27, IFT57, IFT74, and IFT88, were significantly decreased in Ift81 KO mice; however, IFT140 protein did not differ from those expressed in control mice (Fig. 7A).
Fig. 7.
Expression levels of some intraflagellar transport (IFT) components and selective sperm flagellar proteins in the conditional Ift81 knockout (KO) mice. A: examination of selective IFT protein expression levels in the control and conditional Ift81 KO mice by Western blot. Compared with the controls, the expression levels of IFT20, IFT25, IFT27, IFT57, IFT74, and IFT88, but not IFT140, were reduced in the conditional Ift81 KO mice. B: examination of testicular expression levels of ODF2 (a component of sperm tail outer dense fibers), AKAP4 (a major component of fibrous sheath), and SPAG16L (a component of axonemal central apparatus protein) in the control and conditional Ift81 KO mice. There was no difference in ODF2 and SPAG16L expression. However, the processed AKAP4 was significantly reduced in the conditional Ift81 KO mice. GAPDH was used as the control. a: Representative Western blot results. b: Quantitative analysis of indicated protein expression. Three pairs of mice were analyzed. *P < 0.05.
The expression levels for representative sperm flagellar proteins were also examined. IFT81 did not affect testicular expression levels of ODF2, a major component of the sperm tail outer dense fibers, and SPAG16L, a component of the central apparatus of the axoneme. However, AKAP4, a processed form of the major component of the fibrous sheath, was significantly reduced in KO mice (Fig. 7B).
DISCUSSION
In the present study, we investigated the role of IFT81 in mouse male germ cell development and male fertility. Even though the gene was originally cloned from heart (34), in our study the protein was not detected in this tissue by Western blot, indicating that IFT81 protein is less abundant in heart but highly expressed in testis. This is also supported by qPCR, as among all the tissues examined, the expression of Ift81 mRNA was the lowest in heart. Like other IFT genes we analyzed, IFT81 is highly abundant only in the testis, indicating a unique function in sperm formation and male reproduction. Particularly, its expression is highest in the later stages of spermatogenesis, suggesting a more important role in spermiogenesis, during which germ cells undergo dramatic morphological changes, including flagella formation (6, 14). This pattern matches the general role of IFT in ciliogenesis (41, 56). Not surprisingly, the full-length IFT81 protein was also detected in several other tissues, including brain, lung, and kidney, tissues enriched in cilia, indicating that IFT81 plays an essential role in ciliogenesis. Interestingly, even though Ift81 mRNA was not abundant in lung, the protein level was higher than most somatic tissues, suggesting a posttranscriptional regulation of this gene in lung. The smaller proteins detected in liver and kidney may represent new IFT81 isoforms in these tissues.
Even though some IFT components, including IFT20 (54, 65), IFT88 (23), and IFT140 (64) have been described to be present in the manchette, a unique structure only present in male germ cells is believed to play a role in shaping the nucleus and forming the flagella during the late stages of spermiogenesis (22, 28). Like IFT74 (51), IFT81 is not present in this structure, indicating that different IFT components accumulate in the developing sperm flagella for sperm tail formation through different transporting mechanisms. Interestingly, even though it has been shown that IFT74 and IFT81 form a complex for tubulin transport (3, 5, 26), their localizations are different in spermatocytes and round spermatids. IFT81 appears to be evenly distributed in the cytoplasm in these cells, whereas IFT74 is associated with vesicles (51). Given that IFT81 is expressed earlier than IFT74 during germ cell development, it is likely that IFT74 recruits IFT81 to form the IFT74–IFT81 complex in later steps of germ cell differentiation to transport tubulin for sperm flagella formation.
The phenotype of the conditional Ift81 KO mice phenocopied that of the conditional Ift74 KO mice, supporting the concept that the two proteins form a complex. Inactivating each individual gene disrupts spermatogenesis and sperm flagella formation, indicating that the two proteins do not compensate for each other, and normal spermatogenesis/sperm flagella formation needs both proteins. Similar to the conditional Ift74 germ cell KO mice, expression levels of several other IFT components were downregulated, supporting the concept that IFT components form a complex to conduct their function, which is to transport cargos for cilia/flagella formation (57, 62). Missing one core component in the complex will give rise to instability of the IFT complex, and some components will be degraded.
Even though significantly reduced sperm numbers were found in the conditional Ift81 mutant mice, some germ cells were capable of passing through the entire spermatogenesis process, but all sperm produced short tails. One possibility might be that even though IFT74 and IFT81 are likely to function as the main module for IFT of tubulin (3, 26), they may not be the only tubulin‐binding site on the IFT complex (4). Homologs of IFT74 and IFT81 are not present in Drosophila melanogaster and the moss Physcomitrella patens, suggesting that additional IFT components transport tubulin in these species (61). IFT54, IFT57, and FAP22 (flagellar associated protein 22) all have NH2‐terminal domains with significant sequence similarities to calponin‐homology domains (49, 56). Of the three proteins, IFT54 has a verified microtubule/tubulin‐binding activity, which was mapped to the NH2‐terminal part of the human ortholog TRAF3IP1 (MIP‐T3, IFT54; Ref. 31). It is likely that other IFT components partially compensate for the loss of IFT81.
The effect of inactivating IFT81 on flagellar proteins appeared to be similar to that of the conditional Ift74 mutants. However, whereas sperm tail morphology was abnormal, the testicular expression levels of ODF2 and SPAG16L showed nonsignificant fluctuations. However, the pro-AKAP4 was not efficiently processed. Mouse Akap4 gene is translated as a full-length precursor (pro-AKAP4). The pro-AKAP4 should be transported from cytoplasm to the fibrous sheath assembly site, presumably by the IFT. At the fibrous sheath assembly site, the pro-AKAP4 is processed and the 84-kDa AKAP4 is incorporated into sperm fibrous sheath (19, 59). IFT81 might not directly bind to AKAP4 for fibrous sheath assembly. The pro-AKAP4 is presumably transported by other IFT components and processed by an unknown mechanism. Inactivating IFT81 will disrupt the IFT, so pro-AKAP4 is not transported for processing, and the level of the processed 84-kDa AKAP4 is decreased in the absence of IFT81. Thus our observations support the hypothesis that in male germ cells, IFT74 and IFT81 coordinate to transport cargo proteins for sperm flagella formation, an IFT complex process that is driven by motor proteins (58). It has been shown that in Chlamydomonas, IFT81 localization was altered in the absence of the motor proteins (30). A more recent study demonstrated that IFT81 localization was also controlled by RABL2 (33, 39). It remains to be determined whether the localization of the mouse IFT74–IFT81 complex is also controlled by these proteins in male germ cells.
It appears that acrosome biogenesis is not affected in the absence of IFT81, which is also consistent with the conditional Ift74 mutants. The acrosome is a specialized saccular organelle that flattens over the anterior half of the sperm nucleus by linking to a cytoskeletal plate called the acroplaxome at the nuclear membrane (1, 26). This caplike structure is mainly formed by the fusion of proacrosomal vesicles derived from the trans-Golgi network; other resources in addition to the Golgi apparatus include lysosome-associated vesicles (2, 11). Normal acrosome biogenesis in the Ift81 KO mice indicated that IFT81 is not essential for these pathways in acrosome formation.
Significantly reduced sperm number suggests that IFT81 is not only essential for flagella formation, but it might be also important for germ cell survival. However, TUNEL staining did not reveal an increased number of apoptotic cells in testes from the conditional Ift81 KO testis. Other mechanism(s) explaining the observed reduced sperm count might exist, and this requires further investigation.
According to the current literature, no candidate variant of human IFT81 gene has been shown to be associated or responsible for male infertility. Additionally, we have previously sequenced a cohort of 168 infertile individuals affected by multiple morphological abnormalities of the sperm flagella, and no variants were found in any of them that would be predicted to be deleterious for IFT81 protein function (7). All recruited patients were healthy and only recruited for male infertility. The fact that none of the studied individuals harbored deleterious biallelic IFT81 variants is concordant with the expectation that the presence of such variants is expected to induce a severe ciliopathy that would have prevented their recruitment for infertility.
In conclusion, we explored the role of IFT81 in mouse sperm development and male fertility, and our findings support that IFT81, like IFT74, is essential for mouse spermatogenesis and specifically the sperm flagellum via the assembly of microtubules during formation of the axoneme. Together with IFT74, the complex appears to function as a core component of the IFT-B complex to modulate its stability and for transporting tubulin and the fibrous sheath precursor for sperm formation.
GRANTS
This research was supported by National Institute of Child Health and Human Development Grants HD-076257 and HD-090306 and Start-up Fund and Research Fund of Wayne State University (to Z. Zhang), National Natural Science Foundation of China (81671514), Excellent Youth Foundation (2018CFA040) and Youth Foundation (2018CFB114) of Hubei Science and Technology Office, and Special Fund of Wuhan University of Science and Technology for Master Student’s short-term studying abroad, and Institut National de la Santé et de la Recherche Médicale–Bettencourt Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.Z. conceived and designed research; W.Q., S.Y., Q.H., Q.Z., Y.Y., L.S., D.Z., T.G., W.L., S.-P.Y., L.Z., C.C., R.A.H., P.F.R., Z.-E.K., and Z.Z. performed experiments; W.Q., S.Y., Q.H., Q.Z., Y.Y., L.S., D.Z., T.G., W.L., S.-P.Y., L.Z., C.C., R.A.H., P.F.R., Z.-E.K., and Z.Z. analyzed data; W.Q., S.Y., C.Q., Q.H., Q.Z., Y.Y., L.S., D.Z., T.G., W.L., S.-P.Y., L.Z., C.C., R.A.H., P.F.R., Z.-E.K., and Z.Z. interpreted results of experiments; W.Q., S.Y., C.Q., Q.H., Q.Z., Y.Y., L.S., D.Z., T.G., W.L., L.Z., C.C., R.A.H., P.F.R., Z.-E.K., and Z.Z. prepared figures; C.Q. and Z.Z. drafted manuscript; C.Q. and Z.Z. edited and revised manuscript; W.Q., S.Y., C.Q., Q.H., Q.Z., Y.Y., L.S., T.G., W.L., S.-P.Y., L.Z., R.A.H., P.F.R., Z.-E.K., and Z.Z. approved final version of manuscript.
REFERENCES
- 1.Abou-Haila A, Tulsiani DR. Mammalian sperm acrosome: formation, contents, and function. Arch Biochem Biophys 379: 173–182, 2000. doi: 10.1006/abbi.2000.1880. [DOI] [PubMed] [Google Scholar]
- 2.Berruti G, Paiardi C. USP8/UBPy-regulated sorting and the development of sperm acrosome: the recruitment of MET. Reproduction 149: 633–644, 2015. doi: 10.1530/REP-14-0671. [DOI] [PubMed] [Google Scholar]
- 3.Bhogaraju S, Cajanek L, Fort C, Blisnick T, Weber K, Taschner M, Mizuno N, Lamla S, Bastin P, Nigg EA, Lorentzen E. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 341: 1009–1012, 2013. doi: 10.1126/science.1240985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhogaraju S, Weber K, Engel BD, Lechtreck KF, Lorentzen E. Getting tubulin to the tip of the cilium: one IFT train, many different tubulin cargo-binding sites? BioEssays 36: 463–467, 2014. doi: 10.1002/bies.201400007. [DOI] [PubMed] [Google Scholar]
- 5.Brown JM, Cochran DA, Craige B, Kubo T, Witman GB. Assembly of IFT trains at the ciliary base depends on IFT74. Curr Biol 25: 1583–1593, 2015. doi: 10.1016/j.cub.2015.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clermont Y, Oko R, Hermo L. Cell biology of mammalian spermiogenesis. In: Cell and Molecular Biology of the Testis, edited by Desjardins C, Ewing LL. New York; Oxford, UK: Oxford Univ. Press, 1993, p. 332–376. [Google Scholar]
- 7.Coutton C, Martinez G, Kherraf ZE, Amiri-Yekta A, Boguenet M, Saut A, He X, Zhang F, Cristou-Kent M, Escoffier J, Bidart M, Satre V, Conne B, Fourati Ben Mustapha S, Halouani L, Marrakchi O, Makni M, Latrous H, Kharouf M, Pernet-Gallay K, Bonhivers M, Hennebicq S, Rives N, Dulioust E, Touré A, Gourabi H, Cao Y, Zouari R, Hosseini SH, Nef S, Thierry-Mieg N, Arnoult C, Ray PF. Bi-allelic mutations in ARMC2 lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am J Hum Genet 104: 331–340, 2019. doi: 10.1016/j.ajhg.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dharmat R, Liu W, Ge Z, Sun Z, Yang L, Li Y, Wang K, Thomas K, Sui R, Chen R. IFT81 as a candidate gene for nonsyndromic retinal degeneration. Invest Ophthalmol Vis Sci 58: 2483–2490, 2017. doi: 10.1167/iovs.16-19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duran I, Taylor SP, Zhang W, Martin J, Forlenza KN, Spiro RP, Nickerson DA, Bamshad M, Cohn DH, Krakow D. Destabilization of the IFT-B cilia core complex due to mutations in IFT81 causes a spectrum of short-rib polydactyly syndrome. Sci Rep 6: 34232, 2016. doi: 10.1038/srep34232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisa-Beygi S, Benslimane FM, El-Rass S, Prabhudesai S, Abdelrasoul MKA, Simpson PM, Yalcin HC, Burrows PE, Ramchandran R. Characterization of endothelial cilia distribution during cerebral-vascular development in zebrafish (Danio rerio). Arterioscler Thromb Vasc Biol 38: 2806–2818, 2018. doi: 10.1161/ATVBAHA.118.311231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escalier D, Gallo JM, Albert M, Meduri G, Bermudez D, David G, Schrevel J. Human acrosome biogenesis: immunodetection of proacrosin in primary spermatocytes and of its partitioning pattern during meiosis. Development 113: 779–788, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Haimo LT, Rosenbaum JL. Cilia, flagella, and microtubules. J Cell Biol 91: 125s–130s, 1981. doi: 10.1083/jcb.91.3.125s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauer NN, Popp B, Taher L, Vogl C, Dhandapany PS, Büttner C, Uebe S, Sticht H, Ferrazzi F, Ekici AB, De Luca A, Klinger P, Kraus C, Zweier C, Wiesener A, Jamra RA, Kunstmann E, Rauch A, Wieczorek D, Jung AM, Rohrer TR, Zenker M, Doerr HG, Reis A, Thiel CT. Evolutionary conserved networks of human height identify multiple Mendelian causes of short stature. Eur J Hum Genet 27: 1061–1071, 2019. doi: 10.1038/s41431-019-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa. Microsc Res Tech 73: 279–319, 2010. doi: 10.1002/jemt.20783. [DOI] [PubMed] [Google Scholar]
- 15.Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 636: 1–15, 2008. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 16.Heydeck W, Fievet L, Davis EE, Katsanis N. The complexity of the cilium: spatiotemporal diversity of an ancient organelle. Curr Opin Cell Biol 55: 139–149, 2018. doi: 10.1016/j.ceb.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyer-Fender S, Petersen C, Brohmann H, Rhee K, Wolgemuth DJ. Mouse Odf2 cDNAs consist of evolutionary conserved as well as highly variable sequences and encode outer dense fiber proteins of the sperm tail. Mol Reprod Dev 51: 167–175, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa H, Marshall WF. Intraflagellar transport and ciliary dynamics. Cold Spring Harb Perspect Biol 9: a021998, 2017. doi: 10.1101/cshperspect.a021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson LR, Foster JA, Haig-Ladewig L, VanScoy H, Rubin CS, Moss SB, Gerton GL. Assembly of AKAP82, a protein kinase A anchor protein, into the fibrous sheath of mouse sperm. Dev Biol 192: 340–350, 1997. doi: 10.1006/dbio.1997.8767. [DOI] [PubMed] [Google Scholar]
- 20.Jonassen JA, SanAgustin J, Baker SP, Pazour GJ. Disruption of IFT complex A causes cystic kidneys without mitotic spindle misorientation. J Am Soc Nephrol 23: 641–651, 2012. doi: 10.1681/ASN.2011080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev Cell 22: 940–951, 2012. doi: 10.1016/j.devcel.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol 67: 271–284, 2004. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- 23.Kierszenbaum AL. Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Mol Reprod Dev 63: 1–4, 2002. doi: 10.1002/mrd.10179. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi T, Gengyo-Ando K, Ishihara T, Katsura I, Mitani S. IFT-81 and IFT-74 are required for intraflagellar transport in C. elegans. Genes Cells 12: 593–602, 2007. doi: 10.1111/j.1365-2443.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- 25.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA 90: 5519–5523, 1993. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo T, Brown JM, Bellve K, Craige B, Craft JM, Fogarty K, Lechtreck KF, Witman GB. Together, the IFT81 and IFT74 N-termini form the main module for intraflagellar transport of tubulin. J Cell Sci 129: 2106–2119, 2016. doi: 10.1242/jcs.187120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lechtreck KF. IFT-cargo interactions and protein transport in cilia. Trends Biochem Sci 40: 765–778, 2015. doi: 10.1016/j.tibs.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehti MS, Sironen A. Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 151: R43–R54, 2016. doi: 10.1530/REP-15-0310. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Tang W, Teves ME, Zhang Z, Zhang L, Li H, Archer KJ, Peterson DL, Williams DC Jr, Strauss JF 3rd, Zhang Z. A MEIG1/PACRG complex in the manchette is essential for building the sperm flagella. Development 142: 921–930, 2015. doi: 10.1242/dev.119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H, Nauman NP, Albee AJ, Hsu S, Dutcher SK. New mutations in flagellar motors identified by whole genome sequencing in Chlamydomonas. Cilia 2: 14, 2013. doi: 10.1186/2046-2530-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling L, Goeddel DV. MIP-T3, a novel protein linking tumor necrosis factor receptor-associated factor 3 to the microtubule network. J Biol Chem 275: 23852–23860, 2000. doi: 10.1074/jbc.M001095200. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Li W, Zhang Y, Zhang Z, Shang X, Zhang L, Zhang S, Li Y, Somoza AV, Delpi B, Gerton GL, Foster JA, Hess RA, Pazour GJ, Zhang Z. IFT25, an intraflagellar transporter protein dispensable for ciliogenesis in somatic cells, is essential for sperm flagella formation. Biol Reprod 96: 993–1006, 2017. doi: 10.1093/biolre/iox029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo JC, Jamsai D, O’Connor AE, Borg C, Clark BJ, Whisstock JC, Field MC, Adams V, Ishikawa T, Aitken RJ, Whittle B, Goodnow CC, Ormandy CJ, O’Bryan MK. RAB-like 2 has an essential role in male fertility, sperm intra-flagellar transport, and tail assembly. PLoS Genet 8: e1002969, 2012. doi: 10.1371/journal.pgen.1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuda M, Kobayashi K, Horiuchi M, Terazono H, Yoshimura N, Saheki T. A novel gene suppressed in the ventricle of carnitine-deficient juvenile visceral steatosis mice. FEBS Lett 408: 221–224, 1997. doi: 10.1016/S0014-5793(97)00429-8. [DOI] [PubMed] [Google Scholar]
- 35.Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol 248: 331–342, 2002. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
- 36.Mitchison HM, Valente EM. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol 241: 294–309, 2017. doi: 10.1002/path.4843. [DOI] [PubMed] [Google Scholar]
- 37.Mourão A, Christensen ST, Lorentzen E. The intraflagellar transport machinery in ciliary signaling. Curr Opin Struct Biol 41: 98–108, 2016. [Erratum in Curr Opin Struct Biol 41: 255, 2016.] doi: 10.1016/j.sbi.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Navarrete FA, García-Vázquez FA, Alvau A, Escoffier J, Krapf D, Sánchez-Cárdenas C, Salicioni AM, Darszon A, Visconti PE. Biphasic role of calcium in mouse sperm capacitation signaling pathways. J Cell Physiol 230: 1758–1769, 2015. doi: 10.1002/jcp.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishijima Y, Hagiya Y, Kubo T, Takei R, Katoh Y, Nakayama K. RABL2 interacts with the intraflagellar transport-B complex and CEP19 and participates in ciliary assembly. Mol Biol Cell 28: 1652–1666, 2017. doi: 10.1091/mbc.e17-01-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol 157: 103–114, 2002. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 85: 23–61, 2008. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 42.Peng J, Yu L, Horiuchi M, Zhang P, Huang X, Zhang Y, Li D, Jalil MA, Zhao S. Identification of human CDV-1R and mouse Cdv-1R, two novel proteins with putative signal peptides, especially highly expressed in testis and increased with the male sex maturation. Mol Biol Rep 29: 353–362, 2002. doi: 10.1023/A:1021232518628. [DOI] [PubMed] [Google Scholar]
- 43.Perrault I, Halbritter J, Porath JD, Gérard X, Braun DA, Gee HY, Fathy HM, Saunier S, Cormier-Daire V, Thomas S, Attié-Bitach T, Boddaert N, Taschner M, Schueler M, Lorentzen E, Lifton RP, Lawson JA, Garfa-Traore M, Otto EA, Bastin P, Caillaud C, Kaplan J, Rozet JM, Hildebrandt F. IFT81, encoding an IFT-B core protein, as a very rare cause of a ciliopathy phenotype. J Med Genet 52: 657–665, 2015. doi: 10.1136/jmedgenet-2014-102838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pettersson M, Vaz R, Hammarsjö A, Eisfeldt J, Carvalho CMB, Hofmeister W, Tham E, Horemuzova E, Voss U, Nishimura G, Klintberg B, Nordgren A, Nilsson D, Grigelioniene G, Lindstrand A. Alu-Alu mediated intragenic duplications in IFT81 and MATN3 are associated with skeletal dysplasias. Hum Mutat 39: 1456–1467, 2018. doi: 10.1002/humu.23605. [DOI] [PubMed] [Google Scholar]
- 45.Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol 18: 533–547, 2017. doi: 10.1038/nrm.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadate-Ngatchou PI, Payne CJ, Dearth AT, Braun RE. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 46: 738–742, 2008. doi: 10.1002/dvg.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.San Agustin JT, Pazour GJ, Witman GB. Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol Biol Cell 26: 4358–4372, 2015. doi: 10.1091/mbc.E15-08-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satir P. CILIA: before and after. Cilia 6: 1, 2017. doi: 10.1186/s13630-017-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schou KB, Andersen JS, Pedersen LB. A divergent calponin homology (NN-CH) domain defines a novel family: implications for evolution of ciliary IFT complex B proteins. Bioinformatics 30: 899–902, 2014. doi: 10.1093/bioinformatics/btt661. [DOI] [PubMed] [Google Scholar]
- 50.Shao X, Murthy S, Demetrick DJ, van der Hoorn FA. Human outer dense fiber gene, ODF2, localizes to chromosome 9q34. Cytogenet Cell Genet 83: 221–223, 1998. doi: 10.1159/000015183. [DOI] [PubMed] [Google Scholar]
- 51.Shi L, Zhou T, Huang Q, Zhang S, Li W, Zhang L, Hess RA, Pazour GJ, Zhang Z. Intraflagellar transport protein 74 is essential for spermatogenesis and male fertility in mice. Biol Reprod 101: 188–199, 2019. doi: 10.1093/biolre/ioz071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y, Su Y, Lipschutz JH, Lobo GP. Zebrafish as models to study ciliopathies of the eye and kidney. Clin Nephrol Res 1: 6–9, 2017. [PMC free article] [PubMed] [Google Scholar]
- 53.Silva C, Wood JR, Salvador L, Zhang Z, Kostetskii I, Williams CJ, Strauss JF 3rd. Expression profile of male germ cell-associated genes in mouse embryonic stem cell cultures treated with all-trans retinoic acid and testosterone. Mol Reprod Dev 76: 11–21, 2009. doi: 10.1002/mrd.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sironen A, Hansen J, Thomsen B, Andersson M, Vilkki J, Toppari J, Kotaja N. Expression of SPEF2 during mouse spermatogenesis and identification of IFT20 as an interacting protein. Biol Reprod 82: 580–590, 2010. doi: 10.1095/biolreprod.108.074971. [DOI] [PubMed] [Google Scholar]
- 55.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- 56.Taschner M, Bhogaraju S, Lorentzen E. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation 83: S12–S22, 2012. doi: 10.1016/j.diff.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taschner M, Kotsis F, Braeuer P, Kuehn EW, Lorentzen E. Crystal structures of IFT70/52 and IFT52/46 provide insight into intraflagellar transport B core complex assembly. J Cell Biol 207: 269–282, 2014. doi: 10.1083/jcb.201408002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toropova K, Zalyte R, Mukhopadhyay AG, Mladenov M, Carter AP, Roberts AJ. Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nat Struct Mol Biol 26: 823–829, 2019. doi: 10.1038/s41594-019-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turner RM, Eriksson RL, Gerton GL, Moss SB. Relationship between sperm motility and the processing and tyrosine phosphorylation of two human sperm fibrous sheath proteins, pro-hAKAP82 and hAKAP82. Mol Hum Reprod 5: 816–824, 1999. doi: 10.1093/molehr/5.9.816. [DOI] [PubMed] [Google Scholar]
- 60.Turner RM, Johnson LR, Haig-Ladewig L, Gerton GL, Moss SB. An X-linked gene encodes a major human sperm fibrous sheath protein, hAKAP82. Genomic organization, protein kinase A-RII binding, and distribution of the precursor in the sperm tail. J Biol Chem 273: 32135–32141, 1998. doi: 10.1074/jbc.273.48.32135. [DOI] [PubMed] [Google Scholar]
- 61.van Dam TJ, Townsend MJ, Turk M, Schlessinger A, Sali A, Field MC, Huynen MA. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc Natl Acad Sci USA 110: 6943–6948, 2013. doi: 10.1073/pnas.1221011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xin D, Christopher KJ, Zeng L, Kong Y, Weatherbee SD. IFT56 regulates vertebrate developmental patterning by maintaining IFTB complex integrity and ciliary microtubule architecture. Development 144: 1544–1553, 2017. doi: 10.1242/dev.143255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Liu H, Li W, Zhang Z, Shang X, Zhang D, Li Y, Zhang S, Liu J, Hess RA, Pazour GJ, Zhang Z. Intraflagellar transporter protein (IFT27), an IFT25 binding partner, is essential for male fertility and spermiogenesis in mice. Dev Biol 432: 125–139, 2017. doi: 10.1016/j.ydbio.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Liu H, Li W, Zhang Z, Zhang S, Teves ME, Stevens C, Foster JA, Campbell GE, Windle JJ, Hess RA, Pazour GJ, Zhang Z. Intraflagellar transporter protein 140 (IFT140), a component of IFT-A complex, is essential for male fertility and spermiogenesis in mice. Cytoskeleton (Hoboken) 75: 70–84, 2018. doi: 10.1002/cm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64a.Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, Bennett J, Gerton GL, Moss SB, Radice GL, Strauss JF 3rd. Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod 74: 751–759, 2006. doi: 10.1095/biolreprod.105.049254. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Li W, Zhang Y, Zhang L, Teves ME, Liu H, Strauss JF 3rd, Pazour GJ, Foster JA, Hess RA, Zhang Z. Intraflagellar transport protein IFT20 is essential for male fertility and spermiogenesis in mice. Mol Biol Cell 27: 3687–3790, 2016. doi: 10.1091/mbc.e16-05-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Z, Sapiro R, Kapfhamer D, Bucan M, Bray J, Chennathukuzhi V, McNamara P, Curtis A, Zhang M, Blanchette-Mackie EJ, Strauss JF 3rd. A sperm-associated WD repeat protein orthologous to Chlamydomonas PF20 associates with Spag6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Biol 22: 7993–8004, 2002. doi: 10.1128/MCB.22.22.7993-8004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]