Abstract
Age-induced declines in skeletal muscle contractile function have been attributed to multiple cellular factors, including lower peak force (Po), decreased Ca2+ sensitivity, and reduced shortening velocity (Vo). However, changes in these cellular properties with aging remain unresolved, especially in older women, and the effect of submaximal Ca2+ on contractile function is unknown. Thus, we compared contractile properties of muscle fibers from 19 young (24 ± 3 yr; 8 women) and 21 older adults (77 ± 7 yr; 7 women) under maximal and submaximal Ca2+ and assessed the abundance of three proteins thought to influence Ca2+ sensitivity. Fast fiber cross-sectional area was ~44% larger in young (6,479 ± 2,487 µm2) compared with older adults (4,503 ± 2,071 µm2, P < 0.001), which corresponded with a greater absolute Po (young = 1.12 ± 0.43 mN; old = 0.79 ± 0.33 mN, P < 0.001). There were no differences in fast fiber size-specific Po, indicating the age-related decline in force was explained by differences in fiber size. Except for fast fiber size and absolute Po, no age or sex differences were observed in Ca2+ sensitivity, rate of force development (ktr), or Vo in either slow or fast fibers. Submaximal Ca2+ depressed ktr and Vo, but the effects were not altered by age in either sex. Contrary to rodent studies, regulatory light chain (RLC) and myosin binding protein-C abundance and RLC phosphorylation were unaltered by age or sex. These data suggest the age-associated reductions in contractile function are primarily due to the atrophy of fast fibers and that caution is warranted when extending results from rodent studies to humans.
Keywords: aging, calcium, contractile properties, skeletal muscle fibers
INTRODUCTION
Aging is accompanied by decreases in muscle mass and the ability to generate power (9, 50, 70), which can impair mobility and quality of life for older adults (16, 45, 62). Despite these well-recognized reductions of the whole muscle, the data regarding the effect of aging on single muscle fiber contractile properties in saturating Ca2+ (pCa 4.5) are conflicting. Some studies have reported age-related decreases in force, velocity, and power of slow myosin heavy chain (MHC) I and fast MHC II fibers (8, 13, 21, 38, 58), while others have observed no change (20, 56, 69, 74) or even enhanced contractile performance with aging (27, 63). We recently observed an age-induced decline in fiber size, absolute peak force (Po), and power of fast but not slow fibers in men, whereas all other contractile properties were preserved (69). Importantly, the selective loss of fast MHC II muscle was closely associated with the age-related decline in whole-muscle force and power, suggesting that the atrophy of fast fibers is a primary determinant of age-related decrements in contractile function (69). However, it is not known whether single fiber contractile function is impaired in submaximal Ca2+ and if Ca2+ sensitivity is compromised in older men and women.
Findings on aging and Ca2+ sensitivity of human skeletal muscle fibers are equivocal, with some studies observing decreased Ca2+ sensitivity in older compared with young adults (37, 68) and others observing no difference (32, 33). Furthermore, only a single study has tested if there are sex differences in Ca2+ sensitivity with aging (68), and no studies have tested the effect of submaximal Ca2+ on contractile function of human skeletal muscle fibers from young or older adults. Studying contractile function under submaximal Ca2+ is important, because there is evidence of less stored Ca2+ in the sarcoplasmic reticulum (37) and a lower amplitude of the intracellular Ca2+ transient with age (15). The reduced intracellular Ca2+ may result in decreased fiber force, rates of tension development (ktr), and unloaded shortening velocity (Vo) (54, 64), impairing contractile function in fibers from older compared with young adults.
Under submaximal Ca2+ conditions, ktr is depressed and Vo is biphasic, showing a slower shortening velocity at lengths >10% of fiber length (43, 52, 54, 71). The slower velocity at longer shortening lengths has been attributed to an increased internal drag from slower cross-bridge turnover due to binding of myosin binding protein-C (MyBP-C) to actin, and/or the cooperative inactivation of thin filaments decreasing the number of strongly bound cross-bridges (31, 34, 44, 52, 54, 71). Importantly, the contribution of myosin strong binding to activation of the thin filaments is greater in submaximal Ca2+ (25). Thus, if aging decreases myofibrillar Ca2+ sensitivity, the rate and number of myosin strong binding would decrease and, under submaximal Ca2+ conditions, further depress the slow velocity phase of shortening and ktr.
One potential mechanism for age-related differences in Ca2+ sensitivity and contractile function in submaximal Ca2+ could be alterations in the content and/or phosphorylation of key regulatory proteins, such as MyBP-C, troponin I (TnI), and myosin regulatory light chain (RLC) (31, 46, 48). In rodent models, aging has been associated with decreased MyBP-C content (1, 17, 60) and phosphorylation (1), but no age-differences were observed in content and phosphorylation of TnI (76). However, whether the differences, or lack thereof, observed in the content and phosphorylation of these proteins in rodents are reflective of what happens in aging human skeletal muscle is unknown. Moreover, findings on the content and phosphorylation of RLC from aging humans and rodents are equivocal (8, 23, 24, 49, 76). For example, slow RLC (RLCs) content and phosphorylation have been shown to increase in aged rats (23), while fast RLC (RLCf) content and phosphorylation have been shown to decrease with aging in humans (24). In contrast, Brocca et al. (8) found no changes in RLCf content or phosphorylation in older men but found an increase in RLCs phosphorylation, whereas Miller et al. (49) found a decreased RLCf phosphorylation in older women but not men. The explanation for the discrepancies between studies is unclear but may involve the large heterogeneity in fiber type distribution between individuals, as some studies report concurrent changes in both MHC and RLC content with aging (23, 24). Understanding whether aging decreases RLC content and/or phosphorylation is important, because phosphorylation of RLC has been shown to increase 1) Ca2+ sensitivity and ktr (46, 59, 72) and 2) force in submaximal Ca2+ by increasing the disordered array of myosin and thereby the probability of myosin binding to actin (12, 39, 42).
The purpose of the present study was to 1) compare the contractile mechanics of muscle fibers from young and older men and women under both maximal and submaximal Ca2+ conditions and 2) assess the abundance and phosphorylation states of three regulatory proteins thought to influence Ca2+ sensitivity. Because the loss of mobility and risks of debilitating falls are more dependent on age-related changes of large lower limb muscles, we chose to study single fibers isolated from the vastus lateralis muscle. On the basis of our previous findings from men (69), we expected to observe marked atrophy of fast muscle fibers but an overall preservation of contractile function in saturating Ca2+ conditions with age. In contrast, we hypothesized that aging would decrease Ca2+ sensitivity and inhibit ktr and Vo in submaximal Ca2+, with the effect on velocity only observed at shortening lengths >10% of fiber length.
MATERIALS AND METHODS
Subjects.
Nineteen young adults (11 men and 8 women; ages 20–33 yr) and 21 older adults (14 men and 7 women; ages 68–90 yr) volunteered to participate in this study. Participants were given a general health screening that included an assessment of body composition and thigh lean mass with dual X-ray absorptiometry (Lunar iDXA; GE, Madison, WI) (69). Participants were healthy, community-dwelling adults free of any known neurological, musculoskeletal, and cardiovascular diseases and were excluded from participation if they had any major health concerns. All subjects provided written informed consent, and procedures were approved by the Marquette University Institutional Review Board and conformed to the principles in the Declaration of Helsinki.
Physical activity assessment.
Physical activity was quantified for each participant with a triaxial accelerometer (GT3X; ActiGraph, Pensacola, FL) worn around the waist for at least 4 days (2 weekdays and 2 weekend days) as reported previously (30, 69). The data were recorded for each participant if the accelerometer was worn for a minimum of 8 h on at least 3 days (29). Physical activity and anthropometric measurements for the participants are reported in Table 1.
Table 1.
Anthropometries of the young and older adults
| Men |
Women |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Young (11*) | Old (14) | Young (8) | Old (7) | Age | Sex | Age × Sex | |
| Age, yr | 25 ± 4 | 76 ± 7 | 23 ± 2 | 77 ± 7 | 0.000 | 0.757 | 0.383 |
| Height, cm | 178.4 ± 9.8 | 171.9 ± 7.5 | 168.9 ± 7.2 | 162.8 ± 4.1 | 0.018 | 0.001 | 0.944 |
| Weight, kg | 78.7 ± 16.3 | 77.9 ± 8.6 | 62.6 ± 9.2 | 69.4 ± 9.1 | 0.435 | 0.002 | 0.317 |
| Body mass index, kg/m2 | 24.5 ± 2.8 | 26.4 ± 3.2 | 21.8 ± 1.6 | 26.5 ± 4.3 | 0.002 | 0.199 | 0.176 |
| Whole-body fat, % | 18.7 ± 5.3 | 29.4 ± 7.4 | 25.6 ± 3.3 | 38.3 ± 7.4 | 0.000 | 0.000 | 0.619 |
| Thigh lean mass, kg | 15.0 ± 3.5 | 11.7 ± 1.4 | 10.7 ± 1.8 | 9.2 ± 0.9 | 0.002 | 0.000 | 0.230 |
| Physical activity, steps/day | 8,760 ± 4,597 | 9,275 ± 5,178 | 10,151 ± 3,949 | 5,925 ± 2,569 | 0.215 | 0.509 | 0.116 |
Data are presented as means ± SD. Body fat percentage and the combined thigh lean mass of both legs were measured via dual X-ray absorptiometry (Lunar iDXA, GE Healthcare). The sample size (N) for each cohort is reported in parentheses.
One young male did not have physical activity measured (N = 10).
Boldfaced P values highlight statistical significance at P < 0.05.
Muscle biopsy.
Muscle biopsies of the vastus lateralis were performed on all participants using the modified Bergström technique as previously described (69). A portion of the biopsy used for single fiber contractile experiments was placed in ice-cold glycerol skinning solution (see below) and stored at −20°C for up to 4 wks. The remaining portion used for molecular studies was flash-frozen and stored in liquid nitrogen.
Solutions.
Solutions for single fiber contractile experiments were derived from an iterative program using the stability constants adjusted for temperature, pH, and ionic strength (18, 19) and contained (in mM): 20 imidazole, 7 EGTA, 4 free MgATP, 1 free Mg2+, 14.5 creatine phosphate. ATP was added as Na2ATP, Mg as MgCl2, creatine phosphate as Na2 phosphocreatine, and Ca2+ as CaCl2. Sufficient KCl was added to relaxing (79.2 mM) and activating (64 mM) solutions to adjust the ionic strength to 180 mM. All solutions were adjusted to pH 7.0 with KOH. The relaxing solution contained negligible amounts of Ca2+ (pCa 9.0, where pCa = −log10[Ca2+]), while the activating solution contained saturating levels of Ca2+ (pCa 4.5). A range of activating solutions from pCa 6.5 to 5.4 were made by mixing appropriate volumes of pCa 9.0 and 4.5 solutions (77). Glycerol skinning solution was composed of 50% relaxing and 50% glycerol (vol/vol).
Single fiber preparation.
Fibers were prepared as described previously (69). Briefly, single fibers ~2 mm in length were isolated from a biopsy and tied to a force transducer (400A; Aurora Scientific) and a high-speed servomotor (controller 312B; Aurora Scientific). Fibers were kept at 15°C with a temperature-controlled Peltier unit in 120 µL relaxing solution for the duration of the experiment, except when transferred briefly to a second Peltier unit containing 120 µL of activating solution set at 15°C (57). Sarcomere length was adjusted to 2.5 µM, and the fiber length determined by measuring the distance between the points of attachment to the force transducer and motor. Fiber diameter was determined by taking a digital image while the fiber was briefly suspended in air and then measuring the diameter at three points along the length of the fiber. Cross-sectional area (CSA) was calculated from the mean fiber diameter, assuming the fiber forms a cylinder when suspended in air. Fibers were used for multiple experiments if the force remained at >90% of the initial force measured in the first experiment. After completing experiments, we determined the MHC composition of each fiber by SDS-PAGE and silver staining as previously described (69).
Force-pCa relationship.
The force-pCa relationship was determined as described previously (14, 77) on a subset of individuals (10 young men, 6 young women, 9 older men, and 7 older women). Single fibers were activated a total of 10–13 times in a series of solutions with Ca2+ concentrations between pCa 6.5 and 4.5. The forces at each [Ca2+] were fit with Hill plots to determine the half-maximal activation (pCa50), the lowest concentration of Ca2+ that elicits force (activation threshold), and the slope of the pCa-force relationship above (n1) and below (n2) pCa50. The first, seventh, and last contractions were performed at pCa 4.5 to ensure fiber force remained >90% of the initial force.
ktr and Vo.
Rate of force development following a slack re-extension (ktr) and unloaded shortening velocity (Vo) were measured as described previously (69) on fibers at both maximal (pCa 4.5) and submaximal (pCa50) Ca2+. For ktr, slack duration before re-extension was set at 20 ms. For Vo, fibers were activated six to eight times for both pCa 4.5 and pCa50 and slacked to varying distances (100–400 µm), and time of force redevelopment was determined. Slack distances never exceeded >20% of fiber length. Vo was calculated using all slack distances, as well as from slack distances below and above 10% of shortening length (e.g., slack distances of 100–200 µm and 250–400 µm for a 2 mm fiber). Each Vo measurement was calculated as the slope of the least squares regression line between the slack distance and the time required to begin the redevelopment of force. The solution necessary to elicit half-maximal force was determined empirically for each fiber but generally fell between pCa 5.7 and 6.0.
Protein content.
Western blotting was performed on a subset of subjects to examine the content of three proteins that influence Ca2+ sensitivity: RLC, TnI, and MyBP-C. Fast and slow isoforms were compared in the vastus lateralis from the dominant leg of young men (n = 5), young women (n = 6), old men (n = 6), and old women (n = 6). Flash-frozen tissue was weighed (10–30 mg) and homogenized on ice in RIPA buffer (1:30 wt/vol, Thermo Scientific) with a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein concentration was determined using the Bradford assay (7), and samples were diluted to 0.5 μg/μL in SDS sample buffer (10% SDS, 23 mM EDTA, 50% glycerol, 0.4% bromophenol blue, and 5.1% β-mercaptoethanol, pH 6.8) and boiled for 3 min.
After verifying that 4 µg of protein was in the linear range for all antibodies used, we separated proteins on precast polyacrylamide gels (Bio-Rad) with a Mini-PROTEAN Electrophoresis Cell (Bio-Rad). Precast 4–15% polyacrylamide gels (Mini-PROTEAN TGX, Bio-Rad) were run for 1 h at 150 V in Tris-glycine running buffer and then transferred at 100 V for 50 min to 0.2 μM nitrocellulose membranes with a Mini Trans-Blot cell (Bio-Rad) containing Tris-glycine buffer plus 20% methanol. Total protein transferred to the membrane was determined following the procedure for Sypro Ruby Protein Blot Stain (Thermo Fisher Scientific). After being stained, membranes were blocked for 1 h in 5% nonfat dry milk in TBS-0.1% Tween, pH 7.6 (TBST), and rinsed three times for 5 min each in TBST, before being probed with antibodies for fast isoforms: TnIf (Abcam Cat. no. ab184554, 1:2,500), RLCf (Abcam Cat. no. ab135404, 1:2,000), and MyBP-Cf (Thermo Fisher Scientific Cat. no. PA5-20917, RRID: AB 11154456, 1:2,000), or slow isoforms: TnIs (Novus Biologicals Cat. no. NBP1-56641, RRID: AB 11035917, 1:4,000), RLCs (Abcam Cat. no. ab92721, RRID: AB 10563535, 1:5,000), and MyBP-Cs (Novus Biologicals Cat. no. NBP2-41157, 1:10,000). All antibodies were added to 5% nonfat dry milk in TBST, and membranes were incubated overnight at 4°C. Following another set of rinses with TBST as described above, membranes were probed with a horseradish peroxidase-conjugated secondary antibody (Thermo Fisher Scientific Cat. no. G-21234, RRID: AB 2536530, 1:5,000) for 1 h at room temperature in 5% nonfat dry milk in TBST. Membranes were given a final set of rinses before protein signals were detected on autoradiography film (Hyblot CL, Fisher Scientific) by chemiluminescence.
ImageJ (National Institutes of Health) was used for densiometric analysis of the films. To compare data between gels, signals were normalized to total protein and then expressed relative to the pooled homogenate control quantified with Image Studio Lite (Li-Cor) based on calculations in Taylor and Posch (73). Total protein was used as a suitable alternative to housekeeping proteins, such as GAPDH, which may be too abundant for accurate normalization or altered with aging (3, 41, 75). Each sample was run a minimum of two times, and the signal averaged for each participant to compare between cohorts. To minimize the influence of variability between gels, we loaded as many individuals from each cohort on one gel as possible (e.g., 2 young men, 3 old men, 3 young women, and 3 old women), and any participant with considerable differences between gels was rerun. Additionally, the same pooled control homogenate consisting of samples from all four cohorts was loaded at least twice on every gel.
To examine whether variations in the regulatory protein content were due to differences in fiber type distribution, we quantified each participant’s MHC composition by running their homogenate in quadruplicate (50, 60, 80, and 100 ng) on 5% SDS-PAGE gels and silver stained as previously described (69). The dilution series used was based on the stain’s linear range (51). Densitometry analysis was performed in ImageJ (National Institutes of Health) to determine the percentage of MHC slow (I) and fast (IIa and IIa/IIx) in each lane [e.g., %MHC I = SignalMHCI/(SignalMHCI + SignalMHCIIa & IIa/IIx)], and the four replicates averaged per individual.
To evaluate whether age alters the content of the slow and/or fast isoforms of RLC, MyBP-C, or TnI, the Western blot data were analyzed in two ways. First, we compared the protein content between cohorts without accounting for the potential influence of differences in fiber type distribution. As a secondary assessment and to ensure the results were not influenced by variability from the Western blot procedure, we also compared the protein content of RLC from the SYPRO Ruby-stained gels, where the slow and fast isoforms are readily distinguishable based on molecular weights. The results did not differ between the two analyses, and thus, only the Western blot data are presented. And second, to account for the potential influence of differences in fiber type distribution, we expressed each participant’s slow and fast isoforms of the three regulatory proteins relative to the corresponding slow and fast MHC isoform percentages. The results did not differ between these two methods; thus, Western blot data are shown expressed relative to MHC content.
Protein phosphorylation.
Pro-Q Diamond staining was performed to test for differences in the phosphorylation status of RLC, TnI, and MyBP-C between young and old men and women. After determining the load was in the linear range for both stains, we prepared five samples from each cohort as above, loaded at 6 μg on 4–15% gradient precast polyacrylamide gels, and run in Tris-glycine buffer using a Mini-PROTEAN Electrophoresis Cell (Bio-Rad) for 1 h at 150 V. Gels were then stained with Pro-Q Diamond phosphoprotein gel dye (Invitrogen) based on the manufacturer’s instructions and imaged using the UV GelDoc-It2 Imager. The PeppermintStick phosphoprotein molecular weight standard (Invitrogen) was used to verify appropriate exposure time. Following imaging, gels were stained overnight with the SYPRO Ruby protein gel stain (Invitrogen), destained according to the manufacturer’s instructions, and imaged for total protein.
Each sample was run in duplicate on two separate gels, and the same pooled homogenate control was included for comparison across all gels. Densitometry was performed using ImageJ (National Institutes of Health), and phosphorylation level was determined by taking the ratio of each individual’s Pro-Q phosphorylated band intensity by their SYPRO Ruby protein abundance band intensity. After validating the phosphorylation level of the pooled homogenate control was similar between gels for all bands measured (SD < 0.04), we divided the phosphorylation level of each individual by the corresponding phosphorylation level of the control: % Control = (Pro-QInd/Sypro RubyInd)/(Pro-QControl/Sypro RubyControl).
Statistical analysis.
Due to the small number of hybrids (MHC I/IIa and IIa/IIx), only pure MHC I and IIa fibers were included in the analysis. Additionally, some fibers were excluded from analysis if they did not meet quality criteria (see above), which resulted in varying fiber and/or subject number between experiments. A nested ANOVA was used for single fiber data to test for differences in age and sex. To test for an effect of Ca2+ on contractile mechanics, a repeated-measures nested ANOVA was used. Total protein, phosphorylation levels, and MHC content were compared between cohorts using a two-way ANOVA. When necessary, data were transformed to meet assumptions of normality and homogeneity of variance. Statistical analyses were performed using Minitab, version 18.1 (Minitab Inc., State College, PA). Statistical significance was set at P < 0.05. Data are presented as means ± SD in the text and tables. Unless otherwise noted, results comparing the two age groups have men and women combined, sexes have young and old combined, and fiber types have young and old men and women combined.
RESULTS
Cross-sectional area.
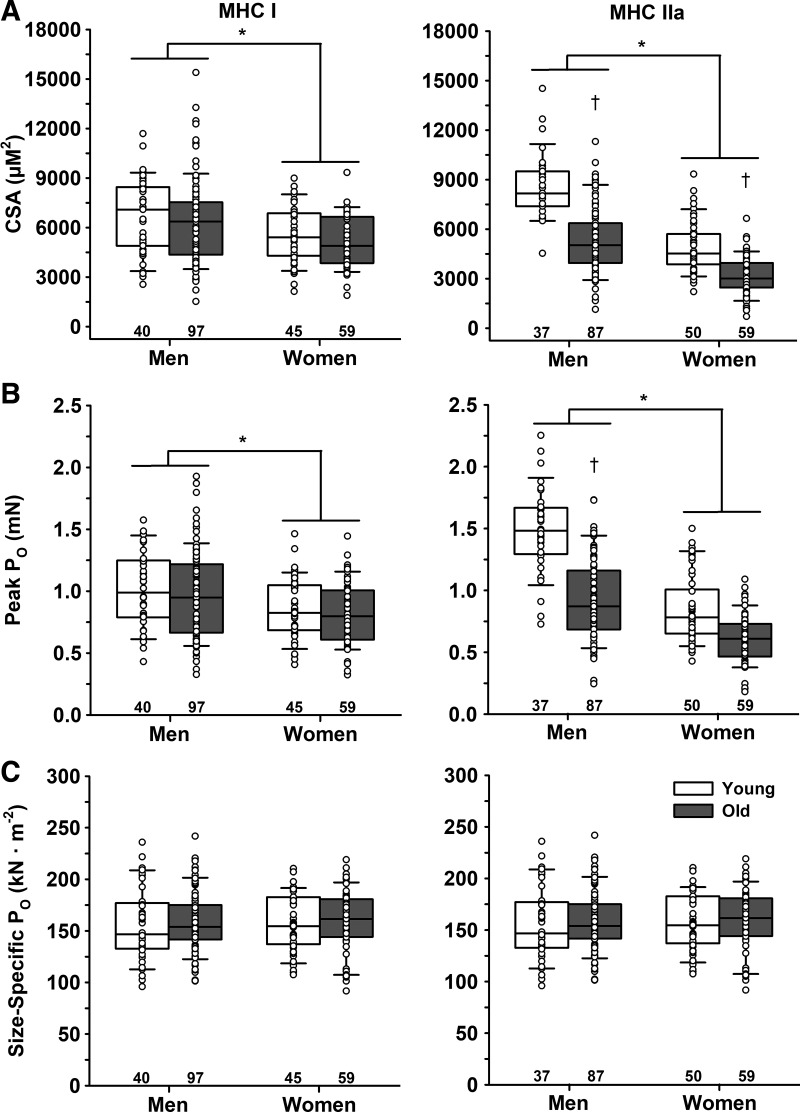
There were no differences in cross-sectional area (CSA) for the MHC I fibers between young (6,116 ± 2,049 µm2) and old (5,870 ± 2,310 µm2, P = 0.543), but men (6,433 ± 2,473 µm2) had ~21% larger MHC I fibers compared with women (5,329 ± 1,648 µm2, P = 0.032) (Fig. 1). CSA was ~44% larger in MHC IIa fibers from young (6,479 ± 2,487 µm2) compared with old (4,503 ± 2,071 µm2, P < 0.001) and ~60% larger in men (6,358 ± 2,483 µm2) compared with women (3,970 ± 1,604 µm2, P < 0.001). Young men (8,599 ± 1,857 µm2) had a larger CSA than young women (4,909 ± 1,515 µm2), old men (5,404 ± 2,049 µm2), and old women (3,174 ± 1,177 µm2). Old men and young women had similar CSAs of MHC IIa fibers, and both were larger than old women.
Fig. 1.
Cross-sectional area (CSA), peak force (Po), and size-specific force of single muscle fibers. A: CSA. Men had greater CSA in both slow MHC I and fast MHC IIa fibers compared with women. There was no age difference in CSA of MHC I fibers; however, MHC IIa fibers from old men and women were smaller than their younger cohort. B: peak force (Po) in maximal Ca2+ (pCa 4.5). Men had greater Po in both MHC I and IIa fibers compared with women. There was no age difference in Po for MHC I fibers; however, Po of MHC IIa fibers was lower in old compared with young, and young men had a greater Po than all other groups. C: size-specific Po in maximal Ca2+ (pCa 4.5). There were no age or sex differences in size-specific Po for either MHC I or IIa fibers, indicating that the age and sex differences in absolute Po were explained by fiber size. *Significant effect of sex. †Significant effect of age. Significance level P < 0.05. The horizontal line in each box plot indicates the median, while the whiskers represent 1.5 times the upper- and lower-interquartile range. Each dot is an individual fiber, with the number of fibers (n) displayed below the box plots. Data were analyzed using a nested ANOVA.
Peak force.
There were no differences in peak force (Po) for the MHC I fibers between young (0.96 ± 0.28 mN) and old (0.91 ± 0.32 mN, P = 0.462), but men (0.98 ± 0.33 mN) had greater Po compared with women (0.83 ± 0.25 mN, P = 0.031) (Fig. 1). Po was greater in MHC IIa fibers from young (1.12 ± 0.43 mN) compared with old (0.79 ± 0.33 mN, P < 0.001) and greater in men (1.09 ± 0.42 mN) compared with women (0.72 ± 0.27 mN, P < 0.001). Po of MHC IIa fibers from young men (1.48 ± 0.34 mN) was higher compared with young women (0.85 ± 0.27 mN), old men (0.92 ± 0.33 mN), and old women (0.60 ± 0.20 mN), but old men, young women, and old women were not different from each other.
Size-specific force.
Size-specific Po of MHC I fibers did not differ between young (157 ± 31 kN/m2) and old (159 ± 29 kN/m2, P = 0.944) or men (158 ± 31 kN/m2) and women (158 ± 28 kN/m2, P = 0.668) (Fig. 1). Similarly, there was no difference in size-specific Po of MHC IIa fibers between young (175 ± 27 kN/m2) and old (183 ± 38 kN/m2, P = 0.154) or men (175 ± 34 kN/m2) and women (186 ± 34 kN/m2, P = 0.286). Irrespective of age or sex, MHC I fibers had a 12% lower size-specific Po compared with IIa fibers (158 ± 30 vs. 180 ± 34 kN/m2, P < 0.001).
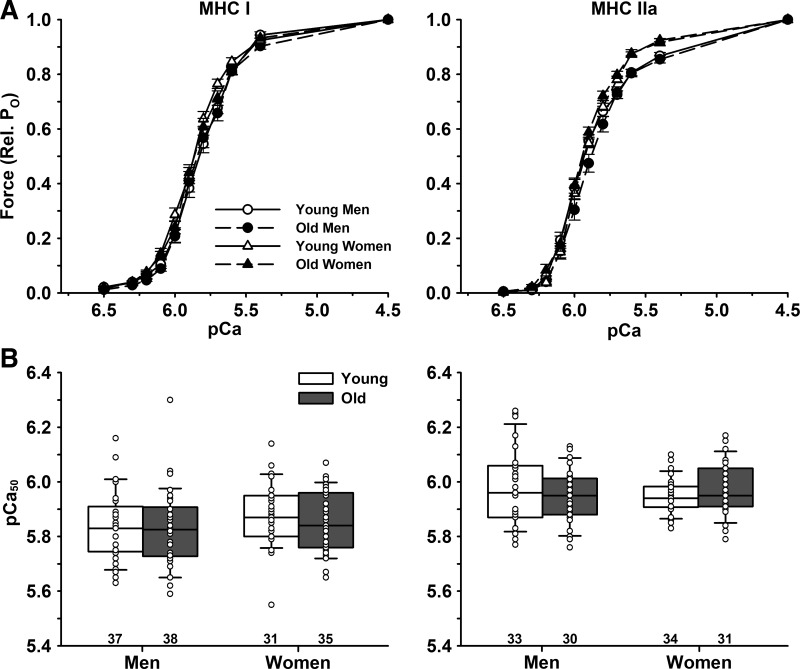
pCa50.
There were no differences in pCa50 of MHC I fibers between young (5.85 ± 0.12) and old (5.84 ± 0.12, P = 0.601) or men (5.83 ± 0.13) and women (5.87 ± 0.11, P = 0.153) (Fig. 2). Similarly, there were no differences in pCa50 of MHC IIa fibers between young (5.96 ± 0.10) and old (5.96 ± 0.09, P = 0.714) or men (5.96 ± 0.12) and women (5.96 ± 0.08, P = 0.834). Irrespective of age or sex, MHC I fibers had a lower pCa50 compared with IIa fibers (5.85 ± 0.12 vs. 5.96 ± 0.10, P < 0.001).
Fig. 2.
Mean force-pCa relationship of single muscle fibers. Single muscle fibers were activated in solutions containing free Ca2+ concentrations ranging from pCa 6.5 to 4.5. A: pCa-force relationship where peak force is expressed as a fraction of maximal Ca2+ activated force. B: pCa50 calculated by fitting the data with Hill plots. There were no age or sex differences for pCa50 in slow MHC I or fast MHC IIa fibers. Significance level P < 0.05. The horizontal line in each box plot indicates the median, while the whiskers represent 1.5 times the upper- and lower-interquartile range. Each dot is an individual fiber, with the number of fibers (n) displayed below the box plots. Data were analyzed using a nested ANOVA.
Activation threshold.
There were no differences in activation threshold (pCa) for MHC I fibers between young (6.75 ± 0.18) and old (6.73 ± 0.17, P = 0.745), but men (6.69 ± 0.18) had a lower activation threshold compared with women (6.79 ± 0.15, P = 0.011). There were no differences in activation threshold for MHC IIa fibers between young (6.43 ± 0.11) and old (6.43 ± 0.12, P = 0.535) or men (6.44 ± 0.13) and women (6.42 ± 0.09, P = 0.801).
n1 and n2.
There were no differences in n1 for MHC I fibers between young (2.09 ± 0.81) and old (2.12 ± 0.72, P = 0.893) or men (2.11 ± 0.80) and women (2.10 ± 0.74, P = 0.876). There were no differences in n1 for MHC IIa fibers between young (1.50 ± 0.62) and old (1.59 ± 0.73, P = 0.528), but men had a lower n1 compared with women (1.27 ± 0.60 vs. 1.81 ± 0.63, P < 0.001). There were no differences in n2 for MHC I fibers between young (2.66 ± 0.63) and old (2.68 ± 0.67, P = 0.983) or men (2.78 ± 0.73) and women (2.55 ± 0.52, P = 0.060). There were also no differences in n2 for MHC IIa fibers between young (4.92 ± 1.00) and old (4.99 ± 1.05, P = 0.868) or men (4.72 ± 0.98) and women (5.17 ± 1.01, P = 0.143). Irrespective of age or sex, MHC I fibers had a lower n2 compared with IIa fibers (2.67 ± 0.65 vs. 4.95 ± 1.02, P < 0.001).
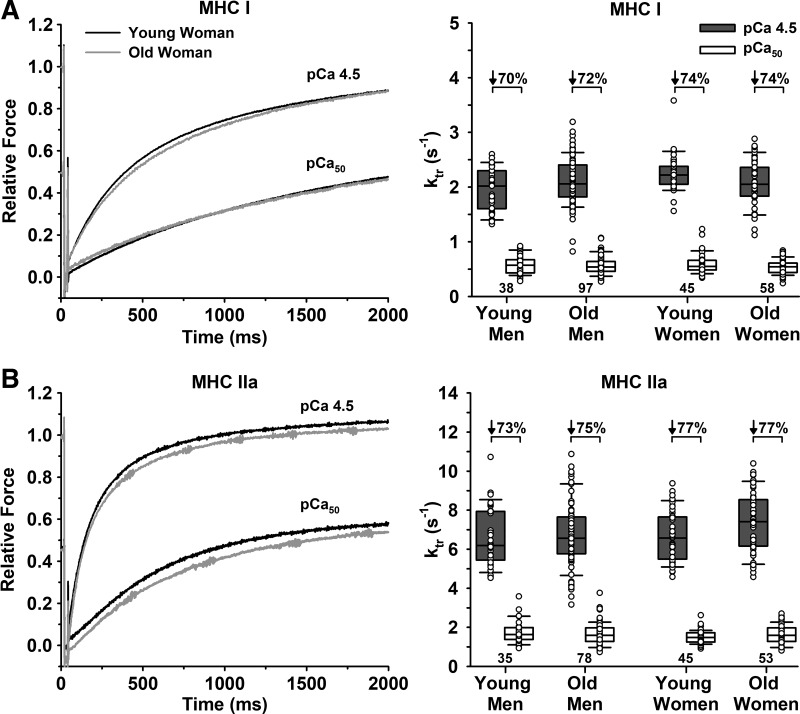
ktr.
There were no differences in ktr at pCa 4.5 or pCa50 between young and old or men and women for either fiber type (Table 2, Fig. 3). Submaximal Ca2+ decreased ktr for both MHC I and IIa fibers (P < 0.001). There were no age differences in the percent decrease in ktr from pCa 4.5 to pCa50 for either MHC I or IIa fibers. However, MHC IIa fibers from men had a lower percent decrease compared with women (74.2 vs. 77.1%, P = 0.033). Compared with MHC IIa fibers, MHC I fibers had a lower ktr at pCa 4.5 (2.10 ± 0.39/s vs. 6.87 ± 1.50/s, P < 0.001) and at pCa50 (0.56 ± 0.16/s vs. 1.64 ± 0.49/s, P < 0.001). MHC I fibers also had a lower percent decrease in ktr from pCa 4.5 to pCa50 compared with IIa fibers (72.5 ± 8.9 vs. 75.6 ± 6.9%, P = 0.012).
Table 2.
ktr and VO at maximal and submaximal Ca2+ levels
| Young | Old | P Value | Men | Women | P Value | |
|---|---|---|---|---|---|---|
| Slow MHC I | ||||||
| n (N) | 83 (15) | 155 (19) | 135 (20) | 103 (14) | ||
| ktr pCa 4.5, /s | 2.12 ± 0.37 | 2.09 ± 0.41 | 0.564 | 2.06 ± 0.41 | 2.15 ± 0.38 | 0.193 |
| ktr pCa50, /s | 0.58 ± 0.17 | 0.55 ± 0.15 | 0.793 | 0.57 ± 0.16 | 0.56 ± 0.15 | 0.862 |
| % Change | −72 ± 9 | −73 ± 9 | 0.803 | −72 ± 11 | −74 ± 6 | 0.257 |
| n (N) | 73 (14) | 131 (19) | 116 (19) | 88 (14) | ||
| VO pCa 4.5, fl/s | 1.14 ± 0.31 | 1.07 ± 0.29 | 0.377 | 1.12 ± 0.35 | 1.06 ± 0.21 | 0.368 |
| VO pCa50, fl/s | 0.45 ± 0.18 | 0.38 ± 0.14 | 0.138 | 0.41 ± 0.17 | 0.41 ± 0.14 | 0.578 |
| % Change | −61 ± 9 | −64 ± 8 | 0.240 | −64 ± 9 | −62 ± 8 | 0.516 |
| Fast MHC IIa | ||||||
| n (N) | 80 (17) | 131 (18) | 113 (20) | 98 (15) | ||
| ktr pCa 4.5, /s | 6.59 ± 1.35 | 7.04 ± 1.57 | 0.252 | 6.69 ± 1.54 | 7.07 ± 1.44 | 0.250 |
| ktr pCa50, /s | 1.60 ± 0.45 | 1.66 ± 0.51 | 0.622 | 1.69 ± 0.55 | 1.59 ± 0.40 | 0.199 |
| % Change | −75 ± 7 | −76 ± 7 | 0.535 | −74 ± 8 | −77 ± 6 | 0.033 |
| n (N) | 58 (15) | 91 (18) | 81 (19) | 68 (14) | ||
| VO pCa 4.5, fl/s | 3.20 ± 1.07 | 3.38 ± 1.09 | 0.563 | 3.46 ± 1.11 | 3.13 ± 1.02 | 0.235 |
| VO pCa50, fl/s | 0.97 ± 0.58 | 0.97 ± 0.44 | 0.839 | 1.03 ± 0.55 | 0.89 ± 0.41 | 0.354 |
| % Change | −72 ± 9 | −71 ± 9 | 0.923 | −71 ± 10 | −72 ± 8 | 0.736 |
Data are presented as means ± SD. The number of fibers (n) and number of subjects (N) are reported for each group and fiber type. Sexes are combined when comparing age groups, and ages are combined when comparing between sexes. ktr, rate of force development; VO, unloaded shortening velocity. Boldfaced P values highlight statistical significance at P < 0.05.
Fig. 3.
Rate of force development (ktr) of single fibers measured in maximal (pCa 4.5) and half-maximal (pCa50) Ca2+. A: slow MHC I fibers. Left, representative force traces of a single fiber from a young and old woman, where ktr is determined from the best fit of an exponential function following a slack and rapid re-extension maneuver. Traces are superimposed to compare between the young and old and between the two Ca2+ levels. Right, group means for ktr. B: fast MHC IIa fibers. Left, representative traces of a single fiber from a young and old woman. Right, group means for ktr. There were no age or sex differences for ktr in pCa 4.5 or pCa50 for either fiber type. Significance level P < 0.05. The horizontal line in each box plot indicates the median, while the whiskers represent 1.5 times the upper- and lower-interquartile range. Each dot is an individual fiber, with the number of fibers (n) displayed below the box plots. Data were analyzed using a nested ANOVA.
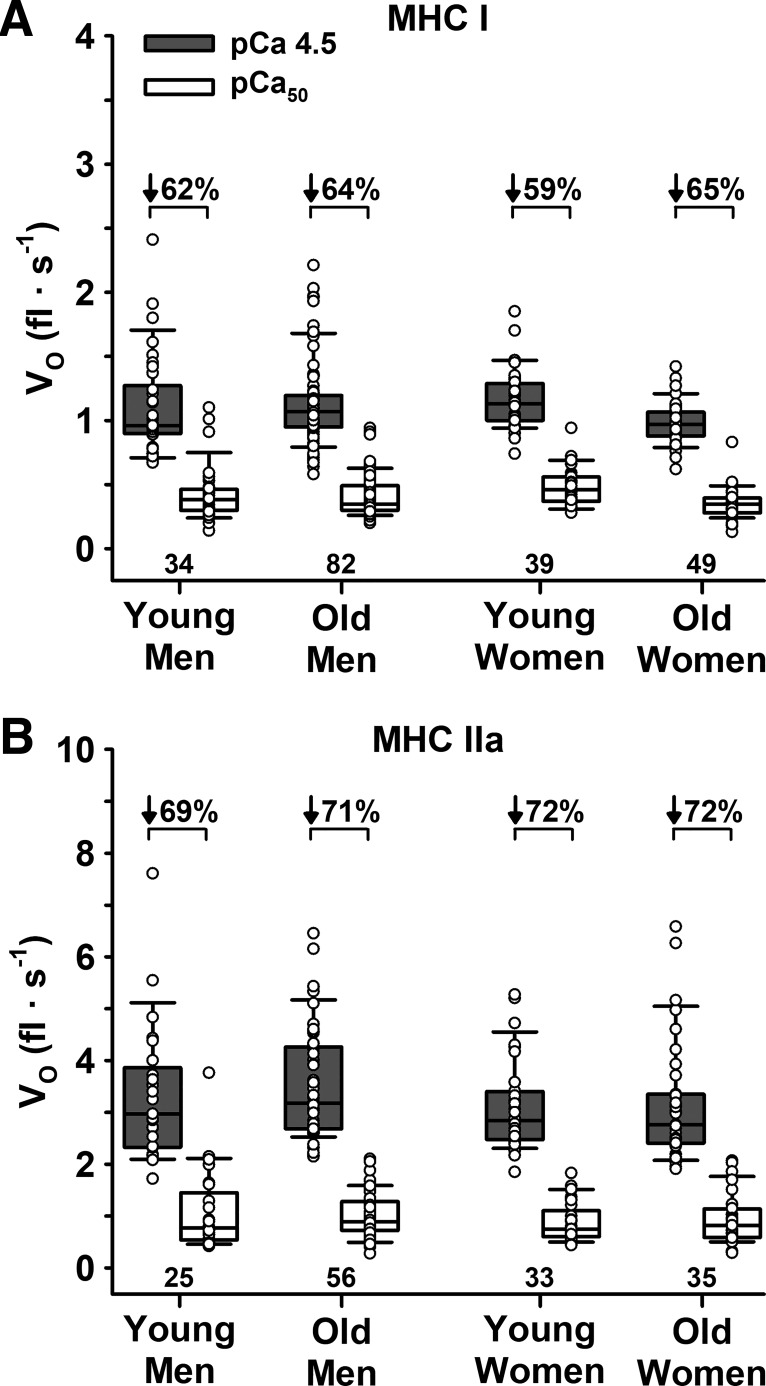
Vo.
There were no differences in Vo at pCa 4.5 or pCa50 between young and old or men and women for either fiber type (Table 2, Fig. 4). Submaximal Ca2+ decreased Vo for both MHC I and IIa fibers (P < 0.001). However, there were no age or sex differences in the percent decrease in Vo from pCa 4.5 to pCa50 for either MHC I or IIa fibers. Compared with MHC IIa fibers, MHC I fibers had a lower Vo at pCa 4.5 (1.10 ± 0.30 vs. 3.31 ± 1.08 fl/s, P < 0.001) and pCa50 (0.41 ± 0.16 vs. 0.97 ± 0.50 fl/s, P < 0.001). MHC I fibers also had a lower percent decrease in Vo from pCa 4.5 to pCa50 compared with IIa fibers (63.0 ± 8.7 vs. 71.0 ± 9.3%, P < 0.001).
Fig. 4.
Unloaded shortening velocity (Vo) measured in maximal (pCa 4.5) and half-maximal (pCa50) Ca2+. A: slow MHC I fibers. B: fast MHC IIa fibers. There were no age or sex differences for Vo in pCa 4.5 or pCa50 for either fiber type. Significance level P < 0.05. The horizontal line in each box plot indicates the median, while the whiskers represent 1.5 times the upper- and lower-interquartile range. Each dot is an individual fiber, with the number of fibers (n) displayed below the box plots. Data were analyzed using a nested ANOVA.
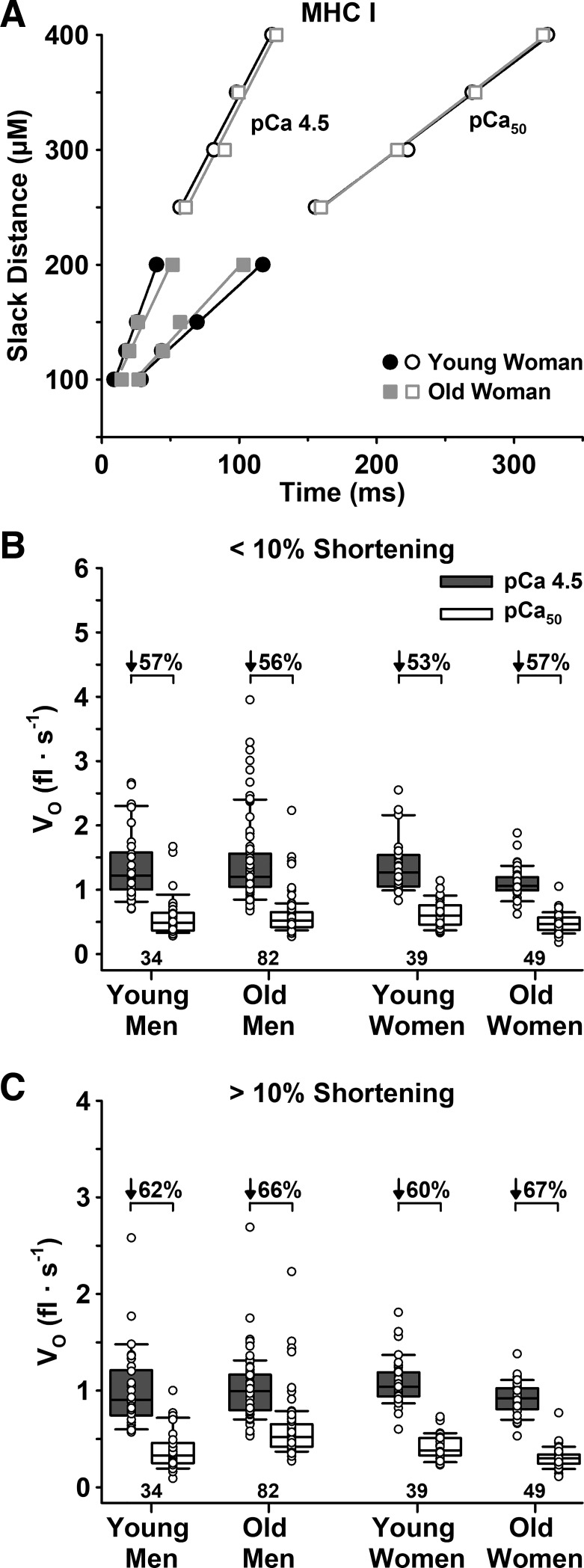
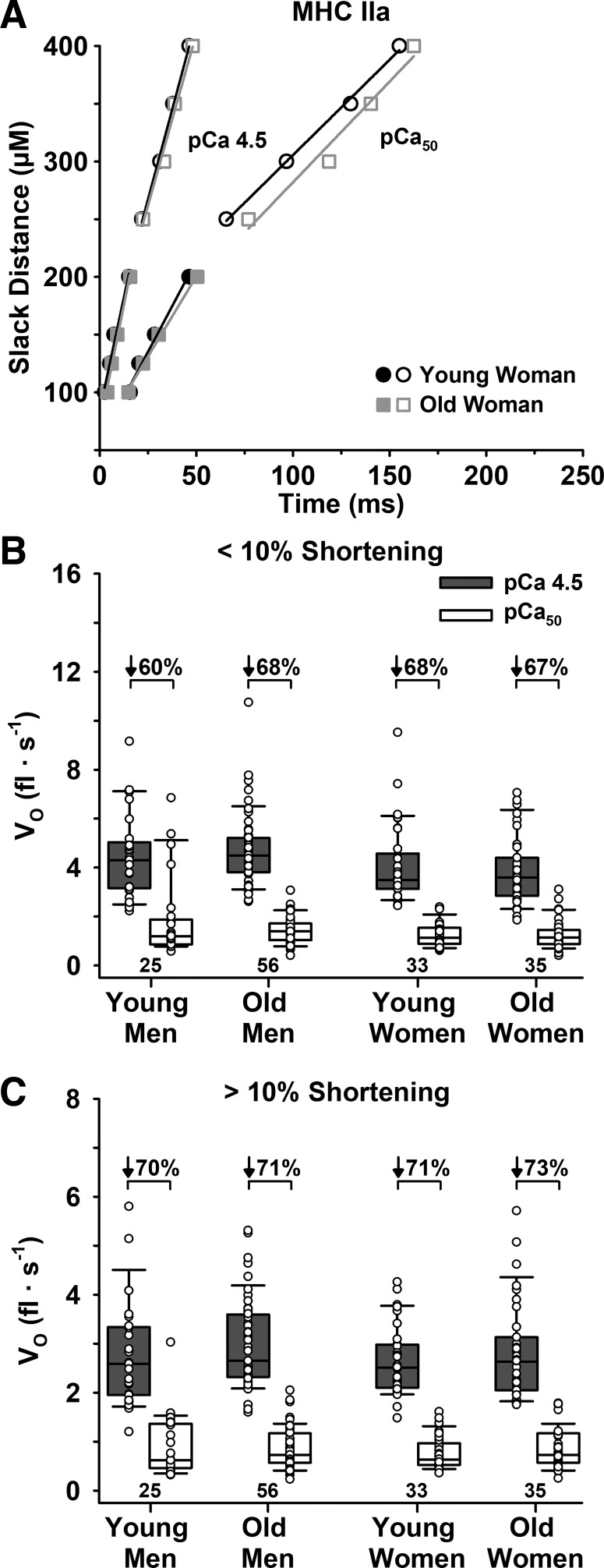
Vo below and above 10% shortening.
There were no age or sex differences in Vo below or above 10% shortening for either Ca2+ level or fiber type (Table 3, Figs. 5 and 6). For all cohorts and both fiber types, Vo decreased with increased shortening length and decreased [Ca2+], such that the Vo below 10% in pCa 4.5 > Vo above 10% in pCa 4.5 > Vo below 10% in pCa50 > Vo above 10% in pCa50. Similarly, the percent decrease in Vo at longer compared with shorter slack distances did not differ with age for either Ca2+ level or fiber type. Specifically, the percent decrease in Vo in pCa 4.5 for young and old adults was 21 ± 17 and 20 ± 17% in slow MHC I (P = 0.672) and 35 ± 14 and 32 ± 13% in fast MHC IIa fibers (P = 0.115), while in pCa50 it was 32 ± 17 vs. 38 ± 14% (P = 0.308) for MHC I and 42 ± 16 vs. 41 ± 13% (P = 0.470) for MHC IIa fibers.
Table 3.
VO below and above 10% shortening at maximal and submaximal Ca2+ levels
| Young | Old | P Value | Men | Women | P Value | |
|---|---|---|---|---|---|---|
| Slow MHC I | ||||||
| n (N) | 73 (14) | 131 (19) | 116 (19) | 88 (14) | ||
| <10% shortening | ||||||
| pCa 4.5, fl/s | 1.36 ± 0.45 | 1.30 ± 0.54 | 0.228 | 1.40 ± 0.60 | 1.22 ± 0.33 | 0.825 |
| pCa50, fl/s | 0.60 ± 0.26 | 0.55 ± 0.26 | 0.358 | 0.59 ± 0.30 | 0.54 ± 0.19 | 0.834 |
| % Change | −55 ± 12 | −56 ± 14 | 0.922 | −56 ± 15 | −55 ± 12 | 0.562 |
| >10% shortening | ||||||
| pCa 4.5, fl/s | 1.04 ± 0.32 | 0.98 ± 0.26 | 0.223 | 1.02 ± 0.34 | 0.99 ± 0.21 | 0.473 |
| pCa50, fl/s | 0.40 ± 0.16 | 0.33 ± 0.12 | 0.076 | 0.35 ± 0.15 | 0.35 ± 0.13 | 0.714 |
| % Change | −61 ± 12 | −67 ± 8 | 0.217 | −65 ± 10 | −64 ± 10 | 0.743 |
| Fast MHC IIa | ||||||
| n (N) | 58 (15) | 91 (18) | 81 (19) | 68 (14) | ||
| <10% shortening | ||||||
| pCa 4.5, fl/s | 4.25 ± 1.62 | 4.37 ± 1.46 | 0.942 | 4.62 ± 1.52 | 3.97 ± 1.46 | 0.064 |
| pCa50, fl/s | 1.51 ± 1.17 | 1.38 ± 0.56 | 0.865 | 1.57 ± 1.02 | 1.26 ± 0.54 | 0.176 |
| % Change | −65 ± 17 | −68 ± 11 | 0.719 | −66 ± 16 | −68 ± 10 | 0.783 |
| >10% shortening | ||||||
| pCa 4.5, fl/s | 2.69 ± 0.88 | 2.90 ± 0.91 | 0.275 | 2.90 ± 0.94 | 2.72 ± 0.85 | 0.635 |
| pCa50, fl/s | 0.82 ± 0.47 | 0.82 ± 0.41 | 0.901 | 0.86 ± 0.47 | 0.77 ± 0.37 | 0.551 |
| % Change | −71 ± 9 | −72 ± 10 | 0.550 | −71 ± 11 | −72 ± 8 | 0.638 |
Data are presented as means ± SD. The number of fibers (n) and number of subjects (N) are reported for each group and fiber type. Sexes are combined when comparing age groups, and ages are combined when comparing between sexes. Significance level P < 0.05.
Fig. 5.
Unloaded shortening velocity (Vo) of slow MHC I fibers measured below and above 10% shortening in maximal (pCa 4.5) and half-maximal (pCa50) Ca2+. A: representative plot of individual fibers from a young and old woman for Vo below (filled symbols) and above (open symbols) 10% shortening in both pCa 4.5 and pCa50. B: group means for Vo below 10% shortening in both pCa 4.5 and pCa50. C: group means for Vo above 10% shortening in both pCa 4.5 and pCa50. There were no age or sex differences in Vo below or above 10% shortening in either pCa 4.5 or pCa50. Significance level P < 0.05. The horizontal line in each box plot indicates the median, while the whiskers represent 1.5 times the upper- and lower-interquartile range. Each dot is an individual fiber, with the number of fibers (n) displayed below the box plots. Data were analyzed using a nested ANOVA.
Fig. 6.
Unloaded shortening velocity (Vo) of fast MHC IIa fibers measured below and above 10% shortening in maximal (pCa 4.5) and half-maximal Ca2+ (pCa50). A: representative plot of individual fibers from a young and old woman for Vo below (filled symbols) and above (open symbols) 10% shortening in both pCa 4.5 and pCa50. B: group means for Vo below 10% shortening in both pCa 4.5 and pCa50. C: group means for Vo above 10% shortening in both pCa 4.5 and pCa50. There were no age or sex differences in Vo below or above 10% shortening in either pCa 4.5 or pCa50. The horizontal line in each box plot indicates the median, while the whiskers represent 1.5 times the upper- and lower-interquartile range. Each dot is an individual fiber, with the number of fibers (n) displayed below the box plots. Data were analyzed using a nested ANOVA.
The percent decrease in Vo from below to above 10% shortening did not differ between men and women in MHC I fibers at pCa 4.5 (23 ± 18 vs. 17 ± 15%, P = 0.068) and pCa50 (37 ± 16 vs. 33 ± 15%, P = 0.244), or in MHC IIa fibers at pCa50 (43 ± 16 vs. 39 ± 12%, P = 0.068). However, the percent decrease in Vo for MHC IIa fibers at pCa 4.5 was greater in men compared with women (36 ± 13 vs. 29 ± 14%, P = 0.013).
Irrespective of age or sex, there were several differences in Vo between fiber types and Ca2+ levels. At both Ca2+ levels, MHC I fibers had a lower Vo below (P < 0.001) and above 10% shortening (P < 0.001) compared with MHC IIa fibers. The percent decrease in Vo from below to above 10% shortening was greater at pCa50 compared with pCa 4.5 for both MHC I (36 ± 16 vs. 20 ± 17%, P < 0.001) and IIa fibers (41 ± 15 vs. 33 ± 14%, P < 0.001), with a lower percent decrease in MHC I compared with IIa fibers at both Ca2+ levels (P < 0.05). The percent decrease in Vo from pCa 4.5 to pCa50 was lower below compared with above 10% shortening in both MHC I (P < 0.001) and IIa fibers (P = 0.001) (Table 3, Figs. 5 and 6) but was lower in MHC I compared with IIa fibers at both shortening lengths (P < 0.001).
Protein content and phosphorylation.
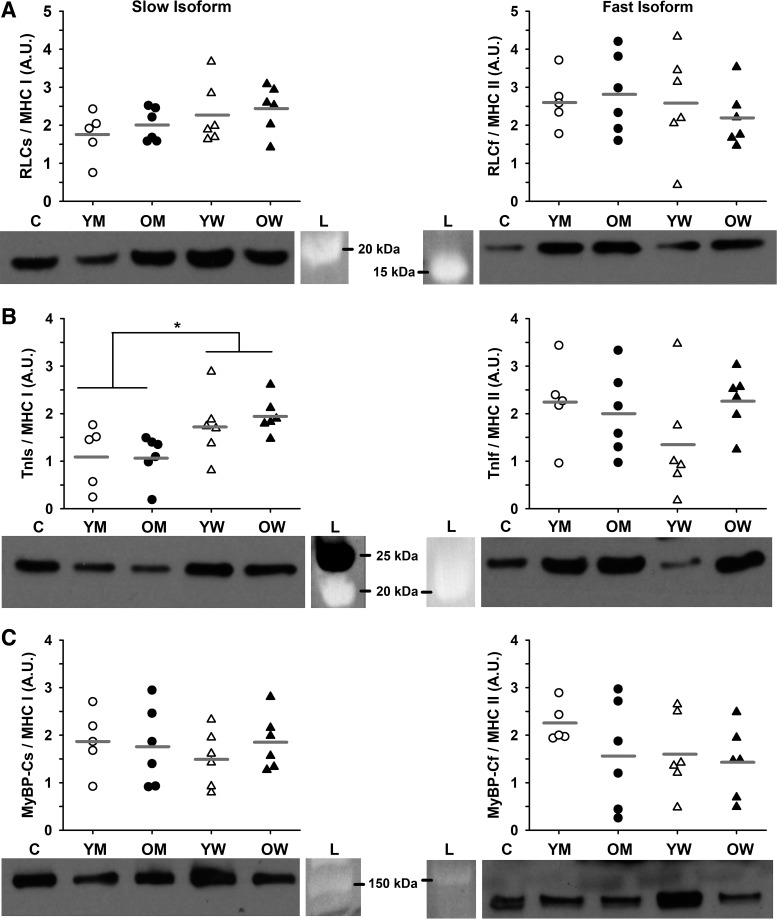
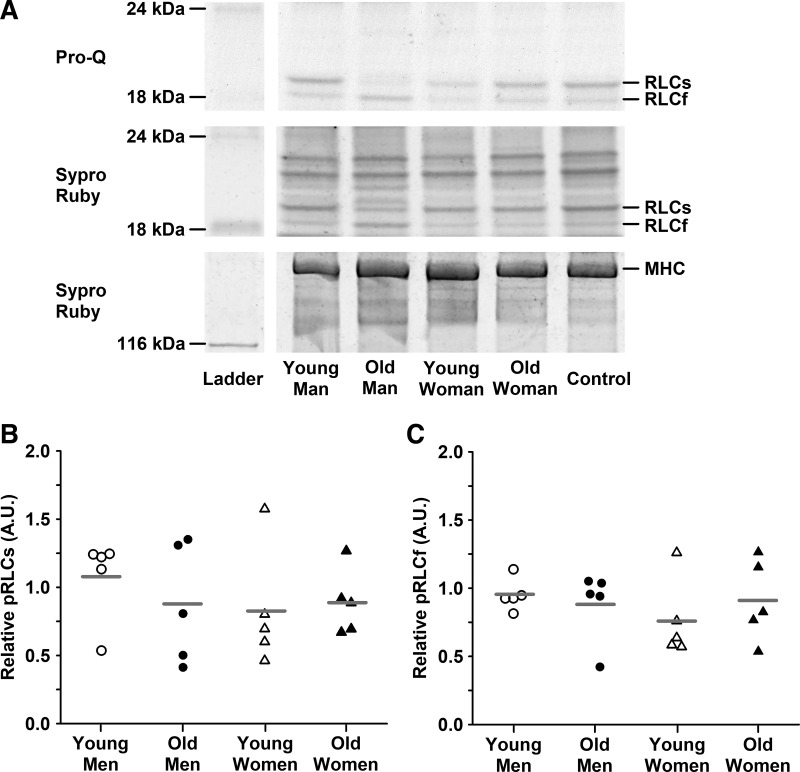
MHC composition was similar between young and old adults (57 ± 15 and 53 ± 13% MHC I in young and old, respectively; P = 0.568) and between men and women (54 ± 16 and 56 ± 12% MHC I in men and women, respectively; P = 0.769). Aging did not alter protein isoform content of RLC, TnI, or MyBP-C (Fig. 7). The only difference in protein content between sexes was in TnI, where men had less of the slow isoform than women (P = 0.005). Using the ProQ Diamond stain, we were unable to detect phosphorylation of TnI or MyBP-C in any of the samples studied and found no age or sex differences in RLC phosphorylation (Fig. 8). For all measurements and in all cohorts, there was large variability between individuals.
Fig. 7.
Relative protein content. A: myosin regulatory light chain (RLC). B: troponin I (TnI). C: myosin binding protein C (MyBP-C). Proteins were identified with antibodies specific to either the slow or fast isoform of each protein. Protein content was normalized to total protein, expressed relative to the pooled homogenate control and then expressed relative to MHC content. Representative Western blots are shown for each protein along with the molecular weight marker from the corresponding Sypro Ruby-stained membrane. There were no age or sex differences except for TnI slow, where men had less content compared with women (P = 0.005). *Significant effect of sex. Significance level P < 0.05. Individual subjects are shown as symbols with group means indicated by gray horizontal lines. N = 6 subjects for each cohort except young men, where N = 5. C, control; L, ladder; OM, old men; OW, old women; YM, young men; YW, young women. Data were analyzed with a two-way ANOVA.
Fig. 8.
Relative phosphorylation of myosin regulatory light chain (RLC). A: representative ProQ Diamond (phosphorylated protein) and Sypro Ruby-stained (total protein) gels are shown with the corresponding molecular weight markers to the left. Phosphorylation signals were normalized to total protein and then to a control homogenate comprising pooled samples from all four cohorts. B: phosphorylation of slow RLC (RLCs). C: phosphorylation of fast RLC (RLCf). Individual subjects are shown as symbols with group means indicated by gray horizontal lines. There were no age or sex differences. Significance level P < 0.05. Data were analyzed with a two-way ANOVA. N = 5 for each cohort.
DISCUSSION
The primary purpose of this study was to test the effects of age and sex on single muscle fiber Ca2+ sensitivity (pCa-force relationship), rate of force development (ktr), and maximal shortening velocity (Vo) at short and long displacements. Importantly, we investigated these contractile properties in both maximal and submaximal Ca2+. Contrary to our hypotheses, we found no effect of age on Ca2+ sensitivity, ktr, or Vo for men or women, suggesting that contractile function is preserved with age in both sexes. Consistent with our previous work in men (69), we found marked atrophy and lower absolute Po in MHC IIa fibers with aging, but no change in size-specific Po, indicating that the age-related differences in Po for both men and women were explained by fiber size. Because fiber CSA and Po are lower in young women compared with young men, the selective atrophy of fast fibers with age may be more detrimental to older women. These data, along with those of Sundberg et al. (69), suggest that the age-associated reductions in contractile function for both men and women are due primarily to the selective atrophy of fast fibers rather than changes in muscle quality.
Age and sex have little to no effect on the force-pCa relationship.
We observed no differences in Ca2+ sensitivity (pCa50) with age, which is in line with some studies (32, 33) but in contrast to others (37, 68). The explanation for the discrepancies between studies is unclear; however, one possibility is that three of the four previous studies did not control the temperature of the experiments or measure sarcomere spacing (32, 33, 37), both factors known to affect Ca2+ sensitivity (65). It is also notable that our study is 1) the largest human aging data set on the force-pCa relationship, examining 68 and 73 MHC I and 61 and 67 MHC IIa fibers from young and older adults, respectively, and 2) includes older adults that were on average 6–10 yr older than previous studies (32, 33, 37, 68). Similar to our findings on pCa50, we found no effect of age on the slopes (n1 and n2) or activation threshold of the force-pCa relationship, which agrees with a majority of studies (32, 33, 37) but not all (68). When the Hill coefficient is calculated, forces less than 50% Po (n2) show greater cooperative activation of thin filaments, increasing the likelihood of myosin strong binding to actin and increasing force at low [Ca2+] (44, 53). Our n2 data suggest that cooperative activation of thin filaments is preserved with aging.
Similar to the effects of age, we found minimal effects of sex on the pCa-force relationship. We did, however, observe that women had a greater n1 in fast MHC IIa fibers and a greater activation threshold in slow MHC I fibers compared with men. An activation threshold at lower [Ca2+] (higher pCa) indicates that more Ca2+ is needed to initiate force in fibers from men compared with women. To our knowledge, the only other study to investigate pCa-force parameters separately for men and women is Straight et al. (68), and they found no sex difference in either young or old participants. Overall, the data show little, if any, effect of age or sex on the force-pCa relationship, suggesting that myofilament Ca2+ sensitivity is not a primary contributor to declines in force with aging in either men or women.
Low- to high-force kinetics of the cross-bridge cycle are preserved with aging.
Similar to our previous work in saturating Ca2+ conditions (69), we found no age differences in ktr and, additionally, no sex differences. To our knowledge, this is the first study on the effects of aging and sex on ktr, and our data show that ktr of fibers from older women are equivalent to the other cohorts in saturating Ca2+. This finding indicates that the net movement of cross-bridges from the low- to high-force state (47) is not affected by sex or age. The only other study on the effect of aging on ktr reported a reduced ktr in slow fibers from older compared with young men (61). Factors that could explain the discrepancy between studies, as mentioned in Sundberg et al. (69), include methods of fiber typing based on Vo rather than MHC composition, experimental temperature (10 vs. 15°C in the current study), fiber compliance due to attachment method, or sarcomere spacing (2.8 vs. 2.5 µm in the current study). Given the robustness of our data (i.e., number of subjects and fibers analyzed), it seems unlikely that aging affects the low- to high-force transition of the cross-bridge cycle in either men or women.
To further test whether aging affects the low- to high-force transition, we also compared ktr of fibers from young and old men and women in submaximal Ca2+. It is known from animal studies that ktr is depressed by submaximal Ca2+, an effect that seems primarily dependent on Ca2+ binding to troponin C (TnC) (10). Contrary to our hypothesis, we observed no age differences in ktr at pCa50, suggesting that any condition that lowers [Ca2+], such as muscle fatigue, would not exacerbate the depression of ktr in older adults. This finding in combination with our observation that aging did not alter the pCa-force relationship, which is also affected by Ca2+ binding to TnC (53, 55), suggests that aging does not alter the ability of Ca2+ to bind to TnC.
Maximal shortening velocity is unaffected by age and sex.
Our finding that Vo is preserved with aging in saturating Ca2+ is in agreement with several previous publications (20, 36, 69, 74), but in contrast to others reporting an age-induced decline in fiber Vo (8, 13, 38, 58, 61). The discrepancies between studies are unclear but may involve the number of subjects, fiber storage conditions, or the statistical analyses (nested vs. nonnested). Similar to the findings on aging, we also observed no sex differences in Vo. Nearly all previous studies on Vo and aging either collected the data only in men or did not indicate whether women were included. To our knowledge, the only study that analyzed men and women is Trappe et al. (74), and in agreement with our data, they did not observe an age- or sex-related decline in Vo. Together, these data suggest that in saturating Ca2+ Vo is preserved with age in both men and women.
Previous studies have focused on Vo in maximal Ca2+ (pCa 4.5), whereas in vivo Ca2+ conditions may at times be subsaturating (e.g., muscle fatigue) (4). Contrary to our hypothesis, we did not observe an age difference in Vo in men or women in submaximal Ca2+, nor with shortening below or above 10% of fiber length. Similar to other studies at pCa 4.5 (52, 54), the Vo determined from the slope of the time to redevelop force and the slack distance was well fit by a simple linear regression. However, further analysis revealed that even in maximal Ca2+ the reduction in Vo at slack distances above compared with below 10% fiber length can be >1 fl/s for fast fibers (Table 3). We also observed a larger reduction in Vo with greater slack distances in pCa50 compared with pCa 4.5, and this reduction was greater for MHC IIa compared with MHC I fibers. Specifically, the percent decrease in Vo with decreased Ca2+ at >10% shortening was 61% for MHC I and 71% for MHC IIa fibers (Table 3). These findings agree qualitatively with data from rabbit muscle (52, 54); however, unlike in rabbit muscle where Vo below 10% shortening was unaltered in pCa 4.5 compared with pCa50, we observed a decreased Vo in both fast and slow fibers from humans (Table 3). Consequently, in pCa50 the decreased slope from below to above 10% shortening was less than observed in rabbit fibers (52, 54). The explanation for the discrepancy between studies is unknown but could be due to species and/or muscle fiber type differences. For example, fibers from the psoas muscle of rabbit are almost exclusively MHC IIb (5), whereas fibers from the vastus lateralis of humans are primarily MHC I and IIa.
The reduction in Vo under low [Ca2+] and high shortening lengths has been attributed to increased internal drag from slower cross-bridge turnover due to MyBP-C binding to actin, and/or the cooperative inactivation of thin filaments decreasing the number of strongly bound cross-bridges (31, 34, 44, 52, 54, 71). The latter is thought to be related to reduced thick filament cooperativity in low [Ca2+] such that the reduced force also slows velocity (25). Consequently, our finding of no age differences in the effects of low [Ca2+] or the degree of shortening on Vo provides further evidence that Ca2+ sensitivity is unaltered by aging. Additionally, it suggests that processes altering the speed of cross-bridge turnover and internal drag, such as MyBP-C tethering the myosin head to actin, are also unaltered.
Protein content and phosphorylation of a few key regulatory proteins are preserved with age.
One focus of this study was to determine if aging induces changes in key regulatory proteins known to affect Ca2+ sensitivity and contractile function in submaximal Ca2+. We found no differences in the protein content of RLC, TnI, or MyBP-C between young and older adults (Fig. 7), which is consistent with our findings of preserved Ca2+ sensitivity and contractile function in both maximal and submaximal Ca2+ with age. These findings, however, are in contrast to other studies that observed an increase in the slow RLC isoform with aging in both rodents (23) and humans (24). The reported increase in slow RLC occurred in conjunction with an age-related increase in the slow MHC isoform. We observed no age difference in the distribution of MHC isoforms, which may explain the discrepancies between studies and is consistent with previous observations from our laboratory (69). The discrepancies may also be due to species differences and/or genetic diversity between individuals, as reflected in the large variation in protein isoform content (Fig. 7). Additionally, physical inactivity and/or denervation, which often occurs in older adults, can increase the expression and coexpression of the MHC IIx isoform (6, 22, 67). In support of this possibility, our single fiber studies tested multiple hybrid IIa/IIx fibers in four of the six older women that were also assessed for protein content, but none of the young women. Given most rodent colonies are inbred, less variation between individual animals would also be expected compared with humans. This genetic diversity, or lack thereof, may in part explain why cross species results do not always agree (40, 66).
In addition to protein content, establishing whether aging alters the phosphorylation levels of key regulatory proteins is important. For example, increased phosphorylation of cardiac TnI is known to decrease Ca2+ sensitivity and increase cross-bridge kinetics (35, 79), while increased phosphorylation of MyBP-C is thought to untether the myosin heads, increasing the probability and kinetics of myosin strong binding to actin (64). Previous studies from mouse skeletal muscle have shown decreased phosphorylation of slow MyBP-C with age (1, 2). However, we were unable to detect phosphorylation of either MyBP-C or TnI from our human samples. The lack of detection could be due to the methods (i.e., ProQ Diamond stain is not sensitive enough) or because these proteins are not phosphorylated in the human vastus lateralis when extracted from quiescent muscle. In agreement with our findings, but in contrast to cardiac TnI (28, 35, 78), proteomic studies have also observed little to no phosphorylation of TnI in either human or rodent skeletal muscle (11, 76). Together, these data suggest that age and sex do not alter the phosphorylation levels of TnI and MyBP-C in human skeletal muscle.
Establishing whether aging alters RLC phosphorylation is also important, as phosphorylation of RLC is known to increase fiber Ca2+ sensitivity and the rate of force development (46). RLC phosphorylation was detectable on the ProQ Diamond-stained gel; however, we found no age or sex differences in the phosphorylation levels of the slow or fast isoforms (Fig. 8). Interestingly, and like our protein content results, we observed large variability between individuals in all four cohorts, suggesting that factors other than age or sex may influence RLC phosphorylation. In partial agreement with our results, Miller et al. (49) reported no age differences in the phosphorylation levels of either RLC isoform in men but found lower phosphorylation of the fast isoform in old women. In contrast, Brocca et al. (8) observed no differences in the fast RLC isoform but an increased phosphorylation of the slow isoform in physically fit older men. Collectively, the data from humans suggest that age-induced decline in RLC phosphorylation either does not occur or is restricted to the fast isoform in women.
In contrast to human studies, studies on aging rodent models report differences in both the content and phosphorylation level of RLC (26, 76). For example, proteomics on the rat gastrocnemius muscle report increases in fast RLC content, but decreased phosphorylation (26, 76), as well as increased phosphorylation levels of the slow isoform with age (23). Gregorich et al. (26) reported the age-induced decline in phosphorylation of the fast RLC isoform was correlated with the loss of MHC IIb fiber function and suggested that interventions designed to maintain phosphorylation of this protein might be protective. Unfortunately, the correlation was not tested in MHC I or IIa fibers, the primary fiber types in humans. In addition, the findings from aging rodent models do not appear to agree with those from humans, suggesting caution is warranted when extending results from rodents to aging humans.
Concluding Remarks
We previously demonstrated that the primary event responsible for declines in skeletal muscle force and power in older men was atrophy of fast MHC II fibers (69). The present study similarly suggests that the age-associated reductions in fiber force in both men and women are primarily due to fast fiber atrophy. While age-induced decreases in limb muscle fiber Ca2+ sensitivity have been reported in humans, we found no effect of age on the pCa-force relationship or ktr and Vo in either maximal or submaximal Ca2+. The older adults in this study represent the healthy physically active population, and thus these findings may not extend to mobility-limited older adults. Similar to rodent skeletal muscle, we observed Vo to be depressed with fiber shortening >10% of fiber length; however, the effect was unaltered with age in men or women. Rodent studies suggest that age-induced losses of muscle protein content or phosphorylation status, particularly reduced RLC phosphorylation, may contribute to the loss in contractile function with age. This is unlikely in humans, as we found RLC content and phosphorylation levels to be unaltered with age in men or women. We conclude that other than the severe age-related atrophy of fast fibers, Ca2+ sensitivity and contractile function of skeletal muscle is preserved in older men and women.
GRANTS
This work was supported by National Institute on Aging Grant R01AG-048262 to R. H. Fitts and S. K. Hunter and American Heart Association postdoctoral fellowship 19POST34380411 to C. W. Sundberg.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.H.F. conceived and designed research; L.E.T., C.W.S., and L.J.K. performed experiments; L.E.T., C.W.S., and L.J.K. analyzed data; L.E.T., C.W.S., L.J.K., and R.H.F. interpreted results of experiments; L.E.T., C.W.S., and L.J.K. prepared figures; L.E.T., C.W.S., L.J.K., and R.H.F. drafted manuscript; L.E.T., C.W.S., L.J.K., S.K.H., and R.H.F. edited and revised manuscript; L.E.T., C.W.S., L.J.K., S.K.H., and R.H.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Mehdi Maadooliat for assistance with the statistical analysis and figure preparation. We also thank Dr. Scott Trappe for comments on an earlier version of this manuscript.
REFERENCES
- 1.Ackermann MA, Kerr JP, King B, W Ward C, Kontrogianni-Konstantopoulos A. The Phosphorylation Profile of Myosin Binding Protein-C Slow is Dynamically Regulated in Slow-Twitch Muscles in Health and Disease. Sci Rep 5: 12637, 2015. [Erratum in Sci Rep 8: 46969, 2018] doi: 10.1038/srep12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann MA, Ward CW, Gurnett C, Kontrogianni-Konstantopoulos A. Myosin Binding Protein-C Slow Phosphorylation is Altered in Duchenne Dystrophy and Arthrogryposis Myopathy in Fast-Twitch Skeletal Muscles. Sci Rep 5: 13235, 2015. doi: 10.1038/srep13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods 172: 250–254, 2008. doi: 10.1016/j.jneumeth.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen DG, Clugston E, Petersen Y, Röder IV, Chapman B, Rudolf R. Interactions between intracellular calcium and phosphate in intact mouse muscle during fatigue. J Appl Physiol (1985) 111: 358–366, 2011. doi: 10.1152/japplphysiol.01404.2010. [DOI] [PubMed] [Google Scholar]
- 5.Bauer HP, Reichmann H, Hofer HW. Perfusion of the psoas muscle of the rabbit. Metabolism of a homogeneous muscle composed of “fast glycolytic” fibres. Int J Biochem 18: 67–72, 1986. doi: 10.1016/0020-711X(86)90010-8. [DOI] [PubMed] [Google Scholar]
- 6.Borina E, Pellegrino MA, D’Antona G, Bottinelli R. Myosin and actin content of human skeletal muscle fibers following 35 days bed rest. Scand J Med Sci Sports 20: 65–73, 2010. doi: 10.1111/j.1600-0838.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Brocca L, McPhee JS, Longa E, Canepari M, Seynnes O, De Vito G, Pellegrino MA, Narici M, Bottinelli R. Structure and function of human muscle fibres and muscle proteome in physically active older men. J Physiol 595: 4823–4844, 2017. doi: 10.1113/JP274148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callahan DM, Kent-Braun JA. Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol (1985) 111: 1345–1352, 2011. doi: 10.1152/japplphysiol.00367.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chase PB, Martyn DA, Hannon JD. Isometric force redevelopment of skinned muscle fibers from rabbit activated with and without Ca2+. Biophys J 67: 1994–2001, 1994. doi: 10.1016/S0006-3495(94)80682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YC, Sumandea MP, Larsson L, Moss RL, Ge Y. Dissecting human skeletal muscle troponin proteoforms by top-down mass spectrometry. J Muscle Res Cell Motil 36: 169–181, 2015. doi: 10.1007/s10974-015-9404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol 588: 981–993, 2010. doi: 10.1113/jphysiol.2009.183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debold EP, Romatowski J, Fitts RH. The depressive effect of Pi on the force-pCa relationship in skinned single muscle fibers is temperature dependent. Am J Physiol Cell Physiol 290: C1041–C1050, 2006. doi: 10.1152/ajpcell.00342.2005. [DOI] [PubMed] [Google Scholar]
- 15.Delbono O, O’Rourke KS, Ettinger WH. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol 148: 211–222, 1995. doi: 10.1007/BF00235039. [DOI] [PubMed] [Google Scholar]
- 16.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol (1985) 95: 1717–1727, 2003. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 17.Doran P, O’Connell K, Gannon J, Kavanagh M, Ohlendieck K. Opposite pathobiochemical fate of pyruvate kinase and adenylate kinase in aged rat skeletal muscle as revealed by proteomic DIGE analysis. Proteomics 8: 364–377, 2008. doi: 10.1002/pmic.200700475. [DOI] [PubMed] [Google Scholar]
- 18.Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol 157: 378–417, 1988. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- 19.Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75: 463–505, 1979. [PubMed] [Google Scholar]
- 20.Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol (1985) 105: 637–642, 2008. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 279: C611–C618, 2000. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher P, Trappe S, Harber M, Creer A, Mazzetti S, Trappe T, Alkner B, Tesch P. Effects of 84-days of bedrest and resistance training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiol Scand 185: 61–69, 2005. doi: 10.1111/j.1365-201X.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- 23.Gannon J, Doran P, Kirwan A, Ohlendieck K. Drastic increase of myosin light chain MLC-2 in senescent skeletal muscle indicates fast-to-slow fibre transition in sarcopenia of old age. Eur J Cell Biol 88: 685–700, 2009. doi: 10.1016/j.ejcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Gelfi C, Vigano A, Ripamonti M, Pontoglio A, Begum S, Pellegrino MA, Grassi B, Bottinelli R, Wait R, Cerretelli P. The human muscle proteome in aging. J Proteome Res 5: 1344–1353, 2006. doi: 10.1021/pr050414x. [DOI] [PubMed] [Google Scholar]
- 25.Gorga JA, Fishbaugher DE, VanBuren P. Activation of the calcium-regulated thin filament by myosin strong binding. Biophys J 85: 2484–2491, 2003. doi: 10.1016/S0006-3495(03)74671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregorich ZR, Peng Y, Cai W, Jin Y, Wei L, Chen AJ, McKiernan SH, Aiken JM, Moss RL, Diffee GM, Ge Y. Top-Down Targeted Proteomics Reveals Decrease in Myosin Regulatory Light-Chain Phosphorylation That Contributes to Sarcopenic Muscle Dysfunction. J Proteome Res 15: 2706–2716, 2016. doi: 10.1021/acs.jproteome.6b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grosicki GJ, Standley RA, Murach KA, Raue U, Minchev K, Coen PM, Newman AB, Cummings S, Harris T, Kritchevsky S, Goodpaster BH, Trappe S; Health ABC Study . Improved single muscle fiber quality in the oldest-old. J Appl Physiol (1985) 121: 878–884, 2016. doi: 10.1152/japplphysiol.00479.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanft LM, Cornell TD, McDonald CA, Rovetto MJ, Emter CA, McDonald KS. Molecule specific effects of PKA-mediated phosphorylation on rat isolated heart and cardiac myofibrillar function. Arch Biochem Biophys 601: 22–31, 2016. doi: 10.1016/j.abb.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act 8: 62, 2011. doi: 10.1186/1479-5868-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassanlouei H, Sundberg CW, Smith AE, Kuplic A, Hunter SK. Physical activity modulates corticospinal excitability of the lower limb in young and old adults. J Appl Physiol (1985) 123: 364–374, 2017. doi: 10.1152/japplphysiol.01078.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann PA, Greaser ML, Moss RL. C-protein limits shortening velocity of rabbit skeletal muscle fibres at low levels of Ca2+ activation. J Physiol 439: 701–715, 1991. doi: 10.1113/jphysiol.1991.sp018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hvid LG, Ortenblad N, Aagaard P, Kjaer M, Suetta C. Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J Physiol 589: 4745–4757, 2011. doi: 10.1113/jphysiol.2011.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hvid LG, Suetta C, Aagaard P, Kjaer M, Frandsen U, Ørtenblad N. Four days of muscle disuse impairs single fiber contractile function in young and old healthy men. Exp Gerontol 48: 154–161, 2013. doi: 10.1016/j.exger.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Iwamoto H. Thin filament cooperativity as a major determinant of shortening velocity in skeletal muscle fibers. Biophys J 74: 1452–1464, 1998. doi: 10.1016/S0006-3495(98)77857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res 88: 1059–1065, 2001. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 36.Korhonen MT, Cristea A, Alén M, Häkkinen K, Sipilä S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol (1985) 101: 906–917, 2006. doi: 10.1152/japplphysiol.00299.2006. [DOI] [PubMed] [Google Scholar]
- 37.Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM, Lamb GD. Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol 593: 2499–2514, 2015. doi: 10.1113/JP270179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol Cell Physiol 272: C638–C649, 1997. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- 39.Levine RJ, Kensler RW, Yang Z, Stull JT, Sweeney HL. Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys J 71: 898–907, 1996. doi: 10.1016/S0006-3495(96)79293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Little TJ, Colegrave N. Caging and Uncaging Genetics. PLoS Biol 14: e1002525, 2016. doi: 10.1371/journal.pbio.1002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowe DA, Degens H, Chen KD, Alway SE. Glyceraldehyde-3-phosphate dehydrogenase varies with age in glycolytic muscles of rats. J Gerontol A Biol Sci Med Sci 55: B160–B164, 2000. doi: 10.1093/gerona/55.3.B160. [DOI] [PubMed] [Google Scholar]
- 42.MacIntosh BR. Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol Sci 18: 222–225, 2003. doi: 10.1152/nips.01456.2003. [DOI] [PubMed] [Google Scholar]
- 43.Martyn DA, Chase PB, Hannon JD, Huntsman LL, Kushmerick MJ, Gordon AM. Unloaded shortening of skinned muscle fibers from rabbit activated with and without Ca2+. Biophys J 67: 1984–1993, 1994. doi: 10.1016/S0006-3495(94)80681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald KS. Ca2+ dependence of loaded shortening in rat skinned cardiac myocytes and skeletal muscle fibres. J Physiol 525: 169–181, 2000. doi: 10.1111/j.1469-7793.2000.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKinnon NB, Connelly DM, Rice CL, Hunter SW, Doherty TJ. Neuromuscular contributions to the age-related reduction in muscle power: Mechanisms and potential role of high velocity power training. Ageing Res Rev 35: 147–154, 2017. doi: 10.1016/j.arr.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol 93: 855–883, 1989. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metzger JM, Moss RL. Kinetics of a Ca(2+)-sensitive cross-bridge state transition in skeletal muscle fibers. Effects due to variations in thin filament activation by extraction of troponin C. J Gen Physiol 98: 233–248, 1991. doi: 10.1085/jgp.98.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metzger JM, Moss RL. Myosin light chain 2 modulates calcium-sensitive cross-bridge transitions in vertebrate skeletal muscle. Biophys J 63: 460–468, 1992. doi: 10.1016/S0006-3495(92)81614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME II, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol (1985) 115: 1004–1014, 2013. doi: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol 5: 369, 2014. doi: 10.3389/fphys.2014.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizunoya W, Wakamatsu J, Tatsumi R, Ikeuchi Y. Protocol for high-resolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Anal Biochem 377: 111–113, 2008. doi: 10.1016/j.ab.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 52.Morris CA, Tobacman LS, Homsher E. Thin filament activation and unloaded shortening velocity of rabbit skinned muscle fibres. J Physiol 550: 205–215, 2003. doi: 10.1113/jphysiol.2003.040899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moss RL. Ca2+ regulation of mechanical properties of striated muscle. Mechanistic studies using extraction and replacement of regulatory proteins. Circ Res 70: 865–884, 1992. doi: 10.1161/01.RES.70.5.865. [DOI] [PubMed] [Google Scholar]
- 54.Moss RL. Effects on shortening velocity of rabbit skeletal muscle due to variations in the level of thin-filament activation. J Physiol 377: 487–505, 1986. doi: 10.1113/jphysiol.1986.sp016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moss RL, Giulian GG, Greaser ML. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. J Gen Physiol 86: 585–600, 1985. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, Reggiani C, Mann M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Reports 19: 2396–2409, 2017. doi: 10.1016/j.celrep.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 57.Nelson CR, Debold EP, Fitts RH. Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. Am J Physiol Cell Physiol 307: C939–C950, 2014. doi: 10.1152/ajpcell.00206.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci 62: 375–381, 2007. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- 59.Persechini A, Stull JT, Cooke R. The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem 260: 7951–7954, 1985. [PubMed] [Google Scholar]
- 60.Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J 19: 1143–1145, 2005. doi: 10.1096/fj.04-3084fje. [DOI] [PubMed] [Google Scholar]
- 61.Power GA, Minozzo FC, Spendiff S, Filion ME, Konokhova Y, Purves-Smith MF, Pion C, Aubertin-Leheudre M, Morais JA, Herzog W, Hepple RT, Taivassalo T, Rassier DE. Reduction in single muscle fiber rate of force development with aging is not attenuated in world class older masters athletes. Am J Physiol Cell Physiol 310: C318–C327, 2016. doi: 10.1152/ajpcell.00289.2015. [DOI] [PubMed] [Google Scholar]
- 62.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12, 2012. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, Frontera WR, Fielding RA. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol 114: 29–39, 2014. doi: 10.1007/s00421-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinett JC, Hanft LM, Geist J, Kontrogianni-Konstantopoulos A, McDonald KS. Regulation of myofilament force and loaded shortening by skeletal myosin binding protein C. J Gen Physiol 151: 645–659, 2019. doi: 10.1085/jgp.201812200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt S. Capturing Genetic Diversity: The Power of the CC and DO Mouse Models. Environ Health Perspect 126: 014003, 2018. doi: 10.1289/EHP2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sonjak V, Jacob K, Morais JA, Rivera-Zengotita M, Spendiff S, Spake C, Taivassalo T, Chevalier S, Hepple RT. Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J Physiol 597: 5009–5023, 2019. doi: 10.1113/JP278261. [DOI] [PubMed] [Google Scholar]
- 68.Straight CR, Ades PA, Toth MJ, Miller MS. Age-related reduction in single muscle fiber calcium sensitivity is associated with decreased muscle power in men and women. Exp Gerontol 102: 84–92, 2018. doi: 10.1016/j.exger.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sundberg CW, Hunter SK, Trappe SW, Smith CS, Fitts RH. Effects of elevated H+ and Pi on the contractile mechanics of skeletal muscle fibres from young and old men: implications for muscle fatigue in humans. J Physiol 596: 3993–4015, 2018. doi: 10.1113/JP276018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sundberg CW, Kuplic A, Hassanlouei H, Hunter SK. Mechanisms for the age-related increase in fatigability of the knee extensors in old and very old adults. J Appl Physiol (1985) 125: 146–158, 2018. doi: 10.1152/japplphysiol.01141.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swartz DR, Moss RL. Strong binding of myosin increases shortening velocity of rabbit skinned skeletal muscle fibres at low levels of Ca(2+). J Physiol 533: 357–365, 2001. doi: 10.1111/j.1469-7793.2001.0357a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sweeney HL, Stull JT. Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal muscle: implications for regulation of actin-myosin interaction. Proc Natl Acad Sci USA 87: 414–418, 1990. doi: 10.1073/pnas.87.1.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor SC, Posch A. The design of a quantitative western blot experiment. BioMed Res Int 2014: 361590, 2014. doi: 10.1155/2014/361590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vigelsø A, Dybboe R, Hansen CN, Dela F, Helge JW, Guadalupe Grau A. GAPDH and β-actin protein decreases with aging, making Stain-Free technology a superior loading control in Western blotting of human skeletal muscle. J Appl Physiol (1985) 118: 386–394, 2015. doi: 10.1152/japplphysiol.00840.2014. [DOI] [PubMed] [Google Scholar]
- 76.Wei L, Gregorich ZR, Lin Z, Cai W, Jin Y, McKiernan SH, McIlwain S, Aiken JM, Moss RL, Diffee GM, Ge Y. Novel Sarcopenia-related Alterations in Sarcomeric Protein Post-translational Modifications (PTMs) in Skeletal Muscles Identified by Top-down Proteomics. Mol Cell Proteomics 17: 134–145, 2018. doi: 10.1074/mcp.RA117.000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Widrick JJ, Norenberg KM, Romatowski JG, Blaser CA, Karhanek M, Sherwood J, Trappe SW, Trappe TA, Costill DL, Fitts RH. Force-velocity-power and force-pCa relationships of human soleus fibers after 17 days of bed rest. J Appl Physiol (1985) 85: 1949–1956, 1998. doi: 10.1152/jappl.1998.85.5.1949. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J, Guy MJ, Norman HS, Chen YC, Xu Q, Dong X, Guner H, Wang S, Kohmoto T, Young KH, Moss RL, Ge Y. Top-down quantitative proteomics identified phosphorylation of cardiac troponin I as a candidate biomarker for chronic heart failure. J Proteome Res 10: 4054–4065, 2011. doi: 10.1021/pr200258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res 76: 1028–1035, 1995. doi: 10.1161/01.RES.76.6.1028. [DOI] [PubMed] [Google Scholar]