Abstract
Both skin and oral mucosa are characterized by the presence of keratinized epithelium in direct apposition to an underlying collagen-dense connective tissue. Despite significant overlap in structure and physiological function, skin and the oral mucosa exhibit significantly different healing profiles in response to injury. The oral mucosa has a propensity for rapid restoration of barrier function with minimal underlying fibrosis, but in contrast, skin is associated with slower healing and scar formation. Modulators of cell function, matricellular proteins have been shown to play significant roles in cutaneous healing, but their role in restoration of the oral mucosa is poorly defined. As will be discussed in this review, over the last 12 years our research group has been actively investigating the role of the profibrotic matricellular protein periostin in tissue homeostasis and fibrosis, as well as healing, in both skin and gingiva. In the skin, periostin is highly expressed in fibrotic scars and is upregulated during cutaneous wound repair, where it facilitates myofibroblast differentiation. In contrast, in gingival healing, periostin regulates extracellular matrix synthesis but does not appear to be associated with the transition of mesenchymal cells to a contractile phenotype. The significance of these findings will be discussed, with a focus on periostin as a potential therapeutic to augment healing of soft tissues or suppress fibrosis.
Keywords: cutaneous healing, drug-induced gingival enlargement, fibrosis, gingiva, oral healing, periostin
INTRODUCTION
Structurally and functionally, skin and the oral mucosa are regarded to be two relatively homologous tissues, with both characterized by the presence of a keratinized epithelium apical to an underlying type I collagen-dense connective tissue, which together function to provide a barrier to microorganisms and other external pathogens. In addition, both skin and oral mucosa show a similar pattern with respect to healing in response to injury. As a result of hemostasis, in mucosal and cutaneous injury, a fibrin-rich granulation tissue forms and matures in composition as the inflammatory phase takes place (92). At the end of inflammation, the wounds transition to the proliferative phase associated with the migration of keratinocytes and fibroblasts onto and into the wound bed, respectively. Fibroblasts secrete extracellular matrix and remodel the granulation tissue, whereas keratinocytes restore barrier function to the region of damage (31, 76).
Although cutaneous and oral tissues follow a similar macroscopic healing process, it has been demonstrated that they in fact exhibit many variations at the cellular and molecular level in relation to cellular processes underlying restoration of tissue architecture and function. Wounds in the oral mucosa have been shown to heal significantly faster, with minimal scar formation in comparison to skin wounds. This observation is concomitant with a reduced inflammatory response primarily attributed to the reduced recruitment of neutrophils, macrophages, and T cells post-injury (15, 33). Several other contributory factors have been proposed to play a role in the speed of healing of the gingiva, including the presence of saliva in the oral cavity, leukocytes, growth factors, and phenotypical differences between oral and cutaneous fibroblasts, as well as the presence of bacteria, that stimulate wound healing (33, 65). However, many of the molecular processes underlying the faster healing of gingiva in comparison with skin remain uncharacterized.
In recent years a plethora of research has demonstrated that the extracellular matrix (ECM) composition is an important determinant of soft tissue healing (116). In particular, matricellular proteins have seen increasing attention due to their role in the direct modification of cell behavior. Defined by Paul Bornstein in 1995 (6), matricellular proteins were classified initially on their ability to modify cell adhesion to the ECM. Surprisingly, initial analysis of matricellular proteins through genetic deletion revealed that many of the mice lacking specific matricellular proteins developed normally and exhibited no major phenotypes in adulthood (80, 96). However, when injured, mice with deletion of several different matricellular proteins exhibited significant defects in healing, which highlighted a potentially important role for matricellular proteins in wound-healing processes.
It is now apparent that whereas fibrin, collagen, and fibronectin provide structural support to tissues during healing, matricellular proteins are upregulated in the granulation tissue, where they modulate the adhesion, migration, proliferation, and differentiation of pericytes, fibroblasts, and epithelial cells up to 15 days post-wounding (62, 76). As a class of proteins, matricellular proteins specifically modulate cell-matrix interactions and cell function (adhesion, spreading, migration, proliferation, and differentiation) (8) by interacting with cell surface receptors (e.g., integrins) and other bioeffector molecules, as well as with structural matrix proteins such as collagens. Certain matricellular proteins exhibit affinity for binding TGFβ1, a growth factor that is important for fibroblast recruitment and differentiation during wound repair. In general, matricellular proteins act in a temporal and spatial manner to control different aspects of wound repair (3, 34, 46, 62, 76, 89, 123).
Because of the relative ease of creating experimental skin injury, much of the research on the role of matricellular proteins in the regulation of healing has focused on skin, rather than tissues such as gingiva. Over the last 12 years, our research group has investigated how matricellular proteins, and in particular periostin, regulate processes underlying the healing of soft connective tissues. As will be discussed in this review, periostin is an important modulator of mesenchymal cell behavior during the proliferative and remodeling phases of healing, although its contrasting effects on dermal and gingival fibroblasts demonstrate the specificity of matricellular protein bioactivity in relatively homologous tissues. Before discussing the role of periostin, we will first define the known differences in fibroblast behavior between skin and gingival connective tissue.
HEALING OF SKIN AND GINGIVAL TISSUE
Despite the apparent similarity of fibroblasts present in connective tissues, not only is there strong evidence now that multiple fibroblast phenotypes exist within one tissue (27, 79), but it also has been shown that cellular phenotypes can be substantially variable among the same tissues but in different anatomic regions (24, 27). Moreover, it is now becoming evident that not all fibroblasts in a tissue arise from the same embryonic origin (17, 93, 107). These phenotypic differences have been proposed to be partially responsible for the different healing patterns of the tissue (65). Recently, Mah et al. (71) investigated the relation between the distinct phenotypes of gingival fibroblasts and skin fibroblasts and their different wound-healing patterns in three-dimensional (3D) cell cultures. Of significance is that they observed that gingival fibroblasts proliferate faster and express higher levels of molecules involved in modulation of inflammation and ECM remodeling (MMP-1, -3, and -10 and TIMP-4) compared with dermal fibroblasts. In contrast, dermal fibroblasts exhibited significantly higher expression of fibrillar (collagens and elastin) and nonfibrillar (SLRPs and matricellular proteins) ECM proteins as well as molecules involved in TGFβ induction of the myofibroblast phenotype and cell contractility [TGFβ1, -β2, and -β3, Smad, α-smooth muscle actin (α-SMA), C-X-C motif chemokine 12 (CXCL12), Cadherin-2 and -11]. Their findings are indicative that gingival fibroblasts display a phenotype that may promote faster resolution of inflammation and ECM remodeling, which is associated with reduced scar formation, whereas skin fibroblasts have a profibrotic, scar-prone phenotype (71).
Another potential difference between skin and gingiva that could account for faster wound healing in the oral mucosa is the observation that mucosal fibroblasts exhibit an increased migration rate. It has been shown that buccal mucosal fibroblasts migrate to a greater extent into a collagen matrix than dermal fibroblasts, and in a 3D culture system, the number of buccal fibroblasts that repopulated the artificial wound bed was found to be significant higher when compared with dermal fibroblasts (103). Faster invasion of granulation tissue in vivo would greatly increase the maturation of the defect, which secondarily would also further enable faster re-epithelialization by providing appropriate ECM to facilitate epithelial migration.
With respect to cell adhesion properties, gingival fibroblasts exhibit higher adherence on vitronectin and collagen type I and IV than dermal fibroblasts (88). Oral fibroblasts have also been reported to be highly responsive to TGFβ1, the major profibrotic cytokine expressed during healing, with TGFβ1 increasing proliferation rates as well as collagen synthesis, when compared with dermal fibroblasts (64). Finally, and most relevant to the topic of this review, differences in ECM composition have been quantified between oral mucosa and skin, with the former found to express more fibronectin, fibronectin ED-A, and chondroitin sulfate and less elastin (29). It has been shown that fibronectin ED-A, an alternative spliced isoform de novo expressed during wound healing and fibrotic changes, is highly upregulated in myofibroblasts (121). Earlier reports from Serini et al. (102) suggested that fibronectin ED-A acts as a necessary ECM component for TGFβ induction of myofibroblast differentiation. Additional evidence for the role of fibronectin ED-A in myofibroblast activation has been demonstrated via the latent TGFβ binding protein-1 (LTBP-1)/EDAFN/integrin interaction network, where fibronectin immobilizes LTBP-1 and thus stores TGFβ1 in the ECM (57).
It is now very apparent that the composition of the matrix, and in particular the presence or absence of matricellular proteins, is a primary determinant of cell phenotype in both health and disease. Over the last decade, the focus of our research has been to understand the cell and molecular mechanisms underlying the healing of soft tissues, with a major emphasis on skin and gingiva. As will be discussed, our focus has been on matricellular proteins and in particular the pro-fibrotic periostin.
PERIOSTIN AS A MODULATOR OF CELL-ECM INTERACTIONS
Matricellular proteins are transiently expressed nonstructural proteins (8). First described in 1995, periostin was initially identified as an 811-amino acid protein secreted by murine osteoblasts that was required for cell adhesion (110). Originally termed osteoblast-specific factor 2 (OSF-2) because of its localization to the periodontal ligament and periosteum of mice (66), it was renamed periostin in 1999 and subsequently classified as a matricellular protein in 2007 by Norris et al. (84). Structurally, periostin is a secreted 90 kDa N-terminus glycosylated protein containing four tandem fascilin domains (60), with several different isoforms described (2, 11, 26, 77). The physiological significance of periostin expression in adult systems has been identified mainly in collagen-rich, biomechanically active tissues (34) such as the skin, bone, and heart, periodontium, and periodontal ligament and within developing teeth (59), highlighting a subtle but complex role for periostin in tissue homeostasis, healing, and pathology.
Although periostin-knockout mice are viable, the deletion manifests in loss of architecture and functional disruption of several collagenous-based tissues, particularly those subject to constant mechanical loading (95). We and others performed microcomputed topography (μCT) imaging analysis on periostin-knockout mice skulls, which revealed the presence of severe periodontal disease, significant reduction in bone density, and structural defects in the incisors and showed that orbital bones are completely missing or fail to fuse properly (34). Removal of masticatory forces reduced this damage, showing that the loss of periostin likely impairs the ability of the tissue to withstand mechanical loading (96). It has been postulated that this relates to collagen fiber assembly, although the exact role of periostin is yet to be definitively proven.
Like many matricellular proteins, periostin has been defined as a nonstructural ECM component, although as research has continued, evidence has accumulated demonstrating that it can modify the biomechanical properties of collagenous based tissues. Periostin’s multidomain structure, with an amino-terminal EMI domain, a tandem repeat of four FAS 1 domains, and a carboxyl-terminal domain, allows for numerous interactions with extracellular/secretory proteins (50). Specifically, periostin has been implicated in processes involved in stabilization of the extracellular matrix, including collagen fibrillogenesis and cross-linking; loss of periostin results in reduced collagen fiber diameter and strength in knockout animals (44, 84). Periostin is also known to act as a scaffold for assembly of several extracellular matrix proteins (type I collagen, fibronectin, tenascin-C, and laminin γ2) as well as accessory proteins (BMP-1 and CCN3) (20, 49, 61, 73, 97), although the binding site for collagens has not been yet identified (50, 84) Among the structural components of periostin, the EMI domain has attracted significant scientific interest, as it is involved in cellular signaling and protein-protein interaction (50). The EMI domain, named after its presence in the EMILIN family of extracellular matrix glycoproteins, is a cysteine-rich sequence of ∼80 amino acids that is most often found at the amino terminus of extracellular proteins that are forming or are compatible with multimer formation (12, 16). Extracellularly, periostin directly binds to fibronectin through the EMI domain (49, 84). Fibronectin has several interaction sites for collagens, suggesting that the EMI domain of periostin may indirectly interact with collagens (50). Intracellularly, a proximal localization between periostin and fibronectin in the endoplasmic reticulum of fibroblastic cells has been observed, indicating that the two proteins interact before fibronectin’s secretion (51), and evidence suggests that periostin enhances secretion of fibronectin from the endoplasmic reticulum into the extracellular environment (51). In totality, this information demonstrates a critical role for periostin in extracellular matrix homeostasis and the regulation of cell phenotype. Our analysis of cutaneous and gingival healing has highlighted further the influence of periostin on cell behavior, demonstrating contrasting roles related to the proliferative phase of healing.
PERIOSTIN IN CUTANEOUS WOUND REPAIR: CONTRACTION
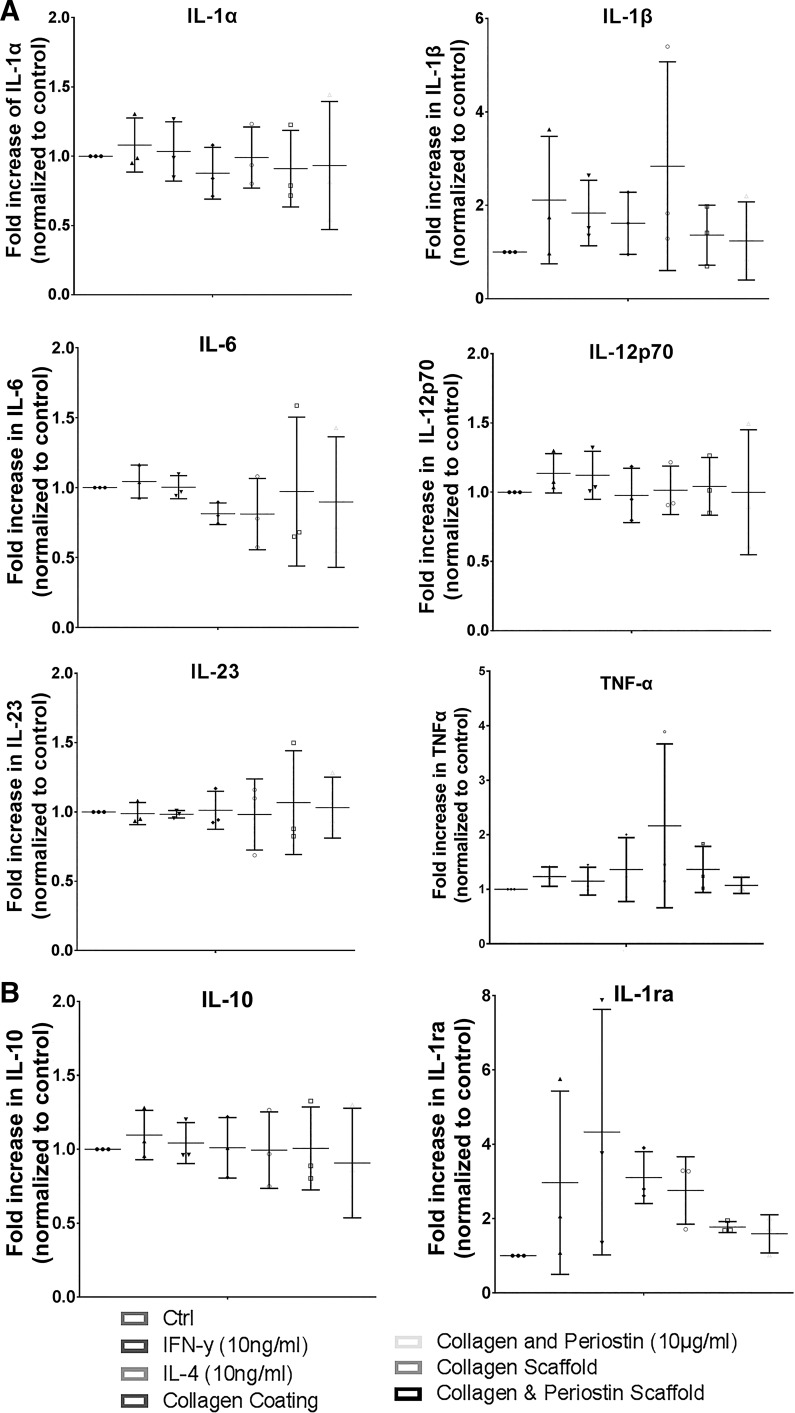
In conjunction with Dr. Simon Conway and colleagues (123) at the University of Indiana, we first described that periostin is prominently expressed during skin development localizing to the dermis, basement membrane, and hair follicles from embryonic through neonatal stages but in postnatal mice only in the basement membrane and hair follicles. More specifically, our findings indicate that in normal, unwounded adult skin, periostin localizes predominantly to the basal epidermis. Using a wild-type mouse model, we first described that after creation of full-thickness excisional wounds, periostin was initially detectable in the developing granulation tissue 3 days post-wounding, with immunoreactivity peaking on day 7, where it was concomitant with maximal α-smooth muscle actin expression, the definitive marker for myofibroblasts (46). The well-established role of periostin in the regulation of inflammation in several pathological conditions in various tissues and systems, such as in the airways (asthmatic and allergic inflammation (1, 5, 45, 74, 81), in hepatic (42), intestinal (58), and renal inflammation (70), and in atherosclerotic lesions (37, 101), has suggested that it could play a role in the inflammatory phase of cutaneous wound healing. However, we found that periostin expression was not colocalized with the highest infiltration of CD68+ macrophages, suggesting that it is not associated with the inflammatory phase of healing in acute wounds (46). The role of periostin in inflammatory processes remains controversial, particularly as it relates to acute healing (18, 21, 23, 34) and fibrosis (53–55). Previous data from excisional skin punch biopsy models demonstrate that macrophages are the dominant immune cells present in skin healing not only during the proliferative phase (67) but also during the remodeling phase during which macrophages ingest cell debris and assist in the degradation of excess ECM in and around the wound bed (31). To assess the role of periostin on macrophage phenotype, we cultured the monocytic cell line THP-1 on periostin-coated plates or electrospun periostin/collagen scaffolds, with a focus on cytokine production associated with M1 or M2 polarization (Fig. 1). In these experiments, THP-1 monocytes were activated using phorbol 12-myristate 13-acetate, which results in cells that more closely resemble peripheral blood mononuclear cell monocyte-derived macrophages. Our analysis shows that the presence of periostin does not influence cytokine or chemokine secretion by activated THP-1 cells, which, in combination with our results in mice demonstrating that inflammatory infiltrate was similar in WT and periostin−/− excisional wounds, suggests an apparent insignificant role for periostin in the inflammatory phase of wound repair process.
Fig. 1.
Cytokine secretion by activated THP-1 monocytes cultured on periostin matrices. Relative levels of cytokines present in cell culture supernatants between 48 and 72 h of culture compared with controls on tissue culture plastic alone. A: M1 polarization-associated cytokines do not differ significantly from controls despite the addition of recombinant IFNγ or IL-4. The presence of collagen coating, periostin/collagen coating, collagen scaffold, or periostin/collagen scaffold did not influence cytokine production. B: cytokines characteristic of M2 polarized macrophages. There was no significant difference in levels of IL-1ra or IL-10 produced in response to IFNγ, IL-4, coatings, or scaffolds. Matched 1-way ANOVA, Bonferroni posttest for multiple comparisons; n = 3. Ctrl, control.
At the FASEB Matricellular meeting in Snowmass in 2010, a meeting with Dr. Akira Kudo, a leading researcher on periostin, led to the serendipitous discovery that both of our research groups were simultaneously assessing the role of periostin deletion on excisional wound healing. A fruitful discussion revealed that we were both focused on different aspects, our group on contraction and the Kudo group on re-epithelialization. Of significance is that both of our research groups were using different derivations of the periostin-knockout (periostin−/−) mouse, although both were in a C57BL/6 genetic background. In the derivation of the Kudo group’s periostin-knockout mice, during the construction the neo gene was removed from the periostin-deficient mice (98). After birth, the periostin−/− mice were healthy, with defects in eruption of incisors noted, although no alterations in cardiac morphology or ventricular performance were described (48). The alterations in tooth morphology and incisor eruption were similarly described in great detail in the knockout mice from the Conway laboratory, with a periodontal disease-like phenotype described (95). In the Conway derivation of the mouse, their deletion removed the DNA encoding the translation start site and the first exon, as well as 300 bp of the first intron of the periostin sequence, replacing it with the bacterial-galactosidase reporter gene. As a result, homozygous embryos with two copies of the lacZ-neo cassette were null for the periostin gene.
First to publish their findings, Nishiyama et al. (83), in 2011, created full-thickness excisional wounds in periostin−/− and wild-type (WT) mice, and they found a delay in wound repair in periostin−/− mice due to impaired re-epithelialization. These authors attributed this defect in re-epithelialization to a reduction in the proliferation of keratinocytes surrounding the hair follicles in periostin−/− mice. However, no direct in vitro test of the loss of periostin on keratinocyte proliferation was reported, and overexpression of murine periostin in a HaCaT cell line resulted only in an increase in proliferation but not in migration, as shown through scratch wound assays (83). Although Nishiyama et al. (83) had described a macroscopic alteration in wound contraction, Dr. Kudo, recognizing the advanced stage of our analysis, agreed that our paper, not theirs, should focus on contraction.
As we had previously shown that periostin expression is absent during inflammation and its onset corresponded with the proliferative and remodeling phases of healing and an increase in α-SMA protein (46, 123), we investigated the role of periostin on cellular processes related to mesenchymal cell recruitment into the granulation tissue and the transition of these cells to contractile, α-SMA-positive myofibroblasts (21). Using the Conway laboratory’s derivation of the periostin−/− mouse strain (95), of importance, we found similar wound closure kinetics to that reported by Nishiyama et al. (83); the different derivations of the mice adding robustness to our mutual findings. More specifically, we showed that the loss of periostin resulted in impaired wound closure repair, which was manifested during the profibrotic phase of healing. The alteration in wound closure corresponded with the onset and peak of periostin expression in periostin WT animals on day 7 post-wounding. Acta2 (the encoding gene of α-SMA) is expressed in and associated with pericytes and myofibroblasts, which are key during wound healing and responsible for ECM synthesis, remodeling, and tissue contraction (25, 39, 111). Assessment of α-SMA in periostin−/− and WT wounds using immunohistochemistry showed that α-SMA was evident at the wound edge and within the granulation tissue of WT wounds on days 5 and 7; increased levels of α-SMA were detected at the wound border throughout the granulation tissue and in blood vessel walls (Fig. 2). Histochemical and mRNA analysis of periostin−/− wounds revealed that α-SMA was strikingly reduced in the granulation tissue on day 7 post-wounding, whereas fibroblast recruitment within the wound, collagen expression, and canonical TGFβ signaling were unaffected. This suggested that altering the defined wound closure kinetics in periostin−/− might be due to a reduction in α-SMA-positive myofibroblasts within the maturing granulation tissue and thus reduced wound contraction. Interestingly, the observed deficit in α-SMA immunoreactivity was specific to the granulation tissue of periostin−/− wounds, with wound borders positive for myofibroblasts (Fig. 2), indicating that the increased stiffness of the wound edge provides a conducive environment for myofibroblast differentiation, even in the absence of periostin (21). Further evidence for this hypothesis was attained by culturing adult dermal fibroblasts derived from the mice on tissue culture plastic, where both cells from WT and periostin−/− animals assumed a myofibroblast phenotype. The impaired contractile phenotype of periostin−/− cells only manifest in vitro when the cells are cultured in collagen gels, with the exogenous addition of human recombinant periostin (rhPN) to the collagen gels sufficient to rescue the defects in collagen gel contraction and myofibroblast differentiation. Addition of antibodies to β1-integrins or PP2 (inhibit Src/FAK signaling) inhibited this exogenous periostin-mediated contraction, demonstrating that periostin induces myofibroblast differentiation through noncanonical TGFβ signaling (21).
Fig. 2.
Localization of α-smooth muscle actin (α-SMA) in periostin−/− and wild-type mice 7 days after creation of full-thickness excisional wounds in the skin. In wild-type mice, α-SMA is present at the edge of the wounds and in the granulation tissue, but in periostin−/− animals, α-SMA is localized to the wound edge only, the region of highest stiffness. KO, knockout.
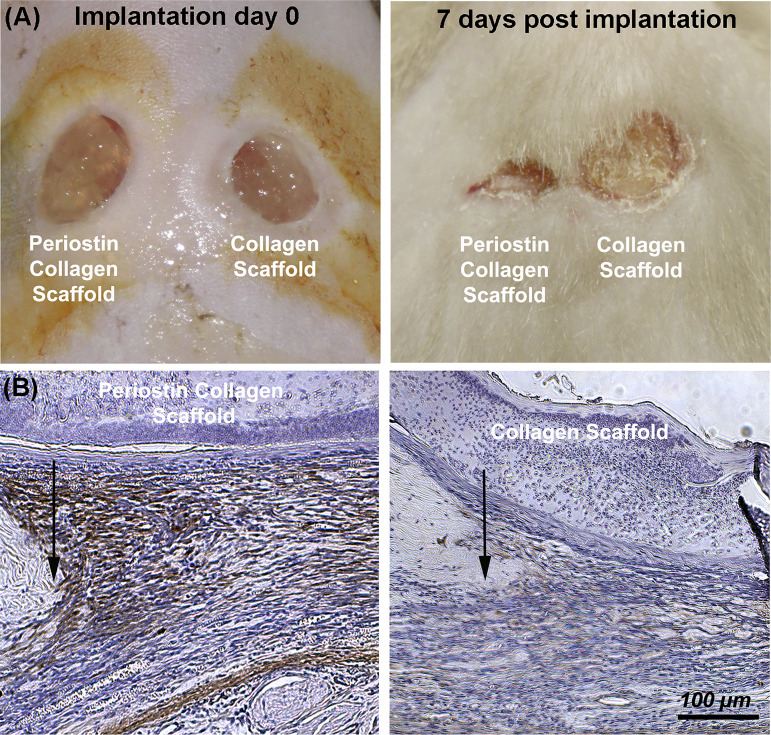
The ability of rhPN to recover the myofibroblast phenotype was extended to in vivo punch wounds in our murine model, where delivery of exogenous rhPN via an electrospun periostin-collagen scaffold resulted in increased α-SMA immunoreactivity in the granulation tissue of day 7 of periostin−/− wounds compared with wounds receiving collagen scaffolds alone (21) (Fig. 3). Further supporting our findings that periostin is involved in the regulation of cellular events required for the proliferative/profibrotic phase of repair of skin healing (including cell recruitment, matrix contraction, and fibroblast to myofibroblast differentiation), we evaluated the effect of periostin/collagen and CCN2/collagen electrospun scaffolds in a murine db/db diabetic model of impaired cutaneous healing, as they represent a significant and growing complication associated with type 2 diabetes (23). These chronic wounds are characterized by a state of persistent hyperinflammation, impaired recruitment of fibroblasts, keratinocytes, pericytes, and endothelial cells, and the inability of fibroblasts to differentiate to myofibroblasts, which prevent the progression of healing to the proliferative and remodeling phases of wound healing (19, 20, 36).
Fig. 3.
Addition of periostin in an electrospun scaffold is sufficient to rescue α-smooth muscle actin (α-SMA) expression in periostin−/− mice. A: representative wounds are shown at 0 and 7 days post-implantation. At 7 days post-implantation, wounds receiving periostin/collagen were significantly smaller than collagen scaffolds alone, which was concomitant with increased α-SMA protein levels (B).
In 2009, we began retrieving human tissue from nonhealing skin ulcers, with our analysis showing that periostin expression is almost completely suppressed in the wound bed of human nonhealing skin lesions and is correlated with a complete absence of myofibroblasts in the tissue (19). Based on our ability to rescue the α-SMA defect in periostin−/− mice with electrospun scaffolds containing periostin, we extended these studies to a murine diabetic model of impaired excisional wounding, demonstrating that both periostin/collagen and CCN2/collagen electrospun scaffolds increased excisional wound closure rates when compared with collagen scaffolds alone or untreated wounds. This observation was associated with reduced neutrophil infiltration and concomitant with an increase in mesenchymal cell infiltration and significantly increased revascularization of the wound bed (23). Therefore, both our in vivo and in vitro results provide compelling evidence that periostin modulates myofibroblast differentiation during excisional would repair in low-stiffness environments (developing granulation tissue), and exogenous rhPN is sufficient to rescue this phenotype through a β1-integrin and focal adhesion kinase (FAK)-dependent mechanism (14, 21, 114, 123).
In contrast to the study of Nishiyama et al. (82), we did not observe a difference in re-epithelialization rates between WT and periostin−/− wounds. Although the percentage of epithelialization appeared to be lower in periostin−/− wounds due to the fact that the wound size was greater in periostin−/− animals, the total epithelial migration distance was actually similar between genotypes. However, this does not reduce the possibility that exogenous periostin could enhance re-epithelialization, although this hypothesis remains yet to be definitively tested.
Aside from the increasing evidence from loss-of-function murine models, the functional role of elevated periostin in the process of wound healing has been only recently investigated, using a periostin transgenic mouse model of constitutively overexpression of periostin (85). Full-thickness excisional wounding in these animals resulted in impaired healing, as demonstrated by the delayed wound closure. These observations were associated with a significant reduction of proinflammatory cytokines, IL-1b and TNFα, and impaired inflammatory cell infiltration before delayed healing, suggesting that regulated spatiotemporal expression of periostin is important for efficient wound healing and that constitutive periostin overexpression interrupts the normal process of wound closure (85).
In summary, the studies to date have shown a pivotal role for periostin in the contraction of granulation tissue in excisional skin models. Based on its apparent regulation of cellular contractility in the dermis, not surprisingly, overexpression of periostin is also associated with skin fibrosis.
PERIOSTIN EXPRESSION AS A MARKER OF SKIN FIBROSIS AND SCARRING
At a base level, fibrosis is characterized by the abnormal production and excessive deposition of ECM molecules, such as collagen, fibronectin, and connective tissue growth factor by myofibroblasts (115). Differentiation of fibroblasts into myofibroblasts requires TGFβ-dependent signaling but also depends heavily on matrix stiffness; increased stiffness in ECM acts as a positive feedback to increase cell contractility (38, 43, 111). Mounting evidence demonstrates a significant role for periostin in this process. Specifically, periostin is one of the highest transcripts associated with excessive scarring and keloid scars (14, 69, 106, 109, 122) and systemic sclerosis (120). A growing body of research, including work from our group (123), demonstrates that periostin protein is overexpressed throughout the dermis of hypertrophic and keloids scars (14, 69). In vitro, keloid and hypertrophic scar fibroblasts also express high levels of periostin compared with normal human dermal fibroblasts, suggesting epigenetic changes in the cells (14, 69). When hypertrophic scar fibroblasts were treated with exogenous rhPN, a significant increase in their proliferation rate was observed, which was not evident in healthy dermal fibroblasts (21). Additionally, periostin induced significant increases in super mature focal adhesion formation, α-SMA levels, and collagen contraction in hypertrophic scar fibroblasts under conditions of increased matrix tension in 3D type-I collagen lattices, effects that were significantly attenuated by the pharmacological inhibition of Rho-associated protein kinase (ROCK) activity. Further evidence supporting the role of periostin in scar fibroblasts emerges from depletion studies of endogenous periostin expression in hypertrophic scar myofibroblasts, which showed a sustained decrease in α-SMA levels under conditions of reducing matrix tension, whereas matrix-associated periostin levels caused the cells to retain high levels of α-SMA under these conditions (14). Parallels can be drawn with our initial assessment of periostin protein in excisional repair, which demonstrated a switch from the intra- to extracellular compartment upon injury, with protein levels returning to baseline at 28 days poswounding (46). As these studies indicate that prolonged extracellular periostin promotes myofibroblast persistence, it demonstrates that the continued presence of periostin protein in the ECM is a powerful regulator of scarring and fibrosis. By acting back on the cells that secreted it, a phenomenon termed dynamic reciprocity by Paul Bornstein (7) and known to play a role in the wound microenvironment (100), it is evident that strategies that reduce or eliminate periostin expression could be a target for the treatment of fibrotic conditions.
The use of neutralizing antibodies against periostin has been investigated mainly in the context of cancer invasion and metastasis. Recent reports provide promising results for the potential use of highly specific anti-periostin antibodies to inhibit metastatic and migratory properties of periostin-expressing cells and stroma in ovarian (124) and breast cancer (113).
More recently, efforts have been shifted to the utilization of aptamers as a therapeutic target for periostin. Aptamers are single-stranded oligonucleotides that bind to specific target molecules. Once bound to a specific target, some aptamers are capable of inhibiting its activity (105). Data from in vivo and in vitro studies demonstrated favorable results for the application of periostin-binding aptamers for the specific inhibition of periostin in peritoneal (82) and renal (112) fibrosis. Local administration of molecules that target and block periostin during wound healing could also be achieved through tissue bioengineering techniques. Biomaterials, such as different types of scaffolds, can be designed to achieve temporal and spatial control over the release of such inhibiting molecules (90) and provide a promising therapeutic solution for cutaneous fibrosis in scarring post-wounding.
Whereas excessive contraction is a hallmark of fibrotic conditions, of equal significance is accumulation of extracellular matrix. Interestingly, neither study on excisional healing in periostin−/− mice defined or characterized a defect in matrix production, suggesting that periostin is not involved in ECM synthesis in acute skin healing. It was at this juncture where we became interested in whether periostin would be expressed in healing of the oral mucosa based on the lack of scarring and contraction evident in this tissue post-injury.
PERIOSTIN IN ORAL CAVITY
Originally described in the oral cavity within the periodontal ligament, periostin is highly expressed in developing teeth at epithelial-to-mesenchymal interaction sites (95) in the periodontal ligament (PDL) and periosteum (Fig. 4) (95, 117). Periostin has also been identified in several pathological conditions, including our analysis of the stroma and differentiated odontogenic epithelium of human ameloblastic fibromas, ameloblastic fibro-odontomas, and odontomas (13), in peripheral and central ossifying fibromas, focal cemento-osseus dysplasia, and fibrous dysplasia (13) as well as oral squamous cell carcinoma (104).
Fig. 4.
Expression of periostin in the healthy periodontium. Periostin immunoreactivity is predominantly associated with the periodontal ligament and alveolar bone. AB, alveolar bone; CT, connective tissue; D, dentine; P, pulp. Black arrows indicate the periodontal ligament.
In contrast to skin, as described previously, gingival tissue typically heals with significantly less scarring, with many similarities evident to the healing of fetal tissue (32, 63, 71, 118). Gingival tissue has been shown to have reduced levels of TGFβ mRNA and protein and exhibits reduced wound contraction compared with skin in pig models (72). Interestingly, gingiva wounds heal faster than skin even in the same animal. As mentioned above, there is considerable amount of evidence showing that fibroblast populations in the oral cavity are inherently phenotypically different from dermal fibroblasts. Oral fibroblasts are less responsive to TGFβ1 stimulation, which results in less α-SMA expression compared with dermal fibroblasts in vitro (68, 75). Studies have also shown that gingival fibroblasts exhibit less of a profibrotic or scarring phenotype in three-dimensional culture, increased synthesis of levels of genes associated with ECM remodeling and inflammation (71). Because the macroscopic pattern of healing is similar in skin and gingival tissue, we investigated the expression of periostin in the repair of gingivectomy defects, which is the oral cavity equivalent of a full-thickness excisional skin wound in rats (55) (Table 1). Although assessment of gingival healing in the periostin−/− mice would be the most direct method, it is technically challenging due to the size of the gingiva, and any defect created in the gingival tissue heals rapidly, limiting time points for analysis. As such, the use of periostin−/− mice in gingival healing experiments has yet to be performed.
Table 1.
Comparison of skin and gingiva wound healing
| Cutaneous Wound Healing | Gingival Wound Healing | |
|---|---|---|
| Fibroblasts | ↑Expression of ECM molecules α-SMA-positive Reduced migratory ability (103) |
Fetal-like phenotype (108) Higher remodeling capacity (108) Increase migration ability (65) Higher proliferation rates (71) |
| Inflammation | Reduced inflammatory response (33) | |
| ECM components (71) | ↑Collagens, SLRPs, matricellular proteins ↑TGFβ1–3, Smad, α-SMA |
↑Fibronectin ED-A ↓Elastin ↓Collagen I, III |
| Epithelial closure | Faster re-epithelialization (99) | |
| Periostin Expression | Granulation tissue-proliferating phase (18, 21) | Granulation tissue-proliferating phase (54) |
| Periostin-null phenotype | Delayed wound closure | Not tested directly |
| Role of periostin during healing | Myofibroblast differentiation and contraction, via β1 integrin, Src and FAK (21) | Fibronectin synthesis via FAK/JNK signaling (55) |
α-SMA, smooth muscle actin; ED-A, extradomain A-containing fibronection; FAK, focal adhesion kinase; SLRPs, small leucine-rich proteoglycans. ↑Higher/more; ↓less/reduced when compared with the other.
PERIOSTIN IN GINGIVAL HEALING: INDUCTION OF ECM SYNTHESIS
Aside from describing periostin expression in gingival healing for the first time, in general, our gingivectomy model provided further evidence that supports that gingival healing differs from skin healing patterns; epithelial proliferation and migration results in restoration of barrier function within 3 days, whereas proliferation in the connective tissue occurs predominantly at 3 and 7 days post-wounding (55). As such, the epithelium has a competitive advantage over fibroblasts, which is known to cause significant problems in restorative and implant dentistry.
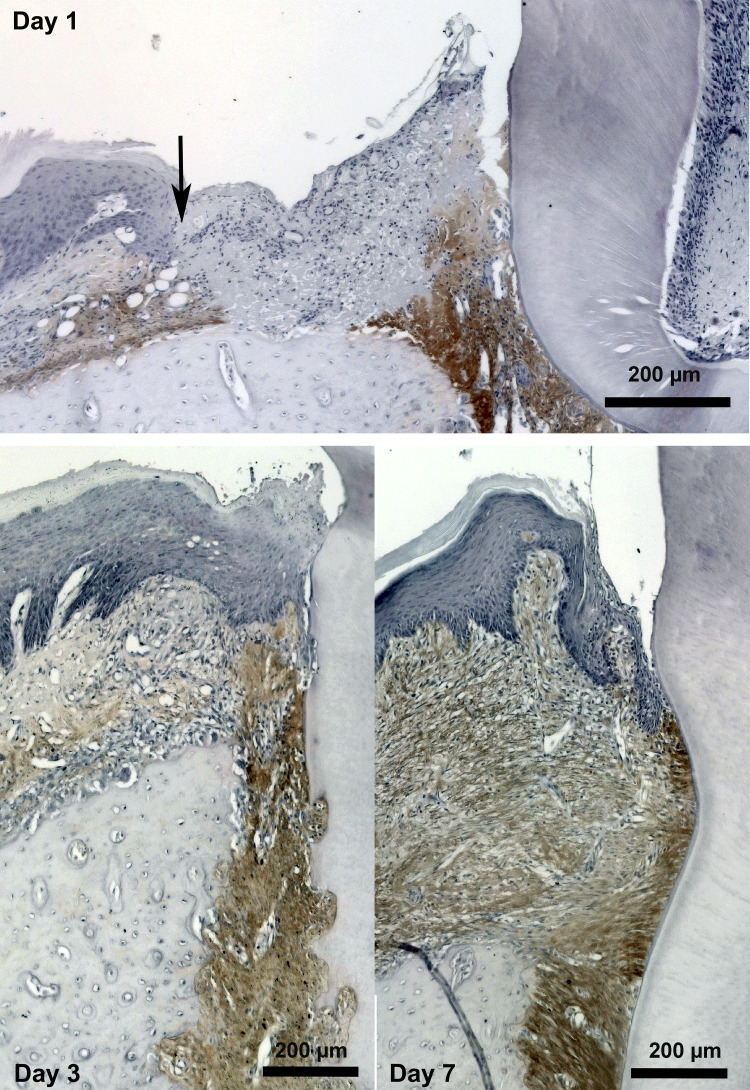
As anticipated, periostin expression was transiently induced during gingival healing (55); immunoreactivity became evident 3 days post-wounding (Fig. 5) and persisted through 14 days, when the tissue was healed and sulcular structure of the tissue re-established. Of surprise is that immunoreactivity for α-SMA was not evident during the healing process, limited to blood vessels only after gingivectomy. In complete contrast to our findings in skin with respect to contraction, we turned to in vitro culture models to investigate the influence of periostin on oral derived fibroblasts. Using a human gingival fibroblast model, we established that exogenous rhPN resulted in increased fibronectin and collagen synthesis, an effect that could be attenuated by pharmacological inhibition of FAK and JNK signaling. Because inhibition of FAK also inhibited fibroblast to myofibroblast transition of dermal fibroblasts, it suggested that fibroblast heterogeneity plays a role in the influence of periostin on cellular function. In addition to FAK, both dermal fibroblasts and gingival fibroblasts predominantly attach to periostin through β1-integrins (21, 55). Of great interest is the finding that the addition of rhPN did not induce myofibroblast differentiation or proliferation of gingival fibroblasts, in contrast to skin and hypertrophic scar fibroblasts as reported by other studies (14, 69). The inability of gingival fibroblasts to transition to myofibroblasts even in the presence of periostin may provide a possible explanation of scarless healing that is evident in attached gingiva compared with skin.
Fig. 5.
Periostin is upregulated in the connective tissue of the oral mucosa after injury. Gingivectomy surgeries were performed in rats, and periostin was assessed using specific antibodies. At 1 day post-surgery, periostin immunoreactivity is evident in the periodontal ligament (PDL) and in the connective tissue at the edge of the wound (black arrow). By day 3, periostin protein is evident in the developing granulation tissue, with increased immunoreactivity on day 7.
Periostin undergoes alternative mRNA splicing, generating several COOH-terminal domain variants (41, 110). In humans and mice, periostin undergoes alternative splicing in its COOH-terminal region, resulting in seven specific isoforms (molecular masses from 80 to 93 kDa) that can be observed in a broad range of tissues such as thyroid gland, PDL and cardiac tissue, as well as in cancers including osteosarcoma, ovarian, pancreatic, colon, breast, and non-small cell lung cancer (2, 26, 28, 40, 41, 78, 91, 110). We recently found that in rat gingiva, two isoforms are detected on days 3, 7 and 14 post-wounding, with the smaller isoform (500 bp size) being more prominent than the isoform that seems to correspond to the full length (55). There is a lack of understanding on types and function of periostin isoforms in rats. Previously performed sequence analysis demonstrated that the dominating periostin isoform encompasses exons 16, 18, 19, 20, 21, and 23 (87). This isoform size corresponds to the prominent periostin isoform found in our study. Results from numerous reports suggest that species- and tissue-specific isoforms of periostin play important roles in the regulation of tissue-specific functions (119). Further studies are needed to focus on the significance of these two different isoforms present in gingival healing, as well as specific isoforms present in cutaneous healing that could provide a potential explanation of the different roles of periostin in these tissues.
From a mechanistic standpoint, as both gingival and dermal fibroblasts express β1 integrins, and all other signal transduction molecules associated with α-SMA expression, alterations in their response to periostin must have other origins. Interestingly, gingival fibroblasts even in culture are very resistant to adopting a myofibroblast phenotype. We have previously shown that when cultured on titanium, only ∼8% of the fibroblast population expresses α-SMA and incorporates it into stress fibers (56). Because surface stiffness is not sufficient to drive myofibroblast differentiation as occurs in dermal fibroblasts, it appears that intracellular alterations that prevent α-SMA expression exist. Of note, Guo et al. (30) demonstrated that microRNAs play a role, with miR-218 required for focal adhesion kinase-dependent TGFβ signaling in gingival fibroblasts. Based on our work, it appears that the presence of periostin is not able to overcome the reduced expression of miR-218 in gingival cells, possibly resulting in reduced α-SMA expression.
From these findings in gingival tissue and the well-described role of periostin in fibrosis, we next investigated whether either periostin or α-SMA is associated with gingival fibrosis.
PERIOSTIN AS A REGULATOR OF FIBROSIS OF THE ORAL MUCOSA
Although gingival tissue typically heals without scarring, it is still associated with several fibrotic conditions, including drug-induced gingival enlargement (DIGE), which we selected as a model system. DIGE is a pathological condition that develops as a side effect from the systemic administration of the antihypertensive drug nifedipine and antiseizure drug phenytoin, and it is classified as a fibrotic lesion (10, 86). As such, it is characterized by imbalance in the remodeling and deposition of ECM as well as the supposed persistence of myofibroblasts (53).
We first confirmed that periostin is indeed associated with gingival connective tissue from patients with nifedipine-induced gingival enlargement when compared with healthy individuals (52). We did not, however, detect the presence of any α-SMA-positive fibroblasts in tissue biopsies from patients with the condition. Labeling of the tissues for the transcription factor p-SMAD2/3, which is associated with canonical TGFβ signaling, demonstrated nuclear localization in both HGFs and oral epithelial cells in gingival tissues obtained from patients with nifedinpide-induced gingival enlargement (NIGE), but not in cells in control healthy tissue demonstrating activity of TGFβ. To establish whether nifedipine could indeed induce periostin upregulation and p-SMAD2/3 nuclear translocation, HGFs treated with different concentrations of nifedipine showed significantly increased periostin mRNA and protein levels, which correlated with increased levels of active TGFβ and increased phosphorylation and nuclear localization of SMAD3. Blocking of canonical TGFβ signaling through inhibition of the TGFβ receptor I with SB431542 significantly reduced nifedipine-induced SMAD3 phosphorylation and periostin expression, demonstrating for the first time that nifedipine upregulates periostin in HGFs in a TGFβ-dependent mechanism (52). More recently, we demonstrated that treatment of HGF culture with different concentrations of phenytoin also resulted in increased periostin protein levels, which correlated with p-SMAD3 phosphorylation, suggesting a common mechanism responsible for DIGE irrespective of the drug (54). Based on the fact that DIGE is defined as a fibrotic lesion, the molecular pathology is in direct contrast to skin scarring in that no differentiation or persistence of myofibroblast is associated with the condition.
CONCLUSIONS
Initially defined as a preosteoblast adhesion factor in 1993, 25 years later, novel roles for the matricellular protein periostin are still being described. As we highlight in this review, through β1-integrin-FAK signaling, periostin stimulates contraction, but not ECM synthesis, in dermal fibroblasts, with the exact opposite finding in the case of gingival fibroblasts. Although our findings provide yet further evidence for fibroblast heterogeneity between skin and the oral mucosa tissues, the absence of myofibroblast differentiation during both healing and scarring of the gingiva suggest significant epigenetic differences compared with dermal fibroblasts. Therefore, it would be intriguing to assess the role of periostin in tissues such as the hard palate, which, although still in the oral cavity, is strongly associated with scarring after injury.
GRANTS
The work is funded by the Canadian Institutes of Health Research Operating Grant to D. W. Hamilton.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.N., K.C., and D.W.H. performed experiments; G.N. and D.W.H. analyzed data; K.C. interpreted results of experiments; K.C. prepared figures; G.N. and D.W.H. drafted manuscript; G.N. and D.W.H. edited and revised manuscript; G.N., K.C., and D.W.H. approved final version of manuscript.
REFERENCES
- 1.Asano T, Kanemitsu Y, Takemura M, Yokota M, Fukumitsu K, Takeda N, Ichikawa H, Uemura T, Takakuwa O, Ohkubo H, Maeno K, Ito Y, Oguri T, Maki Y, Ono J, Ohta S, Nakamura Y, Izuhara K, Suzuki M, Niimi A. Serum Periostin as a biomarker for comorbid chronic rhinosinusitis in patients with asthma. Ann Am Thorac Soc 14: 667–675, 2017. doi: 10.1513/AnnalsATS.201609-720OC. [DOI] [PubMed] [Google Scholar]
- 2.Bai Y, Nakamura M, Zhou G, Li Y, Liu Z, Ozaki T, Mori I, Kakudo K. Novel isoforms of periostin expressed in the human thyroid. Jpn Clin Med 1: 13–20, 2010. doi: 10.4137/JCM.S5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu A, Kligman LH, Samulewicz SJ, Howe CC. Impaired wound healing in mice deficient in a matricellular protein SPARC (osteonectin, BM-40). BMC Cell Biol 2: 15, 2001. doi: 10.1186/1471-2121-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentley JK, Chen Q, Hong JY, Popova AP, Lei J, Moore BB, Hershenson MB. Periostin is required for maximal airways inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 134: 1433–1442, 2014. doi: 10.1016/j.jaci.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 130: 503–506, 1995. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bornstein P, McPherson JG, Sage H. Synthesis and secretion of structural macromolecules by endothelial cells in culture. Pathobiol Endothelial Cell 215–228, 1982. doi: 10.1016/B978-0-12-521980-8.50020-1. [DOI] [Google Scholar]
- 8.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 14: 608–616, 2002. doi: 10.1016/S0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 10.Brown RS, Beaver WT, Bottomley WK. On the mechanism of drug-induced gingival hyperplasia. J Oral Pathol Med 20: 201–209, 1991. doi: 10.1111/j.1600-0714.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 11.Cai L, Brophy RH, Tycksen ED, Duan X, Nunley RM, Rai MF. Distinct expression pattern of periostin splice variants in chondrocytes and ligament progenitor cells. FASEB J 33: 8386–8405, 2019. doi: 10.1096/fj.201802281R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callebaut I, Mignotte V, Souchet M, Mornon JP. EMI domains are widespread and reveal the probable orthologs of the Caenorhabditis elegans CED-1 protein. Biochem Biophys Res Commun 300: 619–623, 2003. doi: 10.1016/S0006-291X(02)02904-2. [DOI] [PubMed] [Google Scholar]
- 13.Chau E, Daley T, Darling MR, Hamilton D. The expression and immunohistochemical localization of periostin in odontogenic tumors of mixed epithelial/mesenchymal origin. Oral Surg Oral Med Oral Pathol Oral Radiol 116: 214–220, 2013. doi: 10.1016/j.oooo.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Crawford J, Nygard K, Gan BS, O’Gorman DB. Periostin induces fibroblast proliferation and myofibroblast persistence in hypertrophic scarring. Exp Dermatol 24: 120–126, 2015. doi: 10.1111/exd.12601. [DOI] [PubMed] [Google Scholar]
- 15.Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res 82: 621–626, 2003. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- 16.Doliana R, Bot S, Bonaldo P, Colombatti A. EMI, a novel cysteine-rich domain of EMILINs and other extracellular proteins, interacts with the gC1q domains and participates in multimerization. FEBS Lett 484: 164–168, 2000. doi: 10.1016/S0014-5793(00)02140-2. [DOI] [PubMed] [Google Scholar]
- 17.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504: 277–281, 2013. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott CG, Kim SS, Hamilton DW. Functional significance of periostin in excisional skin repair: is the devil in the detail? Cell Adhes Migr 6: 319–326, 2012. doi: 10.4161/cam.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott CG, Forbes TL, Leask A, Hamilton DW. Inflammatory microenvironment and tumor necrosis factor alpha as modulators of periostin and CCN2 expression in human non-healing skin wounds and dermal fibroblasts. Matrix Biol 43: 71–84, 2015. doi: 10.1016/j.matbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Elliott CG, Hamilton DW. Deconstructing fibrosis research: do pro-fibrotic signals point the way for chronic dermal wound regeneration? J Cell Commun Signal 5: 301–315, 2011. doi: 10.1007/s12079-011-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott CG, Wang J, Guo X, Xu SW, Eastwood M, Guan J, Leask A, Conway SJ, Hamilton DW. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci 125: 121–132, 2012. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott CG, Wang J, Walker JT, Michelsons S, Dunmore-Buyze J, Drangova M, Leask A, Hamilton DW. Periostin and CCN2 scaffolds promote the wound healing response in the skin of diabetic mice. Tissue Eng Part A 25: 1326–1339, 2019. doi: 10.1089/ten.tea.2018.0268. [DOI] [PubMed] [Google Scholar]
- 24.Gabbiani G. The cellular derivation and the life span of the myofibroblast. Pathol Res Pract 192: 708–711, 1996. doi: 10.1016/S0344-0338(96)80092-6. [DOI] [PubMed] [Google Scholar]
- 25.Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27: 549–550, 1971. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 26.Gadermaier E, Tesarz M, Suciu AAM, Wallwitz J, Berg G, Himmler G. Characterization of a sandwich ELISA for the quantification of all human periostin isoforms. J Clin Lab Anal 32: e22252, 2018. doi: 10.1002/jcla.22252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giannopoulou C, Cimasoni G. Functional characteristics of gingival and periodontal ligament fibroblasts. J Dent Res 75: 895–902, 1996. doi: 10.1177/00220345960750030601. [DOI] [PubMed] [Google Scholar]
- 28.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for αvβ3 and αvβ5 integrins and promotes cell motility. Cancer Res 62: 5358–5364, 2002. [PubMed] [Google Scholar]
- 29.Glim JE, Everts V, Niessen FB, Ulrich MM, Beelen RHJ. Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Arch Oral Biol 59: 1048–1055, 2014. doi: 10.1016/j.archoralbio.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Guo F, Carter DE, Leask A. miR-218 regulates focal adhesion kinase-dependent TGFβ signaling in fibroblasts. Mol Biol Cell 25: 1151–1158, 2014. doi: 10.1091/mbc.e13-08-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration [Online]. Nature 453: 314–321, 2008. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 32.Häkkinen L, Larjava H, Fournier BPJ. Distinct phenotype and therapeutic potential of gingival fibroblasts. Cytotherapy 16: 1171–1186, 2014. doi: 10.1016/j.jcyt.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Häkkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol 2000 24: 127–152, 2000. doi: 10.1034/j.1600-0757.2000.024001127.x. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal 2: 9–17, 2008. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence–implications for treatment. Int Wound J 2: 364–368, 2005. doi: 10.1111/j.1742-4801.2005.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He X, Bao Y, Shen Y, Wang E, Hong W, Ke S, Jin X. Longitudinal evaluation of serum periostin levels in patients after large-artery atherosclerotic stroke: a prospective observational study. Sci Rep 8: 11729, 2018. doi: 10.1038/s41598-018-30121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinz B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol 47: 54–65, 2015. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Hinz B. Myofibroblasts. Exp Eye Res 142: 56–70, 2016. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Hoersch S, Andrade-Navarro MA. Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol Biol 10: 30, 2010. doi: 10.1186/1471-2148-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14: 1239–1249, 1999. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, Liu W, Xiao H, Maitikabili A, Lin Q, Wu T, Huang Z, Liu F, Luo Q, Ouyang G. Matricellular protein periostin contributes to hepatic inflammation and fibrosis. Am J Pathol 185: 786–797, 2015. doi: 10.1016/j.ajpath.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15: 802–812, 2014. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang EY, Jeong MS, Park EK, Kim JH, Jang SB. Structural characterization and interaction of periostin and bone morphogenetic protein for regulation of collagen cross-linking. Biochem Biophys Res Commun 449: 425–431, 2014. doi: 10.1016/j.bbrc.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 45.Izuhara K, Arima K, Ohta S, Suzuki S, Inamitsu M, Yamamoto KI. Periostin in allergic inflammation. Allergol Int 63: 143–151, 2014. doi: 10.2332/allergolint.13-RAI-0663. [DOI] [PubMed] [Google Scholar]
- 46.Jackson-Boeters L, Wen W, Hamilton DW. Periostin localizes to cells in normal skin, but is associated with the extracellular matrix during wound repair. J Cell Commun Signal 3: 125–133, 2009. doi: 10.1007/s12079-009-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kii I, Amizuka N, Minqi L, Kitajima S, Saga Y, Kudo A. Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun 342: 766–772, 2006. doi: 10.1016/j.bbrc.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 285: 2028–2039, 2010. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kii I, Ito H. Periostin and its interacting proteins in the construction of extracellular architectures. Cell Mol Life Sci 74: 4269–4277, 2017. doi: 10.1007/s00018-017-2644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kii I, Nishiyama T, Kudo A. Periostin promotes secretion of fibronectin from the endoplasmic reticulum. Biochem Biophys Res Commun 470: 888–893, 2016. doi: 10.1016/j.bbrc.2016.01.139. [DOI] [PubMed] [Google Scholar]
- 52.Kim SS, Jackson-Boeters L, Darling MR, Rieder MJ, Hamilton DW. Nifedipine induces periostin expression in gingival fibroblasts through TGF-beta. J Dent Res 92: 1022–1028, 2013. doi: 10.1177/0022034513503659. [DOI] [PubMed] [Google Scholar]
- 53.Kim SS, Michelsons S, Creber K, Rieder MJ, Hamilton DW. Nifedipine and phenytoin induce matrix synthesis, but not proliferation, in intact human gingival connective tissue ex vivo. J Cell Commun Signal 9: 361–375, 2015. doi: 10.1007/s12079-015-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SS, Nikoloudaki G, Darling M, Rieder MJ, Hamilton DW. Phenytoin activates smad3 phosphorylation and periostin expression in drug-induced gingival enlargement. Histol Histopathol 33: 1287–1298, 2018. doi: 10.14670/HH-18-015. [DOI] [PubMed] [Google Scholar]
- 55.Kim SS, Nikoloudaki GE, Michelsons S, Creber K, Hamilton DW. Fibronectin synthesis, but not α-smooth muscle expression, is regulated by periostin in gingival healing through FAK/JNK signaling. Sci Rep 9: 2708, 2019. doi: 10.1038/s41598-018-35805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim SS, Wen W, Prowse P, Hamilton DW. Regulation of matrix remodelling phenotype in gingival fibroblasts by substratum topography. J Cell Mol Med 19: 1183–1196, 2015. doi: 10.1111/jcmm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klingberg F, Chau G, Walraven M, Boo S, Koehler A, Chow ML, Olsen AL, Im M, Lodyga M, Wells RG, White ES, Hinz B. The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix. J Cell Sci 131: jcs201293, 2018. doi: 10.1242/jcs.201293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koh SJ, Choi Y, Kim BG, Lee KL, Kim DW, Kim JH, Kim JW, Kim JS. Matricellular protein periostin mediates intestinal inflammation through the activation of nuclear factor κb signaling. PLoS One 11: e0149652, 2016. doi: 10.1371/journal.pone.0149652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S, Markwald RR, Conway SJ. Periostin is expressed within the developing teeth at the sites of epithelial-mesenchymal interaction. Dev Dyn 229: 857–868, 2004. doi: 10.1002/dvdy.10453. [DOI] [PubMed] [Google Scholar]
- 60.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci 68: 3201–3207, 2011. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kudo A, Kii I. Periostin function in communication with extracellular matrices. J Cell Commun Signal 12: 301–308, 2018. doi: 10.1007/s12079-017-0422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost 90: 986–992, 2003. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- 63.Larjava H. Oral Wound Healing: Cell Biology and Clinical Management. Hoboken, NJ: Wiley Blackwell, 2013. [Google Scholar]
- 64.Lee HG, Eun HC. Differences between fibroblasts cultured from oral mucosa and normal skin: implication to wound healing. J Dermatol Sci 21: 176–182, 1999. doi: 10.1016/S0923-1811(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 65.Lepekhin E, Grøn B, Berezin V, Bock E, Dabelsteen E. Differences in motility pattern between human buccal fibroblasts and periodontal and skin fibroblasts. Eur J Oral Sci 110: 13–20, 2002. doi: 10.1034/j.1600-0722.2002.00139.x. [DOI] [PubMed] [Google Scholar]
- 66.Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H, Bednarik DP, Safadi FF. Expression and function of periostin-isoforms in bone. J Cell Biochem 92: 1044–1061, 2004. doi: 10.1002/jcb.20115. [DOI] [PubMed] [Google Scholar]
- 67.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol 184: 3964–3977, 2010. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 68.Lygoe KA, Wall I, Stephens P, Lewis MP. Role of vitronectin and fibronectin receptors in oral mucosal and dermal myofibroblast differentiation. Biol Cell 99: 601–614, 2007. doi: 10.1042/BC20070008. [DOI] [PubMed] [Google Scholar]
- 69.Maeda D, Kubo T, Kiya K, Kawai K, Matsuzaki S, Kobayashi D, Fujiwara T, Katayama T, Hosokawa K. Periostin is induced by IL-4/IL-13 in dermal fibroblasts and promotes RhoA/ROCK pathway-mediated TGF-β1 secretion in abnormal scar formation. J Plast Surg Hand Surg 53: 288–294, 2019. doi: 10.1080/2000656X.2019.1612752. [DOI] [PubMed] [Google Scholar]
- 70.Mael-Ainin M, Abed A, Conway SJ, Dussaule JC, Chatziantoniou C. Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J Am Soc Nephrol 25: 1724–1736, 2014. doi: 10.1681/ASN.2013060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mah W, Jiang G, Olver D, Cheung G, Kim B, Larjava H, Häkkinen L. Human gingival fibroblasts display a non-fibrotic phenotype distinct from skin fibroblasts in three-dimensional cultures. PLoS One 9: e90715, 2014. doi: 10.1371/journal.pone.0090715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mak K, Manji A, Gallant-Behm C, Wiebe C, Hart DA, Larjava H, Häkkinen L. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci 56: 168–180, 2009. doi: 10.1016/j.jdermsci.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem 285: 13294–13303, 2010. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, Takeuchi S, Koike K, Ono J, Noshiro H, Furue M, Conway SJ, Narisawa Y, Izuhara K. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 122: 2590–2600, 2012. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meran S, Thomas D, Stephens P, Martin J, Bowen T, Phillips A, Steadman R. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem 282: 25687–25697, 2007. doi: 10.1074/jbc.M700773200. [DOI] [PubMed] [Google Scholar]
- 76.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 36: 1031–1037, 2004. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Morita H, Komuro I. Periostin isoforms and cardiac remodeling after myocardial infarction: is the dispute settled? Hypertension 67: 504–505, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06449. [DOI] [PubMed] [Google Scholar]
- 78.Morra L, Rechsteiner M, Casagrande S, von Teichman A, Schraml P, Moch H, Soltermann A. Characterization of periostin isoform pattern in non-small cell lung cancer. Lung Cancer 76: 183–190, 2012. doi: 10.1016/j.lungcan.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 79.Moulin V, Castilloux G, Auger FA, Garrel D, O’Connor-McCourt MD, Germain L. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp Cell Res 238: 283–293, 1998. doi: 10.1006/excr.1997.3827. [DOI] [PubMed] [Google Scholar]
- 80.Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol 37: 1–14, 2014. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nair P, Kraft M. Serum periostin as a marker of TH2-dependent eosinophilic airway inflammation. J Allergy Clin Immunol 130: 655–656, 2012. doi: 10.1016/j.jaci.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 82.Nam BY, Park JT, Kwon YE, Lee JP, Jung JH, Kim Y, Kim S, Park J, Um JE, Wu M, Han SH, Yoo TH, Kang SW. Periostin-binding DNA aptamer treatment ameliorates peritoneal dialysis-induced peritoneal fibrosis. Mol Ther Nucleic Acids 7: 396–407, 2017. doi: 10.1016/j.omtn.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One 6: e18410, 2011. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101: 695–711, 2007. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nunomura S, Nanri Y, Ogawa M, Arima K, Mitamura Y, Yoshihara T, Hasuwa H, Conway SJ, Izuhara K. Constitutive overexpression of periostin delays wound healing in mouse skin. Wound Repair Regen 26: 6–15, 2018. doi: 10.1111/wrr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nyska A, Shemesh M, Tal H, Dayan D. Gingival hyperplasia induced by calcium channel blockers: mode of action. Med Hypotheses 43: 115–118, 1994. doi: 10.1016/0306-9877(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 87.Özdemir C, Akpulat U, Sharafi P, Yıldız Y, Onbaşılar I, Kocaefe C. Periostin is temporally expressed as an extracellular matrix component in skeletal muscle regeneration and differentiation. Gene 553: 130–139, 2014. doi: 10.1016/j.gene.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 88.Palaiologou AA, Yukna RA, Moses R, Lallier TE. Gingival, dermal, and periodontal ligament fibroblasts express different extracellular matrix receptors. J Periodontol 72: 798–807, 2001. doi: 10.1902/jop.2001.72.6.798. [DOI] [PubMed] [Google Scholar]
- 89.Puolakkainen PA, Bradshaw AD, Brekken RA, Reed MJ, Kyriakides T, Funk SE, Gooden MD, Vernon RB, Wight TN, Bornstein P, Sage EH. SPARC-thrombospondin-2-double-null mice exhibit enhanced cutaneous wound healing and increased fibrovascular invasion of subcutaneous polyvinyl alcohol sponges. J Histochem Cytochem 53: 571–581, 2005. doi: 10.1369/jhc.4A6425.2005. [DOI] [PubMed] [Google Scholar]
- 90.Rambhia KJ, Ma PX. Controlled drug release for tissue engineering. J Control Release 219: 119–128, 2015. doi: 10.1016/j.jconrel.2015.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ratajczak-Wielgomas K, Dziegiel P. The role of periostin in neoplastic processes. Folia Histochem Cytobiol 53: 120–132, 2015. doi: 10.5603/FHC.a2015.0014. [DOI] [PubMed] [Google Scholar]
- 92.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res 49: 35–43, 2012. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 93.Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348: aaa2151, 2015. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rios H, Koushik SV, Wang H, Wang J, Zhou H-M, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25: 11131–11144, 2005. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rios HF, Ma D, Xie Y, Giannobile WV, Bonewald LF, Conway SJ, Feng JQ. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol 79: 1480–1490, 2008. doi: 10.1902/jop.2008.070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res 102: 752–760, 2008. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sata M, Kudo A, Nakamura K, Saga Y, Fukuda K, Saito M, Kashima T, Amizuka N, Shimazaki M, Fukayama M, Li M, Kitajima S, Kii I, Nishiyama T. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205: 295–303, 2008. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schrementi ME, Ferreira AM, Zender C, DiPietro LA. Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen 16: 80–86, 2008. doi: 10.1111/j.1524-475X.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 100.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 19: 134–148, 2011. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwanekamp JA, Lorts A, Vagnozzi RJ, Vanhoutte D, Molkentin JD. Deletion of periostin protects against atherosclerosis in mice by altering inflammation and extracellular matrix remodeling. Arterioscler Thromb Vasc Biol 36: 60–68, 2016. doi: 10.1161/ATVBAHA.115.306397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J Cell Biol 142: 873–881, 1998. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shannon DB, McKeown STW, Lundy FT, Irwin CR. Phenotypic differences between oral and skin fibroblasts in wound contraction and growth factor expression. Wound Repair Regen 14: 172–178, 2006. doi: 10.1111/j.1743-6109.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 104.Siriwardena BSMS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M, Takata T. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer 95: 1396–1403, 2006. doi: 10.1038/sj.bjc.6603431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song KM, Lee S, Ban C. Aptamers and their biological applications. Sensors (Basel) 12: 612–631, 2012. doi: 10.3390/s120100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song ZH, Qin ZL. [Expression of periostin and the effect of hydrocortisone on it in human fibroblasts of scar]. Beijing Da Xue Xue Bao Yi Xue Ban 40: 301–305, 2008. [PubMed] [Google Scholar]
- 107.Sriram G, Bigliardi PL, Bigliardi-Qi M. Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur J Cell Biol 94: 483–512, 2015. doi: 10.1016/j.ejcb.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 108.Stephens P, Davies KJ, Occleston N, Pleass RD, Kon C, Daniels J, Khaw PT, Thomas DW. Skin and oral fibroblasts exhibit phenotypic differences in extracellular matrix reorganization and matrix metalloproteinase activity. Br J Dermatol 144: 229–237, 2001. doi: 10.1046/j.1365-2133.2001.04006.x. [DOI] [PubMed] [Google Scholar]
- 109.Supp DM, Hahn JM, Glaser K, McFarland KL, Boyce ST. Deep and superficial keloid fibroblasts contribute differentially to tissue phenotype in a novel in vivo model of keloid scar. Plast Reconstr Surg 129: 1259–1271, 2012. doi: 10.1097/PRS.0b013e31824ecaa9. [DOI] [PubMed] [Google Scholar]
- 110.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 294: 271–278, 1993. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 112.Um JE, Park JT, Nam BY, Lee JP, Jung JH, Kim Y, Kim S, Park J, Wu M, Han SH, Yoo TH, Kang SW. Periostin-binding DNA aptamer treatment attenuates renal fibrosis under diabetic conditions. Sci Rep 7: 8490, 2017. doi: 10.1038/s41598-017-09238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Field S, Uyttenhove C, Stroobant V, Cheou P, Donckers D, Coutelier JP, Simpson PT, Cummings MC, Saunus JM, Reid LE, Kutasovic JR, McNicol AM, Kim BR, Kim JH, Lakhani SR, Neville AM, Van Snick J, Jat PS. Novel highly specific anti-periostin antibodies uncover the functional importance of the fascilin 1-1 domain and highlight preferential expression of periostin in aggressive breast cancer. Int J Cancer 138: 1959–1970, 2016. doi: 10.1002/ijc.29946. [DOI] [PubMed] [Google Scholar]
- 114.Walker JT, Kim SS, Michelsons S, Creber K, Elliott CG, Leask A, Hamilton DW. Cell-matrix interactions governing skin repair: matricellular proteins as diverse modulators of cell function. Res Rep Biochem 5: 73–88, 2015. doi: 10.2147/RRBC.S57407. [DOI] [Google Scholar]
- 115.Walraven M, Hinz B. Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer. Matrix Biol 71-72: 205–224, 2018. doi: 10.1016/j.matbio.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 116.Wells A, Nuschke A, Yates CC. Skin tissue repair: matrix microenvironmental influences. Matrix Biol 49: 25–36, 2016. doi: 10.1016/j.matbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wen W, Chau E, Jackson-Boeters L, Elliott C, Daley TD, Hamilton DW. TGF-ß1 and FAK regulate periostin expression in PDL fibroblasts. J Dent Res 89: 1439–1443, 2010. doi: 10.1177/0022034510378684. [DOI] [PubMed] [Google Scholar]
- 118.Wong JW, Gallant-Behm C, Wiebe C, Mak K, Hart DA, Larjava H, Häkkinen L. Wound healing in oral mucosa results in reduced scar formation as compared with skin: evidence from the red Duroc pig model and humans. Wound Repair Regen 17: 717–729, 2009. doi: 10.1111/j.1524-475X.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- 119.Yamada S, Tauchi T, Awata T, Maeda K, Kajikawa T, Yanagita M, Murakami S. Characterization of a novel periodontal ligament-specific periostin isoform. J Dent Res 93: 891–897, 2014. doi: 10.1177/0022034514543015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, Aihara M, Takahashi K. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br J Dermatol 168: 717–725, 2013. doi: 10.1111/bjd.12117. [DOI] [PubMed] [Google Scholar]
- 121.Zent J, Guo LW. Signaling mechanisms of myofibroblastic activation: outside-in and inside-out. Cell Physiol Biochem 49: 848–868, 2018. doi: 10.1159/000493217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Z, Nie F, Chen X, Qin Z, Kang C, Chen B, Ma J, Pan B, Ma Y. Upregulated periostin promotes angiogenesis in keloids through activation of the ERK 1/2 and focal adhesion kinase pathways, as well as the upregulated expression of VEGF and angiopoietin-1. Mol Med Rep 11: 857–864, 2015. doi: 10.3892/mmr.2014.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou H-M, Wang J, Elliott C, Wen W, Hamilton DW, Conway SJ. Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal 4: 99–107, 2010. doi: 10.1007/s12079-010-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu M, Saxton RE, Ramos L, Chang DD, Karlan BY, Gasson JC, Slamon DJ. Neutralizing monoclonal antibody to periostin inhibits ovarian tumor growth and metastasis. Mol Cancer Ther 10: 1500–1508, 2011. doi: 10.1158/1535-7163.MCT-11-0046. [DOI] [PubMed] [Google Scholar]