Abstract
BACKGROUND
Cancer patients experience pathological fractures and the typical poor bone quality frequently complicates stabilization. Methods for overcoming screw failure include utilization of fenestrated screws that permit the injection of bone cement into the vertebral body to augment fixation.
OBJECTIVE
To evaluate the safety and efficacy of cement augmentation via fenestrated screws.
METHODS
A retrospective chart review of patients with neoplastic spinal instability who underwent percutaneous instrumented stabilization with cement augmentation using fenestrated pedicle screws. Patient demographic and treatment data and intraoperative and postoperative complications were evaluated by chart review and radiographic evaluation. Prospectively collected patient reported outcomes (PRO) were evaluated at short (2- <6 mo) and long term (6-12 mo).
RESULTS
Cement augmentation was performed in 216 fenestrated pedicle screws in 53 patients. Three patients required reoperation. One patient had an asymptomatic screw fracture at 6 mo postoperatively that did not require intervention. No cases of lucency around the pedicle screws, rod fractures, or cement extravasation into the spinal canal were observed. Eight cases of asymptomatic, radiographically-detected venous extravasation were found. Systemic complications included a pulmonary cement embolism, a lower extremity deep vein thrombosis, and a postoperative mortality secondary to pulmonary failure from widespread metastatic pulmonary infiltration. Significant improvement in PRO measures was found in short- and long-term analysis.
CONCLUSION
Cement augmentation of pedicle screws is an effective method to enhance the durability of spinal constructs in the cancer population. Risks include cement extravasation into draining blood vessels, but risk of clinically significant extravasation appears to be exceedingly low.
Keywords: Tumor, Fenestrated screws, Spinal instability, PMMA bone cement, Cancer, Spine, Instability, Stabilization
ABBREVIATIONS
- BPI
brief pain inventory
- DVT
deep vein thrombosis
- EBL
estimated blood loss
- K wire
Kirschner wires
- MDASI
MD Anderson symptom inventory
- PE
pulmonary embolus
- PMMA
poly-methyl-methacrylate
- PRO
patient reported outcomes
Cancer-related spinal instability is an independent surgical indication since neither systemic options nor radiation therapy can restore the mechanical integrity of the spinal axis. Spinal instability typically presents with movement-related back pain that is not relieved by pain medication or steroid treatment. The spinal instability neoplastic score1 was developed to facilitate classification of spinal instability and provide a common language across disciplines and among spine surgeons. With improved cancer therapies offering extended survivals for many cancer histologies, the rate of long-term cancer related complications such as spinal instability is expected to grow.
Traditionally, spinal stability was achieved via open surgeries using a variety of techniques. Currently, the most common stabilizing procedure involves cannulation of pedicles above and below the fracture with connecting rods creating a bridge over the fracture level. Bone quality is typically poor in cancer patients due to the osteolytic metastases, chemotherapy, radiation, and other comorbidities. Durability of these constructs is thus compromised in cancer patients. Methods of overcoming screw failure have recently been developed including expandable screws, which increase the screw purchase with the bony interface,2 and fenestrated screws. The latter involves injection of poly-methyl-methacrylate (PMMA) bone cement through the screw into the vertebral body, thus decreasing the risk of screw pull out.3,4 Despite promising biomechanical studies, these techniques have yet to be validated in the cancer population. The objective of this study was to evaluate the safety and efficacy of cement augmentation via fenestrated screws for treatment of neoplastic related instability.
METHODS
This is a retrospective chart and imaging review at a tertiary cancer center between April 2016 and August 2017. The study was approved by the local ethics committee with a waiver for informed consent and all data were kept in accordance with HIPAA regulations. Patients with neoplastic spinal instability who underwent percutaneous instrumented spinal stabilization with cement augmentation using fenestrated pedicle screws (Medtronic, Dublin, Ireland) and were followed up clinically, radiologically or both at least 6 wk postoperatively at time of data collection were included. Patients who underwent open posterior stabilization, those whose constructs were not augmented with PMMA, and those who underwent direct PMMA augmentation without the use of fenestrated pedicle screws were excluded.
Our method for percutaneous spinal stabilization with cement augmentation has been previously described.5 Briefly, patients were placed under general anesthesia and positioned prone. Image guidance was used, either with neuro-navigation (ie, O-Arm computrd tomography with Stealth Navigation [Medtronic]) or standard fluoroscopy, for localization and for pedicle cannulation and screw insertion. After verification of proper screw positioning, PMMA was injected bilaterally at each level through the fenestrated screws under fluoroscopic guidance. In cases where cement extravasation is suspected, as suggested by fluroscopy, injection of cement is stopped. Manufacturer recommended PMMA volumes were injected when feasible. In cases where cement extravasation into blood vessels or posteriorly into the spinal canal were suspected, the injection was halted. Kyphoplasty at the fracture level was performed, except in cases of high-grade epidural tumor extension or fractured osseous fragments into the spinal canal. Sub-fascial, interconnecting rods were placed and secured. Tubular decompression of the spinal cord or exiting nerve roots was then performed when necessary (Figure 1).
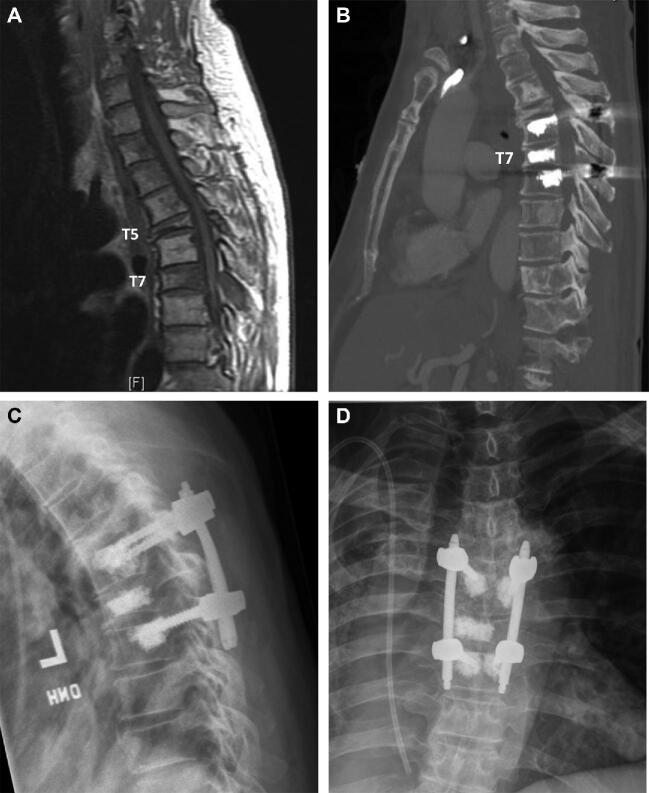
FIGURE 1.
Case example: 50-yr-old male with history of widely metastatic adenocarcinoma of the lung. He presented with severe, debilitating mechanical back pain. Neurologically intact at presentation. Imaging demonstrated multilevel spinal metastases, particularly a progressive lytic compression fracture at T7 with bilateral posterior element involvement (spinal instability neoplastic score 13). There was no apparent spinal cord compression. Notably there was an old planum burst fracture at T5 and sclerotic lesion at T4 providing a rigid junctional level above the planned construct and hence he underwent T6-T8 percutaneous stabilization with T7 kyphoplasty to ensure anterior column support. There were no postoperative complications and the patient was discharged home 3 d after surgery with significant improvement of his back pain at follow-up. A, magnetic resonance imaging T1 noncontrast enhanced demonstrating the hypointense T7 lesion with compression fracture and posterior element involvement. B, Postoperative computed tomography scan demonstrating the PMMA filled vertebral bodies along with the sclerotic and mixed lytic-sclerotic multilevel tumor infiltration. C, Postoperative sagittal and D Anterior-posterior x-rays demonstrating the stabilizing construct.
Patient and tumor data collected included patient demographics, tumor histology, and location of spine tumor. Treatment variables included details of surgical decompression, operative time, estimated blood loss (EBL), number of spinal segments instrumented, number of fenestrated screws implanted, number of suspected cases where PMMA extravasation was suspected, number of intraoperative adverse events and length of hospital stay. Postoperative data included return to the operating room for revision, additional necessary spinal interventions and potential PMMA related complications (pulmonary embolus [PE] or deep vein thrombosis [DVT]). Imaging data reviewed included screw lucency, screw pull out, pedicle fracture, hardware fracture and visible cement extravasation.
Patient reported outcome (PRO) data were prospectively collected and are described in detail in our previous studies.6,7 Briefly, PROs were collected using a web based software while in clinic or as outpatients using an online link to fill out surveys. When necessary, hand-written surveys were collected and data were later transferred to the electronic database. The changes in PROs were compared from preoperative to the short-term postoperative period defined as 2 to up to 6 mo and to long-term follow-up, defined as 6 mo or greater. Two PRO tools were collected for this analysis: the brief pain inventory (BPI) and the MD Anderson symptom inventory (MDASI). The BPI has been previously validated in the cancer population8 and is utilized to assess pain and disease interference.9 Combining 4 pain-related individual items creates a pain construct and combining 7 disease-interference items creates a disease-interference construct. Combining both constructs creates a patient pain experience construct. Similarly, the MDASI is an additional PRO tool that has been validated in cancer patients10 and is particularly useful for spine cancer since it includes a spine specific module (MDASI-sp).11 Combining 13 individual items create the MDASI core symptom construct, combining 6 disease interference items creates a MDASI disease interference construct and combining 5 spine tumor-specific items creates an MDASI spine tumor-specific construct.
Statistical Analysis
Descriptive statistics including medians, means, standard deviations, and frequencies were used to define the cohort. The Wilcoxon Signed-Rank test for matched pairs was used to compare 12 BPI and 24 MDASI preoperative and postoperative individual items. The mean score for each individual item was used to generate 3 BPI and 3 MDASI constructs as previously described. A construct was not calculated for a patient if the majority of each construct's individual items were not answered. The Wilcoxon Signed-Rank test for matched pairs was used to compare all constructs preoperatively to postoperatively. All P-values were 2-sided with an alpha level of significance of <0.0014 calculated using Bonferroni correction for the 36 individual items and an alpha level of significance of <.05 for the 5 constructs. All statistical analyses were done in SAS (version 9.4; SAS Institute, Cary, North Carolina).
RESULTS
Fifty-three patients were included in the analysis (30 male, 57%). Median age at the time of surgery was 63.5 yr. The median radiological follow-up was 148 d (range 2-542). The most common pathologies were lung and renal cell carcinoma with a similar distribution among spine segments across the study cohort with 38% thoracic, 36% lumbar, and 26% in the thoracolumbar region (Table 1).
TABLE 1.
Patient and Tumor Characteristics
| N | % | |
|---|---|---|
| Total | 53 | 100 |
| Gender | ||
| Male | 30 | 57 |
| Female | 23 | 43 |
| Age | ||
| Range | ||
| Min | 19 | |
| Max | 87 | |
| Median | 63.5 | |
| Mean | 61.4 | |
| Fracture level | ||
| Thoracic | 20 | 38 |
| Thoracolumbar | 14 | 26 |
| Lumbar | 19 | 36 |
| Histology | ||
| Lung | 13 | 25 |
| Renal | 7 | 13 |
| Prostate | 5 | 9 |
| Multiple myeloma | 5 | 9 |
| Breast | 4 | 8 |
| Thyroid | 3 | 6 |
| Other | 16 | 30 |
Cement augmentation was performed in 216 fenestrated pedicle screws. Thirty-seven operations (70%) included percutaneous stabilization without decompression while 16 (30%) entailed either a central or a foraminal decompression (Table 2). Median EBL was 100 cc (range 10-800 cc). In most surgeries, 4 pedicle screws were used (mean 4.1) with stabilizing constructs typically placed over 3 contiguous spinal segments (mean 3.3). Intraoperative suspicion of cement extravasation either anteriorly to a draining blood vessel or posteriorly towards the spinal canal was suspected in 17 cases prompting cessation of cement injection. No intraoperative adverse events requiring modification of surgery (such as conversion to an open procedure or abortion of the procedure) were noted (Table 2).
TABLE 2.
Surgical Details
| n | % | |
|---|---|---|
| Total | 53 | 100 |
| Decompression | ||
| None | 37 | 70 |
| Central | 11 | 21 |
| Facetectomy | 5 | 9 |
| Suspected intra-Op PMMA extravasation | 17 | 32 |
| Intra-Op adverse events | 0 | 0 |
| Operative time (min) | ||
| Range | ||
| Min | 83 | |
| Max | 336 | |
| Median | 141 | |
| Mean | 167.5 | |
| Estimated blood loss (mL) | ||
| Range | ||
| Min | 10 | |
| Max | 800 | |
| Median | 100 | |
| Mean | 164.8 | |
| Spinal segments instrumented | ||
| Range | ||
| Min | 3 | |
| Max | 5 | |
| Median | 3 | |
| Mean | 3.3 | |
| Number of fenestrated screws implanted | ||
| Range | ||
| Min | 4 | |
| Max | 6 | |
| Median | 4 | |
| Mean | 4.1 | |
| Total | 216 | |
| Length of hospital stay (days) | ||
| Range | ||
| Min | 2 | |
| Max | 44 | |
| Median | 5 | |
| Mean | 6.7 | |
Patient Reported Outcomes
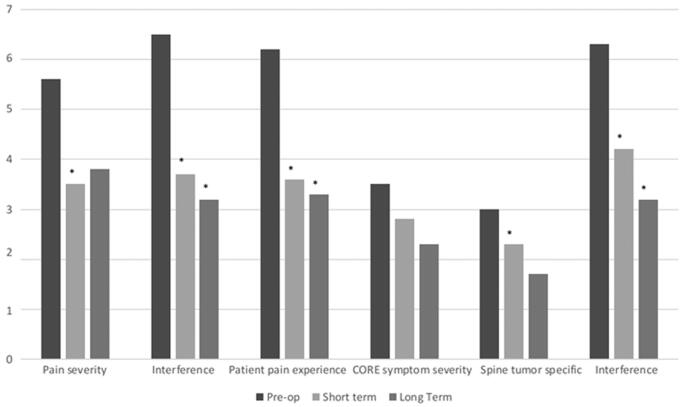
PRO data collected demonstrated statistically significant improvement in the BPI individual items worst pain (P < .0001), average pain (P < .0001), general activity (P < .0001), mood (P = .001), walking ability (P = .0007), normal work (P < .0001), and enjoyment of life (P < .0001) at short-term follow-up and in general activity (P = .0008), normal work (P = .0007), and enjoyment of life (P = .0002) at long-term follow-up. BPI constructs pain severity (P < .0001), interference (P < .0001), and patient pain experience (P < .0001) demonstrated statistically significant improvement at short term with improvement in interference (P = .0002) and patient pain experience (P = .001) remaining durable at long-term follow-up (Figure 2).
FIGURE 2.
Prospective PRO data. Prospectively collected PRO data demonstrating improvement in mean BPI and MDASI construct scores. Short term = 2 to <6 mo, Long term = 6 to 12 mo. (BPI, brief pain inventory; MDASI, MD Anderson symptom inventory) *P < .05
Analysis of MDASI individual items demonstrated statistically significant improvement in pain (P = .0002) and enjoyment of life (P < .0001) at short-term follow-up and in general activity (P = .0012) and work (P < .0001) at long-term follow-up. MDASI spine tumor specific (P = .02) and interference (P = .0006) constructs demonstrated statistically significant improvement at short-term and interference (P = .0008) at long-term follow-up. (Figure 2)
Postoperative Complications
Three patients required a reoperation. One patient presented at 3-mo follow-up with recurrence of mechanical back pain. Radiographic evidence demonstrated pull out of the bottom screws of her construct. Interestingly, intraoperative images demonstrated a paucity of cement injected into these screws as there was suspicion of cement extravasation prompting cessation of cement injection during the initial surgery. She was subsequently taken for hardware revision. Another patient required revision of instrumentation secondary to a fracture of an instrumented pedicle 1 mo after the initial surgery. One patient required a wound revision due to dehiscence while receiving bevacizumab (Table 3).
TABLE 3.
Postoperative Complications
| N | % | |
|---|---|---|
| Total | 53 | 100 |
| Return to OR | ||
| Wound revision | 1 | 2 |
| Hardware revision | 2 | 4 |
| Additional interventions | ||
| Kypho at index level | 1 | 2 |
| Kypho at adjacent level | 1 | 2 |
| PO adverse events | ||
| PE* | 3 | 6 |
| DVT | 1 | 2 |
| Death** | 1 | 2 |
| Imaging review | ||
| Lucency | 0 | 0 |
| Pull out | 1 | 2 |
| Pedicle fracture | 2 | 4 |
| Screw fracture | 1 | 2 |
| Rod fracture | 0 | 0 |
| Cement in vessel | 8 | 15 |
| Radiographic follow-up (days) | ||
| Range | ||
| Min | 2 | |
| Max | 542 | |
| Median | 140 | |
| Mean | 176.0 | |
*Cement found in pulmonary artery on postop chest scans. Did not require anticoagulation and remained asymptomatic for at least 6 wk post operatively.
**Death occurred 1 wk post operatively (still within surgical admission) secondary to respiratory failure from multiple lung metastases. The acute respiratory failure was not related to a pulmonary embolus or cement extravasation.
Radiographic Evaluation
No cases of lucency around the pedicle screws or rod fractures were found. One patient had asymptomatic radiological proof of a broken pedicle screw 6 mo after surgery and did not require intervention. There were no cases of evidence of cement extravasation into the spinal canal. Radiographic evidence of cement extravasation into draining vessels were apparent in 8 cases, of which 1 patient had evidence of cement embolus in the pulmonary artery. This radiological finding did not require intervention as at the time the patient remained asymptomatic. Also noteworthy, this patient had kyphoplasty done along with percutaneous stabilization and it is not possible to determine which level caused this cement embolus. One case of in-house mortality was documented secondary to pulmonary failure from widespread metastatic infiltration of the lungs with no evidence of PE or other cement related toxicities. Lastly, 1 patient had a lower extremity DVT that was likely unrelated to the instrumentation (Table 3).
DISCUSSION
This is the first clinical series demonstrating the safety and efficacy of cement augmentation via fenestrated pedicle screws in cancer-related spinal instability. The data presented demonstrate that when properly placed, the risks of hardware failure including screw pull out, instrumentation breakage, or lucency are low. Taken together with the expected extended survivals in cancer patients and growing need of construct supplementation for improved durability, cement augmentation of pedicle screws seems appealing. The use of fenestrated screws facilitates this process with low morbidity and short operative times.
Traditionally, stabilization has been achieved via open surgery. There are no available guidelines recommending construct length, but several authors have advocated stabilization of at least 2 levels above and 2 levels below the index level in open surgeries. In our previous analysis of 318 patients who underwent separation surgery for solid malignancies, only 2.8% experienced hardware failure.12 Experience with minimal access surgery techniques for spinal stabilization in the trauma and degenerative settings has led to the investigation of these new techniques in the cancer population. Percutaneous instrumentation has revolutionized spine stabilization as it enables preservation of muscle attachments and posterior elements.13 In the cancer population, this has been shown to be a “safe and effective option for palliation of mechanically unstable, cancer-related, vertebral compression fractures with posterior element involvement.”5,13 As previously mentioned, bone quality in cancer patients is typically poor for multiple reasons. Variable fusion rates in this population are reported (36%-100%) and various options for bone graft are used according to surgeon's preference.14 This has brought forth a need for technical innovation to increase construct durability while simultaneously decrease the extent of surgery. The injection of PMMA into the instrumented pedicles is one method to enhance construct durability and use of fenestrated screws to facilitate the PMMA injection is an appealing option. We have previously published a technical guide and outcomes paper using PMMA-augmented screws.5 At the time, “Kirschner wires (K wires) were inserted, and the pedicles were tapped. The K wires were then exchanged for 10-gauge kyphoplasty needles. Subsequently, cement was injected into the tapped vertebral bodies along the screw trajectory. After the injection, kyphoplasty needles were exchanged for K wires, and cannulated screws were put into place.” The recent FDA-approval of fenestrated screws prompted a transition to this method that has significantly stream-lined the procedure. For percutaneous stabilization with cement augmentation we typically instrument 1 level above and 1 below the index level. Rarely, with multilevel fractures or adjacent segment fractures we use longer construct to add anchor points. In our experience, the extent of vertebral body collapse, fractures in junctional areas or degree, of kyphosis typically do not necessitate longer constructs. Augmentation of the anterior column is achieved with kyphoplasty when feasible. Patients with mechanically unstable fractures without posterior element involvement, extensive cortical destruction, or significant epidural extension can often be treated with kyphoplasty alone.
Kyphoplasty and vertebroplasty have generated an abundance of data regarding the safety of PMMA injection into the spinal vertebrae.15-17 According to current literature, “leakage of bone cement into paravertebral venous system occurs in a high percentage of cases.”18 From there, the cement can travel to the right side of the heart and the pulmonary circulation. Several reports have previously described PMMA cement embolus in the pulmonary circulation. Although standard treatment of a pulmonary embolism requires 3 to 6 mo of systemic anticoagulation, some studies show that PMMA is not thrombogenic,19 thus the need for anticoagulation following asymptomatic cement PE remains debatable.20,21 However, for symptomatic cases, surgical removal of the cement embolus has also been advocated.22 One patient in the current series had radiographic evidence of a cement embolus in the pulmonary circulation. As the patient remained a-symptomatic, long-term anticoagulation was not initiated. Further, as kyphoplasty was also performed at the fracture level, it is not possible to determine whether the cement embolus arose from the kyphoplasty needle or fenestrated screws.
Patient reported outcomes are an important method of outcome analysis in the cancer population. The prospective PRO data demonstrated in our current study support the growing body of evidence that percutaneous stabilization provides significant and durable symptom palliation for neoplasia related, mechanically unstable patients. A major goal in the cancer population is rapid return to systemic treatment following surgery. The median length of stay in this series was 5 d, which is shorter than previous reports,23 and the minimally invasive nature of the procedure allows rapid return to oncologic treatment. Furthermore, radiation is a key component in the treatment of spinal metastases. We have previously demonstrated the safety of PMMA injection prior to radiosurgery planning in a clinical and dosimetric analysis.24 The fact that radiation can be delivered to PMMA-filled vertebrae without compromising safety or efficacy allows cement injection in the adjacent segments as well without jeopardizing current or future treatment options.
Limitations
We appreciate the inherent limitations of this study from the retrospective nature of a portion of the data collection. Further, though this is, to our knowledge, the largest series evaluating this new technique, comparative, large-scale, and long-term data will be required for external validation and optimal patient and technique selection guidance.
CONCLUSION
Cement augmentation of pedicle screws in neoplastic spinal instability appears to be safe and effective. When properly positioned, exceedingly low rates of hardware failure occur and may include fracture of screw, rod, or pedicle. Physicians must be aware of the potential to cause cement embolus with potential devastating consequences and hence cement injection should be done under fluoroscopic guidance. Prospective PRO data support previous reports of the palliative benefit of percutaneous stabilization in cancer related spinal instability.
Disclosures
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and in part with support from Medtronic (USA). Dr Lis is a consultant for Medtronic. Dr Bilsky is a consultant for Globus and Brainlab and receives royalties from Depuy/Synthes. Dr Laufer is a consultant for Depuy/Synthes, Globus, and SpineWave.
Notes
The abstract of this study was presented at the 2018 AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves Annual Meeting, Orlando, Florida, March 17, 2018.
COMMENT
In this paper, the authors present their experience with using fenestrated screws for spinal instability related to metastatic cancer. With this technique, the authors demonstrate that cement can be delivered safely and effectively into the vertebral body through fenestrations in the screws via a percutaneous approach. By doing so, the authors have shown that the average length of the constructs is shorter than what has typically been reported and used for stabilization in the oncologic setting. Despite the shorter construct lengths, the authors did not encounter much in the way of instrumentation failure, rod breakage, screw pullout, or need for revision/extension. The complications and morbidity associated with this technique are low. Overall, it seems that a benefit of their technique may be less blood loss and reduced operative times. However, the range in operative times (83-336 minutes) and estimated blood loss (10-800 ml) for this minimally invasive technique where a maximum of 6 screws were placed perhaps reveals the challenges and limitations of this approach in select cases. Further study is needed to better define and clarify the indications for such constructs and whether the anticipated survival and extent of osseous and systemic metastases impacts the decision-making process. The authors have thoughtfully presented their work and are congratulated for an outstanding contribution to the spine oncology literature.
John H. Shin
Boston, Massachusetts
REFERENCES
- 1. Fisher CG, DiPaola CP, Ryken TC et al.. A novel classification system for spinal instability in neoplastic disease. Spine. 2010;35(22):E1221–E1229. [DOI] [PubMed] [Google Scholar]
- 2. Gazzeri R, Roperto R, Fiore C. Surgical treatment of degenerative and traumatic spinal diseases with expandable screws in patients with osteoporosis: 2-year follow-up clinical study. J Neurosurg Spine. 2016;25(5):610–619. [DOI] [PubMed] [Google Scholar]
- 3. Frankel BM, Jones T, Wang C. Segmental polymethylmethacrylate-augmented pedicle screw fixation in patients with bone softening caused by osteoporosis and metastatic tumor involvement: a clinical evaluation. Neurosurgery. 2007;61(3):531–538. [DOI] [PubMed] [Google Scholar]
- 4. Elder BD, Lo SF, Holmes C et al.. The biomechanics of pedicle screw augmentation with cement. Spine J. 2015;15(6):1432–1445. [DOI] [PubMed] [Google Scholar]
- 5. Moussazadeh N, Rubin DG, McLaughlin L, Lis E, Bilsky MH, Laufer I. Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J. 2015;15(7):1609–1617. [DOI] [PubMed] [Google Scholar]
- 6. Barzilai O, Amato M-K, McLaughlin L et al.. Hybrid surgery–radiosurgery therapy for metastatic epidural spinal cord compression: A prospective evaluation using patient-reported outcomes. Neuro Oncol Pract. 2018;5(2):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barzilai O, McLaughlin L, Amato MK et al.. Predictors of quality of life improvement after surgery for metastatic tumors of the spine: prospective cohort study. TSpine J. 2018;18(7):1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tittle MB, McMillan SC, Hagan S. Validating the brief pain inventory for use with surgical patients with cancer. Oncol Nurs Forum. 2003;30(2):325–330. [DOI] [PubMed] [Google Scholar]
- 9. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 10. Cleeland CS, Mendoza TR, Wang XS et al.. Assessing symptom distress in cancer patients. Cancer.2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 11. Armstrong TS, Gning I, Mendoza TR et al.. Reliability and validity of the M. D. Anderson symptom inventory-spine tumor module. J Neurosurg Spine. 2010;12(4):421–430. [DOI] [PubMed] [Google Scholar]
- 12. Amankulor NM, Xu R, Iorgulescu JB et al.. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J. 2014;14(9):1850–1859. [DOI] [PubMed] [Google Scholar]
- 13. Kim CH, Chung CK, Sohn S, Lee S, Park SB. Less invasive palliative surgery for spinal metastases. J Surg Oncol. 2013;108(7):499–503. [DOI] [PubMed] [Google Scholar]
- 14. Elder BD, Ishida W, Goodwin CR et al.. Bone graft options for spinal fusion following resection of spinal column tumors: systematic review and meta-analysis. Neurosurg Focus. 2017;42(1):1–16. [DOI] [PubMed] [Google Scholar]
- 15. Berenson J, Pflugmacher R, Jarzem P et al.. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12(3):225–235. [DOI] [PubMed] [Google Scholar]
- 16. Fourney DR, Schomer DF, Nader R et al.. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1 Suppl):21–30. [DOI] [PubMed] [Google Scholar]
- 17. Qian Z, Sun Z, Yang H, Gu Y, Chen K, Wu G. Kyphoplasty for the treatment of malignant vertebral compression fractures caused by metastases. J Clin Neurosci. 2011;18(6):763–767. [DOI] [PubMed] [Google Scholar]
- 18. Choe DH, Marom EM, Ahrar K, Truong MT, Madewell JE. Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. Am J Roentgenol. 2004;183(4):1097–1102. [DOI] [PubMed] [Google Scholar]
- 19. Blinc A, Bozic M, Vengust R, Stegnar M. Methyl-methacrylate bone cement surface does not promote platelet aggregation or plasma coagulation in vitro. Thromb Res. 2004;114(3):179–184. [DOI] [PubMed] [Google Scholar]
- 20. Krueger A, Bliemel C, Zettl R, Ruchholtz S. Management of pulmonary cement embolism after percutaneous vertebroplasty and kyphoplasty: a systematic review of the literature. Eur Spine J. 2009;18(9):1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang LJ, Yang HL, Shi YX, Jiang WM, Chen L. Pulmonary cement embolism associated with percutaneous vertebroplasty or kyphoplasty: a systematic review. Orthop Surg. 2012;4(3):182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kollmann D, Hoetzenecker K, Prosch H et al.. Removal of a large cement embolus from the right pulmonary artery 4 years after kyphoplasty: consideration of thrombogenicity. J Thorac Cardiovasc Surg. 2012;143(4):e22–e24. [DOI] [PubMed] [Google Scholar]
- 23. Rao PJ, Thayaparan GK, Fairhall JM, Mobbs RJ. Minimally invasive percutaneous fixation techniques for metastatic spinal disease. Orthop Surg. 2014;6(3):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barzilai O, DiStefano N, Lis E et al.. Safety and utility of kyphoplasty prior to spine stereotactic radiosurgery for metastatic tumors: a clinical and dosimetric analysis. J Neurosurg Spine. 2018;28(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]