Abstract
Background:
There are only two anti-trypanocidal drugs available, both have a lot of side effects. This is the pioneer study designed to evaluate the Arthrospira maxima effect in Trypanosoma cruzi -infected mice and macrophages.
Methods:
A. maxima was administered in vivo, and in vitro (120μL/mL; 200 μL/mL; 500 μL/mL; 852 μL/mL) as prophylaxis, and treatment. In vitro, phagocytosis and viability were measured in macrophages cultures supplemented with A. maxima, and T. cruzi-infected. In vivo A. maxima was supplemented to T. cruzi-infected mice in order to obtain the parasitemia curves, parasite amount, and histopathologic changes. This assay was performed in Biological Sciences National School of National Polytechnic Institute, Mexico City, in 2019.
Results:
In vivo, A. maxima administration exacerbates the immune innate host′s response, followed by mice early death. In vitro, A. maxima supplementation promote T. cruzi- macrophage phagocytosis, but also a sooner T. cruzi- infected macrophage death.
Conclusion:
A. maxima administration overactive the immune system, decreasing the parasitemia, but causing a severe tissue damage. Then, this nutraceutical has a paradoxical effect on intracellular parasitic infections such as Chagas disease.
Keywords: Chagas disease, Dietary supplements, Spirulina, Trypanosoma cruzi
Introduction
Chagas’ disease, also known as American Trypanosomiasis, is a potentially life-threatening disease caused by the protozoan parasite, Trypanosoma cruzi. Currently, almost 8 million people are infected. This disease causes an average of 14,000 deaths per year. Its transmission is mostly vector-borne feces ingestion or autoinoculation, followed by vertical transmission, and organ transplant (1)
Chagas’s disease has 3 stages: acute, indeterminate, and chronic. In the acute stage the parasites are in the bloodstream, in the indeterminate stage the individual remains infected, but the parasite is barely bloodstream- founded. In the chronic stage the patients develop cardiomyopathy, heart failure and digestive-tract abnormalities such as megacolon, and megaesophagus. Diagnosis of chronic infection relies on serological testing through detection of immunoglobulin G (IgG) antibodies against T. cruzi (2–4).
There are only two anti-T. cruzi drugs in the health scheme of Chagas’ disease treatment: benznidazole, and nifurtimox. Furthermore, both drugs have low efficacy and numerous side effects that included anorexia, weight loss, neurological disorders, irritability, insomnia, disorientation, mood changes, paresthesia, seizures, and peripheral neuropathy, digestive manifestations such as nausea and vomiting, and occasionally, fever and rash. Besides, only a few people have access to these drugs (1%) (1,4–7).
On the other hand Arthrospira maxima (A. maxima) is a highly nutritional and ecofriendly nutraceutical cyanobacteria thoroughly investigated in the latest 30 years; for its role in human health management, and the US Food and Drug Administration (FDA) has categorized it as GRAS (Generally Recognized as Safe) (8–12).
A. maxima contains phycobiliproteins, allophycocyanin, and phycocyanin (PC), that inhibit the inducible nitric oxide synthase (iNOS), and cyclooxygeanase-2 (COX-2) (13, 14) that stimulates the production of antibodies, and up- or downregulates encoding cytokines genes (15,16), moreover, phycocyanobilin (PCB), inhibits the ONOO − mediated single-strand breaks in supercoiled plasmid DNA (17), allophycocyanin scavenge hydroxyl and peroxyl radicals, and bilirubin functions as a potent inhibitor of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity (18). Moreover, A. maxima is considered as a powerful stimulator of the immune system by increasing the macrophages phagocytic activity, causing natural killer (NK) cells to accumulate in tissues, by stimulating the production of antibodies, and cytokines, and by activating and mobilizing T and B cells (16). All protective properties of A. maxima lead us to try this effect in a murine model infected by T. cruzi, etiological agent of American tripanosomiasis, a parasitosis whit a highly epidemically risk.
Materials and Methods
In 2019, Arthrospira maxima fine powder (Alimentos Esenciales para la Humanidad, Mexico State, Mexico). A. maxima was dissolved in distilled water and sonicated by 5 pulses of 20 sec each, the lysis was observed using an optical microscope at 40X. All was performed by triplicate (19).
Animals: inbred BALB/c male mice were procured and maintained in temperature-controlled rooms (25±2 ºC) with access to water and food Rat Chow (Nestlé Purina, Vevey, Switzerland) ad libitum in the animal facilities of the Physiology Department, Escuela Nacional de Ciencias Biológicas (ENCB), Instituto Politécnico Nacional (IPN).
Mice were handled according the Animal Care and Use Committee Guide. The protocol for all experiments was evaluated and approved by the ENCB Bioethics Committee during 2017.
Parasite: T. cruzi NINOA strain (member of the predominant lineage in Mexico) (20) used in these experiments was kindly maintained and donated by PhD. B Nogueda-Torres (ENCB, IPN). Epimastigote forms were axenically cultured at 28 °C in 50 mL Liver Infusion Tryptose (LIT), the cells were counted every 24 h, using a Neubauer hemocytometer Blaubrand (Merck, Darmstadt, Germany) (19).
Macrophages: macrophages culture were obtained from peritoneal exudate BALB/c mice. Briefly, mononuclear cells separation was carried out by the density centrifugation method (Ficoll), cells were seeded in 9 culture flasks (triplicate) with Dulbecco Modified Eagles Minimal Essential Medium (DMEM) medium 25 mL Gibco BRL (Life Technologies, Grand Island, NY, USA) supplemented with 20% of fetal bovine serum (DMEM-FBS) Gibco BRL (Life Technologies, Grand Island, NY, USA), and incubated 60 min at 37 °C, and 5% CO 2 atmosphere. The culture supernatant was aspirated to eliminate the non-adherent cells (lymphocytes and polymorphonuclear cells), then 12 mL DMEM medium were added (19). The most suitable confluence for each monolayer culture flask was 80% (21).
Macrophages A. maxima phagocytosis in vitro assay
A macrophage culture was incubated 24h before the infection with 852 μg/mL (0.071 g/kg) A. maxima on the conditions mentioned above. This culture, and another four cultures with A. maxima at different concentrations 120 μg/mL (250 mg/kg), 200 μg/ml (500 mg/kg) and 500 μg/mL (1000 mg/kg) were infected with 1×10 3 trypomastigotes. The parasite phagocytosis was counted every 2 h, for 6 h, using an inverted microscope CK2 (Olympus, Tokio, Japan) (100 cells were counted each time (for triplicate) (22).
Parasitemia curves
Male mice were randomly divided into 8 groups of 6 mice each one: an uninfected group, a T. cruzi- parasitized group, 3 groups A. maxima-intragastrical daily treated at doses of 35.7, 71.4, and 142.8 mg/kg for prophylactic treatment two weeks before inoculation, and 3 groups treated with the same doses for post-infection as treatment.
To infect the mice 1×10 3 metacyclic trypomastigotes were inoculated intraperitoneally. Blood parasite counting was done according to Brener method (Brener, 1962). Briefly, 50 μL of mouse peripheral blood obtained through a small cut in the caudal vein, then parasites were counted using a Neubauer camera. Mortality was recorded daily.
Histopathologic Analyzes
Mice were sacrificed employing a CO 2 camera at the maximum parasitemia peak according to the obtained curves, and heart was obtained. The organs were fixed with formaldehyde, paraffin included, cut in slices with a microtome at 5 μm, and each section was dyed with the hematoxylin- eosin (H-E) technique, dehydrated, and mounted in resin. The number of amastigote nests and other changes in the tissue under optical microscopy. Furthermore, the inflammatory infiltrate was ranked as it follows: absent (−), mild (<25% of the microscopic field), moderate (25%–50% of the microscopic field), and severe (>25% of the microscopic field).
Statistical Analyzes
To analysis the parasitemia curves, Area under the curves was obtained, and ANOVA Two Ways Test was performed to correlate the groups’ data. Differences with P<0.05 were considered significant.
ANOVA test was applied to inflammation, and differences with P<0.05 (5%) were considered significant. After, non-parametric test Student-Newman-Keuls (SNK) were used, and results were expressed as mild, moderate and severe values. ANOVA test was also applied to correlate amastigotes nest amount, and differences with P<0.05 (5%) were considered significant. Moreover, to correlate inflammation and amastigotes amount, a post-hoc Principal Components Analysis (PCA) with a Pearson non-parametric rank test correlation was performed to correlate the histopathologic data.
To analyze mortality rates ANOVA Two Ways Test was performed to correlate the groups’ data. Differences with P<0.05 were considered significant. All tests were performed using GraphPad Prism 7® (GraphPad Software, La Jolla, CA, USA).
Results
T. cruzi macrophage phagocytosis
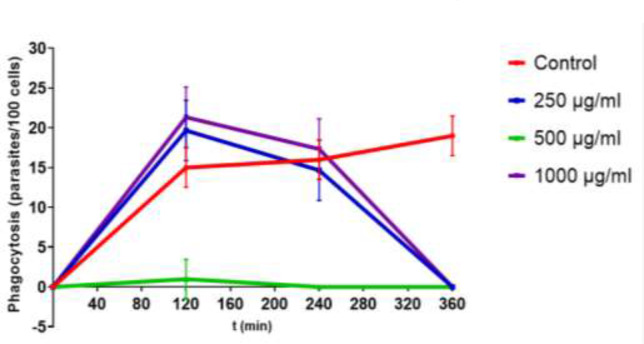
Macrophages without A. maxima had a higher phagocytic activity (70%) that supplemented ones 852 μg/mL with A. maxima as prophylaxis (15%), and 120 μg/mL (21%), 200 μg/ml (2%) and 500 μg/mL (23%) as treatment (Fig. 1).
Fig. 1:
Phagocytosi= of Trypanosoma cruzi-infected macrophages in terms of parasites phagocyted for 100 cells, on medium supplemented with different Arthrospira maxima concentrations. The data is presented as mean ± S.D. of three cultures per group P-value: Infected controls vs. A. maxima 120 μg/mL; A. maxima 200 μg/mL; A. maxima 500 μg/mL (P<0.05)
Parasitemia curves
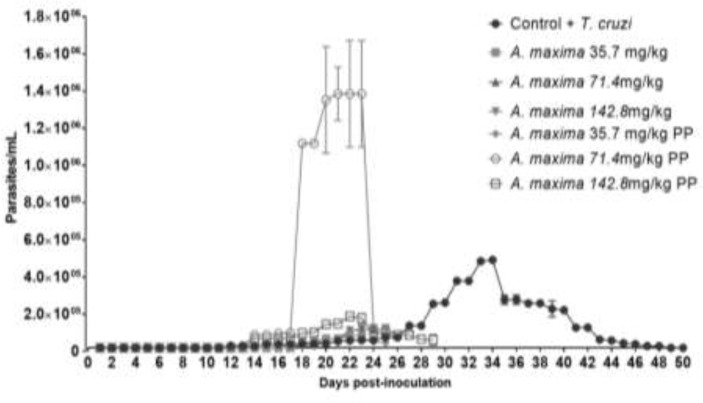
The peak of the T. cruzi- infected group was recorded on day 34 post-infection (p.i.). Nevertheless, the concentration A. maxima administrated as prophylaxis groups has its parasitemia peak at day 25 p.i., and A. maxima (Fig. 2) administrated as treatment groups. All the treated groups showed significant reductions in parasitemia (P<0.05) when they were compared with control group.
Fig. 2:
Parasitemia curves in terms of parasites/mL blood mice on the days post-inoculation, infected and supplemented with different Arthrospira maxima concentrations as treatment, and as prophylaxis (PP). The data is presented as mean ± S.D. of six mice per group: Infected controls vs. A. maxima 35.7 mg/kg; A. maxima 71.4 mg/kg; A. maxima 142.8 mg/kg as treatment; A. maxima 35.7 mg/kg; A. maxima 71.4 mg/kg; A. maxima 142.8 mg/kg as prophylaxis. P<0.05
Parasitemia and mortality
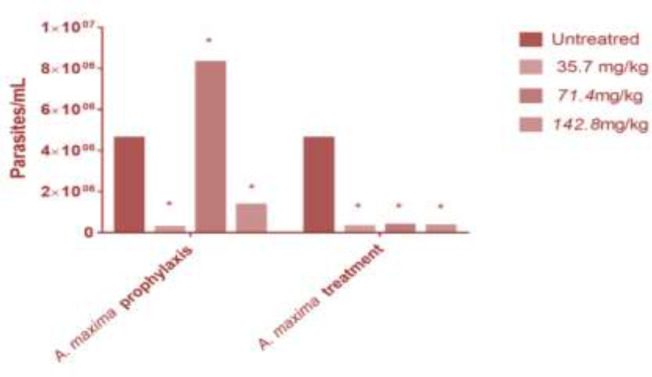
Infected mice parasitemia was 4.5×10 6 parasites/mL, A. maxima administration lower the parasitemia 35.7 mg/kg (2.3×10 5 parasites/mL), 71.4 mg/kg (8.2×10 6 parasites/mL), and 142.8 mg/kg (1.3×10 6 parasites/mL), as prophylaxis with, and 35.7 mg/kg (2.5×10 5 parasites/mL), 71.4 mg/kg (3.0×10 5 parasites/mL), and 142.8 mg/kg (2.6×10 5 parasites/mL), as treatment (Fig. 3).
Fig. 3:
Parasitemia in terms of parasites/mL mice blood on the entire experiment, infected and supplemented with different Arthrospira maxima concentrations as treatment, and as prophylaxis (PP). The data is presented as mean ± S.D. of six mice per group: Infected controls vs. A. maxima 35.7 mg/kg; A. maxima 71.4 mg/kg; A. maxima 142.8 mg/kg as treatment; A. maxima 35.7 mg/kg; A. maxima 71.4 mg/kg; A. maxima 142.8 mg/kg as prophylaxis. P<0.05. The asterisk shows the difference between the untreated group and the supplemented groups
Infected mice mortality rate was 60%. Mortality rises however A. maxima treatment (100%) was performed. It was presented sooner for the 35.7 mg/kg (day 26 p.i.), 71.4 mg/kg (day 28 p.i.), and 142.8 mg/kg (day 30 p.i.) prophylaxis with, and 35.7 mg/kg (day 23 p.i.), 71.4 mg/kg (day 25 p.i.), and 142.8 mg/kg (day 28 p.i.), as treatment than the control group (Fig. 4).
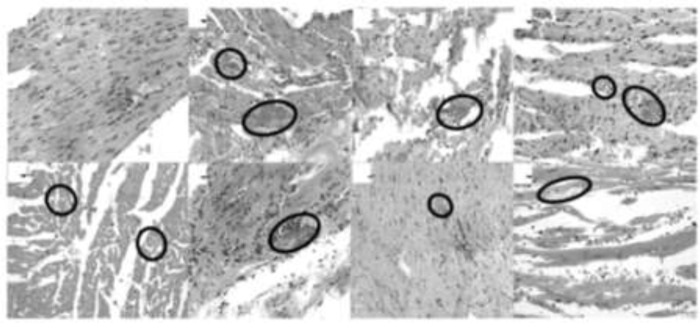
Fig. 4:
Histopathologic Analysis. Murine cardiac muscle infected with T. cruzi and supplemented with different concentrations of A. maxima as prophylaxis and as treatment. A) Cardiac tissue A) Healthy cardiac tissue B) T. cruzi- infected cardiac tissue C) T. cruzi- infected cardiac tissue treated with A. maxima 35.7 mg/kg as prophylaxis D) T. cruzi- infected cardiac tissue treated with A. maxima 71.4 mg/kg as prophylaxis E) T. cruzi- infected cardiac tissue treated with A. maxima 142.8 mg/kg as prophylaxis F) T. cruzi- infected cardiac tissue treated with A. maxima 35.7 mg/kg G) T. cruzi- infected cardiac tissue treated with A. maxima 71.4 mg/kg H) T. cruzi- infected cardiac tissue treated with A. maxima 142.8 mg/kg. 40X. Tec. H-E
Histopathology
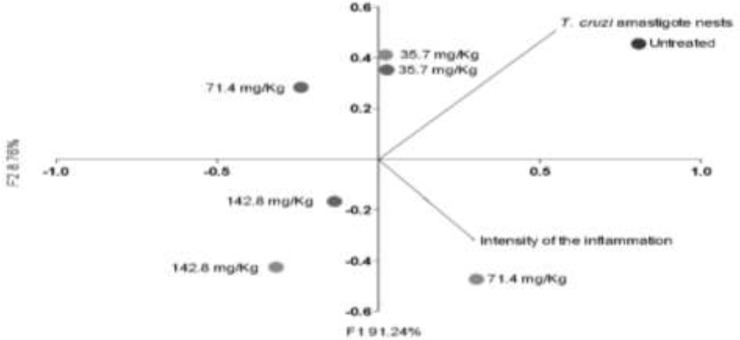
Inflammatory infiltrate was more intense in the supplemented with A. maxima 71.4 mg/kg as treatment, and lower in the A. maxima 71.4mg/kg as prophylaxis. Higher amount of amastigote nest corresponds to the T. cruzi- infected A. maxima-untreated group, and the lower amastigote nests was reported for the A. maxima supplemented mice as treatment and the. Higher A. maxima dosage (142.8 mg/kg) as treatment has minor amount of nest but more inflammation, the same dosage administered as prophylaxis has milder inflammation but major amastigote nest amount. Lower A. maxima dosage as treatment and as prophylaxis behave similar (Figs. 4, 5).
Fig. 5:
Histopathologic Analysis in terms of intensity of the inflammation, and Trypanosoma cruzi amastigote nests amount. The data is presented as mean ± S.D. of six mice per group P-value: Infected controls vs. A. maxima 35.7 mg/kg; A. maxima 71.4 mg/kg; A. maxima 142.8 mg/kg as treatment (gray dots); A. maxima 35.7 mg/kg; A. maxima 71.4 mg/kg; A. maxima 142.8 mg/kg as prophylaxis (black dots), comparison two ways ANOVA, Post-hoc analysis Pearson correlation and analyzed as an principal compounds analysis to correlate experimental groups with controls. P<0.05
Discussion
Macrophages additionally to its phagocytic activity modulates cytokine production, interleukine-1 (IL-1), interleukine-6 (IL-6), and tumor necrosis factor (TNF-α), that derives in the anti-trypanocidal activity due to nitric oxide (NO) production (23).
A. maxima has a complex lipopolysaccharides cell wall that up-regulates the activity of the phagocytic macrophage, but these cells are the primary T. cruzi-niche (24). The main A. maxima compound phycocyanin leads to an immune response exacerbation due to the activation of extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), p38 and IkB could lead to stress pathways activation (25). Besides A. maxima immuline rises macrophage activation through toll-like receptors 2 (TLR-2) receptors, nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) and leading to an interleukine-1β (IL-1β), and TNF-α production (26).
Moreover, reactive oxygen species (ROS) production overstimulation by the presence of T. cruzi itself (27) combined with the A. maxima stimuli lead to an excessive ROS augment that leads to macrophage lysis.
There are two macrophage phenotypes, macrophages phenotype 1 (M1) associated with the Th1 cytokine, and the rising iNOS, NO, ROS, interleukine-2 (IL-2), IL-1β than due to the microorganism death, but if the stimuli are constant could damage the tissue. Usually, macrophage phenotype 2 (M2) are involved in tissue remodeling, intracellular encapsulation, Th2 cytokines and upregulating interleukine-10 (IL-10) and transforming growth factor β (TGF-β) expression. In T. cruzi-infection the parasite leads to a M2 phenotype in order to leading to chronicity (28). Macrophages without A. maxima phagocyte a larger parasite amount in order to employ its cytoplasm to replicate itself due its antioxidant enzymes (29).
Arthrospira exerts an immunostimulatory activity through polysaccharides and glycolipids, which often serve as ligands of Toll receptors, mainly TLR-2 and TLR-4 (26). In the phagocytosis essays the macrophages were activated by the A. maxima lipopolysaccharide (LPS) (24), and they could induce NF-kB pathway, therefore increase both IL-1β and TNF-α (8), and immuline (26). Our results suggest that this cyanobacterium upregulates the pro-inflammatory cytokines together with the pro-inflammatory anti T. cruzi inflammation itself lead to an exacerbated ROS release and could derivate on a tissue damage that exacerbate even more the innate immune response. According to this experiment, A. maxima treated-macrophages lysis occurs sooner that on control ones due a large amount of ROS generated, as was seen in another intracellular protozoa infections, with excessive ROS produces to mitochondrial dysfunction, followed by the caspases activation, and resulted on the cell apoptosis (30). The problem is that macrophage destruction leads T. cruzi (intracellular agent) to invade other cells.
Parasitemia on the A. maxima supplemented groups was lower than in the control due the high oxidative microenvironment generated as response of the parasite presence, and the inflammation upregulation generated by A. maxima. On the other hand, A. maxima contains provitamin A, and vitamin A modulates the IL1-β, IL-2, IL-6, and TNF-α cytokine production (16), but on the proved doses the stimuli were too strong, maybe it activates some stress pathways.
Arthrospira platensis modulates mitogen-activated protein kinases (MAPK) pathway (31), there are no information about A. maxima but as they are of the same genre, they could probably have an immunomodulatory effect employing the same stress pathway. Moreover, T. cruzi rises the inducible nitric oxide synthase (iNOS), and INF-gamma through activation of the NFkB, ERK1/2 MAPK stress pathway (32).
The excessive oxidative stress that is induced into the host because of the parasite presence and the cyanobacteria, exceeds the buffering capacity of the individual to defense its organs, resulting in cell damage and death, that could derivate on the failure of many organs, like the heart (32). T. cruzi cardiomyocytes invasion elicits the proinflammatory mediators (TNF-α, IL-1β, IFN-gamma, NO), increasing the amount of superoxide radical, hydrogen peroxyde, and peroxinitrite generation (33). NINOA-T. cruzi strain is cardiotropic, therefore, presence of the parasite is mostly on the heart, where the oxidative reaction could lead to the death of the mice, the higher the A. maxima concentration, the higher the oxidative stress that individuals suffered.
Mitochondrial stress suppressed the induced up-regulation in MYD88ASC, active caspase 1, and IL-1β in cardiomyocytes liberating mtDNA as a type of damage-associated molecular patterns (DAMPs) to stimulate inflammation through TLR-9 (31).
The uncontrolled macrophage-derived- oxidative burst elicit by T. cruzi by the generation of mitochondrial superoxide radicals leads to host cells to apoptosis (34). Arthrospira promotes cit C release adjuvate to caspase -3, and -9 activation by downregulation Bcl-2 (an anti-apoptotic protein), and upregulating Bax (pro-apoptotic protein) (31). Thus, as a result of the parasite infection, and the presence of A. maxima the M1 died before it has time to change its phenotype.
Arthrospira phycocyanin also induced autophagic cell death by activating p38 and JNK signaling while inhibiting Erk pathway (25), these biliproteins in macrophages LPS activated, inhibits the cyclooxygenase-2 (COX-2) leading to cell apoptosis fragmentation its DNA (13).
In the histopathological analyzes show that highest doses of A. maxima (142.8mg/kg) (regardless the administration scheme) have not a beneficial effect in T. cruzi-infected mice, the following doses (71.4 mg/kg) as treatment diminishes the inflammatory intensity as treatment (downregulate the pro-inflammatory activity) and as prophylaxis augment the intensity of the inflammation (upregulate the pro-inflammatory activity). The best results in this case were obtained with the minimum doses of A. maxima (35.7 mg/kg) however the administration scheme was (treatment or prophylaxis) because this dosage lows the parasite nest amount, and not increase the inflammation so much. The immune system is constantly activated by the cell apoptosis of the infected tissues, this apoptosis is related directly with the phycocyanin concentration (13).
However, as a pioneer experiment the employed doses were too immunostimulant, maybe by employing lower A. maxima doses the immunostimulatory activity could be beneficial for pathogens that do not take advantage of the macrophage activity. A. maxima has not only inflammatory but also anti-inflammatoryy activity by the ERK1/2, JNK y p38 pathways inhibition (12,16). This important issue remains as an interesting investigation subject.
Conclusion
Arthrospira maxima supplementation in macrophages, promotes the phagocytosis, intensifies the ROS production leading them to death, decreasing the intracellular T. cruzi replicative niche, and therefore the parasitosis; however, the continuous exacerbation of the immune system, caused severe tissue damage that derived into individuals′ death.
Then, this nutraceutical has a paradoxical effect on intracellular parasitic infections such as Chagas′ disease.
Acknowledgements
We thank Dr. Benjamín Nogueda-Torres for kindly provided T. cruzi NINOA stain.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Chagas disease [Internet]. Who.int. 2018. [cited 7 December 2018]. Available from: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
- 2.Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat Rev Microbiol. 2005;3(10):819–23. [DOI] [PubMed] [Google Scholar]
- 3.Bern C. Chagas’ Disease. N Engl J Med. 2015;373(19):1882. [DOI] [PubMed] [Google Scholar]
- 4.Molina I, Salvador F, Sánchez-Montalvá A. [Update Chagas Disease]. Enferm Infecc Microbiol Clin. 2016;34(2):132–138. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez J, Davies C, Simonazzi A, et al. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 2016;156:1–16. [DOI] [PubMed] [Google Scholar]
- 6.Carabarin-Lima A, Gonzalez-Vazquez MC, Rodriguez-Morales O, et al. Chagas disease (American trypanosomiasis) in Mexico: an update. Acta Trop. 2013;127(2):126–35. [DOI] [PubMed] [Google Scholar]
- 7.CDC - Chagas Disease - Resources for Health Professionals - Antiparasitic Treatment [Internet]. Cdc.gov. 2018 [cited 7 December 2018]. https://www.cdc.gov/parasites/chagas/health_professionals/tx.html
- 8.Finamore A, Palmery M, Bensehaila S, et al. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid Med Cell Longev. 2017;2017:3247528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miranda MS, Cintra RG, Barros SB, et al. Antioxidant activity of the microalga Spirulina maxima. Braz J Med Biol Res. 1998;31(8):1075–9. [DOI] [PubMed] [Google Scholar]
- 10.Pinero Estrada JE, Bermejo Bescos P, Villar del Fresno AM. Antioxidant activity of the microalga Spirulina maxima. Farmaco. 2001;56(5–7):497–500. [DOI] [PubMed] [Google Scholar]
- 11.Small E. 37. Spirulina – food for the universe. Biodiversity. 2011;12(4):255–265. [Google Scholar]
- 12.Sotiroudis T, Sotiroudis G. Health aspects of Spirulina (Arthrospira) microalga food supplement. Journal of the Serbian Chemical Society. 2013;78(3):395–405. [Google Scholar]
- 13.Reddy CM, Bhat VB, Kiranmai G, et al. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem Biophys Res Commun. 2000;277(3):599–603. [DOI] [PubMed] [Google Scholar]
- 14.Shih CM, Cheng SN, Wong CS, et al. Antiinflammatory and antihyperalgesic activity of C-phycocyanin. Anesth Analg. 2009; 108(4):1303–10. [DOI] [PubMed] [Google Scholar]
- 15.Li YJ, Han Z, Ge L, et al. C-phycocyanin protects against low fertility by inhibiting reactive oxygen species in aging mice. Oncotarget. 2016;7(14):17393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, Liu L, Miron A, et al. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. 2016;90(8):1817–40. [DOI] [PubMed] [Google Scholar]
- 17.Bhat VB, Madyastha KM. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: protection against oxidative damage to DNA. Biochem Biophys Res Commun. 2001;285(2):262–6. [DOI] [PubMed] [Google Scholar]
- 18.McCarty MF. “Iatrogenic Gilbert syndrome”--a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses. 2007;69(5):974–94. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez B, Monteon V, Reyes PA, et al. Standardization of micro-enzyme-linked immunosorbent assay (ELISA) and Western blot for detection of Trypanosoma cruzi antibodies using extracts from Mexican strains as antigens. Arch Med Res. 2001;32(5):382–8. [DOI] [PubMed] [Google Scholar]
- 20.Bosseno MF, Barnabe C, Magallon Gastelum E, et al. Predominance of Trypanosoma cruzi Lineage I in Mexico. J Clin Microbiol. 2002; 40(2), 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias Citalan G. Evaluacion de cinco derivados de los Benzimidazoles contra promastigotes metaciclicos y macrofagos infectados con amastigotes de Leishmania mexicana [Maestria en Ciencias]. Istituto Politecnico Nacional 2009. [Google Scholar]
- 22.Lezama-Davila CM, Isaac-Marquez AP, Barbi J, et al. 17Beta-estradiol increases Leishmania mexicana killing in macrophages from DBA/2 mice by enhancing production of nitric oxide but not pro-inflammatory cytokines. Am J Trop Med Hyg. 2007;76(6):1125–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Dos Santos LM, da Silva TM, Azambuja JH, et al. Methionine and methionine sulfoxide treatment induces M1/classical macrophage polarization and modulates oxidative stress and purinergic signaling parameters. Mol Cell Biochem. 2017;424(1–2):69–78. [DOI] [PubMed] [Google Scholar]
- 24.Volpini X, Ambrosio L, Fozzatti L, et al. Trypanosoma cruzi Exploits Wnt Signaling Pathway to Promote Its Intracellular Replication in Macrophages. Front Immunol. 2018;9,859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao G, Gao B, Gao Y, et al. Phycocyanin Inhibits Tumorigenic Potential of Pancreatic Cancer Cells: Role of Apoptosis and Autophagy. Sci Rep. 2016;6:34564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balachandran P, Pugh ND, Ma G, et al. Toll-like receptor 2-dependent activation of monocytes by Spirulina polysaccharide and its immune enhancing action in mice. Int Immunopharmacol. 2006;6(12):1808–14. [DOI] [PubMed] [Google Scholar]
- 27.Sales Junior PA, Molina I, Fonseca Murta SM, et al. Experimental and Clinical Treatment of Chagas Disease: A Review. Am J Trop Med Hyg. 2017;97(5):1289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanluqui N. Macrophage Polarization in Chagas Disease. J Clin Cellular Immunol. 2015;06(02). [Google Scholar]
- 29.Cardoso MS, Reis-Cunha JL, Bartholomeu DC. Evasion of the Immune Response by Trypanosoma cruzi during Acute Infection. Front Immunol. 2015;6:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, He L, Zhang A, et al. Toxoplasma gondii isolate with genotype Chinese 1 triggers trophoblast apoptosis through oxidative stress and mitochondrial dysfunction in mice. Exp Parasitol. 2015;154:51–61. [DOI] [PubMed] [Google Scholar]
- 31.Saini M, Sanyal S, Vaiphei K. Piroxicam and C-Phycocyanin Mediated Apoptosis in 1,2-Dimethylhydrazine Dihydrochloride Induced Colon Carcinogenesis: Exploring the Mitochondrial Pathway. Nutrition and Cancer. 2012;64(3):409–418. [DOI] [PubMed] [Google Scholar]
- 32.Sadek K, Lebda M, Nasr S, et al. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed Pharmacother. 2017; 92:1085–1094. [DOI] [PubMed] [Google Scholar]
- 33.van der Pol A, van Gilst WH, Voors AA, et al. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019r;21(4):425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Estrada D, Specker G, Martinez A, et al. Cardiomyocyte diffusible redox mediators control Trypanosoma cruzi infection: role of parasite mitochondrial iron superoxide dismutase. Biochem J. 2018;475(7):1235–51. [DOI] [PubMed] [Google Scholar]