Abstract
SARS-CoV-2, the virus that causes COVID-19, has been found in the faeces of infected patients in numerous studies. Stool may remain positive for SARS-CoV-2, even when the respiratory tract becomes negative, and the interaction with the gastrointestinal tract poses a series of questions about wastewater and its treatments. This review aims to understand the viral load of SARS-CoV-2 in faeces and sewage and its fate in wastewater treatment plants (WWTPs).
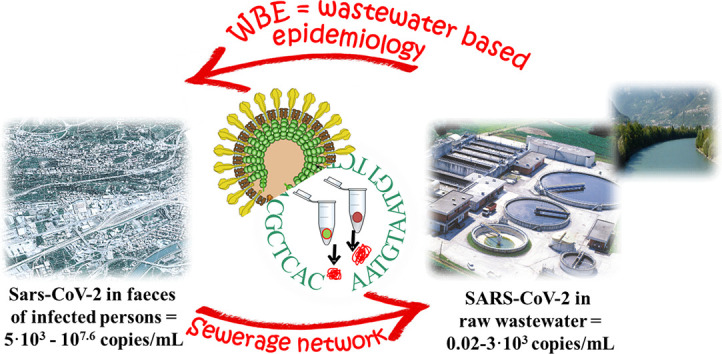
The viral load in the faeces of persons testing positive for SARS-CoV-2 was estimated at between 5·103 to 107.6 copies/mL, depending on the infection course. In the sewerage, faeces undergo dilution and viral load decreases considerably in the wastewater entering a WWTP with a range from 2 copies/100 mL to 3·103 copies/mL, depending on the level of the epidemic. Monitoring of SARS-CoV-2 in sewage, although no evidence of COVID-19 transmission has been found via this route, could be advantageously exploited as an early warning of outbreaks. Preliminary studies on WBE seem promising; but high uncertainty of viral loads in wastewater and faeces remains, and further research is needed.
The detection of SARS-CoV-2 in sewage, based on RNA sequences and RT-PCR, requires a shared approach on sample pre-treatment and on-site collection to ensure comparable results. The finding of viral RNA in stools does not imply that the virus is viable and infectious. Viability of CoVs such as SARS-CoV-2 decreases in wastewater - due to temperature, pH, solids, micropollutants - but high inactivation in WWTPs can be obtained only by using disinfection (free chlorine, UVC light). A reduction in the quantity of disinfectants can be obtained by implementing Membrane-Bioreactors with ultrafiltration to separate SARS-CoV-2 virions with a size of 60–140 nm. In sludge treatment, thermophilic digestion is effective, based on the general consensus that CoVs are highly sensitive to increased temperatures.
Keywords: Coronavirus, Outbreak, SARS-CoV-2, Faeces, Sewage, Wastewater treatment
Graphical abstract
1. Introduction to SARS-CoV-2, the virus responsible for the COVID-19 pandemic outbreak
Until the beginning of this century, coronaviruses (CoVs) were considered minor pathogens for humans. However, CoVs have caused important outbreaks in recent decades: the severe acute respiratory syndrome (SARS) in the years 2002–2003 and the Middle East respiratory syndrome (MERS) in the year 2012 (Yu Chen et al., 2020). At the beginning of the outbreak that occurred in Wuhan at the end of 2019, a novel coronavirus was identified (Lu et al., 2020). It was officially named SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2; formerly called HCoV-19), because the closest relative to the new virus is the SARS-CoV responsible for the SARS outbreak in 2003 (WHO, 2020a; Lu et al., 2020). The genome of the new virus, which has about 80% nucleotide identity with that of human SARS-CoV (Lu et al., 2020; Chan et al., 2020), is more similar to two bat-derived coronavirus strains with 88–89% identity according to Lu et al. (2020) and Chan et al. (2020).
The coronavirus virion is roughly spherical, with a diameter of approximately 60–140 nm and contained within an outer viral envelope covered by projections (9–12 nm) (Zhu et al., 2020) which are arranged in a characteristic external structure similar to a crown (corona in Latin) that gives the name to the family. Envelope proteins are involved in several aspects of the virus life cycle, such as assembly, envelope formation, and pathogenesis. Within the envelope there is the helical capsid containing nucleoprotein and the RNA genome. Fig. 1 shows the virion structure of SARS-CoV/SARS-CoV-2.
Fig. 1.
Virion structure of SARS-CoV/SARS-CoV-2 (permission obtained from Philippe Le Mercier, ViralZone, SIB Swiss Institute of Bioinformatics).
Phylogenetically, SARS-CoV-2 falls within the subgenus Sarbecovirus of the genus Betacoronavirus, which is one of the four genera of CoVs belonging to the Coronavirinae subfamily. CoVs may infect mammals but also birds or fish, showing diverse tissue tropism. Before 2019, only six CoVs were known to be responsible for infections in humans (Yu Chen et al., 2020; Chan et al., 2020) and as causing respiratory diseases: 229E and NL63 (alpha coronavirus), OC43, HKU1, MERS-CoV, SARS-CoV (beta coronavirus). Owing to the typically high mutation rates of RNA viruses in comparison with both DNA viruses and their hosts, CoVs can quickly increase their virulence and adapt to novel hosts (Duffy, 2018; Elena and Sanjuán, 2005).
The respiratory disease caused by SARS-CoV-2 is called COVID-19 (WHO, 2020a). Patients with COVID-19 typically present fever, cough, rhinorrhea, dyspnea, or severe pneumonia (N. Chen et al. 2020; Yeo et al., 2020; Guan et al., 2020). A large percentage of people may remain asymptomatic even if they have tested positive for SARS-CoV-2 (Lai et al., 2020; P. Yu et al. 2020; Liu et al., 2020; Bai et al., 2020; Rothe et al., 2020; X. Pan et al. 2020). Conventional routes of transmission of SARS-CoV-2 are respiratory droplets and direct contact, or indirect contact with contaminated surfaces (Gu et al., 2020; Yeo et al., 2020), similarly to SARS-CoV and MERS-CoV. Without approved and validated treatment options and without a vaccine, the best practices to limit the disease are protection measures and social distancing.
As of June 16, 2020, about 8 million confirmed cases including >400,000 deaths have been reported worldwide, affecting at least 230 Countries and Territories (https://covid19.who.int/ situation report 147).
The aim of this review is to outline the currently available knowledge about the occurrence of the new coronavirus in wastewater and to highlight the areas where further research is needed to answer the following questions: (i) what are the methods for sampling and identifying SARS-CoV-2 in faeces and wastewater? (ii) how large is the viral load of SARS-CoV-2 in faeces and its capacity of active replication? (iii) how large is the concentration of SARS-CoV-2 in municipal wastewater and is there evidence of faecal-oral transmission? (iv) is wastewater monitoring useful in the developing field of Wastewater Based Epidemiology (WBE) for early-warning surveillance of the spread of the virus? (v) what is the role of wastewater treatment plants?
Since the recent onset of the COVID-19 pandemic, some of the investigations reviewed here is preliminary and/or ongoing. However, relevant publicly available papers not yet peer-reviewed have been included.
2. Interaction of SARS-CoV-2 with the gastrointestinal tract and its presence in faeces
In general, viruses detected in faeces can derive from: (1) swallowing of respiratory secretions from the upper respiratory tract; in this case the virus can be damaged by the gastric acidity in the stomach, but protection when mixed with food or potential resistance to low pH may allow its passage in the intestine; (2) residues of infected antigen-presenting immune cells; (3) virus replication in intestinal cells (Gu et al., 2020; Xiao et al., 2020), considering that both avian and human influenza viruses are able to replicate in human intestinal epithelial cells (Minodier et al., 2015).
SARS-CoV and MERS-CoV, which caused the previous outbreaks, were associated with gastrointestinal symptoms in a significant proportion of patients (Zhou et al., 2017; Yeo et al., 2020). During the two SARS-CoV epidemic outbreaks, in 2002 and 2003, up to 73% of patients had gastrointestinal symptoms during the development of the disease, and the presence of SARS-CoV RNA was demonstrated in the stool specimens (WHO, 2003; Zhou et al., 2017). For a small percentage of patients, viral RNA was still present after 30 days of illness (K. H. Chan et al., 2004). The ability of active SARS-CoV to replicate was identified in the intestine specimens. It was consequently speculated that the human gastrointestinal tract could be an infection site of SARS-CoV (Ding et al., 2004; Zhou et al., 2017). During the 2012 MERS-CoV outbreak, a quarter of patients reported symptoms, such as diarrhoea or abdominal pain, before severe respiratory symptoms (Assiri et al., 2013; Mackay and Arden, 2015) and MERS-CoV RNA was found in 14.6% of stool samples (Corman et al., 2016). Zhou et al. (2017) demonstrated that intestinal epithelial cells were highly susceptible to MERS-CoV and could support viral replication.
The new SARS-CoV-2 can affect not only the respiratory system with fever, cough, rhinorrhea, dyspnea or severe pneumonia, but may also cause other clinical symptoms like lethargy, muscle ache, headache, neurologic manifestation or gastrointestinal symptoms such as diarrhoea (Guan et al., 2020; L. Pan et al. 2020). Among the first studies from China, Guan et al. (2020) extracted data on 1099 patients with laboratory-confirmed COVID-19 from 552 hospitals in mainland China through January 29, 2020, and among varying degrees of illness, diarrhoea was considered uncommon (3.8% of 1099 patients). Subsequent observations on 204 patients with COVID-19 from January 18th to March 18th, 2020, highlighted that one-third of patients reported diarrhoea (L. Pan et al. 2020), indicating that digestive issues may be a common early symptom of the disease. The differences between the two studies match the findings of Cheung et al. (2020) who observed a significant heterogeneity among the studies and a significant subgroup difference in the data from and outside Hubei Province. In a meta-analysis of 60 studies and 4243 patients, the pooled prevalences of all gastrointestinal symptoms and viral RNA positive stool were 17.6% and 48.1% (Cheung et al., 2020).
Therefore, SARS-CoV-2 positivity can be observed also in faeces of persons in the absence of gastrointestinal symptoms or diarrhoea: in particular, live SARS-CoV-2 was found in the stool of two patients without diarrhoea (W. Wang et al. 2020).
A synthesis of the incidence of viral RNA positivity in the stools of patients infected with SARS-CoV-2 is reported in Table 1 . In addition, Table 2 reports the loads of SARS-CoV-2 measured in the faeces detected positive by real-time RT-PCR.
Table 1.
Gastrointestinal (GI) symptoms, diarrhoea and viral RNA positivity in stools of patients infected with SARS-CoV-2.
| Reference | Patients |
GI symptoms |
Diarrhoea |
Viral RNA posit. in stool |
Other findings | |||
|---|---|---|---|---|---|---|---|---|
| No. | No. | % | No. | % | No. | % | ||
| (W. Wang et al. 2020) | 153 | – | – | – | – | 44/153 | 29 |
|
| (Lin et al., 2020) | 65 | 42/65 | 64.6 | 31/65 | 47.7 |
|
||
| 95 | 58/95 | 61 | 24.2 | |||||
| (Xiao et al., 2020) | 73 | 39/73 | 53.4 |
|
||||
| (Xu et al., 2020) | 10 | 3/10 | 30 | 3/10 | 30 | 8/10 | 80 |
|
| (Y. Wu et al., 2020) | 74 | 23/74 | 31 | 41/74 | 55 |
|
||
| (Zhang et al., n.d.) | 23 | 10/12 | 83.3 |
|
||||
| (Cheung et al., 2020) - own data | 59 | 15/59 | 25 | 13/59 | 22 | 9/59 | 15.3 | |
| (Cheung et al., 2020) - review | 4243 | – | 17.6 | – | 12.5 | – | 48.1 |
|
| (Wolfel et al., 2020) | 9 | 1/9 | 11 |
|
||||
| (Yu Chen et al., 2020) | 42 | 8 | 19 | 7 | 17 | 28 | 67 |
|
| (Lo et al., 2020) | 10 | 8 | 80 |
|
||||
| (Guan et al., 2020) | 1099 | 55/1099 | 5 | 42/1099 | 3.8 |
|
||
| (L. Pan et al., 2020) | 204 | 103/204 | 50.5 | 35/204 | 17 |
|
||
Table 2.
SARS-CoV-2 loads and viral RNA Ct values in faecal samples detected positive by real-time RT-PCR.
| Reference | No. patients | SARS-CoV-2 load (copies/mL) | Other results |
|---|---|---|---|
| (Wolfel et al., 2020) | 9 | >107 (peak) (range 103–107) |
|
| (Zhang et al., n.d.) | 23 | 5623 (mean) 105.8 (peak) |
|
| (Cheung et al., 2020) | 59 | 104.7 (median) (range 103.4–107.6) |
|
| (W. Wang et al. 2020) | 153 | <2.6·104 copies/mL |
|
| (Y. Wu et al., 2020) | 41 |
|
|
| (Xu et al., 2020) | 10 | 2·103–2·107 (estimated by us) |
|
W. Wang et al. (2020) investigated the biodistribution of SARS-CoV-2 among different tissues of patients with confirmed COVID-19 (bronchoalveolar lavage fluid, blood, sputum, faeces, urine, and nasal samples). In faeces, the positive specimens were 44 of 153, corresponding to 29% of the collected specimens. The mean cycle threshold value for stool specimens was 31.4 (st. dev. = 5.1), indicating a viral load <2.6·104 copies/mL, which was significantly lower than the nasal swabs where the copy number was 1.4·106 copies/mL (Ct mean 24.3) (W. Wang et al. 2020).
Lin et al. (2020) tested faecal samples of 65 hospitalized patients and approximately one half were positive, including cases with and without gastrointestinal symptoms. The authors concluded that the gastrointestinal tract may be a potential transmission route and target organ of SARS-CoV-2. With regard to this latter observation, SARS-CoV-2 uses the ACE2 protein as a receptor (Wan et al., 2020), which is present not only in the respiratory epithelium, but also in the gastro-enteric mucosa (Hamming et al., 2004).
Xiao et al. (2020) examined the viral RNA in faeces from 73 hospitalized patients with SARS-CoV-2 infection and found that a percentage of 53.4% tested positive for viral RNA in stool. Furthermore, >20% of patients with SARS-CoV-2 continued to remain positive in faeces, even after showing negative results in respiratory samples (Xiao et al., 2020).
Another epidemiological and clinical investigation on ten paediatric SARS-CoV-2 infection cases highlighted that some patients were persistently positive on rectal swabs even after their nasopharyngeal testing had become negative (Xu et al., 2020).
Y. Wu et al. (2020), investigating faecal samples from 74 patients, observed that the faecal samples of over half of patients remained positive for SARS-CoV-2 RNA for a mean of 11.2 days after the respiratory tract samples became negative. In certain cases, this duration in faeces persisted for nearly 5 weeks after the respiratory samples tested negative. Y. Wu et al. (2020) suggest that the virus may be actively replicating in the gastrointestinal tract even after viral clearance in the respiratory tract.
Zhang et al. (2020) investigated the faeces of 23 patients, finding 83% positive, with a median duration of virus shedding of 22.0 days for faeces. During this period, the mean virus titre in faeces was 5623 copies/mL, but the highest titre at the peak reached 105.8 copies/mL (Zhang et al., 2020).
As far as we know, only one study - Cheung et al. (2020) - tested viral RNA in stool collected on the day of hospitalization. In this study viral RNA was detected in 15.3% of the patients. In particular, viral RNA was found in 38.5% of patients with diarrhoea and in 8.7% of patients without diarrhoea, confirming again that the presence of SARS-CoV-2 RNA in faeces is not necessarily correlated with diarrhoea. A median viral load of 104.7 copies/mL (range 103.4–107.6) was found in the stool of 9 positive patients (Cheung et al., 2020). The stool viral load (median values) was 105.1 and 103.9 copies/mL in the presence or absence of diarrhoea, respectively.
Frequent measurements of viral RNA concentration were performed on the stool of nine hospitalized patients with COVID-2019 during the course of the disease by Wolfel et al. (2020), who found high viral RNA concentrations in initial samples, with a peak during the first week of symptoms. The viral content then declined gradually over time, but stool samples remained RNA-positive for three weeks in six of the nine patients, in spite of full resolution of symptoms (Wolfel et al., 2020). Maximum viral load over 107 RNA copies/g faeces was measured in some cases; then a progressive decrease by 2–3 orders of magnitude occurred in the subsequent weeks (Wolfel et al., 2020). These results indicate that the viral load in faeces may be highly variable in the range 103–107 RNA copies/g faeces, depending on the day of sampling post onset. To change units from #/mL to #/g, the density of wet human faeces, i.e. about 1.06–1.09 g/mL (Penn et al., 2018), is used.
In a meta-analysis of 60 studies and 4243 patients by Cheung et al. (2020), 70.3% of the stool samples were positive for SARS-CoV-2 even after respiratory specimens tested negative.
Regarding urine, many cases reported negative samples (L. Wang et al. 2020; Yifei Chen et al. 2020; F. Yu et al. 2020; Lescure et al., 2020; Young et al., 2020; Lo et al., 2020; W. Wang et al. 2020). Among the rare positive cases (Xu et al., 2020), viral RNA was found to be still present in urine specimens after throat swabs were negative (Xu et al., 2020).
The presence of SARS-CoV-2 in faeces, based on the detection of viral genetic material, does not necessarily imply viability and infectivity (Y. Wu et al. 2020; W. Wang et al. 2020; Guan et al., 2020; Xiao et al., 2020; Gu et al., 2020; Yuen et al., 2020; Amirian and Susan Amirian, 2020). At the moment, only few studies have been able to indicate the conditions of viability, infectivity or active replication of SARS-CoV-2 in stool:
-
-
live SARS-CoV-2 was detected in the stool samples of 2 patients who did not have diarrhoea (W. Wang et al. 2020);
-
-
the cultivation of SARS-CoV-2 was observed from a single stool specimen, as reported by China CDC (Chinese Center for Disease Control and Prevention) (Zhang et al., 2020);
-
-
the release of the infectious virions in the gastrointestinal tract and the continuous positive detection of viral RNA in faeces was recently suggested by Xiao et al. (2020) (in unpublished data). This means that, after the entry of the virus, its specific RNA and proteins are synthesized in the cytoplasm of the infected gastrointestinal cells to form new virions, which are then released in the gastrointestinal tract (Xiao et al., 2020);
-
-
the possibility of an active viral replication in the gastrointestinal tract could be supported by the finding that faeces may remain positive for SARS-CoV-2 RNA for weeks even after the respiratory tract has resulted negative (Y. Wu et al. 2020);
-
-
no, or only minimal, active replication in stool was reported by Wolfel et al. (2020) on the basis of observations of viral subgenomic messenger RNAs (sgRNA)-containing cells. In fact, viral sgRNA is an indication of actively-infected cells since it is only transcribed in infected cells and is not packaged in virions. Furthermore, Wolfel et al. (2020) observed that the swallowing of respiratory secretions could not be the only passive origin of the virus in the intestine, because a much higher presence of SARS-CoV-2 RNA was found in stool compared to MERS-CoV during its outbreak. This suggested again a potential active replication in the gastrointestinal tract. However, the authors did not experimentally find a replicating form of the virus in stool samples despite the high levels of viral RNA (Wolfel et al., 2020).
3. SARS-CoV-2 RNA in faeces poses a series of questions about potential faecal-oral transmission
The detection of SARS-CoV-2 in the gastrointestinal tract raises the question of a potential faecal-oral transmission (W. Wang et al. 2020; Guan et al., 2020; Xiao et al., 2020; Gu et al., 2020; Yuen et al., 2020; Amirian and Susan Amirian, 2020). The route begins with the transport of the virus in the sewerage and treatment plants, or in pit toilets used in low-income countries for human excreta disposal, or spread through the practice of “open defecation” which concerns about 900 million people worldwide (WHO, 2017). Inadequate sanitation may be a source of contamination by viruses in soil and groundwater.
In previous outbreaks, the prolonged presence of SARS-CoV and MERS-CoV in the environment suggested that faecal excretion, environmental contamination and fomites might contribute to the viral shedding (Yeo et al., 2020; Zhou et al., 2017; Goh et al., 2013). Hence, also the SARS-CoV-2 could be transmitted via this kind of route, but at present no faecal-oral transmission cases have been documented according to (WHO, 2020b). A framework of possible SARS-CoV-2 faecal-oral transmission routes is described in Heller et al. (2020), who unpack the different pathways that may transmit viruses from faeces to mouth. The critical points of the potential ramifications of the COVID-19 pandemic on waste and wastewater services were highlighted by Nghiem et al. (2020).
In wastewater treatment plants (WWTPs), the current need is to understand the fate of SARS-CoV-2 considering the removal in the treatment stages and the emissions in: (i) effluent wastewater that may become reclaimed water; (ii) excess sludge that may become biosolids; (iii) other by-products; (iv) microbial aerosol originated by forced aeration, mixing, pumping, etc. In these complex systems, a prerequisite for the virus to cause infection is its ability to retain viability. The current knowledge is that CoVs viability decreases in wastewater - due to not optimal temperature, acidic pH, light exposure, high content of particulate solids and pollutants - and this gives confidence that the viral infectivity may be attenuated from faeces to sewage, then to WWTPs and then in the environment (La Rosa et al., 2020a). However, as of June 2020, given the limited information on SARS-CoV-2 in sewage and WWTPs, it would be premature to draw any conclusion.
Other routes, such as solid waste or aerosol from toilets to the sewerage, can be hypothesized to originate faecal–oral transmission of SARS-CoV-2. From the state of the art, it is unlikely that these matrices will become an important transmission pathway for SARS-CoV-2, but they direct attention to the need for much more research in this field.
4. Methods for identification of SARS-CoV-2 in wastewater
The presence of SARS-CoV-2 in human samples is confirmed by the detection of specific viral RNA sequences that are unique to SARS-CoV-2 by qPCR. The viral genes included the nucleocapsid protein gene N, the envelope protein gene E, the spike protein gene S, and the RNA-dependent RNA polymerase gene RdRP (also reported as Orf1ab) (Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases, n.d.). Internal controls are required to check for biases introduced from the RNA extraction step onward, and they consist of a nontarget RNA sequence, such as a fragment of 412 bases derived from dengue virus type 2 (Medema et al., 2020). Positive controls include, for instance, plasmids containing the complete SARS-CoV-2 nucleocapsid gene (Y. Wu et al. 2020). SARS-CoV-2 RNA copy number, typically expressed as particles/mL or RNA copies/mL, is estimated by cycle threshold values (Ct), which is inversely related to the viral load. These rRT-PCR assays have not only been applied on respiratory tract swabs or samples; they have also been used to detect viral excretion from the gastrointestinal tract (Xu et al., 2020; W. Wang et al. 2020; Y. Wu et al. 2020; Y. Pan et al. 2020; Ma et al., 2020).
Specific pre-treatments steps are normally performed, in particular during wastewater tests, in order to purify and/or concentrate the virus and thus improve the detection efficacy. However, there is still a lack of agreement on a standard protocol. Different viral enrichment approaches used with wastewater samples in recent SARS-Cov-2 investigations and PCR assays used to detect SARS-CoV-2 RNA are included in Table 3 .
Table 3.
Available methods for the concentration of SARS-CoV-2 from wastewater and assays for PCR detection.
| Reference | Concentration steps | PCR assays |
|---|---|---|
| (F. Wu et al. 2020) |
|
|
| (Ahmed et al., 2020) |
|
RT-qPCR assays were applied in accordance with the recent literature (Corman et al., 2020; Shirato et al., 2020) |
| (Medema et al., 2020) |
|
Four primer sets were selected: the N1, N2, N3 sets (US CDC) targetings different regions of the nucleocapsid (N) gene and the set against the envelope protein (E) gene (Corman et al., 2020) |
| (Wurtzer et al., 2020) | Ultracentrifugation at 200,000g for 1 h at 4 °C |
|
| (La Rosa et al., 2020b) | Adaptation of the standard WHO protocol (WHO, 2003) for Poliovirus surveillance for enveloped viruses. Briefly:
|
RNAs were tested for the presence of SARS-CoV-2 using three different PCR assays:
|
| Randazzo et al. (2020). |
|
RT-qPCR diagnostic panel assays validated by the US Centers for Disease Control and Prevention (CDC, 2020). The first version of the kit with three sets of oligonucleotide primers and probes was used to target three different SARS-CoV-2 regions of the nucleocapsid (N) gene |
| Authors of this study | PEG precipitation |
|
CDC recommends that virus isolation, inactivation and cultures have a Biosafety Level 3 (BSL-3) laboratory, whereas routine diagnostic testing can be conducted in a BSL-2 lab (CDC, 2020).
An issue that should be carefully considered to ensure a robust and reliable characterization of the viral content in sewage is the sampling procedure. Both flow rates and pollutant concentrations in the influent wastewater are subject to strong fluctuations during the day and thus composite samples collected over time are recommended: generally, a time composite sample is acceptable (Lytle and Sagripanti, 2005). For example, 24-h composite samples are formed by fixed aliquots collected at defined time intervals during the day and represent the average wastewater characteristics during the day. Where possible, depending on the presence of specific flow measurement devices in the WWTP, 24-h flow proportional sampling is preferable (EPA, 2013) because they ensure even more accurate and reliable results. Samples should be in any case maintained at low temperature during the period of collection, because this helps to preserve the viral load and viability (Wang et al., 2005b).
In the first case of detection of SARS-CoV-2 in sewage, Medema et al. (2020) collected 24-h -flow-dependent composite samples stored at 4 °C during sampling. Then samples were transported to the lab in melting ice and RNA was isolated on the day of sampling by using volumes of 40–150 mL (Medema et al., 2020). In the investigation by Ahmed et al. (2020), on two Australian WWTPs and a pumping station, samples were collected using automated samplers or grab sampling technique: the samples were transported on ice to the laboratory and stored at 4 °C until further analysis.
5. Concentration of SARS-CoV-2 in wastewater
Faeces reach the sewerage system and undergo a large dilution. Raw wastewater contains organic matter, particulate solids, micropollutants and many pathogens, especially enteric. Viruses contained in faeces may undergo several transformations along the sewer network and possibly a reduction of number and viability, as an effect of solid deposition, decreasing pH, temperature and other factors.
Table 4 summarises the rare studies that have quantified the concentration of SARS-CoV-2 (expressed in copies/mL) in municipal wastewater. F. Wu et al. (2020) reported that the first results in a municipal WWTP in Massachusetts, that could have been affected by a number of factors that are unknown at the moment, were approximately 100 viral particles per mL of sewage, with the lowest observed values of ~10 copies/mL. Wurtzer et al. (2020) measured an increase in the viral load during the exponential growth of the epidemic, with values in the range of 50–3·103 copies/mL (calculated by us). Randazzo et al. (2020) estimated an average viral load of 2.5·102 copies/mL (recalculated by us) in untreated wastewater. Conversely, the viral loads measured by Ahmed et al. (2020) were 1.9 and 12 copies/100 mL of untreated wastewater, which is significantly lower than the values found by (Y. Wu et al. 2020).
Table 4.
Type of raw wastewater collected in different studies and concentrations of SARS-CoV-2 in raw wastewater (n.a. = not available).
| Reference | Geographical area | Points of raw wastewater sampling | No. of samples | SARS-CoV-2 load |
|---|---|---|---|---|
| F. Wu et al. (2020) | Massachusetts (USA) | A major WWTP (split into 2 catchment areas: Southern and Northern). Samples were collected in 7 dates for each catchment. |
14 (4 samples before the first known US SARS-CoV-2 case + 5 samples in March 2020) |
|
| Ahmed et al. (2020) | South-East Queensland (Australia) |
|
2 | 1.9 and 12 copies/100 mL of untreated wastewater |
| (Medema et al., 2020) | Netherlands |
|
24 | Detected, load n.a. |
| (Wurtzer et al., 2020) | Paris (France) |
|
23 | Range 50–3·103 eq/mL (calculated by us; eq/mL = equivalent viral genomes per mL) |
| (La Rosa et al., 2020a) | Milan and Rome (Italy). |
|
12 | Detected, load n.a. |
| Randazzo et al. (2020). | Region of Murcia (Spain) |
|
42 | 5.4 ± 0.2 log10 copies/L on average (2.5·102 copies/mL, recalculated) |
The virus copies in wastewater are largely diluted in comparison to the viral load in the faeces. According to Section 2, viral copies in the faeces of persons testing positive for SARS-CoV-2 varied from 5·103 to 107.6 copies/mL (Zhang et al., n.d.; Cheung et al., 2020). The dilution of positive faeces in wastewater causes a drop in the concentration by 4–5 orders of magnitude or more. This dilution is due to various factors: the daily flow rate discharged into the sewerage by a person (that is about 80% of the average daily supply of drinking water per capita and makes an approximate 103 fold dilution), stormwater or infiltration of parasite inflow in the sewer network. Moreover, not the whole population contributes to the viral load and this depends on the percentage of positive cases among the population served by a WWTP. By way of example, the number of cases in Lombardy, one of the Italian regions most affected to date, was 560 cases in 100,000 people on the 10th of April 2020; this produced a further dilution of 5.6·103 fold.
6. Can the SARS-CoV-2 abundance in wastewater be used for COVID-19 surveillance?
When capillary and frequent individual testing in a population is not possible, aggregate information about the level of the outbreak could be useful for monitoring its evolution and the effectiveness of containment measures. Together with other relevant approaches, additional information could be extrapolated from the viral load in municipal wastewater. This is the focus of the developing field of WBE, which in broad terms means “the application and development of using the quantitative measurement of human biomarkers in sewage to evaluate lifestyle, health and exposure at the community level” (https://score-cost.eu/). It has been so far quite extensively used in studies related to drugs or pharma consumption, but also for poliovirus (Nakamura et al., 2015) and infectious disease surveillance and early warning (Sims and Kasprzyk-Hordern, 2020).
Daughton (2020) suggested that WBE could help in quickly determining an increasing or decreasing trend of SARS-CoV-2 spread. Several research groups are directing the effort in monitoring wastewater for SARS-CoV-2 RNA specifically for this purpose. A WBE tool could be helpful also in regards to the second wave of virus infection or in the case of other future viral epidemics. The level of infection and the temporal trends could be determined by comparing (or associating) the viral load in wastewater with the served population (Daughton, 2020).
However, to elucidate if WBE can be successfully applied, coordination of methodologies and data sharing among different scientists are imperative. Standardization of sampling methods (see Section 4) and sharing analytical methods and collected information would provide a solid basis for estimating the consistency of the population served. In addition to flow and conventional macropollutant loads (COD, TSS, TKN, etc.) largely used to calculate the served population, the inclusion of tracers strictly connected to human discharges could improve the estimation accuracy.
The study performed in Massachusetts in March (F. Wu et al. 2020) on the stool of confirmed COVID-19 patients and on the wastewater produced by the municipality, strongly showed a titre of SARS-CoV-2 in wastewater higher than in clinically confirmed cases. Explaining such discrepancy is not easy, since many factors may be involved such as the underestimation of total positives (asymptomatic) and the population excretion rate.
(Lodder and de Roda Husman, 2020) suggested that wastewater could be a sensitive surveillance system and may act as an early warning tool. Indeed, wastewater samples collected in the Netherlands were found positive within a few days after the first cases of COVID-19 (Lodder and de Roda Husman, 2020).
KWR Netherlands researchers have recently investigated the presence of SARS-CoV-2 in wastewater entering some WWTPs that served 2 large and 3 medium sized cities and the main airport (Medema et al., 2020). They obtained positive signals for the selected primers when the observed COVID-19 prevalence was in the order of 1.0 to 3.5 cases among 100,000 people or more, although not always consistently. On this basis they suggested that, even in cases where the COVID-19 prevalence is low, the monitoring of sewage could be sensitive to predict the circulation of the virus in a community (Medema et al., 2020). Sewage surveillance could complement the current clinical surveillance, which is mostly concentrated on patients with COVID-19 symptoms, while infected but asymptomatic individuals are excluded.
A detailed proof-of-concept study of the WBE approach has been described by Ahmed et al. (2020), who tested 9 wastewater samples, collected from two WWTPs and a pumping station for a period of six days. This study quantified the SARS-CoV-2 RNA copies in raw wastewater, with the aim of estimating, via Monte Carlo simulation, the number of infected individuals in the catchment area. The work by Ahmed et al. (2020) draws attention to the uncertainty of some input parameters. In fact, the viral load in the stool of infected persons is not constant as described in Section 2, and it appears to be a critical parameter. The model of Ahmed et al. (2020) estimated a median range of 171–1090 infected persons in the catchment basin of 600,000 inhabitants. Despite this variation in the estimate, the authors said that this result was in reasonable agreement with the clinical observations that reported the median of 450 cases and a 95% upper confidence bound of 764 cases (Ahmed et al., 2020).
Wurtzer et al. (2020) demonstrated that the increase of the average viral load of SARS-CoV-2 in wastewater samples over time accurately followed the increase in the number of fatal cases of COVID-1. Moreover, the authors indicated that the presence of SARS-CoV-2 was detected before the beginning of the exponential growth of the epidemic (Wurtzer et al., 2020). This aspect was also confirmed by Randazzo et al. (2020) who observed experimentally the circulation of SARS-CoV-2 in wastewater even before the first cases were reported by authorities.
Briefly, a simplified theoretical approach of WBE methodology starts from the concentration of SARS-CoV-2 (converted into copies/m3) measured in municipal wastewater taken along the network or at the inlet of a WWTP, but at a point that represents a known urbanized area drained by the sewer system. The daily viral load in wastewater (expressed in copies/d) is then calculated by multiplying the concentration by the daily flow rate of wastewater (expressed in m3/d). This load is then compared with the viral copies in the faeces of persons testing positive for SARS-CoV-2, to obtain an estimation of the number of positive cases in the urbanized area. Unfortunately, analytical data have demonstrated that the viral load in faeces is highly variable. It is so for up to 4 orders of magnitude, from 5·103 to 107.6 copies/mL (see Section 2), and further research is needed to propose reasonable values that can be used as a reference.
Despite these difficulties, wastewater monitoring could be proposed also as a semi-quantitative detection system or at worst for detecting presence/absence in the early surveillance of COVID-19 diffusion (Nghiem et al., 2020).
In synthesis, WBE could be a promising tool for COVID-19 surveillance, but extensive and highly coordinated studies are required, including the quantification of individual virus load in stool and during the disease, because this information is very uncertain at the moment but is fundamental for obtaining accurate estimations.
7. Fate of SARS-CoV-2 in wastewater treatment plants
Wastewater originating from domestic, commercial and industrial sites flows along the sewerage network with a hydraulic retention time of a few hours and reaches the WWTP. When viruses have survivability (T90, i.e. the time required for the virus titre to decrease by 90%) of hours or days - with T90 that becomes longer at low temperatures - the still infective viruses excreted in faeces can reach the WWTP (Ye et al., 2016).
At present, no comprehensive studies on the fate of SARS-CoV-2 along the entire chain of a WWTP including digested sludge or biosolids are available. Two recent studies reported the investigation of SARS-CoV-2 in treated wastewater (Wurtzer et al., 2020; Randazzo et al., 2020); main findings are summarised in Table 5 . In particular, in the study of (Wurtzer et al., 2020), 6 out of 8 samples of treated wastewater were positive for SARS-CoV-2; the viral load was 1–102 copies/mL and was reduced by 100 times compared to the raw wastewater entering the plant (Wurtzer et al., 2020). In this study, some results were near the quantification limit set at 103 equivalent viral genomes per litre. Randazzo et al. (2020) found positivity only in secondary treated wastewater, with a viral load of 5.40 log10 copies/L, while tertiary treated effluents were all negative. The tertiary treatment in the WWTPs investigated by Randazzo et al. (2020) was based on coagulation/flocculation and/or sand filtration, while all plants implemented disinfection with UV or NaClO. The tertiary treated effluent was directed to reuse for public domain or irrigation.
Table 5.
Studies that report quantification of SARS-CoV-2 in treated wastewater (n.a. = not available).
| Reference | Geographical area of WWTPs | Configuration of WWTPs | Type and No. of samples | SARS-CoV-2 load |
|---|---|---|---|---|
| (Wurtzer et al., 2020) | 3 WWTPs of the Parisian area (France) | n.a. | 8 samples of treated wastewater | From the limit of detection (1 eq/mL) to the maximum value of 102 eq/mL (eq/mL = equivalent viral genomes per mL) |
| Randazzo et al. (2020). | 6 WWTPs serving the major municipalities in the region of Murcia (Spain) | Secondary treatment based on activated sludge | 18 samples of secondary effluents | 2 out of 18 samples were positive (1 with 5.40 log10 copies/L and 1 below quantification limit). |
Tertiary treatment based on:
|
12 samples of tertiary effluents | All negative |
These findings indicate that secondary wastewater treatment may contribute to reduce the virus concentration in wastewater, thanks to the adverse environmental conditions that the virus encounters, but removal is largely variable and thus, to enhance the level of virus inactivation in WWTPs, for example for reuse, disinfection has an important role.
Current knowledge about SARS-CoV-2 in WWTPs is largely based on what is known from the similar CoVs (Nghiem et al., 2020), which are severely affected by several environmental factors (i.e. temperature, solids, pH) or disinfectants. There is evidence that CoVs are less stable in the environment than enteric viruses - such as adenoviruses, norovirus, rotavirus and hepatitis A - for which a wide literature exists in WWTPs (Simmons and Xagoraraki, 2011; Ye et al., 2016; Gundy et al., 2009). In wastewater, T90 ranges from days to months for nonenveloped viruses, whereas it is several hours or days for enveloped viruses (Ye et al., 2018). Viruses of avian influenza, SARS, MERS, Ebola and SARS-CoV-2 are enveloped (Bibby et al., 2017). At the moment, the mechanisms explaining the higher susceptibility of enveloped viruses to be inactivated in aqueous environments are mostly unknown in the literature (Ye et al., 2018). Some environmental factors that may affect the stability of SARS-CoV-2 in water are summarised in Table 6 .
Table 6.
Factors of influence and treatments that contribute to a significant reduction of SARS-CoV-2 or CoVs in WWTPs.
| Factor of influence | Experimental observations about SARS-CoV-2 or derived from other CoVs | Principles, mechanisms and laws | |
|---|---|---|---|
| Environmental factors affecting stability | Stability in water is affected by temperature |
|
|
| Stability in water is affected by contaminants and solids |
|
|
|
| Stability in aerosol |
|
|
|
| Stages of treatment in WWTPS | Treatment by membrane biological reactors |
|
|
| Treatment by disinfection with free chlorine or chlorine dioxide |
|
|
|
| Treatment by UV disinfection (UVC light) |
|
|
|
| Treatment by primary and secondary settling |
|
|
|
| Treatment by mesophilic and thermophilic anaerobic digestion |
|
|
Disinfection treatments implemented in WWTPs are based on hypochlorous acid (free chlorine), chlorine dioxide, ozone and peracetic acid or UV radiation (Naddeo and Liu, 2020). Due to the phylogenetic similarities between SARS-CoV-1 and SARS-CoV-2, disinfection technologies adopted during the SARS epidemic can be implemented also for the inactivation of SARS-CoV-2 in wastewater (J. Wang et al. 2020). The enveloped viruses, having a fragile outer membrane, are more susceptible to oxidant disinfectants, such as chlorine, than nonenveloped human enteric viruses (WHO, 2020c). Among chemical disinfectants, free chlorine proves more effective in inactivating SARS CoV than chlorine dioxide (Wang et al., 2005b), but a continuous determination of the residual chlorine content should be implemented on the effluent, to adjust the disinfectant dosage accordingly. In fact, a free chlorine residual is important to ensure the removal of the virus, but excessive disinfectant applications may cause potential adverse environmental effects, for example on ecosystems or in agriculture (Bruins and Dyer, 1995).
A reduction in the quantity of disinfectants and by-products can be obtained implementing Membrane-Bioreactors equipped with ultrafiltration (UF). The absolute pore size (defined according to Simmons and Xagoraraki, 2011) of UF is from 0.01 μm, permitting to separate SARS-CoV-2 virions with size of 60–140 nm (Table 6).
Water contaminated with sewage discharged from combined sewer overflows (CSO) during events of heavy rainfall, is another issue that poses potential risks, including the definition of specific exposure scenarios (Bibby et al., 2017). During CSO, the flow rate that is above a threshold is discharged directly into the receiving water bodies in order to reduce the impact on public health, since the mix of sewage and rainfall may contain pathogenic microorganisms and other pollutants.
8. Conclusions and research needs
The recent global outbreak of SARS-CoV-2 has highlighted scant knowledge about CoVs in the field of sewage and WWTPs. This review has collected the scientific literature currently available on the route of SARS-CoV-2 from faeces to WWTPs, although the research available in this field is very limited, fragmented or still in the early stage of development.
A percentage from 15 to 83% of patients infected with SARS-CoV-2 have detectable viral RNA in faeces, even in the absence of gastrointestinal symptoms or diarrhoea. Patients may continue to remain positive in the stool, even when respiratory tract samples become negative. Conversely, urine is often negative. The load of SARS-CoV-2 in faeces is highly variable, in the range 5·103–107.6 RNA copies/mL, depending on various factors (i.e. time from onset). This viral load decreases remarkably when faeces are diluted in municipal wastewater, where the concentration of SARS-CoV-2 drops to a range from 2 copies/100 mL to 3·103 copies/mL, depending on the level of the epidemic. Quantitative data on viral load in faeces and wastewater and their relationship, currently uncertain, are fundamental for WBE applications to be used for the early warning of outbreaks. In particular, the large uncertainty in the viral load in faeces makes it difficult to determine a typical value that could be extremely useful in WBE.
The fate of SARS-CoV-2 in WWTPs, because of the actual scarcity of analytical data, is predicted on the basis of similar CoVs that are severely affected by environmental factors (e.g. temperature, solids, pollutants, pH). However, current findings indicate that faecal-oral transmission is not proven. By analogy with the previous studies on SARS and MERS outbreaks, there are reasons to conclude that the viral content may be controlled in WWTPs.
CRediT authorship contribution statement
Paola Foladori: Conceptualization, Methodology, Writing. Francesca Cutrupi: Methodology, Writing. Nicola Segata: Conceptualization, Methodology, Writing. Serena Manara: Methodology, Writing. Federica Pinto: Methodology, Writing. Francesca Malpei: Conceptualization, Methodology, Writing. Laura Bruni: Methodology, Writing. Giuseppina La Rosa: Conceptualization, Methodology, Writing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmed W., Harwood V.J., Gyawali P., Sidhu J.P.S., Toze S. Comparison of concentration methods for quantitative detection of sewage-associated viral markers in environmental waters. Appl. Environ. Microbiol. 2015;81(6):2042–2049. doi: 10.1128/AEM.03851-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Warish, Angel Nicola, Edson Janette, Bibby Kyle, Bivins Aaron, O’Brien Jake W., Choi Phil M., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:1–8. doi: 10.1016/j.scitotenv.2020.138764. 138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirian E. Susan, Susan Amirian E. Potential fecal transmission of SARS-CoV-2: current evidence and implications for public health. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ar Gouilh M., Puechmaille S.J., Diancourt L., Vandenbogaert M., Serra-Cobo J., Lopez Roïg M., et al. SARS-CoV related Betacoronavirus and diverse Alphacoronavirus members found in western old-world. Virology. 2018;517:88–97. doi: 10.1016/j.virol.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri Abdullah, Al-Tawfiq Jaffar A., Al-Rabeeah Abdullah A., Al-Rabiah Fahad A., Al-Hajjar Sami, Al-Barrak Ali, Flemban Hesham, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Yan, Yao Lingsheng, Wei Tao, Tian Fei, Jin Dong-Yan, Chen Lijuan, Wang Meiyun. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environmental Science & Technology. 2013;47(4):1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Viau E., Peccia J. Viral metagenome analysis to guide human pathogen monitoring in environmental samples. Lett. Appl. Microbiol. 2011;52(4):386–392. doi: 10.1111/j.1472-765X.2011.03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby Kyle, Aquino de Carvalho Nathalia, Wigginton Krista. Research needs for wastewater handling in virus outbreak response. Environmental Science & Technology. 2017;51(5):2534–2535. doi: 10.1021/acs.est.6b06492. [DOI] [PubMed] [Google Scholar]
- Brainard J., Pond K., Hunter P.R. Censored regression modeling to predict virus inactivation in wastewaters. Environmental Science & Technology. 2017;51(3):1795–1801. doi: 10.1021/acs.est.6b05190. [DOI] [PubMed] [Google Scholar]
- Bruins G., Dyer J.A. Environmental considerations of disinfectants used in agriculture. Rev. Sci. Tech. Off. Int. Epiz. 1995;14(1):81–94. doi: 10.20506/rst.14.1.826. [DOI] [PubMed] [Google Scholar]
- Casanova L., et al. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2020. Interim laboratory biosafety guidelines for handling and processing specimens associated with coronavirus disease 2019 (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html Available at: [Google Scholar]
- Chan Kwok H., Poon Leo L.L.M., Cheng V.C.C., Guan Yi, Hung I.F.N., Kong James, Yam Loretta Y.C., Seto Wing H., Yuen Kwok Y., Peiris Joseph S. Malik. Detection of SARS coronavirus in patients with suspected SARS. Emerg. Infect. Dis. 2004;10(2):294–299. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Jasper Fuk-Woo, Kok Kin-Hang, Zhu Zheng, Chu Hin, To Kelvin Kai-Wang, Yuan Shuofeng, Yuen Kwok-Yung. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes & Infections. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Yifei, Chen Liangjun, Deng Qiaoling, Zhang Guqin, Wu Kaisong, Ni Lan, Yang Yibin, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Chen Nanshan, Zhou Min, Dong Xuan, Qu Jieming, Gong Fengyun, Han Yang, Qiu Yang, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Yu, Liu Qianyun, Guo Deyin. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Ka Shing, Hung Ivan F.N., Chan Pierre P.Y., Lung K.C., Tso Eugene, Liu Raymond, Ng Y.Y., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A., et al. Stability of SARS-CoV-2 in Different Environmental Conditions. 2020. https://www.medrxiv.org/content/10.1101/2020.03.15.20036673v2.abstract medRxiv. medrxiv.org. Available at. [DOI] [PMC free article] [PubMed]
- Corman Victor M., Albarrak Ali M., Omrani Ali Senosi, Albarrak Mohammed M., Farah Mohamed Elamin, Almasri Malak, Muth Doreen, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2016;62(4):477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman Victor M., Landt Olfert, Kaiser Marco, Molenkamp Richard, Meijer Adam, Chu Daniel Kw, Bleicker Tobias, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton Christian. The international imperative to rapidly and inexpensively monitor community-wide Covid-19 infection status and trends. Sci. Total Environ. 2020;726:1–2. doi: 10.1016/j.scitotenv.2020.138149. 138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Yanqing, He Li, Zhang Qingling, Huang Zhongxi, Che Xiaoyan, Hou Jinlin, Wang Huijun, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.-M., et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomedical and Environmental Sciences: BES. 2003;16(3):246–255. https://www.ncbi.nlm.nih.gov/pubmed/14631830 Available at. [PubMed] [Google Scholar]
- Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16(8) doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena Santiago F., Sanjuán Rafael. Adaptive value of high mutation rates of RNA viruses: separating causes from consequences. J. Virol. 2005;79(18):11555–11558. doi: 10.1128/JVI.79.18.11555-11558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA . 2013. Wastewater Sampling. Number: SESDPROC-306-R3. (28 February 2013) [Google Scholar]
- Goh Gerard Kian-Meng, Dunker A. Keith, Uversky Vladimir. Prediction of intrinsic disorder in MERS-CoV/HCoV-EMC supports a high oral-fecal transmission. PLoS Currents. 2013;5(November) doi: 10.1371/currents.outbreaks.22254b58675cdebc256dbe3c5aa6498b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Jinyang, Han Bing, Wang Jian. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Wei-Jie, Ni Zheng-Yi, Hu Yu, Liang Wen-Hua, Ou Chun-Quan, He Jian-Xing, Liu Lei, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy Patricia M., Gerba Charles P., Pepper Ian L. Survival of coronaviruses in water and wastewater. Food and Environmental Virology. 2008;1(1):10. [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10–14. [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller Léo, Mota César R., Greco Dirceu B. COVID-19 faecal-oral transmission: Are we asking the right questions? Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020 doi: 10.1016/j.watres.2020.115899. Published online 28 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Medrxiv. 2020 doi: 10.1016/j.scitotenv.2020.139652. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases.” n.d. Accessed April 14, 2020. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117.
- Lai Chih-Cheng, Liu Yen Hung, Wang Cheng-Yi, Wang Ya-Hui, Hsueh Shun-Chung, Yen Muh-Yen, Ko Wen-Chien, Hsueh Po-Ren. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi. 2020 doi: 10.1016/j.jmii.2020.02.012. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure Francois-Xavier, Bouadma Lila, Nguyen Duc, Parisey Marion, Wicky Paul-Henri, Behillil Sylvie, Gaymard Alexandre, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30200-0. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Lu, Jiang Xiayang, Zhang Zhenling, Huang Siwen, Zhang Zhenyi, Fang Zhaoxiong, Gu Zhiqiang, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020 doi: 10.1136/gutjnl-2020-321013. April. [DOI] [PubMed] [Google Scholar]
- Liu Ying-Chu, Liao Ching-Hui, Chang Chin-Fu, Chou Chu-Chung, Lin Yan-Ren. A locally transmitted case of SARS-CoV-2 infection in Taiwan. N. Engl. J. Med. 2020;382(11):1070–1072. doi: 10.1056/NEJMc2001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Iek Long, Lio Chon Fu, Cheong Hou Hon, Lei Chin Ion, Cheong Tak Hong, Zhong Xu, Tian Yakun, Sin Nin Ngan. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16(10):1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder Willemijn, de Roda Husman Ana Maria. SARS-CoV-2 in wastewater: potential health risk, but also data source. The Lancet. Gastroenterology & Hepatology. 2020 doi: 10.1016/S2468-1253(20)30087-X. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Roujian, Zhao Xiang, Li Juan, Niu Peihua, Yang Bo, Wu Honglong, Wang Wenling, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle C. David, Sagripanti Jose-Luis. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J. Virol. 2005;79(22):14244–14252. doi: 10.1128/JVI.79.22.14244-14252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Xiang, Su Liang, Zhang Yunkui, Zhang Xiuzhen, Gai Zhongtao, Zhang Zhongfa. Do children need a longer time to shed SARS-CoV-2 in stool than adults? Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi. 2020 doi: 10.1016/j.jmii.2020.03.010. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay Ian M., Arden Katherine E. MERS coronavirus: diagnostics, epidemiology and transmission. Virol. J. 2015;12(December):222. doi: 10.1186/s12985-015-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at Amoy Gardens. J. Environ. Health. 2006;68(9):26–30. (quiz 51-2) [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 in sewage. medRIX. 2020;10:29–20045880. doi: 10.1021/acs.estlett.0c00357. DOI. 2020.03. [DOI] [PubMed] [Google Scholar]
- Minodier Laetitia, Charrel Remi N., Ceccaldi Pierre-Emmanuel, Werf Sylvie van der, Blanchon Thierry, Hanslik Thomas, Falchi Alessandra. Prevalence of gastrointestinal symptoms in patients with influenza, clinical significance, and pathophysiology of human influenza viruses in faecal samples: what do we know? Virol. J. 2015;12(1):215. doi: 10.1186/s12985-015-0448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddeo V., Liu H. Editorial perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci.: Water Res. Technol. 2020;2020 doi: 10.1039/D0EW90015J. Editorial. [DOI] [Google Scholar]
- Nakamura Tomofumi, Hamasaki Mitsuhiro, Yoshitomi Hideaki, Ishibashi Tetsuya, Yoshiyama Chiharu, Maeda Eriko, Sera Nobuyuki, Yoshida Hiromu. Environmental surveillance of poliovirus in sewage water around the introduction period for inactivated polio vaccine in Japan. Appl. Environ. Microbiol. 2015;81(5):1859–1864. doi: 10.1128/AEM.03575-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nao N., Shirato K., Katano H., Matsuyama S., Takeda M. National Institute of Infectious Diseases; Japan: 2020. Detection of second case of 2019-nCoV infection in Japan. Published January 25, 2020. [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. Case Studies in Chemical and Environmental Engineering, Volume 1. 2020. The COVID-19 pandemic: considerations for the waste and wastewater services sector. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Xingfei, Chen Dexiong, Xia Yong, Wu Xinwei, Li Tangsheng, Ou Xueting, Zhou Liyang, Liu Jing. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect. Dis. 2020;20(4):410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Lei, Mu Mi, Yang Pengcheng, Sun Yu, Wang Runsheng, Yan Junhong, Li Pibao, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China. Am. J. Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Yang, Zhang Daitao, Yang Peng, Poon Leo L.M., Wang Quanyi. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Jernigan D.B., 2019-nCoV CDC Response Team Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak - United States, December 31, 2019–February 4, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(5):140–146. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn Roni, Ward Barbara J., Strande Linda, Maurer Max. Review of synthetic human faeces and faecal sludge for sanitation and wastewater research. Water Res. 2018;132(April):222–240. doi: 10.1016/j.watres.2017.12.063. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA titers in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe Camilla, Schunk Mirjam, Sothmann Peter, Bretzel Gisela, Froeschl Guenter, Wallrauch Claudia, Zimmer Thorbjörn, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato Kazuya, Nao Naganori, Katano Harutaka, Takayama Ikuyo, Saito Shinji, Kato Fumihiro, Katoh Hiroshi, et al. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020 doi: 10.7883/yoken.JJID.2020.061. February. [DOI] [PubMed] [Google Scholar]
- Simmons Fredrick James, Xagoraraki Irene. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res. 2011;45(12):3590–3598. doi: 10.1016/j.watres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Simmons F.J., Kuo D.H.-W., Xagoraraki I. Removal of human enteric viruses by a full-scale membrane bioreactor during municipal wastewater processing. Water Res. 2011;45(9):2739–2750. doi: 10.1016/j.watres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Sims Natalie, Kasprzyk-Hordern Barbara. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020;139(April) doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020:1564–1567. doi: 10.1056/nejmc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Yushun, Shang Jian, Graham Rachel, Baric Ralph S., Li Fang. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J., Guo T., Zhen B., Kong Q., Yi B., Li Z., et al. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital of the Chinese People’s Liberation Army. Water Sci. Technol. 2005 doi: 10.2166/wst.2005.0266. [DOI] [PubMed] [Google Scholar]
- Wang X.-W., et al. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Luwen, Li Xun, Chen Hui, Yan Shaonan, Li Dong, Li Yan, Gong Zuojiong. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am. J. Nephrol. 2020:1–6. doi: 10.1159/000507471. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Wenling, Xu Yanli, Gao Ruqin, Lu Roujian, Han Kai, Wu Guizhen, Tan Wenjie. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., et al. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R.L., Ashley C.S. Mode of initiation of cell infection with sludge-associated poliovirus. Appl. Environ. Microbiol. 1979;38(2):329–331. doi: 10.1128/aem.38.2.329-331.1979. https://www.ncbi.nlm.nih.gov/pubmed/229768 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO issues consensus document on the epidemiology of SARS. Releve Epidemiologique Hebdomadaire/Section D’hygiene Du Secretariat de La Societe Des Nations = Weekly Epidemiological Record/Health Section of the Secretariat of the League of Nations. 2003;78(43):373–375. [PubMed] [Google Scholar]
- WHO . 2017. WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. [Google Scholar]
- WHO . World Health Organization; 2020. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It.https://www.Who.Int/emergencies/diseases/novel-Coronavirus-2019/technical-Guidance/naming-the-Coronavirus-Disease-(covid-2019)-and-the-Virus-That-Causes-It [Google Scholar]
- WHO . World Health Organization; 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations: Scientific Brief, 27 March 2020.https://apps.who.int/iris/bitstream/handle/10665/331601/WHO-2019-nCoV-Sci_Brief-Transmission_modes-2020.1-eng.pdf [Google Scholar]
- WHO Water, sanitation, hygiene and waste management for COVID-19. Technical brief, 19 March 2020. 2020. https://covid19-evidence.paho.org/handle/20.500.12663/843 Available at.
- Wigginton K.R., Boehm A.B. Environmental engineers and scientists have important roles to play in stemming outbreaks and pandemics caused by enveloped viruses. Environmental Science & Technology. 2020;54(7):3736–3739. doi: 10.1021/acs.est.0c01476. [DOI] [PubMed] [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environmental Science: Water Research & Technology. Royal Society of Chemistry. 2015;1(6):735–746. doi: 10.1039/C5EW00125K. [DOI] [Google Scholar]
- Wolfel R., Corman V., Guggemos W. 2020. Virological Assessment of Hospitalized Cases of Coronavirus Disease 2019. (DOI.) [Google Scholar]
- Wu Yongjian, Guo Cheng, Tang Lantian, Hong Zhongsi, Zhou Jianhui, Dong Xin, Yin Huan, et al. Prolonged presence of SARS-CoV-2 viral RNA in Faecal samples. The Lancet. Gastroenterology & Hepatology. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Fuqing, Xiao Amy, Zhang Jianbo, Gu Xiaoqiong, Lee Wei Lin, Kauffman Kathryn, Hanage William, et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv. 2020 doi: 10.1128/mSystems.00614-20. (April, 2020.04.05.20051540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer Sebastien, Marechal Vincent, Mouchel Jean-Marie, Moulin Laurent. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. medRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.04.12.20062679v1.abstract [Google Scholar]
- Xiao Fei, Tang Meiwen, Zheng Xiaobin, Liu Ye, Li Xiaofeng, Shan Hong. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Yi, Li Xufang, Zhu Bing, Liang Huiying, Fang Chunxiao, Gong Yu, Guo Qiaozhi, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Yinyin, Ellenberg Robert M., Graham Katherine E., Wigginton Krista R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environmental Science & Technology. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Ye Y., et al. Reactivity of enveloped virus genome, proteins, and lipids with free chlorine and UV254. Environmental Science & Technology. 2018;52(14):7698–7708. doi: 10.1021/acs.est.8b00824. [DOI] [PubMed] [Google Scholar]
- Yeo Charleen, Kaushal Sanghvi, Yeo Danson. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? The Lancet Gastroenterology & Hepatology. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young Barnaby Edward, Ong Sean Wei Xiang, Kalimuddin Shirin, Low Jenny G., Tan Seow Yen, Loh Jiashen, Ng Oon-Tek, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Fengting, Yan Liting, Wang Nan, Yang Siyuan, Wang Linghang, Tang Yunxia, Gao Guiju, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Ping, Zhu Jiang, Zhang Zhengdong, Han Yingjun, Huang Lihong. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa077. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen Kit-San, Ye Zi-Wei, Fung Sin-Yee, Chan Chi-Ping, Jin Dong-Yan. SARS-CoV-2 and COVID-19: the Most important research questions. Cell & Bioscience. 2020;10(March):40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yong, Chen Cao, Zhu Shuangli, Shu Chang, Wang Dongyan, Song Jingdong, Song Yang, et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2(8):123–124. [PMC free article] [PubMed] [Google Scholar]
- Zhang, Ning, Yuhuan Gong, Fanping Meng, Yuhai Bi, Penghui Yang, and Fusheng Wang. n.d. “Virus Shedding Patterns in Nasopharyngeal and Fecal Specimens of COVID-19 Patients.” doi: 10.1101/2020.03.28.20043059. [DOI] [PMC free article] [PubMed]
- Zhou Jie, Li Cun, Zhao Guangyu, Chu Hin, Wang Dong, Yan Helen Hoi-Ning, Poon Vincent Kwok-Man, et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017;3(11) doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]



